Abstract
Background
Checkpoint inhibitors (CPIs) are thought to be effective against cutaneous melanoma in part because of the large burden of somatic mutations (neoantigens) generated from exposure to ultraviolet radiation. However, rare melanoma subtypes arising from acral skin, mucosal surfaces, and the uveal tract are largely sun-shielded. Genomic studies show these sun-shielded melanomas have a paucity of neoantigens and unique biology; they are thought to be largely resistant to immunotherapy. It has not been definitively shown that CPI improves survival in metastatic sun-shielded melanoma.
Methods
We reviewed a single institutional experience using antibodies against CTLA-4, PD-1 and/or PD-L1 to treat patients with metastatic melanoma. Primary tumor histology was categorized as cutaneous, unknown, acral, mucosal, or uveal. We studied demographic data, treatment characteristics, and overall survival (OS) after CPI.
Results
We treated 428 patients with metastatic melanoma from 2007 to 2019. Primary tumors were cutaneous in 283 (66%), unknown in 55 (13%), acral in 22 (5%), mucosal in 38 (9%), and uveal in 30 (7%). Patients with metastatic disease from cutaneous primary tumors had median OS after CPI of 45 months compared with 17 months for acral (p=0.047), 18 months for mucosal (p=0.003), and 12 months for uveal (p<0.001). For all patients with sun-shielded melanoma (n=90), first treatment with anti-PD-1 or anti-PD-L1 was followed by a median OS of 9 months compared with 18 months after anti-CTLA-4 (p=0.010) and 20 months after combination therapy (p=0.003). There were 21 patients who achieved actual 3-year survival; 20 received both anti-CTLA-4 and anti-PD-1, either sequentially or in combination. Over 80% of 3-year survivors with progressive disease were treated with local therapy after CPI.
Conclusions
Long survival in patients with metastatic melanoma from acral, mucosal, and uveal primary tumors was associated with receipt of both anti-CTLA-4 and anti-PD-1 antibodies. Complete responses were rare, and local therapy was frequently employed to control disease progression. While sun-shielded melanomas exhibit worse outcomes after CPI than cutaneous melanomas, with an aggressive multidisciplinary approach, 5-year survival is still possible for 25%–32% of these patients.
Keywords: melanoma, immunotherapy, checkpoint inhibitors, acral lentiginous melanoma, mucosal melanoma, uveal melanoma
Background
Checkpoint inhibitors (CPI), including antibodies against CTLA-4, PD-1 and PD-L1, are a highly effective treatment for metastatic cutaneous melanoma.1–4 Combinations of CPI have been shown to mediate objective response rates exceeding 60%, with dramatic improvements in overall survival (OS). In some cases, treatment with CPI has resulted in complete responses (CRs) that have been durable for years and have been apparently curative.5
An epidemiological association between sun exposure and melanoma has been recognized for over half a century.6 One consequence of mutagenesis by ultraviolet (UV) radiation is the accumulation of a large burden of somatic mutations (neoantigens) that are thought to contribute to the immunogenicity of cutaneous melanoma.7 8 Genomic studies have demonstrated that cutaneous melanomas have an average mutation rate of 16.8 mutations per megabase, one of the highest reported for any cancer type thus far analyzed by The Cancer Genome Atlas Program.7 The mutagenic role of UV radiation in cutaneous melanoma was confirmed by studies showing a high fraction of C>T transitions at dipyrimidines as well as tandem double CC>TT mutations.9 Furthermore, UV mutagenesis in melanoma has been linked to unique tumor biology, such as hot-spot mutations in BRAF or or RAS, as well as loss-of-function mutations in NF1.9
In contrast to the more common cutaneous melanomas, rare subtypes of non-cutaneous melanomas, including mucosal and uveal melanomas, are not driven by sun exposure. In fact, a prevailing hypothesis is that the relative paucity of neoantigens in non-cutaneous melanomas renders these tumors less responsive to immunotherapy. Similarly, cutaneous melanomas that are not exposed to the damages of solar radiation, such as acral lentiginous, have also been shown to behave more aggressively.10 We therefore decided to study these three sites collectively as ‘sun-shielded’ melanomas to gain a better insight into their responses to treatment.
Genomic studies of sun-shielded melanomas show they have a median of 9 non-synonymous somatic mutations, compared with 171 for sun-exposed cutaneous melanomas.8 Not surprisingly, other elements of their biology are unique. For example, 83% of uveal melanomas have driver mutations in GNAQ or GNA11.11 Also, sun-shielded melanomas often lack mutations in BRAF, RAS or NF1 and thus display a ‘triple wild-type’ signature, which is associated with a high proportion of copy number changes and complex structural arrangements.9
Before 2010, the prognosis of metastatic melanoma was universally poor regardless of subtype, as few effective systemic therapies were available. One study reported the median survival of patients with metastatic melanoma from cutaneous, acral, uveal, and unknown primaries was similar, ranging from 10 to 13 months.12 In that series, patients with mucosal melanoma fared slightly worse, with a median survival of 9 months.
However, owing to recent advances in systemic therapy, the biological differences between melanoma subtypes have become clinically consequential. Several reports suggest that patients with sun-shielded melanomas treated with CPI, as compared with their cutaneous counterparts, have low objective response rates and brief progression-free survival ranging from 2 to 5 months.13–15
Consistent with low response rates seen in clinical trials, the survival of patients with mucosal and uveal melanoma has remained poor in the modern era. The median survival of patients with metastatic mucosal melanoma treated with ipilimumab was 6.4 months; after anti-PD-1, it was 12.4 months.15 16 For metastatic uveal melanoma, survival after ipilimumab was 6.8–9.6 months; after anti-PD-1, it was 7.6 months.13 17 18 The dismal outcomes reported in these studies are reminiscent of the pre-CPI era. For example, a study of patients with metastatic uveal melanoma from 1993 reported 9-month median survival.19
Interestingly, while the response rates of patients with metastatic acral melanoma are low, clinical data suggest their survival has improved since the advent of CPIs. Patients with acral melanoma treated with ipilimumab had a median OS of 16.7 months; treatment with anti-PD-1 resulted in median OS of 31.7 months.15 20 These outcomes were unheard of before 2010, when median survival was typically 6–12 months.21–23
These reports show that while there are promising data to support the use of CPI in sun-shielded melanoma, the literature in terms of efficacy has been limited. The extent to which CPI impacts survival in these patients is unclear, and there are few data to guide treatment sequencing or the use of adjunctive therapies. With the advantage of long follow-up and in-depth clinical review, we set out to review our institutional experience using CPI to treat patients with metastatic acral, mucosal, and uveal melanoma. We sought to determine their OS, document treatment patterns, and identify the characteristics of long survivors.
Methods
Treatment with CPIs
Patients in this study had metastatic melanoma and received treatment with antibodies against CTLA-4 (ipilimumab), PD-1 (nivolumab or pembrolizumab) and/or PD-L1 (atezolizumab). Treatment was by recommendation from the Yale multidisciplinary melanoma tumor board or under the auspices of clinical trials which have been previously reported.1 2 4 Patients treated with CPI only in the adjuvant setting did not qualify for this report unless they later were treated for metastatic disease. Some received other forms of immunotherapy before CPI, including interferon, interleukin-2 or adoptive cell transfer.
Statistical analysis
The primary melanoma histology was categorized as cutaneous (ie, sun-exposed), unknown, acral, mucosal, or uveal. Demographic characteristics, treatment patterns and outcomes were recorded per tumor histology. OS was measured from the first dose of CPI given for metastatic disease, and is shown using the Kaplan-Meier method. Univariate comparisons were performed using the log-rank test. Continuous variables are shown as median and range, discrete variables as frequency. Demographic data were compared by Kruskal-Wallis (age) or χ2 methods.
Results
A total of 428 patients with metastatic melanoma were treated with CPI from 2007 to 2018, 187 (44%) of whom were enrolled in a clinical trial. With a median follow-up of 45 months, the median OS for the entire population (n=428) from the first cycle of CPI was 34 months and actuarial 5-year survival was 41%. Median OS was 18 months for patients treated with anti-CTLA-4, 34 months for those treated with anti-PD-1 or PD-L1, and not reached for those receiving combination therapy. For comparison, the median OS of patients treated with ipilimumab, nivolumab and combination therapy in the CheckMate 067 trial were 20 and 37 months and not reached, respectively.24 It is worth noting that in our series, 87 patients (20%) had brain metastases and 30 (7%) had uveal melanomas; most of these patients would have been excluded from the CheckMate 067 study.24
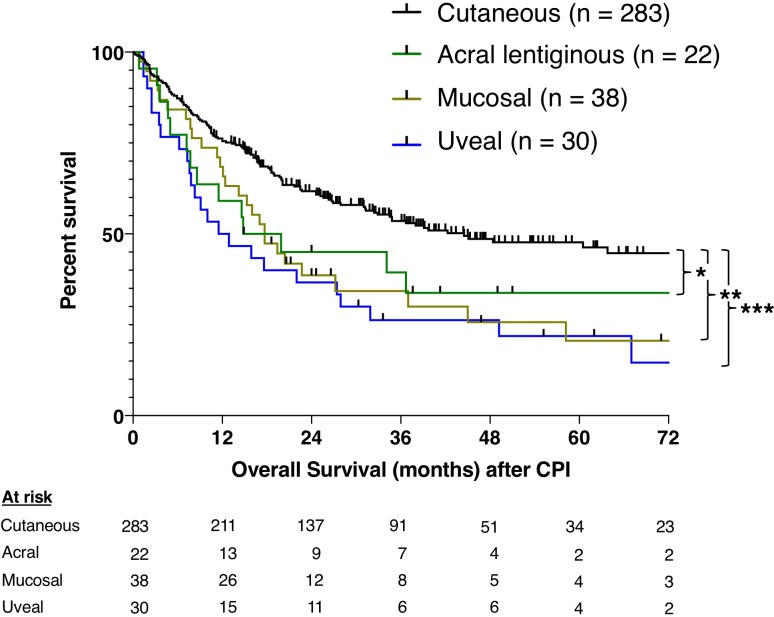
Primary tumors were cutaneous in 283 patients (66%) and unknown in 55 (13%). There were 90 patients with sun-shielded melanomas: 22 acral (5%), 38 mucosal (9%), and 30 uveal (7%) (table 1). Patients with mucosal melanoma had a female preponderance. The proportion staged M1c was high in mucosal (53%) and uveal (83%) patients. Patients with cutaneous primary tumors had a median OS after CPI of 45 months, with 46% 5-year survival (figure 1). Patients with acral melanoma had a 17-month median OS and 34% 5-year survival (acral vs cutaneous, p=0.047). Patients with mucosal melanoma had a median OS of 18 months and 21% 5-year survival (mucosal vs cutaneous, p=0.003). Patients with uveal melanoma had a median OS of 12 months and 22% 5-year survival (uveal vs cutaneous, p<0.001). OS was not statistically different between sun-shielded histologies (acral vs uveal, p=0.342; acral vs mucosal, p=0.842), but small numbers limited the power of this comparison.
Table 1.
Demographics and first treatment
| Cutaneous (n=283) |
Acral (n=22) |
Mucosal (n=38) |
Uveal (n=30) |
|
| Median age (IQR) | 65 (56–75) | 67 (62–73) | 63 (58–71) | 66 (53–72) |
| Gender | 67% male | 59% male | 37% male | 57% male |
| Stage before CPI | ||||
| Stage M1a (%) | 72 (25) | 9 (41) | 6 (16) | 2 (7) |
| Stage M1b (%) | 64 (23) | 6 (27) | 6 (16) | 2 (7) |
| Stage M1c (%) | 87 (31) | 4 (18) | 20 (53) | 25 (83) |
| Stage M1d (%) | 60 (21) | 3 (14) | 6 (16) | 1 (3) |
| First target (%) | ||||
| CTLA-4 | 80 (28) | 6 (27) | 10 (26) | 15 (50) |
| PD-1 or PD-L1 | 95 (34) | 7 (32) | 7 (18) | 4 (13) |
| CTLA-4+PD-1 | 108 (38) | 9 (41) | 21 (55) | 11 (37) |
Figure 1.
Overall survival stratified by histology. Kaplan-Meier curves show overall survival from the first dose of CPI. Comparisons were performed using the log-rank test. *P<0.05, **P<0.01, ***P<0.001. CPI, checkpoint inhibitor.
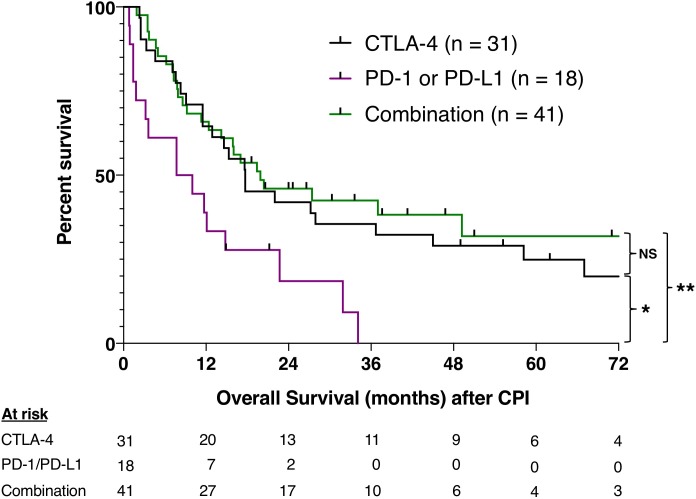
We also evaluated OS stratified by the first type of CPI treatment. For this analysis, we evaluated the 90 patients with sun-shielded melanoma together. The first treatment was with anti-CTLA-4 in 31 patients, anti-PD-1 or anti-PD-L1 in 18, and a combination in 41 patients. There were no statistically significant differences in the type of treatment used per histology (table 1). The median OS after anti-PD-1 or anti-PD-L1 alone was 9 months, compared with 18 months after anti-CTLA-4 alone (p=0.010) and 20 months after combination therapy (p=0.003) (figure 2). It is noteworthy that 20 of 31 (68%) patients treated with anti-CTLA-4 eventually received anti-PD-1 or PD-L1 at a median of 7.4 months after anti-CTLA-4 (IQR 4.9–12.4 months). In contrast, only 5 of 18 (28%) patients treated with anti-PD-1 or PD-L1 monotherapy later received anti-CTLA-4 at a median of 2.6 months (IQR 2.2–8.0 months) (p=0.02).
Figure 2.
Overall survival stratified by first CPI treatment. Kaplan-Meier curves show OS from the first dose of CPI. Some patients treated with anti-CTLA-4 or anti-PD-1/PD-L1 later received the other agent either in combination or as monotherapy. Comparisons were performed using the log-rank test. *P<0.05, **P<0.01. CPI, checkpoint inhibitor; NS, not statistically significant.
Twenty-one patients with sun-shielded melanomas had actual 3-year survival. Ten patients received anti-CTLA-4 followed by anti-PD-1 later, and 10 were treated with a combination upfront. Five patients (5.6%) had a complete response to CPI; one had a short follow-up, while two recurred but have ongoing complete responses after reinduction (table 2). Of the 17 patients with 3-year survival who had disease progression after CPI, 14 (82%) were treated with local therapy (surgery, ablation, or stereotactic radiosurgery) for therapeutic and/or palliative indications. Ten patients had actual 5-year survival; three were complete responders, while six of the seven incomplete responders required local therapy (table 2).
Table 2.
Characteristics of 5-year survivors
| Pt | Primary histology | Dx - CPI (months) | M stage | First CPI | PFS (months) |
Subsequent systemic Rx | Local | Regional | OS (months) |
Vital status |
| 1 | Acral | 34 | M1a | CTLA-4 | 4 | PD-1, chemo, trial | None | ILP | 90 | DOD |
| 2 | Acral | 14 | M1a | CTLA-4 | 8 | PD-1, sorafenib | SRS, surg, SBRT | RT | 79 + | NED |
| 3 | Mucosal | 4 | M1c | CTLA-4+PD-1 | 38 CR | CTLA-4+PD-1 | None | None | 99+ | NED |
| 4 | Mucosal | 37 | M1c | CTLA-4+PD-1 | 18 | None | Surg, SBRT | RT | 71+ | NED |
| 5 | Mucosal | 13 | M1b | CTLA-4+PD-1 | 6 | None | SRS, surg | None | 79+ | NED |
| 6 | Mucosal | 37 | M1a | CTLA-4+PD-1 | 35 | ACT, BRAF-i | Surg | None | 82 | DOD |
| 7 | Uveal | 22 | M1c | CTLA-4 | 80 CR+ | PD-1* | None | None | 80+ | NED |
| 8 | Uveal | 167 | M1c | CTLA-4 | 6 | PD-1 | SRS, surg | RT | 62+ | NED |
| 9 | Uveal | 44 | M1c | CTLA-4 | 15 | PD-1 | SRS | ChEmb, RT | 67 | DOD |
| 10 | Uveal | 199 | M1c | CTLA-4 | 42 CR | PD-1, trial | None | None | 90+ | AWD |
Ten patients with 5-year survival are shown. Dx-CPI indicates the time interval between initial diagnosis and first treatment with CPI. ‘+’ indicates ongoing CR or survival.
*Denotes a patient with stable disease who was started on anti-PD-1 before progression occurred.
AWD, alive with disease; ChEmb, hepatic chemoembolization; CPI, checkpoint inhibitor; CR, complete response; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; DOD, died of disease; Dx, Diagnosis; ILP, isolated limb perfusion; NED, no evidence of disease; OS, overall survival; PD-1, programmed death 1 receptor; PFS, progression-free survival; RT, wide-field radiation therapy; Rx, treatment; SBRT, stereotactic body radiotherapy to extracranial metastases; SRS, stereotactic radiosurgery for central nervous system metastases; Surg, surgery.
Discussion
Here, we present our experience using CPI to treat metastatic melanoma from acral, mucosal, and uveal primary tumors. Our data support literature indicating improved survival of patients with acral melanoma treated with CPI. In our series, median OS for acral patients was 17 months and 5-year survival was 34%; others reported median OS of 16.7 and 31.7 months after treatment with anti-CTLA-4 and anti-PD-1, respectively.15 20 While retrospective, these reports of acral patients treated with CPI show outcomes that are far superior to survival reported in the pre-CPI era.
Our experience suggests the outcomes of patients with mucosal and uveal melanoma are improving as well. Others have reported a median OS of 6.4–12.4 months for stage IV mucosal melanoma, but in our series, median OS after CPI for mucosal patients was 18 months (online supplementary table 1). For patients with uveal melanoma, median survival was 12.2 months and 5-year OS was 22%. That 5-year survival of patients with mucosal and uveal melanoma is exceeding 20% is encouraging. Furthermore, 20 of 21 3-year survivors received anti-CTLA-4 and anti-PD-1, either sequentially or in combination. In contrast, initial treatment with anti-PD-1 or anti-PD-L1 monotherapy was associated with worse outcomes. While this difference may be due to patient selection or small numbers, this observation raises the possibility that CLTA-4 blockade is an integral part of the achievement of long survival in patients with sun-shielded melanomas.
jitc-2019-000341supp001.pdf (69.6KB, pdf)
There is a trend toward moving away from anti-CTLA-4 treatment owing to toxicity concerns, but our data suggest sun-shielded melanomas may require more aggressive treatment. It might be possible to mitigate toxicity by using dose-modified anti-CTLA-4 at 1 mg/kg. Recent trials, including the CheckMate 511 trial, have shown improved safety profiles with this dose, and they are suggestive of preserved efficacy.25 26 However, the primary endpoint for CheckMate 511 was safety but not efficacy, and patients with ocular melanoma were excluded. Longer follow-up and more research will be necessary to determine if low-dose anti-CTLA-4 is effective for patients with sun-shielded melanomas.
There is a clear association between responsiveness of a malignancy to immunotherapy and the tumor mutational burden.7 27 However, other data show that immunotherapy can still be effective against tumors with a paucity of mutations. For example, tumor-reactive lymphocytes have been isolated from uveal melanoma metastases, with adoptive transfer resulting in a 35% objective response rate.28 29 Clear cell renal cell carcinoma only has 1.1 non-silent mutations per megabase (compared with 16.8 mutations per Mb in melanoma) but is very responsive to immune-based treatment.30 Neoantigen-reactive T cell clones have also been reliably isolated from microsatellite-stable tumors of gastrointestinal origin, which have a paucity of neoantigenic mutations.31–33 These studies demonstrate that the burden of somatic mutations is not the sole determinant of the immune-responsiveness of a tumor. Coupled with emerging clinical data suggesting improved survival in patients with sun-shielded melanomas relative to historical data, there is clear scientific and clinical rationale to pursue immune-based treatment strategies for patients with these lethal malignancies.
In this series, we noted that local therapy was used after CPI in over 80% of patients with incomplete responses to control eventual disease progression. These interventions were performed for a variety of therapeutic and/or palliative indications to eliminate metastatic tumors in the body and the central nervous system. In contrast to the high frequency at which local therapy was used in this series, the role of local therapy in patients being treated with immunotherapy has not been extensively studied. We recently reported on our experience with 52 patients who were treated with local therapy for oligoprogression after CPI, 15 of whom had sun-shielded primary melanomas (and are included in the present study).34 Some of these highly selected patients have been disease-free for years after apparently curative resections of immunorefractory metastases. Retrospective data cannot prove local therapy improved the survival of those patients, and patients with indolent biology have more opportunities to be treated with local therapy. Nonetheless, local therapy appears to have an important role in the management of selected patients with metastatic sun-shielded melanoma.
Its retrospective nature and a relatively small patient population limit this study. This report is one of several small series available to date for this rare subgroup of patients with sun-shielded metastatic melanoma. Ongoing multi-institutional efforts will be crucial to meaningfully study the optimal treatments and determine outcomes for these patients. Indeed, there may be intergroup differences that we have not seen because of small numbers. Over time, as we get experience with larger numbers, we will be able to evaluate each site separately to see if there are similarities or disparities among these histological types.
Conclusions
Survival in patients with metastatic acral, mucosal and uveal melanoma was highly associated with blockade of both CTLA-4 and PD-1/PD-L1, either sequentially or in combination. Complete responses were rare, and over 80% of incomplete responders who achieved long survival were treated with local therapy after CPI. These results show that while survival after CPI is worse for patients with sun-shielded melanomas, there is evidence of prolonged survival compared with historical data. Given the unique biology of these rare malignancies, multi-institutional efforts will be essential to determine the optimal treatment approach for these patients.
Footnotes
Twitter: @nicholasklemen
Contributors: Conception by NDK and MS. Analysis of clinical data by NDK and MW. Clinical management performed by KO, JC, SA, CC, SAW, HMK and MS. Manuscript organization, writing and editing by NDK, JCR, KO, SA, SAW, HMK and MS.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: No, there are no competing interests.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. Data included in this study are deidentified subject data.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, et al. . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med Overseas Ed 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolchok JD, Kluger H, Callahan MK, et al. . Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33. 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, et al. . Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–26. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. . Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prieto PA, Yang JC, Sherry RM, et al. . Ctla-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 2012;18:2039–47. 10.1158/1078-0432.CCR-11-1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gellin GA, Kopf AW, Garfinkel L. Malignant melanoma. A controlled study of possibly associated factors. Arch Dermatol 1969;99:43–8. 10.1001/archderm.99.1.43 [DOI] [PubMed] [Google Scholar]
- 7.Lawrence MS, Stojanov P, Polak P, et al. . Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214–8. 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krauthammer M, Kong Y, Ha BH, et al. . Exome sequencing identifies recurrent somatic Rac1 mutations in melanoma. Nat Genet 2012;44:1006–14. 10.1038/ng.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akbani R, Akdemir KC, Aksoy BA, et al. . Genomic classification of cutaneous melanoma. Cell 2015;161:1681–96. 10.1016/j.cell.2015.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bello DM, Chou JF, Panageas KS, et al. . Prognosis of acral melanoma: a series of 281 patients. Ann Surg Oncol 2013;20:3618–25. 10.1245/s10434-013-3089-0 [DOI] [PubMed] [Google Scholar]
- 11.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. . Mutations in GNA11 in uveal melanoma. N Engl J Med 2010;363:2191–9. 10.1056/NEJMoa1000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuk D, Shoushtari AN, Barker CA, et al. . Prognosis of mucosal, uveal, acral, Nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist 2016;21:848–54. 10.1634/theoncologist.2015-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Algazi AP, Tsai KK, Shoushtari AN, et al. . Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016;122:3344–53. 10.1002/cncr.30258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Angelo SP, Larkin J, Sosman JA, et al. . Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. JCO 2017;35:226–35. 10.1200/JCO.2016.67.9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoushtari AN, Munhoz RR, Kuk D, et al. . The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer 2016;122:3354–62. 10.1002/cncr.30259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postow MA, Luke JJ, Bluth MJ, et al. . Ipilimumab for patients with advanced mucosal melanoma. Oncologist 2013;18:726–32. 10.1634/theoncologist.2012-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmer L, Vaubel J, Mohr P, et al. . Phase II DeCOG-Study of ipilimumab in pretreated and treatment-naïve patients with metastatic uveal melanoma. PLoS One 2015;10:e0118564–13 10.1371/journal.pone.0118564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luke JJ, Callahan MK, Postow MA, et al. . Clinical activity of ipilimumab for metastatic uveal melanoma. Cancer 2013;119:3687–95. 10.1002/cncr.28282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kath R, Hayungs J, Bornfeld N, et al. . Prognosis and treatment of disseminated uveal melanoma. Cancer 1993;72:2219–23. [DOI] [PubMed] [Google Scholar]
- 20.Johnson DB, Peng C, Abramson RG, et al. . Clinical activity of ipilimumab in acral melanoma: a retrospective review. Oncologist 2015;20:648–52. 10.1634/theoncologist.2014-0468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkins MB, Hsu J, Lee S, et al. . Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the eastern cooperative Oncology Group. J Clin Oncol 2008;26:5748–54. 10.1200/JCO.2008.17.5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapman PB, Einhorn LH, Meyers ML, et al. . Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 1999;17:2745–51. 10.1200/JCO.1999.17.9.2745 [DOI] [PubMed] [Google Scholar]
- 23.Middleton MR, Grob JJ, Aaronson N, et al. . Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000;18:158–66. 10.1200/JCO.2000.18.1.158 [DOI] [PubMed] [Google Scholar]
- 24.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. . Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebbé C, Meyer N, Mortier L, et al. . Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol 2019;37:867–75. 10.1200/JCO.18.01998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long GV, Atkinson V, Cebon JS, et al. . Standard-Dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1B trial. Lancet Oncol 2017;18:1202–10. 10.1016/S1470-2045(17)30428-X [DOI] [PubMed] [Google Scholar]
- 27.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017;377:2500–1. 10.1056/NEJMc1713444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothermel LD, Sabesan A, Stephens DJ, et al. . Identification of an immunogenic subset of metastatic uveal melanoma. Clinical Cancer Research. American Association for Cancer Research; 2015;:clincanres 2015;2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandran SS, Somerville RPT, Yang JC, et al. . Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol 2017;18:792–802. 10.1016/S1470-2045(17)30251-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43–9. 10.1038/nature12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran E, Turcotte S, Gros A, et al. . Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641–5. 10.1126/science.1251102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran E, Ahmadzadeh M, Lu Y-C, et al. . Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 2015;350:1387–90. 10.1126/science.aad1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran E, Robbins PF, Lu Y-C, et al. . T-Cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med 2016;375:2255–62. 10.1056/NEJMoa1609279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klemen ND, Wang M, Feingold PL, et al. . Patterns of failure after immunotherapy with checkpoint inhibitors predict durable progression-free survival after local therapy for metastatic melanoma. J Immunother Cancer 2019;7:1–9. 10.1186/s40425-019-0672-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2019-000341supp001.pdf (69.6KB, pdf)