Abstract
Background
During metastasis, tumor cells metastasize from primary tumors to distant organs via the circulatory and the lymphatic systems. There is a plethora of information about metastasis through the circulatory system, however not much information is available about the tumor cells dissemination through the lymphatic system or the lymphatic microenvironment that aids in this process in breast cancer metastasis.
Purpose
The study designed to examine the tumor-derived secretome in lymph before reaching the draining lymph nodes.
Methods
Using a microsurgical technique, we have collected the lymph in transit from the primary tumor en route to the regional lymph node in animals with metastatic and non-metastatic mammary carcinoma and healthy controls. The lymph samples were subjected to LC-MS/MS analysis, bioinformatics, and pathway analysis.
Results
The metastatic tumor-draining lymph before its entry into the closest regional lymph node contain 26 proteins with >175-folds in abundance compared to lymph from non-metastatic tumor-bearing animals. Among these proteins were biliverdin reductase B, heat shock protein, coagulation factor XIII, lymphocytes cytosol protein 1, and aldose reductase. These proteins were not identified in the lymph from healthy animals. Pathways analysis revealed that cadherin-mediated endocytosis, acute phase response, junction signaling, gap junction, VEGF singling, and PI3K/AKT singling pathways are overrepresented in the lymph from metastatic tumor-bearing compared to the lymph from non-metastatic tumor-bearing animals. Among the significantly up-regulated proteins in the lymph from metastatic tumor-bearing animals were proteins that identified in exosomes include heat shock protein, enolase 1 alpha, S100, and biliverdin reductase B. One of the proteins significantly down-regulated in lymph from animals with metastasis is Kininogen, a known metastasis inhibitor protein.
Conclusion
Proteins and exosomal proteins in lymph draining a metastatic tumor are different from those in lymph draining non-metastatic tumors, and these proteins involved in pathways that regulate tumor cells migration and invasion.
Keywords: lymph, lymphatic vessels, cancer, breast, lymph node, metastasis, exosome, tumor-derived factors
Introduction
Early diagnosis and systemic adjuvant therapies have improved survival rates in many women with breast cancer; unfortunately, these treatments often fail to treat women with metastatic disease effectively. During metastasis, tumor cells spread from the primary tumor to distant organs forming secondary tumors that reulst in patient death. The tumor cells spread via the circulatory and lymphatic systems.1 Although there is a plethora of information about the tumor cells spread through the circulatory system, little information is available regarding the tumor cells spread through the lymphatic system or the lymphatic microenvironment that aid in this process in breast cancer.
The tumor-draining lymph is shed predominantly at the interstitial periphery of the tumor and collected into lymphatic capillaries that merge into progressively larger vessels, afferent lymphatic vessels, which drain the lymph into the regional lymph node. Upon filtration of proteins and particulate uptake of the lymph by the immune cells in the regional lymph nodes, it returns by efferent lymphatic vessels to the venous blood. This journey makes the lymph rich in proteins from tissue growth and remolding, cellular metabolic/catabolic activities, and cell death. These lymph-collected proteins are organ-specific, suggesting that the lymph proteome reflects ongoing extracellular and intracellular processes in the particular tissue, mirroring the normal or disease conditions.2,3 It has been shown that the lymph in the afferent lymphatic vessels has different proteins from that of the plasma, interstitial fluid, and efferent lymph.4 Accordingly, the proteome (including exosomal proteins) and cellular composition of the tumor-draining afferent lymph before its entry into the draining lymph node differ from that of the efferent lymph or plasma and play a significant role in metastasis.
Furthermore, the tumor microenvironment is known to influence metastasis to the lymph node. The tumor secretes factors such as cytokines, exosomes, and enzymes that pre-condition the microenvironment of lymph nodes, making them a welcoming and supportive metastatic niches for disseminating tumor cells.5,6 Thus, the secreted protein profile, including the tumor-derived factors and exosomes of the afferent lymph, may provide information regarding the physiological and pathological states of the primary tumor and its potential to form metastasis which may provide means to design therapy.
To determine the protein composition and tumor-derived factors of tumor-draining lymph, we have developed and employed a novel microsurgical technique to successfully collect the lymph from the lymphatic vessels exiting the primary tumor before their entry into the draining lymph node in an immunocompetent rat model of breast cancer metastasis. The afferent lymph protein composition of metastatic tumor-bearing, non-metastatic tumor-bearing, compared to no tumor-bearing rats, were characterized and studied to determine unique tumor-derived factors that can serve as discriminating indicators of the metastatic tumor potential.
Materials and Methods
Cell Lines and Culture Condition
The rat metastatic MTLn3 and non-metastatic MTC cells were kindly provided by Dr. Segall (Albert Einstein College of Medicine, Bronx, NY) and approved by Purdue Biosafety Committee. The MTLn3 cells were clonally derived from a lung metastasis of the 13762NF rat mammary adenocarcinoma.7 Both MTLn3 and MTC cells were cultured in Minimal Essential Medium, Alpha (MEM; Sigma, St. Louis, MO), containing nonessential amino acids (Sigma, St Louis, MO), and supplemented with 5% fetal bovine serum (FBS; Hyclone, Logan, UT).
Orthotopic Tumor Growth and Metastasis
All experiments involving rats were conducted according to the National Institutes of Health regulation on the care and use of experimental animals. Purdue University Animal Use and Care Committee approved the study. Immunocompetent syngeneic female Fisher 344 rats were purchased from Harlan (Indianapolis, IN). MTLn3 and MTC cells were grown to 70-80% confluence, and 1X106 cells in 0.1 mL PBS or PBS (vehicle control) were injected into the two left caudal- and rostral-most mammary fat pads to establish primary (MTLn3 and MTC) and lymph node and lung metastasis (MTLn3).
Collection of Lymph and Blood from Tumor-Bearing and Non-Tumor-Bearing Rats
Lymph and blood were collected as previously described by Mohammed et al.8 Briefly MTLn3- and MTC-tumor bearing and PBS-injected animals (no tumor) were anesthetized, and the tumor-draining lymphatic vessels were visualized by injecting Lymphazurin dye (1%, isosulfan blue; United States Surgical Corporation, Ben Venue Laboratories Inc, Ohio). A heparin-treated polyethylene catheter was used to cannulate the afferent vessel at a site distal to the tumor and proximal to the vessel entry site on the sentinel lymph node and taped in place. The lymph from the four tumor cells-injected mammary fat pad sites was collected and pooled for each animal. Blood was collected similarly. The primary tumor, the draining lymph node, and lung tissues were collected and processed for histopathology to confirm metastasis.
Mass Spectrometry (MS) Data Acquisition and Analysis
Lymph and the blood samples were centrifuged, and subsequently, 200 μL of 8 M urea and 10 mM dithiothreitol (DTT) was added to the supernatant, which was then incubated for 30 min at 37°C and then alkylated with 50 mM iodoacetamide for 20 min at room temperature. The urea concentration was adjusted to 2 M, and proteins were digested by trypsin at 37 °C for 6 h in a buffer containing ammonium bicarbonate (50 mM, pH 9). The digestion mixture was then acidified by adding glacial acetic acid to a final concentration of 2% and desalted by ZipTip (Millipore). We used high sensitive reversed-phase liquid chromatography coupled nanospray tandem mass spectrometry (LC-MS/MS) using an LTQ-Orbitrap mass spectrometer (Thermo Fisher)9 to analyze the resultant peptides. The reversed-phase LC column was slurry-packed in-house with 5 μm, 200 Å pore size C18 resin (Michrom BioResources, CA) in a 100 μm i.d. × 10 cm long piece of fused silica capillary (Polymicro Technologies, Phoenix, AZ) with a laser-pulled tip. After packing, the new column, the HPLC system (Surveyor MS Pump Plus from ThermoFisher) and the LTQ-Orbitrap were tested by analyzing 100 fmol “Yeast Enolase Standard & Tryptic Digestion” from Michrom Bioresources, Inc. (catalogue number PTD/00001/46) to ensure obtaining stable ESI, desired mass accuracy, peak resolution, peak intensity and retention time. Additional iterations were performed to ensure reproducibility. We spiked a total of 100 fmol of standard peptide angiotensin I (Ang I) into the sample as an internal standard. After sample injection, we washed the column for 5 min with mobile phase A (0.1% formic acid), and peptides were eluted using a linear gradient of 0% mobile phase B (0.1% formic acid, 80% acetonitrile) to 50% B in 120 min at 200 nL/min, then to 100% B in an additional 10 min for the proteomics analysis. Before and after analyzing one sample, the column was washed with HPLC mobile phase B for 30 min, then mobile phase A for 20 min at a high flow rate (1 μL/min) to reduce potential carryover. The LTQ-Orbitrap mass spectrometer was operated in a data-dependent mode in which eight MS/MS scans followed each full MS scan (60,000 resolving power), and the eight most abundant molecular ions were dynamically selected and fragmented by collision-induced dissociation (CID) using a normalized collision energy of 35%. The Dynamic Exclusion Time was 30 s, and the Dynamic Exclusion Size was 200. The “FT master scan preview mode,” “Charge state screening,” “Monoisotopic precursor selection,” and “Charge state rejection” were enabled so that only the 1+, 2+, and 3+ ions were selected and fragmented by CID.
Tandem mass spectra collected by Xcalibur (version 2.0.2) were searched against the NCBI rodent protein database using SEQUEST (Bioworks software from ThermoFisher, version 3.3.1) with full tryptic cleavage constraints, static cysteine alkylation by iodoacetamide, and variable methionine oxidation. Mass tolerance for precursor ions was 5 ppm, and mass tolerance for fragment ions was 0.25 Da. The SEQUEST search results of proteomics data were filtered by the criteria “Xcorr versus charge 1.9, 2.2, 3.0 for 1+, 2+, 3+ ions; ΔCn > 0.1; probability of randomized identification of peptide <0.01”. Positive peptide identifications were determined using these stringent filter criteria for database match scoring, followed by manual evaluation of the results. The false discovery rate (FDR) was estimated by searching a combined forward-reversed database as described by Elias.10 The SEQUEST search results were exported to Excel files and compared.
Bioinformatic Analysis
For annotation analysis, we uploaded the GI protein accession numbers into the DAVID (Database for Annotation, Visualization, and Integrated Discovery) informatics tool (DAVID Bioinformatics Resources 6.7.11 For GO Term (Gene Ontology) analysis, we studied the Biological Process categories using the GO FAT default settings. For functional annotation searches, we set the following parameters: threshold count 3, EASE score (enrichment probability) 0.1; medium stringency for functional annotation clusters. For KEGG pathway searches, the parameters were: threshold count 5 (minimal count of proteins mapped to the pathway ≥5), enrichment probability ≤0.05 (strong enrichment).
Enrichment values (for GO terms), enrichment scores (for annotation clusters), and statistical determinants (for p values and Benjamini coefficients) are those calculated by DAVID software. The Group Enrichment Score is a geometric mean (in -log scale) of member’s Fisher exact test P-values in a corresponding annotation cluster, where each member’s p-value reflects the probability of enrichment for a particular gene in a given gene list. The Benjamini coefficients are Benjamini-Hochberg-corrected p values adjusted for multiple comparisons to lower the family-wise false discovery rate and thus are more conservative than Fisher exact p values. ToppCluster was used to perform additional functional analysis of differentially expressed proteins.12
Results
Afferent Lymph Collection from Metastatic Mammary Tumor Syngeneic Model
We developed a novel microsurgical technique for the collection of lymph draining a primary tumor before its entry into the regional lymph node (Figure 1A). We used metastatic MTLn3 and non-metastatic MTC cells transplanted orthotopically in mammary fat pads of immunocompetent female Fisher 344 rats for the syngeneic model (Figure 1B). These two cells were isolated from the same parent tumor, 13762NF mammary adenocarcinoma, but differed in their ability to metastasize.13 In approximately 10–14 days after injecting the cells, tumors developed in all MTLn3 and MTC implanted rats, but lymph node and lung metastasis only developed in MTLn3-bearing rats. The PBS injection site was free of tumor (negative controls). Lymph vessels were visualized by using Lymphazurin (Figure 1C), and the lymph and blood were collected as previously described.8
Figure 1.
Experimental plan and concept of lymph collection. (A) Scheme illustrating the tumor cells spread throught the lymphatic and haemotgeous system in women. (B) Outline of the experimental design and tumor implantation. (C) Visuslaiztion of afferent lymphatic vessles (green color).
Tumor-Derived Secretome Profiled in Lymph Before Reaching the Draining Lymph Node
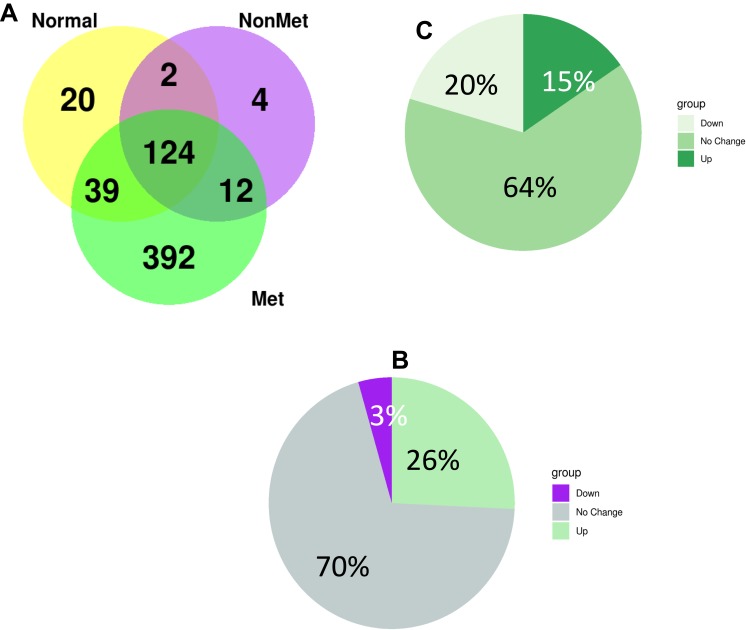
Tumor cells communicate through direct cell-to-cell contact and secretion of soluble protein-based factors such as cytokines and exosomes. To identify these soluble proteins including exosomal proteins in metastatic lymph, we performed liquid chromatography coupled nanospray tandem mass spectrometry (LC-MS/MS) in supernatant of lymph and plasma samples draining the primary tumor before reaching the draining lymph node from metastatic and non-metastatic-tumor bearing, and healthy animals as described by Zhou et al.14 LC-MS/MS analysis identified a total of 598 proteins significantly differentially expressed between lymph samples from animals with metastasis, no metastasis, and healthy control. Of these, 124 proteins were shared between the healthy, non-metastatic, and metastatic lymph samples, while 12 were shared between the metastatic lymph and non-metastatic lymph samples, and 392 proteins were unique to the metastatic lymph samples (Figure 2A). Comparing all proteins found in metastatic lymph to non-metastatic lymph, we found 70% of the proteins were similar in both groups while 26% were up-regulated, and 3 proteins were down-regulated in metastatic lymph (Figure 2B). Additionally, we found 429 proteins to be differentially expressed between lymph and plasma from metastatic tumor-bearing animals; among these, 20% were down-regulated and 15% were up-regulated in lymph (Figure 2C).
Figure 2.
(A) Summary of protein numbers obtained from the lymph from healthy animals, metastatic tumor-bearing and non-metastatic tumor-bearing animals. (B) differntailly expressed protein between lymph and plasma from metastatic tumor-bearing animals. (C) Differntailly expressed protein between lymph from metastatic and non-metastatic tumor-bearing animals.
We found 26 proteins were >175-fold higher in abundance (p-value <0.05) in the lymph from metastatic tumor-bearing compared to lymph from non-metastatic tumor-bearing animals. Among these proteins were biliverdin reductase B, heat shock protein, coagulation factor XIII, lymphocytes cytosol protein 1, and aldose reductase. These proteins were not identified in the lymph from healthy animals (Table 1).
Table 1.
Proteins Differentially (>175 Fold) Expressed in Lymph from Metastatic Tumor-Bearing Compared to Lymph from Non-Metastatic Tumor-Bearing Animals
| Protein | P-value | MW | Accession |
|---|---|---|---|
| Serine (or cysteine) proteinase inhibitor, clade G (C1 inhibitor), member 1, (angioedema, hereditary) | 9.99E-15 | 55,576.2 | 40,018,558 |
| Creatine kinase, muscle | 1.11E-15 | 42,991.8 | 6,978,661 |
| Enolase 1, alpha | 1.13E-09 | 47,086.3 | 6,978,809 |
| Pyruvate kinase, muscle | 7.54E-12 | 57,781.0 | 16,757,994 |
| Guanine deaminase | 1.22E-14 | 50,983.8 | 13,929,094 |
| PREDICTED: similar to tubulin, beta 2 | 8.07E-05 | 49,875.0 | 109,504,787 |
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide | 4.22E-09 | 27,753.7 | 62,990,183 |
| Peptidylprolyl isomerase A | 2.07E-09 | 17,862.8 | 8,394,009 |
| PREDICTED: similar to triosephosphate isomerase | 3.42E-07 | 26,847.8 | 62,663,437 |
| PREDICTED: similar to biliverdin reductase B (flavin reductase (NADPH)) | 1.24E-04 | 22,080.3 | 109,461,493 |
| PREDICTED: similar to Tubulin alpha-3 chain (Alpha-tubulin 3) | 5.66E-13 | 49,927.7 | 109,474,238 |
| Solute carrier family 4, member 1 | 2.56E-13 | 103,178.4 | 76,443,687 |
| PREDICTED: similar to Tubulin alpha-2 chain (Alpha-tubulin 2) | 5.48E-05 | 50,247.7 | 109,481,286 |
| Enolase 2, gamma | 4.47E-11 | 47,111.0 | 26,023,949 |
| Glucose phosphate isomerase | 2.15E-07 | 62,787.2 | 46,485,440 |
| Phosphoglycerate mutase 2 | 1.27E-07 | 28,736.8 | 8,393,948 |
| Enolase 3, beta | 8.77E-10 | 46,931.4 | 6,978,811 |
| Heat shock protein 8 | 3.13E-10 | 70,827.3 | 13,242,237 |
| Coagulation factor XIII, A1 subunit | 1.29E-12 | 82,606.5 | 11,067,435 |
| Transketolase | 1.01E-04 | 71,141.5 | 12,018,252 |
| Xanthine dehydrogenase | 2.47E-04 | 146,148.7 | 8,394,544 |
| Lymphocyte cytosolic protein 1 | 2.59E-08 | 70,077.9 | 58,865,656 |
| Phosphoglycerate mutase 1 | 3.79E-09 | 28,627.7 | 16,757,984 |
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, theta polypeptide | 1.89E-14 | 27,760.8 | 6,981,712 |
| Creatine kinase, brain | 6.01E-11 | 42,685.3 | 31,542,401 |
| PREDICTED: similar to Phosphoglycerate kinase 1 | 5.93E-10 | 43,148.3 | 62,642,907 |
Molecular Pathway Analysis of Lymph Proteins Demonstrate Up Regulation of Key Metastatic Pathways and Immunomodulation Network
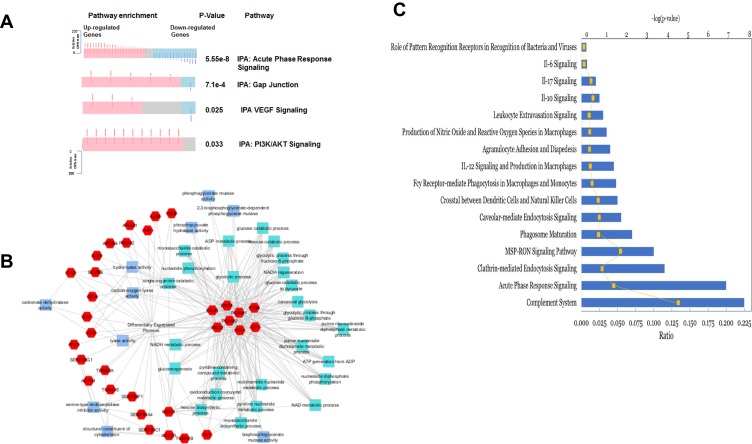
To understand how the tumor-derived proteins in the draining lymph interact to communicate signals which contribute to cancer progression, we examined the major biological pathways involved in lymphatic metastasis using Ingenuity Pathway Analysis (IPA, Qiagen Redwood City). The topmost signaling pathways in lymph from metastatic tumor-bearing animals compared to lymph from non-metastatic tumor-bearing animals were BAG2, Cell cycle G2/M DNA damage checkpoint regulation, p70S6K, protein ubiquitination, and unfolded protein response singling pathways (Figure 3A). These signaling pathways were not identified in plasma from metastatic tumor-bearing or non-metastatic tumor-bearing animals (Figure 3B). These signaling pathways are unique to the lymph from metastatic tumor-bearing animals. We also searched explicitly for pathways overrepresented in metastasis in the IPA and DAVID results. The results showed that pathways that were significantly overrepresented (Fisher’s exact test, p-value<0.001) in the metastatic lymph compared to the non-metastatic lymph included cadherin-mediated endocytosis, acute phase response, junction signaling, gap junction, VEGF singling, and PI3K/AKT singling (Figure 4A)
Figure 3.
(A) Top 10 pathways enriched in differentially expressed proteins in lymph from metastatic tumor-bearing animals versus lymph from non-metastatic tumor-bearing ainmals. (B) Top 10 pathways enriched in differentially expressed proteins in lymph compared to plasma from metastatic tumor-bearing animals. Activation state of the pathway was calculated using an activation z-score. Up-regulated pathways are shown in red, down-regulated are shown in blue, and those in which the activation state could not be determined are shown in grey. Number of proteins in each group is shown for each pathway in the pie chart. All pathways shown have an adjusted p-value < 0.05.
Figure 4.
Protein expressions in tumor-draining lymph from animals with metastatic tumor and the involvement of signaling pathways. (A) A spring layout algorithm was used to show the most significant GO terms associated with proteins that had a more extreme relative difference that + or −50. Molecular function GO terms are light blue, biological process GO terms are teal, and proteins are colored in red. A Bonferroni corrected p-value of 0.01 was used as the cutoff, and the top 25 GO terms were selected. (B) Summary of biological pathways indicating the down-regulated and up-regulated genes and the P value of enrichment. (C) Significantly activated 10 top immune-related pathways that in the metastatic lymph compared to non-metastatic lymph.
Also, a functional analysis performed (Figure 4B) shows the most significantly enriched biological process, and molecular function GO terms associated with the proteins that have extreme relative differences in the range of + 50 or −50. Among the 35 enriched GO terms, 10 are molecular function annotations, and 25 are biological processes. In molecular functions, most terms were associated with metabolic enzymatic activities and cytoskeleton structures, while the most terms in biological processes were associated with glycolysis and nucleotide synthesis. This analysis indicates that the top 25 metastasis-related proteins with extreme relative differences were mostly involved in metabolism and glycolysis.
Figure 4C shows the top 10 immune-related pathways in lymph from metastatic tumor-bearing animals, and these include IL-12, IL-10, IL-17, IL- 6, leukocytes extravasation, and the role of pattern recognition receptors.
Lymph Protein Profile Revealed Exosomal Proteins That Associate with Patients Survival
Among the significantly up-regulated proteins in the metastatic lymph in our study were proteins identified in exosomes, as indicated in the ExoCarta database http://www.exocarta.org/. These proteins include heat shock protein (HSPA8; p-value 3.13e-10), enolase 1 alpha (ENOA; p-value 1.13e-09), S100, and biliverdin reductase B (Blvrb; p-value 1.24E-04). One of the proteins significantly down-regulated in lymph from animals with metastasis is Kininogen (KNG1; p-value 1.00e-30), a known metastasis inhibitor protein.15 To determine whether these tumor-derived proteins are expressed in human breast cancer patients as well, we utilized an in silico approach to analyze the following proteins, Blvrb, HSPA8, and KNG1 in breast cancer using the Human Protein Atlas (http://www.proteinatlas.org) microarray tissue data. We found that Blvrb and HSPA8 are significantly overexpressed in human breast cancer tissues compared to normal, while the opposite is correct for KNG1 protein using three different antibodies.
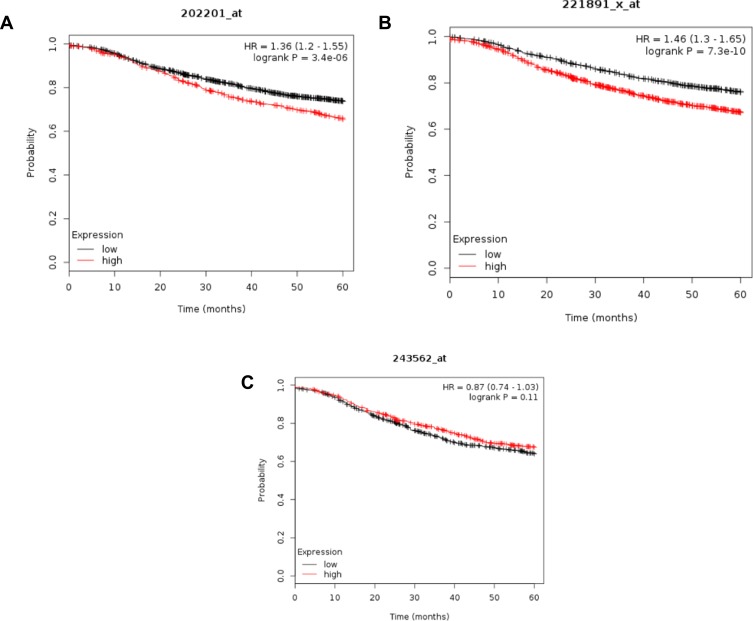
Furthermore, the potential significance of these proteins was evaluated against gene expression data of the human breast cancer from TCGA (https://cancergenome.nih.gov). Kaplan-Meier survival analysis of gene expression, for example, for HSPA8, BLVRB, and KNG1, revealed that a high expression of BLVRB and HSPA8 correlated significantly with shorter relapse-free survival of patients, while the opposite is exact for KNG1 (Figure 5A–C, respectively). Together, these results suggest that the lymph draining from primary tumors before entry into the nearest lymph node contains exosomal proteins, the relative abundance of which may influence the propensity for lymphatic metastasis and tumor aggressiveness.
Figure 5.
Kaplan-Meier survival analysis of human patients (n=3951) with invasive breast cancer divided into two groups based on the expression level of HSPA8 (A), BLVRB (B), and KING1 (C). Log rank test P-value is displayed. HR, hazard ratio.
Discussion
Despite the knowledge regarding the importance of cancer cell dissemination through the lymphatic system, to our knowledge, factors in lymph in transit from the primary tumor to the local draining lymph node were previously unknown. A significant reason that lymph has not been sampled in situ from growing tumors is the microscopic size of lymphatics and the difficulty in identifying and cannulating the lymphatics en route to the regional lymph node. The present study uses a unique microsurgical technique to collect and characterize the lymph draining from the primary tumor. Using an immunocompetent rat model of breast cancer transplanted orthotopically with metastatic MTLn3 and non-metastatic MTC cells; we have isolated and characterized the tumor-draining lymph as it drains from the primary tumor in live immunocompetent animal models of breast cancer metastasis.8
In recent years, accumulating evidence suggested that the primary tumor greatly influences the microenvironment of the regional lymph node. The primary tumor secretes factors that include cytokines, exosomes, or enzymes that condition the lymph node, creating an ideal environment for disseminating tumor cells.5,6 It follows then that the primary tumor-draining lymph should be rich in these premetastatic conditioning factors. These factors can serve as discriminating indicators of the metastatic tumor potential. The identification and monitoring of these premetastatic niche‐inducing materials in situ in lymph draining from primary tumors can provide insights about immune recognition or immune priming in the sentinel lymph nodes, which are highly relevant to tumor treatment. To determine the protein profiles of these tumor-derived factors, we performed a proteomic analysis of the lymph in transit to the regional lymph node. Our study is the first to study the proteomic profiles of the tumor-draining lymph before its entry into the regional lymph node in live animals. Although the lymph proteome has been examined previously in comparison to the plasma in non-cancer and cancerous diseases, the samples used were obtained either from the subject’s legs or mesenteric lymphatic vessels of patients or animals.16–20 Proteomic analysis of exosome in the postoperative lymphatic leak called lymphatic exudate collected after lymphadenectomy inpatient with melanoma was recently examined.21 The drawback of using such lymphatic exudate is the possibility of proteome alteration due to surgery and wound healing. Therefore, our approach is novel and could be applied to breast cancer patients who were undergoing primary and sentinel lymph node dissection comparing women with positive lymph node to those with negative lymph node metastasis permitting a through a comprehensive analysis of specific tumor predictive biomarker signatures.
We show that lymph from metastatic tumor-bearing animals contains distinct proteins compared to lymph from non-metastatic tumor-bearing animals and that these proteins are involved in pathways that modulate the immune system, gap junctions, angiogenesis, and lymphangiogenesis. Significantly altered pathways include BAG2, cell cycle G2/M DNA damage checkpoint regulation, p70S6K - pathways that were not identified in plasma. Notably, Bag2, is a protein of BCl-2 associated athanogene family, the overexpression of which is associated with poor prognosis in triple-negative breast cancer patients. Inhibition of BAG2 gene expression can completely control the growth and metastasis of triple-negative breast cancer cells to the lungs.22,23 Patients with tumors having increased p70S6K phosphorylation had worse disease-free survival and increased metastasis. However, the proteomic profile of extracellular vesicles from lymph node-negative patients’ lymphatic exudate was associated with pathways involved in cellular movement, vascularization, extravasation, and adhesion. These pathways are believed to correlate with the early stages of metastasis.21 In contrast, the proteomic profile of extracellular vesicles isolated from lymphadenectomy following positive lymph node biopsy show up-regulated signaling pathways connected to cell death, proliferation, and cancer, donating of more advanced disease.21
In contrast, extracellular-derived exosome showed pathways related to antigen presentation, endoplasmic reticulum phagosome, G2/M transition, and IL-12 family singling.24 Previously, we have shown that proteomic analysis of tumor cells in lymph draining a tumor enriched in NF-KB, heat shock response, and the unfolded protein response. Also, we have examined the cytokines/chemokines and growth factors in the tumor‐derived factors before lymph node entry to determine which factors may protect tumor cells in lymph, promote their migration to the lymph nodes, and aid in premetastatic niche formation. Of the 27 cytokines/chemokines examined, 19 had > 2‐fold increases in the metastatic lymph relative to the healthy lymph. EGF, TNF‐α, IFN‐γ‐induced protein 10 (IP‐10 or CXCL10), vascular endothelial growth factor (VEGF), and fractalkine (CX3CL1) showed > 10‐fold increases, and interleukin 18 (IL‐18) showed a > 70‐fold increase in the metastatic lymph compared to the lymph from non-metastatic tumor-bearing animals.8 These cytokines/chemokines and growth factors play an essential role in stimulating immune responses, immune cell chemotaxis, and tumor cell migration, invasion, and metastasis.25–28
Among the significantly altered proteins in metastatic lymph were proteins that shown to be carried by exosomes. Tumor-derived exosomes were shown to promote progression, invasion, and metastasis of cancer cells and play a significant role in suppressing the immune responses against the tumor. Although we have not explicitly isolated the exosomes, we have analyzed the lymph supernatants, which included exosomes. Among the exosome proteins that we identified in our study is HSPA8, which is known to activate cancer cell motility, migration and metastasis;29 EnoA, known to promote tumor metastasis30 and EMT; and Blvrb, which regulates the insulin/IGF-1/IRK/PI3K/MAPK, and VEGF pathways.31 Interestingly, among proteins significantly down-regulated in lymph from metastatic-tumor animals, was a metastasis inhibitor protein Kng1, which is known to inhibit migration and invasion of human prostate cancer and has been shown to have an essential role in suppression of cancer cell adhesion, invasion, and angiogenesis.32 Therefore, the metastatic lymph contains exosomal proteins that may influence the outcomes of the tumor cell migration and metastasis.
In summary, we now can routinely characterize the molecular and cellular composition of tumor-derived native lymph in transit to the draining regional lymph node. The molecular characterization of lymph would provide a new level of information of high relevance to the understanding of metastasis biology, particularly to the diagnosis and treatment of the lymphatic spread of human cancers.
Acknowledgments
The authors thank Dr. Mittal for his continuous support. Also, we thank Dr. Segall from Albert Einstein College of Medicine, the Bronx, NY, for providing the MTln3 and MTC cell lines. The work was supported by DOD concept award # W81XWH‐04‐1‐0747 and NIH/NCI grant # 1R21CA199621.
Ethical Concerns
All experiments involving rats were conducted according to the National Institutes of Health regulation on the care and use of experimental animals. Purdue University Animal Use and Care Committee approved the study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr Virginia Espina reports personal fees from Avant Diagnostics Investors Group, LLC., outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Karpanen T, Alitalo K. Lymphatic vessels as targets of tumor therapy? J Exp Med. 2001;194:F37–42. doi: 10.1084/jem.194.6.F37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen KC, D’alessandro A, Clement CC, Santambrogio L. Lymph formation, composition, and circulation: a proteomics perspective. Int Immunol. 2015;27:219–227. doi: 10.1093/intimm/dxv012 [DOI] [PubMed] [Google Scholar]
- 3.Popova TG, Espina V, Zhou W, Mueller C, Liotta L, Popov SG. Whole proteome analysis of mouse lymph nodes in cutaneous anthrax. PLoS One. 2014;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumor microenvironment: linking mechanobiology with immunity. Nat Rev Cancer. 2012;12:210–219. doi: 10.1038/nrc3186 [DOI] [PubMed] [Google Scholar]
- 5.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. doi: 10.1016/S0140-6736(00)49915-0 [DOI] [PubMed] [Google Scholar]
- 6.Sleeman JP. The metastatic niche and stromal progression. Cancer Metastasis Rev. 2012;31:429–440. doi: 10.1007/s10555-012-9373-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neri A, Welch D, Kawaguchi T, Nicolson G. Development and biologic properties of malignant cell sublines and clones of a spontaneously metastasizing rat mammary adenocarcinoma. J Natl Cancer Inst. 1982;68:507–517. [PubMed] [Google Scholar]
- 8.Mohammed SI, Torres-luquis O, Walls E, Lloyd F. Lymph-circulating tumor cells show distinct properties to blood-circulating tumor cells and are efficient metastatic precursors. Mol Oncol. 2019;13(6):1400–1418. doi: 10.1002/1878-0261.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou W, Capello M, Fredolini C, et al. Mass spectrometry analysis of the post-translational modifications of r-enolase from pancreatic ductal adenocarcinoma cells. J Proteome Res. 2010;9:2929–2936. doi: 10.1021/pr901109w [DOI] [PubMed] [Google Scholar]
- 10.Gkountela S, Szczerba B, Donato C, Aceto N. Recent advances in the biology of human circulating tumour cells and metastasis. ESMO Open. 2016;1(4):e000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis G, Sherman BT, Hosack DA, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4. doi: 10.1186/gb-2003-4-9-r60 [DOI] [PubMed] [Google Scholar]
- 12.Kaimal V, Bardes EE, Tabar SC, Jegga AG, Aronow BJ. ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and networkbased dissection of biological systems. Nucleic Acids Res. 2010;38:W96–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyckoff J, Jones J, Condeelis J, Segall J. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–2511. [PubMed] [Google Scholar]
- 14.Zhou W, Capello M, Fredolini C, et al. Proteomic analysis of pancreatic ductal adenocarcinoma cells reveals metabolic alterations. J Proteome Res. 2011;10:1944–1952. doi: 10.1021/pr101179t [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Fang J, Cheng Z, et al. Overexpression of the Kininogen-1 inhibits proliferation and induces apoptosis of glioma cells. J Exp Clin Cancer Res. 2018;37(1):180. doi: 10.1186/s13046-018-0833-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Interewicz B, Olszewski WL, Leak LV, Petricoin EF, Liotta LA. Profiling of normal human leg lymph proteins using the 2-D electrophoresis and SELDI-TOF mass spectrophotometry approach. Lymphology. 2004;37:65–72. [PubMed] [Google Scholar]
- 17.Leak LV, Liotta LA, Krutzsch H, et al. Proteomic analysis of lymph. Proteomics. 2004;4:753–765. doi: 10.1002/(ISSN)1615-9861 [DOI] [PubMed] [Google Scholar]
- 18.Nanjee MN, Cooke CJ, Olszewski WL, Miller NE. Lipid and apolipoprotein concentrations in prenodal leg lymph of fasted humans. Associations with plasma concentrations in normal subjects, lipoprotein lipase deficiency, and LCAT deficiency. J Lipid Res. 2000;41:1317–1327. [PubMed] [Google Scholar]
- 19.Olszewski WL, Pazdur J, Kubasiewicz E, Zaleska M, Cooke CJ, Miller NE. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44:541–549. doi: 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- 20.Ogundiran TO, Huo D, Adenipekun A, et al. Case-control study of body size and breast cancer risk in Nigerian women. Am J Epidemiol. 2010;172:682–690. doi: 10.1093/aje/kwq180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broggi MAS, Maillat L, Clement CC, et al. Tumor-associated factors are enriched in lymphatic exudate compared to plasma in metastatic melanoma patients. J Exp Med. 2019;216:1091–1107. doi: 10.1084/jem.20181618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang KM, Bae E, Ahn SG, et al. Co-chaperone BAG2 determines the pro-oncogenic role of cathepsin B in triple-negative breast cancer cells. Cell Rep. 2017;21:2952–2964. doi: 10.1016/j.celrep.2017.11.026 [DOI] [PubMed] [Google Scholar]
- 23.Yue X, Zhao Y, Liu J, et al. BAG2 promotes tumorigenesis through enhancing mutant p53 protein levels and function. Elife. 2015;4:e08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-silva S, Benito-martín A, Sánchez-redondo S, et al. Use of extracellular vesicles from lymphatic drainage as surrogate markers of melanoma progression and BRAF V600E mutation. J Exp Med. 2019;216(5):1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriai S, Takahara M, Ogino T, et al. Production of interferon-??-inducible protein-10 and its role as an autocrine invasion factor in nasal natural killer/T-cell lymphoma cells. Clin Cancer Res. 2009;15:6771–6779. doi: 10.1158/1078-0432.CCR-09-1052 [DOI] [PubMed] [Google Scholar]
- 26.Ran S, Volk L, Hall K, Flister MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2010;17:229–251. doi: 10.1016/j.pathophys.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei LM, Cao S, Yu WD, Liu YL, Wang JT. Overexpression of CX3CR1 is associated with cellular metastasis, proliferation and survival in gastric cancer. Oncol Rep. 2015;33:615–624. doi: 10.3892/or.2014.3645 [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Cheon S, Jung MK, et al. Interleukin-18 enhances breast cancer cell migration via down-regulation of claudin-12 and induction of the p38 MAPK pathway. Biochem Biophys Res Commun. 2015;459:379–386. doi: 10.1016/j.bbrc.2015.02.108 [DOI] [PubMed] [Google Scholar]
- 29.Becker B, Multhoff G, Farkas B, et al. Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp Dermatol. 2004;13:27–32. doi: 10.1111/exd.2004.13.issue-1 [DOI] [PubMed] [Google Scholar]
- 30.Capello M, Ferri-borgogno S, Cappello P, Novelli F. α-enolase: a promising therapeutic and diagnostic tumor target. FEBS J. 2011;278:1064–1074. doi: 10.1111/j.1742-4658.2011.08025.x [DOI] [PubMed] [Google Scholar]
- 31.Gibbs PEM, Miralem T, Maines MD. Biliverdin reductase: a target for cancer therapy? Front Pharmacol. 2015;6:119. doi: 10.3389/fphar.2015.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colman RW. Role of the light chain of high molecular weight kininogen in adhesion, cell-associated proteolysis and angiogenesis. Biol Chem. 2001;382(1):65–70. [DOI] [PubMed] [Google Scholar]