Abstract
In the present study, modified extraction methods using supercritical CO2 were investigated in order to obtain high-added value compounds from rapeseed oil deodorizer distillate and comparisons were done with modified Soxhlet extraction (solvent extraction + silica). For supercritical fluid extraction (SFE), the optimal extraction parameters were temperature of 40 °C, pressure of 350 bar (for phytosterols), 400 bar (for tocopherol), 5 wt% ethanol as co-solvent, and saponification pretreatment. The optimized SFE procedure led to the recovery of three main phytosterols (50 wt % β-sitosterol, 23.91 wt % Brassicasterol, and 36.25 wt % Campesterol) and only α-tocopherol. Moreover, there was no synergistic effect with saponification pretreatment + co-solvent and the efficiency and concentration of target compounds were less than supercritical CO2 + co-solvent. Also, comparative Data showed that the efficiency of phytosterols and tocopherols was approximately three times higher (p < 0.05) in SFE relative to modified Soxhlet extraction. Furthermore, the use of ethanol (5 wt %) as co-solvent, improved phytosterols and tocopherol efficiency and purity. The SFE technique offers various advantages over the modified Soxhlet extraction technique, including increasing the solubility of tocopherols and sterols by using CO2+ co-solvent, minimized usage of toxic organic solvents and increased purity of extracted products.
Keywords: Tocopherol, Sterols, Solvent extraction, Supercritical fluid extraction, Food science, Food technology
Tocopherol; Sterols; Solvent extraction; Supercritical fluid extraction; Food Science; Food Technology
1. Introduction
Raw edible vegetable oils contain triacylglycerol as the main compound and many minor compounds such as free fatty acids (FFA), tocopherols, sterols and odors, which are removed from the oil during the chemical refining process. The most widely utilized vegetable oil refining process is chemical refining, which reduces the level of FFA and produces a significant amount of by-products such as deodorizer distillate (DOD). As a by-product of the deodorization stage (the final stage of the chemical refining process), DOD contains a mixture of FFA and mono-, di-, and tri-glycerides; its major constituents are phytosterols, tocopherols and hydrocarbons [1]. The most consumable raw edible oils are the oils of soybean and rapeseed. Rapeseed oil is a good source of phytosterols (0.5–0.97 wt %), which is why it has been given more attention by researchers relative to soybean oil [2].
In recent years, hypercholesterolemia has been established as one of the major risk factors for heart disease, with plant sterols having been used for reduction of blood cholesterol since the 1950s. Hence, the enrichment of food products with such compounds is of great importance. Phytosterols are plant sterols that exist in different species of plants [3]. These metabolites are bioactive and can be found in all tissues of the plants as well as in the seeds. Moreover, they are steroidal steroids (C28–C29) that consist of tetracyclic cyclopenta phenanthrene and, usually, a long side chain on C17. Over 200 types of phytosterols are known to exist in plants, with β-sitosterol (24-α-ethyl cholesterol), campesterol (24-α-methyl cholesterol) and stigmasterol (22Δ- and 24-α-ethyl cholesterol) being the most common types [4].
Recently, supercritical fluid extraction (SFE) has received much attention due to featuring greater extraction rates, extraction efficiencies and selective power compared with conventional methods of extraction [5]. One of the characteristics of SFE is that extraction is done using a solvent that has greater permeability and lower viscosity than other solvents. The best supercritical solvent for extraction from natural herbs is CO2 since it is a natural and inert compound with a generally recognized as safe (GRAS) status [6]. The supercritical temperature of CO2 is 31.1 °C, which makes the substance suitable for the extraction of heat-sensitive compounds. Carbon dioxide extraction is a process through which extractable compounds can be separated by the supercritical fluid at optimized temperatures and pressures [7].
Commercially, supercritical carbon dioxide (SC–CO2) extraction is used for the recovery of tocopherols from soybean oil deodorizer distillate [3, 6, 7, 8]. The crystallization process has also been used for the separation of phytosterols from sunflower oil [9], soybean oil deodorizer distillate [10] and simulation of SC-CO2 process was done for the separation of phytosterols from rapeseed oil deodorizer distillate (RODD) [11]. Other techniques such as esterification, water-bathing, alcoholysis, cooling precipitation, distillation and modified silica gel column chromatography have been described in the literature for the recovery of sterols [12]. Column fractionation of canola oil deodorizer distillate using supercritical CO2 has been carried out at 25 MPa and the use of thermal gradient (70–100 °C) [13]. Countercurrent supercritical fluid extraction of tocopherols and sterols from lampante olive oil, sunflower oil deodorizer distillate and esterified olive oil deodorizer distillates was simulated [7]. Although the Recovery of the high aggregated compounds from the deodorizer distillate of the vegetable oils using supercritical fluid has been investigated in previous literature [14, 15, 16] extraction of tocopherols and sterols from modified RODD by supercritical CO2 + co-solvents in countercurrent extraction system has not been reported. The aim of the present study was to evaluate modified SFE methods for their efficiencies and applicability in the recovery of phytosterols and tocopherols from RODD, with the conventional Soxhlet technique being used as the control. The SFE technique was modified in terms of extraction conditions and the use of pretreatments to recover phytosterols and tocopherols from RODD with high efficiency.
2. Materials and methods
2.1. Reagents and chemicals
Rapeseed oil deodorizer distillate was obtained from Shadgol Oil Industry (Neyshabur, Iran). All samples were kept in a refrigerator at 4–8 °C until analysis. Ethanol, potassium hydroxide, chloroform, hexane, sulfuric acid (98%), and anhydride acetic acid were purchased from Merck (Darmstadt, Germany). Cholesterol (GC grade) was obtained from Sigma Chemical Co. (St Louis, USA) with a minimum of 96% purity. All solvents and chemicals used were of analytical grade. Carbon dioxide (99.95% purity) was obtained from Carburos Metálicos S.A. (Madrid, Spain) for use in SC-CO2 extractions.
2.2. Organic solvent extraction
The modified Soxhlet method described by Gunawan et al. [10] was used with some modifications as the control method. Samples of RODD (10–20 mg) were dissolved in 150 mL of ethanol. Silica gel (60 g) was then added, before the mixture was stirred at 300 rpm for 1 h. The temperature of refluxing was set at 40, 60 and 80 °C. Next, the mixture of silica gel and RODD was transferred to the extraction chamber section of a Soxhlet machine. The polar part of the distillate was absorbed by the silica gel then extracted using ethyl ether before analysis via GC-MS.
2.3. Determination of free fatty acids, natural lipids, saponification value and color number in raw RODDs
The percentage of FFAs, expressed in terms of oleic acid, was determined via AOCS methods (Ca. 5a-40) [17]. The composition of natural lipids in the raw sample was determined via an HPLC method using a procedure described elsewhere [18]. Determination of the saponification value was done by the AOAC standard method (920.160) [19]. The color of the samples was determined according to AOCS method (Cc 13c-50) [20] using a Lovibond tintometer in a 1-in. cell on the Lovibond scale in transmittance mode and expressed as (5R + Y) units. All experiments were carried out in triplicates.
2.4. Determination of total tocopherols and sterols
Total tocopherols and sterols were analyzed using the methods described by Wong et al. [21] and Sabir et al. [22], respectively. Moreover, a gas chromatography device (Agilent Technologies, Santa Clara, USA) was used to separate and detect the bioactive compounds in the extracted products. This device was equipped with a quadrupole mass spectrometer detector (5977A-Agilent Technologies, Santa Clara USA). An HP-5MS (5% phenyl methylsiloxane) 30 m × 0.25 mm × 0.25 μm capillary column (Agilent Technologies, Palo Alto, USA) was used to acquire data of the chromatography separations. Helium was utilized as the carrier gas; its flow rate was set at 0.5 ml/min. Injection temperature was 310 °C. A sample injection of 2 μl was performed in a split mode of 1:10. The initial oven temperature was 190 °C; 70 eV worth of ionization energy was utilized. The full scan mode (50–500 Da) was used to detect and acquire the data. Bioactive compounds were identified by comparison of the obtained data against the m/z values reported in the Wiley and NIST mass spectral databases. The Agilent Mass Hunter software (Agilent Technologies, Santa Clara USA) was employed to analyze the data, and the various compounds were quantified by comparison against cholesterol as the internal standard.
2.5. Rapeseed oil deodorizer distillate pre-treatment
Given the low solubility of ODDs in SC-CO2, pretreatment of the raw materials may be required. Pretreatment of RODD was done by allowing it to react with ethanolic potassium hydroxide (1N) for 1 h at 60 °C. For further details of the saponification procedure, see Patterson [23]. The composition (wt %) of the saponified RODD obtained by this method is presented in Table 1. The non-saponified section was recycled and used in the countercurrent-SFE process.
Table 1.
Composition and properties of rapeseed oil deodorizer distillate (∗mean ± standard deviation, n = 3).
| Parameters | RODD |
|---|---|
| Physical appearance (visual) | Dark semisolid |
| Color (1 in. cell, 5R + Y unit) | 75 ± 2∗ |
| Free fatty acid value (as % oleic) | 53 ± 2 |
| Saponification value (mg KOH/g) | 160 ± 1 |
| Neutral oil∗ (wt %) | 7 ± 1 |
| Unsaponifiable matter (wt %) | 40 ± 2 |
| Total tocopherols (wt %) | 14 ± 2 |
| Total phytosterols (wt %) | 26 ± 2 |
Neutral oil % = [100 - (%unsaponifiable matter + %FFA)].
2.6. Countercurrent supercritical carbon dioxide (CC SC-CO2) extraction
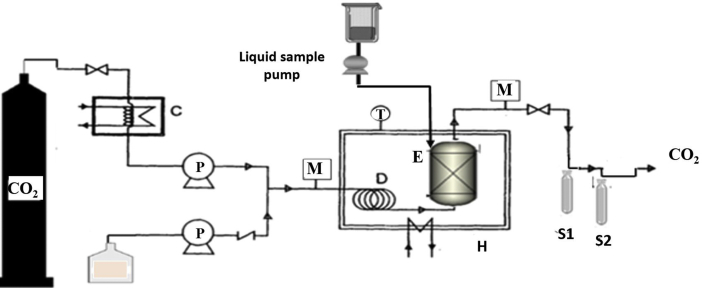
Phytosterols were extracted from RODD via SC-CO2 extraction with an SFX CO-960 CC-SFE system (Valladolid, Spain). The SFE system employed is presented schematically in Figure 1 and features separate sections for fluid delivery, extraction, and collection. The section of fluid delivery is comprised of two pumps that separately transport the liquid carbon dioxide and modifier solvent to the column. Both CO2 and the samples were preheated at the exits of their respective pumps ahead of entering the main part of the CC-SFE device. In the extraction section, SFE was done using ethanol-modified supercritical carbon dioxide within a 316 stainless steel extraction column (17.6 mm i.d., length of 150 cm and volume of 100 ml); CO2 flow rate was 5 mL/min it was introduced into the column, through the bottom. When ethanol was consumed, it was combined into the SC-CO2 stream with a flow rate of 0.5 mL/min. To create a countercurrent flow between the liquid sample (downward) and the SC-CO2 (upward), introduction of the liquid sample was done through the top of the column with a flow rate of 0.5 mL/min. Extractions were performed under modified conditions (first at 40 °C that was suitable for thermo sensitive compounds (according to our pervious study [11] for extraction of sterols and tocopherols [14]), pressure: 100, 200, 250, 300, 350, and 400 bar, CO2 flow: 5 mL/min, modifier flow: 0.5 mL/min and time: 60 min. Then the Extraction efficiencies and concentrations of target compounds were determined under 40, 60, and 80 °C at the other same conditions and specified pressure that have higher efficiencies and concentrations. In the separation vessel (volume of 100 ml) (i.e., the collection section), the pressure was kept at a desired value by a back-pressure regulator. The pressure of the effluent was reduced to atmospheric pressure by flowing it through the back-pressure regulator, and the solubility of solutes in the effluent thereby decreased to near zero. The solutes were consequently deposited in and collected from the separator. Then, the raffinate and liquid fractions (extracted fraction) after ethanol evaporation were weighed and analyzed.
Figure 1.
Schematic flow diagram of the SFE modular system: (C) cooler; (D) temperature equilibration coil; (E) extraction vessel; (H) oven; (S1, S2) sample collection vessel; (F) volumetric flow meter; (M) manometer; (T) thermometer.
2.7. Statistical analysis
All experiments and analyses were performed in triplicates, and data analysis was performed through a completely randomized simple design (CRSD) using Minitab software. Significant differences were determined at p < 0.05 through one-way analysis of variance (ANOVA) and Tukey's test for multiple comparisons. The results were reported as mean values ±standard deviation.
3. Results and discussion
3.1. Physicochemical characteristics of RODD
The RODD which was obtained from the Shadgol Company was dark brown (75 Lovibond color units) and semisolid at room temperature. Other physicochemical characteristics are showed in Table 1. High amount of saponification value in RODD (160.3) indicates a lower amount of glycerides (expressed as neutral oil, 7 wt %). The FFA and saponification values were in good agreement with the stated research [24]. Sherazi et al [1] reported that the amount of non-saponifiable matter in RODD was 33 wt %. Also, their results showed that the content of tocopherols and phytosterols were 18 wt % and 14.60 wt % respectively. They also stated that difference in the range of values to the previous literature was due to variation in processing conditions and raw material variability.
3.2. Modified soxhlet extraction
The experimental results related to the Soxhlet extraction of phytosterols and tocopherols from RODD are summarized in Table 2. The highest extraction efficiency and concentration of target compounds was achieved at 60 °C, which was followed in order by temperatures of 40 and 80 °C (Table1). Due to thermal degradation of tocopherols and phytosterols at higher temperatures [25], the amount of these compounds decreased significantly at 80 °C. Other authors have also shown that high temperatures increase the destruction of tocopherols in oil [26, 27]. The temperature of 60 °C is the boiling point of the hexane solvent. At this temperature, the oil compounds are introduced into the extractor by the solvent; therefore, the extraction efficiency and the amount of separated compounds are also higher. Our results are accordance with the results of Gunawan et al. [10], and Lim et al [28]. Kornsteiner et al. [29] reported that the Soxhlet extraction resulted in the destruction of tocopherols in macadamia oil. Ribeiro et al [30] expressed that the high and prolonged temperatures during the Soxhlet extraction process affect the amount of bioactive compounds. However, Santos et al. [31] identified tocopherols in faveleira seed oil using Soxhlet extraction. Due to the loss of tocopherols and sterols in Soxhlet extraction, an efficient method is needed to control the degradation of bioactive compounds during their extraction from oil waste.
Table 2.
Efficiency of modified Soxhlet extraction of sterols at various experimental temperatures.
| Temperature (°C) | Phytosterol concentration (wt. %) | Tocopherol concentration (wt. %) | Efficiency (wt. %) |
|---|---|---|---|
| 40 | 5 ± 0.8b∗ | 11±1b | 20 ± 0.4b |
| 60 | 10 ± 0.2a | 12±1a | 24 ± 0.9a |
| 80 | 4 ± 0.6c | 7±2c | 12 ± 2c |
Non-similar letters in each column indicate a significant difference according Tukey test (P<0.05).
Mean ± standard deviation.
3.3. Supercritical CO2 extraction (SFE) with co-solvent
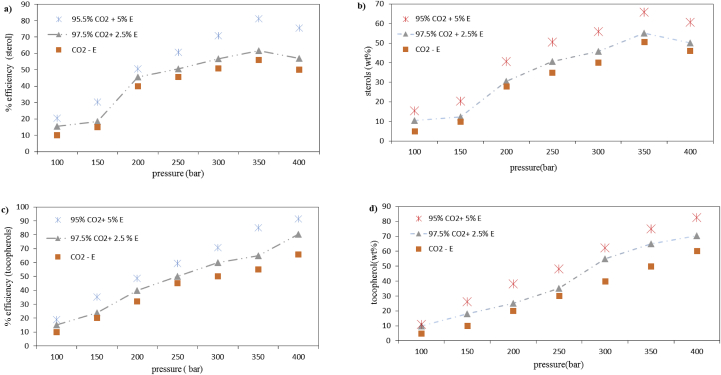
For the SFE of bioactive compounds (tocopherols and phytosterols) from RODD, an appropriate extraction fluid must be selected [32]. According to the results, when pure CO2 was used at 40 °C and 350 bar only 51 wt % of sterols could be extracted from RODD (Figure 2a, b). Once ethanol-modified CO2 was applied, sterols were extracted with greater efficiency. In this technique, the distribution of an analyte between the applied fluid and the sorptive sites within the matrix of the sample determines whether the analyte can be extracted. Despite being the most used supercritical fluid, one major limitation regarding CO2 is its limited ability in the dissolution of polar molecules, even at elevated densities [33]. Alternative pure fluids are generally not opted for due to having high reactivity, flammability, or toxicity. As a solution to this dilemma, solvable compounds can be added to the SC-CO2 to modify its properties during extraction.
Figure 2.
Supercritical fluid extraction of a,b) phytosterols and c,d) tocopherols using different compositions of ethanol co-solvent. Experimental conditions: 60 min extraction at 40 °C; 5 mL/min CO2 flow. (CO2- E: CO2 without ethanol, E: ethanol).
Generally, two main considerations must be made in order to predict the conditions for optimal recovery, including the fluid's ability to replace the analytes at the sorptive sites and, more importantly, the analytes' solubility within the fluid. Co-solvents can dramatically boost the solubility of polar analytes in supercritical fluids. Our findings indicate that the application of 5 wt % ethanol as a co-solvent leads to optimal results in the SFE of phytosterols and tocopherols from RODD. The flow rates of the co-solvent and the SC-CO2 fluid were set at 0.5 and 5.0 mL/min, respectively, leading to an optimal medium (95% CO2 and 5 wt% ethanol) for the SFE of phytosterols and tocopherols.
With the use of 5 wt % ethanol as the co-solvent, 81% of sterols were extracted at 40 °C and 350 bar with a purity of 66 wt %. While at 40 °C and 400 bar 91wt % of tocopherols were extracted with a purity of 83 wt % (Figure 2c, d). The positive effect of co-solvents on the solubility of tocopherols and sterols have been reported and supported in the literature [33, 34, 35, 36].
The determination of optimal SFE conditions is essential for extracting phytosterols and tocopherols from RODD with maximum efficiency. In this regard, the supercritical fluid's temperature and pressure can be regarded as the parameters with the highest significance. To determine the optimal temperature for SFE, the operating temperature was varied between 40 and 80 °C (Table 3). Similar results were obtained by Guclu et al. [33] in the study of solubility of tocopherols and sterols in the multiple system by using of different co-solvents especially ethanol. Porto et al reported [36] that ethanol as co-solvent has significant effect on the tocopherol separation from olive pomace oil.
Table 3.
Supercritical fluid extraction of bioactive compounds using various temperatures. Experimental conditions: 60 min of extraction at 350 bar (for phytosterols) or 400 bar (for tocopherols); CO2 flow 5 mL/min and modifier flow 0.5 mL/min. RSDs based on triplicate extractions under each condition∗.
| Temperature °C | Phytosterols |
Tocopherols |
||
|---|---|---|---|---|
| Efficiency (wt. %) | Concentration (wt. %) | Efficiency (wt. %) | Concentration (wt. %) | |
| 40 | 81 ± 4a | 66 ± 3a | 92 ± 2a | 83 ± 1a |
| 60 | 67 ± 3b | 52 ± 3b | 71 ± 2b | 67 ± 2b |
| 80 | 55 ± 4c | 46 ± 3c | 61 ± 1c | 51 ± 2c |
Non-similar letters in each column indicate a significant difference according Tukey test (P<0.05).
Mean ± standard deviation.
According to our results, the highest recovery was achieved at 40 °C, which was thus selected as the optimal temperature. The extraction efficiency and concentration of sterol and tocopherol compounds were twice higher at 40 °C relative to 80 °C. This can be attributed to the decrease in the SC-CO2 density at high temperatures, which leads to a decrease in solvent solubility and a subsequent reduction in the solubility of the desired material [37].
The density of a supercritical fluid is a determining factor in its ability as a solvent. In turn, pressure has a major impact on this density. In our work, to determine the optimal pressure for SFE, the operating pressure vas varied between 100 and 400 bar. It was found that extraction recovery increased with operating pressure up to 350 bar (Table 4). Above this point, the extraction efficiency decreased, which can be attributed to the reduction of the solvent's selective power for sterols against tocopherols. This phenomenon occurs due to the presence of a phase equilibrium between sterols and other compounds, making isolation difficult [13, 14].
Table 4.
Supercritical fluid extraction of phytosterol and tocopherols at various pressures. Experimental conditions: 60 min extraction at 40 °C; CO2 flow 5.0 mL/min and modifier flow 0.5 mL/min. RSDs based on triplicate extractions under each condition∗.
| Pressure (bar) | Phytosterols |
Tocopherols |
||
|---|---|---|---|---|
| Efficiency (wt. %) | Concentration (wt. %) | Efficiency (wt. %) | Concentration (wt. %) | |
| 100 | 21 ± 3g | 16 ± 3g | 19 ± 1.7g | 11 ± 2g |
| 150 | 30 ± 4f | 20 ± 3f | 35 ± 2f | 26 ± 1f |
| 200 | 51 ± 3e | 41 ± 4e | 49 ± 2e | 38 ± 2e |
| 250 | 61 ± 3d | 51 ± 3d | 59 ± 2d | 48 ± 2d |
| 300 | 71 ± 3c | 56 ± 4c | 71 ± 2c | 62 ± 3c |
| 350 | 81 ± 4a | 66 ± 3a | 86 ± 1b | 75 ± 2b |
| 400 | 76 ± 2b | 61 ± 3b | 92 ± 2a | 83 ± 1a |
Non-similar letters in each column indicate a significant difference according Tukey test (P<0.05).
Mean ± standard deviation.
In this work, a second ethanol composition was trialed as a co-solvent to arrive at optimal extraction conditions (Figure 1). It was found that the percentage of tocopherols and sterols recovered via SFE was higher when 5 wt % ethanol was used in comparison with 2.5 wt % ethanol. Since the effect of the co-solvent depends on its interaction with the main solvent, increasing the percentage of co-solvent increases the extraction efficiency. In other words, the solubility of phytosterols and tocopherols in modified CO2 is affected by the percentage of co-solvent utilized.
3.4. Effect of co-solvent application and saponification pretreatment
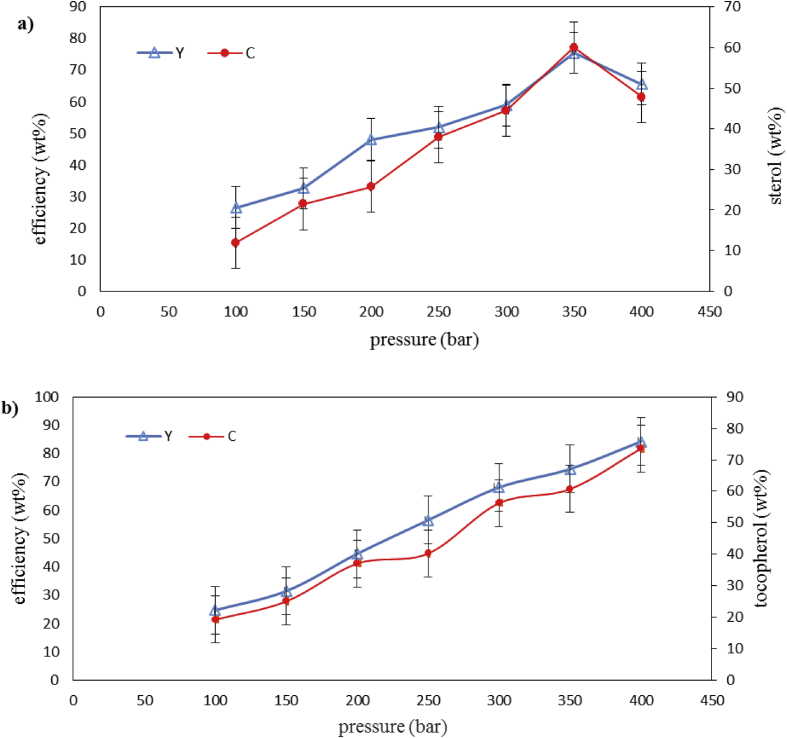
Results of the variance analysis for the fractionation of saponified distillates in the modified SC-CO2 system (5 wt % ethanol as co-solvent) at a constant temperature of 40 °C revealed that the pressure had a significant effect on the extraction efficiency and concentration of the compounds. This is such that the highest values of these parameters were obtained at 350 bar for phytosterols and at 400 bar for tocopherols. As seen in Figure 3a and b, at the mentioned pressure values, the concentrations of sterols and tocopherols were 69 wt % and 74 wt%, respectively. A synergistic effect was expected when saponification pretreatment was combined with the utilization of a co-solvent, but in practice, the efficiency and concentration of the isolated compounds were less compared with when only the co-solvent (ethanol) was used (Table 5). This can be attributed to the formation of a complex between the condensed sterol and tocopherol compounds and the organic solvent in the solid phase, which has an interactive effect on solvent power for the extraction of the desired compounds and decreases the extraction efficiency by reducing the solubility of the compounds in the supercritical fluid [33]. Temelli and et al [38] referred that Self association of ethanol through the increasing of concentration of target samples (as a result of saponification treatment) in separator resulted in the decrease of the amount of extracted tocopherol and sterol. This phenomenon is observed in solvents such as alcohols and acetic acid (co-solvents with hydrogen agents and acceptor groups [39].
Figure 3.
Efficiency (Y) and percentage (C) of a) sterols and b) tocopherols extracted from the saponified rapeseed oil deodorizer distillate with 5% (wt.%) ethanol as co-solvent over 1 h at 40 °C and 5 mL/min CO2 flow. (The drawn bars on each point in the linear graph represent the standard deviation).
Table 5.
Comparison of supercritical fluid extraction and modified soxhlet extraction of phytosterols and tocopherols from RODD.
| Extraction method | Efficiency (%) |
Concentration of sterols (%) | Concentration of tocopherol (%) | |
|---|---|---|---|---|
| Phytosterols | Tocopherols | |||
| Modified Soxhlet | 24 ± 1d∗ | 10 ± 0.2d | 11 ± 1d | |
| Raw RODD + SCF (100% CO2) | 71 ± 3c | 82 ± 2c | 51 ± 1c | 60 ± 1c |
| SCF(95% CO2+ 5% ethanol) | 81 ± 4a | 91 ± 1a | 67 ± 3a | 83 ± 1a |
| Saponified RODD + SCF(95% CO2+ 5% ethanol) | 76 ± 1b | 84 ± 1b | 60 ± 2b | 74 ± 0.3b |
Non-similar letters in each column indicate a significant difference according Tukey test (P<0.05).
Mean ± standard deviation.
3.5. Gas chromatography-mass spectrophotometry analysis and identification of phytosterols and tocopherols
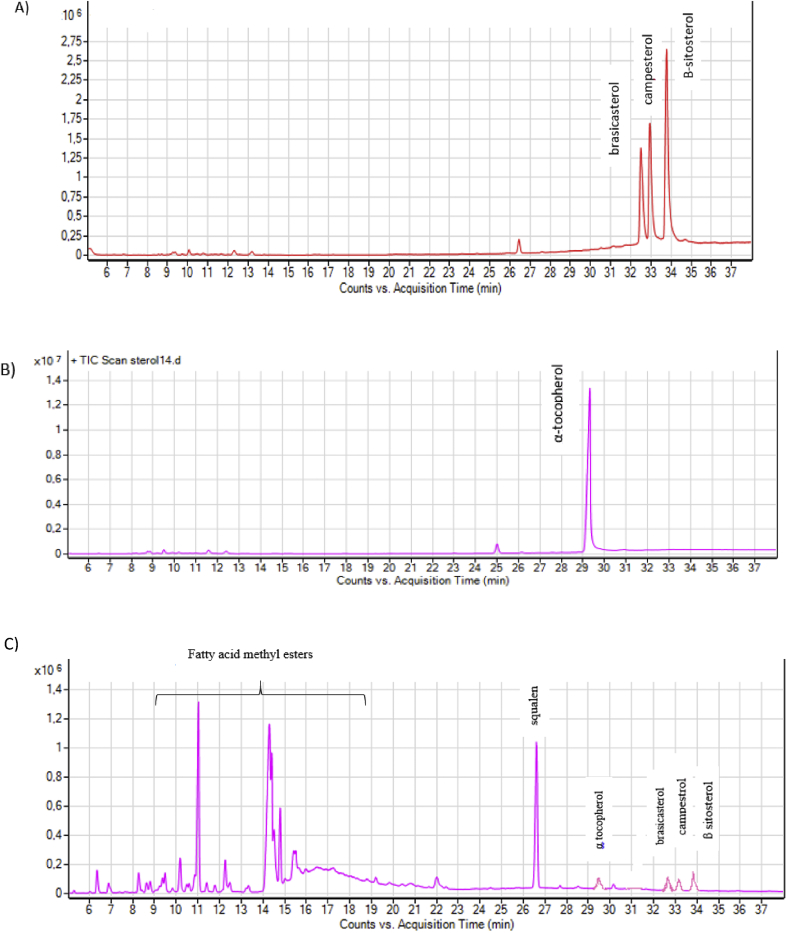
To identify the different phytosterols present in the extracts, GC-MS analysis was conducted. The typical GC-MS chromatogram of the extracts obtained by SFE is shown in Figure 4. The obtained peaks were compared with those obtained via modified Soxhlet extraction. According to Figure 4C, eight different bioactive compounds including squalene, nonacosane, δ-tocopherol, β-tocopherol, α tocopherol, brassicasterol, campesterol, β-sitosterol (in very low quantity), and fatty acid methyl esters were identified in the solvent-extracted (Soxhlet) sample. Lucas et al [40] reported that due to the polar character of tocopherol and the facility of the thermal and oxidative degradation there was no tocopherol content in the hexane extraction.
Figure 4.
GC-MS chromatogram of extracted A) phytosterols and B) tocopherols using SFE with mixed fluid (95% CO2 and 5% ethanol; 40 °C; and 350–400 bar); C) GC-MS chromatogram of extracted rapeseed by-product after modified soxhlet extraction at 60 °C.
From Figure 4, it is noticed that when the samples were extracted by SFE, three different sterols, namely β-sitosterol (50 wt %, the main), brassicasterol (24 wt %) and campesterol (36 wt %) were identified with good separation on the basis of peak area and retention time (Figure 4A). In the case of tocopherols, only α-tocopherol was recognized (Figure 4B). This can be attributed to the higher solubility of α-tocopherol than the other homologues in SC-CO2, which is due its molecular structure. Given that the chroman ring of α-tocopherol has three methyl groups (R1, R2 = CH3), better shielding of the polar –OH group occurs relative to other tocopherols, leading to enhanced solubility in CO2 as a non-polar solvent [12]. This was also demonstrated in the study of Antonie and Pereira [41] where α-tocopherol was found to have greater solubility than the δ-species in the respective binary mixtures.
3.6. Comparison of supercritical fluid and modified soxhlet extraction
In the extraction of phytosterols and tocopherols, the utilized SFE technique has multiple advantages relative to modified Soxhlet extraction (Table 5) the loss of analytes is reduced due to the minimization of sample-handling steps, which also results in shorter analysis time. Given that clean extracts are obtained by SFE with CO2 and ethanol, cleanup steps become unnecessary and GC-MS can be used to directly analyze the extracts. Higher extraction efficiency was obtained when 95% CO2 + 5 wt % ethanol was employed as the mixed supercritical fluid in comparison with other mixtures. Compared with the modified Soxhlet extraction technique, SFE resulted in efficiencies that were three times higher, and a much higher concentration of target compounds was obtained over a specific period (60 min) of extraction time.
The Soxhlet extraction takes an average of 2–72 h because of the production of high volume after completed extraction, the concentrate of dilute solution is necessary prior to analysis. the extraction of thermo sensitive compounds which are degraded at high temperature during long extraction time is one of the main limiting steps of this method [42]. Comparison of supercritical fluid extraction with conventional extraction methods was done by several researchers [43, 44]. They reported that by Soxhlet extraction, higher crude extraction yield could be attained with lower separation of bioactive compounds as they are thermo liable compounds. While applying ultrasound-assisted extraction, SC-CO2 extraction, and SC-CO2 extraction produce lower quantity of crude extraction yield with better quality in terms of valuable bioactive compounds separation.
This study demonstrates that the SFE method is a good alternative for the separation of phytosterols and vitamin E from the waste of vegetable oils; lower extraction times and less use of high-purity solvents are amongst the advantages of this technique relative to conventional methods.
Declarations
Author contribution statement
Parisa J. Asl: Conceived and designed the experiments; Performed the experiments.
Razieh Niazmand: Conceived and designed the experiments, Analyzed and interpreted the data; Wrote the paper.
Franaz Yahyavi: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank the Khorasan Razavi Small Industries and Industrial Parks Organization of Iran and Professor Maria Jose Alonso Cocero for providing valuable technical support and allowing us to use his lab equipments at Valladolid university in Spain.
References
- 1.Sherazi S.T.H., Mahesar S.A. Vegetable oil deodorizer distillate: a dich, source of the natural bioactive components. J. Ole Sci. 2016;65:957–966. doi: 10.5650/jos.ess16125. [DOI] [PubMed] [Google Scholar]
- 2.Naz S., Sherazi S.T.H., Talpour F. Chemical Characterization of Canola and Sunfl ower oil deodorizer distillates. J. Food Nutr. Sci. 2014;17:115–120. [Google Scholar]
- 3.Yang H., Yan F., Wu D., Huo M.J., Li Y. Cao, Recovery of phytosterols from waste residue of soybean oil deodorizer distillate. J. Bio Tech. 2010;101:1471–1476. doi: 10.1016/j.biortech.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Cantrill R. 2008. Phytosterols, Phytostanols and Their Esters Chemical and Technical Assessment. 69th JECFA; pp. 1–13. [Google Scholar]
- 5.Duba K.S., Fiori L. Supercritical CO2 extraction of grape seed oil: effect of process parameters on the extraction kinetics. J. Super Fluids. 2015;98:33–43. [Google Scholar]
- 6.Mendes M.F.F., Pessoa L.P. An economic evaluation based on an experimental study of the vitamin E concentration present in deodorizer distillate of soybean oil using supercritical CO2. J. Super Fluids. 2002;23:257–265. [Google Scholar]
- 7.Fornari T., V´azquez L., Torres C., Ibanez F.E. Countercurrent supercritical fluid extraction of different lipid-type materials: experimental and thermodynamic modeling. J. Super Fluids. 2008;45:206–212. [Google Scholar]
- 8.Chiehming C., Yu-Fang C., Hong-zhi L., Jia-qun L., Po-Wen Y. Supercritical carbon dioxide extraction of high-value substances from soybean oil deodorizer distillate. J. Ind. Eng. Chem. 2000;39:4521–4525. [Google Scholar]
- 9.Gunawan S., Ju Y.H. Vegetable oil deodorizer distillate: characterization, utilization and analysis. J. Sep. Puri Rev. 2009;38:207–241. [Google Scholar]
- 10.Gunawan S., Ismadji S., Ju Y.-H. Design and operation of a modified silica gel column chromatography. J. Chin. Inst. Chem. Eng. 2008;39:625–633. [Google Scholar]
- 11.Asl P.J., Niazmand R., Jahani M. Theoretical and experimental assessment of supercritical CO2 in the extraction of phytosterols from rapeseed oil deodorizer distillates. J. Food Eng. 2019 [Google Scholar]
- 12.Hernandez E.J. High-pressure phase equilibria of squalene + carbon dioxide: new data and thermodynamic modeling. J. Chem. Eng. Data. 2010;40:3606–3611. [Google Scholar]
- 13.Guclu-Ustundag O., Temelli F. Column fractionation of canola oil deodorizer distillate using supercritical carbon dioxide. J. Am. Oil Chem. Soc. 2007;84:953–961. [Google Scholar]
- 14.Mendes M.F., Pessoa F.L.P., Coelho G.V. Recovery of the high aggregated compounds present in the deodorizer distillate of the vegetable oils using supercritical fluids. J. Super Fluids. 2005;34:157–162. [Google Scholar]
- 15.Tai H.f., Brunner G. Extraction of oil and minor compounds from oil palm fruit with supercritical carbon dioxide process. J. Super Fluids. 2019;7:107–115. [Google Scholar]
- 16.Suleiman N., Baharin B., Mirhosseini S., Mohd Helmi A. Optimisation of squalene recovery from palm oil by-product using integrated scco 2 -pressure swing. J. Oil Palm Res. 2019;30:570–578. [Google Scholar]
- 17.AOCS . 1997. Official Method Ce. Preparations of Methyl Esters of Fatty Acids. [Google Scholar]
- 18.Verleyen T., Verhe R., Garcia L., Dewettinck K. Gas chromatographic characterization of vegetable oil deodorization distillate. J. Chrom. A. 2001;921:277–285. doi: 10.1016/s0021-9673(01)00881-0. [DOI] [PubMed] [Google Scholar]
- 19.AOAC . seventeenth ed. Vol. 933. Association of official agricultural chemists; Washington dc: 1997. p. 8. (Official Methods of Analysis). [Google Scholar]
- 20.AOCS (American Oil Chemists’ Society) In: Official Methods and Recommended Practices of the AOCS. fifth ed. Firestone D., editor. AOCS; 2005. [Google Scholar]
- 21.Wong M.L. Colorimetric determination of total tocopherols in palm oil. J. Ole. Stear. 1988;78:258–261. [Google Scholar]
- 22.Sabir S.M., Imran H., Ahmed S.D. Estimation of sterols in edible fats and oils. Pakistan J. Nutr. 2003;2:130–139. [Google Scholar]
- 23.Petterson D.M., Jensen C.M., Hoffman D.L. Oat tocols: saponification vs. Direct extraction and analysis in high-oil genotypes. J Cere. Chem. 2007;84:56–60. [Google Scholar]
- 24.Liu Y., Wang L., Yan Y. Biodiesel synthesis combining pre-esterification with alkali catalyzed process from rapeseed oil deodorizer distillate. J. Fuel. Pro. Technol. 2009;90:857–862. [Google Scholar]
- 25.Santos K.A., Aragão O.P. Fo., Aguiar C.M., Milinsk M.C., Sampaio S.C., Palú F., Silva E.A. Chemical composition, antioxidant activity and thermal analysis of oilextracted from favela (Cnidoscolus quercifolius) seeds. J. Ind. Crops Prod. 2017;97:368–373. [Google Scholar]
- 26.Wang S., Hwang H., Yoon S., Choe E. Temperature dependence of autoxidation of perilla oil and tocopherol degradation. J. Food Sci. 2010;75:498–505. doi: 10.1111/j.1750-3841.2010.01681.x. [DOI] [PubMed] [Google Scholar]
- 27.Busch T.P., King A.J. Stability of cholesterol, 7-ketocholesterol and β-sitosterol during saponification: ramifications for artifact monitoring of sterol oxide products. JAOCS J. Am. Oil Chem. Soc. 2010;87:955–962. doi: 10.1007/s11746-010-1572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lima H., Woob S., Hong S., Seung-Ke Jongb, Junsoo L. Comparison of extraction methods for determining tocopherols in soybeans. Eur. J. Lipid Sci. Technol. 2007;109:1124–1127. [Google Scholar]
- 29.Kornsteiner M., Wagner K., Elmadfa I. Tocopherols and total phenolics in 10 different nut types. J. Food Chem. 2006;98:381–387. [Google Scholar]
- 30.Ribeiro P., Silva D., Dantas M. Determination of copherols and physicochemical properties of faveleira (Cnidoscolus quercifolius) seed oil extracted using different methods. J. Food Sci. Tech. 2019;39:57–62. [Google Scholar]
- 31.Santos K.A., Aragão O.P. Fo., Aguiar C.M., Milinsk M.C., Sampaio S.C., Palú F., Silva E.A. Chemical composition, antioxidant activity and thermal analysis of oilextracted from favela (Cnidoscolus quercifolius) seeds. J. Ind. Crops Prod. 2017;97:368–373. [Google Scholar]
- 32.Turner C., King J., Mathiasson W.L. Supercritical fluid extraction and chromatography for fat-soluble vitamin analysis. J. Chrom. A. 2001;936:215–237. doi: 10.1016/s0021-9673(01)01082-2. [DOI] [PubMed] [Google Scholar]
- 33.Güçlü-Üstündağ O., Temelliو F. Solubility behavior of ternary systems of lipids, co-solvents and supercritical carbon dioxide and processing aspects. J. Supercrit. Fluids. 2005;36:1–15. [Google Scholar]
- 34.Martins P.F., de Melo Marcelo M.R., Pedro S., Carlos M.S. Supercritical fluid extraction of sterols from Eichhornia crassipes biomass using pure and modified carbon dioxide. Enhancement of stigmasterol yield and extract concentration. J. Super Fluids. 2016;107:441–449. [Google Scholar]
- 35.Lee H., Chung B., Park Y. Concentration of tocopherols from soybean sludge bysupercritical carbon dioxide. J. Am Oil Chem Soc. 1991;68:571–573. [Google Scholar]
- 36.Da Porto Carla, Decorti Deborha, Natolino Andrea. Water and ethanol as co-solvent in supercritical fluid extraction of proanthocyanidins from grape marc: a comparison and a proposal. J. Super Fluids. 2014;87:1–8. [Google Scholar]
- 37.Gaset K., Jungfer M., Saure C.G., Brunner Purification of tocochromanols from edible oil. J. Super Fluids. 2005;34:17–25. [Google Scholar]
- 38.Temelli F. Supercritical Fluids Perspectives on supercritical fluid processing of fats and oils. J. Super Fluids. 2009;47:583–590. [Google Scholar]
- 39.Gurina Darya L., Antipova Marina L., Odintsova Ekaterina G., Petrenko Valentina E. Selective solvation in cosolvent-modified supercritical carbon dioxide on the example of hydroxycinnamic acids. The role of cosolvent self-association. J. Super Fluids. 2017;17:896–8446. [Google Scholar]
- 40.de Lucas A., De la Martinez., Rinco N., Ossa b J. Supercritical fluid extraction of tocopherol concentrates from olive tree leaves. J. Super Fluids. 2002;22(2):221–228. [Google Scholar]
- 41.Antonie P., Pereira C.G. Solubility of functional compounds in supercritical CO2: data evaluation and modelling. J. Food Eng. 2019;245:131–138. [Google Scholar]
- 42.Mandana B., Russly A.R., Ali G., Farah S.T., Liza M.D.S. Comparison of different extraction techniques for isolation of major bioactive flavonoid compounds from spearmint (MenthaSpicata L.) leaves. J. Food Bio. Proc. 2011;89:67–72. [Google Scholar]
- 43.Mandana B., Russly A.R., Ali G., Farah S.T., Liza M.D.S. Optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from spearmint (Menthaspicata L.) leaves by using response surface methodology. J. Food Bio. Tech. 2012;5:912–921. [Google Scholar]
- 44.Myer L., Damian J., Liescheski P., Tehrani J. Isco; Lincoln, NE: 1992. Supercritical. Fluid Extraction of Plant Materials. [Google Scholar]