Abstract
Parasitic agents are a common cause of diarrhea in dogs and cats and, thus, determining their prevalence is essential to establish preventive and control measures. This retrospective study examined the fecal tests records from 1111 dogs and 203 cats with diarrhea submitted to a diagnostic laboratory in the city of Medellin between January and May 2018. The detection of parasites was carried out by direct smears and simple flotation methods. Parasitic organisms were detected in feces from 464 (41.7%) dogs and 96 (47.3%) cats. In order of decreasing prevalence, the parasites detected in dogs were: Giardia intestinalis (13%), ancylostomids (12.6%), Entamoeba spp. (6.1%), coccidian oocysts (5.8%), Toxocara spp. (5.6%) and Dipylidium caninum (1.3%). In cats, the prevalence was: Giardia intestinalis (20%), coccidian oocysts (8.9%), Entamoeba spp. (7.9%), ancylostomids (6.4%), Toxocara spp. (2.5%) and Dipylidium caninum (2%). Age, but not gender, was a predisposing factor, as puppies and kittens had significantly higher infection rates that older age categories. The majority of Giardia intestinalis positive cases occurred in puppies (109/145, 75.2%) and kittens (19/36, 52.8%), making this parasite the most prevalent in amongst animals with diarrhea. Out of 117 positive infections in the adult dog population, ancylostomids accounted for 56 cases (47.9%) and was the most common parasite in this age group. In conclusion, although these results do not imply a cause and effect relationship, they are an estimate of the type of parasites that may be most commonly associated with diarrhea in dogs and cats. The lower diagnostic sensitivity of the traditional methods used here as compared to more contemporary techniques like fecal flotation with centrifugation and PCR, may have underestimated the actual prevalence and diminished the detection of co-infections. Future studies should aim to have diagnostic panels that also screen for other enteric pathogens, including bacterial and viral agents.
Keywords: Giardia intestinalis, Dogs, Cats, Diarrhea, Colombia
Highlights
-
•
Prevalence of parasitic infections in dogs and cats can alert of potential zoonotic diseases.
-
•
Giardia was the most common parasitic infection in dogs and cats with diarrhea in the city of Medellin, Colombia.
-
•
Puppies and kittens had greater percentage of infected animals than adults.
1. Introduction
Epidemiological studies are necessary to assess the burden of diseases in a population, compare prevalence in different populations, and examine trends of disease overtime. Unfortunately, although diarrhea is one of the most common problems in dogs and cats, identifying the underlying cause can be frustrating. A number of studies have examined the presence of enteropathogens and associated risk factors in populations of dogs and cats with enteric disease (Hackett and Lappin, 2003; Queen et al., 2012; Paris et al., 2014; Spain et al., 2001). Most of them have found that the presence of putative enteropathogens is often similar in diarrheic and nondiarrheic dogs and cats. Interpretation of the results is further confounded by co-infection with 2 or more enteropathogens that may have a synergistic relationship in causing diarrhea (Paris et al., 2014). In spite of these limitations on the interpretation of results from diagnostic panels, determining the epidemiological and prevalence factors associated with potential diarrheic pathogens can aid veterinarians in their diagnostic approach and estimating risk of infections in their patient community. The purpose of this study was to determine the prevalence of enteric parasites in dogs and cats with diarrhea attending 29 widely distributed veterinary practices in the city of Medellin.
2. Materials and methods
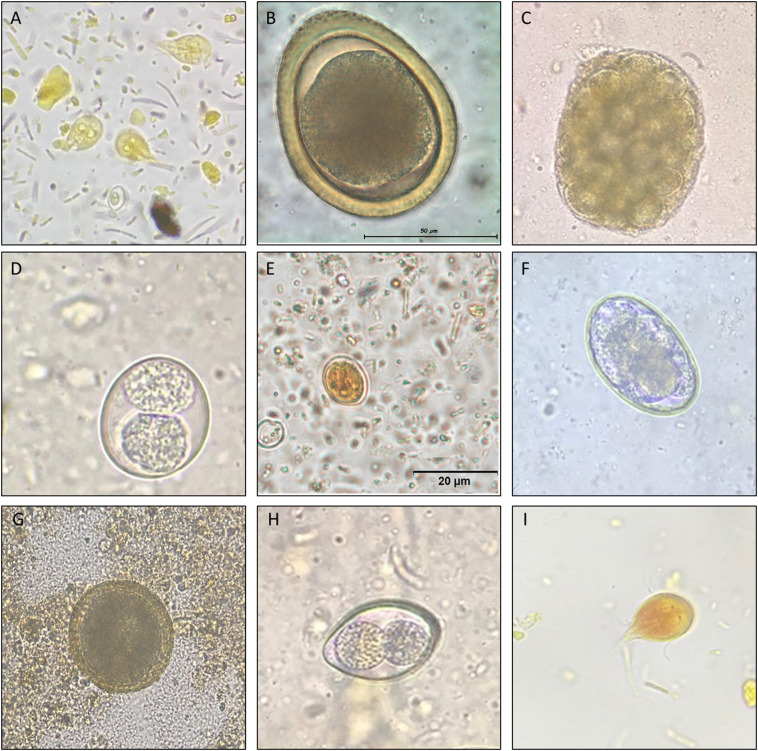
Between January 2018 and May 2018, fecal samples from dogs (n = 1111) and cats (n = 203) were submitted to a diagnostic laboratory in the city of Medellin. The samples were taken directly from the rectum or from already expelled feces and analyzed within 24 h. Additional data recorded from the submission forms (when available) included age, gender and presence of diarrhea. All samples were analyzed with three diagnostic tests: fecal smears (with lugol staining), flotation with saturated sucrose solution (sG1.3; 2 g of sample) and flotation with zinc sulphate solution (sG 1.2; 2 g of sample) (Deplazes et al., 2016). The first two techniques are currently the most commonly used as routine procedures in Colombian diagnostic laboratories. The sample was considered positive if at least one test was positive. Morphological keys were used to identify the type of helminth eggs, protozoan cysts, and yeast cells (Jacobs et al., 2016). Fig. 1 shows images taken from cases for the most common parasites found in this study. For the purpose of examination, a possible relationship between age and the presence or absence of parasitism, dogs were classified as: puppies (<6 months), and adults (1.1 to >7 y). Cats were categorized as kittens (0–6 months) and adults (6 m – 11 y).
Fig. 1.
Panel 1. Dogs (A-F) and Cats (G-I) parasites. A. Giardia intestinalis trophozoites. (100×). B. Toxocara sp. Eggs (100×). C. Dipylidium caninum egg packet (40×). D. Cystoisospora spp. (100×). E. Giardia intestinalis cysts (100×). F. Ancylostomideo egg (100×). G. Toxocara sp. egg (40×). H. Cystoisospora spp. (100×). I. Giardia intestinalis trophozoites. (100×). Technique fecal smear.
3. Results
3.1. Dogs
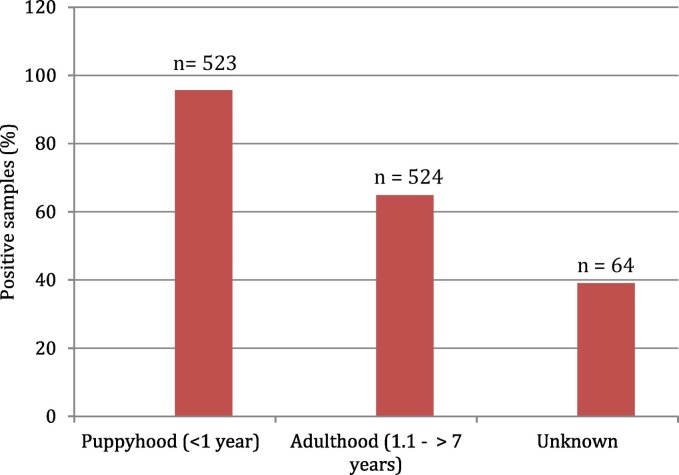
Parasitic organisms were detected in feces from 464 of 1111 (41.7%) dogs. In order of decreasing prevalence, the parasites detected were: G. intestinalis (13%), ancylostomids (12.6%), Entamoeba spp. (6.1%), coccidian oocysts (5.8%), Toxocara spp. (5.6%) and D. caninum (1.3%) (Table 1 ). Co-infections with 2 or more parasites were detected in 30 animals. The Chi-square test showed a significant association between age (excluding unknowns) and presence or absence of parasites (χ2 = 58.8; d.f = 3; P < 0.001) with a moderate size effect of Phi = 0.24. The puppy-age group had the most submissions (n = 369) and also the highest infection rate with 231 positive cases of the 369 (57.4%) fecal samples submitted (Fig. 2 ). This age group also accounted for the majority of animals infected with G. intestinalis and coccidian oocysts, with 109 of the 145 positive G. intestinalis cases, and 41 of the 65 positive coccidian cases (Table 1). The prevalence of Entamoeba spp., Toxocara spp. and D. caninum was also higher in the puppy-age group than the other age categories. Only the ancylostomids were more prevalent in the adult group (56 of 117 infected, 47.9%) than in the puppy group (29 of 231 infected, 12.6%). There was no significant difference (χ2 = 1.1; d.f = 1; p = 0.29) between the prevalence in males (42.2%, 265/349) and females (40.0%, 199/298).
Table 1.
Prevalence (%) of enteric parasites in dogs and cats from the city Medellin according to age category (n = 1111 dogs and n = 203 cats).
| Specie | AGE |
Cystoisospora spp. |
Entamoeba spp. |
Giardia intestinalis |
Ancylostomids |
Toxocara spp |
Dipylidium spp. |
Infected/sampled |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | ||
| Dogs | 6 months-1 year | 46 | (4.1%) | 31 | (2.8%) | 125 | (11.2%) | 51 | (4.6%) | 32 | (2.9%) | 10 | (0.9%) | 295/523 | (26.6%) |
| 1.1- >7 years | 16 | (1.4%) | 34 | (3.0%) | 14 | (1.3%) | 78 | (7.0%) | 28 | (2.4%) | 3 | (0.3%) | 173/524 | (16.5%) | |
| Not known | 3 | (0.3%) | 3 | (0.3%) | 6 | (0.5%) | 11 | (1.0%) | 2 | (0.2%) | 2 | (0.2%) | 27/64 | (2.4%) | |
| TOTAL | 65 | (5.8%) | 68 | (6.1%) | 145 | (13.0%) | 140 | (12.6%) | 62 | (5.6%) | 15 | (1.3%) | 495/1111 | (44.5%) | |
| Cats | < 6 months | 6 | (2.9%) | 6 | (2.9%) | 19 | (9.3%) | 3 | (1.5%) | 2 | (2.0%) | 2 | (2.0%) | 38/67 | (18.7%) |
| 6 m – >10 year | 12 | (5.9%) | 8 | (3.9%) | 17 | (8.3%) | 8 | (3.9%) | 2 | (2.0%) | 2 | (2.0%) | 49/123 | (24.1%) | |
| Not known | 0 | (0.0%) | 2 | (1.0%) | 4 | (2.0%) | 2 | (2.0%) | 1 | (0.5%) | 0 | 0,00% | 9/13 | (4.4%) | |
| TOTAL | 18 | 8.9% | 16 | 7.9% | 40 | 20.0% | 13 | 6.4% | 5 | 2.5% | 4 | 2.0% | 96/203 | (47.3%) | |
Fig. 2.
Prevalence of enteric parasites in dogs according to age categories in the city of Medellin. The total number of animals sampled for each group is depicted on top of the bars (n = 1111 animals).
3.2. Cats
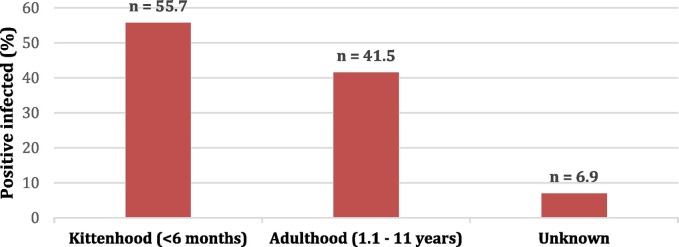
Parasitic organisms were detected in 96 of 203 (47.3%) cats. In order of decreasing prevalence, the type of parasites detected were: G. intestinalis (20%), coccidian oocysts (8.9%), Entamoeba spp. (7.9%), ancylostomids (6.4%), Toxocara spp. (2.5%) and D. caninum (2%) (Table 1). Co-infection with 2 or more agents was detected in 12 cats. The Chi-square test comparing kitten against adult combined showed a significant association between age and presence or absence of parasites (χ2 = 5.0; d.f = 1; P = 0.26) with a weak effect size of Phi = 0.16. The kitten age group had a higher percentage (50.1%; 34/67) of positive samples than the adult age group (40.5%, 47/116) (Fig. 3 ). Of the animals infected with G. intestinalis, this age group also had more animals infected with G. intestinalis (28.3%, 19/67) than adult cats (14.6%, 17/116) (Table 1). For the other parasite groups, the infection rates were similar between age groups. There was no significant difference (χ2 = 0.12; d.f = 1; p = 0.91) between the prevalence in males (43%, 43/60) and females (41.0%, 41/59).
Fig. 3.
Frequency of enteric parasites in cats in the city of Medellin according to age groups. The total number of cats sampled for each group is shown on top of each bar (n = 203).
4. Discussion
The objective of this retrospective study was to determine the prevalence of parasitic infections in a population of dogs and cats in Medellin. Several limitations of the study are important to mention. First, the sensitivity of the fecal tests used (smears and simple flotation) is lower compared to other more contemporary assays (PCR, fecal flotation with centrifugation, antigen detection). Direct examination of the intestinal tracts at necropsy or antigen detection methods have shown that passive techniques will fail to identify numerous infections, even when the evaluations were conducted under ideal conditions (Adolph et al., 2017). Consequently, it is likely that the real number of enteric parasites present in the population studied and the number of cases with co-infections were underestimated. Secondly, most fecal test panels should include assays to detect potential viral (coronavirus and parvovirus) and bacterial (Clostridium spp., Campylobacter spp., E. coli, Salmonella spp.) enteric pathogens. Cryptosporidium spp. Tritrichomonas foetus and Toxoplasma gondii are other protozoal agents that were not detected and have been found with different prevalence in similar epidemiological studies and are associated with diarrhea (Hackett and Lappin, 2003; Queen et al., 2012; Paris et al., 2014). A third limitation was the poor history provided with the submission forms, in particular describing the character of the diarrhea, whether it was acute or chronic (≥3 weeks), or compatible with a small or large bowel origin. Such information may aid interpreting the laboratory findings as the causes for diarrhea are not always infectious. For example, most cases of chronic diarrhea in dogs have been found not to be infectious but rather inflammatory enteropathies that are food, antibiotic, or steroid responsive (Volkman et al., 2017). In addition, this study did not consider the possibility of interference of coprophagic behavior in the dogs. Notwithstanding these limitations, the fecal tests used here can be considered very specific and unlikely to give false positive results. Consequently, and until more sophisticated assays become available in reference laboratories across the country, the results presented here can be used as a rough estimate of the prevalence of the most common parasitic infections in diarrheic dogs and cats in the urban Medellin area.
Of the different parasites detected in this study, G. intestinalis was the most frequent one. Other studies have also shown this parasite to be ubiquitous in dogs and cats, regardless of the presence of clinical signs (Adell-Aledón et al., 2018; Duijvestijn et al., 2016; Paris et al., 2014; Raza et al., 2018). A large scale study across the United States in dogs (n = 16,114) and cats (n = 4978) with gastrointestinal signs found a prevalence of 15.6% and 10.8%, respectively (Carlin et al., 2006). However, differences in prevalence were observed depending on age, history, and geographic location. Because G. intestinalis is also the most common intestinal parasite of humans, the potential zoonotic risk has traditionally been a concern. Recent molecular methods have classified Giardia organisms into assemblages that tend to be species-specific, suggesting low potential zoonotic threat. In people, assemblage clusters place them into A/B assemblages, dogs have C/D assemblages and cats have assemblages F (Adell-Aledón et al., 2018; Raza et al., 2018). However, questions remain as the amount of crossover between people and dogs and cats and what conditions promote this zoonotic transmission, since assemblages A/B have been found in dogs and cats (Adell-Aledón et al., 2018; Raza et al., 2018).
When contrasting our results with two previous studies in dogs from the Medellin area (Caraballo et al., 2007; Sierra-Cifuentes et al., 2015), their results were strikingly similar in spite of the limited number of animals examined. They found that ancylosmotid (Ancylostoma caninum and Uncinaria stenocephala) were the most common parasites in the adult age population, with prevalence ranging between 20.6 and 39.7%. With regards to G. intestinalis, both studies found prevalence between 8.8 and 13.9% that are similar to the 13% observed in this study; however, their puppy age category was under-represented and our results clearly indicate that age is a main predisposing factor as the majority of Giardia intestinalis positive cases occurred in puppies (109/145, 75.2%) and kittens (19/36, 52.8%). They also identified parasites not observed in the present study such as Tritrichomonas spp. and Trichuris spp. In fact, the second most commonly identified parasite in the study conducted exclusively in two shelter dog centers was Trichuris spp., with a 16.2% prevalence (Sierra-Cifuentes et al., 2015). In a review of gastrointestinal parasites from shelter dogs in various locations worldwide, Trichuris spp. are almost always reported (Raza et al., 2018) and are frequently present as the most prevalent parasite for shelter dogs (Scaramozzino et al., 2018). Many kennel and shelter situations are conducive to transmission with contaminated soil in dog runs and cages providing a constant source of infection. In addition, the incidence and parasite burden of trichuriasis seems higher in adult dogs compared to younger animals as there is no transmammary or transplacental routes of transmission.
Although the prevalence of Toxocara spp. were lower than expected (62/1111, 5.6%), particularly in puppies, it is one of the most common and neglected zoonotic parasitic infections worldwide (Rubinsky-Elefant et al., 2010). Ocular toxocariosis has been reported in children and typically presents as a unilateral retinal granuloma in the posterior pole of the peripheral retina (Ahn et al., 2014). In Medellin, 19 of 30 children referred to an ophthalmologic attention service during 2000–2001 had antibodies against T. canis, and of those, 6 were believed to be produced by the migration of T. canis larvae (Botero et al., 2001). Close contact with dogs was the most important risk factor found in the study. Another study conducted in 133 children in the Colombian Caribbean Coast showed a seroprevalence of 42.1% against the antigen secretion/excretion of L2 larvae of T. canis (Mendoza-Mena et al., 2010).
Because many of the parasites detected here have zoonotic potential, there is a need for additional studies to determine the genotype and species, in order to consider the real risk of transmission. In addition, it is important to improve the strategies to proper owner information about such infections risk and the responsibility of exerting proper pet ownership. This may include: deworming schemes with effective anthelmintics, regular fecal examinations, need of preventing their animals from defecating in public areas, and cleaning up feces from soil and pavements. Because there are no practical methods for reducing environmental egg burdens in soils and parks, preventing the initial contamination should be considered the most important control measure. In this respect and as ingestion or contact with items contaminated by dog and cat feces is the mode of transmission, veterinarians play and essential role in educating pet owners.
Ethical statement
The authors declare that written informed consent was obtained from the veterinarians participating in the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
The authors thank all professionals and small animal veterinary hospitals that accepted to collaborate in this study and the CIBAV thanks to the Strategy of consolidation of Research Groups CODI 2018-2019, University of Antioquia, Medellín, Colombia.
References
- Adell-Aledón M., Koster P.C., de Lucio A., Puente P. Occurance and molecular epidemiology of Giardia duodenalis infection in dog populations in eastern Spain. BMC Vet. Res. 2018;14:16. doi: 10.1186/s12917-018-1353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph C., Barnett S., Beall M., Drake J., Elsemore D., Thomas J., Little S. Diagnostic strategies to reveal covert infections with intestinal helminths in dogs. Vet. Parasitol. 2017;247:108–112. doi: 10.1016/j.vetpar.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Ahn S.J., Ryoo N.K., Woo S.J. Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention. Asia. Pac. Allergy. 2014;4:134–141. doi: 10.5415/apallergy.2014.4.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero J.H.M., Cañas L., Bravo J.D., Lopera M.O.N. Frecuencia de toxocarosis ocular en menores de edad remitidos al servicio de parasitología intestinal - Facultad de Medicina, Universidad de Antioquia; 2000-2001. Estudio piloto. Acta Médica Colomb. 2001;26:11–20. [Google Scholar]
- Caraballo A.J., Jaramillo A., Loaiza J. Prevalencia de parásitos intestinales en caninos atendidos en el centro de veterinaria y zootecnia de la Universidad CES. Rev. CES/Med. Vet. y Zoot. 2007;2:24–31. [Google Scholar]
- Carlin E.P., Bowman D.D., Scarlett J.M. Prevalence of Giardia in symptomatic dogs and cats throughout the United States as determined by the IDEXX SNAP Giardia test. Vet. Ther. 2006;7:199–206. [PubMed] [Google Scholar]
- Deplazes P., Eckert J., Mathis A., von Samson-Himmelstjerna G., Zahner H. Wageningen Academic Publishers; The Netherlands: 2016. Parasitology in Veterinary Medicine; p. 650. (978-90-8686-274-0) [Google Scholar]
- Duijvestijn M., Mughini-Gras L., Schuurman N., Schijf W., Wagenaar J.A., Egberink H. Enteropathogen infections in canine puppies: (Co-)occurrence, clinical relevance and risk factors. Vet. Microbiol. 2016;15(195):115–122. doi: 10.1016/j.vetmic.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett T., Lappin M.R. Prevalence of enteric pathogens in dogs of north-Central Colorado. J. Am. Anim. Hosp. Assoc. 2003;39:52–56. doi: 10.5326/0390052. [DOI] [PubMed] [Google Scholar]
- Jacobs D.E., Fox. M., Gibbons L.M., Hermosilla C. Principles of veterinary parasitology. In: West Sussex UK, Hoboken NJ, John Wiley & Sons Ltd, editors. Chichester. Wiley Blackwell; UK: 2016. 312 pp. [Google Scholar]
- Mendoza-Mena D.L., Lozan-Socarras S., Jaimes M.B. Exposición al parásito Toxocara canis en una población escolar de la comuna 7 del distrito de Santa Marta, Colombia. Revista de la Facultad de Ciencias de la Salud. 2010;7:183–190. [Google Scholar]
- Paris J.K., Wills S., Balzer H.J., Shaw D.J., Gunn-Moore D.A. Enteropathogen co-infection in UK cats with diarrhea. BMC Vet. Res. 2014;10:13. doi: 10.1186/1746-6148-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen E.V., Marks S.L., Farver T.B. Prevalence of selected bacterial and parasitic agents in feces from diarrheic and healthy control cats from northern California. J. Vet. Intern. Med. 2012;26:54–60. doi: 10.1111/j.1939-1676.2011.00843.x. [DOI] [PubMed] [Google Scholar]
- Raza A., Rand J., Ghaffar-Qamar A., Jabbar A., Kopp S. Gastrointestinal parasites in shelter dogs: occurrence, pathology, treatment and risk to shelter workers. Animals. 2018;8:108. doi: 10.3390/ani8070108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsky-Elefant G., Hirata C.E., Yamamoto J.H., Ferreira M.U. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann. Trop. Med. Parasitol. 2010;104:3–23. doi: 10.1179/136485910X12607012373957. [DOI] [PubMed] [Google Scholar]
- Scaramozzino P., Carvelli A., Iacoponi F., De Liberto C. Endoparasites in household and shelter dogs from Central Italy. Int. J. Vet. Sci. Med. 2018;6:45–47. doi: 10.1016/j.ijvsm.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Cifuentes V., Jiménez-Aguilar J.D., Alzate-Echeverri A., Cardona-Arias J.A., Ríos-Osorio L.A. Prevalencia de parásitos intestinales en perros de dos centros de bienestar animal de Medellín y el oriente antioqueño (Colombia), 2014. Rev. Med. Vet. 2015;(30):55–66. [Google Scholar]
- Spain C.V., Scarlett J.M., Wade S.E., McDonough P. Prevalence of enteric zoonotic agents in cats less than 1 year old in Central New York state. J. Vet. Intern. Med. 2001;15:33–38. doi: 10.1892/0891-6640(2001)015<0033:poezai>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Volkman M., Steiner J.M., Fosgate G.T., Zentek J., Hartman S., Kohn B. Chronic diarrhea in dogs – retrospective study in 136 cases. J. Vet. Intern. Med. 2017;31:1043–1055. doi: 10.1111/jvim.14739. [DOI] [PMC free article] [PubMed] [Google Scholar]