Abstract
Cardiopulmonary nematodes in cats include different parasite species affecting feline lungs and the heart, with the metastrongyloid Aelurostrongylus abstrusus being the most frequent feline lungworm worldwide. The present case report describes an 11-month-old male neutered European short hair cat which presented with generalised subcutaneous oedema and pleural and peritoneal effusions. According to clinical examination, abdominal imaging and laboratory analyses, a tentative diagnosis of severe glomerulopathy with massive proteinuria was made. Due to worsening of the clinical signs despite therapeutic interventions and a poor prognosis, the cat was euthanised. Necropsy and histological examinations revealed severe bilateral collagenofibrotic glomerulopathy, generalised oedema and a focal verminous pneumonia with thrombosis in arterial lung vessels containing nematode cross sections. A serum sample was tested for the presence of antibodies against the cat lungworm A. abstrusus, resulting negative. Genetic analyses confirmed the presence of nematode DNA; after exclusion of common lung and heart parasites occurring in cats, DNA of the canid heart worm nematode Angiostrongylus vasorum was identified. This is the first description of a naturally occurring infection with A. vasorum in a cat. Previous experimental studies demonstrated the development of adult male and female A. vasorum worms containing eggs in cats, but no larval excretion in the faeces. Although cats did not become patent, A. vasorum infections were clinically relevant. As A. abstrusus and A. vasorum are both gastropod transmitted nematodes, they may share the same intermediate hosts within overlapping areas. In addition, especially chronic A. abstrusus infected cats become non-patent and do not excrete L1. Considering that patent A. vasorum infections are widespread in the dog and fox population in Switzerland (and several other countries) but are apparently not patent in cats, we cannot exclude that infections with A. vasorum may occur more frequently than expected.
Keywords: Angiostrongylus vasorum, Canid heart worm, Felis catus, Aberrant infection, Glomerulopathy, Genetic confirmation
Highlights
-
•
Angiostrongylus vasorum is a cardiopulmonary nematode of dogs and other canids
-
•
First natural infection of a cat (Felis catus) with Angiostrongylus vasorum
-
•
Histological nematode sections identified in pulmonary arteries, confirmed by PCR
-
•
Aberrant, non-patent infection in the cat with clinical relevance
-
•
Such infections may occur more frequently particularly in highly endemic areas
1. Introduction
Cardiopulmonary nematodes in cats include different parasite species affecting feline lungs and the heart, with the metastrongyloid Aelurostrongylus abstrusus being the most frequent feline lungworm worldwide (Giannelli et al., 2017; Scott, 1973). Adult stages (length 5–11 mm, width 0.054–0.08 mm (Gerichter, 1949)) of this parasite are embedded in the lung parenchyma and produce eggs and larval stages causing verminous pneumonia (Hamilton, 1967; Hobmaier and Hobmaier, 1935; Schnyder et al., 2014a). Troglostrongylus sp. was described in the twenties (Vevers, 1923) and then neglected until recently, when the rediscovery of this parasite in single European countries such as Spain (Jefferies et al., 2010), Italy (Brianti et al., 2012; Di Cesare et al., 2014), Greece (Diakou et al., 2014) and Bosnia-Herzegovina (Alic et al., 2015) was reported. The adult nematodes of the latter can be easily differentiated from A. abstrusus: Troglostrongylus sp. parasites are located in bronchi and bronchioli, are larger (length 6.6–16.8 mm, width 0.2–0.4 mm (Gerichter, 1949)), males have clearly longer spicules and females have a different position of the vulva opening. However, the first stage larvae (L1) of both parasites, which are coughed up, swallowed and excreted in the faeces, are very similar (Brianti et al., 2012), potentially leading to diagnostic misinterpretations. A further rarely diagnosed feline lungworm is Oslerus rostratus (previously named Anaphilaroides rostratus): this parasite is also found in the lung parenchyma or embedded in peri-bronchial tissues, being larger than A. abstrusus (length 30–50 mm, width 0.34–0.69 mm (Gerichter, 1949)). In contrast, Capillaria aerophila (syn. Eucoleus aerophilus) is more common than Oslerus rostratus and is frequently observed in trachea, bronchi and bronchioli of foxes (Magi et al., 2015; Saeed et al., 2006), dogs and cats (Giannelli et al., 2017; Traversa et al., 2010). Capillaria sp. are thin filariform parasite with C. aerophila in cats being 12–19 mm long and 0.049–0.102 mm wide (Varcasia et al., 2014a) and diagnosis is accomplished by detection of characteristic eggs in faecal samples.
Regarding heart worms, the Dirofilaria immitis prevalence in endemic areas in cats is supposed to correlate with that in dogs (Venco et al., 2011). Cats are considered imperfect hosts that may harbour immature or adult stages measuring several centimetres which are located in the right heart and the pulmonary artery or at ectopic localisations (Venco et al., 2015). Laboratory diagnosis in cats is challenging as antigen detection is insufficient and because cats are often amicrofilaraemic (Atkins et al., 2008; Genchi et al., 2008), meaning that they cannot be identified based on the detection of microfilariae in blood samples.
Ultimately, Angiostrongylus chabaudi, a heartworm taxonomically related with the now commonly reported canid nematode Angiostrongylus vasorum, has been identified in Italy in the pulmonary arteries of wild (Biocca, 1957; Veronesi et al., 2016) and domestic (Traversa et al., 2015; Varcasia et al., 2014b) cats, and also in wild cats from Romania (Gherman et al., 2016).
In Swiss cats, recently performed serological studies confirmed the wide occurrence of A. abstrusus, with a prevalence of 10.7% based on detection of serological antibodies against the parasite (Gueldner et al., 2019). For the validation of the adopted ELISA and in the frame of a prevalence study performed with stray, shelter and privately owned cats, we confirmed the absence of Troglostrongylus sp. and identified A. abstrusus being the only occurring cardiopulmonary nematode causing patent infections in cats in Switzerland (Zottler et al., 2019).
The present case report describes an 11-month-old male neutered European short hair cat, which presented with generalised subcutaneous oedema and pleural and peritoneal effusions. According to clinical examination, abdominal imaging and laboratory analyses, a tentative diagnosis of severe glomerulopathy with massive proteinuria was made. Due to worsening of the clinical signs despite therapeutic interventions and a poor prognosis, the cat was euthanised. Necropsy followed by further analyses allowed the first description of a naturally occurring infection with A. vasorum in a cat.
2. Material and methods
2.1. Anamnestic data, clinical examination, laboratory analyses and diagnostic imaging
An 11-month-old, outdoor, neutered male domestic shorthair cat with a body weight of 4.7 kg was referred because of a three-day history of inappetence, lethargy and generalised swollen legs and paws. At the time of hospital admission, the cat was alert, responsive, and euhydrated with a body condition score of 4/9. The cat had a body temperature of 38.7 °C. The heart (180 beats/min) and respiratory (34 breaths/min) rates were mildly high, likely attributable to stress or discomfort. On physical examination, the cat showed pain when its neck, back and abdomen was palpated, and presented with generalised limb, head and neck oedema. No abnormalities were detected on examination of the oral cavity.
A CBC revealed a mild microcytic anaemia (Hct, 23%; reference range 33% to 45%) with a reticulocyte count of 69′600/ul, leukocytosis (15′800 leukocytes/ul; reference range 4′600 to 12′800 leukocytes/ul) and eosinophilia (1′420 eosinophils/ul; reference range 100 to 600 eosinophils/ul). The cat had a platelet count of 412′000 platelets/ul, which was within the reference range of 180′000 to 680′000 platelets/ul.
Serum biochemical analysis identified a severe hypoproteinaemia (2.9 g/dl; reference range 6.4 to 8.0 g/dl) and hypoalbuminaemia (1.3 g/dl; reference range 3.2 to 4.2 g/dl). The liver- and kidney values, electrolytes, vitamin B12 and ammonia concentration were within reference range. The cat tested negative for FeLV antigen and FIV antibodies (SNAP FIV/FeLV Combo test, IDEXX Laboratories, Westbrook, Maine, USA). An oral cavity swab to perform PCR to detect calicivirus was negative. Urinalysis revealed a normal specific gravity (specific gravity, 1.050) but the cat was severely proteinuric (UP/C value of 8.96).
On radiographic evaluation of a lateral thoracic and lateral abdominal radiograph there was evidence of a mild pleural and moderate peritoneal effusion. Abdominal ultrasonography was performed for further evaluation of the abdominal pain, the severe panhypoproteinaemia and peritoneal effusion: ultrasonography revealed a large amount of free abdominal fluid (that was subsequently characterized as a low-protein transudate), a moderate lymphadenomegaly, a severe pancreas oedema and a severe stomach wall oedema.
Overnight, the cat was treated with buprenorphine 15 μg/kg QID IV and maropitant 1 mg/kg SID IV. The respiratory rate and effort, the generalised pain and the oedemas deteriorated progressively to the next day. Given the cat's rapid clinical deterioration, the owner elected to have the cat euthanised.
2.2. Pathological and histological examination
Necropsy was performed including a macroscopic evaluation of all inner organs according to standard procedures. Tissue samples of lung, liver, spleen, pancreas and mesenteric lymph nodes were immediately fixed in 10% buffered formalin solution for 48 h. Subsequently, the organ pieces were trimmed and embedded in paraffin blocks (Sakura, Horgen, Switzerland). Then, 2-micrometer sections were prepared and stained with haematoxylin and eosin (HE) according to standard procedures. A Van Gieson special stain was also done.
2.3. ELISAs
One serum sample and tissue fluids from kidney, liver and lung organ pieces were collected at necropsy and stored at −20 °C until further processing. All samples were tested for the presence of antibodies against the cat lungworm A. abstrusus using a validated indirect ELISA (Zottler et al., 2017) according to Gueldner et al. (2019) with minor modifications: the goat anti-feline IgG peroxidase labeled conjugate (Southern Biotech, Birmingham, USA) was used at a dilution of 1:9000. The plate run included a substrate control, two positive controls (sera from experimentally infected cats), two negative controls (from uninfected laboratory cats), a reference serum added twice and a conjugate control. The cut-off values were applied as previously described (Gueldner et al., 2019). The aforementioned ELISA is able to detect antibodies against A. abstrusus the earliest at two weeks after experimental infections and reaches a sensitivity of 100% ten weeks after infection. In randomly sampled, naturally infected cats, the sensitivity of the ELISA was 88.2% and the specificity was 90.0% (Zottler et al., 2017).
In addition, the cat serum sample was tested with ELISAs developed for the detection of circulating antigen of the canine lungworm A. vasorum (Schnyder et al., 2011) and of specific antibodies against A. vasorum (Schucan et al., 2012), this latter test after minor modifications: i.e. the goat anti-dog IgG conjugate was substituted with a goat anti-feline IgG conjugate (Southern Biotech, Birmingham, USA) at six different dilutions, and evaluated at a dilution of 1: 4000. Five proven negative cat sera served as negative controls.
2.4. Genetic analyses
In order to classify the nematodes histologically observed in the lung tissue, DNA was isolated from two histology blocks containing lung tissue (Borel et al., 2018). From both formalin-fixed paraffin-embedded blocks, one 20-μm section was cut and deparaffinized in xylene by centrifugation at 13,800 ×g for 5 min. The supernatant containing xylene was removed, and the sample washed twice in ethanol, centrifuged at 14,800 ×g for 5 min followed by removal of the supernatant. The remaining pellet was lysed with proteinase K (20 mg/ml, Roche Diagnostics, Mannheim, Germany) on a thermomixer at 56 °C and 550 rpm overnight. DNA was extracted using the commercial DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
In a first PCR amplifying the internal transcribed spacer 2 (ITS2) regions in nematodes, primers NC1 (5’-ACGTCTGGTTCAGGGTTGTT-3′; forward) and NC2 (5’TTAGTTTCTTTTCCTCCGCT-3′ reverse) were used (Gasser et al., 1993), and PCR was performed as previously described (Veronesi et al., 2016) using a modified annealing temperature of 54 °C for 60s. A QIAGEN Multiplex PCR Master Mix (QIAGEN, Germany) was used and the reactions were performed in a Thermocycler (Labcycler, SensoQuest GmbH, Göttingen) at 95 °C for 15 min.
In a second PCR for detection of DNA of the feline heartworm A. chabaudi, the primers AChFor (5’-TCAAAGAAATAATCATCGAAC-3′; forward) and NC2 (5’TTAGTTTCTTTTCCTCCGCT-3′ reverse) (Di Cesare et al., 2015) were used and the following modified cycling protocol was adopted: 15 min at 95 °C; 40 cycles at 94 °C for 60s, 52 °C for 60s and 72 °C for 60s; and a final extension at 72 °C for 10 min. The first amplified product was diluted 1:20 and amplified again using the same PCR protocol.
In addition, a third PCR specific for A. vasorum was conducted using the primers COIF (5’-TAAAGAAAGAACATAATGAAAATG 3′) and COIr (5’-TTTTTTGGGCATCCTGAGGTTTAT-3′) targeting a partial region of the mitochondrial COI gene (Bowles et al., 1993; Hu et al., 2002). This PCR was performed as previously described (Jefferies et al., 2009) with the following modifications: reactions were thermo-cycled at 94 °C for 15 min, followed by 40 cycles of (94 °C for 30s, 55 °C for 30s, 72 °C for 30s), and a final extension step at 72 °C for 10 min. This protocol was repeated again with the amplified product using a modified thermo-cycle protocol: 95 °C for 15 min and 40 cycles of (94 °C for 30 s, 50 °C for 45 s, 72 °C for 30s), and a final extension step of 72 °C for 10 min.
All PCR products were electrophoresed on a 1.5% (w/v) agarose gel and visualised using GelRed™ and UV illumination. Positive bands were cut from the agarose gel and the DNA was extracted with a MinElute Gel Extraction Kit (QIAGEN, Germany). Sequencing reactions were performed by Synergene Biotech GmbH, Switzerland and the sequences were analyzed using BLAST (Basic Local Alignment Search Tool).
3. Results
3.1. Pathological and histological examination
3.1.1. Macroscopic findings
The cat was in good body condition. A generalised subcutaneous oedema was present, in particular located in the caudal body part and the paws. The mucous membranes were pale. In the thoracic cavity, a hydrothorax consisting of approximately 12 ml of reddish fluid (specific gravity: 1020) and a mild hydroabdomen were observed. The left lung lobes were of dark red color whereas the right lung lobes were light red mottled, the overall consistency was wet and heavy. The liver had a dark red to brown color and rounded edges, the consistency on the cut surface was firm. Both kidneys had a yellowish to light brown color and a firm consistency, whereas the left kidney seemed slightly enlarged. The mesenteric lymph nodes were enlarged containing round and coalescent 1–2 mm areas on the cut surface.
3.1.2. Histological findings
Histologically, the lungs presented with a generalised oedema and hyperaemia. In one lung area, multifocal foci consisting of inflammatory cells including epitheloid and non-epitheloid macrophages, eosinophils and neutrophils, which were partially degenerated and mixed with fibrin, were observed. These granulomas were frequently associated with vessels, bronchi and bronchioli. In addition, vascular thrombi and adult nematode sections were present in arterial lung vessels (Fig. 1, Fig. 2 ), overall indicating focal verminous pneumonia due to nematodes. The adult nematode stages were characterized due to their size and content. No larvae nor egg stages were observed.
Fig. 1.
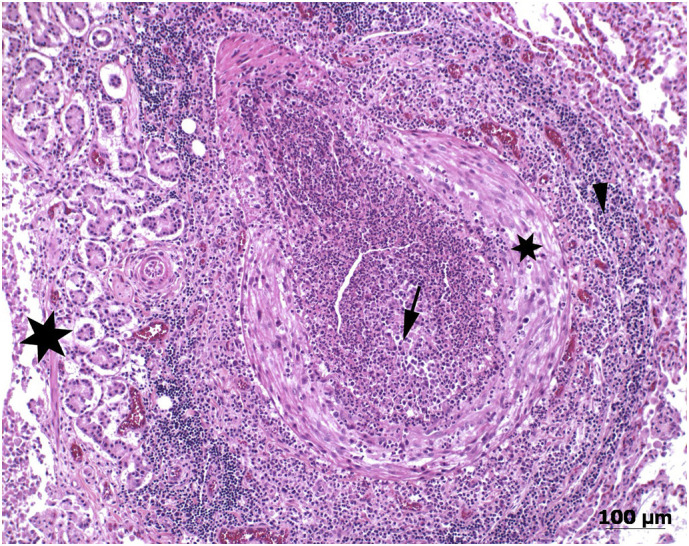
Histological section (haematoxylin and eosin stain) of an enlarged pulmonary artery with thrombosis and massive inflammation in a cat diagnosed with a natural Angiostrongylus vasorum infection. The infection was considered non-patent due to absence of eggs and larval stages in this and further histological sections (not shown). The arrow indicates a mixed cellular inflammatory process in the lumen of the artery (mostly neutrophils and macrophages), the small star points to the Tunica media of the artery; the arrowhead indicates perivascular lymphocytes and plasmacells, while the large star indicates peribronchial glands (right side) and the bronchus lumen (left side).
Fig. 2.
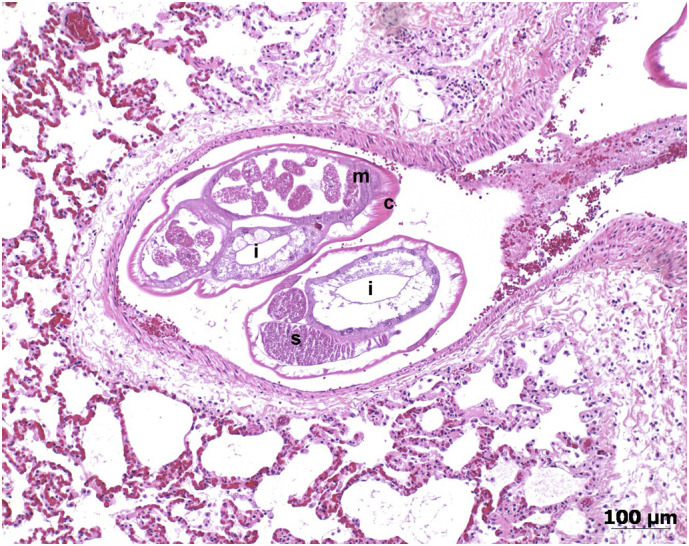
Focal pneumonia and a pulmonary artery containing two nematode cross sections in the lung of a cat diagnosed with Angiostrongylus vasorum infection (histological section, haematoxylin and eosin stain). The letters stand for c: cuticle, m: musculature, i: intestine, s: sperms. Therefore, the size (approx. 0.4–0.5 mm) and content of the nematode sections indicate adult stages.
The fat tissue surrounding the pancreas was multifocally necrotic (fat necrosis) and a mild lymphohistiocytic steatitis was present. The mesenteric lymph nodes showed a reactive hyperplasia.
In both kidneys, hyaline eosinophilic material in all glomeruli that was positive in the Van Gieson special stain was visible and, occasionally, the Bowman's capsule was thickened. Multifocally, mild to moderate proteinuria, tubular calcifications and chronic interstitial nephritis consisting of lymphocytes and plasma cells were observed. These findings led to the tentative diagnosis of a severe, bilateral collagenofibrotic glomerulopathy with proteinuria and a pre-existing mild, chronic interstitial nephritis.
3.2. ELISAs
Serum as well as kidney, liver and lung tissue fluids were negative by the A. abstrusus antibody detection ELISA. The A. vasorum antigen and antibody detection ELISAs were both negative.
3.3. Genetic analyses
The PCR amplifying the ITS2 region performed out of samples extracted from the lung tissue blocks was positive for nematode DNA. Sequence analysis of a 431 base pair (bp) fragment confirmed the presence of A. vasorum DNA with a 100% identity with GenBank accession nos. GU045374.1, EU627595.1, EU627594.1, EU627592.1). The here detected sequence was registered with GenBank accession number MN104952.
Also the PCR that amplifies a partial region of the mitochondrial COI gene (310 bp) was positive and showed 100% identity with previously published sequences of A. vasorum GenBank accession no. EU493163.1. The here detected sequence was registered with GenBank accession number MN104953.
Gel-electrophoresis of the amplified product of the PCR specific for A. chabaudi was negative.
4. Discussion
The clinical signs observed in the here presented case study were consistent with a glomerular disease, possibly caused by an amyloidosis, or an infection with the coronavirus causing feline infectious peritonitis, or a renal lymphoma, or an idiopathic glomerulonephritis or glomerulopathy. Due to an unfavourable prognosis, the cat was euthanised and necropsied allowing further investigations. The histologic examination confirmed a bilateral collagenofibrotic glomerulopathy with a chronic interstitial nephritis, and enabled the detection of an unexpected parasite, A. vasorum, in the lung vessels. The occurrence of this parasite in dogs has been largely described in Europe, especially in the last twenty years, as the cause of canine angiostrongylosis inducing respiratory, neurological and other clinical signs (Koch and Willesen, 2009; Schnyder et al., 2010). Angiostrongylus vasorum-infected foxes, which are considered the natural reservoir of the parasite, are contributing to its spreading and establishment of endemic foci (Gillis-Germitsch et al., 2017a; Taylor et al., 2015). After the first description of A. vasorum in a dog kennel in Switzerland (Wolff et al., 1969), a gradual increase of affected dogs (Staebler et al., 2005) and foxes (Gillis-Germitsch and Schnyder, 2017) was observed approximately 30 years later. Despite its actual widespread occurrence in dogs, an infection with A. vasorum was initially not suspected in the case presented here, given that, to our knowledge, no previous natural infections of cats with this parasite have been described. In cats, A. abstrusus is the most frequent lungworm worldwide including Switzerland (Giannelli et al., 2017; Zottler et al., 2019). However, adult stages of A. abstrusus usually reside in the lung parenchyma of cats and are normally not observed in arterial vessels. Moreover, no antibodies against A. abstrusus were detected in serum or tissue fluid of this cat, excluding an infection with this parasite. Serology, i.e. a serological rapid assay based on a test developed for the detection of A. vasorum in dogs (Schnyder et al., 2014b), was previously used to test blood of badgers and wild cats, identifying animals infected with the nematodes Angiostrongylus daskalovi and Angiostrongylus chabaudi, respectively (Deak et al., 2017). Although A. chabaudi is very rarely described in domestic and wild cats and only in single European countries, the localisation of the nematode sections in the arterial vessels of the cat presented here and the histological absence of larval stages in the lungs were indicative for a potential A. chabaudi infection: in fact only wild but no domestic cats infected with A. chabaudi were patent (Diakou et al., 2016; Varcasia et al., 2014b), indicating that domestic cats are not suitable definitive hosts, not allowing maturation and release of L1 by adult female parasites. However, genetic analyses did not confirm the presence of A. chabaudi, despite the detection of nematode DNA in histological samples. Instead, ITS2 and COI sequencing both surprisingly confirmed an infection with A. vasorum: although patent infections with A. vasorum were observed in a wide range of canids and also in red pandas, badgers, stoats, weasels and even meerkats (Gillis-Germitsch et al., 2017b), the cat was not considered as a suitable definitive host for A. vasorum, as natural infections have never been observed before. Unfortunately, due to the progressing glomerular disease followed by euthanasia, no faecal samples could be collected from the here presented cat for confirmation of absence of larval stages and therefore patency. The tentative ELISAs for A. vasorum detection resulted negative. However, we would like to stress that the A. abstrusus ELISA for cats (Zottler et al., 2017; Gueldner et al., 2019) and the two ELISAs for circulating A. vasorum antigen detection and antibodies against the parasite (Schnyder et al., 2011; Schucan et al., 2012) have been well validated for cats and dogs, respectively, and optimal concentrations of the different test components were determined by a series of titration experiments. In absence of positive control sera of other cats infected with A. vasorum, it was not possible to perform these experiments. We therefore cannot differentiate if the A. vasorum ELISAs performed with the cat material were negative due to unsuitable testing or to true absence of circulating antigens or antibodies.
Experimental studies performed in the seventies demonstrated the development of adult male and female A. vasorum nematodes containing eggs in cats, but no larval excretion in the faeces (Guilhon and Cens, 1970). Of eight cats receiving 1′000–2′000 infective third stage larvae (L3), five developed respiratory clinical signs; six were necropsied and of these, all had pulmonary lesions. Overall, three cats harboured adult worms, of which two had females with eggs (Guilhon and Cens, 1970). In a more recent study, eighteen animals were inoculated with 50 L3: L1 were not observed in faeces, and only 14 worms were recovered, of which 11 were females and five of them were gravid (Dias et al., 2008). Among the above mentioned infected cats, two animals died within two weeks after inoculation: larval stages were identified in the pancreas in one and in the gall bladder of the other cat post mortem (Dias et al., 2008). Therefore, although not becoming patent, A. vasorum infections may be clinically relevant in cats. It was hypothesised that after penetration of the gastric mucosa, the ingested L3 may perform erratic migrations including to the before mentioned organs of cats (Dias et al., 2008). There are manifold descriptions of erratic nematode migrations leading to severe diseases when non-suitable hosts are infected with parasites of other hosts (i.e. the dog and cat ascarids Toxocara canis and T. cati migrating to the eyes or viscera of humans (Deplazes et al., 2011), or the subcutaneous canid filarial Dirofilaria repens causing tumor-like subcutaneous, ocular or pulmonary nodules also in humans (Genchi and Kramer, 2017). In infected dogs, ectopic larval stages of A. vasorum were observed in a number of organs such as brain, diaphragm, liver, pancreas and skin (Oliveira-Junior et al., 2004), and adult stages were found in the pericardial sac, in the bladder (Oliveira-Junior et al., 2004) or in distant blood vessels such as the femoral arteries (Cury and Lima, 1996). However, we were not able to confirm erratic migrations in this cat: several organs were macroscopically and histologically altered in the present case, but no ectopic parasitic stages were found. The cause for the kidney changes is not clear: the only description of a cat with renal glomerular fibrosis postulated a production of collagen type III through activated mesangial cells (Nakamura et al., 1996). In our case, glomerular changes resulted in proteinuria and azotaemia (Hilbe et al., 2019) and did not give evidence for a correlation with the A. vasorum infection.
Inflammatory lung lesions near vessels and vascular thrombi with nematode sections within arterial vessels are comparable with observations made in dogs infected with A. vasorum. In such dogs, nematode sections of eggs and larval stages numerically strongly outrange sections of adult nematodes (Bourque et al., 2008; Schnyder et al., 2010). The same is valid for cats infected with A. abstrusus. In contrast, in the here analysed cat lung, only adult nematodes were observed, supporting the absence of stages that would lead to faecal larval excretion and therefore to patency. Furthermore, lung alterations were observed in a single area, that was observed by histology only and not at dissection. This explains the absence of evident radiological findings by thorax radiography. Infected dogs frequently show respiratory signs and/or abnormal thorax imaging (Dennler et al., 2011; Koch and Willesen, 2009), which was not present in this case. However, this might be not surprising as also cats severely affected by feline lungworms do not necessarily show clinical signs (Crisi et al., 2016; Schnyder et al., 2014a). Interestingly, in cats experimentally infected with A. vasorum the respiratory signs occurred as early as 2–3 weeks after infection, with potentially deadly outcome after five weeks, while other cats remained asymptomatic or presented with mild signs such as transient coughing and tachypnoe (Guilhon and Cens, 1970). The onset of obvious respiratory signs in cats infected with A. vasorum is therefore not predictable or may be even absent; this, together with the potential occurrence of other clinical manifestations in organs unrelated to the cardiopulmonary system, renders the identification of cats infected with A. vasorum highly challenging.
5. Conclusion
Aelurostrongylus abstrusus and A. vasorum are both gastropod transmitted nematodes and they may share the same intermediate hosts within overlapping areas, as it has been observed for Switzerland (Gueldner et al., 2019; Hilbe et al., 2019; Lurati et al., 2015). Especially chronic A. abstrusus infected cats become non-patent over time (Ribeiro and Lima Dos Santos, 2001) and therefore do not excrete L1 in the faeces that are useful for diagnostics. Considering that A. vasorum infections are widespread in dog and fox populations but apparently do not become patent in cats, we cannot exclude that infections with A. vasorum may be misinterpreted and occur more frequently than expected, particularly in highly endemic areas.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgements
Dr. Felix Grimm and Francesca Gori for support with genetic analyses.
References
- Alic A., Traversa D., Duscher G.G., Kadric M., Di Cesare A., Hodzic A. Troglostrongylus brevior in an Eurasian lynx (Lynx lynx) from Bosnia and Herzegovina. Parasit. Vectors. 2015;8:653. doi: 10.1186/s13071-015-1272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins C.E., Arther R.G., Ciszewski D.K., Davis W.L., Ensley S.M., Guity P.S., Chopade H., Hoss H., Settje T.L. Echocardiographic quantification of Dirofilaria immitis in experimentally infected cats. Vet. Parasitol. 2008;158:164–170. doi: 10.1016/j.vetpar.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Biocca E. Angiostrongylus chabaudi n. sp., parassita del cuore e dei vasi polmonari del gatto selvatico (Felis silvestris) Accad. Naz. Lincei. 1957;4:526–532. [Google Scholar]
- Borel N., Marti H., Pospischil A., Pesch T., Prähauser B., Wunderlin S., Seth-Smith H.M.B., Low N., Flury R. Chlamydiae in human intestinal biopsy samples. Pathog. Dis. 2018;76 doi: 10.1093/femspd/fty081. 8, fty081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque A.C., Conboy G., Miller L.M., Whitney H. Pathological findings in dogs naturally infected with Angiostrongylus vasorum in Newfoundland and Labrador. Canada. J. Vet. Diagn. Invest. 2008;20:11–20. doi: 10.1177/104063870802000103. [DOI] [PubMed] [Google Scholar]
- Bowles J., Hope M., Tiu W.U., Liu X., McManus D.P. Nuclear and mitochondrial genetic markers highly conserved between Chinese and Philippine Schistosoma japonicum. Acta Trop. 1993;55:217–229. doi: 10.1016/0001-706x(93)90079-q. [DOI] [PubMed] [Google Scholar]
- Brianti E., Gaglio G., Giannetto S., Annoscia G., Latrofa M.S., Dantas-Torres F., Traversa D., Otranto D. Troglostrongylus brevior and Troglostrongylus subcrenatus (Strongylida: Crenosomatidae) as agents of broncho-pulmonary infestation in domestic cats. Parasit. Vectors. 2012;5:178. doi: 10.1186/1756-3305-5-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisi P.E., Aste G., Traversa D., Di Cesare A., Febo E., Vignoli M., Santori D., Luciani A., Boari A. Single and mixed feline lungworm infections: clinical, radiographic and therapeutic features of 26 cases (2013-2015) J. Fel. Med. Surgery. 2016;19:1017–1029. doi: 10.1177/1098612X16670563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury M.C., Lima W.S. Rupture of femoral artery in a dog infected with Angiostrongylus vasorum. Vet. Parasitol. 1996;65:313–315. doi: 10.1016/s0304-4017(96)00991-0. [DOI] [PubMed] [Google Scholar]
- Deak G., Gherman C.M., Ionica A.M., Daskalaki A.A., Matei I.A., D'Amico G., Domsa C., Pantchev N., Mihalca A.D., Cozma V. Use of a commercial serologic test for Angiostrongylus vasorum for the detection of A. chabaudi in wildcats and A. daskalovi in badgers. Vet. Parasitol. 2017;233:107–110. doi: 10.1016/j.vetpar.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Dennler M., Makara M., Kranjc A., Schnyder M., Ossent P., Deplazes P., Ohlerth S., Glaus T.M. Thoracic computed tomography findings in dogs experimentally infected with Angiostrongylus vasorum. Vet. Radiol. Ultrasound. 2011;52:289–294. doi: 10.1111/j.1740-8261.2010.01776.x. [DOI] [PubMed] [Google Scholar]
- Deplazes P., van Knapen F., Schweiger A., Overgaauw P.A. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet. Parasitol. 2011;182:41–53. doi: 10.1016/j.vetpar.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Di Cesare A., Di Francesco G., Frangipane di Regalbono A., Eleni C., De Liberato C., Marruchella G., Iorio R., Malatesta D., Romanucci M.R., Bongiovanni L., Cassini R., Traversa D. Retrospective study on the occurrence of the feline lungworms Aelurostrongylus abstrusus and Troglostrongylus spp. in endemic areas of Italy. Vet. J. 2014;203:233–238. doi: 10.1016/j.tvjl.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Di Cesare A., Veronesi F., Frangipane di Regalbono A., Iorio R., Traversa D. Novel molecular assay for simultaneous identification of neglected lungworms and heartworms affecting cats. J. Clin. Microbiol. 2015;53:3009–3013. doi: 10.1128/JCM.00901-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakou A., Di Cesare A., Aeriniotaki T., Traversa D. First report of Troglostrongylus brevior in a kitten in Greece. Parasitol. Res. 2014;113:3895–3898. doi: 10.1007/s00436-014-4122-3. [DOI] [PubMed] [Google Scholar]
- Diakou A., Psalla D., Migli D., Di Cesare A., Youlatos D., Marcer F., Traversa D. First evidence of the European wildcat (Felis silvestris silvestris) as definitive host of Angiostrongylus chabaudi. Parasitol. Res. 2016;115:1235–1244. doi: 10.1007/s00436-015-4860-x. [DOI] [PubMed] [Google Scholar]
- Dias S.R., Oliveira E.L., Viana M.H., Lima W.S. Permissivity of the domestic cat (Felis catus) to infection by Angiostrongylus vasorum (Nematoda: Protostrongylidae) Rev. Méd. Vét. 2008;159:87–90. [Google Scholar]
- Gasser R.B., Chilton N.B., Hoste H., Beveridge I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Res. 1993;21:2525–2526. doi: 10.1093/nar/21.10.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchi C., Kramer L. Subcutaneous dirofilariosis (Dirofilaria repens): an infection spreading throughout the old world. Parasit. Vectors. 2017;10:517. doi: 10.1186/s13071-017-2434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchi C., Venco L., Ferrari N., Mortarino M., Genchi M. Feline heartworm (Dirofilaria immitis) infection: a statistical elaboration of the duration of the infection and life expectancy in asymptomatic cats. Vet. Parasitol. 2008;158:177–182. doi: 10.1016/j.vetpar.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Gerichter C.B. Studies on the nematodes parasitic in the lungs of Felidae in Palestine. Parasitology. 1949;39:251–262. doi: 10.1017/s0031182000083827. [DOI] [PubMed] [Google Scholar]
- Gherman C.M., Ionica A.M., D’Amico G., Otranto D., Mihalca A.D. Angiostrongylus chabaudi (Biocca, 1957) in wildcat (Felis silvestris silvestris, S) from Romania. Parasitol. Res. 2016;115:2511–2517. doi: 10.1007/s00436-016-5032-3. [DOI] [PubMed] [Google Scholar]
- Giannelli A., Capelli G., Joachim A., Hinney B., Losson B., Kirkova Z., Rene-Martellet M., Papadopoulos E., Farkas R., Napoli E., Brianti E., Tamponi C., Varcasia A., Margarida Alho A., Madeira de Carvalho L., Cardoso L., Maia C., Mircean V., Mihalca A.D., Miro G., Schnyder M., Cantacessi C., Colella V., Cavalera M.A., Latrofa M.S., Annoscia G., Knaus M., Halos L., Beugnet F., Otranto D. Lungworms and gastrointestinal parasites of domestic cats: a European perspective. Int. J. Parasitol. 2017;47:517–528. doi: 10.1016/j.ijpara.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Gillis-Germitsch N., Schnyder M. Conference Abstracts of the 26th International Conference of the WAAVP. 2017. Increasing prevalence of Angiostrongylus vasorum in wild Swiss red foxes between 2012-2017 and evaluation of serological procedures. Kuala Lumpur, Malaysia, 4-8 September 2017. [Google Scholar]
- Gillis-Germitsch N., Kapel C.M.O., Thamsborg S.M., Deplazes P., Schnyder M. Host-specific serological response to Angiostrongylus vasorum infection in red foxes (Vulpes vulpes): implications for parasite epidemiology. Parasitology. 2017:1–10. doi: 10.1017/S0031182017000427. [DOI] [PubMed] [Google Scholar]
- Gillis-Germitsch N., Manser M.B., Hilbe M., Schnyder M. Meerkats (Suricata suricatta), a new definitive host of the canid nematode Angiostrongylus vasorum. Int. J. Parasitol. Parasites Wildl. 2017;6:349–353. doi: 10.1016/j.ijppaw.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueldner E.K., Gilli U., Strube C., Schnyder M. Seroprevalence, biogeographic distribution and risk factors for Aelurostrongylus abstrusus infections in Swiss cats. Vet. Parasitol. 2019;266:27–33. doi: 10.1016/j.vetpar.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Guilhon J., Cens B. Attempts of transmission of Angiostrongylus vasorum (Baillet 1886) to the cat (in French) Compt. Rendus Hebdom. Seances Acad. Sciences - D: Sciences Naturelles. 1970;271:936–939. [PubMed] [Google Scholar]
- Hamilton J.M. The number of Aelurostrongylus abstrusus larvae required to produce pulmonary disease in the cat. J. Comp. Pathol. 1967;77:343–346. doi: 10.1016/0021-9975(67)90045-x. [DOI] [PubMed] [Google Scholar]
- Hilbe M., Schuppisser C., Hetzel U., Borel N. Collagenofibrotic glomerulopathy in a young cat. Joint Congress ECVP-ESVP-ECVCP-ESVCP, 25-28.9.2019, Arnhem, The Netherlands. 2019 [Google Scholar]
- Hobmaier M., Hobmaier A. Mammalian phase of the lungworm Aelurostrongylus abstrusus in the cat. J. Am. Vet. Med. Ass. 1935;87:191–198. [Google Scholar]
- Hu M., Chilton N.B., Gasser R.B. Long PCR-based amplification of the entire mitochondrial genome from single parasitic nematodes. Mol. Cell. Probes. 2002;16:261–267. doi: 10.1006/mcpr.2002.0422. [DOI] [PubMed] [Google Scholar]
- Jefferies R., Morgan E.R., Shaw S.E. A SYBR green real-time PCR assay for the detection of the nematode Angiostrongylus vasorum in definitive and intermediate hosts. Vet. Parasitol. 2009;166:112–118. doi: 10.1016/j.vetpar.2009.07.042. [DOI] [PubMed] [Google Scholar]
- Jefferies R., Vrhovec M.G., Wallner N., Catalan D.R. Aelurostrongylus abstrusus and Troglostrongylus sp. (Nematoda: Metastrongyloidea) infections in cats inhabiting Ibiza. Spain. Vet. Parasitol. 2010;173:344–348. doi: 10.1016/j.vetpar.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Koch J., Willesen J.L. Canine pulmonary angiostrongylosis: an update. Vet. J. 2009;179:348–359. doi: 10.1016/j.tvjl.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Lurati L., Deplazes P., Hegglin D., Schnyder M. Seroepidemiological survey and spatial analysis of the occurrence of Angiostrongylus vasorum in Swiss dogs in relation to biogeographic aspects. Vet. Parasitol. 2015;212:219–226. doi: 10.1016/j.vetpar.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Magi M., Guardone L., Prati M.C., Mignone W., Macchioni F. Extraintestinal nematodes of the red fox Vulpes vulpes in north-West Italy. J. Helminthol. 2015;89:506–511. doi: 10.1017/S0022149X1400025X. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Shibata S., Shirota K., Abe K., Uetsuka K., Nakayama H., Goto N., Doi K. Renal glomerular fibrosis in a cat. Vet. Pathol. 1996;33:696–699. doi: 10.1177/030098589603300609. [DOI] [PubMed] [Google Scholar]
- Oliveira-Junior S.D., Barcante J.M., Barcante T.A., Ribeiro V.M., Lima W.S. Ectopic location of adult worms and first-stage larvae of Angiostrongylus vasorum in an infected dog. Vet. Parasitol. 2004;121:293–296. doi: 10.1016/j.vetpar.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Ribeiro V.M., Lima Dos Santos W. Larval production of cats infected and re-infected with Aelurostrongylus abstrusus (Nematoda: Protostrongylidae) Rev. Méd. Vét. 2001;152:815–820. [Google Scholar]
- Saeed I., Maddox-Hyttel C., Monrad J., Kapel C.M. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006;139:168–179. doi: 10.1016/j.vetpar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Schnyder M., Fahrion A., Riond B., Ossent P., Webster P., Kranjc A., Glaus T., Deplazes P. Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitol. Res. 2010;107:1471–1480. doi: 10.1007/s00436-010-2021-9. [DOI] [PubMed] [Google Scholar]
- Schnyder M., Tanner I., Webster P., Barutzki D., Deplazes P. An ELISA for sensitive and specific detection of circulating antigen of Angiostrongylus vasorum in serum samples of naturally and experimentally infected dogs. Vet. Parasitol. 2011;179:152–158. doi: 10.1016/j.vetpar.2011.01.054. [DOI] [PubMed] [Google Scholar]
- Schnyder M., Di Cesare A., Basso W., Guscetti F., Riond B., Glaus T., Crisi P., Deplazes P. Clinical, laboratory and pathological findings in cats experimentally infected with Aelurostrongylus abstrusus. Parasitol. Res. 2014;113:1425–1433. doi: 10.1007/s00436-014-3783-2. [DOI] [PubMed] [Google Scholar]
- Schnyder M., Stebler K., Naucke T.J., Lorentz S., Deplazes P. Evaluation of a rapid device for serological in-clinic diagnosis of canine angiostrongylosis. Parasit. Vectors. 2014;7:72. doi: 10.1186/1756-3305-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schucan A., Schnyder M., Tanner I., Barutzki D., Traversa D., Deplazes P. Detection of specific antibodies in dogs infected with Angiostrongylus vasorum. Vet. Parasitol. 2012;185:216–224. doi: 10.1016/j.vetpar.2011.09.040. [DOI] [PubMed] [Google Scholar]
- Scott D.W. Current knowledge of aelurostrongylosis in the cat. Lit. Rev. Case Rep. 1973;63:483–500. [PubMed] [Google Scholar]
- Staebler S., Ochs H., Steffen F., Naegeli F., Borel N., Sieber-Ruckstuhl N., Deplazes P. Autochthonous infections with Angiostrongylus vasorum in dogs in Switzerland and Germany (in German) Schweiz. Arch. Thkd. 2005;147:121–127. doi: 10.1024/0036-7281.147.3.121. [DOI] [PubMed] [Google Scholar]
- Taylor C.S., Garcia Gato R., Learmount J., Aziz N.A., Montgomery C., Rose H., Coulthwaite C.L., McGarry J.W., Forman D.W., Allen S., Wall R., Morgan E.R. Increased prevalence and geographic spread of the cardiopulmonary nematode Angiostrongylus vasorum in fox populations in Great Britain. Parasitology. 2015;142:1190–1195. doi: 10.1017/S0031182015000463. [DOI] [PubMed] [Google Scholar]
- Traversa D., Di Cesare A., Conboy G. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasit. Vectors. 2010;3:62. doi: 10.1186/1756-3305-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa D., Lepri E., Veronesi F., Paoletti B., Simonato G., Diaferia M., Di Cesare A. Metastrongyloid infection by Aelurostrongylus abstrusus, Troglostrongylus brevior and Angiostrongylus chabaudi in a domestic cat. Int. J. Parasitol. 2015;45:685–690. doi: 10.1016/j.ijpara.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Varcasia A., Brianti E., Tamponi C., Pipia A.P., Cabras P.A., Mereu M., Dantas-Torres F., Scala A., Otranto D. Simultaneous infection by four feline lungworm species and implications for the diagnosis. Parasitol. Res. 2014;114:317–321. doi: 10.1007/s00436-014-4207-z. [DOI] [PubMed] [Google Scholar]
- Varcasia A., Tamponi C., Brianti E., Cabras P.A., Boi R., Pipia A.P., Giannelli A., Otranto D., Scala A. Angiostrongylus chabaudi Biocca, 1957: a new parasite for domestic cats? Parasit. Vectors. 2014;7:588. doi: 10.1186/s13071-014-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venco L., Genchi M., Genchi C., Gatti D., Kramer L. Can heartworm prevalence in dogs be used as provisional data for assessing the prevalence of the infection in cats? Vet. Parasitol. 2011;176:300–303. doi: 10.1016/j.vetpar.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Venco L., Marchesotti F., Manzocchi S. Feline heartworm disease: A’Rubik’s-cube-like’ diagnostic and therapeutic challenge. J. Vet. Cardiol. 2015;17(Suppl. 1):S190–S201. doi: 10.1016/j.jvc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Veronesi F., Traversa D., Lepri E., Morganti G., Vercillo F., Grelli D., Cassini R., Marangi M., Iorio R., Ragni B., Di Cesare A. Occurrence of lungworms in European wildcats (Felis Silvestris Silvestris) of Central Italy. J. Wildl. Dis. 2016;52:270–278. doi: 10.7589/2015-07-187. [DOI] [PubMed] [Google Scholar]
- Vevers G.M. On the parasitic nematoda collected from mammalian hosts which died in the gardens of the Zoological Society of London during the years 1919-1921; with a description of three new genera and three new species. Proc. Zool. Soc. London. 1923;61:901–919. [Google Scholar]
- Wolff K., Eckert J., Leemann W. Dtsch Vet-med Ges; Zürich: 1969. Beitrag zur Angiostrongylose des Hundes. [Google Scholar]
- Zottler E.M., Strube C., Schnyder M. Detection of specific antibodies in cats infected with the lung nematode Aelurostrongylus abstrusus. Vet. Parasitol. 2017;235:75–82. doi: 10.1016/j.vetpar.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Zottler E.M., Bieri M., Basso W., Schnyder M. Intestinal parasites and lungworms in stray, shelter and privately owned cats of Switzerland. Parasitol. Int. 2019;69:75–81. doi: 10.1016/j.parint.2018.12.005. [DOI] [PubMed] [Google Scholar]


