Abstract
Pneumonia is still the number one killer of young children globally, accounting for 18% of mortality in children under 5 years of age. An estimated 120 million new cases of pneumonia occur globally each year. In developing countries, management and prevention efforts against pneumonia have traditionally focused on bacterial pathogens. More recently however, viral pathogens have gained attention as a result of improved diagnostic methods, such as polymerase chain reaction, outbreaks of severe disease caused by emerging pathogens, discovery of new respiratory viruses as well as the decrease in bacterial pneumonia as a consequence of the introduction of highly effective conjugate vaccines. Although the epidemiology, etiology and clinical characterization of viral infections are being studied extensively in the developed world, little data are available from low- and middle-income countries. In this paper, we review the epidemiology, etiology, clinical and radiological features of viral pneumonia in developing countries.
Keywords: acute respiratory infections, adult, children, developing countries, pneumonia, viral
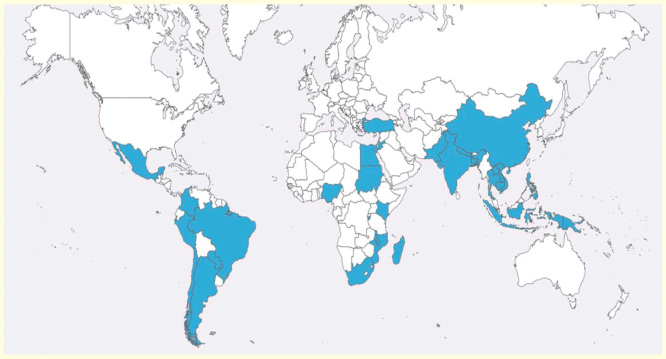
Despite significant reductions in child mortality over the last decade, pneumonia remains the leading cause of childhood mortality worldwide, accounting for 18% of deaths in children under 5 years of age [1]. An estimated 120 million new cases of pneumonia occur globally each year [2]. In addition, an estimated 14.9 million (95% CI: 12.4–18.1 million) episodes of severe and very severe acute lower respiratory infections (ALRI) in young children result in hospital admissions [3]. In developing countries, management and prevention efforts against pneumonia have traditionally focused on bacterial pathogens, known to cause between 30 and 77% of the most severe pneumonia cases [4–6]. Among these pathogens, two bacteria stand out as the major causes of disease: Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) [5–9]. The scarcity of routine microbiology facilities in most resource-constrained settings and the low sensitivity of current bacterial identification methods hinder adequate surveillance, which may in turn contribute to an underestimation of the real burden of these pathogens. The relevance and impact of bacteria as a cause of pneumonia was clearly established prior to the introduction of the pneumococcal and Hib conjugate vaccines [8–11]. More recently, however, attention has shifted toward non-bacterial causes of pneumonia, namely, to viral respiratory infections. Respiratory syncytial virus (RSV), widely studied in both developed and developing countries, is estimated to cause over 22% of all ALRI and up to 200,000 deaths annually [12]. In addition to RSV, other viruses have emerged as major causes of pneumonia and other potentially life-threatening respiratory syndromes. Viral pathogens have gained recent interest as a result of improved diagnostic methods such as PCR, outbreaks of severe disease caused by emerging pathogens such as avian influenza A (H5N1) and pandemic influenza A (H1N1) and the discovery of new respiratory viruses such as human metapneumovirus (hMPV), human coronavirus (hCoV), human bocavirus (hBoV) and human rhinovirus (HRV) species C. Furthermore, the introduction of highly effective conjugate vaccines globally has led to a decrease in bacterial pneumonia, and consequently, a rise in the prominence of viruses. Although the epidemiology, etiology and clinical characterization of such infections in children are currently being studied extensively in the developed world, very little microbiologically confirmed data are available from low- and even middle-income countries [13]. In this paper, we review the epidemiology, etiology, clinical and radiological features of viral pneumonia in developing countries. Data have been retrieved from countries highlighted in Figure 1.
Figure 1.
Countries with data on viral pneumonia included in the review.
Methods
Definitions
A major challenge of reviewing published literature on acute respiratory infections (ARI) in children is the lack of a gold standard, universally accepted case definition for pneumonia [14]. The lack of a gold standard definition has hampered a homogenous approach to pneumonia research for decades. Therefore, we decided to use a non-restrictive approach during this systematic literature search to incorporate the wide variety of utilized case definitions for pneumonia (Table 1).
Table 1.
Definitions of ‘pneumonia’ used in different studies.
| Study (year) | Age group, years | Nomenclature | Case definition | Ref. |
|---|---|---|---|---|
| Turner et al. (2013) | <2 | Pneumonia | WHO criteria † | [26] |
| Lamarao et al. (2012) | <3 | CAP | Two clinical findings: cough, history of fever, pleuritic pain, crackles or bronchial breath sounds AND one radiological finding: focal airspace consolidation or patchy increased interstitial markings | [96] |
| Mathisen et al. (2010) | <3 | Pneumonia | WHO criteria | [108] |
| Mathisen et al. (2009) | <3 | Pneumonia | WHO criteria | [85] |
| Guerrier et al. (2013) | <5 | LRTI | Cough or respiratory distress associated with tachypnea, with or without fever | [44] |
| Brooks et al. (2010) | <5 | Pneumonia | Age-specific tachypnea AND crackles | [20] |
| Munywoki et al. (2013) | <5 | Pneumonia | History of cough or difficulty breathing AND one of the following: age-specific tachypnea, lower chest indrawing, oxygen saturation ≤90%, or inability to feed, prostration or unconsciousness | [105] |
| Nokes et al. (2009) | <5 | Pneumonia | History of cough or difficulty in breathing for less than 30 days | [109] |
| Katz et al. (2012) | All ages | LRTI | Patients <5 years: cough or difficulty breathing AND at least one of the following: oxygen saturation ≤90%, or maternal report of convulsions, or inability to drink/breast-feed, or lethargy, lower-chest indrawing or estridor Patients >5 years: cough, difficulty breathing or chest pain AND documented fever or oxygen saturation ≤90% |
[30] |
| Fry et al. (2011) | All ages | ALRI | Fever or abnormal white blood cell count AND cough or abnormal breath sounds | [22] |
| Bagget et al. (2012) | All ages | ALRI | At least one of the following: reported fever, reported chills, measured temperature >38.2 or <35°C, or abnormal white blood cell count or differential AND at least one of the following: abnormal breath sounds, documented tachypnea, cough, sputum production or dyspnea | [19] |
| Vong et al. (2013) | >5 | ALRI | Symptoms onset ≤14 days AND fever ≥38°C or history of fever during the last 3 days AND cough AND one of the following: dyspnea, chest pain or crackles on auscultation | [79] |
| Feikin et al. (2012) | >5 | ALRI | Cough or difficulty breathing or chest pain AND fever ≥38°C or oxygen saturation ≤90% or hospitalization | [28] |
Only studies including at least 1000 subjects published during the last 5 years have been considered.
†WHO case definition: age-specific tachypnea AND cough and/or difficulty in breathing.
ALRI: Acute lower respiratory infection; CAP: Community-acquired pneumonia; LRTI: Lower respiratory tract infection.
We defined ‘developing country’ using the World Bank and International Monetary Found (IMF) classifications and included studies performed in countries in the newly industrialized category as of 2011, including Brazil, People’s Republic of China, India, Mexico, Philippines, South Africa, Thailand and Turkey (IMF 2011).
Search strategy
We undertook a systematic literature review using various search terms in Medline and Embase (Appendix 1) as well as hand-searched online journals so as to include the different age ranges, inclusion criteria (ARI, ALRI, severe ALRI, very severe ALRI, influenza-like illness, radiologically confirmed pneumonia and WHO-defined pneumonia), the different detection techniques utilized for viral detection (PCR, immunofluorescent antibody test, enzyme-linked immunosorbent assay, shell-vial and viral culture); the different number of virus studied (from single virus detection studies to multiple-viral detection studies) and finally the different samples studied (nasopharyngeal aspirates [NPA], nasal and pharyngeal swabs, bronchial aspirates and lung punctures).
Epidemiology
To address public health issues, a thorough knowledge of the epidemiology of viral pneumonia is mandatory. While the epidemiology of ARI in the developed world has been adequately characterized, this is not the case for most developing countries where there is a scarcity of reliable surveillance data. In resource-constrained settings, incidence calculations are either roughly estimated or based on a limited number of geographically limited areas under demographic surveillance systems (DSS) set up in the context of research efforts. Furthermore in such settings, data from adults and elderly patients are extremely scarce, if not, unexistent. In developed countries, the incidence of ARI is highest among children younger than 5 years of age, decreasing greatly after 15 years of age and increasing again among adults older than 75 years of age [13]. The few available data from the developing world suggests similar age-distribution tendencies, although a rise in pneumonia incidence has been observed among young adults in settings with high HIV prevalence [15].
Incidence of ALRI & specific virus-related ALRI
A total of 21 studies from South America [16–18], Asia [19–27] and Africa [25,28–35] presented incidence data on ALRI and virus-specific associated ALRI, 4 of which presented incidences in adults [19,22,24,30]. Incidence rates are summarized in Tables 2–5. In the pediatric populations from African settings, the incidence of ALRI ranged from 90 to 1029 cases per 1000 children-year-at-risk (CYAR) for all ALRI, and from 13.2 to 560 cases per 1000 CYAR for severe ALRI. In Asian settings, the incidence of ALRI and severe ALRI ranged from 21.7 to 730 and from 16 to 210 cases per 1000 CYAR, respectively. In South America, only incidences of all ALRI cases (and not for severe cases) were available, ranging from 64 to 1710 cases per 1000 CYAR. Discrepancies between the different settings may in part be explained by differences in age groups, year of study and definitions hampering accurate epidemiological comparisons among regions. Children under 1 year of age have the highest incidence of ALRI and severe ALRI. This declines with age and is lowest between 15 and 65 years, increasing again in the elderly although never attaining the figures observed in the under one populations. This trend was observed in influenza and HRV-related ALRI [19,22], but not in RSV-related ALRI [24]. Few studies presented incidence rates for viruses other than RSV and influenza [22,23,33].
Table 2.
Descriptors of studies reporting incidence of acute lower respiratory infection in children younger than 5 years of age from developing countries.
| Study (year) |
Country (study site) Date of the study |
Denominator (age group) |
Diagnostic tests | Control group | Ref. |
|---|---|---|---|---|---|
| Weber et al. (2002) | Gambia (Western region) 1994 |
20,338 (<1 y) |
IFA, serology | None | [34] |
| Robertson et al. (2004) | Mozambique (Manhiça) 1999 |
1342 (<1 y) |
ELISA | None | [25] |
| Mozambique (Manhiça) 1999 |
6020 (<5 y) |
ELISA | None | ||
| Nigeria (Ibadam) 1999 |
1579 (<5 y) |
ELISA | None | ||
| South Africa (Agincourt) 1999 |
8258 (<5 y) |
ELISA | None | ||
| Nokes et al. (2008) | Kenya (Kilifi) 2002 |
655 (birth cohort † , 4 y) |
IFA | None | [31] |
| Nokes et al. (2004) | Kenya (Kilifi) 2002 |
338 (birth cohort, 1 y) |
IFA | None | [32] |
| O’Callaghan et al. (2011) | Mozambique (Manhiça)
‡
2006 |
807 (<5 y) |
PCR | None | [33] |
| Onyango et al. (2012) | Kenya (Kilifi) 2007 |
2002 (<13 y) |
PCR | None | [35] |
| Feikin et al. (2013) | Kenya (Western region) 2007 |
2973 (<5 y) |
PCR | Asymptomatic outpatients who presented for immunizations or medicine refills | [29] |
| Berman et al. (1983) | Colombia (Cali) 1977 |
8748 (<5 y) |
Culture, serology | None | [16] |
| Borrero et al. (1990) | Colombia (Cali) 1986 |
340 (birth cohort, 17 months) |
IFA, culture | None | [17] |
| Sutmoller et al. (1995) | Brazil (Rio de Janeiro) 1987 |
262 (<5 y) |
IFA, culture | None | [18] |
| Robertson et al. (2004) | Indonesia (Bandung) 1999 |
1420 (<5 y) |
ELISA | None | [25] |
| Djelantik et al. (2003) | Indonesia (Lombok Island) 2000 |
30,000 (<2 y) |
ELISA | None | [21] |
| Brooks et al. (2010) | Bangladesh (Kamalupur) 2004 |
2370 (<5 y) |
Culture | None | [20] |
| Turner et al. (2013) | Thailand (Myanmar Border) 2007 |
1067 (<2 y) |
PCR | None | [26] |
| Yoshida et al. (2013) | Vietnam 2007 |
1992 (<2 y) |
PCR | Community controls randomly selected | [27] |
| Hasan et al. (2013) | Thailand (SaKaeo) 2005–2010 |
28,523 (<5 y) |
PCR | Outpatients without ALRI | [23] |
†Birth cohort: study participants recruited in the maternity ward and monitored over the specified period.
‡Results in HIV-infected children are presented in the first line, whereas results in HIV-uninfected children are in the second line.
IFA: Immunofluorescent antibody analysis; y: Years.
Table 3.
Incidence of ALRI and specific virus-associated ALRIs in children younger than 5 years of age from developing countries.
| Study (year) | ALRI incidence | RSV-associated ALRI incidence | Influenza-associated ALRI incidence | RSV-associated ALRI incidence | ADV-associated ALRI incidence | hMPV-associated ALRI incidence | PIV-associated ALRI incidence | Case fatality rate | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Weber et al. (2002) | 96 | 8 | [34] | ||||||
| Robertson et al. (2004) | 509 | 30 | [25] | ||||||
| 126
†
(<1 y) 68 † (<5 y) |
15
†
(<1 y) 5 † (<1 y) |
||||||||
| 323 (<1 y) 270 (<5 y) |
116 (<1 y) 94 (<5 y) |
||||||||
| 332
†
(<1 y) 80 † (<5 y) |
15
†
(<1 y) 9 † (<5 y) |
||||||||
| Nokes et al. (2008) | 90/43 ‡ (<2.5 y) | 12/8 ‡ (<2.5 y) | RSV 0% | [31] | |||||
| Nokes et al. (2004) | 1029/463 ‡ (<1 y) | 154/100 ‡ (<1 y) | RSV 0% | [32] | |||||
| O’Callaghan et al. (2011)†† | 45.6–125
¶
7.8–8.3 ¶ |
3.3–9.1 1.4–1.5 |
4.1–11.4 0.5–0.6 |
24.9–68.2 3.5–3.7 |
11.6–31.8 2.1–2.3 |
6.6–18.2 0.8–0.9 |
3.3–9.1 0.7–0.8 |
Viral ALRI 3% | [33] |
| Onyango et al. (2012) | 39
§
(<1 y) 13.2 § (<5 y) 5.9 § (<13 y) |
1.5
§
(<1 y) 0.6 § (<5 y) 0.3 § (<13 y) |
Influenza 4% | [35] | |||||
| Feikin et al. (2013) | 560 † | 71 † | 58 † | [29] | |||||
| Berman et al. (1983) | 70 | 6 | RSV 0.94% | [16] | |||||
| Borrero et al. (1990) | 1710 (<1.5 y) | 198 (<1.5 y) | [17] | ||||||
| Sutmoller et al. (1995) | 64 | 14 | [18] | ||||||
| Robertson et al. (2004) | 178/25
‡
(<1 y) 191/22 ‡ (<5 y) |
41/16
‡
(<1 y) 34/10 ‡ (<5 y) |
[25] | ||||||
| Djelantik et al. (2003) | 60 | 16 | RSV 1.9% | [21] | |||||
| Brooks et al. (2010) | 511 | 102 | Influenza 0% | [20] | |||||
| Turner et al. (2013) | 730/210 ‡ | 284 | RSV 0% | [26] | |||||
| Yoshida et al. (2013) # | 21.7 | 4.3 | 2.6 | [27] | |||||
| Hasan et al. (2013) | 57.7 | 11 | 4.6 | 10.8 | Viral ALRI 0.1% | [23] |
All incidences are expressed in cases per 1000 person-years at risk unless indicated otherwise.
†Severe ALRIs.
‡All ALRIs/severe ALRIs.
§Severe and very severe ALRIs.
¶Viral pneumonia.
#Incidence expressed in cases per 1000 persons.
††Results in HIV-infected children are presented in the first line, whereas results in HIV-uninfected children are in the second line.
ALRI: Acute lower respiratory infection; y: Years.
Table 4.
Descriptors of studies reporting incidence of acute lower respiratory infection from developing countries (studies including adults).
| Study (year) | Country (study site) date |
Denominator (age group) |
Diagnostic tests | Control group | Ref. |
|---|---|---|---|---|---|
| Katz et al. (2012) | Kenya (Kibera, Lwak) 2007 | 10,873 (children and adults) |
PCR | None | [30] |
| Feikin et al. (2012) | Kenya (Western region) 2007 | 3406 (>5 y) |
PCR | Asymptomatic outpatients who presented for immunizations or medicine refills | [28] |
| Fry et al. (2011) | Thailand (SaKaeo) 2003 | 1919 (children and adults) |
PCR | Outpatients without fever, cough, sore throat or diarrhea within the previous 3 days | [22] |
| Olsen et al. (2010) | Thailand (2 rural provinces) 2003 | 1730 (children and adults) |
PCR, culture | None | [24] |
| Bagget et al. (2012) | Thailand (SaKaeo, Nakhon) 2009 | 7207 (children and adult) |
PCR | None | [19] |
Table 5.
Incidence of ALRI and specific virus-associated ALRIs in studies from developing countries.
| Study (year) | ALRI incidence | RSV-associated ALRI incidence | Influenza-associated ALRI incidence | HRV-associated ALRI incidence | Case fatality rate | Ref. |
|---|---|---|---|---|---|---|
| Katz et al. (2012) | 36.7 (<1 y) 25.3 (5–17 y) 8.1 (18–34 y) 3 (≥50 y) |
Influenza 0.3% | [30] | |||
| Feikin et al. (2012) | 120 | 4.4 | 26 | RSV 3% Influenza 0% HRV 2% |
[28] | |
| Fry et al. (2011) | 36.2 (<1 y) 16 (1–4 y) 3.1 (20–65 y) 18 (≥65 y) |
10.4 (<1 y) 4.6 (1–4 y) 0.3 (20–65 y) 1.6 (≥65 y) |
HRV 1.6% | [22] | ||
| Olsen et al. (2010) | 4.2 (<5 y) 0.03 (5–49 y) 0.12 (≥50 y) |
1.5 (<5 y) 0.06 (5–49 y) 0.4 (≥50 y) |
2.4 (<5 y) 0.07 (5–49 y) 0.37 (≥50 y) |
[24] | ||
| Bagget et al. (2012) | 4.8 (<5 y) <1 (15–60 y) 4.1 (≥75 y) |
Influenza 0.4% | [19] |
Studies including adults; all incidences are expressed in cases per 1000 person-years at risk.
ALRI: Acute lower respiratory infection; y: Years.
Seasonality
Before 2000, few studies recruited for more than 12 consecutive months and hence seasonality could not always be inferred [36]. Since then, there have been several reports on site-specific virus seasonality, especially for RSV, influenza virus and hMPV (Table 6). RSV and hMPV in particular seem to be more frequently detected during the rainy season in America, Africa and Asia, with the exception of Nigeria and South Africa where RSV showed higher incidence during the dry season [25] and Kenya where several peaks of epidemics occurred without following any clear seasonal pattern. HRV and adenovirus (ADV) were detected throughout the year. Influenza viruses showed different seasonal patterns (throughout the year in Vietnam in 2004–2008, or restricted to the cool season in 2007–2008; cool season in Chile, and during the rainy season in India, Mozambique and Kenya). Limited data are available for parainfluenza virus (PIV), hCoV and hBoV. Interestingly, seasonality of RSV, hMPV and PIV in Chile differed from that of the other countries, most likely due to its temperate instead of tropical climate with two distinctive seasons.
Table 6.
Seasonality of specific viruses.
| Virus | General pattern | Reported exceptions (country) |
|---|---|---|
| Respiratory syncytial virus | Rainy season | Dry season (Nigeria, South Africa) Peaks without clear pattern (Kenya) |
| Influenza virus | Rainy season | Cool season (Chile, Vietnam) Throughout all year (Vietnam) |
| Human rhinovirus | Throughout all year | |
| Adenovirus | Throughout all year | |
| Human metapneumovirus | Rainy season | Cool season (Chile) |
Diagnosis, detection rates & attributable causes of viral pneumonia
Microbiologic confirmation of viral pneumonia relies on the detection of a specific virus, usually in upper respiratory samples using either culture, antigen detection by immunofluorescence microscopy or, more recently, PCR methods. In developing countries, lower respiratory samples such as induced sputum or lung aspiration are seldom collected because health facilities are rarely equipped for such invasive, often iatrogenic procedures. In a study of 95 cases of pediatric radiologically confirmed pneumonia in Malawi, PCR on lung aspirates identified S. pneumoniae in 41% of samples, Hib in 6%, ADV in 16% and hBoV in 4% [37]. Pneumothorax occurred in two children, consistent with previously reported complication rates related to lung punctures [38]. Establishing the etiology of pneumonia is challenging because viral–viral and viral–bacterial co-infections are frequently identified in upper respiratory samples and the pathogenic role of each microorganism is difficult to determine. Viruses identified in NPA samples of patients with pneumonia could either be pathogenic, bystanders or the cause of initial damage in the respiratory epithelium leading to a subsequent bacterial infection. The recent adoption of highly sensitive methods such as PCR could overestimate the etiologic role of respiratory viruses if causality is inferred in all positive results. Prolonged shedding of viruses is poorly understood and could overestimate the relevance of viral identification in pneumonia cases. In a study in Wisconsin, 285 children were followed-up during their first year of life and serial NPAs were obtained during moderate to severe respiratory illnesses and during pre-scheduled visits. The authors reported that while ADV was frequently detected more than 2 weeks after the initial onset, HRV rarely caused persistent infection [39]. In a study of healthy adults who underwent experimental inoculation of HRV-16, viral shedding was still detectable in 54% subjects 2 weeks after inoculation [40]. Detection of respiratory viruses in a control group with asymptomatic nasopharyngeal infection could be useful in clarifying the burden of virus-specific attributable disease at a population level, but only 12 studies have used this approach [5,22,27–29,41–47].
Pediatric studies
In the majority of pediatric studies, the detection of at least one virus among ALRI cases was a common finding. In studies where culture or antigen detection were the only diagnostic methods used, 19–35% of the samples were positive for at least one virus [4,7]. However, when PCR methods were utilized, detection rates increased to 35–85% in all ALRI cases and to 47–60% in severe ALRI [45,48–50]. Overall among the 21 studies reviewed, RSV was the most frequently detected virus, especially in children under the age of 2, with detection rates of 15–64 and 15–34% in all ALRI and severe ALRI, respectively (Table 7) [51,52]. Interestingly, 30% of lung samples from 98 fatal pneumonia episodes in children under the age of 2 from Mexico were positive for RSV [53]. HRV was the second more frequently reported virus, although in some studies it ranked first, especially in children over the age of 2 [52]. In all ALRI and in severe ALRI, HRV was detected in 21–40 and 20–41%, respectively. In studies that further typed HRV, HRV-A was the most frequently identified species in both severe and non-severe ALRI while HRV-B was rarely detected [47,54–59]. HRV species distribution was consistent among African, Asian and American settings, although recent reports from non-developing settings, namely Australia, have highlighted the pathogenic role of the newly described HRV-C and its potential impact as a cause of pediatric severe respiratory disease and hospital admissions [60]. ADV, influenza and PIV were recorded in 4–37, 3–16 and 5–23% of ALRI cases, respectively. However, ADV was consistently more frequently identified than RSV in samples from Mozambique in three large studies [33,48,61], two of which were in severe ALRI cases in children under the age of 5. This trend was not confirmed by other studies with the exception of a Malawian study where 16% of the lung puncture samples in radiologically confirmed pneumonia were positive to ADV. In those populations, there is a high burden of HIV and the importance of this condition needs to be further investigated. Of the newly described respiratory viruses, hMPV was recorded in 3–27.2% of ALRI cases, hCoV in 0–9% and hBoV in 0.7–5%. Finally, only two studies reported polyomavirus infection in 3.6 and 25% of ALRI in children under 13 years of age in Philippines, and in children younger than 12 years of age in Thailand, respectively [57,59]. When considering only the most comprehensive studies (detection by PCR of at least 10 viruses in study populations larger than 500 patients over a minimum 12-month period), the general prevalence of viral infections ranged from 47 to 69%, with HRV, RSV, influenza, ADV and hMPV being the most commonly detected pathogens [29,33,42,44,45,48,59,62].
Table 7.
Detection rates for specific viruses in pediatric population.
| Virus | ALRI (%) | Severe ALRI (%) |
|---|---|---|
| Respiratory syncytial virus | 15–64 | 15–34 |
| Human rhinovirus | 21–40 | 20–41 |
| Adenovirus | 4–37 | |
| Influenza virus | 3–16 | |
| Parainfluenza virus | 5–23 | |
| Human metapneumovirus | 3–27 | |
| Human coronavirus | 0–9 | |
| Human bocavirus | 0.7–5 |
ALRI: Acute lower respiratory infection.
Viral etiology can only be inferred if proper case–control studies including healthy and/or asymptomatic children are conducted to compare the prevalence of viral pathogens between both groups and to calculate the attributable fraction. Such a study design, currently being utilized by the Pneumonia Etiology Research for Child Health (PERCH) study [63], could help clarify the role of some respiratory viruses such as HRV in viral pneumonia. Eleven studies conducted in countries including the Gambia, Kenya, Nepal, Thailand and Vietnam included control groups to compare virus prevalence in patients with either pneumonia, severe pneumonia or radiologically confirmed pneumonia. The only virus indisputably associated with pneumonia was RSV, which was significantly more prevalent in all three pneumonia case definitions and for different age groups. Influenza was related to hospitalized pneumonia in children under the age of 3 [27], but not in children younger than 12 years of age with severe pneumonia. Two studies conducted in Kenya with 810 and 2973 patients with severe pneumonia under the age of 5, respectively, demonstrated variable results with a significant association between influenza and severe pneumonia in the larger study [29,45]. HRV, hMPV and PIV were mostly unrelated to pneumonia in different case–control studies, although a significant association was found between HRV and pneumonia in the 1–4 age group (odds ratio [OR]: 2.3; 95% CI: 1.0–5.2) with the exception of one study of infants from Thailand [22]. In this study, HRV genotypes were typed and significance was only attained for species A and C, but not B. Interestingly, hMPV was only related to pneumonia in children older than 5 years of age, but not in those younger than 5 or in those younger than 12 [28]. Only PIV-3 was associated with an increased risk of pneumonia (OR: 13; 95% CI: 6.0–28.0) in children under the age of 3 [46]. ADV, hCoV and hBoV were not related to pneumonia in any of the case–control studies that included their detection [27–29,41,43,45].
Viral pneumonia in human immunodeficiency virus-infected children
Pneumonia is a major cause of death and hospital admissions in HIV-infected children [64]. In sub-Saharan Africa, where the pediatric HIV epidemic is essentially circumscribed, studies describe singularities in HIV-associated pneumonia. Cytomegalovirus (CMV), a viral pathogen responsible for mononucleosis-like or influenza-like illnesses in immunocompetent children, is a leading cause of pneumonia in HIV-infected children. In a study of children with severe ALRI from South Africa, CMV was identified in 36% of HIV-infected children (and in 15% of uninfected children, most of whom were severely malnourished). Furthermore, CMV was more common than Pneumocystis jirovecii pneumonia (27%) and other viral-associated pneumonia (19%) [65]. In another study, hMPV was indentified as the only infection in 3.7% of HIV-infected and 9.1% of HIV-uninfected children [66]. Other respiratory viruses are also commonly identified among HIV-infected patients with severe ALRI, although less frequently than among HIV-uninfected children. One likely explanation for this is the role of other pathogens in severe pneumonia in such immunocompromised patients. In a large study of children younger than 2 years of age admitted for severe pneumonia, viruses and bacteria were identified in 15.7 and 12.5% of HIV-infected patients compared with 34.8 and 5.8% of HIV-uninfected cases, respectively [67]. P. jirovecii was only detected among HIV-infected children. In this study, CMV and hMPV were not tested for, possibly resulting in an underestimation of the relevance of viral pathogens causing ALRI in HIV-infected patients. In such children, polymicrobial infections were more common than in HIV-uninfected patients and also appeared to be associated with a worse prognosis [68]. Clinical features were similar in hMPV-associated ALRI [66], but not in PIV and influenza-associated ALRI where wheezing was less frequent and alveolar consolidation was more frequent in HIV-infected than in HIV-uninfected children [69,70]. Fatal outcome was recorded in 0–35% of HIV-infected and in 0–11% of HIV-uninfected children with viral pneumonia, with CMV causing worse prognosis than any other virus (Table 8) [65,67,69,70]. Lymphoid interstitial pneumonitis (LIP) is a lymphoproliferative response to HIV and/or Epstein–Barr virus common in HIV-infected children older than 2 years of age without proper antiretroviral treatment [71–73]. Although LIP is a chronic lung disease, it may present intermittent episodes of ALRI with fever and tachypnea mostly due to bacterial super-infections [74]. Digital cubbling, generalized lymphadenopathy and hepatomegaly are common in children with LIP, and the characteristic radiological findings include persistent, diffuse bilateral reticulonodular pattern [74,75].
Table 8.
Viral acute lower respiratory infection in HIV-infected children.
| Study (year) |
Country Study date |
Study group age pathology n (HIV+/HIV-) |
Prevalence of pathogen |
Case fatality rate |
Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| HIV positive | HIV negative | OR (95% CI) or p-value | HIV positive | HIV negative | OR (95% CI) or p-value | ||||
| Zampoli et al. (2011) | South Africa Dec 2006–Jun 2008 |
<2 y Severe ALRI 200 (124/76) |
CMV 36% |
15% |
OR: 3 (1.3–7.4) |
Severe ALRI 35% |
11% |
OR: 4.5 (1.9–11.8) |
[65] |
| Madhi et al. (2003) | South Africa Mar 2000–Sep 2000 |
<1 y ALRI 812 (326/486) |
hMPV 3.7% |
9.1% |
p = 0.24 |
hMPV- ALRI 0% |
0% |
NA |
[66] |
| Madhi et al. (2002) | South Africa Mar 1997–Mar 1999 |
<5 y Influenza severe ALRI 116 (25/91) |
Influenza severe ALRI 8% |
2.2% |
p = 0.2 |
[69] | |||
| Madhi et al. (2002) | South Africa Mar 1997–Mar 1999 |
<5 y PIV severe ALRI 80 (24/56) |
PIV severe ALRI 21% |
0% |
p = 0.001 |
[70] | |||
| Madhi et al. (2000) | South Africa Mar 1997–Mar 1998 |
<5 y Severe ALRI 946 (440/506) |
Virus 15.7% Bacteria 12.5% |
34.8% 5.8% |
p = 0.00001 p = 0.0001 |
Severe viral ALRI 7.5% |
0% |
p = 0.001 |
[67] |
OR: Odds ratio; y: Years.
Studies in adult population
We identified 13 studies that included adults, 4 of which reported detection rates for both adults and children (Table 9). Viral detection rates were 20–39% [76–79] in adult ALRI with the exception of a study in Laos of 34 patients hospitalized with ALRI where 67% of samples were positive to at least one virus [80]. This value must be considered with caution because of the small size of the study. HRV was the most frequently reported virus (6–11.5%) and was detected in all studies where it was tested for. Influenza was detected in 3.1–13% of ALRI adult cases and was usually the second most commonly identified virus after HRV, with the exception of a study conducted in Vietnam during the H1N1 pandemic where influenza was the most frequently detected virus [81]. RSV was documented in 0–13% of cases, hMPV in 0–11.5%, ADV in 0.7–3% and hBoV in 0.7% of adult ALRI cases [43]. Two studies reported data stratified according to age groups for influenza-related ALRI and HRV-related ALRI, showing a decrease of viral detection with age [22,30].
Table 9.
Detection rates for specific viruses in adult population.
| Virus | ALRI (%) |
|---|---|
| Human rhinovirus | 6–11.5 |
| Influenza virus | 3.1–13 |
| Respiratory syncytial virus | 0–13 |
| Human metapneumovirus | 0–11.5 |
| Adenovirus | 0.7–3 |
| Human bocavirus | 0.7 |
ALRI: Acute lower respiratory infection.
Viral co-infections
Since the advent of multiplex-PCR allowing the simultaneous detection of up to 18 respiratory viruses, multiple viruses in the same specimen have been increasingly identified in pneumonia etiological studies. Multiple viral infections were reported in 5.8–8% of adults and 11–56% of children [80,82–86]. Both the lowest and the highest co-infection rates in children were reported from studies using an 18-virus multiplex PCR in similar age groups (<5 years vs <3 years of age), so disparity may be due to epidemiological differences in the various settings. The most frequently detected combination usually included the most prevalent viruses identified in the study (RSV-hMPV, RSV-HRV, RSV-influenza and HRV-ADV). hBoV is frequently detected in association with other respiratory viruses (70–89%), followed by polyomavirus (73%), ADV, hCoV and PIV (60% each) and HRV, hMPV and RSV (50% each) [29,50,87]. The clinical relevance of those associations remains unclear and some studies did not find differences in severity due to viral co-detection compared with single virus identification [50,79,88]. However, two studies in Mozambique reported multiple viral infections in 11–20% of cases of severe pneumonia and found significant associations with nasal flaring (OR: 2.7; 95% CI: 1.1–6.5), lower chest indrawing (OR: 3.8; 95% CI: 1.4–9.9) and fatal outcome (OR: 2.2; 95% CI: 1.0–4.7) [33,61]. Other studies have reported increased risk of developing ALRI in children with RSV-HRV, RSV-hMPV and RSV-PIV-3 co-infections but not with RSV-influenza co-infection [27], and longer hospitalization length and oxygen requirements in RSV-HRV co-infected toddlers [49]. In a large study of children and adults with radiologically confirmed pneumonia in Thailand, single hBoV infection was not associated with ALRI hospitalization. However, hBoV co-detection with RSV, HRV and PIV was associated with wheezing and signs of respiratory distress more frequently than RSV, HRV and PIV single infections [43].
Clinical & radiological features
The WHO-proposed case definition for pneumonia, part of the integrated management of childhood illnesses (IMCI) strategy that has saved the lives of millions of children in the developing world [89], is a highly sensitive albeit poorly specific case definition aimed to maximize the detection of potentially life-threatening ALRI episodes and prompt the rapid initiation of life-saving antibiotic therapy. Respiratory and non-respiratory illnesses might both fulfill this all-embracing definition, but not all patients with this clinical presentation may benefit from antibiotic therapy, particularly if the cause of their ALRI is exclusively a viral infection. Furthermore, in malaria-endemic countries, severe malaria might also present with respiratory distress or tachypnea [48]. For these reasons, given the scarcity of antibiotics (particularly wide-spectrum ones such as third-generation cephalosporins) in many developing settings, together with the emergence of antimicrobial resistance to those commonly available [90], it is important to identify features that could reliably distinguish viral from bacterial pneumonia, which could clearly have relevant management and public health implications for clinicians and policy makers in developing countries.
Radiological features
In developed countries, the diagnosis of pneumonia relies on a combination of clinical signs and symptoms, radiological results and laboratory tests [91,92]. In developing countries, hospitals outside urban areas rarely have x-ray facilities or access to laboratory tests such as procalcitonin (PCT) or C-reactive protein (CRP) and diagnostic biomarkers are usually limited to clinical research. Thus, in such contexts, diagnosis is often purely clinical, although standardized x-ray-based case definitions have been proposed by WHO for their use in epidemiological studies or clinical trials [93]. A study in Sweden with 346 pediatric cases with confirmed pneumonia who underwent extensive microbiological tests concluded that x-ray features had no relation to etiology [94]. Authors described hyperinflation and interstitial infiltrates more frequently in viral pneumonia and more alveolar infiltrates in bacterial pneumonia, but those descriptions were not specific. However, they found a relation with age and concluded that age might be a confounder in x-ray interpretation. A study carried out in China describing the x-ray features of 210 pediatric cases less than 15 years of age with viral pneumonia reported that 63% of patients had patchy areas of consolidation, 15% had interstitial infiltrates and 7% had lobar consolidation [95]. Bilateral affectation of lower lobes was frequent. The authors found that ADV-related pneumonia was associated with interstitial infiltrates and seasonal influenza A (H1N1) with diffuse areas of air space consolidation. They found no specific features related to influenza B or PIV pneumonia. However, respiratory viruses commonly causing viral pneumonia were not tested for. Another study in Thailand of 1067 children under the age of 2 years described a significant relationship between RSV-associated pneumonia and interstitial infiltrates [26]. Interestingly, 30% of the pneumonia cases showing lobar consolidations were positive for RSV. In a similar study from Brazil, 54% of RSV-positive patients and 51% of RSV-negative patients had identical interstitial infiltrates and no relation could be established between clinical features and etiology [96]. Most of the studies carried out in a large variety of settings have reported similar results; the most frequent pattern of viral pneumonia (with RSV being the most studied virus) includes interstitial or peribronchial infiltrates, but alveolar or lobar consolidation is not a rare finding (7–30% of viral pneumonias), and mixed patterns are frequent both in viral and bacterial-confirmed pneumonia episodes [21,97–100]. Thus, it is fair to conclude that etiologic diagnosis (specific respiratory virus or virus vs bacteria) cannot and should not be made on the basis of x-ray features alone in pediatric pneumonia [101,102].
In adults, radiologic findings in viral pneumonia are variable and highly overlapping [103]. Moreover, the corroboration of a lobar consolidations and/or pleural effusion is not rare in adult viral pneumonia [79,81]. In a series of Kenyan adult patients with viral pneumonia, lobar and multilobar consolidation patterns were seen in 15 patients while patchy consolidation was seen only in one [78]. Although the study was small, it is noteworthy that lung aspirates were performed to rule out bacterial disease. However, 11 patients were HIV-infected and the radiological pattern could have been altered by this condition.
Clinical features
Most clinicians in developing countries rely on their clinical evaluation for an initial orientation of the probable etiology of their patients, despite the poor specificity of clinical symptoms for that purpose. A study carried out at Turku University Hospital (Finland) of 4277 children with laboratory-confirmed viral respiratory infections compared clinical features of eight different respiratory viruses (HRV, RSV, ADV, PIV-1, PIV-2, PIV-3, influenza virus A and influenza virus B), and found that overlapping symptomatology was frequent [13]. Overlapping clinical presentation is also common in developing countries, and more importantly, viral pneumonia signs and symptoms seem to overlap with those of bacterial pneumonia. In a study of 226 children less than 5 years of age with ALRI from Thailand, the authors were unable to identify clinical signs to differentiate viral from bacterial etiology [100]. This was also observed in a study of 214 children under 14 years of age in Sudan [104]. Other studies have compared virus-positive pneumonia versus virus-negative pneumonia and failed to identify significant differences. Low sensitivity of bacterial diagnosis, mostly based on blood cultures, may underestimate viral-bacterial co-infections in the virus-positive group leading to conflicting results [44,105]. However, wheezing has shown significant association with viral pneumonia in some studies, and was present in 17–64% of children and 32% of adults [79,106]. RSV-related ALRI usually presents with crackles, wheezing, nasal flaring, lower chest indrawing, rhinorrhea and nasal obstruction more frequently than other viruses [96,107–109]. Unfortunately, none of those clinical features are clearly specific of RSV infection and wheezing has also been associated with HRV (particularly to HRV-C), hMPV and hBoV [43,58,60,61,110]. Reactive airway disease as a sequel of previous viral infections leading to repeated wheezing episodes and readmissions has been described in RSV infections, but also after influenza-related ALRI [20,105]. In most studies, RSV has been characterized as having more severe presentation in terms of oxygen requirements, hypoxemia and signs of respiratory distress [50,58,108], but other studies found HRV infections to be more severe in both children and adults [49,79], and finally some studies concluded severity was not associated with any virus [44,111,112]. Case fatality rates have been reported for RSV-associated ALRI (1.9–7.5% in children from different settings), severe influenza-associated ALRI (4% in <12 years in rural Kenya), influenza-associated ALRI (0.3–0.4% in children and adults series in Kenya) and hMPV-associated ALRI (5.9% in Indonesia) among the most pathogenic respiratory viruses [35,59,106–108]. In addition, avian influenza A (H5N1) may be associated with a case fatality rate as high as 33% with early antiviral treatment or 80–93% without treatment [113–116].
Serum biomarkers
In developed countries, serum biomarkers such as PCT and CRP are used for distinguishing viral from bacterial infections, especially in children. Serum PCT and CRP levels increase in bacterial infections above a cutoff of 1–5 ng/ml and 15–100 mg/l, respectively [117]. Other commonly studied biomarkers, including IL-6, IL-8, IL-18, IFN-α, TNF-γ, lipopolysaccharide-binding protein or heparin-binding protein have not shown higher sensitivity, specificity or predictive value than PCT or CRP for that purpose [118–120]. In a meta-analysis comparing PCT and CRP for differentiating bacterial from viral infections, PCT was more sensitive and specific and had a higher false discovery rate (Q value) than CRP [117]. In developing countries, affordable, rapid and simple diagnostic tools for that purpose are crucial to assure adequate management of antibiotics, especially in view of the low specificity of clinical features. PCT and CRP have proved to be useful markers for bacterial diagnosis in African settings [121–124]. However, serum levels of PCT and CRP increase in patients with malaria infection and hence may compromise their utility in malaria-endemic settings [125–127]. Further research in this area should be undertaken with the aim of developing a rapid diagnostic test, ideally a point-of-care test that is capable of identifying the underlying etiology of infection in severely ill patients at admission.
Treatment & prevention
Antivirals are rarely used in developing countries, except for avian influenza A (H5N1) cases where oseltaminvir, amantadine, rimantadine and rivabirin have been tested with good results if initiated promptly [116]. Lack of rapid etiologic diagnosis renders this option unsuitable for clinicians from resource-constrained areas. Following WHO recommendations [128], antibiotics are initiated in patients fulfilling the WHO clinical pneumonia case definition.
Passive immunization through the use of specific monoclonal antibodies has been used in developed countries for the management of RSV infections, particularly among highly selected high-risk populations such as extreme preterms and congenital heart defect patients during epidemics. Its prohibitive price, however, makes it an unrealistic option in developing countries. Thus, prevention and control of viral respiratory diseases should primarily be focused on vaccines. Unfortunately, there are no registered vaccines against respiratory viruses suitable for use in children younger than 6 months of age, which is the population at highest risk [31,129]. For older children and adults, the only available vaccine in the market is that against seasonal influenza, although this vaccine has not been included in the expanded program of immunization for children. Immunization of pregnant women relying on the protectiveness of mother-to-child transferred antibodies, and immunization of post-partum mothers and household contacts have been studied as a strategy to reduce influenza virus burden in young children with promising results [130–132]. A polyvalent vaccine against RSV and PIV-3 (MEDI-534) is being evaluated in Phase I trials with good safety profile [133]. Vaccines against hMPV and HRV are still in pre-clinical stages and their widespread availability cannot be expected in the short-term [134,135].
Expert commentary & five-year view
In developing countries, similar to what occurs in more developed settings, respiratory viruses are an important cause of pneumonia, particularly among young children. However, their real burden is presumably heavily underestimated. The pathogenic role of specific viruses (or viral subtypes) detected in the upper respiratory tract of patients with pneumonia still requires further clarification. Thus, well-designed case–control studies providing robust data and pathogen-specific attributable disease fractions are required, in addition to new diagnostic methods or biomarkers clearly differentiating pathogens which colonize from pathogens which cause disease. Homogenization in clinical and radiological definitions, inclusion criteria and laboratory tests are also necessary to allow comparisons among studies and clearly detect priorities for public health policy makers. Only then, the real and underexposed burden of viral pneumonia in resource-constrained settings will emerge in its true magnitude and significance.
Appendix 1. Search strategy in Medline (Ovid) and Embase (Ovid) (number of references retrieved).
(pneumonia or respiratory infection* or respiratory tract infection* or bronchiolitis).mp (202,707 and 192,748).
(adenovirus or bocavirus or citomegalovirus or coronavirus or enterovirus or influenza or metapneumovirus or parainfluenza or picornavirus or respiratory syncitial virus or rhinovirus or RSV).mp or virus pneumonia/ (154,170 and 225,549).
(developing countries or developing world or Africa or South Asia or Southeast Asia or “South and Central America” or Latin America).mp (155,984 and 284,753).
1 and 2 and 3 (391 and 542).
Limit 4 to human and (English, French, Portuguese, Spanish) and year = 1990-Current and article (323 and 281).
We included all studies regardless of sampling and detection methods. After exclusion of duplicates (140), we further excluded guidelines, studies focused on specific population (e.g., leukemia patients, ventilator-associated pneumonia, travellers, nosocomial pneumonia) and small series of cases. Data was extracted for study location, period of study, study design, sample size, clinical diagnosis, sample type, diagnostic test, aetiological agents investigated, and incidence of viral infections. Studies that did not report data on a complete year (or multiples of a year) were not included in the Epidemiology section.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
Key issues
Despite great reductions in child mortality over the last decade, pneumonia remains the major killer of young children globally, accounting for 18% of the under-5 mortality. In recent years, attention has shifted toward non-bacterial causes of pneumonia, and more specifically, to viral-attributable respiratory infections. Data of viral pneumonia from developing countries are scarce.
In developed countries, the highest incidence of acute respiratory infections (ARIs) is evidenced among children younger than 5 years of age, falling dramatically after 15 years of age and increasing again among adults older than 75 years of age. The few available data from the developing world suggests similar age-distribution tendencies.
When considering only the most comprehensive studies (detection by PCR of at least 10 viruses in study populations larger than 500 patients over a minimum 12-month period), the prevalence of viral infections in pediatric pneumonia cases ranged from 47 to 69%, with human rhinovirus, respiratory syncytial virus, influenza virus, adenovirus and human metapneumovirus being the most commonly detected pathogens. In adult pneumonia cases, human rhinovirus and influenza are the most frequently detected viruses.
Causality cannot be inferred from a positive viral isolate in a respiratory sample, especially from upper respiratory tract samples, given lack of controls in most studies.
Overlapping clinical and radiological presentation is common in viral pneumonia cases caused by different respiratory virus, and more importantly, viral pneumonia signs and symptoms seem to overlap with those of bacterial pneumonia.
Serum biomarkers used in developed countries to distinguish between viral and bacterial infection such as procalcitonin and C-reactive protein increase in patients with malaria infection compromising their utility in malaria-endemic settings.
There are no registered vaccines against respiratory viruses suitable for use in children younger than 6 months of age, which is the population at highest risk.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Liu L, Johnson HL, Cousens S et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379(9832), 2151–2161 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Rudan I, O’Brien KL, Nair H et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J. Glob. Health 3(1), 10401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive review of the global burden of childhood pneumonia.
- 3.Nair H, Simoes EA, Rudan I et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 381(9875), 1380–1390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forgie IM, Campbell H, Lloyd-Evans N et al. Etiology of acute lower respiratory tract infections in children in a rural community in The Gambia. Pediatr. Infect. Dis. J. 11(6), 466–473 (1992). [DOI] [PubMed] [Google Scholar]
- 5.Forgie IM, O’Neill KP, Lloyd-Evans N et al. Etiology of acute lower respiratory tract infections in Gambian children: I. Acute lower respiratory tract infections in infants presenting at the hospital. Pediatr. Infect. Dis. J. 10(1), 33–41 (1991). [DOI] [PubMed] [Google Scholar]
- 6.Shann F, Gratten M, Germer S, Linnemann V, Hazlett D, Payne R. Aetiology of pneumonia in children in Goroka Hospital, Papua New Guinea. Lancet 2(8402), 537–541 (1984). [DOI] [PubMed] [Google Scholar]
- 7.Forgie IM, O’Neill KP, Lloyd-Evans N et al. Etiology of acute lower respiratory tract infections in Gambian children: II. Acute lower respiratory tract infection in children ages one to nine years presenting at the hospital. Pediatr. Infect. Dis. J. 10(1), 42–47 (1991). [DOI] [PubMed] [Google Scholar]
- 8.O’Brien KL, Wolfson LJ, Watt JP et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374(9693), 893–902 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Watt JP, Wolfson LJ, O’Brien KL et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 374(9693), 903–911 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Roca A, Sigauque B, Quinto L et al. Estimating the vaccine-preventable burden of hospitalized pneumonia among young Mozambican children. Vaccine 28(30), 4851–4857 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Madhi SA, Levine OS, Hajjeh R, Mansoor OD, Cherian T. Vaccines to prevent pneumonia and improve child survival. Bull. World Health Organ. 86(5), 365–372 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair H, Nokes DJ, Gessner BD et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375(9725), 1545–1555 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet 377(9773), 1264–1275 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott JA, Wonodi C, Moisi JC et al. The definition of pneumonia, the assessment of severity, and clinical standardization in the Pneumonia Etiology Research for Child Health study. Clin. Infect. Dis. 54(Suppl. 2), S109–S116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tornheim JA, Manya AS, Oyando N, Kabaka S, Breiman RF, Feikin DR. The epidemiology of hospitalized pneumonia in rural Kenya: the potential of surveillance data in setting public health priorities. Int. J. Infect. Dis. 11(6), 536–543 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Berman S, Duenas A, Bedoya A et al. Acute lower respiratory tract illnesses in Cali, Colombia: a two-year ambulatory study. Pediatrics 71(2), 210–218 (1983). [PubMed] [Google Scholar]
- 17.Borrero I, Fajardo L, Bedoya A, Zea A, Carmona F, de Borrero MF. Acute respiratory tract infections among a birth cohort of children from Cali, Colombia, who were studied through 17 months of age. Rev. Infect. Dis. 12(Suppl. 8), S950–956 (1990). [DOI] [PubMed] [Google Scholar]
- 18.Sutmoller F, Ferro ZP, Asensi MD, Ferreira V, Mazzei IS, Cunha BL. Etiology of acute respiratory tract infections among children in a combined community and hospital study in Rio de Janeiro. Clin. Infect. Dis. 20(4), 854–860 (1995). [DOI] [PubMed] [Google Scholar]
- 19.Baggett HC, Chittaganpitch M, Thamthitiwat S et al. Incidence and epidemiology of hospitalized influenza cases in rural Thailand during the influenza A (H1N1)pdm09 pandemic, 2009–2010. PLoS ONE 7(11), e48609 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks WA, Goswami D, Rahman M et al. Influenza is a major contributor to childhood pneumonia in a tropical developing country. Pediatr. Infect. Dis. J. 29(3), 216–221 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Djelantik IG, Gessner BD, Soewignjo S et al. Incidence and clinical features of hospitalization because of respiratory syncytial virus lower respiratory illness among children less than two years of age in a rural Asian setting. Pediatr. Infect. Dis. J. 22(2), 150–157 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Fry AM, Lu X, Olsen SJ et al. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS ONE 6(3), e17780 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasan R, Rhodes J, Thamthitiwat S et al. Incidence and etiology of acute lower respiratory tract infections in hospitalized children younger than five years in rural Thailand. Pediatr. Infect. Dis. J. doi:10.1097/INF.0000000000000062 (2013) (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 24.Olsen SJ, Thamthitiwat S, Chantra S et al. Incidence of respiratory pathogens in persons hospitalized with pneumonia in two provinces in Thailand. Epidemiol. Infect. 138(12), 1811–1822 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Robertson SE, Roca A, Alonso P et al. Respiratory syncytial virus infection: denominator-based studies in Indonesia, Mozambique, Nigeria and South Africa. Bull. World Health Organ. 82(12), 914–922 (2004). [PMC free article] [PubMed] [Google Scholar]
- 26.Turner C, Turner P, Carrara V et al. High rates of pneumonia in children under two years of age in a South East Asian refugee population. PLoS ONE 8(1), e54026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida LM, Suzuki M, Nguyen HA et al. Respiratory syncytial virus, its co-infection and paediatric lower respiratory infections. Eur. Respir. J. 42(2), 461–469 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Feikin DR, Njenga MK, Bigogo G et al. Etiology and Incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010. PLoS ONE 7(8), e43656 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Very well-designed study reporting etiologies by age and odds ratio of acute respiratory infections (ARIs) case by virus.
- 29.Feikin DR, Njenga MK, Bigogo G et al. Viral and bacterial causes of severe acute respiratory illness among children aged less than 5 years in a high malaria prevalence area of western Kenya, 2007–2010. Pediatr. Infect. Dis. J. 32(1), e14–e19 (2013). [DOI] [PubMed] [Google Scholar]; • Complementary report of [28] on children aged less than 5 years of age.
- 30.Katz MA, Lebo E, Emukule G et al. Epidemiology, seasonality, and burden of influenza and influenza-like illness in urban and rural Kenya, 2007–2010. J. Infect. Dis. 206(Suppl. 1), S53–S60 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Nokes DJ, Okiro EA, Ngama M et al. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi District, Kenya. Clin. Infect. Dis. 46(1), 50–57 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nokes DJ, Okiro EA, Ngama M et al. Respiratory syncytial virus epidemiology in a birth cohort from Kilifi district, Kenya: infection during the first year of life. J. Infect. Dis. 190(10), 1828–1832 (2004). [DOI] [PubMed] [Google Scholar]
- 33.O’Callaghan-Gordo C, Bassat Q, Morais L et al. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: a malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr. Infect. Dis. J. 30(1), 39–44 (2011). [DOI] [PubMed] [Google Scholar]; • Study providing important data on incidences of various respiratory viruses in HIV-infected and -uninfected children.
- 34.Weber MW, Milligan P, Sanneh M et al. An epidemiological study of RSV infection in the Gambia. Bull. World Health Organ. 80(7), 562–568 (2002). [PMC free article] [PubMed] [Google Scholar]
- 35.Onyango CO, Njeru R, Kazungu S et al. Influenza surveillance among children with pneumonia admitted to a district hospital in coastal Kenya, 2007–2010. J. Infect. Dis. 206(Suppl. 1), S61–S67 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips PA, Lehmann D, Spooner V et al. Viruses associated with acute lower respiratory tract infections in children from the eastern highlands of Papua New Guinea (1983–1985). Southeast Asian J. Trop. Med. Public Health 21(3), 373–382 (1990). [PubMed] [Google Scholar]
- 37.Carrol ED, Mankhambo LA, Guiver M et al. PCR improves diagnostic yield from lung aspiration in Malawian children with radiologically confirmed pneumonia. PLoS ONE 6(6), e21042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott JA, Hall AJ. The value and complications of percutaneous transthoracic lung aspiration for the etiologic diagnosis of community-acquired pneumonia. Chest 116(6), 1716–1732 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Jartti T, Lee WM, Pappas T, Evans M, Lemanske RF Jr, Gern JE. Serial viral infections in infants with recurrent respiratory illnesses. Eur. Respir. J. 32(2), 314–320 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosser AG, Vrtis R, Burchell L et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am. J. Respir. Crit. Care Med. 171(6), 645–651 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Adegbola RA, Falade AG, Sam BE et al. The etiology of pneumonia in malnourished and well-nourished Gambian children. Pediatr. Infect. Dis. J. 13(11), 975–982 (1994). [DOI] [PubMed] [Google Scholar]
- 42.Berkley JA, Munywoki P, Ngama M et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA 303(20), 2051–2057 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fry AM, Lu X, Chittaganpitch M et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J. Infect. Dis. 195(7), 1038–1045 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guerrier G, Goyet S, Chheng ET et al. Acute viral lower respiratory tract infections in Cambodian children: clinical and epidemiologic characteristics. Pediatr. Infect. Dis. J. 32(1), e8–13 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Hammitt LL, Kazungu S, Morpeth SC et al. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin. Infect. Dis. 54(Suppl. 2), S190–S199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Study including a comparison of different diagnosis methods and odds ratios of severe and very severe pneumonia by virus.
- 46.Mathisen M, Strand TA, Valentiner-Branth P et al. Respiratory viruses in nepalese children with and without pneumonia: a case-control study. Pediatr. Infect. Dis. J. 29(8), 731–735 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Onyango CO, Welch SR, Munywoki PK et al. Molecular epidemiology of human rhinovirus infections in Kilifi, coastal Kenya. J. Med. Virol. 84(5), 823–831 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bassat Q, Machevo S, O’Callaghan-Gordo C et al. Distinguishing malaria from severe pneumonia among hospitalized children who fulfilled integrated management of childhood illness criteria for both diseases: a hospital-based study in Mozambique. Am. J. Trop. Med. Hyg. 85(4), 626–634 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.da Silva ER, Pitrez MC, Arruda E et al. Severe lower respiratory tract infection in infants and toddlers from a non-affluent population: viral etiology and co-detection as risk factors. BMC Infect. Dis. 13, 41 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan NM, Dove W, Abd-Eldayem SA, Abu-Zeid AF, Shamoon HE, Hart CA. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J. Med. Virol. 80(1), 168–174 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehmann D, Michael A, Omena M et al. Bacterial and viral etiology of severe infection in children less than three months old in the highlands of Papua New Guinea. Pediatr. Infect. Dis. J. 18(10 Suppl.), S42–S49 (1999). [DOI] [PubMed] [Google Scholar]
- 52.Marcone DN, Ellis A, Videla C et al. Viral etiology of acute respiratory infections in hospitalized and outpatient children in Buenos Aires, Argentina. Pediatr. Infect. Dis. J. 32(3), e105–e110 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Bustamante-Calvillo ME, Velazquez FR, Cabrera-Munoz L et al. Molecular detection of respiratory syncytial virus in postmortem lung tissue samples from Mexican children deceased with pneumonia. Pediatr. Infect. Dis. J. 20(5), 495–501 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Espinola EE, Russomando G, Aquino C, Basualdo W. Phylogeny-based classification of human rhinoviruses detected in hospitalized children with acute lower respiratory infection in Paraguay, 2010–2011. J. Med. Virol. 85(9), 1645–1651 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Esposito S, Daleno C, Baggi E et al. Circulation of different rhinovirus groups among children with lower respiratory tract infection in Kiremba, Burundi. Eur. J. Clin. Microbiol. Infect. Dis. 31(11), 3251–3256 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Fuji N, Suzuki A, Lupisan S et al. Detection of human rhinovirus C viral genome in blood among children with severe respiratory infections in the Philippines. PLoS ONE 6(11), e27247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linsuwanon P, Payungporn S, Samransamruajkit R et al. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J. Infect. 59(2), 115–121 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller EK, Khuri-Bulos N, Williams JV et al. Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J. Clin. Virol. 46(1), 85–89 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki A, Lupisan S, Furuse Y et al. Respiratory viruses from hospitalized children with severe pneumonia in the Philippines. BMC Infect. Dis. 12, 267 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Interesting study including novel respiratory viruses and case-fatality rates of different single virus infection.
- 60.Cox DW, Bizzintino J, Ferrari G et al. HRV-C Infection in Young Children with Acute Wheeze is Associated with Increased Acute Respiratory Hospital Admissions. Am. J. Respir. Crit. Care Med. doi:10.1164/rccm.201303-0498OC (2013) (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 61.O’Callaghan-Gordo C, Diez-Padrisa N, Abacassamo F et al. Viral acute respiratory infections among infants visited in a rural hospital of southern Mozambique. Trop. Med. Int. Health 16(9), 1054–1060 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Yoshida LM, Suzuki M, Yamamoto T et al. Viral pathogens associated with acute respiratory infections in central vietnamese children. Pediatr. Infect. Dis. J. 29(1), 75–77 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Deloria-Knoll M, Feikin DR, Scott JA et al. Identification and selection of cases and controls in the Pneumonia Etiology Research for Child Health project. Clin. Infect. Dis. 54(Suppl. 2), S117–S123 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Punpanich W, Groome M, Muhe L, Qazi SA, Madhi SA. Systematic review on the etiology and antibiotic treatment of pneumonia in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 30(10), e192–202 (2011). [DOI] [PubMed] [Google Scholar]
- 65.Zampoli M, Morrow B, Hsiao NY, Whitelaw A, Zar HJ. Prevalence and outcome of cytomegalovirus-associated pneumonia in relation to human immunodeficiency virus infection. Pediatr. Infect. Dis. J. 30(5), 413–417 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Madhi SA, Ludewick H, Abed Y, Klugman KP, Boivin G. Human metapneumovirus-associated lower respiratory tract infections among hospitalized human immunodeficiency virus type 1 (HIV-1)-infected and HIV-1-uninfected African infants. Clin. Infect. Dis. 37(12), 1705–1710 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madhi SA, Schoub B, Simmank K, Blackburn N, Klugman KP. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J. Pediatr. 137(1), 78–84 (2000). [DOI] [PubMed] [Google Scholar]; •• Excellent study reporting relative risk and incidence of various respiratory viruses in a large series of HIV-infected children.
- 68.Gray DM, Zar HJ. Community-acquired pneumonia in HIV-infected children: a global perspective. Curr. Opin. Pulm. Med. 16(3), 208–216 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Madhi SA, Ramasamy N, Bessellar TG, Saloojee H, Klugman KP. Lower respiratory tract infections associated with influenza A and B viruses in an area with a high prevalence of pediatric human immunodeficiency type 1 infection. Pediatr. Infect. Dis. J. 21(4), 291–297 (2002). [DOI] [PubMed] [Google Scholar]
- 70.Madhi SA, Ramasamy N, Petersen K, Madhi A, Klugman KP. Severe lower respiratory tract infections associated with human parainfluenza viruses 1–3 in children infected and noninfected with HIV type 1. Eur. J. Clin. Microbiol. Infect. Dis. 21(7), 499–505 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Graham SM. Impact of HIV on childhood respiratory illness: differences between developing and developed countries. Pediatr. Pulmonol. 36(6), 462–468 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Sharland M, Gibb DM, Holland F. Respiratory morbidity from lymphocytic interstitial pneumonitis (LIP) in vertically acquired HIV infection. Arch. Dis. Child. 76(4), 334–336 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toro AA, Altemani AM, da Silva MT, Morcillo AM, Vilela MM. Epstein-Barr virus (EBV) gene expression in interstitial pneumonitis in Brazilian human immunodeficiency virus-1-infected children: is EBV associated or not? Pediatr. Dev. Pathol. 13(3), 184–191 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Graham SM. Non-tuberculosis opportunistic infections and other lung diseases in HIV-infected infants and children. Int. J. Tuberc. Lung Dis. 9(6), 592–602 (2005). [PubMed] [Google Scholar]
- 75.Weber HC, Gie RP, Cotton MF. The challenge of chronic lung disease in HIV-infected children and adolescents. J. Int. AIDS Soc. 16, 18633 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartung TK, Chimbayo D, van Oosterhout JJ et al. Etiology of suspected pneumonia in adults admitted to a high-dependency unit in Blantyre, Malawi. Am. J. Trop. Med. Hyg. 85(1), 105–112 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luchsinger V, Ruiz M, Zunino E et al. Community-acquired pneumonia in Chile: the clinical relevance in the detection of viruses and atypical bacteria. Thorax 68(11), 1000–1006 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Scott JA, Hall AJ, Muyodi C et al. Aetiology, outcome, and risk factors for mortality among adults with acute pneumonia in Kenya. Lancet 355(9211), 1225–1230 (2000). [DOI] [PubMed] [Google Scholar]
- 79.Vong S, Guillard B, Borand L et al. Acute lower respiratory infections in >/= 5 year -old hospitalized patients in Cambodia, a low-income tropical country: clinical characteristics and pathogenic etiology. BMC Infect. Dis. 13, 97 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vongphrachanh P, Simmerman JM, Phonekeo D et al. An early report from newly established laboratory-based influenza surveillance in Lao PDR. Influenza Other Respir. Viruses 4(2), 47–52 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi K, Suzuki M, Minh le N et al. The incidence and aetiology of hospitalised community-acquired pneumonia among Vietnamese adults: a prospective surveillance in Central Vietnam. BMC Infect. Dis. 13, 296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shafik CF, Mohareb EW, Yassin AS et al. Viral etiologies of lower respiratory tract infections among Egyptian children under five years of age. BMC Infect. Dis. 12, 350 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pretorius MA, Madhi SA, Cohen C et al. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness--South Africa, 2009–2010. J. Infect. Dis. 206(Suppl. 1), S159–S165 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Bharaj P, Sullender WM, Kabra SK et al. Respiratory viral infections detected by multiplex PCR among pediatric patients with lower respiratory tract infections seen at an urban hospital in Delhi from 2005 to 2007. Virol. J. 6, 89 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mathisen M, Strand TA, Sharma BN et al. RNA viruses in community-acquired childhood pneumonia in semi-urban Nepal; a cross-sectional study. BMC Med. 7, 35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roy Mukherjee T, Chanda S, Mullick S, De P, Dey-Sarkar M, Chawla-Sarkar M. Spectrum of respiratory viruses circulating in eastern India: prospective surveillance among patients with influenza-like illness during 2010–2011. J. Med. Virol. 85(8), 1459–1465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoffmann J, Rabezanahary H, Randriamarotia M et al. Viral and atypical bacterial etiology of acute respiratory infections in children under 5 years old living in a rural tropical area of Madagascar. PLoS ONE 7(8), e43666 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Do AH, van Doorn HR, Nghiem MN et al. Viral etiologies of acute respiratory infections among hospitalized Vietnamese children in Ho Chi Minh City, 2004–2008. PLoS ONE 6(3), e18176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gove S. Integrated management of childhood illness by outpatient health workers: technical basis and overview. The WHO Working Group on Guidelines for Integrated Management of the Sick Child. Bull. World Health Organ. 75(Suppl. 1), 7–24 (1997). [PMC free article] [PubMed] [Google Scholar]
- 90.Mandomando I, Sigauque B, Morais L et al. Antimicrobial drug resistance trends of bacteremia isolates in a rural hospital in southern Mozambique. Am. J. Trop. Med. Hyg. 83(1), 152–157 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Armitage K, Woodhead M. New guidelines for the management of adult community-acquired pneumonia. Curr. Opin. Infect. Dis. 20(2), 170–176 (2007). [DOI] [PubMed] [Google Scholar]
- 92.Harris M, Clark J, Coote N et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 66(Suppl. 2), ii1–ii23 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Cherian T, Mulholland EK, Carlin JB et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull. World Health Organ. 83(5), 353–359 (2005). [PMC free article] [PubMed] [Google Scholar]
- 94.Wahlgren H, Mortensson W, Eriksson M, Finkel Y, Forsgren M, Leinonen M. Radiological findings in children with acute pneumonia: age more important than infectious agent. Acta. Radiol. 46(4), 431–436 (2005). [DOI] [PubMed] [Google Scholar]
- 95.Guo W, Wang J, Sheng M, Zhou M, Fang L. Radiological findings in 210 paediatric patients with viral pneumonia: a retrospective case study. Br. J. Radiol. 85(1018), 1385–1389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lamarao LM, Ramos FL, Mello WA et al. Prevalence and clinical features of respiratory syncytial virus in children hospitalized for community-acquired pneumonia in northern Brazil. BMC Infect. Dis. 12, 119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aderele WI, Johnson WB, Osinusi K et al. Respiratory syncytial virus--associated lower respiratory diseases in hospitalised pre-school children in Ibadan. Afr. J. Med. Med. Sci. 24(1), 47–53 (1995). [PubMed] [Google Scholar]
- 98.Hasan K, Jolly P, Marquis G et al. Viral etiology of pneumonia in a cohort of newborns till 24 months of age in Rural Mirzapur, Bangladesh. Scand. J. Infect. Dis. 38(8), 690–695 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Hatipoglu N, Somer A, Badur S et al. Viral etiology in hospitalized children with acute lower respiratory tract infection. Turk. J. Pediatr. 53(5), 508–516 (2011). [PubMed] [Google Scholar]
- 100.Sunakorn P, Chunchit L, Niltawat S, Wangweerawong M, Jacobs RF. Epidemiology of acute respiratory infections in young children from Thailand. Pediatr. Infect. Dis. J. 9(12), 873–877 (1990). [DOI] [PubMed] [Google Scholar]
- 101.Klig JE, Chen L. Lower respiratory infections in children. Curr. Opin. Pediatr. 15(1), 121–126 (2003). [DOI] [PubMed] [Google Scholar]
- 102.Padilla Ygreda J, Lindo Perez F, Rojas Galarza R. et al. [Etiology of community acquired pneumonia in children 2–59 months old in two ecologically different communities from Peru]. Arch Argent Pediatr. 108(6), 516–523 (2010). [DOI] [PubMed] [Google Scholar]
- 103.Kim EA, Lee KS, Primack SL et al. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics 22(Spec No), S137–149 (2002). [DOI] [PubMed] [Google Scholar]
- 104.Salih MA, Herrmann B, Grandien M et al. Viral pathogens and clinical manifestations associated with acute lower respiratory tract infections in children of the Sudan. Clin. Diagn. Virol. 2(3), 201–209 (1994). [DOI] [PubMed] [Google Scholar]
- 105.Munywoki PK, Ohuma EO, Ngama M, Bauni E, Scott JA, Nokes DJ. Severe lower respiratory tract infection in early infancy and pneumonia hospitalizations among children, Kenya. Emerg. Infect. Dis. 19(2), 223–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weber MW, Dackour R, Usen S et al. The clinical spectrum of respiratory syncytial virus disease in The Gambia. Pediatr. Infect. Dis. J. 17(3), 224–230 (1998). [DOI] [PubMed] [Google Scholar]
- 107.Loscertales MP, Roca A, Ventura PJ et al. Epidemiology and clinical presentation of respiratory syncytial virus infection in a rural area of southern Mozambique. Pediatr. Infect. Dis. J. 21(2), 148–155 (2002). [DOI] [PubMed] [Google Scholar]
- 108.Mathisen M, Strand TA, Sharma BN et al. Clinical presentation and severity of viral community-acquired pneumonia in young Nepalese children. Pediatr. Infect. Dis. J. 29(1), e1–e6 (2010). [DOI] [PubMed] [Google Scholar]
- 109.Nokes DJ, Ngama M, Bett A et al. Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin. Infect. Dis. 49(9), 1341–1349 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bizzintino J, Lee WM, Laing IA et al. Association between human rhinovirus C and severity of acute asthma in children. Eur. Respir. J. 37(5), 1037–1042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sentilhes AC, Choumlivong K, Celhay O et al. Respiratory virus infections in hospitalized children and adults in Lao PDR. Influenza Other Respir. Viruses 7(6), 1070–1078 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnson BR, Osinusi K, Aderele WI, Tomori O. Viral pathogens of acute lower respiratory infections in pre-school Nigerian children and clinical implications of multiple microbial identifications. West Afr. J. Med. 12(1), 11–20 (1993). [PubMed] [Google Scholar]
- 113.Hui DS. Review of clinical symptoms and spectrum in humans with influenza A/H5N1 infection. Respirology 13(Suppl. 1), S10–S13 (2008). [DOI] [PubMed] [Google Scholar]
- 114.Kawachi S, Luong ST, Shigematsu M et al. Risk parameters of fulminant acute respiratory distress syndrome and avian influenza (H5N1) infection in Vietnamese children. J. Infect. Dis. 200(4), 510–515 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tran TH, Nguyen TL, Nguyen TD et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350(12), 1179–1188 (2004). [DOI] [PubMed] [Google Scholar]
- 116.Yu H, Gao Z, Feng Z et al. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS ONE 3(8), e2985 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin. Infect. Dis. 39(2), 206–217 (2004). [DOI] [PubMed] [Google Scholar]
- 118.Chalupa P, Beran O, Herwald H, Kasprikova N, Holub M. Evaluation of potential biomarkers for the discrimination of bacterial and viral infections. Infection 39(5), 411–417 (2011). [DOI] [PubMed] [Google Scholar]
- 119.Gendrel D, Raymond J, Coste J et al. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs. viral infections. Pediatr. Infect. Dis. J. 18(10), 875–881 (1999). [DOI] [PubMed] [Google Scholar]
- 120.ten Oever J, Tromp M, Bleeker-Rovers CP et al. Combination of biomarkers for the discrimination between bacterial and viral lower respiratory tract infections. J. Infect. 65(6), 490–495 (2012). [DOI] [PubMed] [Google Scholar]
- 121.Carrol ED, Mankhambo LA, Jeffers G et al. The diagnostic and prognostic accuracy of five markers of serious bacterial infection in Malawian children with signs of severe infection. PLoS ONE 4(8), e6621 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheung YB, Zaman SM, Ruopuro ML et al. C-reactive protein and procalcitonin in the evaluation of the efficacy of a pneumococcal conjugate vaccine in Gambian children. Trop. Med. Int. Health 13(5), 603–611 (2008). [DOI] [PubMed] [Google Scholar]
- 123.Madhi SA, Heera JR, Kuwanda L, Klugman KP. Use of procalcitonin and C-reactive protein to evaluate vaccine efficacy against pneumonia. PLoS Med. 2(2), e38 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Madhi SA, Kohler M, Kuwanda L, Cutland C, Klugman KP. Usefulness of C-reactive protein to define pneumococcal conjugate vaccine efficacy in the prevention of pneumonia. Pediatr. Infect. Dis. J. 25(1), 30–36 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Diez-Padrisa N, Bassat Q, Machevo S et al. Procalcitonin and C-reactive protein for invasive bacterial pneumonia diagnosis among children in Mozambique, a malaria-endemic area. PLoS ONE 5(10), e13226 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Diez-Padrisa N, Bassat Q, Morais L et al. Procalcitonin and C-reactive protein as predictors of blood culture positivity among hospitalised children with severe pneumonia in Mozambique. Trop. Med. Int. Health 17(9), 1100–1107 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Diez-Padrisa N, Bassat Q, Roca A. Serum biomarkers for the diagnosis of malaria, bacterial and viral infections in children living in malaria-endemic areas. Drugs Today 47(1), 63–75 (2011). [DOI] [PubMed] [Google Scholar]
- 128.World Health Organization Pocket Book for Hospital Care of Children: Guidelines for the Management of Common Illness with Limited Resources. WHO, Geneva, Switzerland; (2005). [Google Scholar]
- 129.Beeler JA, Eichelberger MC. Influenza and respiratory syncytial virus (RSV) vaccines for infants: safety, immunogenicity, and efficacy. Microb. Pathogene. 55, 9–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maltezou HC, Fotiou A, Antonakopoulos N et al. Impact of Postpartum Influenza Vaccination of Mothers and Household Contacts in Preventing Febrile Episodes, Influenza-like Illness, Healthcare Seeking, and Administration of Antibiotics in Young Infants During the 2012–2013 Influenza Season. Clin. Infect. Dis. 57(11), 1520–1526 (2013). [DOI] [PubMed] [Google Scholar]
- 131.Poehling KA, Szilagyi PG, Staat MA et al. Impact of maternal immunization on influenza hospitalizations in infants. Am. J. Obstet. Gynecol. 204(6 Suppl. 1), S141–148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zaman K, Roy E, Arifeen SE et al. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 359(15), 1555–1564 (2008). [DOI] [PubMed] [Google Scholar]
- 133.Bernstein DI, Malkin E, Abughali N, Falloon J, Yi T, Dubovsky F. Phase 1 study of the safety and immunogenicity of a live, attenuated respiratory syncytial virus and parainfluenza virus type 3 vaccine in seronegative children. Pediatr. Infect. Dis. J. 31(2), 109–114 (2012). [DOI] [PubMed] [Google Scholar]
- 134.Edlmayr J, Niespodziana K, Linhart B et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J. Immunol. 182(10), 6298–6306 (2009). [DOI] [PubMed] [Google Scholar]
- 135.Le Bayon JC, Lina B, Rosa-Calatrava M, Boivin G. Recent developments with live-attenuated recombinant paramyxovirus vaccines. Rev. Med. Virol. 23(1), 15–34 (2013). [DOI] [PubMed] [Google Scholar]