ABSTRACT
Introduction: Influenza-Like Illness is a leading cause of hospitalization in children. Disease burden due to influenza and other respiratory viral infections is reported on a population level, but clinical scores measuring individual changes in disease severity are urgently needed.
Areas covered: We present a composite clinical score allowing individual patient data analyses of disease severity based on systematic literature review and WHO-criteria for uncomplicated and complicated disease. The 22-item ViVI Disease Severity Score showed a normal distribution in a pediatric cohort of 6073 children aged 0–18 years (mean age 3.13; S.D. 3.89; range: 0 to 18.79).
Expert commentary: The ViVI Score was correlated with risk of antibiotic use as well as need for hospitalization and intensive care. The ViVI Score was used to track children with influenza, respiratory syncytial virus, human metapneumovirus, human rhinovirus, and adenovirus infections and is fully compliant with regulatory data standards. The ViVI Disease Severity Score mobile application allows physicians to measure disease severity at the point-of care thereby taking clinical trials to the next level.
KEYWORDS: Disease severity, influenza-like illness, influenza, respiratory syncytial virus, human metapneumovirus, human rhinovirus, adenovirus, seasonality, antivirals, clinical trials
1. Introduction
Influenza-like illness (ILI) and acute respiratory infections (ARI) in children are common. The clinical presentation may range from subtle to severe symptoms requiring advanced medical care [1,2]. The wide spectrum of disease presentations and the role of risk factors (RFs) in terms of disease severity are poorly understood. Laboratory diagnostics are not usually ordered in routine care [3–6].
Surveillance programs should rely on laboratory-confirmed cases rather than clinical suspicion to solve the denominator problem. This will allow the timely detection of virus-specific seasonality in a given (sub)population [7].
An even greater challenge will present itself when investigators wish to determine the impact of different respiratory viruses on disease burden [8]. A deeper understanding of disease severity in relation to specific respiratory viruses will help in the monitoring of the real-world impact of ‘natural’ or untreated disease as well as preventive measures and therapeutic interventions such as vaccines and antivirals. The timely detection of seasonality will help with the targeted and cost-effective use of viral diagnostics in hospital-based surveillance settings. Ideally, viral diagnostics should be aligned with simultaneous standardized disease severity assessments.
Standardized measures of disease severity are urgently needed for clinical trials of vaccines and antivirals currently in development for ARI caused by influenza (FLU), respiratory syncytial virus (RSV), human metapneumovirus (HMPV), adenovirus (ADV), or human rhinovirus (HRV) [9–17]. Furthermore, it would be desirable to assess, at the point of care, which patients are suffering from severe disease in relation to their perceived RF profile, and to use such point-of-care assessments to individualize the use of anti-infective therapy. Experience during the recent influenza pandemic has shown that influenza disease severity appears rather unpredictable, especially in young patients. Whilst the majority of adults with severe disease did have previously identifiable RFs, the majority of children affected by severe disease did not [18,19]. The expected or perceived risk of severe outcomes may also influence a physician’s decision to test a patient for influenza and other respiratory viruses [20]. There is little consensus on which symptoms should trigger a physician’s suspicion, and local practices differ significantly from site to site and from season to season [19,21–24].
Comprehensive reviews of the published literature and disease severity measurements used in clinical trials and surveillance systems are lacking. The numerous observational studies and clinical trials assessing the prevention and treatment influenza and other respiratory viruses have been rather inconsistent. Commonly used indicators of disease severity such as ‘hospitalization,’ a diagnosis of ‘pneumonia,’ and other adverse outcomes including mortality are known to be highly dependent on the studied population, the medical setting, the choice of data sources, and the availability of resources [25]. Head-to-head comparisons and meta-analyses comparing different preventive and treatment interventions will require universally accepted disease severity measurements.
Sentinel surveillance systems tend to focus on private practices and laboratory testing based on clinical suspicion on behalf of primary care providers working at surveillance sites [26]. With children being the most prominent transmitters of influenza, pediatric emergency rooms and large tertiary care hospitals are ideal sites to monitor seasonality covering the entire spectrum of clinical presentations [27–29]. To create a model system free of selection bias, a perennial quality management (QM) program was instituted at a large pediatric academic center in collaboration with the National Reference Centre for Influenza and Other Respiratory Viruses [30–34].
The specific aims of the presented analyses are
to develop a standardized approach to measuring ILI disease severity based on literature review and WHO guidelines and
to apply new mathematical models to the real-time surveillance of ILI in large tertiary care centers.
2. Methods
2.1. Literature review
To understand which disease severity parameters have been used in clinical trials and observational studies, a systematic literature search of the PubMed database was performed using the following search terms: ‘(disease severity[Title/Abstract] OR illness severity[Title/Abstract]) AND (influenza[Title/Abstract] OR rhinoviruses[Title/Abstract] OR adenovirus[Title/Abstract] OR human metapneumovirus[Title/Abstract] OR Respiratory Syncytial Virus[Title/Abstract] OR Coronavirus[Title/Abstract] OR bocavirus[Title/Abstract] OR parainfluenza virus[Title/Abstract] OR respiratory virus[Title/Abstract]).’ For the purposes of this expert review, the literature review was updated covering publications dating from 1 January 2006 to 8 June 2016. Searches were limited to human studies published in English. Abstracts were screened manually and excluded according to the following criteria: (1) studies were not pediatric or study subjects were, in the majority, >18 years old; (2) studies were not one of the following: randomized clinical trials, non-randomized clinical trials, observational studies, or epidemiological studies; and (3) studies lacked any clinical criterion for disease severity. Animal studies, adult studies, meta-analysis, and review papers were also excluded.
2.2. The ViVI Disease Severity Score
Based on the systematic literature review, the ViVI Disease Severity Score was developed as a 22-item weighed clinical composite score, according to WHO-criteria of uncomplicated and complicated disease [35]. The ViVI Disease Severity Score is comprised of 9 items describing signs and symptoms of uncomplicated disease (Disease Severity, Uncomplicated: DSU, weighed single-fold) reflecting ‘regular’ ILI activity, whereas the 13 items describing parameters consistent with complicated disease (Disease Severity, Complicated: DSC, weighed threefold) indicate high-impact clinical presentations in the target population (Textbox 1). The ViVI Disease Severity Score was subsequently user tested as a web–user interface as well as a mobile application for tablet computers, to be used at the point of care.
Textbox 1.
The ViVI Disease Severity Score.
|
The ViVI Disease Severity Score (ViVI Score) = Disease Severity with Signs and Symptoms of Uncomplicated disease (DSU; weighed 1×) PLUS Disease Severity with Signs and Symptoms of Complicated disease (DSC; weighed 3×) | |
|---|---|
| SU 1–9: | |
| DSU 1: | Fever |
| - Evidence of fever (defined as any measurement in current disease episode ≥38°C) | |
| DSU 2: | Cough |
| - Evidence of cough | |
| DSU 3: | Pharyngitis |
| - Evidence of sore throat or inflamed throat on exam | |
| DSU 4: | Rhinitis |
| - Evidence of coryza/rhinitis on exam | |
| DSU 5: | Headache |
| - Evidence of headache or pain in head/neck area on exam (using age-appropriate techniques) | |
| DSU 6: | Myalgia |
| - Evidence of muscle pain on exam (incl. age appropriate techniques in infants and young children) | |
| DSU 7: | Malaise |
| - Level of reduction in general well-being ≥5 on a scale from 0 to 10 | |
| DSU 8: | Diarrhea |
| - Evidence of diarrhea ≥3 bowel movements (or ≥3 more/day or baseline) | |
| DSU 9: | Vomiting |
| - Evidence of vomiting (at least once) | |
| DSC 1–13: | |
| DSC 1: | High and prolonged fever |
| - Body temperature >40°C for 3 days or more | |
| DSC 2: | Dyspnea |
| One or more of the following: | |
| - Evidence of shortness of breath (dyspnea, labored breathing, resp. distress) | |
| - Evidence of difficulty breathing | |
| - Evidence of tachypnea (using age-appropriate standards) | |
| - Need for mechanical ventilation or ECMO | |
| DSC 3: | Hypoxia |
| One or more of the following: | |
| - Evidence of cyanosis (including turning blue during seizures) | |
| - Evidence of hypoxia (O2 sat <93%) | |
| - Evidence of O2 requirement (incl. blow-by oxygen) | |
| - Evidence of respiratory failure and/or need for medical ventilation or ECMO | |
| DSC 4: | Hemoptysis |
| - Evidence of bloody/colored sputum | |
| DSC 5: | Altered/ loss of consciousness |
| One or more of the following: | |
| - Evidence of CNS involvement (e.g. encephalopathy, encephalitis) | |
| - Evidence of altered mental status | |
| - Evidence of GCS (Glasgow Coma Scale) or IFS (Infant Face Scale) <15 and/or marked personality change | |
| - Evidence of unconsciousness (other than postictal) or/and | |
| - Evidence of drowsiness or difficult to arouse (including lethargy and/or markedly decreased levels of activity) | |
| - Evidence of dizziness | |
| - Evidence of confusion | |
| - Evidence of severe weakness (including floppiness in infants) | |
| - Evidence of paralysis | |
| DSC 6: | Seizure |
| - Evidence of seizures | |
| DSC 7: | Dehydration |
| One or more of the following: | |
| - Evidence of severe dehydration (documented dehydration, need for IV-therapy or Base Excess <−7 on BGA) | |
| - Evidence of decreased urine output and/or need for hemofiltration/dialysis | |
| DSC 8: | Exacerbation of chronic disease |
| - Exacerbation of chronic disease (incl. asthma, chronic hepatic cardiovascular or renal disease, diabetes or metabolic disease) | |
| DSC 9: | Septic shock or multi-organ failure |
| One or more of the following: | |
| - Evidence of septic shock | |
| - Evidence of secondary complications (renal/multi-organ failure, rhabdomyolysis, myocarditis) | |
| - Evidence of hypotension and/or need for vasopressor support | |
| DSC 10: | Need for hospitalization |
| - Assessor’s judgment that the patient should be admitted to an inpatient ward (regardless of cost, availability of hospital beds, and other outside factors) | |
| DSC 11: | Lower respiratory tract infection/superinfection |
| One or more of the following: | |
| - Evidence of lower respiratory tract disease (pneumonia, bronchitis, pulmonary rales, wheezing/obstruction, need mechanical ventilation/ECMO incl. clinical, radiological) | |
| - Evidence of bacterial superinfection in the lower respiratory tract (clinical, laboratory, radiological) | |
| DSC 12: | Upper respiratory tract infection/superinfection |
| One or more of the following: | |
| - Evidence of upper respiratory tract disease (cough, coryza, red/sore throat, ear ache) | |
| - Evidence of upper RT bacterial superinfection (incl. laboratory, radiological, or clinical findings, such as purulent drainage, bulging tympanic membrane, positive StrepA rapid test or microbiology result) | |
| DSC 13: | Need for ICU admission |
| One or more of the following: | |
| - Assessor’s judgment that patient would benefit from admission to the ICU (including intermediate care) | |
| - Assessor’s judgment that patient would benefit from assisted respiration (incl. BiPAP, CPAP) | |
| - Assessor’s judgment that patient would benefit from mechanical ventilation or ECMO | |
2.3. The ViVI Risk Factor Score
Based on the 16 most commonly cited RFs for severe disease in the pediatric or adolescent age group, a simple RF score was composed [35–38]. The ViVI Risk Factor Score (Textbox 2) was implemented on the same mobile application to allow the reporting of disease severity in relation to previously identifiable RFs in the individual patient.
Textbox 2.
The ViVI Risk Factor Score.
| The ViVI Risk Factor (RF) Score | |
|---|---|
| RF 1: | Infant <2 years of age |
| RF 2: | Pulmonary condition |
| RF 3: | Cardiac condition |
| RF 4: | Diabetes |
| RF 6: | Obesity |
| RF 7: | Other metabolic condition |
| RF 8: | Chronic renal disease |
| RF 9: | Chronic hepatic disease |
| RF 10: | Chronic neurological conditions |
| RF 11: | Hemoglobinopathies |
| RF 12: | Congenital immunosuppression |
| RF 13: | Acquired immunosuppression |
| RF 14: | Aspirin therapy |
| RF 15: | Pregnancy |
| RF 16: | Prematurity <33 weeks gestational age |
2.4. The consultation index
The Consultation Index is an epidemiological indicator reported weekly by the National Reference Centre for Influenza and Other Respiratory Viruses and the Influenza Working Group, based on the proportion of ARI at representative sentinel practices across the country [39].
The Consultation Index represents a timely indicator of any deviation from a baseline rate of ARI cases presenting to the respective sentinel practices. A ‘normal ARI activity’ is assumed if the Consultation Index remains below 115. Increased activities are typically measured during the winter months, when seasonal viruses circulate in the community.
Fluctuations in ARI activity as measured by the Consultation Index represent a useful indicator of disease burden based on actual case numbers. Reporting of the number of cases, however, does not reveal information on disease severity with each individual case. By plotting the Consultation Index with the corresponding average ViVI Disease Severity Score in the same graph, we obtain a comprehensive picture of ARI disease burden that is based on both actual case numbers and case severity. Figure 4(a) illustrates that disease severity does not always follow the peaks and troughs of case numbers as measured using the Consultation Index [39]. The ViVI Score and the Consultation Index are therefore measuring opposing end points; one is based on individual disease severity per patient (ViVI Disease Severity Score) and the other serves as an epidemiological indicator of ARI activity and the overall disease burden within the national surveillance system (Consultation Index).
Figure 4.
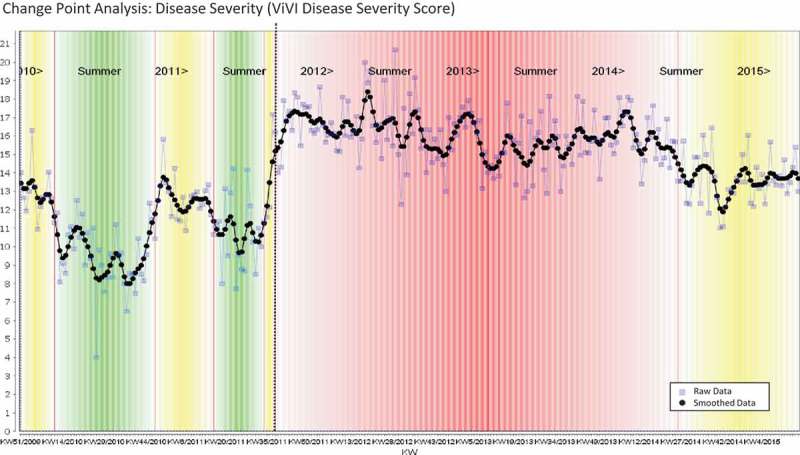
Average weekly Disease Severity in the ED (ViVI Disease Severity Score, black line). Change Point Analysis: Disease Severity (ViVI Disease Severity Score).
2.5. Cohort design and patient population
The ViVI Disease Severity and Risk Factor Score were user tested in the context of a QM program for children with ILI at a large pediatric hospital in Germany as described previously [30–34]. According to the standard operating procedures, patients with a physician diagnosis of ILI and/or fulfilling predefined case criteria (body temperature ≥38°C and ≥1 respiratory symptom) admitted to the emergency department (ED) or pediatric inpatient wards, participated in the QM program [30–34]. Independent of routine clinical care, a specifically trained QM team obtained nasopharyngeal samples and performed standardized clinical assessments using the ViVI Disease Severity Score in line with WHO criteria for uncomplicated and complicated influenza [30,35,40].
The ViVI Disease Severity Score was recorded at the first consultation with patients participating in the QM Program. Physicians in routine care were blinded to the results of the clinical assessments by QM staff, and they were unaware of the ViVI Disease Severity Scores assigned by the QM team. QM staff on the other hand assessed patients prior to allocation and treatment decisions on behalf of the clinical team in routine care [30]. Nasopharyngeal specimens were delivered to the National Reference Centre for Influenza and Other Respiratory Viruses for individual RT-PCR testing influenza virus A and B, RSV, HMPV, HRV, and ADV as described below.
From December 2009 until April 2015, a total of 6073 children aged 0–18 years participated in the QM program. The QM program included both in- and outpatients to represent the broadest possible spectrum of disease severity. From 2009 to 2015, all patients presenting the ED were screened for ILI criteria once weekly, regardless of whether they were subsequently admitted to the hospital or not. From 2011 onward, daily screenings of all inpatients were added (including weekends and holidays). The QM team performed the disease severity assessments independently and the results remained unknown to the routine staff. Hence, the data acquired by the QM team did not have any influence on treatment or hospitalization decisions. Also, the treating physician did not know the result of the RT-PCR testing when deciding on neuraminidase inhibitor treatment.
Patients with laboratory-confirmed influenza infection were invited to participate in follow-up assessments whenever feasible. Follow-up visits in the QM program were voluntary and scheduled according to the parent’s preferences. During follow-up visits, the ViVI Disease Severity Score assessment was repeated and recorded by the QM team using the same procedure as during the initial assessment. Nasopharyngeal samples were repeated and sent for analogous RT-PCR testing [32]. The QM program was approved by the Institutional Review Board (EA 24/008/10). Informed consent procedures were waived for enhanced quality of care and infection control [30–34,40].
2.6. Laboratory methods
Nasopharyngeal swabs were washed out in a total volume of 3 ml of cell culture medium either individually or pooled per patient. RNA was extracted from 300 µl of patient specimen using the MagAttract Viral RNA M48 Kit (Qiagen, Hilden, Germany) and eluted in 80 µl elution buffer. Alternatively, RNA was extracted using the MagNA Pure 96 DNA and Viral NA Small Volume Kit (Roche Deutschland Holding GmbH, Mannheim, Germany) from 200 µl specimen with an elution volume of 50 µl. A volume of 25 µl of extracted RNA was subjected to cDNA synthesis applying 200 U M-MLV Reverse Transcriptase (Invitrogen, Karlsruhe, Germany) in a total volume of 40 µl. All cDNA samples were analyzed by RT-PCR for the presence of each of the pathogens influenza virus A and B, RSV, HMPV, HRV, and ADV as published previously [41–45].
2.7. Statistical analysis
A descriptive analysis of the study sample was performed by calculating proportions and summarizing continuous variables using mean (standard deviation and range) and median (interquartile range). Histograms and box plots were used to illustrate the distribution of ViVI Disease Severity Scores. Correlations between the ViVI Disease Severity Score and the Consultation Index were assessed using scatter plots and Pearson’s correlation coefficient. The mean difference in ViVI Disease Severity Scores was compared across patient and clinical characteristics. Statistical significance was assessed using the t-test or the chi-squared test as appropriate. To test whether patients with elevated ViVI Disease Severity Scores also had elevated RF scores, we performed correlation analysis using Pearson’s correlation coefficient. These analyses were conducted using Stata version 14 (Statacorp LP, Texas, USA).
We further performed regression analyses to identify a set of influential RFs that could model a linear correlation: ViVI Disease Severity Score = w1 × RF 1, w2 × RF 2, …, wn × RF n. Here, wi is the respective weight factor for feature i in the regression model [46].
In a subset of patients with laboratory-confirmed influenza infection during the 2011/12 and 2012/13 winter seasons, the ViVI Disease Severity Score was also used to follow patients longitudinally with respect to viral load and disease severity over time [32]. To assess the relationship between ViVI Disease Severity Score and virus load, we performed Pearson correlation analyses for all records, for which more than two follow-up time point with virology and ViVI Disease Severity Scores was available. Decision tree analysis [47] was performed to study the relationship between subgroups with a strong positive and negative correlation between disease severity and virus load.
2.8. Time series analysis with change point detection
As an objective and data-driven measure to detect seasonality of respiratory viral infections in acute care settings, we introduced time series analysis with change point (CP) detection. The goal of CP detection algorithms is to identify changes in the dynamical behavior within a time series [48]. The main difference to a statistically oriented analysis is that it assumes that an intrinsic dynamics model generates the data. CP detection therefore identifies those time points, when time series trends start differing significantly from previous data. This procedure allows identification of critical time points when weekly average numbers of laboratory-confirmed influenza infections start to increase (or decrease) compared to preceding weeks. For further detail on CP detection, please refer to the Supplemental Data.
In this paper, we used the CP detection approach to analyze the QM dataset, which allowed computing averages of target variables assigned to respective calendar weeks (such as average rates of laboratory-confirmed influenza infections per calendar week, average disease severity per calendar week, etc.).
For the detection of seasonal patterns, we used the following three-step algorithm. (1) The data were clustered using k-means clustering [49] into potential seasons. We used k = 3 to model two main seasons (high and low) and a transition between those seasons. (2) We assigned a preliminary CP to a week wt, if the cluster assignment c(wt) to the respective week differed from the cluster assignment to the preceding week, i.e. if c(wt) ≠ c(wt−1). (3) Finally, we computed a list of preliminary CPs that would split the dataset into time frames tf1…tfn, where each time frame ti was defined to lie between two consecutive CPs. We then checked for each preliminary CP, whether the values before and after the CP (for the two time frames tfi−1…tfi) differed significantly (p < 0.05) based on a t-test. This procedure ensures that two regions separated by a CP are indeed different. All preliminary CPs fulfilling the above criteria were reported.
3. Results
3.1. Literature review
The systematic literature search yielded 613 potentially relevant articles. Among these, 529 articles were excluded based on the criteria mentioned above. An additional 56 studies lacked specific criteria for disease severity. Finally, a total number of 84 eligible articles were identified, the characteristics of which are summarized in Textbox 3.
Textbox 3.
Systematic review of the literature.
| Reference | Country | Age | Name of the score | Viruses | Clinical parameters of disease severity |
Number of patientsa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospitalization | (P)ICU admission | Oxygen requirement | Mechanical ventilation/intubation/respiratory failure | Death/Mortality | Respiratory rate/tachypnea | Dyspnea/labored breathing (incl. retractions) | Feeding problems/vomiting/dehydration | Fever | Wheezing/Abnormal breath sounds | Others | ||||||
| Tief et al. [30] | Germany | Children (<18 years) | ViVI Disease Severity Score | Respiratory viruses | Yes | Yes | Yes | Yes | – | Yes | Yes | Yes | Yesb | Yes | 22 Clinical parameters based on WHO criteria for uncomplicated and complicated disease | 3106 |
| Sung et al. [50] | Taiwan | Children (<3 years) | Bronchiolitis Clinical Score System | Respiratory viruses | Yes | – | – | – | – | Yes | Yes | – | – | Yes | Length of hospitalization, respiratory support, nasal flaring | 48 |
| Reed et al. [51] | South Africa | Children (<2 years) | RISC | Respiratory viruses | – | – | Yes | – | – | – | Yes | Yes | – | Yes | Weight, age, and HIV clinical classification | 4148 |
| Valet et al. [52] | Latino | Infants | Tal Score | Respiratory viruses | – | Yes | – | – | – | Yes | – | – | – | Yes | Flaring | 674 |
| Pedraza-Bernal et al. [53] | Colombia | Children (<5 years) | – | Respiratory viruses | Yes | Yes | Yes* | Yes | Yes | – | – | – | – | – | Length of hospitalization, use of drugs (antibiotics) | 1180 |
| Skjerven et al. [54] | Norway | Infants | – | Respiratory viruses | Yes | Yes | Yes | – | – | – | – | – | – | – | Length of hospitalization, nasogastric tube feeding | 363 |
| Moesker et al. [55] | Netherlands | Children (≤18 years) | – | Respiratory viruses | Yes | Yes | – | – | – | – | – | – | – | – | Length of hospitalization, length of PICU stay, noninvasive or invasive respiratory support, sever acute respiratory tract infections | 84 |
| Dong et al. [56] | China | Children | – | Respiratory viruses | Yes | Yes | – | – | – | – | – | – | – | – | Length of hospitalization | 1000 |
| Martin et al. [57] | United States | Children (≤21 years) | – | Respiratory viruses | Yes | – | Yes | Yes | – | – | – | – | – | – | Length of hospitalization | 893 |
| Franz et al. [58] | Germany | Children (≤16 years) | – | Respiratory viruses | Yes | – | Yes | – | – | – | – | – | Yes | – | Length of hospitalization, use of drugs (antibiotics/bronchodilators/systemic corticosteroids) or chest radiography examination | 404 |
| Turunen et al. [59] | Finland | Infants (3–23 months) | – | Respiratory viruses | Yes | – | – | – | – | – | – | – | – | Yes | Length of hospitalization, duration of wheezing and cough, inpatient status, severity score | 125 |
| Brand et al. [60] | Netherlands | Children (<2 years) | – | Respiratory viruses | – | – | Yes | Yes | – | – | – | – | – | – | Nasogastric feeding | 142 |
| Petrie et al. [61] | United States | Children and adults | – | Respiratory viruses | – | – | – | – | – | – | – | – | – | Pre-ARI general health, health at enrollment, ability to perform usual activities, sleep quality, employment status, hours of work missed due to ARI, work productivity loss due to ARI, subjective social position | 6766 | |
| Baird et al. [62] | United States | Children (≤18 years) | Pediatric Risk of Mortality III Score | Influenza A H1N1 | – | Yes | Yes | Yes | Yes | – | – | – | – | – | length of PICU stay | 7519 |
| Doshi et al. [63] | United States | Children and adults | – | Influenza A H1N1 | Yes | Yes | – | – | Yes | – | – | – | – | – | Influenza-like illness in the community, emergency department visits | 264,250 |
| Chiaretti et al. [64] | Italy | Children | – | Influenza A H1N1 | – | Yes | Yes | Yes | – | – | – | – | Yes | – | Fever >39°C at admission, duration of cough, abnormal-specific radiologic findings | 45 |
| Miroballi et al. [65] | United States | Children | – | Influenza A pandemic (H1N1) 2009 | Yes | Yes | – | Yes | Yes | – | – | – | – | – | length of hospitalization, bacteria superinfection, respiratory failure | 3750 |
| Kohet al. [66] | Malaysia | Children (≤12 years) | – | influenza A pandemic (H1N1) 2009 | – | Yes | Yes* | Yes | Yes | – | Yes | – | Yes | Yes | Pneumonia, abnormal chest radiograph, encephalitis and encephalopathy, shock and organ failure, myocarditis, rhabdomyolysis | 77 |
| Xu et al. [67] | China | Children and adults | – | Influenza A (H7N9) | Yes | – | – | – | Yes | – | – | – | – | – | Antiviral treatment | 701 |
| Virlogeux et al. [68] | China | Infants (<2 years) | – | Influenza A (H7N9) | – | – | Yes* | Yes | Yes | – | – | – | – | – | NA | 395 |
| Yang et al. [69] | China | Adolescents and adults | PSI | Influenza | Yes | – | – | – | – | – | – | – | – | – | Length of hospitalization | 188 |
| Tasher et al. [70] | Israel | Children (≤18 years) | – | Influenza | Yes | Yes | Yes* | Yes | – | – | – | – | – | – | Length of hospitalization | 880 |
| Burton et al. [71] | Canada | Children (≤16 years) | – | Influenza | Yes | Yes | – | Yes | – | – | – | – | – | – | Length of hospitalization, length of ICU stay, seizures | 1991 |
| Garcia et al. [72] | United States | Children (≤18 years) | – | Influenza | Yes | Yes | – | – | – | – | – | – | – | – | NA | 696 |
| Launes et al. [73] | Spain | Children (<18 years) | – | Influenza | – | – | – | Yes | – | – | – | – | – | – | NA | 93 |
| Hayward et al. [74] | United Kingdom | Children and adults | – | Influenza | – | – | – | – | – | – | – | Yes | – | – | Headache, muscle aches, cough, sore throat, runny nose, blocked nose, sneezing | 5548 |
| Oliveira et al. [75] | Brazil | Children | – | Influenza | – | – | – | – | – | – | – | – | – | – | Prematurity | 128 |
| Bamberger et al. [76] | Israel | Infants (<2 years) | CSS | RSV | Yes | Yes | Yes | – | – | – | – | – | – | – | Length of hospitalization, duration of oxygen requirement | 366 |
| Zhang et al. [77] | China | Children (<14 years) | CSS | RSV | Yes | Yes | Yes | – | – | – | – | – | – | – | Length of hospitalization | 894 |
| Vieira et al. [78] | Brazil | Infants (<3 months) | Clinical Scoring System | RSV | – | – | Yes* | Yes | – | – | – | – | – | – | Duration of invasive or noninvasive ventilatory support | 30 |
| Mejias et al. [79] | United States and Finland | Children (<2 years) | Clinical Score | RSV | – | – | Yes | – | – | Yes | Yes | Yes | – | – | Level of activity | 220 |
| Mellaet al. [80] | United States | Children (<2 years) | Clinical Disease Severity Score | RSV | – | – | Yes | – | – | Yes | Yes | – | – | Yes | Other auscultatory findings, need for intravenous fluids, duration of supplemental oxygen | 37 |
| Aydin et al. [81] | Turkey | Newborns (<30 days) | Downes’ Score | RSV | – | – | – | – | – | Yes | Yes | – | – | – | Cyanosis, degree of air entry, grunt | 54 |
| Mosalli et al. [82] | Saudi Arabia | Infants (<2 years) | Kristjansson Clinical Respiratory Score | RSV | – | – | – | – | – | Yes | Yes | – | – | Yes | Skin color | 77 |
| Schene et al. [83] | Netherlands | Infants (<1 year) | PIMS | RSV | – | Yes | Yes | Yes | Yes | – | – | – | – | – | Length of PICU stay | 129 |
| Borckink et al. [84] | Netherlands and France | Infants (<6 months) | PRIMS | RSV | – | Yes | Yes* | – | Yes | Yes | – | – | – | Yes | Nasal flaring | 133 |
| Kong et al. [85] | United States | Children | PRIMS | RSV | – | Yes | Yes* | Yes | – | – | – | – | – | – | Length of intubation, length of PICU stay | 117 |
| Grimwood et al. [86] | New Zealand | Infants (<2 years) | Severity Index Score | RSV | Yes | – | Yes | Yes | – | – | – | – | – | – | Length of hospitalization | 141 |
| Gilca et al. [87] | Canada | Children (≤3years) | Severity Index | RSV | Yes | Yes | Yes | – | – | – | – | – | – | – | Length of hospitalization | 448 |
| Panayiotou et al. [88] | Cyprus | Children (<12 years) | Severity Score | RSV | – | – | Yes | – | – | Yes | Yes | Yes | – | Yes | Nasal flaring | 391 |
| Tran et al. [89] | Vietnam | Children (≤15 years) | Scoring of Disease Severity | RSV | – | – | – | – | – | Yes | Yes | Yes | Yes◊ | Yes | Apnea, cough, rhinorrhea, hoarseness, duration of illness >4 days, cyanosis | 1082 |
| Houben et al. [90] | Netherlands | Infants | Scoring of Disease Severity | RSV | – | – | – | – | – | Yes | Yes | Yes | Yes◊ | Yes | Apnea, cough, rhinorrhea, hoarseness, duration of illness >4 days, cyanosis | 82 |
| Suarez-Arrabal et al. [91] | United States | Infants (1.5–4.4 months) | Standardized Clinical Disease Score | RSV | Yes | Yes | Yes | Yes | – | – | – | – | Yes◊ | – | Length of hospitalization, duration of invasive or noninvasive ventilatory support | 136 |
| Hasegawa et al. [92] | United States and Finland | Children (<2 years) | – | RSV | Yes | Yes | Yes* | Yes | – | – | – | – | – | – | Length of hospitalization | 2615 |
| Moreno-Perez et al. [93] | Spain | Children (<5 years) | – | RSV | Yes | Yes | Yes | Yes | – | – | – | – | – | – | Length of hospitalization, length of PICU stay, duration of oxygen therapy, duration of mechanical ventilation, venous line, nasogastric tube feeding, use of drugs (antibiotics) | 1763 |
| Somech et al. [94] | Israel | Infants (<1year) | – | RSV | Yes | Yes | Yes | – | – | – | Yes | Yes | – | – | NA | 195 |
| Fodha et al. [95] | Tunisia | Children (≤12 years) | – | RSV | Yes | Yes | – | Yes | – | Yes | – | – | – | – | Length of hospitalization | 81 |
| El Saleeby et al. [96] | United States | Infants (≤2 years) | – | RSV | Yes | Yes | – | Yes | – | – | – | – | – | – | Length of hospitalization | 291 |
| El Saleeby et al. [97] | United States | Children (<2 years) | – | RSV | Yes | Yes | – | Yes | – | – | – | – | – | – | Length of hospitalization | 219 |
| Somerset al. [98] | United States | Children (<2 years) | – | RSV | Yes | Yes | – | Yes | – | – | – | – | – | – | Duration of mechanical ventilation | 40 |
| Kurji et al. [99] | Canada | Children | – | RSV | Yes | Yes | – | Yes | – | – | – | – | – | – | Length of hospitalization, length of ICU stay | 590 |
| Kim et al. [100] | United States | Children | – | RSV | Yes | Yes | – | Yes | – | – | – | – | – – |
– | Length of hospitalization | 149 |
| Tabarani et al. [101] | United States | Children (<5 years) | – | RSV | Yes | Yes | – | – | – | – | – | – | – | – | NA | 851 |
| Garcia et al. [102] | United States | Children (<2 years) | – | RSV | Yes | – | Yes | Yes | Yes | – | – | – | – | – | Length of hospitalization, length of ICU stay, duration of oxygen requirement or mechanical Ventilation | 1777 |
| Brand et al. [103] | Netherlands | Children | – | RSV | Yes | – | Yes* | – | – | – | – | Yes | – | – | nasogastric feeding | 106 |
| Dotan et al. [104] | Israel | Infants | – | RSV | Yes | – | – | – | – | – | – | – | – | – | NA | 875 |
| Thompson et al. [105] | United States | Infants | – | RSV | Yes | – | – | – | – | – | – | – | – | – | NA | 67 |
| Stagliano et al. [106] | United States | Children (<3 years) | – | RSV | Yes | – | – | – | – | – | – | – | – | – | Length of hospitalization, age of hospitalization | 633,200 |
| Gijtenbeek et al. [107] | Netherlands | Infants (43–49months) | – | RSV | Yes | – | – | – | – | – | – | – | – | – | Length of hospitalization, the type of treatment | 2060 |
| Faber et al. [108] | Netherlands | Children (<1year) | – | RSV | – | Yes | Yes* | Yes | – | – | – | – | – | – | Nasal flaring | 465 |
| Forbeset al. [109] | United States | Infants | – | RSV | – | Yes | Yes | Yes | – | – | – | – | – | – | Duration of mechanical ventilation or oxygen requirement | 48 |
| Goncalves et al. [110] | Portugal | Newborns (≤28 days) | – | RSV | – | Yes | Yes | Yes | – | – | – | – | – | – | Length of ICU stay, duration of respiratory support | 259 |
| Semple et al. [111] | United Kingdom | Infants (<2 years) | – | RSV | – | Yes | Yes | – | – | – | – | – | – | – | NA | 197 |
| Schuurhof et al. [112] | Netherlands | Infants (<13 months) | – | RSV | – | Yes | – | Yes | – | – | – | – | – | – | NA | 465 |
| Vissers et al. [113] | Netherlands | Infants | – | RSV | – | – | Yes | Yes | – | – | – | – | – | – | NA | 105 |
| Thorburn et al. [114] | United Kingdom | Children | – | RSV | – | – | Yes | Yes | – | – | – | – | – | – | NA | 34 |
| Kaplan et al. [115] | Jordan | Children (<5 years) | – | RSV | – | – | Yes | – | – | Yes | Yes | – | – | – | NA | 326 |
| Faneye et al. [116] | Nigeria | Children (<5 years) | – | RSV | – | – | – | – | – | – | – | – | – | – | Lastly, bronchiolitis, pneumonia | 280 |
| Garcia et al. [117] | United States | Children (<2 years) | Modified Clinical Disease Severity Score | RSV and HRV | Yes | Yes | Yes | Yes | – | Yes | Yes | – | – | Yes | Length of hospitalization, need for intravenous fluids | 37 |
| Papenburg et al. [118] | Canada | Children (<3 years) | Severity Index (for hospitalized children) | RSV and HMPV | Yes | Yes | Yes | – | – | – | – | – | – | – | Length of hospitalization | 1039 |
| Midulla et al. [119] | Italy | Infants (7 days–11months) | Clinical Severity Score | RSV, HRV, and HBoV | – | – | Yes | – | – | Yes | Yes | Yes | – | – | NA | 182 |
| Martin et al. [120] | United States | Children (≤21 years) | – | RSV and HMPV | Yes | Yes | Yes | Yes | – | – | – | – | Yes◊ | – | Length of hospitalization, use of drugs (bronchodilators), or chest radiographs examination | 418 |
| Hahn et al. [121] | United States | Children (≤15 years) | – | HMPV | Yes | Yes | Yes | Yes | Yes | – | – | – | – | – | Length of hospitalization | 238 |
| Roussy et al. [122] | Canada | Children (<3 years) | – | HMPV | Yes | Yes | Yes | – | – | Yes | Yes | – | Yes | Yes | Length of hospitalization, hoarseness of voice, cough, duration of illness >4 days, rhinorrhea | 118 |
| Davis et al. [123] | United States | Children (≤17 years) | – | HMPV | Yes | Yes | – | Yes | – | – | – | – | – | – | NA | 815 |
| Caracciolo et al. [124] | Italy | Children (<5 years) | – | HMPV | Yes | – | Yes | – | – | – | – | – | – | – | Duration of oxygen requirement | 347 |
| Schuster et al. [125] | Jordan | children (<2 years) | – | HMPV | – | Yes | Yes | Yes | – | – | – | – | – | – | Abnormal chest X-ray | 3168 |
| Costa et al. [126] | Brazil | Children (≤5 years) | – | HRV | Yes | – | Yes | Yes | – | – | – | – | – | – | ICD-10 | 434 |
| Xiao et al. [127] | China | Children (1 month–16 years) | – | HRV | – | Yes | Yes | Yes | – | – | – | – | – | – | NA | 1742 |
| Chen et al. [128] | United States | Children and adults | – | HRV | – | – | – | – | – | – | Yes | – | – | – | Earache, runny nose, sore throat, sneezing, cough, hoarseness, chest pain, muscle ache, fatigue, headache, chills | 160 |
| Asner et al. [129] | Canada | Children | – | HRV/Enterovirus | Yes | Yes | Yes | – | Yes | – | – | – | – | – | Length of hospitalization | 380 |
| Zhao et al. [130] | China | Children (<5 years) | Index of Severity (IOS) | HBoV | Yes | – | Yes | Yes | – | – | – | – | – | – | Hydrogen, length of hospitalization, partial CO2 pressure | 554 |
| Tran et al. [131] | Japan | Children (<15 years) | – | HBoV | Yes | – | Yes | – | – | – | – | – | Yes | Yes | Length of hospitalization, pneumonia | 1082 |
| Jean et al. [132] | Canada | Children | Symptom Score | HCoV-OC43 | – – |
– | – | – | – | – | – | Yes | Yes | – | Cough, rhinorrhea, headache | 3847 |
RISC: Respiratory Index of Severity in Children; PSI: Pneumonia Severity Index; CSS: Clinical Severity Scores; PMS: Pediatric Index of Mortality Score; PRIMS: Pediatric Risk of Mortality Scores.
aNumber of patients where disease severity was measured or scored.
bFever defined as >=38°C.
*Includes the following items: supplemental oxygen and clinical parameters, such as: oxygen saturation (O2Sat), O2Sat-to-FiO2 ratio (O2Sat/ FiO2), or hypoxia.
It became evident that several clinical parameters were shared by multiple studies, as for example hospitalization, oxygen requirement, labored breathing, (P)ICU admission, mortality, feeding problems/dehydration/vomiting, fever, wheezing or abnormal breath sounds, etc. All of these commonly used criteria were included in the ViVI Disease Severity Score (see also: Tief et al. [30], in Textbox 3) except for mortality, which is usually recorded separately in hospital records.
3.2. Patient baseline demographics and hospital course
The ViVI Disease Severity Score was validated in the full QM Cohort comprised of 6073 patients aged 0–18 years (mean: 3.13 years; SD: 3.89; range: 0–18.79 years). A percentage of 33.6 of the QM program participants was under the age of 1 year, 51.0% were aged 1–5 years, 13.6% were in the age group 6–15 years, and 1.8% were aged 16–18 years. A total of 3399 (56.0%) of the participants were male. A total of 1685 (27.8%) participants were prescribed antibiotics while only 202 (3.3%) were prescribed antivirals in hospital.
At presentation, 3172 (52.2%) were assessed as being in need of hospitalization with 997 (16.4%) being in need of intensive care (including assisted ventilation and extracorporeal membrane oxygenation). With regard to viral etiology, in decreasing order of frequency, we identified rhinovirus (22.9%), RSV (17.2%), ADV (9.6%), A(H1N1) influenza virus (4.5%), metapneumovirus (4.4%), A(H3N2) influenza virus (2.8%), influenza B viruses of the Victoria-lineage influenza (1.7%), and type B viruses of the Yamagata-lineage (2.0%). In 5.8% of the cases, there was more than 1 concurrent viral infection. Table 1 summarizes the findings from the RF assessment exercise carried out as part of the quality monitoring and Table 2 summarizes the clinical symptoms at presentation.
Table 1.
Risk factors assessed as part of the quality monitoring (n = 6073).
| Risk factor | Number (%) |
|---|---|
| RF 1: Infant <2 years of age | 3471 (57.2) |
| RF 2: Pulmonary condition | 494 (8.1) |
| RF 3: Cardiac condition | 488 (8.0) |
| RF 4: Diabetes | 18 (0.3) |
| RF 6: Obesity | 76 (1.3) |
| RF 7: Other metabolic condition | 157 (2.6) |
| RF 8: Chronic renal disease | 152 (2.5) |
| RF 9: Chronic hepatic disease | 47 (0.8) |
| RF 10: Chronic neurological condition | 338 (5.6) |
| RF 11: Hemoglobinopathies | 50 (0.8) |
| RF 12: Congenital immunosuppression | 47 (0.8) |
| RF 13: Acquired immunosuppression | 47 (0.8) |
| RF 14: Aspirin therapy | 58 (1.0) |
| RF 15: Pregnancy | 2 (0.03) |
| RF 16: Prematurity <33 weeks gestational age | 320 (5.3) |
Table 2.
Clinical symptoms at presentation (n = 6073).
| Presenting symptom | Number () |
|---|---|
| DSU 1: Fever | 5225 (86.0) |
| DSU 2: Cough | 3805 (62.7) |
| DSU 3: Pharyngitis | 3702 (61.0) |
| DSU 4: Coryza/Rhinitis | 3210 (52.9) |
| DSU 5: Headache | 412 (6.8) |
| DSU 6: Myalgia | 118 (1.9) |
| DSU 7: Malaise | 1399 (23.0) |
| DSU 8: Diarrhea | 511 (8.4) |
| DSU 9: Vomiting | 1270 (20.9) |
| DSC 1: High and prolonged fever | 521 (8.6) |
| DSC 2: Dyspnea | 2223 (36.6) |
| DSC 3: Hypoxia | 1098 (18.1) |
| DSC 4: Hemoptysis | 91 (1.5) |
| DSC 5: Altered or loss of consciousness | 352 (5.8) |
| DSC 6: Seizure | 502 (8.3) |
| DSC 7: Dehydration | 577 (9.5) |
| DSC 8: Exacerbation of chronic disease | 112 (1.8) |
| DSC 9: Septic shock or multi-organ failure | 38 (0.6) |
| DSC 10: Need for hospitalization | 3172 (52.2) |
| DSC 11: Lower respiratory tract infection/superinfetcion | 1681 (27.7) |
| DSC 12: Upper respiratory tract infection/superinfetcion | 3823 (63.0) |
| DSC 13: Need for ICU admission | 997 (16.4) |
A total of 702 patients (11.6%) had chest-radiography in the ED. Chest radiography findings showed that 438 (7.2%) had pneumonia, 84 (1.4%) had bronchitis, 1 (0.02%) had bronchiectasis, 3 (0.05%) had bronchiolitis, and 33(0.5%) had other non-pneumonia abnormalities. One hundred and nineteen (2.0%) had a lumbar puncture done in the ED and 113 (1.9%) had cerebrospinal fluid (CSF) chemistry done. Sixty-nine (1.1%) had CSF cultures done with only four (0.07%) sample positive for bacteria (1 Bacillus species, 1 Staphylococcus epidermidis, 1 Staphylococcus hominis, and 1 unspecified bacteria positive). No cases of Streptococcus pneumoniae were identified on culture.
During hospitalization, 119 (2.0%) had a lumbar puncture and 97 (1.6%) had CSF chemistry and culture done. Four (0.08%) samples were positive for bacteria including Escherichia coli, Micrococcus, Staphyococcus epidermidis and Streptococcus salivarius, Streptococcus mitis/oralis as well as Enterovirus in two cases. No cases of Streptococcus pneumoniae were identified on culture. A total of 603 (9.9%) had a chest radiograph during their inpatient stay. Inpatient chest radiography findings showed that 354 (5.8%) had pneumonia, 48 (0.8%) had bronchitis, 2 (0.03%) had bronchiolitis, 3 (0.05%) had bronchiectasis, and 53 (0.9%) had other non-pneumonia abnormalities. In total, 698 (11.5%) of the study participants had been diagnosed with pneumonia on chest radiography at some point during hospitalization. There were two (0.03%) deaths recorded in the emergency room. One of the deaths was attributed to encephalitis and sepsis following infection. The cause of death in the second patient was related to serious underlying cardiac disease in a young infant.
3.3. Using the ViVI Score for cross-cohort comparison
The ViVI Disease Severity Scores showed a normal distribution with a mean score of 14.5 (SD: 6.0; range 0–34) at initial assessment (Figure 1). The ViVI Disease Severity Score was significantly higher in patients with the need for hospitalization (mean difference [95% CI]: −7.51 [−7.76 to −7.26]; p < 0.001), with a need for critical care facilities (mean difference [95% CI]: −6.24 [−6.58 to −5.91]; p < 0.001) as well as in those with signs of primary or secondary bacterial lower respiratory tract infections (mean difference [95% CI]: −6.46 [−6.71 to −6.20]; p < 0.001). The median Risk Factor Score in this cohort was 1 (IQR: 0–1); the median RF score was 0.88 (SD: 0.78) and scores ranged from 0 to 6.
Figure 1.
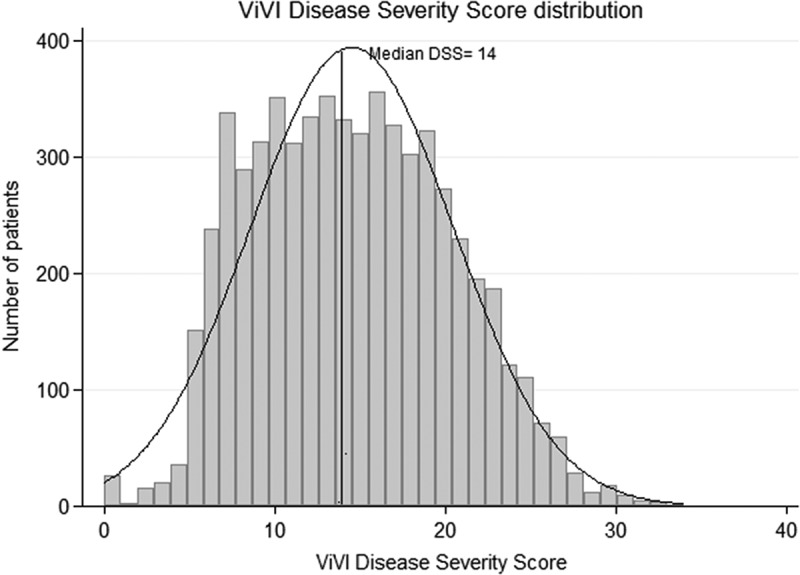
Distribution of disease severity (ViVI Scores) across the QM cohort.
3.4. Seasonality of respiratory viral infections
The CP analysis was applied to detect seasonal patterns for each virus detected in the QM Cohort. We define a virus to be seasonal if it is not present during the whole year. With this definition, we found that influenza viruses (Figure 2), as well as RSV and HMPV, showed a strong seasonal behavior (Figure 3) with predominance during the Northern Hemisphere winter months. ADV and HRV were ‘rapid cyclers’ with frequent and brief peaks throughout the year (Figure 3). The CP method showed that in a hospital-based syndromic surveillance system, seasons can be detected and defined in real time for each of the respiratory viruses. During the post-pandemic 2010/11 season for example, influenza A(H1N1)pdm09 viruses continued to predominate in the QM cohort. Influenza A(H3N2) viruses, on the other hand, were absent during the 2009/10 and 2010/11 seasons but replaced pandemic H1N1 strains during the subsequent season (see Figure 2). Also, differentiation of influenza B lineages revealed that Influenza B Yamagata and Victoria viruses did not always circulate annually but instead showed alternating patterns.
Figure 2.
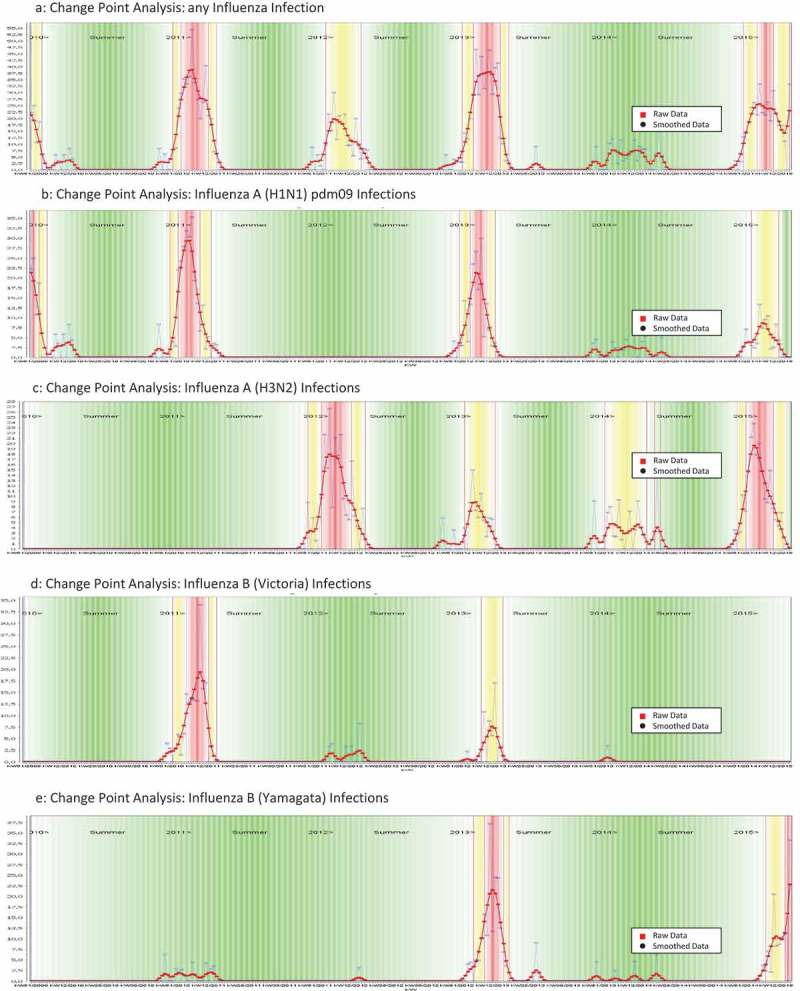
CP Analyses identifying seasonality of influenza and influenza (sub)types. (a) Change Point Analysis: any Influenza Infection. (b) Change Point Analysis: Influenza A (H1N1) pdm09 Infections. (c) Change Point Analysis: Influenza A (H3N2) Infections. (d) Change Point Analysis: Influenza B (Victoria) Infections. (e) Change Point Analysis: Influenza B (Yamagata) Infections.
Figure 3.
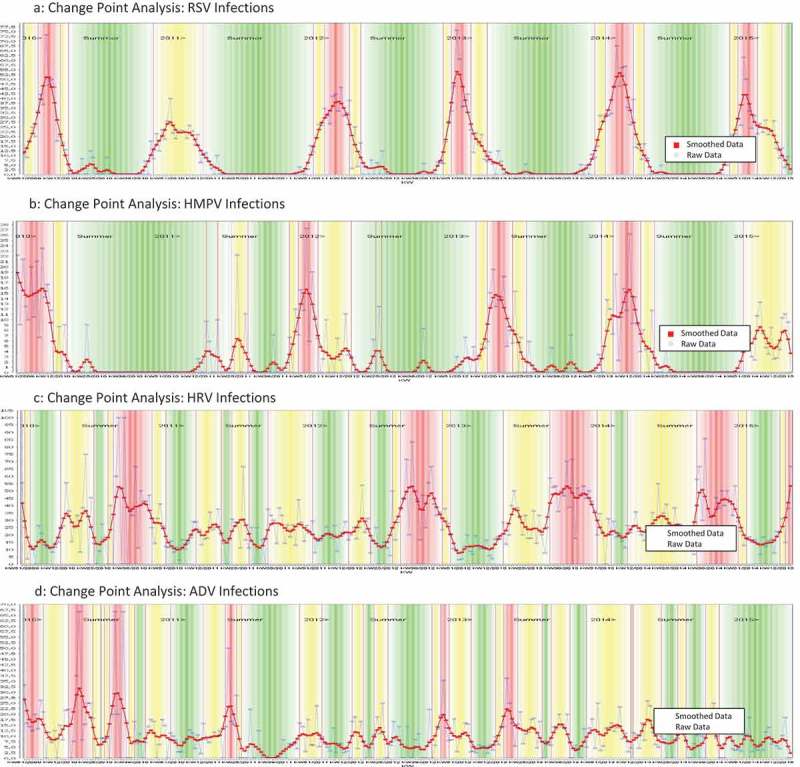
CP Analyses identifying seasonality of ADV, HRV, RSV, HMPV. (a) Change Point Analysis: RSV Infections. (b) Change Point Analysis: HMPV Infections. (c) Change Point Analysis: HRV Infections. (d) Change Point Analysis: ADV Infections.
3.5. Seasonality of disease severity
The CP analysis was also used to identify fluctuations in average disease severity per calendar week in the QM program (Figure 4). The initial period until summer of 2011, when the QM program was restricted to once-weekly screening of in- and outpatients in the ED, is visually separated from the full surveillance phase beginning with the 2011/12 winter season, when daily screenings of all inpatients hospitalized with suspected ILI were added. The use of ViVI Disease Severity Scores during perennial, hospital-based surveillance provided standardized disease severity reports throughout the course of the year.
3.6. Disease severity with different respiratory viral (co)infections
There was a small but significant difference in ViVI Disease Severity Scores between those subjects where no viral etiology could be detected (mean ViVI Disease Severity Score: 13.85; SD 5.81), those identified with a single viral infection (mean ViVI Disease Severity Score: 14.90; SD 6.00) and those with more than 1 concurrent viral infection (mean ViVI Disease Severity Score: 15.73; SD 6.10); p (ANOVA) < 0.001. For each patient in the QM Cohort, we computed the overall ViVI Disease Severity Scores as well as the component of the DSU and DSC symptom category, respectively (Table 3). Average disease severity with different respiratory viral infections revealed that RSV induced the highest level of disease severity followed by HMPV, influenza A(H3N2), and HRV infections. Disease severity with ADV, influenza B, and influenza A (H1N1)pdm09 viruses remained below average (Table 3). The ViVI Disease Severity Score distributions including viral coinfections are displayed in Figure 5.
Table 3.
Distribution of ViVI Disease Severity Score by different viral etiologies.
| Disease | Mean ViVI Score | Mean ViVI Score for uncomplicated disease | Mean ViVI Score for complicated disease |
|---|---|---|---|
| Respiratory Syncytial Virus | 16.87† | 3.20 | 3.52† |
| Metapneumovirus | 16.18† | 3.35† | 3.35† |
| A(H3N2) influenza virus | 15.08 | 3.29 | 2.48† |
| Rhinovirus | 14.92† | 3.17 | 3.09† |
| Adenovirus | 13.64† | 3.39† | 2.56† |
| Influenza B (Yamagata-lineage) | 12.51† | 3.21 | 2.16† |
| A(H1N1)pdm09 influenza virus | 12.39† | 3.42† | 1.96† |
| Influenza B (Victoria-lineage) | 11.51† | 3.69† | 2.01† |
†Statistically significant difference (t-test) between ViVI Disease Severity Score for the given virus as compared to those for all other etiologies combined.
Figure 5.
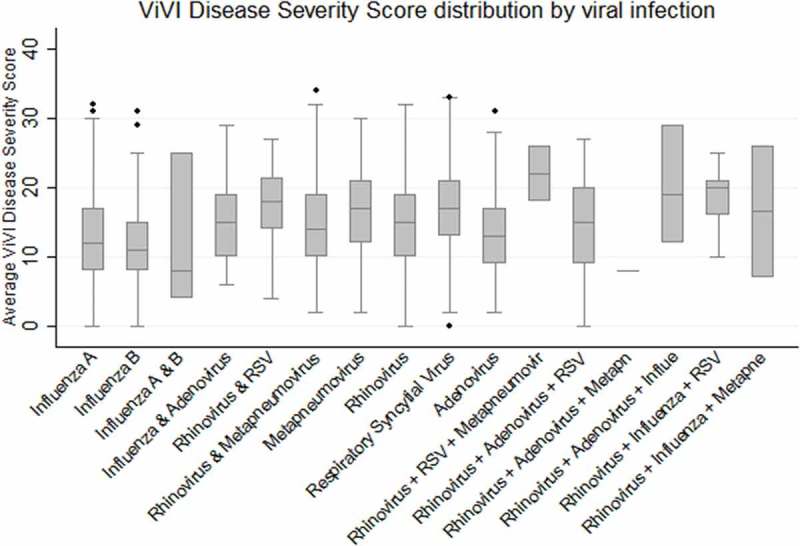
Average ViVI Disease Severity Scores for patients infected by different respiratory viruses.
3.7. Comparison between disease severity and the consultation index
To illustrate the comparison, we computed the average ViVI Disease Severity Score per calendar week and compared to the Consultation Index during the same week (Figure 6(A)). As expected, no significant correlation was observed between weekly ViVI Disease Severity Scores and the Consultation Index (Pearson’s correlation coefficient r = 0.10; p = 0.1309) during corresponding weeks, indicating disease severity and case numbers are not linked.
Figure 6.
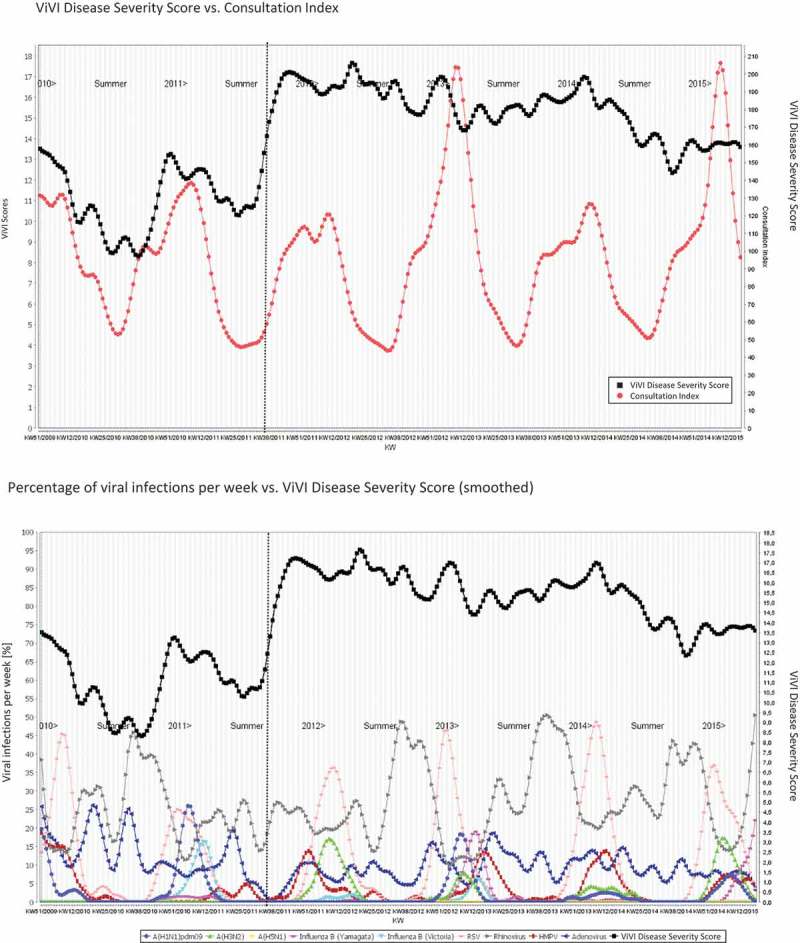
Average ViVI Disease Severity Score vs. Consultation Index and Weekly Virus Infections. ViVI Disease Severity Score vs. Consultation Index. Percentage of viral infections per week vs. ViVI Disease Severity Score (smoothed).
To allow visual interpretation, we also plotted the weekly average ViVI Disease Severity Score (in the ED prior to October 2011 and in ED and inpatient units thereafter) against the time course of the respective seasonal viruses. The results are shown in Figure 6(b). The viruses circulating (represented in % of all QM patients tested: y axis) are shown in relationship to the average ViVI Disease Severity Score during the respective calendar week. Some viruses peaked simultaneously with the average disease severity but a cumulative effect was more common. The effect of viruses prevalent during the summer months was more pronounced when inpatients were included in the QM Program, thus including severe cases requiring hospital admission.
3.8. RFs influencing disease severity
The Pearson’s correlation coefficient r was 0.1923 indicating a statistically significant but weak positive correlation between ViVI Disease Severity and Risk Factor Scores (p < 0.001). The distribution of ViVI Disease Severity Scores by different RFs is illustrated in Table 4.
Table 4.
Distribution of ViVI Disease Severity Scores by different risk factors.
| Risk factor | Mean ViVI Score in patients with the risk factor (95% CI) | Mean ViVI Score in patients without the risk factor (95% CI) | Mean difference in ViVI Scores (95% CI) |
|---|---|---|---|
| RF 1: Infant <2 years of age | 14.91 (14.71, 15.10) | 14.01 (13.77, 14.25) | −0.89(−1.20, −0.59)† |
| RF 2: Pulmonary condition | 18.41 (17.89, 18.93) | 14.18 (14.02, 14.33) | −4.24 (−4.78, −3.70) |
| RF 3: Cardiac condition | 17.02 (16.50, 17.54) | 14.30 (14.15, 14.46) | −2.71(−3.26, −2.17)† |
| RF 4: Diabetes* | 14.83 (11.67, 18.00) | 14.52 (14.37, 14.67) | −0.31 (−3.07, 2.44) |
| RF 6: Obesity* | 15.03 (13.40, 16.66) | 14.52 (14.37, 14.67) | −0.51 (−1.86, 0.84) |
| RF 7: Other metabolic condition | 15.96 (15.04, 16.87) | 14.48 (14.33, 14.64) | −1.47(−2.42, −0.53)† |
| RF 8: Chronic renal disease | 15.30 (14.35, 16.24) | 14.50 (14.35, 14.65) | −0.79 (−1.75, 0.17) |
| RF 9: Chronic hepatic disease* | 14.98 (13.59, 16.37) | 14.52 (14.37, 14.67) | −0.46 (−2.17, 1.25) |
| RF 10: Chronic neurological condition | 17.52 (16.83, 18.22) | 14.35 (14.19, 14.50) | −3.18(−3.82, −2.53)† |
| RF 11: Hemoglobinopathies* | 14.84 (13.47, 16.21) | 14.52 (14.37, 14.67) | −0.32 (−1.98, 1.34) |
| RF 12: Congenital immunosuppression* | 15.19 (13.64, 16.74) | 14.52 (14.37, 14.67) | −0.67 (−2.39, 1.04) |
| RF 13: Acquired immunosuppression* | 13.97 (12.89, 15.05) | 14.53 (14.38, 14.68) | 0.56 (−0.62, 1.74) |
| RF 14: Aspirin therapy* | 17.17 (15.57, 18.78) | 14.50 (14.35, 14.65) | −2.68(−4.22, −1.14)† |
| RF 15: Pregnancy** | 7.50 (1.15, 13.85) | 14.52 (14.37, 14.67) | 7.02 (−1.24, 15.29) |
| RF 16: Prematurity <33 weeks gestational age | 16.53 (15.88, 17.18) | 14.41 (14.26, 14.56) | −2.12(−2.79, −1.45)† |
†Statistically significant mean differences are highlighted in bold (t-test p value < 0.05).
*The interpretation of this risk factor was limited or **very limited by a low (*n < 100) or very low (**n < 10) prevalence rate in the QM population (see also Table 1).
To evaluate which of the RFs as defined by WHO [35] (Textbox 2) had the highest impact on disease severity (i.e. ViVI Disease Severity Score), we performed regression analysis as follows: ViVI Disease Severity Score = w1 × RFs 1, w2 × RF 2, …, wn × RF n. Regression analysis revealed that there was no specific set of variables that could be used to model this relationship significantly well. The best subset of RF variables was ‘RF 1: Infant <2 years of age,’ ‘RF 3: Cardiac condition,’ ‘RF 2: Pulmonary condition,’ ‘RF 6: Obesity,’ and ‘RF 4: Diabetes’ together yielded a R2 goodness-of-fit of 0.06. Using all RF variables yielded a R2 of 0.07.
To further explore the relationship between age and RF, we studied median and mean ViVI Disease Severity Score in infants in children below 5 years of age, and in children aged 6 years and above (Table 5).
Table 5.
Distribution of ViVI Disease Severity Scores by Age.
| Age category | Median ViVI Score (IQR) | Mean ViVI Score (SD); range |
|---|---|---|
| <1 year (n = 2040) | 14 (10–19) | 14.6 (5.6); 0–33 |
| 1–5 years (n = 3094) | 15 (10–19) | 14.8 (6.0); 0–33 |
| 6–18 years (n = 939) | 12 (8–18) | 13.4 (6.3); 0–34 |
We then performed Pearson correlation to test for a potential relationship between ViVI Disease Severity Score and patient age. The Pearson collation revealed r = −0.073, suggesting that there is in fact no significant correlation between the ViVI Disease Severity Score and patient age.
3.9. Disease severity in patients with and without antiviral/antibiotic prescription
An increasing ViVI Disease Severity Score indicates increasing disease severity. The key aspect of the ViVI Disease Severity Score is that it provides data standardization across the full spectrum of severity as well as comparison within a cohort, and between different seasons or sites. In the future, this may allow the comparison of various treatment decisions in clinical trials and observational settings.
As described above, physicians in routine care were unaware of the results of ViVI Disease Severity Score assessments by QM staff and reversely, QM staff were unaware of treatment decisions when assessing a patient. Analysis of ViVI Disease Severity Score results revealed that disease severity in patients (with any virus) who were prescribed neuraminidase inhibitors was 19.18 (95% CI: 17.62–20.74) compared to 14.52 (95% CI: 14.37–14.67) in individuals who were not prescribed neuraminidase inhibitors at the time of presentation to the ED (mean difference [95% CI]: −4.66 [−6.24 to −3.09]; p < 0.001). In patients with PCR-confirmed influenza infection, this difference upheld: The mean ViVI Disease Severity Score in patients where physicians had decided to prescribe neuraminidase inhibitors was 20.33 (95% CI: 11.22–29.45) compared to 12.87 (95% CI: 12.40–13.33) in patients without antiviral therapy. The mean difference was −7.47 (95% CI: −12.28 to −2.65); p = 0.0024.
Similarly, patients, who had been prescribed antibiotics in routine care, also showed higher ViVI Disease Severity Score 16.73 (95% CI: 16.47–16.99) compared to a mean score of 13.51 (95% CI: 13.33–13.69) in individuals without antibiotic prescription (mean difference [95% CI]: −3.22 [−3.53 to −2.91]; p < 0.001).
3.10. Using the ViVI Disease Severity Score to follow individual patients over time
Considering the variability in disease presentations and courses of illness with influenza and other respiratory viral infections in children, the ViVI Disease Severity Score is not intended to be validated against future clinical events or outcomes. To assess whether the ViVI Disease Severity Score could be used to standardize consecutive follow-up visits in clinical trials, a total number of 216 QM patients with influenza diagnoses were followed longitudinally with virology (PCR) and disease severity assessments over time.
The overall Pearson Correlation between ViVI Disease Severity Score and virus load (using cycle threshold = CT values) over time was 0.501. A closer look at the correlation histogram (Figure 7) revealed three major subgroups: The largest group of 161 patients can be categorized as having a moderate to strong positive correlation (r ≥ 0.5) between disease severity and viral load over time; a second group of 35 patients showed a strong negative correlation (r ≤ −0.5). A third group of 20 patients showed a weak (positive or negative) correlation (−0.5 < r < 0.5). Preliminary decision tree analysis of these groups suggested that a ViVI Disease Severity Score below 11 and the RF ‘infant below 2 years of age’ were connected to a negative correlation between virus load and disease severity.
Figure 7.
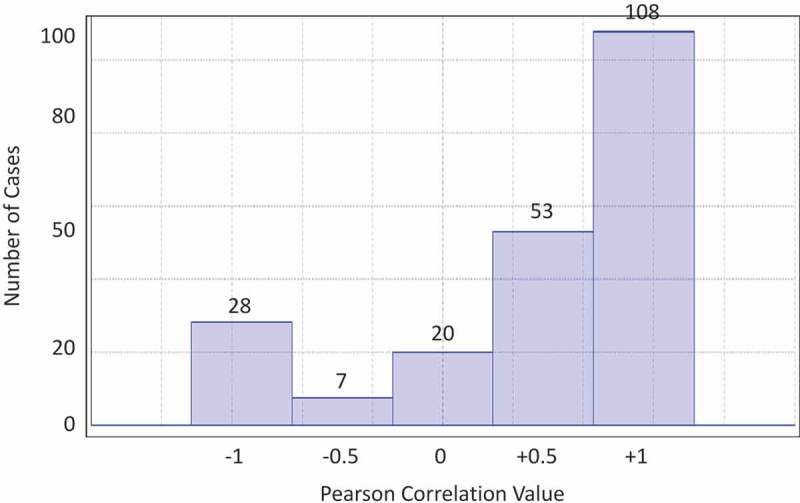
Histogram of Pearson Correlation between ViVI Disease Severity Score and Viral Load (CT Value).
4. Discussion
Respiratory infections are among the most common reasons for children to be admitted to pediatric hospitals. Hospital-based surveillance of respiratory viral infections is of great value to understand the full disease spectrum, from mild symptoms to serious presentations. Children are the most avid transmitters of respiratory viral infections, and the information gained from syndromic surveillance in children’s hospitals can complement decentralized sentinel surveillance systems in a meaningful way [133]. With the advent of rapid diagnostics and mobile health applications, it has now become possible to monitor virological and clinical end points in real time [134–140].
Traditionally, disease activity is monitored based on epidemiological parameters such as ILI or ARI incidence, hospitalization rates, or mortality [141,142]. The Consultation Index was developed by the Robert Koch Institute and has proven to be a sophisticated epidemiological tool to assess background ARI activity at representative sentinel practices. Fluctuations in ARI activity in private practices represent a useful indicator of disease burden based on actual case numbers. Reporting of the number of cases, however, does not reveal information on disease severity with each individual case [143].
The ViVI Disease Severity Score aims to fill this gap. The ViVI Disease Severity Score is a 22-item weighed clinical composite score consisting of DSU items reflecting ‘regular’ ILI activity and DSC items indicating 'high-impact' clinical presentations in the target population [144]. The ViVI Disease Severity Score opens avenues to new individual patient data (IPD) analyses, for example to identify clinically relevant seasonal patterns of disease severity linked to different viral diagnoses confirmed in the same group of patients.
The ViVI Disease Severity Score also allows consistent measurements of disease severity when following individual patients over time, as would be the case in clinical trials [40]. Follow-up assessments are useful whenever standardized severity data need to be recaptured over time. When frequent ‘snap shots’ of disease severity are combined with virology data, it may be possible to generate a ‘moving image’ with interesting new applications in clinical research. The introduction of standardized disease severity scores will facilitate head-to-head comparisons and the ‘meta-analyzability’ of clinical trials and observational studies. Full compliance of the ViVI Disease Severity Score mobile application with Clinical Data Interchange Standards Consortium (CDISC) standards further expands data interoperability and compliance with reporting formats to regulatory agencies [145–150].
It is important to note the scope of the proposed disease severity measure. This expert review does not intend to raise expectations that a disease severity score could or should be used to predict future events or physician behavior. Instead, we introduce a simple 22-item weighted clinical composite score allowing the assessor to translate the current condition of the patient into a two-digit number, which allows comparison of one patient to another, regardless of the setting. To this end, the paper provides the descriptive account of how a standardized score can be utilized to assess the relationships observed between the score and various (independent) treatment and management decisions for readers to draw their own conclusions about how they may in turn use the ViVI Disease Severity Score in clinical practice or research.
This expert review also introduces Time Series Analysis with Change Point Detection as a mathematical model applied, for the first time, to determining the timing and seasonality or respiratory viruses circulating in a hospital ad emergency room. While the observation that several viruses may circulate in a seasonal pattern is not new, the authors demonstrate that purely data-inherent definitions of seasonality could be an interesting addition to traditionally used methods.
Comparisons of average ViVI Disease Severity Score in the hospital system with the Consultation Index in the same calendar week (i.e. simultaneous ARI consultations in sentinel practices) revealed that the two parameters are intrinsically different. A ‘heavy’ season with a high frequency of ARI consultations is not the same as a ‘light’ season with fewer but more severe cases. The individual assessments in the QM cohort detected fluctuations in disease severity at a time when increases in overall ARI incidence in the general population were not evident. Especially during atypical influenza seasons with unusually few or unusually severe cases, the monitoring of disease severity in addition to incidence rates will provide important complementary information. Standardized disease severity assessments also enable the cross-cohort comparison of disease burden between different viral pathogens. It is not surprising that RSV was identified as a key contributor to disease severity in a tertiary children’s hospital, followed by HMPV disease. A better understanding of the real-world impact of different respiratory viruses on child health will help in the prioritization of drug and vaccine development.
The development of the ViVI Disease Severity Score is based on a systematic review of the published literature. The review showed that severity assessments have been inconsistent. Four clinical management parameters were used commonly as indicators of disease severity: hospitalization, intensive care treatment, oxygen supplementation, and mechanical ventilation (both invasive or noninvasive). The availability of any such measure, however, is highly dependent on the setting. The ViVI Disease Severity Score therefore uses the ‘need for hospitalization’ or ‘need for ICU admission’ (as determined by the assessor) instead. If the assessor determines that a patient would benefit from any such measures, the item can be scored regardless of the availability of ICU or hospital beds at the respective time or location.
To ensure inter-rater consistency, the QM team was specifically trained to apply established WHO definitions and standard criteria for the assessment of each aspect of the ViVI Disease Severity Score. For example, fever was defined according to Marcy et al. [151] and acute lower respiratory tract infection as per Roth et al. [152]. For use in multicenter settings, the ViVI Disease Severity Score App will include help menus in the user interface to ensure that assessors are aware of the same criteria and age-appropriate values. Acknowledging that the content, structure, and quality of standardized data are of paramount importance, the development team worked closely with the CDISC to ensure full compliance of terminologies and data elements with industry and regulatory guidance.
The literature review showed that grading severity is not the same as predicting severity. Especially in young children, disease severity will fluctuate over time, until the episode is resolved eventually. The course of illness may or may not be linear. The ViVI Disease Severity Score is designed to help the physician measure and monitor the situation ad hoc, or repeatedly over time, but not to predict the future of the patient. Several scores have been designed, not to measure severity ad hoc, but to predict the likelihood of fatal outcomes in the future as is the case with the respiratory index of severity in children [153], the Pediatric Index of Mortality Score (PIMS) [83], and the pediatric risk of mortality score (PRIMS) [84,85]. These latter two scores (PIMS and PRIMS) were specific to RSV infections [83] in infants [84,85]. The Kristjansson Clinical Respiratory Score for RSV Infections in Children [82] was designed to include children beyond the infant age group. The index of severity was studied in bocavirus infections in infants and children <5 years [130], as was the symptom score for coronavirus infections in children [132]. Very few scores were developed to measure disease severity regardless of the type of respiratory virus causing the disease. The Clinical Severity Score was used to monitor RSV, HRV, and HMPV infections in children <3 years [91,117,122]. The systematic literature review, updated in 2016, confirmed that ViVI Disease Severity Score was the only score covering all pediatric age groups and any respiratory virus encompassing any of the key parameters outlined in the published literature to date [18,30]. The ViVI Disease Severity Score was also the only composite score that has been validated in a prospective cohort of more than 6000 children and adolescents from 0 to 18 years, yielding a normal distribution.
Regular severity assessments over time can be combined with CP detection methodology to detect of significant changes in disease severity in cohorts. Hospital surveillance will thus become feasible in real time, as rapid-turnaround diagnostic tests are evolving [154–157]. The use of rapid diagnostic tests can then be targeted according to the local surveillance information. Bioinformatics analyses and machine learning algorithms may provide new avenues for the identification of virus-specific seasonality patterns [158].
During past influenza seasons, differences in the composition of subtypes and disease severity have been significant. The linkage of simultaneous virus surveillance with point-of-care disease severity assessments will advance the understanding of local epidemiology. Local epidemiology is key to understanding the impact of different strains on different populations. In North America, influenza A H3N2 viruses reappeared 1 year sooner than in Europe, i.e. in 2010/11 [159] followed by an unusually ‘light’ season with few or late cases during the winter of 2011/12 [160]. Public health agencies in the UK reported a particularly ‘severe’ season in 2010/11 [161,162], whereas Australia reported increased rates of severe influenza disease in 2014 [163–165], similar to Mexico during 2013/14. Classically, seasons have been regarded as ‘severe’ when coinciding with high overall case numbers, hospitalization rates, or mortality [166,167]. In the future, it will be important to distinguish the impact of fluctuations in influenza (sub)types on disease severity in specific patient groups, based on IPD.
The use of standardized measures of severity may also be helpful in the study of medical decision-making and diagnostic algorithms. Physicians in routine care often report that their decision to order virus diagnostics is often dependent on a variety of factors such as levels of training, media attention [168,169], specific requests by patients or parents, ‘typical’ versus ‘atypical’ disease presentations, availability and cost of diagnostic tests, insurance status of the patient, time constraints, etc. [20]. The same applies to the decision to hospitalize a patient. It is safe to assume that testing and rates of hospitalization are not the same at the beginning, peak, and end of an influenza season. Standardized disease severity scores may allow hospitals to set objective thresholds for diagnostic testing or admission decisions, depending on local conditions and epidemiology.
When population-based indicators are used instead of individual clinical outcome parameters, considerable bias may be introduced due to differences in patient reporting, access to health care [170] as well as physician awareness and reimbursement [171] creating challenges in global surveillance systems [172,173]. Some surveillance programs use retrospective chart reviews and ICD coding. ICD codes, however, do not always distinguish between laboratory-confirmed cases and clinical diagnoses [174].
Interpersonal variability and the unpredictable nature of respiratory viral infections pose a challenge to surveillance and preparedness programs [175]. Influenza seasons in particular vary with respect to case numbers and disease severity attributable to various viral subtypes and population strata [67,68,113,127]. The prospective monitoring of disease severity associated with laboratory-confirmed diagnoses will help to delineate vulnerable subpopulations expressing disease severity differently compared to the population average. Real-time surveillance of disease severity may provide public health stakeholders with crucial information to adjust the allocation of hospital beds and resources [52]. The introduction of IPD disease severity assessments in a hospital-based surveillance system facilitates the timely identification of abnormal patterns of disease severity, i.e. though network analysis [176] or during time periods when the overall ILI disease severity is different from previous seasons or the rest of the year. Importantly, fluctuations in disease severity measured by the ViVI Disease Severity Score are independent of incidence-based surveillance indices.
Traditional disease severity estimates have focused on extreme presentations such as mortality rates [177,178] or ICU admission [179] but were not designed to monitor the full spectrum of mild-to-severe disease presentations. Additional granularity will be required for clinical trials. When the ViVI Disease Severity Score was used to follow patients longitudinally, disease severity was measured consistently from the time of initial presentation until resolution of symptoms. The ViVI Disease Severity Score has also been used to measure of subtle changes in disease severity in ICU patients requiring organ replacement therapy [40]. Once standardized scores are used consistently, this will open the path to head-to-head comparisons of antivirals and vaccines and to prospective IPD meta-analyses.
It will be important to investigate the complex relationship between virus load and disease severity and expected outcomes, which would provide important clues for clinical trial design [40]. Patients showing atypical patterns of disease severity for example (such as a negative correlation between virus load and disease severity) may represent individuals where antivirals do not exert the desired effect. Additional analyses are underway to understand this relationship better. Standardized disease severity measures will facilitate biomarkers studies and the identification of viral and host factors associated with severe outcomes [180]. A precision medicine approach would lead to individualized risk communication strategies to improve the acceptance of vaccines and antivirals where they are most effective. The low uptake in influenza vaccines in the QM Cohort indicates that significant numbers of symptomatic influenza cases might have been prevented through immunization [181].
The presented work has several limitations: The current experience with the ViVI Disease Severity Score is based on a single-center tertiary care setting. Additional decentralized studies will be needed to validate the ViVI Disease Severity Score in international settings and in private practice networks, where severity may be lower. Further studies are planned in adults and the elderly, including the development of a compatible score for patient-reported outcomes. It is possible that different populations yield different results, but standardization is the prerequisite to study any such difference. Mobile applications will be particularly useful in low-resource settings, where disease severity may be higher and decisions have to be taken instantly. Finally, the effect of antiviral treatment or vaccine prevention on disease severity could not be assessed due to a minimal use of neuraminidase inhibitors and influenza vaccines in the current setting [182]. The only conclusion that can be drawn is that physicians in this setting hardly ever used antivirals but were more likely to prescribe antibiotics if a patient appeared severely ill, as expressed in significantly higher ViVI Disease Severity Scores [30]. It will be interesting to study decision-making processes and the impact of different forms of medical interventions on disease severity in a variety of settings in the future.
5. Expert commentary
At this point, the majority of sentinel surveillance systems are laboratory based yielding limited clinical information but important data with respect to the evolution of influenza viruses, subtypes, resistance, seasonality, and transmissibility. It would be of great benefit to monitor disease severity individually, along with regional and geographic differences in virus circulation, using standardized disease severity measurements such as the ViVI Disease Severity Score.
Our contributions are the following: (A) The design of a hospital-based surveillance program and a unique QM cohort of more than 6000 children, where an independent QM team monitored patients daily using standardized clinical assessments and virology at the National Reference Centre for Influenza and Other Respiratory Viruses. (B) A novel disease severity score (the ViVI Disease Severity Score) and mobile application to detect specific changes in IPD and the individual course of illness in pediatric clinical trials and observational settings.
The presented tools are In line with the priorities issued by regulatory agencies with regards to data standardization and the development of clinical outcome measures for the development of new antivirals. With composite disease severity scores, the focus will shift from virological to clinical end points, and the impact of therapeutic interventions on the quality of disease presentations. Only the systematic unbiased and prospective assessment of all cases, whether mild or severe, throughout several seasons, will provide objective insight into the actual disease burden with influenza and other respiratory viruses.
6. Five-year view
Mobile health technologies enable new precision medicine approaches not only in clinical trials but also in routine patient care. Individualized disease severity assessments in children with influenza and other respiratory viruses will allow the physician to communicate better with the parent or patient, providing the current status as a validated measure of disease severity compared to similar age and population strata. In patents receiving antiviral therapy, progress can be measured and communicated accordingly and again, individually.
Most importantly, with the availability of validated disease severity measures and standardized datasets, the physician will be able to determine which patients may be ‘lagging behind’ in their response to therapeutic interventions. A better understanding of the complex relationship between virus load and disease severity in children with different respiratory viruses will provide important clues for a personalized approach to antiviral therapy.
Biomarker analyses linked to standardized disease severity assessments will help to elucidate why some patients improve rapidly as soon as virus loads decline, whereas a smaller group of patients does not improve as expected. This latter subgroup of patients may benefit from different therapeutic approaches, for example immunomodulation. Precision medicine tools such as the ViVI Disease Severity Score mobile application will provide important tools for the objective evaluation of new antivirals for soon-to-be treatable respiratory viruses.
Key issues
Regulatory agencies and public health stakeholders have repeatedly called for international consensus on disease severity measures in influenza and other respiratory viruses.
This need has become imminent with the rapid development of new anti-infective therapies for respiratory viral infections in children and adults.
The challenge of data standardization is greatest in infants and young children, who may present with subtle and atypical symptoms.
Based on a systematic review of the literature we developed a 22-item composite clinical score (the ViVI Disease Severity Score) for the immediate measurement of disease severity with acute reparatory infections the point-of-care.
The ViVI Disease Severity Score was made available as a web-user interface and mobile application for validation in a quality management program including more than 6000 children 0–18 years of age.
Linking standardized diseases severity scores with rapid diagnostics will allow the instantaneous monitoring of incidence rates of acute respiratory viral infections along with the severity of each case.
With this comprehensive manuscript, we are providing insight into the future of observational studies and clinical trials of antivirals for soon-to-be-treatable acute respiratory diseases in children.
The reader is guided through novel analytic approaches that have become possible through rigorously standardized individual patient-data (IPD) analyses of disease severity.
Acknowledgments
BR wrote the initial draft of the manuscript. MA, FT, XC and PO were in charge of data aggregation, acquisition and QC/QA, and provided important input into the manuscript. XM conducted and interpreted the systematic literature review. BK and CH were in charge of data standardization, CH provided database maintenance and management. BS designed and supervised the laboratory analyses. TC, PM conducted the data analysis. BR designed the QM Program and the ViVI Disease Severity Score and supervised the project. All Authors take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have seen and approved the final version of the manuscript.
The authors would like to express their gratitude to the team at the Robert Koch Institute for providing virology testing in-kind and to the Vienna Vaccine Safety Initiative for providing the ViVI Disease Severity Score and mobile application. TC was funded by the German Ministry of Research and Education (BMBF) project grant 3FO18501 (Forschungscampus MODAL). The authors would also like to express their thanks to members of the ViVI Think Tank for their expert feedback and encouragement throughout the course of the project.
Supplemental meterial
Supplemental data for this article can be accessed here.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Moral L, Marco N, Toral T, et al. Burden of severe 2009 pandemic influenza A (H1N1) infection in children in Southeast Spain. Enferm Infecc Microbiol Clin. 2011;29(7):497–501. [DOI] [PubMed] [Google Scholar]
- 2.Miller EK, Griffin MR, Edwards KM, et al. Influenza burden for children with asthma. Pediatrics. 2008;121(1):1–8. [DOI] [PubMed] [Google Scholar]
- 3.Gunson RN, Carman WF.. During the summer 2009 outbreak of “swine flu” in Scotland what respiratory pathogens were diagnosed as H1N1/2009? BMC Infect Dis. 2011;11:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361(26):2507–2517. [DOI] [PubMed] [Google Scholar]; •• Unique data describing clinical features of pandemic influenza infection (see Supplementary Data).
- 5.Babcock HM, Merz LR, Dubberke ER, et al. Case-control study of clinical features of influenza in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(10):921–926. [DOI] [PubMed] [Google Scholar]
- 6.Babcock HM, Merz LR, Fraser VJ.. Is influenza an influenza-like illness? Clinical presentation of influenza in hospitalized patients. Infect Control Hosp Epidemiol. 2006;27(3):266–270. [DOI] [PubMed] [Google Scholar]
- 7.Mizuta K, Abiko C, Aoki Y, et al. Seasonal patterns of respiratory syncytial virus, influenza A virus, human metapneumovirus, and parainfluenza virus type 3 infections on the basis of virus isolation data between 2004 and 2011 in Yamagata, Japan. Jpn J Infect Dis. 2013;66(2):140–145. [DOI] [PubMed] [Google Scholar]
- 8.Chan KP, Wong CM, Chiu SS, et al. A robust parameter estimation method for estimating disease burden of respiratory viruses. Plos One. 2014;9(3):e90126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blair W, Cox C. Current landscape of antiviral drug discovery. F1000Res. 2016;5 doi: 10.12688/f1000research.7665.1. eCollection 2016. PMID: 26962437 PMCID: PMC4765712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonelli M, Cichero E. Fight against H1N1 influenza a virus: recent insights towards the development of druggable compounds. Curr Med Chem. 2016;23(18):1802–1817. [DOI] [PubMed] [Google Scholar]
- 11.Cox R, Plemper RK. The paramyxovirus polymerase complex as a target for next-generation anti-paramyxovirus therapeutics. Front Microbiol. 2015;6:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden FG. Advances in antivirals for non-influenza respiratory virus infections. Influenza Other Respir Viruses. 2013;7(Suppl 3):36–43. . [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Key review paper on antiviral drug development for non-influenza respiratory viruses
- 13.Dropulic LK, Cohen JI. Update on new antivirals under development for the treatment of double-stranded DNA virus infections. Clin Pharmacol Ther. 2010;88(5):610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thibaut HJ, Lacroix C, De Palma AM, et al. Toward antiviral therapy/prophylaxis for rhinovirus-induced exacerbations of chronic obstructive pulmonary disease: challenges, opportunities, and strategies. Rev Med Virol. 2015;26(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Der Linden L, Wolthers KC, Van Kuppeveld FJ. Replication and inhibitors of enteroviruses and parechoviruses. Viruses. 2015;7(8):4529–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thibaut HJ, De Palma AM, Neyts J. Combating enterovirus replication: state-of-the-art on antiviral research. Biochem Pharmacol. 2012;83(2):185–192. [DOI] [PubMed] [Google Scholar]
- 17.De Clercq E. Chemotherapy of Viral Infections In: Baron S, editor. Medical microbiology. Galveston (TX): University of Texas Medical Branch at Galveston. The University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- 18.Rath B, Tief F, Karsch K, et al. Towards a personalised approach to managing influenza infections in infants and children - food for thought and a note on oseltamivir. Infect Disord Drug Targets. 2013;13(1):25–33. [DOI] [PubMed] [Google Scholar]; • Position paper describing the overall concept of the quality management in children with ILI, including systematic disease severity assessments and point-of-care diagnostics.
- 19.Matias G, Taylor RJ, Haguinet F, et al. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016;16(1):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh P. The continued rise of respiratory viruses. MLO Med Lab Obs. 2011;43(9):8, 10–2, 14. [PubMed] [Google Scholar]
- 21.Wongsawat J, Chittaganpitch M, Ampornareekul S, et al. The validity of clinical practice guidelines for empirical use of oseltamivir for influenza in Thai children. Paediatr Int Child Health. 2016;36(4):275–281. [DOI] [PubMed] [Google Scholar]
- 22.Abraham MK, Perkins J, Vilke GM, et al. Influenza in the emergency department: vaccination, diagnosis, and treatment: clinical practice paper approved by american academy of emergency medicine clinical guidelines committee. J Emerg Med. 2016;50(3):536–542. [DOI] [PubMed] [Google Scholar]
- 23.Shrestha S, Foxman B, Berus J, et al. The role of influenza in the epidemiology of pneumonia. Sci Rep. 2015;5:15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell AP, Guthrie KA, Englund JA, et al. Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clin Infect Dis. 2015;61(2):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trachtenberg AJ, Dik N, Chateau D, et al. Inequities in ambulatory care and the relationship between socioeconomic status and respiratory hospitalizations: a population-based study of a canadian city. Ann Fam Med. 2014;12(5):402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Example of a study illustrating the limitations of using hospitalization as an indicator of disease severity.
- 26.Al-Samarrai T, Wu W, Begier E, et al. Evaluation of a pilot respiratory virus surveillance system linking electronic health record and diagnostic data. J Public Health Manag Pract. 2013;19(4):322–329. [DOI] [PubMed] [Google Scholar]
- 27.Fairbrother G, Cassedy A, Ortega-Sanchez IR, et al. High costs of influenza: direct medical costs of influenza disease in young children. Vaccine. 2010;28(31):4913–4919. [DOI] [PubMed] [Google Scholar]
- 28.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355(1):31–40. [DOI] [PubMed] [Google Scholar]
- 29.Poeppl W, Hell M, Herkner H, et al. Clinical aspects of 2009 pandemic influenza A (H1N1) virus infection in Austria. Infection. 2011;39(4):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tief F, Hoppe C, Seeber L, et al. An inception cohort study assessing the role of pneumococcal and other bacterial pathogens in children with influenza and ILI and a clinical decision model for stringent antibiotic use. Antivir Ther. 2016;21(5):413–424. [DOI] [PubMed] [Google Scholar]; •• Key publication illustrating the use of the ViVI Score in the QM program.
- 31.Tuttle R, Weick A, Schwarz WS, et al. Evaluation of novel second-generation RSV and influenza rapid tests at the point of care. Diagn Microbiol Infect Dis. 2015;81(3):171–176. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Pouran Yousef K, Duwe S, et al. Quantitative influenza follow-up testing (QIFT)–a novel biomarker for the monitoring of disease activity at the point-of-care. Plos One. 2014;9(3):e92500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rath B, Tief F, Obermeier P, et al. Early detection of influenza A and B infection in infants and children using conventional and fluorescence-based rapid testing. J Clin Virol. 2012;55(4):329–333. [DOI] [PubMed] [Google Scholar]
- 34.Rath B, Von Kleist M, Tief F, et al. Virus load kinetics and resistance development during oseltamivir treatment in infants and children infected with Influenza A(H1N1) 2009 and Influenza B viruses. Pediatr Infect Dis J. 2012;31(9):899–905. [DOI] [PubMed] [Google Scholar]
- 35.WHO Clinical management of human infection with pandemic (H1N1) 2009: revised guidance; 2009. [cited 2015 December12] Available from: http://www.who.int/csr/resources/publications/swineflu/clinical_management_h1n1.pdf.; •• WHO criteria for uncomplicated and complicated disease as formulated at the time of the 2009 influenza pandemic.
- 36.Gill PJ, Ashdown HF, Wang K, et al. Identification of children at risk of influenza-related complications in primary and ambulatory care: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(2):139–149. [DOI] [PubMed] [Google Scholar]
- 37.Ma HY, Wu JL, Lu CY, et al. Risk factors associated with severe influenza virus infections in hospitalized children during the 2013 to 2014 season. J Microbiol Immunol Infect. 2016;49(3):387–393. [DOI] [PubMed] [Google Scholar]
- 38.Meerhoff TJ, Simaku A, Ulqinaku D, et al. Surveillance for severe acute respiratory infections (SARI) in hospitals in the WHO European region - an exploratory analysis of risk factors for a severe outcome in influenza-positive SARI cases. BMC Infect Dis. 2015;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uphoff H, Buchholz U, Lang A, et al. Calculation of the incidence of primary care visits due to acute respiratory infections. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2004;47(3):279–287. [DOI] [PubMed] [Google Scholar]; •• RKI publication describing the Consultation Index.
- 40.Karsch K, Chen X, Miera O, et al. Pharmacokinetics of oral and intravenous oseltamivir treatment of severe influenza B virus infection requiring organ replacement therapy. Eur J Drug Metab Pharmacokinet. 2016;42(1):155–164. [DOI] [PubMed] [Google Scholar]
- 41.Biere B, Bauer B, Schweiger B. Differentiation of influenza B virus lineages Yamagata and Victoria by real-time PCR. J Clin Microbiol. 2010;48(4):1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiche J, Schweiger B. Genetic variability of group A human respiratory syncytial virus strains circulating in Germany from 1998 to 2007. J Clin Microbiol. 2009;47(6):1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulze M, Nitsche A, Schweiger B, et al. Diagnostic approach for the differentiation of the pandemic influenza A(H1N1)v virus from recent human influenza viruses by real-time PCR. Plos One. 2010;5(4):e9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chmielewicz B, Nitsche A, Schweiger B, et al. Development of a PCR-based assay for detection, quantification, and genotyping of human adenoviruses. Clin Chem. 2005;51(8):1365–1373. [DOI] [PubMed] [Google Scholar]
- 45.Reiche J, Jacobsen S, Neubauer K, et al. Human metapneumovirus: insights from a ten-year molecular and epidemiological analysis in Germany. Plos One. 2014;9(2):e88342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedmann J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 47.Quinlan JR. C 4.5: programs for machine learning. San Francisco (CA): Morgan Kaufmann Publishers; 1993. [Google Scholar]
- 48.Meerbach E, Latorre J, Schütte C. Sequential change point detection in molecular dynamics trajectories. Multicale Model Sim. 2012;10(4):1263–1291. [Google Scholar]
- 49.Forgy EW. Cluster analysis of multivariate data: efficiency versus interpretability of classifications. Biometrics. 1965;21:768–769. [Google Scholar]
- 50.Sung CC, Chi H, Chiu NC, et al. Viral etiology of acute lower respiratory tract infections in hospitalized young children in Northern Taiwan. J Microbiol Immunol Infect. 2011;44(3):184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed C, Madhi SA, Klugman KP, et al. Development of the Respiratory Index of Severity in Children (RISC) score among young children with respiratory infections in South Africa. Plos One. 2012;7(1):e27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valet RS, Gebretsadik T, Carroll KN, et al. Increased healthcare resource utilization for acute respiratory illness among Latino infants. J Pediatr. 2013;163(4):1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pedraza-Bernal AM, Rodriguez-Martinez CE, Acuna-Cordero R. Predictors of severe disease in a hospitalized population of children with acute viral lower respiratory tract infections. J Med Virol. 2016;88(5):754–759. [DOI] [PubMed] [Google Scholar]
- 54.Skjerven HO, Megremis S, Papadopoulos NG, et al. Virus type and genomic load in acute bronchiolitis: severity and treatment response with inhaled adrenaline. J Infect Dis. 2016;213(6):915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moesker FM, Van Kampen JJ, Van Rossum AM, et al. Viruses as sole causative agents of severe acute respiratory tract infections in children. Plos One. 2016;11(3):e0150776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong L, Dai L, Fan J, et al. Epidemiologic characteristics and the relationship with disease severity of respiratory syncytial virus genotypes from children with lower respiratory tract infection in the southern Zhejiang province. Zhonghua Er Ke Za Zhi. 2015;53(7):537–541. [PubMed] [Google Scholar]
- 57.Martin ET, Kuypers J, Wald A, et al. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses. 2012;6(1):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franz A, Adams O, Willems R, et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010;48(4):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Example of a study exploring the complex relationship between virus load and disease severity.
- 59.Turunen R, Koistinen A, Vuorinen T, et al. The first wheezing episode: respiratory virus etiology, atopic characteristics, and illness severity. Pediatr Allergy Immunol. 2014;25(8):796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brand HK, De Groot R, Galama JM, et al. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol. 2012;47(4):393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Example of a study exploring the complex relationship between viral coinfections and disease severity in children.
- 61.Petrie JG, Cheng C, Malosh RE, et al. Illness severity and work productivity loss among working adults with medically attended acute respiratory illnesses: US influenza vaccine effectiveness network 2012-2013. Clin Infect Dis. 2016;62(4):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baird JS, Buet A, Hymes SR, et al. Comparing the clinical severity of the first versus second wave of 2009 Influenza A (H1N1) in a New York City pediatric healthcare facility. Pediatr Crit Care Med. 2012;13(4):375–380. [DOI] [PubMed] [Google Scholar]
- 63.Doshi SS, Stauffer KE, Fiebelkorn AP, et al. The burden and severity of illness due to 2009 pandemic influenza A (H1N1) in a large US city during the late summer and early fall of 2009. Am J Epidemiol. 2012;176(6):519–526. [DOI] [PubMed] [Google Scholar]
- 64.Chiaretti A, Pulitano S, Conti G, et al. Interleukin and neurotrophin up-regulation correlates with severity of H1N1 infection in children: a case-control study. Int J Infect Dis. 2013;17(12):e1186–93. [DOI] [PubMed] [Google Scholar]
- 65.Miroballi Y, Baird JS, Zackai S, et al. Novel influenza A(H1N1) in a pediatric health care facility in New York City during the first wave of the 2009 pandemic. Arch Pediatr Adolesc Med. 2010;164(1):24–30. [DOI] [PubMed] [Google Scholar]
- 66.Koh MT, Eg KP, Loh SS. Hospitalised Malaysian children with pandemic (H1N1) 2009 influenza: clinical characteristics, risk factors for severe disease and comparison with the 2002-2007 seasonal influenza. Singapore Med J. 2016;57(2):81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu C, Iuliano AD, Chen M, et al. Characteristics of hospitalized cases with influenza A (H1N1)pdm09 infection during first winter season of post-pandemic in China. Plos One. 2013;8(2):e55016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Virlogeux V, Yang J, Fang VJ, et al. Association between the severity of influenza A(H7N9) virus infections and length of the incubation period. Plos One. 2016;11(2):e0148506. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Example of a study exploring the complex relationship between incubation time and disease severity.
- 69.Yang SQ, Qu JX, Wang C, et al. Influenza pneumonia among adolescents and adults: a concurrent comparison between influenza A (H1N1) pdm09 and A (H3N2) in the post-pandemic period. Clin Respir J. 2014;8(2):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tasher D, Stein M, Solomon C, et al. Children hospitalised with influenza-associated pneumonia during the 2009 pandemic displayed increased disease severity. Acta Paediatr. 2015;104(3):e100–5. [DOI] [PubMed] [Google Scholar]
- 71.Burton C, Vaudry W, Moore D, et al. Burden of seasonal influenza in children with neurodevelopmental conditions. Pediatr Infect Dis J. 2014;33(7):710–714. [DOI] [PubMed] [Google Scholar]
- 72.Garcia MN, Philpott DC, Murray KO, et al. Clinical predictors of disease severity during the 2009-2010 A(HIN1) influenza virus pandemic in a paediatric population. Epidemiol Infect. 2015;143(14):2939–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Launes C, Garcia-Garcia JJ, Jordan I, et al. Viral load at diagnosis and influenza A H1N1 (2009) disease severity in children. Influenza Other Respir Viruses. 2012;6(6):e89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Example of a study exploring the complex relationship between virus load and disease severity.
- 74.Hayward AC, Fragaszy EB, Bermingham A, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014;2(6):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Example of a study of disease burden based on epidemiological criteria.
- 75.Oliveira TF, Freitas GR, Ribeiro LZ, et al. Prevalence and clinical aspects of respiratory syncytial virus A and B groups in children seen at Hospital de Clinicas of Uberlandia, MG, Brazil. Mem Inst Oswaldo Cruz. 2008;103(5):417–422. [DOI] [PubMed] [Google Scholar]
- 76.Bamberger E, Srugo I, Abu Raya B, et al. What is the clinical relevance of respiratory syncytial virus bronchiolitis?: findings from a multi-center, prospective study. Eur J Clin Microbiol Infect Dis. 2012;31(12):3323–3330. [DOI] [PubMed] [Google Scholar]
- 77.Zhang RF, Jin Y, Xie ZP, et al. Human respiratory syncytial virus in children with acute respiratory tract infections in China. J Clin Microbiol. 2010;48(11):4193–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vieira RA, Diniz EM, Ceccon ME. Correlation between inflammatory mediators in the nasopharyngeal secretion and in the serum of children with lower respiratory tract infection caused by respiratory syncytial virus and disease severity. J Bras Pneumol. 2010;36(1):59–66. [DOI] [PubMed] [Google Scholar]
- 79.Mejias A, Dimo B, Suarez NM, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. Plos Med. 2013;10(11):e1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Example of a study exploring the complex relationship between biomarkers and disease severity.
- 80.Mella C, Suarez-Arrabal MC, Lopez S, et al. Innate immune dysfunction is associated with enhanced disease severity in infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2013;207(4):564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aydin B, Zenciroglu A, Dilli D, et al. Clinical course of community-acquired respiratory syncytial virus pneumonia in newborns hospitalized in neonatal intensive care unit. Tuberk Toraks. 2013;61(3):235–244. [PubMed] [Google Scholar]
- 82.Mosalli R, Abdul Moez AM, Janish M, et al. Value of a risk scoring tool to predict respiratory syncytial virus disease severity and need for hospitalization in term infants. J Med Virol. 2015;87(8):1285–1291. [DOI] [PubMed] [Google Scholar]
- 83.Schene KM, Van Den Berg E, Wosten-Van Asperen RM, et al. FiO2 predicts outcome in infants with respiratory syncytial virus-induced acute respiratory distress syndrome. Pediatr Pulmonol. 2014;49(11):1138–1144. [DOI] [PubMed] [Google Scholar]
- 84.Borckink I, Essouri S, Laurent M, et al. Infants with severe respiratory syncytial virus needed less ventilator time with nasal continuous airways pressure then invasive mechanical ventilation. Acta Paediatr. 2014;103(1):81–85. [DOI] [PubMed] [Google Scholar]
- 85.Kong MY, Clancy JP, Peng N, et al. Pulmonary matrix metalloproteinase-9 activity in mechanically ventilated children with respiratory syncytial virus. Eur Respir J. 2014;43(4):1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grimwood K, Cohet C, Rich FJ, et al. Risk factors for respiratory syncytial virus bronchiolitis hospital admission in New Zealand. Epidemiol Infect. 2008;136(10):1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gilca R, De Serres G, Tremblay M, et al. Distribution and clinical impact of human respiratory syncytial virus genotypes in hospitalized children over 2 winter seasons. J Infect Dis. 2006;193(1):54–58. [DOI] [PubMed] [Google Scholar]
- 88.Panayiotou C, Richter J, Koliou M, et al. Epidemiology of respiratory syncytial virus in children in Cyprus during three consecutive winter seasons (2010-2013): age distribution, seasonality and association between prevalent genotypes and disease severity. Epidemiol Infect. 2014;142(11):2406–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tran DN, Pham TM, Ha MT, et al. Molecular epidemiology and disease severity of human respiratory syncytial virus in Vietnam. Plos One. 2013;8(1):e45436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Houben ML, Coenjaerts FE, Rossen JW, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82(7):1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suarez-Arrabal MC, Mella C, Lopez SM, et al. Nasopharyngeal bacterial burden and antibiotics: influence on inflammatory markers and disease severity in infants with respiratory syncytial virus bronchiolitis. J Infect. 2015;71(4):458–469. [DOI] [PubMed] [Google Scholar]
- 92.Hasegawa K, Jartti T, Mansbach JM, et al. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis. 2015;211(10):1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moreno-Perez D, Calvo C, Five Study G. Epidemiological and clinical data of hospitalizations associated with respiratory syncytial virus infection in children under 5 years of age in Spain: FIVE multicenter study. Influenza Other Respir Viruses. 2014;8(2):209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Somech R, Tal G, Gilad E, et al. Epidemiologic, socioeconomic, and clinical factors associated with severity of respiratory syncytial virus infection in previously healthy infants. Clin Pediatr (Phila). 2006;45(7):621–627. [DOI] [PubMed] [Google Scholar]
- 95.Fodha I, Vabret A, Ghedira L, et al. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. J Med Virol. 2007;79(12):1951–1958. [DOI] [PubMed] [Google Scholar]
- 96.El Saleeby CM, Li R, Somes GW, et al. Surfactant protein A2 polymorphisms and disease severity in a respiratory syncytial virus-infected population. J Pediatr. 2010;156(3):409–414. [DOI] [PubMed] [Google Scholar]
- 97.El Saleeby CM, Bush AJ, Harrison LM, et al. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis. 2011;204(7):996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Somers CC, Ahmad N, Mejias A, et al. Effect of dexamethasone on respiratory syncytial virus-induced lung inflammation in children: results of a randomized, placebo controlled clinical trial. Pediatr Allergy Immunol. 2009;20(5):477–485. [DOI] [PubMed] [Google Scholar]
- 99.Kurji A, Tan B, Bodani J, et al. Children hospitalized with respiratory syncytial virus infection in Saskatchewan pediatric tertiary care centers, 2002-2005. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16(10):1005–1013. [PubMed] [Google Scholar]
- 100.Kim Y-I, Murphy R, Majumdar S, et al. Relating plaque morphology to respiratory syncytial virus subgroup, viral load, and disease severity in children. Pediatr Res. 2015;78(4):380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tabarani CM, Bonville CA, Suryadevara M, et al. Novel inflammatory markers, clinical risk factors and virus type associated with severe respiratory syncytial virus infection. Pediatr Infect Dis J. 2013;32(12):e437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.García CG, Bhore R, Soriano-Fallas A, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126(6):e1453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brand HK, Ahout IM, De Ridder D, et al. Olfactomedin 4 serves as a marker for disease severity in pediatric Respiratory Syncytial Virus (RSV) infection. Plos One. 2015;10(7):e0131927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dotan M, Ashkenazi-Hoffnung L, Samra Z, et al. Hospitalization for respiratory syncytial virus bronchiolitis and disease severity in twins. Isr Med Assoc J. 2013;15(11):701–704. [PubMed] [Google Scholar]
- 105.Thompson TM, Roddam PL, Harrison LM, et al. Viral specific factors contribute to clinical respiratory syncytial virus disease severity differences in infants. Clin Microbiol. 2015;4(3):pii:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stagliano DR, Nylund CM, Eide MB, et al. Children with Down syndrome are high-risk for severe respiratory syncytial virus disease. J Pediatr. 2015;166(3):703–9e2. [DOI] [PubMed] [Google Scholar]
- 107.Gijtenbeek RG, Kerstjens JM, Reijneveld SA, et al. RSV infection among children born moderately preterm in a community-based cohort. Eur J Pediatr. 2015;174(4):435–442. [DOI] [PubMed] [Google Scholar]
- 108.Faber TE, Schuurhof A, Vonk A, et al. IL1RL1 gene variants and nasopharyngeal IL1RL-a levels are associated with severe RSV bronchiolitis: a multicenter cohort study. Plos One. 2012;7(5):e34364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Forbes ML, Kumar VR, Yogev R, et al. Serum palivizumab level is associated with decreased severity of respiratory syncytial virus disease in high-risk infants. Hum Vaccin Immunother. 2014;10(10):2789–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goncalves A, Rocha G, Guimaraes H, et al. Value of chest radiographic pattern in RSV disease of the newborn: a multicenter retrospective cohort study. Crit Care Res Pract. 2012;2012:861867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Semple MG, Dankert HM, Ebrahimi B, et al. Severe respiratory syncytial virus bronchiolitis in infants is associated with reduced airway interferon gamma and substance P. Plos One. 2007;2(10):e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schuurhof A, Bont L, Hodemaekers HM, et al. Proteins involved in extracellular matrix dynamics are associated with respiratory syncytial virus disease severity. Eur Respir J. 2012;39(6):1475–1481. [DOI] [PubMed] [Google Scholar]
- 113.Vissers M, Ahout IM, Van Den Kieboom CH, et al. High pneumococcal density correlates with more mucosal inflammation and reduced respiratory syncytial virus disease severity in infants. BMC Infect Dis. 2016;16(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thorburn K, Eisenhut M, Shauq A, et al. Right ventricular function in children with severe respiratory syncytial virus (RSV) bronchiolitis. Minerva Anestesiol. 2011;77(1):46–53. [PubMed] [Google Scholar]
- 115.Kaplan NM, Dove W, Abd-Eldayem SA, et al. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J Med Virol. 2008;80(1):168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Faneye A, Motayo BO, Adesanmi A, et al. Evaluation of IgG antibodies against Respiratory Syncytial Virus (RSV), and associated risk factors for severe respiratory tract infections in pre-school children in north-central, Nigeria. Afr J Infect Dis. 2014;8(2):36–39. [PMC free article] [PubMed] [Google Scholar]
- 117.Garcia C, Soriano-Fallas A, Lozano J, et al. Decreased innate immune cytokine responses correlate with disease severity in children with respiratory syncytial virus and human rhinovirus bronchiolitis. Pediatr Infect Dis J. 2012;31(1):86–89. [DOI] [PubMed] [Google Scholar]
- 118.Papenburg J, Hamelin ME, Ouhoummane N, et al. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis. 2012;206(2):178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Midulla F, Scagnolari C, Bonci E, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 2010;95(1):35–41. [DOI] [PubMed] [Google Scholar]
- 120.Martin ET, Kuypers J, Heugel J, et al. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis. 2008;62(4):382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hahn A, Wang W, Jaggi P, et al. Human metapneumovirus infections are associated with severe morbidity in hospitalized children of all ages. Epidemiol Infect. 2013;141(10):2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roussy JF, Carbonneau J, Ouakki M, et al. Human metapneumovirus viral load is an important risk factor for disease severity in young children. J Clin Virol. 2014;60(2):133–140. [DOI] [PubMed] [Google Scholar]
- 123.Davis CR, Stockmann C, Pavia AT, et al. Incidence, morbidity, and costs of human metapneumovirus infection in hospitalized children. J Pediatric Infect Dis Soc. 2016;5(3):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caracciolo S, Minini C, Colombrita D, et al. Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease: virologic and clinical features. Pediatr Infect Dis J. 2008;27(5):406–412. [DOI] [PubMed] [Google Scholar]
- 125.Schuster JE, Khuri-Bulos N, Faouri S, et al. Human metapneumovirus infection in jordanian children: epidemiology and risk factors for severe disease. Pediatr Infect Dis J. 2015;34(12):1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Costa LF, Queiroz DA, Lopes Da Silveira H, et al. Human rhinovirus and disease severity in children. Pediatrics. 2014;133(2):e312–21. [DOI] [PubMed] [Google Scholar]
- 127.Xiao Q, Zheng S, Zhou L, et al. Impact of human rhinovirus types and viral load on the severity of illness in hospitalized children with lower respiratory tract infections. Pediatr Infect Dis J. 2015;34(11):1187–1192. [DOI] [PubMed] [Google Scholar]
- 128.Chen WJ, Arnold JC, Fairchok MP, et al. Epidemiologic, clinical, and virologic characteristics of human rhinovirus infection among otherwise healthy children and adults: rhinovirus among adults and children. J Clin Virol. 2015;64:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Asner SA, Petrich A, Hamid JS, et al. Clinical severity of rhinovirus/enterovirus compared to other respiratory viruses in children. Influenza Other Respir Viruses. 2014;8(4):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao B, Yu X, Wang C, et al. High human bocavirus viral load is associated with disease severity in children under five years of age. Plos One. 2013;8(4):e62318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tran DN, Nguyen TQ, Nguyen TA, et al. Human bocavirus in children with acute respiratory infections in Vietnam. J Med Virol. 2014;86(6):988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jean A, Quach C, Yung A, et al. Severity and outcome associated with human coronavirus OC43 infections among children. Pediatr Infect Dis J. 2013;32(4):325–329. [DOI] [PubMed] [Google Scholar]
- 133.Ziemann A, Fouillet A, Brand H, et al. Success factors of European syndromic surveillance systems: a worked example of applying qualitative comparative analysis. Plos One. 2016;11(5):e0155535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoppe C, Obermeier P, Muehlhans S, et al. Innovative digital tools and surveillance systems for the timely detection of adverse events at the point of care: a proof-of-concept study. Drug Saf. 2016;39(10):977–988. [DOI] [PubMed] [Google Scholar]
- 135.Obermeier P, Muehlhans S, Hoppe C, et al. Enabling precision medicine with digital case classification at the point-of-care. EBioMedicine. 2016;4:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Atkinson KM, Westeinde J, Ducharme R, et al. Can mobile technologies improve on-time vaccination? A study piloting maternal use of ImmunizeCA, a Pan-Canadian immunization app. Hum Vaccin Immunother. 2016;12(10):2654–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Manaktala S, Claypool SR. Evaluating the impact of a computerized surveillance algorithm and decision support system on sepsis mortality. J Am Med Inform Assoc. 2016;23(6):1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Donaldson RI, Ostermayer DG, Banuelos R, et al. Development and usage of wiki-based software for point-of-care emergency medical information. J Am Med Inform Assoc. 2016;23(6):1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wilson K, Atkinson KM, Westeinde J, et al. An evaluation of the feasibility and usability of a proof of concept mobile app for adverse event reporting post influenza vaccination. Hum Vaccin Immunother. 2016;12(7):1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ginsburg AS, Delarosa J, Brunette W, et al. mPneumonia: development of an Innovative mHealth application for diagnosing and treating childhood pneumonia and other childhood illnesses in low-resource settings. Plos One. 2015;10(10):e0139625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Evans B, Charlett A, Powers C, et al. Has estimation of numbers of cases of pandemic influenza H1N1 in England in 2009 provided a useful measure of the occurrence of disease? Influenza Other Respi Viruses. 2011;5(6):e504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Study exploring various parameters commonly used for disease burden estimates.
- 142.Skowronski DM, Janjua NZ, De Serres G, et al. A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010-2011 season. Clin Infect Dis. 2012;55(3):332–342. [DOI] [PubMed] [Google Scholar]
- 143.Lambert SB, Faux CE, Grant KA, et al. Influenza surveillance in Australia: we need to do more than count. Med J Aust. 2010;193(1):43–45. [DOI] [PubMed] [Google Scholar]
- 144.Tief F, Hoppe C, Seeber L, et al. An inception cohort study assessing the role of pneumococcal and other bacterial pathogens in children with influenza and ILI and a clinical decision model for stringent antibiotic use. Antivir Ther. 2016;21(5):413-424. [DOI] [PubMed] [Google Scholar]
- 145.Jiang G, Evans J, Endle CM, et al. Using Semantic Web technologies for the generation of domain-specific templates to support clinical study metadata standards. J Biomed Semantics. 2016;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.El Fadly A, Daniel C, Bousquet C, et al. Electronic Healthcare Record and clinical research in cardiovascular radiology. HL7 CDA and CDISC ODM interoperability In: AMIA Annu Symp Proc. 2007. p. 216–220. PMID: 18693829 PMCID: PMC2655824. [PMC free article] [PubMed] [Google Scholar]
- 147.De Moor G, Sundgren M, Kalra D, et al. Using electronic health records for clinical research: the case of the EHR4CR project. J Biomed Inform. 2015;53:162–173. [DOI] [PubMed] [Google Scholar]
- 148.Hume S, Aerts J, Sarnikar S, et al. Current applications and future directions for the CDISC Operational Data Model standard: A methodological review. J Biomed Inform. 2016;60:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hochedlinger N, Nitzlnader M, Falgenhauer M, et al. Standardized data sharing in a paediatric oncology research network–a proof-of-concept study. Stud Health Technol Inform. 2015;212:27–34. [PubMed] [Google Scholar]
- 150.Jiang G, Evans J, Oniki TA, et al. Harmonization of detailed clinical models with clinical study data standards. Methods Inf Med. 2015;54(1):65–74. [DOI] [PubMed] [Google Scholar]
- 151.Marcy SM, Kohl KS, Dagan R, et al. Fever as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22(5–6):551–556. [DOI] [PubMed] [Google Scholar]
- 152.Roth DE, Caulfield LE, Ezzati M, et al. Acute lower respiratory infections in childhood: opportunities for reducing the global burden through nutritional interventions. Bull World Health Organ. 2008;86(5):356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Randolph AG, Agan AA, Flanagan RF, et al. Optimizing virus identification in critically ill children suspected of having an acute severe viral infection. Pediatr Crit Care Med. 2016;17(4):279–286. [DOI] [PubMed] [Google Scholar]
- 154.Su S, Fry AM, Kirley PD, et al. Survey of influenza and other respiratory viruses diagnostic testing in US hospitals, 2012-2013. Influenza Other Respir Viruses. 2016;10(2):86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chartrand C, Tremblay N, Renaud C, et al. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta-analysis. J Clin Microbiol. 2015;53(12):3738–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Timbrook T, Maxam M, Bosso J. Antibiotic discontinuation rates associated with positive respiratory viral panel and low procalcitonin results in proven or suspected respiratory infections. Infect Dis Ther. 2015;4(3):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eggers M, Enders M, Terletskaia-Ladwig E. Evaluation of the becton dickinson rapid influenza diagnostic tests in outpatients in Germany during seven influenza seasons. Plos One. 2015;10(5):e0127070. . [DOI] [PMC free article] [PubMed] [Google Scholar]; • Example of a study exploring the use of rapid diagnostics in respiratory virus surveillance in Germany
- 158.Tamerius J, Nelson MI, Zhou SZ, et al. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ Health Perspect. 2011;119(4):439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Radin JM, Hawksworth AW, Myers CA, et al. Influenza vaccine effectiveness: maintained protection throughout the duration of influenza seasons 2010-2011 through 2013-2014. Vaccine. 2016;34(33):3907–3912. [DOI] [PubMed] [Google Scholar]
- 160.CDC Update: influenza activity - United States, 2011-12 season and composition of the 2012-13 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2012;61(22):414–420. [PubMed] [Google Scholar]
- 161.Bolotin S, Pebody R, White PJ, et al. A new sentinel surveillance system for severe influenza in England shows a shift in age distribution of hospitalised cases in the post-pandemic period. Plos One. 2012;7(1):e30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mytton OT, Rutter PD, Donaldson LJ. Influenza A(H1N1)pdm09 in England, 2009 to 2011: a greater burden of severe illness in the year after the pandemic than in the pandemic year. Euro Surveill. 2012;17(14):pii: 20139. [PubMed] [Google Scholar]
- 163.Vyas A, Ingleton A, Huhtinen E, et al. Influenza outbreak preparedness: lessons from outbreaks in residential care facilities in 2014. Commun Dis Intell Q Rep. 2015;39(2):E204–7. [DOI] [PubMed] [Google Scholar]
- 164.WHO Review of the 2014 influenza season in the southern hemisphere. Wkly Epidemiol Rec. 2014;89(48):529–541. [PubMed] [Google Scholar]
- 165.Cheng AC, Holmes M, Senenayake S, et al. Influenza epidemiology in adults admitted to sentinel Australian hospitals in 2014: the Influenza Complications Alert Network (FluCAN). Commun Dis Intell Q Rep. 2015;39(3):e355–60. [DOI] [PubMed] [Google Scholar]
- 166.Davila J, Chowell G, Borja-Aburto VH, et al. Substantial morbidity and mortality associated with pandemic A/H1N1 influenza in Mexico, Winter 2013-2014: gradual age shift and severity. Plos Curr. 2014. March 26;6 pii:ecurrents.outbreaks.a855a92f19db1d90ca955f5e08d6631. PMID: 24744975 PMCID: PMC3967911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Davila-Torres J, Chowell G, Borja-Aburto VH, et al. Intense seasonal A/H1N1 influenza in Mexico, winter 2013-2014. Arch Med Res. 2015;46(1):63–70. [DOI] [PubMed] [Google Scholar]
- 168.Moss R, Zarebski A, Dawson P, et al. Forecasting influenza outbreak dynamics in Melbourne from Internet search query surveillance data. Influenza Other Respir Viruses. 2016;10(4):314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang S, Santillana M, Kou SC. Accurate estimation of influenza epidemics using Google search data via ARGO. Proc Natl Acad Sci U S A. 2015;112(47):14473–14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kumar S, Quinn SC, Kim KH, et al. The impact of workplace policies and other social factors on self-reported influenza-like illness incidence during the 2009 H1N1 pandemic. Am J Public Health. 2012;102(1):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Slaon-Gardner T, Stirzaker S, Knuckey D, et al. Australia’s notifiable disease status, 2009: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell. 2011;35(2):61–131. [DOI] [PubMed] [Google Scholar]
- 172.Hanvoravongchai P, Coker R. Early reporting of pandemic flu and the challenge of global surveillance: a lesson for Southeast Asia. Southeast Asian J Trop Med Public Health. 2011;42(5):1093–1099. [PubMed] [Google Scholar]
- 173.Escorcia M, Attene-Ramos MS, Estrada MJ, et al. Improving global influenza surveillance: trends of A(H5N1) virus in Africa and Asia. BMC Res Notes. 2012;5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chuang JH, Huang AS, Huang WT, et al. Nationwide surveillance of influenza during the pandemic (2009-10) and post-pandemic (2010-11) periods in Taiwan. Plos One. 2012;7(4):e36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kasowski EJ, Garten RJ, Bridges CB. Influenza pandemic epidemiologic and virologic diversity: reminding ourselves of the possibilities. Clin Infect Dis. 2011;52(Suppl 1):S44–9. [DOI] [PubMed] [Google Scholar]
- 176.Chan J, Holmes A, Rabadan R. Network analysis of global influenza spread. Plos Comput Biol. 2010;6(11):e1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Regan J, Fowlkes A, Biggerstaff M, et al. Epidemiology of influenza A (H1N1)pdm09-associated deaths in the United States, September-October 2009. Influenza Other Respi Viruses. 2012;6(6):e169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reich NG, Lessler J, Cummings DA, et al. Estimating absolute and relative case fatality ratios from infectious disease surveillance data. Biometrics. 2012;68(2):598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Adlhoch C, Wadl M, Behnke M, et al. Pandemic influenza A(H1)pdm09 in hospitals and intensive care units - results from a new hospital surveillance, Germany 2009/2010. Influenza Other Respi Viruses. 2012;6(6):e162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dobrovolny HM, Baron MJ, Gieschke R, et al. Exploring cell tropism as a possible contributor to influenza infection severity. Plos One. 2010;5(11):e13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Godinho CA, Yardley L, Marcu A, et al. Increasing the intent to receive a pandemic influenza vaccination: testing the impact of theory-based messages. Prev Med. 2016;89:104–111. [DOI] [PubMed] [Google Scholar]
- 182.Dharan NJ, Beekmann SE, Fiore A, et al. Influenza antiviral prescribing practices during the 2007-08 and 2008-09 influenza seasons in the setting of increased resistance to oseltamivir among circulating influenza viruses. Antiviral Res. 2010;88(2):182–186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


