Abstract
Background
Faecal incontinence is a common and potentially distressing disorder of childhood.
Objectives
To assess the effects of behavioural and/or cognitive interventions for the management of faecal incontinence in children.
Search methods
We searched the Cochrane Incontinence Group Specialised Trials Register (searched 28 October 2011), which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and CINAHL, and handsearching of journals and conference proceedings, and the reference lists of relevant articles. We contacted authors in the field to identify any additional or unpublished studies.
Selection criteria
Randomised and quasi‐randomised trials of behavioural and/or cognitive interventions with or without other treatments for the management of faecal incontinence in children.
Data collection and analysis
Reviewers selected studies from the literature, assessed study quality, and extracted data. Data were combined in a meta‐analysis when appropriate.
Main results
Twenty one randomised trials with a total of 1371 children met the inclusion criteria. Sample sizes were generally small. All studies but one investigated children with functional faecal incontinence. Interventions varied amongst trials and few outcomes were shared by trials addressing the same comparisons.
Combined results of nine trials showed higher rather than lower rates of persisting symptoms of faecal incontinence up to 12 months when biofeedback was added to conventional treatment (OR 1.11 CI 95% 0.78 to 1.58). This result was consistent with that of two trials with longer follow‐up (OR 1.31 CI 95% 0.80 to 2.15). In one trial the adjunct of anorectal manometry to conventional treatment did not result in higher success rates in chronically constipated children (OR 1.40 95% CI 0.72 to 2.73 at 24 months).
In one small trial the adjunct of behaviour modification to laxative therapy was associated with a significant reduction in children's soiling episodes at both the three month (OR 0.14 CI 95% 0.04 to 0.51) and the 12 month assessment (OR 0.20 CI 95% 0.06 to 0.65).
Authors' conclusions
There is no evidence that biofeedback training adds any benefit to conventional treatment in the management of functional faecal incontinence in children. There was not enough evidence on which to assess the effects of biofeedback for the management of organic faecal incontinence. There is some evidence that behavioural interventions plus laxative therapy, rather than laxative therapy alone, improves continence in children with functional faecal incontinence associated with constipation.
Plain language summary
Behavioural interventions for the treatment of faecal incontinence in children
Children with "faecal incontinence" cannot control their bowel movements and so they soil their underwear. Sometimes people use the word "soiling" or "encopresis" to mean the same thing. Faecal incontinence can be caused by either physical or psychological problems. The term "organic faecal incontinence" is used when faecal incontinence is due to a physical damage or abnormality whilst "functional faecal incontinence" is used when faecal incontinence is caused by non‐organic/psychological factors. Behavioural interventions (toilet training, rewards) are used to reduce children's anxiety and to restore normal bowel habits. Biofeedback is a technique that can be used to teach children how to control the muscles around their back passage.
This review identified 21 studies with a total of 1371 children. Behavioural interventions when used together with laxative therapy may improve continence in children with non‐organic faecal incontinence and constipation whilst biofeedback does not add any long‐term benefit. Children who received biofeedback treatment had not always been evaluated beforehand for the suitability of the treatment.
There was not enough evidence to assess the effects of biofeedback in children with organic faecal incontinence.
Background
Faecal incontinence in children is defined as loss of stool, solid or liquid, from the bowel in inappropriate places at an undesirable time. It can be the consequence of physical abnormalities (congenital malformations, acquired neurological deficits, post‐surgery defects) or emotional and psychological disturbances. The term "encopresis" (from "kopros", Greek for stool) was initially coined by Weissenberg (Weissenberg 1926) to describe functional (psychological) soiling while the term faecal incontinence was reserved for organic (physiological) conditions. Latterly however it has been used to indicate any form of soiling (Levine 1982). Although a universally accepted definition has still to be agreed upon, encopresis is generally described as the repeated expulsion of faeces, whether involuntary or intentional, in inappropriate places (e.g. clothing, floor) in a child at least four years of age (or equivalent developmental level) (DSM‐IV 1994). The Paris Consensus on Childhood Constipation Terminology (PACCT) Group has recently suggested that the term "faecal incontinence" should be adopted in place of the terms "encopresis" and "soiling" (PACCT Group 2005). Therefore the terms "faecal incontinence", "encopresis", and "soiling" will be used synonymously to imply the undesired passing of faecal material regardless of the subtle differences in their meaning proposed by some trials' authors. Faecal incontinence can be sub‐divided in: i) organic faecal incontinence, which results from neurological damage, sphincter abnormalities, and other organic causes; and ii) functional faecal incontinence, which includes constipation‐associated faecal incontinence and non‐retentive faecal incontinence.
In most cases faecal incontinence develops as a result of faecal constipation or faecal retention, often potentiated by phobic conditioning, and is manifested as overflow soiling or staining. Faecal constipation can arise from organic causes such as Hirschsprung's disease, malabsorption syndromes, hypothyroidism, hypercalcaemia, diabetes insipidus, or neurological conditions. However, most retentive disorder is functional with no obvious cause. According to the PACCT Group criteria, chronic constipation can be defined as the occurrence of two or more of the following events during the last eight weeks: less than three bowel movements per week; more than one episode of faecal incontinence per week; large stools in the rectum or palpable in abdomen; passing of large or jagged stool (sometimes exacerbated by an anal fissure: a small tear or rip in the anorectal margin); retentive posture and withholding behaviour; and painful defaecation (PACCT Group 2005). This comprehensive definition replaces the distinction between functional constipation and functional faecal retention that was still present in the Pediatric Rome II diagnostic criteria (Rasquin‐Weber 1999).
Children who have developed chronic constipation often do not feel the urge to go to the toilet. By withholding stool for long periods they cause the rectum to accommodate to distension and the nerves, which send signals to the brain, become insensitive and habituated. When this happens the children may "leak" soft or liquid faeces into underclothing and may feel the urge to go to the toilet only when their large intestine gets critically stretched to the point where they feel pain and cramps. In some children with chronic constipation, abnormally low frequency of evacuation may lead to impaction and megacolon (the large intestine enlarges because it is continuously stretched). Eventually these children will pass a very large and very hard bowel movement and subsequently experience pain, which in turn creates a vicious circle of pain, leading to withholding, constipation and further pain, irrespective of whether eventual evacuation takes place naturally or with the aid of laxatives. In certain unusual cases, constipation results from atypical contraction of the external anal sphincter while straining on the toilet. This condition is known as pelvic floor dyssynergia, anismus, or paradoxical sphincter contraction. The PACCT Group recommends that the term "pelvic floor dyssynergia" be used instead of anismus. Non‐retentive faecal incontinence is often observed as a manifestation of emotional disturbance in school‐age children. Children with non‐retentive disorders may have either never succeeded in establishing control of bowel function, typically because of lack of adequate bowel training and support from their family, or have attained a period of at least six months of independent bowel control that is subsequently lost. Loss of control is often the child's reaction to stressful events (e.g. coercive measures toward toilet training, family disharmony, parental separation, the birth of a sibling or sibling antagonism) or "toilet phobia" (e.g. excessive fear of passing stool or using the toilet). Both the PACCT Group and the Pediatric Rome II diagnostic criteria agree in defining functional non‐retentive faecal incontinence as the passage of stools in places and at times inappropriate to the age of the child and in the absence of inflammatory disease and evidence of constipation (PACCT Group 2005; Rasquin‐Weber 1999).
Faecal incontinence in its various forms affects about 1.5% of children in western countries and is a clinical problem that makes considerable demands on primary care and paediatric services (Bellman 1966, Levine 1983, Kelly 1996). Soiling incidents occur most often during the day, often after school in the afternoon (Fielding 1988). Unlike enuresis, nocturnal episodes of faecal incontinence are rare but can occur reflexively in cases characterised by extreme retention during the day, long‐standing constipation or a large rectal faecaloma (Benninga 1996). Boys are affected four/five times more than girls (Fritz 1982).
Childhood functional faecal incontinence with or without constipation is a condition difficult to treat due to the complex nature of the problem and the potentially high level of psychological distress affecting not only the children but also their families.
Dietary modifications and medical treatments (principally enemas and laxatives, though occasionally manual evacuation under anaesthetic) aim at softening the stool and emptying the intestine so that constipation does not perpetuate 'retentive' behaviour. Behavioural (toilet training, incentive and reward schemes, desensitisation of toilet phobia, and environmental management), cognitive (psychotherapy, cognitive and family therapy), and educational interventions aim at lowering the level of distress and developing or restoring normal bowel habits through training and education. The management of children with functional faecal incontinence associated with constipation might comprise a combination of medical treatments, dietary modifications and behavioural interventions (e.g. use of laxatives together with toilet training and dietary advice). Biofeedback training (a procedure that allows the muscle tone of the external anal sphincter to be displayed on a screen or presented as sound modulations) can be used either with children with functional non‐retentive faecal incontinence or children with constipation‐associated faecal incontinence to teach them how to tighten and relax their perianal muscles in order to pass bowel movements more efficiently (Kohlenberg 1973; Olness 1980a). Although behavioural and cognitive interventions are commonly used to treat children with functional faecal incontinence, the relative effectiveness of different treatment options has yet to be established.
Functional faecal incontinence tends to resolve spontaneously by adolescence. However, treatments aimed at accelerating voluntary bowel control are justified insofar as they give the growing child more adaptive and socially acceptable patterns of personal hygiene and they help avoid the possible adverse effects of soiling on mobility and personality development. The proportion of children whose problems persist into their adult life is still unknown but it is noteworthy that adults suffering from megacolon often present with a history of constipation and bowel problems from childhood.
Children whose faecal incontinence is due to physical abnormalities (e.g. Hirschsprung's disease, myelomeningocele, spina bifida, spinal cord trauma and imperforate anus) may require surgery, but as they may still be incontinent after surgery they may benefit from additional medical and behavioural interventions.
The aim of this review is to summarise systematically evidence from all relevant randomised controlled trials on the effects of behavioural (including biofeedback training) and cognitive therapies with or without other treatments for the management of children defaecation disorders in order to provide the best evidence currently available on which to base recommendations for clinical practice.
Objectives
To determine the effects of behaviour and/or cognitive interventions for the treatment of defaecation disorders in children. The following comparisons have been considered:
whether a particular behavioural or cognitive intervention is more effective than no treatment or a sham procedure for treating defaecation disorders in children;
whether a particular combination of behavioural and/or cognitive interventions is more effective than any other combination of interventions for treating defaecation disorders in children;
whether a combination of behavioural and/or cognitive interventions is more effective than each intervention alone for treating defaecation disorders in children and when this is the case;
whether a particular behavioural or cognitive intervention is more effective than another intervention for treating defaecation disorders in children.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials comparing:
a single behavioural or cognitive intervention with another behavioural or cognitive intervention;
a combination of behavioural and/or cognitive interventions with another combination of behavioural and/or cognitive interventions;
behavioural and/or cognitive interventions with conventional treatment (e.g. use of laxatives together with toilet training and dietary advice);
behavioural and/or cognitive interventions with other interventions or no‐intervention for the management of defaecation disorders in children.
Trials that used a quasi‐randomised approach (e.g. allocation by date of birth) were also included. Controlled clinical trials without randomisation were excluded.
Trials assessing the effects of dietary fibres and/or fluid intake on colonic transit time were not deemed suitable for inclusion.
Types of participants
Children (as defined by trials' authors) with a history of faecal incontinence with and without constipation. Faecal incontinence is defined here as imperfect control of defaecation to the point where the disorder is troublesome to child and carers. The terms "encopresis" and "soiling" are used synonymously for faecal incontinence irrespective of the distinction that some authors make between functional and organic causes of incontinence, complete or incomplete rectal emptying, or formed or unformed stool.
The term "constipation" is used to indicate difficulty or delay in the passage of stools (not a description of the consistency of stool). Infants were not regarded as suitable participants.
Types of interventions
All interventions described by the authors of individual trials as behavioural or cognitive were included. Among behavioural interventions we expected to include: contingency management programmes (reinforcement and incentive schemes), desensitisation methods for toilet phobia, imposition of toileting routines, biofeedback, and environmental management. Among non‐behavioural (i.e. educational and cognitive) interventions we expected to include: parental instructions on bowel function and continence, counselling, psychotherapy, cognitive and family therapies.
Types of outcome measures
1. Children's symptoms:
number of children cured or improved
number of voluntary bowel movements per week
number of soiling episodes per week
number of self‐initiated trips to the toilet
number of parent‐initiated trips to the toilet
number of self‐toileting episodes per week
rate of improvement in incontinence status (by means of continence scales)
self‐reported defaecation pain
parent‐rated defaecation pain
adjunctive use of medication such as laxatives, suppositories, and enemas
2. Anorectal physiology:
resting anal pressure
maximum squeeze pressure
sensory threshold
rectal inhibitory reflex
defaecation dynamics
saline retention test
3. Health status measures:
psychological measures (e.g. Child Behavior Checklist, Achenbach 1987)
4. Health economics
resource implications
resources required to provide the intervention resource consequences of long‐term care
costs of intervention
costs of resources cost falling on health services cost falling on patients, families or carers
cost‐effectiveness of interventions
cost per episode of soiling avoided cost per unit of health gain/preference (e.g. cost per QALY, Weinstein 1977)
5. Other outcomes:
outcome measures quoted in individual trials and judged to be important by the reviewers
Search methods for identification of studies
We did not impose any language restriction or other limits on the searches.
Electronic searches
This review has drawn on the search strategy developed for the Incontinence Group as a whole. Relevant trials have been primarily identified from the Cochrane Incontinence Group Specialised Trials Register. The methods used to derive this, including the search strategy, are described under the Group's module in The Cochrane Library. The register contains trials identified from MEDLINE, CINAHL and the Cochrane Central Register of Controlled Trials (CENTRAL) and handsearching of journals and conference proceedings.
The Incontinence Group's Trials Register was searched using the Group's own keyword system. The search terms used are given in Appendix 1. Date of the most recent search of the register for this review: 28 October 2011. The trials in the Incontinence Group's Specialised Register are also contained in CENTRAL.
Searching other resources
We perused all reference lists of identified trials. We contacted authors and well‐known experts in the field to identify any additional or unpublished studies.
Data collection and analysis
Selection of studies
The review authors independently examined all the citations derived from the original search strategy performed in July 2000. One reviewer (MB) screened the results of the updated search strategy performed in January 2006. Two reviewers screened the results of the updated search strategy performed in October 2011. Reports of potential, eligible trials were discarded if it emerged from the title and/or the abstract that they did not draw on a randomised or quasi‐randomised design or if they included participants other than children, or did not include either behavioural or cognitive therapies. When the title/abstract could not be rejected with certainty the report of the trial was retrieved in full. Review authors were not blind to the names of the trial's investigators, institutions or journals. Any disagreement about trial selection and inclusion was resolved by discussion. A third independent opinion was sought if disagreement persisted. Where possible, studies in foreign languages were translated prior to the methodological assessment.
Data extraction and management
Information on the characteristics of participants and interventions as well as on the pre‐specified outcome measures was extracted for each trial.
Assessment of risk of bias in included studies
Information on concealment of allocation, blind assessment of outcomes, compliance with treatment, number of withdrawals and dropouts, adequacy of controls, comparability of groups and proportion of people analysed according to randomisation at the end of the treatment period and at follow‐up (intention‐to‐treat analysis) was extracted for each trial. Since inadequate concealment can lead to the introduction of bias in the results of studies (Schulz 1995), the quality of allocation was considered using the following criteria: adequately concealed trials (central randomisation; serially numbered opaque, sealed envelopes; numbered or coded bottles or containers; other examples of adequate concealment); unclearly concealed trials (authors did not report an allocation concealment approach or reported an approach which did not fall into one of the other categories); inadequately concealed trials (alternation or reference to case record numbers or dates of birth). trials with no attempt at allocation concealment Studies were excluded if they were not randomised or quasi‐randomised or if they made comparisons other than those pre‐specified. These studies are listed in the Table of Excluded Studies.
Measures of treatment effect
Data were analysed using the MetaView statistical package in Review Manager. For dichotomous variables, odds ratios (OR) and 95% confidence intervals (CI) were derived for each study. For continuous variables mean differences were calculated for each study. Continuous data from rating scales were analysed only if the validity and reliability of such scales had been well demonstrated in the available literature.
If appropriate, the results of included studies were combined for each outcome in a formal meta‐analysis to produce an overall estimate of treatment effect. For dichotomous data "typical" odds ratios and 95% confidence intervals were derived using a fixed effects model and for continuous data weighted mean differences (WMD ‐ weighted by the inverse of the variance) or standardised mean differences (SMD) were calculated. Both the weighted mean difference and standardised mean difference methods assume that the outcome measurements within each trial have a normal distribution. When these distributions are skewed the results of these methods may be misleading. Continuous data were analysed only if there was no clear evidence of skew in the distribution. One reviewer (MB) assessed the skewness of continuous data in the original studies (in particular if parametric tests were used for non‐parametric data).
Data from studies showing high drop‐out rates in experimental and/or control groups (50% or more) were not included in the meta‐analysis.
Heterogeneity amongst studies was explored by means of a visual inspection of the graphical plot of the results and formally by the Chi‐squared test and I square test. In case of considerable statistical heterogeneity (e.g. significance level less than 0.10) with no clear explanation, the reviewers adopted the following options:
to exclude the results of studies that contributed most variation and repeat the analysis (recalculating the summary measure of effect and the heterogeneity statistics for the remaining studies) until no heterogeneity is present;
to use both a fixed and a random effects model to see if they give substantially different results.
All outcomes were reported in terms of unfavourable events (e.g. children not cured or improved, number of soiling episodes, etc.). This implies that beneficial results for the 'experimental' intervention (i.e. an odd ratio less than one or a WMD less than 0) are displayed to the left of the line of no effect.
Results
Description of studies
The search strategy identified 59 reports of potentially eligible trials. When full‐text papers were retrieved, 34 reports were found not to meet the inclusion criteria and were excluded. Reasons for exclusion can be found in the Characteristics of excluded studies table. The remaining 24 reports of 21 papers met our inclusion criteria. The total number of participants across trials was 1371. The flow of the literature through the assessment process is shown in Figure 1. For a detailed description of individual studies please refer to the Characteristics of included studies table.
1.
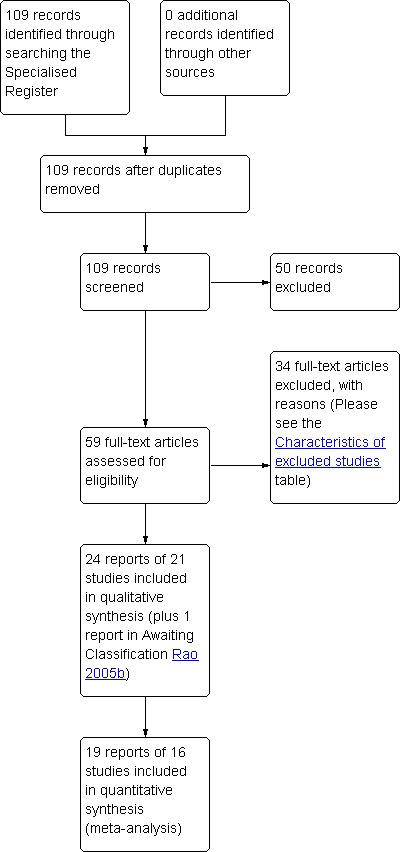
PRISMA study flow diagram.
Design
Nineteen were randomised controlled trials (Berg 1983, Borowitz 2002, Davila 1992, Demirogullari 2007Loening‐Baucke 1988, Loening‐Baucke 1990, Mellon 1996, Nolan 1991, Nolan 1998, Pieczarkowski 2005, van Dijk 2008, van der Plas 1996, van der Plas 1996a, van Ginkel 2000, Wald 1987; Ritterband 2003; van Ginkel 2001; Sunic‐Omejc 2002; Croffie 2005) one was a quasi‐randomised controlled trial (Taitz 1986), and one reported the findings of a series of single case experiments (Latimer 1984) in which participants were randomly allocated to treatment A, B, C, or D (ABACADA or ACABADA design).
Location/setting
All trials but one (Ritterband 2003) were conducted in single centres. Seven trials were carried out in the United States of America, five in the Netherlands, two in Australia, two in the United Kingdom, one in Croatia, one in Canada, one in South America, one in Poland and one in Turkey.
Sample size
In most trials the number of participants was less than 50. The largest trial included 212 children and the smallest eight. Only four trials reported a sample size calculation for statistical power.
Diagnosis
Nineteen trials enrolled children with functional faecal incontinence and two trials children with faecal incontinence due to congenital abnormalities (imperforate anus and myelomeningocele). Amongst the 19 trials assessing children with functional faecal incontinence, fourteen trials studied children with a history of constipation and/or faecal impaction, four trials children with "primary and secondary encopresis" and one trial children with "faecal soiling with or without constipation".
Intervention
The 21 identified trials entailed the following comparisons: 1) conventional treatment (use of laxatives, toilet training, and dietary advice) versus conventional treatment plus biofeedback (nine trials) 2) conventional treatment (use of laxatives, toilet training, and dietary advice) versus conventional treatment plus two sessions of anorectal manometry (one trial) 3) biofeedback plus oral laxatives versus biofeedback (one trial) 4) behaviour modifications plus laxative therapy versus behaviour modifications alone (two trials) 5) web‐based behaviour modifications versus no web‐based behaviour modifications (one trial) 6) behaviour modifications versus mineral oil plus rewards (one trial) 7) behaviour modifications versus behaviour modifications plus psychotherapy (one trial) 8) biofeedback at home compared with biofeedback in the laboratory (one trial) 9) exercise training versus sensory discrimination training versus biofeedback (one trial) 10) laxative therapy plus behaviour modifications versus laxative therapy alone (one arm of an already included trial)
11) conventional treatment versus bevioural modifications plus laxative
All trials but two (Loening‐Baucke 1988; Latimer 1984) investigated children with functional faecal incontinence.
Length of treatment
Eight trials lasted between two to six weeks, nine between seven to 24 weeks and two trial lasted up to 12 months. In one trial duration of the intervention was not clearly reported.
Follow‐up
One trial had a follow‐up assessment at four months, five trials at six months, seven trials at 12 months, three trials at 18 months, one trial 22 weeks after 6 month treatment and one trial at 24 months. In two trials no further follow‐up was reported after the post‐treatment assessment at 3 and 4 months.
Outcomes
Common reported outcomes were: number of children cured or improved, number of bowel movements in toilet, soiling episodes, use of laxatives, and anorectal manometric measurements.
Only a supplementary report of the Borowitz trial (Cox 1998) reported data relating to costs (average cost of the therapy) .
Risk of bias in included studies
Allocation
Randomisation concealment was judged to be adequate in four trials (low risk of bias), unclear in 16 trials (unclear risk) and inadequate in one trial (high risk of bias). In this last trial allocation of participants to intervention groups was performed on 'alternate basis'. In one trial investigators stated that the participants were assigned to intervention groups in a 'non‐blinded fashion'.
Blinding
The nature of the interventions (behaviour modifications such as toilet training, reward systems) and the study population (children) precluded blinding of the participants in most of the included studies. In many trials blinding of treatment was not mentioned. Only one trial adopted a double‐blind design. In two trials outcome assessors were reported to be blind.
Incomplete outcome data
Number of withdrawals and dropouts was clearly reported in 12 of the included trials. In 10 of these trials the small proportion of withdrawals and dropouts was similar (or reported to be similar) in both treatment groups and the analysis of data was deemed in line with an intention‐to‐treat approach. The remaining trial had a high attrition rate with 32.5% of the children lost at the 12‐month follow‐up. Five trials had no apparent withdrawals or dropouts. In one trial information on withdrawals and dropouts was not clearly provided but investigators stated that 'there were no group differences in the number of children who dropped out of treatment (p=0.29)'. In one trial 17 children of the 47 enrolled were found to be non‐compliant and results were analysed by investigators according to compliance with treatment.
Other potential sources of bias
Most of the trials were small. Only six trials included over 50 participants.
Non‐parametric tests were used appropriately in many of the included trials. Four trials chose median values to assess clinical symptoms because of the skewed distribution of the continuous variables (van der Plas 1996; van der Plas 1996a; van Ginkel 2000; van Ginkel 2001). One trial (Latimer 1984) did not report measures of central tendency but provided individual patient data by means of bar graphs. In one trial (Pieczarkowski 2005) measures of variance were not reported. In one trial (Davila 1992) statistical methods were not adequately described.
Effects of interventions
Behavioural or cognitive intervention versus no treatment or sham procedure
No trials were found.
Combination of behavioural and/or cognitive interventions versus another combination of interventions
(i) Ten trials compared conventional treatment (laxatives, dietary advice and toilet training) with conventional treatment plus biofeedback; (ii) one trial evaluated the effect of conventional treatment (laxatives, dietary advice and toilet training) with two anorectal manometry sessions versus conventional treatment alone; (iii) one trial investigated whether biofeedback training plus laxatives is better than biofeedback training alone; (iv) two trials compared behaviour modifications (dietary advice, toilet training, incentive programme, general counselling) with behaviour modification plus laxatives; (v) one trial compared a web‐based approach to deliver information about enhanced toilet training with a no‐web intervention (routine care from primary care physicians); (vi) one trial compared behaviour modifications (diet modification and scheduled toileting) with mineral oil use plus rewards for toileting; (vii) one trial assessed the effects of behaviour modification (star chart system, dietary advice) with behaviour modification plus psychotherapy; (viii) one trial evaluated the effects of home biofeedback versus biofeedback in the laboratory; (ix) one trial evaluated three separate components of biofeedback
(x) one trial evaluated conventional treatment versus bevioural modifications plus laxative therapy
(xi) behavioural therapy versus imapramine
(i) Conventional treatment plus biofeedback versus conventional treatment alone (comparison 01 in the Tables of Comparisons)
Success rate
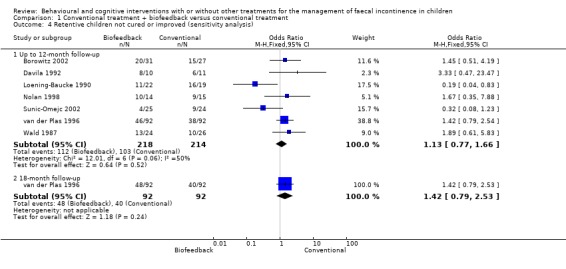
Data from nine trials were combined in a meta‐analysis (Borowitz 2002; Davila 1992; Loening‐Baucke 1988; Loening‐Baucke 1990; Nolan 1998; van der Plas 1996; van der Plas 1996a; Wald 1987; Sunic‐Omejc 2002). All trials but one (Loening‐Baucke 1988) investigated children with functional faecal incontinence. Four trials included children with faecal incontinence associated with chronic constipation, three trials children with faecal incontinence associated with constipation and pelvic floor dyssynergia (anismus), and one trial children with non‐retentive faecal incontinence. The Loening‐Baucke 1988 trial focused on children with faecal incontinence due to myelomeningocele. Children amongst whom biofeedback was tried had not always evaluated beforehand for the suitability of the treatment. The choice of outcomes varied among trials. Only one outcome was shared by all the nine trials: number of children not cured or improved. Data for follow‐up periods within one year and data for longer follow‐up periods (18 months) were analysed separately. For trials reporting more than one follow‐up assessment within a year, the longest period of time was chosen. For five of the nine trials follow‐up assessment at one year was available. For the remaining four trials follow‐up assessment was performed within 6 months. There was no statistically significant pooled effect of the adjunct of biofeedback to conventional treatment at both the 'within 12‐month' follow‐up (OR 1.11 95% CI 0.78 to 1.58) and at 18‐month follow‐up (OR 1.31 95% CI 0.80 to 2.15). However, it should be noted that the test for heterogeneity displayed borderline significance (p = 0.09) for the 12‐month results, which casts some doubt about the statistical validity of the pooling. The analysis was therefore performed using both fixed and random effects models. Results from both methods were consistent. The two studies which showed a different trend from the other seven were Loening‐Baucke 1990 and Sunic‐Omejc 2002. It is unclear how these trials may differ from the remaining trials as their study design and the characteristics of their participants are apparently similar to those reported in some of the other trials. A sensitivity analysis excluding these two trials eliminated heterogeneity among trials (p=0.77) and led to a non‐statistically significant difference in favour of the conventional treatment group (OR 1.44 95% CI 0.98 to 2.13). A further sensitivity analysis excluding trials which enrolled either children with non‐retentive encopresis (van der Plas 1996a) or children with faecal incontinence due to congenital causes (Loening‐Baucke 1988) showed a non‐statistically significant difference in favour of the conventional management group at both the 12‐month assessment (OR 1.13 95% CI 0.77 to 1.66) and the 18‐month follow‐up (OR 1.42 95% CI 0.79 to 2.53).
Normal defaecation dynamics
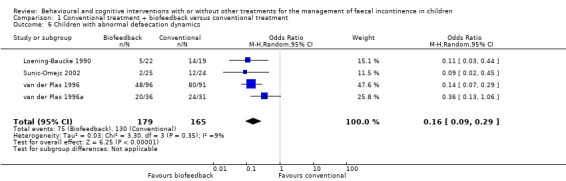
Four trials (Loening‐Baucke 1990; van der Plas 1996; van der Plas 1996a; Sunic‐Omejc 2002) assessed the number of children who were able to achieve normal defaecation dynamics (to relax the external anal sphincter) after conventional treatment or conventional treatment plus biofeedback. Significantly more children in the conventional plus biofeedback group achieved normal defecation dynamics at the end of the treatment period (OR 0.16 95% CI 0.09 to 0.29). This short‐term benefit of biofeedback did not correspond, however, with later treatment success (both trials by van der Plas did not show any additional benefit of biofeedback training to conventional treatment ‐ Figure 1 of the Table of Analyses).
Anorectal manometry
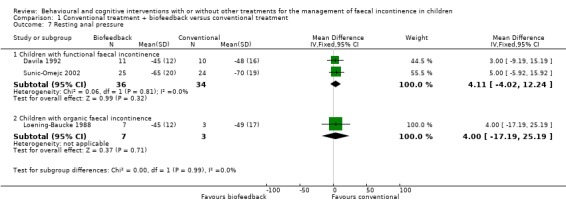
Anorectal manometric measures (resting and squeeze anal pressure, rectal sensation, rectosphincter reflex, saline retention test) were reported in six small trials (Davila 1992; Loening‐Baucke 1988; Loening‐Baucke 1990; Nolan 1998; Pieczarkowski 2005; Sunic‐Omejc 2002). Five trials focused on children with functional faecal incontinence whilst one trial on children with organic faecal incontinence. Overlapping of outcomes was only partial and none of the outcomes were reported by all five trials. One trial (Pieczarkowski 2005) did not report measures of variance hence the data could not be used. Results were presented according to the etiology of faecal incontinence. There was no evidence of effect of biofeedback on any of the considered anorectal measures compared with conventional treatment (the confidence intervals around the estimates of effect were wide).
Other outcomes
The following outcomes were reported in the Borowitz trial (Borowitz 2002): bowel movements in the toilet, self‐initiated trips to the toilet, number of children still using laxatives, number of medical assessments. Fewer children in the conventional group required laxative therapy at the 12‐month follow‐up (9/27 versus 17/31 in the biofeedback group) but the difference was not statistically significant (OR 2.43 95% CI 0.83 to 7.07). No effect of biofeedback over conventional treatment was observed in the frequency of bowel movements, self‐initiated toileting, and number of medical assessments. As continuous data were expressed as mean values and SD and there were some doubts about whether or not the distributions were positively skewed, analyses have been tabulated separately (Additional Tables:Table 9). One study (Pieczarkowski 2005) assessed the number and quality of children's stools, but data were insufficiently reported to be analysed in RevMan.
1. Results of the Borowitz trial at 12 months.
| Outcome | Biofeedback (N 31) | Conventional (N 27) | Results |
| Bowels movements in the toilet ‐ Mean (SD) | 1.16 (0.67) | 1.01 (0.51) | MD 0.15 (95% CI ‐0.15 to 0.45) |
| Self‐initiated trips to the toilet ‐ Mean (SD) | 1.31 (0.69) | 1.31 (0.83) | MD 0.00 (95% CI ‐0.40 to 0.40) |
| Children who remained on laxatives ‐ (n/N) | 17/31 | 9/27 | OR 2.43 (95% CI 0.83 to 7.07) |
| Number of medical assessments ‐ Mean (SD) | 3.42 (1.70) | 2.96 (2.00) | MD 0.46 (95% CI ‐0.50 to 1.42) |
(ii) Conventional treatment plus anorectal manometry versus conventional treatment alone (comparison 02 in the Tables of Comparisons)
One trial evaluated the effect of conventional treatment plus two anorectal manometry sessions versus conventional treatment alone (van Ginkel 2001). No statistically significant difference in the number of children treated successfully was observed between groups at both the 12‐month follow‐up (OR 1.09 95% CI 0.58 to 2.05) and the 24‐month follow‐up (OR 1.40 95% CI 0.72 to 2.73). The proportion of children who showed a significant increase in defaecation frequency, a significant decrease in incontinence frequency, and who were still on laxatives at 12 months was similar between groups, albeit with wide confidence intervals.
(iii) Biofeedback training plus laxatives versus biofeedback training alone (comparison 03 in the Tables of Comparisons)
One trial (van Ginkel 2000) studied the effects of biofeedback training with and without laxatives in 45 children with non‐retentive encopresis. In addition to biofeedback training all randomised children received advice about a high‐fibre diet and toilet training instructions. The proportion of unsuccessful children was higher in the biofeedback and laxatives group both at the 12‐week (OR 8.25 95% CI 1.58 to 43.02) and the 12‐month follow‐up (OR 5.91 95% CI 1.12 to 31.20).
(iv) Behaviour modifications plus laxatives versus behaviour modifications alone (comparison 04 in the Tables of Comparisons)
Success rate
Two trials evaluated behaviour modifications plus laxatives versus behaviour modifications alone (Berg 1983, Nolan 1991). Succes rate was the only outcome measure available in both trials. The larger trial (Nolan 1991) showed better results in the combination group. In contrast, the results of the Berg trial, which included a considerably smaller number of participants (about 15 in each arm), favoured the behaviour modifications alone group. The reason for the significant heterogeneity between the two studies is not clear but may be due to the higher drop‐out rate in the Berg trial or the different choice of laxative.
Number of relapses
In the Nolan trial information was also available about the number of children who relapsed. More children in the behaviour modifications plus laxatives group experienced more than one relapse but the difference was not statistically significant between intervention groups (6/83 versus 1/86 ‐ OR 6.62 95% CI 0.78 to 56.26) and the confidence intervals were wide.
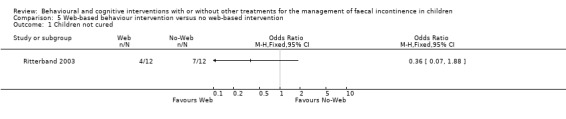
(v) Web‐based approach to deliver information about enhanced toilet training with a no‐web intervention (comparison 05 in the Tables of Comparisons)
One small trial evaluated the effects of information delivered through the Internet on toilet training and behavioural modifications versus routine medical care (primary care physician visits) for children with functional faecal incontinence (Ritterband 2003). The proportion of children who had no soiling accidents post‐intervention was higher in the web group but not statistically significant between groups (children who were not cured 4/12 versus 7/12 ‐ OR 0.36 95% CI 0.07 to 1.88). A significant reduction in the average weekly number of soiling episodes was observed in the web group compared to the no‐web group (mean 0.50 SD 0.85 versus mean 8.27 SD 13.83 ‐ mean difference of ‐7.77 95% CI ‐15.61 to 0.07) but confidence intervals were wide and the distribution probably skewed. The trialists reported also that the web participants demonstrated significant improvements in terms of bowel movements in the toilet, self‐initiated trips to the toilet, and trips to toilet with parental prompts (p<0.02) but data were provided in a format unsuitable for further statistical analyses.
(vi) Behaviour modifications (diet modifications and scheduled toileting) versus mineral oil use (laxative) plus rewards for toileting
One trial evaluating behaviour modifications versus mineral oil was found (Mellon 1996) but incomplete reporting of data (i.e. the number of randomised children in each treatment group, mean values and measures of variance) hampered any statistical analyses. According to the trialists there were no significant group differences at the end of the six‐week treatment and through the six‐month follow‐up.
(vii) Behaviour modifications plus psychotherapy versus behaviour modifications alone
One trial evaluating behaviour modifications with and without psychotherapy was identified (Taitz 1986) but data were reported in a format unsuitable for statistical analyses in RevMan (measures of variance not provided). Results of treatment in the group of children receiving behaviour modifications were reported to be similar to those receiving additional psychotherapy. In a post hoc analysis, significantly more adverse psychosocial factors were noticed in the families of children who either did not comply or did not respond to the treatment.
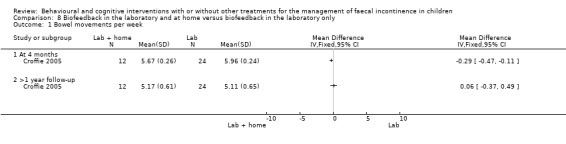
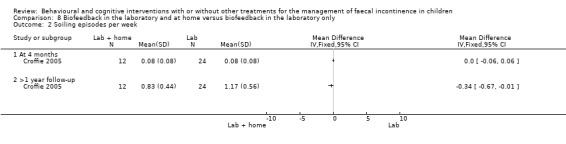
(viii) Home biofeedback versus biofeedback in the laboratory (comparison 08 in the Tables of Comparisons)
One trial evaluated the effects of biofeedback for children with pelvic floor dyssynergia and constipation (Croffie 2005). Children were randomised to biofeedback in the laboratory or to biofeedback in the laboratory and at home. The trialists reported that all children improved after treatment in terms of bowel movements in the toilet, soiling accidents, and use of laxatives. No apparent differences were found between the home group and the laboratory group at the 4‐month assessment. Marginally significant reductions were observed in the number of soiling episodes (mean difference of ‐0.34 95% CI ‐0.67 to ‐0.01) and the weekly use of laxatives at > 1 year follow‐up (mean difference of ‐0.41 95% CI ‐0.70 to ‐0.12) for children using biofeedback at home.
(ix) One modality of biofeedback versus any other modality
One trial (Latimer 1984) compared three different components of biofeedback in a group of 4 children with organic faecal incontinence (three with imperforate anus and one with Hirschsprung's disease) and a group of 4 incontinent adults. This trial adopted a single‐case cross‐over design (see Table of Characteristics of Included Studies for details). Data were insufficiently reported to be analysed in RevMan. The trial's authors reported that all children became more continent during treatment. Two children responded well to sensory discrimination training, one to exercise training and one child initially responded to each treatment phase (exercise training, sensory discrimination training and biofeedback) with a reduction in soiling accidents but then partially relapsed during each no‐treatment phase.
(x) Conventional treatment versus behaviour modifications plus laxative therapy
One trial (van Dijk 2008) evaluated the addition of behaviour modifications to laxative therapy compared with conventional treatment. The primary outcomes were defecation frequency, fecal incontinence and success rate. Success rate was defined as defecation frequency of more than three times per week and fecal incontinence of once or less per two weeks. Secondary outcomes were stool withholding behaviour and behaviour problems. Unfortunately the data presented in the report were not observed raw data but estimates based upon an analysis which adjusted for baseline and for all time points. Missing data were also imputed by the trial's investigators. The trial's authors reported that they found no advantage of behavior modifications with laxatives over conventional treatment.
Combination of behavioural and/or cognitive interventions versus a single intervention
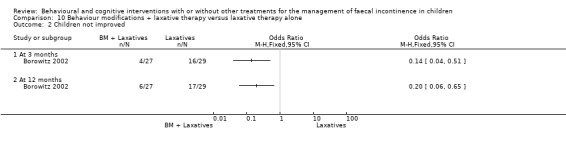
(i) Behaviour modifications plus laxative therapy versus laxative therapy alone (comparison 10 in the Tables of Comparison)
The trial by Borowitz (Borowitz 2002) compared behaviour modifications (incentive programmes, toilet training) plus laxatives with laxatives alone. A third arm of the trial evaluated also the effects of biofeedback and its results are reported above (conventional treatment plus biofeedback versus conventional treatment alone comparison). The number of children who experienced a total cure was slightly higher in the behaviour modifications plus laxative group but not significantly different from that of the laxative therapy group (OR 0.66 95% CI 0.22 to 1.93).The proportion of children who responded to treatment (improvement rate) was significantly higher in the behaviour modifications plus laxative group at both the three month assessment (OR 0.14 95% CI 0.04 to 0.51) and the 12‐month follow‐up (OR 0.20 95% CI 0.06 to 0.65). Fewer children in the behaviour modifications plus laxatives group continued to receive laxatives at 12 months, but again the difference was not statistically significant (OR 0.35 95% CI 0.12 to 1.05). No strong evidence of effect of the addition of laxative therapy to behaviour modifications was observed on the frequency of bowel movements in the toilet and the frequency of self‐initiated trips to the toilet (Additional Tables: Table 10). Children receiving behaviour modifications plus laxative, however, required significantly fewer medical assessments than those receiving laxative therapy alone ‐ mean difference of ‐0.96, 95% CI ‐1.87 to 0.05 (Additional Tables: Table 10). Consequently the estimated average cost of the behaviour modifications plus laxatives approach was lower ($213 versus $246 in Cox 1998, a supplementary report of the Borowitz trial).
2. Results of the Borowitz trial at 12 months.
| Outcome | BM+Laxatives (N 27) | Laxatives (N 29) | Results |
| Bowel movements in the toilet ‐ Mean (SD) | 1.01 (0.51) | 1.30 (0.61) | MD ‐0.29 (95% CI ‐0.58 to 0.00) |
| Self‐initiated toileting ‐ Mean (SD) | 1.31 (0.83) | 1.40 (0.76) | MD ‐0.09 (95% CI ‐0.51 to 0.33) |
| Number of medical assessments ‐ Mean (SD) | 2.96 (2.00) | 3.92 (1.40) | MD ‐0.96 (95% CI ‐1.87 to ‐0.05) |
(ii) Behaviour modifications therapy versus imapramine
One study (Demirogullari 2007) evaluated the role of anorectal physiology and bowel habits when comparing behavioural therapy versus imapramine (an antidepressant). The outcomes recorded were colonic transit time, anorectal manometry, weekly defecation and number of fecal and urinary incontinence episodes. The original report was written in Turkish and included an English abstract. We managed to translate part of the main text but were still unclear as to whether measures of variance were reported. Therefore we could not analyse these data in RevMan.The trialists concluded that imipramine may be a suitable adjunct therapy to behavioral therapy.
Behavioural or cognitive intervention versus another intervention
No trials were found.
Discussion
The management of faecal incontinence in children is conventionally based on both medical and behavioural interventions. In particular, the use of softening agents and laxatives and increasing daily fibre intake aim at promoting evacuation of retained stool. Behavioural approaches aim at restoring normal bowel habits and providing support to the child. Biofeedback has been employed to teach children how to control their sphincter muscles and primarily to teach children with pelvic floor dyssynergia (anismus) to relax their sphincter muscles. However, neither the clinical effectiveness nor the cost‐effectiveness of biofeedback training have yet been established. It is also unclear whether the conventional medical‐behavioural approach is superior to a single behavioural or single medical intervention in the management of children with faecal incontinence. In particular it is difficult to single out the contribution of behavioural/education programmes (instructions to parents and children on the psychophysiology of constipation and faecal incontinence and on regular toileting in order to establish normal bowel habits) from the effects of medical treatments such as laxative therapy, a common problem in the evaluation of complex healthcare interventions.
The 18 studies in the present review were not homogeneous. They encompassed different diagnoses, treatment options, outcome measures and follow‐up periods. The majority of trials assessed the effects of biofeedback for the management of constipation‐associated faecal incontinence. We were unable to evaluate the effects of biofeedback in children with organic faecal incontinence as only two very small trials included participants with such a diagnosis. It is noteworthy that none of the included trials had considered the impact on the wider family. Only five trials enrolled more than 50 participants. The combination of data from small trials may not lead to meaningful summary estimates of effects. In the performed meta‐analyses, however, all trials but two showed a trend in the same direction giving robustness to the conclusions.
Conventional treatment (laxatives, dietary advice and toilet training) versus conventional treatment plus biofeedback
Nine trials, which provided information on the number of children successfully treated, were combined in a meta‐analysis. All trials but one investigated children with functional faecal incontinence. The observed trend favoured the conventional approach (OR 1.11 CI 95% 0.78 to 1.58). This could not be explained by different numbers of dropouts in the trial groups. Two trials, one by Loening‐Baucke and colleagues (Loening‐Baucke 1990) and one by Sunic‐Omejc and colleagues (Sunic‐Omejc 2002), showed results which appeared clearly inconsistent with the overall treatment effect. The reasons why the results of these two small trials were out of line with the others are not clear. The study by Loening‐Baucke was restricted to children with pelvic floor dyssynergia (anismus). One possible explanation is that children with normal expulsion patterns tend to respond better to conventional treatment whilst children with abnormal expulsion patterns show greater improvement with biofeedback training (Wald 1987). Another trial which specifically evaluated children with pelvic floor dyssynergia (Nolan 1998) showed that those receiving conventional treatment plus biofeedback training were significantly more likely to achieve relaxation of the sphincter muscles after treatment. The small trial by Sunic‐Omejc showed that a higher proportion of children responded successfully to biofeedback when they were evaluated soon after treatment (12‐week assessment). Another small trial evaluating the effects of biofeedback in the laboratory and at home (Croffie 2005) found that all children ‐independently of their group allocation ‐ learned to relax the external anal sphincter after five sessions of biofeedback. All these findings are in line with those of larger studies including children with and without normal defaecation dynamics (van der Plas 1996; van der Plas 1996a) However, the observed physical improvement did not appear to be translated into a functional improvement in the children's continence status (see the Analyses Tables) and both the Nolan and the van der Plas trials (Nolan 1998; van der Plas 1996) reported that success rates after cessation of the intervention (12 and 18‐month follow‐ups) were similar in the trial groups. Pelvic floor dyssynergia therefore does not seem to play a crucial role in the aetiological mechanisms of childhood constipation and biofeedback training appears to have no long‐term effects. The follow‐up of both the Loening‐Baucke trial and the Sunic‐Omejc trial was relatively short and the observed positive effect of biofeedback could be interpreted merely as the consequence of a transient clinical improvement in children with constipation and abnormal expulsion patterns. It could be argued that the absence of a significant effect of biofeedback over conventional treatment in large trials is due to a sort of therapeutic, "demystifying" effect of performing anorectal manometry ‐ the manometric evaluation of anorectal function without any specific training on how to relax and control sphincter muscles ‐ in children receiving conventional treatment. The trial by van Ginkel and colleagues (van Ginkel 2001) specifically evaluated the effects of conventional treatment plus two sessions of anorectal manometry with conventional treatment alone (dietary advice, toilet training, oral laxatives and enemas) and did not find any higher success rate in children who underwent anorectal manometry. This observation suggests that anorectal manometry does not play any therapeutic role in constipated children and that the lack of benefits in biofeedback trials could not be attributed to its alleged demystifying effect.
A single trial (van Ginkel 2000) assessed adding laxative therapy to biofeedback training amongst children with non‐retentive faecal incontinence (non‐constipated children). The use of stimulant laxatives for non‐retentive faecal incontinence is based on the assumption that children often have relatively dull sensation of the urgency of defaecation and a stimulant laxative may help to intensify this sensation. The dose for an individual child, however, is critical as any increase in rapidity of defaecation is likely to make the situation worse. The significantly lower success rate in the group receiving biofeedback training together with oral laxatives in the van Ginkel trial may therefore be due to the unnecessary softening of stools resulting in more soiling accidents. This interpretation is strengthened by the finding of a higher number of dropouts in the biofeedback plus laxatives group (9 children) compared with the biofeedback training alone group (no dropouts).
In conclusion, the analyses of the available randomised trials do not support the claim based on short‐term observational studies that biofeedback training provides additional benefit to conventional treatment in the management of faecal incontinence associated with constipation. Furthermore it is an invasive and expensive procedure. The positive trend in favour of the conventional intervention cannot be explained by methodological problems such as a difference in the proportion of dropouts between treatment groups amongst trials. The exclusion of trials which studied children with non‐retentive faecal incontinence (one trial) as well as children with faecal incontinence due to organic causes (one small trial) did not alter the above conclusions. The positive short‐term effects of biofeedback reported in the literature could make people rely on an invasive technique which proves ineffective in the long run. Overall our findings are akin to those of a recent systematic review on the effects of biofeedback for gastrointestinal conditions which concluded that "there is no strong evidence to support biofeedback as a useful treatment for gastrointestinal diseases" (Coulter 2002).
Behaviour modifications and laxative therapy
The results of two trials (Berg 1983; Nolan 1991) of adding laxative therapy (enemas and/or oral laxatives) to a structured behavioural programme (toilet training, positive reinforcing scheme with or without dietary advice) were not consistent. The larger trial (Nolan 1991) showed a significantly higher proportion of children cured or improved. The results from one trial (van Dijk 2008) indicated that behavioural modifications with laxatives had no clear benefit over conventional treatment in treating childhood constipation. On the other hand the results of the Borowitz trial (Borowitz 2002) suggested that the adjunct of behaviour components to laxative therapy may improve symptoms of children with constipation‐associated faecal incontinence. These findings are in line with the current clinical recommendations for the management of childhood faecal incontinence associated with constipation that favour the individual‐adjusted use of laxatives with behavioural approaches (Clayden 1996; Baker 1999). However, it should be noticed that "laxative therapy" is an umbrella term for a range of drugs, the effectiveness of each of which has not yet been adequately addressed in randomised trials. In particular, to our knowledge, there are no well‐designed randomised controlled trials comparing the effectiveness of one laxative (or regimen of laxatives) with another. Furthermore, the specific contribution of laxative therapy should be established independently from any behavioural interventions. A recent Cochrane review on "Stimulant laxatives for constipation and soiling in children" (Price 2001) failed to identify any suitable randomised controlled trial in the existing literature demonstrating the lack of evidence to guide the use of stimulant laxatives as well as the urgent need for further research in this subject.
Authors' conclusions
Implications for practice.
The data available suggest that biofeedback does not provide any additional benefit to conventional treatment (laxative, toilet training, dietary advice) for the management of children with functional faecal incontinence. There is a suggestion that children with pelvic floor dyssynergia receiving biofeedback are more likely to achieve normal defaecation dynamics in the short‐term. This achievement, however, is not associated with a clinical improvement in continence status and the evidence available indicates that it is not maintained in the long‐term. There is no enough evidence on which to assess the effects of biofeedback for the treatment of organic faecal incontinence. There is no evidence that anorectal manometry (i.e. manometric evaluation of anorectal function) plays a therapeutic role in constipated children. No trials have yet attempted to weigh the costs of biofeedback against its putative benefits. There is some evidence, albeit weak, that the addition of behavioural interventions (toilet training, incentive scheme, dietary advice) to laxative therapy produces higher success rates in children with faecal incontinence associated with constipation.
Implications for research.
Well‐conducted and better‐reported randomised controlled trials are needed which include larger samples, longer follow‐up periods, and cover both socially and clinically relevant outcomes. Data about children's emotions, social interactions, scholastic performance, and family education would be useful as well as information regarding the impact of the problem on family functioning. Moreover, as some medical and behavioural procedures may be stressful or upsetting (e.g. the prolonged use of laxatives, dietary manipulation, invasive anorectal examinations, biofeedback), acceptability is a component of intervention that needs more consideration. Future trials should also include when possible an appropriate spectrum of severity and history of incontinence (such as children with and without chronic constipation) and provide subgroup analyses based on the etiology of the condition. Economic implications relating to the choice of intervention (especially when an expensive procedure such as biofeedback is employed) should be also thoroughly addressed. We found no trials comparing behavioural or cognitive interventions alone with no‐treatment or with other interventions alone. The evidence about combinations of behavioural interventions and laxatives would be enhanced by replication of the trials that are currently available. The specific contribution of laxative therapy should be evaluated independently from behavioural interventions. The effects of different laxative regimens and combinations of laxatives should also be properly addressed during both the initial disimpaction phase and the maintenance phase.
What's new
| Date | Event | Description |
|---|---|---|
| 8 November 2011 | New citation required but conclusions have not changed | three studies added |
| 8 November 2011 | New search has been performed | three new studies added |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 16 September 2008 | Amended | Converted to new review format. |
| 22 February 2006 | New citation required and conclusions have changed | Substantive amendment |
| 28 October 2004 | New search has been performed | minor update |
Acknowledgements
We thank Sheila Wallace for help and advice with the electronic searches and for checking the references, Claire Short for compiling the initial literature database, June Cody and Adrian Grant for providing support and advice, Marion Campbell for statistical assistance, Graham Clayden, Janet Hiller and Marc Benninga for their comments on the first version of the manuscript.
We also thank Karolina Kazimierczai, Dr Raif Yucel and Cakil Sarac for help with translation of non‐english reports.
Appendices
Appendix 1. Search strategy for the Specialised Register
The Incontinence Group's Trials Register was searched using the Group's own keyword system. The search terms used were:
(design.rct* or design.cct*) AND ({TOPIC.FAECAL.bowelfunction.} OR {TOPIC.FAECAL.CONSTIPATION*} OR {TOPIC.FAECAL.ENCOPRESIS*} OR {TOPIC.FAECAL.INCON.} OR {TOPIC.FAECAL.INCON.CONSTIPATION.} OR {TOPIC.FAECAL.INCON.CONSTIPATION.NEUROGENIC.} OR {TOPIC.FAECAL.INCON.multiplesclerosis.} OR {TOPIC.FAECAL.INCON.neuropathic.} OR {TOPIC.FAECAL.INCON.PROLAPSERECTUM} OR {TOPIC.FAECAL.INCON.PROLAPSERECTUM.CONSTIPATION.} OR {TOPIC.FAECAL.NEUROGENIC.} OR {TOPIC.FAECAL.INCON.CONSTIPATION.NEUROGENIC.} OR {TOPIC.FAECAL.INCON.neuropathic.} OR {TOPIC.NOT INCON.FAECAL.CONSTIPATION.NEUROLOGICAL.}) AND ({INTVENT.PSYCH*} OR {intvent.phys*}) (All searches were of the keyword field of Reference Manager 12, Thomson Reuters). Date of the most recent search of the register for this review: 28 October 2011.
Data and analyses
Comparison 1. Conventional treatment + biofeedback versus conventional treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Children not cured or improved (fixed effects) | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to 12‐month follow‐up | 9 | 510 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.78, 1.58] |
| 1.2 18‐month follow‐up | 2 | 251 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.80, 2.15] |
| 2 Children not cured or improved (random effects) | 9 | 510 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.63, 1.84] |
| 3 Children not cured or improved (sensitivity analysis) | 7 | 420 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.98, 2.13] |
| 4 Retentive children not cured or improved (sensitivity analysis) | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Up to 12‐month follow‐up | 7 | 432 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.66] |
| 4.2 18‐month follow‐up | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.79, 2.53] |
| 6 Children with abnormal defaecation dynamics | 4 | 344 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.09, 0.29] |
| 7 Resting anal pressure | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Children with functional faecal incontinence | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | 4.11 [‐4.02, 12.24] |
| 7.2 Children with organic faecal incontinence | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐17.19, 25.19] |
| 8 Maximum squeeze anal pressure | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children with functional faecal incoontinence | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Children with organic faecal incontinence | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Rectal sensation | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Children with functional faecal incontinence | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐2.74 [‐7.73, 2.25] |
| 9.2 Children with organic faecal incontinence | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐32.05, 12.05] |
| 10 Rectosphincter reflex | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children with functional faecal incontinence | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Children with organic faecal incontinence | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Saline retention test (ml) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children with organic faecal incontinence | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 Conventional treatment + biofeedback versus conventional treatment, Outcome 1 Children not cured or improved (fixed effects).
1.2. Analysis.

Comparison 1 Conventional treatment + biofeedback versus conventional treatment, Outcome 2 Children not cured or improved (random effects).
1.3. Analysis.

Comparison 1 Conventional treatment + biofeedback versus conventional treatment, Outcome 3 Children not cured or improved (sensitivity analysis).
1.4. Analysis.
Comparison 1 Conventional treatment + biofeedback versus conventional treatment, Outcome 4 Retentive children not cured or improved (sensitivity analysis).
1.6. Analysis.
Comparison 1 Conventional treatment + biofeedback versus conventional treatment, Outcome 6 Children with abnormal defaecation dynamics.
1.7. Analysis.
Comparison 1 Conventional treatment + biofeedback versus conventional treatment, Outcome 7 Resting anal pressure.
1.8. Analysis.
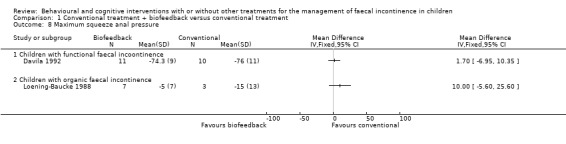
Comparison 1 Conventional treatment + biofeedback versus conventional treatment, Outcome 8 Maximum squeeze anal pressure.
1.9. Analysis.

Comparison 1 Conventional treatment + biofeedback versus conventional treatment, Outcome 9 Rectal sensation.
1.10. Analysis.

Comparison 1 Conventional treatment + biofeedback versus conventional treatment, Outcome 10 Rectosphincter reflex.
1.11. Analysis.
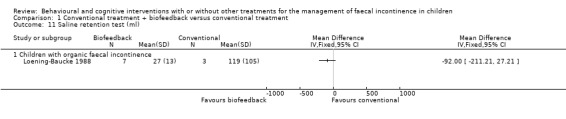
Comparison 1 Conventional treatment + biofeedback versus conventional treatment, Outcome 11 Saline retention test (ml).
Comparison 2. Conventional treatment + anorectal manometry versus conventional treatment alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Children not cured or improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 12‐month follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 24‐month follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Children who did not show a significant decrease in encopresis episodes | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Children who were still using laxatives at 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Children who did not show a significant increase in defaecation frequency | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.

Comparison 2 Conventional treatment + anorectal manometry versus conventional treatment alone, Outcome 1 Children not cured or improved.
2.2. Analysis.
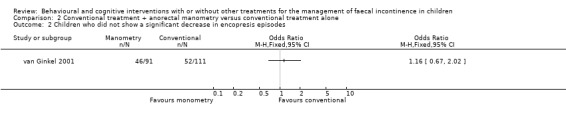
Comparison 2 Conventional treatment + anorectal manometry versus conventional treatment alone, Outcome 2 Children who did not show a significant decrease in encopresis episodes.
2.3. Analysis.
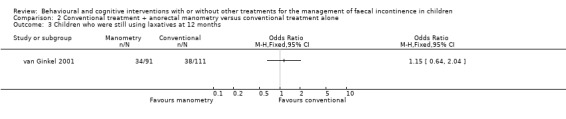
Comparison 2 Conventional treatment + anorectal manometry versus conventional treatment alone, Outcome 3 Children who were still using laxatives at 12 months.
2.4. Analysis.
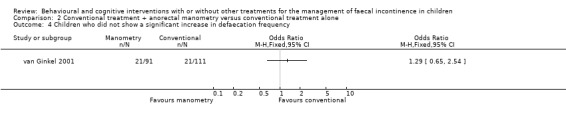
Comparison 2 Conventional treatment + anorectal manometry versus conventional treatment alone, Outcome 4 Children who did not show a significant increase in defaecation frequency.
Comparison 3. Biofeedback + laxative therapy versus biofeedback.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Children not cured or improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 12‐week follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 12‐month follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
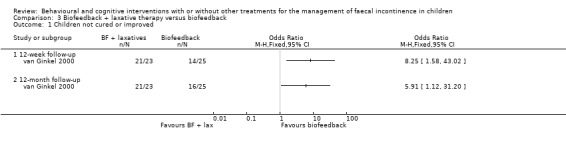
Comparison 3 Biofeedback + laxative therapy versus biofeedback, Outcome 1 Children not cured or improved.
Comparison 4. Behaviour modifications + laxatives versus behaviour modifications alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Children not cured or improved | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 6‐month follow‐up | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 12‐month follow‐up | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Children who had more than one relapse | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
4.1. Analysis.
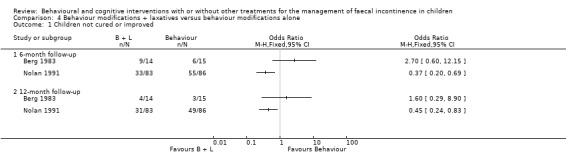
Comparison 4 Behaviour modifications + laxatives versus behaviour modifications alone, Outcome 1 Children not cured or improved.
4.2. Analysis.
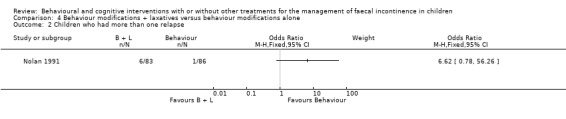
Comparison 4 Behaviour modifications + laxatives versus behaviour modifications alone, Outcome 2 Children who had more than one relapse.
Comparison 5. Web‐based behaviour intervention versus no web‐based intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Children not cured | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
5.1. Analysis.
Comparison 5 Web‐based behaviour intervention versus no web‐based intervention, Outcome 1 Children not cured.
Comparison 8. Biofeedback in the laboratory and at home versus biofeedback in the laboratory only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bowel movements per week | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 >1 year follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Soiling episodes per week | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 >1 year follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Days of laxatives use per week | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 >1 year follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
Comparison 8 Biofeedback in the laboratory and at home versus biofeedback in the laboratory only, Outcome 1 Bowel movements per week.
8.2. Analysis.
Comparison 8 Biofeedback in the laboratory and at home versus biofeedback in the laboratory only, Outcome 2 Soiling episodes per week.
8.3. Analysis.

Comparison 8 Biofeedback in the laboratory and at home versus biofeedback in the laboratory only, Outcome 3 Days of laxatives use per week.
Comparison 10. Behaviour modifications + laxative therapy versus laxative therapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Children not cured | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Children not improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Children on laxatives at 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
10.1. Analysis.

Comparison 10 Behaviour modifications + laxative therapy versus laxative therapy alone, Outcome 1 Children not cured.
10.2. Analysis.
Comparison 10 Behaviour modifications + laxative therapy versus laxative therapy alone, Outcome 2 Children not improved.
10.6. Analysis.
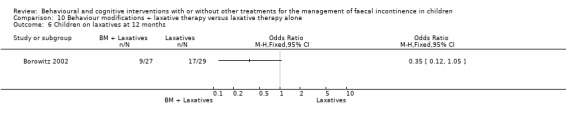
Comparison 10 Behaviour modifications + laxative therapy versus laxative therapy alone, Outcome 6 Children on laxatives at 12 months.
Comparison 11. protocol behav ther vs conventional therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bowel movements per week | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 soiling per week | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Children not cured | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berg 1983.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Not stated BLINDING PROCEDURES: Double‐blind trial SAMPLE CALCULATION: No DURATION: Three months FOLLOW‐UP: 6, 12 months WITHDRAWALS/ DROPOUTS: Four children dropped out at the beginning of the treatment period. Further three children where not available at the 6‐month follow‐up and other 10 were lost at the 12‐month follow‐up ITT: No GEOGRAPHICAL LOCATION: UK SETTING: Outpatients/ Soiling clinic | |
| Participants | DIAGNOSIS: Functional faecal incontinence often with a history of constipation ELIGIBLE: Unknown ENROLLED: 44 COMPLETED: 40 AGE: Mean 7.9 years (SD 2.3) GENDER: Unclear | |
| Interventions | 1) Behaviour modifications (toilet training, rewards, counselling) + laxative (Senokot) 2) Behaviour modifications + placebo 3) Behaviour modifications + no medications | |
| Outcomes | Soiling episodes | |
| Notes | Small sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four children dropped out at the beginning of the treatment period. Further three children where not available at the 6‐month follow‐up and other 10 were lost at the 12‐month follow‐up |
Borowitz 2002.
| Methods | DESIGN: Randomised controlled trial, block randomisation used ALLOCATION CONCEALMENT: Not stated BLINDING PROCEDURES: Provider and participants not blind. Outcome data collected by means of a computerized voice mail data collection system SAMPLE CALCULATION: No DURATION: Twelve months FOLLOW‐UP: Three, six, and twelve months. WITHDRAWALS/ DROPOUTS: Not stated. ITT: Yes (no reported dropouts) GEOGRAPHICAL LOCATION: USA SETTING: Outpatients | |
| Participants | DIAGNOSIS: Chronic faecal incontinence (weekly episodes of faecal soiling for at least six months) ELIGIBLE: 105 ENROLLED: 87 COMPLETED: 87 AGE: Mean 8.6 years; range 5‐13 GENDER: 72 boys and 15 girls | |
| Interventions | 1) Intensive medical care (laxatives) 2) Intensive medical care + enhanced toilet training 3) Intensive medical care + enhanced toilet training + biofeedback | |
| Outcomes | Child's self‐initiated and parent‐initiated trips to the toilet, number of voluntary bowel movements in the toilet, pain with defecation, soiling episodes, number of teaspoons of laxatives and number of enemas or suppositories | |
| Notes | It was difficult from the reporting of results to establish whether the data were adjusted for clustering effect. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | block randomisation |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Provider and participants not blind. Outcome data collected by means of a computerized voice mail data collection system |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not stated |
Croffie 2005.
| Methods | DESIGN: randomised controlled trial (randomisation 2:1 to intervention and comparison group) ALLOCATION CONCEALMENT: Not stated BLINDING PROCEDURES: Not stated. Long‐term follow‐up data were obtained through telephone questionnaire performed by a physician who was not involved in the care of the patients. SAMPLE CALCULATION: No DURATION: Two months FOLLOW‐UP: 2 and 4 months after the last biofeedback session follow‐up WITHDRAWALS/ DROPOUTS: None ITT: Yes GEOGRAPHICAL LOCATION: USA SETTING: Outpatients/ Paediatric Gastroenterology Unit | |
| Participants | DIAGNOSIS: Recalcitrant faecal incontinence and constipation with dyssynergic defecation at anorectal manometry ELIGIBLE: Unknown ENROLLED: 36 COMPLETED: 36 AGE:Mean 9.2 years (6‐14) GENDER: 30 boys and 6 girls | |
| Interventions | 1) Biofeedback in the laboratory alone 2) Biofeedback in the laboratory and at home | |
| Outcomes | Number of bowels movements, soiling episodes, use of laxatives | |
| Notes | All patients used laxatives daily. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomisation 2:1 to intervention and comparison group |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not stated. Long‐term follow‐up data were obtained through telephone questionnaire performed by a physician who was not involved in the care of the patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none |
Davila 1992.
| Methods | DESIGN: Randomised controlled trial, block randomisation ALLOCATION CONCEALMENT: not stated BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: 4 weeks FOLLOW‐UP: No follow‐up WITHDRAWALS/ DROPOUTS: None ITT: Yes GEOGRAPHICAL LOCATION: South America SETTINGS: Outpatients/ Gastroenterology Unit | |
| Participants | DIAGNOSIS: Faecal incontinence and constipation ELIGIBLE: Unknown ENROLLED: 21 COMPLETED: 21 AGE: Mean 9 years GENDER: 14 boys and 7 girls | |
| Interventions | 1) Conventional treatment (enemas for three days + dietary advice + use of laxatives + toilet training) 2) Conventional treatment + biofeedback | |
| Outcomes | Number of children cured or improved, number of bowel movements, abdominal pain, anorectal manometric assessment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none |
Demirogullari 2007.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Not stated BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: Not stated in English abstract FOLLOW‐UP: Not stated in English abstract WITHDRAWALS/ DROPOUTS: Not stated in english abstract ITT: not stated in english abstract GEOGRAPHICAL LOCATION: Turkey SETTINGS: Dept of Pediatrics and Pediatric Psychiatry | |
| Participants | DIAGNOSIS: Faecal incontinence and constipation ELIGIBLE: Unknown ENROLLED: 47 COMPLETED: Not stated in english abstract AGE: 5 to 14 years GENDER: 36 boys and 11 girls | |
| Interventions | 1) Behaviour modifications 2) Imipramine | |
| Outcomes | Colonic transit time, anorectal manometry, weekly defecation numbers, fecal and urinary incontinence episodes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not stated in english abstract |
Latimer 1984.
| Methods | DESIGN: Single case experiments ‐ ABACADA or ACABADA Following baseline period participants were randomly allocated to treatment options. ALLOCATION CONCEALMENT: Not stated BLINDING PROCEDURES: Not stated DURATION: 3 four‐week periods FOLLOW‐UP: 6 months WITHDRAWALS/DROPOUTS: None ITT: Yes GEOGRAPHICAL LOCATION: Canada SETTING: Outpatients | |
| Participants | DIAGNOSIS: Children with faecal incontinence due to imperforate anus or Hirschsprung's disease ELIGIBLE: Unknown ENROLLED: 8 people (4 children and 4 adults) COMPLETED: 8 people AGE: Mean 11 years; range 8‐14 GENDER: 3 boys and one girl with imperforate anus and one boy with Hirschsprung's disease | |
| Interventions | Three components of biofeedback were investigated. TREATMENT B: exercise training TREATMENT C: sensory discrimination training TREATMENT D: biofeedback (which incorporates both treatment B and C) | |
| Outcomes | Number of bowel movements in a toilet, stained underwear, changes of underwear, "accidents", use of laxatives or enemas, anorectal manometric assessment | |
| Notes | Following the baseline period participants were randomly assigned to either treatment B or C. Each treatment was conducted for 4 weeks. This initial treatment period was followed by a 4‐ week baseline period. The second treatment period was then employed in like fashion and followed again by a 4‐week baseline period. Finally all patients received treatment D. Data not suitable for statistical analyses (presented by means of graphs for each single patient or reported for all included patients at the end of the study and not for each single phase of treatment) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | none |
Loening‐Baucke 1988.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Sealed envelopes BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: Unclear. Three biofeedback sessions and perineal exercises at least every other day. FOLLOW‐UP: 6, 12 months (by questionnaire) WITHDRAWALS/ DROPOUTS: One child in the conventional group and one child in the biofeedback group were not available at follow‐up for anorectal measurements ITT: Yes GEOGRAPHICAL LOCATION: USA SETTINGS: Outpatients/ Myelodysplastic Clinic | |
| Participants | DIAGNOSIS: Faecal incontinence due to myelomeningocele ELIGIBLE: Unknown ENROLLED: 12 children (16 non‐randomised controls were also included) COMPLETED: 12 AGE: Mean 12 years; range 7‐21 GENDER: 8 boys and 4 girls | |
| Interventions | 1) Conventional treatment (toilet training) 2) Conventional treatment + biofeedback Behaviour modifications were used for both groups to increase self‐initiation of bowel movements. Laxatives were given as needed | |
| Outcomes | Soiling episodes, anorectal manometric assessment | |
| Notes | Unbalanced randomisation (4 people in one treatment group and 8 in the other). Anorectal measurements were not significant different between treatment groups at both the initial or follow‐up assessment (p>0.3). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One child in the conventional group and one child in the biofeedback group were not available at follow‐up for anorectal measurements. Carried out an intention to treat analysis |
Loening‐Baucke 1990.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Sealed envelopes BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: Yes DURATION: 6‐month protocol. Biofeedback group received up to six sessions of therapy 7 +/‐ 2 days apart. FOLLOW‐UP: 7, 12 months ITT: Yes WITHDRAWALS/ DROPOUTS: One child in the conventional treatment group and one child in the biofeedback group withdrew before completion of the treatment GEOGRAPHICAL LOCATION: USA SETTING: Outpatients | |
| Participants | DIAGNOSIS: Chronic constipation and faecal incontinence with pelvic floor dyssynergia (anismus) ELIGIBLE: Unknown ENROLLED: 43 COMPLETED: 41 AGE: Mean 8.9 years; range 5‐16 GENDER: 31 boys and 10 girls | |
| Interventions | 1) Conventional treatment (use of laxatives, increase of dietary fibre and scheduled toileting) 2) Conventional treatment + biofeedback | |
| Outcomes | Rate of improvement, anorectal manometric assessment | |
| Notes | Significantly more girls in the biofeedback group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One child in the conventional treatment group and one child in the biofeedback group withdrew before completion of the treatment. Carried out an intention to treat analysis |
Mellon 1996.
| Methods | DESIGN: Randomised controlled trial, coin toss ALLOCATION CONCEALMENT: not stated BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: 18 weeks FOLLOW‐UP: 6 months ITT: No WITHDRAWALS/ DROPOUTS: Not clearly reported. No significant group differences in the number of children who dropped out of treatment p = 0.29 GEOGRAPHICAL LOCATION: USA SETTING: Outpatients, Gastroenterology Clinic | |
| Participants | DIAGNOSIS: Functional faecal incontinence (primary and secondary encopresis as defined by the paper's authors) ELIGIBLE: Unknown ENROLLED: 25 COMPLETED: Not clearly reported AGE: Mean 8.3 years GENDER: 23 boys and 2 girls | |
| Interventions | 1) Dietary modifications and scheduled toileting 2) Mineral oil and rewards | |
| Outcomes | Success and failure rate, soiling accidents, number and type of bowel movements in the toilet | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly reported. No significant group differences in the number of children who dropped out of treatment p = 0.29 |
Nolan 1991.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Stratified blocked schedule by a person not connected with the study. Opaque numbered sealed envelopes BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: Yes DURATION: 12 days FOLLOW‐UP: 6,12,18 months ITT: Yes WITHDRAWALS/ DROPOUTS: Three children in the multimodel therapy group and 4 in the behaviour modification group GEOGRAPHICAL LOCATION: Australia SETTING: Outpatients | |
| Participants | DIAGNOSIS: Functional faecal incontinence ELIGIBLE: 181 ENROLLED: 169 COMPLETED: 162 AGE: range 3‐16 GENDER: 124 boys 45 girls | |
| Interventions | 1) Behaviour modifications (positive reinforcement, toilet training, dietary advice) 2) Multimodal therapy (behaviour modifications + use of laxatives ‐ 'Agarol', senna granules and/or bisacodyl tablets) | |
| Outcomes | Time to remission from encopresis, reduction in stool retention | |
| Notes | Compliance with toileting was similar in the two groups (11 children in the behaviour modifications group and 13 in the behaviour modifications + laxatives group were poorly compliant. 17 children in the behaviour modifications group and 17 in the behaviour modifications + laxatives group had nocturnal enuresis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Low risk | Stratified blocked schedule by a person not connected with the study. Opaque numbered sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three children in the multimodel therapy group and 4 in the behaviour modification group. Carried out an intention to treat analysis |
Nolan 1998.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Stratified blocked schedule by a person not connected with the study. Opaque numbered sealed envelopes stored sequentially BLINDING PROCEDURES: Outcome assessor blind SAMPLE CALCULATION: Yes DURATION: 4 weeks FOLLOW‐UP: 6 months ITT: Yes WITHDRAWALS/ DROPOUTS: One child in the biofeedback group and 2 children in the conventional treatment group were not available for EMG biofeedback evaluation at the 6‐month follow‐up GEOGRAPHICAL LOCATION: Australia SETTING: Outpatients, Encopresis and Gastroenterology Clinics | |
| Participants | DIAGNOSIS: Faecal incontinence associated with constipation and with pelvic floor dyssynergia ELIGIBLE: 68 ENROLLED: 29 COMPLETED: 29 AGE: Mean 8.8 years; range 4.8 ‐ 14.9 GENDER: 24 boys and 5 girls | |
| Interventions | 1) EMG biofeedback training 2) Conventional treatment (laxatives) | |
| Outcomes | Anorectal manometric assessment, Child Behavior Checklist standardised score | |
| Notes | Children were stratified on the basis of whether they were soiling or were laxative‐dependent remission. More children with primary encopresis in the biofeedback group. 13 children had nocturnal and three children had diurnal enuresis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Low risk | Stratified blocked schedule by a person not connected with the study. Opaque numbered sealed envelopes stored sequentially |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One child in the biofeedback group and 2 children in the conventional treatment group were not available for EMG biofeedback evaluation at the 6‐month follow‐up. Intention to treat analysis |
Pieczarkowski 2005.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Not stated BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: Six months FOLLOW‐UP: 1, 3 and 6 months WITHDRAWALS/ DROPOUTS: Two withdrawals ITT: No GEOGRAPHICAL LOCATION: Poland SETTINGS: Dept of Pediatrics and Pediatric Psychiatry | |
| Participants | DIAGNOSIS: Chronically constipated children with anal‐rectal dyssynergy; recto‐sigmoid transit time in Hinton's test with radio‐paque markers at least 28.8 h, paradoxical external anal sphincter contraction during defecation ELIGIBLE: Not stated ENROLLED: 24 COMPLETED: 22 AGE: Mean 8.1 years; SD 2.8 GENDER: 16 boys and 6 girls | |
| Interventions | 1) Conventional treatment (toilet training, high fibre diet, lactulose) 2) Conventional traditional treatment plus biofeedback | |
| Outcomes | Manometric parameters, number of stools | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two withdrawals |
Ritterband 2003.
| Methods | DESIGN: Randomised controlled trial (children were matched on the basis of soiling frequency before randomisation) ALLOCATION CONCEALMENT: Not stated BLINDING PROCEDURES: Not clearly stated. Participants not blinded. SAMPLE CALCULATION: No DURATION: 3 weeks FOLLOW‐UP: Post‐treatment assessment at 3 weeks, no further follow up ITT: Yes (no reported dropouts) WITHDRAWALS/ DROPOUTS: None reported GEOGRAPHICAL LOCATION: USA SETTING: Outpatients | |
| Participants | DIAGNOSIS: Faecal incontinence and constipation DURATION OF SYMPTOMS: Mean length of laxatives regimen = 20 months ELIGIBLE: Unknown ENROLLED: 24 COMPLETED: 24 AGE: Mean 8.5 years GENDER: 19 boys and 5 girls | |
| Interventions | 1) Internet version of enhanced toilet training
2) No‐Internet intervention All children in the study were encouraged to visit their physicians regularly |
|
| Outcomes | Success rate, number of soiling episodes per week, number of bowel movements in the toilet, self‐initiated trips to the toilet, parent‐initiated trips to the toilet | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clearly stated. Participants not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reported |
Sunic‐Omejc 2002.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Not stated BLINDING PROCEDURES: Not stated. Participants not blinded. SAMPLE CALCULATION: No DURATION: 12 weeks FOLLOW‐UP: 12‐week assessment and not further follow‐up. ITT: Yes WITHDRAWALS/ DROPOUTS: None reported. GEOGRAPHICAL LOCATION: Croatia SETTING: Outpatient clinic | |
| Participants | DIAGNOSIS: Faecal incontinence associated with chronic constipation MEAN DURATION OF SYMPTOMS: 33 months ELIGIBLE: Unknown ENROLLED: 49 COMPLETED: 49 AGE: Mean 7.8 years GENDER: 27 boys and 22 girls | |
| Interventions | 1) Conventional treatment (toilet training, dietary advice, use of laxatives) 2) Conventional treatment plus biofeedback | |
| Outcomes | Success rate, anorectal manometric assessment. | |
| Notes | Children with Hirschsprung's disease, spina bifida, hypothyroidism, metabolic or renal disorders, mental retardation, and those taking drugs that influence gastrointestinal function (expect laxatives) were excluded. Treatment was considered successful if a frequency of three or more stools per week and less than two episodes of soiling per month were achieved without laxatives. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. Participants not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reprted |
Taitz 1986.
| Methods | DESIGN: Quasi‐randomised clinical trial ALLOCATION CONCEALMENT: Allocation on alternate basis BLINDING PROCEDURES: Participants and outcome assessors not blind SAMPLE CALCULATION: No DURATION: 12 months FOLLOW‐UP: No ITT: Yes WITHDRAWALS/ DROPOUTS: 17 children were non‐compliant GEOGRAPHICAL LOCATION: UK SETTING: Outpatients, Paediatric Outpatient Clinic | |
| Participants | DIAGNOSIS: Faecal incontinence with or without constipation ELIGIBLE: Unknown ENROLLED: 47 COMPLETED: Not clearly reported AGE: Mean 6 years; range 2‐14 GENDER: 26 boys and 21 girls | |
| Interventions | 1) Behaviour modifications (toilet training, dietary advices, use of star chart) 2) Behaviour modifications + psychotherapy Laxatives and enemas were used in cases of severe constipation and faecal impaction | |
| Outcomes | Number of children cured, improved or unchanged, number of bowel movements, soiling episodes, use of laxatives | |
| Notes | Results were analysed according to compliance with treatment and children social class | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised clinical trial |
| Allocation concealment (selection bias) | High risk | Allocation on alternate basis |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants and outcome assessors not blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17 children were non‐compliant |
van der Plas 1996.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Not stated BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: Yes DURATION: 6 weeks FOLLOW‐UP: 6,12, 18 months ITT: Yes WITHDRAWALS/ DROPOUTS: Three children in the conventional treatment group and 2 children in the biofeedback group GEOGRAPHICAL LOCATION: Netherlands SETTING: Outpatients | |
| Participants | DIAGNOSIS: Faecal incontinence associated with chronic constipation ELIGIBLE: 220 ENROLLED: 192 COMPLETED: 187 AGE: Median 8 years; range 5‐16 GENDER: 126 boys and 66 girls | |
| Interventions | 1) Conventional treatment (toilet training, dietary advice, use of laxatives) 2) Conventional treatment plus biofeedback | |
| Outcomes | Success rate, anorectal manometric assessment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three children in the conventional treatment group and 2 children in the biofeedback group. Intention to treat |
van der Plas 1996a.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Sealed envelopes using a schedule generated by a person not involved with the treatment BLINDING PROCEDURES: Not stated SAMPLE CALCULATION: No DURATION: 6 weeks FOLLOW‐UP: 6,12,18 months ITT: Yes WITHDRAWALS/ DROPOUTS: One child in the biofeedback group and one child in the conventional treatment group did not complete the intervention programme. Another two children in the biofeedback group withdrew at six months. One child in the conventional treatment group was lost to follow‐up at 18 months GEOGRAPHICAL LOCATION: Netherlands SETTING: Outpatients | |
| Participants | DIAGNOSIS: Faecal incontinence in the absence of any other signs of constipation ELIGIBLE: 88 ENROLLED: 71 COMPLETED: 69 AGE: Median 9 years; range 5‐16 GENDER: proportion of boys/girls not reported | |
| Interventions | 1) Conventional treatment (toilet training, dietary advice, use of laxatives) 2) Conventional treatment plus biofeedback | |
| Outcomes | Success rate, anorectal manometric assessment, Child Behaviour Checklist scale | |
| Notes | At the end of the treatment period (6 weeks) biofeedback training was more successful than use of laxatives but no additional value of biofeedback was found with further follow‐up. Results were presented by means of median and SD values (not suitable for statistical analyses in RevMan) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes using a schedule generated by a person not involved with the treatment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One child in the biofeedback group and one child in the conventional treatment group did not complete the intervention programme. Another two children in the biofeedback group withdrew at six months. One child in the conventional treatment group was lost to follow‐up at 18 months |
van Dijk 2008.
| Methods | DESIGN: Randomised controlled parallel group trial DURATION: Treatment 22 weeks FOLLOW‐UP: At end of treatment (22 weeks) and six months after 22 weeks GEOGRAPHICAL LOCATION: Netherlands SETTING: Children referred to gastrointestinal outpatient clinic | |
| Participants | DIAGNOSIS: Patients to meet two of four criteria: Defacation frequency less than three times per week, fecal incontinence more than two times per week, passage of large amounts of stool at least once every 7 to 30 days, palpable abdominal or rectal fecal mass ELIGIBLE: 146 ENROLLED: 134 COMPLETED: 114 AGE: 4 to 18 years GENDER: 76 boys, 58 girls | |
| Interventions | 1) Conventional treatment (laxatives, bowel diary discussed, patients and their parents received education 2) Behaviour modifications (teaching parents behavioural procedures and behavioural play therapy with child in presence of his/her parents, laxative therapy) | |
| Outcomes | Primary outcome defecation frequency per week, fecal incontinence per week and successful treatment. Secondary outcomes stool with holding behaviour and behaviour problems | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer based system to generate random group assignment |
| Allocation concealment (selection bias) | Low risk | Research Assistant called randomisation centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Research assistant revealed allocation to parents immediately. Unclear who assessed primary outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 56 of 67 in the Behaviour modifications group and 58 of 67 in conventional treatment group had assessment at 6 months. To address this imputation regression modelling was used in the analysis. |
van Ginkel 2000.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Not described BLINDING PROCEDURES:Not blind SAMPLE CALCULATION: No DURATION: 7 weeks FOLLOW‐UP: 6, 12, 26 weeks and at 12 months ITT: No WITHDRAWALS/ DROPOUTS: One child after 3 weeks discontinued laxative treatment and 8 children after 6 weeks were not motivated to continue laxatives. GEOGRAPHICAL LOCATION: Netherlands SETTING: Outpatients, Division of Pediatric Gastroenterology and Nutrition | |
| Participants | DIAGNOSIS: Non‐retentive faecal incontinence. Children with constipation were excluded ELIGIBLE: Unknown ENROLLED: 49 COMPLETED: 48 AGE: Median 8.0 years; range 5 ‐ 17 GENDER: 41 boys and 7 girls | |
| Interventions | 1) Biofeedback training alone 2) Oral laxatives and biofeedback training | |
| Outcomes | Success rate, total number of encopresis episodes, anorectal manometric assessment | |
| Notes | 22 children had diurnal enuresis (12 in the BF group and 10 in the BF + LAX group) and 19 children had nocturnal enuresis (11 in the BF group and 8 in the BF + LAX group). One child discontinued laxatives after 3 weeks because an increase in the number of encopresis episodes. At he 6‐week and 12‐week follow‐up assessments, 8 and 14 children respectively were not motivated to continue laxative treatment due to lack of results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | not blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One child after 3 weeks discontinued laxative treatment and 8 children after 6 weeks were not motivated to continue laxatives. |
van Ginkel 2001.
| Methods | DESIGN: Parallel randomised controlled trial ALLOCATION CONCEALMENT: Electronic randomisation BLINDING PROCEDURES:Not blind SAMPLE CALCULATION: Yes DURATION: 6 weeks FOLLOW‐UP: 6, 12, 26 weeks, 12 and 24 months ITT: Yes WITHDRAWALS/ DROPOUTS: 4 children in the conventional treatment group and 6 in the conventional treatment + manometry group GEOGRAPHICAL LOCATION: Netherlands SETTING: Outpatients, Pediatric Gastroenterology Practice | |
| Participants | DIAGNOSIS: Faecal incontinence and constipation. ELIGIBLE: 212 ENROLLED: 212 COMPLETED: 202 at 6 months. AGE: Median 7.5 years; range 5 ‐ 17 GENDER: 137 boys and 65 girls | |
| Interventions | 1) Conventional treatment (toilet training, dietary advice, use of oral laxatives) 2) Conventional treatment + 2 sessions of manometry | |
| Outcomes | Success rate, defecation frequency, encopresis frequency, use of laxatives. | |
| Notes | Children with Hirschsprung's disease, spina bifida, hypothyroidism, metabolic or renal disorders, mental retardation, and those taking drugs that influence gastrointestinal function (expect laxatives) were excluded. More boys included in the conventional treatment + manometry group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronic randomisation |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 children in the conventional treatment group and 6 in the conventional treatment + manometry group |
Wald 1987.
| Methods | DESIGN: Randomised controlled trial ALLOCATION CONCEALMENT: Not stated BLINDING PROCEDURES: Outcome assessor blind SAMPLE CALCULATION: No DURATION: 12 weeks FOLLOW‐UP: 3,6,12 months ITT: No WITHDRAWALS/ DROPOUTS: In the biofeedback group one child withdrew at three months, 2 children at six months and 3 further children at twelve months. In the conventional treatment group one child was lost at the three‐month follow‐up, one at six months and two at twelve months GEOGRAPHICAL LOCATION: USA SETTING: Outpatients | |
| Participants | DIAGNOSIS: Faecal incontinence and constipation ELIGIBLE: Unknown ENROLLED: 50 COMPLETED: 48 AGE: Mean 8.4 years; range 6‐15 GENDER: 40 boys and 10 girls | |
| Interventions | 1) Conventional treatment (toilet training, use of mineral oil as laxative) 2) Conventional treatment + biofeedback | |
| Outcomes | Number of children cured or improved, number of bowel movements, soiling episodes, anorectal manometric assessment | |
| Notes | All dropouts were designated as treatment failures for each assessment point. Results presented by means of graphs. For statistical analyses raw data were approximated from graphical presentation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not stated |
| Allocation concealment (selection bias) | Unclear risk | not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the biofeedback group one child withdrew at three months, 2 children at six months and 3 further children at twelve months. In the conventional treatment group one child was lost at the three‐month follow‐up, one at six months and two at twelve months |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Borowitz 1999 | Not a RCT |
| Byrne 2002 | Participants: adults |
| Chiarioni 2006 | Participants: adults |
| Chiarioni 2010 | Participants: adults |
| Dehli 2009 | Participants: adults |
| Heymen 1999 | Participants: adults |
| Heymen 2005 | Participants: adults |
| Hinninghofen 2003 | Participants: women |
| Houts 1988 | Not a RCT. Multiple baseline across subjects design. Three children studied. |
| Jeon 2005 | Effects of abdominal massage in in stroke patients |
| Klijn 2003 | Participants: children with non‐neuropathic bladder sphincter dysfunction and urinary tract infection |
| Loening‐Baucke 1995 | Non randomised follow‐up of a previous study (Loening‐Baucke 1990) |
| Marques 2008 | Comparison of techniques of biofeedback on children. No patient treatment outcomes reported. |
| Marshall 1997 | Intervention: electrical stimulation versus sham procedure |
| Miner 1990 | Participants: adults |
| Mooren 1996 | Intervention: evaluation of feeding patterns in children with chronic constipation and their influence on colonic transit time |
| Norton 2002 | Participants: adults |
| Norton 2003 | Participants: adults |
| Olness 1980b | Not a RCT |
| Pager 2002 | Participants: adults |
| Pages 2003 | Participants: adults |
| Peterson 1986 | Not a RCT |
| Rao 2005a | Participants: adults |
| Solomon 2000a | Participants: adults |
| Solomon 2000b | Participants: adults |
| Solomon 2003 | Participants: adults |
| Sprague‐McRae 1993 | Intervention: oral laxatives versus rectal cathartics |
| Stark 1990 | Not a RCT |
| Stark 1997 | Not a RCT |
| Surh 1998 | Participants: adults |
| van Kuyk 2001a | No indication of randomisation |
| van Kuyk 2001b | No indication of randomisation |
| Vasconcelos 2006 | Participants: children with urinary incontinence |
| Whitehead 1986 | Not a RCT |
Characteristics of studies awaiting assessment [ordered by study ID]
Rao 2005b.
| Methods | Randomized controlled trial |
| Participants | Age not clear |
| Interventions | Biofeedback therapy |
| Outcomes | Dyssynergia pattern, defecation index, colonic transit, bowel satisfaction |
| Notes |
Contributions of authors
Original Review One review author (M. Brazzelli) wrote the initial protocol. Two review authors (M. Brazelli, P Griffiths) independently selected relevant trials from the literature search. One reviewer (M. Brazzelli) assessed the methodological quality of eligible trials and extracted data. All review authors contributed to the interpretation of analyses and writing of the final version of the review.
First Update One reviewer (M. Brazzelli) selected studies from the updated search strategy and assessed study quality. Five new studies were included. Both reviewers revised the text of the review.
Second update
Two review authors (D. Tappin, J. Cody) selected studies from the updated search and assessed study quality. Three new studies were added. All reviewers revised the text of the review.
Sources of support
Internal sources
Chief Scientist Office, Scottish Executive Health Department, UK.
External sources
National Health Service R&D Programme for People with Physical and Complex Disabilities, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Berg 1983 {published data only}
- Berg I, Forsythe I, Holt P, Watts J. A controlled trial of 'Senokot' in faecal soiling treated by behavioural methods. Journal of Child Psychology Psychiatry & Allied Disciplines 1983;24(4):543‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borowitz 2002 {published data only}
- Borowitz SM, Cox DJ, Sutphen JL, Kovatchev B. Treatment of childhood encopresis: a randomized trial comparing three treatment protocols. J Pediatr Gastroenterol Nutr 2002;34(4):378‐84. [DOI] [PubMed] [Google Scholar]
- Cox DJ, Sutphen J, Borowitz S, Kovatchev B, Ling W. Contribution of behavior therapy and biofeedback to laxative therapy in the treatment of pediatric encopresis. Annals of Behavioral Medicine 1998;20(2):70‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cox DJ, Sutphen J, Ling W, Quillian W, Borowitz S. Additive benefits of laxative, toilet training, and biofeedback therapies in the treatment of pediatric encopresis. Journal of Pediatric Psychology 1996;21(5):659‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Croffie 2005 {published data only}
- Croffie JM, Ammar MS, Pfefferkorn MD, Horn D, Klipsch A, Fitzgerald JF, Gupta SK, Molleston JP, Corkins MR. Assessment of the effectiveness of biofeedback in children with dyssynergic defecation and recalcitrant constipation/encopresis: does home biofeedback improve long‐term outcomes. Clinical Pediatrics 2005;44(1):63‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davila 1992 {published data only}
- Davila E, Rodriguez GG, Adrianza A, Pereira Y, Toro J, Gonzalez I, et al. [The usefulness of biofeedback in children with encopresis. A preliminary report] [Spanish] [Utilidad del biofeedback en ninos con encopresis. Comunication preliminar]. GEN Revista de la Sociedad Venezolana de Gastroenterologia 1992;46(4):297‐301. [MEDLINE: ] [PubMed] [Google Scholar]
Demirogullari 2007 {published data only}
- Demirogullari B, Bagbanci B, Ozdemir DF, Iseri E, Gokce E, Kockar AI, et al. [The role of bowel habits and anorectal region functions on determining behaviour vs drug therapy on pediatric patients with fecal incontinence: A prospective clinical trial]. Cocuk Cerrahisi Dergisi 2007;21(1):15‐9. [SR‐INCONT28038] [Google Scholar]
Latimer 1984 {published data only}
- Latimer PR, Campbell D, Kasperski J. A components analysis of biofeedback in the treatment of fecal incontinence. Biofeedback and Self Regulation 1984;9(3):311‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Loening‐Baucke 1988 {published data only}
- Loening‐Baucke V, Desch L, Wolraich M. Biofeedback training for patients with myelomeningocele and fecal incontinence. Developmental Medicine Child Neurology 1988;30(6):781‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Loening‐Baucke 1990 {published data only}
- Loening‐Baucke V. Modulation of abnormal defecation dynamics by biofeedback treatment in chronically constipated children with encopresis. Journal of Pediatrics 1990;116(2):214‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mellon 1996 {published data only}
- Mellon MW, Houts AC, Lazar LF. Treatment of retentive encopresis with diet modification and scheduled toileting vs. mineral oil and rewards for toileting: a clinical decision. Ambulatory Child Health 1996;1:214‐22. [Google Scholar]
Nolan 1991 {published data only}
- Nolan T, Debelle G, Oberklaid F, Coffey C. Randomised trial of laxatives in treatment of childhood encopresis. Lancet 1991;338(8766):523‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nolan 1998 {published data only}
- Nolan T, Catto‐Smith T, Coffey C, Wells J. Randomised controlled trial of biofeedback training in persistent encopresis with anismus. Archives of Disease in Childhood 1998;79(2):131‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pieczarkowski 2005 {published data only}
- Pieczarkowski S, Fyderek K. [Usefulness of biofeedback treatment in children with chronic functional constipation]. [Polish]. Przeglad Lekarski 2005;62(9):851‐4. [SR‐INCONT21810] [PubMed] [Google Scholar]
Ritterband 2003 {published data only}
- Ritterband LM, Cox DJ, Walker LS, Kovatchev B, McKnight L, Patel K, Borowitz S, Sutphen J. An Internet intervention as adjunctive therapy for pediatric encopresis. Journal of Consulting & Clinical Psychology 2003;71(5):910‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sunic‐Omejc 2002 {published data only}
- Sunic‐Omejc M, Mihanovic M, Bilic A, Jurcic D, Restek‐Petrovic B, Maric N, Dujsin M, Bilic A. Efficiency of biofeedback therapy for chronic constipation in children. Collegium Antropologicum 2002;26 Suppl:93‐101. [MEDLINE: ] [PubMed] [Google Scholar]
Taitz 1986 {published data only}
- Taitz LS, Wales JK, Urwin OM, Molnar D. Factors associated with outcome in management of defecation disorders. Archives of Disease in Childhood 1986;61(5):472‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Plas 1996 {published data only}
- Plas RN, Benninga MA, Buller HA, Bossuyt PM, Akkermans LMA, Redekop WK. Biofeedback training in treatment of childhood constipation: a randomised controlled study. Lancet 1996;348(9030):776‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van der Plas 1996a {published data only}
- Plas RN, Benninga MA, Redekop WK, Taminiau JA, Buller HA. [Encopresis; het effect van behandeling en de relatie met psychosociale problemen (Abstract)]. Tijdschrift voor Kindergeneeskunde. 1996 (Suppl):72‐3.
- Plas RN, Benninga MA, Redekop WK, Taminiau JA, Buller HA. Randomised trial of biofeedback training for encopresis. Archives of Disease in Childhood 1996;75(5):367‐74. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dijk 2008 {published data only}
- Dijk M, Bongers ME, Vries GJ, Grootenhuis MA, Last BF, Benninga MA. Behavioral therapy for childhood constipation: a randomized, controlled trial. Pediatrics 2008;121(5):e1334‐41. [SR‐INCONT27089] [DOI] [PubMed] [Google Scholar]
van Ginkel 2000 {published data only}
- Ginkel R, Benninga MA, Blommaart PJ, Plas RN, Boeckxstaens GE, Buller HA, et al. Lack of benefit of laxatives as adjunctive therapy for functional nonretentive fecal soiling in children. Journal of Pediatrics 2000;137(6):808‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Ginkel 2001 {published data only}
- Ginkel R, Buller HA, Boeckxstaens GE, Plas RN, Taminiau JA, Benninga MA. The effect of anorectal manometry on the outcome of treatment in severe childhood constipation: a randomized, controlled trial. Pediatrics 2001;108(1):E9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wald 1987 {published data only}
- Wald A, Chandra R, Gabel Stewart, Chiponis D. Evaluation of biofeedback in childhood encopresis. Journal of Pediatric Gastroenterology and Nutrition 1987;6(4):554‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Borowitz 1999 {published data only}
- Borowitz SM, Cox DJ, Sutphen JL. Differences in toileting habits between children with chronic encopresis, asymptomatic siblings, and asymptomatic nonsiblings. Journal of Developmental & Behavioral Pediatrics 1999;20(3):145‐9. [DOI] [PubMed] [Google Scholar]
Byrne 2002 {published data only}
- Byrne CM, Pager CK, Rex J, Roberts R, Solomon MJ. Assessment of quality of life in the treatment of patients with neuropathic fecal incontinence. Diseases of the Colon & Rectum 2002;45(11):1431‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chiarioni 2006 {published data only}
- Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130(3):657‐64. [SR‐INCONT21687] [DOI] [PubMed] [Google Scholar]
Chiarioni 2010 {published data only}
- Chiarioni G, Nardo A, Vantini I, Romito A, Whitehead WE. Biofeedback is superior to electrogalvanic stimulation and massage for treatment of levator ani syndrome. Gastroenterology 2010;138(4):1321‐9. [SR‐INCONT39447] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dehli 2009 {published data only}
- Dehli T, Vonen B, Stordahl A, Jacobsen A, Lindsetmo RO, Mevik K. A randomized, controlled, clinical trial of biofeedback and anal injections as first treatment of fecal incontinence. ControlledTrials.com (www.controlled‐trials.com) [accessed 2 February 2009] 2009. [SR‐INCONT29646] [Google Scholar]
Heymen 1999 {published data only}
- Heymen S, Wexner SD, Vickers D, Nogueras JJ, Weiss EG, Pikarsky AJ. Prospective, randomized trial comparing four biofeedback techniques for patients with constipation. Diseases of the Colon & Rectum 1999;42(11):1388‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heymen 2005 {published data only}
- Heymen S, Scarlett Y, Jones K, Drossman D, Ringel Y, Whitehead WE. Randomized controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssenergia‐type constipation (Abstract number S1838). Gastroenterology 2005;128(Suppl 2):A266. [SR‐INCONT23014] [DOI] [PubMed] [Google Scholar]
Hinninghofen 2003 {published data only}
- Hinninghofen H, Wietek B, Jehle EC, Enck P. [Quality of life in patients with incontinence of faeces: Bio‐feedback training versus surgical measures] (Abstract number P103). Zeitschrift fur Gastroenterologie 2003;41(8):783. [SR‐INCONT28050] [Google Scholar]
Houts 1988 {published data only}
- Houts AC, Mellon MW, Whelan JP. Use of dietary fiber and stimulus control to treat retentive encopresis: a multiple baseline investigation. Journal of Pediatric Psychology 1988;13(3):435‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jeon 2005 {published data only}
- Jeon SY, Jung HM. [The effects of abdominal meridian massage on constipation among CVA patients]. [Korean]. Daehan Ganho Haghoeji 2005;35(1):135‐42. [SR‐INCONT21708] [DOI] [PubMed] [Google Scholar]
Klijn 2003 {published data only}
- Klijn AJ, Winkler‐Seinstra PL, Vijverberg MA, Uiterwaal CS, Jong TP. Results of behavioral therapy combined with homeflow biofeedback for non‐neuropathic bladder sphincter dysfunction, a prospective randomized study in 143 patients (Abstract). Proceedings of the International Continence Society (ICS), 33rd Annual Meeting, Florence, 5‐9 Oct. 2003:176‐7. [MEDLINE: ]
Loening‐Baucke 1995 {published data only}
- Loening‐Baucke V. Biofeedback treatment for chronic constipation and encopresis in childhood: long‐term outcome. Pediatrics 1995;96(1):105‐10. [MEDLINE: ] [PubMed] [Google Scholar]
Marques 2008 {published data only}
- Marques GM, Martins JL, Nobre VD. Comparison between perfusion and balloon techniques for performing anorectal manometry in children with intestinal constipation. Acta Cirurgica Brasileira 2008;23(5):405‐11. [SR‐INCONT28632] [DOI] [PubMed] [Google Scholar]
Marshall 1997 {published data only}
- Marshall DF, Boston VE. Altered bladder and bowel function following cutaneous electrical field stimulation in children with spina bifida. Interim results of a randomized double‐blind placebo‐controlled trial. European Journal of Pediatric Surgery 1997;7, Suppl 1:41‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miner 1990 {published data only}
- Miner PB, Donnelly TC, Read NW. Investigation of mode of action of biofeedback in treatment of fecal incontinence. Digestive Diseases & Sciences 1990;35(10):1291‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mooren 1996 {published data only}
- Mooren GC, Plas RN, Bossuyt PM, Taminiau JA, Buller HA. [The relationship between intake of dietary fiber and chronic constipation in children]. [Dutch]. Nederlands Tijdschrift voor Geneeskunde 12‐10‐1996;140(41):2036‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Norton 2002 {published data only}
- Norton C, Chelvanayagam S, Kamm M. Randomised controlled trial of biofeedback for faecal incontinence (Abstract). Neurourology and Urodynamics 2002;21(4):295‐6. [MEDLINE: ] [Google Scholar]
Norton 2003 {published data only}
- Norton C, Chelvanayagam S, Wilson‐Barnett J, Redfern S, Kamm MA. Randomized controlled trial of biofeedback for fecal incontinence.[see comment]. Gastroenterology 2003;125(5):1320‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olness 1980b {published data only}
- Olness K, McParland FA, Piper J. Biofeedback: A new modality in the management of children with fecal soiling. Journal of Pediatrics 1980;96(3):505‐9. [DOI] [PubMed] [Google Scholar]
Pager 2002 {published data only}
- Pager CK, Solomon MJ, Rex J, Roberts RA. Long‐term outcomes of pelvic floor exercise and biofeedback treatment for patients with fecal incontinence. Diseases of the Colon & Rectum 2002;45(8):997‐1003. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pages 2003 {published data only}
- Pages I‐H, Lemke J, Grundel K. Comparison of biofeedback and electrostimulation for treatment of fecal incontinence. [German]. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin 2003;13(6):325‐9. [SR‐INCONT25376] [Google Scholar]
Peterson 1986 {unpublished data only}
- Peterson JK. Treatment of three types of encopresis with contingency management and diet and stimulus control. PHD Thesis. Memphis, USA: Memphis State University, 1986. [Google Scholar]
Rao 2005a {published data only}
- Rao SS, Kinkade KJ, Schulze KS, Nygaard II, Brown K, Stumbo PI, et al. Biofeedback therapy (bt) for dyssynergic constipation ‐ randomized controlled trial (Abstract number S1851). Gastroenterology 2005;128(Suppl 2):A269. [SR‐INCONT23015] [Google Scholar]
Solomon 2000a {published data only}
- Solomon M, Rex J, Manning J, Eyers A, Stewart P, Roberts R. Randomised controlled trial of biofeedback (manometry vs transanal US) compared with pelvic floor exercises for faecal incontinence (Abstract). Australian & New Zealand Journal of Surgery 2000;70(Suppl May):A56. [MEDLINE: ] [Google Scholar]
Solomon 2000b {published data only}
- Solomon MJ, Rex J, Eyers AA, Stewart P, Roberts R. Biofeedback for fecal incontinence using transanal ultrasonography: novel approach. Diseases of the Colon & Rectum 2000;43(6):788‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Solomon 2003 {published data only}
- Solomon MJ, Pager CK, Rex J, Roberts R, Manning J. Randomized, controlled trial of biofeedback with anal manometry, transanal ultrasound, or pelvic floor retraining with digital guidance alone in the treatment of mild to moderate fecal incontinence. Diseases of the Colon & Rectum 2003;46(6):703‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sprague‐McRae 1993 {published data only}
- Sprague‐McRae JM, Lamb W, Homer D. Encopresis: a study of treatment alternatives and historical and behavioral characteristics. Nurse Practitioner 1993;18(10):52‐3, 56‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stark 1990 {published data only}
- Stark LJ, Owens‐Stively J, Spirito A, Lewis A, Guevremont D. Group behavioral treatment of retentive encopresis. Journal of Pediatric Psychology 1990;15(5):659‐71. [DOI] [PubMed] [Google Scholar]
Stark 1997 {published data only}
- Stark LJ, Opipari LC, Donaldson DL, Danovsky MB, Rasile DA, DelSanto AF. Evaluation of a standard protocol for retentive encopresis: a replication. Journal of Pediatric Psychology 1997;22(5):619‐33. [DOI] [PubMed] [Google Scholar]
Surh 1998 {published data only}
- Surh S, Kienle P, Stern J, Herfarth C. [Passive electrostimulation therapy of the anal sphincter is inferior to active biofeedback training]. [German]. Langenbecks Archiv fur Chirurgie ‐ Supplement ‐ Kongressband 1998;115:976‐8. [PubMed] [Google Scholar]
van Kuyk 2001a {published data only}
- Kuyk EM, Wissink‐Essink M, Brugman‐Boezeman AT, Oerlemans HM, Nijhuis‐van der Sanden MW, Severijnen RS, Festen C, Bleijenberg G. Multidisciplinary behavioral treatment of defecation problems: a controlled study in children with anorectal malformations. Journal of Pediatric Surgery 2001;36(9):1350‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Kuyk 2001b {published data only}
- Kuyk EM, Brugman‐Boezeman AT, Wissink‐Essink M, Oerlemans HM, Severijnen RS, Bleijenberg G. Defecation problems in children with Hirschsprung's disease: a prospective controlled study of a multidisciplinary behavioural treatment. Acta Paediatrica 2001;90(10):1153‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vasconcelos 2006 {published data only}
- Vasconcelos M, Lima E, Caiafa L, Noronha A, Cangussu R, Gomes S, et al. Voiding dysfunction in children. Pelvic‐floor exercises or biofeedback therapy: a randomized study. Pediatric Nephrology 2006;21(12):1858‐64. [SR‐INCONT22131] [DOI] [PubMed] [Google Scholar]
Whitehead 1986 {published data only}
- Whitehead WE, Parker L, Bosmajian L, Morrill‐Corbin ED, Middaugh S, Garwood M, Cataldo MF, Freeman J. Treatment of fecal incontinence in children with spina bifida: comparison of biofeedback and behavior modification. Archives of Physical Medicine & Rehabilitation 1986;67:218‐24. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Rao 2005b {published data only}
- Rao SSC, Kinkade KJ, Miller MJ, Brown K, Stumbo PE, Zimmerman BM, et al. Randomized controlled trial of long term outcome of biofeedback therapy (BT) for dyssynergic defecation (Abstract number 386). American Journal of Gastroenterology 2005;100(s9):S150. [SR‐INCONT23006] [Google Scholar]
Additional references
Achenbach 1987
- Achenbach TM, Verhulst FC, Baron GD, Akkerhuis GW. Epidemiological comparisons of American and Dutch children: I. Behavioral/emotional problems and competencies reported by parents for ages 4 to 16. Journal of the American Academy of Child & Adolescent Psychiatry 1987;26(3):317‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baker 1999
- Baker SS, Liptak GS, Colletti RB, Croffie JM, Lorenzo C, Ector W. Constipation in infants and children: evaluation and treatment. A medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 1999;29(5):612‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bellman 1966
- Bellman M. Studies on encopresis. Acta Paediatrica Scandinavica 1966;170 Suppl:1‐151. [MEDLINE: ] [PubMed] [Google Scholar]
Benninga 1996
- Benninga MA, Buller HA, Tytgat GN, Akkermans LM, Bossuyt PM, Taminiau JA. Colonic transit time in constipated children: does pediatric slow‐transit constipation exist?. Journal of Pediatric Gastroenterology Nutrition 1996;23(3):241‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clayden 1996
- Clayden G. A guide for good paediatric practice: childhood constipation. Ambulatory Child Health 1996;1:250‐5. [Google Scholar]
Coulter 2002
- Coulter ID, Favreau JT, Hardy ML, Morton SC, Roth EA, Shekelle P. Biofeedback interventions for gastrointestinal conditions: a systematic review. Alternative Therapies 2002;8(3):76‐83. [PubMed] [Google Scholar]
DSM‐IV 1994
- American Psychiatry Association. Diagnostic and statistical manual of mental disorders: DSM‐IV. 4th Edition. Washington DC: American Psychiatry Association, 1994:106‐7. [Google Scholar]
Fielding 1988
- Fielding DM, Doleys DM. Elimination problems: enuresis & encopresis. In: Mash EJ, Terdahl LG editor(s). Behavioural assessment of childhood disorders. 2nd Edition. New York: Guildford Press, 1988. [Google Scholar]
Fritz 1982
- Fritz GK, Armbrust J. Enuresis and encopresis. Psychiatric Clinics of North America 1982;5(2):283‐96. [MEDLINE: ] [PubMed] [Google Scholar]
Kelly 1996
- Kelly C. Chronic constipation and soiling in children: a review of the psychological and family literature. Child Psychology and Psychiatry Review 1996;1:59‐66. [Google Scholar]
Kohlenberg 1973
- Kohlenberg RJ. Operant conditioning of human anal sphincter pressure. Journal of Applied Behaviour Analysis 1973;6:201‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 1982
- Levine MD. Encopresis: its potentiation, evaluation, and alleviation. Pediatric Clinics of North America 1982;29(2):315‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levine 1983
- Levine MD, Carey WB, Crocker AC, Gross RT. Developmental‐behavioural paediatrics. Philadelphia: Saunders, 1983. [Google Scholar]
Olness 1980a
- Olness K, McParland FA, Piper J. Biofeedback: a new modality in the management of children with fecal soiling. Journal of Pediatrics 1980;96((3 Pt 1)):505‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PACCT Group 2005
- Benninga M, Candy DCA, Catto‐Smith AG, Clayden G, Loening‐Baucke V, Lorenzo C, Nurko S. The Paris Consensus on Childhood Constipation Terminology (PACCT) Group. Journal Of Pediatric Gastroenterology and Nutrition 2005;40:273‐5. [DOI] [PubMed] [Google Scholar]
Price 2001
- Price KJ, Elliott TM. Stimulant laxatives for constipation and soiling in children. Cochrane Database of Systematic Reviews 2001, Issue 3. [Art. No.: CD002040. DOI: 10.1002/14651858.CD002040] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rasquin‐Weber 1999
- Rasquin‐Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, Staiano A. Childhood functional gastrointestinal disorders. Gut 1999;45 (Suppl II):1160‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weinstein 1977
- Weinstein MC, Stason WB. Foundations of cost‐effectiveness analysis for health and medical practices. New England Journal of Medicine 1977;296(13):716‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weissenberg 1926
- Weissenberg S. [Uber enkopresis]. Zeitschrift fur kinderheilkunde 1926;40:674‐7. [Google Scholar]


