Abstract
Medicinal plants have been used worldwide for centuries to maintain health and to treat diseases, more so chronic diseases. However, adulteration and use of spurious materials as substitutes have become a major concern for users and industry for reasons of safety and efficacy. Therefore, authentication of medicinal plants is of utmost importance. Morphological, anatomical, chemical and DNA markers solve the problem by differentiating the genuine material from the adulterants, substitutes and spurious drugs. DNA markers use nucleotide sequences to identify species; it takes preference over the other two markers being not age dependent, tissue specific and having a higher discriminating power. Therefore, characterization of plants with such markers is an ideal approach for identification of medicinal plant species and populations/varieties of the same species. Availability of certified taxonomic specimens in herbaria is certainly required for unambiguous confirmation through final visual comparison and analysis.
Keywords: Adulteration, Authentication, DNA markers, Medicinal plants
Highlights
-
•
DNA markers have upper hand in indentifying medicinal plants compared to other markers
-
•
Of the various DNA markers, LAMP, SCAR and DNA barcoding are ideal for authentication
-
•
DNA markers are used to build a reference library of traditional medicine.
1. Introduction
Medicinal plant species are known to be in use since time immemorial for the treatment and cure of human and animal ailments. Though their use and practice registered a decline with the advent of antibiotics and sulfa-drugs, the toxicity and harmful effects associated with synthetic drugs and antibiotics have brought the herbal systems of medicine again to the forefront of healthcare system. According to WHO and other reports (WHO, 2002, Willcox and Bodeker, 2004), around 80% of the global population still relies on botanical drugs and there is speculation that more than two billion people may be heavily reliant on medicinal plants (Lambert et al. 1997). This system plays prominent role in the strategy to contain and treat severe acute respiratory syndrome (Wen et al., 2003). Vinblastin and Vincristine of Catharanthus roseus G. are potent anti-cancer agents and are the first agents to advance into clinical use for the treatment of cancer (Cragg and Newman 2005). The discovery of paclitaxel derived from Taxus brevifolia Nutt. (Taxaceae), is another evidence of the success in natural product drug discovery. Two most important anti-malarial drugs quinine and artemisinin are derived from traditional medical knowledge in Peru and China respectively. Survey reports showed that 78% of patients living with HIV/AIDS in the USA use medicinal herbs (WHO 2002) and similar patterns have been reported in other countries. Other systematic studies on efficacy are slowly emerging suggesting antiretroviral, immunomodulatory and opportunistic infection reducing effects of traditional management methods (Liu 2007). Apart from general healers, traditional orthopedic practitioners, birth attendants, poison healers, spiritual therapists, mental health providers, healers specialized in eye, pediatric conditions, skin diseases etc., are some of the specialty areas (Payyappallimana 2010).
The international trade of herbal products is one of the major forces in the global economy and the demand is increasing in both developed and developing countries. Survey of literature reveals that more than 1000 companies are engaged in the production of herbal products with the annual revenues in excess of US$60 billion (Newmaster et al. 2013). In North America, herbal medicinal market is considered to constitute the mostly rapidly growing segment (Gutierrez et al. 2004), with over 29,000 herbal substances (Astin et al., 1998, Kaye et al., 2000) generating billions of dollars in trade. These statistics are the direct indication of the rapid growth (approximately 15% per year) in the market place from natural plant products and broadening consumer base which show interest in herbal products from different countries including India. The value of botanical related trade in India is about US$10 billion per annum with the annual export of US$1.1 billion (Singh et al. 2003). China's annual herb drug production is worth US$48 billion with annual export of US$3.6 billion (Handa 2004). India is ranked third in the herbal medicine category with less than 2% global market share. The Indian market is growing at 15–20% per annum- Rs 7000 million or $150 million (www.indianmedicine.nic.in). Therefore, massive demand of herbal medicinal system at both national and international level has resulted in renewed interest of biologists in this field to maintain the quality and purity of herbal raw material and finished products.
The prime cause of the problems associated with the standardization of medicinal plants is due to reasons of complex composition of drugs used in the form of whole plants, plant parts or extracts obtained there from. Therefore, first step is to make reproducibility and minimal batch-to-batch variation a desirable quality of any herbal remedy, and side-by-side initiating steps towards use of authentic starting material. In ancient days, the activity of herb procurement, storage, preparation of drugs and its distribution remained mainly the responsibility of local physicians (Vaidyas and Hakims). They were very well versed with the identity of medicinal plants as they had very close contact with nature. But gradually in the process of urbanization this contact with nature was lost and, consequently, the knowledge about the identification of medicinal plants has also deteriorated to a great extent.
A perusal of literature available on medicinal plants (Sivarajan and Balachandran 1994) reveals that different workers have interpreted ancient texts differently resulting in coining of same Unani or Ayurvedic name for more than one plant and different names for the same drug plant. This way a number of medicinal plants have controversial botanical identity due to the existence of homonyms (same name for different drug plants) and synonyms (different names for same drug plants). Such situation has also been compounded by linguistic diversity, and prevalence of local dialects. For example, different botanically identified plants are being used for the same traditional drug and vice-versa in different parts of India. Thus, there exists at present, a chaotic state of botanical and vernacular nomenclature with regards to medicinal plants. Due to ambiguity of plant names, over-lapping of data occur in chemical or pharmacological information where the botanical identity of market samples of crude drugs taken up for research is not ascertained. This has put many drug plants in controversial position and their identity has become doubtful. In addition to ambiguity in nomenclature, the crude drugs sold in the market are adulterated or substituted by quite unrelated plant materials. Many researchers have examined a number of market samples of crude drugs used in Indian systems of medicine (Afaq 1999) and observed that the prevalence of adulteration is such that quite unrelated plants are being sold in the crude drug markets in place of genuine ones. For example, Anemone flowers are sold as ‘Banafsa’ (Viola spp.); paper colored, scented and cut into small pieces as ‘Saffron’ (Crocus sativa); Malva rotundifolia as ‘Brahmi’ (Centella asiatica); Parthenium hysterophorus, an obnoxious weed, as Shahtara ‘(Fumaria indica); culm of different grasses as ‘Chirayata’ (Swertia spp.); Leea alata as ‘Manjith’ (Rubia cordifolia), and so on. It is invariably found that the adverse effects/reports are not due to the intended herb, but rather due to the presence of an unintended herb (De Smet et al., 1992). The credibility of any system of medicine depends not only on the skill of the physician but also on the quality of medicine administered to the patients. Correct identification of plants forming the drug is a prerequisite and fundamental to whole realm of medicine and science. Thus, authentication of botanical source of plant taken up for research or medicinal use is a necessity to achieve satisfactory results and also to maintain efficacy and therapeutic property of the preparations in which these plants are used. We have therefore, tried to present a comprehensive review on strategies related to identification of medicinal plants.
1.1. Authentication of medicinal plants by molecular markers
DNA- based markers that are based on analysis of the unique genetic structure are undoubtedly higher level markers and have the upper hand over other marker systems because these are not affected by age, environmental factors and physiological conditions. Moreover, these markers are not tissue specific and thus can be detected at any stage of plant development. Compared with phenotypic and chemical markers, DNA- based technology can provide an efficient, accurate and cheaper means of testing the authenticity of hundreds of samples simultaneously as these can be amenable to automation. DNA based authentication of medicinal plants can be useful as a tool for quality control and safety monitoring of herbal pharmaceuticals and neutraceuticals and will significantly add to the medical potential and commercial profitability of herbal products.
A genetic marker may be defined as gene or a nucleotide sequence on a chromosome that has the potential to differentiate cells, individuals or species. As the DNA sequences are highly specific, they can be identified with the help of the known molecular markers which can find out a particular sequence of DNA from a group of unknown.
1.1.1. Types of molecular markers.
A vast numbers of molecular markers (Table 1 ) are available of which the more common are Restriction Fragment Length Polymorphisms (RFLPs), Amplified Fragment Length Polymorphisms (AFLPs), Randomly Amplified Polymorphic DNA (RAPD), Simple Sequence Repeats (SSRs), Inter Simple Sequence Repeats (ISSR), Sequence Characterized Amplified Regions (SCAR), Loop Mediated Isothermal Amplification (LAMP), Single Nucleotide Polymorphisms (SNPs) and others. DNA barcoding, microarrays based markers and Next Generation Sequencing (NGS) based markers are of relatively recent development. Each of the techniques are targeted towards a particular component of the genome or is completely arbitrary, face unique methodological, technical and material challenges; thus no DNA marker can be considered ideal. The use of a particular marker is therefore, dependent on objectives of the researcher.
Table 1.
Comparison of different DNA markers used in biological sciences.
| RFLP | RAPD | AFLP | ISSR | SSR | SCAR | LAMP | DNA barcoding | |
|---|---|---|---|---|---|---|---|---|
| Genomic abundance | High | Very high | Very high | Medium | medium | High | High | high |
| Genomic DNA required | 2–5 μg | 15–30 ng | 200–300 ng | 15–30 ng | 30–50 ng | 30–50 ng | 10–20 ng | 30–50 ng |
| inheritance | Co-dominant | Dominant | Dominant | Dominant | Co dominant | Co dominant | Co dominant | NA |
| Primers/probes used | Specific | Random | Specific to adapter sequence | Specific to repeats | Specific | specific | Specific | Specific |
| Type of polymorphism | Nucleotide base change that affect specificity of restriction endo-nucleases | Nucleotide base change at primer binding sequences | Nucleotide base changes that affect specificity of restriction endonucleases and presence/absence of nucleotide complementary to selective nucleotides | Nucleotide base change at primer binding sequences | Complete presence/absence of DNA fragment | Complete presence/absence of DNA fragment | Complete presence/absence of DNA fragment | Nucleotide changes in universal genes. |
| Reproducibility | Very high | Very low | High | Medium | Very high | Very high | Very high | Very high |
| Applicability in Plant authentication | Yes | Yes, but should not be used (It is genetic diversity marker) | Yes, but should not be used (It is a perfect genetic diversity marker) | Yes, but should not be used (It is genetic diversity marker) | Yes | Yes | Yes | Yes |
| Cloning/sequencing | Yes | No | No | No | Yes | Yes | Yes | Yes |
| Use of radioactivity | Yes, but not in PCR-RFLP | No | Yes | No | No | No (required in AFLP based SCAR) | No (required in AFLP based LAMP) | No |
| Detection of alleles | Yes | Generally no | Generally no | Generally no | Yes | Yes | Yes | Yes |
| Cost | High | low | High | Medium | medium | high | Medium | medium |
| Ease of use of automation | Not easy (PCR based AFLP is easy) | Very easy | difficult if radioactivity is used (Licor system is easy) | Easy | Very easy | Easy | Easy (However, primer designing sometimes becomes difficult) | Easy |
2. Restriction fragment length polymorphisms (RFLP)
RFLPs (Fig. 1 ) are considered as one of the first developments in the field of genetic markers, responsible for initiating the field of molecular genetics. The technique is based on the principle of variation that is present due to occurrence of mutations in restriction enzyme binding and cleavage sites; additionally, any re-organization in the genomic region flanked by restriction sites that also disrupts their distribution and thus causes polymorphism also contributes to RFLP. The digested fragments vary in size, have to be separated using Southern blot analysis and accordingly visualized by hybridization to specific probes which could be homologous or heterologous in nature. Alternatives of Southern analysis in RFLPs are polymerase chain reactions. When the flanking regions of nucleotide sequence are known, the region meant for RFLPs could be amplified through polymerase chain reaction. However, it must be kept in mind that If the length polymorphism is caused by a relatively large (> approx. 100 bp depending on the size of the undigested PCR product) deletion or insertion, gel electrophoresis of the PCR products should reveal the size difference and when the length polymorphism is caused by base substitution at a restriction site, PCR products must be digested with a restriction enzyme to reveal the RFLP. The positive point of this marker is its co-dominant nature. Like other DNA based markers, there is ample literature (mainly PCR-RFLP) that suggests use of this technique in the identification of medicinal plants. For example, Six plant species — Desmodium giganicum, Aegle marmelos, Solanum xanthocarpum, Solanum indicum, Tribulus terresteris, Oroxylum indicum were identified through polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) and the regions amplified were Internal transcribed spacer (ITS) with the aid of ITS1 (F) and ITS4 (R) primers (Biswas and Biswas 2013). The same technique has been applied on Boerhavia diffusa L. in which 700 bp of ITS region was obtained via polymerase chain reaction and when this region was restricted with Msp I, provided four unique fragments that differentiated this specie from T. portulacastrum and T. monogyna (Biswas et al. 2013). Feng et al. (2010) differentiated Angelica sinensis from its seven different adulterant Angelica species; here a pair of primers specific for ITS region were designed which amplified 520 bp from the adulterants and no products was amplified with the DNA of A. sinensis.
Fig. 1.
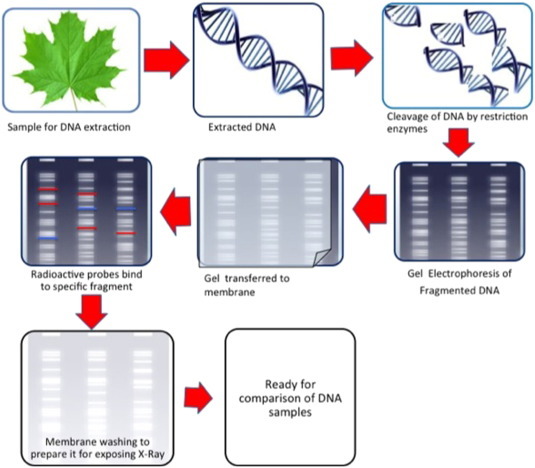
Pictorial view methodology of RFLP.
2.1. Limitation of RFLPs
-
•
The limited sensitivity of detection associated with RFLPs is the serious problem; because it is very difficult to get valid profiles from trace biological evidence or from samples that are too aged or have significantly compromised environmental insults.
-
•
RFLP is time consuming, involves radioactivity, and is laborious and difficult to automate. However, this can be overcome by PCR-based DNA typing systems.
3. Simple sequence repeats (SSR)
Simple Sequence Repeats (Fig. 2 ) are comprised of 2–5 bp DNA monomeric units that are repeated multiple times at a specific locus. Such markers are completely co-dominant; used for fingerprinting, marker assisted selection, kinship, breeding behavior such as selfing and outcrossing, establish population structure etc. Microsatellites are locally tandemly duplicated, and are also found dispersed throughout the genome. Thus it becomes important to amplify a specific microsatellite in a locus specific manner using locus-specific primers. The primer designing involves identifying microsatellites, either is manual examination in case the sequences are small or of limited length, or using automated tools for locating microsatellite such as microsatellite finders. However, depending upon the research objectives, either unique flanking regions can be used for primer designing where the products are co-dominant, or microsatellites can themselves be used as primers where the products generated act as dominant markers. Microsatellite discovery also involves cloning microsatellite-enriched library and sequence analysis. The clones containing repeats can also be identified through hybridization of fluorescent labeled probe with the repeats. DNA segment containing the microsatellites is then sequence verified and employed for primer designing. Microsatellite markers are valued for their hyper-variability which occurs because these regions can add or delete one or two nucleotides because of the slippage during replication.
Fig. 2.
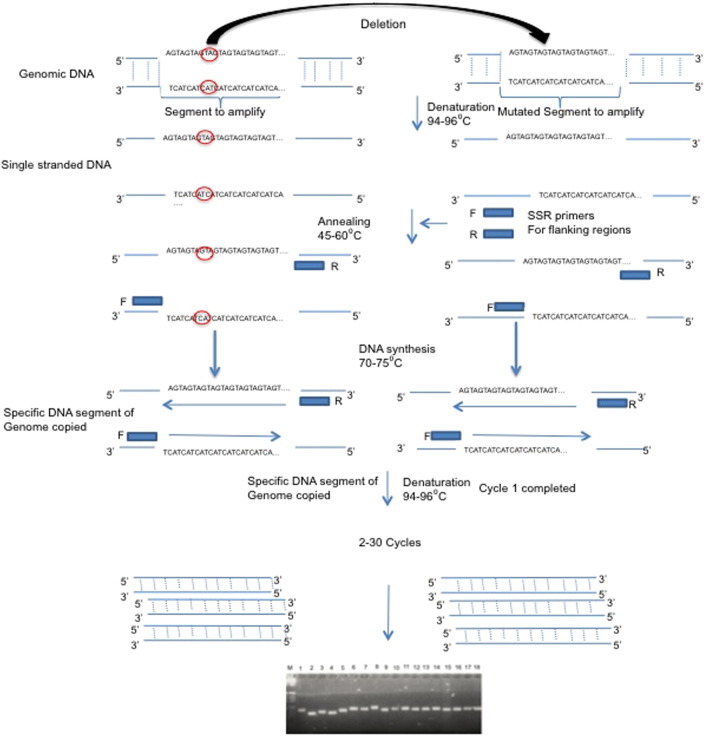
Pictorial view methodology of SSR.
Research reports suggest involvement of microsatellites in authentication and identification of medicinal plants. Hon et al. (2003) applied 16 microsatellites to analyze 150 and 40 American ginseng and Oriental ginseng roots respectively. Of the 16 microsatellites, 9 could differentiate Chinese samples from American ginseng. Capsicum species which are commonly used as spices have been analyzed through microsatellites; the study involves 800 lines collected from different locations of Central and South America. SSR primers (5751 pairs) were designed and similarity search with tomato genome resulted successful mapping of 2245 C. annuum markers onto the tomato genome. Ninety six were found to span entire tomato genome and selected for further analysis. Sixty markers showed polymorphism in Capsicum lines and based on this, the 192 lines were grouped into five clusters. Additionally, plastid genes, matK and rbcL, divided the Capsicum lines into 3 main groups; over all, 19 marker loci reveal genotype specificity to species and clusters (Shirasawa et al., 2013).
3.1. Limitation of SSR
-
•
It requires much time and cost to isolate and characterize each SSR locus when the DNA sequence of a plant species is not available.
-
•
Another drawback is the occurrence of null alleles. This may be due to the poor primer annealing because of nucleotide sequence divergence, inconsistent DNA quality or low DNA quantity (Ellegren 2004) or it might be due to mutations in the primer binding site. This can cause difficulty in the determination of allelic and genotypic frequencies and an underestimation of heterozygosity (Kumar et al. 2009).
4. Random amplification of polymorphic DNA (RAPD):
In RAPD technology (Fig. 3 ), random short synthetic oligonucleotide primers (10–12 base pairs) are used to amplify the genomic DNA through polymerase chain reaction under low annealing temperature. The amplicons generated are separated on agarose gels based on sizes. As stated that primer size is short, therefore annealing temperature range is 28–38 °C. At this temperature range, primers anneal wherever they find complementary sequences from the genome and the profile of amplified DNA vary in size that depends on nucleotide sequence homology and the primer at the end of each amplified product. As it is obligatory that primers for RAPD are arbitrarily chosen, therefore, a minimum of 40% GC and the absence of palindrome sequence is a prerequisite for RAPDs.
Fig. 3.
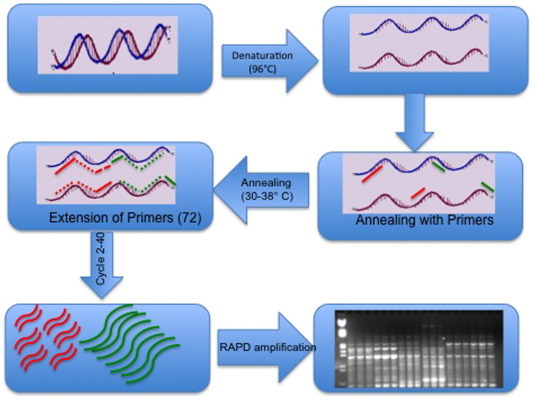
Pictorial view methodology of RAPD.
Majority of the RAPD fragments are due to the amplification of one locus, furnishing two types of polymorphisms — the band could be present (1) or absent (0). The bands obtained may vary in intensity which could be due to the differences in copy number or relative sequence abundance; therefore, may work for distinguishing homozygote dominant from heterozygote because more bright bands are expected for the former. However, some workers (Thormann et al. 1994) have found no correlation between copy number and band intensity. The possible occurrence of fainter bands could be due to the varying degree of primer mismatch. The other drawback associated with RAPD markers is the low reproducibility. However, this problem could be overcome through the choice of an appropriate DNA extraction protocol to remove any contaminants, by optimizing the polymerase chain reaction parameters, by testing several oligonucleotide primers and most importantly scoring only reproducible DNA fragments.
RAPDs have been widely used for authentication of plant species of medicinal importance. Two varieties of S. marianum which are very difficult to distinguish in dried conditions could be differentiated with RAPD. The banding pattern amplified with primer OPP-10 contained two (600 and 1000 bp) characteristic bands; primers OPG-03, OPC-17 generated two unique bands (1000 and 300 bp). This profile specific for S. marianum var. album could differentiate it from S. marianum var. purple (Abouzid 2014). Similarly, two species of the parasite Cuscuta (C. reflexa and C. chinensis) have been distinguished with primers OPC-1, OPC-02, OPC-03, OPC-04, OPC-05, OPC-06, OPC-07 and OPC-08 (Khan et al. 2010a). Ali et al. (2013), while characterizing Clitoria ternatea at inter-zonal level identified complete monomorphism with primer OPN-02, therefore, the identification of this herb with OPN-02 is the good choice. Molecular characterization of Convolvulus pluricaulis revealed that primer OPN-09 is the specie specific primer for the herb (Ganie et al., 2015). A common band of 2.2 kb amplified by Primer OPN-05 was found when different accessions of Evolvulus alsinoides were studied through RAPD analysis (Ganie and Sharma 2014).
Laboratories with limited budget prefer RAPDs as the entire process is only dependent on thermal cycler and gel electrophoresis unit. RAPDs can efficiently differentiate taxa below the species level (Choo et al. 2009), because it reflects both coding and non-coding regions of the genome.
4.1. Limitations of RAPDs
-
•
RAPD is a less reproducible marker. Because the annealing temperature for such marker is low (28–38°C) therefore, there are chances of wrong annealing.
-
•
It is a dominant marker
5. Amplified fragment length polymorphism (AFLP)
This technique (Vos et al., 1995) which is a combination of both RFLP and RAPD is based on the detection of restriction fragments by PCR amplification and can be used for DNA of any origin or complexity, and quality (Fig. 4 ). The fingerprints are produced without any prior knowledge of sequence using a limited set of primers. Fragments are generated with two different end sequences which form template for adapter ligation. Such adapters then form the basis for PCR which is performed using end-labeled nested primers with selective nucleotides. The amplified products are electrophoresed in a 6% denaturing polyacrylamide gel and autoradiographed or detected through fluroscent detection. AFLPs are generally considered highly valued marker for analyzing the genetic diversity for both animal and plant systems compared to authentication reports. Seven specific fragments identified with the primer combinations E-ATC + M- AG and E-GCA + M-GT could serve as the genetic markers for Aloe vera (Tripathi et al. 2011). Although the three species of the genus Echinacea could easily be distinguishable in fresh and intact conditions, but it becomes very tough to differentiate them in commercial preparations particularly ground, dry plant parts of E. purpurea (valuable species for chemotherapeutic properties) mixed with the other two species. Therefore, Russi et al. (2009) used fresh material collected from cultivated Echinacea spp. for the discrimination of these species by AFLP. E + CAC/M + AAT and E + CAC/M + AGC generated 13, 9, and 4 or 7, 5, and 5 specific fragments for E. purpurea, E. angustifolia, and E. pallida, respectively. Passinho-Soares et al. (2006) identified specie specific bands of different species of the genus Plectranthus. During the study 2, 2, 1, unique bands were confirmed for P. barbatus, P. grandis and P. ornatus respectively with the primer combination EcoR 1-CAC + Msc 1-GCA. Applying AFLP technique, Gowda et al. (2010) using P + GC/M + CTA combination, found three bands of the range 500–700 bp in Embelica ribes and could be considered unique to this species. In the same study, P + CA/M + CTG combination yielded two unique bands between 500 and 517 bp for E. tsjeriam-cottam.
Fig. 4.
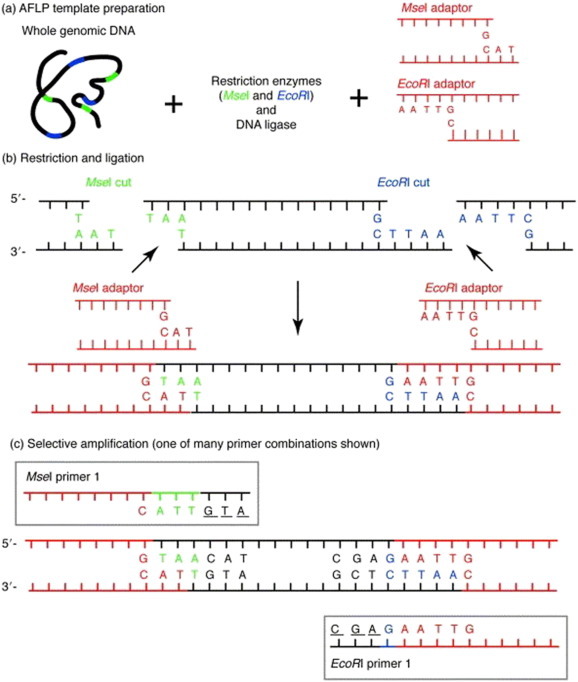
Pictorial view methodology of AFLP. DNA digestion with restriction enzymes (a), adaptor ligation (b) Selective amplification (c); [Prior to selective amplification, pre amplification is carried out with a single nucleotide extension, followed by selective amplification using three 3-bp extension] (Mueller and Wolfenbarger 1999).
5.1. Limitations of AFLPs
-
•
Like RAPD markers AFLPs are also dominant.
-
•
The method needs purified and high molecular weight DNA. Also, the procedure involves harmful radioactive materials; however, this can be overcome by using fluorescent tags.
6. Inter-simple sequence repeats (ISSR)
ISSR (Fig. 5 ) is one of dominant markers involved in amplification of DNA segment present at an amplifiable distance in between two identical microsatellite repeat regions oriented in opposite direction. This technique relies on the principle of using microsatellite as primers which target multiple genomic loci to amplify inter simple sequence repeats of the genomic DNA of different sizes. Microsatellite used as primers for ISSRs are di-penta nucleotide. The primers for the ISSRs could either be unanchored or anchored at 3`-5` end having 1–4 degenerate bases extended in to the flanking sequences. Compared with RAPDs the size of the primers in ISSRs are much longer (18–25 mers), which permit the annealing of the primers at higher temperatures leading to higher stringency. The DNA fragments obtained are generally 0.5–2.0 kb long and could be detected both by agarose and polyacrylamide gel electrophoresis. Survey of literature reveals that ISSR markers show high reproducibility than RAPDs (Kojima et al. 1998) although it varies with the detection method used. Fang and Roose (1997) reported greater than 99% reproducibility level after performing repeatedly tests for ISSR markers with the DNA samples of the same cultivar grown in different locations, DNA extracted from different aged leaves of the same individual, and by performing separate PCR runs. However, in other studies, the reproducibility of ISSRs amplification products are in the range of 86–94%, with the maximum when the priority is given to polyacrylamide gel electrophoresis and AgNO3 staining over agarose gel electrophoresis and faint bands are totally excluded while scoring the bands.
Fig. 5.
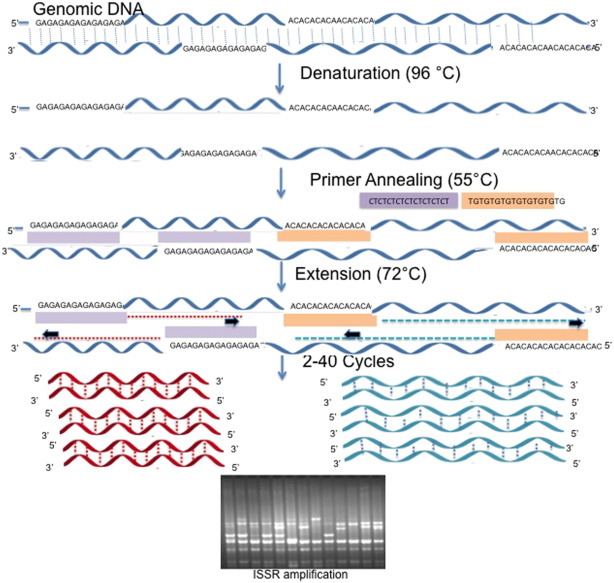
Pictorial view methodology of ISSR.
The species of Rheum viz. — Rheum officinale Baill., Rheum palmatum L., and Rheum tanguticum Maxim. ex Balf are very difficult to distinguish on the basis of arial morphological and anatomical studies. Though root and rhizome is prescribed to have medicinal importance, however to distinguish these plant species, the aerial parts leave doubt regarding the differentiation of the three species. Wang (2011) used ISSRs to authenticate these Rheum species with different ISSR primers. A successful attempt by Tamhankar et al. (2009) was also made for authentication of samples of Chirayat complex (Swertia angustifolia Ham. ex D. Don Nauni, S. chirayita (Roxb. ex Fleming) Karsten, S. cordata Wall, S. densifolia Griseb, S. lurida Clarke, S. ciliate, S. paniculata Wall, S. alata Clark Mirik, S. bimaculata, Hook f. & Thoms, Andrographis paniculata Nees, Enicostemma axillare, Exacum tetragonum Roxb). The DNA of the market samples and the three authentic species was amplified using ISSR primers and the profile obtained was compared and the differentiations were obtained with primers 808 and 809. The roots of Cissampelos pareira L. var. hirsuta (Buch.-Ham. ex DC.) generally named as Patha in India is substituted with two other species, viz., Cyclea peltata (Lam.) Hook.f. & Thomson and Stephania japonica (Thunb.) Miers. ISSR profiles (Vijayan et al. 2014) distinguished genuine raw drug of ‘Patha’ from its substitutes/adulterants to guarantee the quality and legitimacy of this drug in the market.
6.1. Limitations of ISSRs
-
•
ISSR markers do have more value compared to RAPDs; however, the marker has the reproducibility issues.
-
•
This marker is also dominant.
7. Sequence characterization of amplified regions (SCAR)
The most important marker for authentication of medicinal plants is SCAR (Fig. 6 ). It is sequence-based mono-locus and a co-dominant marker in which forward and reverse primers are designed from the particular region of a cloned AFLP, RAPD and ISSR DNA fragment linked to a trait of interest. SCAR may be a specific gene or random DNA fragment in the genome of an organism and the primers for amplification are located at any suitable position within or flanking the unique AFLP, RAPD, ISSR amplicon. SCAR is fast, reliable and highly reproducible marker used in molecular biology. The designed primers are used to identify the target species from the pool of related species by the presence of a single, distinct and bright band in the desired sample (Kiran et al. 2010). The length and GC content is also concern for SCAR markers and generally 20–25 oligonucleotide bases are sequence specific (Kiran et al. 2010). SCAR markers developed from AFLP and SSR though is more reproducible but simultaneously such markers are costly, time consuming and more difficult (particularly in AFLP where silver staining is required for the elution of DNA fragment from polyacrylamide gels). To convert a selected unique RAPD, AFLP, ISSR or SSR band to a SCAR marker, each unique band is eluted, cloned and sequence verified. The nucleotide sequence of the unique DNA band is analyzed for uniqueness by comparing with the known DNA sequences available at various databases for synthesizing specific SCAR primers. These markers have the advantage of being co-dominant and are highly reproducible.
Fig. 6.
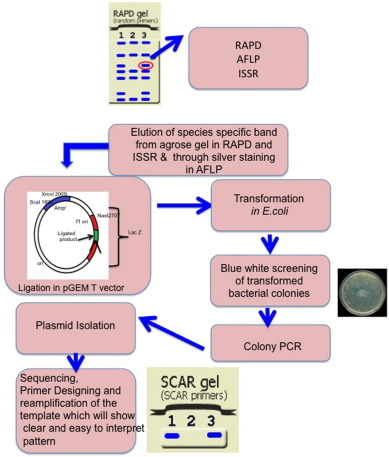
Pictorial view methodology of SCAR.
Molecular biologists prefer these markers and the most recent data shows that these markers are actively used to authenticate medicinal plants. Yadav et al. (2012) confirmed a 589 bp species specific band with RAPD primer OPAA-3 in Bacopa monnieri accessions and not found in other adulterant candidates. For further processing a pair of SCAR primers (between 406 bp of 589 bp sequence of RAPD amplicon) was designed. The PCR confirmation resulted a distinct band from Bacopa monnieri and not in the adulterants. Seethapathy et al. (2014) authenticate Ativisha (Aconitum heterophyllum) and Musta (Cyperus rotundus) using nrDNA ITS sequence based SCAR markers and when market samples were examined it was found that SCAR primers (Cyr-FP and Cyr-RP) could identify tissue sample containing 750 μg to 4.76 mg/100 mg of Musta in complex mixtures of DNA extracted from commercial herbal drugs and it was also found Ativisha was not be identified through SCAR markers confirming that this authentic species is not used to prepare herbal drugs despite its being labeled as one of the ingredients in formulations. Abdin (2013) aimed to develop RAPD based SCAR markers for the genuine herbs, C. angustifolia and C. acutifolia and their adulterants, C. sophera and C. tora. Analysis of results confirmed that these samples were more than 50% adulterated. Fu et al. (2013) studied Lonicera japonica, a traditionally used medicinal plant by an improved random amplified polymorphic DNA (RAPD) analysis and SCAR markers developed differentiated the different accessions/populations. Similarly Li and Park (2012) while using SCAR markers “SA06 and SB05” differentiated Ophiopogon japonicas with an amplicon of 460 and 553-bp respectively from Liriope platyphylla; the marker SA12 amplified a 485-bp fragment specific to Liriope platyphylla. Another case is the Phyllanthus amarus Schum. & Thonn used as antipyretic, diuretic, to treat liver diseases and viral infections. Theerakulpisut et al. (2008) efficiently differentiated this specie from the less effective species which include P. debilis L. and P. urinaria Klein ex Willd.
7.1. Limitations of SCAR markers
-
•
The need for sequence data to design the PCR primers is prerequisite for SCAR markers.
8. DNA barcoding:
DNA barcoding may be defined as use of short nuclear or organelle DNA sequences for the identification of organisms. DNA barcoding has a clear goal to identify an unknown sample utilizing pre-existing classification and not to determine patterns of relationship. This technique is over now a decade old when Hebert et al. (2003) proposed that the mitochondrial enzyme CO 1 (Cytochrome oxidase 1) coding DNA could be applied to generate DNA barcode in animals. As mitochondrial genes in plant systems are slowly evolving with very low substitution rates, therefore these are not considered suitable for barcoding (Techen et al. 2014). An alternative is the use of chloroplast/nuclear genomes which have high substitution rates. Many chloroplast genomic regions (rbcL, matK, trnH-psbA, trnL-F, rpl36-rps8, ITS and 5S rRNA) have been evaluated in plant systems by- “The Consortium for the Barcode of Life Plant Working Group (CBOL)” (CBOL 2009), of which rbcl is considered as universal but is having low species resolution and reverse is the case with matK; combination of these two markers could show better results. Cameron and Chase (1999) showed less specie discriminating ability with rbcl and matK when very close taxa are concerned. Hence, Li et al. (2011) included nucleur ITS to the combination matK + rbcL with the aim to have better discriminating ability in closely related species. In DNA barcoding the main focus is to find out a universal DNA sequence that must a balance of conserved sequence as well as harbor enough diversity in order to differentiate organisms. Primers are therefore designed which are complementary to the conserved sequences and flank the variable barcode sites. This process becomes problematic when the primer binding sequences have also accumulated sufficient sequence divergence over evolutionary time. It is therefore; quite difficult to identify universal primer sets that satisfy all taxonomic hierarchies. To overcome this dilemma, barcode primers should accommodate sequence variation, or degeneracy, at one or several nucleotide positions. As is obvious that there are enough chloroplast genomes in a single plant cell, so, amplification of barcode regions of rbcL and COI are higher than nuclear loci, thus minute material provides ample template to get higher quantities of product simultaneously lowering contaminant concentration that could otherwise affect PCR. The DNA sequence of chosen DNA amplicon (e.g. rbcL, COI or ITS) is required in both orientation through bi-directional sequencing to obtain a reliable DNA barcode.
Several excellent reports have appeared towards authentication of medicinal plants through DNA barcoding. A very good example is of Crocus sativus, one of the most important and expensive medicinal spice products in the world. Because of its high market value, this herb is often adulterated with other spurious materials- Carthamus tinctorius L. and Calendula officinalis L. flowers, Hemerocallis L. petals, Daucus carota L. fleshy root, Curcuma longa L. rhizomes, Zea mays L., and Nelumbo nucifera Gaertn. stigmas (Jiang et al. 2014). Jiang et al. (2014) developed accurate detection of these adulterants in traded saffron with the application of barcoding melting curve analysis method (Bar-MCA). Another example is the discrimination of Schisandra chinensis (Li et al. 2013) at the species and population levels with internal transcribed spacer 2 (ITS2). Li et al. (2013) observed C → A substitution at site 86-bp in wild populations when compared with cultivated populations. Further, ITS 2 region clearly differentiated S. chinensis from S. sphenanthera. Other study was aimed to identify Astragalus (Gao et al. 2009), many of its species share morphological similarity. During this study four coding (trnH-psbA, rpoC1, rbcL, matK) and two non-coding regions (ITS, ITS2) were compared among 319 species; ITS2 and ITS barcodes were more productive towards discriminating the species. Moving forward is the case in which Peucedanum praeruptorum has been discriminated by DNA barcoding. Zhou et al. (2014) used ITS and nrDNA barcodes to distinguish it from common substitutes and adulterants. Further study about the applications of DNA markers is mentioned in Table 2 .
Table 2.
Molecular markers used for authentication of medicinal plant species.
| Name of the authentic drug | Part used | Medicinal uses | Substituent/adulterants if any | Technique used | Study | References |
|---|---|---|---|---|---|---|
| Litchi chinensis Sonn. | Fruit | Fruit and its secondary metabolic products have been reported to have anticancer, anti-inflammatory, antifungal, antiviral, antioxidant, antiplatelet and anticoagulant, and antidiabetic activities | None | RAPD-SCAR | Development and significance of RAPD-SCAR markers for the identification of Litchi chinensis Sonn. by improved RAPD amplification and molecular cloning | Cheng et al. (2015) |
| Akebia quinata | Stem | Antiphlogistic, diuretic, analgesic | Akebia trifoliata, Aristolochia manshuriensis, and Clematis armandii. | RAPD-SCAR | Authentication of Akebia quinata DECNE.from its common adulterant medicinal plant species based on the RAPD-derived SCAR markers and multiplex-PCR | Moon et al. (2015) |
| Sida cordifolia | Root, leaves | Anti-oxidant, anti-inflammatory, anti-diabetic | Abuliton indicum, Sida rhombifolia | DNA barcoding | DNA barcoding for species identification from dried and powdered plant parts: a case study with authentication of the raw drug market samples of Sida cordifolia. | Vassou et al. (2015) |
| Zanthoxylum acanthopodium and Zantho xylum oxyphyllum | Whole plant | Useful against stomach trouble, blood purifier, reducing the incidence of leucoderma | None | AFLP | Species Specific AFLP Markers for authentication of Zanthoxylum acanthopodium & Zanthoxylum oxyphyllum | Gupta and Mandi (2014) |
| Cissampelos Pereira | whole plant | Stomach pain, fever, skin conditions, cardiac pain | Cyclea peltata, Stephania japonica | RAPD | Developing RAPD markers for identification of three source plants of Ayurvedic raw drug ‘Patha. | Vijayan et al. (2013) |
| Dracocephalum oldavica L. | Whole plant | Coronary diseases, pain relief, | M. officinalis, N. Cataria L. | RFLP | Genetic authentication by RFLP versus ARMS? The case of Moldavian dragonhead (Dracocephalum moldavica L.) | Horn et al. (2014) |
| Podophyllum hexandrum Royle | Root, rhizome | Anti-cancer | Podophyllum peltatum L | RAPD-SCAR | SCAR Molecular Markers for identification and authentication of medicinal plants Podophyllum hexandrum Royle | Al-Shaqha et al. (2014) |
| Bulbus Fritillariae Cirrhosae | Bulb | Antitussive and expectorant | Bulbus Fritillariae Pallidiflorae (Yibeimu), Bulbus Fritillariae Thunbergii (Zhebeimu), Bulbus Fritillariae Hupehensis (Hubeibeimu); and Bulbus Fritillariae Ussuriensis (Pingbeimu). | RAPD-SCAR | Authentication of Bulbus Fritillariae cirrhosae by RAPD-Derived DNA Markers | Xin et al. (2014) |
| Peucedanum praeruptorum L. | Roots | Expectorant | Anthricus sylvestris | DNA-barcoding | Molecular authentication of the traditional medicinal plant Peucedanum praeruptorumand its substitutes and adulterants by dna - barcoding technique | Zhou et al. (2014) |
| Ginko biloba | Leaves | Used for treatment of degenerative diseases of the brain, dementia, and for slowing down the progression of Parkinson's disease and Alzheimer's disease | None | DNA-barcoding | Authentication of Ginkgo biloba herbal dietary supplements using DNA barcoding | Little (2014) |
| Phoenix dactylifera L | Fruits | Fruits are antimutagenic and anti-oxidants | None | DNA-barcoding | DNA barcoding based on plastid matK and RNA polymerase for assessing the genetic identity of date (Phoenix dactylifera L.) cultivars | Enan and Ahamed (2014) |
| Piper nigrum L. | Fruit | Antimicrobial, antioxidant, antiinflammatory and antitoxic | Carica papaya L. | DNA-barcoding | DNA barcoding to detect chili adulteration in traded black pepper powder | Parvathy et al. (2014) |
| Indirubin (Isatis tinctoria, Polygonum tinctorium, and Strobilanthes cusia) | Leaves | Treatment of chronic myelocytic leukemia | P. hydropiper, P. chinense, Clerodendrum cyrtophyllum, Indigofera tinctoria, and S. dimorphotricha and roots of the geniuine drug. | DNA-barcoding | Rapid Identification and Verification of Indirubin-Containing Medicinal Plants | Hu et al. (2014) |
| Angelica acutiloba Kitagawa var. acutiloba Kitagawa, A. acutiloba Kitagawa var. sugiyamae Hikino | Root | To treat gynecological diseases | A. acutiloba Kitagawa var. sugiyamae Hikino | RAPD | Authentication and Genetic Origin of Medicinal Angelica acutiloba Using Random Amplified Polymorphic DNA Analysis. | Matsubara et al. (2013) |
| M. ilicifolia and M. Aquifolia | M. ilicifolia- leaf, M. Aquifolia- leaf, flower | Treatment of ulcer, gastritis and indigestion | Sorocea bonplandii | PCR-RFLP | Molecular authentication of Maytenus sp. by PCR-RFLP | Nakamura et al. (2013) |
| Knema andamanica | Whole plant | Antimicrobial and therapeutic | None | RAPD-SCAR | RAPD, SCAR and conserved 18S rDNA markers for a red-listed and endemic medicinal plant species, Knema andamanica (Myristicaceae) | Sheeja et al. (2013) |
| Schisandra chinensis | Fruit | Astringent, treat diarrhea, arrest excessive sweating | S. sphenanthera | RAPD-SCAR | Development of RAPD-Derived SCAR Markers and Multiplex-PCR for Authentication of the Schisandrae fructus | Lee et al. (2013) |
| Lonicera japonica | Leaves, flowers | Possess h heat-clearing, detoxifying, and anti-inflammatory effects | L. japonica var. chinensis, L. similis, and L. acuminate | DNA-barcoding | Stability and Accuracy Assessment of Identification of Traditional Chinese Materia Medica Using DNA Barcoding: A Case Study on Flos Lonicerae Japonicae | Hou et al. (2013) |
| Gentiana scabra, Gentiana triflora, Gentiana manshurica and Gentiana rigescens | Leaves, roots | Treating liver diseases and hepatoprotective against acetaminopheninduced acute toxicity | Gentiana rhodantha and Podophyllum hexandrum | DNA-barcoding | Evaluation of seven DNA barcodes for differentiating closely related medicinal Gentiana species and their adulterants | Wong et al. (2013) |
| Croton bonplandianum Baill | Seeds | Treatment of jaundice, acute constipation abdominal dropsy and internal abscesses | None | DNA-barcoding | MATK gene based molecular characterization of medicinal plant — Croton bonplandianum Baill | Chandramohan et al. (2013) |
| Shankhpushpi (Convolvulus pluricaulis) | Whole plant | Memory enhancer | Cansicora decussata, Clitoria ternatea, Evolvulus alsinoides | RAPD | Authentication of shankhpushpi by RAPD markers | Ganie et al. (2012) |
| Paris polyphylla Smith var. yunnanensis | Tuber | Anti-tumor, analgesia, anti-inflammatory, and Antifungal | None | PCR-RFLP | Molecular authentication of the medicinal plant Paris polyphylla Smith var. yunnanensis (Melanthiaceae) and its related species by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) | Liu and Ji (2012) |
| Fallopia multiflora | Root | Counteracting toxicity, cures carbuncles, and relaxes the bowels | C. auriculatum | PCR-RFLP | Molecular authentication of Fallopia multiflora by PCR-RFLP based on the trnL-trnF analysis | Zheng et al. (2012) |
| Catharanthus roseus L. Don. | whole plant | Anti-cancer | Solanum melongena, Lycopersicon esculentum | RT-SCAR | Real time sequence characterized amplified region (RT-SCAR) marker: Development and its application for authentication and quantification of Catharanthus roseus L. Don. | Chaudhary et al. (2013) |
| Asparagus racemosus Willd. | Root | Used for dyspepsia, lactogogue, anti- diarrhoeal, anti-septic, diuretic etc. | Asparagus gonoclados Baker | DNA-barcoding | DNA barcoding of authentic and substitute samples of herb of the family Asparagaceae and Asclepiadaceae based on the ITS2 region | Rai et al. (2012) |
| Eleutherococcus senticosus | Root, stem and leaf | Adaptogen used to cure many cancers | Periploca sepium | PCR-RFLP | Genetic and chemical diversity of Eleutherococcus senticosus and molecular identification of Siberian ginseng by PCR-RFLP analysis based on chloroplast trnK intron Sequence | Zhu et al. (2011) |
| Zingiber Officinale | Rhizome | Antioxidant, antitumor, and anti-inflammatory | Z. montanum, Z. zerumbet | AFLP | Species-specific AFLP markers for identification of Zingiber officinale, Z. montanum and Z. zerumbet (Zingiberaceae) | Ghosh et al. (2011) |
| Ipomoea mauritiana | Roots, leaves | Aphrodisiac, cardiotonic, demulcent, diuretic, refrigerant and galactogogue | Pueraria tuberosa (Roxb. ex Willd.) DC (Fabaceae), Adenia hondala (Gaertn.) de Wilde (Passifloraceae) and pith of Cycas circinalis L. | RAPD-SCAR | Development of Randomly Amplified Polymorphic DNA Based SCAR Marker for Identification of Ipomoea mauritiana Jacq (Convolvulaceae) | Devaiah et al. (2011) |
| Scrophularia ningpoensis | Root | To treat inflammation, laryngitis, tonsillitis, abscesses of carbuncles | None | ISSR-SCAR | Molecular authentication of geo-authentic Scrophularia ningpoensis | Chen et al. (2011) |
| Scutellaria baicalensis | Root | Treatment of hepatitis, jaundice, diarrhea, and inflammatory diseases. | S. amoena, S. rehderiana, and S. viscidula | DNA- barcoding | DNA barcodes for discriminating the medicinal plant Scutellaria baicalensis (Lamiaceae) and its adulterants. | Guo et al. (2011) |
| Ruta graveolens L | leaves, stems, flowers | Fertility regulation, menstrual cramps, earache, headache, nose bleed, and insect repellent | Euphorbia dracunculoides Lam. | DNA-barcoding (ITS) | Authentication of Ruta graveolens and its adulterant using internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA | Al-Qurainy (2010) |
| Pluricaria crispa, Pluricaria undulata | arial parts | Used against Gram positive bacteria | None | RAPD | Genetic relationship of two Pluricaria species and identification of their putative hybrids using RAPD markers | El-Kamali et al. (2010) |
| Piper nigrum | Fruits | Anti-bacterial, anti-inflammatory, antioxidant | Carica papaya | RAPD | Development of RAPD markers for authentication of Piper nigrum (L.) | Khan et al. (2010b) |
| Swertia chirayita | Seeds | Treating asthma and liver disorders | Andrographis paniculata, Exacum tetragonum, E. pedunculatum, Slevolgia orientalis, S. alata, S. angustifolia, S. bimaculata, S. ciliata, S. densifolia, S. elegans, S. lawii, S. minor, S. paniculata, S. multiflora, S. cordata | AFLP | AFLP markers for identification of Swertia species (Gentianaceae) | Misra et al. (2010a) |
| Aconitum heterophyllum | Root, stem | Used against stomach ache and fever | Cyperus rotundus | AFLP | AFLP markers for the identification of Aconitum species | Misra et al. (2010b) |
| Curcuma comosa | Rhizome | anti-inflammatory | Curcuma latifolia | AFLP | Identification and characterization of Curcuma comosa Roxb., phytoestrogens-producing plant, using AFLP markers and morphological characteristics | Keeratinijakal et al. (2010) |
| Rheum officinale Baill., Rheum palmatum L., and Rheum tanguticum Maxim. ex Balf. | roots, rhizome | Purgative, anti-inflammatory, antibacterial, purging heat, curing renal disorders, antitumor and antimutagenicity | R. hotaoense, R. compactum, R. undulatum and R. emodi | ISSR | Inter-simple sequence repeats (ISSR) molecular fingerprinting markers for authenticating the genuine species of rhubarb | Wang (2011) |
| Cynanchum wilfordii, Cynanchum auriculatum, and Polygonum multiflorum | Roots | Cynanchum wilfordii tonic to promote renal function, Cynanchum auriculatum- antidepressant, Polygonum multiflorum- protects against free radical damage induced by ultraviolet B irradiation of the skin | Cynanchum auriculatum is the substitute of Cynanchum wilfordii and Cynanchum wilfordii, Cynanchum auriculatum are the substitutes of Polygonum multiflorum | RAPD-SCAR | Rapid molecular authentication of three medicinal plant species, Cynanchum wilfordii, Cynanchum auriculatum, and Polygonum multiflorum (Fallopia multiflorum), by the development of RAPD-derived SCAR markers and multiplex-PCR | Moon et al. (2010) |
| Taxillus chinensis | Branches, leaves | Kidney reinforcement, tendons and bones strengthening, relief for rheumatic conditions and abortion prevention | Thuja, sutchuenensis, Scurrula parasitica, and Scurrula parasitica var. graciliflora | DNA-barcoding | Authentication of Taxillus chinensis using DNA barcoding technique | Li et al. (2010) |
| Cinnamomum osmophloeum Kaneh. | Leaves | Anti-diabetic, anti-inflammatory, astringent and diuretic | None | DNA-barcoding | DNA Barcoding Cinnamomum osmophloeum Kaneh. Based on the Partial Non-Coding ITS2 Region of Ribosomal Genes | Lee et al. (2010) |
8.1. Limitations of DNA barcoding
-
•
It has been found that DNA barcoding fail to distinguish recently diverged species (Kerr et al. 2007).
-
•
It is also to mention that use of barcoding depends to a large extent over the amplification of degraded DNA. This is one of the most difficult aspects because sequences characterized are longer than 500 bp and therefore, create problem in amplification (Min and Hickey 2007). Hence, DNA barcoding shall not be considered universal like other conventional markers.
9. Loop mediated isothermal amplification (LAMP)
Loop-mediated isothermal amplification (LAMP) is a molecular biology technique (Notomi et al. 2000) that relies on auto-cycling strand displacement DNA synthesis with Bst DNA polymerase. Unlike other DNA markers, here two primer pairs (inner and outer) are required to amplify the target gene. It has very high specificity, efficiency and rapidity and the molecular biology reports have shown these markers could amplify a specific gene from the whole genome discriminating a single nucleotide difference (Parida et al. 2008). DNA synthesis occurs within 1 h at a single temperature which is in range of 60–66°C. Further, this technique does not demand thermo-cycler as simple incubators and block heaters are good enough to furnish the temperature for successful amplification. Gel electrophoresis is also not mandatory since LAMP products can be detected by the turbidity that come to light due to a large amount of by-product, pyrophosphate ion, being produced, yielding an insoluble white precipitate of magnesium pyrophosphate in the reaction mixture (Mori et al. 2001). The applications of LAMP markers for medicinal plant identification, although is scarce; however, there are reports which confirm its utilization in plant authentication studies. Ganie et al. (2013) developed RAPD based LAMP markers to develop molecular ID for Nigella sativa. Taraxacum formosanum is substituted by many plant species which include Taraxacum officinale, Ixeridium laevigatum, Youngia japonica, Ixeris chinensis and Emilia sonchifolia var. javanica. Lai et al. (2015) designed four specific LAMP primers based on the nucleotide sequence of the internal transcribed spacer 2 (ITS2) and nuclear ribosomal DNA (nrDNA). LAMP amplicons were amplified and detected; here amplification of authentic plant species (Taraxacum formosanum) occurred and no such amplification in non-targeted adulterant plant DNA was observed. Twelve samples of Zingiber officinale from different regions of India were collected and screened with RAPD. A prominent specie specific DNA fragment of 780 bp was cloned and sequence verified with LAMP primers (Chaudhary et al. 2014). Other examples that support application of LAMP markers in plant medicinal biology include Curcuma longa (Sasaki and Nagumo 2007), Panex ginseng (Sasaki et al. 2008), Catharanthus roseus (Chaudhary et al., 2012).
9.1. Limitations of LAMP markers
-
•
Primer designing in LAMP technology is complex; a minimum of two primer pairs is required to identify six different regions of target gene/DNA sequence.
10. Next generation sequencing (NGS)
The advent of reversible chain- termination reaction in sequencing coupled with high resolution detection, popularly termed as Next Generation Sequencing or NGS allows concurrent sequencing of a large number of molecules therefore generating vast amount of sequence data simultaneously. The technique (Fig. 7 ) is based on the principle that DNA templates are first fragmented and thereafter immobilized on a solid support. These fragments are to be amplified and sequenced. Three main technologies/strategies that are in practice for NGS are commercialized by Roche/454 Life Sciences (Indianapolis, IN), Illumina/Solexa Genome Analyzer (San Diego, CA) and Applied iosystems/SOLiD System (orange county, CA). All of them retain their distinctive enzyme systems, sequencing chemistry, hardware and software engineering (Mardis, 2008, Shendure and Ji, 2008, Metzker, 2010); also, the sequencing reads obtained with these technologies varies in total sequencing output. Among these Illumina/Solexa and Applied Biosystems/SOLiD platforms have the ability to generate tens of millions of short reads (25–35 bp) per run; whereas Roche/454, might generate a few hundred thousand to 1 million reads of 400–500 bp DNA fragments per run (Sarwat and Yamdagni 2015). However, it must be kept in mind that Roche/454 is more advantageous regarding the identification of species because this system is more rapid and produces longer read lengths (Metzker 2010). The technology uses pyrosequencing in which pyrophosphate ions released are detected during the addition of nucleotides by DNA polymerase (Ronaghi 2001). The release of these ions starts off a series of reactions of which end product is to be detected by the formation of light by fire-fly luciferase.
Fig. 7.
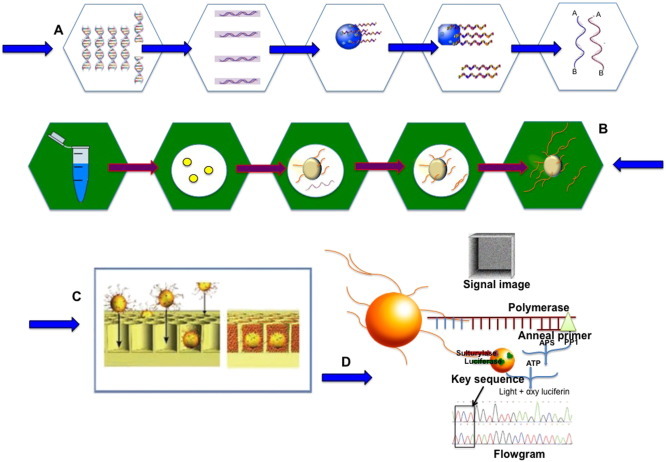
Outline of the Roche/454 sequencer workflow (A) Single-strand template DNA library preparation (B) Emulsion-based clonal amplification (C) Depositing DNA beads into the PicoTiter Plate device (D) Sequencing by synthesis. [Next Generation Sequencing and Whole Genome Selection in Aquaculture, Ed. Zhanjiang (John) Liu (2011) Blackwell Publishing Ltd.]
NGS technology relies extensively on use of complex algorithms for filtering, assembly and sequence analysis. Most of the commercially available tools such as DNAStar, CLCBio, GeneSpring are now quiet capable of analyzing NGS data. The advent of such rapid and in-expensive method of data generation has now allowed analysis of transcriptomes of medicinal plant species through the sequencing of their cDNA (RNA sequencing) rather to enter whole genome profiling. Transcriptome data could be helpful to provide the details of plant's response to developmental cues and the related environment. With the aid of RNA-sequencing methodologies it is possible to address questions encompassing cell type-specific transcriptomics, transcript secondary structure and gene mapping (Sangwan 2014). Transcriptome analyses in medicinal plants furnish enormous data to screen the entire proposed metabolic pathways with massive flexibility to examine the data.
Next Generation Sequencing has been employed for identifying molecular signatures in the transcriptome related to physiological functions of plant tissues. For example the leaf transcriptome of Costus pictus was sequenced applying Illumina reversible dye terminator sequencing technology and using combination of bioinformatics tools, transcripts related to anti-diabetic properties of this plant species was identified (Annadurai et al. 2012). Yun et al. (2015) applied NGS (Roche 454 GS-FLX Titanium Platform) and developed 9 microsatellite markers for Aconitum austrokoreense, an endangered medicinal plant. The same platform was applied to find out the prime components of the biosynthetic pathway in Dendrobium officinale through the formation of ESTs (Guo et al. 2013). Luo et al. (2010) identified the genes and their transcriptome regulation meant for the biosynthetic pathway in Huperzia serrata and Phlegmariurus carinatus with the aid of Roche/454 Titanium platform. Two sesquiterpenes (VoTPS1, VoTPS2) have been identified from one million transcript reads in the roots of Valeriana officinalis (Pyle et al. 2012). Ghangal et al. (2013) identified 13,299 SSRs of the 10,980 (12.4%) seabuckthorn (Hippophae rhamnoides L.) transcripts of which the mononucleotide SSRs represented the largest fraction (56.4%) followed by di-nucleotide repeats (21.5%) and the tri nucleotide repeats constitute the least (18.9%). It is mandatory to mention that traditional full length sequencing is a better choice when the aim is to obtain full length barcodes; however, for the degraded (Shokralla et al. 2009) mixed species samples (Ivanova et al. 2009), species phylogeny (Liu et al. 2013) Roche/454 pyrosequencing or Illumina NGS is the best option. Further reports are mentioned in Table 3 .
Table 3.
Applications of next generation sequencing in medicinal plant research.
| S. no. | Plant | Biological uses | Platform used in NGS | Study | Reference |
|---|---|---|---|---|---|
| 1 | Ocimum sanctum | Used for cough, Cold, bronchitis, expectorant. | Illumina HiSeq2000 | Unraveling the genome of Holy basil: an “incomparable” “elixir of life” of traditional Indian medicine | Rastogi et al. (2015) |
| 2 | Beta vulgaris | For the treatment of fevers and constipation, disorders of the respiratory tract, fevers, and infections | Illumina HiSeq2000 | The genome of the recently domesticated crop plant sugar beet (Beta vulgaris) | Dhom et al. (2014) |
| 3 | Panax ginseng | To stimulate immune system, anticancer, and anti-hyperlipidemic | Illumina HiSeq | Transcriptome profiling and comparative analysis of Panax ginseng adventitious roots |
Jayakodi et al. (2014) |
| 4 | Elaeis guineensis | Used as laxative and diuretic, as a poison antidote, as a cure for gonorrhea, menorrhagia, and bronchitis | Roche/454 | “Oil palm genome sequence reveals divergence of interfertile species in Old and New worlds.” | Singh et al. (2013), |
| 5 | Curcuma longa | Anti-malarial, anti-inflammatory and antitumor | Illumina GAIIx | De Novo Transcriptome Assembly (NGS) of Curcuma longa L. Rhizome Reveals Novel Transcripts Related to Anticancer and Antimalarial Terpenoids |
Annadurai et al. (2013) |
| 6 | Catharanthus roseus | Anti-cancer | Illumina HiSeq2000 | CathaCyc, a Metabolic Pathway Database Built from Catharanthus roseus RNA-Seq Data | Van Moerkercke et al. (2013) |
| 7 | Withania somnifera | Restorative tonic, stress, nerve disorder, aphrodiasiac. | 454-GS FLX sequencing (454 Life Sciences, Roche, USA) | De Novo Assembly, Functional Annotation and Comparative Analysis of Withania somnifera Leaf and Root Transcriptomes to Identify Putative Genes Involved in the Withanolides Biosynthesis | Gupta et al. (2013) |
| 8 | Azadirachta indica | Sedative, analgesic, epilepsy, hypertensive. | Pyro-sequencing | A draft of the genome and four transcriptomes of a medicinal and pesticidal angiosperm Azadirachta indica | Krishnan et al. (2012) |
| 9 | Cannabis sativa | Hallucinogenic, hypnotic, sedative, analgesic, and anti-inflammatory agent | Illumina, Roche | “The draft genome and transcriptome of Cannabis sativa” | van Bakel et al. (2011) |
| 10 | Populus trichocarpa | Antipyretic, analgesic and to control inflammation | Expressed sequence tag based methods | “The genome of black cottonwood, Populus trichocarpa (Torr. & Gray)”. | Tuskan et al. (2006) |
11. Issues of DNA markers towards authentication
DNA based technology though is superior has certain drawbacks. One requirement is of high quality DNA while analyzing samples, which might be a problem for dried or processed materials. During drug-processing, there could be a change in temperature and pH that may lead to degradation (fragmentation) of the DNA, rendering PCR analysis difficult. However, depending on the degree of degradation of DNA some methods can still be used in processed materials. Again, even low content of secondary metabolites (polysaccharides, tannins, essential oils, phenolics, alkaloids, etc.) may inhibit PCR or might affect DNA isolation. As secondary metabolite content increases with the age and this becomes severe as the material gets older. Such contaminations are problematic, either they stop or minimize the activity of many enzymes, such as polymerases, ligases, and restriction endonucleases. The market samples might be contaminated with endophytic fungi that could shatter the results of dominant markers like RAPD, AFLP and ISSR and might also influence DNA sequencing; however, this can be overcome with a plant-specific primer design. DNA related methods totally fail when the herb is in capsule form or an extract. Many markers, like ITS region of the 18S, 5.8S, and 26S nuclear ribosomal cistron, might show intra-specific sequence variation because of non functional paralogous sequences (pseudogenes) and for DNA barcoding, only orthologous DNA sequences is the choice. Consequently cloning of PCR products is sometimes inevitable. In order to develop a marker for identification of taxa, DNA analysis of closely related species and/or varieties and common botanical contaminants and adulterants is necessary, which is a costly and time-consuming process.
12. Conclusions
The genetic profile of the medicinal plant species will find applications in the quality control of the drugs obtained from these species not only by the pharmaceutical industries, but also Govt, agencies responsible for monitoring their quality. DNA markers which fill the maximum gap in solving the problem of identification, off-course is a boom to the molecular biology but is dependent over other two markers because you cannot find the efficiency of an authentic drug without chemical analysis. DNA markers are not tissue specific, is an advantage but, the same statement creates a problem when adulterants of the same plant are sold (Sometimes the flower of the plant species has the medicinal properties but instead of flowers some other part of the same plant e.g. roots, leaves etc. are sold), in such cases you simply cannot judge it by DNA markers because it will provide the same profile to all the different tissues of the same plant. In our viewpoint RAPD, AFLP, SAMPL and ISSR should not be allowed for authentication unless and until these markers are converted either into LAMP or SCAR. These markers can determine efficiently the genetic diversity of plant species. SCAR, LAMP and DNA bar-coding are ideal for medicinal plant authentication besides morphological, anatomical and chemical markers. In future, DNA markers can be used to build a reference library of traditional medicine (such as DNA sequences and fingerprints), in order to get complete rid of adulterants and spurious materials that has ruined this medicine.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgment
Dr. Showkat Hussain Ganie gratefully acknowledges the Science & Engineering Research Board, DST for providing scholarship (Grant NO. SB/YS/LS-118/2014).
References
- Abdin M.Z. Authentication of Cassia angustifolia and Cassia acutifolia in herbal medicines employing SCAR markers. Planta Med. 2013 doi: 10.1055/s-0033-1336562. [DOI] [Google Scholar]
- Abouzid S. Authentication of Silybum marianum varieties using RAPD analysis. Plant Tissue Cult. Biotechnol. 2014;24:57–63. [Google Scholar]
- Afaq S.H. A comparative introduction of the Unani and Tibetan medical traditions. Ayur Vijnana. 1999;6 [Google Scholar]
- Ali Z., Ganie S.H., Narula A., Sharma M.P., Srivastava P.S. Intra-specific genetic diversity and chemical profiling of different accessions of Clitoria ternatea L. Ind. Crop. Prod. 2013;43:768–773. [Google Scholar]
- Al-Qurainy F. Application of inter simple sequence repeat (ISSR marker) to detect genotoxic effect of heavy metals on Eruca sativa (L) Afr. J. Biotechnol. 2010;9:467–474. [Google Scholar]
- Al-Shaqha W.M., Khan M., Chaudhary A.A. SCAR molecular markers for identification and authentication of medicinal plants Podophyllum hexandrum Royle. Asian J. Biochem. Pharm. Res. 2014;4:66–75. [Google Scholar]
- Annadurai R.S., Jayakumar V., Mugasimangalam R.C., Katta M.A., Anand S., Gopinathan S., Sarma S.P., Fernandes S.J., Mullapudi N., Murugesan S., Rao S.N. Next generation sequencing and de novo transcriptome analysis of Costus pictus D. Don, a non-model plant with potent anti-diabetic properties. BMC. Genomics. 2012 doi: 10.1186/1471-2164-13-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annadurai R.S., Neethiraj R., Jayakumar V., Damodaran A.C., Rao S.N. De novo transcriptome assembly (NGS) of Curcuma longa L. rhizome reveals novel transcripts related to anticancer and antimalarial terpenoids. PLoS. ONE. 2013;8:e56217. doi: 10.1371/journal.pone.0056217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astin J.A., Marie A., Pelletier K.R., Hansen E., Haskell W.L. A review of the incorporation of complementary and alternative medicine by mainstream physicians. Arch. Intern. Med. 1998;158:2303–2310. doi: 10.1001/archinte.158.21.2303. [DOI] [PubMed] [Google Scholar]
- Biswas K., Biswas R. Identification of medicinal plants using PCR-RFLP in Dasamula — an Ayurvedic drug. J. Pharm. BioSci. 2013;3:94–99. [Google Scholar]
- Biswas K., Kapoor A., Biswas R. Authentication of herbal medicinal plant — Boerhavia diffusa L. Using PCR-RFLP. Curr. Trends Biotechnol. Pharm. 2013;7:716-124. [Google Scholar]
- Cameron K.M., Chase M.W. Phylogenetic relationships of Pogoniinae (Vanilloideae, orchidaceae): an herbaceous example of the eastern North America–eastern Asia phytogeographic disjunction. J. Plant Res. 1999;112:317–329. [Google Scholar]
- CBOL Plant working group a DNA barcode for land plants. Proc. Natl. Acad. Sci. USA. 2009;106:12794–12797. doi: 10.1073/pnas.0905845106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramohan A., Divya S.R., Dhanarajan M.S. Matk gene based molecular characterization of medicinal plant — Croton bonplandianum Baill. Int. J. Biosci. Res. 2013;2:1–7. [Google Scholar]
- Chaudhary A., Khan M., Al-Shaqha W.M., Alharbi M., Al-Khamees O.A. Rapid and easy molecular authentication of medicinal plant Zingiber officinale Roscoe by loop-mediated isothermal amplification (LAMP)-based marker. J. Med. Plants. Res. 2014;8:756–762. [Google Scholar]
- Chaudhary A.A., Hemant, Mohsin M., Ahmad A. Application of loop-mediated isothermal amplification (LAMP)-based technology for authentication of Catharanthus roseus (L.) G. Don. Protoplasma. 2012;249:417–422. doi: 10.1007/s00709-011-0293-2. [DOI] [PubMed] [Google Scholar]
- Chaudhary A.A., Yadav D., Hemant, Jamil S.S., Asif M. Real time sequence characterized amplified region (RT-SCAR) marker: development and its application for authentication and quantification of Catharanthus roseus L. Don. J. Med. Plants. Res. 2013;7:1154–1160. [Google Scholar]
- Chen C., Duan L.N., Zhou X.L., Chen B.L., Fu C.X. Molecular authentication of geo-authentic Scrophularia ningpoensis. J. Zheijang Univ. Sci. B. 2011;12:393–398. doi: 10.1631/jzus.B1000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Long Y., Khan M.A., Wei C., Shelly F., Junjiang F. Development and significance of RAPD-SCAR markers for the identification of Litchi chinensis Sonn. By improved RAPD amplification and molecular cloning. Electron. J. Biotechnol. 2015;18:35–39. [Google Scholar]
- Choo B.K., Moon B.C., Ji Y., Kim B.B. Development of SCAR markers for the discrimination of three species of medicinal plants, Angelica decursiva (Peucedanum decursivum), Peucedanum praeruptorum and Anthricus sylvestris, based on the internal transcribed spacer (ITS) sequence and random amplified polymorphic DNA (RAPD) Biol. Pharm. Bull. 2009;32:24–30. doi: 10.1248/bpb.32.24. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Plants as source of anticancer agents. J. Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- De Smet P.A.G.M., Keller K., Hansel R., Chandler R.F. Vol. 1. Springer-Verlag; Heidelberg: 1992. (Adverse effects of herbal drugs). [Google Scholar]
- Devaiah K.M., Balasubramani S.P., Venkatasubramanian P. Development of randomly amplified polymorphic DNA based SCAR marker for identification of Ipomoea mauritiana Jacq (Convolvulaceae) Evid. Based Complement. Alternat. Med. 2011 doi: 10.1093/ecam/neq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhom J.C., Minoche A.E., Holtgrawe D. The genome of the recently domesticated crop plant sugar beet (Beta vulgaris) Nature. 2014;505:546–549. doi: 10.1038/nature12817. [DOI] [PubMed] [Google Scholar]
- El-Kamali H.H., Habeballa R., Abdalla I., Mohammed A.Y., Abdelkarim N.D., Abbas I.M., Ali S.M. Genetic relationships of two Pulicaria species and identification of their putative hybrids using RAPD markers. J. Appl. Sci. 2010;8:687–693. [Google Scholar]
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat. Rev. Genet. 2004;5:435–445. doi: 10.1038/nrg1348. [DOI] [PubMed] [Google Scholar]
- Enan M.R., Ahamed A. DNA barcoding based on plastid matK and RNA polymerase for assessing the genetic identity of date (Phoenix dactylifera L.) cultivars. Genet. Mol. Res. 2014;13:3527–3536. doi: 10.4238/2014.February.14.2. [DOI] [PubMed] [Google Scholar]
- Fang D.Q., Roose M.L. Identification of closely related Citrus cultivars with inter-simple sequence repeat markers. Theor. Appl. Genet. 1997;95:408–417. [Google Scholar]
- Feng T., Liu S., Xing-jin H. Molecular authentication of the traditional Chinese medicinal plant Angelica sinensis based on internal transcribed spacer of nrDNA. Electron. J. Biotechnol. 2010;13(1) [Google Scholar]
- Fu J., Yang L., Khan M.A., Mei Z. Genetic characterization and authentication of Lonicera japonica thunb. By using improved RAPD analysis. Mol. Biol. Rep. 2013;40:5993–5999. doi: 10.1007/s11033-013-2703-3. [DOI] [PubMed] [Google Scholar]
- Ganie S.H., Ali Z., Das S., Srivastava P.S., Sharma M.P. Molecular characterization and chemical profiling of different populations of Convolvulus pluricaulis (Convolvulaceae); an important herb of Ayurvedic medicine. 3. Biotechnology. 2015;5:295–302. doi: 10.1007/s13205-014-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganie S.H., Sharma M.P. Molecular and chemical profiling of different populations of Evolvulus alsinoides (L.) L. Int. J. Agricul. Crop. Sci. 2014;7:1322–1331. [Google Scholar]
- Ganie S.H., Srivastava P.S., Narula A., Ali Z., Sharma M.P. Authentication of shankhpushpi by RAPD markers. EurAsia J. BioSci. 2012;6:39–46. [Google Scholar]
- Ganie S.H., Yadav D., Ahmad A., Chadhry A., Asif A. Authentication of traditional crop Kalongi (Nigella sativa L.) by LAMP marker. Ind. J. Res. Pharm. Biotechnol. 2013;1:765–771. [Google Scholar]
- Gao T., Pang X.H., Chen S.L. Authentication of plants in Astragalus by DNA barcoding technique. Planta Med. 2009 doi: 10.1055/s-0029-1234433. [DOI] [Google Scholar]
- Ghangal R., Chaudhary S., Jain M., Purty R.S., Sharma P.C. Optimization of de novo short read assembly of seabuckthorn (Hippophae rhamnoides L.) transcriptome. PLoS. ONE. 2013;8:e72516. doi: 10.1371/journal.pone.0072516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Majumdar P.B., Sen M.S. Species-specific AFLP markers for identification of Zingiber officinale, Z. montanum and Z. zerumbet (Zingiberaceae) Genet. Mol. Res. 2011;10:218–229. doi: 10.4238/vol10-1gmr1154. [DOI] [PubMed] [Google Scholar]
- Gowda B., Chandrika K., Prasanna K.T., Kirana V.C. Authentication of Embelica ribes Brrm F. and Embelica tsjeriam-cottam A. DC. Int. J. Sci. Nat. 2010;1:58–60. [Google Scholar]
- Guo X., Li Y., Li C. Analysis of the Dendrobium officinale transcriptome reveals putative alkaloid biosynthetic genes and genetic markers. Gene. 2013;527:131–138. doi: 10.1016/j.gene.2013.05.073. [DOI] [PubMed] [Google Scholar]
- Guo X., Wang X., Su W., Zhang G., Zhou R. DNA barcodes for discriminating the medicinal plant Scutellaria baicalensis (Lamiaceae) and its adulterants. Bio. Pharm. Bull. 2011;34:1198–1203. doi: 10.1248/bpb.34.1198. [DOI] [PubMed] [Google Scholar]
- Gupta D.D., Mandi S.S. Species specific AFLP markers for authentication of Zanthoxylum acanthopodium & Zanthoxylum oxyphyllum. J. Med. Plants. Stud. 2014;1:1–9. [Google Scholar]
- Gupta P., Goel R., Pathak S., Srivastava A., Singh S.P., Sangwan R.S., Asif M.H., Trivedi P.K. De novo assembly, functional annotation and comparative analysis of Withania somnifera leaf and root transcriptomes to identify putative genes involved in the withanolides biosynthesis. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0062714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez S., Ang-Lee M.K., Walker D.J., Zacny J.P. Assessing subjective and phychomoter effects of the herbal medication valerian in healthy volunteers. Pharmacol. Biochem. Behav. 2004;78:57–64. doi: 10.1016/j.pbb.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Handa S. proceedings of the first joint workshop on quality control and standardization of traditional medicine. Indo-China experience. 2004. Indian efforts for quality control and standardization of herbal drug products; pp. 8–10. [Google Scholar]
- Hebert P.D.N., Ratnasingham S., deWaard J.R. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. B. 2003;270:S96–S99. doi: 10.1098/rsbl.2003.0025. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon C.C., Chow Y.C., Zeng F.Y., Leung F.C. Genetic authentication of ginseng and other traditional Chinese medicine. Acta Pharmacol. Sin. 2003;24:841–846. [PubMed] [Google Scholar]
- Horn T., Völker J., Rühle M., Häser A., Jürges G., Nick P. Genetic authentication by RFLP versus ARMS? The case of Moldavian dragonhead (Dracocephalum moldavica L.) Eur. Food Res. Technol. 2014;238:93–104. [Google Scholar]
- Hou D.Y., Song J.Y., Shi L.C., Ma X.C., Xin T.Y., Han J.P. Stability and accuracy assessment of identification of traditional Chinese materia medica using DNA barcoding: a case study on Flos Lonicerae japonica. Bio. Med. Res. Int. 2013 doi: 10.1155/2013/549037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Yuan T., Ye X., Peipei C., Wei S., Yuhua S., Licheng G., Haibo H., Chao X., Shilin C., Xiuqiao Z. Rapid identification and verification of indirubin-containing medicinal plants. Evid. Based Complement. Alternat. Med. 2014 doi: 10.1155/2015/484670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N.V., Borisenko A.V., Hebert P.D.N. Express barcodes: racing from specimen to identification. Mol. Ecol. Resour. 2009;9:35–41. doi: 10.1111/j.1755-0998.2009.02630.x. [DOI] [PubMed] [Google Scholar]
- Jayakodi M., Lee S.C., Park H.S., Jang W., Choi B.S., Nah G.J., Kim D.S., Natesan S., Sun C., Lee Y.S., Yang T.J. Transcriptome profiling and comparative analysis of Panax ginseng adventitious roots. J. Ginseng. Res. 2014;38:278–288. doi: 10.1016/j.jgr.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Cao L., Yuan Y., Chen M., Jin Y., Huang L. Barcoding melting curve analysis for rapid, sensitive, and discriminating authentication of saffron (Crocus sativus L.) from its adulterants. BioMed. Res. Int. 2014 doi: 10.1155/2014/809037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye A., Clarke R., Sabar R., Vig S., Dhawan K., Hofbauer R., Kaye A.M. Herbal medicines: current trends in anaesthesiology practice — a hospital survey. J. Clin. Anesth. 2000;12:468–471. doi: 10.1016/s0952-8180(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Keeratinijakal V., Kladmook M., Laosatit V. Identification and characterization of curcuma comosa roxb. Phytoestrogens-producing plant, using AFLP markers and morphological characteristics. J. Med. Plants. Res. 2010;4:2651–2657. [Google Scholar]
- Kerr K.C., Stoeckle M.Y., Dove C.J. Comprehensive DNA barcode coverage of north American birds. Mol. Ecol. Notes. 2007;7:35–43. doi: 10.1111/j.1471-8286.2007.01670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Mirza K.J., Abdin M.Z. Development of RAPD markers for authentication of medicinal plant Cuscuta Reflexa. EurAsia J. BioSci. 2010;4:1–7. [Google Scholar]
- Khan S., Mirza K.J., Anwar F., Abdin M.Z. Development of RAPD markers for authentication of Piper nigrum (L.) Environ. Int. J. Sci. Tech. 2010;5:47–56. [Google Scholar]
- Kiran U., Khan S., Mirza K.J., Ram M. SCAR markers: a potential tool for authentication of herbal drugs. Fitoterapia. 2010;81:969–976. doi: 10.1016/j.fitote.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Kojima T., Nagaoka T., Noda N., Ogihara Y. Genetic linkage map of ISSR and RAPD markers in Einkorn wheat in relation to that of RFLP markers. Theor. Appl. Genet. 1998;96:37–45. [Google Scholar]
- Krishnan N.M., Pattnaik S., Jain P., Gaur P., Choudhary R., Srividya V., Sa D., Arun K.H., Krishna P.G.B., Nair J., Varghese L., Valivarthi N.K., Dhas K., Ramaswamy K., Panda B. A draft of the genome and four transcriptomes of a medicinal and pesticidal angiosperm Azadirachta indica. BMC Genomics. 2012;13:464. doi: 10.1186/1471-2164-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Gupta V.K., Misra A.K., Modi D.R., Pandey B.K. Potential of molecular markers in plant biotechnology. Plant. OMICS J. 2009;2:141–162. [Google Scholar]
- Lai G.H., Chao J., Lin M.K., Chang W.T., Peng W.H., Sun F.C. Rapid and sensitive identification of the herbal tea ingredient Taraxacumformosanum using loop-mediated isothermal amplification. Int. J. Mol. Sci. 2015;16:1562–1575. doi: 10.3390/ijms16011562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J., Srivastava J., Vietmeyer N. The World Bank; Washington, DC: 1997. Medicinal Plants: Rescuing a Global Heritage. [Google Scholar]
- Lee S.C., Shu-Jiau C., Jui-Hung Y., Tsai-Yun L., Kun-Ting H., Jeng-Chuan Y. DNA barcoding Cinnamomum osmophloeum Kaneh. Based on the partial non-coding ITS2 region of ribosomal. Genes. J. Food Drug Anal. 2010;18:128. [Google Scholar]
- Lee Y.M., Cheol M.B., Yunui J., Seok S.H., Kyoung K.H. Development of RAPD-derived SCAR markers and multiplex-PCR for authentication of the Schisandrae Fructus. Korean J. Med. Crop. Sci. 2013;3:165–173. [Google Scholar]
- Li G., Park Y.J. SCAR markers for discriminating species of two genera of medicinal plants, Liriope and Ophiopogon. Genet. Mol. Res. 2012;11:2987–2996. doi: 10.4238/2012.May.18.14. [DOI] [PubMed] [Google Scholar]
- Li M., Jiang R.W., Hon P.M., Cheng L., Li L.L., Zhou J.R., Shaw P.C., But P.P.H. Authentication of the anti-tumor herb baihuasheshecao with bioactive marker compounds and molecular sequences. Food Chem. 2010;119:1239–1245. doi: 10.1016/j.foodchem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang B., Han R., Zheng Y., Yin H.Y., Xu L. Identification of medicinal plant schisandra chinensis using a potential DNA barcode ITS2. Acta Soc. Bot. Pol. 2013;82:283–288. [Google Scholar]
- Li Y., Ruan J., Chen S., Song J. Authentication of Taxillus chinensis using DNA barcoding technique. J. Med. Plants. Stud. 2011;4:2706–2709. [Google Scholar]
- Little D.P. Authentication of Ginkgo biloba herbal dietary supplements using DNA barcoding. Genome. 2014;57:513–516. doi: 10.1139/gen-2014-0130. [DOI] [PubMed] [Google Scholar]
- Liu Z.J. Wiley-Blackwell; 2011. Next Generation Sequencing and Whole Genome Selection in Aquaculture. [Google Scholar]
- Liu J. An overview of clinical studies on complementary and alternative medicine in HIV infection and AIDS. In: Bodeker G., Burford G., editors. Traditional, Complementary and Alternative Medicine Policy and Public Health Perspectives. Imperial College Press; 2007. pp. 295–310. [Google Scholar]
- Liu T., Ji Y. Molecular authentication of the medicinal plant Paris polyphylla smith var. yunnanensis (melanthiaceae) and its related species by polymerase chain reaction– restriction fragment length polymorphism (PCR-RFLP) J. Med. Plants. Res. 2012;6:1181–1186. [Google Scholar]
- Liu Y., Huo N., Dong L. Complete chloroplast genome sequences of Mongolia medicine Artemisia frigida and phylogenetic relationships with other plants. PLoS ONE. 2013;8:e57533. doi: 10.1371/journal.pone.0057533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Li Y., Sun C. Comparison of 454-ESTs from Huperzia serrata and Phlegmariurus carinatus reveals putative genes involved in lycopodium alkaloid biosynthesis and developmental regulation. BMC. Plant Biol. 2010;10:209. doi: 10.1186/1471-2229-10-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis E.R. Next-generation DNA sequencing methods. Annu. Rev. Genomics Hum. Genet. 2008;9:387–402. doi: 10.1146/annurev.genom.9.081307.164359. [DOI] [PubMed] [Google Scholar]
- Matsubara K., Shindo S., Watanabe H., Ikegami F. Authentication and genetic origin of medicinal Angelica acutiloba using random amplified polymorphic DNA analysis. Am. J. Plant. Sci. 2013;4:269–273. [Google Scholar]
- Metzker M.L. Sequencing technologies the next generation. Nat. Rev. Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- Min X.J., Hickey D.A. Assessing the effect of varying sequence length on DNA barcoding of fungi. Mol. Ecol. Notes. 2007;7:365–373. doi: 10.1111/j.1471-8286.2007.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A., Shasany A.K., Shukla A.K., Darokar M.P. AFLP markers for identification of Swertia species (Gentianaceae) Genet. Mol. Res. 2010;9:1535–1544. doi: 10.4238/vol9-3gmr785. [DOI] [PubMed] [Google Scholar]
- Misra A., Shukla A.K., Shasany A.K., Sundaresan V., Jain S.P., Singh S.C., Bagchi G.D., Khanuja S.P.S. AFLP markers for identification of Aconitum species. Med. Aromat. Plant Sci. Biotechnol. 2010;4:15–19. [Google Scholar]
- Moon B.C., Choo B.K., Cheon M.S., Yoon T., Ji Y., Kim B.B., Lee A.Y., Kim H.K. Rapid molecular authentication of three medicinal plant species Cynanchum wilfordii, Cynanchum auriculatum, and Polygonum multiflorum (Fallopia multiflorum), by the development of RAPD-derived SCAR markers and multiplex-PCR. Plant Biotechnol. Rep. 2010;4:1–7. [Google Scholar]
- Moon B.C., Yunui J., Young M.L., Kang Y.M., Kim H.K. Authentication of Akebia quinata DECNE.From its common adulterant medicinal plant species based on the RAPD-derived SCAR markers and multiplex-PCR. Genes. Genomics. 2015;37:23–32. [Google Scholar]
- Mori Y., Nagamine K., Tomita N., Notomi T. Detection of loop-mediated isothermal amplication by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001;289:150–154. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- Mueller U.G., Wolfenbarger L.L. AFLP genotyping and fingerprinting. Trends Ecol. Evol. 1999;14:389–394. doi: 10.1016/s0169-5347(99)01659-6. [DOI] [PubMed] [Google Scholar]
- Nakamura S.S., do Valle J.S., Jacomassi E., Linde G.A., Colauto N.B. Vol. 34. 2013. Semina: ciências. Agrárias. Londrina; pp. 627–634. [Google Scholar]
- Newmaster S.G., Grguric M., Shanmughanandhan D., Ramalingam S., Ragupathy S. DNA barcoding detects contamination and substitution in North American herbal products. BMC. Med. 2013;11:222. doi: 10.1186/1741-7015-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Notomi T., Okayama H., Masubuchi H., Yonekawa T., Wananabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28 doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida M., Sannarangaiah S., Dash P.K., Rao P.V.L., Morita K. Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 2008;18:407–421. doi: 10.1002/rmv.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathy V.A., Swetha V.P., Sheeja T.E., Leela N.K., Chempakam B., Sasikumar B. DNA barcoding to detect chilli adulteration in traded black pepper powder. Food Biotechnol. 2014;28:25–40. [Google Scholar]
- Passinho-Soares H., Felix D., Kaplan M.A., Margis-Pinheiro M. Authentication of medicinal plant botanical identity by amplified fragmented length polymorphism dominant DNA marker: inferences from the Plectranthus genus. Planta Med. 2006;72:929–931. doi: 10.1055/s-2006-946673. [DOI] [PubMed] [Google Scholar]
- Payyappallimana U. Role of traditional medicine in primary health care: an overview of perspectives and challenges. Yokohama J. Soc. Sci. 2010;14:723–743. [Google Scholar]
- Pyle B.W., Tran H.T., Pickel B., Haslam T.M., Gao Z., Macnevin G., Vederas J.C., Kim S.U., Ro D.K. Enzymatic synthesis of valerena-4,7(11)-diene by a unique sesquiterpene synthase from the valerian plant (Valeriana officinalis) FEBS J. 2012;279:3136–3146. doi: 10.1111/j.1742-4658.2012.08692.x. [DOI] [PubMed] [Google Scholar]
- Rai P.S., Bellampalli R., Dobriyal R.M., Agarwal A., Satyamoorthy K., Narayana D.B.A. DNA barcoding of authentic and substitute samples of herb of the family Asparagaceae and Asclepiadaceae based on the ITS2 region. J. Ayurveda Integr. Med. 2012;3:136–140. doi: 10.4103/0975-9476.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S., Kalra A., Gupta V., Khan F., Lal R.K., Tripathi A.K., Parameswaran S., Gopalakrishnan C., Ramaswamy G., Shasany A.K. Unravelling the genome of Holy basil: an “incomparable” “elixir of life” of traditional Indian medicine. BMC Genomics. 2015;16:413. doi: 10.1186/s12864-015-1640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaghi M. Pyrosequencing sheds light on DNA sequencing. Genome Res. 2001;11:3–11. doi: 10.1101/gr.11.1.3. [DOI] [PubMed] [Google Scholar]
- Russi L., Moretti C., Raggi L., Albertini E., Falistocco E. Identifying commercially relevant Echinacea species by AFLP molecular markers. Genome. 2009;52:912–918. doi: 10.1139/g09-066. [DOI] [PubMed] [Google Scholar]
- Sangwan N.S. Transcriptomics in aid to the establishment of secondary metabolic pathways in non-model plants. Next Gener. Seq. 2014 doi: 10.4172/jngsa.1000e101. [DOI] [Google Scholar]
- Sarwat M., Yamdagni M.M. DNA barcoding, microarrays and next generation sequencing: recent tools for genetic diversity estimation and authentication of medicinal plants. Crit. Rev. Biotechnol. 2015 doi: 10.3109/07388551.2014.947563. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Nagumo S. Rapid identification of Curcuma longa and C. aromatica by LAMP. Biol. Pharm. Bulletin. 2007;30:2229–2230. doi: 10.1248/bpb.30.2229. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Komatsu K., Nagumo S. Rapid detection of Panax ginseng by loop-mediated isothermal amplification and its application to authentication of ginseng. Biol. Pharm. Bull. 2008;31:1806–1808. doi: 10.1248/bpb.31.1806. [DOI] [PubMed] [Google Scholar]
- Seethapathy G.S., Balasubramani S.P., Venkatasubramanian P. NrDNA ITS sequence based SCAR marker to authenticate Aconitum heterophyllum and Cyperus rotundus in ayurvedic raw drug source and prepared herbal products. Food Chem. 2014;145:1015–1020. doi: 10.1016/j.foodchem.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Sheeja T.E., Rosana O.B., Swetha V.P., Shalini R.S., Siju S., Dhanya R., Rahul P.R., Krishnamoorthy B. The 18S rDNA gene discriminates between red-listed and unexplored ethnomedicinal species of Myristicaceae restricted to humid tropics of India. Genet. Resour. Crop. Evol. 2013 doi: 10.1007/s10722-013-0060-7. [DOI] [Google Scholar]
- Shendure J., Ji H. Next-generation DNA sequencing. Nat. Biotechnol. 2008;26:1135–1145. doi: 10.1038/nbt1486. [DOI] [PubMed] [Google Scholar]
- Shokralla S., Meusnier I., Janzen D.H. Third International Barcode of Life Conference; 2009 Nov 7–12; Mexico city, Mexico. 2009. Pyrosequencing for rapid mini-barcoding. [Google Scholar]
- Shirasawa K., Ishii K., Kim C., Ban T., Suzuki M., Ito T., Muranaka T., Kobayashi M., Nagata N., Isobe S., Tabata S. Development of Capsicum EST-SSR markers for species identification and in silico mapping onto the tomato genome sequence. Mol. Breed. 2013;31:101–110. doi: 10.1007/s11032-012-9774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Singh A., Khanuja S. Medicinal plants: India's opportunities. Pharm. Bioworld. 2003;1:59–66. [Google Scholar]
- Singh R., Ong-Abdullah M., Low E.T., Manaf M.A., Rosli R., Nookiah R., Ooi L.C., Ooi S.E., Chan K.L., Halim M.A., Azizi N., Nagappan J., Bacher B., Lakey N., Smith S.W., He D., Hogan M., Budiman M.A., Lee E.K., DeSalle R., Kudrna D., Goicoechea J.L., Wing R.A., Wilson R.K., Fulton R.S., Ordway J.M., Martienssen R.A., Sambanthamurthi R. Oil palm genome sequence reveals divergence of inter fertile species in old and new worlds. Nature. 2013;500:335–339. doi: 10.1038/nature12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarajan V.V., Balachandran I. Oxford and IBH Publications Co; New Delhi: 1994. Ayurvedic Drugs and Their Plant Sources. [Google Scholar]
- Tamhankar S., Ghate V., Raut A., Rajput B. Molecular profiling of “Chirayat” complex using inter simple sequence repeat (ISSR) markers. Planta Med. 2009;75:1266–1270. doi: 10.1055/s-0029-1185543. [DOI] [PubMed] [Google Scholar]
- Techen N., Parveen I., Pan Z., Khan I.A. DNA barcoding of medicinal plant material for identification. Curr. Opin. Biotechnol. 2014;25:103–110. doi: 10.1016/j.copbio.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Theerakulpisut P., Kanawapee N., Maensiri D., Bunnag S., Chantaranothai P. Development of species-specific SCAR markers for identification of three medicinal species of Phyllanthus. J. Syst. Evol. 2008;46:614–621. [Google Scholar]
- Thormann C.E., Ferreira M.E., Camargo L.E.A., Tivang J.G., Osborn T.C. Comparison of RFLP and RAPD markers to estimating genetic relationships within and among cruciferous species. Theor. Appl. Genet. 1994;88:973–980. doi: 10.1007/BF00220804. [DOI] [PubMed] [Google Scholar]
- Tripathi N., Saini N., Tiwari S. Assessment of genetic diversity among Aloe vera accessions using amplified fragment length polymorphism. Int. J. Med. Arom. Plants. 2011;1:115–121. [Google Scholar]
- Tuskan G.A., Di-Fazio S. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- Van Bakel H., Stout J.M., Cote A.G., Tallon C.M., Sharpe A.G., Hughes T.R., Page J.E. The draft genome and transcriptome of Cannabis sativa. Genome Biol. 2011 doi: 10.1186/gb-2011-12-10-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Moerkercke A., Fabris M., Pollier J., Baart G.J., Rombauts S., Hasnain G., Rischer H., Memelink J., Oksman-Caldentey K.M., Goossens A. CathaCyc, a metabolic pathway database built from Catharanthus roseus RNA-Seq data. Plant Cell Physiol. 2013;54:673–685. doi: 10.1093/pcp/pct039. [DOI] [PubMed] [Google Scholar]
- Vassou S.L., Kusuma G., Parani M. DNA barcoding for species identification from dried and powdered plant parts: a case study with authentication of the raw drug market samples of Sida cordifolia. Gene. 2015;559:86–93. doi: 10.1016/j.gene.2015.01.025. [DOI] [PubMed] [Google Scholar]
- Vijayan D., Cheethaparambil A., Pillai G.S., Balachandran I. Developing RAPD markers for identification of three source plants of ayurvedic raw drug ‘Patha’. Int. J. Phytomed. Relat. Ind. 2013;5:55–58. [Google Scholar]
- Vijayan D., Cheethaparambil A., Pillai G.S., Balachandran I. Molecular authentication of Cissampelos pareira L. var. hirsuta (Buch.-Ham. ex DC.) Forman, the genuine source plant of ayurvedic raw drug ‘Patha’, and its other source plants by ISSR markers. 3. Biotech. 2014;4:559–562. doi: 10.1007/s13205-013-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P., Hogers R., Bleeker M., Reijans M., Lee T. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.M. Inter-simple sequence repeats (ISSR) molecular fingerprinting markers for authenticating the genuine species of rhubarb. J. Med. Plant Res. 2011;5:758–764. [Google Scholar]
- Wen C.C., Kuo Y.H., Jan J.T. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- WHO . WHO; Geneva: 2002. Traditional medicine strategy 2002–2005. [Google Scholar]
- Willcox M.L., Bodeker G. Traditional herbal medicines for malaria. Br. Med. J. 2004;329:1156–1159. doi: 10.1136/bmj.329.7475.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.L., But P.P.H., Shaw P.C. Evaluation of seven DNA barcodes for differentiating closely related medicinal Gentiana species and their adulterants. Chin. Med. 2013;8:16. doi: 10.1186/1749-8546-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin G.Z., Lam Y.C., Maiwulanjiang M., Chan G.K., Zhu K.Y., Tang W.L., Dong T.T., Shi Z.Q., Li P., Tsim K.W. Authentication of Bulbus Fritillariae Cirrhosae by RAPD-derived DNA markers. Molecules. 2014;19:3450–3459. doi: 10.3390/molecules19033450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A., Ahmad J., Chaudhary A.A., Altaf A. Development of sequence characterized amplified region (SCAR) marker for the authentication of Bacopa monnieri (L.) Wettst. Eur. J. Med. Plants. 2012;2:186–198. [Google Scholar]
- Yun Y.E., Yu J.N., Nam G.H., Ryu S.A., Kim S., Oh K., Lim C.E. Next-generation sequencing identification and characterization of microsatellite markers in Aconitum austrokoreense Koidz., an endemic and endangered medicinal plant of Korea. Genet. Mol. Res. 2015;14:4812–4817. doi: 10.4238/2015.May.11.13. [DOI] [PubMed] [Google Scholar]
- Zheng C.J., Sheng S.J., Zhao S.J. Molecular authentication of fallopia multiflora by PCR-RFLP based on the trnL–trnF analysis. Zhong Yao Cai. 2012;35:543–547. [PubMed] [Google Scholar]
- Zhou J., Wang W., Liu M., Liu Z. Molecular authentication of the traditional medicinal plant Peucedanum praeruptorum and its substitutes and adulterants by DNA - barcoding technique. Pharmacogn. Mag. 2014;10:385–390. doi: 10.4103/0973-1296.141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Bai Y., Oya M., Tanaka K., Komatsu K., Maruyama T., Goda Y., Kawasaki T., Fujita M., Shibata T. Genetic and chemical diversity of Eleutherococcus senticosus and molecular identification of Siberian ginseng by PCR-RFLP analysis based on chloroplast trnK intron sequence. Food Chem. 2011;129:1844–1850. [Google Scholar]


