Abstract
Ultrahigh sensitivity and specificity assays that detect Ebola virus disease or other highly contagious and deadly diseases quickly and successfully upstream in Spatial Care Paths™ can stop outbreaks from escalating into devastating epidemics ravaging communities locally and countries globally. Even had the WHO and CDC responded more quickly and not misjudged the dissemination of Ebola in West Africa and other world regions, mobile rapid diagnostics were, and still are, not readily available for immediate and definitive diagnosis, a stunning strategic flaw that needs correcting worldwide. This article strategizes point-of-care testing for diagnosis, triage, monitoring, recovery and stopping outbreaks in the USA and other countries; reviews Ebola molecular diagnostics, summarizes USA FDA emergency use authorizations and documents why they should not be stop-gaps; and reduces community risk from internal and external infectious disease threats by enabling public health at points of need.
Keywords: Ebola diagnostic center, Ebola, emergency use authorization, facilitated-access self-testing, point-of-care, public health resilience, ultrahigh sensitivity, ultrahigh specificity
Building on a foundation
This special report builds on and expands the knowledge base comprising: established principles of needs assessment (Box 1) [1] that can be used to create a logic web for future planning [2]; use of the critical path method to assess the validity of specifications for diagnostics, such as those published recently for Ebola virus disease (‘Ebola’) by the WHO (Table 1) [3,4]; the theory of the Spatial Care PathTM (SCP), described recently by Kost et al. [5,6]; the pivotal advantages of point-of-care testing (POCT) for diagnosis, triage, monitoring, recovery, derisking and stopping outbreaks; and a real sense of urgency, as more highly infectious disease outbreaks emerge to threaten the world.
Box 1. Needs assessment principles for developing point-of-care diagnostics.
Develop a logic model for systematic pursuit of project goals and timeline efficiency.
Clearly identify the goals, objectives and terms of a needs assessment survey after assessing results already published from previous surveys.
After designing the survey (e.g., observation, intervention, epidemiological or other), secure ethics approval at the appropriate administrative level and/or locale.
Draw respondents randomly from geographically dispersed sites, or from well-defined experts, professionals, laypersons and focus groups.
Avoid surveying special interest groups, which later courts of law or other legal bodies may deem hearsay not representative of randomly sampled larger populations, and therefore, invalid.
Perform surveys of adequate breadth, depth and sample size to yield significant comparisons of predefined variables in a comprehensive balance of closed- and open-ended questions.
Achieve an adequate response rate through use of facile media (e.g., SurveyMonkey), personal interviews, repetitive encouragement, follow-up communications and appropriate incentives.
Use original methods, such as trade-off questions, statistically validate results and base claims only on comparisons with p values <0.05.
Consider the broader context of national policies and guidelines, cost–effectiveness and market sustainability while simultaneously targeting outcomes people want, business realities and technical feasibility.
Integrate small-world network analysis with consideration of disasters, emergencies, outbreaks, and public health resilience.
Table 1.
WHO point-of-care target product profile.
|
Target population |
Patients presenting with fever to health care facilities for
assessment. |
|
|---|---|---|
| Key features | Desired | Acceptable |
| Priority | ||
| Target use setting | Decentralized health care facilities with no laboratories infrastructure available | Decentralized health care facilities with minimum laboratory infrastructures available. |
| Intended use | In Ebola outbreak setting, distinguish between symptomatic patients with acute Ebola virus infection and non-Ebola virus infection without the need for confirmatory testing | In Ebola outbreak setting, distinguish between symptomatic patients with acute Ebola virus infection and non-Ebola virus infection with the need for confirmatory testing |
| Clinical sensitivity†,‡ | >98% | >95% |
| Analytical specificity | >99% | >99% |
| Type of analysis | Qualitative or quantitative | Qualitative |
| Sample type | Capillary whole blood from finger stick once/if the use of this type of samples has been validated. Other less invasive sample types (e.g., saliva, buccal) once/if their use has also been validated | Whole blood from phlebotomy, in particular if collection is simple and automated to reduce biosafety requirements |
| Test procedure | ||
| Number of steps to be performed by operator (use of different reagents/incubation steps) | <3 0 timed steps |
<10 1 timed step |
| Biosafety§ | No additional biosafety in addition to PPE§ | No additional biosafety in addition to PPE§ |
| Need for operator to transfer a precise volume of sample | No | Acceptable if adequate disposable blood transfer device is provided |
| Time to result | <30 min | <3 h |
| Internal control | included | Included |
| Sample preparation Need to process sample prior to performing the test |
None or fully integrated | None or fully integrated |
|
Operational characteristics | ||
| Operating conditions | 5–50°C 90% RH |
5–40°C 90% RH |
| Reagent storage (stability) | 24 months at 40°C + 90% RH; no cold chain should be
required. Should be able to tolerate stress during transport (3 days at 50°C) |
12 months at 30°C + 70% RH including 3 months at 40°C, no
cold chain should be required. Should be able to tolerate stress during transport (3 days at 50°C) |
| In use stability (under tropical conditions) | >1 h for single-use test after opening the pouch | >0.5 h for single-use test after opening the pouch |
| Reagents reconstitution Need to prepare the reagents prior utilization |
All reagents ready to use | Reconstitution acceptable if very simple to do.
All liquids, including water, already in kit |
| Training needs Time dedicated to training session for end users |
Less than half a day for any level health care worker. Job aid provided. | Less than 2 days for any level of health care worker. Job aid provided. |
| Equipment (if needed) | Small and portable, handheld instrument Weight <2 kg |
Small, table top device, portable |
| Power requirements | None required Optional: 110–220 V AC current DC power with rechargeable battery lasting up to 8 h of testing |
110–220 V AC current DC power with rechargeable battery lasting up to 8 h of testing |
| Need for maintenance/spare parts | None | 1 annual calibration ideally by operator |
Detection should occur prior to presenting with fever to health care facilities in order to stop the spread of an outbreak.
†Clinical sensitivity in first 10 days of presentation. Allow for repeat testing as per WHO guidelines.
‡Reference test: Lab validated quantitative PCR assay on blood sample (whole blood or plasma) drawn by phlebotomy.
§Biosafety resources for Ebola [70,71].
The following organizations contributed to the development of this target Product Profile: WHO, MSF, FIND, BMGF, US DoD, US CDC, NIH, and PATH. Editorial comment regarding early detection to stop outbreaks in the target population added by the POCT•CTR.
PPE: Personal protective equipment.
Mapping the future
We extrapolate from stop-gap emergency use authorizations (EUAs) for Ebola and middle east respiratory syndrome Coronavirus (MERS-CoV) detection issued by the FDA in the USA onward to future sustainable POC molecular diagnostics that could be used for facilitated-access self-testing point of care (‘FAST POCTM’), a new solution for enhancing resilience [6]. This community-based strategy fills gaps needed in order for local population clusters of people to respond to both external and internal threats from old, new and often unpredictable, infectious diseases (Box 2).
Box 2. The internal threat from highly infectious diseases: demographics, incidents, secrecy & community risks in the USA.
Demographics
Over 200 high containment laboratories operated by government agencies, universities and private companies are scattered throughout the USA and the District of Columbia.
Experiments underway involve drug-resistant TB, plague, anthrax, botulism, ricin, exotic flu, Severe Acute Respiratory Syndrome, Middle East Respiratory Syndrome and Ebola and Marburg hemorrhagic fever viruses.
Incidents
More than 100 laboratories experimenting with potential bioterror agents have been cited by regulators at the CDC and USDA for serious safety and security failings and are facing sanctions.
According to limited information released for 2006–13 through the Freedom of Information Act, laboratories notified federal regulators of 1500 incidents, more than 800 workers received medical treatment or evaluation, 15 people contracted laboratory-acquired infections, animals also became infected, 79 laboratories were referred for potential enforcement actions and fines levied against 19 totaled $2.4 million.
Additionally, 33 laboratories have been placed on performance improvement plans, 7 are under scrutiny for select agent performance, 5 have been barred from using select agents, 5 had multiple enforcement actions, and 2 were expelled.
Accidents in the last year have compromised security for Ebola, avian influenza and smallpox at the NIH and CDC collectively; flu viruses are engineered to be more contagious than those found in nature, but so far, no accidental outbreaks have been reported.
Secrecy
Identities, affiliations and locations of laboratories out of compliance are kept secret under a 2002 bioterrorism law, and not even the names of the two laboratories expelled have been revealed to the public.
There is no publicly available list of high containment laboratories nor their research activities, scope of research or safety records, all largely unknown to state officials charged with protecting public health.
Community risks
Oversight is fragmented and self-policing is marginally effective, in view of hundreds of safety accidents at laboratories nationwide.
Deliberate theft and misuse of deadly pathogens; release of viruses, bacteria, and toxins; accidental and intentional outbreaks; and lack of transparency for residents living near biolabs represent sources of mistrust, frustration and community risk.
Information from [47].
Reflecting on the past
One of the greatest victories in global health, between 1796 and 1979, smallpox was eradicated through worldwide vaccination, mostly following World War II, because in nature variola infected only humans. With zoonotic diseases, such as Ebola and MERS-CoV, animals provide a reservoir from which the viruses can leap to humans, for the most part defeating attempts at eradication and upscaling perpetual need for surveillance, prevention and importantly, early detection to prevent the spread of outbreaks.
Derisking infectious threats
The Ebola crisis demonstrated the intrinsic value of testing at points of need, such as in diagnostic centers (Figure 1). In order to assure timely patient results during infectious disease crises, diagnostic centers, even mobile transportable ones, can be placed strategically within or near hospitals, next to or inside alternate care facilities [6], or logically situated along SCPs in regional small-world networks of healthcare [7]. Outbreaks should not be underestimated. They wreak havoc on health small-world networks, social networks and national economies. The World Bank estimates the financial burden of the Ebola epidemic in West Africa to be $32.6 billion by 2017 [8].
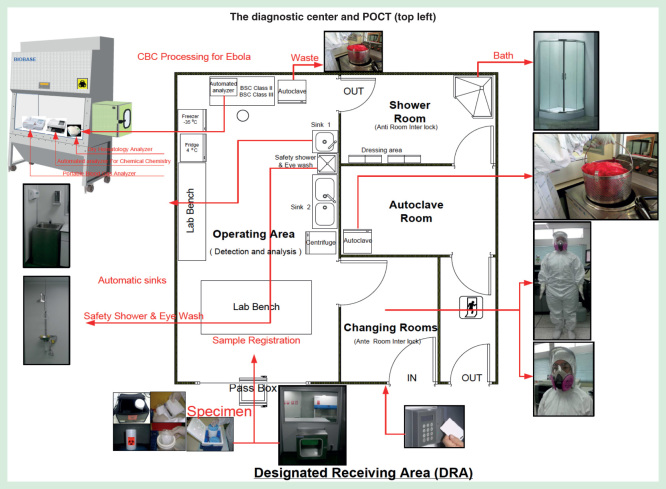
Figure 1.
The Ebola diagnostic center.
This schematic illustrates the floor plan and component design of a newly created diagnostic center. The left area is at negative pressure with a temperature of 22°C and relative humidity at 55%, a clean room class 10K. On the right, the shower room and changing room are at positive pressure, the latter with an interlocking anteroom. The top left inset shows POCT in the biosafety cabinet (BSL-3). Refer to the text for test clusters (test menus). Specimen preparation, then routing through a pass box are shown along the bottom, and PPE used in SE Asia, on the right. Staff circulate clockwise as they don PPE, perform work, and doff PPE before exiting to avoid exposure and contamination. This diagnostic center has been deployed in Bangkok, Thailand, but could be on a mobile vehicle moved to hotspots to break the log jam of slow or no testing in tiered (frontline, assessment and treatment) approaches. Figures provided courtesy and permission of Knowledge Optimization®, Davis, California, and Visual Logistics, a division of Knowledge Optimization®.
Investment in POCT to stop Ebola and other infectious disease outbreaks can be viewed, therefore, as having high value and a substantial financial rate of return abroad and in the USA as well. Collective action will produce economies of scale and increase value even more. On 18 February 2015, the CDC listed 55 USA Ebola treatment centers (see [9]), but they are unevenly distributed geographically, fixed in location and for some states, simply not there at all. Thus, the nation is not adequately prepared for challenges like Ebola, which masquerades among medical mimics causing febrile illnesses that should be sorted out with a probabilistic differential diagnosis attained in minutes, that is, as quickly as possible to mitigate risk.
Enabling public health
Striking gaps in preparedness, here and abroad, generate uncertainty, which leads to unpredictability. Suppose, as some suggest, that Ebola becomes more contagious, albeit perhaps less deadly, in a genetic give and take. Common sense dictates that distributing resources will enhance future resilience. Thus, POCT [10,11] has blossomed to become an innovative, ubiquitous and rapidly evolving point-of-need resource for resilience [12–18]. It is transforming not only disaster medicine [2], but also public health practice at points of contact worldwide. An obvious benefit of POCT rests with its utility ‘24/7’. That is, once in place and used regularly by well-trained operators, POCT simultaneously meets the needs of emergency, urgent and routine care. Now, it should fold in public health.
Key for the future is integrating public health practitioners in the needs fulfillment POCT scheme. Rather than depending on distant reference laboratories, borrowing from national disaster caches [13,18] or using non-dedicated externalities, such as conventional hospital main clinical laboratories, and also faced with extraordinary risk, demands for speed and epidemiological containment, hospitals that admitted Ebola patients or individuals suspected of being infected rapidly implemented broad-spectrum POCT directly within isolation areas [19,20] and containment units [21,22]. It is our overriding goal here to detail strategies for a new POC public health framework, which should be put in place nationwide now to transform and better enable public health practice, while there is still time, rather than later, after the next crisis hits.
Design criteria & device specifications
Extensive formal needs assessment surveys have defined specifications, namely high sensitivity, safety and user friendliness, for portable molecular pathogen detection with biohazard containment [1,23–29]. In an urgent call [3], the WHO published acceptance criteria and priority features for Ebola diagnostics in a ‘target product profile’ (Table 1) [4]. These WHO features comprise high clinical sensitivity in the first 10 days of patient presentation (>98% desired, >95% acceptable), extremely high analytical specificity (>99% desired, >99% acceptable) and minimally invasive and validated sample types (e.g., fingerstick capillary blood, saliva, or buccal) [4].
These specifications present serious challenges for technology designers, because implicit in them is low cost. However, our global field experience has shown that cost–effectiveness is attained by assessing the overall value of POC diagnostics, not just the costs, despite the practical challenges and economic realities of enabling appropriate value propositions. Also, the WHO target population [4], “Patients presenting with fever to health care facilities for assessment,” misses the point, a serious error. To stop an outbreak, POC detection should be substantially upstream at the first point of contact before sick febrile patients spread the disease to others. The first point of contact is anywhere that POCT can be used. The WHO concept of waiting until the patient presents with fever is too far downstream in the SCP and therefore too late to stop outbreaks.
Additional WHO features include efficient process steps performed by the operator (<3 desired, <10 acceptable with no and 1 timed steps, respectively), no or facilitated transfer of a precise volume of sample, quick analysis (<30 min desired, <3 h acceptable), internal controls, no or automated sample preparation and several delineated operational characteristics, such as robust operating conditions (0–50°C desired, 0–40°C acceptable; both up to 90% relative humidity), durable reagents and minimal training requirements [4].
The WHO temperature specification of 50°C may encounter bubble formation in reagent cassettes and other physical alterations in the sample, but temperature can be controlled in a diagnostic center, although in limited-resource settings, it will be challenging to do so. The WHO format is a small handheld (<2 kg, desired) or tabletop (acceptable) portable instrument with 30–60 min time limits for using opened reagents and ideally, solar, alternate or rechargeable battery power operating up to 8 h [4]. Well-configured, portable, robust and ergonomic POC devices would be ideal for isolation areas, alternate care facilities, mobile venues and the field.
Point-of-care testing in isolation areas
Table 2 lists POCT selected for the specialized isolation area at Emory University Hospital (2A) [19], used in the biocontainment unit of the University of Nebraska Medical Center (2B) [20] and designed for other Ebola containment centers [21] and suites [22] (2C & 2D). Additional POC test clusters can be selected by the reader from web sources [30] and lists of Clinical Laboratory Improvement Act-waived POC tests, which are the simplest to use [31]. One should be aware of which tests are FDA cleared for use with critically ill patients, an important consideration for Ebola patients, and identify clusters of tests that fulfill needs cost-effectively, ideally, on as few instruments as possible. POC staff [32] and other POC device operators must be trained in the use of personal protective equipment, sample handling procedures and the care of patients with Ebola.
Table 2.
Point-of-care tests implemented for Ebola secured settings.
| 2A. Emory university hospital
specialized isolation area | ||
|---|---|---|
| Manufacturer website | Instrument | Tests/status |
| Abaxis www.abaxis.com |
Piccolo Express | Chemistry profiles, Magnesium, Phosphate, liver enzyme assays, others available† |
| Instrumentation Laboratory www.instrumentationlaboratory.com |
GEM Premier 4000 | pH, pC02, p02, Na+, K+, Ca++, Cl-, Glu, Lac, Hct, THb, CO-Oximetry, TBil |
| Siemens www.healthcare.siemens.com |
CLINITEK Status automated urinalysis |
Albumin, Bilirubin, Cr, Glu, Ketone, Leukocytes, Nitrite, pH, Protein, Specific Gravity, Urobilinogen, others available‡ |
| Hoffman-La Roche www.coaguchek.com |
CoaguChek | PT/INR§ |
| Sysmex www.sysmex.com |
pocH-100i | CBC: WBC (3-part differential), RBC, Hb, Hct, MCV, MCH, MCHC, Platelets¶ |
| Alere www.alere.com/us/en/product-details/binaxnowmalaria.html |
BinaxNOW | Malaria |
| BioFire Diagnostics www.biofiredx.com |
FilmArray | Infectious diseases including Ebola FDA Ebola EUA 10/25/14, reissue 3/2/15 |
| 2B. University of nebraska
medical center biocontainment BSL-3 laboratory | ||
|---|---|---|
| Manufacturer website | Instrument/method | Tests |
| Abbott www.Abbott.com |
i-Stat | G3+ cartridge (pH, pCO2, pO2) & Chem8+ cartridge (Na+, K+, Cl-, TCO2, Ca++, Glu, UN, Cr, Hct) |
| International Technidyne Corp. www.itcmed.com |
Hemochron signature elite | Citrate prothrombin time (PT), citrate-activated partial thromboplastin time (aPTT) |
| Slide agglutination | Manual | Blood & serum antibody typing (for transfusion) |
| Slide preparation | Manual | Malaria—modified for the slide to be fixed in methanol 15 min before delivering to Core Lab for staining & interpretation |
| NS | Rapid manual assay | HIV Ab/Ag |
| Urine dipstick | Manual dipstick | For tests not on strip, specimen transferred with precautions to Core Lab for non-decapped DxI800 & DXC800i# analysis |
| NS | RPR | Syphilis (card assay) |
| 2C. Ebola holding units (4)
in Sierra Leone, West Africa†† | ||
| Developer website | Method | Performance |
| United Kingdom’s Defense Science & Technology Laboratory https://www.gov.uk/government/organisations/defence-scienceand-technology-laboratory | Rapid diagnostic antigen test |
Sensitivity 100, 95% CI: 78.2–100. Specificity: 96.6, 95%
CI: 91.3–99.1. Positive and negative predictive values: 79.0% (95% CI: 54.4–93.8)/100% (95% CI: 96.7–100) |
| 2D. Suite environment, ARUP
Institute for clinical & experimental pathology | ||
| Manufacturer website | Instrument/method | Tests, Evaluation study objectives |
| Abaxis www.abaxis.com |
Piccolo Express | Liver Panel Plus‡‡ using disposable exact volume transfer pipettes and BSL-2 cabinet in BSL-3 suite environment for Ebola patient workup. Checked device air flow characteristics are suitable. |
†See [72] for test clusters.
‡See [73].
§FDA-cleared for warfarin monitoring only.
¶See [74] for list of variables and parameters.
#Beckman-Coulter, La Brea, California, manufactures the DxI800 and DXC800i.
††See [21].
‡‡See [22] and [75], for evaluation study details and panel details, respectively. See [73] for test cluster lists.
ARUP: Associated regional and university pathologists; BSL: Biosafety level; Ca++: Ionized calcium; CBC: Complete blood count; Cr: Creatinine; EUA: Emergency use authorization; Glu: Glucose; Hb: Hemoglobin; Hct: Hematocrit; Lac: Lactate; MCV: Mean corpuscular volume; MCH: Mean corpuscular hemoglobin; MCHC: Mean corpuscular hemoglobin concentration; NS: Not specified in reference no. 15; pCO2: Partial pressure of carbon dioxide; pO2: Partial pressure of oxygen; PT/INR: Prothrombin time/international normalized ratio; RBC: Red blood cell; TBil: Total bilirubin; TCO2: Total carbon dioxide content; THb: Total hemoglobin; TP: Total protein; UN: Urea nitrogen; WBC: White blood cell.
Researchers are innovating novel technologies, such as surface acoustic wave detection [33], a recombinase polymerase amplification panel [34] and multicolored silver nanoparticles [35]. Manufacturers are encouraged to respond to the threat of future outbreaks by producing user-friendly POC cassettes/cartridges/cuvettes with multiplex test clusters designed specifically for Ebola patients (‘Ebola test clusters’). For example, coagulation tests on handheld devices are cleared by the FDA for anticoagulation (warfarin) monitoring, not for diagnosing disseminated intravascular coagulation, one of the most serious complications of Ebola. Clearly, these gaps need to be closed to improve the survival, assuming suitable therapy is available, of Ebola patients, who develop rampant bleeding, septic shock, multiorgan failure and acute hepatic necrosis leading to fatal outcomes.
Evolving molecular diagnostics & emergency use authorizations
Saijo et al. [36] reviewed laboratory diagnostics for Ebola and Marburg hemorrhagic fever in 2006. Now nearly a decade later, the FDA is accelerating the ongoing development, validation and approval of new diagnostic tests for Ebola by issuing EUAs more or less continuously since Fall 2014. Table 3 presents the EUAs in chronological order with their respective methods and status of development. Ebola-specific challenges for molecular diagnostics include: i) reduction in initial false negatives, FN = FN(t), as a function of time, to ramp up sensitivity, {TP/[TP + FN(t)]}, to ultrahigh levels in infected patients during the first 72 h when symptoms may be mild or absent, in order to avoid shunting false-negative cases to community hospitals ill prepared to receive high-risk patients (TP is true positive); ii) automation of totally self-contained and sealable specimen cassettes and cartridges to eliminate need for expensive high level biosafety cabinets; iii) proof of effectiveness in controlling internal contamination in portable instruments, thereby sustaining ultrahigh specificity [TN/(TN + FP)] and minimizing false positives, which place people at risk when near infected patients (TN is true negative and FP false positive); and as more sophisticated but compact technologies become available; and iv) determination of quantitative viral genome titers, which will be useful for early detection of exposure in small volumes of specimen and also for de-escalating the level of care and quarantine as the patient improves.
Table 3.
The rapid evolution of diagnostics for Ebola virus disease.
|
Instrument(s) &/or Assay/Kit Manufacturer |
Principle | Sample(s) | Time to Results | FDA Status |
|---|---|---|---|---|
| Xpert Ebola Assay Cepheid | rRT-PCR Cartridge-based | Blood | 2 h | EUA 3/23/15 |
| Corgenix ReEBOV & Fio Corp† | Lateral flow Ag immunoassay, Deki reader, smartphone data capture, & case tracking | Blood or plasma | 15 min | EUA 3/16/15 [eligible for WHO procurement] |
| LIghtMix Roche cobas z480 | rRT-PCR | Blood | Over 3 h | EUA 12/23/14 |
| QIAamp Viral Kit RealStar Filovirus: ABI Prism 7500
SDS LightCycler 480 II CFX96/Dx RT Sys |
rRT-PCR (Kit 1.0) | Blood, plasma | Varies with instrument | EUA 11/26/14 [eligible for WHO‡ procurement] |
| BioFire Defense Biothreat-E/NGDS bioMerieux§ [in 300 hospitals] | Film Array EZV Auto’d. rRT-PCR | Blood, urine (if matched to blood) | 1 h | EUA 10/25/14 3/2/15 (RI) |
| MagMax Pathogen Kit, Dynal Bead Re. ABI 7500 BioRad CFX96 | CDC NP rRT-PCR VP40 rRT-PCR |
Blood, plasma, serum, urine (if matched) | NS | EUA 10/10/14 3/2/15 (RI) |
| ABI 7500 LightCycler 480 JBAIDS |
DOD EZ1 rRT-PCR TaqMan Assay |
Inactivated whole blood & plasma |
Varies with instrument | EUA 10/10/14 |
| Nanomix [Corgenix & Tulane University] | Carbon nanotube biosensor¶ Handheld multiplex cartridge-based | Pinprick capillary blood | 10 min | No EUA# (see above) |
| Lucigen AmpliFire [Douglas Sci., UTMB, CDC] | LAMP (isothermal) 1-step, battery-operated, portable†† | RNA extract [plan 50 μL POC fingerstick capillary blood] | 40 min | No EUA# |
| Biomarkers USAMRIID/ECBC/TFS | Mass spectrometry | In development | NS | No EUA# |
| OraQuick‡‡ Orasure | CLF Ag assay [EZV, SEV, & BEV not differentiated] | In development: saliva sample | Est. 20 min | EUA# 7/31/15 [venous WB & fingerstick WB; not for screening, e.g., in airports; not for contact tracing] |
US FDA Emergency Use Authorization (EUA) status can be found at [76].
†See [77]. The WHO states, “The antigen test is rapid, easy to perform and does not require electricity-it can therefore be used at lower health care facilities or in mobile units for patients in remote settings. Where possible, results from ReEBOV antigen Rapid Test Kit should be confirmed by testing a new blood sample using an approved Ebola NAT”.
‡See [78].
§See [79].
#Instrumentation and corporate/academic relationships may have changed. See “Letters of Authorization” on the FDA EUA webpage for details [82]. Contact company and investigator sources for updates.
‡‡See [85].
CDC: Centers for disease control and prevention; DOD: Department of defense; ECBC: Edgewood Chemical Biological Center, US Army; EUA: Emergency use authorization; EZV: Ebola zaire virus; FDA: Food and drug administration; LAMP: Loop-mediated isothermal amplification; NA: Not available; NS: Not specified in the EUA; RI: Reissued by the FDA on the given date, as explained by the FDA on the EUA webpage-only latest date listed if reissuance within 3 months; rRT-PCR: Real-time reverse transcriptase polymerase chain reaction; TFS: Thermo fisher scientific; USAMRIID: US Army medical research institute of infectious diseases; WHO: World health organization.
When performed properly with biohazard precautions in the near-patient testing area of the diagnostic center, results will be available much more quickly than sending specimens to a public health laboratory or to the CDC [37,38]. The gain in time can be substantial, less than 1 h needed to obtain an answer (see Table 3), which facilitates rapid screening, focused triage and effective workflow. In West Africa, these practical efficiencies can help offset professional shortages in countries with large numbers of people per physician, such as 70,000:1 in Liberia (fatality rate 43.0%), 45,000:1 in Sierra Leone (28.7%) and 10,000:1 in Guinea (61.9%) [39,40]. Last Fall, the WHO urgently pleaded for rapid diagnostics to meet needs and help make up for these shortfalls, even as physicians and nurses in West Africa contracted Ebola and died.
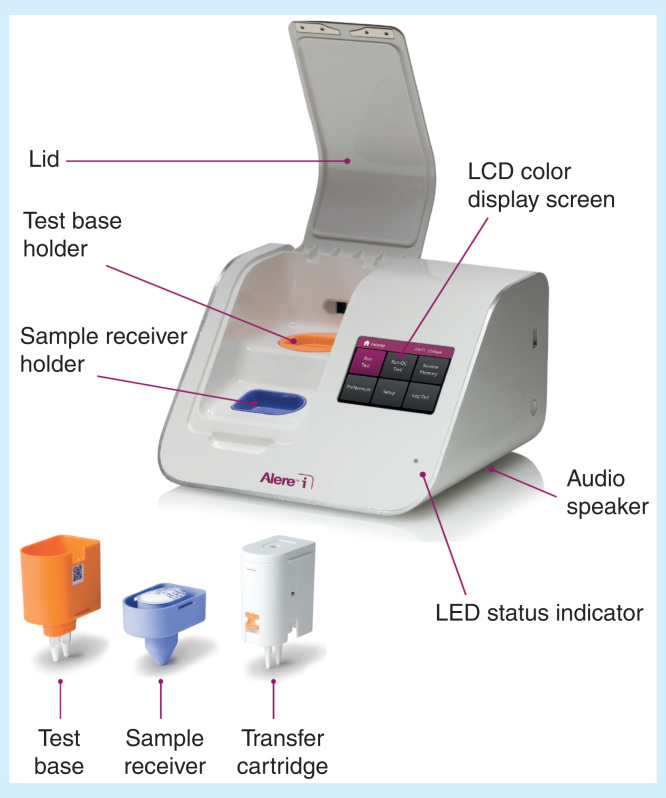
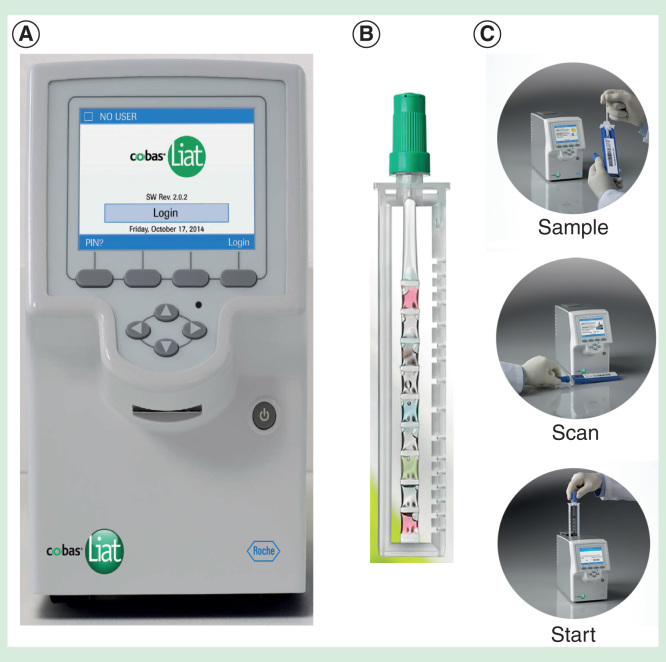
Self-contained cartridge/cassette-based rapid molecular tests are available on small portable platforms that test for infectious diseases. Development of POC molecular diagnostics for high-risk infectious diseases (see Table 3) forecasts the feasibility of introducing Ebola assays on lightweight platforms like the Alere i (Figure 2) [41] and the tiny Roche Diagnostics cobas Liat (Figure 3) [42], both Clinical Laboratory Improvement Act-waived. If tests satisfy certain conditions, they can be waived, that is, cleared by the FDA for use in clinics and at home. Most are simple to carry out and use operator-friendly equipment, which improves chances of accurate test results. We will see these potential solutions emerge as FAST POCTM when industry moves forward in the chronological progression of Ebola EUAs-inexpensive, portable, safe and appropriate for detection of viruses in the early stages of clinical illness.
Figure 2.
The Alere i molecular diagnostics platform and sample processing components (inset).
Figures provided courtesy and permission of Knowledge Optimization®, Davis, California, and Visual Logistics.
Figure 3.
The Roche Diagnostics cobas Liat. (A) Instrument; (B) PCR-based sample processor; (C) Workflow.
Figures provided courtesy and permission of Knowledge Optimization®, Davis, California, and Visual Logistics.
Facilitated-access self-testing point of care
FAST POCTM means that the patient obtains his or her own (capillary) blood (or other) sample with an automatic retractable lancet (or other sampling device) built into a self-aspirating and self-contained microcassette/microcuvette/cartridge, which then seals for automated processing and automatic testing on an integrated POC instrument, while another person, the ‘facilitator’, instructs and guides (hands off), so there is extremely limited or no exposure to infectious agents. Technological developments in POCT indicate that FAST POCTM is becoming a reality and will complement POC culture well [43,44]. Like something hidden in plain view, FAST POCTM offers a culturally adaptable solution for stopping future outbreaks, whether Ebola, MERS-CoV or novel new threats, because of minimally significant exposure to others or of oneself to infected patients and hence, little risk.
Similar to self-monitoring of blood glucose by individuals with diabetes, family members can instruct each other in homes and obtain results without actually contacting any blood, other specimens or testing components. Alternately, visiting local public health workers can educate, instruct, guide and facilitate self-testing in primary care sites without necessarily touching the patient or assay components. FAST POCTM will help eliminate fear of going to treatment centers, being separated from family and assigning loved ones to lonely deaths, when they may have Dengue, hepatitis, HIV, influenza, malaria, syphilis, tuberculosis or other common diseases in limited-resource settings or elsewhere, but not Ebola or MERS-CoV. In West Africa, FAST POCTM will enable primary care sites, clinics and treatment centers to be sought out as sites for speedy diagnosis [45] and excellent care with improved chances of survival.
Conclusions, strategies, & recommendations
POCT is improving global health [46], and now, a global health problem, Ebola, has propelled POCT and its urgent implementation worldwide. Needs are clear, as documented by several surveys worldwide [1]. Additionally, recent revelations of accidents, secrecy and adverse outcomes from highly infectious diseases made public by investigative journalists [47] highlight potential for outbreaks locally in USA communities.
World financial losses from Ebola and other outbreaks warrant investment [48], which should be directed not just to vaccines [49], but also to the development POC molecular diagnostics, for which there is precedent [50–57].
POC diagnostics should be available upstream for immigration screening, on cruise ships, in industrial sites abroad and at other points of first encounter worldwide. Companion diagnostics, such as coagulopathy test clusters [PT/INR (prothrombin time/international normalized ratio), D-dimer, fibrinogen and platelets], viral load assays and digital PCR will streamline therapeutic monitoring downstream.
SCPs consolidate process steps and ultimately will help stop the spread of outbreaks. Diagnostic centers with controlled environmental conditions placed along SCPs will motivate industry to respond to WHO calls [3] for robust diagnostic tests [4] and consolidate community efforts on a cost-effective broader scale.
Construction of approximately 1400 specialized isolation units in Hong Kong was motivated historically by the deaths of patients and healthcare workers from Severe Acute Respiratory Syndrome, which infected 1800 and killed 299 people, and Avian Influenza [58,59]. In Guangzhou, China, Severe Acute Respiratory Syndrome killed 8000 [60].
These disasters should not be repeated. The USA is vulnerable to an unending series of threats [61]. These threats include not just ones from abroad, but also from within, as documented in Box 2. Recent journalistic research revealed shocking non-disclosure of communities at risk.
Adaptations in SE Asia and in individual USA hospitals, such as isolation areas in Atlanta, Dallas, New York, Bethesda (NIH Clinical Center) and Omaha, are notable, but have not yet generated isolation bed capacity nor adequate experience quickly enough to deal with potentially large numbers of cases from infectious disease outbreaks in the future.
CDC-approved hospital treatment centers are distributed unevenly. The recent launch and funding of the National Ebola Training and Education Center by the CDC and the Health and Human Services Office of the Assistant Secretary for Preparedness and Response comprising Emory University Medical Center in Atlanta, Georgia; the University of Nebraska Medical Center in Omaha, Nebraska and the Bellevue Hospital Center in New York City will facilitate the development of additional treatment centers [62]. Nine of the current treatment centers will have enhanced capabilities.
Alternate care facilities and diagnostic centers with integrated logistics for community small-world networks, as the CDC recommends be engaged, will allow USA communities to respond efficiently and effectively.
In regard to internal threats, the government, academia and private companies must disclose information about problems that have occurred at secured biolabs, so that communities can set medical, financial and public health priorities. People have a right to know when safety violations occur. In fact, laypersons should be given more weight on oversight committees.
For external threats, such as Ebola or new infectious disease threats, strategic planers must reshape public health for resilience at points of need. In June 2015, MERS-CoV, for which a feasible rapid POC molecular diagnostic [63] and one FDA EUA [64] exist, but for which there is no vaccine or definitive treatment, was spreading throughout South Korea [65,66] 1369 in quarantine, 30 cases confirmed (since 5/15/15), 2 dead, 1 traveled sick to China (exposing all on an airplane), 540 schools closed, facilities closed and according to the Korean Health Minister, not enough done to detect the first wave, stop spread and end the outbreak. Later, when Koreans were told to ‘rest easy’, 36 had died, 186 had been infected,17,000 had been placed in quarantine and 12 remained hospitalized [67]. Second quarter South Korean growth was the worst in 6 years, the government announced a 22-trillion won stimulus package and the economic growth forecast was cut from 3.1 to 2.8%. Hence, we can predict heavy economic impact from future threats in ASEAN + 6 countries.
MERS-CoV was not suspected and health care workers did not treat the first patient in isolation. Some of the infected people occupied the same room as the first patient, and others had been in the same ward for times ranging from 5 min to several hours. Said the Prime Minister, Park Geun-hye, “there were some insufficiency in the initial response, including the judgment on its contagiousness [65].” How many times will this scenario repeat, at what economic and medical costs, and to whom, before responses are properly strategized, restructured and enabled at the points of first contact, need and care?
Therefore, in summary, we recommend accelerating proactive planning, national preparedness, development of new POC technologies [68] and especially field evaluation, which, in the case of Ebola, has demonstrated high sensitivity and interesting POC results in clinic settings [69].
Expert commentary & five-year view
This future paradigm of POC diagnostics is to think globally and act globally, but test locally where the people are in the context of their own cultural settings. Intrinsic to emerging POC culture [5,43,44] is the popular expectation of rapid diagnosis [45] of high-risk viruses using disposable test strips, self-contained automated technologies and other mobile cartridge-, cassette- or cuvette-based approaches. Diagnostics that properly fulfill these expectations, and those popular expectations are the keys to motivation, in the next 5 years while delivering ultrahigh sensitivity, specificity and predictive values will help stifle outbreaks in the USA and other countries before they succumb to future threats and untoward economic losses.
Multiplex PCR assays are particularly beneficial in cases when samples are difficult to collect or short on volume, and when different pathogens create the same clinical constellation of symptoms and signs. Additionally, the instant traceability of infected or potentially infected individuals by knowing multiplex results, locations, and movement over time and across borders can help contain the spread of highly contagious diseases. FAST POCTM, self-knowledge and enabled people who take ownership of personalized diagnostic testing will interrupt the rapid spread of outbreaks, an impactful shift in public health to immediate diagnosis at points of need and a new POC culture in public health.
Disclaimer
Devices must comply with jurisdictional regulations in specific countries, operator use limitations based on patient conditions, federal and state legal statutes and hospital accreditation requirements. Not all POC devices presented in this article are FDA cleared for use in the USA. FDA emergency use authorization is limited in scope and term. Please check with manufacturers for the current status of Ebola, MERS-CoV and other threat detection diagnostics and POC tests within the relevant domain of use.
Acknowledgements
The authors are grateful to the creative students who participate in the POCT•CTR and contribute substantially to knowledge in point-of-care.
Financial & competing interests disclosure
This work was supported by the Point-of-Care Testing Center for Teaching and Research (POCT•CTR) and by GJ Kost, Director. Spatial Care Path™ is a trademark by W Ferguson and GJ Kost, Knowledge Optimization®, Davis, CA, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Key issues.
Zoonotic reservoirs sustain major virus threats while enabling jumps to humans, and although vaccination can provide protection, animal reservoirs generally cannot be eliminated, so rapid detection of high morality diseases at points of need is necessary to stop new outbreaks – facilitated-access self-testing point of care (FAST POCTM) can enable this type of resolution.
Detection should occur upstream early in the spatial care path in homes (using FAST POCTM), primary care sites (e.g., clinics) and emergency rooms (to prevent spread like occurred with MERS-CoV in South Korea), before patients disseminate the disease throughout the community, and in particular, waiting for symptomatic patients to present at treatment centers perpetuates outbreaks and unnecessary mortality.
The value proposition for early rapid diagnosis should be formulated in the context of extraordinary financial losses (e.g., billions of dollars lost in West Africa) incurred when whole nations are afflicted with epidemics, in order to assess cost–effectiveness and return on investment in point-of-care testing.
The recent Ebola and MERS-CoV outbreaks have motivated significant rethinking, which ideally should manifest as national policies for POC testing, so that appropriate infrastructure can be designed, funded and distributed evenly geographically.
Public health practitioners, the CDC, the WHO and other organizations should anticipate points of first critical need by assimilating POC know-how and technologies, such as molecular diagnostics and mobile testing, in order to acquire knowledge bases of sensitivity, specificity and predictive values while epidemics are underway – these data will facilitate real-world diagnostic device and assay maturation, refine evidence-based medicine for target diseases and prepare first responders for future outbreaks.
The advent of hospital isolation units, Ebola treatment centers, national Ebola training sites and community preparation through PPE rehearsals should be coordinated with FDA education in emergency use authorizations, so that as they emerge, the new diagnostic technologies, such as the Orasure POC and Cepheid molecular diagnostic tests for Ebola (see Table 3), can be integrated systematically and efficiently.
Point-of-care instrument experts, professionally certified POC coordinators and geospatial scientists designing spatial care paths should contribute to collaborative leadership for the development of cohesive national strategies. Transforming public health must occur through education, deep understanding of point-of-care testing and professionally integrated teamwork. Stopping future outbreaks more quickly in the future will demand redefinition of preparedness and current public health practice.
Ultimately, communities worldwide would be wise to develop alternate care sites equipped with POC testing and diagnostic centers in order to enhance resilience in the event of widespread outbreaks that require quarantine of large groups of people, in part in order to ameliorate civil rights issues by providing well thought out and equitable care.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Kost GJ, Louie RF, Curtis CM. Needs assessment for rapid decision making in pandemics, complex emergencies, and disasters: A global perspective Chapter 43. : , editor Kost GJ, Curtis CM, Assoc. editor Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. p 3-22. [Google Scholar]; •• Comprehensive description of how to perform needs assessment for POCT.
- 2.Kost GJ, Curtis CM, editor Global point of care: strategies for disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. Available from: https://www.aacc.org/store/books/9200/global-point-of-care-strategies-for-disastersemergencies-and-public-health-resilience [Google Scholar]; •• Over 105 authors who cover all aspects of global point of care and its future.
- 3.WHO Urgently needed: rapid, sensitive, safe, and simple Ebola diagnostic tests. November 18, 2014. Available from: www.who.int/mediacentre/news/ebola/18-november-2014-diagnostics/en/ [Last accessed 14 July 2015]
- 4.WHO Target product profile for zaire ebolavirus rapid, simple test to be used in the control of the Ebola outbreak in West Africa. October 3, 2014. Available from: www.who.int/medicines/publications/target-product-profile.pdf?ua=1 [Last accessed 14 July 2015]
- 5.Kost GJ, Ferguson WJ. Principles of point of care culture, the spatial care path, TM and enabling community and global resilience. Elect J Intl Fed Clin Chem. 201425:4-23. Available from: www.ifcc.org/media/260912/eJIFCC%20August%202014.pdf [Last accessed 14 July 2015] [Google Scholar]; • Introduces the new Spatial Care PathTM concept.
- 6.Kost GJ, Ferguson WJ, Hoe J, et al. The Ebola Spatial Care Path™: Accelerating point-of-care diagnosis, decision making, and community resilience in outbreaks. Am J Disaster Med 2015; in press [DOI] [PubMed] [Google Scholar]; •• Key paper on Ebola, what to do about it, and how to enhance community resilience.
- 7.Kost GJ. Using small-world networks to optimize preparedness, response, and resilience. : Kost GJ, Curtis CM, editor Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. p 539-68 [Google Scholar]
- 8.The World Bank Ebola: New World bank group study forecasts billions in economic loss if epidemic lasts longer, Spreads in West Africa. Available from: www.worldbank.org/en/news/press-release/2014/10/08/ebola-new-world-bank-group-study-forecasts-billions-in-economic-loss-if-epidemic-lasts-longer-spreads-in-west-africa [Last accessed July 14, 2015]
- 9.Hospital preparedness: A tiered approach. Available from: www.cdc.gov/vhf/ebola/healthcare-us/preparing/current-treatment-centers.html [Last accessed August 19, 2015]
- 10.Kost GJ, Ehrmeyer SS, Chernow B, et al. The laboratory-clinical interface: Point-of-care testing. Chest 1999;115:1140-54 [DOI] [PubMed] [Google Scholar]
- 11.Kost GJ. Principles and Practice of Point-of-Care Testing. Lippincott Williams and Wilkins, Philadelphia, PA, 2002. p 654 [Google Scholar]
- 12.Kost GJ, Sakaguchi A, Curtis C, et al. Enhancing crisis standards of care using innovative point-of-care testing. Am J Disaster Med 2011;6:351-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis CM, Louie RF, Vy JH, et al. Innovations in point-of-care testing for United States disaster caches. Am J Disaster Med 2013;8:181-204 [DOI] [PubMed] [Google Scholar]
- 14.Kost GJ, Curtis C, Louie R, Sakaguchi A. Disaster point-of-care testing: Fundamental concepts and new technologies. : Arora R, Arora P, Disaster Management: Medical Preparedness, Response, and Homeland Security. CABI International, Wallingford, England, 2013. p 119-50 [Google Scholar]
- 15.Kost GJ, Curtis CM, Ferguson WJ, et al. The role of point-of-care testing in complex emergency and disaster resilience. : Raskovic B, Mrdja S, Natural Disasters: Prevention, Risk Factors and Management. Nova Science Publishers, Hauppauge, New York, 2013. p 73-110 [Google Scholar]
- 16.Kost GJ, Curtis CM, Ferguson WJ, et al. Emergency and disaster point-of-care testing in southern Thailand and Phang Nga Province: Tsunami impact, needs assessment, geospatial preparedness, and future resilience. : Cai T, editor Tsunamis: Economic Impact, Disaster Management, and Future Resilience. Nova Science Publishers, Hauppauge, New York, 2013. p 1-39 [Google Scholar]
- 17.Kost GJ, Curtis CM. Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. p 701 [Google Scholar]
- 18.Curtis CM, Louie RF, Kost GJ. Current and future design of point of care in national disaster caches. : Kost GJ, Curtis CM, Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. p 413-32 [Google Scholar]
- 19.Hill CE, Burd EM, Kraft CS, et al. Laboratory test support for Ebola patients within a high-containment facility. Lab Med 2014;45:e109-11 [DOI] [PubMed] [Google Scholar]; • This paper and the three below present solutions for the care of Ebola patients.
- 20.Iwen PC, Garrett JL, Gibbs SG, et al. An integrated approach to laboratory testing for patients with Ebola virus disease. Lab Med 2014;45:e146-51 [DOI] [PubMed] [Google Scholar]
- 21.Walker NF, Brown CS, Youkee D, et al. Evaluation of a point-of-care blood test for identification of Ebola virus disease at Ebola holding units, Western Area, Sierra Leone, January to February 2015. Euro Surveillance 2015;20(12):pii 21073. [DOI] [PubMed] [Google Scholar]
- 22.Owen WE, Caron JE, Genzen JR. Liver function testing on the Abaxis Piccolo Xpress: Use in Ebola virus disease protocols. Clin Chim Acta 2015;446:119-27 [DOI] [PubMed] [Google Scholar]
- 23.Kost GJ, Hale KN, Brock TK, et al. Point-of-care testing for disasters: Needs assessment, strategic planning, and future design. Clin Lab Med 2009;29:583-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brock TK, Mecozzi DM, Sumner S, Kost GJ. Evidence-based point-of-care tests and device designs for disaster preparedness. Am J Disaster Med 2010;5:285-94 [PMC free article] [PubMed] [Google Scholar]
- 25.Mecozzi DM, Brock TK, Tran NK, et al. Evidence-based point-of-care device design for emergency and disaster care. Point Care 2010;9:65-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kost GJ, Katip P, Curtis CM. Strategic point-of-care requirements of hospitals and public health for preparedness in regions at risk. Point Care 2012;11:114-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kost GJ, Mecozzi DM, Brock TK, Curtis CM. Assessing point-of-care device specifications and needs for pathogen detection in emergencies and disasters. Point Care 2012;11:119-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kost GJ, Katip P, Vinitwatanakhun C. Diagnostic testing strategies for healthcare delivery during the Great Bangkok Flood and other weather disasters. Point Care 2012;11:191-9 [Google Scholar]
- 29.Kost GJ, Katip P, Kulrattanamaneeporn S, Gentile N. Point-of-care testing value proposition for disaster preparedness in small-world networks: Post-tsunami Phang Nga Province, coastal Thailand. Point Care 2013;12:9-22 [Google Scholar]
- 30.PointofCare.net: The point-of-care coordinators online resource, Point-of-care vendors. Available from: www.pointofcare.net/vendors/index.htm [Last accessed 14 July 2015]
- 31.Centers for disease control and prevention Clinical laboratory improvements amendments: Waived tests. Available from: wwwn.cdc.gov/clia/Resources/WaivedTests [Last accessed 14 July 2015]
- 32.Mann PA. Describing a Point-of-care Coordinator’s potential role in disasters and complex emergencies. Kost GJ, Curtis CM, Global point of care: Strategies for disasters, emergencies, and public health resilience. AACC Press; Washington DC: 2015. p. 347-56 [Google Scholar]
- 33.Baca JT, Servens V, Lovato D, et al. Rapid detection of Ebola virus with a reagent-free biosensor. Sensors 2015;15:8605-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Euler M, Wang Y, Heidenreich D, et al. Development of a panel of recombinase polymerase amplification assays for detection of biothreat agents. J Clin Microbiol 2013;51:1110-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yen CW, de Puig H, Tam J, et al. Multicolored silver nanoparticles for multiplexed disease diagnostics: Distinguishing dengue, Yellow fever, and ebola viruses. Lab Chip 2015;15:1538-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saijo M, Niikura M, Ikegamni T, et al. Laboratory diagnostic systems for Ebola and Marburg Hemorrhagic Fevers developed with recombinant proteins. Clin Vaccine Immunol 2006;13:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CDC. Ebola (Ebola Virus Disease) Interim Guidance for Specimen Collection, Transport, Testing, and Submission for Persons Under Investigation (PUIs) for Ebola Virus Disease in the United States. Available from: www.cdc.gov/vhf/ebola/hcp/interim-guidance-specimen-collection-submission-patients-suspected-infection-ebola.html [[Last accessed 31 December 2014] [Google Scholar]
- 38.Centers for Disease Control (CDC) Ebola (Ebola Virus Disease). Diagnosis. Available from: www.cdc.gov/vhf/ebola/diagnosis/ [Last accessed 14 July 2015]
- 39.CNN library Ebola fast facts. Available from: www.cnn.com/2014/04/11/health/ebola-fast-facts/ [Last accessed 14 July 2015]
- 40.BBC News Africa Ebola: Mapping the outbreak. Available from: www.bbc.com/news/world-africa-28755033 [Last accessed 14 July 2015]
- 41.Alere i. Available from: http://www.alere-i.com/en/index.html
- 42.Roche Diagnostics USA. Available from: https://usdiagnostics.roche.com/en/instrument/cobas-liat.html
- 43.Kost GJ, Katip P, Vansith K, Negash H. The final frontier for point of care: Performance, resilience, and culture. Point Care 2013;12:1-8 [Google Scholar]; •• This paper and the one below innovate ‘point of care culture’ and explain its significance for the future of synoptic diagnostics in world societies.
- 44.Kost GJ, Zhou Y, Katip P. Understanding point of care culture improves resiliency and standards of care in resource-limited countries. : Kost GJ, Curtis CM, Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015;p 471-90 [Google Scholar]
- 45.Baker A. Stopping Ebola: The risks and realities of the outbreak-The race to diagnose: Better and faster Ebola testing in West Africa would save lives and could help bring an end to the outbreak. Time 2014;184:28-29. Available from: http://timereaders.web.fc2.com/20151b.html [Last accessed 14 July 2015
- 46.Kost GJ. Point-of-care testing and public health: A global challenge for the 21st Century. Point Care 2006;5:137 [Google Scholar]
- 47.Young A, Penzenstadler N. 40 Gannett Newspaper Journalists. Biolabs in your backyard: Hundreds of accidents, safety violations and close calls put people at risk. USA Today Weekend 2015;33(181):1A- 4A,- 5A [Google Scholar]
- 48.Leonard K. Obama praises ebola vaccine testing, calls for more funding ($6 billion). US. News. December 2, 2014. Available from: www.usnews.com/news/articles/2014/12/02/obama-praises-ebola-vaccine-testing-calls-for-more-funding [Last accessed 14 July 2015]
- 49.Leonard K. Obama praises ebola vaccine testing, calls for more funding ($6 billion). US. News. December 2, 2014. Available from: www.usnews.com/news/articles/2014/12/02/obama-praises-ebola-vaccine-testing-calls-for-more-funding [Last accessed 14 July 2015]
- 50.Bissonette L, Bergeron MG. Infectious disease management through point-of-care personalized medicine molecular diagnostic technologies. J Pers Med 2012;2:50-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bissonnette L, Chapdelaine S, Peytavi R, et al. A revolutionary microfluidic stand-alone platform (GenePOC) for nucleic-acid-based point-of-care diagnostics. : Kost GJ, Curtis CM, Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. p 235-48 [Google Scholar]
- 52.Mahmoudzadeh S, Tran NK, Louie RF, et al. Rapid molecular diagnosis of sepsis in critical, emergency, and disaster care. : Kost GJ, Curtis CM, Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. p 191-220. [Google Scholar]; • A current in-depth review of POC molecular diagnostics. Covers all aspects of technology, application, and the spectrum of organisms currently detected by NAT.
- 53.He J, Hoe J. Rapid molecular diagnostics for major respiratory viruses. : Kost GJ, Curtis CM, Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. p 221-34 [Google Scholar]
- 54.Crowther Z, Powell S. Needs-centered multiplex molecular testing for time-critical infectious diseases. : , editor Kost GJ, Curtis CM, Assoc. editor Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press; Washington DC: 2015;p 249-60 [Google Scholar]
- 55.Sesler C, Green J, Malone L, et al. Target enriched multiplex PCR (TEM-PCRTM) for rapid detection of bloodstream infections. : Kost GJ, Curtis CM, Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. p 261-8 [Google Scholar]
- 56.Charnot-Katsikas A, Tesic V, Beavis KG. Rapid detection of microorganisms using MALDI-TOF MS in the clinical microbiology laboratory. : Kost GJ, Curtis CM, Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. p 269-76 [Google Scholar]
- 57.Vandiver TKB. Collaborative medicine: Weaving microbiology into point-of-care clinical responsiveness. : Kost GJ, Curtis CM, Global Point of Care: Strategies for Disasters, Emergencies, and Public Health Resilience. AACC Press, Washington DC, 2015. p 277-89 [Google Scholar]
- 58.Bradsher K. As Ebola spreads, Asia senses vulnerability. International New York Times. October 26, 2014. Available from: www.nytimes.com/2014/10/27/world/asia/as-ebola-spreads-asia-senses-vulnerability.html?_r=0 [Last accessed 14 July 2015]
- 59.Legacy of SARS leaves Hong Kong better prepared for Ebola fight South China Morning Post. November 2, 2014. Available from: www.scmp.com/news/hong-kong/article/1630457/legacy-sars-leaves-hong-kong-better-prepared-ebola-fight [Last accessed 14 July 2015
- 60.Macauley R. China is imposing an unofficial Ebola quarantine in its “Little Africa.” Quartz. November 26, 2014. Available from: http://qz.com/303070/china-is-imposing-an-unofficial-ebola-quarantine-in-its-little-africa/ [Last accessed 14 July 2015]
- 61.Pifer LLW, Hicks W. Emerging viruses of North America: are labs ready? Medical Laboratory Observer 2015;47(3):10. [PubMed] [Google Scholar]
- 62.US. Department of Health and Human Services HHS Launches National Ebola Training and Education Center. July 1, 2015. Available from: www.hhs.gov/news/press/2015pres/07/20150701a.html [Last accessed July 14, 2015]
- 63.Wahed AAE, Patel P, Heidenriech D, et al. Reverse transcription recombinase polymerase amplification assay for the detection of Middle East Respiratory Syndrome Coronavirus. PLoS Currents 2013;12:1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.FDA 2013 Coronavirus Emergency Use Authorization (Potential Emergency). Available from: www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm#coronavirus [Last accessed 14 July 2015]
- 65.Park M. South Korea grapples to contain MERS as 1,369 in quarantine. CNN Breaking News. June 3, 2015. Available from: www.cnn.com/2015/06/03/world/south-korea-mers/index.html [Last accessed 14 July 2015]
- 66.Szabo L. South Korean MERS outbreak likely to spread, health officials say. USA Today. June 3, 2015. Available from: www.usatoday.com/story/news/2015/06/02/mers-spreads-south-korea/28373087/ [Last accessed 14 July 2015]
- 67.Koreans told to “rest easy” over MERS The Strait Times. July 25, 2015: A14. Available from: www.straitstimes.com/asia/east-asia/koreans-told-to-rest-easy-over-mers [Last accessed 2 August 2015]
- 68.Developing a qPCR point-of-care diagnostic for Ebola: The Ubiquitome Freedom4 device and PrimeTime® qPCR Assays Integrated DNA Technologies. July 14, 2015. Available from: http://sg.idtdna.com/pages/decoded/decoded-articles/pcr-qpcr/decoded/2015/07/14/developing-a-qpcr-point-of-care-diagnostic-for-ebola [Last accessed 2 August 2015]
- 69.Broadhurst MJ, Kelly JD, Miller A, et al. ReEBOV Antigen Rapid Test kit for point-of-care and laboratory-based testing for Ebola virus disease: a field validation study. Lancet (online first). 2015. Epub ahead of print Available from: 10.1016/S0140-6736(15)61042-X [Last accessed 27 July 2015] [DOI] [PubMed]; • This brief report points to what to look for in the future of POC response in field setting evaluations.
- 70.WHO Ebola outbreak-home. Available from: www.who.int/csr/disease/ebola/en
- 71.WHO Infection prevention and control guidance for care of patients in health-care settings, with focus on Ebola. Available from: www.who.int/csr/resources/publications/ebola/filovirus_infection_control/en
- 72.Piccolo Xpress product menu. Available from: www.piccoloxpress.com/products/panels/menu
- 73.Siemens CLINITEK Status+ analyzer. Available from: www.healthcare.siemens.com/point-of-care/urinalysis/clinitek-status-analyzer/technical-specifications
- 74.Sysmex pocH-100i™ Automated hematology analyzer. Available from: www.sysmex.com/us/en/Brochures/Brochure_pocH-100i_MKT-10-1025.pdf [PubMed]
- 75.Piccolo® Liver Panel Plus. Available from: www.piccoloxpress.com/wp-content/uploads/Liver-Panel-Plus.pdf
- 76.US FDA Current EUAs. Available from: www.fda.gov/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/MCMLegalRegulatoryandPolicyFramework/ucm182568.htm#current
- 77.Corgenix Corgenix and fio combine rapid ebola test with automated analysis and data capture to improve frontline care and case tracking. Available from: www.corgenix.com/news-releases/corgenix-and-fio-combine-rapid-ebola-test-with-automated-analysis-and-data-capture-to-improve-frontline-care-and-case-tracking/
- 78.WHO Emergency Quality Assessment Mechanism for EVD IVDs PUBLIC REPORT, Product: RealStar® Filovirus Screen RT-PCR Kit 1.0. Available from: www.who.int/diagnostics_laboratory/procurement/141125_evd_public_report_altona_v1.pdf?ua=1
- 79.Fierce medical devices, One-hour ebola test from bioMérieux receives FDA emergency use authorization. Available from: www.fiercemedicaldevices.com/story/one-hour-ebola-test-biom-rieux-receives-fda-emergency-use-authorization/2014-10-27
- 80.Jones A, Boisen M, Radkey R, et al. Development of a multiplex point of care diagnostic for differentiation of Lassa fever, Dengue fever and Ebola hemorrhagic fever. American Association for Clinical Chemistry Poster.
- 81.Nanomix Discussion of PCR testing and immunoassay testing using the Nanomix system for the detection of Ebola virus. Available from: www.nano.com/downloads/Ebola%20testing_PCR%20vs%20Immunoassay.pdf
- 82.US FDA Emergency use authorization. Available from: www.fda.gov/EmergencyPreparedness/Counterterrorism/ucm182568.htm
- 83.Benzine J, Manna D, Mire C, et al. Rapid point of care molecular diagnostic test for Ebola virus. Poster. 2015, ASM-Biodefense. Available from: www.lucigen.com/docs/posters/ASM-Biodefense-2015-ebola.pdf
- 84.Johnson M. Lucigen to seek FDA emergency use approval for isothermal Point-of-care ebola test. Available from: www.douglasscientific.com/NewsEvents/News/2014-10-21%20Lucigen%20to%20Seek%20FDA%20Emergency%20Use%20Approval%20for%20Isothermal%20Point-of-Care%20Ebola%20Test.pdf 2014
- 85.Mangan D. Ebola test: Quick, cheap, easy help in stopping disease. Available from: www.cnbc.com/2015/06/12/ebola-test-quick-cheap-easy-help-in-stopping-disease.html 2015