Abstract
OBJECTIVE:
Colorectal cancer (CRC) is the third most common type of cancer observed in cancer-related mortality because it has a high metastasis ratio. This study aims to investigate the expression levels of several genes, including metastasis-related colon cancer 1 (MACC1), Filamin A (FLNA), F-box/WD repeat-containing protein 7 (FBXW7), which has an important role in cell signaling, migration and adhesion through the remodeling of the cell skeleton.
METHODS:
In this study, 21 patients with a precise diagnosis of CRC and 21 controls were included. Gene expressions were examined using the RT-PCR technique. To define the relationship of the genes with metastasis, blood samples were collected from all patients with colon/rectal cancer diagnosis without metastasis at six months before and after the medication with Xelox.
RESULTS:
Our findings showed that no significant difference was observed in the pre-treatment values compared to the control group, whereas FLNA (p=0.001) expression was observed to be significantly increased following treatment with Xelox.
CONCLUSION:
To our knowledge, our study is the first study to investigate the effects of Xelox treatment on the expression levels of MACC1, FBXW7 and FLNA genes in non-metastatic colorectal cancer patients in Turkey.
Keywords: FBXW7, FLNA, MACC1
Colorectal cancer that forms an important part of the gastrointestinal system diseases originates at colon or rectum and is named according to its origin [1]. Many studies indicate a correlation between colorectal cancer and various risk factors, such as age, family history, inflammatory bowel disease, smoking, alcohol consumption, obesity and nutrition [2]. Colorectal cancer is a heterogeneous disease that has molecular pathways, including different phenotypes and develops due to the accumulation of genetic and epigenetic changes that lead to the transformation of the normal colon and rectum mucosa into invasive cancer [3–5].
MACC1 acts as a key regulator of tumor development and growth through the activation of the HGF/MET signal pathway [6–8]. It plays an important role in the epithelial-mesenchymal transition to provide cell proliferation, motility, invasiveness and diffusion. Over-expression of MACC1 mRNA is an important indicator of invasion of tumor tissue and recurrence of colon cancer independent from the American Joint Commission of Cancer (AJCC/TNM) tumor staging [9, 10].
Stein et al. reported that expression of metastasis-related colon cancer 1 (MACC1) mRNA located in chromosome 7 (7 p 21.1), which is responsible for cell migration, invasion, colony formation and proliferation, could be a prognostic indicator of disease-free survival, recurrence and metastasis in colorectal carcinoma [6, 9]. Patients with high MACC1 and low expression in the primary tumor were compared, and the 5-year-survival rate without metastasis was observed to be 80% [10]. Clinical studies suggest MACC1 as an indicator of colorectal cancer with metastasis and high potential of diffusion [11] because MACC1 was determined to be overexpressed in the metastatic type compared to primary colon cancer, and colon cancer compared to normal tissues [8, 11–13].
The F-box/WD repeat-containing protein 7 (FBXW7) gene codes a member of the F-box protein family characterized by F-box, including 40 amino acids [14, 15]. FBXW7 is argued to be a tumor suppressor gene that can be inactivated in the progression of human tumorigenesis via mutation, deletion or hypermethylation, and that leads to an increase in various oncoproteins [16, 17].
The FLNA gene may lead to two divergent outcomes of cancer, depending on its subcellular localization. Whole length FLNA in the cytoplasm is bound to actin to provide a structure for the cell skeleton and remodeling, and more importantly, acts as a framework molecule that facilitates protein interactions. FLNA, which interacts with cell signal molecules, may increase cell growth and metastasis [18, 19]. On the other hand, FLNA within the nucleus is related to transcription factors and leads to inhibition of cell growth and prevents metastasis. FLNA expression has been related to hepatic and lymph node metastasis and rectal invasion independent from gender, age, tumor location, size, or the histological type of colorectal carcinoma. Low FLNA expression is closely related to the formation and development of colorectal cancer [20].
Chemotherapy is a therapy that is performed via anticancer drugs that inhibit cell division to kill cancer cells or to block growth and diffusion of these cells. Agents used in chemotherapy and the route depending on the localization and stage of the tumor and on the conditions patients are in. Recently, the importance of pharmacogenetics has increased with the increase in the importance of personal therapy, and possible response to chemotherapeutic agents is being considered before the therapy.
In the light of these findings, in this study, we aimed to compare the expression levels of the oncogene MACC1, the tumor suppressor gene FBXW7 and the FLNA gene, which have important roles in cell signaling, migration and adhesion through remodeling of the cellular skeleton, both before and after medication with Xelox in patients with colon cancer without metastasis.
MATERIALS AND METHODS
Patient Selection
This study included 21 patients (12 male, nine female) with colon cancer without metastasis and 21 healthy individuals with no personal or family history of cancer. Written informed consent of all participants was obtained, and this study was approved by the local ethical committee. Age, gender, medical history (obesity, type 2 diabetes and others), smoking/alcohol consumption, date of colon/rectal cancer, previous medications and surgical operations and family history were recorded on admission. Patients, consents on medication with Xelox and with the same treatment protocol were also obtained.
RNA Isolation
Peripheral blood samples collected from both the patient (before therapy and six months after the therapy) and control groups into dry EDTA tubes were centrifuged at 3000 rpm for 10 minutes, and sera were obtained. RNA isolation was performed using QIAzol.
cDNA Recovery
cDNAs were recovered from the RNAs using the RT2 First Strand Kit.
Gene Expression Analysis
A PCR mix of 25 µl was prepared with 12.5 µl of RT SYBR-Green Mastermix, HMBS selected for internal control (house-keeping), 1 µl of each primer specific to all three genes (300 mM of each primer), and 10.5 µl of RNase-free water and 1 µl of cDNA (for each sample 700 ng/ml). It was incubated in the RT-PCR device 95°C for 10 minutes at (1 repeat), at 95°C for 15 seconds, and 60°C for 1 minute (44 repeats). Reading was performed at all 60°C stages. Real-time amplification and melting curve analyses were performed for all genes.
Statistical Analysis
The SPSS 21.0 (SPSS Inc.; Chicago, IL, the USA) program package was used for the statistical analysis. Significance was accepted as a p-value of <0.05. The significance of the demographic and the clinical data obtained from the participants was tested using the χ2, Fischer Exact and the Mann-Whitney U tests.
RESULTS
The demographic and clinical data have been presented in Table 1. No significant difference was observed in the mean ages between the groups (p>0.05).
TABLE 1.
Demographical and clinical parameters of study groups
| Parameters | Patients (n=21) | Control (n=21) |
|---|---|---|
| Age | 61.76±10.11 | 57.19±6.86 |
| Sex (n, F) | 9 | 9 |
| Smoking (%) | 23.8 | 19 |
| Alcohol consumption (%) | 19 | 9.5 |
| Family (%) | 33.3 | 0 |
| Differentiation (%) | Good: 62.5 Bad: 37.5 |
– |
n: Number of subjects.
Table 2 and Figure 1 present the fold changes in the expressions of the genes MACC1, FLNA and FBXW7 in patients with cancer compared to healthy controls. No significant difference was observed in the pre-treatment findings between the groups; however, a significant increase was observed in the expressions of the FLNA gene (p=0.001; 95% CI: 5.84-10.63) following the Xelox treatment.
TABLE 2.
Fold changes of MACC1, FLNA and FBXW7 genes on before and after Xelox treatment
| Fold change | p | 95% Cl | |
|---|---|---|---|
| Before treatment MACC1 | 13.07 | 0.22 | -1.43–5.92 |
| After treatment MACC1 | 11.94 | 0.36 | -1.49–3.92 |
| Before treatment FLNA | 5.13 | 0.85 | -2.32–1.92 |
| After treatment FLNA | 13.63 | 0.001* | 5.84–10.63 |
| Before treatment FBXW7 | 2.39 | 0.56 | -0.77–1.39 |
| After treatment FBXW7 | 2.46 | 0.06 | 1.31–4.83 |
MACC1: Metastasis-related colon cancer 1; FLNA: Filamin A; FBXW7: F-box/WD repeat-containing protein 7; CI: Confidence interval.
FIGURE 1.
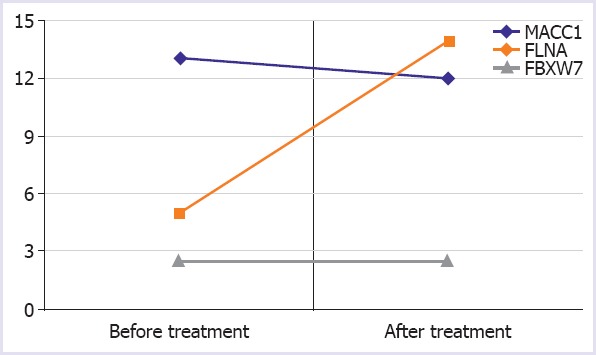
Changes of fold change values of MACC1, FLNA and FBXW7 genes on before and after Xelox treatment.
No significant difference was observed in the change between the pre- and post-treatment expressions of the genes MACC1, FLNA and FBXW7 in the study group (p>0.05) (Table 3).
TABLE 3.
Comparison of MACC1, FLNA and FBXW7 gene expressions before and after Xelox treatment in patients group
| Fold change | p | 95% Cl | |
|---|---|---|---|
| MACC1 after treatment- before treatment | -1.13 | 0.82 | 11.26–9.29 |
| FLNA after treatment- before treatment | 8.50 | 0.07 | -0.94–17.94 |
| FBXW7 after treatment- before treatment | 0.06 | 0.91 | -1.20–1.33 |
MACC1: Metastasis-related colon cancer 1; FLNA: Filamin A; FBXW7: F-box/WD repeat-containing protein 7; CI: Confidence interval.
DISCUSSION
Our study aimed to investigate the expression levels of several genes including the oncogene MACC1, which is observed in patients with colorectal cancer without metastasis, the tumor suppressor gene FBXW7, and FLNA gene, which has an important role in cell signaling, migration and adhesion through the remodeling of the cell skeleton. Xelox is a chemotherapy regimen consisting of capecitabine (Pyrimidine Analog) and oxaliplatin (a platinum alkylating agent) used for the treatment of advanced-stage colorectal cancer and is also known as CAPOX. We investigated the correlation between the expression levels of these genes and the response to treatment and metastasis. No significant difference was observed between the groups concerning the pre-treatment findings; however, a significant increase was observed in the expressions of the genes FLNA (p=0.001) following the Xelox treatment. No significant differences were detected between the pre- and post-treatment levels of MACC1, FLNA and FBXW7 expressions in the study group (p>0.05). Babaei-Jadidi et al. [21] reported that the expression level of FBXW7 gene was lower in colorectal cancer tissue compared to normal tissue and that the low expression level of FBXW7 was related to a poor prognosis. FBXW7 inhibition and drug response-related FBXW7 mutations were reported to increase resistance against some therapeutic agents, whereas there was increased susceptibility to some others. Recently, Wertz et al. demonstrated that wild-type colon cancer cells were more susceptible to the chemotherapeutic agent Taxol compared to the FBXW7- null type [22]. The relation of FLNA with the prognosis was investigated in 46 colorectal cancer and normal tissues, and the expression of FLNA in cancer tissues was found to be lower compared to that in the normal mucosa. The expression of FLNA was found to be related to hepatic metastasis, lymph node metastasis, depth of rectal invasion, gender, age, tumor localization, tumor size, gross size, histological type independent from colorectal carcinoma; and FLNA was demonstrated to be an independent risk factor for the postoperative survival of the patients with colorectal adenocarcinoma. Additionally, survival analysis demonstrated that the survival among patients with low expression of FLNA protein was shorter compared to those with high expression [23].
The increased post-treatment expression level of the FLNA gene was found to be correlated to the previously defined physiological roles of these genes in the study group compared to the control. No significant difference was observed between the pre- and post-treatment levels of gene expressions in the study group; however, the FLNA level was observed to be increased at a level close to significance following the treatment. This may be accepted as a positive outcome of the treatment for the FLNA gene low expression level, which is related to a poor prognosis. In our study, no correlation was observed between the clinical parameters and the expressions of MACC1, FBXW7 and the FLNA genes. This study also sought to investigate the effects of Xelox treatment on MACC1, FBXW7, FLNA expression levels and to investigate whether these genes have a role on the prognosis of CRC treatment. However, no differences were detected between pre- and post-treatment levels of genes in patients.
One of the limitations of this study is the relatively small size of the study population. Another limitation is that we could not use any other techniques, such as western blotting, for the confirmation of expression analysis.
To our knowledge, there is no study in the literature evaluating the expression levels of MACC1, FBXW7 and FLNA genes at the same time in patients with non-metastatic CRC, both before and after the treatment with Xelox. Therefore, we believe that the findings obtained in our study, particularly FLNA, will contribute to the knowledge in the literature on tumor progression and prognosis issues argued in the literature, in addition to their physiological roles.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Istanbul Faculty of Medicine (date: 27.06.2016, number: 855).
Conflict of Interest: Authors have no conflict of interest to declare.
Financial Disclosure: The present work was supported by the Scientific Research Fund of Istanbul University. Project No. 22887.
Authorship Contributions: Concept – AE; Design – FY; Supervision – SP; Fundings – SP; Materials – DT; Data collection and/or processing – DT; Analysis and/or interpretation – FY, HAS; Litarature review – FY; Writing – FY; Critical review – AE.
REFERENCES
- 1.Akın T. Uzmanlık Tezi. İstanbul: Dr.Lütfi Kırdar Kartal Eğitim ve Araştırma Hastanesi; 2009. Rektum ve Rektosigmoid Kanserlerin Tedavisinde Laparoskopik Cerrahinin Perioperatif ve Erken Dönem Onkolojik Sonuçlarıİle Yaşam Kalitesine Etkisi. [Google Scholar]
- 2.Haraldsdottir S, Einarsdottir HM, Smaradottir A, Gunnlaugsson A, Halfdanarson TR. Colorectal cancer - review [Article in Icelandic] Laeknabladid. 2014;100:75–82. doi: 10.17992/lbl.2014.02.531. [DOI] [PubMed] [Google Scholar]
- 3.Takayama T, Miyanishi K, Hayashi T, Sato Y, Niitsu Y. Colorectal cancer:genetics of development and metastasis. J Gastroenterol. 2006;41:185–92. doi: 10.1007/s00535-006-1801-6. [DOI] [PubMed] [Google Scholar]
- 4.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–7. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 5.Al-Sohaily S, Biankin A, Leong R, Kohonen-Corish M, Warusavitarne J. Molecular pathways in colorectal cancer. J Gastroenterol Hepatol. 2012;27:1423–31. doi: 10.1111/j.1440-1746.2012.07200.x. [DOI] [PubMed] [Google Scholar]
- 6.Stein U, Burock S, Herrmann P, Wendler I, Niederstrasser M, Wernecke KD, et al. Circulating MACC1 transcripts in colorectal cancer patient plasma predict metastasis and prognosis. PLoS One. 2012;7:e49249. doi: 10.1371/journal.pone.0049249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein U, Smith J, Walther W, Arlt F. MACC1 controls Met:what a difference an Sp1 site makes. Cell Cycle. 2009;8:2467–9. doi: 10.4161/cc.8.15.9018. [DOI] [PubMed] [Google Scholar]
- 8.Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59–67. doi: 10.1038/nm.1889. [DOI] [PubMed] [Google Scholar]
- 9.Stein U, Dahlmann M, Walther W. MACC1 - more than metastasis?Facts and predictions about a novel gene. J Mol Med (Berl) 2010;88:11–8. doi: 10.1007/s00109-009-0537-1. [DOI] [PubMed] [Google Scholar]
- 10.Schmid F, Wang Q, Huska MR, Andrade-Navarro MA, Lemm M, Fichtner I, et al. SPON2, a newly identified target gene of MACC1, drives colorectal cancer metastasis in mice and is prognostic for colorectal cancer patient survival. Oncogene. 2016;35:5942–52. doi: 10.1038/onc.2015.451. [DOI] [PubMed] [Google Scholar]
- 11.Shirahata A, Shinmura K, Kitamura Y, Sakuraba K, Yokomizo K, Goto T, et al. MACC1 as a marker for advanced colorectal carcinoma. Anticancer Res. 2010;30:2689–92. [PubMed] [Google Scholar]
- 12.Zhang Y, Wang Z, Chen M, Peng L, Wang X, Ma Q, et al. MicroRNA-143 targets MACC1 to inhibit cell invasion and migration in colorectal cancer. Mol Cancer. 2012;11:23. doi: 10.1186/1476-4598-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galimi F, Torti D, Sassi F, Isella C, Corà D, Gastaldi S, et al. Genetic and expression analysis of MET, MACC1, and HGF in metastatic colorectal cancer:response to met inhibition in patient xenografts and pathologic correlations. Clin Cancer Res. 2011;17:3146–56. doi: 10.1158/1078-0432.CCR-10-3377. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Inuzuka H, Fukushima H, Wan L, Gao D, Shaik S, et al. Emerging roles of the FBW7 tumour suppressor in stem cell differentiation. EMBO Rep. 2011;13:36–43. doi: 10.1038/embor.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Inuzuka H, Zhong J, Wan L, Fukushima H, Sarkar FH, et al. Tumor suppressor functions of FBW7 in cancer development and progression. FEBS Lett. 2012;586:1409–18. doi: 10.1016/j.febslet.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon TI, Esquejo RM, Roqueta-Rivera M, Phelan PE, Moon YA, Govindarajan SS, et al. An SREBP-responsive microRNA operon contributes to a regulatory loop for intracellular lipid homeostasis. Cell Metab. 2013;18:51–61. doi: 10.1016/j.cmet.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arabi A, Ullah K, Branca RM, Johansson J, Bandarra D, Haneklaus M, et al. Proteomic screen reveals Fbw7 as a modulator of the NF-κB pathway. Nat Commun. 2012;3:976. doi: 10.1038/ncomms1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou AX, Hartwig JH, Akyürek LM. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010;20:113–23. doi: 10.1016/j.tcb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Yue J, Huhn S, Shen Z. Complex roles of filamin-A mediated cytoskeleton network in cancer progression. Cell Biosci. 2013;3:7. doi: 10.1186/2045-3701-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Bigas MA, Stoler DL, Bertario L, Anderson GR, Baba S. Colorectal cancer:how does it start?How does it metastasize?Surg Oncol Clin N Am. 2000;9:643–52. [PubMed] [Google Scholar]
- 21.Babaei-Jadidi R, Li N, Saadeddin A, Spencer-Dene B, Jandke A, Muhammad B, et al. FBXW7 influences murine intestinal homeostasis and cancer, targeting Notch, Jun, and DEK for degradation. J Exp Med. 2011;208:295–312. doi: 10.1084/jem.20100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–4. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 23.Tian ZQ, Shi JW, Wang XR, Li Z, Wang GY. New cancer suppressor gene for colorectal adenocarcinoma:filamin A. World J Gastroenterol. 2015;21:2199–205. doi: 10.3748/wjg.v21.i7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]


