Graphical abstract
Abbreviations: RA, rheumatoid arthritis; TNF-α, tumour necrosis factor; IL, interleukin; GC, glucocorticoid; COX- 1, cyclooxygenase - 1; COX- 2, cyclooxygenase - 2; Ig, immunoglobulin; HA- AuNP/TCZ, hyaluronate gold nanoparticle/Tocilizumab; HUVEC, human umbilical vein cells; VEGF, vascular endothelial growth factor; CEL-TS-LN, celecoxib loaded tristearin based lipidic nanoparticles; CHNP, chitosan nanoparticle; HEKcells, human embryonic kidney cells; RGD, arginine-glycine aspartic acid; DMARD, disease modifying antirheumatic drugs; TAC-HSA-NPs, tacrolimus human serum albumin nanoparticle; AIA, antigen-induced arthritis; LDE, lipidic nanoemulsion; SLN, solid lipid nanoparticles; C-SLN, curcumin loaded solid lipid nanoparticles; CFA, complete freund’s adjuvant; Pir-SLN, piroxicam solid lipid nanoparticles; P-SLN, piperine loaded solid lipid nanoparticle; ACF-SLN, aceclofenac loaded solid lipid nanoparticles; A-SLN, actarit loaded solid lipid nanoparticles; PSA-PCL-CyA-NMs, polysialic acid- polycaprolactone cyclosporine A nanomicelles; DEX-PMs, dexamethasone-loaded polymeric micelles; IND-NMs, indomethacin loaded polymeric micelles; DEX, dexamethasone; PCL-PEG, poly (ethylene glycol)-block-poly (ε-caprolactone); LX-NMs, larnoxicam loaded nanomicelles; PSA, polysialic acid; PCL, polycaprolactone; Ind-NCs, indomethacin-loaded nanocapsules; TAC-LCNCs, tacrolimus loaded lipidic core nanocapsules; MTX-LCNCs, methotrexate-loaded lipidic core nanocapsules; ALP, alkaline phosphate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FA, folic acid; HSA-NCs, human serum albumin nanocapsules; CLSM, confocal laser scanning microscopy; FR-β, folate receptor-beta; VIP, vasoactive intestinal peptide; VCAM-1, vascular cell adhesion molecule-1; mRNA, messenger RNA; shRNA, short hairpin RNA; siRNA, small interfering RNA; RNAi, RNA interference; NSAIDs, non steroidal anti-inflammatory drugs
Keywords: Rheumatoid arthritis, Nanoformulation, Inflammation, Nanoparticles
Abstract
Rheumatoid arthritis (RA) is the most common complex multifactorial joint related autoimmune inflammatory disease with unknown etiology accomplished with increased cardiovascular risks. RA is characterized by the clinical findings of synovial inflammation, autoantibody production, and cartilage/bone destruction, cardiovascular, pulmonary and skeletal disorders. Pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and IL-10 were responsible for the induction of inflammation in RA patients. Drawbacks such as poor efficacy, higher doses, frequent administration, low responsiveness, and higher cost and serious side effects were associated with the conventional dosage forms for RA treatment. Nanomedicines were recently gaining more interest towards the treatment of RA, and researchers were also focusing towards the development of various anti-inflammatory drug loaded nanoformulations with an aid to both actively/passively targeting the inflamed site to afford an effective treatment regimen for RA. Alterations in the surface area and nanoscale size of the nanoformulations elicit beneficial physical and chemical properties for better pharmacological activities. These drug loaded nanoformulations may enhances the solubility of poorly water soluble drugs, improves the bioavailability, affords targetability and may improve the therapeutic activity. In this regimen, the present review focus towards the novel nanoparticulate formulations (nanoparticles, nanoemulsions, solid lipid nanoparticles, nanomicelles, and nanocapsules) utilized for the treatment of RA. The recent advancements such as siRNA, peptide and targeted based nanoparticulate systems for RA treatment were also discussed. Special emphasis was provided regarding the pathophysiology, prevalence and symptoms towards the development of RA.
1. Introduction
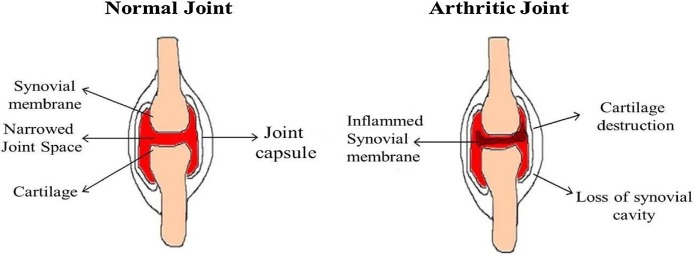
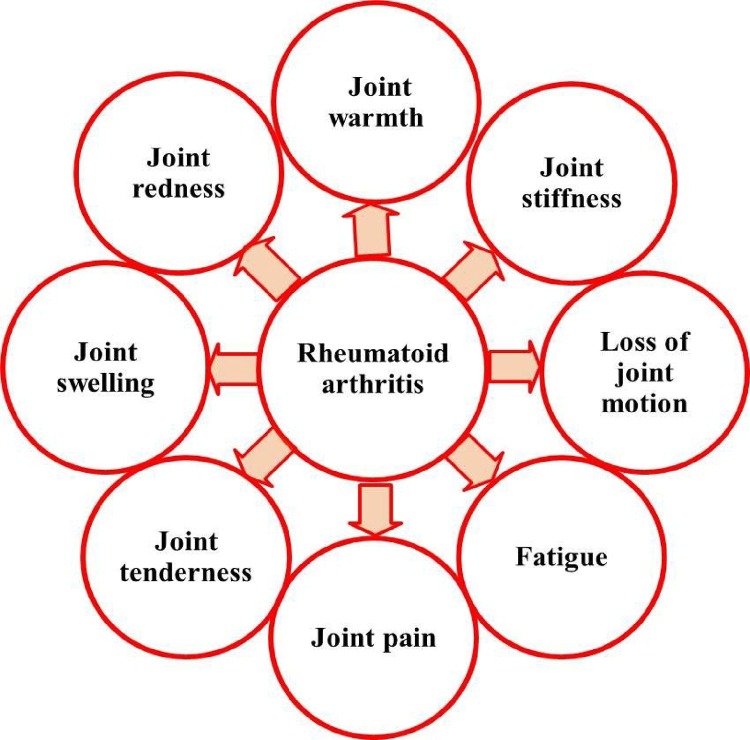
Rheumatoid arthritis (RA) is an autoimmune disease characterized with joint pain (functional joint failure), swelling/destruction of bone and cartilage, with an estimated reported global prevalence of around 0.3–1.0 % [1]. RA is the commonest inflammatory arthritis affecting up to 0.75% of the Indian population. Various factors such as disease activity, socioeconomic, educational status, body mass index, spirituality, age and gender affects RA patient’s quality of life [2]. RA varies significantly from osteoarthritis, which is a degenerative joint disease affects only joint function (Fig. 1 ). Gastrointestinal disturbance, renal malfunctioning and increased cardio vascular risk were also associated with RA [3]. Systemic manifestations such as subcutaneous nodules, pleuritis, pericarditis, and vasculitis, contribute to morbidity and mortality of RA development [4]. RA development seems to be stronger for men’s associated with cigarette smoking than womens. The occurrence of RA is unidentified, but it is presumed that the environmental factors may contribute to its development in genetically susceptible individuals [5]. The genetic components and environmental factors influences that subsequent immune response and studies have reported that multiple cell types (including B cells, T cells, macrophages/synoviocytes) serves as key regulators for immunologic events in RA over the years [6]. The interactions between the environmental factors along with the genetics of patients were associated with RA development. The other important environmental risk factors for RA development were coffee/alcohol intake, oral contraceptive usage, birth weight irregularities and breast feeding [7]. The various symptoms associated with the RA development were shown in Fig. 2 .
Fig. 1.
Comparison between (A) normal and (B) rheumatoid arthritis joints.
Fig. 2.
Symptoms associated with Rheumatoid arthritis development.
2. Pathogenesis of rheumatoid arthritis
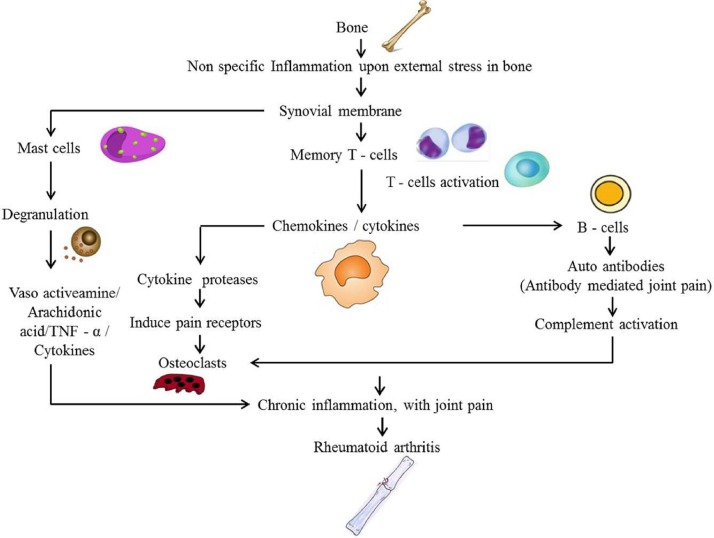
Pathogenesis of RA evokes that, cytokines control a wide variety of inflammatory processes in rheumatoid joints, where the imbalance between pro and anti-inflammatory cytokine activities induces autoimmunity, chronic inflammation and thereby causes joint damage (Fig. 1) [8]. T cells proliferated as a cascade of immune system release activates B cells and macrophages, thereby induce cytokines such as (TNF) and interleukin (IL). Once RA diagnosis is confirmed, the pathologic inflammatory response may leads to joint destruction and extra-articular complications. These extra-articular complications may occur over time and induce infections, lymphomas, cardiovascular disease, and osteoporosis.
Cytokines plays major role due to its destructive effects on bones and articular cartilage, which provide the rationale for current RA treatment strategies, towards the usage of monoclonal antibodies against TNF-α, IL-1β, and IL-6 [6]. Tumor necrosis factor (TNF) is a proinflammatory cytokine which plays major role in regulating the inflammatory responses especially during RA conditions [9]. There is no treatment option for the complete recovery of RA, but treatment may address to decrease the joint pain, inflammation and for the protection of articular structures. Management of arthritic condition involves an interdisciplinary approach which includes physical therapy modalities associated with medical management [10]. During the past 10 years there have been major advances for the treatment of RA, including aggressive use of disease-modifying anti-rheumatic drugs and the development of immune therapies targeting towards cells specific to RA immunopathogenesis [11]. The pathogenesis of RA development is displayed in Fig. 3 .
Fig. 3.
Pathogenesis of rheumatoid arthritis development.
3. Manifestations associated with rheumatoid arthritis
The major manifestations of RA are categorized in to three, which include bone, airway and cardiovascular system. In case of bone manifestations, bones are affected both locally and systemically. Local, factors that stimulate osteoclasts results in increased bone resorption and these osteoclasts were released from inflammatory and fibroblastic pannus cells [12]. Mostly, RA patients were prone to bisphosphonate therapy for osteoporosis or prevention of glucocorticoid-mediated bone loss. Airway manifestations of RA include cricoarytenoid arthritis, pulmonary fibrosis and small airway disease, typically seen as bronchiolitis with obstructive abnormalities in lung functions. Lung disease is more predominant in RA patients especially in male seropositive smokers. RA patient’s holds 40% increased risk of mortality which leads to an increased incidence of cardiovascular disease with elevated level of inflammatory markers [13].
4. Treatment options for rheumatoid arthritis
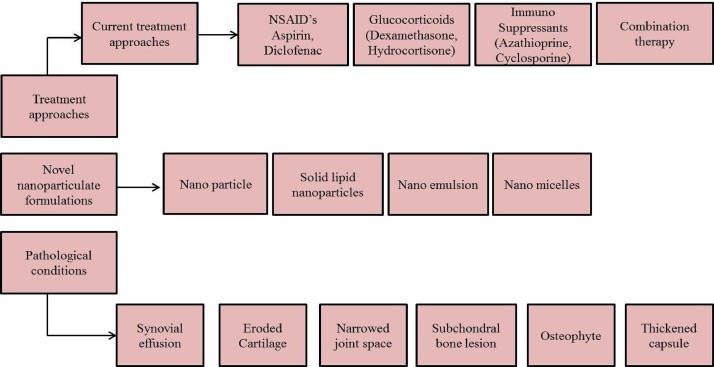
RA can be effectively managed by anti-rheumatic drugs (such as, Methotrexate (MTX), leflunomide, sulfasalazine, and hydroxy-chloroquine [14] combined with/without different non-steroidal anti-inflammatory drug (aspirin, celecoxib, diclofenac, ibuprofen, indomethacin, ketoprofen etc.,) among these drugs, MTX was considered as an effective drug since 1980 [15]. Inspite, we are also developing a formulation using MTX with another drug combination for the effective treatment of RA. In addition glucocorticoid (GC) also widely used for the effective management of RA. GC can be used as monotherapy as well as in combination with other agents. Long term administration of GCs may leads to anti-inflammatory remunerations and action against structural damage. The most widely utilized GCs were methylprednisolone, triamcinolone, prednisone, and hydrocortisone [16]. Glucocorticoidal injections, TNF-α inhibitors, T-cell activation inhibitors, B-cell depletes, IL-6 inhibitors, JAK inhibitors, immunosuppressant’s and steroids are also highly effective for the treatment of RA. The use of combination therapy for RA treatment were followed during past years (10 years), but now at least one third of patients with RA are treated by combination therapy in India. Traditional approach for the management of RA has also been changed from monotherapy to combination approach. The different treatment methods adopted for RA is shown in Fig. 4 .
Fig. 4.
Treatment methods and pathological conditions for rheumatoid arthritis.
5. Problems associated with conventional dosage forms
The various conventional dosage forms available for the treatment of RA includes tablets, capsules, oral liquids, topical products, parenterals, paediatric/geriatric products and transdermal patches. Topical dosage forms for the treatment of RA includes ointment, cream, gels or paste. Transdermal patches are topical drug delivery system which delivers medication through the skin in a non-invasive manner. The major drawbacks associated with the conventional dosage forms for the treatment of RA were poor patience compliance, short half-life, low bioavailability and poor solubility which may be improved by modified novel dosage forms [17]. The novel dosage form available for RA treatments includes microparticles, nanoparticles, nanoemulsions, nanomicelles, nanodispersions, nanocapsules, nanosuspensions etc., the conventional dosage forms available for the treatment of RA are displayed in Table 1 .
Table 1.
Conventional dosage forms for the treatment of rheumatoid arthritis.
| S. No | Pharmaceutical Dosage Form | Therapeutic drugs with anti-rheumatic properties |
Brand Names | Mode of action | References |
|---|---|---|---|---|---|
| 1. | Tablets | Aspirin | Bayer, Empirin | Inhibits the activity of cyclooxygenase thereby decrease the prostaglandins production | [18] |
| Celecoxib | Celebrex | Specially inhibits COX-2 and analgesic properties with low toxicity | [19] | ||
| Sulfasalazine | Azulfidine | Inhibits dihydrofolate reductase | [20] | ||
| Leflunomide | Arava | Immune modulatory effect by inhibiting mitochondrial enzyme dihydro-orotate dehydrogenase. | [21] | ||
| Indomethacin | Indocin, Indocin-SR |
Analgesic, antipyretic and anti-inflammatory and decreases prostaglandin synthesis. | [22] | ||
| 2. | Capsules | Minocycline | Minocin, Dynacin | Binds to bacterial 30 s ribosomal subunit and inhibit the synthesis of protein. | [23] |
| Oxaprozin | Daypro | Induces cyclooxygenase inhibition analgesic and anti-inflammatory activities. | [24] | ||
| Cyclosporine | Gengraf, Neoral | Exhibits potent immunosuppressive properties and blocks the transcription of cytokines in activated T cells. | [25] | ||
| Sulfasalazine | Azulfidine | Exhibits antibacterial and anti- inflammatory effects and acts as immuno modulators. Scavenging reactive oxygen metabolites, blocks the production of prostaglandins and leukotriene’s | [20] | ||
| Tofacitinib | Xeljanz, Xeljanz XR | Inhibits janus kinase enzyme and interferes JAK-STAT signaling pathway and thereby influencing DNA transcription process. | [26] | ||
| 3. | Oral liquids | Azathioprine | Azasan, Imuran | Acts as immunosuppressive agent and blocks the de novo pathway of purine synthesis thereby it stops DNA replication process. | [27] |
| Doxorubicin | Adriamycin, Doxil, Caelyx | Intercalates into DNA, distorts the polynucleotide structure and result in enzyme inhibition related to the DNA replication and transcription. | [28] | ||
| 4. | Topical (Oinment, Gel) |
Hydroxy chloroquine | Plaquenil | Acts as antimalarial agents and also exerts beneficial effect of lupus and elicits acute or chronic rheumatoid arthritis. | [29] |
| Piroxicam | Feldene | Inhibit prostaglandin synthesis and decrease the creation of IgM rheumatoid factor and inhibitor of the cyclo-oxygenase in arachidonic acid metabolism. | [30] | ||
| MTX | Oterxup Rheumatrex | Exhibits antagonistic effect on folic acid metabolism and function as an antimetabolite and inhibits dihydrofolate reductase enzyme which participates in the tetrahydrofolate synthesis. | [31] | ||
| Ketoprofen | Fastum | Inhibits prostaglandin synthesis through the L-arginine-nitric oxide pathway. | [32] | ||
| 5. | Transdermal patches | Diclofenac sodium | Voltaren-XR | Induce anti-inflammatory, antipyretic, and analgesic action and inhibits prostaglandins synthesis. | [33] |
| Teriflunomide | Aubagio | Proliferation of activated lymphocytes depends on a new pyrimidine synthesis by dihydro-orotate dehydrogenase and inflammatory activity likely involving both T and B cells. | [34] |
6. Nanotechnology- an overview
Nanotechnology deals with the manipulation of issues at nuclear level to create newer novel nano materials due to its ability to produce sophisticated nanomaterials, processes and products designated at nanoscale, which is creating an additional increment over the recent years [35]. Recently many techniques were developed to study the physical phenomena and constructs (typically 1–100 nanometers) of different nanomaterials [36]. The term "smart materials" has been utilized to describe nanoparticles to focus delivery of drugs to particular organs of the body. This technology paved its way for the past several years towards the expansion in the potential uses of nanoparticles as cosmetic products. Liposomal technologies were utilized to adjust optical properties, in order to improve its solubility and to alter its physical properties, provided for hydrophilic vesicles with phosphatidylcholine membrane(s) [37]. Nanomaterials have been used in medicinal field for therapeutic drug delivery with the focus for treatments of various diseases/disorders. Micro and nano scale systems can maximize the efficacy of therapeutic treatments in numerous ways because they paves the ability to rapidly detect and respond to disease states directly at the site by improving the patient’s quality of life [38].
6.1. Nanoparticles for the treatment of RA
Nanoparticles are spherically shaped particles [39]. The size, surface characteristics and morphology of nanoparticles possess essential role towards the biodistribution of nanoparticle for RA treatment [40]. Nanoparticles (NPs) are utilized as therapeutic/imaging agents, for theranostic applications. The encapsulated drug containing particles helps to afford targeted delivery/controlled release of encapsulated drugs. Physicochemical properties associated with, passive targeting of drugs for RA treatment, includes particle size, charge shape and surface characteristics. Especially, nanoparticles due to their biocompatibility and biodegradability properties hold its vital role in pharmaceutical industries. Nanoparticles conjugated with specific ligand targets and facilitate cellular penetration [41]. The most commonly reported liposomes, micelles, metallic nanoparticles, and polymeric nanoparticle affords efficient delivery towards the treatment of RA. Nanoparticles can be taken by systemic circulation through different process such as adsorption, ligand receptor attachment, covalent coupling, and internalization [42].
NSAIDs based delivery systems were widely reported for RA, which decrease pain (analgesia) associated with early stage of RA through its anti-inflammatory mechanisms without loss of articular function, additionally; it blocks COX-1 and COX-2 enzymes which play an essential role in the generation of prostaglandins. Drug containing nanoparticle systems were delivered therapeutically to inflamed synovium [43]. Metal oxide nanoparticles reveals various desired characteristics such as drug carriers with incredible higher surface area and huge pore sizes for drug encapsulation, intrinsic biodegradability characteristics due to its labile metal-ligand bonds, and versatile functionality for post synthetic grafting of drug molecules [44]. Rutin stabilized silver nanoparticles elicits anti-inflammatory activity in chronic inflammation by its critical inhibition of the creation of pro-inflammatory cytokines (tumour necrotic factor-α (TNF-α) and interleukin-6 (IL-6). Silver nanoparticles have been also utilized for therapeutic benefits in RA patients [45].
Lee et al. [46] developed a hyaluronate gold nanoparticle/Tocilizumab (HA-AuNP/TCZ) complex for the treatment of RA. They utilized an immunosuppressive tocilizumab as a monoclonal antibody, targeting the interleukin-6 (IL-6) receptor. Synthesis of gold nanoparticles was performed using citrate method. They attained a particle diameter of 64.83 nm with a polydispersity index of 0.18 for HA-AuNP/TCZ complex. They found that TCZ was steadily released from the HA-AuNP/TCZ complex up to 80% for about 8 days after incubation in the bovine serum albumin. Further they observed anti-angiogenic effect of the developed HA-AuNP/TCZ complex upon proliferation of HUVEC cells by binding to VEGF receptor. At in-vivo level they found that treatment with HA-AuNP/TCZ complex in RA induced mice exhibits clear interface between cartilage and bone comparable to the normal group (without RA induced). Further their confirmation by western blot analysis showed significant upregulated expression levels of IL-6 and CD68, which indicates that the treatment with HA-AuNP/TCZ complex resulted in significantly reduced levels of IL-6 and CD68. Finally they conclude that, HA-AuNP/TCZ complex might be useful for the dual targeting drug candidate to VEGF and IL-6R receptors for the treatment of RA. Celecoxib loaded tristearin based lipidic nanoparticles (CEL-TS-LN) was synthesized by Kishore et al. [47] and they checked for its effective management in RA treatment by in-vitro/in-vivo studies. They utilized celecoxib as the selective cyclooxygenase-2 (COX-2) inhibitor, orally for the treatment of RA. They utilized statistical experimental design to optimize the nanoparticle using the independent variables (homogenization speed, quantity of tristearin and quantity of emulsifier) depend on desired particle size, loading efficiency and drug release. They observed a particle size of 188.6 nm with a PDI 0.26, obtaining a spherical morphology. XRD results indicated that the drug is dispersed at a molecular level in the lipid matrix and also rapid quenching of the micro emulsion prevents the drug to crystallize stage. In-vitro drug release study revealed a continuous drug release of 62% within 36 h; and they found that drug release follows a zero order and Fickian diffusion pattern. In-vivo studies revealed the significant reduction in inflammation with improvement in lysosomal enzymes, collagen and glycosamino glycan levels in CEL-TS-LN treated rats compared to the pure drug (CEL) treated group. Finally, they suggested that the poorly soluble celecoxib encapsulated tristearin solid lipid nanoparticles facilitated safe, efficacious, prolonged release characteristics with enhanced bioavailability.
The chitosan nanoparticle (CHNP) loaded with dexamethasone (DEX)/(MTX) was developed by Kumar et al. [48] for the efficient treatment of RA. They prepared chitosan nanoparticle using ionic gelation method. The entrapment efficiency of MTXCHNPs and DEXCHNPs was found to be from ∼55% and ∼10% respectively. They achieved size and charge for CHNPs, MTXCHNPs, DEXCHNPs about 299.4 nm (PDI 0.437), 217.4 nm (PDI 0.275), 329.8 nm (PDI 0.286) and +35.7 mV, +26.9 mV, +19.5 mV respectively. The FTIR results confirmed that phosphoric group of tripolyphosphate with amine groups of chitosan plays the major role for hydrogen bond in the formulation of MTXCHNP and DEXCHNP. They observed a controlled release patterns coupled with diffusion of drug in two different buffers at pH 7.4 and pH 5.8 respectively. They studied the cellular behaviour in HEK and murine macrophage cell line RAW264.7 cell lines. The IC50 value for MTXCHNP in human Embryonic Kidney cells was 26.1 μg/mL and 7.7 μg/mL for RAW cell lines. The IC50 value for MTXCHNP was 20.12 μg/mL in HEK cells and 7.37 μg/mL in RAW264.7 cells. Cellular uptake studies indicate the internalization of nanoparticles by phagocytosis process. The drug release gets enhanced at lower pH with increased cytotoxicity. Insignificant ex-vivo haemolysis indicated the higher biocompatibility of the nanoparticles. The synthesized CHNP exhibited maximum absorption in blood circulation within 3 h, followed by hepatic metabolism and renal clearance. Finally, they observed higher anti-arthritic activity with antioxidant potential for the developed nanoparticulate systems. Dewangan et al. [49] synthesized curcumin loaded carboxy methyl cellulose acetate butyrate nanoparticles (CMCAB) by flash nano precipitation method using multi inlet vortex mixer for the treatment of diseases like colon cancer, pancreatic cancer, RA and Alzheimer’s disease. They varied the reynolds number of the inlet streams to optimize the nano particle size and average diameter and observed a size around 155–400 nm. They observed that the synthesized nanoparticle were amorphous nature. They found that, nanoparticles have been demonstrated especially to expand absorption and bioavailability of drugs, leading to effective drug administration.
Lee et al. [50] examined the effect of arginine-glycine aspartic acid (RGD) attached gold half shell nanoparticle containing MTX as category of DMARDs for the treatment of RA. They utilized RGD peptide as targeting moiety for inflammation. Near-infrared irradiation, upon the development of nanoparticles generates heat due to gold half shells, and MTX gets rapidly released. Synovial inflammation at multiple joints may be targeted with a penetration depth of near infrared light using this therapy. Nanoparticle with smaller dosage (1/930 of MTX solution) treatment would contribute to collagen-induced arthritic mice. They emphasized that multifunctional nanoparticle were effective for maximizing the therapeutic efficacy and minimizing dosage-related side effects in the treatment of RA. Furthermore, they showed that the nanoparticle could be applied for DMARD for the treatment of RA and other diseases. Tacrolimus (TAC-HSA-NPs) loaded albumin nanoparticles was developed by Thao et al. [51] using nanoparticle albumin-bound technology to improve targetability and anti-arthritic efficacy. They observed an average diameter and charge of 185 nm and +30.5 mV respectively with spherical morphology. The encapsulation efficacy of TAC, within the nanoparticles was 79% and the water solubility of the developed nanoparticles gets increased 46 times than that of free TAC. TAC gets steadily discharged from the developed NPs over 24 h, which provides enough time for targeting and treatment in inflamed arthritic site by intravenous injection. In-vitro studies reported the anti-proliferative activities of TAC HSA-NPs monitored upon activated T cells compared with non-activated T cells. TAC HSA-NPs displayed significant higher anti-arthritic potential than TAC formulations compared to intravenously administered TAC solution or oral TAC suspension, as reflected by the incidence of arthritis and clinical score (1.6 vs. 3.2 and 5.0, respectively). Hence they conclude that novel TAC HSA-NPs may be an efficient drug delivery system for enhanced solubility with increased accumulation of drugs at joints for RA treatment. The different nanoparticulate based delivery system adopted using various drugs are showed in Table 2 .
Table 2.
Nanoparticle based anti-rheumatic arthritic drugs.
| S. No | Drugs | Nano carrier | Particle size (nm) / Zeta potential (mV) | Therapeutic effects | References |
|---|---|---|---|---|---|
| 1. | MTX | Gold half shell nanoparticle | 100-115/-- | Anti-rheumatic arthritic effect | [46] |
| 2. | Celecoxib | Solid lipid nanoparticle (Tristearin) | 188/+9.12 | Anti-inflammatory, Anti-rheumatic arthritic | [47] |
| 3. | Dexamethasone and MTX | Chitosan Nanoparticles | 299.4/+35.7 | Anti-arthritic, anti-inflammatory and antioxidant potential | [48] |
| 4. | Curcumin | Carboxy methyl cellulose acetate butyrate (CMCAB) | CMCAB172.3/-47.8 PLGA 201.8/ −34.7 |
Anti-rheumatic arthritic effect | [49] |
| 5. | MTX | (Arginine glycine aspartic acid) peptide conjugated gold shell nanoparticle | 100/-11.5 | Anti-inflammatory, anti-rheumatic arthritic effects | [50] |
| 6. | Tacrolimus | Human serum albumin | 185/-30.5 | Anti-rheumatic arthritic effect | [51] |
The significance of nanoparticles based formulation for the treatment of RA may afford improved bioavailability, increased accumulation of drug at the diseased inflamed site and prolonged release characteristics. At the superior level, these nanoparticulate based formulation may also elicits targetability potential with specific receptors.
6.2. Nanoemulsion for the treatment of RA
Nanoemulsions are isotropic, transparent systems consisting of oil, water and emulsifier and hold an average diameter of 20–500 nm. Emulsifiers plays essential role in settling nanoemulsions through repulsive electrostatic interactions and steric hindrance. The expansion of an emulsifier is mandatory for the production of smaller sized droplets as it decreases the interfacial tension and surface energy per unit range, between the oil and water phases of the emulsion [52]. Nanoemulsions have been reported to enhance bioavailability and efficacy of most anti-inflammatory agents [53]. Nanoemulsions have been used in food industries as flavored nanoemulsions and in cosmetic industries for skin hydration with ease of application. In pharmaceutical field, nanoemulsions have been utilized for drug delivery systems especially for parenteral, oral, ocular, and topical administration [54]. In addition to that, nanoemulsions contain building blocks for complex material such as compartmentalized nanoparticles and encapsulated oil droplets [55]. The properties associated with nanoemulsion were, high surface area per unit volume, robust stability, optically transparency, and tunable rheology.
MTX loaded lipidic nanoemulsion for the treatment of RA was developed by Mello et al. [56] and they determined the plasma decaying curves and biodistribution of MTX loaded nanoemulsion by radioactive counting method in antigen induced arthritis rabbits. In-vivo studies revealed that, MTX loaded nanoemulsion was taken up mainly by the liver and the uptake by arthritic joints was twofold greater than that of control joints. MTX loaded nanoemulsion treatment reduces leukocyte influx into the synovial fluid (65%) in particular, mononuclear and polymorphonuclear cells was reduced by 47 and 72%, respectively. Protein leakage into the arthritis induced rabbit knees was also more limited for MTX loaded nanoemulsion treatment, than commercial MTX treatment. These results indicate the superior anti-inflammatory effects in the joints of rabbits with antigen-induced arthritis compared to commercial MTX Hoscheid et al. [57] developed an oil based novel therapy for RA treatment. Since, Pterodon pubescens oil is commonly used due to its anti-rheumatic, antinociceptive and anti-inflammatory activities. They optimized a nanoemulsion comprising P. pubescens oil for intramuscular administration. They estimated the influence of altered ingredients such as polysorbates and PEG castor oils, upon physicochemical properties of formulations and found that droplet size of the nanoparticle to be around 199 nm. They observed a pseudoplastic behavior enabling effect, and this may be found suitable for nano emulsion based RA treatment.
Meloxicam loaded nanoemulsions for transdermal delivery of RA was developed by Pathan et al. [58]. They determined the solubility of the meloxicam in different excipients (oils, surfactants and cosurfactants) and found that the viscosity of nanoemulsion gets increased with increasing oil content. They observed an average particle size of 60.6 nm with polydispersity index of 0.22 and zeta potential of 0.86 mV. The percutaneous permeation of meloxicam loaded nanoemulsion through the skin was observed. They concluded that the developed meloxicam loaded nanoemulsions are promising vehicles for transdermal delivery in RA treatment and joint diseases. Mello et al. [59] formulated lipidic nanoemulsions containing MTX where increased MTX intra-articular actions were observed in antigen-induced arthritis (AIA) rabbits by subsequent intra-articular injection with the antigen. After 24 h treatment the dose level increased for LDE-MTX (0.0625-0.5 μmol/kg), compared to commercial MTX (0.5 μmol/kg), LDE alone, and saline (controls). Uptake of radioactive LDE by arthritic joints was 2.5-fold greater than normal joints. Treatment with intra-articular LDE-MTX elicited a clear dose responsive pattern by reducing the synovial leukocyte infiltrate (P = 0.004) and protein leakage (P = 0.032) when compared with arthritic non-treated joints. In contrast, the intra-articular injection of commercial MTX and LDE did not reduce leukocyte infiltrate or protein leakage without toxicity issues during treatment period. The association between lipidic nanoemulsions and MTX presented a marked anti-inflammatory effect that was absent after intra-articular commercial MTX treatment. The different nanoemulsions based delivery system adopted using various drugs are showed in Table 3 .
Table 3.
Nano emulsion based anti-rheumatic arthritic drugs.
| S. No | Drug Name | Nano carrier | Particle size (nm)/Zeta potential (mV) | Therapeutic effects | References |
|---|---|---|---|---|---|
| 1. | MTX | Lipoprotein | – | Anti-inflammatory effects | [56] |
| 2. | Pterodon. pubescens oil | Poly ethylene glycol | 200/ −11.38 to −30.93 |
Anti-rheumatic arthritic and anti-inflammatory effects | [57] |
| 3. | Meloxicam | Labrafil 1944CS | 60.6 - 195/-- | Anti-rheumatic arthritic effect | [58] |
| 4. | MTX | Low density Lipoprotein (cholesteryl oleate, egg phosphatidylcholine and cholesterol) | 60/-- | Anti-inflammatory effect | [59] |
The significance of nanoemulsions based formulation for the treatment of RA may afford stability, optically transparency, inhibits p-glycoprotein mediated drug efflux, promotes lymphatic transport and exhibits tunable rheology for an effective treatment regimen.
6.3. Solid lipid nanoparticle for the treatment of RA
Solid lipid nanoparticles (SLNs) are colloidal carriers with particle size ranging from 120 to 200 nm, widely utilized for controlled drug delivery which merges the benefits of polymeric nanoparticles and oil in water emulsions [60]. SLNs possess remarkable properties such as a good tolerability, protection of incorporated active compounds against chemical degradation, higher bioavailability with incorporation of both lipophilic and hydrophilic drugs, higher drug loading capacity and relatively safe for biological applications [61]. Due to its unique size range SLNs rarely undergoes blood clearance by the reticulo endothelial system. SLNs were made up of physiological lipids, fatty acids, phospholipids and mono/di/triglycerides. SLNs may be prepared by various techniques such as, high shear homogenization, ultrasound, high pressure homogenization, hot homogenization, cold homogenization, solvent emulsification and evaporation methods. In recent years, greater attention has been focused towards lipid based formulations for the improved oral bioavailability of poorly water soluble drugs using SLNs [62]. The drug carrier combines the advantage of polymeric nanoparticles, fat emulsions and liposome; due to its improved physical stability, low cost, ease of scale-up, and producing [63].
Curcumin loaded solid lipid nanoparticles (C-SLNs) for inflammation by overcoming the poor bioavailability issues of curcumin was developed by Arora et al. [64]. They observed an average particle size, drug content and entrapment efficiency of 134 nm, 3.78 mg/mL and 81.92%. They examined the effect of pure curcumin and C-SLNs in complete freund’s adjuvant (CFA) – induced arthritic rats. They showed that C-SLNs act as carrier for effective delivery of curcumin to RA patients. They elicited that C-SLNs showed its anti-arthritic mechanism through oxido-inflammatory and immunomodulatory cascade in CFA induced arthritis model. Peng et al. [65] developed SLNs for the sustained release and transdermal delivery of piroxicam (Pir), as well as to elucidate the anti-inflammation effect of developed SLNs. They observed that, Pir loaded SLNs possess average particle size of 102 nm with a PDI of 0.262 and charge of +30.21 mV. However, their optimized formulation showed that Pir-SLNs were spherical shaped with higher entrapment efficiency (87%). They demonstrated that, SLNs were best carrier for encapsulation and sustained release of drugs. Pir-SLNs illustrated the anti-inflammatory responses in edematous site, by reducing the secretion of inflammatory cytokines.
Piperine loaded solid lipid nanoparticle (P- SLNs) by melt emulsification method for RA treatment was formulated by Bhalekar et al. [66]. They found an average diameter of 128.80 nm, with an encapsulation of 78.71% and charge of −23.34 mV. In-vivo pharmacodynamic studies in complete freund’s adjuvant induced arthritic rats showed significant reduction of TNF-α in treated rat which might be the mechanism behind the DMARD action of P-SLNs. Raj et al. [67] developed aceclofenac loaded solid lipid nanoparticles (ACF-SLNs) by incorporating into hydrogels by ultrasonic emulsification method using glyceryl monostearate as lipid. They optimized on the basis of lipid and stirring speed and found the average particle size (189 nm), polydispersity index (0.162 nm) and zeta potential (-32.51 mV) respectively for optimized formulation. They observed an entrapment efficiency of about 85%. In-vivo studies, showed better inhibition of edema with the magnitude of 81% after 6 h, as compared to that of plain ACF hydrogel. Furthermore, optimum size of the SLNs and close contact with the stratum corneum improved the enhanced skin deposition of drug. Thereby they emphasized that ACF-SLNs could have beneficial effect for RA treatment.
Injectable actarit loaded solid lipid nanoparticles (A-SLNs) as passive targeting agent for RA treatment was developed by Ye et al. [68]. In this method A-SLNs were used to target spleen and to eliminate the adverse effects (nephrotoxicity) upon oral administration. Their optimized formulation showed an average size of 241 nm with a charge of -17.14 mV. They showed that entrapment efficiency and loading for A-SLNs were 50.87% and 8.48%, respectively. They achieved that, the plasma concentration of A-SLNs were 1.88 times better than that of the actarit in 50% propylene glycol solution. However, targeting efficiency of A-SLNs was enhanced from 6.31% to 16.29% in spleen while the renal distribution of drug gets significantly reduced as compared to that of the drug solution, after intravenous administration to mice. These results suggests that injectable A-SLNs acts as promising passive targeting therapeutic agents, with reduced doses, decreased dosing frequency and lowered toxicity for RA treatment. The different SLNs based delivery system adopted using various drugs were showed in Table 4 .
Table 4.
Solid Lipid Nanoparticle based anti-rheumatic arthritic drugs.
| S. No | Drug Name | Carrier | Particle size (nm) / Zeta potential (mV) | Therapeutic effects | References |
|---|---|---|---|---|---|
| 1. | Curcumin | Indian Gold | 134/-- | oxido-inflammatory and immunomodulatory cascade | [64] |
| 2. | Piroxicam | Glycerol monostearate | 102/ 30.21 |
Anti-inflammatory effect | [65] |
| 3. | Piperine | Glycerol monostearate | 128.80/ −23.34 |
Anti-rheumatic arthritic effect | [66] |
| 4. | Aceclofenac | Glycerol monostearate | 189/ −32.51 |
Anti-rheumatic arthritic effect | [67] |
| 5. | Actarit | Stearic acid | 241/ −17.14 |
Anti-rheumatic arthritic effect | [68] |
The significance of solid lipid nanoparticles based formulation for the treatment of RA may afford controlled/sustained release pattern with lowered dosing frequencies. These solid lipid based nanoparticles may also have the capacity to enhance the bioavailability of the encapsulated drug in RA treatment.
6.4. Nanomicelles for the treatment of RA
Nanomicelles are amphiphilic molecules or surfactant monomers that have a polar head and a lipophilic tail. The properties related with amphiphilic molecules in solution results in developmental structures termed as micelles. These micelles hold internally hydrophobic core and externally a hydrophilic surface [69]. Micelles are generally made up of 50–200 monomers. The parameter which affects the micelle formation was the size of the hydrophobic area of amphiphilic molecule, amphiphile concentration, temperature, and solvent [70]. Polymeric micelle holds the diameter of 10–100 nm. Micellar core serves as a compatible microenvironment with center point for joining water insoluble guest molecules. Hydrophobic molecules can be covalently coupled to the block copolymers or it can be physically incorporated into the hydrophobic micelle core. Solubilization process of nanomicelles leads to enhancement of their water solubility and bioavailability.
Cyclosporine A loaded polysialic acid- polycaprolactone micelles (PSA-PCL-CyA-NMs) for the treatment of RA was developed using self-assembly process using grafting technology by Wilson et al. [71]. However, PSA-PCL-CyA-NMs possess a loading capacity and loading efficiency of 0.09% and 29.3% precisely. In-vitro cellular behaviour studies in SW982 (synovial fibroblast cell line) indicated that, CyA gets released from PSA-PCL micelle by subsequent uptake of synovial fibroblasts through a non-receptor mediated endocytosis and partitioning of CyA into phospholipid membrane. Crielaard et al. [72] developed dexamethasone-loaded polymeric micelles (DEX-PMs) for the treatment of RA. They observed an average diameter of 70 nm and PDI of 0.1. However, highly efficient encapsulation of the DEX derivatives (>80%) was attained at a drug/polymer ratio of 10% (w/w) and they confirmed the effect of developed formulation in-vivo in adjuvant induced arthritis model. Finally, they suggests that treatment of DEX-PMs showed an immediate and prolonged anti-arthritic effect in animal model and emphasized that DEX-PMs were significantly more effective than free DEX in terms of inhibition of arthritis score (p < 0.05 on day 16 and 20; p < 0.001 on day 21 onwards) by minimizing the degree of ankle swelling. Zhang et al. [73] developed indomethacin loaded polymeric micelles (IND-NMs) by conjugating amphiphilic polyphosphazenes with poly (N-isopropylacrylamide) and ethyl 4-aminobenzoate as adjacent groups by thermal ring opening polymerization. In-vivo pharmacodynamic studies performed on both acute paw edema and adjuvant arthritis models indicated that a sustained therapeutic efficacy could be achieved through local or intra-articular injection of IND-NMs. Furthermore, they suggest that amphiphilic copolymers could be utilized as injectable drug carriers for hydrophobic drugs. Wang et al. [74] developed low-dose glucocorticoidal therapy by utilizing micelle loaded with dexamethasone (DEX). They synthesized the polymeric micelle using poly (ethylene glycol)-block–poly (ε-caprolactone) (PCL-PEG) by self-assembly process. DEX incorporated into the PCL-PEG micelles showed an encapsulation of 94.2% and critical micellar concentration of 7.2 μg/mL. Cellular behaviour in Raw 264.7 cells showed a viability of 90%, whereas the free drug showed a viability of 70%. These results confirmed the conjugation of PCL-NPC and PCL-PEG in NMR analysis. They found that, DEX-NMs may afford a targeted delivery to inflamed joints. These indicate the potential of DEX-NMs at lower dose for the treatment of inflammatory diseases. Helmy et al. [75] developed larnoxicam loaded nanomicelles (LX-NMs) by direct equilibrium technique for RA treatment. The hydrodynamic diameter and poly dispersive index of LX-NMs were found to be 169.45 nm and 0.243 respectively. In-vivo study revealed that LX-NMs exhibits better inhibitory effect on carrageenan-induced edema than free LX. LX-NMs showed effects comparable to that of diclofenac. In study period after 14th day, there was significant reduction in edema percentage and increased weight growth. They emphasized that, LX-NMs can be considered for RA treatment and inflammatory disorders. The different nanomicellar based delivery system adopted using various drugs were showed in Table 5 .
Table 5.
Nanomicelle based anti-rheumatic arthritic drugs.
| S. No | Drug Name | Carrier | Particle size (nm) / Zeta potential (mV) | Therapeutic effects | References |
|---|---|---|---|---|---|
| 1. | Cyclosporine A | Polysialic acid (PSA) -polycaprolactone (PCL) | PSA (73.8–107.5)/-- PCL (138.4–195.3)/-- |
Anti-rheumatic arthritic effect | [71] |
| 2. | Dexamethasone | Polymeric micelles (PEG) and pHPMAmLac | 50-100/-- | Anti-rheumatic arthritic effect | [72] |
| 3. | Indomethacin | PNIPAAm/EAB-PPPs | – | Anti-rheumatic arthritic effect | [73] |
| 4. | Dexamethasone | Polycaprolactone -Polyethlene glycol | 45/-- | Anti-rheumatic arthritic effect | [74] |
| 5. | Larnoxicam | Polymeric nanomicelle | 169.45/-- | Anti-inflammatory arthritic effect | [75] |
The significance of nanomicelles based formulation for the treatment of RA may afford tendency to break beyond critical micellar concentration, longer circulation behaviour, sustained release effect, improved bioavailability, enhanced solubility, increased accumulation of drug at the diseased inflamed site and prolonged effect.
6.5. Nanocapsules for the treatment of RA
Nanocapsules are sub-micron sized formulations ranging from 10 to 1000 nm, which contains one or more active materials (core) which forms a protective matrix (shell) [76]. The therapeutic substance may be in the form of liquid/solid/molecular dispersion surrounded by a polymeric membrane. Nanocapsules have recently attracted significant interest due to its protective coating, which is generally pyrophoric and gets effectively oxidized [[77], [78], [79]]. The main advantages of nanocapsules towards drug delivery applications were sustained release property, incremental drug selectivity, improved bioavailability and alleviation of drug toxicity [80]. In addition, polymeric nanocapsules enhance the safety and efficacy of drugs by increasing their aqueous solubility, protecting them from degradation, controlling release, enhancing bioavailability and tissue selectivity [[81], [82], [83], [84]]. The diversified applications of nanocapsules in various fields includes, agrochemicals, genetic engineering, cosmetics, wastewater treatments, adhesive component applications, delivery of the drug to tumours, radiotherapy and as liposomal nanocapsules in food science/ agriculture [85].
Bernardi et al. [86] synthesized indomethacin-loaded nanocapsules (Ind-NCs) and evaluated its acute and sub chronic edema in experimental rat models by comparing their results with free indomethacin by employing in-vivo models in carrageenan-induced acute oedema, sub-chronic oedema and CFA-induced arthritis. The developed Ind-NCs were more potent in both sub-chronic and in CFA induced the arthritis model, when compared to pure indomethacin. However, in CFA arthritis model, treated with Ind-NCs markedly inhibited the serum levels of the pro-inflammatory cytokines tumour necrosis factor A and IL-6 (83% and 84% sequentially), while the levels of the anti-inflammatory cytokine IL-10 were significantly increased. This indicates that gastrointestinal damage in Ind-NCs treated animals were significantly lesser in comparison to Indomethacin treated groups and these results suggested that these formulation might represent a promising alternative for treating chronic inflammatory diseases, with decreased undesirable effects. Friedrich et al. [87] developed tacrolimus loaded lipidic core nanocapsules (TAC-LCNCs) to afford systemic anti-arthritic properties using self-assembly process and they showed unimodal particles size distribution with an average particle size of 212 nm (PDI < 0.12) at an encapsulation of 99%. In-vivo studies elicits that the animals treated with TAC-LCNCs showed significant inhibition of paw oedema after intraperitoneal administration. They suggested that these LCNCs have potential to prevent hyperglycemia mediated adverse effect of the drug and they conclude that TAC-LCNCs served as novel nanomedicine for the treatment of inflammatory disease.
Boechat et al. [88] developed MTX-loaded lipidic core nanocapsules (MTX-LCNCs) and observed an averaged particle size and encapsulation efficiency of 172 nm and 20.0% respectively. They observed that MTX-LCNCs reduced both TNF-α and serum levels. MTX-LCNCs showed its efficacy by decreasing the pro-inflammatory cytokines and T-cell-derived cytokines such as interferon-gamma and interleukin-17 A than MTX solution. Furthermore, they concluded that these formulations were more effective towards inflammation control at doses 75% lower than conventional MTX.
In-vivo antioedematogenic activity for polyphenols (resveratrol and quercetin) co-encapsulated in lipid-core nanocapsules in complete freund’s adjuvant induced arthritis models was studied by Coradini et al. [89] and they observed inhibition of 37% to 55% among day 16th and 22nd day after arthritis induction with major histological changes (fibrosis in synovial tissue, cartilage and bone loss). Unlike conventionally arthritis treatment, drugs co-encapsulated in lipid-core nanocapsules did not alter hepatic biochemical markers (ALP, Alkaline phosphate; ALT, alanine aminotransferase; AST, aspartate aminotransferase). Finally, they conclude that lipid-core nanocapsule may be their efficacious as oedematogenic agents without hepatotoxic effects.
Rollett et al. [90] developed folic acid (FA) functionalized human serum albumin nanocapsules (HSA- NCs) for the targeted delivery to chronically activated macrophages specific for RA treatment using method avoiding toxic crosslinking chemicals and emulsifiers. The optimized formulation showed an average size of 443 nm with polydispersity index 0.066. They confirmed the FA distribution on the surface of HSA nanocapsules three-dimensionally after fluorescence labelling in confocal laser scanning microscopy (CLSM). Besides, particular binding and internalization of HSA-NCs by FRβ – positive and FRβ- negative macrophages, acquired from human peripheral blood mononuclear cells, was demonstrated by flow cytometry. FRβ- communicating macrophages demonstrated and expand binding for FA modified capsules compared with those without FA. This folate based nanocarrier may be used to target activated macrophages without affecting normal cells in RA treatment. The different nanocapsules based delivery system adopted using various drugs were showed in Table 6 .
Table 6.
Nanocapsules based anti-rheumatic arthritic drugs.
| S. No | Drugs | Carrier | Particle size (nm) /Zeta potential (mV) | Therapeutic effects | References |
|---|---|---|---|---|---|
| 1. | Indomethacin | Polymeric nanocapsules | 240/-6.9 | Anti-inflammatory and Anti-rheumatic arthritic effect | [86] |
| 2. | Tacrolimus | Lipid-core nanocapsules | 210/-13.05 | Anti-rheumatic arthritic | [87] |
| 3. | MTX | Lipid-core nanocapsules poly (ε-caprolactone) | 172/-13.1 | Anti-rheumatic arthritic and Anti-inflammatory effects | [88] |
| 4. | Resveratrol and Quercetin | Lipid-core Nano capsules | 200/-- | Anti-rheumatic arthritic effect | [89] |
| 5. | Human serum Albumin | Nano capsules | 443/-20 | Anti-rheumatic arthritic effect | [90] |
The significance of nanocapsules based formulation for the treatment of RA may afford longer site specific dose retention, rapid absorption of drug, improved bioavailability, higher dose loading, controlled release effect, enhanced solubility and improved therapeutic effect.
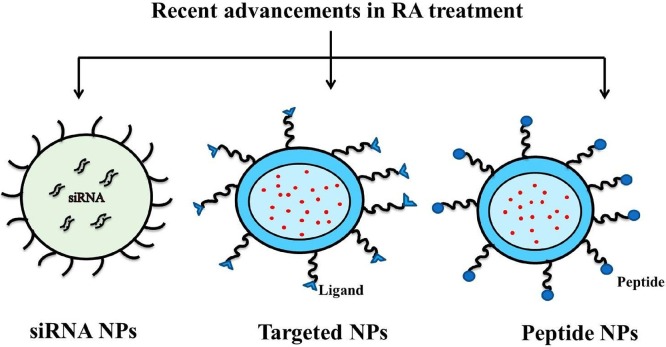
7. Recent advancement for the treatment of RA
Recently siRNA, peptides and targeted approaches based nanoformulation approaches were attempted for the effective treatment of RA (Fig. 5 ). The different approaches focussed towards the treatment of RA were shown below.
Fig. 5.
Recent advancements in RA treatment.
7.1. siRNA based nanoparticulate systems for the treatment of RA
Cellular mechanism responsible for post-transcriptional gene silencing acting on messenger RNA (mRNA) is termed as RNA interference (RNAi). Silencing attained with small interference RNA (siRNA) is transient; hence novel strategies were developed and reported to achieve more permanent silencing. Vectors encoded strategy such as short hairpin RNA (shRNA) may be also used for long-term stable cell silencing approach. Due to the excellent gene silencing potential of RNAi, it has attracted broad attention in terms of high specificity, significant effect, minor side effects and ease of synthesis. siRNA has the capacity for silencing a specific gene of interest. The problem associated with naked siRNA upon systemic administration was degradation with nucleases which ultimately shortens the siRNA circulation time in the blood stream. Moreover, siRNA gets engulfed through receptor mediated endocytosis, thereby escapes from the endosomal compartment with decreased therapeutic efficacy of naked siRNA. However, these issues have been recently addressed through the development of nanotechnology based products. In order to afford a better protection the siRNA has been encapsulated in to positively charged particles which effectively protects from serum degradation and off-target immunological effects. Recently, siRNA based delivery systems were utilized for the treatment of RA, especially providing an efficient distribution into peripherally inflamed tissue.
RNA interference is major phenomena mediated by small interfering RNA (siRNA), which is effective in gene silencing with a high degree of specificity. Komano et al. [91] developed wrapsome (WS) containing siRNA (core composed of a cationic lipid bilayer and siRNA complex enveloped in a neutral lipid bilayer in polyethylene glycol over the surface) (SiRNA/WS) and they checked its therapeutic activity in collagen induced arthritis (CIA) mice model, at an intravenous dose of Cy5-labeled siRNA/WS. They showed that the efficacies of siRNA-targeting tumor necrosis factor (TNF-α/WS) in CIA mice by arthritis score. They further quantified TNF-α and mRNA levels in the joints by real-time reverse transcriptase-polymerase chain reaction and observed that the intensity of the fluorescence remained higher up to 48 h after injection in Cy5-siRNA/WS. The siRNA/WS combined with CD11b+ macrophages and neutrophils in the inflamed synovium, suggested that the expression of various molecules is associated with the pathogenesis of RA. Hence, they conclude that treatment with TNF-α siRNA/WS reduces TNF-α mRNA level in the joints compared to control group. Finally, they emphasized the efficient delivery of siRNA to arthritic joints.
Scheinman et al. [92] developed RGD functionalized siRNA-loaded poly (lactide-co-glycolide) (PLGA) nanoparticles (NPs) as a nanosystem for delivery of STAT1 siRNA to joint tissues of CIA mouse model. They observed the nanoparticles property and stability of siRNA after encapsulation. Morphological examination reveals size range of RGD-NPs of 100–200 nm with net positive charge, which may be due to RGD functionalization. PLGA NPs protects the siRNA from serum degradation and presence of the RGD peptide on the NPs outer surface increases tissue uptake by 10–200 folds in arthritic mice. RGD functionalization increased lung delivery of nanoparticles in arthritic mice compared to control group. RGD functionalized PLGA nanoparticles encapsulated STAT1-targeted siRNAs may be more effective for the treatment of arthritis, possibly through a selective inhibition of macrophage and dendritic cell activation.
Lee et al. [93] developed nanocomplex of polymerized siRNA (poly-siRNA) focusing on TNF-α with thiolated glycol chitosan (tGC) polymers for the treatment of RA. They showed that, Poly-siRNA formed by self-polymerization of thiol groups at the 5′ end of sense and antisense strand of siRNA encapsulated with tGC polymers, results in polysiRNA- tGC nanoparticles (psi-tGC-NPs) with an average diameter of 370 nm. The macrophage culture system, psi-tGC-NPs showed that rapid cellular uptake and excellent in-vitro TNF-α gene silencing efficacy. In addition, psi-tGC-NPs showed higher accumulation at arthritic joint sites in CIA mice. The matrix metalloproteinase 3-specific nanoprobe and micro computed tomography results showed that intravenous injection of psi-tGC-NPs significantly inhibits inflammation and bone erosion in CIA mice, comparable to MTX (5 mg/kg). Therefore, the availability of psi-tGC-NP therapy that target specific cytokines may herald new era in the treatment of RA.
Park et al. [94] developed PLGA nanoparticles loaded with dexamethasone and siRNA for the treatment of RA. Here, dexamethasone was initially loaded into PLGA nanoparticles, and then drug-loaded PLGA nanoparticles were complexed with poly (-ethyleneimine) (PEI)/siRNA. For the assessment of co-delivery of siRNA and dexamethasone into human chondrocyte cell line (C28/I2), cells were transfected with green fluorescence protein siRNA (GFP siRNA) and drugs. After transfection with GFP siRNA, 70% reduction of C28/I2 cells demonstrated GFP expression, whereas MOCK carrying PLGA nanoparticles and PLGA nanoparticles without siRNA does not showed any remarkable differences of GFP expressions. Combined dexamethasone and COX-2 siRNA treatment clearly reduced the expression of inflammatory and apoptosis related factors produced in C28/I2 cells, which were induced to enter an inflammatory state by TNF-a (10 ng/mL). COX-2 and inducible nitric oxide (iNOS) productions in C28/I2 cells were examined after TNF- α pre-treatment to induce expression of arthritis-related molecules in-vitro. Finally, they conclude that the reduction of gene and protein expression associated with arthritis by transfection with dexamethasone-loaded and COX-2 siRNA-complexed PLGA nanoparticles.
Desai et al. [95] designed cyclic cationic head lipid-polymer hybrid nanocarriers (CyLiPns) encapsulated with anti-nociception agent Capsaicin (Cap) and anti-TNFα siRNA (siTNFα) for the treatment of chronic skin inflammations. Physico-chemical characterizations including hydrodynamic size, surface potential and entrapment efficacies of CyLiPns were found to be 163 ± 9 nm, 35.14 ± 8.23 mV and 92% respectively for Cap. In-vitro skin distribution studies showed that CyLiPns could effectively deliver FITC-siRNA up to 360 μm skin depth. Further, enhanced Cap permeation from CyLiPns was observed compared to capsaicin-solution and capzasin-HP. Therapeutic efficacies of CyLiPns were assessed using imiquamod-induced psoriatic plaque model. CyLiPns carrying both Cap and siTNFα showed significant reduced expression of TNF-α, NF-κB, IL-17, IL-23 and Ki-67 genes compared to either drugs alone and were in close comparison with Topgraf®.
Duan et al. [96] designed core shell nanoparticles loaded with siRNA for the effective treatment of arthritis. They prepared the nano carrier by (polyethyleneimine [PEI]-super paramagnetic iron oxide nanoparticle [SPIO]), composed of a core of iron oxide with a shell of PEI for the delivery of SiRNA. Nanoparticles were administered intravenously to arthritic rats to analyze cellular uptake, tissue distribution and the therapeutic effect of siRNA against the IL-2/-15 receptor b chain (IL-2/IL-15R). PEI-SPIO-delivered siRNA accumulated easily in inflamed joints and was efficiently taken up by joint macrophages and T cells. In-vitro studies suggested that PEI-SPIOs loaded with siRNA exhibited negligible cytotoxicity, improved siRNA stability and induces specific gene silencing in-vitro. PEI-functionalized SPIOs used for systemic siRNA delivery in RA with enhanced therapeutic benefit by application of external magnetic field.
7.2. Targeted nanoparticulate systems for the treatment of RA
Drug delivery systems can be further improved for its therapeutic efficacy and specificity towards the treatment of various disorders using active targeting ligands such as antibody, peptide and polysaccharides. The two modes of targeting were active and passive. In the treatment of RA, the nanoparticulate formulation has been targeted towards the selectively expressed CD44 surface receptors. Mediators such as growth factors, pro-inflammatory cytokines, chemokines, cell adhesion molecules, and proteases plays vital role in the RA development. In this strategy angiogenesis and inflammation were the conditions associated with RA progression. The CD44, CD64, folate receptor-beta (FR-β), vasoactive intestinal peptide (VIP) receptor, and scavenger receptor class A, toll-like receptors, transforming growth factor-beta receptors were over expressed in macrophages. Whereas αvβ3 integrins, E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intercellular cell adhesion molecule-1 were predominantly expressed during angiogenic conditions.
MTX-loaded poly (lactic-co-glycolic acid, PLGA) gold (Au)/iron (Fe)/gold (Au) half-shell nanoparticles (NPs) combined with arginine-glycine-aspartic acid (RGD), was developed by Kim et al. [97]. They accomplished average diameter of NPs about 135 nm with estimated MTX loading of 2 wt %. During near-infrared (NIR) irradiation, heat generated at the inflammed site due to the NIR resonance of Au half-shells which MTX release from PLGA nanoparticles. They found that, Fe half-shell layer inserted between the Au half-shell layers facilitates in-vivo T2-magnetic resonance (MR) imaging besides to NIR absorbance imaging. Moreover, the distribution of the nanoparticles to the inflammed region in collagen-induced arthritic (CIA) mice, and their retention can be enhanced under the influence of external magnetic field. When combined with successive NIR irradiation and external magnetic field results in higher therapeutic effects compared to conventional treatment despite the use of MTX dosages 0.05% dosage of NPs.
Jain et al. [98] developed an alginate based nanoparticles encapsulated with anti-inflammatory (IL-10) cytokine encoding plasmid DNA. They found that the average particle size of the unmodified NPs was 470 nm. They modified the surface of the nano-carriers by scrambled and tuftsin peptide and particle size was found to be 327.5 ± 3.5 nm and 299.7 ± 2.2 nm respectively with a spherical morphology. Nanoparticle treatment in arthritic rats significantly reduces systemic and joint tissue pro-inflammatory cytokines (TNF-α, IL-1b, and IL-6) expression, which prevents the progression of inflammation, joint damage as revealed by magnetic resonance imaging and histology. They observed an enhanced localization in tuftsin-peptide modified alginate nanoparticles into the limbs of arthritic animals when compared to unmodified and scrambled-peptide modified nanoparticles. Consequently, single dose of the targeted formulation loaded with IL-10 plasmid DNA demonstrated superior transfection efficiency with sustained local and systemic IL-10 expression (leading to alleviation of pro-inflammatory cytokines), with reduction in paw edema. These targeted alginate nanoparticle loaded with IL-10 plasmid DNA can efficiently re-polarize macrophages from M1 to an M2 state, offering a novel treatment for chronic inflammatory diseases.
In general normal inflammatory process, resolution was mediated by several agonists, among which the glucocorticoid - regulated protein is called annexin A1 also plays vital role. The pro-resloving action of annexin A1was mediated by N-formyl peptide receptor 2 (FPR2/ALX). Fredman et al. [99] mimicked a FPR2/ALX receptor activity by amino-terminal peptide encompassing amino acids 2-26 (Ac 2-26) co-loaded with collagen IV (Col IV)-targeted nanoparticles (NPs). They accomplished particle size of around ∼100 nm with charge of +30 mV for Col IV-Ac 2-26 NPs. Col IV-Ac 2-26 NPs were administered to preexisting lesions leads to improvement in protective collagen layer overlying lesions with decreased lesional collagenase activity, decrease in plaque necrosis and suppression of oxidative stress. Thus, administration of a resolution-mediating peptide (Col IV-Ac 2-26 NPs) activates its receptor on myeloid cells to stabilize advanced atherosclerotic lesions. In-vivo studies suggested that mice lacking FPR2/ALX in myeloid cells doesn’t showed these improvements.These findings support the concept that defective inflammation resolution plays a role in advanced atherosclerosis, and suggest a new form of therapy.
Yang et al. [100] selectively target synovial joints in autoimmune arthritis of joint vasculature. They utilized the adjuvant induced arthritis model of human rheumatoid arthritis, and profiled the synovial vasculature (both ex-vivo and in-vivo) of a defined phage peptide-display library. They found that synthetic peptides showed binding to the joint-derived endothelial cells, as well as specificity in inhibiting binding of the respective phage to the synovial vasculature. Intriguingly, the treatment of arthritic rats with one such peptide resulted in efficient inhibition of arthritis progression. However, the suppression of arthritis was achieved via peptide-induced reduction of T-cell trafficking into the joints and inhibition of angiogenesis. Furthermore peptide differed in sequence, in receptor binding specificity, and in angiogenesis/inflammation-related cell signaling from the previously characterized arginine-glycine-aspartic acid-containing peptide. They showed that, peptides further exploited for the selective distribution of anti-arthritic agents into the inflamed joints to improving its efficacy, and reduces systemic toxicity.
7.3. Peptide based nanoparticulate systems for the treatment of RA
Peptides were recently gaining keen interest towards drug delivery for various therapeutic approaches. Bioactive peptides derived from natural proteins sources (milk, egg, plants, fish, meat etc) upon enzymatic proteolysis exert biological activities for various disorders. Most peptides exhibit antihypertensive, anti-inflammatory, antidiabetic, anticancer, antimicrobial and antioxidant properties. Casein hydrolysate has been effective against inflammation. The problems associated with peptides were low bioavailability, metabolic liability, degradation in gastro intestinal tract, low absorption, inability to cross epithelial barriers. Solid-phase peptide synthesis, ring-opening polymerization, and protein engineering techniques were utilized to incorporate/assemble the peptides in to the nanostructures. Nanoparticulate self-assemblies of different forms of peptides such as dipeptides, cyclic peptides, amphiphilic peptides, α-helical peptides, and β-sheet peptides have been utilized. Recently, peptides based delivery systems were utilized for the treatment of RA, especially from natural sources and synthetic peptides.
Zhou et al. [101] found that, melittin-derived cationic amphipathic peptide combined with siRNA acts as active targeting agent for p65 subunit of NF-KB (p5RHH-p65). Administration of p5RHH-p65 siRNA nanocomplexes down modulate inflammatory cytokines production and the expression of p65 specifically in the joints, which protect against bone erosions and preserved cartilage integrity. They identified that, p5RHH-p65 siRNA nanocomplexes potently suppressed early inflammatory arthritis without affecting p65 expression in off-target organs or elicits a humoral immune response after serial injections. Their result suggested that self-assembled p5RHH-p65 siRNA nanocomplexes act as nontoxic platform for specific delivery of siRNA to target and constrain inflammatory processes for the treatment of a variety of diseases.
Protein citrullination is an important posttranslational modification that has increased attention particularly for its involvement RA Tutturen et al. [102] evaluated the citrullinome in RA synovial fluid by direct LC–MS/MS analysis and performed an enrichment approach based on citrulline specific biotinylation technique. They analysed that, RA synovial fluid was depleted with abundant proteins, total and depleted fractions. They suggested that, total and depleted with synovial fluid after enrichment was found to be 3600 and 2100, respectively whereas without enrichment it shows 119 and 157 citrullinated peptides. Frequency of citrullinated peptides and their degree of citrullination were determined for four known RA autoantigens, as well as a novel in-vivo auto citrullination site of peptidylarginine deiminase 4. Their result indicated that direct analysis allows identification of one a fraction of the citrullinated proteins present in synovial fluid and that specific enrichment is still needed for a comprehensive in-depth elucidation of the citrullinome.
Shen et al. [103] designed immunomodulatory peptide that targets T-Cell receptor (TCR) which critically plays major role in immune diseases such as autoimmune arthritis. They used Signaling Chain HOmo OLigomerization (SCHOOL) approach and acute respiratory syndrome coronavirus (SARS-CoV) fusion peptide sequence for targeting TCR. In addition TCR Core peptide has been shown for the treatment for human T cell-mediated dermatoses that can substitute for corticosteroids. Incorporation of the peptide into self-assembling lipopeptide nanoparticles that mimic native human high density lipoproteins essentially increases peptide dosage efficacy. They found that, peptide improves collagen-induced arthritis in DBA/1 J mice and ensures against bone and cartilage damage.
Rush et al. [104] developed degradable and non-degradable poly (NIPAm-AMPS) nanoparticles encapsulated with cell-penetrating anti-inflammatory peptide (KAFAK) to suppress proinflammatory cytokines such as TNF-α and IL-6 in inflamed cartilage explants. They checked KAFAK-loaded poly (NIPAm-AMPS) activity in in-vitro in human macrophage model (THP1 human monocytes) and ex-vivo in bovine knee cartilage tissue poly (NIPAm-AMPS). Their results showed a dose-dependent suppression of pro-inflammatory cytokines upon treatment with KAFAK-loaded poly (NIPAm-AMPS) nanoparticles. Further, they demonstrated selective targeting and therapeutic efficacy of KAFAK when released from both degradable and non-degradable poly (NIPAm-AMPS) nanoparticles in in-vitro and ex-vivo models. They emphasized that, poly (NIPAm-AMPS) nanoparticles loaded with KAFAK could be an effective tool for the treatment of osteoarthritis.
8. Future perspectives for the treatment of RA
The natural therapies such as physical therapy, occupational therapy and psychosocial therapy relevantly supports the cure of autoimmune inflammatory diseases RA at present. Eventhough combinatorial therapies are providing a better response, the aspects of selectivity and toxicity to the healthy cells creates a major issues. The future treatment options for RA treatment apart from nanoparticulate formulations may focus towards the development of selective inhibitors of proinflammatory cytokines by the virtue applications of monoclonal antibodies, bioactive peptides and siRNA based delivery systems. Molecular biology and computational chemistry oriented focussing may offers a better design for developing formulations that specifically target pro-inflammatory cytokines.
9. Conclusions
The interest towards the development of nanoformulations for the treatment of RA has been increased in the recent years due to its versatile applications. These nanoformulations possess versatile properties such as biocompatibility, reduced dosing frequency, lowered doses, improved efficacy, sustained effect and decreased adverse effects. These nanosystems provide systemic, topical and oral delivery of most anti-rheumatic arthritic drugs (glucocorticoids/ NSAIDs etc). This review may permits the basic idea to focus nano based therapeutic approaches using pharmaceutical drugs, siRNA, peptides or phytoconstituents for the effective treatment of RA.
Acknowledgements
The authors gratefully acknowledge Department of Science and Technology (GoI), New Delhi supported National Facility for Drug Development for Academia, Pharmaceutical and Allied Industries (NFDD) (Ref No. VI- D&P/349/10-11/TDT/1 Dt: 21.10.2010). Author Mr. J. Kumar gratefully acknowledges the financial support (Senior Research Fellowship) received from Indian council of medical research, New Delhi (Ref No. 45/21/2018/NAN/BMS. Dt. 05.06.2018).
References
- 1.Taylor P.C., Moore A., Vasilescu R., Alvir J., Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol. Int. 2016;36(5):685–695. doi: 10.1007/s00296-015-3415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorner T., Strand V., Cornes P., Goncalves J., Gulacsi L., Kay J., Kvien T.K., Smolen J., Tanaka Y., Burmester G.R. The changing landscape of biosimilars in rheumatology. Ann. Rheum Dis. 2016;75(6):974–982. doi: 10.1136/annrheumdis-2016-209166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2014. Pfizer Value of Medicines, Rheumatoid Arthritis and the Value of Treatment Global Policy and International Public Affairs.https://www.pfizer.com/files/health/VOMPaper_RheumatoidArthritis_11-3-2016.pdf (Accessed 12 December 2017. [Google Scholar]
- 4.Fearon U., Canavan M., Biniecka M., Veale D.J. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 2016;12:385–397. doi: 10.1038/nrrheum.2016.69. [DOI] [PubMed] [Google Scholar]
- 5.Svendsen A.J., Junker P., Houen G., Kyvik K.O., Nielsen C., Skytthe A., Holst R. Incidence of chronic persistent rheumatoid arthritis and the impact of smoking: a historical twin cohort study. Arthritis Care Res. 2016;69(5):616–624. doi: 10.1002/acr.22987. [DOI] [PubMed] [Google Scholar]
- 6.Yang M., Feng X., Ding J., Chang F., Chen X. Nanotherapeutics relieve rheumatoid arthritis. J. Control Release. 2017;252:108–124. doi: 10.1016/j.jconrel.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 7.McInnes I.B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–2337. doi: 10.1016/S0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 8.Mateen S., Zafar A., Moin S., Khan A.Q., Zubair S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta. 2016;455:161–171. doi: 10.1016/j.cca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Ursini F., Leporini C., Bene F., D’Angelo S., Mauro D., Russo E., De Sarro G., Olivieri I., Pitzalis C., Lewis M., Grembiale R.D. Anti-TNF-alpha agents and endothelial function in rheumatoid arthritis: a systematic review and meta-analysis. Sci. Rep. 2017;7:5346. doi: 10.1038/s41598-017-05759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka Y. Current concepts in the management of rheumatoid arthritis. Korean J. Intern. Med. 2016;31(2):210–218. doi: 10.3904/kjim.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McInnes I.B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet . 2017;389:2328–2337. doi: 10.1016/S0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 12.Kraft C.T., Agarwal S., Ranganathan K., Wong V.W., Loder S., Li J., Delano M.J., Levi B. Trauma induced heterotopic bone formation and the role of the immune system: a review. J Trauma Acute Care Surg. 2016;80(1):156–165. doi: 10.1097/TA.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones G., Nash P., Hall S. Advances in rheumatoid arthritis. Med. J Aust. 2017;206(5):221–224. doi: 10.5694/mja16.01287. [DOI] [PubMed] [Google Scholar]
- 14.Schett G., Emery P., Tanaka Y., Burmester G., Pisetsky D.S., Naredo E., Fautrel B., Vollenhoven R.V. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann. Rheum Dis. 2016;75:1428–1437. doi: 10.1136/annrheumdis-2016-209201. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi G., Caporali R., Todoerti M., Mattana P. Methotrexate and rheumatoid arthritis: current evidence regarding subcutaneous versus oral routes of administration. Adv. Ther. 2016;33(3):369–378. doi: 10.1007/s12325-016-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrappa M., Biswas S. Glucocorticoids in management of adult rheumatoid arthritis - current prescribing practices and perceptions of physicians in India: glumar survey. Rheumatol.: Curr. Res. 2017;7(2):220. [Google Scholar]
- 17.Movahedi M., Beauchamp M.E., Abrahamowicz M., Ray D.W., Michaud K., Pedro S., Dixon W.G. Risk of incident diabetes mellitus associated with the dosage and duration of oral glucocorticoid therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2016;68(5):1089–1098. doi: 10.1002/art.39537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cadavid A.P. Aspirin: the mechanism of action revisited in the context of pregnancy complications. Front. Immunol. 2017;8(261):1–27. doi: 10.3389/fimmu.2017.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Rashed F., Calay D., Lang M., Thornton C.C., Bauer A., Kiprianos A., Haskard D.O., Seneviratne A., Boyle J.J., Schonthal A.H., Wheeler-Jones C.P., Mason J.C. Celecoxib exerts protective effects in the vascular endothelium via COX-2-independent activation of AMPK-CREB-Nrf2 signalling. Sci. Rep. 2018;8(1):6271. doi: 10.1038/s41598-018-24548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown P.M., Pratt A.G., Isaacs J.D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. 2016;12:731. doi: 10.1038/nrrheum.2016.175. [DOI] [PubMed] [Google Scholar]
- 21.Comi G., Freedman M.S., Kappos L., Olsson T.P., Miller A.E., Wolinsky J.S., O’Connor P.W., Benamor M., Dukovic D., Truffinet P., Leist T.P. Pooled safety and tolerability data from four placebo-controlled teriflunomide studies and extensions. Multiple Scler. Relat. Disorders. 2016;5:97–104. doi: 10.1016/j.msard.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Lucas S. The pharmacology of indomethacin. Headache. 2016;56(2):436–446. doi: 10.1111/head.12769. [DOI] [PubMed] [Google Scholar]
- 23.Chukwudi C.U. Ribosomal RNA binding sites and the molecular mechanism of action of the tetracyclines. Antimicrob. Agents Chemother. 2016;60(8):4433–4441. doi: 10.1128/AAC.00594-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nissen S.E. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N. Engl. J. Med. 2017;376(14):1390. doi: 10.1056/NEJMc1702534. [DOI] [PubMed] [Google Scholar]
- 25.Ma C., Li F., Musharrafieh R.G., Wang J. Discovery of cyclosporine a and its analogs as broad-spectrum anti-influenza drugs with a high in vitro genetic barrier of drug resistance. Antiviral Res. 2016;133:62–72. doi: 10.1016/j.antiviral.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodge J.A., Kawabata T.T., Krishnaswami S., Clark J.D., Telliez J.B., Dowty M.E., Menon S., Lamba M., Zwillich S. The mechanism of action of tofacitinib – an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 2016;34(2):318–328. [PubMed] [Google Scholar]
- 27.Morisset J., Johannson K.A., Vittinghoff E., Aravena C., Elicker B.M., Jones K.D., Fell C.D., Manganas H., Dube B.P., Wolters P.J., Collard H.R., Ryerson C.J., Ley B. Use of mycophenolate mofetil or azathioprine for the management of chronic hypersensitivity pneumonitis. Chest. 2017;151(3):619–625. doi: 10.1016/j.chest.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meredith A.M., Dass C.R. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J. Pharm. Pharmacol. 2016;68(6):729–741. doi: 10.1111/jphp.12539. [DOI] [PubMed] [Google Scholar]
- 29.Li H.Z., Xu X.H., Lin N., Lu H.D. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann. Rheum Dis. 2018;77(1):98–103. doi: 10.1136/annrheumdis-2017-211836. [DOI] [PubMed] [Google Scholar]
- 30.Garg V., Singh H., Bhatia A., Raza K., Singh S.K., Singh B., Beg S. Systematic development of transethosomal gel system of piroxicam: formulation optimization, in vitro evaluation, and ex vivo assessment. AAPS PharmSciTech. 2017;18(1):58–71. doi: 10.1208/s12249-016-0489-z. [DOI] [PubMed] [Google Scholar]
- 31.Brown P.M., Pratt A.G., Isaacs J.D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. Rheumatol. 2016;12(12):731–742. doi: 10.1038/nrrheum.2016.175. [DOI] [PubMed] [Google Scholar]
- 32.Rius B., Claria J. Principles, mechanisms of action, and future prospects of anti-inflammatory drugs. NSAIDs Aspirin. 2016:17–34. [Google Scholar]
- 33.Scavone C., Bonagura A.C., Fiorentino S., Cimmaruta D., Cenami R., Torella M., Fossati T., Rossi F. Efficacy and safety profile of diclofenac/cyclodextrin and progesterone/cyclodextrin formulations: a review of the literature data. Drugs R D. 2016;16(2):129–140. doi: 10.1007/s40268-016-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan A., de Seze J., Comabella M. Teriflunomide in patients with relapsing–remitting forms of multiple sclerosis, CNS. Drugs. 2016;30(1):41–51. doi: 10.1007/s40263-015-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahadar H., Maqbool F., Niaz K., Abdollahi M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed. J. 2016;20(1):1–11. doi: 10.7508/ibj.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu L., Qi X., Li X., Bai Y., Liu H. Recent advances in applications of nanomaterials for sample preparation. Talanta. 2016;146:714–726. doi: 10.1016/j.talanta.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 37.Panahi Y., Farshbaf M., Mohammadhosseini M., Mirahadi M., Khalilov R., Saghfi S., Akbarzadeh A. Recent advances on liposomal nanoparticles: synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017;45(4):788–799. doi: 10.1080/21691401.2017.1282496. [DOI] [PubMed] [Google Scholar]
- 38.Heloise R., Danhier F., Preat V., Langer R., Anderson D.G. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert Opion Drug. Deliv. 2017;14(7):851–864. doi: 10.1080/17425247.2016.1244187. [DOI] [PubMed] [Google Scholar]
- 39.Dolati S., Sadreddini S., Rostamzadeh D., Ahmadi M., Jadidi-Niaragh F., Yousefi M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed. Pharmacother. 2016;80:30–41. doi: 10.1016/j.biopha.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Nogueira E., Gomes A.C., Preto A., Cavaco-Paulo A. Folate-targeted nanoparticles for rheumatoid arthritis therapy. Nanomedicine. 2016;12(4):1113–1126. doi: 10.1016/j.nano.2015.12.365. [DOI] [PubMed] [Google Scholar]
- 41.Thao le Q., Byeon H.J., Lee C., Lee S., Lee E.S., Choi H.G., Park E.S., Youn Y.S. Pharmaceutical potential of tacrolimus-loaded albumin nanoparticles having targetability to rheumatoid arthritis tissues. Int. J Pharm. 2016;497(1–2):268–276. doi: 10.1016/j.ijpharm.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Behzadi S., Serpooshan V., Tao W., Hamaly M.A., Alkawareek M.Y., Dreaden E.C., Brown D., Alkilany A.M., Farokhzad O.C., Mahmoudi M. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 2017;46(14):4218–4244. doi: 10.1039/c6cs00636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolati S., Sadreddini S., Rostamzadeh D., Ahmadi M., Jadidi-Niaragh F., Yousefi M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed. Pharmacother. . 2016;80:30–41. doi: 10.1016/j.biopha.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed T.M., Aljaeid B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug. Des. Devel Ther. 2016;10:483–507. doi: 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao S.D. Rheumatoid arthritis (ra) disease treatment with rutin stabilized nanoparticles. Austin J. Biotechnol. Bioeng. 2015;2(2):1043. [Google Scholar]
- 46.Lee S.N., Kim H.J., Ha Y.J., Park Y.N., Lee S.K., Park Y.B., Yoo K.H. Targeted chemo-photothermal treatments of rheumatoid arthritis using gold half-shell multifunctional nanoparticles. ACS. 2012;7(1):50–57. doi: 10.1021/nn301215q. [DOI] [PubMed] [Google Scholar]
- 47.Kishore N., Raja M.D., Kumar C.S., Dhanalekshmi U., Srinivasan R. Lipid carriers for delivery of celecoxib: in-vitro, in-vivo assessment of nanomedicine in rheumatoid arthritis. Eur. J. Lipid Sci. Technol. 2016;118:949–958. [Google Scholar]
- 48.Kumar V., Leekha A., Tyagi A., Kaul A., Mishra A.K., Verma A.K. Preparation and evaluation of biopolymeric nanoparticles as drug delivery system in effective treatment of rheumatoid arthritis. Pharm. Res. 2017;34(3):654–667. doi: 10.1007/s11095-016-2094-y. [DOI] [PubMed] [Google Scholar]
- 49.Dewangan A.K., Varkey S., Mazumder S. Synthesis of curcumin loaded CMCAB nanoparticles for the treatment of rheumatoid arthritis. IJCEBS. 2015:18–19. [Google Scholar]
- 50.Lee S.M., Kim H.J., Ha Y.J., Park Y.N., Lee S.K., Park Y.B., Yoo K.H. Targeted chemo-photothermal treatments of rheumatoid arthritis using gold half-shell multifunctional nanoparticles. ACS Nano. 2013;7(1):50–57. doi: 10.1021/nn301215q. [DOI] [PubMed] [Google Scholar]
- 51.Thao L.Q., Byeon H.J., Lee C., Lee S., Lee E.S., Choi H.G., Park E.S., Youn Y.S. Pharmaceutical potential of tacrolimus-loaded albumin nanoparticles having targetability to rheumatoid arthritis tissues. Int. J. Pharm. 2016;497:268–276. doi: 10.1016/j.ijpharm.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Gupta A., Eral H.B., Hatton T.A., Doyle P.S. Nanoemulsions: formation, properties and applications. RSC. 2016;1:1–17. doi: 10.1039/c5sm02958a. [DOI] [PubMed] [Google Scholar]
- 53.Tang S.Y., Sivakumar M., Ng A.M., Shridharan P. Anti-inflammatory and analgesic activity of novel oral aspirin-loaded nanoemulsion and nano multiple emulsion formulations generated using ultrasound cavitation. Int. J. Pharm. 2012;430:299–306. doi: 10.1016/j.ijpharm.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 54.Salim N., Basri M., Rahman M.B., Abdullah D.K., Basri H. Modification of palm kernel oil esters nanoemulsions with hydrocolloid gum for enhanced topical delivery of ibuprofen. Int. J. Nanomed. 2012;7:4739–4747. doi: 10.2147/IJN.S34700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ostertag F., Weiss J., McClements D.J. Low-energy formation of edible nanoemulsions: factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012;388(1):95–102. doi: 10.1016/j.jcis.2012.07.089. [DOI] [PubMed] [Google Scholar]
- 56.Mello S.B., Tavares E.R., Guido M.C., Maranhao R.C. Anti-inflammatory effects of intravenous methotrexate associated with lipid nanoemulsions on antigen-induced arthritis. Clinics. 2016;71(1):54–58. doi: 10.6061/clinics/2016(01)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoscheid J., Outuki P.M., Kleinubing S.A., Silva M.F., Bruschi M.L., Cardoso M.L.C. Development and characterization of Pterodon pubescens oil nanoemulsions as a possible delivery system for the treatment of rheumatoid arthritis. Colloids Surf. A. Physicochem. Eng. Aspects. 2015;484:19–27. [Google Scholar]
- 58.Pathan I., Mangle M., Bairagi S. Design and characterization of nanoemulsion for transdermal delivery of meloxicam. Anal. Chem. Lett. 2016;6(3):286–295. [Google Scholar]
- 59.Mello S.B., Tavares E.R., Bulgarelli A., Bonfa E., Maranhao R.C. Intra-articular methotrexate associated to lipid nanoemulsions: anti-inflammatory effect upon antigen-induced arthritis. Int. J. Nanomed. 2013;8:443–449. doi: 10.2147/IJN.S29392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar R., Singh A., Garg N., Siril P.F. Solid lipid nanoparticles for the controlled delivery of poorly water soluble non-steroidal anti-inflammatory drugs. Ultrason. Sonochem. 2018;40(Pt A):686–696. doi: 10.1016/j.ultsonch.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Geszke Moritz M., Moritz M. Solid lipid nanoparticles as attractive drug vehicles: composition, properties and therapeutic strategies. Mater. Sci. Eng. C. 2016;68:982–994. doi: 10.1016/j.msec.2016.05.119. [DOI] [PubMed] [Google Scholar]
- 62.Garg N.K., Singh B., Tyagi R.K., Sharma G., Katare O.P. Effective transdermal delivery of methotrexate through nanostructured lipid carriers in an experimentally induced arthritis model. Colloids Surf. B Biointerfaces. 2016;147:17–24. doi: 10.1016/j.colsurfb.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 63.Chavan D., Gangode B., Jadhav A., Patil M., Kshirsagar S. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. IJOD. 2017;5(2):56–70. [Google Scholar]
- 64.Arora R., Kuhad A., Kaur I.P., Chopra K. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur. J. Pain. 2014;19(7):940–952. doi: 10.1002/ejp.620. [DOI] [PubMed] [Google Scholar]
- 65.Peng L.H., Wei W., Shan Y.H., Chong Y.S., Yu L., Gao J.Q. Sustained release of piroxicam from solid lipid nanoparticle as an effective anti-inflammatory therapeutics in-vivo. Drug. Dev. Ind. Pharm. 2016;43(1):55–66. doi: 10.1080/03639045.2016.1220563. [DOI] [PubMed] [Google Scholar]
- 66.Bhalekar M.R., Madgulkar A.R., Desale P.S., Marium G. Formulation of piperine solid lipid nanoparticles (SLN) for treatment of rheumatoid arthritis. Drug. Dev. Ind Pharm. 2017;43(6):1003–1010. doi: 10.1080/03639045.2017.1291666. [DOI] [PubMed] [Google Scholar]
- 67.Raj R., Mongia P., Ram A., Jain N.K. Enhanced skin delivery of aceclofenac via hydrogel-based solid lipid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016;44(6):1434–1439. doi: 10.3109/21691401.2015.1036997. [DOI] [PubMed] [Google Scholar]
- 68.Ye J., Wang Q., Zhou X., Zhang N. Injectable actarit-loaded solid lipid nanoparticles as passive targeting therapeutic agents for rheumatoid arthritis. Int. J. Pharm. 2008;352:273–279. doi: 10.1016/j.ijpharm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Nunes S., Madureira A.R., Campos D., Sarmento B., Gomes A.M., Pintado M., Reis F. Solid lipid nanoparticles as oral delivery systems of phenolic compounds: overcoming pharmacokinetic limitations for nutraceutical applications. Crit. Rev. Food Sci. Nutr. 2017;57(9):1863–1873. doi: 10.1080/10408398.2015.1031337. [DOI] [PubMed] [Google Scholar]
- 70.Cagel M., Tesan F.C., Bernabeu E., Salgueiro M.J., Zubillaga M.B., Moretton M.A., Chiappetta D.A. Polymeric mixed micelles as nanomedicines: achievements and perspectives. Eur. J. Pharm. Biopharm. 2017;113:211–228. doi: 10.1016/j.ejpb.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 71.Wilson D.R., Zhang N., Silvers A.L., Forstner M.B., Bader R.A. Synthesis and evaluation of cyclosporine A – loaded polysialic acid-poly caprolactone micelles for rheumatoid arthritis. Eur. J. Pharm. Sci. 2014;51:146–156. doi: 10.1016/j.ejps.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 72.Crielaard B.J., Rijcken C.J., Quan L., van der Wal S., Altintas I., van der Pot M., Kruijtzer J.A., Liskamp R.M., Schiffelers R.M., van Nostrum C.F., Hennink W.E., Wang D., Lammers T., Storm G. Glucocorticoid-loaded core-cross-linked polymeric micelles with tailorable release kinetics for targeted therapy of rheumatoid arthritis. Angew. Chem. Int. Ed. 2012;51:1–6. doi: 10.1002/anie.201202713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang J.X., Yan M.Q., Li X.H., Qiu L.Y., Li X.D., Li X.J., Jin Y., Zhu K.J. Local delivery of indomethacin to arthritis-bearing rats through polymeric micelles based on amphiphilic polyphosphazene. Pharm. Res. 2007;24(10):1944–1953. doi: 10.1007/s11095-007-9322-4. [DOI] [PubMed] [Google Scholar]
- 74.Wang Q., Jiang J., Chen W., Jiang H., Zhang Z., Sun X. Targeted delivery of low-dose dexamethasone using PCL-PEG micelles for effective treatment of rheumatoid arthritis. J. Control Release. 2016;230:64–72. doi: 10.1016/j.jconrel.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 75.Helmy H.S., El-Sahar A.E., Sayed Shamma R.N., Salama A.H., Elbaz E.M. Therapeutic effects of lornoxicam-loaded nanomicellar formula in experimental models of rheumatoid arthritis. Int. J. Nanomed. 2017;2:7015–7023. doi: 10.2147/IJN.S147738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bale S., Khurana A., Reddy A.S., Singh M., Godugu C. Overview on therapeutic applications of microparticulate drug delivery systems. Crit. Rev. Ther. Drug. Carr. Syst. 2016;33(4):309–361. doi: 10.1615/CritRevTherDrugCarrierSyst.2016015798. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y., Li P., Truong-Dinh Tran T., Zhang J., Kong L. Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer. Nanomaterials (Basel) 2016;6(2):pii: E26. doi: 10.3390/nano6020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.J.P. Devissaguet, H. Fessi, F. Puisieux, Process for the preparation of dispersible colloidal systems of a substance in the form of nanocapsules. US Patent 5049322 (1991).
- 79.Radtchenko I., Sukhorukov G., Mohwald H. A novel method for encapsulation of poorly water-soluble drugs: precipitation in polyelectrolyte multilayer shells. Int. J. Pharm. 2002;242:219–223. doi: 10.1016/s0378-5173(02)00161-8. [DOI] [PubMed] [Google Scholar]
- 80.Jager A., Stefani V., Guterres S.S., Pohlmann A.R. Physico-chemical characterization of nanocapsule polymeric wall using fluorescent benzazole probes. Int. J Pharm. 2007;338(1-2):297–305. doi: 10.1016/j.ijpharm.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 81.Bernardi A., Frozza R.L., Meneghetti A., Hoppe J.B., Battastini A.M., Pohlmann A.R., Guterres S.S., Salbego C.G. Indomethacin-loaded lipid-core nanocapsules reduce the damage triggered by Aβ1-42 in Alzheimer’s disease models. Int. J Nanomed. 2012;7:4927–4942. doi: 10.2147/IJN.S35333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dimer F.A., Ortiz M., Pase C.S., Roversi K., Friedrich R.B., Pohlmann A.R., Burger M.E., Guterres S.S. Nanoencapsulation of olanzapine increases its efficacy in antipsychotic treatment and reduces adverse effects. JBN. 2014;10:1137–1145. doi: 10.1166/jbn.2014.1817. [DOI] [PubMed] [Google Scholar]
- 83.Haas S.E., Bettoni C.C., de Oliveira L.K., Guterres S.S., Dalla Costa T. Nanoencapsulation increases quinine antimalarial efficacy against plasmodium berghei in-vivo. Int. J. Antimicrob. Agents. 2009;34(2):156–161. doi: 10.1016/j.ijantimicag.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 84.Benvegnu D.M., Barcelos R.C., Boufleur N., Pase C.S., Reckziegel P., Flores F.C., Ourique A.F., Nora M.D., da Silva Cde B., Beck R.C., Burger M.E. Haloperidol-loaded polysorbate-coated polymeric nanocapsules decrease its adverse motor side effects and oxidative stress markers in rats. Neurochem. Int. 2012;61:623–631. doi: 10.1016/j.neuint.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 85.Kothamasu P., Kanumur H., Ravur N., Maddu C., Parasuramrajam R., Thangavel S. Nanocapsules: the weapons for novel drug delivery systems. Bio Impacts. 2012;2(2):71–81. doi: 10.5681/bi.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bernardi A., Zilberstein A.C., Jager E., Campos M.M., Morrone F.B., Calixto J.B., Pohlmann A.R., Guterres S.S., Battastini A.M. Effects of indomethacin-loaded nanocapsules in experimental models of inflammation in rats. Br. J. Pharmacol. 2009;158:1104–1111. doi: 10.1111/j.1476-5381.2009.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Friedrich R.B., Coradini K., Fonseca F.N., Guterres S.S., Beck R.C., Pohlmann A.R. Lipid-core nanocapsules improved anti edematogenic activity of tacrolimus in adjuvant-induced arthritis model. J. Nanosci. Nanotechnol. 2016;16(2):1265–1274. doi: 10.1166/jnn.2016.11673. [DOI] [PubMed] [Google Scholar]
- 88.Boechat A.L., de Oliveira C.P., Tarrago A.M., da Costa A.G., Malheiro A., Guterres S.S., Pohlmann A.R. Methotrexate-loaded lipid-core nanocapsules are highly effective in the control of inflammation in synovial cells and a chronic arthritis model. Int. J. Nanomed. 2015;10:6603–6614. doi: 10.2147/IJN.S85369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coradini K., Friedrich R.B., Fonseca F.N., Vencato M.S., Andrade D.F., Oliveira C.M., Battistel A.P., Guterres S.S., da Rocha M.I., Pohlmann A.R., Beck R.C. A novel approach to arthritis treatment based on resveratrol and curcumin co-encapsulated in lipid-core nanocapsules: in-vivo studies. Eur. J. Pharm. Sci. 2015;78:163–170. doi: 10.1016/j.ejps.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 90.Rollett A., Reitera T., Nogueira P., Cardinale M., Loureiro A., Gomes A., Cavaco-Paulo A., Moreira A., Carmo A.M., Guebitz G.M. Folic acid-functionalized human serum albumin nanocapsules for targeted drug delivery to chronically activated macrophages. Int. J. Pharm. 2012;427(2):460–466. doi: 10.1016/j.ijpharm.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 91.Komano Y., Yagi N., Onoue I., Kaneko K., Miyasaka N., Nanki T. Arthritic joint-targeting small interfering rna-encapsulated liposome: implication for treatment strategy for rheumatoid arthritis. J. Pharmacol. Exp. Ther. 2012;340(1):109–113. doi: 10.1124/jpet.111.185884. [DOI] [PubMed] [Google Scholar]
- 92.Scheinman R.I., Trivedi R., Vermillion S., Kompella U.B. Functionalized STAT1 siRNA nanoparticles regress rheumatoid arthritis in a mouse model. Nanomedicine. 2011;6(10):1669–1682. doi: 10.2217/nnm.11.90. [DOI] [PubMed] [Google Scholar]
- 93.Lee S.J., Lee A., Hwang S.R., Park J.S., Jang J., Huh M.S., Jo D.G., Yoon S.Y., Byun Y., Kim S.H., Kwon I.C., Youn I., Kim K. TNF-α gene silencing using polymerized siRNA/thiolated glycol chitosan nanoparticles for rheumatoid arthritis. Mol. Ther. 2014;22(2):397–408. doi: 10.1038/mt.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park J.S., Yang H.N., Jeon S.Y., Woo D.G., Kim M.S., Park K.H. The use of anti-COX2 siRNA coated onto PLGA nanoparticles loading dexamethasone in the treatment of rheumatoid arthritis. Biomaterials. 2012;33:8600–8612. doi: 10.1016/j.biomaterials.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 95.Desai P.R., Marepally S., Patel A.R., Voshavar C., Chaudhuri A., Singh M. Topical delivery of anti-TNFα siRNA and capsaicin via novel lipid-polymer hybrid nanoparticles efficiently inhibits skin inflammation in-vivo. J. Control Release. 2013;170:51–63. doi: 10.1016/j.jconrel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duan J., Dong J., Zhang T., Su Z., Ding J., Zhang Y., Mao X. Polyethyleneimine-functionalized iron oxide nanoparticles for systemic siRNA delivery in experimental arthritis. Nanomedicine. 2014;9(6):789–801. doi: 10.2217/nnm.13.217. [DOI] [PubMed] [Google Scholar]
- 97.Kim H.J., Lee S.M., Park K.H., Mun C.H., Park Y.B., Yoo K.H. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemophotothermal treatment of rheumatoid arthritis. Biomaterials. 2015;61:95–102. doi: 10.1016/j.biomaterials.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 98.Jain S., Tran T.H., Amiji M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials. 2015;61:162–177. doi: 10.1016/j.biomaterials.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fredman G., Kamaly N., Spolitu S., Milton J., Ghorpade D., Chiasson R., Kuriakose G., Perretti M., Farokhzad O., Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 2015;7(275):275ra20. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yanga Y.H., Rajaiaha R., Ruoslahtib E., Moudgil K.D. Peptides targeting inflamed synovial vasculature attenuate autoimmune arthritis. PNAS. 2011;108(31):12857–12862. doi: 10.1073/pnas.1103569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou H.F., Yan H., Pan H., Hou K.K., Akk A., Springer L.E., Hu Y., Allen J.S., Wickline S.A., Pham C.T.N. Peptide-siRNA nanocomplexes targeting NF-κB subunit p65 suppress nascent experimental arthritis. J. Clin. Invest. 2014;124(10):4363–4374. doi: 10.1172/JCI75673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tutturen A.E.V., Fleckenstein B., de Souza G.A. Assessing the citrullinome in rheumatoid arthritis synovial fluid with and without enrichment of citrullinated peptides. J. Proteome Res. 2014;13:2867–2873. doi: 10.1021/pr500030x. [DOI] [PubMed] [Google Scholar]
- 103.Shen Z.T., Sigalov A.B. SARS coronavirus fusion peptide-derived sequence suppresses collagen-induced arthritis in DBA/1J Mice. Sci. Rep. 2016;28(6):28672. doi: 10.1038/srep28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bartlett R.L., II, Sharma S., Panitch A. Cell-penetrating peptides released from thermo sensitive nanoparticles suppress pro-inflammatory cytokine response by specifically targeting inflamed cartilage explants. Nanomed.: Nanotechnol. Biol. Med. 2013;9:419–427. doi: 10.1016/j.nano.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]