Abstract
On-site multiplex biosensors for innate immunity antibodies are ideal tools for monitoring health status of individuals against various diseases. This study introduces a novel antibody immunoassay testing platform incorporating microfiber-based arrays of antigens to capture specific antibodies. The fabrication and setup of the device revolved around electrospun polystyrene (ESPS) microfibers that act as three-dimensional membrane filters, capable of rapid and multifold analyte capture. In particular, the ESPS microfibers were patterned through localized oxygen plasma to create hydrophilic zones that facilitate fluid flows and immobilizations of antigens. The bulk of this robust antibody immunoassay platform could be installed into a compact syringe-driven cassette device, which could perform multiplex antibody immunoassay for antibodies specifically against Middle East respiratory syndrome coronavirus (MERS-CoV) with rapid preparation amounting to a total of 5 min, as well as high sensitivity and specificity for the MERS-CoV down to 200 μg/mL.
Keywords: MERS, Multiplex immunoassay, Microfiber, POC, Rapid
Highlights
-
•
3D electrospun polystyrene microfibers were successfully patterned with O2 plasma.
-
•
Antigen adsorption were controlled via plasma-treatment time and blocking reagents.
-
•
Above multiplex microfiber mats could capture multiple antibodies simultaneously.
1. Introduction
For the clinical use of immunoassays, these platforms are expected to be high-throughput, rapid and highly sensitive, as these are basic requirements for the necessary performance. Thus, assays combining these characteristics are proposed and often realized by the use of microfluidics and nanotechnology [[1], [2], [3], [4], [5], [6], [7], [8]]. Furthermore, many types of multiplex immunoassay platforms have been reported, including microelectromechanical systems (MEMS) [9,10], paper-based [[11], [12], [13], [14]], bead-based [[15], [16], [17]] and array-based platforms [[18], [19], [20], [21]]. In recent years, one of the most prominent application of such immunoassay platforms from the perspective of healthcare has been virus detection [22,23].
Since 2012, MERS-CoV has become a prevalent issue affecting multiple countries [[24], [25], [26]]. This coronavirus has affected individuals with a high mortality rate of about 30% which was reported by the World Health Organization (WHO) [27]. Furthermore, MERS-CoV being a relatively recent outbreak, there has been no effective vaccine which is clinically approved to treat an individual [28]. Therefore, a means for antibody diagnostics in a simple, robust and rapid manner is necessary to test vaccine or drug efficacies upon patients while also preventing the spread of this infectious disease.
In the world of vaccination, blood antibody titres often in the form of enzyme-linked immunosorbent assays (ELISA) are common methods to determine whether a patient currently has the necessary antibodies at adequate concentrations. ELISA is a highly sensitive method that can achieve limits of detection (LOD) below 1 μg/mL for MERS-CoV, but the test can take days to produce diagnostic results due to the requirements for specialists and large equipment [[29], [30], [31], [32], [33], [34], [35], [36]]. Thus, a more rapid and effective testing methodology that does not require large testing apparatus is desirable to test for vaccine or drug efficacy. In particular, it is important to emphasize the need for low-cost, simple and robust point-of-care (POC) device manufacturing in order to improve global healthcare.
Here, a rapid immunoassay methodology for antiviral antibody testing that can be conducted within minutes will be introduced. This work illustrates the construction of a novel multiplex antibody immunoassay test which leverages the ESPS microfiber mats as the capture membrane and pressure-driven convection for ultra-rapid testing. In addition to the previously established fluorescently-linked immunosorbent assay (FLISA) microfiber platform [28,37], the microfiber mats were pre-patterned with O2 plasma to create multiple hydrophilic zones with different antigens, and thus creating a multiplex detection system.
As a proof of concept, two different antigens designed to individually capture their corresponding antibodies were tested: human serum albumin (HSA) and MERS-CoV. HSA is a representative protein that exists in human plasma samples, while MERS is a viral agent currently lacking a means of vaccination [28,38]. The simplistic design, ease of fabrication and setup, the rapid speeds at which diagnostics can be performed for antibody detection with this system can be an integral step forward in creating a low cost commercial device for protecting the general public health in case of MERS outbreaks.
2. Materials and methods
2.1. FLISA reagents and materials
For the polymeric electrospinning processing, pelletized PS (PS Japan) dissolved in a solution containing THF (Sigma-Aldrich) and DMF (Sigma-Aldrich). HSA full rapid FLISA operations included Goat anti-Human Albumin (Bethyl Laboratories, A80-129A), Human Reference Serum (Bethyl Laboratories, RS10-110) and FITC-conjugated Goat anti-Human Albumin (Bethyl Laboratories, A80-129F), noted as ‘anti-HSA’, ‘HSA’ and ‘FITC anti-HSA’ in this study, respectively. For antibody testing both the HSA reagents mentioned and the following MERS reagents: His-MERS-NP antigen protein (Yokohama City University) and anti-MERS-NP (mAb #20, Yokohama City University) were utilized and noted in this study as the ‘MERS’ and ‘FITC anti-MERS’ reagents, respectively. Anti-MERS-NP was labeled by the Fluorescein Labeling Kit – NH2 (Dojindo Inc.). Phosphate buffered saline (PBS, pH 7.4, Gibco) with Tween 20 (PBS-T, pH 7.4, 0.1 w/v% Tween 20) and skim milk (Yukijirushi Inc.) were utilized for different purposes like diluents, washes and blocking agents. Additionally, FITC-conjugated bovine serum albumin (BSA-FITC, Sigma-Aldrich, A9771-1G) and BSA (Sigma Aldrich, A3608-50G) were utilized in combination with ESPS fiber mats for fluorescence microscopy.
2.2. Electrospinning technique
Pre-pelletized PS at 10 vol%/vol% was dissolved into a solution of 1:1 THF/DMF. The solutions were left to stir mildly at room temperature for 24 h to allow for the PS to dissolve entirely. The PS solutions were then loaded through a syringe to the electrospinning device. The environmental and processing conditions were set as noted in Supplementary Material Table S1. After electrospinning, a ‘wet-press’ technique reported by Wu et al. was applied to the microfibers [39]. This technique layered ESPS fiber mats by stacking 8 layers of fibers atop one another, soaking them in ethanol (EtOH) then sandwiching the stack between glass sheets and pressed by a 2 kg weight for 24 h before use.
2.3. O2 plasma spot treatment
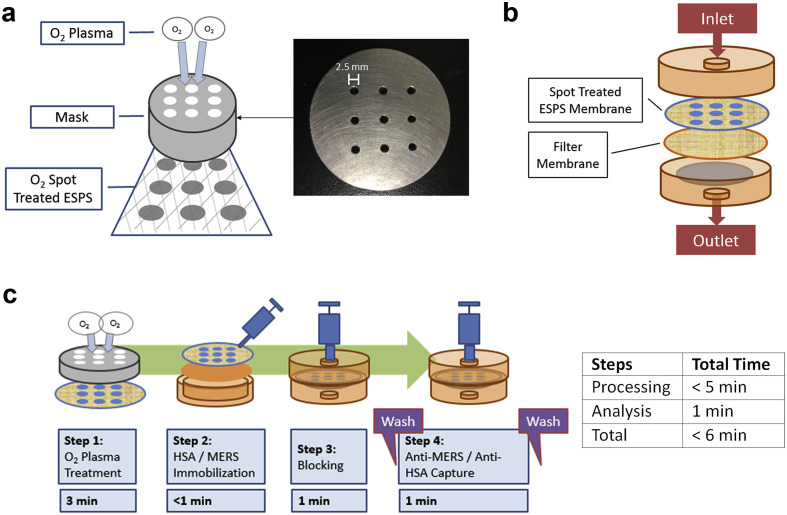
An O2 plasma cleaner (PDC-001, Expanded Plasma Cleaner, Harrick Plasma) paired with a gas flowmeter (PDF-FMG, PlasmaFlo Gas Flow Mixer, Harrick Plasma) were utilized to treat the ESPS fiber mats with O2 plasma. A steel mask with 9 holes (spot size = 2.5 mm) was custom-made to create patterns of O2 plasma treatment on the ESPS fiber mats. ESPS 10 wt% fiber mats that were wet-pressed at 8-layers were utilized for spot treatment experimentation. 8-Layered 10 wt% ESPS was used rather than 4-layer samples since the 4-layer samples showed a spreading of added reagents into non-hydrophilic spot regions over time. 8-Layer samples showed minimal spread or leak of reagents beyond the scope of the O2 plasma treated hydrophilic spots.
8-layer ESPS fiber mats after spot treatment were tested for both protein adsorption and blocking preference by fluorescence microscopy. For protein adsorption ESPS fiber mats were submerged in a FITC-BSA solution (10 wt% FITC-BSA to BSA in 20 mL PBS) for 1 h. Samples were then dip-washed in PBS-T 20 times. Blocking effectiveness was tested by comparing BSA, BlockingONE and skim milk as blocking agents, where three separate ESPS fiber mats were soaked in the respective blocking solutions: BSA (2 w/v), BlockingONE (20 v/v), and skim milk (2 w/v) for 1 h before being dip washed in PBS-T 20 times. Then the samples were soaked in the same FITC-BSA solution as the previous experiment for 1 h and dip-washed again in PBS-T 20 times. All fluorescence images were measured under an inverted microscope (CKX53, Olympus) paired with a fluorescence light source and filter (U-HGLGPS, Olympus). Images were taken by the cellSens (Olympus) image processing software and measured by ImageJ (National Institutes of Health) under the ROI manager tool.
2.4. Cassette-device-based immunoassay system
A 37-mm monitor (Advantec) was adapted as the cassette device by replacing the internal membranes with the 8-layered 10 wt% ESPS samples together with an appropriately cut and shaped filter paper (40 mm, No.5B, Kiriyama Rohto) layered inside the 37-mm monitor housing environment. The setup had the lower layer as the filter paper with the upper layer as the O2 plasm-treated ESPS fiber mat. Through the inlet, bulk reagents (PBS wash, antigen, and secondary antibody) were inserted and flushed out the opposite end outlet through syringe pressure.
2.5. Rapid MERS immunoassay testing protocol
In all immunoassays, the 8-layered 10 wt% ESPS membranes, which were treated with O2 plasma treatment for 3 min at 100W, were utilized. Rapid immunoassay testing using the cassette platform were performed in following steps: (1) antigen immobilization, (2) blocking, and (3) antibody capture. First, HSA and MERS concentrations were optimized independently on the treated ESPS membranes. HSA concentration in PBS was varied in the range from 0 to 1000μg/mL, using 6 μL per spot. Blocking by 2 mL of skim milk at 2% w/v was then applied through the syringe directly into the cassette, where the solution was held for 1 min then pulled through the outlet. Finally, 1 mL of FITC-conjugated anti-HSA antibody solution at 10 μg/mL was held in the cassette for 1 min and also flushed through. Similarly, MERS was tested first by immobilizing His-MERS-NP at concentrations ranging from 0 to 500 μg/mL. After the skim milk blocking procedure, 1 mL of FITC-conjugated anti-MERS NP #20 solution at 11 μg/mL was held in the cassette for 1 min and flushed through. For both HSA and MERS testing, washing was conducted with PBS-T with 1 mL volumes flushed through three times through the cassette both after the blocking step and FITC-conjugated antibody step.
For the multiplex immunoassay, it was conducted identically to the above immunoassay protocol, but with different antigens patterned separately on a single ESPS membrane: 3 spots were treated with MERS (200 μg/mL; 6 μL), 3 spots treated with HSA (200 μg/mL; 6 μL) and 3 spots with no antigen immobilized. Again, the blocking was performed with 2 mL of skim milk at 2% w/v was held for 1 min in the cassette and then flushed through. Finally, three different test solutions of antibodies were prepared and tested through the multiplex ESPS membrane: 1 mL of FITC-conjugated anti-HSA solution at 1:100 dilution, 1 mL of FITC-conjugated anti-MERS NP #20 solution at 1:50 dilution, and a 1 mL mixture of FITC-conjugated anti-HSA (1:100 dilution) and FITC conjugated-anti-MERS NP #20 (1:50 dilution), where the concentrations for each antibody were identical in all cases. Fig. 1 and Supplementary Material Table S2 illustrate the overall procedure as well as the final concentrations used for the test protocols.
Fig. 1.
(a) Schematic representation of the O2 plasma spot treatment setup. (b) Schematic representation of the housing environment and membrane setup. (c) Overview of steps taken in the rapid MERS immunoassay protocol.
3. Results
3.1. O2 plasma spot treatment
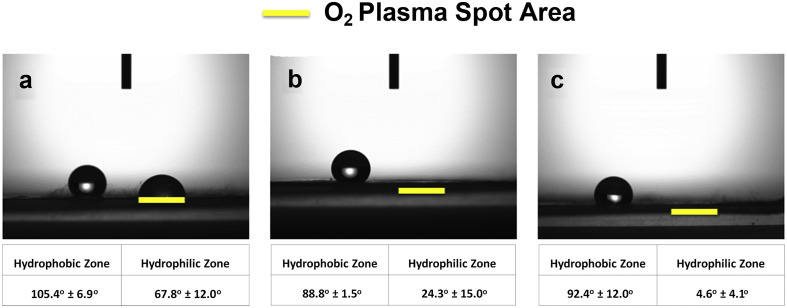
It is critical that as much antigens are immobilized to the surface to enhance the signal of the immunoassay system for the antiviral antibodies. Therefore, the amount of protein immobilization was optimized through the microfiber surface hydrophobicity. The 8-layered 10 wt% ESPS fiber mat samples were treated by O2 plasma spot treatment for different time lengths, then tested for protein adsorption to the surface. To determine the optimum condition for the largest antibody immobilization to the surface, BSA was used as the model protein. First, the contact angles were measured to confirm the effect of O2 plasma treatments (Fig. 2 ). Overall, it was confirmed that the hydrophilic areas treated by O2 plasma showed a decreasing trend in contact angle with increasing treatment time. On the other hand, the contact angles of hydrophobic zones were not significantly altered among the samples.
Fig. 2.
Contact angle measurements for (a) 1 min, (b) 3 min, and (c) 5 min of O2 spot plasma treatments.
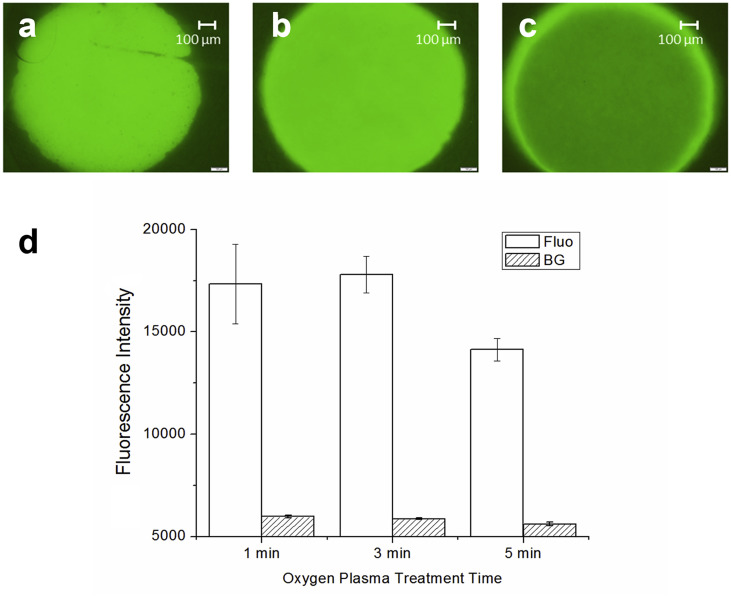
Next, the actual amount of BSA adsorption on plasma-treated samples were analyzed to estimate the antigen immobilization capacity. The results for varied O2 plasma treatment conditions are shown in Fig. 3 , where the samples were treated for 1, 3 and 5 min at 100W. It can be seen that the most amount of BSA was adsorbed to the 3-min-treated samples. The lack of sufficient O2 plasma time at 1 min likely did not allow the oxygen to fully penetrate the fiber thus not yielding adequately-hydrophilic fiber surfaces for the BSA solution to penetrate the fiber and come in contact with the fiber surfaces. On the other hand, the excessive 5-min-treated samples over treated the samples to become super hydrophilic, preventing any hydrophobic bonding between the surface and protein samples. Thus, for subsequent experiments, 3 min was chosen as the optimal time for O2 plasma treatment for protein immobilization to the fiber surfaces.
Fig. 3.
Fluorescence images labeled BSA adsorbed onto the 8-layered 10 wt% ESPS treated with spot O2 plasma for (a) 1 min, (b) 3 min and (c) 5 min. (d) For quantitation of the BSA adsorption, fluorescence (Fluo) as well as background (BG) intensities were quantified. (n = 4; error bars are standard error).
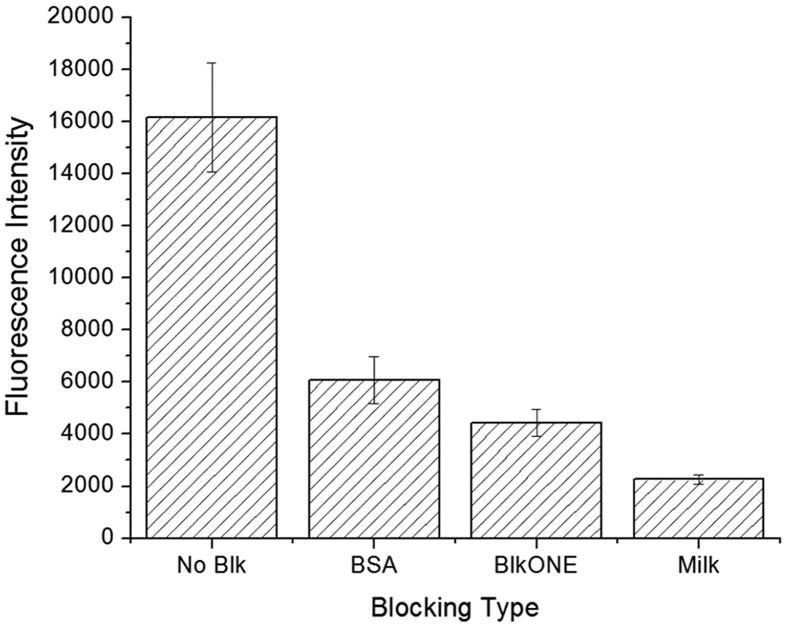
In addition, blocking agents were optimized for the ESPS samples and thus a similar protein adsorption test was conducted with three types of blocking agents: BSA, BlockingONE and skim milk (Fig. 4 ). Although all of them displayed efficient blocking of fluorescently-labeled BSA adsorption, it was demonstrated that skim milk performed the best. Therefore, in subsequent experiments, skim milk was used as the blocking agent.
Fig. 4.
Fluorescence quantification of the ability of blocking agents to prevent the adsorption of fluorescently-labeled BSA, comparing BSA, BlockingONE (BlkONE) and Skim milk (Milk).
3.2. Multiplex rapid MERS immunoassay
To provide a proof of concept study for clinical application as to whether the fabricated ESPS platform together with the design rapid immunoassay protocol within a device-based environment could be applied to a rapid MERS immunoassay, antibodies against HSA (control) and MERS-CoV (target) were tested using this platform. As mentioned in the introduction, by attaining a rapid detection of desired analytes, in this case antibodies to examine the immune system, a progressive step forward can be made towards preventative diagnostics.
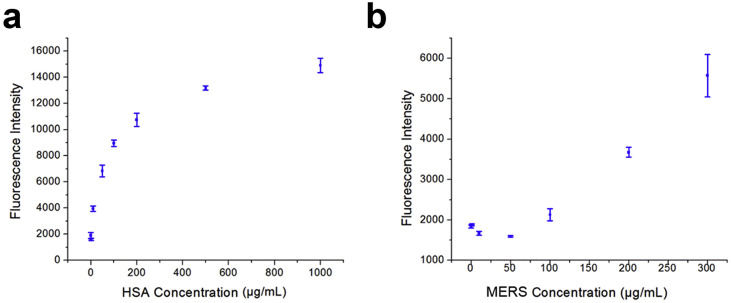
First, the optimization of the protocol for antigen immobilization to the ESPS platform was performed. Here, both HSA and MERS antigens were tested separately. Optimization of HSA adsorption indicated a rapidly increasing trend up until about 200 μg/mL to where increasing amounts of HSA immobilized to the surface has a plateaued effect of detection intensity (Fig. 5 ). Meanwhile, the MERS adsorption test demonstrated that detection was limited at concentrations less than 100 μg/mL, thus concentrations above this value were used for subsequent studies.
Fig. 5.
Varied immobilized antigen concentrations to the surface for (a) HSA and (b) MERS utilizing the rapid immunoassay protocol. (n = 3; error bars are standard errors).
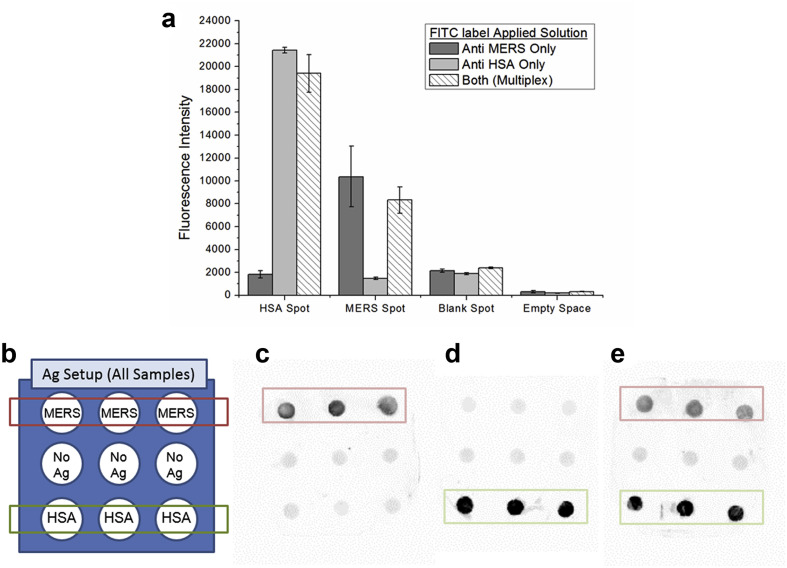
Lastly, multiplex rapid immunoassay using 200 μg/mL of HSA and MERS antigens was performed (Fig. 6 ). Three separate ESPS platforms, each with 9 O2-plasma treated spots, were all processed under the same conditions with the only difference being the antibody solution having: (1) only FITC-conjugated anti-HSA antibodies, (2) only FITC-conjugated anti-MERS antibodies, or (3) a mix of both FITC conjugate anti-HSA and FITC-conjugated anti-MERS antibodies. It was successfully observed that, upon the ESPS platforms tested with FITC-conjugated anti-MERS antibody and anti-HSA antibody solutions, each of MERS and HSA antibodies could be selectively detected. Additionally, when a mixed solution of FITC-conjugated anti-MERS and anti-HSA antibodies was added, both antibodies could be simultaneously detected with the multiplex setup. It was also noted that the signals for both antibodies slightly decreased in the mixture test, in comparison to the individual tests, possibly due to the crowding.
Fig. 6.
(a) Quantification of the multiplex MERS immunoassay, (b) schematic of the antigen immobilization setup, (c) FITC-conjugated anti-MERS only solution, (d) FITC-conjugated anti-HSA only solution, and (e) both anti-HSA and anti-MERS. (n = 3; error bars are standard errors).
4. Discussion
This multiplex microfiber-based immunoassay platform hinges on several key factors including the patterned O2 plasma treatment and efficient blocking that warrant some discussions. First, the rationale behind how proteins such as the immobilized antibodies/antigens preferentially adsorb to just the hydrophilic spots is of great importance. Generally, the protein adsorption on flat surface is affected by the hydrophobicity and surface potential [40]. In the case of EPSP fiber mats, the nature of hydrophobicity arises from Cassie-Baxter regime consisting of two kinds of hydrophobic materials, which are the untreated PS and air [41]. The O2 plasma treatment modifies the surface properties of PS rendering it hydrophilic. For flat PS plates, the surface wettability of water changed from 77.7° ± 3.0°–25.2° ± 3.9° after treatment with O2 plasma for 5 min. Interestingly, in stark contrast to the flat plates, the O2-plasma-treated PS microfibers resulted in greater levels of hydrophilicity (4.5° ± 4.1°) after the same plasma treatment, as illustrated in Fig. 2. This hydrophilic nature of the ESPS microfiber mat after O2 plasma treatment can be explained by the Wenzel regime, describing the reduced apparent water contact angles for rough surfaces [41]. Indeed, the patterned O2 plasma treatment on the ESPS microfiber mats resulted in separate regions with these two different regimes, where only the hydrophilic surfaces promoted solution flow through the plasma-treated spots. In other words, O2 plasma treatment of PS was suitable for preventing air pockets within microfibers, and thus the protein solution could contact the fiber surface through wetting. On the contrary, the unexposed, hydrophobic surfaces of ESPS did not allow wetting, so the protein solution could not contact the fiber surfaces in these regions. Thus, although the total amount of protein adsorption is regulated by the true contact angle of materials, the apparent contact angle arising of the porous structure of ESPS microfiber is also a crucial parameter in dictating protein adsorption.
Next, a brief discussion of skim milk as the blocking agent is of importance. Often times, immunoassays are conducted with blocking agents commonly those of proteins which can adhere well to the surface without preventing any other adsorption of proteins to the surface. Among the common choices, skim milk, BSA or BSA-based products, such as BlockingONE exist. As noted in Fig. 5, skim milk had the best results for blocking in the case of the O2-plasma-treated samples. The mix of variety of proteins that exist within skim milk could beneficially be penetrating the fiber and thus blocking the surface most effectively [[42], [43], [44]]. It has been found that casein, within milk, can be an effective blocking agent due to its small protein size. Together with other proteins within skim milk a closely packed surface of proteins can effectively prevent non-specific adsorption to the surface. In contrast, BSA has relatively large molecular weight components which can make random close packing of blocking to the surface difficult in comparison to that of skim milk. Another interesting point is that skim milk is considered amphipathic having both hydrophilic and hydrophobic parts. As O2 treatment is introduced to the surface, the hydrophilic nature may have allowed milk to be effectively bound to the surface.
Lastly, the comparison of the multiplex microfiber platform used in this study to other conventional assays and devices is important. A comparable work to this study was conducted by Sato et al. [45], where a combinatorial assay was conducted utilizing a microfluidic chip together with integrated polystyrene beads to measure IgA antibody concentration. Similar to how microfibers were utilized in this study to increase the surface to volume ratios, microbeads were packed into a microfluidic device with a filling factor of 60% (bead diameter of 46 μm). Although they were able to achieve a high level of sensitivity below 10 μg/mL, their straightforward step-by-step immunoassay procedure of antigen immobilization and then capture of colloid gold labeled-IgA antibodies took roughly 1 h, compared to several minutes in this study. Thus, sensitivity and speed is often a fine balance for these immunoassays, and priorities should be chosen based on the applications of these assays. The multiplex microfiber platform in this study is intended for antibody detection for POC monitoring of antibody levels. It has recently been reported that the antibody titers of patients infected by MERS-CoV range from 1: 80 to 1: 800, depending on the time post infection, with the neutralizing antibody titer being more than 1: 800 [[46], [47], [48]]. Considering that ELISA is often used for determining antibody titers and its LOD is around 1 μg/mL [36], it is suggested that the required antibody concentration for neutralizing MERS-CoV would be above 800 μg/mL. Thus, the LOD of 200 μg/mL for the anti-MERS-CoV antibody by the multiplex microfiber platform, although not yet as low as other established platforms, would be sufficient to detect the necessary antibody production in MERS-infected patients.
5. Conclusion
The multiplex ESPS fiber system with a housing suited for POC was introduced as a means for rapid MERS antibody detection. The capability of a rapid FLISA was exhibited by utilizing 8-layered ESPS fiber mats treated with O2 plasma. The patterned O2 plasma treatment method with developed mask showed optimal protein adsorptive results at 3 min at 100W. This in turn created a hydrophilic surface capable of antibody immobilization and, further, rapid FLISA testing. In terms of the detection surface, effective detection could be achieved upon this platform within 1 min of operation time. Furthermore, the actual setup and preparation of the device could be completed in 5 min, which includes capture molecule immobilization, blocking and washing steps. The results demonstrated the capability of the device for multiplex analysis by detecting antibodies for both HSA and MERS-CoV concurrently. This microfiber-based multiplex immunoassay platform serves as a stepping stone for the next-generation POC device that would enable preventative diagnostics by accurately and rapidly determining the existence of adequate antibodies and aid general healthcare through appropriate vaccination schemes.
Author contributions
C.F.O.H. and Y.Y. performed experiments; C.F.O.H. and K.K. analyzed data; K.K., A.R. and M.T. designed research; C.F.O.H., K.K., Y.Y., A.R., and M.T. wrote manuscript.
Acknowledgements
We would like to thank Dr. Satoko Matsunaga at the Yokohama City University for her kind donations of MERS reagents. This work was supported by the Grant-in-Aid for Exploratory Research [16K13623] from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sbsr.2019.100304.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wilkins J., Gallimore J.R., Moore E.G., Pepys M.B. Rapid automated high sensitivity enzyme immunoassay of C-reactive protein. Clin. Chem. 1998;44:1358–1361. [PubMed] [Google Scholar]
- 2.Chon H., Lee S., Son S.W., Oh C.H., Choo J. Highly sensitive immunoassay of lung cancer marker carcinoembryonic antigen using surface-enhanced Raman scattering of hallow gold nanospheres. Anal. Chem. 2009;81:3029–3034. doi: 10.1021/ac802722c. [DOI] [PubMed] [Google Scholar]
- 3.Tajima N., Takai M., Ishihara K. Significance of antibody orientation unraveled: well-oriented antibodies recorded high binding affinity. Anal. Chem. 2011;83:1969–1976. doi: 10.1021/ac1026786. [DOI] [PubMed] [Google Scholar]
- 4.Lim C.T., Zhang Y. Bead-based microfluidic immunoassays: the next generation. Biosens. Bioelectron. 2007;22:1197–1204. doi: 10.1016/j.bios.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Takai M. Highly sensitive and rapid biosensing on a three-dimensional polymer platform. Polym. J. 2018;50:847–855. [Google Scholar]
- 6.Li Z.H., Wang Y., Wang J., Tang Z.W., Pounds J.G., Lin Y.H. Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal. Chem. 2010;82:7008–7014. doi: 10.1021/ac101405a. [DOI] [PubMed] [Google Scholar]
- 7.Qu H.H., Zhang Y., Qu B.P., Kong H., Qin G.F., Liu S.C. Rapid lateral-flow immunoassay for the quantum dot-based detection of puerarin. Biosens. Bioelectron. 2016;81:358–362. doi: 10.1016/j.bios.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Lin C.C., Wang J.H., Wu H.W., Lee G.B. Microfluidic immunoassays. Jala-J Lab. Autom. 2010;15:253–274. [Google Scholar]
- 9.Auroux P.A., Iossifidis D., Reyes D.R., Manz A. Micro total analysis systems. 2. Analytical standard operations and applications. Anal. Chem. 2002;74:2637–2652. doi: 10.1021/ac020239t. [DOI] [PubMed] [Google Scholar]
- 10.Reyes D.R., Iossifidis D., Auroux P.A., Manz A. Micro total analysis systems. 1. Introduction, theory, and technology. Anal. Chem. 2002;74:2623–2636. doi: 10.1021/ac0202435. [DOI] [PubMed] [Google Scholar]
- 11.Martinez A.W., Phillips S.T., Whitesides G.M., Carrilho E. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal. Chem. 2010;82:3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 12.Yamada K., Henares T.G., Suzuki K., Citterio D. Paper-based inkjet-printed microfluidic analytical devices. Angew. Chem. Int. Ed. 2015;54:5294–5310. doi: 10.1002/anie.201411508. [DOI] [PubMed] [Google Scholar]
- 13.Abe K., Suzuki K., Citterio D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal. Chem. 2008;80:6928–6934. doi: 10.1021/ac800604v. [DOI] [PubMed] [Google Scholar]
- 14.Li X., Ballerini D.R., Shen W. A perspective on paper-based microfluidics: current status and future trends. Biomicrofluidics. 2012;6 doi: 10.1063/1.3687398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato K., Tokeshi M., Kimura H., Kitamori T. Determination of carcinoembryonic antigen in human sera by integrated bead-bed immunoassay in a microchip for cancer diagnosis. Anal. Chem. 2001;73:1213–1218. doi: 10.1021/ac000991z. [DOI] [PubMed] [Google Scholar]
- 16.Swartzman E.E., Miraglia S.J., Mellentin-Michelotti J., Evangelista L., Yuan P.M. A homogeneous and multiplexed immunoassay for high-throughput screening using fluorometric microvolume assay technology. Anal. Biochem. 1999;271:143–151. doi: 10.1006/abio.1999.4128. [DOI] [PubMed] [Google Scholar]
- 17.Lee B.S., Lee J.N., Park J.M., Lee J.G., Kim S., Cho Y.K. A fully automated immunoassay from whole blood on a disc. Lab Chip. 2009;9:1548–1555. doi: 10.1039/b820321k. [DOI] [PubMed] [Google Scholar]
- 18.Kellar K.L., Kalwar R.R., Dubois K.A., Crouse D., Chafin W.D., Kane B.E. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry. 2001;45:27–36. doi: 10.1002/1097-0320(20010901)45:1<27::aid-cyto1141>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Bruls D.M., Evers T.H., Kahlman J.A.H., van Lankvelt P.J.W., Ovsyanko M., Pelssers E.G.M. Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab Chip. 2009;9:3504–3510. doi: 10.1039/b913960e. [DOI] [PubMed] [Google Scholar]
- 20.Fu Q., Zhu J., Van Eyk J.E. Comparison of multiplex immunoassay platforms. Clin. Chem. 2010;56:314–318. doi: 10.1373/clinchem.2009.135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smits G.P., van Gageldonk P.G., Schouls L.M., van der Klis F.R.M., Berbers G.A.M. Development of a bead-based multiplex immunoassay for simultaneous quantitative detection of IgG serum antibodies against measles, mumps, rubella, and varicella-zoster virus. Clin. Vaccine Immunol. 2012;19:396–400. doi: 10.1128/CVI.05537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X., Xia Y.Q., Tang Y., Zhang W.L., Yeh Y.T., Lu H.G. A nanostructured microfluidic immunoassay platform for highly sensitive infectious pathogen detection. Small. 2017;13 doi: 10.1002/smll.201700425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaminska A., Witkowska E., Winkler K., Dziecielewski I., Weyher J.L., Waluk J. Detection of Hepatitis B virus antigen from human blood: SERS immunoassay in a microfluidic system. Biosens. Bioelectron. 2015;66:461–467. doi: 10.1016/j.bios.2014.10.082. [DOI] [PubMed] [Google Scholar]
- 24.Yamaoka Y., Matsuyama S., Fukushi S., Matsunaga S., Matsushima Y., Kuroyama H. Development of monoclonal antibody and diagnostic test for Middle East respiratory syndrome coronavirus using cell-free synthesized nucleocapsid antigen. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y., Chan K.H., Kang Y.H., Chen H.L., Luk H.K.H., Poon R.W.S. A sensitive and specific antigen detection assay for Middle East respiratory syndrome coronavirus. Emerg. Microb. Infect. 2015;4 doi: 10.1038/emi.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsolamy S., Arabi Y.M. Infection with Middle East respiratory syndrome coronavirus. Can. J. Respir. Ther. 2015;51:102. [PMC free article] [PubMed] [Google Scholar]
- 28.Hotez P.J., Bottazzi M.E., Tseng C.T.K., Zhan B., Lustigman S., Du L.Y. Calling for rapid development of a safe and effective MERS vaccine. Microb. Infect. 2014;16:529–531. doi: 10.1016/j.micinf.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Haas R., van den Hof S., Berbers G.A., de Melker H.E., Conyn-van Spaendonck M.A. Prevalence of antibodies against rubella virus in The Netherlands 9 years after changing from selective to mass vaccination. Epidemiol. Infect. 1999;123:263–270. doi: 10.1017/s0950268899002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Hof S., Beaumont M.T., Berbers G.A., de Melker H.E. Antibodies against mumps in The Netherlands as assessed by indirect ELISA and virus neutralization assay. Epidemiol. Infect. 2003;131:703–709. doi: 10.1017/s0950268803008641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harmsen T., Jongerius M.C., Vanderzwan C.W., Plantinga A.D., Kraaijeveld C.A., Berbers G.A.M. Comparison of a neutralization enzyme-immunoassay and an enzyme-linked-immunosorbent-assay for evaluation of immune status of children vaccinated for mumps. J. Clin. Microbiol. 1992;30:2139–2144. doi: 10.1128/jcm.30.8.2139-2144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celikbas A., Ergonul O., Aksaray S., Tuygun N., Esener H., Tanir G. Measles, rubella, mumps, and varicella seroprevalence among health care workers in Turkey: is prevaccination screening cost-effective? Am. J. Infect. Contr. 2006;34:583–587. doi: 10.1016/j.ajic.2006.04.213. [DOI] [PubMed] [Google Scholar]
- 33.Craft J.E., Grodzicki R.L., Steere A.C. Antibody-response in lyme-disease - evaluation of diagnostic-tests. JID (J. Infect. Dis.) 1984;149:789–795. doi: 10.1093/infdis/149.5.789. [DOI] [PubMed] [Google Scholar]
- 34.Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titer. Scand. J. Immunol. 1973;2:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 35.Fukushi S., Fukuma A., Kurosu T., Watanabe S., Shimojima M., Shirato K. Characterization of novel monoclonal antibodies against the MERS-coronavirus spike protein and their application in species-independent antibody detection by competitive ELISA. J. Virol. Methods. 2018;251:22–29. doi: 10.1016/j.jviromet.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly-Cirino C., Mazzola L.T., Chua A., Oxenford C.J., Van Kerkhove M.D. An updated roadmap for MERS-CoV research and product development: focus on diagnostics. BMJ Glob. Health. 2019;4 doi: 10.1136/bmjgh-2018-001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoy C.F.O., Kushiro K., Takai M. Fabrication and assessment of an electrospun polymeric microfiber-based platform under bulk flow conditions with rapid and efficient antigen capture (vol 143, pg 865. Analyst. 2018;143(2018):1713. doi: 10.1039/c8an90024h. [DOI] [PubMed] [Google Scholar]
- 38.Choi S., Choi E.Y., Kim D.J., Kim J.H., Kim T.S., Oh S.W. A rapid, simple measurement of human albumin in whole blood using a fluorescence immunoassay (I) Clin. Chim. Acta. 2004;339:147–156. doi: 10.1016/j.cccn.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Wu D.P., Han D.W., Steckl A.J. Immunoassay on free-standing electrospun membranes. Acs Appl. Mater. Inter. 2010;2:252–258. doi: 10.1021/am900664v. [DOI] [PubMed] [Google Scholar]
- 40.Attwood S.J., Kershaw R., Uddin S., Bishop S.M., Welland M.E. Understanding how charge and hydrophobicity influence globular protein adsorption to alkanethiol and material surfaces. J. Mater. Chem. B. 2019;7:2349–2361. doi: 10.1039/c9tb00168a. [DOI] [PubMed] [Google Scholar]
- 41.Whyman G., Bormashenko E., Stein T. The rigorous derivation of Young, Cassie-Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon. Chem. Phys. Lett. 2008;450:355–359. [Google Scholar]
- 42.Peterfi Z., Kocsis B. Comparison of blocking agents for an ELISA for LPS. J. Immunoass. 2000;21:341–354. doi: 10.1080/01971520009349541. [DOI] [PubMed] [Google Scholar]
- 43.Kim W.S., Nishizawa T., Yoshimizu M. Non-specific adsorption of fish immunoglobulin M (IgM) to blocking reagents on ELISA plate wells. Dis. Aquat. Org. 2007;78:55–59. doi: 10.3354/dao01843. [DOI] [PubMed] [Google Scholar]
- 44.Contarini G., Povolo M. Phospholipids in milk fat: composition, biological and technological significance, and analytical strategies. Int. J. Mol. Sci. 2013;14:2808–2831. doi: 10.3390/ijms14022808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato K., Tokeshi M., Odake T., Kimura H., Ooi T., Nakao M. Integration of an immunosorbent assay system: analysis of secretory human immunoglobulin A on polystyrene beads in a microchip. Anal. Chem. 2000;72:1144–1147. doi: 10.1021/ac991151r. [DOI] [PubMed] [Google Scholar]
- 46.Corman V.M., Albarrak A.M., Omrani A.S., Albarrak M.M., Farah M.E., Almasri M. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin. Infect. Dis. 2016;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Said M.Y. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992-2013. Emerg. Infect. Dis. 2014;20:1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mubarak A., Alturaiki W., Hemida M.G. Middle East respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J. Immunol. Res. 2019;2019:11. doi: 10.1155/2019/6491738. 6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.