Abstract
Cases of vaping-induced lung injury have increased in the USA, resulting in a heterogeneous collection of pneumonitis patterns in persons who used electronic cigarettes. Hypersensitivity pneumonitis has been documented in several cases of first-hand electronic cigarette use; however, secondhand smoke health-related consequences have not been fully understood. We present a case of the patient who developed hypersensitivity pneumonitis secondary to exposure to secondhand smoke from electronic cigarette. We summarise the presentation and diagnostic investigation, as well as the management of this case.
Keywords: interstitial lung disease, bronchopulmonary dysplasia, tobacco related disease
Background
The cases of vaping-induced lung injury have increased in identification in the USA.1–3 These cases demonstrate a diverse collection of pneumonitis patterns ranging from lipoid pneumonia to hypersensitivity pneumonitis to acute respiratory distress syndrome. In regard to electronic cigarette or vaping product use-associated lung injury (EVALI), while 82% of the cases in the USA were found to be associated with tetrahydrocannabinol-containing products, there is a minority of EVALI where the toxins were unknown.2 Electronic cigarette fluids contain potentially toxic compounds including nicotine, carbonyls, volatile organic compounds and aldehydes.4 Further, the mixing of primary active compounds with various contaminants may result in the production of new potentially toxic agents.5 While the exact compound or compounds have not been identified to be the primary cause of these lung injuries, the use of electronic cigarettes appears to be the initial inciting circumstance.
Hypersensitivity pneumonitis has been described as a complication of electronic cigarette use.1 3 Hypersensitivity pneumonitis is a complex pulmonary syndrome mediated by an already sensitised immune system reacting to inhaled antigens.6 There are both acute and chronic manifestations of the disease, whereby the characteristic bronchiolocentric granulomatous lymphocytic alveolitis seen on pathology may evolve to fibrosis in advanced disease.7 The definitive diagnosis requires identifying a known antigen in conjunction with supporting data from clinical history and symptoms, radiological findings, laboratory data and, oftentimes, biopsy of the lung. While a diagnosis of hypersensitivity pneumonitis has been identified in electronic cigarette use, there are no reported cases currently of the development of this syndrome from secondhand smoke exposure to the aerosol from electronic cigarettes.
In this report, we present the case of a patient with dyspnea on exertion associated with a diagnosis of hypersensitivity pneumonitis from secondhand smoke exposure of electronic cigarettes. The diagnosis proved challenging, ultimately necessitating a careful review of antigen exposure and surgical biopsy of the lung. Further, we discuss in detail the electronic cigarette aerosol content that pose a significant risk to the development of hypersensitivity pneumonitis, in both first-hand and secondhand exposure.
Case presentation
A 37-year-old woman presented with 2 years of wheezing. Her medical history was only significant for premature births, as the patient was born at 26 weeks gestation and required supplemental oxygen.
In 2016, the patient (then 34 years of age) was found to have expiratory wheezing and was diagnosed with ‘asthma’ during her annual physical examination. She had no active pulmonary symptoms such as dyspnea on exertion or cough and was reluctant to start an inhaler. However, despite the initiation of an inhaler (an inhaled corticosteroid), she continued to have wheezing and self-discontinued the treatment. Given the persistent wheezing, she underwent formal imaging at an outside facility of her chest via a non-contrast CT scan. The imaging showed ‘diffuse mosaic attenuation throughout the lung fields’, which describes alternating low-attenuation and high-attenuation areas within the lungs often present in small airways disease. She underwent a bronchoscopy in 2016 at an outside facility and was told that the findings were ‘non-diagnostic’. With the concern of her CT scan findings, and without a definitive aetiology, she sought a second opinion.
In 2018, she presented to our clinic for further discussion of the abnormal CT scan findings as well as newly developed dyspnea on exertion. The patient denied any symptoms of orthopnea, paroxysmal nocturnal dyspnea, lower extremity oedema, coughing or sputum production. She also denied joint pain or rashes. Her modified Medical Research Council (mMRC) Dyspnea Scale was +2 at the time of the clinic visit (meaning that the patient walked slower than the people of the same age due to dyspnea). On pulmonary function testing, the patient demonstrated airflow limitation (forced expiratory volume in 1 s (FEV1) 1.69 (59%), forced vital capacity (FVC) 2.98 (87%) and FEV1/FVC 57%) and a decreased diffusing capacity of the lungs for carbon monoxide (DLCO) (16.37 mL/min/mm Hg (76%)). She underwent a non-contrast CT scan of the chest with both inspiratory and expiratory views (figure 1). Expiratory phase images confirmed multifocal air-trapping, demonstrated by increased conspicuity of mosaicism, consistent with small airways disease. Findings of fibrosis, such as reticulations, traction bronchiectasis and architectural distortion, were absent on imaging. Further, no supraclavicular, mediastinal or hilar lymphadenopathy was present on imaging. Given the indication of small airways disease, an extensive medical history and exposure as well as laboratory data were reviewed.
Figure 1.
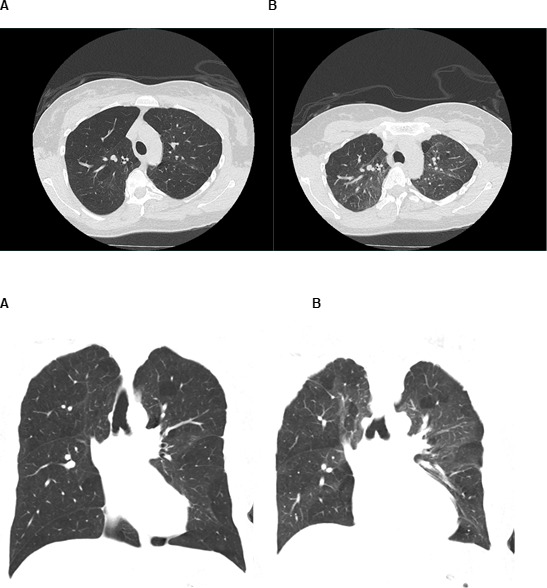
Coronal CT images of the chest with both inspiration (A) and expiration (B). Patchy segmental/subsegmental zones of decreased attenuation (mosaic attenuation) is present throughout the bilateral lungs and more conspicuous on the expiratory phase, confirming air trapping secondary to small airways disease.
The patient endorsed being a never smoker. She had three children, all delivered vaginally without any complications. The patient discussed an active childhood (played a significant amount of sports) without any hospitalisations other than at the time of her own birth. She denies having any pets and no exposure to farm animals. However, the only identification of a risk factor discovered was that of secondhand vaping aerosol exposure by her live-in partner who had used electronic cigarettes since 2015.
Investigations
In regard to laboratory testing, an autoimmune-related lung disease panel was investigated that could result in small airways disease: anti-neutrophil cytoplasmic antibody (ANCA), anti-double-stranded DNA antibody, anti-nuclear antibody, anti-Ro antibody, anti-La antibody, anti-glomerular basement membrane antibody, anti-phospholipid antibody, anti-Smith and anti-ribonucleoprotein (RNP) antibodies. These were all negative. Fungal serologies were also negative. A hypersensitivity panel to common pathogens was also sent and found to be negative. Further, the patient’s differential cell count was unremarkable.
Within a month after the patient’s initial clinic visit, she underwent a video-assisted thoracoscopic wedge resection of her right middle lobe. The pathology demonstrated lung overinflation and mild emphysematous changes and focal predominantly subpleural small, well-formed, non-necrotising granulomas (figure 2). The biopsy further revealed patchy mild bronchiolitis with bronchioles containing vacuolated macrophages.
Figure 2.
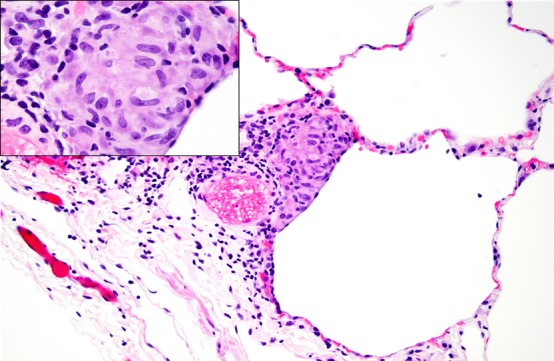
Portion of lung with mild emphysematous changes, focal predominantly subpleural small, well-formed, non-necrotising granulomas, and mild focal chronic bronchiolitis.
Differential diagnosis
The emphysema seen in the patient’s biopsy was thought to be related to perinatal bronchopulmonary dysplasia caused by premature birth at 26 weeks.
In regard to the non-necrotising granulomas, laboratory data and radiological findings, the differential diagnosis was limited to sarcoidosis or hypersensitivity pneumonitis.
Treatment
After consultation with several other experts, hypersensitivity pneumonitis was recognised as the patient’s most likely diagnosis. An extensive discussion occurred with the likelihood that the resulting cause of the condition was secondhand aerosol exposure from the electronic cigarettes of the patient’s partner.
For treatment, we initiated steroids at 0.5 mg/kg/day with plans to titrate after 3 months time and repeat pulmonary function testing in addition to reassessing symptoms. Further, we strongly recommended to avoid any exposure to electronic cigarette aerosol.
Outcome and follow-up
After 3 months of steroids at 0.5 mg/kg/day, pulmonary function testing did not show any significant changes: FEV1 1.66 (59%) and DLCO 16.68 mL/min/mm Hg (77%). Her total lung capacity (TLC) was 4.29 L and her alveolar volume (VA) was 4.24 L. However, it was disclosed that she was still exposed to secondhand smoke exposure. After a conversation with the patient’s partner, assistance was offered to help discontinue electronic cigarette usage.
At 9 months, the patient had completed 6 months of high-dose steroids ultimately tapered off and had avoided secondhand smoke exposure for 4 months. Her dyspnea on exertion had resolved (mMRC Dyspnea Scale 0) and her pulmonary function tests remained stable: FEV1 1.64 (58%) and DLCO 15.55 mL/min/mm Hg (73%). It should be noted that during her inspiratory attempt to measure the DLCO, she had an inadequate inspired volume (as evident by a reduced VA value of 4.07 L while the TLC was measured at 4.27 L), which may have underestimated the true value of the DLCO.
Moving forward, we emphasised ongoing avoidance of any first-hand use or secondhand exposure to electronic cigarette aerosol.
Discussion
Hypersensitivity pneumonitis is the result of a cell-mediated immune response to a diverse group of inhaled antigens. The syndrome manifests commonly to antigens associated with microbial agents, such as mould or animal proteins.7 However, reactions to chemicals after exposure have also been documented.6 8 In regard to electronic cigarettes and their causality in hypersensitivity pneumonitis, sound plausible discussions could point to both microbial agents as well as chemical ones.
In regard to chemicals, low-molecular-weight substances may result in hypersensitivity pneumonitis. One such chemical is isocyanate, where cases have been identified prior whereby isocyanate was the inciting antigen.8–10 Isocyanates are not antigenic by themselves; however, they may combine with host proteins forming haptens, which in turn are antigenic.7 Further, various aerosolised chemical substances can form bonds with human proteins and induce an exaggerated immune response in susceptible individuals, resulting in hypersensitivity pneumonitis. Electronic cigarettes contain various chemicals, some with isocyanate,4 which likely pose a risk for the development of this immune reaction in susceptible individuals, such as our patient. While commercial laboratories are available to test for up to 10 common causes of hypersensitivity pneumonitis, disease may occur from exposure to substances not included in these panels. This is especially true in this day and age where the list of inhaled substances or mixtures known to cause hypersensitivity pneumonitis continues to grow, limiting the ability to diagnose this syndrome by the detection of precipitating antibodies.
Another potential culprit to the causation of hypersensitivity pneumonitis from electronic cigarettes could be a microbial growth antigen. Metalworking fluids may contain carbon (in the form of lipids) and water, whereby water-based metalworking fluids routinely sustain microbial growth.11 For instance, nontuberculosis mycobacteria have been identified in water-based metalworking fluids and resulting hypersensitivity pneumonitis in employees exposed to such aerosols.11 12 Nontuberculosis mycobacteria, such as Mycobacterium avium, are well known to grow in high water temperature and within aerosols of water droplets. Therefore, it is plausible that microbial antigens could induce similar hypersensitivity pneumonitis in users of electronic cigarettes, as well as secondhand smoke exposure.
To date, we do not have an extensive understanding of the number of toxins present in the secondhand aerosol of electronic cigarettes. Even secondhand smoke from combustible cigarettes poses challenges to field studies attempting to comprehensively evaluate all of the potential toxins in secondhand smoke.13 In regard to the secondhand aerosol composition of electronic cigarettes, there is evidence that particulate matter and volatile organic compounds are present, though it is often cited as ‘not significant amounts’ since larger concentrations are found in combustible cigarettes.14 However, there is no certain level of ‘safe exposure’ to such aerosols, especially in the ability to induce the cell-mediated immune response needed to develop hypersensitivity pneumonitis.
One differential that should be considered is sarcoidosis. However, there are several findings that make sarcoidosis less likely. First, there was no finding of perilymphatic nodularity or any lung nodularity for that matter on imaging. Nodules in sarcoidosis represent aggregates in granulomas and are seen in 80%–100% of patients with sarcoidosis.15 Second, there were no findings of mediastinal or hilar lymphadenopathy, both of which are commonly seen in sarcoidosis.15 Finally, while mosaic attenuation can occasionally be seen in sarcoidosis, it does so in the context of other pulmonary findings (eg, nodules and/or masses) which were not present on this patient’s imaging.15 Therefore, while sarcoidosis was in the differential diagnosis given the imaging findings, the patient’s history, symptoms and pathology more strongly support hypersensitivity pneumonitis as the appropriate diagnosis.
In regard to the management of our patient, we initiated both steroids and avoidance of the potential trigger. Three months into treatment, she did not find a significant improvement with the steroid management alone, since exposure to the secondhand aerosol continued. Once the patient was no longer exposed to the secondhand aerosol, she did identify significant changes in her symptoms, even after the steroids had been tapered off. There was no evidence of lung fibrosis from the patient’s hypersensitivity pneumonitis, both on biopsy and on imaging; therefore, the patient’s subacute presentation as well as resolution appear to be consistent with the gathered clinical data.
In conclusion, our case demonstrates the development of hypersensitivity pneumonitis in an adult patient due to secondhand smoke exposure from electronic cigarettes. While no definitive identification of an antigen was found through traditional laboratory data, the patient’s radiographic findings, biopsy results, as well as management indicate that the secondhand aerosol is the most likely culprit. Steroids may have helped with regard to the patient’s syndrome, though the improvement was seen once the patient was able to completely avoid secondhand aerosol from the electronic cigarettes. Further, it is unclear if this pulmonary ramification was due to vaping aerosol exposure in an individual with underlying susceptibility factors, such as bronchopulmonary dysplasia. More information is needed to understand the composition of secondhand aerosol from electronic cigarettes, especially how it varies with regard to brand and device.
Patient’s perspective.
Doctors should ask their patients if they are around electronic cigarette smoke, in addition to if they actually use the devices.
Learning points.
Hypersensitivity pneumonitis has been reported in first-hand users of electronic cigarettes; however it is unclear if it can develop from passive exposure to secondhand aerosol of electronic cigarettes.
Hypersensitivity pneumonitis from electronic cigarettes is likely due to both microbial and non-microbial antigens in the aerosol formation found in the secondhand aerosol.
Definitive management of hypersensitivity pneumonitis from secondhand aerosol exposure from electronic cigarettes warrants further investigation, but is likely to consist of oral steroids and avoidance of the secondhand aerosol.
Footnotes
Contributors: PG, PS and EN helped design the case report and review the case thoroughly. EG and CL and PBI all contributed in reviewing the case, the manuscript draft and providing thorough edits, as well as insight from their respective fields (radiology and pathology). All authors assured the manuscripts final draft was well-written and edited thoroughly.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Layden JE, Ghinai I, Pray I, et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Preliminary Report. N Engl J Med 2019. [DOI] [PubMed] [Google Scholar]
- 2.Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury - United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep 2020;69:90–4. 10.15585/mmwr.mm6903e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sommerfeld CG, Weiner DJ, Nowalk A, et al. Hypersensitivity pneumonitis and acute respiratory distress syndrome from e-cigarette use. Pediatrics 2018;141. doi: 10.1542/peds.2016-3927. [Epub ahead of print: 17 May 2018]. [DOI] [PubMed] [Google Scholar]
- 4.Klager S, Vallarino J, MacNaughton P, et al. Flavoring chemicals and aldehydes in e-cigarette emissions. Environ Sci Technol 2017;51:10806–13. 10.1021/acs.est.7b02205 [DOI] [PubMed] [Google Scholar]
- 5.Christiani DC, Injury V-IL. Vaping-Induced acute lung injury. N Engl J Med 2020;382:960–2. 10.1056/NEJMe1912032 [DOI] [PubMed] [Google Scholar]
- 6.Spagnolo P, Rossi G, Cavazza A, et al. Hypersensitivity pneumonitis: a comprehensive review. J Investig Allergol Clin Immunol 2015;25:237–50. [PubMed] [Google Scholar]
- 7.Selman M, Pardo A, King TE. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med 2012;186:314–24. 10.1164/rccm.201203-0513CI [DOI] [PubMed] [Google Scholar]
- 8.Wild LG, Lopez M. Hypersensitivity pneumonitis: a comprehensive review. J Investig Allergol Clin Immunol 2001;11:3–15. [PubMed] [Google Scholar]
- 9.Axford AT, McKerrow CB, Jones AP, et al. Accidental exposure to isocyanate fumes in a group of firemen. Br J Ind Med 1976;33:65–71. 10.1136/oem.33.2.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lhoumeau A, Pernot J, Georges M, et al. Hypersensitivity pneumonitis due to isocyanate exposure in an airbag "welder". Eur Respir Rev 2012;21:168–9. 10.1183/09059180.00008811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckett W, Kallay M, Sood A, et al. Hypersensitivity pneumonitis associated with environmental mycobacteria. Environ Health Perspect 2005;113:767–70. 10.1289/ehp.7727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreiss K, Cox-Ganser J. Metalworking fluid-associated hypersensitivity pneumonitis: a workshop summary. Am J Ind Med 1997;32:423–32. [DOI] [PubMed] [Google Scholar]
- 13.Moritsugu KP. The 2006 report of the surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med 2007;32:542–3. 10.1016/j.amepre.2007.02.026 [DOI] [PubMed] [Google Scholar]
- 14.Czogala J, Goniewicz ML, Fidelus B, et al. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res 2014;16:655–62. 10.1093/ntr/ntt203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunes H, Uzunhan Y, Gille T, et al. Imaging of sarcoidosis of the airways and lung parenchyma and correlation with lung function. Eur Respir J 2012;40:750–65. 10.1183/09031936.00025212 [DOI] [PubMed] [Google Scholar]


