Significance
Small GTPases of the Rab family play a crucial role in the intracellular organization of eukaryotic cells. These molecular switches transition between active guanosine triphosphate- and inactive guanosine diphosphate-bound states in concert to regulate membrane trafficking in space and time. Despite its ubiquity, the collective network dynamics of the Rab activity switch are unknown. Here, we use in vitro reconstitution and mathematical modeling to answer fundamental long-standing questions of small GTPase regulation. We discover that the minimal Rab5 regulatory system operates as an intrinsically bistable and stochastic switch, which can precisely tune the collective GTPase state transitions in the cytoplasm. Furthermore, the indispensable positive feedback of Rab5 activation drives emergent spatial activity patterns, a general small GTPase system behavior.
Keywords: Rab5, bistability, positive feedback, stochasticity
Abstract
The eukaryotic endomembrane system is controlled by small GTPases of the Rab family, which are activated at defined times and locations in a switch-like manner. While this switch is well understood for an individual protein, how regulatory networks produce intracellular activity patterns is currently not known. Here, we combine in vitro reconstitution experiments with computational modeling to study a minimal Rab5 activation network. We find that the molecular interactions in this system give rise to a positive feedback and bistable collective switching of Rab5. Furthermore, we find that switching near the critical point is intrinsically stochastic and provide evidence that controlling the inactive population of Rab5 on the membrane can shape the network response. Notably, we demonstrate that collective switching can spread on the membrane surface as a traveling wave of Rab5 activation. Together, our findings reveal how biochemical signaling networks control vesicle trafficking pathways and how their nonequilibrium properties define the spatiotemporal organization of the cell.
Various cellular processes such as cell polarization (1), oocyte maturation (2), and cell cycle progression (3) rely on decisive signaling reactions in the form of bistable switches, where a biochemical system rapidly transitions between an ON and OFF state (4). Protein–protein interaction networks regulating the activity of small GTPases are also thought to produce bistable behavior (5). In the case of proteins of the Rab family, these biochemical networks could control the vectorial flow in membrane traffic pathways (6), endosome maturation (7), and the formation of Rab membrane domains (6). While theoretical studies (8, 9) motivated by biochemical experiments (10, 11) support the idea of ultrasensitivity and bistable switching as an emergent property of these networks, experimental evidence in vitro or in vivo is so far missing.
The reason for this lack of data is that the characterization of small GTPase networks on a systems level has remained challenging. First, the inherent complexity of the living cell makes in vivo control over reaction conditions and precise experimental readouts challenging. Second, small GTPases are often lipidated to reversibly bind to membranes (12). However, in vitro activity studies were commonly performed with soluble, unprenylated proteins in the absence of lipid bilayers (11, 13). Accordingly, the emergent, out-of-equilibrium properties of small GTPase networks have not been studied (10, 14, 15) and their input–output relationship under physiological conditions is currently unknown.
Arguably the best-characterized Rab GTPase is Rab5, which controls the maturation of early endosomes toward the lysosomal system (16). Rab5 possesses two lipophilic geranylgeranyl chains on its C terminus for membrane binding (17) and, like all small GTPases, can exist in either active guanosine triphosphate (GTP)- or inactive guanosine diphosphate (GDP)-bound conformation (18). In the active state, they stably bind to the membrane surface, recruiting downstream effectors to control vesicle budding, transport, and fusion (18). The transition between nucleotide states is controlled by guanine nucleotide exchange factors (GEFs) that catalyze exchange of GDP with GTP and GTPase activating proteins (GAPs) catalyzing GTP hydrolysis (12). In their inactive GDP-bound state, the Rab GDP-dissociation inhibitor (GDI) extracts the Rab GTPase from the membrane and keeps it soluble in the cytoplasm (19). As a result, nucleotide exchange and hydrolysis can drive a dynamic cycling of the GTPase to and from the membrane away from biochemical equilibrium. To achieve collective switching and rapid Rab GTPase accumulation on the membrane, the biochemical network controlling activation likely contains nonlinear interactions such as a positive feedback loop (6, 10, 11, 20). Indeed, positive feedback regulation has been postulated to exist for Rab5, as its cognate GEF Rabex5 was found to form a complex with the Rab5 effector Rabaptin5 (21). Consequently, Rab5 is thought to recruit its own activator, which was suggested to result in ultrasensitive activation (5), stable membrane accumulation (6, 10, 11, 20), and also hysteretic behavior, where the active state is maintained even as the input GEF concentration decreases. Although this concept has helped to explain the directionality of signaling cascades and vesicle transport, if such a feedback indeed exists, how exactly it is implemented on a molecular level and if it produces bistable, collective switching remains unclear (22).
Here, to study its emergent properties, we rebuild the dynamic biochemical network underlying Rab5 activation using a minimal set of purified components (Fig. 1A and SI Appendix, Fig. S1): fluorescently labeled, prenylated Rab5 in complex with GDI, a complex of the full-length proteins Rabex5 and Rabaptin5, and biomimetic membranes. In combination with computational modeling, this experimental approach gave us full control over the reaction conditions and allowed us to assay collective Rab5 activation in an out-of-equilibrium setting. We demonstrate that this minimal biochemical system is able to act in a switch-like, bistable manner. Furthermore, we found conditions that allow the system to form spatial patterns of Rab5 activation, which share features of Rab domains found on endosomes in vivo (23, 24).
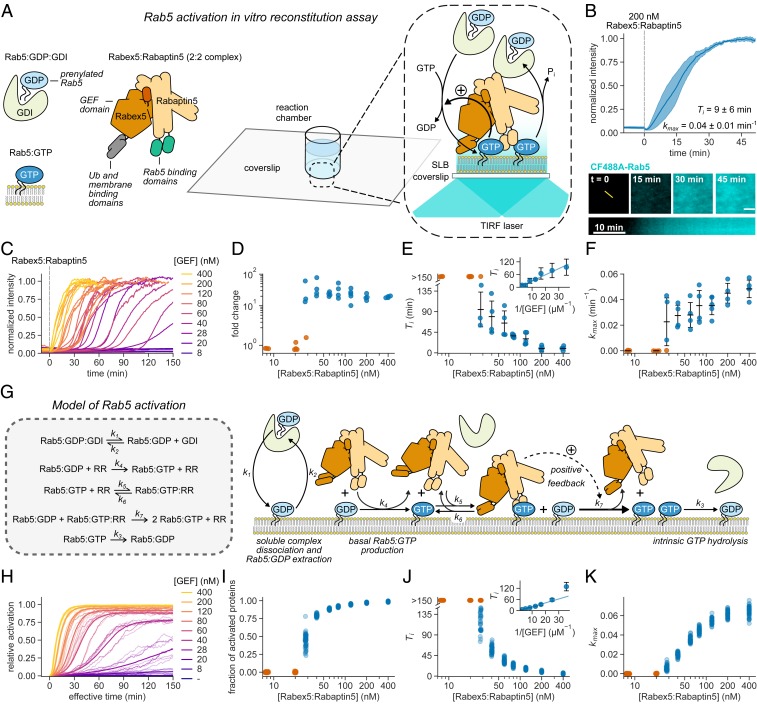
Fig. 1.
Rab5:GDI activation on phospholipid membranes is ultrasensitive and stochastic. (A) Schematic of Rab5 activation reconstitution assay on an SLB. (B, Top) Addition of Rabex5:Rabaptin5 triggers nucleotide exchange by CF488A-Rab5, which can be followed by an increase of fluorescence intensity on the membrane surface. Solid line is mean normalized intensity; shaded area corresponds to SD (n = 4). (B, Bottom) Micrographs of CF488A-Rab5 binding to the SLB after addition of 200 nM GEF complex and corresponding kymograph (below) taken along the yellow line (scale bar, 5 μm). (C) Rab5 intensity traces obtained at increasing Rabex5:Rabaptin5 concentrations. (D) Rab5:GDI activation response diagram with Rabex5:Rabaptin5. The fold change was calculated by dividing the fluorescence intensity at steady state with the average signal 10 min before GEF addition. (E) Activation delay Ti decreases with higher Rabex5:Rabaptin5 concentration. Where no detectable activation was observed within 150 min, the Tis are denoted as >150 min and shown in orange. Error bars are SD. (F) Relative maximum rates kmax against the GEF complex concentration reveal cooperativity of Rab5 activation. Without detectable activation within 150 min, the activation rate was determined to be 0 and the corresponding points are depicted in orange. Error bars are means ± SD. (G) Schematic representation of modeled molecular interactions. We constructed a model of the minimal Rab5 activation network based on the known literature (9–11, 21, 53). The ordinary differential equations were derived from mass action kinetics. (H) Stochastic model simulations of Rab5 activation at increasing Rabex5:Rabaptin5 particle numbers. Shown are average curves from 50 individual runs in bold and 10 random traces per condition. The effective simulation time was scaled to align with experimental results. (I–K) Signal fold change, temporal delays, and relative maximum rates from the stochastic simulations in H. We ran 50 individual stochastic simulations per condition.
Results and Discussion
For the in vitro reconstitution of the biochemical network controlling Rab5 activation, we first set out to verify the activity of purified Rabex5:Rabaptin5 on native Rab5:GDP in complex with GDI. To this end, we loaded lipid-modified Rab5 with the fluorescent GDP analog mant-GDP and used its fluorescence intensity as a real-time readout of nucleotide exchange (11, 21). With 60 nM GEF and in the absence of membranes we could not detect nucleotide exchange on 250 nM Rab5:mant-GDP:GDI. However, we found robust activation in the presence of small unilamellar vesicles (SUVs) (SI Appendix, Fig. S2), confirming that the phospholipid bilayer is essential for activation of Rab:GDP in a complex with GDI (25).
To investigate the role of biological membranes for Rab5 activation, we utilized glass supported lipid bilayers (SLBs) as membrane substrates, combined with fluorescently labeled proteins and total internal reflection fluorescence (TIRF) microscopy (Fig. 1A) (26). To recapitulate the intracellular preactivation state, we first incubated the SLB with inactive CF488A-Rab5:GDI (500 nM), 0.5 mM GTP, and 0.05 mM GDP. We included free GDI (2 µM) to mimic cellular stoichiometric excess of RabGDI (27). We then initiated nucleotide exchange by adding 200 nM Rabex5:Rabaptin5 and followed the fluorescence of CF488A-Rab5 on the membrane. Starting from a low basal level of fluorescence on the membrane surface, the addition of the GEF complex produced a characteristic rise in fluorescence intensity until the signal saturated after about 40 min (Fig. 1B and Movie S1), consistent with an accumulation of Rab5:GTP on the membrane. Accordingly, this shows that SLBs can act as a membrane substrate for prenylated Rab5, allowing us to follow its collective activation and membrane binding in real time.
Positive feedback regulation typically gives rise to sigmoidal signal–response curves (28). To test for the presence of positive feedback in the Rabex5:Rabaptin5:Rab5 activation network we recorded Rab5 membrane binding after adding increasing amounts of the GEF complex (Fig. 1C and Movie S2). We found that this titration resulted in a pronounced two-state response profile: While there was no activation at GEF concentrations below 20 nM even 150 min after Rabex5:Rabaptin5 injection, we found a 10- to 80-fold increase of fluorescence on the membrane with higher concentrations of Rabex5:Rabaptin5 (Fig. 1D). From the temporal activation curves, we extracted the relative maximal rate of Rab5 activation (kmax) as well as the time delay needed to reach this rate (Ti) (SI Appendix, Fig. S3 and Materials and Methods) (29). High GEF complex concentrations (400 nM) gave rise to an immediate rise in Rab5 fluorescence intensity, which saturated within 30 min. Strikingly, at the intermediate GEF concentrations tested (between 28 nM and 60 nM), we observed nearly flat intensity profiles for up to 2 h, before Rab5 abruptly switched to the ON state (Fig. 1E). At lower GEF concentrations, we observed no response within the measurement window of 150 min (orange circles, Fig. 1E). We also performed extended time recordings at 8 nM GEF and saw no response even after up to 12 h (SI Appendix, Fig. S4). Interestingly, the temporal delays needed to reach maximal activation increased linearly with the inverse of GEF complex concentrations (Fig. 1 E, Inset) and despite a wide range of delay times all activation profiles had a similar sigmoidal shape (SI Appendix, Fig. S5). By plotting kmax against GEF concentration, we found that nucleotide exchange showed highly ultrasensitive behavior (Fig. 1F) with a critical GEF concentration of around 28 nM. At this concentration we observed significant variations between the response curves: While some measurements showed no response over the course of the experiment, others showed fast activation with delay times between 50 and 140 min.
To better understand the observed dynamic response curves and the origin of activation delays, we constructed a model of the minimal reaction network similar to a previously suggested mechanism (5). Importantly, our model assumes transient binding of the nonactivated GTPases to the membrane surface and includes cooperative activation due to a direct interaction of Rab5:GTP with its GEF complex (Fig. 1G and SI Appendix, Supplementary Text). As the precise details of this cooperative interaction are still under debate (30), we took a conservative approach in the model, whereby the positive feedback is relatively weak. We estimate the number of membrane-bound Rab5 molecules to be around ∼20 to 200 per µm2 (Materials and Methods). Therefore, given particle diffusion and randomness in binding kinetics, we expect stochasticity to be important, particularly near the GEF threshold concentration.
Given the above, we solved the model using the Gillespie algorithm to incorporate biochemical noise (stochasticity) in the reactions (31), producing dynamics and time delays similar to those observed experimentally (Fig. 1 H–K). In the absence of stochasticity, however, the predicted response curves deviated from the experiments: 1) At early times the intensity profiles were not flat, unlike those measured experimentally, and 2) near the critical Rab5 concentration (∼30 nM) the model cannot replicate the broad range of activation times (SI Appendix, Fig. S6). We cannot discount potential variations (e.g., precise initial protein concentrations) between each experiment playing a role in the observed results. However, given the highly controlled nature of our reconstituted experiment, we expect these fluctuations to be small. To test this assumption, we deterministically solved a coarse-grained (phenomenological) model of the network with positive feedback but varying the input parameters in each simulation (model details are given below and in SI Appendix, Supplementary Text). The deterministic model, even assuming varying parameters between simulations, did not behave similarly to the experimental observations (SI Appendix, Fig. S7). Together, our experimental and theoretical results provide evidence for the postulated positive feedback within a minimal Rab activation network to be sufficient for generating switch-like, ultrasensitive behavior. Furthermore, we find that near the critical concentration stochasticity becomes relevant for the system response.
What could be the origin for the observed cooperativity? It has been proposed that GTP-dependent, effector-mediated GEF recruitment results in cooperative Rab5 activation (10, 11, 21). Alternatively, effector dimerization (32) or direct binding of Rabex5 to the negatively charged membrane could also enhance nucleotide exchange by retaining the GEF complex on the membrane (33, 34). To test if GEF recruitment is the reason for cooperativity, we prepared ΔRBDRabaptin5, a truncated version of Rabaptin5 that lacks its Rab5 binding domains (RBDs) (22). We also prepared ΔRabex5, which misses putative membrane targeting motifs (11). We then used these different protein versions to prepare four different GEF complexes (Fig. 2A), which all demonstrated nucleotide exchange activity on soluble Rab5:mant-GDP (SI Appendix, Fig. S8). However, in our in vitro reconstitution assay using the Rab5:GDI complex, we detected efficient Rab5 activation only for full-length Rabex5:Rabaptin5 and ΔRabex5:Rabaptin5, while ΔRabex5 alone (i.e., in the absence of Rabaptin5) and even the GEF complex without the Rabaptin5 RBDs (Rabex5:ΔRBDRabaptin5) failed to activate Rab5 (Fig. 2B). These results show that collective activation depends on the binding of Rabaptin5 to Rab5:GTP, but not on direct membrane interactions. The same dependence on Rab5:Rabaptin5 interaction was also apparent in our computational model, where we could not reproduce switching without Rab5:GTP:GEF complex formation (Fig. 2C). Rab5:GTP-mediated GEF recruitment was also confirmed by dual-color imaging experiments (Fig. 2D and Movie S3) and in our model (Fig. 2E), which both show similar membrane localization behavior of Rabaptin5 and Rab5. This further demonstrates that Rabex5:Rabaptin5 is retained on the membrane surface by active Rab5:GTP to engage the positive feedback loop (9, 35). Together, these results demonstrate that Rabaptin5 not only enhances the GEF activity of Rabex5 (11) but also that direct interactions between GTPase and its GEF and effector in a ternary complex are essential for the cooperative activation of Rab5 and its collective binding to the membrane.
Fig. 2.
Positive feedback of Rab5 activation depends on GEF recruitment. (A) Illustration of protein interactions responsible for collective Rab5 switching. Positive feedback originates from a direct interaction between Rabex5:Rabaptin5 and Rab5:GTP. (B) Fluorescence intensity traces obtained from experiments depicted in A. Solid lines are mean normalized intensities; shaded areas are SD (Rabex5:Rabaptin5, ΔRabex5:Rabaptin5 n = 4; ΔRabex5, Rabex5:ΔRBDRabaptin5 n = 3). (C) Stochastic model simulations with and without Rabex5:Rabaptin5:Rab5:GTP complex formation (k5, k6 = 0) for 200 Rabex5:Rabaptin5 particles. Average curves from 50 individual runs are depicted in bold with 10 random traces per condition. The effective simulation time was scaled to align with experimental results. (D) Kinetic traces of CF488A-Rab5 and Rabex5:sCy5-Rabaptin5 activation. Solid line is mean normalized fluorescence intensity; shaded area is SD (n = 5). (Inset) Ti for CF488A-Rab5 (blue) and Rabex5:sCy5-Rabaptin5 (orange). (E) Stochastic model simulations for Rab5 and Rabex5:Rabaptin5 membrane binding for 200 Rabex5:Rabaptin5 particles. Shown are curves from 50 independent runs; the mean line is depicted bold with 10 random traces per condition. (F) Schematic of the reconstitution experiment with preactivated SLB-immobilized Rab5Q80L-His10:GTP. (G) Collective switching is faster with preactivated Rab5. (Left) Rab5 switching time courses in presence of 500 nM Rab5Q80L-His10 with increasing DOGS-NTA lipid concentration in the SLB. Solid line is mean normalized fluorescence intensity over time, shaded area is SD (n = 3). (Right) Corresponding time delays Ti and relative maximum rates kmax.
The long delay times and stochastic switching observed at intermediate concentration of the GEF complex were unexpected. What could be the reason for this behavior? Typically, long lag phases are related to processes that rely on random nucleation events triggering phases of rapid growth (36, 37). Importantly, these lag phases can be dramatically shortened in the presence of activation seeds. To test this prediction, we added seeds of GTP-loaded constitutively active Rab5Q80L-His10 on the SLB with nickel-chelating lipids (DOGS-NTA) before injecting 80 nM Rabex5:Rabaptin5 (Fig. 2F). Without preactivated Rab5 on the membrane (no DOGS-NTA in the SLB), activation occurred 20 min after GEF addition. In contrast, the time delays with Rab5Q80L-His10 on 2% DOGS-NTA membranes were three times shorter and completely absent with 5% DOGS-NTA (Fig. 2G), while the maximal activation rates were not significantly changed. These data show that membrane-bound Rab5:GTP can indeed act as a nucleation seed for Rab5 activation and collective membrane binding.
We found that Rab5 activation is enhanced on membranes with Rab5:GTP, but what initiates the Rab5 activation switch on membranes without any preexisting cues? Previous models suggested that rapid reversible membrane binding precedes activation by the GEF, which is in agreement with our observation that membranes are required to activate the Rab5:GDI complex, despite its nanomolar affinity (38). Importantly, this suggests that there should be a small amount of inactive Rab5:GDP existing on the membrane, which is the actual substrate for nucleotide exchange (38). Indeed, with small amounts of sCy5-Rab5:GDI in a background of CF488A-labeled Rab5:GDI, we detected individual sCy5-labeled proteins on the membrane even before adding Rabex5:Rabaptin5 (Fig. 3A). Using single-molecule tracking, we found that nonactivated sCy5-Rab5 diffused rapidly on the membrane and had a mean residence time of 0.8 ± 0.4 s (Fig. 3B). After addition of the GEF complex, we observed a sudden increase in sCy5-Rab5 particle counts, along with a sigmoidal increase of membrane-bound CF488A-Rab5. The frequency diagram of Rab5:GTP membrane residence times and corresponding fits revealed two populations: a short-lived population with a residence of 0.7 ± 0.3 s, similar to Rab5:GDP, and a long-lived population with a 10-times-longer residence time (7.4 ± 3.2 s) (Fig. 3B and Movie S4). A similar dwell time distribution was observed for membrane-locked Rab5 with the nonhydrolyzable GTP analog GMP-PNP (SI Appendix, Fig. S9), indicating that the values for activated Rab5 are influenced by fluorophore bleaching and represent a lower bound of residence time. Together, these results confirm the idea that Rab5 first transiently binds to the cellular membranes nonspecifically in its GDP-bound state. However, it is converted to the long-lived GTP-bound state by Rabex5:Rabaptin5 only on the endosomal surface (22, 33, 39, 40). This Rab5:GTP seed can then retain the GEF complex on the membrane (Fig. 2D) and initiate the positive feedback. Furthermore, this mechanism can help to maintain the biochemical identity of endosomes, as Rab5:GDP would first sample different membrane surfaces but is only activated on membranes with Rab5:GTP already present.
Fig. 3.
Rab5:GDI activation is tuned by free Rab5:GDP abundance. (A) Rab5 cycles between the membrane and solution before and after nucleotide exchange. (Top) sCy5-Rab5 molecule counts per frame and collective CF488A-Rab5 activation. (Bottom) Snapshots of the activation reaction. sCy5- and CF488A-Rab5 are depicted in yellow and cyan, respectively. The sCy5 channel was smoothed before merging to reduce nonspecific high-frequency noise (scale bar, 10 μm). (B) Rab5 single-molecule trajectories reveal GDP- and GTP-bound proteins on the membrane. (Top) Five hundred tracks of membrane-bound sCy5-Rab5 particles before (GDP) and after (GTP) activation. (Bottom) Frequency diagram identifies two populations with distinct lifetimes. A monoexponential decay with lifetime τGDP and two-exponential decay with lifetimes τ1GTP and τ2GTP was fitted to grouped data from n = 5 independent experiments, respectively (nGDP = 4829, τGDP = 0.58 ± 0.04 s; nGTP = 3090, τ1GTP = 1.20 ± 0.35 s, τ2GTP = 10.6 ± 3.3 s; errors are 95% CI). Box plot with mean lifetimes, **P < 0.01, two-sided Student’s t test (n = 5). (C) Parameter phase space of the phenomenological model for Rab5 switching, depending on the basal rate of activation and the strength of positive feedback . Switching is defined as the relative difference in steady-state Rab5 concentration on the membrane relative to the scenario with no positive feedback. (Inset) Fold activation along the red line in the diagram. Stochasticity was introduced by solving the phenomenological model within a Fokker–Planck framework. See text for parameter definitions. (D) Stoichiometric GDI excess over Rab5 affects delay of Rab5 activation in vitro. (Left) Solid lines are mean normalized intensities over time, shaded areas correspond to SD (n = 3). (Right) Corresponding activation Ti and relative maximum rates kmax. (E) Stochastic simulations of the full model for varying initial amounts of GDI excess (0 to 2,000 particle number). Shown are curves from 10 random runs per condition; the mean line from 50 runs is depicted in bold. The effective simulation time was scaled to align with experimental results. (F) PRA1 in the membrane enhances Rab5 activation at low GEF concentrations. Solid lines are mean normalized fluorescence intensities; shaded areas correspond to SD (n = 3).
Our observations suggest that initial random activation events of individual Rab5 proteins on the membrane are the likely cause of the observed stochasticity in the collective transition to the active state. We then asked how initial levels of membrane-bound Rab5 and the strength of the positive feedback affects the transition between the OFF and ON states. To answer this question, we used the coarse-grained version of our model, which incorporated binding (a0, basal binding rate) and unbinding (a2) of Rab5 (R) on the membrane, along with positive feedback (a1, with activation concentration K): (SI Appendix, Fig. S7). Here, the binding term a0 effectively includes the contributions of Rab5:GDI disassociation and a basal rate of GEF-mediated nucleotide exchange. The parameter space that leads to GTPase switching shows that the network response (i.e., the fold change in membrane-bound Rab5) after activation is small when the basal binding rate is set high (Fig. 3C and SI Appendix, Fig. S10). Conversely, if the basal rates are too low, the critical threshold for switching fails to occur, even with stochastic fluctuations. This reveals that the system is potentially highly tunable, and that switching depends on both the basal binding rate and positive feedback strength.
To experimentally test the model predictions, we first varied the amount of Rab5:GDP extraction by changing the amount of free GDI in our experiments (Fig. 3D). We found that increasing the stoichiometric GDI excess over Rab5 lowered the basal background fluorescence prior to activation, prolonged activation delay times after GEF addition, and limited kmax, consistent with a decreased basal binding rate due to perturbed Rab5:GDI dissociation equilibrium. Using our full model, we also see similar results when altering the level of free GDI (Fig. 3E), confirming that continuous Rab5:GDP membrane extraction causes long delay times and stochastic activation (41).
Conversely, to prevent GDI extraction of Rab5 after GTP hydrolysis, we replaced GTP with GMP-PNP. As this GTP analog inhibits Rab5’s high intrinsic GTPase activity (42), it should increase the amount of active GTPase on the membrane and lead to a more robust transition into the ON state. In agreement with this prediction, we observed immediate collective Rab5 membrane binding after adding 80 nM GEF complex with GMP-PNP and 2 µM GDI, while the delay time was more than 36 min when we used GTP (Fig. 3D, magenta curve). Preventing Rab5 membrane extraction and keeping other parameters fixed, we see that our full model displays similar behavior for the GMP-PNP nucleotide exchange (Fig. 3E, magenta curve). Next, we added the Rab5-specific GDI dissociation factor (GDF) PRA1 to our experiments, which has been suggested to accelerate the release of Rab5:GDP from the GDI complex (43) or prevent GTPase reextraction (44). Accordingly, it should also either increase the basal binding rate or reduce the unbinding of Rab5:GDP and facilitate the collective activation switch. Indeed, with PRA1 in the membrane, we observed fast Rab5 activation with short delay times even at a Rabex5:Rabaptin5 concentration too low to support Rab5 activation on PRA1-free membranes (8 nM) (Fig. 3F). These findings show that although it is not strictly required for Rab5 activation (38, 40) the presence of PRA1 in the endosomal membrane can lower the threshold for positive feedback initiation, making collective Rab5 activation more likely.
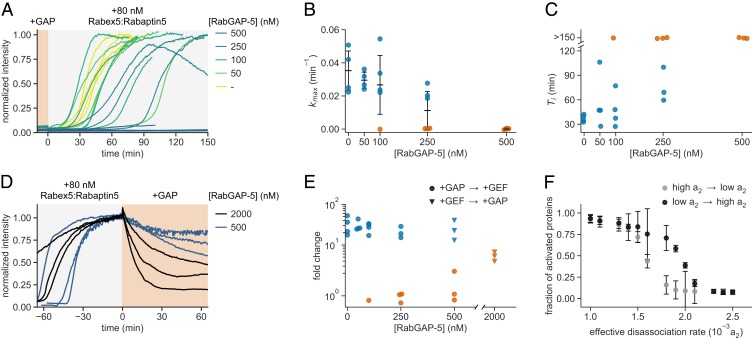
Conversely, further increasing Rab5’s GTPase activity above its intrinsic hydrolysis rate should inhibit collective switching as it prevents effector recruitment of the GEF complex and enables extraction of inactivated Rab5 from the membrane (Fig. 3C; moving to the left along the red line). To test this prediction, we performed experiments in the presence of purified full-length RabGAP-5 (SGSM3), a Rab5-specific GAP (45), which stimulates GTP hydrolysis by Rab5. We recorded the signaling response after addition of 80 nM Rabex5:Rabaptin5 in the presence of increasing RabGAP-5 amounts (Fig. 4A) and found that the reconstituted system either eventually collectively switched or remained inactive for highest inactivator concentrations (Fig. 4 B and C). At RabGAP-5 between 100 and 250 nM, the reconstituted network showed both successful and unsuccessful systemic activation events at identical initial conditions. Importantly, at higher GAP concentrations we found that the observed response depended on its starting point, that is, showed hysteretic behavior. While 500 nM RabGAP-5 prevented Rabex5:Rabaptin5 activation, we found that once the system was switched ON addition of 500 nM or even 2 μM GAP (Fig. 4 D and E) did not completely reverse the system to its preactivated state. Similarly, by increasing the dissociation rate a2 in our phenomenological model, we observed clear difference in switching responses after 150 min—depending on the initial network state (Fig. 4F). This hysteretic response not only confirms the bistable behavior of the Rab5 activation network but also provides a mechanism to maintain the active state under GTPase cycling conditions.
Fig. 4.
GAP activity reveals bistability of the reconstituted network. (A) Effect of RabGAP-5 on Rab5 activation. Different concentrations of RabGAP-5 were present in experiments with 500 nM CF488A-Rab5:GDI and 2 μM GDI, before adding 80 nM Rabex5:Rabaptin5. Shown are time courses at increasing GAP concentrations. (B) Maximal rates kmax of Rab5 activation for curves shown in A. Without detectable activation within 150 min, the activation rate was set to 0 and the corresponding points are depicted in orange. Error bars are SD. (C) Activation delay Ti for data presented in A. Without detectable activation, the times to inflection point are denoted as >150 min (orange). (D) GAP addition after activation with 80 nM GEF. RabGAP-5 was added to 500 nM and 2 µM final concentration, respectively. The traces were offset to the point of GAP addition. (E) GAP titration response curve. The fold change was calculated by dividing the fluorescence intensity at steady state with the average fluorescence signal 10 min before GEF addition. The +GAP → +GEF fold values (circles) are calculated based on traces in A and +GEF → +GAP values are taken from D. Activation is depicted in blue and inactive reactions in orange (fold <10). (F) Changing the dissociation rate in opposite directions reveals hysteresis in switching of the phenomenological model after 150 min. Shown are means of 20 simulations; error bars are SD.
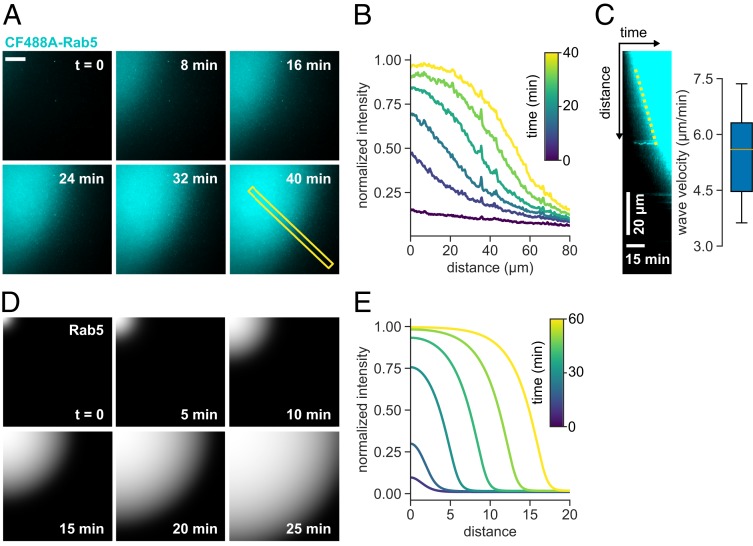
Strikingly, at RabGAP-5 concentrations of 50 nM, we observed Rab5 activation fronts on the membrane, where Rab5 self-organized into defined areas of high density which grew into low-GTPase-density areas (in 9 out of 13 experiments; in 3 experiments no obvious waves were noticed during activation, and in 1 experiment no activation occurred). This spatiotemporal activation pattern existed for more than 30 min, during which the activation wave traveled at 5 μm/min before the system settled into a fully active state (Fig. 5 A–C and Movies S5 and S6). What could explain the emergence of this spatial pattern? Under global GAP inhibition, random fluctuations in protein amounts spur local Rab5 activation, which is reinforced and stabilized by positive feedback via Rabex5:Rabaptin5 engagement. This region of initial Rab5:GTP activation will have higher probability of additional Rab5:GTP recruitment at its boundary, giving rise to a propagating activation front. The emergent property can be captured in our phenomenological model by introducing a diffusive term, where Rab5 activation can spread at constant rate by propagating the positive feedback activation in presence of a GAP via an activation front (46). Such a front is dependent on the GAP activity and the threshold Rab5:GTP density that can sustain the positive feedback activation (Fig. 5 D and E and SI Appendix, Supplementary Text). It is well known that dynamic biochemical systems composed of locally acting cooperative actuators and long-ranged inhibitors can give rise to chemical waves on the cellular and tissue level (47–49). In our system, RabGAP-5 acts as a global inhibitor, rather than a long-ranged diffusing inhibitor, resulting in simpler spatiotemporal activation pattern. Despite the large difference in scales, the Rab5 patches we observed experimentally might originate from a similar mechanism as endosomal Rab domains (23, 24).
Fig. 5.
Rab5 activation wave spreads on membrane in presence of GAP. (A) Micrographs of CF488A-Rab5 activation wave spreading across the membrane (scale bar, 20 μm). Times indicate relative duration after start of acquisition, not time after addition of GEF complex. (B) Fluorescence intensity time profile of the indicated area in A. (C) Kymograph of the indicated area in A and mean wave velocity. Wave velocity was determined from the slope of fluorescence increase in generated kymographs (n = 6). (D) Simulated Rab5 activation front with diffusion inclusion in the phenomenological model (SI Appendix, Supplementary Text and SI Appendix, Eq. 6). (E) Solution in terms of the dimensionless distance .
To summarize, using in vitro reconstitution and computational modeling, we provide the experimental evidence for a long-postulated positive feedback in the regulatory network controlling Rab5 activation (20). We find that this feedback results in ultrasensitivity and bistable behavior, the prerequisites for decisive signaling reactions in vesicle traffic control. Furthermore, we confirmed a hypothetical model of Rab GTPase activation, according to which Rab GTPases rapidly sample the membrane surfaces, before they are activated by the cognate GEF (5, 38). We have also demonstrated that the architecture of the Rab5 activation network, consisting of a local positive feedback and global inhibition, supports the formation of spatiotemporal patterns. We also found that at critical parameter conditions the activation in minimal Rab5 network can occur stochastically, with restrictive amounts of nonactive GTPase as a potential source for this behavior. While stochasticity and long delay times are generally disadvantageous for intracellular signaling reactions that rely on tight control, our in vitro experiments demonstrate that it is possible to tune the network response, not only in time but also to shape the associated spatial pattern. Indeed, GDFs or phosphoregulation (50) are likely cellular mechanisms that take advantage of the discovered intrinsic stochastic bistability (51) to direct GTPase signaling at limiting GEF concentrations by tuning Rab:GDI stability.
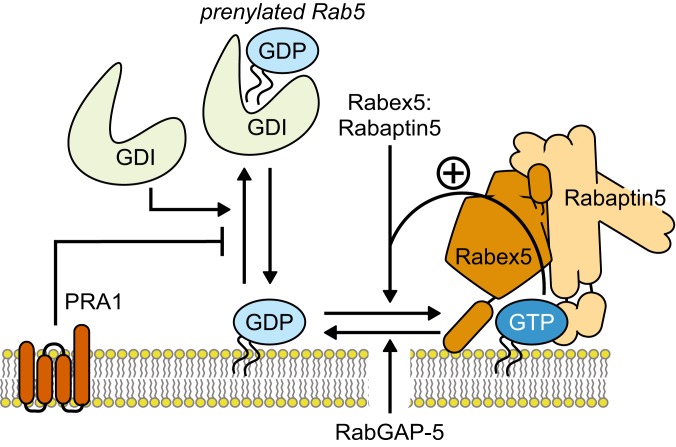
Our study represents a demonstration of a minimal biochemical circuit of Rab GTPase activation (Fig. 6). We also provide a systematic characterization of additional regulatory interactions and show how they can be employed to direct and tune small GTPase activation in space and time. Of course, the complexity of the cell allows for more modes of regulation, both on the protein and membrane level. Our in vitro system can be further extended to include other effectors or membrane compositions, making it an excellent test bed for probing the mechanisms of organelle identity formation during vesicle trafficking and compartmentalization of the cell. Furthermore, our approach can also be used to study the dynamic networks of other small GTPase families, such as Arf, Rac, and Rho.
Fig. 6.
The reconstituted Rab5 regulatory network. The Rab5 activation network of Rab5:GDI, Rabex5:Rabaptin5, and the membrane is intrinsically stochastic and bistable. The switching probability depends on the amount of membrane-bound Rab5:GDP, which in turn is controlled by GDI extraction and the presence of a GDF such as PRA1 that counters the GDI activity. Furthermore, RabGAP-5 can suppress activation by limiting the amount of active Rab5:GTP on the membrane. This effect increases the bistability of the system and can give rise to waves of Rab5 activation on the membrane. The interplay of the network’s components enables the cell to precisely trigger collective Rab activation in space and time.
Materials and Methods
Protein Purification and Labeling.
See SI Appendix, Supplementary Methods for a detailed description.
Coverslip Treatment and Reaction Chamber Immobilization.
We used 24- × 50-mm-high precision coverslips (no. 1.5H; Marienfeld) in our fluorescence microscopy assays. First, coverslips were cleaned by 1-h incubation in piranha solution (1:3 volume ratio of 30% H2O2; Sigma-Aldrich and 98% H2SO4; Merck) and extensive washing in Milli-Q grade water. The cleaned coverslips were stored in Milli-Q water for up to 2 wk. Immediately before use, the coverslips were dried and further cleaned in a Zepto B (Diener Electronic) plasma oven for 10 min at 30 W under 1 normal liter per h airflow. We immobilized the microscopy reaction chambers by attaching a cut PCR tube on the cleaned coverslip glass using ultraviolet (UV) curing glue (Norland optical adhesive 63) under 365-nm UV light for 5 to 10 min. The attached reaction chambers were then ready for SLB preparation.
SLB Preparation.
To mimic the intracellular membranes, we prepared SLBs on clean microscope slides. The SLBs are made by mixing synthetic lipids with intended composition in chloroform at 0.5 mM concentration per 1-mL volume. In this study, we used 79.9% DOPC, 20% DOPS, 0.1% DMPE-PEG2000, and varying amounts of DOGS-NTA[Ni2+] lipids (Avanti Polar Lipids). For the experiments with DOGS-NTA, we decreased the ratio of DOPC proportionally. The lipid mixture was dried under nitrogen flow in a glass vial and kept under vacuum for at least 1 h. The dried lipids were hydrated in vesicle buffer (20 mM Hepes-KOH, pH 7.4, and 150 mM KOAc) by vortexing to produce multilamellar vesicles (MLVs). In the following step, we prepared SUVs by passing the MLV solution over five freeze–thaw cycles using liquid nitrogen and extrusion through 100-nm polycarbonate membrane 21 times (LiposoFast; Avestin). The produced SUVs were stored at 4 °C for up to 5 d. Similarly, we used DOGS-NTA SUVs within 2 d after production. A notable decrease in His10-tagged protein recruitment efficiency was observed for older NTA–lipid preparations. Finally, the SLB was formed in immobilized plastic reaction chambers on glass surface by inducing fusion of the SUVs with 3.33 mM CaCl2. The SLBs were left to form for at least 45 min at 37 °C. Later, the unfused vesicles were washed away with vesicle buffer, Milli-Q water, and reaction buffer.
TIRF Microscopy.
TIRF microscopy experiments were done on a 1) Zeiss Axio Observer.Z1 inverted microscope with Visitron iLas2 illumination module (Gataca), Zeiss Definite Focus 2 and Plan-APOCHROMAT 63×/numerical aperture (N.A.) 1.46 immersion objective and 2) inverted Olympus IX83 with Cell^TIRF system and Olympus UApo N 100×/N.A. 1.49 oil objective. Imaging on the Zeiss system was performed on two Photometrics Evolve-EM 512 D electron-multiplying charge-coupled device (EMCCD) cameras. The Olympus setup was equipped with water-cooled Hamamatsu C9100-13 EMCCD camera. On both systems, the imaging was done with 200 EM camera gain and 30-ms exposure time with varying laser intensities unless stated otherwise.
Rab5 Activation Reconstitution Assays on SLB.
For the in vitro reconstitution assays on the SLBs, purified proteins were incubated in the immobilized reaction chambers with glass-supported membranes in Rab reaction buffer (20 mM Hepes-KOH, pH 7.4, 150 mM KOAc, 1 mM MgCl2, 1 mM dithiothreitol [DTT], and 50 µM GDP), supplemented with 0.5 mM GTP, in 30 µL volume. To track membrane localization of fluorescently labeled components we used TIRF microscopy with <100-nm penetration depth of the evanescent excitation field at the SLB focal plane. We recorded the fluorescence signal in 30-s intervals for at least 10 min after equilibration of the reconstituted system to obtain stable baseline intensity. Then, we injected GEF in 20 µL Rab reaction buffer and mixed the contents of the reaction chamber to initiate the nucleotide exchange (50 µL final volume). We continued the recording in 30-s intervals until the fluorescence signal reached steady state. For the hysteresis assay, we first injected 10 µL 80 nM Rabex5:Rabaptin5 and allowed the activation reaction to plateau. We induced the reverse reaction by addition of 10 µL RabGAP-5 to a final concentration of 500 or 2,000 µM in 50 µL. In cases where no increase in signal was detected after at least 150 min postinduction, the recording was stopped and the activation delay time was taken to be >150 min. Additionally, we recorded the camera noise by closing the microscope shutter and collecting the detector readout.
Microscopy Data Analysis.
The collected microscopy data were analyzed using Fiji ImageJ 1.52i package. The membrane localization of the selected fluorescently labeled component was determined by measuring the mean fluorescence intensity at the SLB and subtracting the recorded camera noise value for each recorded frame. The point of GEF addition was set as t = 0. To obtain the time of inflection Ti and maximum growth rate kmax, we fitted a Gompertz function (29) to the subtracted fluorescence intensity values between the GEF addition and the onset of steady state:
where I(t) is the measured fluorescence intensity at time t, A and B are the upper and lower fit asymptotes, respectively, kmax is the relative maximum signal growth rate, and Ti is the temporal delay to reach the kmax after the GEF addition. For the reactions where no signal increase was observed after 150 min after GEF addition, the activation delay Ti was taken to be >150 min and the kmax was set to 0. To normalize the data, we divided the fluorescence intensities by the upper fitted asymptote A. The traces that did not result in collective activation were normalized by dividing the signal intensities with the mean of upper asymptotes A at the corresponding microscope setup. This workflow is also summarized in SI Appendix, Fig. S3. The normalized fluorescence intensities were used to group independent replicates. This way, we obtained the mean intensity traces and SDs for selected conditions. Similarly, the fold change in fluorescence signal was calculated by taking the upper asymptote value A and dividing it with the mean value of the baseline signal before the GEF injection.
Single-Particle Tracking.
For single-particle tracking we used CF488A-Rab5:GDI, supplemented with small amounts of sCy5-Rab5:GDI. The particle diffusion of sCy5-Rab5 on the SLB was captured using the Zeiss Axio Observer.Z1 inverted TIRF microscope. We used 100% 640-nm laser power, 30-ms exposure time, 300 EMCCD camera gain, and 100-ms acquisition interval. To capture single particles landing on the SLB before nucleotide exchange, we incubated the glass-supported membrane with 500 nM CF488A-Rab5:GDI, 2 µM GDI, 50 µM GDP, 500 µM GTP, and ca. 1 nM sCy5-Rab5:GDI. To limit the effects of photobleaching, we also included an oxygen scavenging system with 60 mM d-glucose, 0.1 mg/mL glucose oxidase (SERVA), 0.32 mg/mL catalase (Sigma-Aldrich), and 2 mM Trolox (Sigma-Aldrich). We built the particle trajectories using the TrackMate ImageJ plugin v.3.7.0 (52). There, we used simple LAP tracker with 0.7-µm particle diameter, 15 threshold value with median filter, signal-to-noise ratio >0.6, 2-µm maximum linking distance, and up to two frame gaps with 3-µm closing distance to account for fluorophore blinking. To image particles after the nucleotide exchange with GTP or GMP-PNP, we first triggered the Rab5 activation by injecting 200 nM Rabex5:Rabaptin5 into 500 nM CF488A-Rab5:GDI, 2 µM GDI, 50 µM GDP, 500 µM GTP/GMP-PNP, and ca. 50 fM sCy5-Rab5:GDI mixture. We followed the progression of collective activation by measuring the CF488A-Rab5 fluorescence intensity at the SLB. When the reaction reached steady state, we supplemented the sample with fresh oxygen scavengers and imaged sCy5-Rab5 particles. Before GEF addition, we observed 5 Rab5 particles on average per 51- × 51-µm field of view and 50 particles at steady state. Taking into account 104 times dilution ratio of the sCy5-labled GTPase, we estimate the number of membrane bound Rab5 molecules to rise from 20 to 200 µm−2 over the course of the experiment. The TrackMate trajectories were built as before, with 1-µm maximum linking distance and at most two frame gaps for 1.5-µm closing distance. For GMP-PNP–bound sCy5-Rab5, ≤0.8-µm and ≤1.2-µm distances were used to link particles and close up to two frame gaps, respectively. We analyzed only trajectories with three spots or longer. To calculate the membrane residence lifetimes for sCy5-Rab5:GDP τGDP, we fitted a monoexponential function to the logarithmic probability density values in a trajectory duration histogram:
where ln(y) is natural logarithm, p(t) is the trajectory probability density at a given duration t, and τ is the mean lifetime. Conversely, a two-exponential function was better at fitting the sCy5-Rab5:GTP/GMP-PNP trajectory histogram. This gave us τ1GTP/GMP-PNP, τ2GTP/GMP-PNP and amplitudes A1 and A2:
We excluded the rare stuck particles from the analysis by limiting the fitting range up to the 98th percentile of the trajectory duration distribution. The calculated lifetimes are not corrected for photobleaching and thus represent a lower estimate of the actual membrane residence lifetimes.
Activation Wave Velocity.
Activation waves were observed several minutes after 80 nM Rabex5:Rabaptin5 injection to 500 nM CF488A-Rab5:GDI, 2 µM GDI, 50 µM GDP, 500 µM GTP, and 50 nM RabGAP-5. The collected time series were first corrected for uneven illumination profile. To this end, we prepared the SLB, doped with 0.25 µg/mL DiO tracer (Sigma-Aldrich). We analyzed the acquired images using ImageJ 1.52i. First, we generated an illumination profile reference image by Gaussian filtering of the DiO-labeled SLB snapshot with FFT Bandpass Filter. We then divided the wave time series with the illumination reference to produce corrected images. These images were used to produce a kymograph along a line across the field of view. Finally, we estimated the activation wave velocity from the slope of the kymograph fluorescence profile.
Size-Exclusion Chromatography–Multiangle Light Scattering.
The oligomeric state of the purified Rabex5:Rabaptin5 sample was analyzed with size-exclusion chromatography–multiangle light scattering. A 100-µL sample at 1.0 mg/mL in 50 mM Tris⋅HCl, pH 7.5, 150 mM KCl, 5 mM MgCl2, 2 mM TCEP, and 10 vol% glycerol was run in duplicate on Superdex 200 Increase 10/300 column at 0.5 mL/min and 35 °C. We used OMNISEC RESOLVE for sample separation and the REVEAL module (Malvern Instruments) for multiangle light scattering and refractive index detection. Prior to the runs, the samples were stored at 6 °C in the autosampler. The recorded data were analyzed using the OMNISEC v10.41 software package.
GEF Assay.
Activity assays of purified GEFs were performed in 384-well plates (black nonbinding surface microplate; Corning). First, prenylated Rab proteins were loaded with mant-GDP by 1-h incubation at 37 °C in the presence of 2 mM EDTA and 20 times molar excess of the labeled nucleotide during purification. Similarly, soluble GTPase was loaded with mant-GDP on the day of experiment. The exchange reaction was then quenched with 5 mM MgCl2 and the buffer was exchanged to the reaction buffer using desalting columns; 250 nM Rab5:mant-GDP:GDI, Rabex5:Rabaptin5 and 500 µM SUVs were added to the microplate and incubated in reaction buffer (20 mM Hepes-KOH, pH 7.4, 150 mM KOAc, 1 mM MgCl2, and 1 mM DTT) for 10 min. Alternatively, 300 nM soluble Rab5:mant-GDP with the GEF complex in reaction buffer was used. The measurements of mant-GDP fluorescence were performed on Biotek Synergy H1 plate reader (excitation 355 nm, emission 450 nm). After acquiring the baseline fluorescence, the GEF exchange reaction was induced by injecting GTP to the wells at 1 mM final concentration. Finally, to determine observed exchange rates kobs, the measured time courses were fitted with a monoexponential function:
where I(t) is the measured fluorescence intensity at time t. The catalytic efficiency kcat/Km was obtained from the slope of a linear fit to kobs([GEF]) plot, where kobs is the observed exchange rate at the given Rabex5:Rabaptin5 molar concentration [GEF] and k0 is the intrinsic Rab5 nucleotide exchange rate:
Computational Models.
A detailed description of the full stochastic and coarse-grained phenomenological models is given in SI Appendix, Supplementary Text.
Data and Materials Availability.
All presented protocols, codes, and materials are available from the corresponding authors (M.L. for experimental data, T.E.S. for theoretical work) upon reasonable request. The raw data for Figs. 1–5 and uncompressed Movies S1–S6 files are available online at https://figshare.com/collections/Stochastic_activation_and_bistability_in_a_Rab_GTPase_regulatory_network/4835259/1 (54).
Supplementary Material
Acknowledgments
We thank B. Simons (Gurdon Institute, Cambridge, UK), K. Kruse, Aurélien Roux (University of Geneva, Switzerland), M. Howard (John Innes Centre Norwich, UK), E. Hannezo (IST Austria, Austria), A. Yap (University of Queensland, Australia), T. Lecuit (Aix Marseille Université, France), and J. Brugués (MPI-CPG, Dresden, Germany) for discussions and valuable feedback on the manuscript. Additionally, we thank C. Mieck, K. Loibl, M. Lopez Pelegrin, and other M.L. laboratory members and the Bioimaging and Life Science Facilities at IST Austria for their support. This work was supported by a Young Investigator Grant of the Human Frontier Science Program to M.L. and T.E.S. (HFSP RGY0083/2016). T.E.S. thanks the Kavli Institute, Santa Barbara, CA, which supported his visit during part of the manuscript preparation (supported in part by National Science Foundation Grant PHY-1748958, National Institute of Health Grant R25GM067110, and Gordon and Betty Moore Foundation Grant 2919.01).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The raw data for Figs. 1–5 and uncompressed Movies S1–S6 files are available online at https://figshare.com/collections/Stochastic_activation_and_bistability_in_a_Rab_GTPase_regulatory_network/4835259/1.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921027117/-/DCSupplemental.
References
- 1.Altschuler S. J., Angenent S. B., Wang Y., Wu L. F., On the spontaneous emergence of cell polarity. Nature 454, 886–889 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C. Y., Ferrell J. E. Jr, Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. U.S.A. 93, 10078–10083 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pomerening J. R., Sontag E. D., Ferrell J. E. Jr, Building a cell cycle oscillator: Hysteresis and bistability in the activation of Cdc2. Nat. Cell Biol. 5, 346–351 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Gardner T. S., Cantor C. R., Collins J. J., Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Barr F. A., Review series: Rab GTPases and membrane identity: Causal or inconsequential? J. Cell Biol. 202, 191–199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zerial M., McBride H., Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Huotari J., Helenius A., Endosome maturation. EMBO J. 30, 3481–3500 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Conte-Zerial P., et al. , Membrane identity and GTPase cascades regulated by toggle and cut-out switches. Mol. Syst. Biol. 4, 206 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H., Qian H., Li G., Delayed onset of positive feedback activation of Rab5 by Rabex-5 and Rabaptin-5 in endocytosis. PLoS One 5, e9226 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippé R., Miaczynska M., Rybin V., Runge A., Zerial M., Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol. Biol. Cell 12, 2219–2228 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delprato A., Lambright D. G., Structural basis for Rab GTPase activation by VPS9 domain exchange factors. Nat. Struct. Mol. Biol. 14, 406–412 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherfils J., Zeghouf M., Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Langemeyer L., et al. , Diversity and plasticity in Rab GTPase nucleotide release mechanism has consequences for Rab activation and inactivation. eLife 3, e01623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohya T., et al. , Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature 459, 1091–1097 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Langemeyer L., Perz A., Kümmel D., Ungermann C., A guanine nucleotide exchange factor (GEF) limits Rab GTPase-driven membrane fusion. J. Biol. Chem. 293, 731–739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeigerer A., et al. , Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature 485, 465–470 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Kalinin A., et al. , Expression of mammalian geranylgeranyltransferase type-II in Escherichia coli and its application for in vitro prenylation of Rab proteins. Protein Expr. Purif. 22, 84–91 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Stenmark H., Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Gavriljuk K., Itzen A., Goody R. S., Gerwert K., Kötting C., Membrane extraction of Rab proteins by GDP dissociation inhibitor characterized using attenuated total reflection infrared spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 110, 13380–13385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiuchi H., et al. , A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90, 1149–1159 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z., et al. , Molecular mechanism for Rabex-5 GEF activation by Rabaptin-5. eLife 3, 1–19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kälin S., Hirschmann D. T., Buser D. P., Spiess M., Rabaptin5 is recruited to endosomes by Rab4 and Rabex5 to regulate endosome maturation. J. Cell Sci. 128, 4126–4137 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Franke C., et al. , Correlative single-molecule localization microscopy and electron tomography reveals endosome nanoscale domains. Traffic 20, 601–617 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sönnichsen B., De Renzis S., Nielsen E., Rietdorf J., Zerial M., Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149, 901–914 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas L. L., Fromme J. C., GTPase cross talk regulates TRAPPII activation of Rab11 homologues during vesicle biogenesis. J. Cell Biol. 215, 499–513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen P. A., Field C. M., Groen A. C., Mitchison T. J., Loose M., Using supported bilayers to study the spatiotemporal organization of membrane-bound proteins. Methods Cell Biol. 128, 223–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeffer S. R., Dirac-Svejstrup A. B., Soldati T., Rab GDP dissociation inhibitor: Putting rab GTPases in the right place. J. Biol. Chem. 270, 17057–17059 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Ferrell J. E., Xiong W., Bistability in cell signaling: How to make continuous processes discontinuous, and reversible processes irreversible. Chaos 11, 227–236 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Tjørve K. M. C., Tjørve E., The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS One 12, e0178691 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauer J., et al. , Auto-regulation of Rab5 GEF activity in Rabex5 by allosteric structural changes, catalytic core dynamics and ubiquitin binding. eLife 8, 562504 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landeros A., et al. , BioSimulator.jl: Stochastic simulation in Julia. Comput. Methods Programs Biomed. 167, 23–35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumas J. J., et al. , Multivalent endosome targeting by homodimeric EEA1. Mol. Cell 8, 947–958 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Zhu H., et al. , Rabaptin-5-independent membrane targeting and Rab5 activation by Rabex-5 in the cell. Mol. Biol. Cell 18, 4119–4128 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christoforidis S., McBride H. M., Burgoyne R. D., Zerial M., The Rab5 effector EEA1 is a core component of endosome docking. Nature 397, 621–625 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Jilkine A., Angenent S. B., Wu L. F., Altschuler S. J., A density-dependent switch drives stochastic clustering and polarization of signaling molecules. PLOS Comput. Biol. 7, e1002271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arosio P., Knowles T. P. J., Linse S., On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 17, 7606–7618 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishida E., Sakai H., Kinetic analysis of actin polymerization. J. Biochem. 93, 1011–1020 (1983). [DOI] [PubMed] [Google Scholar]
- 38.Wu Y.-W., et al. , Membrane targeting mechanism of Rab GTPases elucidated by semisynthetic protein probes. Nat. Chem. Biol. 6, 534–540 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Mattera R., Bonifacino J. S., Ubiquitin binding and conjugation regulate the recruitment of Rabex-5 to early endosomes. EMBO J. 27, 2484–2494 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blümer J., et al. , RabGEFs are a major determinant for specific Rab membrane targeting. J. Cell Biol. 200, 287–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchler N. E., Cross F. R., Protein sequestration generates a flexible ultrasensitive response in a genetic network. Mol. Syst. Biol. 5, 272 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rybin V., et al. , GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature 383, 266–269 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Sivars U., Aivazian D., Pfeffer S. R., Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature 425, 856–859 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Hutt D. M., Da-Silva L. F., Chang L. H., Prosser D. C., Ngsee J. K., PRA1 inhibits the extraction of membrane-bound rab GTPase by GDI1. J. Biol. Chem. 275, 18511–18519 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Haas A. K., Fuchs E., Kopajtich R., Barr F. A., A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat. Cell Biol. 7, 887–893 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Jörg D. J., et al. , The proneural wave in the Drosophila optic lobe is driven by an excitable reaction-diffusion mechanism. eLife 8, 1–34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gierer A., Meinhardt H., A theory of biological pattern formation. Kybernetik 12, 30–39 (1972). [DOI] [PubMed] [Google Scholar]
- 48.Deneke V. E., Di Talia S., Chemical waves in cell and developmental biology. J. Cell Biol. 217, 1193–1204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turing A. M., The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 237, 37–72 (1952). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steele-Mortimer O., Gruenberg J., Clague M. J., Phosphorylation of GDI and membrane cycling of rab proteins. FEBS Lett. 329, 313–318 (1993). [DOI] [PubMed] [Google Scholar]
- 51.Bishop L. M., Qian H., Stochastic bistability and bifurcation in a mesoscopic signaling system with autocatalytic kinase. Biophys. J. 98, 1–11 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tinevez J.-Y., et al. , TrackMate: An open and extensible platform for single-particle tracking. Methods 115, 80–90 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Stenmark H., Vitale G., Ullrich O., Zerial M., Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell 83, 423–432 (1995). [DOI] [PubMed] [Google Scholar]
- 54.Bezeljak U., Loya H., Kaczmarek B., Saunders T. E., Loose M., Stochastic activation and bistability in a Rab GTPase regulatory network. Figshare. https://figshare.com/collections/Stochastic_activation_and_bistability_in_a_Rab_GTPase_regulatory_network/4835259/1. Deposited 29 January 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.