Abstract
Background Middle East respiratory syndrome coronavirus (MERS-CoV), is an emerging virus respiratory infection. It has a high mortality rate and a wide spectrum of clinical features. This study describes the clinical characteristics and outcome of MERS infected patients.
Methods A retrospective study was conducted of all confirmed MERS-CoV infections from March 2014 to May 2014 at two tertiary care hospitals in Al-Madinah region (Saudi Arabia). We gathered data about demographic, clinical presentation, and factors associated with severity and mortality.
Results A total of 29 cases were identified; 20 males (69%) and nine females (31%), age 45 ± 12 years. The death rate was higher for men (52%) than for women (23%). Initial presentation was fever in 22 (75%) cases, cough in 20 (69%) cases, and shortness of breath in 20 (69%) cases. Associated comorbidities were diabetes mellitus in nine (31%) patients and chronic kidney disease (CKD) in eight (27%) patients. Duration of symptoms before hospitalization ranged from 2.9 days to 5 days. Elevated liver enzymes were present in 14 (50%) patients and impaired renal profile present in eight (27%) patients. We also describe in this study radiological patterns and factors associated with mortality.
Conclusion MERS-CoV infection transmission continues to occur as clusters in healthcare facilities. The frequency of cases and deaths is higher among men than women and among patients with comorbidities.
Keywords: Characteristics, Coronavirus, Epidemiology, MERS, Saudi Arabia, Survival
1. Introduction
In Saudi Arabia, a beta new coronavirus was isolated for the first time at the end of 2012 from a patient who presented with acute community acquired pneumonia [1]. He died 11 days later from progressive severe respiratory failure and acute renal failure (ARF) and his sputum sample was negative for respiratory viruses commonly tested. Epidemiology of Middle East respiratory syndrome coronavirus (MERS-CoV) was expanded after exploring the large hospital outbreak in Al-Hasa, Saudi Arabia [2]. Subsequently, the virus was designated as MERS-CoV [3]. The geographic distribution of the cases has been mainly linked to the Arabian Peninsula particularly from Saudi Arabia where most of the cases were reported [4–7]. However, in some countries in North America, Europe, Africa, and Asia, the disease has been detected in some travelers from endemic countries [3,7–13]. The initial occurrence of MERS-CoV was thought to have particular predominance for male patients and those with comorbid diseases. The male-to-female ratio was between 2.8:1 and 3.3:1 [2,6]; this male predominance might have been related to the nature of the outbreak. Initial cases were reported among elderly patients with a median age of 56 years. MERS-CoV has a very high mortality rate, and complications arising from infection can result in severe respiratory and renal failure [2]. Symptoms of MERS-CoV range from mild upper respiratory symptoms to rapidly progressive severe pneumonia requiring intubation, and multiorgan failure. A significant number of patients may present with nonrespiratory symptoms such as headache, myalgia, and gastrointestinal symptoms of nausea, diarrhea, or vomiting [2,14]. This study describes the demographic, clinical characteristics, and outcome of MERS-CoV in Al-Madinah region, Saudi Arabia.
2. Materials and methods
A retrospective chart review study of all confirmed MERS-CoV cases recorded by two tertiary hospitals from the Madinah region from March 2014 to May 2014. Institutional Review Board approval was obtained for our study from authorities of both hospitals. A case was confirmed as having infection if MERS-CoV real-time polymerase chain reaction was positive, using the recommended sampling technique (nasopharyngeal swab and tracheal aspirates or bronchoalveolar lavage in intubated patients). Extraction of RNA was performed with Roche MagNa Pure LC (RNA viral isolation Kit). Samples were pretreated with lysis according to the manufacturer’s instructions [15]. We obtained data about demographic characteristics, clinical presentation, laboratory results, diagnosis, incubation period, smoking history, comorbidities, and history of contact with camels or MERS-CoV positive patients in regions within the Madinah area. We recorded the duration of the patient’s illness, microbiological test results, and reviewed imaging and treatments received. We also recorded the following outcomes: duration of mechanical ventilation, intensive care unit (ICU) length of stay, and survival during hospitalization until the patient is discharged from hospital.
2.1. Statistical analysis
Data were analyzed using IBM SPSS for Windows, version 18.0. The frequency of cases of MERS-CoV infection and percentage of resulting deaths were calculated. Statistical analyses of demographics, clinical, and laboratory descriptive data are tabulated. Descriptive statistics such as means and standard deviation mean (±SD) were used to describe the age of the patients, laboratory test results, and duration of illness. Frequencies and percentages n (%) were used to describe demographic and MER-COV outcomes. We also did a correlation with the outcome using the t test and Fisher’s exact test as appropriate with a significant value at p ⩽ 0.05.
3. Results
The total number of cases with confirmed MERS-CoV infection reported from April 2014 to May 2014 was 29. The majority of patients (60%) were men, and the median age was 45 years. The most common symptoms were fever (75.9%) and cough (69%), shortness of breath (69%), and vomiting and diarrhea (27%). The average duration of symptoms prior to hospitalization was 5 days (range, 1–12 days). Demographic and clinical characteristics of patients with confirmed MERS-CoV are shown in Table 1. Mortality rate among patients with confirmed MER-CoV was 34%. Mortality from MERS-CoV was significant (p < 0.05) and associated with older age, the presence of gastrointestinal symptoms, longer duration of symptoms prior to hospitalization (8 ± 2.5 days), diabetes mellitus, chronic kidney disease (CKD), smokers, and lower blood pressure, as shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection according to outcome.
| Clinical characteristics | Died (n = 10) | Survived (n = 19) | p | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age (y) Mean ± SD | 54.6 ± 13.0 | 40.7 ± 8.5 | 0.002* | ||
| Gender | |||||
| Male | 6 | 60.0 | 14 | 73.7 | 0.675 |
| Female | 4 | 40.0 | 5 | 26.3 | |
| Symptoms | |||||
| Fever | 9 | 90.0 | 13 | 68.4 | 0.367 |
| Cough | 7 | 70.0 | 13 | 68.4 | 1.0 |
| Shortness of breath | 8 | 80.0 | 12 | 63.2 | 0.431 |
| Vomiting | 7 | 70.0 | 1 | 5.3 | 0.001* |
| Diarrhea | 7 | 70.0 | 1 | 5.3 | 0.001* |
| History of chronic diseases | |||||
| Diabetes mellitus | 7 | 70.0 | 2 | 10.5 | 0.002* |
| Chronic kidney disease | 8 | 80.0 | 0 | 0.0 | <0.0001* |
| Smoking habit | |||||
| Smoker | 10 | 100.0 | 9 | 47.4 | 0.005* |
| Nonsmoker | 0 | 0.0 | 10 | 52.6 | |
| Duration of disease before hospitalization (d) | |||||
| Min–Max | 4–12 | 1–6 | <0.0001* | ||
| Mean ± SD | 8.1 ± 2.5 | 3.4 ± 1.3 | |||
| Vital signs | |||||
| Pulse | |||||
| Min–Max | 98–154 | 82–142 | <0.0001* | ||
| Mean ± SD | 132.5 ± 17.0 | 101.0 ± 21.5 | |||
| Temperature (°C) | |||||
| Min–Max | 37.1–39.0 | 37.0–39.1 | 0.415 | ||
| Mean ± SD | 38.2 ± 0.5 | 38.0 ± 0.6 | |||
| Systolic blood pressure (mmHg) | |||||
| Min–Max | 90–105 | 125–140 | <0.0001* | ||
| Mean ± SD | 98.0 ± 4.8 | 132.6 ± 5.6 | |||
| Diastolic blood pressure (mmHg) | |||||
| Min–Max | 55–65 | 70–87 | <0.0001* | ||
| Mean ± SD | 59.0 ± 3.2 | 78.3 ± 6.4 | |||
SD = standard deviation.
Denotes significant p value.
Most of the patients were coming from Hanakia 11 (38%) and all 11 patients had contact with camels Table 2. Close contact with confirmed index MERS-CoV was documented in five patients, all of whom were healthcare professionals; three staff nurses and two clinicians.
Table 2.
Residence within Al-Madinah area of patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection – according to outcome.
| Demographic characteristics | Died (n = 10) | Survived (n = 19) | p | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Residence | |||||
| Al-Madinah | 0 | 0.0 | 6 | 31.6 | 0.045* |
| Hanakia | 6 | 60.0 | 5 | 26.3 | |
| Yanbu | 1 | 10.0 | 3 | 15.8 | |
| Wadi Alfara | 0 | 0.0 | 3 | 15.8 | |
| Khaibar | 1 | 10.0 | 2 | 10.5 | |
| Al Mahd | 2 | 20.0 | 0 | 0.0 | |
Denotes significant p value.
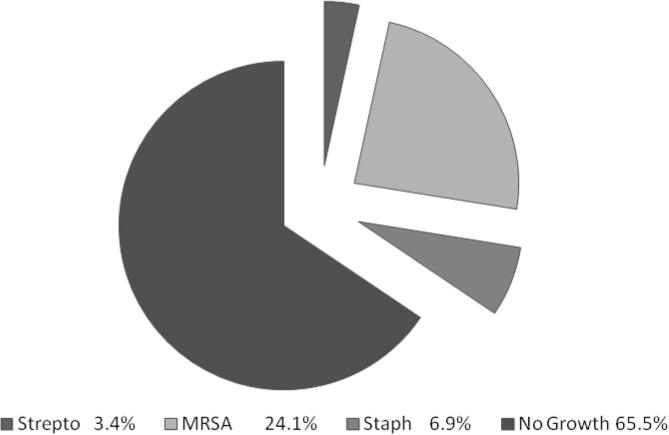
Most of the patients were mildly hypoxic; oxygen saturation was 88.9 ± 5.4 at presentation with a picture of respiratory acidosis (pH 7.3 ± 0.1 and pCO2 57.1 ± 8.2). The basic liver functions show elevated alanine transaminase (98.4 ± 105.4 U/L), aspartate aminotransferase (86.3 ± 93.5 U/L), and creatinine (225.0 ± 115.3 µmol/L). As shown in Table 3, deaths among patients with MERS-CoV was significantly associated with worse gas exchanges pH 7.2 ± 0.1, SPO2 84.8 ± 3.5, lower WBC 3.3 ± 0.9, lower hemoglobin 11.7 ± 1 g/dL, worse liver enzymes, and kidney function (all p < 0.05). Other important findings are the sputum culture results illustrated in Fig. 1, which were collected during the patient’s initial assessment.
Table 3.
Laboratory findings of Middle East respiratory syndrome coronavirus (MERS-CoV) patients according to outcome, April–May 2013/Al-Madinah.
| Laboratory investigations | Studied patients (n = 29) | p | |
|---|---|---|---|
| Mean ± SD | |||
| Died (n = 10) | Survived (n = 19) | ||
| Hematologic parameters | |||
| WBC | 3.3 ± 0.9 | 9.0 ± 4.0 | <0.0001* |
| Hemoglobin | 11.7 ± 1.5 | 13.2 ± 1.8 | 0.033* |
| Platelets | 253.9 ± 81.5 | 313.8 ± 78.1 | 0.064 |
| ABG | |||
| pH | 7.2 ± 0.1 | 7.4 ± 0.1 | <0.0001* |
| PCO2 | 64.1 ± 5.2 | 53.5 ± 7.1 | <0.0001* |
| PO2 | 80.3 ± 1.3 | 82.0 ± 1.8 | 0.012* |
| SPO2 | 84.8 ± 3.5 | 91.2 ± 5.0 | 0.001* |
| HCO3 | 22.8 ± 1.3 | 24.5 ± 1.4 | 0.004* |
| Chemistry | |||
| ALT | 203.1 ± 125.9 | 43.3 ± 8.8 | <0.0001* |
| AST | 169.6 ± 123.8 | 42.5 ± 6.9 | <0.0001* |
| Bilirubin (total) | 23.0 ± 3.9 | 13.3 ± 2.1 | <0.0001* |
| Bilirubin (direct) | 15.9 ± 2.2 | 12.2 ± 1.3 | <0.0001* |
| Creatinine | 370.8 ± 60.2 | 148.3 ± 29.3 | <0.0001* |
| Urea | 22.5 ± 1.8 | 14.2 ± 2.1 | <0.0001* |
| Sodium | 146.1 ± 7.9 | 139.7 ± 3.9 | 0.033* |
| Potassium | 3.9 ± 0.3 | 3.7 ± 0.3 | 0.065 |
| Magnesium | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.323 |
ABG = arterial blood gases; ALT = alanine transaminase; AST = aspartate aminotransferase.
Denotes significant p value.
Fig. 1.
Sputum culture results. MRSA = methicillin-resistant Staphylococcus aureus; Staph = Staphylococcus; Strepto = Streptococcus.
All patients had abnormal initial chest radiographs. The predominant finding was bilateral basal consolidations with ground-glass opacities, nodular or/and reticular pattern, and total diffuse multilobar involvement. All patients had appropriate supportive management and received a broad spectrum antibiotic and readjusted based on sputum cultures. Among patients who required ICU, the mean time of ICU stay ranged from 9 days to 55 days (13.7 ± 4.0 days), and mechanical ventilation support was used in nine (31%) patients. Mechanical ventilation support and longer stay in ICU were significantly associated with death (p < 0.05) (see Table 4).
Table 4.
Management of Middle East respiratory syndrome coronavirus (MERS-CoV) patients according to outcome, April–May 2013/Al-Madinah.
| Management | Died (n = 10) | Survived (n = 19) | p | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Medication received | |||||
| Meropenem | 8 | 80.0 | 12 | 63.2 | 0.431 |
| Linezolid | 4 | 40.0 | 13 | 68.4 | 0.236 |
| Levofloxacin | 7 | 70.0 | 8 | 42.1 | 0.245 |
| Piperacillin | 4 | 40.0 | 11 | 57.9 | 0.450 |
| Ribavirin | 3 | 30.0 | 7 | 36.8 | 1.0 |
| Azithromycin | 6 | 60.0 | 13 | 68.4 | 0.698 |
| Interferon | 6 | 60.0 | 13 | 68.4 | 0.698 |
| Steroids | 10 | 100.0 | 19 | 100.0 | NA |
| Use of mechanical ventilation | 8 | 80.0 | 1 | 5.3 | <0.0001* |
| Duration of ICU stay (d), mean ± SD | 18.5 ± 2.3 | 11.1 ± 1.6 | <0.0001* | ||
ICU = intensive care unit; NA = not applicable; SD = standard deviation.
Denotes significant p value.
4. Discussion
Emerging viral respiratory infections are causing a significant burden on public health and causing significant morbidity and mortality. Over the past decades, many viral infection outbreaks have been reported including influenza H7N9 such as H1N1, SARS-CoV, and the most recent MERS-CoV infection. The World Health Organization reported 1368 laboratory-confirmed cases of human infection with MERS-CoV including at least 487 deaths between 2012 and July 2015 [7].
They reported that 65% of cases were male (n = 1359) and the median age was 50 years (n = 1365) which is similar to our study. Similar to a study reported by Assiri et al. [2], our study showed more cases among older patients, but our study showed an association of death with older age.
Since 2012, 26 countries have been affected, including countries in the Middle East, Africa, Europe, Asia, and North America as reported from the World Health Organization. The majority of cases (∼75%) have been reported from Saudi Arabia. In Saudi Arabia, mortality secondary to MERS-CoV was 35% [16] and in our study it was 37% (Al-Madinah).
Camels have been confirmed as a reservoir for MERS-CoV, and many hypotheses are behind this zoonotic (camels) transmission [17,18]. In our study, only five healthcare employees acquired infection from documented contact with an infected patient, but another 24 patients were coming from areas around Al-Madinah; the largest number of infected patients was from the Alhenakia area, where camels are prevalent. The second important mode of transmission is person-to-person transmission (travelers returning from the Middle East and close contacts with MERS-CoV cases) [19]. This type of transmission was confirmed by genome sequencing of MERS-CoV [19] and isolates from the Al-Hasa healthcare-associated outbreak [16]. In the UK, MERS-CoV was transmitted to a family member who visited a patient with confirmed infection and another report from France described patient-to-patient nosocomial transmission of MERS-CoV. In our study, five (18%) patients had transmission through close contact.
Approximately 30% of MERS-CoV patients in our study reported diabetes and CKD, which is similar to those from other observational epidemiological studies in Saudi Arabia [2,16]. The high mortalities reported early were probably due to a delay in the diagnosis and presence of comorbidities [20]. However, the large number of MERS-CoV cases and CKD might have been based on the Al-Hasa hospital outbreak, which mainly happened in the dialysis center [2]. We may consider the existence of chronic illnesses such as diabetes, hypertension, and CKD increasing the risk of acquiring this infection and categorize them as a high risk group for more complications and worse outcome as was revealed [21] also in our study.
We and others have found that the severity of illness associated with MERS-CoV infection ranges from mild to fulminant [22]. The severity of the respiratory infections caused by MERS-CoV can progress to hypoxemic respiratory failure which requires the use of mechanical ventilation and death [23]. All of our patients had significant respiratory manifestations requiring admission to the ICU but 30% only required mechanical ventilation and died [24]. MERS-CoV is known to infect cell lines of the intestinal tract [25], but it is not yet known what proportion of ill patients shed the virus in their stools, which is why some patients presented with gastrointestinal symptoms. Identification of the full range of clinical presentations is important so that the mild cases are not missed.
MERS-CoV is detected by reverse transcription polymerase chain reaction. To date, laboratory testing for MERS-CoV remains not very accurate; the sensitivity and negative predictive values are unknown. Development of rapid and accurate diagnostic tests is needed urgently. Results of throat swabs were occasionally negative and repeat testing for MERS-CoV is recommended. It seems difficult to conclude that one negative sample is enough to rule out MERS-CoV disease when a patient presents with respiratory symptoms and history of exposure. It is also not clear whether nasopharyngeal samples might be superior to throat samples or whether virus is shed more abundantly later in the course of the illness as it is in SARS. There is evidence that repeat testing and tests on sputum or bronchoalveolar lavage fluid are of value in improving diagnostic accuracy.
Microbiological investigations were done routinely to exclude bacterial copathogens with community acquired pneumonia (CAP). We had seven patients with methicillin-resistant Staphylococcus aureus (MRSA) coinfection, two with Streptococcus and one with methicillin-sensitive Staphylococcus aureus (MSSA) in our study population. Assiri et al. [2] stated that none of the 47 samples screened was positive. Other investigators found that one patient was coinfected with MSSA and influenza B and another with Streptococcus pneumonia [23].
There might have been a selection bias because we were only screening critically ill cases, which in turn will lead to detection of more severe cases of MERS-CoV infection; mild cases may not come to hospital or may not be screened for MERS-CoV and could lead to false high case-fatality rates. Clinical symptoms, laboratory investigations, and imaging findings of MERS-CoV are similar to those noted in other community-acquired respiratory tract infections.
Radiological findings in MERS-patients tended to range from unilateral focal air-space opacities to multifocal or bilateral lower lobe involvement was seen with a picture of organizing pneumonia which was noted in our patients and other reports [25,26].
On the basis of findings until now, the clinical features of MERS-CoV infection have similarities to those seen in patients with SARS-CoV infection. The initial phase of nonspecific fever, cough, and shortness of breath are the major symptoms in those admitted to hospital; other common symptoms include chills, rigor, headache, myalgia, and malaise which may last for several days before progressing to pneumonia [21,22]. A significant number of patients had GI symptoms, another important similarity to SARS. We found patients with MERS-CoV who had GI manifestation tend to progress to severe illness and this may be considered one of the poor prognostic factors. The disease may progress rapidly to a critical respiratory failure, requiring mechanical ventilation and lead to death in the ICU [5]. In our observations, all of the patients started with symptoms of fever, cough for 5–7 days.
Our study design has several limitations including that it is a retrospective chart review study with known inherited problems; these include missing data regarding contact with camels and documentation of all comorbidities and availability of follow up data after discharge. Despite these limitations, we have been able to highlight some features in the epidemiological, demographic, and clinical characteristics of patients with MERS-CoV infection in Al-Madinah regions.
5. Conclusion
The epidemiology and the transmission pattern of MERS-CoV to date indicate that the majority of cases occur in the healthcare setting. Strengthening the infection control measures in the healthcare setting is of great importance. Since about 25% of cases are community based, there is a real need to further prevent the animal-to-human transmission of MERS-CoV. The frequency of cases and deaths is higher among men than women and those around 45 years of age are the most affected patients. The disease had higher mortality in older patients with comorbidities. Also, the presence of gastrointestinal manifestations, high liver enzymes, and need for mechanical ventilation or longer stay in ICU are all associated with high mortality.
There are gaps in our knowledge of the epidemiology, prevalence, clinical characteristics, prognostic factors, and nature of the disease. It is also important to further delineate the transmission routes and the presence of any other animal or intermediate hosts. The influence of geographical distribution and comorbidities on the incidence and outcome of MERS-CoV patients should be studied further.
Acknowledgment
This study did not receive funding.
Footnotes
Peer review under responsibility of Ministry of Health, Saudi Arabia.
Contributor Information
Nahid Sherbini, Email: drnahed@yahoo.com.
Ayman Iskandrani, Email: monmonsa@hotmail.com.
Ayman Kharaba, Email: a7yman@hotmail.com.
Ghalilah Khalid, Email: Kghalilah@yahoo.com.
Mohammed Abduljawad, Email: samamohammedhabeb@yahoo.com.
Hamdan AL-Jahdali, Email: jahdalih@gmail.com.
Conflicts of interest
The authors report no conflicts of interest in this work.
References
- [1].Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. doi: 10.1056/nejmoa1211721. [DOI] [PubMed] [Google Scholar]
- [2].Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–61. doi: 10.1016/s1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–2. doi: 10.1128/jvi.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Al-Tawfiq JA, Memish ZA. Managing MERS-CoV in the healthcare setting. Hosp Pract. 2015;43:158–63. doi: 10.1080/21548331.2015.1074029. [DOI] [PubMed] [Google Scholar]
- [5].Ganczak M. Etiological, epidemiological and clinical aspects of coronavirus infection MERS-CoV. Pol Merkur Lekarski. 2015;38:46–50. [in Polish] [PubMed] [Google Scholar]
- [6].Al-Tawfiq JA, Assiri A, Memish ZA. Middle East respiratory syndrome novel corona MERS-CoV infection. Epidemiology and outcome update. Saudi Med J. 2013;34:991–4. doi: 10.1016/b978-0-12-416975-3.00014-5. [DOI] [PubMed] [Google Scholar]
- [7].World Health Organization (WHO) Middle East respiratory syndrome coronavirus (MERS-CoV), Summary of current situation, literature update and risk assessment. Geneva: WHO; 2015. WHO/MERS/RA/151. [DOI] [Google Scholar]
- [8].Khan A, Farooqui A, Guan Y, Kelvin DJ. Lessons to learn from MERS-CoV outbreak in South Korea. J Infect Dev Ctries. 2015;9:543–6. doi: 10.3855/jidc.7278. [DOI] [PubMed] [Google Scholar]
- [9].Park HY, Lee EJ, Ryu YW, Kim Y, Kim H, Lee H, et al. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. Euro Surveill. 2015;20:1–6. doi: 10.2807/1560-7917.es2015.20.25.21169. [DOI] [PubMed] [Google Scholar]
- [10].Parry-Ford F, Boddington N, Pebody R, Phin N. Public health response to two incidents of confirmed MERS-CoV cases travelling on flights through London Heathrow Airport in 2014. Lessons learnt. Euro Surveill. 2015;20:1–7. doi: 10.2807/1560-7917.es2015.20.18.21114. [DOI] [PubMed] [Google Scholar]
- [11].Fanoy EB, van der Sande MA, Kraaij-Dirkzwager M, Dirksen K, Jonges M, van der Hoek W, et al. Travel-related MERS-CoV cases: an assessment of exposures and risk factors in a group of Dutch travellers returning from the Kingdom of Saudi Arabia, May 2014. Emerg Themes Epidemiol. 2014;11:16. doi: 10.1186/1742-7622-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].EDs on heightened alert for MERS-CoV as first cases reach the US. ED Manag. 2014;26:73–7. [PubMed] [Google Scholar]
- [13].Kraaij-Dirkzwager M, Timen A, Dirksen K, Gelinck L, Leyten E, Groeneveld P, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) infections in two returning travellers in the Netherlands, May 2014. Euro Surveill. 2014;9 doi: 10.2807/1560-7917.es2014.19.21.20817. [DOI] [PubMed] [Google Scholar]
- [14].Omrani AS, Shalhoub S. Middle East respiratory syndrome coronavirus (MERS-CoV): what lessons can we learn? J Hosp Infect. 2015;91:188–96. doi: 10.1016/j.jhin.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Memish ZA, Al-Tawfiq JA, Makhdoom HQ, Al-Rabeeah AA, Assiri A, Alhakeem RF, et al. Screening for Middle East respiratory syndrome coronavirus infection in hospital patients and their healthcare worker and family contacts: a prospective descriptive study. Clin Microbiol Infect. 2014;20:469–74. doi: 10.1111/1469-0691.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–16. doi: 10.1056/nejmoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Memish ZA, Cotten M, Meyer B, Watson SJ, Alsahafi AJ, Al Rabeeah AA, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20:1012–5. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Memish ZA, Alsahly A, Masri MA, Heil GL, Anderson BD, Peiris M, et al. Sparse evidence of MERS-CoV infection among animal workers living in Southern Saudi Arabia during 2012. Influenza Other Respir Viruses. 2015;9:64–7. doi: 10.1111/irv.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–94. doi: 10.1056/nejmoa1303729. [DOI] [PubMed] [Google Scholar]
- [20].Alqurashi KA, Aljabri KS, Bokhari SA. Prevalence of diabetes mellitus in a Saudi community. Ann Saudi Med. 2011;31:19–23. doi: 10.4103/0256-4947.75773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hui DS, Chan PK. Severe acute respiratory syndrome and coronavirus. Infect Dis Clin North Am. 2010;24:619–38. doi: 10.1016/j.idc.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Drosten C, Seilmaier M, Corman VM, Hartmann W, Scheible G, Sack S, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13:745–51. doi: 10.1016/s1473-3099(13)70154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–97. doi: 10.7326/m13-2486. [DOI] [PubMed] [Google Scholar]
- [24].Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–94. doi: 10.1056/nejmoa030685. [DOI] [PubMed] [Google Scholar]
- [25].Fan CK, Yieh KM, Peng MY, Lin JC, Wang NC, Chang FY. Clinical and laboratory features in the early stage of severe acute respiratory syndrome. J Microbiol Immunol Infect. 2006;39:45–53. [PubMed] [Google Scholar]
- [26].Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Madani TA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014;203:782–7. doi: 10.2214/ajr.14.13021. [DOI] [PubMed] [Google Scholar]