Abstract
Introduction: Pyrazolines are well-known and important nitrogen-containing five-membered ring heterocyclic compounds. Various methods have been worked out for their synthesis. Several pyrazoline derivatives have been found to possess diverse biological properties, which has stimulated research activity in this field.
Areas covered: The present review sheds light on the recent therapeutic patent literature (2000 – 2011) describing the applications of pyrazolines and their derivatives on selected activities. Many of the therapeutic applications of pyrazoline derivatives have been discussed, either in the patent or in the general literature areas in this review. In addition to selected biological data, a wide range of pharmaceutical applications and pharmaceutical compositions are also summarized.
Expert opinion: Pyrazoline derivatives have numerous prominent pharmacological effects, such as antimicrobial (antibacterial, antifungal, antiamoebic, antimycobacterial), anti-inflammatory, analgesic, antidepressant and anticancer. Further pharmacological effects include cannabinoid CB1 receptor antagonists, antiepileptic, antitrypanosomal, antiviral activity, MAO-inhibitory, antinociceptive activity, insecticidal, hypotensive, nitric oxide synthase inhibitor, antioxidant, steroidal and antidiabetic. Lastly, they also effect ACAT inhibition, urotensin II and somatostatin-5 receptors, TGF-β signal transduction inhibitors and neurocytotoxicity inhibitors activities. Many new pyrazoline derivatives have been synthesized and patented, but there are still new aspects to explore and work on.
Keywords: 2-pyrazolines, biological activity, dihydropyrazoles, pharmacological activity
1. Introduction
Pyrazoline is a five-membered ring heterocycle having two adjacent nitrogen atoms within the ring. It has only one endocyclic double bond and is of basic in nature [1]. They are also known as dihydropyrazoles and their chemistry are closely related to pyrazoles. According to heterocyclic nomenclature, pyrazolines require that the nitrogen atoms to be numbered 1 and 2 in each structure. There are three well-known tautomeric structures for pyrazolines namely: 1-pyrazoline, 2-pyrazoline and 3-pyrazoline (Figure 1). However, among these tautomeric structures, 2-pyrazoline is the most common.
Figure 1.
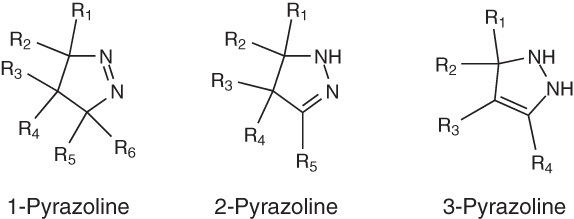
Tautomeric structures for pyrazolines.
Pyrazoline derivatives are electron-rich nitrogenous heterocycles, which play an important role in the diverse biological activities. Among its derivatives, 3-substituted pyrazolines seem to be the most attractive pyrazoline-type derivatives [2]. These heterocyclic compounds are widely occurring in nature in the form of alkaloids, vitamins, pigments and as constituents of plant and animal cell.
According to the X-ray analysis, pyrazoline ring has the structure of the five-membered dihydropyrazole ring and it has an envelope conformation [3]. The carbon atom at position-5 is deviated from the almost planar system of the other four atoms of the heterocyclic ring [4]. 2-Pyrazoline is insoluble in water but soluble in propylene glycol because of its lipophilic character [4].
Diversely substituted pyrazolines and their derivatives embedded with variety of functional groups are important biologically active agents and a huge amount of research activities have been directed toward this class of compounds. Pyrazoline derivatives are typical ICT (intramolecular charge transfer) compounds [5], and are known as a kind of fluorescent brightening agents because they have strong blue fluorescence in solution.
A classical synthesis of these compounds has been stimulated after the pioneering work of Fischer and Knöevenagel in the late 19th century [6]. The reaction of α,β-unsaturated aldehydes and ketones with hydrazines became one of the most popular methods for the preparation of 2-pyrazolines [7,8]. In this method, hydrazones are formed as intermediates, which can be subsequently cyclized to 2-pyrazolines in the presence of a suitable cyclizing reagent like acetic acid. An alternative route involves 1,3-dipolar cycloaddition of nitrileimines to carbon–carbon double bond of arylmethylene (arylidene derivatives of active methylene) compounds as well as acrylic and cinnamic acid derivatives [9-15]. The discovery of this class of compounds and considering their efficiency as drugs provides an outstanding case history of modern drug developments and also points out the unpredictability of pharmacological activity from structural modification of a prototype drug molecule. In general, pyrazoline derivatives display a broad spectrum of potential pharmacological activities and have wide medical applications.
2. Pyrazoline patents
2.1. Overview of patent activity
In the last decade, a significant interest of research in heterocyclic chemistry has been directed to substituted 2-pyrazoline derivatives. Section 2.2 of this review focuses on the recent development on pyrazolines along with their biological properties in both regular literature and patents field. Recently, some important reviews have been published on pyrazoline compounds [16,17].
The present review casts light on the recent patents and literature that is directed toward the therapeutic applications of pyrazolines during the period 2000 – 2011. The patents activities that have been reviewed include the World Intellectual Proprietary Organization (WIPO), United States Patent Trademark Office (USPTO), The European Patent Office (EP), German. Offen. (DE), Spain (ES), China (CN) and Korea (KR) patents. Figure 2 illustrates the distribution of patents over the period 2000 – 2010. Obviously, 2007 has the highest activity in the therapeutic applications of pyrazolines.
Figure 2.
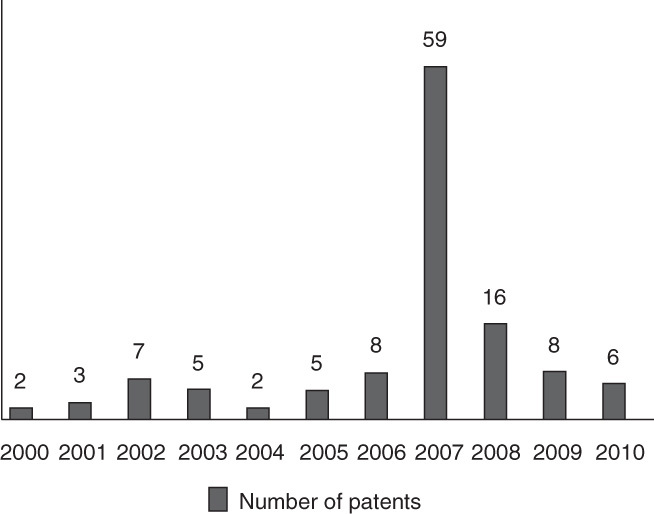
The distribution of patents on pyrazolines over the period 2000 – 2010.
2.2. Biological and therapeutic applications of pyrazolines
2.2.1. Cannabinoid CB1 receptor antagonists
One of the most attractive subjects of intensive research on pyrazoline derivatives is cannabinoid receptor type 1 (CB1) antagonists due to their highly fruitful therapeutic features. CB1 receptor antagonists have good prospects in many therapeutic areas, such as smoking and alcohol addiction as well as cognitive impairment. Recently, new chemical entities (NCEs) with CB1 antagonistic properties, structurally related to rimonabant 1, have been disclosed by some academic research groups and several pharmaceutical companies as well. There are a considerable number of CB1 antagonists that are bioisosteres and derived from rimonabant (1) by replacement of the pyrazole moiety with other heterocyclic analogs like pyrazoline.
 |
Recently, Marti et al. reported one-pot preparation of cinchonidine salt of (4S,5S)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-4,5-dihydro-1H-pyrazole-3-carboxylic acid (2), which is useful as an intermediate in the preparation of cannabinoid CB1 neutral antagonists. The method is efficient to avoid isolation of intermediates and produces product in good yield and enantiomeric excess [18].
 |
Also, synthesis of 3,4-diphenyl-4,5-dihydro-pyrazole-1-carboxylic acid hydrazide 3, which is used as CB1 modulators was achieved by Yoo et al. [19]. In CB1 receptor binding assay, pyrazoline 4 hydrochloride showed 97% affinity at 3 μM. So far, pyrazoline derivatives of the general structure 5 are claimed to be useful for the treatment of obesity and schizophrenia.
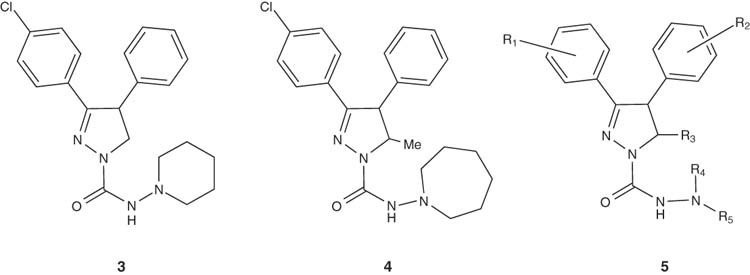 |
Preparation of the cannabinoid CB1 receptor ligand 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-N-(cis-2,6-dimethylpiperidin-1-yl)-4-methyl-4,5-dihydro-1H-pyrazole-3-carboxamide (6a) have been reported by Soler et al. [20,21]. Solid solutions and/or solid dispersions of 6a as racemate, or the (S)-enantiomer 6b were prepared efficiently in such patents. The same results have been achieved by Vela Hernandez for the preparation of (4S,5S)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-N-(cis-2,6-dimethylpiperidin-1-yl)-4-methyl-4,5-dihydro-1H-pyrazole-3-carboxamide (6b) as a cannabinoid CB1 neutral antagonist for treatment of food intake disorders [28,29].
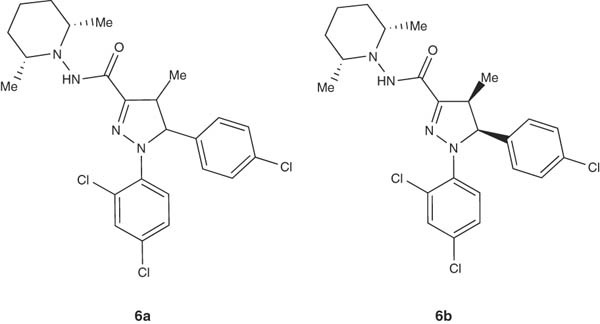 |
In the same context, Mcelroy and Chorvat have achieved the preparation of substituted N-phenyl-5-phenyl-pyrazolin-3-yl amides as cannabinoid receptor antagonists/inverse agonists useful for treating obesity, diabetes, dyslipidemias, cardiovascular disorders and/or hepatic disorders [22].
3,4-Diarylpyrazoline derivatives 7 have been reported by Lange et al. as potent CB1 receptor antagonists. Compound 7 showed lower lipophilic characters. The dramatic change was the replacement of the arylsulfonyl group by a dialkylaminosulfonyl moiety. One of these compounds exhibited the highest CB1 receptor affinity as well as very potent CB1 antagonistic activity and a high CB1/CB2 subtype selectivity [23].
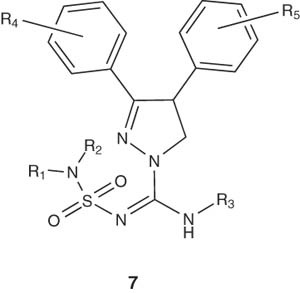 |
Sulfonamide-containing pyrazoline derivatives of general structure 8 were prepared by Buschmann et al. [24] and were used as CB1 modulators. Compounds 9 have been prepared via a multistep synthesis starting from 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1H-pyrazole-3-carboxylic acid. The pyrazoline 9 showed a high affinity to the CB1 receptor and IC50 value of 14.1 nM.
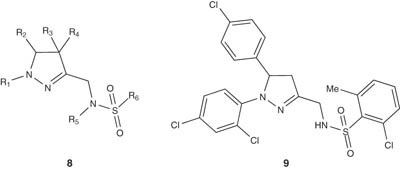 |
A number of analogs of diaryl-2-pyrazoline-3-carboxamides 10 have been prepared by Srivastava et al. and were evaluated for appetite suppression and body weight reduction in animal models. Both of the bisulfate salt of (±)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1H-pyrazole-3-carboxylic acid morpholin-4-ylamide and (-)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydro-1H-pyrazole-3-carboxylic acid morpholin-4-ylamide (11) showed significant body weight reduction in vivo. This was attributed to their CB1 antagonistic activity together with favorable pharmacokinetic profiles [25].
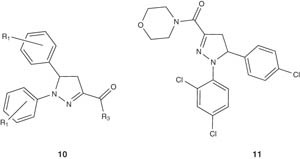 |
Donohue et al. used the radio-labeled ligands of (-)-3-(4-chlorophenyl)-N-[(4-cyanophenyl)sulfonyl]-4-phenyl-4,5-dihydro-1H-pyrazole-1-carboxamidine (12) for imaging of CB1 receptors in vivo with positron emission tomography (PET) for understanding their importance in neuropsychiatric disorders [26].
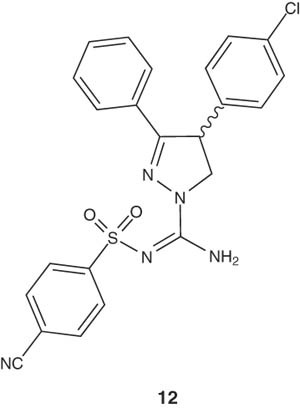 |
Also, some substituted pyrazoline derivatives 13 have been reported by Fisas-Escasany and Buschmann [27] and were evaluated for preventing weight gain. They reported a multistep synthesis of pyrazoline derivatives 14, starting from 4-chlorobenzaldehyde and ethyl pyruvate. Compounds 14 showed IC50 value of 26 nM when tested in vitro for the rat CB1 receptor subtype.
In the same consequence, Buschmann et al. reported the preparation of 4-substituted pyrazoline derivatives 13 as cannabinoid receptor inhibitors for treating various diseases [34].
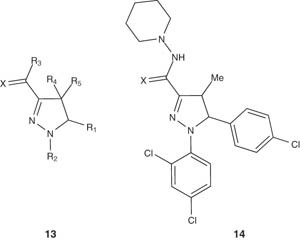 |
Lange et al. [30] reported the reaction of (pentylhydrazono)acetic acid ethyl ester, with N-chlorosuccinimide (NCS) followed by in situ treatment with styrene, hydrolysis and amidation with 2-adamantanamine hydrochloride to afford the pyrazoline 16. The latter compound showed high affinity for cannabinoid receptors and agonistic activity on CB1 receptor, which is also useful for the treatment of multiple sclerosis and traumatic brain injury.
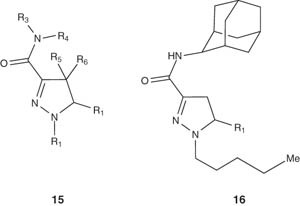 |
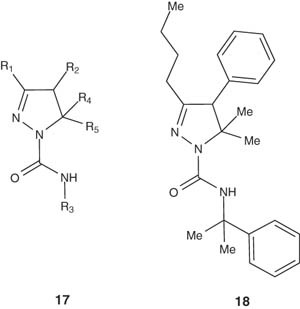
4,5-Dihydro-(1H)-pyrazole derivatives 17 and 18 have been prepared by Yildirim et al. [31]. Compounds containing the pyrazoline structure 17 are used as CB1 receptor modulators. Pyrazoline 18 exhibited pKi values of 8.1 and 8.4 for CB1 and CB2, respectively, in cannabinoid receptor affinity assays.
 |
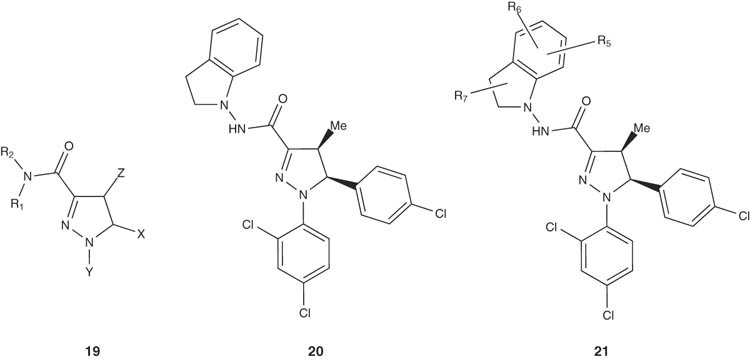
Indoline-substituted pyrazoline derivatives 20 and 21 have been reported as cannabinoid receptor modulators by Torrens-Jover et al. [32]. Compounds of the general structure 19 were tested for their cannabinoid activity in vivo.
 |
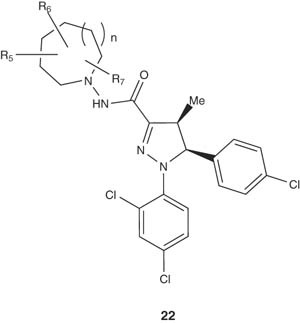
In addition, azepane- or azocane-substituted pyrazoline derivatives 22 have been also prepared by Torrens-Jover et al. [33].
 |
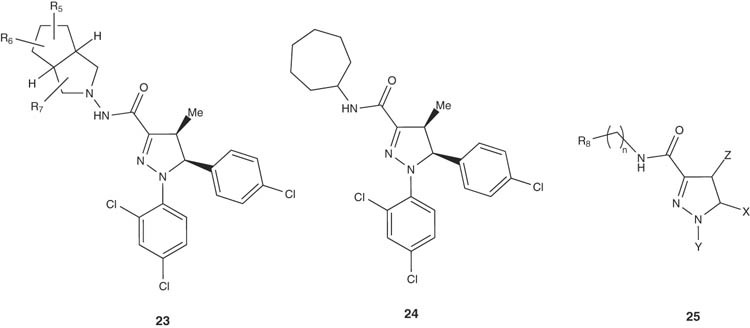
Preparation of octahydropentalene- and cycloalkane-substituted pyrazoline derivatives 23 and 24, respectively have been achieved by Torrens-Jover et al. [36,37]. Some of the selected compounds of the general formula 25 were tested for their in vivo cannabinoid activity. Also, some prodrugs of pyrazoline compounds 25 have been prepared by Torrens-Jover et al. as CB1 receptor antagonists [38].
 |
2.2.2. Anticancer activity
Recently, syntheses of 4-substituted 1H-pyrazolines have been achieved by Yenes-Minguez and Torrens-Jover [39]. The synthesized pyrazoline derivatives 26 have been investigated for cancer treatment or prophylaxis. Pyrazolines such as 27 and 28 could be successfully employed in treatment and/or cancers prophylaxis of many types ranging from the brain, bone, mouth, esophagus, stomach, liver, bladder, pancreas, cervix, lung, breast, colon, rectum or prostate cancers.
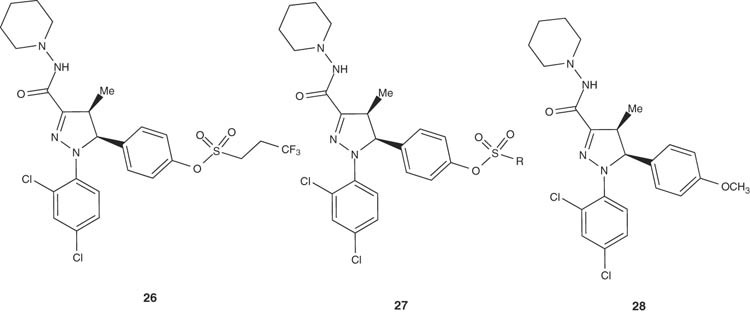 |
Several novel thiazolone-based compounds containing 5-aryl-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl framework 29a have been reported by Havrylyuk et al. [40]. The synthesized compounds were evaluated in vitro for their cyctotoxic activity. Most of the tested compounds displayed promising anticancer activity versus variety of cancer types including leukemia, melanoma, lung, colon, ovarian, renal, prostate and breast cancer cell lines. Among these series, compound 29b showed the most efficient anticancer potency and was found to be active with selective influence on colon cancer cell lines, especially on HT-29 (log GI50 = -6.37).
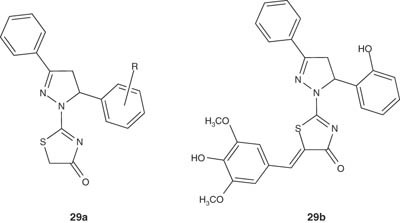 |
Bhat et al. [41] prepared some substituted pyrazoline derivatives 30 and evaluated for their in vitro cytotoxic activity against a panel of human cancer cell lines. Only eight compounds showed marked activity out of 93 screened compounds.
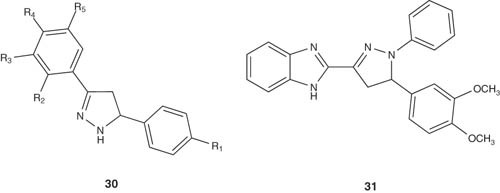 |
The antineoplastic activities a series of pyrazoline-bearing benzimidazoles versus full NCI 60 cell panel have been reported by Shahrayar et al. [42]. Compound 31 demonstrated the best cytotoxic properties. It has high selectivity against certain cell lines including Leukemia CCRF-CEM and RPMI-8226 cell lines with GI50 values of 2.23 and 2.76 μM, respectively [42].
1-Acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives 32 and 33 have been reported by Manna et al. [43], evaluated for their antineoplastic activity, and for their inhibitory effect on P-glycoprotein-mediated multidrug resistance protein (MDR1); which confers resistant to tumor cells by decreasing drug accumulation within tumor cells [44]. Both compounds 32 and 33 have been found to be active as MDR1 blockers.
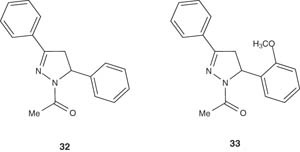 |
Johnson et al. synthesized the pyrazoline derivatives 34 in analogy to the natural cis-stilbine derivative combretastatin-A4 35, by replacement the ethylene-bridge with pyrazoline heterocycle, and tested for their anticancer activity [45].
 |
1,4-Diaryl-4,5-dihydropyrazoles 36 were reported by Roecker et al. to be mitotic kinesin spindle protein (KSP) inhibitors [46] with IC50 value of 0.2 nM and cell EC50 values of 3.2 nM. Some of the fused pyrazoline derivatives of cyclolignans 37 have been reported and evaluated for their cytotoxic activities in culture cells of P-388 murine leukemia, HT-29 colon carcinoma and A-549 lung carcinoma. Indene fused series of 3-(4-chlorophenyl)-[1,2-c]pyrazolines substituted with benzene sulfonamides, N1,N3-disubstituted sulfonylurea and sulfonylthiourea scaffolds 38,39 and some derived thiazolidinone and thiazoline ring systems have been synthesized by Rostom and evaluated for their antitumor activity. Eight compounds showed promising broad-spectrum antitumor activity against most of the tested sub-panel tumor cell lines [47].
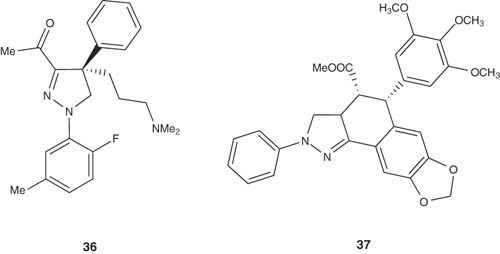 |
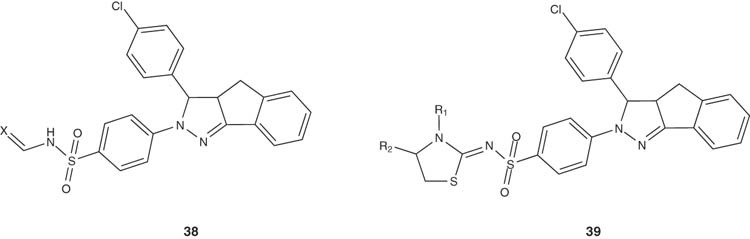 |
Preparation of some pyrazole derivatives of related structure to the targeted pyrazolines 40 have been achieved by Coleman et al. [48]. The dihydropyrazole compounds of formula 40 are useful for treating cellular proliferative diseases, for treating disorders associated with KSP kinesin activity, and for inhibiting KSP kinesin.
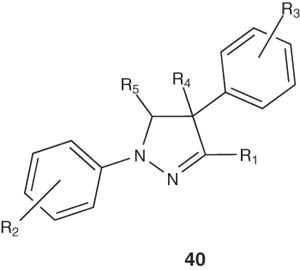 |
Breslin et al. [49] reported the preparation of 4,5-dihydro-1H-pyrazole derivatives 41 as potent mitotic kinesin inhibitors. The antineoplastic activities of compounds 41 have been evaluated by kinesin ATPase in vitro assay using human KSP motor domain, and it revealed potent binding affinity. Compound 42 was prepared from the starting precursor 43, and demonstrated IC50 value ≤ 50 μM.
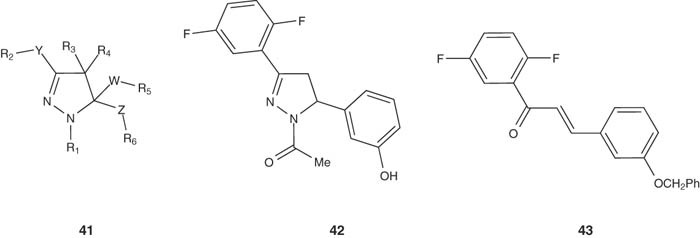 |
GLI proteins play pivotal roles in both cell proliferations and apoptosis [50]. It is also reported that blocking GLI genes is important in the initiation of DNA damage in early S-phase, leading to cell death in some human carcinomas [51]. He et al. [52,53] reported the synthesis of dihydropyrazole carboxamides 44 that were used as kits for the diagnosis and treatment of cancers expressing a GLI polypeptide; in particular a GLI1, GLI2 or GLI3 polypeptide. All compounds were evaluated for their GLI polypeptide inhibitory activity.
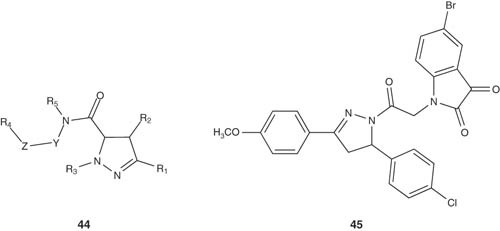 |
4,5-Dihydropyrazolyl-indole-2,3-dione 45 was identified as an active antitumor candidate by Havrylyuk et al. with selective influence on leukemia subpanel tumor cell lines (GI50 = 0.69 – 3.35 μM) [54].
TGF-β is another cancer attractive target, since it is directly involved in apoptosis induction process [55-58]. The preparation of fused dihydropyrazoles 46 as TGF-β signal transduction inhibitors have been synthesized by Sawyer et al. [59]. For instance, 1-{[2-(6-bromoquinolin-4-yl)-1-(pyridin-2-yl)ethylidene]amino}pyrrolidin-2-one was treated with sodium hydride (NaH) in dimethylformamide (DMF) at 80 – 85°C for 18 h to afford the product 47 in 54% yield. The selected pyrazolines had IC50 value below 20 μM for the TGF-β type I receptor.
 |
2.2.3. CNS effects
The reported central nervous system (CNS) pharmacological actions of pyrazoline derivatives include antiepileptic and antidepressant effects; in addition to neurodegenerative disorders.
2.2.3.1. Antiepileptic activity
Ozdemir et al. [60] prepared 1-phenyl-1-thiocarbamoyl- and 1-N-substituted thiocarbamoyl-3-(2-furyl)-5-phenyl/(2-furyl)-2-pyrazoline derivatives 48 – 50 and studied their antiepileptic action by maximal electroshock seizure (MES) and subcutaneous pentylenetetrazole tests. Compounds 48 – 50 were found to be protective against MES and subcutaneous metrazole (scMet) at 30 – 300 mg/kg dose levels.
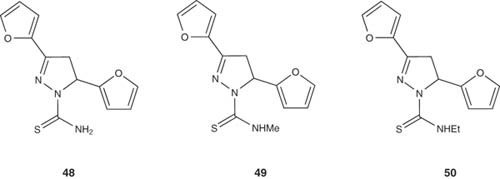 |
Several 3-(3-acetoamino)phenyl-1,5-substituted phenyl-2-pyrazolines 51 – 53 were synthesized by Singh et al. [61] and evaluated for their anticonvulsant activity. The synthesized pyrazolines 51 – 53 exhibited anticonvulsant activity, which was reflected by 60 – 80% protection observed versus PTZ-induced seizures. Those compounds exhibit their anticonvulsant potentials via inhibiting oxidation of certain nicotinamide–adenine–dinucleotide substrates.
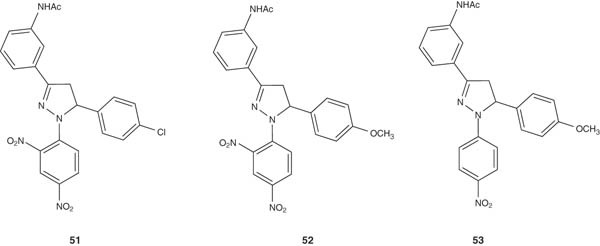 |
A set of pyrazoline derivatives have been prepared by Shishikura et al. [62] and were used as kainic acid neurocytotoxicity inhibitors. The synthesized derivatives showed non-competitive antagonism versus the non-N-methyl-d-aspartate (NMDA)-type ionotropic transmembrane receptor for glutamate AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors and are useful as nerve cell protectors, or in antiepileptic therapy. Particularly, the pyrazoline derivative; 1-benzoyl-4,5-dihydro-3,5-diphenyl-1H-pyrazole 54 and (+)-3-(1-benzoyl-5-phenyl-4,5-dihydro-1H-pyrazol-3-yl)pyridine 55 showed IC50 of 2.6 and 1.3 μM versus AMPA receptors, respectively.
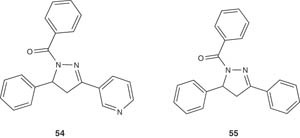 |
Some quaternary ammonium salts of substituted pyrazoline compounds have been prepared by Torrens-Jover et al. [63] and evaluated for their use as medicaments for the treatment of humans and animals neurocytotoxicity. The pyrazoline derivative 1 was prepared by methylation of N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-dihydropyrazole-3-carboxamide with methyl iodide. All of the synthesized pyrazolines were evaluated for their neurocytotoxicity inhibitory effect.
2.2.3.2. Antidepressant activity
3,5-Diphenyl-2-pyrazoline derivatives 56 – 58 showed decent antidepressant activities [64]. 3-(4-Methoxyphenyl)-5-(3,4-dimethoxyphenyl)-2-pyrazoline (56), 3-(4-methoxyphenyl)-5-(2-chloro-3,4-dimethoxyphenyl)-2-pyrazoline (57) and 3-(4-chlorophenyl)-5-(2-chloro-3,4-dimethoxyphenyl)-2-pyrazoline (58) reduced 41.9 – 48.6% immobility times at 100 mg/kg dose level. From the structure–activity relationship (SAR) point of view, it was found that 4-methoxy and 4-chloro substituents on the phenyl ring at position-3 of the pyrazoline ring increased the antidepressant activity. Replacement of these groups by bromo and methyl substituents has negative impacts on the antidepressant properties.
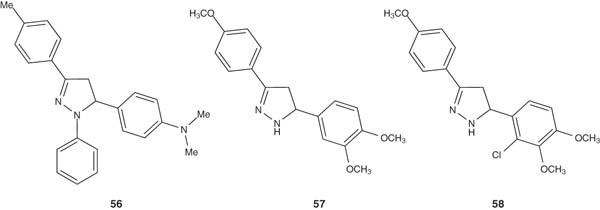 |
Prasad et al. [65] synthesized some 1,3,5-triphenyl-2-pyrazolines having the general formula 59, and 3-(2′′-hydroxynaphthalen-1′′-yl)-1,5-diphenyl-2-pyrazolines 60 and evaluated their antidepressant activity.
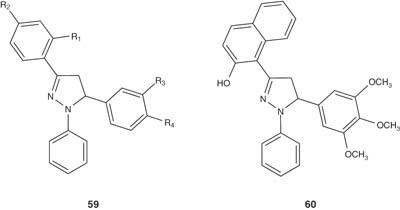 |
The pyrazoline derivative 61 was found to reduce the immobility times by 25 – 59% at 100 mg/kg dose level. It was found that compounds having electron-releasing groups on both aromatic rings at pyrazoline positions 3 and 5 dramatically enhanced the antidepressant activity when compared with the pyrazolines having no substituents on the aromatic rings [65].
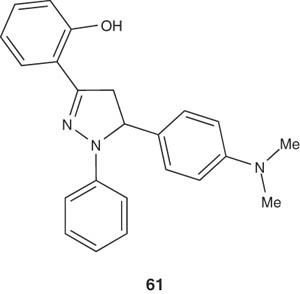 |
Similarly, some 1-phenyl-, 1-thiocarbamoyl- and 1-N-substituted thiocarbamoyl-3-(2-furyl)-5-phenyl/(2-furyl)-2-pyrazoline derivatives 62 and 63 were synthesized and investigated for their antidepressant activity by the Porsolt test (forced swimming test) in albino mice [66].
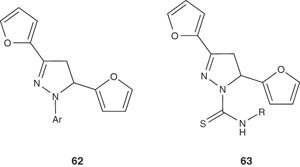 |
Monoamine oxidase (MAO) inhibitors are well-established antidepressant agents [67-77]. Gokhan-Kelekci et al. [78,79] synthesized several pyrazoline derivatives such as 64a and evaluated their antidepressant potentials via measuring their MAO inhibitory activities. In addition, the anxiolytic and monoamine oxidase-A (MAO-A) and monoamine oxidase-B (MAO-B) inhibitory activities have been also evaluated by in vivo and/or in vitro tests. The synthesized compounds showed high activity against both MAO-A and MAO-B isoforms.
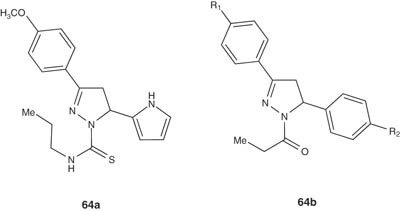 |
Chimenti et al. [66] reported the synthesis of series of N1-propanoyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives 64b and tested their pharmacological activities as MAO inhibitors. Most of the tested compounds showed inhibitory activity within the micromolar range and high selectivity versus the more clinically important isozyme, that is, MAO-A [80].
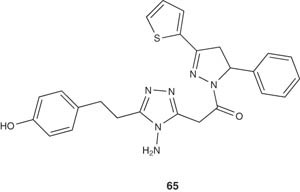 |
The antidepressant-like activities of compound X analogs were found to be favorably comparable with the clinically used fluoxetine [81]. This action was suggested to be mediated by modulating the CNS serotonin pathway [81].
Jayaprakash et al. [82] synthesized a series of 3,5-diaryl carbothioamide pyrazoline derivatives 66 designed as mycobactin analogs (mycobacterial siderophore), and evaluated their antidepressant and MAO inhibitory activities. Interestingly, it was found that compound 67, which has antitubercular potential, acts also as a selective inhibitor of rat liver MAO-B.
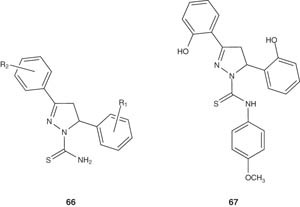 |
The substituted 1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazole derivatives 66 was found to selectively inhibit MAO-A and MAO-B isoforms [83].
Manna et al. [43] synthesized a novel series of 1-acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives and studied their ability to selectively inhibit MAOs, swine kidney oxidase and bovine serum amine oxidase. The pyrazoline 68 showed a potent MAO inhibitor with a Ki value of about 10 – 8 nM.
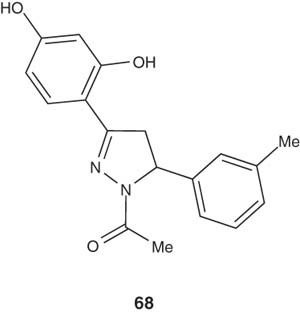 |
2.2.3.3. Antineurodegenerative effects
A series of pyrazoles 69 were synthesized by Chimenti et al. [66,84] and assayed as MAO inhibitors. Compound 69a showed inhibitory activity in micromolar range and high selectivity toward MAO-A isozyme. In addition, it was found to be useful as adjuvant therapy in the treatment of neurodegenerative disorders such as Parkinson's disease and Alzheimer's disease.
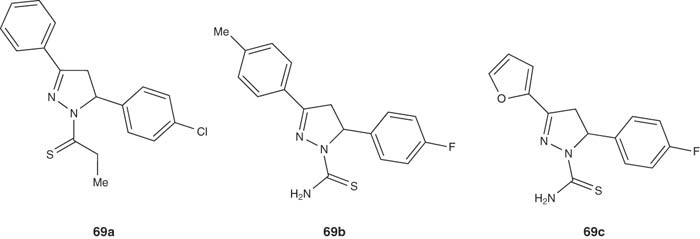 |
2.2.4. Anti-inflammatory, antipyretic and analgesic activities
The bis(3-aryl-4,5-dihydro-1H-pyrazole-1-carboxamide) derivatives 70 – 71 were synthesized and screened for their anti-inflammatory properties utilizing in vivo acute carrageenan-induced paw edema standard method in rats [85]. This set of pyrazolines also demonstrated a decent inhibitory activity versus prostaglandin E2 (PGE2) that is responsible for fever [86-88], at a dose level of 50 mg/kg [85]. The bis-pyrazoline derivative 70 exhibited considerable anti-inflammatory properties [85]. Compounds 70 and 71 showed remarkable anti-inflammatory activities relative to indomethacin, as a standard reference, with a lower ulcer index values [85].
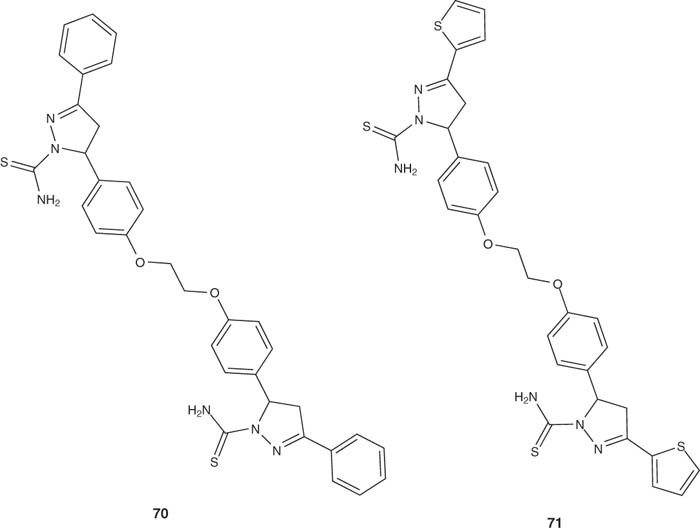 |
Also, some novel bis(1-acyl-2-pyrazolines) derivatives 72 were synthesized by the same research group and screened for their anti-inflammatory and ulcerogenic activities as well. Some of the synthesized compounds showed advanced anti-inflammatory properties with lower ulcerogenic liability than the standard used drug [89].
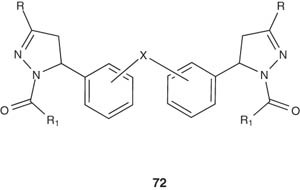 |
A series of 3-(4-biphenyl)-5-substituted phenyl-2-pyrazolines 73a and 1-benzoyl-3-(4-biphenyl)-5-substituted phenyl-2-pyrazolines 73b have been reported by Amir et al. and were screened for their anti-inflammatory and analgesic activities [90].
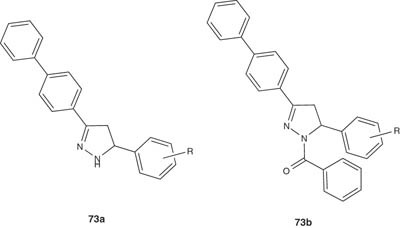 |
2-Pyrazoline-bearing benzenesulfonamide derivatives 74 have been synthesized by Rathish et al. [91] and screened for their anti-inflammatory activity. Pyrazoline derivatives 74 were found to be more active than celecoxib throughout their study.
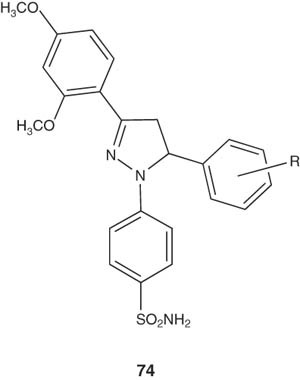 |
Rani et al. [92] synthesized a series of pyrazoline derivatives pendent to an indole moiety and reported their evaluation for their anti-inflammatory activity against carrageenan-induced edema in albino rats at an oral dosage regimen of 50 mg/kg. All of the synthesized pyrazolines showed promising anti-inflammatory activity. 3-[1-Acetyl-5-(p-hydroxyphenyl)-2-pyrazolin-3-yl]indole (75) was found to be the most potent derivative in this series. It showed higher percentile of edema inhibition, along with lower ulcerogenic liability and acute toxicity than phenylbutazone as a standard drug [92].
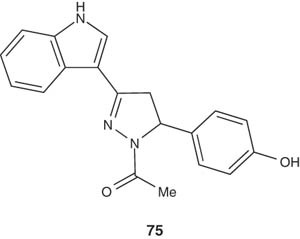 |
Compound 76 that bears an electron withdrawing nitro group in the aryl moiety showed good activity comparable with that of standard drugs pentazocin and diclofinac sodium [93].
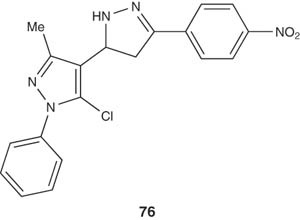 |
Rathish et al. synthesized a series of 1,3,5-trisubstituted pyrazolines-bearing benzene sulfonamides 77 and evaluated their anti-inflammatory activity. Among the tested compounds, several showed promising anti-inflammatory activity [91]. Some 1-(4-sulfamylphenyl)-3-trifluoromethyl-5-indolylpyrazoline derivatives 78 were prepared and screened for cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitory activities [94].
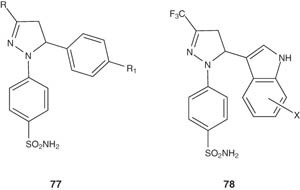 |
Phenyl-5-(2-pyrrolyl)-4,5-dihydro-(1H)-pyrazole derivatives containing a thiocarbamoyl group such as 64 have been reported by Gokhan-Kelekci et al. [79]. The synthesized series of pyrazolines were tested for their in vivo anti-inflammatory activity by two different bio-assays namely, carrageenan-induced edema and acetic acid-induced increase in capillary permeability in mice. Moreover, analgesic and ulcerogenic activities were also investigated.
A series of 5-(substituted) aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines 79 have been synthesized by Khode et al. [95] and screened for in vivo anti-inflammatory and analgesic activities. Among the 12 prepared compounds, the pyrazoline derivatives 79 exhibited significant anti-inflammatory activity in a model of acute inflammation.
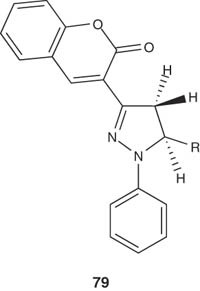 |
Shoman et al. [96] synthesized a group of NO-donating 2-pyrazoline derivatives 80 and evaluated for their anti-inflammatory activity using carrageenan-induced rat paw edema and compared with indomethacin as a known standard. The ability of the prepared derivatives to induce gastric toxicity was also evaluated. Most of the prepared series showed significant anti-inflammatory activity, with higher safety margins than indomethacin in regard to gastric toxicity. When NO-donating group was incorporated into the parent pyrazoline derivatives, they caused a non-significant reduction in the anti-inflammatory activity and a marked decrease in gastric ulcerations induced by pyrazolines that lack the same group.
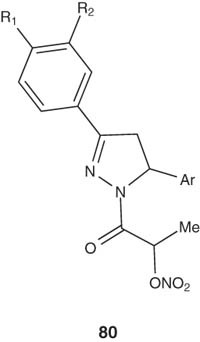 |
Pyrazoline derivatives containing benzoxazole and benzimidazole moiety 81 have been reported by Kaplancikli et al. [97]. The synthesized compounds were evaluated for antinociceptive activities. Compounds 81 exhibited significant antinociceptive activities in both hot plate- and acetic acid-induced writhing tests.
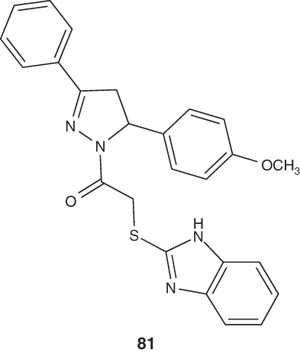 |
The pyrazolines 82 and 83 were synthesized by Godoy et al. [98] and tested for their antinociceptive activity [98]. Moreover, Godoy et al. has also investigated whether the pain relief effect is mediated by spinal noradrenergic or serotonergic systems. The results showed that, unlike the standard dipyrone, both spinal serotonin receptors and α2-adrenoceptors are involved in the antinociception induced by 82 and 83 [98].
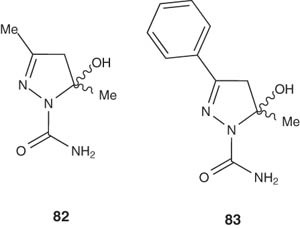 |
2.2.5. Antimicrobial activities
Pyrazoline ring represents a central scaffold for diverse antimicrobials that include antibacterials, antifungals, antivirals and antiamoebics. However, antitubercular could be considered a sub-class from antibacterials, we opted here to separate it under a specific subtitle, because of its clinical importance.
2.2.5.1. Antibacterial and antifungal activities
Several 1-(4-aryl-2-thiazolyl)-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives 84 have been synthesized by Ozdemir et al. [99] and were evaluated for their antimicrobial activities against Escherichia coli, Bacillus cereus, Salmonella typhimurium, Streptococcus faecalis, Staphylococcus aureus, Aeromonas hydrophila, Candida glabrata and Candida albicans. A significant level of activity was observed.
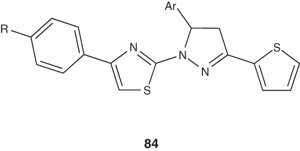 |
In the same context, Abdel-Wahab et al. [100] reported the synthesis of several 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles 85 and evaluated their antibacterial and antifungal activities. Some of the synthesized compounds showed enhanced antimicrobial activities than control drugs.
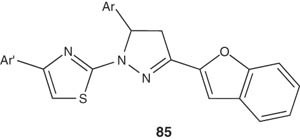 |
1,3-Diaryl-5-(cyano-, aminocarbonyl- and ethoxycarbonyl)-2-pyrazoline 86 and 1,3,4,5-tetra-aryl-2-pyrazoline derivatives 87 have been prepared by Abunada et al. [101] and screened for their antimicrobial activities against E. coli, S. aureus, Asperagillus flavus and C. albicans.
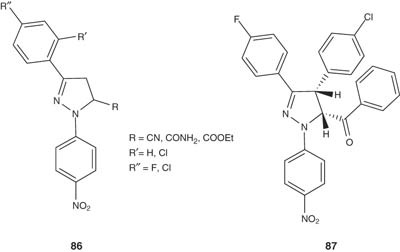 |
Bhatt et al. [102] synthesized several substituted pyrazolines 88 as potential antimicrobial agents. The synthesized compounds were found to have remarkable activity against Bacillus megaterium Bacillus subtilis, E. coli and Mycobacterium tuberculosis H37Rv.
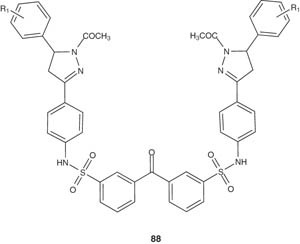 |
The synthesis of pyrazoline derivatives of naproxen, as represented by compound 89, have been achieved by Udupi et al. [103]. The biological evaluation showed that some compounds of the series showed significant dual antimicrobial and anti-inflammatory activities.
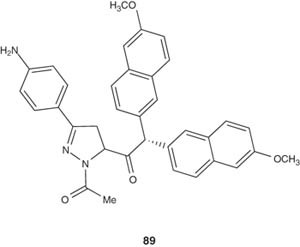 |
Bharmal et al. [104] synthesized the pyrazoline derivative 90 with antimicrobial activity against Salmonella typhosa and Aspergillus niger.
 |
Basawaraj et al. [105] reported the synthesis of some 1H-pyrazolines pendent to benzofuran 91a and 91 b and tested their antimicrobial activity against both Gram-positive and Gram-negative bacteria represented by S. aureus and E. coli. Compounds 91a and 91b revealed potent antibacterial effect versus Gram-positive bacteria. By contrast, their antimicrobial effect has been significantly reduced in case of Gram-negative strains.
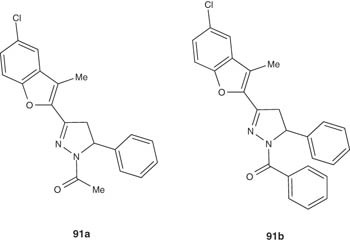 |
Some new pyrazolines and N-phenylpyrazolines, 92a and 92 b have been prepared by Desai et al. [106] and evaluated for their antimicrobial activities. The synthesized compounds exhibited activity against Gram-positive bacteria.
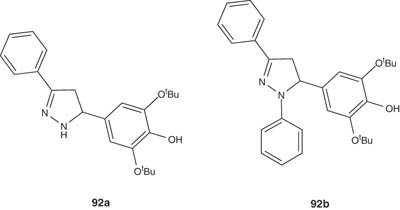 |
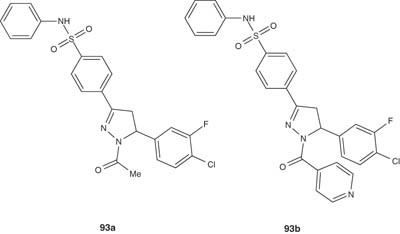
Jamode et al. [107] reported the synthesis of the 1-isonicotinoyl/carboxamido-2-pyrazolines 93a and 93 b and evaluated their antimicrobial properties against S. aureus, E. coli, Proteus mirabilis and Pseudomonas aeruginosa. Most of the pyrazoline derivatives were found to have moderate antibacterial activity.
 |
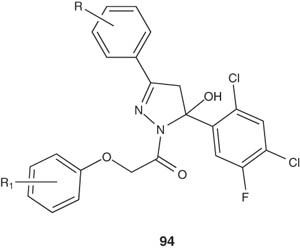
A series of chlorofluoropyrazolines 94 were prepared by Karthikeyan et al. [108]. Some of such pyrazolines showed significant antibacterial and antifungal activity.
 |
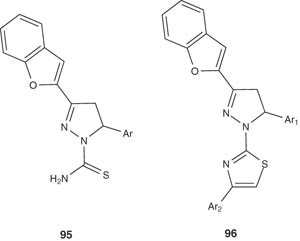
Treatment of the chalcones with nitromethane under Michael addition condition, followed by subsequent cyclization with thiosemicarbazide under basic reflux conditions gave 3-(benzofuran-2-yl)-5-(4-aryl)-4,5-dihydropyrazole-1-carbothioamides 95. These pyrazolines were further reacted with phenacyl bromides to afford thiazole-substituted pyrazolines 96. Some of the compounds showed a significant antimicrobial activity against E. coli and A. niger [100].
 |
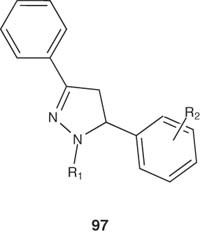
Some N1-substituted 3,5-diphenylpyrazoline derivatives 97 have been synthesized by Chimenti et al. [83] and evaluated for their in vitro antibacterial activity against Helicobacter pylori. Pyrasolines with an N1-acetyl group and 4-methoxy substituent in the 5-phenyl ring showed the highest activity against H. pylori metronidazole-resistant strains with minimum inhibitory concentration (MIC) value of 1 – 4 µg/ml [83].
 |
Mogilaiah et al. [109] evaluated the antibacterial activities of the pyrazoline derivatives containing 1,8-naphthyridine moiety as represented by compound 98. The prepared compounds showed less activity than the standard aminogylcoside gentamycin.
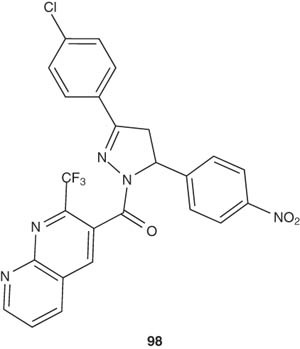 |
Vijayvergiya et al. [110] synthesized some 3,5-diaryl-1-phenyl/isonicotinoyl-2-pyrazoline derivatives 99 and evaluated their antibacterial activity. The new pyrazolines showed remarkable antibacterial activity against Gram-positive bacteria S. aureus, Staphylococcus albus, Streptococcus pyogenes, Streptococcus viridans and Gram-negative bacteria E. coli and S. typhosa [110].
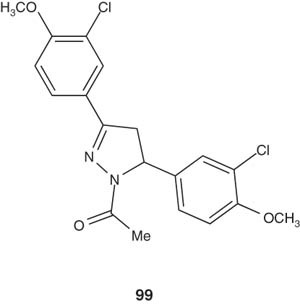 |
Waheed and Khan [111] synthesized some derived substituted 1,2-pyrazolines 100 from nalidixic acid as antibacterial and analgesic agents. These pyrazolines were found to have a significant antibacterial activity against Gram-negative bacteria and possessed appreciable analgesic activity.
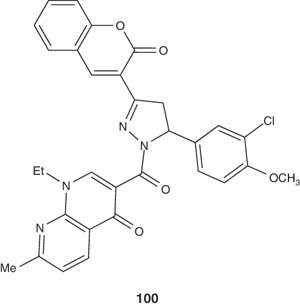 |
2.2.5.2. Antimycobacterial activity
Stirrett et al. [112] synthesized some pyrazolines represented by compound 101 with structural similarities to siderophores and evaluated their ability as novel antimicrobials against M. tuberculosis and Yersinia pestis.
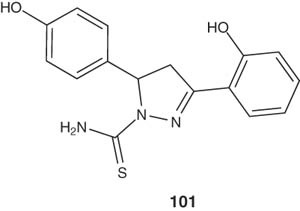 |
1,3,5-Trisubstituted-2-pyrazolines 102 were reported by Shenoy et al. [113] and were tested for their antimicrobial activity. Some of the compounds exhibited potential antitubercular activity.
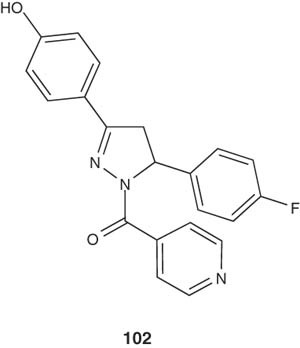 |
Zampieri et al. [118] reported the synthesis of 1-(3,5-diaryl-4,5-dihydro-1H-pyrazol-4-yl)-1H-imidazole derivatives 103 and tested them against strains of C. albicans and a strain of M. tuberculosis H37Rv.
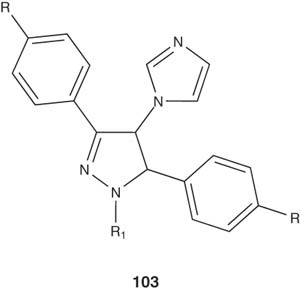 |
Shaharyar et al. presented a series of N1-nicotinoyl-3-(4-hydroxy-3-methylphenyl)-5-(substituted phenyl)-2-pyrazolines 104 and tested them in vitro for their antimycobacterial activity. Some of these pyrazolines were found to be an active agent against different strains of TB, with MIC value of 0.26 μM [114].
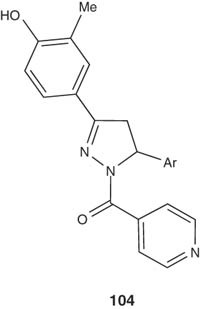 |
5-Aryl-1-isonicotinoyl-3-(pyridin-2-yl)-4,5-dihydro-1H-pyrazole derivatives 105 have been synthesized by Mamolo et al. [115] and tested for their in vitro antimycobacterial activity. The latter pyrazoline derivative showed an interesting activity against different strains of M. tuberculosis.
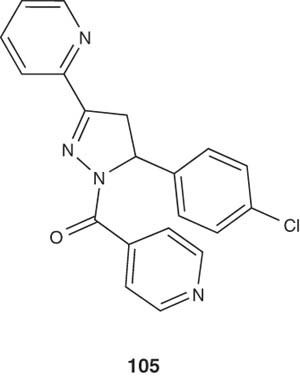 |
Several 1-[(N,N-disubstituted thiocarbamoylthio)acetyl]-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives 106 were synthesizes by Ozdemir et al. [116] and Shaharyar et al. [114] and evaluated for their in vitro antimycobacterial activity against H37Rv strain.
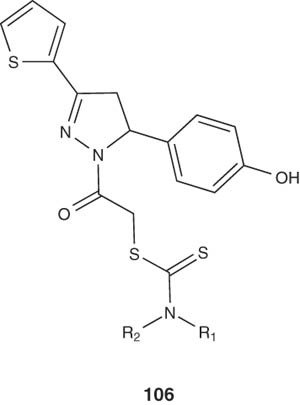 |
The potential benefits of medicaments endowed with both antimycobacterial and antifungal characters represent a valuable goal, since the association between TB and mycotic infections often occurs in immunocompromised patients [117]. So far, some 1,3,5-triryl-4,5-dihydro-1H-pyrazoles, as represented by structure 107, pendent to 1H-imidazole ring have been reported by Zampieri et al. [118] and tested for their in vitro antifungal and antimycobacterial activities. These imidazole-substituted pyrazole derivatives showed significant antifungal activity against a clinical strain of C. albicans and remarkable antitubercular activity against M. tuberculosis H37Rv.
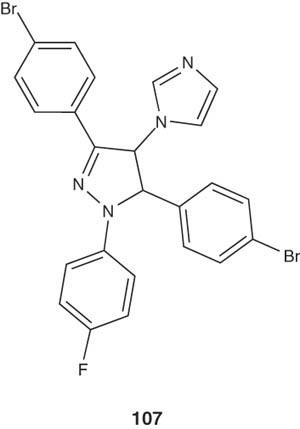 |
A series of heterocyclic-substituted diphenyl ether derivatives of pyrazoles 108 have been synthesized and evaluated for their activity against H37Rv strain of Mycobacterium. Ten compounds inhibited the growth at concentrations as low as 1 µg/ml. This level of activity was found comparable with the reference drugs rifampicin and isoniazid at the same concentration [119].
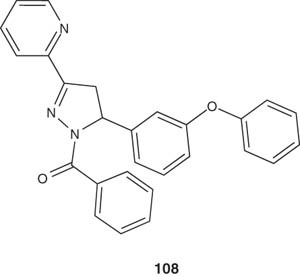 |
Another series of 5-(4-(substituted)phenyl)-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-2-toluidinomethanethione and 5-(substituted)phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-2-methoxyanilino methanethione derivatives were synthesized by Ali et al. [120] and tested for their in vitro antitubercular activity against M. tuberculosis H37Rv. Compound 109 was found to be the most active with MIC value of 0.0034 µM [120].
 |
Babu et al. [121] evaluated the biological activity of 1,3,5-trisubstituted pyrazolines-bearing benzofuran moiety 110a and 110b. These compounds have been found to have some antitubercular effect.
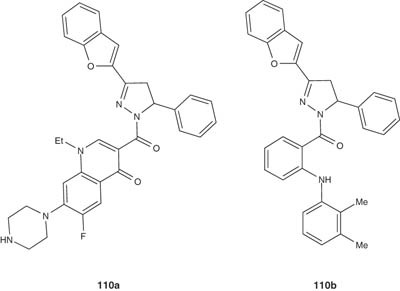 |
2.2.5.3. Antiamoebic activity
Compounds 111 have been synthesized by Hayat et al. and tested in vitro for their antiamoebic activity versus HM1:IMSS strain of Entamoeba histolytica [122]. The results showed that these compounds exhibited promising antiamoebic activity (IC50 = 0.05, 0.31, 0.06 and 0.29 μM, respectively) [122].
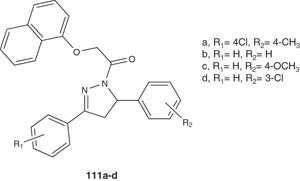 |
A variety of 3-(3-bromophenyl)-5-phenyl-1-(thiazolo[4,5-b]quinoxaline-2-yl)-2-pyrazoline derivative, as represented by compound 112, have been achieved by Budakoti et al. [123], and screened for their antiamoebic activity against HMI:IMSS strain of E. histolytica by micro-dilution method which compared the IC50 values with the standard drug metronidazole [123].
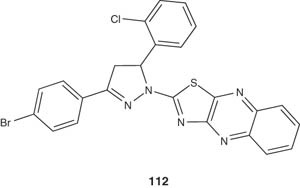 |
Also, the same authors have synthesized a novel Pd(II) complexes with 1-N-substituted thiocarbamoyl-3,5-diphenyl-2-pyrazoline (Budakoti et al. [124]) and evaluated their antiamoebic activity by micro-dilution method against HM1:IMSS strain of E. histolytica and compared the results with the standard drug metronidazole. These palladium complexes showed better activity than their corresponding ligands. The pyrazoline 113a showed better inhibitory activity as indicated by their lower IC50 values (IC50 = 0.05 µM) as compared with metronidazole (IC50 = 1.82 µM) [124]. Similarly, Husain et al. reported another series of Pd(II) complexes, represented below by the most active compound 113b [125]. But this series of compounds were less active than 113a in terms of IC50 values; however, complex 113b (IC50 = 0.37 µM) is more active than metronidazole [125].
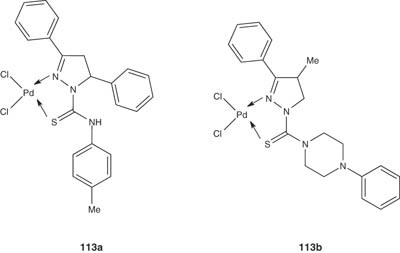 |
Abid et al. [126,127] synthesized a series of 1-N-substituted thiocarbamoyl-3-phenyl-2-pyrazoline derivatives 114 by cyclization of Mannich bases with thiosemicarbazide and evaluated their in vitro antiamoebic activities against E. histolytica in comparison with metronidazole as reference drug. The preliminary SARs indicated that the substitution of 3-chloro or 3-bromo on the phenyl ring at pyrazoline position-3 markedly enhanced the antiamoebic activity. Moreover, compound 115 showed the most promising antiamoebic activity with an IC50 value of 0.6 versus 1.8 μM of metronidazole.
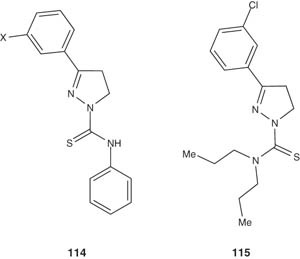 |
Another new series of pyrazoline derivatives were synthesized by cyclization of Mannich bases with thiosemicarbazides being substituted by different cyclic and aromatic amines and screened for in vitro antiamoebic activity against E. histolytica. The pyrazoline 116 was found to be the most active compound in this series [126]. The Pd(II) complexes of these derivatives have been also prepared and evaluated for their antiamoebic activity [128]. As observed previously, the Pd(II) complexes exhibited stronger antiamoebic activity [128].
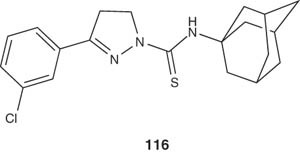 |
Bhat et al. reported some interesting bis-pyrazolines 117 prepared by cyclization of chalcones with N-4-substituted thiosemicarbazides under basic conditions. Investigation of the antiamoebic activity showed that compounds with aromatic substituents at the thiocarbamoyl group were more active than those with the cyclic groups [129].
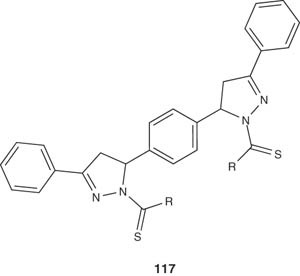 |
2.2.5.4. Antitrypanosomal activity
Semicarbazone-based pyrazoline derivatives, represented by compound 118, have been designed as drug candidates for Chagas' disease (American trypanosomiasis) [130]. Compound 118 showed inhibitory activity versus Trypanosoma cruzi as low as 80 nM. Compound 118 and its analogs exhibited their antitrypanosomal activities by targeting cysteine protease cruzain, interestingly, with low cellular toxicity [130].
Compound 119 was prepared by Seebacher et al. in 2003 together with few others. Compound 119 showed moderate activity against T. cruzi [131].
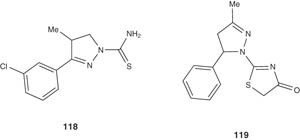 |
2.2.5.5. Antiviral activity
Some new N-acetyl and N-thiocarbamoyl derivatives of 4,5-dihydropyrazoles were synthesized by El-Sabbagh et al. and were tested against a broad panel of viruses in different cell cultures [132]. N-Acetyl-4,5-dihydropyrazole was the only active compound at sub-toxic concentrations against vaccinia virus (Lederle strain) in HEL cell cultures with an EC50 value of 7 μg/ml [132].
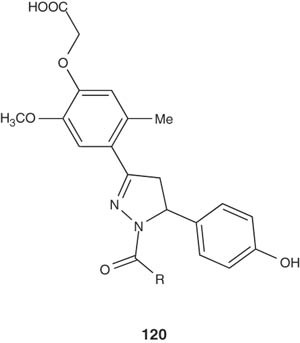 |
Yar et al. reported the synthesis of new pyrazolines derived from phenoxyacetic acid. The synthesized derivatives were tested for their in vitro cytotoxicity and antiviral activity. In addition to the antiviral activity of 2-{4-[3-(2,4-dihydroxyphenyl)-1-(2-hydroxybenzoyl-4,5-dihydro-1H-5-pyrazolyl]-2-methoxyphenoxy}acetic acid (120), it showed the maximum cytotoxicity of the series [133-135].
A series of trisubstituted pyrazolines 121a and 121b showed an inhibition for flavivirus infection in cell culture and was identified through high-throughput screening of a compound library using a luciferase-expressing West Nile virus (WNV) infection assay [136]. The aryl-rings in such pyrazolines are essential for the activity against WNV. The pyrazolines inhibitors of RNA synthesis that were investigated pointed the viral RNA polymerase, RNA helicase or other viral replication enzymes as potential targets [137].
 |
Liang et al. [138] reported the synthesis of the 3,5-diaryl-4,5-dihydropyrazole derivatives 122, which inhibits the activity of picornavirus and coronavirus simultaneously. These compounds could be used for preventing and treating diseases caused by pecorino virus and coronavirus, such as influenza, foot-and-mouth disease, epidemic keratoconjunctivitis, aseptic meningitis, myocarditis, hepatitis A and severe acute respiratory syndrome (SARS).
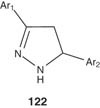 |
2.2.6. Insecticidal activity
Silver and Soderlund [139] synthesized some insecticides that are related to the pyrazolines 123a and 123 b and examined their mechanism of action based on electrophysiological, pharmacological and toxicological information. Eventually, it was found that these compounds exert their insecticidal activity via neuronal targets [139].
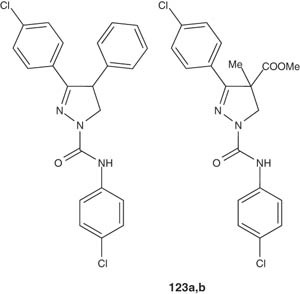 |
2.2.7. Hypotensive activity
Turan-Zitouni et al. [140] synthesized several 1-(4-arylthiazol-2-yl)-3,5-diaryl-2-pyrazolines 124 and studied their hypotensive activity by the tail-cuff method. All the synthesized pyrazolines showed appreciable hypotensive activities comparable with clonidine as a reference drug.
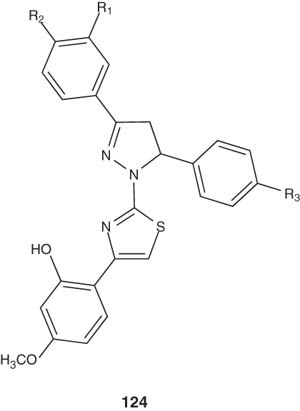 |
2.2.8. Cholesterol metabolism modulators (ACAT inhibition activity)
A series of 3-(3,5-dialkyl-4-hydroxyphenyl)-5-(multi-substituted-4-hydroxyphenyl)-2-pyrazolines were prepared and evaluated their inhibitory action on acyl-coenzyme A cholesterol acyltransferase (ACAT), which is responsible for formation of cholesterol precursor acetoacetyl CoA in the mevalonate pathway [141]. The pyrazoline 125 as an example of this series showed in vitro inhibitory activity on hACAT-1 and -2 [142].
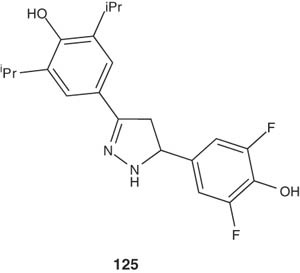 |
Substituted pyrazoline derivatives of the general formula 126 with ACAT inhibition activity were prepared and their pharmaceutical compositions and uses in the treatment of dyslipidemia have been reported by Yenes-Minguez and Torrens-Jover [134,143].
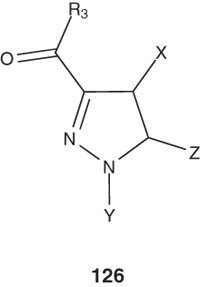 |
2.2.9. Nitric oxide synthase inhibitors
Camacho et al. synthesized a new series of neural nitric oxide synthase (nNOS) inhibitors with 4,5-dihydro-1H-pyrazole structure 127 in an attempt to find new compounds with neuroprotective activity. The pyrazolines 128 and 129 showed the highest activities with inhibition percentages of 70 and 62%, respectively [144].
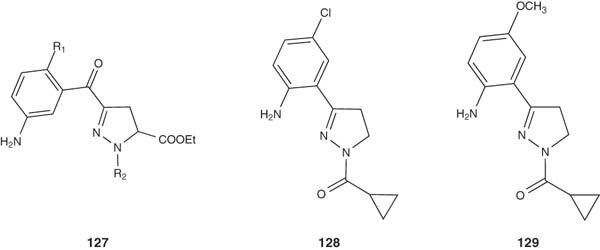 |
Also, Carrión et al. synthesized and evaluated a series of 1-alkyl-3-benzoyl-4,5-dihydro-1H-pyrazole derivatives 130 and 1-alkyl-3-benzoyl-1H-pyrazoles 131 as potential inhibitors of both neuronal and inducible nitric oxide synthases (nNOS and iNOS) [145].
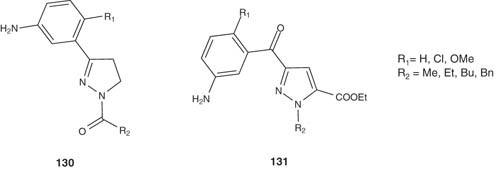 |
2.2.10. Antioxidant activity
A novel series of pyrazoline derivatives have been synthesized by Babu et al. [146] and evaluated for their antioxidant activity at various concentrations against standard ascorbic acid. The pyrazoline 132 among the series of the synthesized compounds showed excellent antioxidant activity as compared with ascorbic acid.
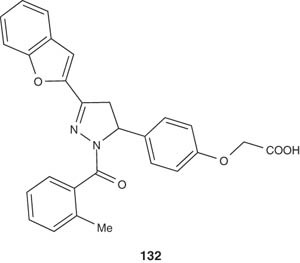 |
2.2.11. Steroidal hormones modulators
Zhang et al. designed several pyrazolines of the general formula 133 and evaluated them by in vivo screening as tissue selective androgen receptor modulators (SARMs). SARs were investigated at the R1 to R6 positions as well as the core pyrazoline ring and the anilide linker. It was found that, strong electron-withdrawing groups at the R1 and R2 positions and small groups at the R5 and R6 positions were optimal for androgen receptor agonist activity [147].
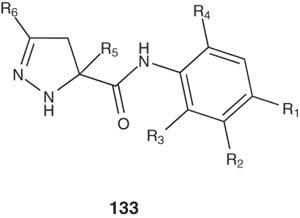 |
Jones et al. achieved the synthesis of 4-substituted pyrazoline derivatives 134 and studied their docking into a protein receptor homology model. The results of the study revealed that the synthesized compounds exhibited functional antagonism of protein receptor [148].
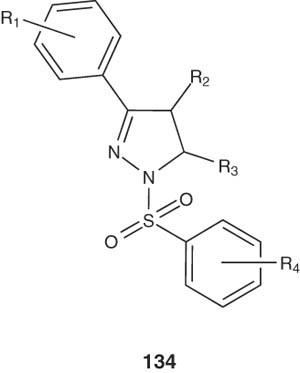 |
A series of interesting androstano[17,16-c]pyrazolines and their oxidized derivatives 135 have been synthesized and evaluated for their anti-androgenic activity compared with that of cyproterone as a positive control. Some of these compounds showed better anti-androgenic activity than the reference drug [149].
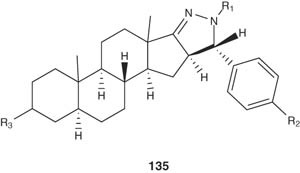 |
2.2.12. Antidiabetic agents
Fisas-Escasany et al. [150,151] achieved preparation of substituted pyrazolines 136 in order to be used in combination therapy with other antidiabetic agents.
 |
2.2.13. Urotensin II and somatostatin-5 receptors modulators
The human urotensin II receptor (h-UTR) is a member of the family of rhodopsin-like G-protein-coupled receptors (GPCRs) involved in the modulation of the functionality of many tissues and organs. A combinatorial scaffold approach has been reported by Olsson [152] to build a library of compounds having four diversity points. The synthesized compounds provide the mapping of urotensin II and somatostatin-5 receptors by differential binding of the receptors.
3. Expert opinion
From the aforementioned examples, pyrazoline heterocyclic ring was mainly used as a structural core for building huge variety of biologically active compounds. The versatile pyrazoline structure with semi-saturated status provides a unique spatial configuration which allows various substitution patterns. Figure 3 shows eight possible patterns of only two substitutions. One can imagine the diversity in cases of three or more substituents.
Figure 3.
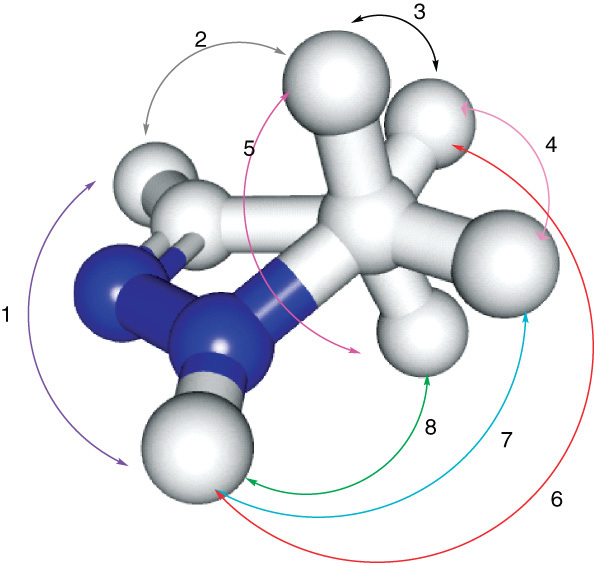
Possible patterns of two substituents in pyrazoline ring.
Among the reported activities, there are some important notes; pyrazoline-containing cytotoxic compounds are not only useful in treatment of various cancer types, but also some of them act as cancer chemopreventive agents. Concerning the reported antimycobaterium activity of pyrazoline-containing compounds, all reports have been assayed utilizing the vaccine-strain H37Rv and not the infectious strains, which make the biological impacts doubtful. Evaluating those compounds versus multidrug-resistant (MR-TB) or extreme drug-resistant (XDR-TB) mycobacterium strains will add huge value to the present work.
The reported articles are hampered by lacking molecular target identification. Most of the reported biological activities for pyrazoline-containing compounds were based on cell-based assays, and the authors have not reported anything about the possible molecular target. So far, these kinds of drug development protocols do not allow conducting structure-based drug design approaches. From our point of view, more attention has to be given for the molecular basis of mode of actions. Hence, rational drug design methods could be applied and more drug-like candidates will likely be obtained.
Article highlights.
Pyrazoline heterocycle provides a structural core for building huge variety of biologically active compounds.
The pharmacological activity of pyrazoline-based compounds extends from central nervous system (CNS) applications to antimicrobials.
The most predominant pharmacological activity was observed for the class of ‘antimicrobials'.
Cannabinoid receptor type 1 (CB1) pyrazoline modulators are very useful in treatment of obesity and schizophrenia.
Pyrazoline-containing cytotoxic compounds are not only useful in treatment of cancer, but also some of them act as cancer chemopreventive agents.
There are many reports centered on the antimycobaterium activity of pyrazoline-containing compounds, most of them have been assayed versus the vaccine-strain H37Rv.
This box summarizes key points contained in the article.
Declaration on interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.ElShora A. Crystal and molecular structure of 3-hydrazino-1-hydrazinothiocarbonyl pyrazoline (TNT3). Egypt J Sol 2000;23:251-4 [Google Scholar]
- 2.Li JT, Zhang XH, Lin ZP. An improved synthesis of 1,3,5-triaryl-2-pyrazolines in acetic acid aqueous solution under ultrasound irradiation. Beilstein J Org Chem 2007;3:(doi:10.1186/1860-5397-3-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sobhi HR, Yamini Y, Esrafili A, Adib M. Extraction and determination of 2-pyrazoline derivatives using liquid phase microextraction based on solidification of floating organic drop. J Pharm Biomed 2008;48:1059-63 [DOI] [PubMed] [Google Scholar]
- 4.Eisinger J, Boens N, Flores J. Fluorescence polarization study of human-erythrocyte membranes with 1-Phenyl-3-(2-Naphthyl)-2-Pyrazoline as orientational probe. Biochim Biophys Acta 1981;646:334-43 [DOI] [PubMed] [Google Scholar]
- 5.Li JF, Guan B, Li DX, Dong C. Study on the fluorescence properties of a new intramolecular charge transfer compound 1,5-diphenyl-3-(N-ethylcarbazole-3-yl)-2-pyrazoline. Spectrochim Acta A 2007;68:404-8 [DOI] [PubMed] [Google Scholar]
- 6.oFischer E, Kn evenagel O. Compounds of phenylhydrazine with acrolein, mesityloxide, and allylbromide. Justus Liebigs Ann Chem 1887;239:194-206 [Google Scholar]
- 7.Levai A. Synthesis of pyrazolines by the reactions of alpha,beta-enones with diazomethane and hydrazines (review). Chem Heterocycl Compd 1997;33:647-59 [Google Scholar]; •• This review, along with [8] describes the general methods for preparation of 2-pyrazolines.
- 8.Leavai A. Synthesis of 2-pyrazolines by the reactions of alpha,beta-unsaturated aldehydes, ketones, and esters with diazoalkanes, nitrile imines, and hydrazines. J Heterocyclic Chem 2002;39:1-13 [Google Scholar]
- 9.Farag AM, Abbas IM, Abdallah MA,. Regioselective synthesis of novel 1-Methyl-2-(4-Aryl-5-Cyano-1,3-Diphenyl-4,5-Dihydropyrazol-5-Yl)benzimidazoles and 2-(4-Aryl-5-Cyano-1,3-Diphenyl-4,5-Dihydropyrazol-5-yl)benzothiazoles via benzonitrilium N-Phenylimide. J Chem Res S 1994;286-7 [Google Scholar]
- 10.Farag AM, Kheder NA, Budesinsky M. Regioselective synthesis of polysubstituted 3,3'-bi-1H-pyrazole derivatives via 1,3-dipolar cycloaddition reactions. Tetrahedron 1997;53:9293-300 [Google Scholar]; •• This paper, along with [11] describes a convenient method for the synthesis of bi(2-pyrazolines).
- 11.Farag AM, Shawali AS, Abed NM, Dawood KM. 1,3-Dipolar cycloaddition syntheses of 3,3'-Bi(2-Pyrazolines), 3,3'-Bipyrazoles and 3,3'-Bi(1,2,4-Triazoles). Gazz Chim Ital 1993;123:467-70 [Google Scholar]
- 12.Hassaneen HM, Algharib MS, Farag AM, Shawali AS. Direct synthesis of pyrazoles via reaction of C-ethoxycarbonyl-N-arylnitrilimines with benzalmalononitriles and some alpha-cyanocinnamic acid-derivatives. J Prakt Chem 1988;330:558-62 [Google Scholar]
- 13.Hassaneen HM, Farag AM, Algharib MS,. Selectivity in cyclo-additions of nitrilimines to geminal dinitriles and alpha-beta-disubstituted acrylonitriles. Gazz Chim Ital 1988;118:569-72 [Google Scholar]
- 14.Hassaneen HM, Farag AM, Shawali AS, Algharib MS. Regioselectivity in dipolar cycloaddition reactions of N-Phenylcinnamonitrilimine. J Heterocyclic Chem 1987;24:577-80 [Google Scholar]
- 15.Shawali AS, Farag AM, Algharib MS, Albar HA. 1,3-Dipolar cycloaddition reactions of benzonitrilium N-Phenylimide with 3-Arylmethylene-5-Phenylfuran-2(3H)-ones. J Chem Res S 1993;80-1 [Google Scholar]
- 16.Kumar S, Bawa S, Drabu S,. Biological activities of pyrazoline derivatives: a recent development. Recent Patents Anti Infect Drug Discov 2009;4:154-63 [DOI] [PubMed] [Google Scholar]; •• This recent review, along with [17] focuses on the research and development of biologically active pyrazoline derivatives as therapeutic agents.
- 17.Rahman MA, Siddiqui AA. Pyrazoline derivatives: a worthy insight into the recent advances and potential pharmacological activities. Int J Pharm Sci Drug Res 2010;2:165-75 [Google Scholar]
- 18.Marti BS, Ramon BM, Jordi LT. One-pot preparation of cinchonidine salt of (4S,5S)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-4,5-dihydro-1Hpyrazole-3-carboxylic acid. Eur Pat Appl. EP2314578A120110427; 2011
- 19.Yoo JU, Lee DW, Choi JG,. Preparation of 3,4-diphenyl-4,5-dihydro-pyrazole-1-carboxylic acid hydrazide compounds as cannabinoid 1 (CB1) modulators. Repub Korean Kongkae Taeho Kongbo. KR2010095277A20100830; 2010
- 20.Lambert W, Crouse G, Sparks T, Cudworth D. Pesticidal compositions of prepared heteroaryl-N-aryl carbamates and their use in pest control. Eur Pat Appl. EP2151234A120100210; 2010
- 21.Soler Ranzani L, Casadevall Pujals G, Santanach Delisau A. Preparation of the cannabinoid CB1 receptor ligand 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-N-(cis-2,6-dimethylpiperidin-1-yl)-4-methyl-4,5-dihydro-1H-pyrazole-3-carboxamide and pharmaceutical formulations thereof in solid solutions and/or solid dispersions. PCT Int Appl. WO2010012437A120100204; 2010
- 22.Mcelroy JF, Chorvat RJ. Substituted N-phenyl-5-phenyl-pyrazolin-3-yl amides as cannabinoid receptor antagonists/inverse agonists useful for treating obesity. US20090286758A120091119; 2009
- 23.Lange JHM, van Stuivenberg HH, Veerman W,. Novel 3,4-diarylpyrazolines as potent cannabinoid CB1 receptor antagonists with lower lipophilicity. Bioorg Med Chem Lett 2005;15:4794-8 [DOI] [PubMed] [Google Scholar]
- 24.Buschmann HH, Torrens-Jover A, Mas-Prio J, Yenes-Minguez S. Preparation of sulfonamide substituted pyrazoline compounds as CB1 modulators. PCT Int Appl. WO2008043544A120080417; 2008
- 25.Srivastava BK, Joharapurkar A, Raval S,. Diaryl dihydropyrazole-3-carboxamides with significant in vivo antiobesity activity related to CBI receptor antagonism: synthesis, biological evaluation, and molecular modeling in the homology model. J Med Chem 2007;50:5951-66 [DOI] [PubMed] [Google Scholar]
- 26.Donohue SR, Pike VW, Finnema SJ,. Discovery and labeling of high-affinity 3,4-diarylpyrazolines as candidate radioligands for in vivo imaging of cannabinoid subtype-1 (CB(1)) receptors. J Med Chem 2008;51:5608-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisas-Escasany MA, Buschmann HH. Preparation of substituted pyrazoline for preventing weight gain. Eur Pat Appl. EP1946777A120080723; 2008
- 28.Vela Hernandez JM, Yenes-Minguez S. Preparation of (4S,5S)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-N-(cis-2,6-dimethylpiperidin-1-yl)-4-methyl-4,5-dihydro-1H-pyrazole-3-carboxamide as a cannabinoid CB1 neutral antagonist for treatment of food intake disorders. PCT Int Appl. WO2009124950A220091015; 2009
- 29.Vela Hernandez JM, Yenes-Minguez S. Preparation of (4S,5S)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-N-(cis-2,6-dimethylpiperidin-1-yl)-4-methyl-4,5-dihydro-1H-pyrazole-3-carboxamide as a cannabinoid CB1 neutral antagonist for treatment of food intake disorders. Eur Pat Appl. EP2108643A120091014; 2009
- 30.Lange JHM, Zilaout H, Van Vliet BJ. Preparation of 5-aryl-4,5-dihydro-(1H)-pyrazoles as cannabinoid CB1 receptor agonists. PCT Int Appl. WO2009037244A220090326; 2009
- 31.Yildirim M, Wals HC, Van Vliet BJ, Lange JHM. Preparation of 4,5-dihydro-(1H)-pyrazole derivatives as cannabinoid CB1 receptor modulators. PCT Int Appl. WO2008152086A220081218; 2008
- 32.Torrens-Jover A, Yenes-Minguez S. Preparation of indoline-substituted pyrazoline derivatives as cannabinoid receptor modulators. PCT Int Appl. WO2007009721A220070125; 2007
- 33.Torrens-Jover A, Yenes-Minguez S. Preparation of azepane- or azocane-substituted pyrazoline derivatives as cannabinoid receptor modulators. PCT Int Appl. WO2007009723A2 20070125; 2007
- 34.Buschmann H, Torrens-Jover A, Mas Prio J,. Preparation of 4,5-dihydro-1H-pyrazole derivatives as cannabinoid receptor inhibitors for treating various diseases. PCT Int Appl. WO2007009689A120070125; 2007
- 35.Torrens-Jover A, Mas Prio J, Buschmann HH,. 4,5-Dihydro-1H-pyrazole derivatives, their preparation and use as medicaments. Eur Pat Appl. EP1743890A120070117; 2007
- 36.Torrens-Jover A, Yenes-Minguez S. Preparation of octahydropentalene-substituted pyrazoline derivatives as cannabinoid receptor modulators. PCT Int Appl. WO2007009722A120070125; 2007
- 37.Torrens-Jover A, Yenes-Minguez S. Preparation of cycloalkane-substituted pyrazoline derivatives as cannabinoid receptor modulators. PCT Int Appl. WO2007009724A220070125; 2007
- 38.Torrens-Jover A, Yenes-Minguez S. Prodrugs of pyrazoline compounds as CB1-receptor antagonists, their preparation and use as medicaments. PCT Int Appl. WO2007009720A220070125; 2007
- 39.Yenes-Minguez S, Torrens-Jover A. Preparation and anti-tumor analysis of 4-substituted 1H-pyrazolines for the treatment or prophylaxis of human cancers. Span ES2341522A120100621; 2010
- 40.Havrylyuk D, Zimenkovsky B, Vasylenko O,. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur J Med Chem 2009;44:1396-404 [DOI] [PubMed] [Google Scholar]
- 41.Bhat BA, Dhar KL, Puri SC,. Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic agents. Bioorg Med Chem Lett 2005;15:3177-80 [DOI] [PubMed] [Google Scholar]
- 42.Shaharyar M, Abdullah MM, Bakht MA, Majeed J. Pyrazoline bearing benzimidazoles: search for anticancer agent. Eur J Med Chem 2010;45:114-19 [DOI] [PubMed] [Google Scholar]
- 43.Manna F, Chimenti F, Bolasco A,. Inhibition of amine oxidases activity by 1-acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives. Bioorg Med Chem Lett 2002;12:3629-33 [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi H, Dorai T, Holland JF, Ohnuma T. Reversal of drug-sensitivity in multidrug-resistant tumor-cells by an MDR1 (Pgy1) Ribozyme. Cancer Res 1994;54:1271-5 [PubMed] [Google Scholar]
- 45.Johnson M, Younglove B, Lee L,. Design, synthesis, and biological testing of pyrazoline derivatives of combretastatin-A4. Bioorg Med Chem Lett 2007;17:5897-901 [DOI] [PubMed] [Google Scholar]
- 46.Roecker AJ, Coleman PJ, Mercer SP,. Kinesin spindle protein (KSP) inhibitors. Part 8: design and synthesis of 1,4-diaryl-4,5-dihydropyrazoles as potent inhibitors of the mitotic kinesin KSP. Bioorg Med Chem Lett 2007;17:5677-82 [DOI] [PubMed] [Google Scholar]
- 47.Rostom SAF. Synthesis and in vitro antitumor evaluation of some indeno[1,2-c]-pyrazol(in)es substituted with sulfonamide, sulfonylurea(-thiourea) pharmacophores, and some derived thiazole ring systems. Bioorg Med Chem 2006;14:6475-85 [DOI] [PubMed] [Google Scholar]
- 48.Coleman PJ, Mercer SP, Roecker AJ. Preparation of pyrazole derivatives as mitotic kinesin inhibitors. PCT Int Appl. WO2006068933A220060629; 2006
- 49.Breslin MJ, Coleman PJ, Cox CD,. 4,5-Dihydro-1H-pyrazole derivatives useful as mitotic kinesin inhibitors, and their pharmaceutical compositions and use in the treatment of cancer. PCT Int Appl. WO2003079973A220031002; 2003
- 50.Villavicencio EH, Yoon JW, Frank DJ,. Cooperative E-box regulation of human GLI1 by TWIST and USF. Genesis 2002;32:247-58 [DOI] [PubMed] [Google Scholar]
- 51.Mazumdar T, DeVecchio J, Agyeman A,. Blocking hedgehog survival signaling at the level of the GLI genes induces DNA damage and extensive cell death in human colon carcinoma cells. Cancer Res 2011;71:5904-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He B, Fujii N, You L,. Dihydropyrazolecarboxamides and their preparation, pharmaceutical compositions and use in the targeting GLI proteins in human cancer by small molecules. US7714014B220100511; 2010
- 53.He B, Fujii N, You L,. Dihydropyrazolecarboxamides and their preparation, pharmaceutical compositions and use in the targeting GLI proteins in human cancer by small molecules. Int Appl. WO2007067814A220070614; 2007
- 54.Havrylyuk D, Kovach N, Zimenkovsky B,. Synthesis and anticancer activity of isatin-based pyrazolines and thiazolidines conjugates. Arch Pharm (Weinheim) 2011;344:514-22 [DOI] [PubMed] [Google Scholar]
- 55.Buske C, Becker D, FeuringBuske M,. TGF-beta inhibits growth and induces apoptosis in leukemic B cell precursors. Leukemia 1997;11:386-92 [DOI] [PubMed] [Google Scholar]
- 56.Jang CW, Chen CH, Chen CC,. TGF-beta induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol 2002;4:51-8 [DOI] [PubMed] [Google Scholar]
- 57.Schlapbach R, Spanaus KS, Malipiero U,. TGF-beta induces the expression of the FLICE-inhibitory protein and inhibits Fas-mediated apoptosis of microglia. Eur J Immunol 2000;30:3680-8 [DOI] [PubMed] [Google Scholar]
- 58.Totth A, Sebestyen A, Barna G,. TGF-beta 1 induces caspase-dependent but death-receptor independent apoptosis in lymphoid cells. Anticancer Res 2001;21:1207-12 [PubMed] [Google Scholar]
- 59.Sawyer JS, Beight DW, Ciapetti P,. Preparation of substituted 5,6-dihydro-4H-pyrrolo[1,2-b]pyrazoles as TGF-beta signal transduction inhibitors. PCT Int Appl. WO2002094833A120021128; 2002
- 60.Ozdemir Z, Kandilci HB, Gumusel B,. Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur J Med Chem 2007;42:373-9 [DOI] [PubMed] [Google Scholar]
- 61.Singh SP, Chaudhari A, Barthwal JP, Parmar SS. Anticonvulsant activity and selective-inhibition of nicotinamide adenine dinucleotide-dependent oxidations by 1,3,5-Trisubstituted Pyrazolines. J Pharm Sci 1974;63:1948-50 [DOI] [PubMed] [Google Scholar]
- 62.Shishikura J-I, Inami H, Kaku H,. Preparation of acylazole derivatives as kainic acid neurocytotoxicity inhibitors. PCT Int Appl. WO2001032173A120010510; 2001
- 63.Torrens-Jover A, Mas Prio J. Quaternary ammonium salts of substituted pyrazoline compounds, their preparation and use as medicaments. PCT Int Appl. WO2007009697A120070125; 2007
- 64.Palaska E, Aytemir M, Uzbay IT, Erol D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur J Med Chem 2001;36:539-43 [DOI] [PubMed] [Google Scholar]
- 65.Prasad YR, Rao AL, Prasoona L,. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2''-hydroxynaphthalen-1''-yl)-1,5-diphenyl-2-pyrazolines. Bioorg Med Chem Lett 2005;15:5030-4 [DOI] [PubMed] [Google Scholar]
- 66.Chimenti F, Fioravanti R, Bolasco A,. Synthesis, molecular modeling studies and selective inhibitory activity against MAO of N1-propanoyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem 2008;43:2262-7 [DOI] [PubMed] [Google Scholar]
- 67.Henkel V, Mergl R, Hegerl U. Atypical depression: is there still evidence for superiority of MAO-inhibitors'? Eur Psychiatry 2005;20:S134 [Google Scholar]
- 68.Enserink M. Drug therapies for depression: from MAO inhibitors to substance P. Science 1999;284:238-4010232967 [Google Scholar]
- 69.Murasaki M. The role of MAO inhibitors on the treatment of depression. Jpn J Neuropsychopharmacol 1997;19:685-700 [Google Scholar]
- 70.Amrein R, Uchida C, Stabl M. Efficacy of reversible inhibitors of MAO-A (Rima) in various forms of depression. Int Congr Ser 1991;968:850-4 [Google Scholar]
- 71.Larsen JK. The concept of atypical depression and its relation to successful treatments with MAO-Inhibitors. Psychopharmacology (Berl) 1991;103:B6-B [Google Scholar]
- 72.Nolen WA, Vandeputte JJ, Dijken WA,. Treatment strategy in depression. 2. MAO inhibitors in depression resistant to cyclic antidepressants - 2 controlled crossover studies with tranylcypromine versus L-5-Hydroxytryptophan and Nomifensine. Acta Psychiatr Scand 1988;78:676-83 [DOI] [PubMed] [Google Scholar]
- 73.Haffmans J, Nolen WA, Vanderham E. MAO-inhibitors and sleep in major depression. Pharmacopsychiatry 1988;21:61 [Google Scholar]
- 74.Davis JM, Janicak PG, Bruninga K. The efficacy of MAO inhibitors in depression – a metaanalysis. Psychiatr Annu 1987;17:825-31 [Google Scholar]
- 75.Raskin DE. MAO inhibitors in chronic pain and depression. J Clin Psychiatr 1982;43:122. [PubMed] [Google Scholar]
- 76.Abdala EN. Combination of tricyclic anti-depressants and MAO inhibitors in treatment of depression. Acta Psiquiat Psicol 1975;21:52-5 [PubMed] [Google Scholar]
- 77.Purgyi P. Correlation between glucose-tolerance and depression and its treatment with Mao inhibitors and lithium salts. Nervenarzt 1972;43:379-82 [PubMed] [Google Scholar]
- 78.Gokhan-Kelekci N, Koyunoglu S, Yabanoglu S,. New pyrazoline bearing 4(3H)-quinazolinone inhibitors of monoamine oxidase: synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. Bioorg Med Chem 2009;17:675-89 [DOI] [PubMed] [Google Scholar]
- 79.Gokhan-Kelekci N, Yabanoglu S, Kupeli E,. A new therapeutic approach in Alzheimer disease: some novel pyrazole derivatives as dual MAO-B inhibitors and antiinflammatory analgesics. Bioorg Med Chem 2007;15:5775-86 [DOI] [PubMed] [Google Scholar]
- 80.Lensch K, Fuchs G, Boning J, Milech U. A clinical-study of the selective MAO-A inhibitor Moclobemide (RO11-1163) – a comparison of 2 different dosages with particular reference to platelet Mao-activity and urinary Mhpg-excretion. Int Clin Psychopharm 1987;2:165-71 [DOI] [PubMed] [Google Scholar]
- 81.Kaplancikli ZA, Ozdemir A, Turan-Zitouni G,. New pyrazoline derivatives and their antidepressant activity. Eur J Med Chem 2010;45:4383-7 [DOI] [PubMed] [Google Scholar]
- 82.Jayaprakash V, Sinha BN, Ucar G, Ercan A. Pyrazoline-based mycobactin analogues as MAO-inhibitors. Bioorg Med Chem Lett 2008;18:6362-8 [DOI] [PubMed] [Google Scholar]
- 83.Chimenti F, Bizzarri B, Manna F,. Synthesis and in vitro selective anti-Helicobacter pylori activity of pyrazoline derivatives. Bioorg Med Chem Lett 2005;15:603-7 [DOI] [PubMed] [Google Scholar]
- 84.Chimenti F, Carradori S, Secci D,. Synthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem 2010;45:800-4 [DOI] [PubMed] [Google Scholar]
- 85.Barsoum FF, Girgis AS. Facile synthesis of bis(4,5-dihydro-1H-pyrazole-1-carboxamides) and their thio-analogues of potential PGE(2) inhibitory properties. Eur J Med Chem 2009;44:2172-7 [DOI] [PubMed] [Google Scholar]
- 86.Coceani F, Bishai I, Hynes N,. Prostaglandin-E2 as a central messenger of fever - mechanism of formation. News Trend L 1989;3:183-6 [Google Scholar]
- 87.Coceani F, Bishai I, Lees J, Sirko S. Prostaglandin-E2 in the pathogenesis of Pyrogen fever - validation of an intermediary role. Adv Prostaglandins Thromb L 1989;19:394-7 [PubMed] [Google Scholar]
- 88.Rotondo D, Abul HT, Milton AS, Davidson J. Pyrogenic immunomodulators increase the level of prostaglandin-E2 in the blood simultaneously with the onset of fever. Eur J Pharmacol 1988;154:145-52 [DOI] [PubMed] [Google Scholar]
- 89.Barsoum FF, Hosni HM, Girgis AS. Novel bis(1-acyl-2-pyrazolines) of potential anti-inflammatory and molluscicidal properties. Bioorg Med Chem 2006;14:3929-37 [DOI] [PubMed] [Google Scholar]
- 90.Amir M, Kumar H, Khan SA. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg Med Chem Lett 2008;18:918-22 [DOI] [PubMed] [Google Scholar]
- 91.Rathish IG, Javed K, Ahmad S,. Synthesis and antiinflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg Med Chem Lett 2009;19:255-8 [DOI] [PubMed] [Google Scholar]
- 92.Rani P, Srivastava VK, Kumar A. Synthesis and antiinflammatory activity of heterocyclic indole derivatives. Eur J Med Chem 2004;39:449-52 [DOI] [PubMed] [Google Scholar]
- 93.Girisha KS, Kalluraya B, Narayana V, Padmashree P.. Synthesis and pharmacological study of 1-acetyl/propyl-3-aryl-5-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)-2-pyrazoline. Eur J Med Chem 2010;45:4640-4 [DOI] [PubMed] [Google Scholar]
- 94.Reddy MVR, Billa VK, Pallela VR,. Design, synthesis, and biological evaluation of 1-(4-sulfamylphenyl) 3-trifluoromethyl-5-indolyl pyrazolines as cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitor. Bioorg Med Chem 2008;16:3907-16 [DOI] [PubMed] [Google Scholar]; •• This paper describes the integrated effect in trifluoromethylpyrazoline derivatives and their evaluation for COX-2 and LOX inhibitory activities.
- 95.Khode S, Maddi V, Aragade P,. Synthesis and pharmacological evaluation of a novel series of 5-(substituted) aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur J Med Chem 2009;44:1682-8 [DOI] [PubMed] [Google Scholar]
- 96.Shoman ME, Abdel-Aziz M, Aly OM,. Synthesis and investigation of anti-inflammatory activity and gastric ulcerogenicity of novel nitric oxide-donating pyrazoline derivatives. Eur J Med Chem 2009;44:3068-76 [DOI] [PubMed] [Google Scholar]
- 97.Kaplancikli ZA, Turan-Zitouni G, Ozdemir A,. Synthesis and antinociceptive activities of some pyrazoline derivatives. Eur J Med Chem 2009;44:2606-10 [DOI] [PubMed] [Google Scholar]
- 98.Godoy MCM, Fighera MR, Souza FR,. alpha(2)-adrenoceptors and 5-HT receptors mediate the antinociceptive effect of new pyrazolines, but not of dipyrone. Eur J Pharmacol 2004;496:93-7 [DOI] [PubMed] [Google Scholar]
- 99.Ozdemir A, Turan-Zitouni G, Kaplancikli ZA,. Synthesis and antimicrobial activity of 1-(4-aryl-2-thiazolyl)-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives. Eur J Med Chem 2007;42:403-9 [DOI] [PubMed] [Google Scholar]
- 100.Abdel-Wahab BF, Abdel-Aziz HA, Ahmed EM. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur J Med Chem 2009;44:2632-5 [DOI] [PubMed] [Google Scholar]
- 101.Abunada NM, Hassaneen HM, Kandile NG, Miqdad OA. Synthesis and biological activity of some new pyrazoline and pyrrolo[3,4-c]pyrazole-4,6-dione derivatives: Reaction of nitrilimines with some dipolarophiles. Molecules 2008;13:1011-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhatt AH, Parekh HH, Parikh KA, Parikh AR. Synthesis of pyrazolines and cyanopyridines as potential antimicrobial agents. Indian J Chem B 2001;40:57-61 [Google Scholar]
- 103.Udupi RH, Kushnoor AS, Bhat AR. Synthesis and biological evaluation of certain pyrazoline derivatives of 2-[6-methoxy naphthyl]-propionic acid (Naproxen). Indian J Heterocyclic Chem 1998;8:63-6 [Google Scholar]
- 104.Bharmal FM, Kaneriya DJ, Parekh HH. Synthesis of some pyrazoline derivatives as biologically active agents. Indian J Heterocyclic Chem 2001;10:189-92 [Google Scholar]
- 105.Basawaraj R, Yadav B, Sangapure SS. Synthesis of some 1H-pyrazolines bearing benzofuran as biologically active agents. Indian J Heterocyclic Chem 2001;11:31-4 [Google Scholar]
- 106.Desai J, Nair KB, Misra AN. Synthesis and antimicrobial activities of some new pyrazolines phenyl pyrazolines, flavanones and related compounds. Indian J Heterocyclic Chem 2001;10:261-6 [Google Scholar]
- 107.Jamode VS, Chandak HS, Bhagat PR, Tambekar DH. Synthesis and antimicrobial evaluation of some 1-isonicotinoyl/carboxamido-2-pyrazolines. Indian J Heterocyclic Chem 2003;12:323-6 [Google Scholar]
- 108.Karthikeyan MS, Holla BS, Kumari NS. Synthesis and antimicrobial studies on novel chloro-fluorine containing hydroxy pyrazolines. Eur J Med Chem 2007;42:30-6 [DOI] [PubMed] [Google Scholar]
- 109.Mogilaiah K, Sakram B. Synthesis and antibacterial activities of 1,3,4-oxadiazole and pyrazoline derivatives containing 1,8-naphthyridine moiety. Indian J Heterocyclic Chem 2004;13:289-92 [Google Scholar]
- 110.Vijayvergiya D, Kothari S, Verma BL. Synthesis and biological activity of some new 3,5-diaryl-1-phenyl/isonicotinoyl-2-pyrazolines and 3,5-diaryl-6-carbethoxy cyclohexenone derivatives. Indian J Heterocyclic Chem 2003;13:105-10 [Google Scholar]
- 111.Waheed A, Khan SA. Synthesis of certain substituted 1,2 pyrazolines from nalidixic acid as antibacterial and analgesic agents. Indian J Heterocyclic Chem 2001;11:59-62 [Google Scholar]
- 112.Stirrett KL, Ferreras JA, Jayaprakash V,. Small molecules with structural similarities to siderophores as novel antimicrobials against Mycobacterium tuberculosis and Yersinia pestis. Bioorg Med Chem Lett 2008;18:2662-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shenoy GG, Bhat AR, Bhat GV, Kotian M. Synthesis and antimicrobial activities of 1,3,5 trisubstituted 2-pyrazolines. Indian J Heterocyclic Chem 2001;10:197-200 [Google Scholar]
- 114.Shaharyar M, Siddiqui AA, Ali MA,. Synthesis and in vitro antimycobacterial activity of N-1-nicotinoyl-3-(4'-hydroxy-3'-methylphenyl)-5-[(sub)phenyl]-2-pyrazolines. Bioorg Med Chem Lett 2006;16:3947-9 [DOI] [PubMed] [Google Scholar]
- 115.Mamolo MG, Zampieri D, Falagiani V,. Synthesis and antimycobacterial activity of 5-aryl-1-isonicotinoyl-3-(pyridin-2-yl)-4,5-dihydro-1H-pyrazole derivatives. Farmaco 2001;56:593-9 [PubMed] [Google Scholar]
- 116.Ozdemir A, Turan-Zitouni G, Kaplancikli ZA. Novel analogues of 2-Pyrazoline: synthesis, characterization, and antimycobacterial evaluation. Turk J Chem 2008;32:529-38 [Google Scholar]
- 117.Guardiola-Diaz HM, Foster LA, Mushrush D, Vaz ADN. Azole-antifungal binding to a novel cytochrome P450 from Mycobacterium tuberculosis: implications for treatment of tuberculosis. Biochem Pharmacol 2001;61:1463-70 [DOI] [PubMed] [Google Scholar]
- 118.Zampieri D, Mamolo MG, Laurini E,. Antifungal and antimycobacterial activity of 1-(3,5-diaryl-4,5dihydro-1H-pyrazol-4-yl)-1H-imidazole derivatives. Bioorg Med Chem 2008;16:4516-22 [DOI] [PubMed] [Google Scholar]
- 119.Kini SG, Bhat AR, Bryant B,. Synthesis, antitubercular activity and docking study of novel cyclic azole substituted diphenyl ether derivatives. Eur J Med Chem 2009;44:492-500 [DOI] [PubMed] [Google Scholar]
- 120.Ali MA, Shaharyar M, Siddiqui AA. Synthesis, structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives. Eur J Med Chem 2007;42:268-75 [DOI] [PubMed] [Google Scholar]
- 121.Babu VH, Manna SK, Srinivasan SKK, Bhat GV. Synthesis and biological evaluation of 1,3,5-trisubstituted pyrazolines bearing benzofuran. Indian J Heterocyclic Chem 2004;13:253-6 [Google Scholar]
- 122.Hayat F, Salahuddin A, Umar S, Azam A. Synthesis, characterization, antiamoebic activity and cytotoxicity of novel series of pyrazoline derivatives bearing quinoline tail. Eur J Med Chem 2010;45:4669-75 [DOI] [PubMed] [Google Scholar]
- 123.Budakoti A, Bhat AR, Athar F, Azam A. Syntheses and evaluation of 3-(3-bromo phenyl)-5-phenyl-1-(thiazolo[4,5-b]quinoxaline-2-yl)-2-pyrazoline derivatives. Eur J Med Chem 2008;43:1749-57 [DOI] [PubMed] [Google Scholar]
- 124.Budakoti A, Abid M, Azam A. Syntheses, characterization and in vitro antiamoebic activity of new Pd(II) complexes with 1-N-substituted thiocarbamoyl-3,5-diphenyl-2-pyrazoline derivatives. Eur J Med Chem 2007;42:544-51 [DOI] [PubMed] [Google Scholar]; • This paper describes the unique Pd(II) complexes with 1-N-substituted thiocarbamoyl-3,5-diphenyl-2-pyrazoline and evaluation of their antiamoebic activity.
- 125.Husain K, Abid M, Azam A. Novel Pd(II) complexes of 1-N-substituted 3-phenyl-2-pyrazoline derivatives and evaluation of antiamoebic activity. Eur J Med Chem 2008;43:393-403 [DOI] [PubMed] [Google Scholar]
- 126.Abid M, Bhat AR, Athar F, Azam A. Synthesis, spectral studies and antiamoebic activity of new 1-N-substituted thiocarbamoyl-3-phenyl-2-pyrazolines. Eur J Med Chem 2009;44:417-25 [DOI] [PubMed] [Google Scholar]
- 127.Abid M, Azam A. Synthesis and antiamoebic activities of 1-N-substituted cyclised pyrazoline analogues of thiosemicarbazones. Bioorg Med Chem 2005;13:2213-20 [DOI] [PubMed] [Google Scholar]
- 128.Asha B, Mohammad A, Amir A. Synthesis and antiamoebic activity of new 1-N-substituted thiocarbamoyl-3,5-diphenyl-2-pyrazoline derivatives and their Pd(II) complexes. Eur J Med Chem 2006;41:63-70 [DOI] [PubMed] [Google Scholar]
- 129.Bhat AR, Athar F, Azam A. Bis-pyrazolines: synthesis, characterization and antiamoebic activity as inhibitors of growth of Entamoeba histolytica. Eur J Med Chem 2009;44:426-31 [DOI] [PubMed] [Google Scholar]
- 130.Du X, Guo C, Hansell E,. Synthesis and structure-activity relationship study of potent trypanocidal thio semicarbazone inhibitors of the trypanosomal cysteine protease cruzain. J Med Chem 2002;45:2695-707 [DOI] [PubMed] [Google Scholar]
- 131.Seebacher W, Belaj F, Saf R,. New 1,3-Thiazoles and 1,3-Thiazines from 1-Thiocarbamoylpyrazoles. Monatsh Chem 2003;134:1623-8 [Google Scholar]
- 132.El-Sabbagh OI, Baraka MM, Ibrahim SM,. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur J Med Chem 2009;44:3746-53 [DOI] [PubMed] [Google Scholar]
- 133.Torrens-Jover A. Substituted pyrazoline compounds with ACAT inhibition activity, their preparation and use as medicaments in the treatment of dyslipidemia. PCT Int Appl. WO2008087030A120080724; 2008
- 134.Yenes-Minguez S, Torrens-Jover A. Substituted pyrazoline compounds with ACAT inhibition activity and their preparation, pharmaceutical compositions and use in treatment of dyslipidemia. PCT Int Appl. WO2008087029A120080724; 2008
- 135.Yar MS, Bakht MA, Siddiqui AA,. Synthesis and evaluation of in vitro antiviral activity of novel phenoxy acetic acid derivatives. J Enzyme Inhib Med Chem 2009;24:876-82 [DOI] [PubMed] [Google Scholar]
- 136.Puig-Basagoiti F, Tilgner M, Forshey BM,. Triaryl pyrazoline compound inhibits flavivirus RNA replication. Antimicrob Agents Chemother 2006;50:1320-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goodell JR, Puig-Basagoiti F, Forshey BM,. Identification of compounds with anti-West Nile Virus activity. J Med Chem 2006;49:2127-37 [DOI] [PubMed] [Google Scholar]
- 138.Liang P-H, Ku C-C, Liu H-K,. Pyrazole derivatives for preventing and treating diseases caused by picornavirus and coronavirus. Repub Korean Kongkae Taeho Kongbo. KR2010066142A20100617; 2010
- 139.Silver KS, Soderlund DM. Action of pyrazoline-type insecticides at neuronal target sites. Pest Biochem Phys 2005;81:136-43 [Google Scholar]
- 140.Turan-Zitouni G, Chevallet P, Kilic FS, Erol K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur J Med Chem 2000;35:635-41 [DOI] [PubMed] [Google Scholar]
- 141.Li L, Pownall HJ. Regulation of acyl-coenzyme A: cholesterol acyltransferase (ACAT) synthesis, degradation, and translocation by high-density lipoprotein(2) at a low concentration. Arterioscler Thromb Vasc 2000;20:2636-42 [DOI] [PubMed] [Google Scholar]
- 142.Jeong TS, Kim KS, An SJ,. Novel 3,5-diaryl pyrazolines as human acyl-CoA: cholesterol acyltransferase inhibitors. Bioorg Med Chem Lett 2004;14:2715-17 [DOI] [PubMed] [Google Scholar]
- 143.Yenes-Minguez S, Torrens-Jover A. Substituted pyrazoline compounds with ACAT inhibition activity and their preparation, pharmaceutical compositions and use in treatment of dyslipidemia. Eur Pat Appl. EP1947087A120080723; 2008
- 144.Camacho ME, Leon J, Entrena A,. 4,5-Dihydro-1H-pyrazole derivatives with inhibitory nNOS activity in rat brain: Synthesis and structure-activity-relationships. J Med Chem 2004;47:5641-50 [DOI] [PubMed] [Google Scholar]
- 145.Carrion MD, Cara LCL, Camacho ME,. Pyrazoles and pyrazolines as neural and inducible nitric oxide synthase (nNOS and NOS) potential inhibitors (III). Eur J Med Chem 2008;43:2579-91 [DOI] [PubMed] [Google Scholar]
- 146.Babu VH, Sridevi C, Joseph A, Srinivasan K. Synthesis and biological evaluation of some novel pyrazolines. Indian J Pharm Sci 2007;69:470-3 [Google Scholar]
- 147.Zhang XQ, Li XJ, Allan GF,. Design, synthesis, and in vivo SAR of a novel series of pyrazolines as potent selective androgen receptor modulators. J Med Chem 2007;50:3857-69 [DOI] [PubMed] [Google Scholar]
- 148.Jones DG, Liang X, Stewart EL,. Discovery of non-steroidal mifepristone mimetics: pyrazoline-based PR antagonists. Bioorg Med Chem Lett 2005;15:3203-6 [DOI] [PubMed] [Google Scholar]
- 149.Amr AEE, Abdel-Latif NA, Abdalla MM. Synthesis and antiandrogenic activity of some new 3-substituted androstano[17,16-c]-5'-aryl-pyrazoline and their derivatives. Bioorg Med Chem 2006;14:373-84 [DOI] [PubMed] [Google Scholar]; • In this paper, the unique androstano[17,16-c]pyrazolines and their oxidized derivatives have been synthesized and evaluated for their anti-androgenic activity.
- 150.Fisas-Escasany MA, Buschmann HH. Preparation of substituted pyrazoline for use in combination therapy with at least one antidiabetic agent. Span. ES2328653A120091116; 2009
- 151.Fisas-Escasany MA, Buschmann HH. Preparation of substituted pyrazoline for use in combination therapy with at least one antidiabetic agent. Eur Pat Appl. EP1946778A120080723; 2008
- 152.Olsson R. A combinatorial scaffold approach towards the pharmacophores of ligands to urotensin II and somatostatin 5 receptors. PCT Int Appl. WO2004073642A220040902; 2004; •• A combinatorial scaffold approach has been reported to build a library of compounds having four diversity points. The synthesized compounds provide the mapping of urotensin II and somatostatin-5 receptors by differential binding of the receptors.


