Abstract
Bone regeneration remains a great clinical challenge. Two-dimensional materials, especially graphene and its derivative graphene oxide, have been widely used for bone regeneration. Since its discovery in 2014, black phosphorus (BP) nanomaterials including BP nanosheets and BP quantum dots have attracted considerable scientific attention and are considered as prospective graphene substitutes. BP nanomaterials exhibit numerous advantages such as excellent optical and mechanical properties, electrical conductivity, excellent biocompatibility, and good biodegradation, all of which make them particularly attractive in biomedicine. In this review, we comprehensively summarize recent advances of BP-based nanomaterials in bone regeneration. The advantages are reviewed, the different synthesis methods of BP are summarized, and the applications to promote bone regeneration are highlighted. Finally, the existing challenges and perspectives of BP in bone regeneration are briefly discussed.
Keywords: 2D materials, black phosphorus nanomaterials, bone regeneration, nanomedicine
Introduction
Bone regeneration has been a major challenge in clinical surgery owing to conditions such as trauma, tumors and diseases (eg, osteomyelitis and osteitis).1 Many therapeutic strategies such as autografts, allografts, and artificial bone scaffolds have been employed.2,3 Autografts are considered as the gold standard in clinical practice, but they pose significant limitations, including limited bone mass, size mismatch, low availability, and donor site damage.4,5 Allografts also frequently carry potential risks such as disease transmission, contamination, and immune response.6,7 Thus, development of an artificial bone substitute with excellent osteoconduction, osteoinduction, and osteointegration is urgently needed.8–10
Black phosphorus (BP) nanomaterials, also called phosphorene, a novel member of the two-dimensional (2D) materials family, have sparked considerable research interest since BP nanosheets were first exfoliated from bulk BP in 2014.11 Since then, BP nanomaterials including BP nanosheets and BP quantum dots (BPQDs) have been extensively investigated.12–15 Compared with other 2D nanomaterials, BP nanomaterials exhibit puckered structures along the armchair direction and its bilayer configuration along the zigzag direction.16 This structural anisotropy leads to some unique properties of BP, such as electronic conductivity, optical, thermoelectric, and topological features, and unusual mechanical behavior.17–19 Due to their excellent properties, BP nanomaterials have attracted attention for a variety of biomedical applications such as photothermal therapy (PTT), photodynamic therapy (PDT), drug delivery, and bioimaging.20–22 Especially, there are several advantages of BP nanomaterials for bone regeneration according to recent studies. Firstly, compared to other 2D materials, they offer good biocompatibility and are biodegradable in the physiological environment.23 The degradation product of BP is nontoxic phosphate anions, which are a component of bone tissue and can be used for mineralization.24 Wang et al demonstrated that BP incorporated hydrogels can induce mineralization and enhance osteogenic cell differentiation and bone regeneration.25 Other studies showed that BP-based materials in bone defects can produce local mild heat and accelerate bone regeneration.24,26 BP nanomaterials also exhibit excellent PTT and PDT effects, which can be used to enhance bone regeneration, even under disease conditions such as tumors and infections. For instance, Yang et al designed BP-reinforced, 3D-printed scaffolds, and the intra-scaffold BP enabled both photothermal ablation of osteosarcoma and subsequent material-guided bone regeneration.27 Finally, compared to other 2D materials, BP nanomaterials have good drug-delivery capacity, and various drugs, biomolecules, and nanoparticles can be loaded on BP.28,29 Considering these advantages, BP nanomaterials could have promising applications in bone regeneration.
To date, the biomedical uses of BP nanomaterials, especially for anti-tumor therapy, have been well reviewed.14,19,30,31 However, their use for bone regeneration has not been extensively described. In this review, we systematically summarize the latest advances of BP nanomaterials in bone repair and regeneration. First, the composition of bone tissue and the requirements of scaffolds for bone regeneration are discussed. Second, the advantages of BP nanomaterials in bone regeneration are reviewed. Thirdly, different synthesis methods of BP are summarized. Lastly, the applications of BP nanomaterials to improve bone regeneration are highlighted. The existing challenges and opportunities of BP in bone regeneration are also discussed.
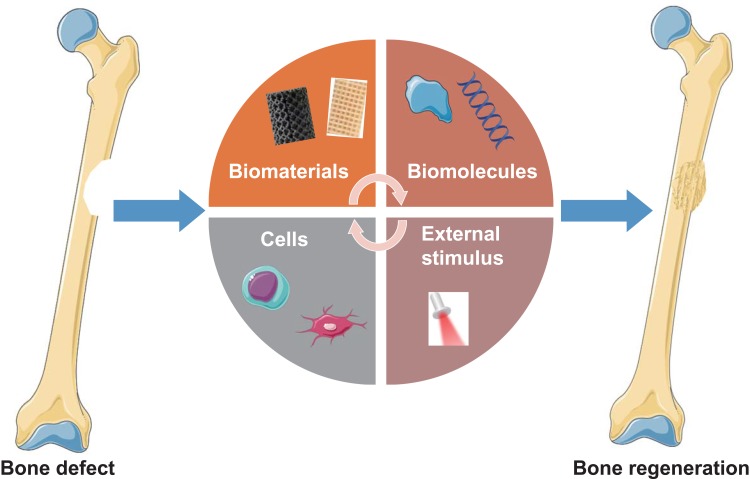
Bone Regeneration Strategies
Bone is composed of different cell types including osteocytes, osteoblasts, and osteoclasts embedded in a highly complex microenvironment.32 Osteoblasts and osteocytes play key roles in bone formation, while osteoclasts are involved in the reabsorption of existing bone tissue and regeneration.10 The structure of bone is mainly composed of organic type I collagen and the inorganic minerals calcium (Ca2+) and phosphate (PO43-), which provide a platform effectively promote cell proliferation and differentiation.33 Although bone tissue normally exhibits some degree of self-healing ability, it is limited when the bone defect exceeds a critical value (2 cm) or the defect is due to orthopedic diseases such as tumor or infection.34–36 Failure of bone healing will result in nonunion of the bone due to ischemia, osteonecrosis, and bone loss.37 To prevent these problems, additional treatments are required to stimulate bone regeneration. In the past few decades, there has been great success in artificial bone substitutes with excellent osteoconduction, osteoinduction, and osteointegration. Strategies to promote bone regeneration can be divided into four categories (Figure 1): (1) biomaterials, (2) biomolecules, (3) cells, and (4) external stimuli.38 They can be used separately or in combination to design scaffolds that mimic bone extracellular matrix (ECM) to promote regeneration. The designed scaffolds should meet four requirements. (1) Biocompatibility: the scaffolds can support cellular activity and molecular signaling without causing inflammatory reactions or toxic effects.39 (2) Mechanical properties: the scaffold should satisfy the mechanical strength needs and provide transfer properties.40 (3) Vesicular structure: interconnectedness, volume, and pore size are thought to provide space for bone growth.41 (4) Bioabsorbability: this provides space for new tissue ingrowth.42 An ideal scaffold should be degraded in vivo with a controllable rate, and the degradation products must be non-cytotoxic.43 Numerous strategies have been applied to construct biomimetic scaffold platforms for bone regeneration. For instance, graphene oxide (GO) loaded with micro-RNAs can be introduced to a silk fibroin/hydroxyapatite scaffold.44 Without loading osteoblast cells, this scaffold can increase osteogenic differentiation and mineralized bone formation in defects. Despite some success, the design of multi-scale porous scaffolds with ideal composition, loaded biomolecules, appropriate mechanical properties, and good bioresorbability still faces many challenges.
Figure 1.
Strategies for bone regeneration that can be used separately or in combination to design scaffolds that mimic bone extracellular matrix and promote bone regeneration.
The Advantages of BP Nanomaterials for Bone Regeneration
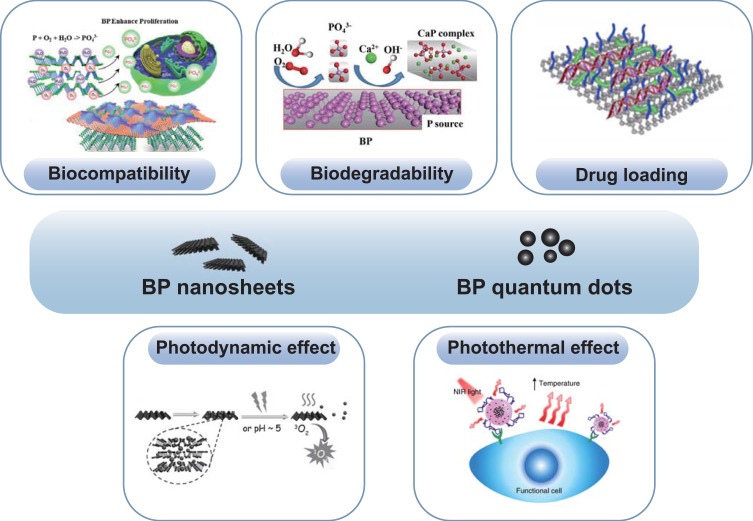
BP nanomaterials, an emerging two-dimensional metal-free layered material, has been sparked enormous research interest since discovery in 2014.11 It exhibit numerous superior properties, such as distinct pleated structures in layers, an adjustable direct band gap, high carrier mobility, and many interesting in-layer anisotropies.45 Thus, it has been applied in many fields including batteries, field-effect transistors, electronics, and nanomedicine in recent years.46,47 Notably, BP nanomaterials exhibit excellent biocompatibility, biodegradability, PTT and PDT effects, and high drug-loading capacity, which meet the above requirements and are beneficial for bone regeneration (Figure 2).
Figure 2.
The advantages of BP nanomaterials for bone regeneration, including biocompatibility,101 biodegradability,25 drug loading,71 and photothermal26 and photodynamic effects.55,68 Reproduced with permission from Liu X, Miller AL, II, Park S, et al. Two-Dimensional Black Phosphorus and Graphene Oxide Nanosheets Synergistically Enhance Cell Proliferation and Osteogenesis on 3D Printed Scaffolds. Acs Appl Mater Inter. 2019;11(26):23558–23572. Copyright 2019, ACS publications.101 Reproduced with permission Wang ZM, Zhao, Tang WZ et al Multifunctional Nanoengineered Hydrogels Consisting of Black Phosphorus Nanosheets Upregulate Bone Formation. Small. 2019;15(41). Copyright 2019, Wiley-VCH.25 Reproduced with permission from Chen L, Chen C, Chen W. et al Biodegradable Black Phosphorus Nanosheets Mediate Specific Delivery of hTERT siRNA for Synergistic Cancer Therapy. ACS Appl Mater Interfaces. 2018;10(25):21137–21148. Copyright 2018, ACS publications.71 Reproduced with permission from Chen W, Ouyang J, Liu H. et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv Mater. 2017;29(5). Copyright 2017, Wiley-VCH,68 Reproduced with permission from Xie HH, Shao JD, Ma YF et al. Biodegradable near-infrared-photoresponsive shape memory implants based on black phosphorus nanofillers. Biomaterials. 2018;164:11–21. Copyright 2018, Elsevier.55 Reproduced from Wang, Y., Hu, X., Zhang, L. et al. Bioinspired extracellular vesicles embedded with black phosphorus for molecular recognition-guided biomineralization. Nat Commun.10, 2829 (2019).This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.26
Abbreviation: BP, black phosphorus.
Biocompatibility and Biodegradability
Biocompatibility is a basic requirement in biomedicine. In general, the biocompatibility of 2D nanomaterials is material, size, dose, and cell dependent.48 A previous study demonstrated that BP showed less cytotoxicity than graphene, but more cytotoxicity than molybdenum disulfide and tungsten diselenide.49 BP with larger lateral size (884.0 ± 102.2 nm) and thickness (91.9 ± 32.0 nm) has higher cytotoxicity than small BP (lateral size: 208.5 ± 46.9 nm, thickness: 17.4 ± 9.1 nm).50 BP nanosheets can induce cytotoxicity at a high concentration (>50 μg/mL), but smaller BPQDs (<10 nm) were considered nontoxic up to the concentration of 1000 µg/mL.51 Thus, for further biomedical application, parameters of BP nanomaterials such as size and dose need to be optimized.
Due to its high reactivity with both oxygen and water, BP is easily degraded in the physiological environment, which may limit its application in some aspects.52–55 However, biodegradability limits the cytotoxicity caused by material accumulation in the body. Thus, compared to other 2D materials, this shortcoming is an advantage for biomedical applications. Moreover, the final degradation product of BP is nontoxic PO43-, which is a component of bone tissue and is safe to the human body and can be a resource for mineralization.23,56 In short, BP nanomaterials have good biocompatibility and biodegradability and are suitable for biomedical applications.
Phototherapy Effect
Previous researches suggested that PTT and PDT effect can be used to anti-tumor,57 anti–infection,58 and improve tissue regeneration.59 Sun et al showed that ultrasmall BPQDs exhibited an excellent near-infrared (NIR) photothermal performance with a large extinction coefficient of 14.8 L g(−1) cm (−1) at 808 nm, and photothermal conversion efficiency up to 28.4%.13 This indicates that BP is an effective photothermal agent. Recently, BP nanomaterials have been widely used for anti-tumor therapies through PTT effect. For instance, BPQDs were loaded into poly(lactic-co-glycolic acid) (PLGA) with additional conjugation of the chemotherapeutic agent docetaxel.60 This system exhibits outstanding controllable chemo-photothermal combinatory therapeutics that synergistically incurs apoptosis-dependent cell death, resulting in the elimination of lung metastases. Polymer encapsulation has also been used to improve the stability of BP nanosheets in the ambient environment. Zeng et al adopted a polydopamine (PDA) modification to enhance the stability and photothermal performance of bare BP nanosheets.61 The rise of 27.1 °C in temperature for BP@PDA was higher than that for bare BP (temperature change of 24.1 °C).
Besides PTT ability, BP also exerts PDT effects. Compared to other PDT agents, BP nanosheets are biocompatible and have higher singlet oxygen generation efficiency; they produce singlet oxygen with a quantum yield up to 9.1 and show notable cancer therapy ability.62 To enhance the penetration depth of the irradiation wavelength in the visible light region, upconversion nanoparticles (UCNP)-conjugated BP nanosheets (UCNP-BPs) were proposed, and different laser irradiations (650, 808, and 980 nm wavelength) were applied to explore PDT performance. Both in vitro and in vivo experiments revealed that the group pretreated with UCNP-BP composites, 808-nm NIR light irradiation generated the largest amount of reactive oxygen species (ROS) and exerted the strongest tumor inhibition effect. Similar to BP nanosheets, BPQDs (5.4 nm) with rapid renal clearance showed excellent PDT effects under light irradiation and could effectively generate ROS to kill cancer cells.63
Based on the above analysis, the excellent PTT and PDT abilities of BP nanomaterials make it a promising application for promoting bone regeneration, especially in disease conditions such as cancer and infection.
Drug-Loading Capacity
2D nanomaterials for drug delivery systems have been extensively studied due to their high specific surface area and unique surface chemistry functions.64,65 The surface of BP is negatively charged and can bond with positively charge drugs.66 Various functional biomolecules and nanoparticles can be adsorbed onto the surface of BP nanomaterials.30 For example, after modification with positively charged polyethylene glycol (PEG) to enhance physiological stability and biocompatibility, the chemotherapy drug doxorubicin (DOX) can be loaded on BP nanosheets.28 In combination with chemotherapy and PTT, DOX-loaded BP nanosheets induced significant tumor cell death. Moreover, compared to other 2D materials (eg, graphene), BP has a higher surface-to-volume ratio due to its puckered lattice configuration, which allows for effective drug loading.67 BP can hold higher amounts of DOX on the sheet surface (950% in weight), which is far superior to other reported 2D materials.68 In another study, iron oxide nanoparticles and Au nanoparticles were assembled on BP sheets.20 This novel composite is highly biocompatible and exhibits excellent tumor inhibition efficacy due to synergistic PTT and PDT induced by NIR laser treatment. In addition to chemotherapeutic drugs and nanoparticles, BP can also carry biomolecules such as small interfering RNA (siRNA) that can regulate the expression of specific genes by degrading messenger RNA after transcription.69 Wang et al designed a survivin siRNA delivery system based on PEG functionalized BP nanosheets, which showed excellent anti-tumor effects through synergistic PTT and gene therapy.70 In another study, Chen et al presented a drug delivery system based on BP nanosheets.71 After functionalization with PEG and polyethylenimine, the BP nanosheets exhibited high siRNA loading capacity. This delivery system efficiently inhibited tumor growth and metastasis due to the synergistic combination of specific gene therapy, PDT, and PTT.71 In summary, BP is an excellent drug delivery platform as reflected by its strong drug-loading capacity and photo-responsive drug release properties.
Synthesis of BP Nanomaterials
The fabrication of thin 2D nanostructures can be categorized into “top-down methods” (mechanical exfoliation, liquid phase exfoliation, plasma etching, etc.) and “bottom-up methods” (chemical vapor deposition [CVD], wet-chemistry, etc.).72 In this section, we summarize the methods used for preparing BP, particularly the most commonly used approaches of liquid phase exfoliation and mechanical exfoliation.
Liquid Phase Exfoliation
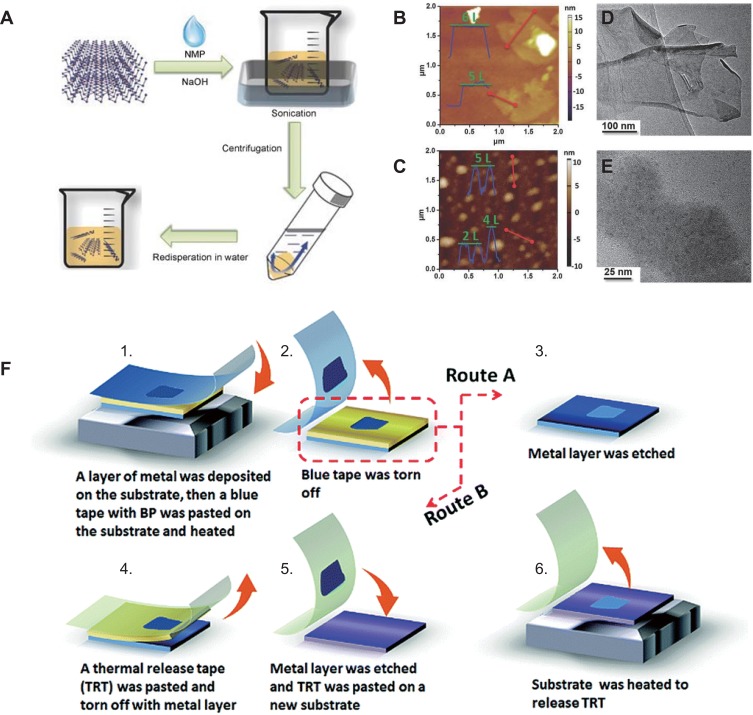
Liquid phase exfoliation is the most popular method for manufacturing BP nanomaterials and is based on the balanced surface energy between the exfoliation solvent and layer material.73,74 The most commonly used solvent is N-methyl-2-pyrrolidone (NMP);75 other solvents including N-vinyl pyrrolidone (NVP),76 dimethyl formamide (DMF),77 dimethyl sulfoxide (DMSO),78 and isopropyl alcohol (IPA)79 are also employed to prepare BP nanomaterials. In 2014, Brent et al80 first reported liquid-phase exfoliation, BP was exfoliated in NMP using bath ultrasonication, and both 3- to 5-layered phosphorene and smaller 1- to 3-layered phosphorene were obtained. Ultrasonic waves produced large amounts of cavitation bubbles that collapse into high-energy jets, destructing the weak van der Waals interaction between adjacent sheets of bulk BP to induce the formation of monolayer or few-layered BP.47 Guo et al81 added NaOH into NMP during the exfoliation process, which enhanced the dispersion ability and long-term stability of BP in water (Figure 3A). According to atomic force microscope and transmission electron microscope results, phosphorene obtained by centrifugation at 12,000 rpm had an average diameter of about 670 nm and a thickness of 5.3 ± 2.0 nm (Figure 3B and D). Smaller phosphorene with an average diameter of around 210 nm and a thickness of 2.8 ± 1.5 nm can be obtained via centrifugation at 18,000 rpm (Figure 3C and E). To avoid the potential reaction of BP with oxygen during preparation, a novel sealed-tip ultrasonication system was adopted.82 High-powered tip ultrasonication can greatly improve the efficiency of liquid phase exfoliation.
Figure 3.
(A) Schematic illustration of the fabrication process of basic-NMP-exfoliated phosphorene.81 (B–E) Characterization of phosphorene obtained by centrifugation of phosphorene dispersion at different speeds.81 Height-mode AFM images and TEM images: (B, D) centrifugation at 12,000 rpm, (C, E) centrifugation at 18,000 rpm. (F) Illustration of the metal-assisted exfoliation progress for few-layered BP.88 Reproduced with permission from Guo ZN, Zhang H, Lu SB, et al. From Black Phosphorus to Phosphorene: Basic Solvent Exfoliation, Evolution of Raman Scattering, and Applications to Ultrafast Photonics. Adv Funct Mater. 2015;25(45):6996–7002. Copyright 2017, Wiley-VCH.81 Reproduced with permission from Guan L, Xing BR, Niu XY, et al. Metal-assisted exfoliation of few-layer black phosphorus with high yield. Chem Commun. 2018;54(6):595–598. Copyright 2015, Royal Society of Chemistry.88
Abbreviation: NMP, N-methyl-2-pyrrolidone.
BPQDs can also be synthesized using the liquid exfoliation method. Zhang et al first synthesized BPQDs with a median size of 4.9 ± 1.6 nm and a thickness of 1.9 ± 0.9 nm by liquid phase ultrasonic exfoliation.83 The BP solution obtained after probe sonication treatment was more convenient for the subsequent bath sonication. Through combining probe and water bath sonication, Sun et al obtained BPQDs with a median size of 2.6 nm and a medial thickness of 1.5 nm.13 Moreover, a higher sonication power and extended sonication time can promote monodispersity of BPQDs.
Mechanical Exfoliation
Mechanical exfoliation is another common method to prepare thin-layer materials. Compared with other 2D materials, BP can be easily exfoliated and layered by mechanical exfoliation due to its weak interlayer van der Waals force.45 Mechanical exfoliation, also called Scotch-tape delamination, usually with the aid of some adhesive tape (Scotch tape/blue Nitto tape).84 A single-layer BP nanosheet with a thickness of 0.85 nm is created with adhesive tape.85 Then BP nanosheets are transferred onto a silicon-based substrate and cleaned with alcohol to remove the residual adhesive tape. However, the normal “Scotch-tape” method has some drawbacks such as low yield and inability to control the shape, size, and thickness. Polydimethylsiloxane (PDMS) substrates and tape can be used together to strip BP to obtain nano BP with high yield.86 Transferring the stripped BP from PDMS substrates with fully dry transfer technology can solve the problem of adhesive residue.87 Recently, metal-assisted mechanical exfoliation was introduced to prepare few-layer BP with a lateral size >50 μm and an area 100× larger than those exfoliated using the normal “Scotch-tape” technique (Figure 3F).88 Although some success has been achieved, the low yield and uncontrollable morphology of BP still need to be addressed.
Other Synthetic Methods
Electrochemical exfoliation is a liquid exfoliation technique that can also be used to synthesize BP nanomaterial.89 In this method, a bulk BP crystal was used as a working electrode while a platinum wire was used as a counter electrode.84 By applying a positive bias on the working electrode, water molecules were oxidized to generate •OH and •O radicals that accumulate around the BP crystal.90 When the free radical was oxidized to generate oxygen, BP nanosheets were successfully peeled off from the bulk BP.
Plasma etching can be used to prepare BP nanomaterial by thermal ablation. Jia et al suggested that modulating the etching time can effectively control the thickness of layered BP.91 The BP nanomaterials maintained excellent crystallinity with this plasma etching technique. In another study, the use of oxygen plasma dry etching can fabricate air-stable, high-quality BP films with a designated number of layers from a few down to a monolayer.92
CVD growth is another effective strategy for synthesizing high-quality 2D materials with superior electro-optical performance.93 When performed on a given substrate with precursors at high temperature, it is possible to obtain ultrathin nanomaterial with a large surface area, tunable size and thickness, and excellent crystal quality.94 Jiang et al reported that they directly grew BP on an aluminum foil matrix with a novel thermal-vaporization transformation approach.95 This method can produce BP in various forms including BP thin film and BP particles on supports.
The Application of BP Nanomaterials for Bone Regeneration
Owing to the aforementioned advantages, BP nanomaterial-based scaffolds to stimulate bone regeneration have been widely investigated in past 2 years. The mechanism of BP to promote bone regeneration can be classified as the effects of PO43-, PTT, and PDT.
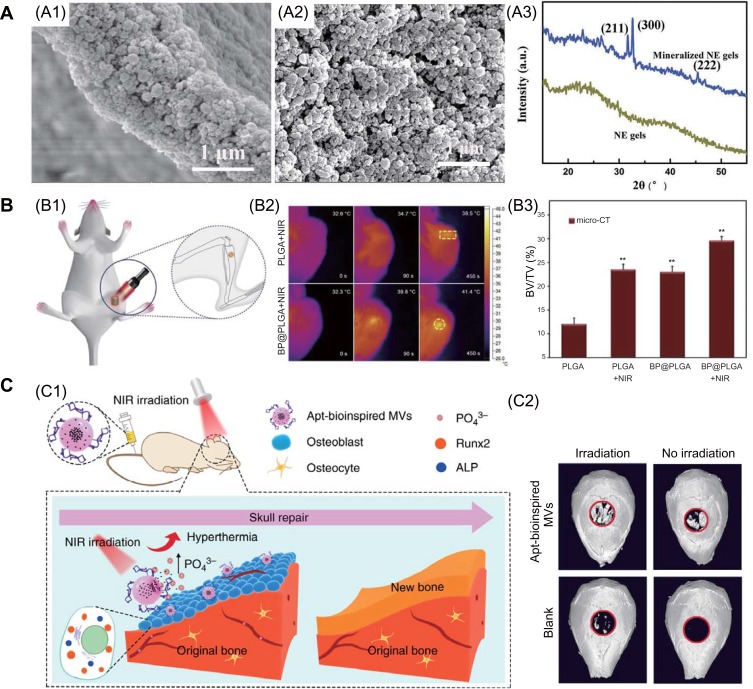
Firstly, phosphorus is a vital elements in the human body and accounts for approximately 1% of the total body weight as a bone constituent.56 It is known that BP can moderate oxidation and degradation in ambient conditions, and the major degradation product is PO43-, which is nontoxic.52 In addition, PO43- is the resource for mineralization, it can capture Ca2+ in vivo to form calcium phosphate (CaP) deposits that accelerate bone repair.96,97 Various studies have demonstrated that phosphorus-rich materials can stimulate mineralization and bone regeneration.98,99 Wang et al introduced ultrathin BP nanosheets into double network hydrogels to induce tissue regeneration.25 From the electron microscopy and X-ray diffraction results, the incorporated BP nanosheets in the hydrogel exhibit intrinsic properties for induced CaP crystal particle formation after soaked in simulated body fluid solution (Figure 4A). Thus, this platform provides a favorable ECM microenvironment to promote greater osteogenic cell differentiation and bone regeneration. Huang et al fabricated BP-based hydrogel scaffolds and showed that the introduction of BP nanosheets enhanced hydrogel mechanical performance.100 These hydrogels not only accelerated biomineralization in bone defects by the degraded phosphorus ion from BP nanosheets to capture Ca2+, they also stimulated osteogenic differentiation of stem cells via the bone morphogenic protein-2 pathway. Similar results were reported by Liu and colleagues, who constructed a composite of GO and BP on 3D scaffolds to stimulate bone regeneration.101 The introduced GO nanosheets enhance cell attachment at the initial stage and wrap BP to achieve continuous PO43- release that stimulates cell osteogenesis toward new bone formation.
Figure 4.
(A) The introduction of BP nanosheets to double network hydrogels could facilitate inorganic matrix formation: SEM (A1, A2) and (A3) XRD results.25 (B) Photothermal stimulation of bone regeneration: (B1) illustration the progress of rat tibia implantation and subsequent NIR irradiation; (B2) infrared thermo-graphic maps in irradiated areas; (B3) BV/TV ratio results, ** denotes p < 0.01 compared with the PLGA group.24 (C) Apt-bioinspired MVs can stimulate bone regeneration: (C1) overview of Apt-bioinspired MVs in cell mineralization; (C2) micro-CT reconstruction of the bone defect.26 Reproduced with permission Wang ZM, Zhao, Tang WZ et al. Multifunctional Nanoengineered Hydrogels Consisting of Black Phosphorus Nanosheets Upregulate Bone Formation. Small. 2019;15(41). Copyright 2019, Wiley-VCH.25 Reproduced with permission from Tong LP, Liao Q, Zhao YT et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials. 2019;193:1–11. copyright 2019, Elsevier.24 Reproduced from Wang, Y., Hu, X., Zhang, L. et al. Bioinspired extracellular vesicles embedded with black phosphorus for molecular recognition-guided biomineralization. Nat Commun.10, 2829 (2019). This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.26
Abbreviations: ALP, alkaline phosphatase; BV, bone volume; NE, nanoengineered; NIR, near infrared; PLGA, poly(lactic-co-glycolic acid); Runx2, runt-related transcription factor 2; TV, tissue volume.
Previous researches have demonstrated that mild localized heat can efficiently stimulate cell proliferation and induce rapid in situ bone regeneration in vivo.102–104 BP nanomaterials have high photothermal conversion efficiency. This property of BP nanomaterials can be used for both anti-tumor and tissue regeneration applications. Tong et al assed the osteogenetic performances of BP@PLGA osteoimplant with NIR light irradiation.24 BP@PLGA with only 0.2 wt% BP show the highly-efficient NIR photothermal response even when being covered by a biological tissue as thick as 7 mm. Under NIR irradiation, the BP@PLGA generate mild heat (40–42 °C) which up-regulate the expression of heat shock proteins (HSPs) and stimulate bone regeneration (Figure 4B). In another interesting study, Wang et al synthesized bioinspired matrix vesicles (MVs) by encapsulating BPQDs into a PLGA nanosphere and functionalizing with an osteoblast-specific aptamer.26 The aptamer directs bioinspired MVs to targeted cells, and the increasing local PO43- concentration facilitates cell biomineralization. Then, the photothermal effect of BPQDs can promote bone regeneration by up-regulating expression of HSPs and alkaline phosphatase (Figure 4C). It is well known that bioactive ions such as strontium (Sr2+), magnesium, and silicon can significantly stimulate osteoblast differentiation and bone formation, and they have been widely used for tissue regeneration.7,105-107 BP exhibits high drug-loading capacity. Wang et al incorporated BP and SrCl2 into PLGA microspheres, and local Sr2+ release was controlled by NIR irradiation.108 The synergistic effect of Sr2+ and PO43- can significantly promote bone regeneration.
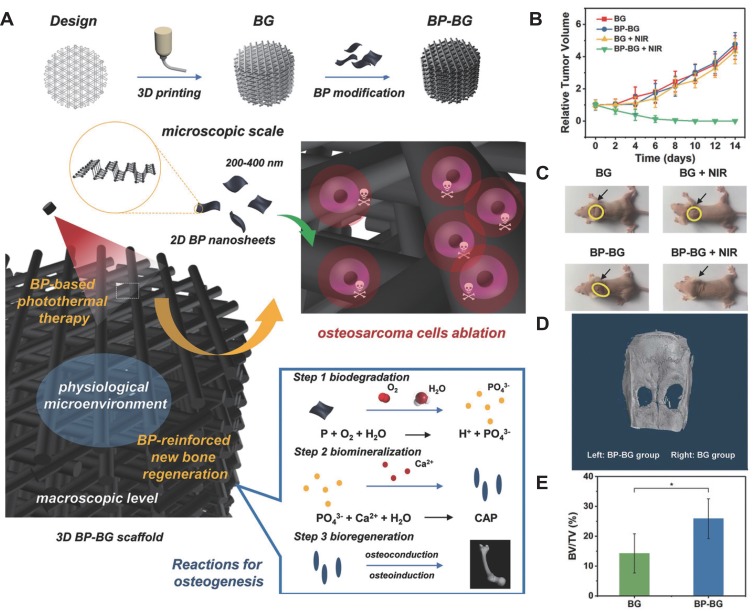
BP also exhibits excellent PTT and PDT effects, so they can be used to stimulate bone regeneration even under disease conditions such as tumor and infection. It is well known that bone is one of the most frequent sites of metastasis.109 The treatment of bone metastatic cancer or bone tumor usually causes significant damage to surrounding bone tissue.110 Thus, a multifunctional platform which can not only to eliminate the tumor but also promote the bone regeneration is strongly needed.111 As the schematic illustration in Figure 5A, Yang et al integrating BP nanosheets into 3D printed bioglass (BG) scaffold, this bifunctional BP-BG scaffold enables both against osteosarcoma and enhance bone regeneration.27 Under NIR irradiation, tumors on mice of BP-BG scaffold group were eliminated completely, without reoccurrence in the observation period of 14 d (Figure 5B and C). In addition, the micro-CT results confirmed that BG-BP scaffolds showed significantly better repairing outcome for bone defects than pure BG scaffolds (Figure 5D and E). In another research, Raucci et al demonstrated that BP nanosheets exerted opposite effects on tumor cells and osteoblasts; BP nanosheets inhibited the metabolic activity of osteosarcoma cells, but induced both the proliferation and osteogenic differentiation of human preosteoblast cells.112 Therefore, BP can serve as a promising candidate for bone regenerative and anticancer applications.
Figure 5.
BP-based scaffolds can eliminate osteosarcoma and enhance bone regeneration. (A) Schematic illustration of the fabrication process for BP-BG scaffolds and the stepwise therapeutic strategy. (B) Time-dependent tumor-growth curves of the mice after different treatments as described in the figure. (C) Photographs of osteosarcoma-bearing mice after different treatments on day 14, the yellow circle and black arrow indicate the location of the tumor. (D) Micro-CT images of the cranium based on the density variations of osseous tissue. (E) The percentage of newborn osseous tissue volume over the entire defect space (*p < 0.05). Reproduced with permission from Yang B, Yin J, Chen Y. et al. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A Stepwise Countermeasure for Osteosarcoma. Adv Mater. 2018;30(10). Copyright 2018, Wiley-VCH.27
Abbreviations: BG, bioglass; BP, black phosphorus; BV, bone volume; CaP, calcium phosphate; NIR, near-infrared; TV, tissue volume.
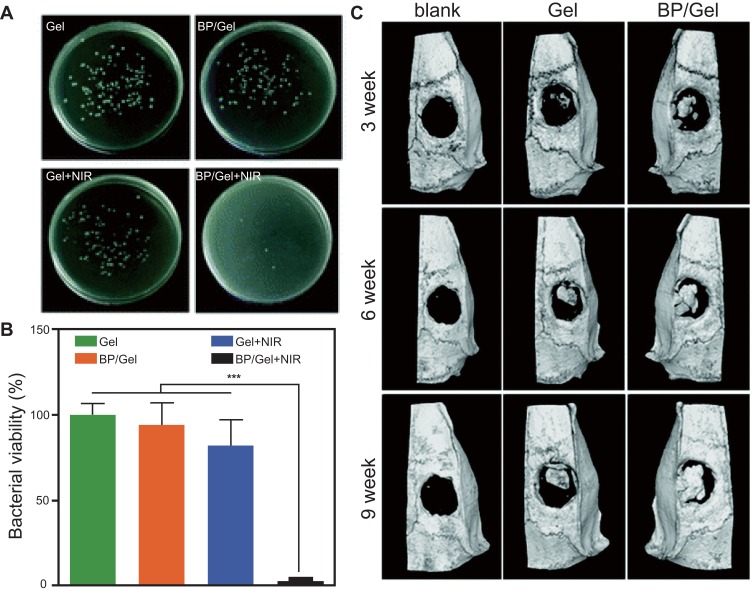
Implant-related infection is a common postoperative complication of orthopedic surgery which often causing serious problems.113 BP nanosheets showed antibiotic-like behavior against both Gram negative Escherichia coli and Gram-positive Bacillus subtilis. With time- and concentration-dependent antibacterial behavior, the maximum bactericidal efficiency of BP nanosheets against E. coli and B. subtilis was 91.65% and 99.69%, respectively.114 What’s more, through generate amount ROS, BP nanosheets can effectively fight bacterial infections without causing antibiotic resistance.115 To simultaneously combat infection and improve bone regeneration, a multi-functional, therapeutic, BP-based hydrogel nanocomposite was designed.116 Upon NIR irradiation, the nanocomposite hydrogel demonstrated efficient photothermal antibacterial features (Figure 6A and B). What’s more, this nanocomposite hydrogel can promote osteogenesis in the absence of osteoinductive factors in vitro, and in the meantime induce newborn cranial bone tissue formation (Figure 6C). In short, as an emerging material, BP nanomaterials offers new perspectives for future bone regeneration.
Figure 6.
(A, B) Photothermal antibacterial performance: optical images and bacterial viability of different groups (***p <0.001). (C) Micro-CT images of the harvested cranium obtained from rats at different times. Reproduced with permission from Miao YL, Shi XT, Li QT, et al. Engineering natural matrices with black phosphorus nanosheets to generate multi-functional therapeutic nanocomposite hydrogels. Biomater Sci. 2019;7(10):4046–4059. Copyright 2019, Royal Society of Chemistry.116
Abbreviations: BP, black phosphorus; NIR, near-infrared.
Conclusion and Outlook
In conclusion, this review provides a comprehensive summary on recent progress regarding the use of BP nanomaterials for bone regeneration, including their advantages, synthesis methods, and applications. The fascinating properties of BP nanomaterials (eg, biocompatibility, biodegradability, PTT and PDT abilities, and high drug-loading capacity) play key roles in bone regeneration application. Therefore, BP could be considered an ideal candidate to promote bone regeneration.
Despite the above-described progress, the application of BP nanomaterials in bone regeneration from basic research to clinical application still faces many challenges. Compared to other well-explored 2D nanomaterials, BP is still in its infancy. Liquid phase exfoliation and mechanical exfoliation are widely used to prepare BP nanomaterials, but both methods offer low yields. It is therefore urgent to explore effective approaches to obtain high-quality BP nanomaterials. While the biodegradability of BP can hamper its PTT effect, it is beneficial to bone regeneration. Physical encapsulation with PLGA or PDA has been applied to protect BP from degradation. Future studies should assess strategies to optimize the balance between stability and biodegradability to achieve the best therapeutic effects. As a candidate agent, BP is commonly encapsulated into polymers or introduced onto scaffolds via immersion to promote bone regeneration. These approaches are less efficient, and other options should be explored. In addition, more drugs, biomolecules and nanoparticles should be loaded on to BP nanomaterials to achieve synergistic therapy of bone regeneration. The application of BP in bone regeneration is still in its initial stage. With more scientist participate in the research, the application of BP nanomaterials will definitely promote to a higher level.
Acknowledgment
This work is supported by the National Natural Science Foundation of China (Nos. 81772456, 51627805).
Abbreviations
BG, bioglass; BP, black phosphorus; BPQD, black phosphorus quantum dots; Ca2+, calcium; CaP, calcium phosphate; CVD, chemical vapor deposition; DMF, dimethyl formamide; DMSO, dimethyl sulfoxide; DOX, doxorubicin; ECM, extracellular matrix; GO, graphene oxide; HSP, heat shock protein; IPA, isopropyl alcohol; MV, matrix vessel; NIR, near-infrared; NMP, N-methyl-2-pyrrolidone; NVP, N-vinyl pyrrolidone; PDA, polydopamine; PDMS, polydimethylsiloxane; PDT, photodynamic therapy; PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic acid); PO43-, phosphate; PTT, photothermal therapy; ROS, reactive oxygen species; siRNA, small interfering RNA; Sr2+, strontium; UCNP, upconversion nanoparticles.
Disclosure
The authors have declared that there are no competing interests. This article does not deal with any ethical issues.
References
- 1.Bhattacharjee P, Naskar D, Maiti TK, Bhattacharya D, Kundu SC. Investigating the potential of combined growth factors delivery, from non-mulberry silk fibroin grafted poly(epsilon-caprolactone)/hydroxyapatite nanofibrous scaffold, in bone tissue engineering. Appl Mater Today. 2016;5:52–67. doi: 10.1016/j.apmt.2016.09.007 [DOI] [Google Scholar]
- 2.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995 [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Zhang K, Zhao R, et al. Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials. 2017;147:133–144. doi: 10.1016/j.biomaterials.2017.09.018 [DOI] [PubMed] [Google Scholar]
- 4.Cao HJ, Guan HF, Lai YX, Qin L, Wang XL. Review of various treatment options and potential therapies for osteonecrosis of the femoral head. J Orthop Transl. 2016;4:57–70. doi: 10.1016/j.jot.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma HS, Feng C, Chang J, Wu CT. 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta Biomater. 2018;79:37–59. doi: 10.1016/j.actbio.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 6.Tang D, Tare RS, Yang LY, Williams DF, Ou KL, Oreffo ROC. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials. 2016;83:363–382. doi: 10.1016/j.biomaterials.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 7.Wang S-J, Jiang D, Zhang -Z-Z, et al. Biomimetic nanosilica-collagen scaffolds for in situ bone regeneration: toward a cell-free, one-step surgery. Adv Mater. 2019:e1904341–e1904341. doi: 10.1002/adma.201904341 [DOI] [PubMed] [Google Scholar]
- 8.Olszta MJ, Cheng XG, Jee SS, et al. Bone structure and formation: A new perspective. Mat Sci Eng R. 2007;58(3–5):77–116. doi: 10.1016/j.mser.2007.05.001 [DOI] [Google Scholar]
- 9.Seyednejad H, Gawlitta D, Kuiper RV, et al. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(epsilon-caprolactone). Biomaterials. 2012;33(17):4309–4318. doi: 10.1016/j.biomaterials.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 10.Torgbo S, Sukyai P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl Mater Today. 2018;11:34–49. doi: 10.1016/j.apmt.2018.01.004 [DOI] [Google Scholar]
- 11.Li LK, Yu YJ, Ye GJ, et al. Black phosphorus field-effect transistors. Nat Nanotechnol. 2014;9(5):372–377. doi: 10.1038/Nnano.2014.35 [DOI] [PubMed] [Google Scholar]
- 12.Nakhanivej P, Yu X, Park SK, et al. Revealing molecular-level surface redox sites of controllably oxidized black phosphorus nanosheets. Nat Mater. 2019;18(2):156–162. doi: 10.1038/s41563-018-0230-2 [DOI] [PubMed] [Google Scholar]
- 13.Sun ZB, Xie HH, Tang SY, et al. Ultrasmall black phosphorus quantum dots: synthesis and use as photothermal agents. Angew Chem Int Edit. 2015;54(39):11526–11530. doi: 10.1002/anie.201506154 [DOI] [PubMed] [Google Scholar]
- 14.Choi JR, Yong KW, Choi JY, et al. Black phosphorus and its biomedical applications. Theranostics. 2018;8(4):1005–1026. doi: 10.7150/thno.22573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayorga-Martinez CC, Latiff NM, Eng AYS, Sofer Z, Pumera M. Black phosphorus nanoparticle labels for immunoassays via hydrogen evolution reaction mediation. Anal Chem. 2016;88(20):10074–10079. doi: 10.1021/acs.analchem.6b02422 [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Zhou XY, Zhang D, Lou WK, Zhai F, Chang K. Electronic and magneto-optical properties of monolayer phosphorene quantum dots. 2D Mater. 2015;2(4). doi: 10.1088/2053-1583/2/4/045012 [DOI] [Google Scholar]
- 17.Choi SJ, Kim B-K, Lee T-H, et al. Electrical and thermoelectric transport by variable range hopping in thin black phosphorus devices. Nano Lett. 2016;16(7):3969–3975. doi: 10.1021/acs.nanolett.5b04957 [DOI] [PubMed] [Google Scholar]
- 18.Viti L, Hu J, Coquillat D, et al. Black phosphorus terahertz photodetectors. Adv Mater. 2015;27(37):5567–5572. doi: 10.1002/adma.201502052 [DOI] [PubMed] [Google Scholar]
- 19.Gui RJ, Jin H, Wang ZH, Li JH. Black phosphorus quantum dots: synthesis, properties, functionalized modification and applications. Chem Soc Rev. 2018;47(17):6795–6823. doi: 10.1039/c8cs00387d [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Yang GX, Yang PP, et al. Assembly of Au plasmonic photothermal agent and iron oxide nanoparticles on ultrathin black phosphorus for targeted photothermal and photodynamic cancer therapy. Adv Funct Mater. 2017;27(18). doi: 10.1002/adfm.201700371 [DOI] [Google Scholar]
- 21.Mao CY, Xiang YM, Liu XM, et al. Repeatable photodynamic therapy with triggered signaling pathways of fibroblast cell proliferation and differentiation to promote bacteria-accompanied wound healing. ACS Nano. 2018;12(2):1747–1759. doi: 10.1021/acsnano.7b08500 [DOI] [PubMed] [Google Scholar]
- 22.Sun ZB, Zhao YT, Li ZB, et al. TiL4-coordinated black phosphorus quantum dots as an efficient contrast agent for in vivo photoacoustic imaging of cancer. Small. 2017;13(11). doi: 10.1002/smll.201602896 [DOI] [PubMed] [Google Scholar]
- 23.Shao JD, Xie HH, Huang H, et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat Commun. 2016;7. doi: 10.1038/ncomms12967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong LP, Liao Q, Zhao YT, et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials. 2019;193:1–11. doi: 10.1016/j.biomaterials.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 25.Wang ZM, Zhao TWZ, Tang W, et al. Multifunctional nanoengineered hydrogels consisting of black phosphorus nanosheets upregulate bone formation. Small. 2019;15(41). doi: 10.1002/smll.201901560 [DOI] [PubMed] [Google Scholar]
- 26.Wang YQ, Hu XX, Zhang LL, et al. Bioinspired extracellular vesicles embedded with black phosphorus for molecular recognition-guided biomineralization. Nat Commun. 2019;10. doi: 10.1038/s41467-019-10761-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang B, Yin J, Chen Y, et al. 2D-black-phosphorus-reinforced 3D-printed scaffolds: a stepwise countermeasure for osteosarcoma. Adv Mater. 2018;30(10). doi: 10.1002/adma.201705611 [DOI] [PubMed] [Google Scholar]
- 28.Tao W, Zhu XB, Yu XH, et al. Black phosphorus nanosheets as a robust delivery platform for cancer theranostics. Adv Mater. 2017;29(1). doi: 10.1002/adma.201603276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin F, Hu K, Chen S, et al. Black phosphorus quantum dot based novel siRNA delivery systems in human pluripotent teratoma PA-1 cells. J Mater Chem B. 2017;5(27):5433–5440. doi: 10.1039/c7tb01068k [DOI] [PubMed] [Google Scholar]
- 30.Cheng L, Wang XW, Gong F, Liu T, Liu Z. 2D nanomaterials for cancer theranostic applications. Adv Mater. 2019. doi: 10.1002/adma.201902333 [DOI] [PubMed] [Google Scholar]
- 31.Luo M, Fan T, Zhou Y, Zhang H, Mei L. 2D black phosphorus-based biomedical applications. Adv Funct Mater. 2019;29(13). doi: 10.1002/adfm.201808306 [DOI] [Google Scholar]
- 32.Mohammadi M, Shaegh SAM, Alibolandi M, et al. Micro and nanotechnologies for bone regeneration: recent advances and emerging designs. J Controlled Release. 2018;274:35–55. doi: 10.1016/j.jconrel.2018.01.032 [DOI] [PubMed] [Google Scholar]
- 33.Jang JH, Castano O, Kim HW. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliver Rev. 2009;61(12):1065–1083. doi: 10.1016/j.addr.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 34.Wagner JM, Reinkemeier F, Wallner C, et al. Adipose-derived stromal cells are capable of restoring bone regeneration after post-traumatic osteomyelitis and modulate B-cell response. Stem Cell Transl Med. 2019;8(10):1084–1091. doi: 10.1002/sctm.18-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma HS, Jiang CA, Zhai D, et al. A bifunctional biomaterial with photothermal effect fortumor therapy and bone regeneration. Adv Funct Mater. 2016;26(8):1197–1208. doi: 10.1002/adfm.201504142 [DOI] [Google Scholar]
- 36.Yang Y, Chu LY, Yang SB, et al. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018;79:265–275. doi: 10.1016/j.actbio.2018.08.015 [DOI] [PubMed] [Google Scholar]
- 37.Loi F, Cordova LA, Pajarinen J, et al. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes D, Martins-Cruz C, Oliveira MB, Mano JF. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials. 2018;185:240–275. doi: 10.1016/j.biomaterials.2018.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31(3):461–466. doi: 10.1016/j.biomaterials.2009.09.063 [DOI] [PubMed] [Google Scholar]
- 40.Yi H, Ur Rehman F, Zhao C, Liu B, He N. Recent advances in nano scaffolds for bone repair. Bone Res. 2016;4:16050. doi: 10.1038/boneres.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou S, Niu XF, Li LH, et al. Simultaneous nano- and microscale structural control of injectable hydrogels via the assembly of nanofibrous protein microparticles for tissue regeneration. Biomaterials. 2019;223:119458. doi: 10.1016/j.biomaterials.2019.119458 [DOI] [PubMed] [Google Scholar]
- 42.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–2953. doi: 10.1016/j.biomaterials.2008.04.023 [DOI] [PubMed] [Google Scholar]
- 43.Mondschein RJ, Kanitkar A, Williams CB, Verbridge SS, Long TE. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials. 2017;140:170–188. doi: 10.1016/j.biomaterials.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 44.Ou L, Lan Y, Feng Z, et al. Functionalization of SF/HAP Scaffold with GO-PEI-miRNA inhibitor complexes to enhance bone regeneration through activating transcription factor 4. Theranostics. 2019;9(15):4525–4541. doi: 10.7150/thno.34676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing C, Zhang JH, Jing JY, Li JZ, Shi F. Preparations, properties and applications of low-dimensional black phosphorus. Chem Eng J. 2019;370:120–135. doi: 10.1016/j.cej.2019.03.177 [DOI] [Google Scholar]
- 46.Hanlon D, Backes C, Doherty E, et al. Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics. Nat Commun. 2015;6. doi: 10.1038/ncomms9563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ge X, Xia Z, Guo S. Recent advances on black phosphorus for biomedicine and biosensing. Adv Funct Mater. 2019;29(29):1900318. doi: 10.1002/adfm.201900318 [DOI] [Google Scholar]
- 48.Kenry LCT. Biocompatibility and nanotoxicity of layered two-dimensional nanomaterials. Chemnanomat. 2017;3(1):5–16. doi: 10.1002/cnma.201600290 [DOI] [Google Scholar]
- 49.Latiff NM, Teo WZ, Sofer Z, Fisher AC, Pumera M. The cytotoxicity of layered black phosphorus. Chem-Eur J. 2015;21(40):13991–13995. doi: 10.1002/chem.201502006 [DOI] [PubMed] [Google Scholar]
- 50.Zhang XJ, Zhang ZM, Zhang SY, et al. Size effect on the cytotoxicity of layered black phosphorus and underlying mechanisms. Small. 2017;13(32). doi: 10.1002/smll.201701210 [DOI] [PubMed] [Google Scholar]
- 51.Lee HU, Park SY, Lee SC, et al. Black phosphorus (BP) nanodots for potential biomedical applications. Small. 2016;12(2):214–219. doi: 10.1002/smll.201502756 [DOI] [PubMed] [Google Scholar]
- 52.Island JO, Steele GA, van der Zant HSJ, Castellanos-Gomez A. Environmental instability of few-layer black phosphorus. 2D Mater. 2015;2(1):011002. doi: 10.1088/2053-1583/2/1/011002 [DOI] [Google Scholar]
- 53.Walia S, Sabri Y, Ahmed T, et al. Defining the role of humidity in the ambient degradation of few-layer black phosphorus. 2D Mater. 2017;4(1). doi: 10.1088/2053-1583/4/1/015025 [DOI] [Google Scholar]
- 54.Zhang TM, Wan YY, Xie HY, et al. Degradation chemistry and stabilization of exfoliated few-layer black phosphorus in water. J Am Chem Soc. 2018;140(24):7561–7567. doi: 10.1021/jacs.8b02156 [DOI] [PubMed] [Google Scholar]
- 55.Xie HH, Shao JD, Ma YF, et al. Biodegradable near-infrared-photoresponsive shape memory implants based on black phosphorus nanofillers. Biomaterials. 2018;164:11–21. doi: 10.1016/j.biomaterials.2018.02.040 [DOI] [PubMed] [Google Scholar]
- 56.Childers DL, Corman J, Edwards M, Elser JJ. Sustainability challenges of phosphorus and food: solutions from closing the human phosphorus cycle. Bioscience. 2011;61(2):117–124. doi: 10.1525/bio.2011.61.2.6 [DOI] [Google Scholar]
- 57.Wang Z, Gai SL, Wang CQ, et al. Self-assembled zinc phthalocyanine nanoparticles as excellent photothermal/photodynamic synergistic agent for antitumor treatment. Chem Eng J. 2019;361:117–128. doi: 10.1016/j.cej.2018.12.007 [DOI] [Google Scholar]
- 58.Yuan Z, Tao BL, He Y, et al. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials. 2019;223:119479. doi: 10.1016/j.biomaterials.2019.119479 [DOI] [PubMed] [Google Scholar]
- 59.Lu ZH, Liu SJ, Le YG, et al. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials. 2019;218:119190. doi: 10.1016/j.biomaterials.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 60.Wang S, Shao J, Li Z, Ren Q, Yu X-F LS. Black phosphorus-based multimodal nanoagent: showing targeted combinatory therapeutics against cancer metastasis. Nano Lett. 2019;19(8):5587–5594. doi: 10.1021/acs.nanolett.9b02127 [DOI] [PubMed] [Google Scholar]
- 61.Zeng XW, Luo MM, Liu G, et al. Polydopamine-modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments. Adv Sci. 2018;5(10). doi: 10.1002/advs.201800510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Yang XZ, Shao W, et al. Ultrathin black phosphorus nanosheets for efficient singlet oxygen generation. J Am Chem Soc. 2015;137(35):11376–11382. doi: 10.1021/jacs.5b06025 [DOI] [PubMed] [Google Scholar]
- 63.Guo T, Wu Y, Lin Y, et al. Black phosphorus quantum dots with renal clearance property for efficient photodynamic therapy. Small. 2018;14(4):1702815. doi: 10.1002/smll.201702815 [DOI] [PubMed] [Google Scholar]
- 64.Yadav V, Roy S, Singh P, Khan Z, Jaiswal A. 2D MoS2-based nanomaterials for therapeutic, bioimaging, and biosensing applications. Small. 2019;15(1). doi: 10.1002/smll.201803706 [DOI] [PubMed] [Google Scholar]
- 65.Ji DK, Menard-Moyon C, Bianco A. Physically-triggered nanosystems based on two-dimensional materials for cancer theranostics. Adv Drug Deliver Rev. 2019;138:211–232. doi: 10.1016/j.addr.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 66.Tayari V, Hemsworth N, Fakih I, et al. Two-dimensional magnetotransport in a black phosphorus naked quantum well. Nat Commun. 2015;6. doi: 10.1038/ncomms8702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu F, Zhang M, Chu X, et al. Black phosphorus nanosheets-based nanocarriers for enhancing chemotherapy drug sensitiveness via depleting mutant p53 and resistant cancer multimodal therapy. Chem Eng J. 2019;370:387–399. doi: 10.1016/j.cej.2019.03.228 [DOI] [Google Scholar]
- 68.Chen W, Ouyang J, Liu H, et al. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv Mater. 2017;29(5). doi: 10.1002/adma.201603864 [DOI] [PubMed] [Google Scholar]
- 69.Agrawal N, Dasaradhi PVN, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK. RNA interference: biology, mechanism, and applications. Microbiol Mol Biol R. 2003;67(4):657–685. doi: 10.1128/Mmbr.67.4.657-685.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Zhong L, Liu Y, et al. A black phosphorus nanosheet-based siRNA delivery system for synergistic photothermal and gene therapy. Chem Commun. 2018;54(25):3142–3145. doi: 10.1039/c8cc00931g [DOI] [PubMed] [Google Scholar]
- 71.Chen L, Chen C, Chen W, et al. Biodegradable black phosphorus nanosheets mediate specific delivery of hTERT siRNA for synergistic cancer therapy. ACS Appl Mater Interfaces. 2018;10(25):21137–21148. doi: 10.1021/acsami.8b04807 [DOI] [PubMed] [Google Scholar]
- 72.Qiu M, Ren WX, Jeong T, et al. Omnipotent phosphorene: a next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem Soc Rev. 2018;47(15):5588–5601. doi: 10.1039/c8cs00342d [DOI] [PubMed] [Google Scholar]
- 73.Li Q, Zhou QH, Shi L, Chen Q, Wang JL. Recent advances in oxidation and degradation mechanisms of ultrathin 2D materials under ambient conditions and their passivation strategies. J Mater Chem A. 2019;7(9):4291–4312. doi: 10.1039/c8ta10306b [DOI] [Google Scholar]
- 74.Peng XH, Wei Q, Copple A. Strain-engineered direct-indirect band gap transition and its mechanism in two-dimensional phosphorene. Phys Rev B. 2014;90(8). doi: 10.1103/PhysRevB.90.085402 [DOI] [Google Scholar]
- 75.Liu G, Tsai H-I, Zeng X, et al. Black phosphorus nanosheets-based stable drug delivery system via drug-self-stabilization for combined photothermal and chemo cancer therapy. Chem Eng J. 2019;375:121917. doi: 10.1016/j.cej.2019.121917 [DOI] [Google Scholar]
- 76.Wang W, Niu XY, Qian HL, et al. Surface charge transfer doping of monolayer molybdenum disulfide by black phosphorus quantum dots. Nanotechnology. 2016;27(50):505204. doi: 10.1088/0957-4484/27/50/505204 [DOI] [PubMed] [Google Scholar]
- 77.Yasaei P, Kumar B, Foroozan T, et al. High-quality black phosphorus atomic layers by liquid-phase exfoliation. Adv Mater. 2015;27(11):1887–1892. doi: 10.1002/adma.201405150 [DOI] [PubMed] [Google Scholar]
- 78.Passaglia E, Cicogna F, Costantino F, et al. Polymer-based black phosphorus (bP) hybrid materials by in situ radical polymerization: an effective tool to exfoliate bP and stabilize bP nanoflakes. Chem Mater. 2018;30(6):2036–2048. doi: 10.1021/acs.chemmater.7b05298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen W, Li KW, Wang Y, et al. Black phosphorus quantum dots for hole extraction of typical planar hybrid perovskite solar cells. J Phys Chem Lett. 2017;8(3):591–598. doi: 10.1021/acs.jpclett.6b02843 [DOI] [PubMed] [Google Scholar]
- 80.Brent JR, Savjani N, Lewis EA, Haigh SJ, Lewis DJ, O’Brien P. Production of few-layer phosphorene by liquid exfoliation of black phosphorus. Chem Commun. 2014;50(87):13338–13341. doi: 10.1039/c4cc05752j [DOI] [PubMed] [Google Scholar]
- 81.Guo ZN, Zhang H, Lu SB, et al. From black phosphorus to phosphorene: basic solvent exfoliation, evolution of raman scattering, and applications to ultrafast photonics. Adv Funct Mater. 2015;25(45):6996–7002. doi: 10.1002/adfm.201502902 [DOI] [Google Scholar]
- 82.Kang J, Wood JD, Wells SA, et al. Solvent exfoliation of electronic-grade, two-dimensional black phosphorus. ACS Nano. 2015;9(4):3596–3604. doi: 10.1021/acsnano.5b01143 [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Xie H, Liu Z, et al. Black phosphorus quantum dots. Angew Chem Int Ed. 2015;54(12):3653–3657. doi: 10.1002/anie.201409400 [DOI] [PubMed] [Google Scholar]
- 84.Anju S, Ashtami J, Mohanan PV. Black phosphorus, a prospective graphene substitute for biomedical applications. Mater Sci Eng C. 2019;97:978–993. doi: 10.1016/j.msec.2018.12.146 [DOI] [PubMed] [Google Scholar]
- 85.Liu H, Neal AT, Zhu Z, et al. Phosphorene: an unexplored 2D semiconductor with a high hole mobility. ACS Nano. 2014;8(4):4033–4041. doi: 10.1021/nn501226z [DOI] [PubMed] [Google Scholar]
- 86.Castellanos-Gomez A, Vicarelli L, Prada E, et al. Isolation and characterization of few-layer black phosphorus. 2D Mater. 2014;1(2):025001. doi: 10.1088/2053-1583/1/2/025001 [DOI] [Google Scholar]
- 87.Castellanos-Gomez A, Buscema M, Molenaar R, et al. Deterministic transfer of two-dimensional materials by all-dry viscoelastic stamping. 2D Mater. 2014;1(1):011002. doi: 10.1088/2053-1583/1/1/011002 [DOI] [Google Scholar]
- 88.Guan L, Xing BR, Niu XY, et al. Metal-assisted exfoliation of few-layer black phosphorus with high yield. Chem Commun. 2018;54(6):595–598. doi: 10.1039/c7cc08488a [DOI] [PubMed] [Google Scholar]
- 89.Chen YT, Ren R, Pu HH, Chang JB, Mao S, Chen JH. Field-effect transistor biosensors with two-dimensional black phosphorus nanosheets. Biosens Bioelectron. 2017;89:505–510. doi: 10.1016/j.bios.2016.03.059 [DOI] [PubMed] [Google Scholar]
- 90.Erande MB, Pawar MS, Late DJ. Humidity sensing and photodetection behavior of electrochemically exfoliated atomically thin-layered black phosphorus nanosheets. Acs Appl Mater Inter. 2016;8(18):11548–11556. doi: 10.1021/acsami.5b10247 [DOI] [PubMed] [Google Scholar]
- 91.Jia J, Jang SK, Lai S, et al. Plasma-treated thickness-controlled two-dimensional black phosphorus and its electronic transport properties. ACS Nano. 2015;9(9):8729–8736. doi: 10.1021/acsnano.5b04265 [DOI] [PubMed] [Google Scholar]
- 92.Pei JJ, Gai X, Yang J, et al. Producing air-stable monolayers of phosphorene and their defect engineering. Nat Commun. 2016;7(1). doi: 10.1038/ncomms10450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang J, Li N, Zou J, et al. Synergistic additive-mediated CVD growth and chemical modification of 2D materials. Chem Soc Rev. 2019;48(17):4639–4654. doi: 10.1039/c9cs00348g [DOI] [PubMed] [Google Scholar]
- 94.Batmunkh M, Bat-Erdene M, Shapter JG. Phosphorene and phosphorene-based materials - prospects for future applications. Adv Mater. 2016;28(39):8586–8617. doi: 10.1002/adma.201602254 [DOI] [PubMed] [Google Scholar]
- 95.Jiang QQ, Xu L, Chen N, Zhang H, Dai LM, Wang SY. Facile synthesis of black phosphorus: an efficient electrocatalyst for the oxygen evolving reaction. Angew Chem Int Edit. 2016;55(44):13849–13853. doi: 10.1002/anie.201607393 [DOI] [PubMed] [Google Scholar]
- 96.Penido MG, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012;27(11):2039–2048. doi: 10.1007/s00467-012-2175-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rashdan NA, Rutsch F, Kempf H, Varadi A, Leftheriotis G, MacRael VE. New perspectives on rare connective tissue calcifying diseases. Curr Opin Pharmacol. 2016;28:14–23. doi: 10.1016/j.coph.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 98.Kim HD, Jang HL, Ahn HY, et al. Biomimetic whitlockite inorganic nanoparticles-mediated in situ remodeling and rapid bone regeneration. Biomaterials. 2017;112:31–43. doi: 10.1016/j.biomaterials.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 99.Diaz ECG, Shih YRV, Nakasaki M, Liu MQ, Varghese S. Mineralized biomaterials mediated repair of bone defects through endogenous cells. Tissue Eng Pt A. 2018;24(13–14):1148–1156. doi: 10.1089/ten.tea.2017.0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang KQ, Wu J, Gu ZP. Black phosphorus hydrogel scaffolds enhance bone regeneration via a sustained supply of calcium-free phosphorus. Acs Appl Mater Inter. 2019;11(3):2908–2916. doi: 10.1021/acsami.8b21179 [DOI] [PubMed] [Google Scholar]
- 101.Liu X, Miller AL, Park S, et al. Two-dimensional black phosphorus and graphene oxide nanosheets synergistically enhance cell proliferation and osteogenesis on 3D printed scaffolds. Acs Appl Mater Inter. 2019;11(26):23558–23572. doi: 10.1021/acsami.9b04121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang XG, Cheng G, Xing X, et al. Near-infrared light-triggered porous AuPd alloy nanoparticles to produce mild localized heat to accelerate bone regeneration. J Phys Chem Lett. 2019;10(15):4185–4191. doi: 10.1021/acs.jpclett.9b01735 [DOI] [PubMed] [Google Scholar]
- 103.Yanagi T, Kajiya H, Kawaguchi M, Kido H, Fukushima T. Photothermal stress triggered by near infrared-irradiated carbon nanotubes promotes bone deposition in rat calvarial defects. J Biomater Appl. 2015;29(8):1109–1118. doi: 10.1177/0885328214556913 [DOI] [PubMed] [Google Scholar]
- 104.Shui CX, Scutt A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J Bone Miner Res. 2001;16(4):731–741. doi: 10.1359/jbmr.2001.16.4.731 [DOI] [PubMed] [Google Scholar]
- 105.Lin S, Yang G, Jiang F, et al. A magnesium-enriched 3D culture system that mimics the bone development microenvironment for vascularized bone regeneration. Adv Sci. 2019;6(12):1900209. doi: 10.1002/advs.201900209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang S, Li R, Li D, et al. Fabrication of bioactive 3D printed porous titanium implants with Sr ion-incorporated zeolite coatings for bone ingrowth. J Mater Chem B. 2018;6(20):3254–3261. doi: 10.1039/c8tb00328a [DOI] [PubMed] [Google Scholar]
- 107.Wang XY, Gao L, Han Y, et al. Silicon-enhanced adipogenesis and angiogenesis for vascularized adipose tissue engineering. Adv Sci. 2018;5(11). doi: 10.1002/advs.201800776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang XZ, Shao JD, Abd El Raouf M, et al. Near-infrared light-triggered drug delivery system based on black phosphorus for in vivo bone regeneration. Biomaterials. 2018;179:164–174. doi: 10.1016/j.biomaterials.2018.06.039 [DOI] [PubMed] [Google Scholar]
- 109.Kuchimaru T, Kataoka N, Nakagawa K, et al. A reliable murine model of bone metastasis by injecting cancer cells through caudal arteries. Nat Commun. 2018;9. doi: 10.1038/s41467-018-05366-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Makhoul I, Montgomery CO, Gaddy D, Suva LJ. The best of both worlds - managing the cancer, saving the bone. Nat Rev Endocrinol. 2016;12(1):29–42. doi: 10.1038/nrendo.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xue Y, Niu W, Wang M, Chen M, Guo Y, Lei B. Engineering a biodegradable multifunctional antibacterial bioactive nanosystem for enhancing tumor photothermo-chemotherapy and bone regeneration. ACS Nano. 2019. doi: 10.1021/acsnano.9b06145 [DOI] [PubMed] [Google Scholar]
- 112.Raucci MG, Fasolino I, Caporali M, et al. Exfoliated black phosphorus promotes in vitro bone regeneration and suppresses osteosarcoma progression through cancer-related inflammation inhibition. Acs Appl Mater Inter. 2019;11(9):9333–9342. doi: 10.1021/acsami.8b21592 [DOI] [PubMed] [Google Scholar]
- 113.Qing YA, Cheng L, Li RY, et al. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomed. 2018;13:3311–3327. doi: 10.2147/Ijn.S165125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xiong Z, Zhang X, Zhang S, et al. Bacterial toxicity of exfoliated black phosphorus nanosheets. Ecotoxicol Environ Saf. 2018;161:507–514. doi: 10.1016/j.ecoenv.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 115.Li B, Lai C, Zeng G, et al. Black phosphorus, a rising star 2D nanomaterial in the post-graphene era: synthesis, properties, modifications, and photocatalysis applications. Small. 2019;15(8):e1804565. doi: 10.1002/smll.201804565 [DOI] [PubMed] [Google Scholar]
- 116.Miao YL, Shi XT, Li QT, et al. Engineering natural matrices with black phosphorus nanosheets to generate multi-functional therapeutic nanocomposite hydrogels. Biomater Sci. 2019;7(10):4046–4059. doi: 10.1039/c9bm01072f [DOI] [PubMed] [Google Scholar]