Abstract
Aim
To investigate the prevalence of urinary tract infections in hospitalized patients with type 2 diabetes mellitus and identify corresponding risk factors.
Methods
We conducted a cross-sectional study on 7.347 patients with type 2 diabetes mellitus as the principal diagnosis, using hospitalization discharge summary data from January 1 to December 31, 2015. Disease stages were classified as stages 1, 2, and 3.
Results
Of 7.347 patients, 16.2% had urinary tract infections. The urinary tract infection prevalence was 24.4% in 428 patients in stage 1 and 4.8% in 2.840 patients in stage 2; it was higher among patients who underwent medical procedures than among those who underwent surgery (24.4% vs 4.8%). In multivariate regression analysis, age (OR = 1.031; 95% CI = 1.02–1.04), length of hospitalization (OR = 1.018; 95% CI = 1.013–1.024), sex (woman) (OR = 2.248; 95% CI = 1.778–2.842), comorbidity of stage 3 cerebrovascular disease (OR = 1.737; 95% CI = 1.111–2.714), and comorbidity of stage 1 colorectal cancer (OR = 2.417; 95% CI = 1.152–5.074) were found to be the risk factors of urinary tract infection in the ten hospitals considered.
Conclusions
Our findings suggest that urinary tract infection prevalence was higher in women without evidence of organ injury and those receiving medical treatment. Comorbidities (cerebrovascular disease and colorectal cancer) were identified as risk factors.
Keywords: Type 2 diabetes mellitus, Urinary tract infections, In-hospital, Prediction model, Cross-sectional study
Abbreviations: T2DM, type 2 diabetes mellitus; UTI, urinary tract infection; COC, complications-of-care
Highlights
-
•
Patients with stage 1 diabetes had higher UTI rates than those with stage 2 diabetes.
-
•
Women were at a higher risk of urinary tract infection than men.
-
•
Cerebrovascular disease and colorectal cancer were risk factors.
-
•
The UTI was significantly higher in those who received medical treatment than those who received surgical treatment.
1. Introduction
The prevalence of type 2 diabetes mellitus (T2DM) has increased in recent years (Zheng et al., 2018; isk Factor Collabora, 2016). In 2015, the International Diabetes Federation reported that approximately 415 million adults (aged 20–79 years) had T2DM; this number is expected to increase to 642 million by 2040 (Zheng et al., 2018). Globally, the prevalence of age-standardized adult diabetes increased from 4.3% in 1980 to 9.0% in 2014 in men and from 5.0% to 7.9% in women in the same time frame (isk Factor Collabora, 2016). By 2015, T2DM had occurred in 12.1% of men and 7.7% of women in Portugal (Sociedade Portuguesa de Diabetologia, 2015); only 7.3% of these patients received primary care (Sociedade Portuguesa de Diabetologia, 2015).
T2DM and its complications have contributed to an increase in the rate of admissions (Zheng et al., 2018). The admission rates for patient with T2DM are two to six times higher than those for patients without diabetes (Lara-Rojas et al., 2019). In Portugal, 88-870 adults had T2DM in 2006, and hospital admission rates increased by 82.7% between 2006 and 2015, with acute myocardial infarction being the primary cause of hospitalization (Sociedade Portuguesa de Diabetologia, 2015). Microangiopathic disease and stroke were identified as predictors of hospitalization within Portugal's National Health System (Sociedade Portuguesa de Diabetologia, 2015). In contrast, cardiovascular disease is the primary cause of hospitalization in Spain (Lara-Rojas et al., 2019).
In Portugal, urinary tract infections (UTIs) are frequent adverse events during hospitalization (https://apurologia.pt/. A, 2008), for which T2DM is a major risk factor (Wilke et al., 2015; Hirji et al., 2012). However, the cause of UTI is considered to be multifactorial (https://apurologia.pt/. A, 2008). Studies (Wilke et al., 2015; Hirji et al., 2012) have reported that UTI is prevalent in those with diabetes, particularly in older women. Wilke et al. (2015) showed that 3.86% of UTI events were associated with inpatient hospitalization and 35.6% of UTI events were caused by more than one reported bacterium. It is difficult to determine the actual incidence of UTIs in Portugal, but approximately 15% of women aged 70 years have asymptomatic bacteriuria; this rate increases to 30–40% in elderly women admitted to geriatric institutions and practically to 100% in patients with permanent urinary tubes (https://apurologia.pt/. A, 2008).
Mazzi et al. (2017) reported that other risk factors, such as severe hypoglycemic events, advanced age, and the presence of comorbidities, may increase the occurrence of infection in diabetes patients. Another study (de Decker et al., 2017) found that a high burden of comorbidities (stroke, heart failure, myocardial infarction, and functional cognitive impairment) increased the risk of hypoglycemic events in elderly patients (aged over 80 years) with diabetes. Mendez-Bailon et al. (2017) studied 715 patients with diabetes admitted for cardiovascular diseases who underwent invasive cardiac procedures, and found that obesity (18%), arterial hypertension (67.3%), lipid abnormalities (45.8%), renal failure (14.07%), and intermittent claudication (1.65%) were risk factors for adverse events during hospitalization.
Recently, a meta-analysis (Rodríguez-Acelas et al., 2017) revealed that the duration of surgery, duration of central venous catheterization, number of re-operations, type of hospitalization, admission to the intensive care unit, use of immunosuppressants, and use of cephalosporins were risk factors for adverse events during hospitalization in patients with diabetes. Akirov et al. (2017) reported that variability in glycemic levels (glucose ≥180 mg/dl) during hospitalization increases the length of stay, rate of infections and complications, and mortality risk.
This study aimed to investigate the prevalence of UTI events during hospitalization and corresponding risk factors in those with diabetes that were hospitalized.
1.1. Subjects
The study was performed in 32 public hospitals in Portugal. The medical data of 7.347 patients with T2DM between January 1 and December 31, 2015 were reviewed.
Inclusion criteria were hospitalized patients with T2DM as the principal diagnosis, older than 18 years. Exclusion criteria included patients with type 1 of diabetes mellitus and patients with T2DM admitted to hospitals providing oncology services and mental health.
2. Materials and methods
This retrospective study used medical data from the National Hospital Morbidity Database provided by the Central Administration of the Health System. We used (1) the Diagnosis Related Group (DRG) to identify episodes of T2DM (codes DX 250.00) as principal diagnoses during hospitalization and (2) Staging [13] version 5.26 to identify the severity of T2DM, its comorbidities and stages, and UTI events.
Staging uses a specific methodology – MEDSTAT-COC [13] – that identifies potential UTI events (designated as complications-of-care [COC] Urinary) through principal and secondary diagnostic codes, ICD-9-CM, as well as ICD-9-CM procedure codes, age, sex, and the number of days of hospitalization. The parameters for COC Urinary were as follows: code 59010 – acute pyelonephritis not specified; code 5909 – infection of the kidney not specified; code 5950 – acute cystitis; code 5953 – trigonitis; code 5959 – cystitis not specified; and code 5990 – urinary tract infection not specified. UTI events that started from day 1 of admission until discharge from the hospital were investigated in this study.
2.1. Statistical analysis
Bivariate analysis was used to determine the prevalence of UTIs. The distribution and the rate of COC Urinary were calculated using the following formula14:
-
•
COC Urinary infection rate = total number of patients with infection/sum of patients at risk without infection and at risk with infection * 100.
Through the Staging software (Thomson Reuteurs, 2009), COC risk was explicitly assessed according to the relationship between the principal and secondary diagnosis codes, ICD-9-CM, and defined as (i) a patient at risk for a particular infection and (ii) a patient with evidence of potential infection of care during hospitalization.
To determine the risk factors of UTI, we used multivariate logistic regression analysis. The risk adjustment model was used to estimate the correlation between the occurrence of COC Urinary (dependent variable) and the various risk factors (independent variables). The forward conditional method was used for stepwise selection of the variables, whereas the Hosmer and Lemeshow (H-L) test was used to adjust the model with the independent variables. For the validation of the model, its discriminatory capability, sensitivity, and specificity were analyzed using the area under the curve of the receiver operating characteristic curve (AUROC) (c). COC Urinary was set as the adverse outcome and dependent variable that assumed the values: 0 (without infection) and 1 (with infection). Independent variables were selected from variables previously reported to be associated with less favorable health outcomes (Iezzoni and Iezzoni, 2003) as follows:
-
(1)
Length of hospitalization: the length of stay causes adverse outcomes because patients are more exposed to hospital infections. The variable was constructed based on the total number of days in-hospital.
-
(2)
Severity of primary disease: the severity of disease increases the risk of death or organ failure and weakens the immune system. We considered 3 stages (Thomson Reuteurs, 2009): stage 1, initial phase of the disease without evidence of organ injury; and stages 2 and 3, more advanced severity levels of the disease with evidence of organ injury and micro- and macrovascular complications, respectively.
-
(3)
The comorbidities and stage: comorbidities increase the disease burden. We focused on the most relevant comorbidities (defined according to the number of in- and out-of-hospital deaths previously reported to represent a significant burden in those with diabetes. The following comorbidities were included: lipid abnormalities (NUT82); essential hypertension (CVS13); obesity (NUT02); coronary artery disease without prior coronary revascularization (CVS11); cerebrovascular disease (NEU04); congestive heart failure (CVS09); pneumonia: bacterial (RES15); renal failure (GUS08); other disorders of the respiratory system (RES83); rhino, adeno, and corona virus infections (RES24); neoplasm, malignant: colon and rectum (GIS27); neoplasm, malignant: lungs, bronchi, or mediastinum (RES13); and neoplasm, malignant: stomach (GIS30). For all comorbidities, we considered three stages (Thomson Reuteurs, 2009): stage 1, known diagnosis, without local or systemic complications; stage 2, disease limited to an organ or system, increased risk of adverse outcomes; and stage 3, generalized involvement of the system, with systemic complications and with even higher risk of adverse outcomes.
-
(4)
Treatment type: the treatment type may increase the risk of bacterial infections, particularly if preventive procedures are not taken. This was categorized as surgical (surgical treatments) and medical (other invasive diagnostic and therapeutic procedures) and was constructed based on the admission episode and was classified according to the DRG criteria.
-
(5)
Hospitals: some hospitals do not apply infection control programs. This variable was constructed based on the volume of hospitalization (episodes/population) in descending order: hospital (1) represents the highest-volume hospital, and hospital (32) represents the lowest-volume hospital.
Age and sex.
OR values were presented with their respective 95% confidence intervals (CI) or p-values. The p-value set to indicate statistical significance was p < 0.005. All statistical analyses were performed using the IBM SPSS Statistics for Windows, Version 22.0. (Armonk, NY: IBM Corp.).
2.2. Ethical considerations
This study was approved by the Central Administration of the Health System. The need for written informed consent from all participants was waived because this was a retrospective study with anonymized patient data.
3. Results
3.1. Study population
Of the 7.347 patients with T2DM who were admitted, 3.757 (51.1%) patients were men, and 3.590 (48.9%) were women. The majority of patients were elderly, with 71.1% above 65 years. The median duration of hospitalization was 8 days (average 12 ± 18 days).
The total UTI rate was 16.2%. Patient characteristics are shown in Table 1 .
Table 1.
Demographic characteristics.
| Population | Total (%) | Age (years) |
|||||
|---|---|---|---|---|---|---|---|
| 18–64 | 65–74 | 75–84 | >85 | Mean ± SD | |||
| Sex | Men | 3757 (51.1) | 1284 (60.7) | 1053 (57.5) | 1066 (45.8) | 354 (33.0) | 69 ± 13 |
| Women | 3590 (48.9) | 832 (39.3) | 779 (42.5) | 1259 (54.2) | 720 (67.0) | 73 ± 13 | |
| Severity of T2DM | Stage 1 | 428 (5.8) | |||||
| Stage 2 | 2840 (38.7) | ||||||
| Stage 3 | 4079 (55.5) | ||||||
| Treatment type | Surgical | 1813 (24.7) | |||||
| Medical | 5534 (75.3) | ||||||
Abbreviations: T2DM, type 2 of diabetes mellitus; SD, standard deviation.
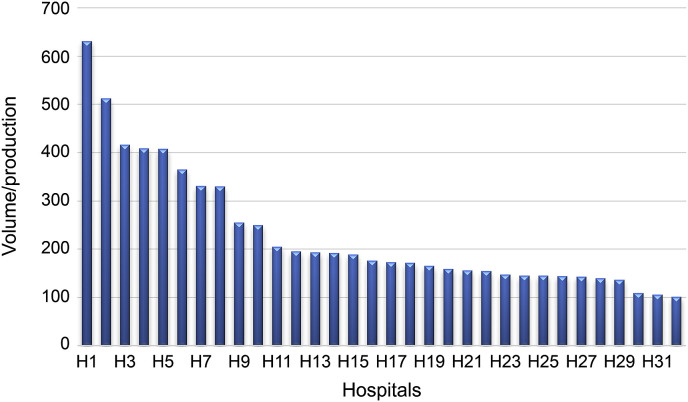
Fig. 1 shows the proportion of T2DM patients by hospital. In hospital (1), 631 patients were admitted, corresponding to 7.9% of the population; meanwhile, in hospital (32), 101 patients where admitted, corresponding to 1.3% of the hospital production.
Fig. 1.
Frequency of admitted T2DM patients by hospital
Abbreviations: T2DM, type 2 of diabetes mellitus.
3.2. Urinary tract infection rate
The UTI infection rate was significantly higher in women than in men (23.6% vs 10.5%; OR = 2.65; p < 0.0001), in those aged above 85 years than in those aged 18–64 years (27.5% vs 9%; OR = 3.84; p < 0.0001), and in those with stage 1 disease than in those with stage 2 (24.4% vs 4.8%; OR = 0.16; p < 0.0001). It was also significantly higher in those who received medical treatment than those who received surgical treatment (24.6% vs 4.8%; OR = 6.52; p < 0.0001). Table 2 shows the values of UTI rate by sex, age (stratified), severity of T2DM, comorbidities, and treatment type.
Table 2.
COC Urinary rate by sex, age, severity of T2DM, comorbidities, and treatment type.
| COC Urinary | ||||
|---|---|---|---|---|
| Variables | Rate (%) | OR | P-value | |
| Sex | Men (ref.) | 10.5 | 2.65 | <0.0001 |
| Age (years) | 18–64 (ref.) | 9.0 | ||
| 65–74 | 12.6 | 1.47 | 0.011 | |
| 75–84 | 22.2 | 2.9 | <0.0001 | |
| Above 85 | 27.5 | 3.84 | <0.0001 | |
| Severity of T2DM | Stage 1 (ref.) | 24.4 | ||
| Stage 2 | 4.8 | 0.16 | <0.0001 | |
| Stage 3 | 24.7 | 1.02 | 0.9445 | |
| Comorbidities | NUT82 (ref.) | 14.6 | ||
| CVS13 | 21.5 | 1.6 | 0.0005 | |
| NUT02 | 16.8 | 1.18 | 0.2614 | |
| CVS11 | 14.8 | 1.02 | 0.9266 | |
| NEU04 | 22.7 | 1.72 | 0.0008 | |
| CVS09 | 20.7 | 1.52 | 0.0423 | |
| RES15 | 24.6 | 1.91 | 0.0006 | |
| GUS08 | 23.1 | 1.76 | 0.0034 | |
| RES83 | 25.5 | 2.01 | 0.0003 | |
| RES24 | 18.5 | 1.32 | 0.4 | |
| GIS27 | 28.2 | 2.29 | 0.0031 | |
| RES13 | 8.3 | 0.53 | 0.5468 | |
| GIS30 | 12.5 | 0.84 | 0.8133 | |
| Treatment type | Surgical (ref.) | 4.8 | 6.52 | <0.0001 |
| Medical | 24.6 | |||
COC Urinary, urinary tract infection after procedure; NUT82, lipid abnormalities; CVS13, essential hypertension; NUT02, obesity; CVS11, coronary artery disease without prior coronary revascularization; NEU04, cerebrovascular disease; CVS09, congestive heart failure; RES15, pneumonia: bacterial; GUS08, renal failure; RES83, other disorders of the respiratory system; RES24, rhino, adeno, and corona virus infections; GIS27, neoplasm, malignant: colon and rectum; RES13, neoplasm, malignant: lungs, bronchi, or mediastinum; GIS30, neoplasm, malignant: stomach. Rate (%) = Total number of patients with infection/sum of patient at risk without infection and at risk with infection *100, Abbreviation: T2DM, type 2 of diabetes mellitus; OR, odds ratio; ref., reference group.
3.3. Risk factors
Multivariate regression analysis with COC Urinary as the dependent variable showed that the following variables were significant predictors of UTIs: age of at least 18 years (OR = 1.031; 95% CI = 1.02–1.041); length of hospitalization (OR = 1.018; 95% IC = 1.013–1.024); sex (woman) (OR = 2.248; 95% IC = 1.778; 2.842); comorbidity of stage 3 cerebrovascular disease (OR = 1.737; 95% IC = 1.111–2.714); and comorbidity of stage 1 colorectal cancer (OR = 2.417; 95% IC = 1.152–5.074). Table 3 shows the result of the logistic regression analysis with COC Urinary as the dependent variable.
Table 3.
Logistic regression analysis for COC Urinary.
| COC Urinary | ||
|---|---|---|
| Independent variables | OR | (95% CI) |
| Age | 1.031 | (1.02–1.041) |
| Duration of hospitalization (days) | 1.018 | (1.013–1.024) |
| Sex | ||
| Men (Ref.) | 1 | |
| Women | 2.248 | (1.778–2.842) |
| Stages NEU04 | ||
| Without (Ref.) | 1 | |
| Stage 1 | 1.12 | (0.68–1.843) |
| Stage 3 | 1.737 | (1.111–2.714) |
| Stages GIS27 | ||
| Without (Ref.) | 1 | |
| Stage 1 | 2.417 | (1.152–5.074) |
| Stage 2 | 2.936 | (0.782–11.024) |
| Stage 3 | 2.024 | (0.171–24.013) |
| Hospital | ||
| 1 (Ref.) | 1 | |
| 5 | 4.024 | (2.319–6.983) |
| 8 | 3.149 | (1.643–6.035) |
| 11 | 2.995 | (1.337–6.712) |
| 12 | 3.339 | (1.589–7.015) |
| 13 | 2.176 | (1.039–4.558) |
| 19 | 2.167 | (1.088–4.315) |
| 20 | 3.06 | (1.23–7.617) |
| 27 | 2.828 | (1.05–7.618) |
| 28 | 4.528 | (1.949–10.518) |
| 32 | 4.741 | (1.988–11.309) |
| Severity of T2DM | ||
| Stage 1 (Ref.) | 1 | |
| Stage 2 | 0.604 | (0.304–1.199) |
| Constant | <0.001 | |
NEU04, cerebrovascular disease; GIS27, neoplasm, malignant: colon and rectum.
Abbreviations: OR, odds ratio; CI, confidence interval; Ref., reference group; T2DM, type 2 of diabetes mellitus.
Among hospitals, hospitals 19 and 32 showed the lowest and higher OR value of 2.167 and 4.741, respectively. Meanwhile, among the three stages of disease severity, stage 2 showed the highest OR of 0.604 (0.304,1.199).
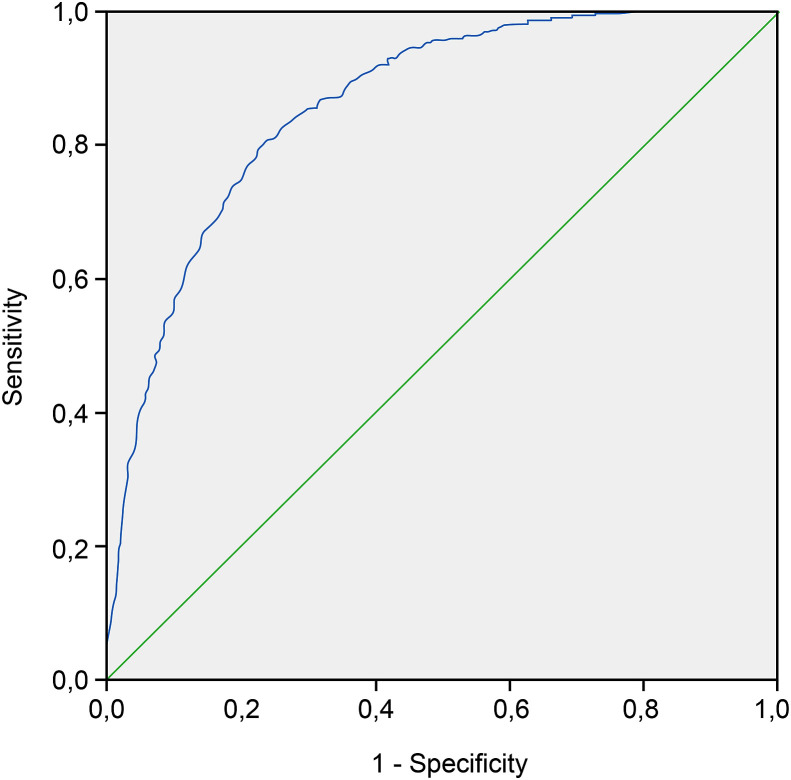
The p-value found for the model was p < 0.0001 in Table 4 . The H-L test revealed a p-value of 0.848 in Table 5 , and the area under curve was 0.862, indicating that the model had a good sensitivity and specificity in Fig. 2 .
Table 4.
Omnibus tests of model coefficients.
| Chi-square | Df | Sig. | ||
|---|---|---|---|---|
| Step 1 | Step | 540.183 | 50 | .000 |
| Block | 540.183 | 50 | .000 | |
| Model | 540.183 | 50 | .000 | |
| Step 2 | Step | 45.478 | 1 | .000 |
| Block | 585.662 | 51 | .000 | |
| Model | 585.662 | 51 | .000 | |
| Step 3 | Step | 54.315 | 1 | .000 |
| Block | 639.976 | 52 | .000 | |
| Model | 639.976 | 52 | .000 | |
| Step 4 | Step | 42.777 | 1 | .000 |
| Block | 682.753 | 53 | .000 | |
| Model | 682.753 | 53 | .000 | |
| Step 5 | Step | 69.087 | 30 | .000 |
| Block | 751.840 | 83 | .000 | |
| Model | 751.840 | 83 | .000 | |
| Step 6 | Step | 15.208 | 2 | .000 |
| Block | 767.048 | 85 | .000 | |
| Model | 767.048 | 85 | .000 | |
| Step 7 | Step | 9.890 | 1 | .002 |
| Block | 776.937 | 86 | .000 | |
| Model | 776.937 | 86 | .000 | |
| Step 8 | Step | 6.763 | 1 | .009 |
| Block | 783.700 | 87 | .000 | |
| Model | 783.700 | 87 | .000 | |
| Step 9 | Step | 11.956 | 3 | .008 |
| Block | 795.656 | 90 | .000 | |
| Model | 795.656 | 90 | .000 | |
| Step 10 | Step | 5.263 | 1 | .022 |
| Block | 800.919 | 91 | .000 | |
| Model | 800.919 | 91 | .000 | |
Abbreviations: df, degrees of freedom; Sig., significance.
Table 5.
Hosmer and Lemeshow test.
| Step | Chi-square | Df | Sig. |
|---|---|---|---|
| 1 | .000 | 7 | 1.000 |
| 2 | 12.967 | 8 | .113 |
| 3 | 7.956 | 8 | .438 |
| 4 | 3.705 | 8 | .883 |
| 5 | 5.943 | 8 | .654 |
| 6 | 7.156 | 8 | .520 |
| 7 | 8.076 | 8 | .426 |
| 8 | 4.112 | 8 | .847 |
| 9 | 5.147 | 8 | .742 |
| 10 | 4.095 | 8 | .848 |
Abbreviations: df, degrees of freedom; Sig., significance.
Fig. 2.
Receiver operating characteristics curve.
Collectively, the results of this model show that (1) the risk of UTIs was higher in hospitals 5, 8, 11, 12, 13, 19, 20, 27, 28, and 32; (2) the comorbidity of stage 3 cerebrovascular disease and comorbidity of stage 1 colorectal cancer are risk factors for UTIs; and (3) the severity of T2DM is not an explanatory factor of UTIs. Our study showed that UTI rate is higher in women without evidence of organ injury and in those receiving medical treatment.
4. Discussion
A recent epidemiological study (Tandogdu and Wagenlehner, 2016) showed significant geographic variations in the frequency of UTIs in those with diabetes, with the rate being the highest in developing countries (24%) compared with that in the US (12.9%) and Europe (19.6%) (Tandogdu and Wagenlehner, 2016). In our study, the rate of UTIs was 16.2%, indicating differences in healthcare delivery and systems between developing and developed countries.
We found a higher rate of UTIs in women than in men (UTI rate, 23.6% vs 10.5%). Further, it was also higher in those aged at least 85 years (27.5%). Popejoy et al. (2017) found that 48.5% of women with diabetes and aged above 65 years had UTIs. The discrepancy in the UTI rates may imply better healthcare in Portugal.
UTIs were also more common after medical treatment than after surgical treatment (24.6% vs 4.8%), consistent with the findings reported in a previous study (Shah et al., 2018). This was attributed to (i) the excessive use of certain medical devices involving urologic manipulation (e.g. catheters); (ii) failure to follow aseptic techniques; and (iii) procedures in patients with diabetes with poor glycemic control and/or with asymptomatic bacteriuria. The difference in the frequency of UTIs between the type of treatment may be due to the different therapeutic strategies employed; for example, antibiotic prophylaxis was administered in patients undergoing surgery.
With respect to the severity of diabetes, we found a higher UTI rate in patients with stage 2 diabetes than that of those with stage 1 diabetes (4.8% vs 24.4% respectively; OR = 0.16), contradicting previous studies (isk Factor Collabora, 2016; Ljungqvist et al., 2005). Increased hyperglycemia may occur after surgical stress (Ljungqvist et al., 2005), and, thus, our results may indicate a non-effective glycemic control in those with stage 1 severity of diabetes. Typically, patients with diabetes are only diagnosed with UTI during their first hospital visit in the health services of the Portuguese National Health System because of the gap in follow-up of these patients in primary care (Sociedade Portuguesa de Diabetologia, 2015). This may have caused a higher risk of UTIs in those with stage 1 severity T2DM than those with stage 2, because patients with stage 2 T2DM have previous glycemic control.
Logistic regression analysis showed the influence of hospitalization on the risk of UTI. This is consistent with the findings of some studies (https://apurologia.pt/. A, 2008; Wilke et al., 2015; Hirji et al., 2012; Mazzi et al., 2017; de Decker et al., 2017; Mendez-Bailon et al., 2017; Rodríguez-Acelas et al., 2017; Shwartz et al., 1994) that have reported a high number of unnecessary hospitalizations. One study (Rosenthal et al., 2014) showed that marked differences exist in the use of medical resources, hospital beds, diagnostic exams, and medical treatments putting the patient at risk of infection. The findings in the current study may be due to (1) discrepancies in the use of hospital resources; (2) concentration of specific procedures to certain patient groups; and (3) differences in the clinical management of patients. Further, some studies (Galiczewski and Shurpin, 2017; Kummerow Broman et al., 2018; Fairfield et al., 2018) have reported unsafe treatments, inadequate human and technical resources, and, consequently, poor healthcare practices, leading to variations in the frequency of UTIs among geographic areas. Some hospitals do not observe safety protocols in the insertion, maintenance, and aseptic removal of devices and do not comply with guidelines for the prevention of UTIs, such as the assessment of the need for catheterization (based on individual risk); do not select the type of catheter according to the predicted duration of catheterization; do not implement prevention and control policies for asymptomatic bacteriuria; and do not follow clinical practice guidelines for managing hospitalized patients with diabetes who have UTIs.
With respect to sex, women were at a higher risk of UTIs than men (OR = 2.248), consistent with other studies (Popejoy et al., 2017; Costantini et al., 2017). This may be due to (i) hypotonic neurogenic bladder and (2) physiological changes in bladder associated with aging. This raises a question about the efficacy of treatment of urinary tract alterations.
With respect to diabetes severity, no difference was observed in the risk of UTIs between those with stage 2 and stage 1 T2DM, in contrast to other studies that reported that the severity of diabetes is a predictor of COC Urinary (isk Factor Collabora, 2016; Popejoy et al., 2017). These findings may be due to the differences in clinical coding between health institutions and the definition of the severity of diabetes. This result raises a question about the use of the guidelines for T2DM diagnosis.
With respect to the comorbidities, we found that cerebrovascular disease and malignant neoplasm of the colon and rectum are predictors of UTIs, consistent with previous studies (Kwaan et al., 2017; Mi et al., 2018; Vermeij et al., 2018). Particularly, the association between stage 3 cerebrovascular disease and UTI may be due to the use of ineffective prophylactic procedures and differences in clinical coding. These findings indicate the need for more effective clinical safety protocols for nursing staff. Meanwhile, the comorbidity of malignant neoplasm of the colon and rectum may be due to (1) ineffective prophylactic measures, (2) differences in clinical practice, (3) differences in clinical coding, and (4) differences in the use of hospital resources, as previously reported (Kwaan et al., 2017; D'Hondt et al., 2017; Eveno et al., 2010). Some hospitals do not offer specialized care, and thus, we could assess what treatments are available in these hospitals.
Our study has several limitations. First, biochemical data, particularly, the glycemic values in pre- and post-treatment that may result in less favorable outcomes, were lacking. Second, inconsistences may have been present in the clinical coding of medical data (codes ICD-9-CM) among hospitals. Therefore, random audits of medical processes have caused better handling of medical data, in Portugal. Third, we do not know if the cause of UTIs was preventable or not. Lastly, this study has all the limitations and risks of bias inherent to cross-sectional study design, thus limiting the generalizability of the conclusions to other populations. Given the increasing incidence of T2DM worldwide, particularly in Portugal, diabetes-specific healthcare guidelines during hospitalization are needed, and the number of physicians and nurses in primary care should be increased to meet the increased need for proper management of comorbidities, in order to avoid more hospitalizations.
In this study, we identified individuals with diabetes, who were at a higher risk of UTI, which is the most common adverse event during hospitalization in patients with diabetes. Our findings suggest that women in stage 1 of T2DM have the highest prevalence of UTIs and those who have received medical treatment with fewer comorbidities. More effective health policies should be implemented to manage T2DM, particularly in stage 1 disease. Home visits by primary care physicians and nurses are crucial for those with limited healthcare access for better diabetes management and for reducing admission rates. In hospitals, health professionals should control hyperglycemia before and after medical treatments and should adopt safe protocols to reduce UTIs. Further, health education on diet and nutrition should be provided to reduce comorbidities due to increased risk for UTIs. This study can be beneficial for risk stratification of these patients and for the early identification of those at higher risk of infection, and the findings can be used to implement optimal healthcare measures to prevent UTIs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
None of the authors has any declarations of interest.
Acknowledgments
None.
References
- Akirov A., Grossman A., Shochat T. Hyperglycemia on admission and hospitalization outcomes in patients with atrial fibrillation. Clin. Cardiol. 2017;40:1123–1128. doi: 10.1002/clc.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini E., Illiano E., Giannitsas K. Urological dysfunction in young women: an inheritance of childhood? BJU Int. 2017;121:453–457. doi: 10.1111/bju.14081. [DOI] [PubMed] [Google Scholar]
- D Hondt M., Yoshihara E., Dedrye L. Transanal endoscopic operation for benign rectal lesions and T1 carcinoma. J. Soc. Laparoendosc. Surg. 2017;21 doi: 10.4293/JSLS.2016.00093. pii: e2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Decker L., Hanon O., Boureau A.S. Association between hypoglycemia and the burden of comorbidities in hospitalized vulnerable older diabetic patients: a cross-sectional, population-based study. Diabetes Ther. 2017;8:1405–1413. doi: 10.1007/s13300-017-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveno C., Lamblin A., Mariette C. Sexual and urinary dysfunction after proctectomy for rectal cancer. J. Vis. Surg. 2010;147:e21–e30. doi: 10.1016/j.jviscsurg.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Fairfield K.M., Black A.W., Lucas F.L. Behavioral risk factors and regional variation in cardiovascular health care and death. Am. J. Prev. Med. 2018;54:376–384. doi: 10.1016/j.amepre.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Galiczewski J.M., Shurpin K.M. An intervention to improve the catheter associated urinary tract infection rate in a medical intensive care unit: direct observation of catheter insertion procedure. Intensive Crit. Care Nurs. 2017;40:26–34. doi: 10.1016/j.iccn.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Hirji I., Guo Z., Andersson S.W. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD) J. Diabet. Complicat. 2012;26:513–516. doi: 10.1016/j.jdiacomp.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Associação Portuguesa de Urologia Guia de prática clínica: cistite não complicada na mulher. 2008. https://apurologia.pt/wp-content/uploads/2018/10/Guia-cistite.pdf [Cited 2019 August 6] Available from:
- Iezzoni L.I. Reasons for risk adjustment. In: Iezzoni L.I., editor. Lit. – Risk Adjustment for Measuring Health Care Outcomes. third ed. Health Administration Press. American College of Healthcare Executives; Chicago: 2003. pp. 1–17. [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530s. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummerow Broman K., Ward M.J., Poulose B.K. Surgical transfer decision making: how regional resources are allocated in a regional transfer network. Joint Comm. J. Qual. Patient Saf. 2018;44:33–42. doi: 10.1016/j.jcjq.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaan M.R., Fan Y., Jarosek S. Long-term risk of urinary adverse events in curatively treated patients with rectal cancer: a population-based analysis. Dis. Colon Rectum. 2017;60:682–690. doi: 10.1097/DCR.0000000000000788. [DOI] [PubMed] [Google Scholar]
- Lara-Rojas C.M., Pérez-Belmonte L.M., López-Carmona M.D. National trends in diabetes mellitus hospitalization in Spain 1997-2010: analysis of over 5.4 millions of admissions. Eur. J. Intern. Med. 2019;60:83–89. doi: 10.1016/j.ejim.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Ljungqvist O., Nygren J., Soop M. Metabolic perioperative management: novel concepts. Curr. Opin. Crit. Care. 2005;11:295–299. doi: 10.1097/01.ccx.0000166395.65764.71. [DOI] [PubMed] [Google Scholar]
- Mazzi S., Ravasio R., Forlani G. Estimating the risk of severe hypoglycemic event related to glucose-lowering treatment among Italian patients with diabetes: the HYPOTHESIS database. Clinicoecon. Outcomes Res. 2017;9:711–720. doi: 10.2147/CEOR.S148368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Bailon M., Lorenzo-Villalba N., Muñoz-Rivas N. Transcatheter aortic valve implantation and surgical aortic valve replacement among hospitalized patients with and without type 2 diabetes mellitus in Spain (2014-2015) Cardiovasc. Diabetol. 2017;16:144. doi: 10.1186/s12933-017-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Li S., Li H. The effects of infection on severe stroke patients in the neurological intensive care unit in China. Int. J. Neurosci. 2018;128:715–720. doi: 10.1080/00207454.2017.1412966. [DOI] [PubMed] [Google Scholar]
- Popejoy M.W., Long J., Huntington J.A. Analysis of patients with diabetes and complicated intra-abdominal infection or complicated urinary tract infection in phase 3 trials of ceftolozane/tazobactam. BMC Infect. Dis. 2017;17:316. doi: 10.1186/s12879-017-2414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Acelas A.L., de Abreu Almeida M., Engelman B. Risk factors for health care-associated infection in hospitalized adults: systematic review and meta-analysis. Am. J. Infect. Contr. 2017;45:e149–e156. doi: 10.1016/j.ajic.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Rosenthal V.D., Maki D.G., Mehta Y. International Nosocomial Infection Control Consortium (INICC) report, data summary of 43 countries for 2007-2012. Device-associated module. Am. J. Infect. Contr. 2014;42:942–956. doi: 10.1016/j.ajic.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Shah K.J., Cherabuddi K., Shultz J. Ampicillin for the treatment of complicated urinary tract infections caused by vancomycin resistant enterococcus spp (VRE): a single-center university hospital experience. Int. J. Antimicrob. Agents. 2018;51:57–61. doi: 10.1016/j.ijantimicag.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Shwartz M., Ash A.S., Anderson J. Small area variations in hospitalization rates: how much you see depends on how you look. Med. Care. 1994;32:189–201. doi: 10.1097/00005650-199403000-00001. [DOI] [PubMed] [Google Scholar]
- Sociedade Portuguesa de Diabetologia Relatório Anual do Observatório da Diabetes: “Diabetes Factos e Números – o ano 2015. http://spd.pt/images/OND/DFN2015.pdf [Cited 2019 August 3] Available from:
- Tandogdu Z., Wagenlehner F.M. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 2016;29:73–79. doi: 10.1097/QCO.0000000000000228. [DOI] [PubMed] [Google Scholar]
- Thomson Reuteurs . The MEDSTAT Group, Inc.; Ann Arbor, Michigan: 2009. Disease Staging: Clinical and Coded Criteria.www.hcup-us.ahrg.gov/db/nation/nis/DiseaseStagingV5.27ClinicalandCodedCriteria.pdf [Cited 2019 May 6] Available from: [Google Scholar]
- Vermeij J.D., Westendorp W.F., Dippel D.W. Antibiotic therapy for preventing infections in people with acute stroke. Cochrane Database Syst. Rev. 2018;1:CD008530. doi: 10.1002/14651858.CD008530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke T., Boettger B., Berg B. Epidemiology of urinary tract infections in type 2 diabetes mellitus patients: an analysis based on a large sample of 456,586 German T2DM patients. J. Diabet. Complicat. 2015;29:1015–1023. doi: 10.1016/j.jdiacomp.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]