Abstract
Clinical testing for MLH1 promoter hypermethylation status is important in the workup of patients with MLH1-deficient colorectal and uterine carcinomas when evaluating patients for Lynch syndrome. Current assays use single gene–based methods to assess promoter hypermethylation. Herein, we describe the development and report the performance of a clinical assay for MLH1 promoter hypermethylation using the Infinium methylationEPIC (850k) bead-array platform. Using four cytosine-guanine dinucleotide (CpG) sites within the MLH1 gene promoter, a qualitative MLH1 promoter hypermethylation assay was developed and validated using 63 gastrointestinal and uterine carcinoma samples of known hypermethylation status based on a pyrosequencing reference test. The array-based method achieves clinically robust and reproducible results at an analytical sensitivity level of 8%. Of importance, the 850k array contains probes targeting >850,000 additional CpG sites across the genome, covering sites in most known genes as well as important enhancer regions provided by the Encyclopedia of DNA Elements and Functional Annotation of The Mammalian Genome projects. Thus, the testing modality presented may also be applied to determine the methylation status of other clinically relevant genes or regulatory regions, potentially providing a single laboratory testing workflow for all clinical methylation assays. Furthermore, the concomitant acquisition of genome-wide methylation information provides a workflow that seamlessly enables wider translational epigenetic research.
The DNA mismatch repair (MMR) system is composed of four key genes: mutL homologue 1 (MLH1), postmeiotic segregation increased 2 (PMS2), mutS homologue 2 (MSH2), and mutS homologue 6 (MSH6).1 These genes encode proteins that function in heterodimers (MLH1/PMS2 and MSH2/MSH6) to repair and prevent DNA mutations. Defects in the system result in a dramatic increase in single-nucleotide variants and small insertion and deletion mutations, a phenotype termed mismatch repair deficiency or microsatellite instability–high. MMR deficiency results from a loss of gene function due to either epigenetic silencing or loss-of-function mutations.
MLH1 promoter methylation with consequential gene silencing is the most common cause of the MMR deficiency/microsatellite instability–high phenotype in colorectal and uterine carcinomas, occurring in approximately 10% to 20% of cases.2, 3, 4, 5 Germline mutations in any of the four MMR genes result in Lynch syndrome (alias hereditary nonpolyposis colorectal cancer), a hereditary cancer predisposition syndrome that carries an elevated risk of colorectal, uterine, and genitourinary cancers.6,7 Universal routine clinical testing for MSI status/MMR protein expression is recommended for all colorectal adenocarcinomas and endometrial carcinomas.8, 9, 10 MLH1 promoter hypermethylation testing is frequently performed for tumors that demonstrate loss of MLH1 expression because somatic MLH1 promoter hypermethylation supports sporadic microsatellite instability–high/MMR deficiency rather than Lynch syndrome.11,12 Rarely, MLH1 promoter methylation may occur as a constitutional event or in the setting of Lynch syndrome.13
DNA methylation is an important epigenetic regulatory process with key functions in development, tissue differentiation, and disease.14 Methylation most commonly occurs on the fifth carbon of cytosines within cytosine-guanine dinucleotide (CpG) sites.15 A variety of analytic methods have been developed to assay MLH1 promoter hypermethylation status, including real-time PCR, methylation-specific PCR, and pyrosequencing.16, 17, 18, 19, 20, 21, 22, 23 The single gene–based methods are primarily based on the evaluation of a 78-bp region in the MLH1 promoter containing eight CpG sites that are associated with loss of MLH1 protein expression.24,25 Recently, a variety of sequencing- and array-based high-throughput platforms have been developed to assess methylation status in a genome-wide manner. The Illumina (San Diego, CA) Infinium bead array–based methylation platform is a widely used and cost-effective platform. The current iteration of the array technology (850k) profiles the methylation status of approximately 850,000 CpG sites across all 23 chromosomes. CpG sites in >95% of known genes as well as important enhancer and intergenic regions are covered on the platform.26 Several methods have been developed on the platform for clinical testing, such as a logistic regression–based model for MGMT promoter hypermethylation testing.27,28 Herein, we establish a clinically robust test for MLH1 promoter hypermethylation using four CpG sites targeted by the 850k array.
Materials and Methods
Samples were procured under institutional review board number 12-245, and the study was conducted as a clinical laboratory assay validation for submission to the New York State Department of Health.
DNA Samples
Sixty-three DNA samples (38 positive/hypermethylated and 25 negative/not hypermethylated), extracted from formalin-fixed, paraffin-embedded (FFPE) tissue, were obtained from our archive of clinical samples at Memorial Sloan Kettering Cancer Center (New York, NY). The samples were selected in two phases. First, a group of 24 uterine and gastrointestinal samples was obtained, followed by a group of 38 colorectal samples (see Validation Process below).
The samples comprised 45 colorectal carcinomas, 15 uterine carcinomas, 2 gastric carcinomas, and 1 appendiceal carcinoma that previously underwent clinical testing for MLH1 promoter hypermethylation using a pyrosequencing-based method. The samples were ensured to span a range of methylation levels (positive samples, 14%–71%; negative samples, 2%–8%) routinely present in clinical specimens, particularly near 10%, which is the level of sensitivity of the reference method. A summary of the samples is available in Supplemental Table S1. Human Methylated & Non-methylated DNA Set (catalog number D5014; Zymo Research, Irvine, CA), fully methylated and fully nonmethylated DNA derived from human cell lines, was used to assess the performance characteristics of the platform.
850k Protocol
Genomic DNA was extracted from FFPE tissue using the Chemagic DNA Tissue kit (PerkinElmer chemagen Technologie, GmbH, Baesweiler, Germany) after manual macrodissection to ensure at least 10% tumor content. For each sample, 250 ng of input was used for bisulfite conversion (EZ DNA Methylation Kit; Zymo Research; catalog number D5002), followed by an FFPE restoration step using the Infinium HD FFPE DNA Restore Kit (Illumina; catalog number WG-321-1002). All samples were processed on the Infinium 850k array and scanned using the Illumina iScan, according to the manufacturer's recommended protocol. Each CpG site interrogated by the Infinium array is identified by a unique cg identifier in the format of cg#, where # is a number (eg, cg23658326 is the cg identifier of an MLH1 promoter CpG site).
Reference Method and 850k CpG Sites
A previously validated pyrosequencing-based assay used in the Clinical Laboratory Improvement Amendments–accredited Diagnostic Molecular Genetic Laboratory at Memorial Sloan Kettering Cancer was used as the reference method for all samples.20, 21, 22, 23 This method, using the PyroMark (Qiagen, Hilden, Germany) system, quantitatively measures the methylation level of five CpG sites within the MLH1 promoter and defines a sample as MLH1 promoter hypermethylated if all five CpG sites demonstrate >10% methylation. The five CpG sites are highly correlated; they are either all methylated or all unmethylated.
The 850k array targets four (cg identifiers cg23658326, cg11600697, cg21490561, and cg00893636) of the eight CpG sites associated with MLH1 silencing. One of these CpG sites (cg23658326) overlaps with a site interrogated by the reference method and, of note, one CpG site's (cg00893636) methylation status was previously associated with decreased MLH1 gene expression.29 Table 1 describes the genomic positions with respect to Genome Reference Consortium Human Build 37 and Deng et al24 of the eight CpG sites associated with MLH1 gene silencing and describes which CpG sites are used by the reference method and the 850k array method for clinical testing. Of note, these CpG sites are also contained on Illumina's targeted methylation sequencing platform (using the TruSeq Methyl Capture EPIC Library Prep Kit). The MLH1 probes chosen do not meet criteria for poor probe performance (eg, cross-reactivity or the presence of single-nucleotide polymorphisms near the CpG site), as defined by previous studies.30,31
Table 1.
Description of CpG Sites Associated with MLH1 Gene Repression
| CpG site | Illumina cg identifier | GRCh37 coordinate on chromosome 3 | Position as designated in Deng et al24,25 |
|---|---|---|---|
| 1∗ | NA | 37034770 | –248 |
| 2∗ | NA | 37034777 | –241 |
| 3∗ | cg23658326 | 37034787 | –231 |
| 4∗ | NA | 37034789 | –229 |
| 5∗ | NA | 37034795 | –223 |
| 6 | cg11600697 | 37034814 | –204 |
| 7 | cg21490561 | 37034825 | –193 |
| 8 | cg00893636 | 37034840 | –178 |
CpG, cytosine-guanine dinucleotide; GRCh37, Genome Reference Consortium Human Build 37; NA, not targeted by array.
Sites interrogated by the pyrosequencing reference method.
Bioinformatic and Statistical Analysis
Bioinformatic and statistical analyses were performed with R version 3.5.2 (https://www.R-project.org; last accessed November 27, 2019) using the minfi version 1.22.1 package.32 Green and red channel intensities were normalized using the preprocessIllumina function with background correction. The methylation level for each CpG site was quantified using β values, calculated as the ratio of methylated signal/total signal plus an offset (100 is the Illumina recommended offset). β Values are continuous values between zero and one. Values closer to zero indicate low to absent methylation, and values closer to one indicate high levels of methylation; values between the two extremes indicate partial methylation (eg, a mixture of cell types with methylation in a subset of cells or hemimethylation of a CpG site).33
Sensitivity and specificity were estimated with 95% binomial proportion CIs with the Jeffreys prior interval using the binom R package version 1.1-1.33
Quality Control
Each array contains several internal control probes to assess sample-independent and sample-dependent steps. Among these are a set of internal bisulfite conversion controls that target unmethylated cytosines (nonpolymorphic non–CpG-associated cytosines). These controls consist of unconverted probes and converted probes that target unconverted and converted DNA, respectively. As the probes intentionally target unmethylated cytosines, signal from the converted probes should be greater than the signal from the unconverted probes in a successfully converted sample. The control performance can be assessed quantitatively by taking the ratio of converted/unconverted signals. Values greater than one signify successful conversion. According to the manufacturer's recommendations, non-FFPE controls (eg, mixtures of fully methylated and unmethylated cell lines) are useful in evaluating the conversion because FFPE samples often demonstrate depressed ratios and when bisulfite conversion fails, it typically occurs across all samples in a batch. Thus, a non-FFPE sample is useful to assess the experiment-wide bisulfite conversion efficiency. All the controls were evaluated using Illumina's BeadArray Controls Reporter software version 1.1 and visualized with Genome Studio 2.0, according to the manufacturer's recommendations.
Individual MLH1 probe failures were assessed using the detectionP minfi function. The function calculates P values for each CpG site based on the CpG site's total intensity under a background null hypothesis distribution, defined by a set of internal negative control probes and assuming a normal distribution. Probes with P > 0.001 (three SDs from the mean) were considered failures.
Validation Process
Quantitative and qualitative performance characteristics of the array were first assessed by looking at the distribution of β values across predefined methylation levels (100%, 50%, 25%, 12.5%, 6.3%, 3.1%, and 0%) using samples generated from serial dilutions of fully methylated DNA into fully nonmethylated DNA and run in triplicate.
The method for clinical determination of MLH1 promoter hypermethylation status was developed and validated in a two-step process. First, 24 DNA samples (14 positive and 10 negative) extracted from FFPE tissue were analyzed as a training study to establish interpretive criteria for calling hypermethylation status based on the performance characteristics of the samples in the study. The accuracy, interassay and intra-assay reproducibility, analytical sensitivity, and limit of detection were then evaluated on the remaining samples (n = 38; 23 positive and 15 negative) using the interpretive criteria, as defined by the training study. Particular attention was paid to samples at low levels of methylation (approximately 10%) to ensure the platform could accurately assay samples with low tumor content that are routinely seen in clinical settings.
Interassay and intra-assay variability was assessed by running three positive (methylation levels, 15%, 24%, and 26%) and three negative FFPE samples (methylation levels, 3%, 4%, and 5%) in triplicate on one run and across three different runs. Analytical sensitivity was performed by diluting a positive FFPE sample (average methylation level of 66% by pyrosequencing) into a negative FFPE sample to achieve 100%, 50%, 25%, 12.5%, 6.3%, 3.2%, and 0% levels of the positive sample (corresponding to methylation levels of 66%, 33%, 17%, 8%, 4%, 2%, and 0%). A limit of detection experiment was performed by running a positive sample (methylation level, 40%) at DNA concentration inputs of 250, 200, 150, 100, 50, and 25 ng before bisulfite conversion.
Results
The dilution study using samples generated from fully methylated and fully unmethylated human cell line DNA (Figure 1) demonstrated three important performance characteristics of the MLH1 CpG sites assayed by the array: i) each CpG site generates a unique β value distribution for a given methylation level, ii) β values measured on the array are not a reliable quantitative measure of methylation, and iii) the array can distinguish unmethylated CpG sites from CpG sites with methylation level >6% in high-quality DNA samples.
Figure 1.
Performance characteristics study using cell lines at varying methylation levels run in triplicate. The four cytosine-guanine dinucleotide (CpG) sites from the triplicate samples are represented with different colors. Each CpG site demonstrates a unique β distribution. Variance of the β distributions is largest for intermediate methylation levels and smallest for 0% and 100% levels. A small amount of jitter is added to methylation level for visualization purposes.
Results of the training study are shown in Figure 2A. Informed by these data, a qualitative cutoff was defined for each CpG site such that a CpG site was designated as methylated if its β value is greater than or equal to the cutoff and unmethylated if it is less than the cutoff. The cutoffs were defined as the means plus three SDs of the negative samples' β values (Table 2 and Figure 2). The methylation state of the four CpG sites was highly correlated; the CpG sites were either all methylated or all unmethylated. On the basis of these data, interpretive criteria for determining promoter hypermethylation status using the four MLH1 β values were defined such that all four CpG sites are required to be qualitatively methylated (ie, above their respective cutoffs) to call a sample positive for MLH1 promoter hypermethylation (Table 3).
Figure 2.
Results of training (A), validation (B), and analytical sensitivity (C) studies. In each graph, the four MLH1 cytosine-guanine dinucleotide (CpG) sites are represented on the x axis (with a small amount of jitter for ease of visualization) and their respective β values are represented on the y axis. Line segments connect β values from the same sample. A: The training study was used to establish qualitative cutoffs (blue line segments in A and B; black line segments in C) and interpretive criteria. B: The interpretive criteria were tested in the validation study. Box-and-whisker plots are shown for the negative samples only for each CpG site in the training and validation studies (A and B). C: Analytical sensitivity study using a sample with 66% methylation by quantitative pyrosequencing diluted into a negative sample shows consistent hypermethylation detection when the methylation level is ≥8%. The >8% category contains triplicates of samples with methylation levels of 66%, 33%, and 17%.
Table 2.
Empirically Determined Qualitative β Value Cutoffs for the Four MLH1 CpG Sites
| CpG site | Qualitative β value cutoff |
|---|---|
| cg23658326 | 0.18 |
| cg11600697 | 0.27 |
| cg21490561 | 0.11 |
| cg00893636 | 0.10 |
CpG, cytosine-guanine dinucleotide.
Table 3.
Interpretive Criteria for Reporting MLH1 Promoter Hypermethylation Status
| Analytical result | Clinical interpretation |
|---|---|
| β Values for all four MLH1 CpG sites greater than or equal to their respective cutoffs | MLH1 promoter hypermethylation positive |
| β Values of less than four of the MLH1 CpG greater than or equal to their respective cutoffs | MLH1 promoter hypermethylation negative |
CpG, cytosine-guanine dinucleotide.
These criteria were validated on the second group (validation group) of 38 FFPE samples with known MLH1 promoter hypermethylation status. The array demonstrated 100% concordance with respect to the reference method (Figure 2B) using the samples tested. The estimated sensitivity was 98% (95% CI, 92%–100%), and the estimated specificity was 97% (95% CI, 88%–100%). All β values for the four CpG sites were above their qualitative cutoff for all the positive samples. Negative samples showed larger variation of β values in the validation group than in the training group (Supplemental Tables S2 and S3); however, this did not affect the final designation of the samples on the basis of the a priori defined criteria. For example, five negative samples had a single CpG site above the respective cutoff (two samples with an elevated cg23658326 site and three samples with an elevated cg21490561 site). One negative sample had two CpG sites above their respective cutoffs (cg11600697 and cg21490561). The four CpG sites for the remaining eight negative samples were all below their respective qualitative cutoffs. A comparison of the methylation level of the one CpG site on the pyrosequencing assay that overlaps with the array site was performed and supports the qualitative discriminative ability of the platform (Supplemental Figure S1).
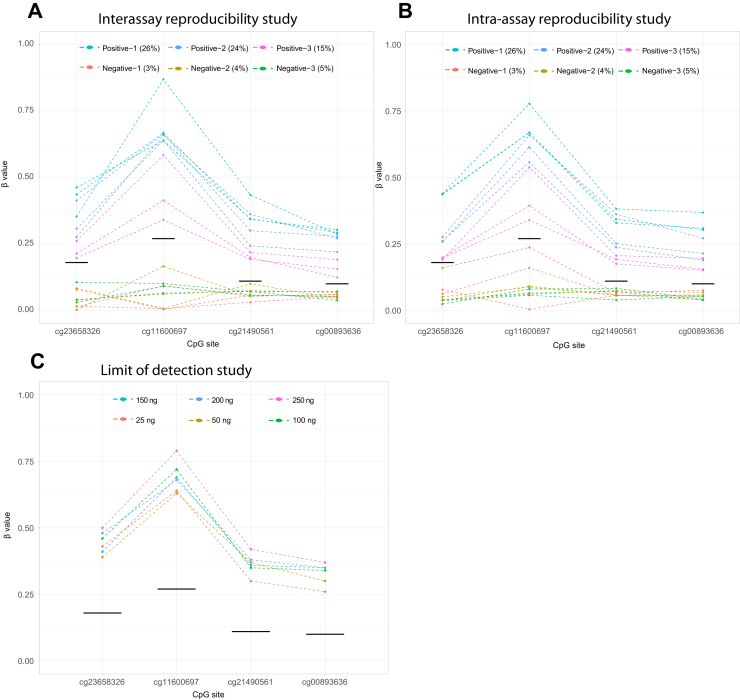
Consistent with the cell line experiment, the analytical sensitivity study using FFPE demonstrated the ability to detect promoter hypermethylation when the level is >8% (Figure 2C). Interassay and intra-assay variability studies demonstrated reproducibility of the assay within and across multiple runs (Figure 3, A and B), particularly at low methylation levels routinely seen in clinical settings (10%). Limit of detection demonstrated good performance of the assay down to 25 ng input DNA before bisulfite conversion (Figure 3C). The β values of the four MLH1 CpG sites across all experiments are provided in the Supplemental Tables S4–S10.
Figure 3.
Interassay reproducibility (A), intra-assay reproducibility (B), and limit of detection (C) studies. In each graph, the four MLH1 cytosine-guanine dinucleotide (CpG) sites are represented on the x axis and their respective β values are represented on the y axis. Line segments connect β values from the same sample. Black line segments designate the CpG-specific qualitative cutoff, as defined by the training study. A and B: Samples with methylation levels near the limit of sensitivity were run across three runs (A) and in triplicate on the same run (B). Results demonstrate accurate and reproducible results across and within experimental runs. C: Limit of detection study demonstrated the ability to detect hypermethylation in a positive sample using as little as 25 ng of DNA input before bisulfite conversion.
Sixteen samples (from the training group) were repeated because of depressed bisulfite conversion control ratios (ratio of one or less) in 12 of the 16 samples tested on a single run, consistent with experiment-wide suboptimal conversion. The run was deemed a failure and repeated with successful bisulfite conversion. All remaining samples' internal controls showed acceptable performance, particularly the bisulfite conversion controls (ie, the ratio of converted probe signals/unconverted probe signals was greater than one). No individual probes failed (P > 0.001) in any of the samples tested.
Discussion
This study demonstrates the ability of the Infinium methylation array to achieve robust and reproducible results for clinical MLH1 promoter hypermethylation testing on FFPE tissue at an analytical sensitivity of 8%. Consistent with previous studies, the β values were heteroscedastic across methylation levels and demonstrated low variance at extremes of methylation (0% and 100%).34 Although this technical behavior precludes a quantitative assay, the qualitative cutoff–based approach developed herein shows excellent performance characteristics using β values.
Interpretive criteria that require all four MLH1 CpG sites to be above their respective cutoffs provide robustness against experimental variation inherent to the array, particularly for negative samples for which one to two CpG sites showed mild elevation above their cutoffs (Figure 2B). Used in isolation, a result based on a single CpG site may cause false-positive results. By using four separate CpG sites in the interpretive criteria, no false-positive results occurred. No probe failures were observed in the experiments; however, it is recommended to repeat samples in which a probe failure occurs and the failure affects the clinical interpretation.
A limitation of this study is the use of a single scanner for all experiments. Experimental variation across different instruments was, therefore, not evaluated. We surmise that the β value cutoffs established in this study may be influenced by scanner-dependent technical variables and, therefore, recommend validating the cutoffs independently on individual scanners.
This same approach can be expanded to assay any site covered on the array. For example, a similar method could be applied to CpG sites within the BRCA1 gene promoter for development of a clinical assay that assesses epigenetic BRCA1 silencing.35 Of importance, the use of methylation arrays enables a single clinical laboratory testing workflow for all methylation assays, with potential advantages in turnaround time and more efficient use of technologist time because of the ability to batch samples across different assays. For example, MLH1, MGMT, BRCA1, and methylation-based central nervous system tumor classification tests can be performed simultaneously and analyzed on a single batch instead of multiple runs.36
Finally, the use of genome-wide methylation arrays provides a large amount of additional data that can separately be used for a variety of epigenetic research purposes (on appropriate institutional review board approval). The use of genome-wide methylation profiles as an ancillary diagnostic tool and as potential prognostic and/or predictive biomarkers is a rich area of current investigation.36, 37, 38, 39 Thus, this platform could potentially provide a powerful parallel research platform for brain, gastrointestinal, endometrial, and other tumors in which the hypermethylation status of genes is clinically relevant.
Footnotes
Supported by National Cancer Institute Cancer Center support grant P30 CA008748.
Disclosures: None declared.
Supplemental material for this article can be found at https://doi.org/10.1016/j.jmoldx.2019.11.005.
Supplemental Data
Comparison of the β values and quantitative methylation levels of the CpG site (cg23658326) that is interrogated by both the methylation array and the pyrosequencing reference method.
References
- 1.Richman S. Deficient mismatch repair: read all about it. Int J Oncol. 2015;47:1189–1202. doi: 10.3892/ijo.2015.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman J.G., Umar A., Polyak K., Graff J.R., Ahuja N., Issa J.P., Markowitz S., Willson J.K., Hamilton S.R., Kinzler K.W., Kane M.F., Kolodner R.D., Vogelstein B., Kunkel T.A., Baylin S.B. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagle C.M., O'Mara T.A., Tan Y., Buchanan D.D., Obermair A., Blomfield P., Quinn M.A., Webb P.M., Spurdle A.B., Australian Endometrial Cancer Study Group Endometrial cancer risk and survival by tumor MMR status. J Gynecol Oncol. 2018;29:e39. doi: 10.3802/jgo.2018.29.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black D., Soslow R.A., Levine D.A., Tornos C., Chen S.C., Hummer A.J., Bogomolniy F., Olvera N., Barakat R.R., Boyd J. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol. 2006;24:1745–1753. doi: 10.1200/JCO.2005.04.1574. [DOI] [PubMed] [Google Scholar]
- 5.Moreira L., Balaguer F., Lindor N., de la Chapelle A., Hampel H., Aaltonen L.A., Hopper J.L., Le Marchand L., Gallinger S., Newcomb P.A., Haile R., Thibodeau S.N., Gunawardena S., Jenkins M.A., Buchanan D.D., Potter J.D., Baron J.A., Ahnen D.J., Moreno V., Andreu M., Ponz de Leon M., Rustgi A.K., Castells A., EPICOLON Consortium Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonadona V., Bonaïti B., Olschwang S., Grandjouan S., Huiart L., Longy M., Guimbaud R., Buecher B., Bignon Y.J., Caron O., Colas C., Noguès C., Lejeune-Dumoulin S., Olivier-Faivre L., Polycarpe-Osaer F., Nguyen T.D., Desseigne F., Saurin J.C., Berthet P., Leroux D., Duffour J., Manouvrier S., Frébourg T., Sobol H., Lasset C., Bonaïti-Pellié C, French Cancer Genetics Network Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 7.Dowty J.G., Win A.K., Buchanan D.D., Lindor N.M., Macrae F.A., Clendenning M., Antill Y.C., Thibodeau S.N., Casey G., Gallinger S., Marchand L.L., Newcomb P.A., Haile R.W., Young G.P., James P.A., Giles G.G., Gunawardena S.R., Leggett B.A., Gattas M., Boussioutas A., Ahnen D.J., Baron J.A., Parry S., Goldblatt J., Young J.P., Hopper J.L., Jenkins M.A. Cancer risks for MLH1 and MSH2 mutation carriers. Hum Mutat. 2013;34:490–497. doi: 10.1002/humu.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network . National Comprehensive Cancer Network; Plymouth Meeting, PA: 2019. Clinical Practice Guidelines in Oncology: Uterine Neoplasms (Version 4.2019) [Google Scholar]
- 9.National Comprehensive Cancer Network . National Comprehensive Cancer Network; Plymouth Meeting, PA: 2019. Clinical Practice Guidelines in Oncology: Colon Cancer (Version 3.2019) [Google Scholar]
- 10.Sepulveda A.R., Hamilton S.R., Allegra C.J., Grody W., Cushman-Vokoun A.M., Funkhouser W.K., Kopetz S.E., Lieu C., Lindor N.M., Minsky B.D., Monzon F.A., Sargent D.J., Singh V.M., Willis J., Clark J., Colasacco C., Rumble R.B., Temple-Smolkin R., Ventura C.B., Nowak J. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J Mol Diagn. 2017;19:187–225. doi: 10.1016/j.jmoldx.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons M.T., Buchanan D.D., Thompson B., Young J.P., Spurdle A.B. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. J Med Genet. 2012;49:151–157. doi: 10.1136/jmedgenet-2011-100714. [DOI] [PubMed] [Google Scholar]
- 12.Newton K., Jorgensen N.M., Wallace A.J., Buchanan D.D., Lalloo F., McMahon R.F., Hill J., Evans D.G. Tumour MLH1 promoter region methylation testing is an effective prescreen for Lynch syndrome (HNPCC) J Med Genet. 2014;51:789–796. doi: 10.1136/jmedgenet-2014-102552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazzoli I., Loda M., Garber J., Syngal S., Kolodner R.D. A hereditary nonpolyposis colorectal carcinoma case associated with hypermethylation of the MLH1 gene in normal tissue and loss of heterozygosity of the unmethylated allele in the resulting microsatellite instability-high tumor. Cancer Res. 2002;62:3925–3928. [PubMed] [Google Scholar]
- 14.Robertson K.D. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 15.Illingworth R.S., Bird A.P. CpG islands: “a rough guide.”. FEBS Lett. 2009;583:1713–1720. doi: 10.1016/j.febslet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Bettstetter M., Dechant S., Ruemmele P., Grabowski M., Keller G., Holinski-Feder E., Hartmann A., Hofstaedter F., Dietmaier W. Distinction of hereditary nonpolyposis colorectal cancer and sporadic microsatellite-unstable colorectal cancer through quantification of MLH1 methylation by real-time PCR. Clin Cancer Res. 2007;13:3221–3228. doi: 10.1158/1078-0432.CCR-06-3064. [DOI] [PubMed] [Google Scholar]
- 17.Grady W.M., Rajput A., Lutterbaugh J.D., Markowitz S.D. Detection of aberrantly methylated hMLH1 promoter DNA in the serum of patients with microsatellite unstable colon cancer. Cancer Res. 2001;61:900–902. [PubMed] [Google Scholar]
- 18.Herman J.G., Graff J.R., Myohanen S., Nelkin B.D., Baylin S.B. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogino S., Kawasaki T., Brahmandam M., Cantor M., Kirkner G.J., Spiegleman D., Makrigiorgos G.M., Weisenberger D.J., Laird P.W., Loda M., Fuchs C.S. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–217. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colella S., Shen L., Baggerly K.A., Issa J.P., Krahe R. Sensitive and quantitative universal pyrosequencing methylation analysis of CpG sites. Biotechniques. 2003;35:146–150. doi: 10.2144/03351md01. [DOI] [PubMed] [Google Scholar]
- 21.Tost J., Dunker J., Gut I.G. Analysis and quantification of multiple methylation variable positions in CpG islands by pyrosequencing. Biotechniques. 2003;35:152–156. doi: 10.2144/03351md02. [DOI] [PubMed] [Google Scholar]
- 22.Ronaghi M., Uhlen M., Nyren P. A sequencing method based on real-time pyrophosphate. Science. 1998;281:363–365. doi: 10.1126/science.281.5375.363. [DOI] [PubMed] [Google Scholar]
- 23.Dejeux E., Audard V., Cavard C., Gut I.G., Terris B., Tost J. Rapid identification of promoter hypermethylation in hepatocellular carcinoma by pyrosequencing of etiologically homogeneous sample pools. J Mol Diagn. 2007;9:510–520. doi: 10.2353/jmoldx.2007.060209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng G., Chen A., Hong J., Chae H.S., Kim Y.S. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999;59:2029–2033. [PubMed] [Google Scholar]
- 25.Deng G., Peng E., Gum J., Terdiman J., Sleisenger M., Kim Y.S. Methylation of hMLH1 promoter correlates with the gene silencing with a region-specific manner in colorectal cancer. Br J Cancer. 2002;86:574–579. doi: 10.1038/sj.bjc.6600148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moran S., Arribas C., Esteller M. Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics. 2016;8:389–399. doi: 10.2217/epi.15.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bady P., Sciuscio D., Diserens A.C., Bloch J., van den Bent M.J., Marosi C., Dietrich P.Y., Weller M., Mariani L., Heppner F.L., McDonald D.R., Lacombe D., Stupp R., Deorenzi M., Hegi M.E. MGMT methylation analysis of glioblastoma on the infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol. 2012;124:547–560. doi: 10.1007/s00401-012-1016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bady P., Delorenzi M., Hegi M.E. Sensitivity analysis of the MGMT-STP27 model and impact of genetic and epigenetic context to predict the MGMT methylation status in gliomas and other tumors. J Mol Diagn. 2016;18:350–361. doi: 10.1016/j.jmoldx.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou W., Laird P.W., Shen H. Comprehensive characterization, annotation and innovative use of infinium DNA methylation BeadChip probes. Nucleic Acids Res. 2017;45:e22. doi: 10.1093/nar/gkw967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y.A., Lemire M., Choufani S., Butcher D., Grafodatskaya D., Zanke B., Gallinger S., Hudson T., Weksberg R. Discovery of cross-reactive probes and polymorphic CpGs in the illumina infinium HumanMethylation450 microarray. Epigenetics. 2013;8:203–209. doi: 10.4161/epi.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fortin J.P., Triche T.J., Jr., Hansen K.D. Preprocessing, normalization and integration of the illumina HumanMethylationEPIC array with minfi. Bioinformatics. 2017;33:558–560. doi: 10.1093/bioinformatics/btw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown L.D., Cai T.T., DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16:101–133. [Google Scholar]
- 34.Du P., Zhang X., Huang C.C., Jafari N., Kibbe W.A., Hou L., Lin S.M. Comparison of beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. doi: 10.1186/1471-2105-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee S., Kaye S.B., Ashworth A. Making the best of PARP inhibitors in ovarian cancer. Nat Rev Clin Oncol. 2010;7:508–519. doi: 10.1038/nrclinonc.2010.116. [DOI] [PubMed] [Google Scholar]
- 36.Capper D., Jones D.T.W., Sill M., Hovestadt V., Schrimpf D, Sturm D. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran S., Martínez-Cardús A., Sayols S., Musulén E., Balañá C., Estival-Gonzalez A., Moutinho C., Heyn H., Diaz-Lagares A., de Moura M.C., Stella G.M., Comoglio P.M., Ruiz-Miró M., Matias-Guiu X., Pazo-Cid R., Antón A., Lopez-Lopez R., Soler G., Longo F., Guerra I., Fernandez S., Assenov Y., Plass C., Morales R., Carles J., Bowtell D., Mileshkin L., Sia D., Tothill R., Tabernero J., Llovet J.M., Esteller M. Epigenetic profiling to classify cancer of unknown primary: a multicentre, retrospective analysis. Lancet Oncol. 2016;17:1386–1395. doi: 10.1016/S1470-2045(16)30297-2. [DOI] [PubMed] [Google Scholar]
- 38.Dogan S., Vasudevaraja V., Xu B., Serrano J., Ptashkin R., Jae Jung H., Chiang S., Jungbluth A., Cohen M., Ganly I., Berger M., Boroujeni A.M., Ghossein R., Ladanyi M., Chute D., Snuderl M. DNA methylation-based classification of sinonasal undifferentiated carcinoma. Mod Pathol. 2019;32:1447–1459. doi: 10.1038/s41379-019-0285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boussios S., Ozturk M.A., Moschetta M., Karathanasi A., Zakynthinakis-Kyriakou N., Katsanos K.H., Christodoulou D.K., Pavlidis N. The developing story of predictive biomarkers in colorectal cancer. J Pers Med. 2019;9:12. doi: 10.3390/jpm9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the β values and quantitative methylation levels of the CpG site (cg23658326) that is interrogated by both the methylation array and the pyrosequencing reference method.