Abstract
Nucleic acid amplification techniques such as PCR have facilitated rapid and accurate diagnosis in central laboratories over the past years. PCR-based amplifications require high-precision instruments to perform thermal cycling reactions. Such equipment is bulky, expensive and complex to operate. Progressive advances in isothermal amplification chemistries, microfluidics and detectors miniaturisation are paving the way for the introduction and use of compact ‘sample in-results out’ diagnostic devices. However, this paradigm shift towards decentralised testing poses diverse technological, economic and organizational challenges both in industrialized and developing countries. This review describes the landscape of molecular isothermal diagnostic techniques for infectious diseases, their characteristics, current state of development, and available products, with a focus on new directions towards point-of-care applications.
Keywords: ASSURED, diagnostic, isothermal amplification, molecular test, point-of care
Diverse technologies are currently available for diseases diagnostics in laboratory environments. Culture remains the gold standard of bacteriological diagnosis despite being moderately sensitive, time-consuming and expensive. Culturing also requires specific laboratory infrastructure as well as trained operators. Rapid antigen tests have been described to provide modest diagnostic sensitivity, particularly for vaccinated populations, but they can deliver results in minutes with relatively simple equipment and are less expensive than culture. Tests that are based on nucleic acid amplification (NAAT), such as PCR, are available for many infectious diseases. PCR techniques provide high accuracy with a time to results of few hours and allow multianalyte testing. However, these techniques involve performance of thermal cycling amplifications in high-precision instruments. Such equipment is bulky, expensive and complex to operate, requiring specially trained operators and stringent conditions of laboratory compartmentalization. All these factors constrain performance of PCR out of centralized laboratories.
An ideal diagnostic test should meet the ‘ASSURED’ criteria: affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free and delivered to those who need it (accessible quality-assured diagnostics – 2009 annual report) [1]. Less than US$10 per test and less than US$500 per device could be considered affordable prices for developing countries [2]. New directions are being explored to improve accessibility, rapidity and simplicity of diagnostic tests. Advances in microfluidics and miniaturization of signal detectors have allowed the integration of molecular diagnostics into microscale lab-on-a-chip devices that perform all necessary PCR steps automatically, from sample intake to cell lysis, DNA extraction, purification and amplification. However, there continues to be a gap between first-generation PCR-based point-of-care (POC) tests and ideal ‘ASSURED’ tests in terms of portability, affordability and instrumentation requirements.
Isothermal DNA amplification technologies are emerging as a promising driver of molecular POC diagnostics aiming to meet ‘ASSURED’ attributes. Such techniques have been reported to have analytical sensitivities and specificities comparable to PCR and a higher tolerance to inhibitory compounds, while allowing shorter time to results easier use. Some of these chemistries have the capability of processing raw samples directly without previous DNA purification, thus contributing increased simplicity and additional reductions in cost and time [3].
Although currently isothermal reagents are not cheaper than those for PCR, isothermal techniques avoid the use of bulky, complex and expensive devices in contrast to PCR. Additionally, some of these techniques do not require DNA purification, allowing the use of raw samples. Therefore, isothermal assays pave the way for the development and introduction of inexpensive POC devices with prices that will progressively tend to be closer to lateral flow antigen tests than to PCR.
For example, a device for the detection of Salmonella by PCR (TaqMan® Salmonella enterica Detection Kit; Thermo Fisher Scientific, Life Technologies; CA, USA) costs about US$20,000–30,000, whereas a device for detection of the same pathogen by recombinase polymerase amplification (RPA) (TwistFlow® Salmonella kit, TwistDx; Cambridge, UK) is estimated to cost US$4500. It is also remarkable that those isothermal techniques processing raw samples skip purification steps, reducing by US$4–10 the total cost per sample in comparison with PCR. Overall, isothermal techniques have the potential to make performance easier, bringing additional savings in labor costs.
Diverse studies have described the evolution of isothermal molecular techniques [4,5]. This review describes the state-of-the-art and new directions in the development of isothermal amplification technologies for diagnosis of infectious diseases with particular focus on those susceptible to be integrated in inexpensive molecular POC tests. Most promising techniques, their basic design, state of development and applications are classified and discussed. We also provide a glimpse at the evolution of these applications in the near future and the barriers to overcome for their successful deployment.
Molecular isothermal techniques currently used for diagnosis
Nucleic acid sequence-based amplification
Nucleic acid sequence-based amplification (NASBA) [6] and related techniques, such as transcription mediated amplification [7] and self-sustained sequence replication [8], were the first isothermal amplification reactions mimicking retroviral RNA replication. Emergence of HIV in the 1990s propelled the development of more rapid and reliable HIV detection by NASBA in the early dawn of isothermal NAATs [9]. NASBA is based on three different enzymes participating in the amplification process (Supplementary Figure 1; supplementary material can be found online at www.informahealthcare.com/suppl/10.1586/14737159.2014.940319).
The range of currently commercialized NASBA tests covers solutions integrated into a lateral flow format method (named ‘oligochromatography’) and fluorescent detection assays (Table 1). Among NASBA tests, PANTHER (Gene-Probe) system and TGRIS devices are already available for fully automated process from sample to result. However, because of their large volume and weight, as well as long time consumed to results, such solutions cannot be characterized as ASSURED tests.
Table 1.
Main marketed diagnostic test based on isothermal techniques.
| Target |
Amplex Biosystem (LAMP) |
Eiken Chem. (LAMP) | Biomerieux (NASBA) | Twistdx (RPA) | Envirologix (NEAR) | Coris bioconcept (NASBA) | Meridian Bioscience (LAMP) | Gen-Probe (TMA) | BioHelix† (HDA) | Alere (NEAR) | BD Probetec (SDA) |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Bacteria | |||||||||||
| A. baumannii | √ | ||||||||||
| Campylobacter | √ | ||||||||||
| Chlamydia trachomatis | √ | √ | |||||||||
| Clavibacter michiganesis | √ | ||||||||||
| Clostridium difficile | √ | √ | |||||||||
| Enterovirus | √ | ||||||||||
| Escherichia coli O157 | √ | ||||||||||
| Group A Streptococcus | √ | ||||||||||
| Group B Streptococcus | √ | ||||||||||
| Legionella sp. | √ | ||||||||||
| M. tuberculosis | √ | ||||||||||
| Mycoplasma pneumonia | √ | ||||||||||
| Neisseria gonorrhoeae | √ | √ | √ | ||||||||
| Salmonella sp | √ | √ | √ | ||||||||
| Staphylococcus aureus | √ | √ | |||||||||
| Virus | |||||||||||
| Herpes simplex Type 1&2 | √ | √ | |||||||||
| HIV | √ | √ | √ | ||||||||
| HPV | √ | ||||||||||
| Human papillomavirus | √ | √ | |||||||||
| Influenza A&B virus | √ | ||||||||||
| Listeria monocytogenes | √ | ||||||||||
| Parasite | |||||||||||
| Cryptosporidium | √ | ||||||||||
| Giardia | √ | ||||||||||
| Leishmania sp. | √ | ||||||||||
| Plasmodium sp. | √ | ||||||||||
| Trichomonas vaginales | √ | ||||||||||
| Trypanosomabruceil cruzi | √ | ||||||||||
|
Others | |||||||||||
| Carbapenemase resistant | √ | √ | |||||||||
| Red snapper | √ | ||||||||||
| Amplex Biosystem | www.hyplex.info/ | ||||||||||
| Eiken Chemical Co. | www.eiken.co.jp/en/ | ||||||||||
| Biomerieux | www.biomerieux.com/ | ||||||||||
| Twistdx | www.twistdx.co.uk/ | ||||||||||
| Envirologix | http://envirologix.com/ | ||||||||||
| Coris bioconcepet | www.corisbio.com/ | ||||||||||
| Meridian bioscience | www.meridianbioscience.com/ | ||||||||||
| Gen-Probe | www.gen-probe.com/ | ||||||||||
| Biohelix | www.biohelix.com/ | ||||||||||
| Alere | www.alere-i.com/ | ||||||||||
| BD Probetec | www.bd.com/ | ||||||||||
†Discontinued.
HDA: Helicase-dependent amplification; LAMP: Loop-mediated isothermal amplification; NASBA: Nucleic acid sequence-based amplification; NEAR: Nicking and extension amplification reaction; RPA: Recombinase polymerase amplification; SDA: Strand displacement amplification; TMA: Transcription mediated amplification.
Some attempts have been made for developing POC tests based on NASBA. An integrated system combining RNA purification (previously extracted from bacteria), NASBA and amplification detection [10] was described for detection of Escherichia coli RNA. Recently, advances toward a ‘sample-in, answer-out’ NASBA POC instrument have been done integrating preconcentration, nucleic acid extraction, amplification and real-time fluorescent detection into a single sample device [11].
Strand displacement amplification
Strand displacement amplification (SDA) is an isothermal amplification method that combines the action of a restriction endonuclease and the strand displacing DNA polymerase to amplify a DNA target sequence [12] (Supplementary Figure 2). The amplification reaction takes place at low astringency conditions (37°C), which produce nonspecific primer binding and nonspecific amplification, as occurs in the case of NASBA. Such mechanism is especially problematic for diagnostic devices utilized in clinical environments since samples contain more abundant human DNA than the desired target. Additionally, initial amplification time of 2 h makes SDA inadequate for POC applications.
At present, a laboratory diagnostic device named BD ProbeTec™ ET system for Chlamydia trachomatis and Neisseria gonorrhoeae detection is in commercialization by BD Diagnostics. Nonetheless, this device requires intensive hand labor (Table 1). Approaches to SDA integration into a POC device have been done through development of an automated instrument combining sample preparation from whole cells, SDA and detection [13]. Long time for detection (2.5 h) is an obstacle for using SDA in ASSURED tests.
Loop-mediated isothermal amplification
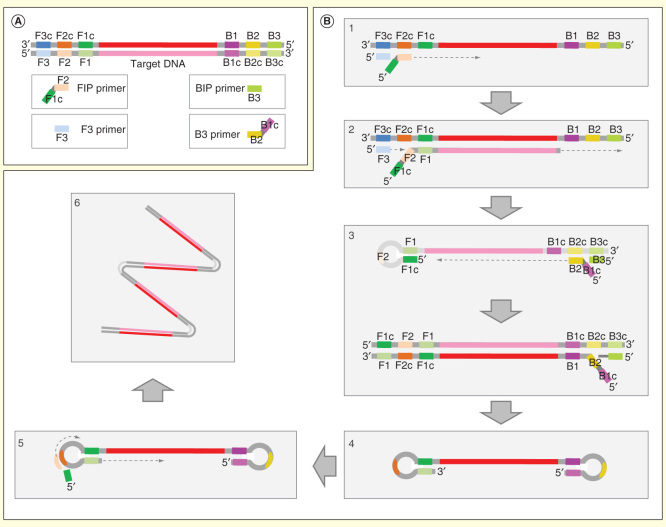
Loop-mediated isothermal amplification (LAMP) [14] is one of the most widely described one-step amplification techniques. This technology is based on the strand-displacement activity of the Bst DNA polymerase, which avoids a continuous DNA amplification reaction. Four primers are necessary for the reaction: F3/B3, FIP/BIP (Figure 1A); additional LF/LB primers accelerate the reaction [15] (Figure 1B). This pool of primers combined with the restrictive annealing and a reaction temperature of 60–65°C makes the reaction highly specific, allowing an ‘amplification is detection’ scheme in 15–60 min [16]. The common template for LAMP is DNA, but RNA template could also be detected by adding the reverse transcriptase together with DNA polymerase. This specific variant has been termed reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) [17].
Figure 1.
Loop-mediated isothermal amplification. For clarity, only the process initiated in one strand is shown. (A) Primer design with inner primers (F1c-F2 and B1c-B2) and outer primers (F3 and R3). Loop primers are not shown. (B) Amplification process. (1) F2 region of the FIP primer anneals with DNA template by its homologous sequence (F2c) and strand extension take place by the polymerase. (2) DNA polymerase synthesizes a new strand from F3 and displaces the previous DNA strand. (3) F1c segment of F3 primer hybridizes with F1. At other end, B3 and BIP primers hybridize with their homologues sequences and process 1 and 2 are repeated. (4) Formation of loop DNA molecule. (5) DNA molecule steam loops on both ends enter in a cycling step and a concatemere final product with several inverted repeats is produced (6).
Image adapted from Eiken Genome site (http://loopamp.eiken.co.jp/e/lamp/principle.html).
White precipitates of magnesium pyrophosphate produced during LAMP reaction allow turbidimetric detection of DNA amplification by both qualitative visual methods and real-time quantitative turbidimetry [18]. Several detection methods such as intercalating fluorescence dyes [19], lateral flow [20], bioluminescence [21] and electrochemical [22] detection have been performed. In addition, Tomita et al. developed a simple visual detection method [23] that could be useful especially in developing countries. A recent multiplexing assay permits real-time detection of 1–4 target sequences in a single LAMP reaction tube utilizing a standard real-time fluorimeter [24]. Similarly, a 3-plex LAMP has been developed in order to detect influenza A (H1 and H3) and B [25].
LAMP does not need initial denaturation at 95°C to allow strand separation, which is an interesting characteristic for POC applications. Nevertheless, higher levels of sensitivity (both analytical and diagnostic) have been obtained when including heating denaturation [26] with detection limits as low as one copy per reaction [27]. Importantly, the Bst DNA polymerase used in LAMP reaction shows high tolerance to inhibitory substances commonly present in biological samples, in contrast to PCR and others isothermal NAATs. Robust LAMP performance following little or no sample preparation has been proved in blood and stool [28], serum [29], cerebrospinal fluid [30], nasopharyngeal swabs [31] and urine samples [32].
LAMP background has been built upon more than 1000 scientific articles since the year 2000, which describe a diversity of applications targeting bacteria [19], viruses [33], viral variations [29], fungi [34] and parasites [35].
Eiken Chemical Company (Tokyo, Japan) has developed and commercialized the LAMP method for both DNA and RNA amplification kits targeting laboratories that develop home-made techniques. In addition, this company offers a variety of kits for the detection of human pathogens in food or environment such as Salmonella, E. coli O157, Listeria monocytogenes, Campylobacter, Legionella, Giardia and Cryptosporidium. In addition, Eiken Chemical in collaboration with Foundation for Innovative New Diagnostics and the Hospital for Tropical Diseases has recently developed a field-stable LAMP kit for malaria for the detection of all species of human malaria parasites [36,37]. The validation of a commercial loop-mediated isothermal amplification assay (TB-LAMP) for the detection of tuberculosis has been recently reported by Eiken and is expected to be launched into the market soon together with a kit for human African trypanosomiasis. The LAMP technology has also been implemented in the Illumigene (Meridian Bioscience, Cincinnati, OH, USA) assay for rapid diagnosis of Clostridium difficile, Mycoplasma pneumonia, Group A Streptococcus and Group B Streptococcus. In addition, Amplex Biosystems (Gießen, Germany) have commercialized the eazyplex® SuperBug that detects carbapenemases antibiotic resistances (covering KPC, NDM, OXA-48, VIM and the Acinetobacter baumannii belonging Oxa-groups OXA-23 like, OXA-40 like and OXA-58 like) and the eazyplex SuperBug CRE, which also includes ESBL genes of CTX-M-1 and CTX-M-9 families. All these tests take between 15 and 30 min for results. It is also of interest to note that real-time PCR devices can be used for isothermal amplification and detection although less complex instruments are specifically aimed for this purpose. Among others, both Instrument Genie® II (Optigene, Horsham, UK) and Instrument Genie III allow isothermal amplification and detection by fluorescence, whereas Ilumipro-10 (Meridian Bioscience, Cincinnati, OH) detects amplification products by turbidity.
Due to its rapidness, specificity, robustness, simple equipment and the possibility to process almost raw samples, LAMP is a suitable method for low-cost POC utilization, in combination with other technologies such as microfluidics. Since a first microfluidic disc for detection of hepatitis B virus reached an analytical sensitivity of about 50 copies per reaction within 60 min measured by turbidity [38], a significant number of POC devices based on LAMP have been developed: that is, a multichannel microfluidic device based on LAMP turbidity with a need of only 0.4 µl for detection of the swine virus in less than 1 h [39] or a multiplex microfluidic RT-LAMP device able to distinguish three types of influenza A substrains with a detection limit of 20 copies per reaction in 30 min [40]. In a similar way, a colorimetric device based on a RT-LAMP assay has been reported to detect hepatitis E virus by visual detection with a limit of detection of 10 copies and low handling and equipment requirement [41]. Last year a completely automated POC device termed Microfluidic Biomolecular Amplification Reader was reported to process raw samples through combination of molecular, microfluidic, optical and electronic technologies. This battery-powered instrument, which uses light emitting diodes and phototransistors, is able to detect the HIV-1 integrase gen in 70 min [42]. Recently, a novel microfluidic biochip has been manufactured that integrates LAMP, line probes assay and giant magnetoresistive sensors, having high analytical sensitivity for hepatitis B virus detection (10 copies per ml-1) in 1 h [41].
Helicase-dependent amplification
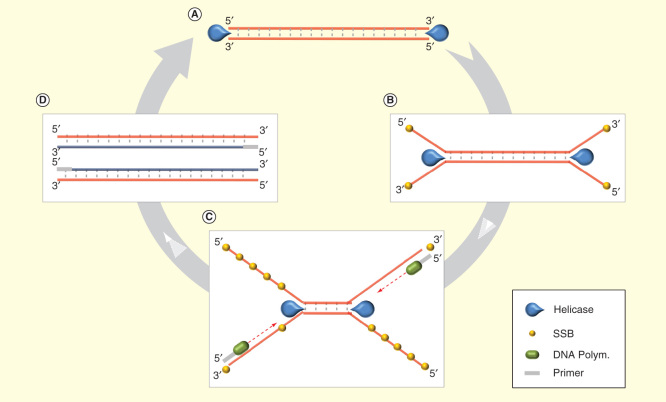
Helicase-dependent amplification (HDA) [43] is an elegant method based on a natural DNA replication mechanism, without the need of a denaturation step like PCR. Originally HDA process was performed through three different proteins (plus Polymerase): the DNA helicase, which unwinds the dsDNA, and two additional proteins (Figure 2). Nevertheless, the new thermostable helicase Tte-UvrD from Thermoanaerobacter tengcongenesis is able to work at 65°C, allowing more specific reactions without both MutL and SSB [44]. HDA amplification usually takes 60–120 min, at least for low-copy numbers, but possible modifications for optimization can be done [45]. Although only a single set of primers is needed for HDA, few multiplex HDA applications have been developed so far such as a duplex-detection of pathogens N. gonorrhoeae and Staphylococcus aureus by combining HDA with microarray-based detection [46], and a 4-plex detection assay for four different genes from the same organisms [47]. HDA is compatible with diverse detection mechanisms like fluorescent detection [48] or electrochemical DNA detection with gold nanoparticles [49]. This technology has been successfully used for different types of samples including urine [45], stool [50], blood [3] and plasma [51].
Figure 2.
Helicase-dependent amplification. (A) Helicase unwinds the dsDNA. (B) SSB protein prevents premature strand reassociation. (C) Primer hybridizes with the target and is extended by the DNA polymerase while helicase continues unwinding the dsDNA remaining. (D) New dsDNA copy is formed as a product and is able to go into to a new cycle.
BioHelix (Beverly, MA, USA), the developers of HDA technology, commercialize diverse kits for end point or real-time HDA detection such as IsoAmp® II Universal tHDA and IsoAmp III Universal tHDA Kit. These kits are aimed for further development of home-made techniques. In addition, a BESt Cassette Type II for lateral flow detection in 5–10 min has reached the stage of early commercialization. Several assays have being developed for the detection of, for example, S. aureus [52], or HIV-1 [51] by lateral flow with a time of detection of 20–40 min. However, only the kit for Herpes simplex virus detection (IsoAmp HSV) by Biohelix is currently available in the market (Table 1).
Andresen et al. [46] developed a HDA/microarray for the detection of N. gonorrhoeae and S. aureus in 2 h, in single or a duplex way and therefore suitable for the simultaneous detection of various pathogens. This microarray could be regarded as the first fully integrated microfluidic device that combines sample preparation and real-time HDA with capability to detect 100 genomic copies. Nonetheless, this method does not improve time to detection of PCR techniques and neither does the device developed by Mahalanabis et al. for E. coli detection [50]. In contrast, Ramalingam et al. [53] designed a microfluidic device where all flow is by capillarity and absorbent pad, without pumps or valves. Such device allows for HDA real-time detection of SARS-coronavirus in 30 min. In addition, Zhang et al. [54] presented a droplet microfluidic integrative technique where isolation, HDA amplification and detection occur in the same chip. Finally, steps in the direction of free-electricity pathogen detection have been done combining a nearly power-free nucleic acid extraction with an electricity-powered isothermal amplification assay for diarrhea diagnosis [55].
HDA seems to be an appropriate method for low-cost POC utilization. However, the relatively modest detection limit <100 copies achieved demands further improvement in sensitivity.
Recombinase polymerase amplification
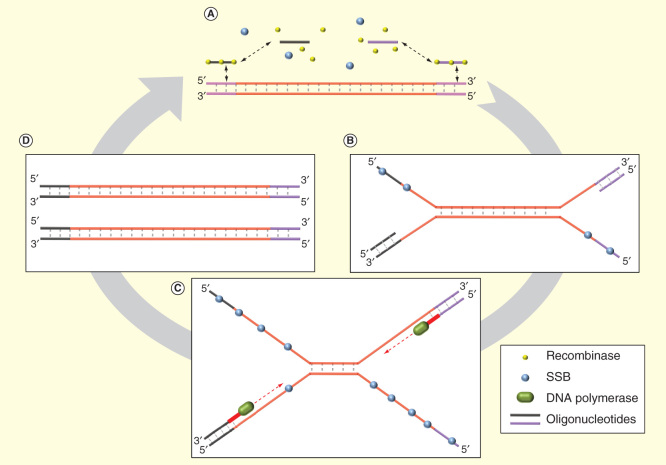
Recombinase polymerase amplification is a low-temperature (37–42°C) isothermal amplification method that uses three proteins in the reaction [56]: recombinase protein, DNA polymerase and DNA-binding protein (gp32) to prevent reannealing. RPA facilitates amplification in 20–40 min (Figure 3). Detection methods such as intercalating dyes, molecular beacons and TaqMan technologies are incompatible with RPA. For this reason, other detection strategies such as Twist-Ampexo and TwistAmp fpg have been developed by using sequence-specific probes.
Figure 3.
Recombinase polymerase amplification. (A) Recombinase exchanges primers with the target DNA template. (B) SSB prevents premature strand reassociation while primes anneal with target DNA. (C) DNA polymerase synthesizes new DNA strands unwinding remaining dsDNA. (D) Two dsDNA molecules are generated and can again repeating the cycle.
Successful detection of less than 10 copies of HIV-1 DNA within 20 min has been documented for a RPA assay [57]. Likewise, a real-time reverse transcription recombinase polymerase amplification assay has been developed for fast detection of foot-and-mouth disease virus within 10 min [58] or bovine coronavirus in 10–20 min [59] as well as for targeting different viruses and bacteria biothreat agents [60].
TwistDX (Cambridge, UK) commercializes a variety of RPA products for developing both end point and real-time assays such as TwisAMP and TwistAmp® exo (probe included), but primers and template have to be supplied by the user. This company also has commercial kits for detection of Salmonella, L. monocytogenes, Campylobacter and Red snapper (fish) determination (Table 1), together with some devices to perform amplification and detection like the T-16 Isothermal Device, Twirla™ and Twista® portable real-time fluorometer.
Being a novel technology, there is little background about RPA. However, it appears to be one of the fastest amplification techniques, which is an appealing feature for adaptation to POC devices. In this regard, a fully automated chip with a centrifugal disc format has been developed by Lutz et al. This device, with pre-stored freeze-dried RPA reagents, has been described to allow detection of gene mecA of S. aureus in 20 min [61]. Novel development of digital RPA on a SlipChip indicates that RPA may potentially be fast and sensitive with low volumes [62].
Nicking & extension amplification reaction
Nicking and extension amplification reaction (NEAR) is based on an isothermal method termed exponential amplification reaction [63]. Exponential amplification reaction uses nicking-enzymes that by nicking the DNA target expose sites for primer annealing and allow DNA amplification in a few minutes. This method seems to be strongly limited by the requirement of native restriction flanking the target sequence to be amplified [64]. By contrast, NEAR allows amplification of any target by adding restriction sites in flank regions. Only one strand is nicked in NEAR while, comparatively, SDA avoids the requirement of dATPαS (5′-O-l-thiotriphosphate) to prevent double-stranded cleavage [65]. NEAR meets two basic criteria that are desirable for integration into POC tests: no DNA denaturation is needed and higher tolerance to inhibition than PCR is achieved [65].
Little is known about the NEAR technology, although first reports have described high analytical sensitivity for detection of N. gonorrhoeae and C. trachomatis (10 DNA copy number) and Clavibactermichiganesis and Ralstoniasolnacearum (50 copies of detection level), both of them with time to results within 10 min [66,67].
First commercial tests based on NEAR have been launched by EnviroLogix (Portland, ME, USA) for rapid detection (within 15 min) of the plant pathogen Clavibacter michiganesis and Salmonella, with a sensitivity of 103 cfu per reaction. Recently, Alere (Waltham, MA, USA) has released a detection kit that in combination with the Alere-i POC detects influenza A & B viruses in 15 min with a total cost around US$40 (Table 1).
Proof-of-concept molecular isothermal techniques
Rolling circle amplification
Rolling circle amplification (RCA) [68] is an isothermal method based on strand displacing DNA polymerase ϕ29DNAP that is able to amplify continuously circular ssDNA (Supplementary Figure 3). A peculiar feature of RCA is that the unique concatenated DNA molecule that results as a product of the reaction allows in situ amplification [69]. This characteristic could be interesting for applications in which maintenance of cellular morphology is important. Another interesting application of RCA is the ultrasensitive protein detection by sandwich immunoassay [70]. Intriguingly, in spite of having been described almost 20 years ago, no RCA diagnostic test targeting pathogens has reached the market yet.
Different approaches to integrate RCA into a chip have been performed up to now. Sato et al. created a microchip that integrates all operating processes, including RCA [71]. Mahmoudian et al. combined RCA amplification followed by electrophoresis in a lab-on-a-chip assay for Vibrio cholerae detection in less than 65 min [72]. Reiss et al. performed a RCA in a flow-through system that allows stretching the amplified product and visualizing it under fluorescence microscopy [73].
Smart amplification process & signal mediated amplification of RNA technology
The novel LAMP-based smart amplification process (SMAP) v.2 technology [74] should not be confounded with the Signal mediated amplification of RNA technology (SmartAmp2). SMAP2 primes and enzymes are similar to LAMP, but it adopts an asymmetric primer design. SMAP2 is used to distinguish single nucleotide polymorphism clones differing by only one nucleotide, in contrast to LAMP reaction where targets have lower identity and primer design is more flexible. Therefore, in SMAP2 reactions, primers could anneal with wild-type and single nucleotide polymorphism variant through different amplification pathways by two priming event: primer priming (specific) and self-priming (nonspecific). An asymmetrical design of primers allows reducing the self-primer event that contributes to the ‘background/wild-type’ amplification product.
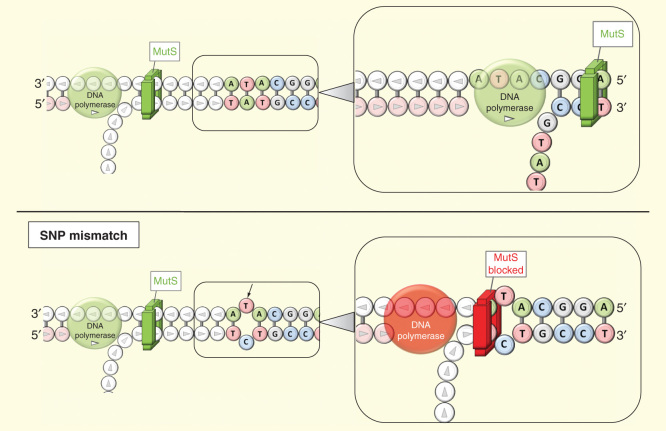
This specific design takes into account that wild-type and target sequences could just differ in one nucleotide. Five primers are required: turn-back primers (TP), folding primers (FP), boost primer (BP) and two outer primers (OP1 and OP2). A second special feature of SMAP2 is the use of Thermusaquaticus MutS, an enzyme that scans DNA and identifies mismatches with single-nucleotide sensitivity (Figure 4). When MutS finds a mismatch, it binds to it in an irreversible way, blocking the action of the DNA polymerase and, consequently, DNA amplification. Both asymmetric primer design and MutS allow single-nucleotide discrimination, features that make this technique very useful for single nucleotide polymorphism identification [75].
Figure 4.
Smart amplification process v.2. MutS goes over template DNA strand, and DNA polymerase drives the extension (on top). If MutS find a mismatch (black arrow), it is blocked and both MutS nether DNA polymerase are able to continue the process (bottom).
Little information can be found in the literature about SMAP2, but this technology has been documented to have not only high specificity but also high analytical sensitivity (three copies) [74]. Precise design of primers is needed to achieve desirable specificity and sensitivity, especially in relation to TP primers [76] and FP primers [77]. The use of SMAP2 for infectious disease diagnosis is still limited. A first progress has been made in the detection of pandemic flu within 40 min based on this technique coupled to a reverse transcriptase reaction (RT-SMAP2) [78]. However, as far as we know, kits for research or diagnostic utilization have not been commercialized yet.
Isothermal & chimeric primer-initiated amplification of nucleic acids
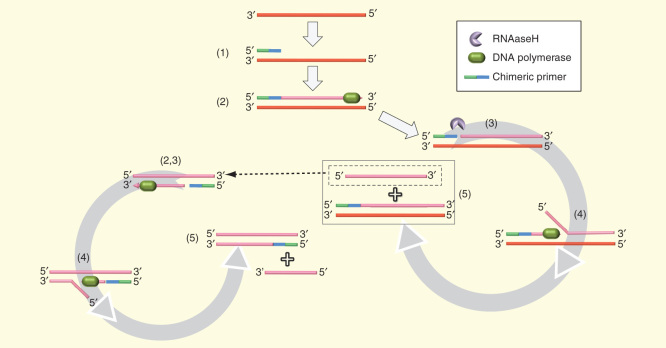
Isothermal and chimeric primer-initiated amplification of nucleic acids (ICAN) [79] is an isothermal method (around 55°C) based on 5′-DNA-RNA-3′ chimeric primers, thermo-stable RNaseH and BcaBEST DNA polymerase with strand-displacing activity. Chimeric primers bind to ssDNA template, after initial dsDNA denaturation by heat, starting the synthesis of the new DNA strands. These strands are straightaway nicked by the RNase action at the penultimate 3′ RNA residue allowing DNA amplification in a repetitive cycle until the primer is too short (Figure 5).
Figure 5.
Isothermal and chimeric primer-initiated amplification of nucleic acid. For clarity, the process is shown only from one strand. (A and B) Polymerase extension from the chimeric primer produces a dsDNA molecule. (C) RNAseH nicks the original primer into the 3′ extreme. (D and E) Strand displacing polymerase extension from nick site release a shorter ssDNA that goes into the same process until generated molecule is too short.
The amplification product generated by ICAN technology can be detected in real time in a more sensitive way (25 times) than the conventional PCR method [80]. At least 2-plex detection systems have been described for simultaneous detection of C. trachomatis and N. gonorrhoeae, by luminescent probe [81]. In this case, analytical sensitivity between 10 and 100 copies was achieved after 3.5 h for detection.
A few approaches have been done in order to incorporate this technology into POC assays. A semiautomated POC test able to detect spores of Bacillus subtilis by fluorescence has been described [82] as an example of biological warfare agents detection. Nonetheless, low analytical sensitivity (104 spores) and long time for detection (2 h) require further improvement for effective POC application. Another POC approach is the ICAN-chromatostrip for detecting Salmonella invA, which was documented to have higher diagnostic sensitivity than a standard PCR assay [83]. An N. gonorrhoeae test kit (ICAN NG-QR) and a Salmonella Detection Kit for DNA chips (ICAN™ DNA) were ICAN-based products commercialized by Takara Bio (Otsu, Japan). However, no ICAN products for research or diagnostic use are available in the market at present.
Expert commentary
The current landscape of emerging isothermal diagnostic solutions for infectious diseases covers a variety of technologies (Table 2) that allow amplification of both DNA (ssDNA or dsDNA) or RNA (with a previous reverse transcriptase step). Interestingly, some methods such as LAMP, RCA and SMAP2 produce a continuous concatemeric DNA strand that could be useful for in situ detection. It is noteworthy that the majority of isothermal techniques have been attributed high analytical sensitivity within a range of 1–10 copies in previous studies, whereas SDA, ICAN and NEAR seem to be less sensitive. Nevertheless, since most evaluations have been performed at the development stage in academic laboratories, further studies on precommercial or available products should be undertaken to confirm these data. Certain techniques (LAMP, SMAP2, SDA, HDA, RPA and NEAR) appear to show mid-to-high tolerance to biological compounds and allow a low- or non-pretreated sample process. This could be an interesting characteristic for POC use and procedural simplicity. Denaturation is a previously needed step for some technologies as it obviously is for PCR. Since heating requirements at 95°C for DNA extraction and denaturation are moderate, this factor may facilitate their integration into power-free POC instruments that would be particularly suitable for use in remote settings [55].
Table 2.
Table of iNAATs features.
| Property | NASBA | SDA | LAMP | RPA | HDA | NEAR | RCA | SMAP | ICAN |
|---|---|---|---|---|---|---|---|---|---|
| Temperature (s) (ºC) | 37–42 | 37 | 60–65 | 37–42 | 60–65 | 55 | 30–60 | 60–65 | 55 |
| Time to detection (min) | 60 | 120 | 15–60 | 10–20 | 60–120 | 10–15 | 10–40 | 15–60 | 60 |
| Number of enzyme(s) | 2–3 | 2 | 1 | 3 | 2 | 2 | 1 | 2 | 2 |
| Product detection method | Fluorescence | Fluorescence | Intercalating dye, fluorescence, turbimetric | Proprietary fluorescence probe | Intercalating DNA dye, fluorescence probe, lateral flow strip | Fluorescence | Fluorescence | Intercalating dye, fluorescence, turbimetric | Fluorescence |
| Tolerance to biological components | NO | YES | YES | YES | YES | YES | NO | YES | NO |
| Need to template denaturation | YES | YES | NO | NO | NO | NO | NO | NO | YES |
| Denaturating agent (s) |
RNAseH | Restriction enzymes, bumper primers | Betaine | Recombinase enzyme | Helicase | Restriction enzymes | Strand–displacement ϕ29 DNA polymerase | Betaine | Heat |
| Analytical sensitivity (copies) | 1–10 | >10 | 1–10 | 1–10 | 100 | >10 | >10 | 1–10 | 10–100 |
| Multiplex demonstrated | YES | NO | YES | NO | YES | YES | NO | NO | YES |
| Primer required | 2 | 2 DNA/RNA chimeric + 2 bumpers | 4 | 2 | 2 | 2 DNA/RNA chimeric | 1 | 4 | 2 DNA/RNA chimeric |
| First publication | 1991 | 1992 | 2000 | 2006 | 2004 | 2007 | 1996 | 2007 | 2002 |
| Intellectual property | Cangene Corp, Winnipeg, Canada | Becton, Dickinson & Company. USA | Eiken Chemical Co, Japan | TwistDX, UK | Biohelix, USA | Ionian Technologies, San Diego, USA | Various | NA | Takara Bio (Otsu, Japan) |
| Type of diagnostic product (marketed) | Reagents/ device |
Reagents | Reagents/ device |
Reagents/ device |
Reagents/ device |
Reagents/ device |
NA | NA | NA |
| Type of detection (marketed) | End point/real time | Real time | End point/real time | Real time | End point/real time | End point/real time | NA | NA | NA |
| Estimated cost per test† (US$) | 34–45 | N.A | 25–65 | 7–19 | 9–20 | 34–47 | NA | NA | NA |
| Articles number | >900 | >150 | >900 | <20 | >40 | <10 | >250 | <10 | <10 |
Grey cells, techniques with no commercial diagnostic test currently available.
†Estimated costs per test are subject to variations by countries, distribution logistics and companies, among other multiples factors.
HDA: Helicase-dependent amplification; ICAN: Isothermal & chimeric primer-initiated amplification of nucleic acids; LAMP: Loop-mediated isothermal amplification; NA: Not available; NASBA: Nucleic acid sequence-based amplification; NEAR: Nicking and extension amplification reaction; RCA: Rolling circle amplification; RPA: Recombinase polymerase amplification; SDA: Strand displacement amplification; SMAP: Smart amplification process.
The integration of isothermal techniques into POC devices offers the advantages of simple instrumentation and the possibility of certain technologies to process raw samples, thus reducing costs of products and processes involved. However, most emerging isothermal near-POC or POC applications have only reached a proof-of-concept stage. Further evaluation studies are needed to determine their feasibility for commercialization.
Five-year view
Interest and expectation around POC disease diagnostics are increasing. Specifically, there has been an exponential growth of near-POC or POC developments based on PCR and isothermal amplification technologies for diagnosis of infectious diseases in the last years. Because of their competitive advantages, isothermal technologies are likely to become reference chemistries for future POC applications and could gradually displace PCR-based solutions. Diverse isothermal-based technologies are tacking technical challenges at the micro-scale and finding their way to market. Low sample volume handling, low concentration of target, complex signal amplification due to low concentration, target instability and compatibility and integration of the various device subsystems have been identified as major hurdles for the development of POC assays.
It appears that LAMP has reached a higher degree of maturity than other isothermal techniques to overcome such hurdles, according to the number of related developments and products. However, technical potentialities of LAMP or any other competing chemistries do not automatically guarantee their future adoption. In addition to further validation in clinical labs to obtain solid results, a range of cultural, organizational, economic and regulatory barriers may undermine successful deployment of diagnostics at the point of care, either isothermal or based on other technologies. One of the main challenges for effective implementation in the upcoming years will be to gain a positive perception from end users and healthcare providers. Since rapid diagnosis by POC assays alleviates patient anxiety and eliminates return visits for results, it appears that delivery of diagnosis on site will be well accepted by end users. However, acceptance of decentralized diagnostics by healthcare providers will imply important changes in clinical practices, workloads and logistics. New frameworks will need to be designed at the point of contact between the patient and the healthcare system to ensure adequate on-site sample collection, test performance and quality control of results [84].
Similarly, protocols of communication between healthcare centers and public health agencies will have to be put in place so that results of POC assays are properly captured and registered for adequate centralized surveillance and control of infectious diseases. Another challenge to be met in the near future will be to establish the final price for a POC test that is acceptable to payers, not only in terms of low cost but also of willingness to pay. It will be important to optimize costs of reagents, materials and systems of mass production and assembly of device components. Nonetheless, the final decision to purchase a POC test or a centralized laboratory test will not strictly be which test is cheapest or faster but rather the cost of the test needed to provide the desired clinical benefit [85]. On the other hand, future widespread use of POC devices will be constrained by nonharmonized regulatory conditions for commercialization of POC devices in diverse geographical markets, especially in Western countries.
Being aware that unexpected events may encourage or limit evolution toward near-patient diagnostics, we envision two scenarios where initial adoption of isothermal POC tests is more plausible within a timeframe of 5 years. Core laboratories, which are usually located in referral hospitals of industrialized countries, will presumably be the first market segment to be penetrated. Such environments are already familiarized with performance of PCR and other NAATs and can easily evaluate characteristics of isothermal POC solutions against current methods. Minor organizational changes may be needed for implementation of isothermal assays in specialized laboratories, but it will be fundamental that they prove to have sensitivity and specificity comparable to gold standard techniques for pathologies of complex etiology, most prevalent in referral hospitals. The demonstration of cost–effectiveness will also be required prior to adoption to ensure sustainability of diagnostic services. POC applications that would be best valued referral hospitals could be assays targeting diagnosis of time-critical, chronic or highly transmissible infections, as well as differential diagnosis of infections with common symptoms and different treatments and those requiring complex, toxic or expensive therapies. In a mid-term time, POC applications may be able to migrate from core laboratories to other areas within the referral hospital (emergency wards, intensive care units) and beyond (second-level hospitals, primary care), provided that they maintain diagnostic accuracy, rapidity and cost–effectiveness and allow use by nonspecifically trained personnel.
A second scenario for the implementation of POC testing in the next years will be the global health market. The WHO estimated that in 2008 resource-limited countries experienced 241 million cases of malaria, 2.8 million new infections with HIV, 7.8 million cases of reactivated TB and 217 million cases of bacterial pneumonia [86]. In fact, simple inexpensive POC tests based on antibody detection that are close to the ‘ASSURED’ ideal are already available in most developing countries in a lateral-flow or dipstick format (i.e., for the diagnosis of human immunodeficiency virus infection). It also needs to be highlighted that the majority of population in the developing world resides in remote rural areas, so patients often travel long distances to primary healthcare settings and thus may be unable to return for test results. Moreover, primary care infrastructures may have poor availability of trained technicians and limited electricity supply for equipment or refrigerators for the storage of reagents. Portable, extremely low-cost, very easy-to-use isothermal POC tests that provide rapid visual readouts with minimal instrumentation and reagents stable at room temperature would have wide acceptance in this scenario. In particular, it is expectable that mobile phones, which are prevalent in the developing world, will become fundamental complements of POC applications since they can contribute capabilities of battery-powered supply, readout and connectivity for transmission of results. The big size of the global health market and its urgent need for more accessible diagnostics offer attractive opportunities for manufacturers that have the ability to scale up POC applications and reduce prices. While margins may be low in the poorest countries, POC products may allow profitable commercialization in emerging middle-income countries. Those applications that target infections with a heavy burden on global health or that match with those occurring in developed countries may have higher potential for success in the short term.
Supplementary Material
Financial & competing interests disclosure
The authors acknowledge support from The Seventh framework programme of the European Union (FP7-SME-2013, grant 606488). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Key issues
An ideal diagnostic test should meet the ‘ASSURED’ criteria: affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free and delivered to those who need it.
Current PCR-based devices, even those intended for application at the point of care, need to be instrumented for performing thermal cycling amplification steps and have limited portability and affordability.
Molecular isothermal amplification technologies may facilitate development of electricity-free, non- or minimally instrumented point-of-care devices that could be particularly suitable for use in developing countries.
Proof-of-concept studies in academic laboratory environments have reported that most isothermal techniques have similar or better sensitivity, specificity and rapidness than PCR assays.
Further studies in real field conditions are needed to validate the promising diagnostic characteristics of isothermal techniques.
Loop-mediated isothermal amplification, smart amplification process & signal mediated amplification of RNA technology, helicase-dependent amplification, strand displacement amplification, recombinase polymerase amplification and nicking and extension amplification reaction are isothermal techniques with mid/high tolerance to inhibitory compounds that allow the use of raw samples without any pretreatment step, which may be an interesting feature for PCR-based point-of-care (POC) testing.
Isothermal techniques such as loop-mediated isothermal amplification, nicking and extension amplification reaction and recombinase polymerase amplification could be adequate for integration into POC tests due to their rapidness.
The introduction and use of isothermal POC applications will need to meet the diverse organizational, economic and regulatory challenges before successful adoption.
Central laboratories in industrialized countries and primary health centers in resource-limited countries are the most plausible scenarios for the deployment of isothermal POC tests in a 5-year view.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Accessible quality-assured diagnostics. 2009 annual report. Available from: www.who.int/tdr/publications/about-tdr/annual-reports/bl7-annual-report/en/
- 2.Low-cost tools for diagnosing and monitoring HIV infection in low-resource settings. Available from: www.who.int/bulletin/volumes/90/12/12-102780/en/ [DOI] [PMC free article] [PubMed]
- 3.Li Y, Kumar N, Gopalakrishnan A, et al.. Detection and species identification of malaria parasites by isothermal tHDA amplification directly from human blood without sample preparation. J Mol Diagn 2013;15(5):634-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craw P, Balachandran W. Isothermal nucleic acid amplification technologies for point-of-care diagnostics: a critical review. Lab Chip 2012;12(14):2469-86 [DOI] [PubMed] [Google Scholar]
- 5.Asiello PJ, Baeumner AJ. Miniaturized isothermal nucleic acid amplification, a review. Lab Chip 2011;11(8):1420-30 [DOI] [PubMed] [Google Scholar]
- 6.Compton J. Nucleic acid sequence-based amplification. Nature 1991;350(6313):91-2 [DOI] [PubMed] [Google Scholar]
- 7.Pasternack R, Vuorinen P, Miettinen A. Evaluation of the Gen-Probe Chlamydia trachomatis transcription-mediated amplification assay with urine specimens from women. J Clin Microbiol 1997;35(3):676-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gingeras TR, Whitfield KM, Kwoh DY. Unique features of the self-sustained sequence replication (3SR) reaction in the in vitro amplification of nucleic acids. Ann Biol Clin 1990;48(7):498-501 [PubMed] [Google Scholar]
- 9.van Gemen B, Kievits T, Nara P, et al.. Qualitative and quantitative detection of HIV-1 RNA by nucleic acid sequence-based amplification. AIDS 1993;7(Suppl 2)):S107-10 [DOI] [PubMed] [Google Scholar]
- 10.Dimov IK, Garcia-Cordero JL, O’Grady J, et al.. Integrated microfluidic tmRNA purification and real-time NASBA device for molecular diagnostics. Lab Chip 2008;8(12):2071-8 [DOI] [PubMed] [Google Scholar]
- 11.Gulliksen A, Keegan H, Martin C, et al.. Towards a "Sample-In, Answer-Out" Point-of-Care Platform for Nucleic Acid Extraction and Amplification: Using an HPV E6/E7 mRNA Model System. J Oncol 2012;905024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker GT, Fraiser MS, Schram JL, et al.. Strand displacement amplification–an isothermal, in vitro DNA amplification technique. Nucleic Acids Res 1992;20(7):1691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JM, Bell J, Huang Y, et al.. An integrated, stacked microlaboratory for biological agent detection with DNA and immunoassays. Biosens Bioelectron 2002;17(6-7):605-18 [DOI] [PubMed] [Google Scholar]
- 14.Notomi T, Okayama H, Masubuchi H, et al.. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 2000;28(12)):E63. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study describing the molecular isothermal loop-mediated isothermal amplification technology.
- 15.Nagamine K, Hase T, Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes 2002;16(3):223-9 [DOI] [PubMed] [Google Scholar]
- 16.Lalande V, Barrault L, Wadel S, et al.. Evaluation of a loop-mediated isothermal amplification assay for diagnosis of Clostridium difficile infections. J Clin Microbiol 2011;49(7):2714-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoneda A, Taniguchi K, Torashima Y, et al.. The detection of gastric cancer cells in intraoperative peritoneal lavage using the reverse transcription-loop-mediated isothermal amplification method. J Surg Res 2013;187(1)):E1-6 [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Zhu J, Ren H, et al.. Rapid visual detection of highly pathogenic Streptococcus suis serotype 2 isolates by use of loop-mediated isothermal amplification. J Clin Microbiol 2013;51(10):3250-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soli KW, Kas M, Maure T, et al.. Evaluation of colorimetric detection methods for Shigella, Salmonella, and Vibrio cholerae by loop-mediated isothermal amplification. Diagn Microbiol Infect Dis 2013;77(4):321-3 [DOI] [PubMed] [Google Scholar]
- 20.Kaewphinit T, Arunrut N, Kiatpathomchai W, et al.. Detection of Mycobacterium tuberculosis by using loop-mediated isothermal amplification combined with a lateral flow dipstick in clinical samples. Biomed Res Int 2013;2013:926230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McElgunn CJ, Pereira CR, Parham NJ, et al.. A low complexity rapid molecular method for detection of clostridium difficile in stool. PLoS One 2014;9(1)):e83808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Jiang DN, Xiang GM, et al.. DNA detection of Clostridium difficile infection based on real-time resistance measurement. Genet Mol Res 2013;12(3):3296-304 [DOI] [PubMed] [Google Scholar]
- 23.Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc 2008;3(5):877-82 [DOI] [PubMed] [Google Scholar]
- 24.Tanner NA, Zhang Y, Evans TC. Simultaneous multiple target detection in real-time loop-mediated isothermal amplification. Biotechniques 2012;53(2):81-9 [DOI] [PubMed] [Google Scholar]
- 25.Mahony J, Chong S, Bulir D, et al.. Multiplex loop-mediated isothermal amplification (M-LAMP) assay for the detection of influenza A/H1, A/H3 and influenza B can provide a specimen-to-result diagnosis in 40 min with single genome copy sensitivity. J Clin Virol 2013;58(1):127-31 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki R, Ihira M, Enomoto Y, et al.. Heat denaturation increases the sensitivity of the cytomegalovirus loop-mediated isothermal amplification method. Microbiol Immunol 2010;54(8):466-70 [DOI] [PubMed] [Google Scholar]
- 27.Pyrc K, Milewska A, Potempa J. Development of loop-mediated isothermal amplification assay for detection of human coronavirus-NL63. J Virol Methods 2011;175(1):133-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francois P, Tangomo M, Hibbs J, et al.. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol Med Microbiol 2011;62(1):41-8 [DOI] [PubMed] [Google Scholar]
- 29.Ihira M, Sugiyama H, Enomoto Y, et al.. Direct detection of human herpes virus 6 DNA in serum by variant specific loop-mediated isothermal amplification in hematopoietic stem cell transplant recipients. J Virol Methods 2010;167(1):103-6 [DOI] [PubMed] [Google Scholar]
- 30.Kim DW, Kilgore PE, Kim EJ, et al.. Loop-mediated isothermal amplification assay for detection of Haemophilus influenzae type b in cerebrospinal fluid. J Clin Microbiol 2011;49(10):3621-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotoh K, Nishimura N, Takeuchi S, et al.. Assessment of the loop-mediated isothermal amplification assay for rapid diagnosis of Mycoplasma pneumoniae in pediatric community-acquired pneumonia. Jpn J Infect Dis 2013;66(6):539-42 [DOI] [PubMed] [Google Scholar]
- 32.Koizumi N, Nakajima C, Harunari T, et al.. A new loop-mediated isothermal amplification method for rapid, simple, and sensitive detection of Leptospira spp. in urine. J Clin Microbiol 2012;50(6):2072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malik YS, Sharma K, Kumar N, et al.. Rapid detection of human rotavirus using NSP4 gene specific reverse transcription loop-mediated isothermal amplification assay. Indian J Virol 2013;24(2):265-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moradi A, Almasi MA, Jafary H, Mercado-Blanco J. A novel and rapid loop-mediated isothermal amplification assay for the specific detection of Verticillium dahliae. J Appl Microbiol 2014;116(4):942-54 [DOI] [PubMed] [Google Scholar]
- 35.Verma S, Avishek K, Sharma V, et al.. Application of loop-mediated isothermal amplification assay for the sensitive and rapid diagnosis of visceral leishmaniasis and post-kala-azar dermal leishmaniasis. Diagn Microbiol Infect Dis 2013;75(4):390-5 [DOI] [PubMed] [Google Scholar]
- 36.Hopkins H, Gonzalez IJ, Polley SD, et al.. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis 2013;208(4):645-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polley SD, Gonzalez IJ, Mohamed D, et al.. Clinical evaluation of a loop-mediated amplification kit for diagnosis of imported malaria. J Infect Dis 2013;208(4):637-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SY, Lee CN, Mark H, et al.. Efficient, specific, compact hepatitis B diagnostic device: optical detection of the hepatitis B virus by isothermal amplification. Sens Actuators B Chem 2007;127(2):598-605 [Google Scholar]
- 39.Fang X, Liu Y, Kong J, Jiang X. Loop-mediated isothermal amplification integrated on microfluidic chips for point-of-care quantitative detection of pathogens. Anal Chem 2010;82(7):3002-6 [DOI] [PubMed] [Google Scholar]
- 40.Fang X, Chen H, Yu S, et al.. Predicting viruses accurately by a multiplex microfluidic loop-mediated isothermal amplification chip. Anal Chem 2011;83(3):690-5 [DOI] [PubMed] [Google Scholar]
- 41.Chen Q, Yuan L, Wan J, et al.. Colorimetric detection of hepatitis E virus based on reverse transcription loop mediated isothermal amplification (RT-LAMP) assay. J Virol Methods 2014;197:29-33 [DOI] [PubMed] [Google Scholar]
- 42.Myers FB, Henrikson RH, Bone J, Lee LP. A handheld point-of-care genomic diagnostic system. PLoS One 2013;8(8)):e70266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent M, Xu Y, Kong H. Helicase-dependent isothermal DNA amplification. EMBO Rep 2004;5(8):795-800 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study describing the molecular isothermal helicase-dependent amplification technology.
- 44.An L, Tang W, Ranalli TA, et al.. Characterization of a thermostable UvrD helicase and its participation in helicase-dependent amplification. J Biol Chem 2005;280(32):28952-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong Y, Lemieux B, Kong H. Multiple strategies to improve sensitivity, speed and robustness of isothermal nucleic acid amplification for rapid pathogen detection. BMC Biotechnol 2011;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andresen D, von Nickisch-Rosenegk M, Bier FF. Helicase dependent OnChip-amplification and its use in multiplex pathogen detection. Clin Chim Acta 2009;403(1-2):244-8 [DOI] [PubMed] [Google Scholar]
- 47.Doseeva V, Forbes T, Wolff J, et al.. Multiplex isothermal helicase-dependent amplification assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. Diagn Microbiol Infect Dis 2011;71(4):354-65 [DOI] [PubMed] [Google Scholar]
- 48.Ramalingam N, Liu HB, Dai CC, et al.. Real-time PCR array chip with capillary-driven sample loading and reactor sealing for point-of-care applications. Biomed Microdevices 2009;11(5):1007-20 [DOI] [PubMed] [Google Scholar]
- 49.Torres-Chavolla E, Alocilja EC. Nanoparticle based DNA biosensor for tuberculosis detection using thermophilic helicase-dependent isothermal amplification. Biosens Bioelectron 2011;26(11):4614-18 [DOI] [PubMed] [Google Scholar]
- 50.Mahalanabis M, Do J, ALMuayad H, et al.. An integrated disposable device for DNA extraction and helicase dependent amplification. Biomed Microdevices 2010;12(2):353-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang W, Chow WH, Li Y, et al.. Nucleic acid assay system for tier II laboratories and moderately complex clinics to detect HIV in low-resource settings. J Infect Dis 2010;201(Suppl 1):S46-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frech GC, Munns D, Jenison RD, Hicke BJ. Direct detection of nasal Staphylococcus aureus carriage via helicase-dependent isothermal amplification and chip hybridization. BMC Res Notes 2012;5:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramalingam N. Microfluidic devices harboring unsealed reactors for real-time isothermal helicase-dependent amplification. Microfluid Nanofluid 2009;7(3):325-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Park S, Liu K, et al.. A surface topography assisted droplet manipulation platform for biomarker detection and pathogen identification. Lab Chip 2011;11(3):398-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang S, Do J, Mahalanabis M, et al.. Low cost extraction and isothermal amplification of DNA for infectious diarrhea diagnosis. PLoS ONE 2013;8(3):e60059. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report about nearly electricity-free nucleic acid extraction process in combination with an electricity-free isothermal amplification assay.
- 56.Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS Biol 2006;4(7):e204. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study describing the molecular isothermal recombinase polymerase amplification technology.
- 57.Boyle DS, Lehman DA, Lillis L, et al.. Rapid detection of HIV-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification. MBio 2013;4(2):e00135-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abd El Wahed A, El-Deeb A, El-Tholoth M, et al.. A portable reverse transcription recombinase polymerase amplification assay for rapid detection of foot-and-mouth disease virus. PLoS One 2013;8(8):e71642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amer HM, Abd El Wahed A, Shalaby MA, et al.. A new approach for diagnosis of bovine coronavirus using a reverse transcription recombinase polymerase amplification assay. J Virol Methods 2013;193(2):337-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Euler M, Wang Y, Heidenreich D, et al.. Development of a panel of recombinase polymerase amplification assays for detection of biothreat agents. J Clin Microbiol 2013;51(4):1110-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lutz S, Weber P, Focke M, et al.. Microfluidic lab-on-a-foil for nucleic acid analysis based on isothermal recombinase polymerase amplification (RPA). Lab Chip 2010;10(7):887-93 [DOI] [PubMed] [Google Scholar]
- 62.Shen F, Davydova EK, Du W, et al.. Digital isothermal quantification of nucleic acids via simultaneous chemical initiation of recombinase polymerase amplification reactions on SlipChip. Anal Chem 2011;83(9):3533-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Ness J, Van Ness LK, Galas DJ. Isothermal reactions for the amplification of oligonucleotides. Proc Natl Acad Sci USA 2003;100(8):4504-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jia H, Li Z, Liu C, Cheng Y. Ultrasensitive detection of microRNAs by exponential isothermal amplification. Angew Chem Int Ed Engl 2010;49(32):5498-501 [DOI] [PubMed] [Google Scholar]
- 66.Roth U, Richard B. Rapid isothermal nucleic acid assays for the detection of pathogens. Lab Automation; San Diego, CA, USA; 2008 [Google Scholar]
- 67.Spenlinahauer TR, Estock ML, McFadd T, et al.. Nicking Enzyme Amplification Reaction (NEAR), an Isothermal Nucleic Acid Based Technology for Point-of-Testing of Plant Pathogens. American Phytopathological Society Annual meeting; 1 – 5 August 2009; Portland, Oregon, USA [Google Scholar]
- 68.Liu D, Daubendiek SL, Zillman MA, et al.. Rolling Circle DNA Synthesis: small Circular Oligonucleotides as Efficient Templates for DNA Polymerases. J Am Chem Soc 1996;118(7):1587-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henriksson S, Blomström AL, Fuxler L, et al.. Development of an in situ assay for simultaneous detection of the genomic and replicative form of PCV2 using padlock probes and rolling circle amplification. Virol J 2011;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan J, Song S, Li B, et al.. An on-nanoparticle rolling-circle amplification platform for ultrasensitive protein detection in biological fluids. Small 2012;6(22):2520-5 [DOI] [PubMed] [Google Scholar]
- 71.Sato K, Tachihara A, Renberg B, et al.. Microbead-based rolling circle amplification in a microchip for sensitive DNA detection. Lab Chip 2010;10(10):1262-6 [DOI] [PubMed] [Google Scholar]
- 72.Mahmoudian L, Melin J, Mohamadi MR, et al.. Microchip electrophoresis for specific gene detection of the pathogenic bacteria V. cholerae by circle-to-circle amplification. Anal Sci 2008;24(3):327-32 [DOI] [PubMed] [Google Scholar]
- 73.Reiss E, Hölzel R, Bier FF. Synthesis and stretching of rolling circle amplification products in a flow-through system. Small 2009;5(20):2316-22 [DOI] [PubMed] [Google Scholar]
- 74.Mitani Y, Lezhava A, Kawai Y, et al.. Rapid SNP diagnostics using asymmetric isothermal amplification and a new mismatch-suppression technology. Nat Methods 2007;4(3):257-62 [DOI] [PubMed] [Google Scholar]; •• First study describing the molecular isothermal smart amplification process v.2 technology.
- 75.Azuma K, Lezhava A, Shimizu M, et al.. Direct genotyping of Cytochrome P450 2A6 whole gene deletion from human blood samples by the SmartAmp method. Clin Chim Acta 2011;412(13-14):249-51 [DOI] [PubMed] [Google Scholar]
- 76.Kimura Y, de Hoon MJ, Aoki S, et al.. Optimization of turn-back primers in isothermal amplification. Nucleic Acids Res 2011;39(9):e59-2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kimura Y, Oguchi-Katayama A, Kawai Y, et al.. Tail variation of the folding primer affects the SmartAmp2 process differently. Biochem Biophys Res Commun 2009;383(4):455-9 [DOI] [PubMed] [Google Scholar]
- 78.Kawai Y, Kimura Y, Lezhava A, et al.. One-step detection of the 2009 pandemic influenza A(H1N1) virus by the RT-SmartAmp assay and its clinical validation. PLoS One 2012;7(1)):e30236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimada M, Hino F, Sagawa H, et al.. Development of the detection system for Mycobacterium tuberculosis DNA by using the isothermal DNA amplification method ICAN. Rinsho Byori 2002;50(5):528-32 [PubMed] [Google Scholar]; •• First detection system based on isothermal and chimeric primer-initiated amplification of nucleic acid technology.
- 80.Urasaki N, Kawano S, Mukai H, et al.. Rapid and sensitive detection of “Candidatus Liberibacter asiaticus” by cycleave isothermal and chimeric primer-initiated amplification of nucleic acids. J Gen Plant Pathol 2008;74(2):5 [Google Scholar]
- 81.Shimada M, Hino F, Yamamoto J, et al.. Evaluation of the simultaneous detection system for Chlamydia trachomatis/Neisseria gonorrhoeae DNA by the isothermal and chimeric primer-initiated amplification of nucleic acids (ICAN). Rinsho Byori 2003;51(11):1061-7 [PubMed] [Google Scholar]
- 82.Inami H, Tsuge K, Matsuzawa M, et al.. Semi-automated bacterial spore detection system with micro-fluidic chips for aerosol collection, spore treatment and ICAN DNA detection. Biosens Bioelectron 2009;24(11):3299-305 [DOI] [PubMed] [Google Scholar]
- 83.Isogai E, Makungu C, Yabe J, et al.. Detection of Salmonella invA by isothermal and chimeric primer-initiated amplification of nucleic acids (ICAN) in Zambia. Comp Immunol Microbiol Infect Dis 2005;28(5-6):363-70 [DOI] [PubMed] [Google Scholar]
- 84.Jani IV, Peter TF. How point-of-care testing could drive innovation in global health. N Engl J Med 2013;368(24):2319-24 [DOI] [PubMed] [Google Scholar]
- 85.Chin CD, Linder V, Sia SK. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012;12(12):2118-34 [DOI] [PubMed] [Google Scholar]
- 65.Brian K Maples, Rebecca C Holmberg, Andrew P Miller, et al.. Nicking and extension amplification reaction for the exponential amplification of nucleic acids. US20090017453; 2007; • Patent describing the molecular isothermal nicking and extension amplification reaction technology.
- 86.The global burden of disease. 2004 Update. Available from: www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.