Abstract
OBJECTIVE:
There are many instruments to measure disease activity in ulcerative colitis. While determining clinical activity according to these instruments, many clinical and laboratory parameters are needed to be followed. Determination of disease activity with non-invasive and objective inflammatory indicators may be a practical and objective way. CRP/Albumin ratio (CAR) is an inflammatory marker that is considered to have prognostic value in various cancers, sepsis and acute pancreatitis. In this study, we aim to investigate diagnostic performance CAR in determining the clinical severity of ulcerative colitis.
METHODS:
Between November 2011 and February 2017, hospital records and follow-up cards of patients with ulcerative colitis were reviewed retrospectively. One hundred forty-nine patients were included in this study. Patient’s demographic data, laboratory values, clinical disease activity, according to Truelove & Witts criteria and endoscopic activity according to the Mayo sub-score and treatments, were recorded. Diagnostic performance of CAR analyzed to determine the clinical severity.
RESULTS:
Of the patients included in this study, 99 (62%) were male, and 50 (38%) were female. Mean age was 45.22±14 years. When patients were grouped into remission, mild, moderate and severe disease according to disease activity, there was a statistically significant difference between CRP, CAR, erythrocyte sedimentation rate (ESR) and albumin levels (p=0.001; p<0.05). Area under the curve (AUC) values for the diagnosis of severe disease were 0.941, 0.931, 0.888 and 0.883 for CAR, CRP, ESR and albumin levels, respectively. Cut-off value to determine severe disease for CAR was 0.6 (sensitivity: 88.9%, specificity of 90.3%, positive predictive value (PPV) 85.1%, negative predictive value (NPV) 92.8%, AUC: 0.941, p<0.001).
CONCLUSION:
There was a significant relationship between CAR, CRP, ESR and albumin levels and clinical disease severity in patients with ulcerative colitis. CAR is a cheap and practical marker for the diagnosis of acute severe ulcerative colitis.
Keywords: Albumin, CRP, CRP/albumin ratio, disease activity, ulcerative colitis
Ulcerative colitis (UC) is a chronic, idiopathic, inflammatory disease characterized by colonic involvement. UC is typically characterized by relapsing and remitting mucosal inflammation that starts from the rectum and extends to the proximal colon [1, 2]. The optimal treatment plan is determined according to the site of involvement and disease activity.
There are many instruments to measure disease activity for the UC. Generally, these measures are nonspecific. Thus, patients with other disorders, such as irritable bowel syndrome, could attain high scores even in the absence of any inflammation [3]. The Truelove–Witts criteria are one of the frequently used clinical indices and mainly used to determining acute severe UC [4, 5]. While determining disease activity, according to Truelove-Witts criteria, systemic inflammation findings (temperature and number of pulses per minute), the number of bloody defecations within 24 hours, erythrocyte sedimentation rate (ESR) and hemoglobin levels (Hb) are used [6]. Therefore, many clinical and laboratory parameters need to be followed. Determining disease activity through non-invasive markers might be useful to make appropriate decisions, such as hospitalization or intravenous steroid treatment.
Low serum albumin and high C-reactive protein (CRP) levels are particularly associated with severe disease activity. These indicators are also associated with unresponsiveness to corticosteroid and anti-tumor necrosis factor-alpha (anti-TNF) therapies and increased risk for colectomy [7–10].
CRP to albumin ratio (CAR) is an inflammatory marker that has been recently shown to have a prognostic value in various cancer types, sepsis and acute pancreatitis [11, 12]. The high level of CAR value is associated with increased inflammatory burden, poor prognosis and mortality [13]. In this study, we aim to investigate the diagnostic performance of CAR in determining clinical disease severity in the UC.
MATERIALS AND METHODS
In this study, clinical and follow-up data stored in the hospital database of 251 patients diagnosed with UC based on endoscopic, clinical, and pathological findings in the gastroenterology clinic at the Umraniye Training and Research Hospital, a tertiary health care institution, between November 2011 and February 2017 were retrospectively evaluated.
The following patients were excluded from this study: patients with (at diagnosis or presentation to the hospital) malignancy, connective tissue disease, vacuities, celiac disease, chronic liver disease, chronic kidney disease, heart failure; patients with a history of systemic infection within the last four weeks, patients receiving steroid therapy, patients with unidentified disease severity according to Truelove–Witts criteria and patients with missing data related to the inflammatory indicators that were being analyzed. Additionally, infectious etiology was evaluated (based on results of stool examination, culture, indirect haemagglutination test for entamoeba histolitica, histopathological findings and immunohistochemistry results for cytomegalovirus colitis who receiving immunosuppressive therapy) in all patients who presented with acute exacerbation. Patients with active disease associated with infectious causes were excluded from this study.
A total of 149 patients, comprising 118 with clinically active disease and 31 in remission, were included in this study. The demographic characteristics of the patients at presentation, the treatments they received, disease activity, site of involvement, endoscopic activity one week before or after the visit at which clinical activity was assessed, and CRP, erythrocyte sedimentation rate (ESR), and albumin values tested during the visit were recorded.
Acute severe UC was defined as the patient passing more than six bloody stools/day plus one or more of the following: temperature >37,8 °C; pulse >90 bpm; Hemoglobin level (Hb) <10.5 g/dL or ESR >30 mm/h. Moderate activity was defined as patient passing more than four or more bloody stools/day plus all of the following: temperature ≤37,8 °C; pulse ≤90 bpm; Hb≥10.5 g/dL, ESR ≤30 mm/h. Mild activity was defined as patient passing less than four bloody stools/day plus all of the following: temperature <37,8 °C; pulse <90 bpm; Hb>11.5 g/dL, ESR <20 mm/h according to Truelove–Witts criteria [6]. Patients with severe colitis who appear toxic, with a fever higher than 38,3°C, tachycardia, abdominal distention, signs of localized or generalized peritonitis, and leukocytosis, were considered to have fulminant colitis. Remission was defined as defecation without blood three times daily and a lack of abdominal pain and fecal urgency [3].
The endoscopic activity was assessed by experienced gastroenterology physicians using standard-definition colonoscopies (VP-4450HD system and EC-590WL Fujinon, Fujifilm, Tokyo, Japan). According to the mayo endoscopic activity index; normal endoscopic mucosal appearance was defined as Mayo:0. The presence of mucosal erythema decreased vascular pattern and mild friability were defined as Mayo: 1. The presence of marked erythema, the absence of vascular pattern, friability and erosions were defined as Mayo: 2. The presence of spontaneous bleeding, ulceration were defined as Mayo: 3 [14].
Statistics
The IBM SPSS Statistics 22 (IBM SPSS, Turkey) program was used to evaluate the data. Normal distribution of parameters was evaluated by the Shapiro–Wilks test. The Kruskal–Wallis test was used for comparing quantitative data and non-normally distributed variables in descriptive statistics, whereas the Mann–Whitney test was used to determine intergroup differences. Spearman’s rho correlation analysis was used to examine the relationship among parameters that were not normally distributed. The optimal cut-off point was selected according to the receiver operating curve (ROC) analysis. Significance was set at a p-value of <0.05.
RESULTS
A total of 149 patients, 118 of which had clinically active disease and 31 were in remission, were included in this study. Fifty (33.8%) patients were female and 99 (66.2%) were male. The mean age was 45.22±14 years. The number of patients with mild clinical activity was 39 (26.1%), patients with moderate clinical activity were 33 (22.1%), and patients with severe disease activity were 46 (31%). There was no patient with fulminant colitis in the severe active group. Demographic characteristics and clinical and endoscopic activities of the patients are shown in Table 1.
TABLE 1.
Demographic and clinical characteristics of the patients
| Parameters | (n=149) | |
|---|---|---|
| n | % | |
| Age | 45.22±14 | |
| Sex | ||
| Male | 99 | 66.2 |
| Female | 50 | 33.8 |
| Disease location | ||
| Proctitis | 12 | 8 |
| Left-sided | 64 | 43 |
| Extensive | 73 | 49 |
| Disease severity of UC (Mayo Subscore) | ||
| 0 | 12 | 9.1 |
| 1 | 10 | 7.5 |
| 2 | 16 | 12.1 |
| 3 | 94 | 71.2 |
| Clinical activity | ||
| Remission | 31 | 20.8 |
| Mild activity | 39 | 26.1 |
| Moderate activity | 33 | 22.1 |
| Severe activity | 46 | 31 |
| Treatment | ||
| Mesalazine | 140 | 94.1 |
| Sulfasalazine | 9 | 5.8 |
| Azathiopyrine | 44 | 29.4 |
| Anti-TNF | 5 | 3.3 |
| Cyclosporine | 2 | 1 |
| CRP (mg/dl) | 2.95±4.73 | |
| Albumin (g/dl) | 3.75±0.82 | |
| CAR | 1.01±1.77 | |
| ESR (mm/h) | 32.41±25.18 | |
UC: Ulcerative colitis; Anti-TNF: Anti-tumor necrosis factor; CRP: C-reactive protein; CAR: CRP/albumin ratio; ESR: Erythrocyte sedimentation rate.
There was a statistically significant difference among groups concerning CAR, CRP, albumin, and ESR values (p<0.001) when patients were separated into groups as remission, mild, moderate, and severe according to their clinical activities. Comparisons of CAR, CRP, albumin, and ESR values among clinical activity groups are shown in Table 2. As disease activity increased from remission to severe activity, CRP, CAR and ESR significantly increased, whereas albumin level significantly decreased (p=0.001; p<0.05).
TABLE 2.
Comparison of CAR, CRP, Albumin and ESR in different clinical activity of ulcerative colitis
| Clinical activity | p | ||||
|---|---|---|---|---|---|
| Remission Mean±SD | Mild Mean±SD | Moderate Mean±SD | Severe Mean±SD | ||
| CRP (mg/dl) | 0.25±0.23 | 0.43±0.37 | 2.07±2.82 | 7.57±5.94 | 0.001* |
| CAR | 0.05±0.07 | 0.11±0.12 | 0.60±0.82 | 2.73±2.32 | 0.001* |
| Albumin (g/dl) | 4.45±0.19 | 4.20±0.60 | 3.67±0.61 | 2.97±0.55 | 0.001* |
| ESR (mm/h) | 13.78±9.71 | 16.14±12.58 | 32.07±17.56 | 57.63±23.15 | 0.001* |
Kruskal Wallis Test
p<0.05. CRP: C-reactive protein; CAR: CRP/albumin ratio; ESR: Erythrocyte sedimentation rate; SD: Standard deviation.
The endoscopic activity was documented by colonoscopy or rectosigmoidoscopy in 132 (88%) (25 patients (80.6%) in the remission group and 107 (90.6%) patients in the clinical active group) patients. There was a statistically significant positive correlation between the endoscopic activity of the patients and CAR, CRP, albumin, and ESR values. The correlation coefficient of CAR was higher than that of CRP and ESR. The correlation between endoscopic activity index and CAR, CRP, and ESR is shown in Table 3.
TABLE 3.
Relationship of endoscopic activity with CRP, CAR and ESR
Spearman Rho Correlation Analysis
p<0.05. CRP: C-reactive protein; CAR: CRP/albumin Ratio; ESR: Erythrocyte sedimentation rate.
Patients were separated into two groups as severe clinical activity (n: 46, 39%) and non-severe (n: 72, 61%) clinical activity while excluding patients in remission. CAR, CRP, and ESR values were statistically significantly higher, whereas albumin levels were significantly lower in the severe clinical activity group than in the other groups (p=0.001; p<0.05; Table 4). ROC analysis was performed for CAR, CRP, albumin, and ESR to determine the cut-off point for severe disease episode.
TABLE 4.
CRP, CAR, albumin levels and ESR in severe and non-severe clinical activity
| Clinical activity | p | ||
|---|---|---|---|
| Non-severe Mean±SD | Severe Mean±SD | ||
| CRP (mg/dl) | 1.19±2.09 | 7.57±5.94 | 0.001* |
| CAR | 0.34±0.61 | 2.73±2.32 | 0.001* |
| Albumin (g/dl) | 3.95±0.65 | 2.98±0.55 | 0.001* |
| ESR (mm/h) | 23.36±16.92 | 57.63±23.15 | 0.001* |
Mann Whitney U Test
p<0.05. CRP: C-reactive protein; CAR: CRP/albumin ratio; ESR: Erythrocyte sedimentation rate; SD: Standard deviation.
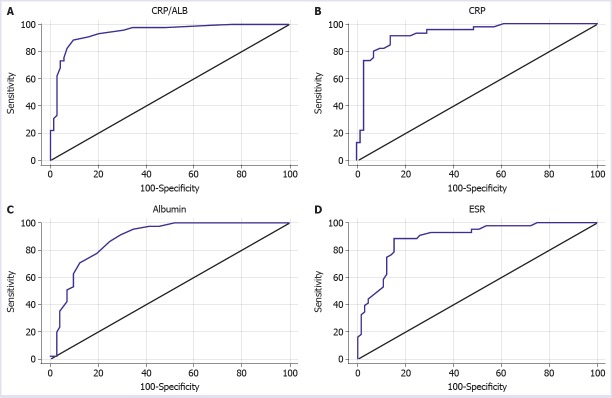
The area under the ROC curve (AUC) for CAR in severe disease episodes was found to be 0.941 (standard error 0.022) (Fig. 1A). The cut-off point for CAR in the case of severe disease episode was 0.6. The sensitivity, specificity, positive predictive value, and negative predictive value for this cut-off point were 88.9%, 90.3%, 85.1%, and 92.8%, respectively.
FIGURE 1.
Receiver operator curve for CAR (A), CRP (B), Albumin (C) and ESR (D) in the diagnosis of severe clinical activity.
AUC for CRP in severe disease episodes was 0.931 (standard error 0.025) (Fig. 1B). In case of severe disease episode, the cut-off point for CRP was found to be 2. The sensitivity, specificity, positive predictive value and negative predictive value for this cut-off point were 91.1%, 86.1%, 80.4%, and 93.9%, respectively. AUC for albumin in severe disease episode was 0.883 (standard error 0.03) (Fig. 1C). The cut-off point for albumin in the presence of severe disease episode was 3.6. The sensitivity, specificity, positive predictive value, and negative predictive value for this cut-off point were 91.1%, 70.8%, 66.1%, and 92.7%, respectively.
AUC for ESR in severe disease episodes was 0.888 (standard error 0.032) (Fig. 1D). The cut-off point for ESR in the case of severe disease episode was 36. The sensitivity, specificity, positive predictive value, and negative predictive value for this cut-off point were 88.4%, 84.6%, 79.2%, and 91.7%, respectively. The summary of ROC analysis is shown in Table 5.
TABLE 5.
ROC analysis of CAR, CRP, and ESR in the diagnosis of severe disease
| Parametre | Cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | p |
|---|---|---|---|---|---|---|---|
| CAR | 0.6 | 88.9 | 90.3 | 85.1 | 92.8 | 0.941 | 0.001 |
| CRP | 2 | 91.1 | 86.1 | 80.4 | 93.9 | 0.931 | 0.001 |
| Albumin | 3.6 | 91.1 | 70.8 | 66.1 | 92.7 | 0.883 | 0.001 |
| ESR | 36 | 88.4 | 84.6 | 79.2 | 91.7 | 0.888 | 0.001 |
CRP: C-reactive protein; CAR: CRP/Albumin ratio; ESR: Erythrocyte sedimentation rate; PPV: Positive predictive value; NPV: Negative predictive value; AUC: The area under the receiver operating characteristic.
Additionally, the diagnostic performance of CAR was evaluated with ROC analysis to differentiate patients who have severe from moderate clinical activity. The area under the ROC curve (AUC) for CAR in severe disease episodes was found to be 0,877 (standard error 0.041) (Fig. 2). The cut-off point for CAR to differentiate severe from moderate disease episode was 0.12. The sensitivity, specificity, positive predictive value, and negative predictive value for this cut-off point were 97%, 79%, 62%, and 87%, respectively.
FIGURE 2.
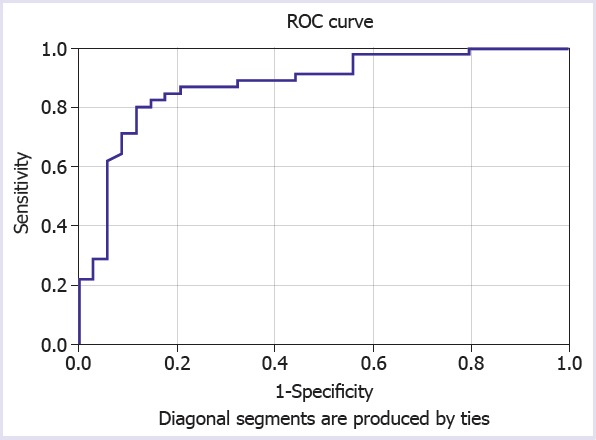
Receiver operator curve of CAR in differentiating severe from moderate clinical activity.
DISCUSSION
UC is an inflammatory bowel disease characterized by relapses and remissions. Although the best indicator of disease activity is an endoscopic activity, it is important to determine the clinical severity while planning optimal treatment [5, 6].
There are many instruments to measure disease activity for UC. Truelove–Witts criteria provide objective criteria to identify the severity of colitis and have been widely used to define the need for hospital admission and intravenous steroids [15].
Inflammation indicators are instruments that show inflammation noninvasively and objectively and are also used to evaluate disease activity. The most useful biomarkers in ulcerative colitis are levels of CRP and faecal calprotectin. Faecal calprotectin level is a useful noninvasive tool for monitoring disease activity over time. This biomarker has been shown to predict persistent inflammation and the risk of relapse [15]. CRP, an acute-phase protein synthesized from the liver in response to inflammatory cytokines, such as interleukin-6 (IL-6), TNF alpha, and interleukin-1 (IL-1), is one of the most commonly used nonspecific inflammatory indicators, and it has a half-life of approximately 19 h [16]. CRP concentration is less useful in quiescent, mild, or moderately active UC, as it correlates weakly with endoscopic disease activity and may be normal in some patients with active UC [15, 17]. However, there is a standard cut-off point for steroid unresponsiveness in patients with acute severe ulcerative colitis. A CRP value >4.5 mg/dl and the stool frequency of 3–8/day are strongly predictive of colectomy [4, 18, 19]. In addition, CRP has been found to be superior to erythrocyte sedimentation rate, albumin, and procalcitonin in reflecting disease severity in UC [17–20].
CRP response may be influenced by genetic factors (single-nucleotide polymorphisms regulating CRP production), the extent of involvement, and endoscopic and histological activity [21–23]. Rodgers et al. have stated that there is a positive and linear correlation between disease activity assessed by Lichtiger score and log (CRP) and a negative linear correlation with serum albumin level in patients with extensive and left colonic involvement [20]. In another study, Karoui et al. have stated that there is a positive correlation between clinical and endoscopic activity and CRP levels [24].
Albumin level is inversely related to the extent of the inflammatory response, which arises from the decrease in albumin synthesis in the liver because of the hypercatabolic state associated with the inflammatory process and down regulation of synthesis by cytokines, such as TNF alpha and IL-6 [25]. Albumin level is also associated with nutritional status [26]. In many studies, hypoalbuminemia was found to be associated with disease activity, unresponsiveness to treatment, and increased risk of colectomy in patients with acute severe UC [9, 10].
A high CRP and low albumin level may correlate with high level of inflammatory response. Chronic and high levels of inflammation refer to hypercytokinemia, which can lead to weight loss and malnutrition [27]. CAR reflects the combination of systemic inflammation and nutritional status. It has been shown that it can be used in predicting patient outcomes in many diseases, such as sepsis, acute pancreatitis, hepatocellular carcinoma, small cell lung, pancreatic and esophageal cancer and soft tissue sarcoma [12, 27].
In this study, 110 patients had endoscopically active disease (73.8%, Mayo subscores 2 and 3), 137 patients (92%) had extensive left colon involvement. Because of the high rate of endoscopically active patients, a low proportion of patients with proctitis, we think that the inflammatory burden is higher in patients not in remission. We found statistically significant differences concerning CRP, CAR, albumin, and ESR values of low, moderate, and severe activity groups created according to the remission and Truelove–Witts criteria. These markers show a statistically significant increase, whereas disease activity shifted from remission to severe clinical activity. When the correlation with endoscopic activity was evaluated, the correlation coefficient of CAR was higher than that of CRP. There was a significant positive correlation among CAR, CRP, and ESR and endoscopic activity. Effectiveness of CAR, CRP, albumin and ESR in assessing disease severity according to clinical activity score was compared. The specificity (90.3% vs. 86.1%, 70.8%, 84.6%), positive predictive value (85.1% vs 80.4%, 66.1%, 79.2%), and AUC value (0.941 vs 0.931, 0.883, 0.888) of CAR in determining severe activity were higher than those of other parameters.
Limited information is available in the literature on the use of CAR in inflammatory bowel disease. Qin et al. reported a positive association between disease activity and CAR in patients with CD. [28]. In the second study, Gibson et al. found that CAR measured on the 3rd day of treatment in patients with acute severe UC was strongly predictive of non-responsiveness to steroid therapy that was superior to CRP and albumin. Gibson et al. found that the optimal cut-off point for the non-response of the steroid was 0.85 [29]. In our study, we found the cut-off point to be 0.6 for diagnosing acute severe UC.
The first weakness of this study is retrospective design, inter-observer variability in determining clinical and endoscopic activity may affect the outcome of this study. Second, Truelove & Witts criteria are the gold standard for determining only severe activity, so the study was limited to only detecting the diagnostic performance of the severe clinical activity. In addition, due to the small number of patients, we could not investigate the prognostic value of CAR concerning colectomy risk. Third, the most important indicator of disease activity is endoscopic activity. However, there was a moderate correlation between CRP and CAR with endoscopic activity in this study. Given that endoscopic activity was not assessed by the score-based other activity indices may have affected the correlation analysis result. Another weakness is that the nutritional status of the patients is uncertain due to the retrospective nature of the study.
In conclusion, the present study found a significant relationship between CAR and disease activity, and CAR showed higher specificity and positive predictive value than CRP in predicting severe ulcerative colitis. CAR is a cheap, simple and easily calculated parameter that can be used to identify severe clinical activity.
Footnotes
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – SS, RK; Design – HLD, SS; Supervision – KO; Materials – KK, ZC; Data collection and/or processing – KK, SS; Analysis and/or interpretation – SS, OO; Literature review – RA; Writing – SS; Critical review – KO, SS.
REFERENCES
- 1.Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis as a progressive disease:the forgotten evidence. Inflamm Bowel Dis. 2012;18:1356–63. doi: 10.1002/ibd.22839. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE):Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324–38. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 3.Osterman MT, Lichtenstein GR. Feldman M, Friedman LS, Brandt JL, editors. Ulcerative Colitis. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. (10 th ed) 2016:2023–61. [Google Scholar]
- 4.Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1:definitions and diagnosis. J Crohns Colitis. 2012;6:965–90. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1:Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649–70. doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 6.Truelove SC, Witts LJ. Cortisone in ulcerative colitis;final report on a therapeutic trial. Br Med J. 1955;2:1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Travis SP, Farrant JM, Ricketts C, Nolan DJ, Mortensen NM, Kettlewell MG, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38:905–10. doi: 10.1136/gut.38.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda-García P, Chaparro M, Gisbert JP. Correlation between serological biomarkers and endoscopic activity in patients with inflammatory bowel disease. Gastroenterol Hepatol. 2016;39:508–15. doi: 10.1016/j.gastrohep.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Hindryckx P, Jairath V, D'Haens G. Acute severe ulcerative colitis:from pathophysiology to clinical management. Nat Rev Gastroenterol Hepatol. 2016;13:654–64. doi: 10.1038/nrgastro.2016.116. [DOI] [PubMed] [Google Scholar]
- 10.García-Bosch O, Aceituno M, Ordás I, Etchevers J, Sans M, Feu F, et al. Long-Term Follow-Up of Patients Treated with Infliximab for Ulcerative Colitis:Predictive Factors of Response-An Observational Study. Dig Dis Sci. 2016;61:2051–9. doi: 10.1007/s10620-016-4089-2. [DOI] [PubMed] [Google Scholar]
- 11.Xu HJ, Ma Y, Deng F, Ju WB, Sun XY, Wang H. The prognostic value of C-reactive protein/albumin ratio in human malignancies:an updated meta-analysis. Onco Targets Ther. 2017;10:3059–70. doi: 10.2147/OTT.S137002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan M, Ates I, Akpinar MY, Yuksel M, Kuzu UB, Kacar S, et al. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2017;16:424–30. doi: 10.1016/S1499-3872(17)60007-9. [DOI] [PubMed] [Google Scholar]
- 13.Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One. 2013;8:e59321. doi: 10.1371/journal.pone.0059321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis A randomized study. N Engl J Med. 1987;317:1625–9. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 15.Iborra M, Beltrán B, Nos P. Noninvasive Testing for Mucosal Inflammation in Inflammatory Bowel Disease. Gastrointest Endosc Clin N Am. 2016;26:641–56. doi: 10.1016/j.giec.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. 2016;13:567–79. doi: 10.1038/nrgastro.2016.128. [DOI] [PubMed] [Google Scholar]
- 17.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD:useful, magic, or unnecessary toys? Gut. 2006;55:426–31. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermeire S, Van Assche G, Rutgeerts P. The role of C-reactive protein as an inflammatory marker in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:580–6. doi: 10.1038/ncpgasthep0359. [DOI] [PubMed] [Google Scholar]
- 19.Henriksen M, Jahnsen J, Lygren I, Stray N, Sauar J, Vatn MH, et al. IBSEN Study Group. C-reactive protein:a predictive factor and marker of inflammation in inflammatory bowel disease Results from a prospective population-based study. Gut. 2008;57:1518–23. doi: 10.1136/gut.2007.146357. [DOI] [PubMed] [Google Scholar]
- 20.Rodgers AD, Cummins AG. CRP correlates with clinical score in ulcerative colitis but not in Crohn's disease. Dig Dis Sci. 2007;52:2063–8. doi: 10.1007/s10620-006-9691-2. [DOI] [PubMed] [Google Scholar]
- 21.Brull DJ, Serrano N, Zito F, Jones L, Montgomery HE, Rumley A, et al. Human CRP gene polymorphism influences CRP levels:implications for the prediction and pathogenesis of coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:2063–9. doi: 10.1161/01.ATV.0000084640.21712.9C. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg L, Nanda KS, Zenlea T, Gifford A, Lawlor GO, Falchuk KR, et al. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clin Gastroenterol Hepatol. 2013;11:991–6. doi: 10.1016/j.cgh.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg L, Lawlor GO, Zenlea T, Goldsmith JD, Gifford A, Falchuk KR, et al. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2013;19:779–84. doi: 10.1097/MIB.0b013e3182802b0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karoui S, Laz S, Serghini M, Bibani N, Boubaker J, Filali A. Correlation of C-reactive protein with clinical and endoscopic activity in patients with ulcerative colitis. Dig Dis Sci. 2011;56:1801–5. doi: 10.1007/s10620-010-1496-7. [DOI] [PubMed] [Google Scholar]
- 25.Chojkier M. Inhibition of albumin synthesis in chronic diseases:molecular mechanisms. J Clin Gastroenterol. 2005;39:S143–6. doi: 10.1097/01.mcg.0000155514.17715.39. [DOI] [PubMed] [Google Scholar]
- 26.Don BR, Kaysen G. Serum albumin:relationship to inflammation and nutrition. Semin Dial. 2004;17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 27.Tominaga T, Nonaka T, Sumida Y, Hidaka S, Sawai T, Nagayasu T. The C-Reactive Protein to Albumin Ratio as a Predictor of Severe Side Effects of Adjuvant Chemotherapy in Stage III Colorectal Cancer Patients. PLoS One. 2016;11:e0167967. doi: 10.1371/journal.pone.0167967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin G, Tu J, Liu L, Luo L, Wu J, Tao L, et al. Serum Albumin and C-Reactive Protein/Albumin Ratio Are Useful Biomarkers of Crohn's Disease Activity. Med Sci Monit. 2016;22:4393–400. doi: 10.12659/MSM.897460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson DJ, Hartery K, Doherty J, Nolan J, Keegan D, Byrne K, et al. CRP/Albumin Ratio:An Early Predictor of Steroid Responsiveness in Acute Severe Ulcerative Colitis. J Clin Gastroenterol. 2018;52:e48–52. doi: 10.1097/MCG.0000000000000884. [DOI] [PubMed] [Google Scholar]