Abstract
Coronavirus disease 2019 (COVID-19) is a highly infective disease caused by the severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2). Previous studies of the COVID-19 pneumonia outbreak were based on information from the general population. Limited data are available for hemodialysis patients with COVID-19 pneumonia. This report describes the clinical characteristics of COVID-19 in an in-center hemodialysis patient, as well as our experience in implementing steps to prevent the spread of COVID-19 pneumonia among in-center hemodialysis patients. The diagnosis, infection control, and treatment of COVID-19 in hemodialysis patients are discussed in this report, and we conclude with recommendations for how a dialysis facility can respond to COVID-19 based on our experiences.
Index Words: Coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2), hemodialysis, epidemic, lopinavir/ritonavir
Introduction
In late December 2019, an outbreak of a novel coronavirus disease (coronavirus disease 2019 [COVID-19]) was reported in Wuhan, a city in the Hubei Province of China. It rapidly spread, resulting in an epidemic throughout China, with sporadic cases reported globally, becoming a global health emergency.1 COVID-19 pneumonia has had a significant impact on people's health and social activity behavior and on economic development. The Chinese government reported that 78,195 cases of COVID-19 had been confirmed, 2,491 cases were suspected, and 2,718 patients had died as of February 26, 2020.2 The outbreak of COVID-19 led to the closure of Wuhan, workers were delayed to work, and schools were closed across China. Although the majority of cases are mild, a certain proportion of patients develop critical illness, with a documented case-fatality rate of 2.3% in China.1 Reports to date indicate that although patients of all ages are susceptible to the disease, individuals developing critical illness were older and had a greater number of comorbid conditions,3 suggesting that patients with comorbid conditions have high mortality rates and poor outcomes.
Kidney failure is a severe medical condition with a high prevalence of comorbid conditions, including diabetes and heart disease, disproportionately affecting older adults.4 According to the China National Data System,5 there were 579,381 hemodialysis patients in China in 2018, including 33,795 hemodialysis patients in Hubei Province. Because hemodialysis patients are older and have more underlying diseases, they likely are more susceptible to severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2) pneumonia than the general population. This report describes the clinical characteristics of COVID-19 pneumonia in a hemodialysis patient and reviews our management of this individual, as well as measures to reduce transmission to other hemodialysis patients and staff.
Case Report
A man in his 50s receiving maintenance hemodialysis presented to the emergency department with a 7-day history of a nonproductive cough. His medical history included kidney failure due to diabetes maintained on thrice-weekly in-center hemodialysis, hypertension, and chronic hepatitis B virus infection. Epidemiologic survey showed that he drove to Wuhan in mid-January 2020, visited his relatives, and drove back to Zhongshan City of Guangdong province in China 1 week later.
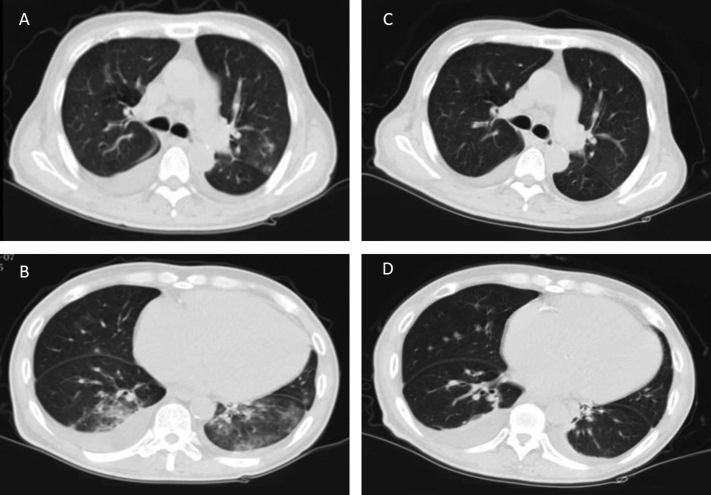
The patient was admitted to the hospital 2 weeks later with hypoxemia (blood oxygen saturation of 90% while breathing room air) without fever or myalgia. Physical examination results showed temperature of 36.9°C, blood pressure of 184/107 mm Hg, heart rate of 74 beats/min, and bilateral normal lung respiratory sounds. Laboratory tests showed white blood cell count of 3.38 ×109/L with 77.5% neutrophils, 15.7% lymphocytes, and 0% eosinophils. C-Reactive protein and procalcitonin levels were 40.1 mg/L and 0.73 ng/mL, respectively. Liver function and cardiac enzyme levels were within the normal range. Computed tomography of the chest showed bilateral multiple ground-glass opacities (Fig 1). The Chinese Guangdong Center for Disease Prevention and Control reported that SARS-CoV-2 nucleic acid tests were positive in throat swab samples 2 times. Based on epidemiologic characteristics and these findings, we diagnosed COVID-19 pneumonia.
Figure 1.
Coronavirus disease 2019 (COVID-19) pneumonia chest computed tomographic images. Computed tomographic scans of the chest show (A, B) bilateral multiple ground-glass opacities, prominent on the left, and (C, D) bilateral pleural effusion. (C, D) Repeated computed tomographic scans show decreases in the size of ground-glass opacities in the lungs.
As soon as COVID-19 pneumonia was diagnosed, the patient was transferred to a specialty hospital and received hemodialysis in a room with isolation facilities designated for patients with COVID-19 infection. Although all hemodialysis patients wear a face mask during dialysis, the other 42 patients who had a potential exposure to COVID-19 due to receiving dialysis in the same room were also transferred to the isolation ward of the specialty hospital with regular dialysis for 14 days. These hemodialysis patients were tested for SARS-CoV-2 nucleic acid at admission and at 14 days, remaining negative. Because of universal precautions taken by the medical staff, the staff tested for SARS-CoV-2 nucleic acid had negative results and were not isolated.
The patient was treated with oxygen support by nasal cannula, regular hemodialysis, antihypertensive medications, moxifloxacin (400 mg daily), and lopinavir/ritonavir (2 tablets twice daily) antiviral therapy. The potential side effects of lopinavir/ritonavir, such as nausea, diarrhea, and occasional dizziness, were not observed during treatment. After 8 days of treatment, the SARS-CoV-2 nucleic acid test results were negative in throat swab samples twice, cough had improved, and laboratory tests results and computed tomographic chest images had improved (Fig 1). The patient was discharged from the hospital.
Discussion
In this report, we describe the clinical course of a hemodialysis patient who developed COVID-19 infection. The clinical presentation of the patient was similar to patients with atypical symptom COVID-19 infection. Although the patient did not have many of the typical symptoms, including fever, sore throat, nasal congestion, malaise, headache, or myalgia, he had cough, dyspnea, and typical changes of bilateral multiple ground-glass opacities on computed tomographic images of the chest.
According to the Chinese criteria for diagnosis of pneumonia caused by novel coronavirus (trial version 6),6 suspected cases can be divided into 2 case definitions: the first requires epidemiologic history of likely exposure and any 2 clinical criteria (fever and/or respiratory symptoms, imaging characteristics of pneumonia, and normal or decreased total number of white blood cells or lymphocytes in early onset), and the second requires no clear epidemiologic history of exposure but presents with at least 3 of the clinical manifestations described. Confirmation requires a positive SARS-CoV-2 nucleic acid test result.
Because hemodialysis patients have disorders of B- and T-cell function,7,8 patients may have atypical presentations. Lymphopenia is common in patients with COVID-19 infection and might be a critical factor associated with disease severity and mortality in general patients.9 Huang and colleagues reported that the most common laboratory abnormalities were depressed total lymphocytes, prolonged prothrombin time, and elevated lactate dehydrogenase levels in COVID-19 pneumonia.10 Given low lymphocyte counts in hemodialysis patients chronically, lymphopenia likely is not helpful for identifying individuals with SARS-CoV-2 virus infection. Procalcitonin has similar limitations, with levels chronically elevated in hemodialysis patients even in the absence of severe acute illness.11 Given these limitations and the high prevalence of comorbid conditions, COVID-19 pneumonia diagnosis in hemodialysis patients is dependent on clinical epidemiology, radiographic findings, and viral nucleic acid testing.
Lopinavir/ritonavir is a combination antiviral medicine used to treat human immunodeficiency virus infection that is metabolized by the liver. The active ingredient, lopinavir, is a protease inhibitor.12 Because coronavirus replication requires the action of viral proteases, lopinavir/ritonavir may be effective by binding to the coronavirus protease to inhibit viral replication. Previous studies13,14 have shown that lopinavir/ritonavir can inhibit MERS-CoV (Middle East respiratory syndrome coronavirus) and SARS-CoV replication. Our results suggest that lopinavir/ritonavir may improve the course of COVID-19 pneumonia in patients receiving hemodialysis, although the lack of a control patient makes this conclusion somewhat subjective. Because of the liver clearance and higher protein-binding capacity of lopinavir/ritonavir, no dosing adjustments are necessary in the treatment of hemodialysis patients.15
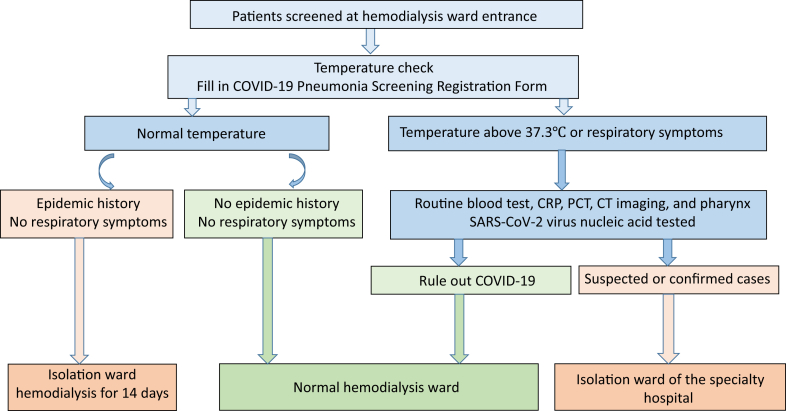
Given the likely immunosuppression and the high prevalence of comorbid conditions among dialysis patients, the hemodialysis population may be particularly vulnerable to COVID-19 pneumonia, making efforts to promptly identify individuals with SARS-CoV-2 virus infection and implement steps to limit the spread of the virus critical (Fig 2). This begins with strengthening prescreening triage of hemodialysis patients.5 Persons without face masks are not allowed to enter the hemodialysis ward. We also implemented a policy whereby it was mandated that the “COVID-19 Pneumonia Screening Registration Form” be completed (Box 1). All patients had their temperature checked at the entrance to the hemodialysis unit and were surveyed regarding any recent fever, cough, or exposure to epidemic areas or to individuals who have visited epidemic areas within the last 2 weeks. If available, particularly within epidemic areas, we suggest promptly assessing for SARS-CoV-2 virus nucleic acid with swabs, especially in those with respiratory symptoms. For nonsuspected patients with potential COVID-19 exposure or from epidemic areas within the last 2 weeks, we performed hemodialysis in an isolation area within the hemodialysis facility.
Figure 2.
Screening triage and processing in hemodialysis patients. These incorporated epidemiology and clinical symptoms and signs to identify suspected cases of coronavirus disease 2019 (COVID-19) infection. Abbreviations: CRP, C-reactive protein; CT, computed tomographic; PCT, procalcitonin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2 virus.
Box 1. COVID-19 Pneumonia Screening Registration Form.
| Basic Information | Name | Gender | Age | Time | Case number | Tel number | Address | |
|---|---|---|---|---|---|---|---|---|
| Epidemiological survey | Have you been to epidemic areas such as Hubei Province within 2 weeks? | Y/N | ||||||
| Have you ever been to a place where COVID-19 pneumonia is endemic? | Y/N | |||||||
| Do you have friends or family with COVID-19 pneumonia? | Y/N | |||||||
| Do you have coworkers with COVID-19 pneumonia? | Y/N | |||||||
| Is there anyone infected with COVID-19 pneumonia in your community? | Y/N | |||||||
| Clinical symptoms | Fever | Y/N | Cough | Y/N | Sputum | Y/N | Myalgia | Y/N |
| Abnormal breathing | Y/N | Wheezing | Y/N | Sneezing | Y/N | Headache | Y/N | |
| Sore throat | Y/N | Fatigue | Y/N | Vomit | Y/N | Diarrhea | Y/N | |
Abbreviations: COVID-19, coronavirus disease 2019; N, no; Tel, telephone; Y, yes.
All patients with temperatures > 37.3°C or respiratory symptoms were considered as suspected cases and were referred to the emergency department for evaluation. This assessment included routine serum chemistry tests and a complete blood count, C-reactive protein level, procalcitonin level, computed tomography of the chest and pharynx, and SARS-CoV-2 nucleic acid testing to rule out SARS-CoV-2 infections. These suspected or confirmed cases were not brought into the dialysis treatment area, but rather were transferred to the isolation ward of the specialty hospital and received dialysis treatment in an isolation room until the SARS-CoV-2 nucleic acid test was negative.
SARS-CoV-2 is transmitted in most instances from person to person, either through inhalation or through deposition on mucosal surfaces of large respiratory droplets. Dialysis facility staff adhered to infection control measures recommended by European Centre for Disease Prevention and Control,16 including use of a waterproof disposable gown, cap, gloves, face shields, and an N95 face mask. After each hemodialysis treatment, the dialyzer and all blood tubing were discarded as infectious waste. The dialysis machine was disinfected using sodium hypochlorite solution according to the manufacturer’s instruction. The dialysis machine was kept in a room in the COVID-19 isolation ward between dialysis sessions and was only used for patients who had contracted COVID-19 infection. Because of these rigorous practices by medical staff, they were allowed to continue to work and return home per their usual routines.
In summary, we describe a hemodialysis patient with COVID-19 pneumonia. This particular patient had a relatively mild course despite multiple comorbid conditions including chronic hepatitis B virus infection and type 2 diabetes. With appropriate infection control measures, the staff who cared for this patient were not infected. Lopinavir/ritonavir therapy was used in this patient; whether this resulted in less severe disease remains uncertain but warrants further study. Last, through rigorous triage measures, we were able to limit the transmission of disease to other patients.
Article Information
Authors’ Full Names and Academic Degrees
Bin Tang, MD, PhD, Sijia Li, MD, Yuwan Xiong, MD, MS, Ming Tian, MD, MS, Jianbin Yu, MD, MS, Lixia Xu, MD, PhD, Li Zhang, MD, PhD, Zhuo Li, MD, MS, Jianchao Ma, MD, PhD, Feng Wen, MD, PhD, Zhonglin Feng, MD, Xinling Liang, MD, PhD, Wei Shi, MD, PhD, and Shuangxin Liu, MD, PhD.
Support
This study was supported by Guangzhou City Science and Technology Project (no. 201707010009), National Natural Science Foundation of China (no. 81670656, 81870508), and Guangdong Province High-level Hospital Construction Project (no. DFJH201901). The funders had a role in study design, data collection, analysis, and reporting.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in the report.
Peer Review
Received March 5, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from the Editor-in-Chief. Accepted in revised form March 9, 2020.
Footnotes
B.T., S.Li, and Y.X. are joint first authors.
Complete author and article information provided before references.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print February 24, 2020]. JAMA. https://doi.org/10.1001/jama.2020.2648. [DOI] [PubMed]
- 2.https://news.ifeng.com/c/specialClient/7tPlDSzDgVk?needpage=1&webkit=1. Accessed March 3, 2020.
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [published online ahead of print February 7, 2020]. JAMA. https://doi.org/10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed]
- 4.US Renal Data System. https://www.usrds.org/Default.aspx Accessed March 3, 2020.
- 5.Chinese National Renal Data System. http://www.cnrds.net/Static/OfficialDocumentDown.html
- 6.National Health Commission of People’s Republic of China Diagnosis and treatment of pneumonia caused by novel coronavirus (trial version 6) http://www.nhc.gov.cn/yzygj/s7652m/202002/54e1ad5c2aac45c19eb541799bf637e9.shtml
- 7.Borges A., Borges M., Fernandes J. Apoptosis of peripheral CD4(+) T-lymphocytes in end-stage renal disease patients under hemodialysis and rhEPO therapies. Ren Fail. 2011;33(2):138–143. doi: 10.3109/0886022X.2011.553300. [DOI] [PubMed] [Google Scholar]
- 8.Freitas G.R.R., da Luz Fernandes M., Agena F. Aging and end stage renal disease cause a decrease in absolute circulating lymphocyte counts with a shift to a memory profile and diverge in Treg population. Aging Dis. 2019;10(1):49–61. doi: 10.14336/AD.2018.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome [published online ahead of print February 18, 2020]. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed]
- 10.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meisner M., Lohs T., Huettemann E. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur J Anaesthesiol. 2001;18(2):79–87. doi: 10.1046/j.0265-0215.2000.00783.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan S.S., Hicks C.B. Lopinavir/ritonavir in the treatment of human immunodeficiency virus infection. Expert Opin Pharmacother. 2005;6(9):1573–1585. doi: 10.1517/14656566.6.9.1573. [DOI] [PubMed] [Google Scholar]
- 13.de Wilde A.H., Jochmans D., Posthuma C.C. Screening of an FDA-approved compound library identifies four small molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58(8):4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu C.M., Cheng V.C., Hung I.F. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S.K., Rosenkranz S.L., Cramer Y.S. The pharmacokinetics and pharmacogenomics of efavirenz and lopinavir/ritonavir in HIV-infected persons requiring hemodialysis. AIDS. 2008;22(15):1919–1927. doi: 10.1097/QAD.0b013e32830e011f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Centre for Disease Prevention and Control Infection prevention and control for the care of patients with 2019-nCoV in healthcare settings. https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-care-patients-2019-ncov-healthcare-settings