Abstract
Viruses still pose a significant threat to human and animal health worldwide. In the fight against viral infections, high-purity viral stocks are needed for manufacture of safer vaccines. It is also a priority to ensure the viral safety of biopharmaceuticals such as blood products. Chromatography techniques are widely implemented at both academic and industrial levels in the purification of viral particles, whole viruses and virus-like particles to remove viral contaminants from biopharmaceutical products. This paper focuses on polysaccharide adsorbents, particulate resins and membrane adsorbers, used in virus purification/removal chromatography processes. Different chromatographic modes are surveyed, with particular attention to ion exchange and affinity/pseudo-affinity adsorbents among which commercially available agarose-based resins (Sepharose®) and cellulose-based membrane adsorbers (Sartobind®) occupy a dominant position. Mainly built on the development of new ligands coupled to conventional agarose/cellulose matrices, the development perspectives of polysaccharide-based chromatography media in this antiviral area are stressed in the conclusive part.
Keywords: Polysaccharide, Chromatography, Virus particle, Biopharmaceuticals purification, Vaccine
Graphical abstract
Highlights
-
•
Chromatography has been and is still extensively implemented in virus purification/removal downstream processes.
-
•
Typical application fields are the manufacturing of purified viral vaccines and virus-free biopharmaceuticals.
-
•
Agarose and cellulose remain the primary polysaccharide bases for chromatography adsorbents in such virus-related applications.
-
•
Present R&D studies mainly focus on multimodal chromatography and affinity ligands.
1. Introduction
Whether transmitted through water, air, blood or animal vectors (insects, rodents), viral infections still remain a significant threat to life worldwide. Besides the common viral illnesses of childhood such as measles, rubella, varicella and mumps, influenza, severe acute respiratory syndrome (SARS), hemorrhagic fevers such as yellow fever, Ebola and dengue, acquired immune deficiency syndrome (AIDS) and chronic hepatitis (B, C) are some examples of severe viral diseases. Hence, the fight against viral contamination is actually a great concern, as shown by a number of prevention approaches being implemented. Among these are the routine viral monitoring of environmental and drinking waters to detect waterborne viruses such as enteric viruses (e.g., noroviruses) causing acute gastrointestinal illness and limit waterborne viral outbreaks [1]; the implementation of emerging air decontamination technologies for preventing aerosol transmission of infection in indoor environments [2]; the development of effective and robust procedures to ensure the viral safety of biologicals such as blood components and plasma derivatives during manufacturing [3]; the control of vectors such as mosquitos to reduce the potential for biting nuisance and transmission of vector-borne pathogens (e.g., dengue and yellow fever viruses) [4]. On an individual level, besides good hygiene practices, protective measures include the use of individual equipment such as facemasks to reduce the spread of airborne viruses with pandemic potential, e.g., influenza A virus (IAV), condoms to prevent the transmission of human immunodeficiency virus (HIV) during sexual intercourse, or sterile syringes and needles to reduce transmission of blood-borne viruses, e.g., HIV, hepatitis B/hepatitis C virus (HBV/HCV), among injecting drug users. Active immunization, i.e., vaccination, is an indispensable treatment to prevent viral infection [5]. While vaccines against childhood viral illnesses, influenza, polio or hepatitis A/B are routinely administered, the research for efficient and safe prophylactic vaccines against life-threatening diseases such as dengue [6] and Ebola [7] fevers, AIDS [8] and hepatitis C [9], is in continuous progress.
Polysaccharide (PS)-based materials offer significant potential in the fight against viruses considering several of the above-mentioned aspects. Virus filters made from cellulose (CEL) and its derivatives have been implemented over the past twenty years for the capture of viral pathogens from environmental water and blood product samples [10]. PS micro/nanoparticles, particularly chitosan and derivatives, have been extensively tested in viral vaccine formulations, allying immunological adjuvant and antigen carrier properties [11]. The present review examines the contribution of PS-based chromatographic adsorbents to this antiviral warfare. Associated with filtration procedures, (liquid) chromatography technologies are now widely used to reduce viral contamination of biological/biopharmaceutical products such as monoclonal antibodies (mAbs) [12] and blood products [3]. They have also become a major step in bioseparation schemes for the recovery/purification of viruses or virus-like particles, with a view to large-scale production of high-purity viral stocks needed for manufacture of safer vaccines and viral vectors for gene therapy [13,14]. In the search for improved tools against viral contamination and infections, chromatographic adsorbents based on PS – agarose (AG) and CEL, essentially, and dextran, to a lesser extent (Table 1) – have proven to be materials of choice for virus purification and virus removal. Recent developments involve both conventional packed beds and membrane adsorbers (MAs).
Table 1.
The three main polysaccharides used as base materials for chromatography adsorbents.
| Polysaccharides | Origins |
|---|---|
Agarose 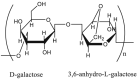 Linear polysaccharide made up from alternating d-galactose and 3,6-anhydro-alpha-l-galactopyranose residues joined by α(1 → 3)- and β(1 → 4)-linkages |
Marine red algae (Rhodophyceae) |
Cellulose  β(1 → 4)-linked d-glucose residues (cellobiose units) |
Plants (flax, cotton…) Bacterial fermentation («bacterial cellulose») (Gluconacetobacter xylinus) |
Dextran 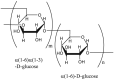 Main chains consist of α(1 → 6)-linked d-glucose residues while side chains begin from α(1 → 3) linkages |
Bacterial fermentation (Leuconostoc mesenteroides, Streptococcus mutans) |
2. Viral particle purification
The vast majority of viral vaccines manufactured at present include whole-cell vaccines in which viruses are either live attenuated or inactivated, and virus-like particles (VLPs), subunit vaccines containing specific parts of the virus with immunogenic properties [5,15]. Examples of attenuated vaccines are those against smallpox, polio (oral vaccine), measles, mumps and rubella, rotavirus gastroenteritis and yellow fever. Inactivated vaccines include rabies, polio, hepatitis A and influenza vaccines. Licensed VLP vaccines against hepatitis B, human papillomavirus (HPV) [16,17] and hepatitis E [18] infections are also currently available. Furthermore, a number of VLP vaccines are under clinical development [16,17].
The manufacturing of whole-cell vaccines, based initially on viruses grown in vivo in whole animals or in ovo in embryonated eggs, is now moving towards animal cell culture systems allowing virus propagation in vitro. Chicken embryo fibroblast (CEF) primary cells, human lung-derived Medical Research Council 5 (MRC5) diploid cells, the African green monkey kidney-derived (Vero) and Madin Darby canine kidney (MDCK) cells (continuous cell lines) are the most common cell substrates currently used for vaccine production [5,19]. VLPs can be produced in a variety of cell culture systems including microbial (bacteria and yeasts, mainly Escherichia coli and Saccharomyces cerevisiae), insect (e.g., High Five™ cells from Trichoplusiani used in the Baculovirus Expression Vector System), mammalian (e.g., Chinese hamster ovary (CHO) cells) and –to a lesser extent– plant cells [16,17]. Therefore, both whole viruses and VLPs designed for vaccine formulations are derived from complex media containing biological impurities such as cell debris and host cell (HC)-derived contaminants (e.g., proteins, DNA, endotoxins), and their downstream processing must comply with strict purity requirements [13,14,20,21] detailed in regulatory guidelines [22]. Typical downstream production processes of viral particles involve three main steps (Fig. 1). Initial clarification of the virus/VLP loaded bulk medium ensures the removal of cell debris and other large aggregates. Centrifugation and (micro)filtration techniques are most commonly utilized in this initial step. Clarification is followed by a concentration/purification step and a final polishing step that both make extensive use of a variety of chromatography techniques, in particular ion exchange, affinity, hydrophobic interaction and size exclusion chromatography. Endonuclease (e.g., Benzonase®) is eventually added to the clarified virus broth to ensure degradation of contaminant nucleic acids (HC DNA). A preconcentration step of the clarified virus broth and final concentration of the purified virus suspension using chromatography techniques are also frequently included in the process. Chromatography stages have been largely performed using PS-based packed beds and MAs operated in the positive (bind-and-elute) or negative (flow-through) mode. The efficiency of these chromatographic purification steps is usually assessed by the recovery yield (% virus recovered) and purity (only controlled or quantified as % virus in the viral product with remaining contaminants) of viral particles, with efforts made to achieve the best trade-off between these two parameters. Data published so far are highly variable with no reference parameter such as a yield vs. purity ratio that could allow an easier comparison of the purification performance, but difficult to standardize. Common recovery yields range around 50%, with purities, the prominent parameter of the compromise, frequently over 90%.
Fig. 1.
General scheme of virus downstream production processing. Adapted from Ref. [20] with permission from Elsevier.
2.1. Packed-bed column chromatography
Table 2 [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]] gathers a variety of packed-bed column chromatography procedures applied to viral particle purification in which the stationary phase consists of AG – essentially Sepharose® (“Separation-Pharmacia-AG”; GE Healthcare, Chicago, Ill.) (Seph) – or CEL – e.g., Cellufine™ (JNC Corporation, Tokyo, Japan) – gel beads, modified to fulfill varying separation modes, i.e., ion exchange, size exclusion and affinity (Table 3 [[77], [78], [79], [80], [81]]). Among these, anion exchange (AE) has been most frequently implemented for virus purification over the past decades [82], mainly in association with other chromatographic steps. A few examples of virus/VLP purification by expanded bed chromatography using AG-based adsorbents are also mentioned in Table 2.
Table 2.
Column chromatography procedures using PS-based materials for viral particle purification [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]].
| Chromatographic purification step (s)a | Stationary phaseb | Target viral particlesc | Virus production systemd | Ref. |
|---|---|---|---|---|
| AEC | DEAE Seph FF, Q Seph XL | (Mo)MLV derived vector | TE fly A7 | [23] |
| Q Seph XL | Norovirus VLPs | Sf9 | [24] | |
| AEC (EBAC) | Streamline Q XL | AdV-5-derived vector | HEK-293 | [25] |
| Streamline DEAE | HBV VLPs | Escherichia coli | [26] | |
| CEC | P11 phosphocellulose | HPV VLPs | Saccharomyces cerevisiae | [27,28] |
| SEC | Sephacryl™ S-500 | Rotavirus VLPs | Sf9 | [29] |
| Seph CL-4B | rBV vector | Sf9 | [30] | |
| Superdex™ 200, Sephacryl S-1000 | rBmNPV | Silkworm larvae | [31] | |
| Seph 6 FF | EV71 vaccine strain | Vero | [32] | |
| Seph 4 FF | CSFV | PK-15 | [33] | |
| SEC (SMBC) | Seph 4 FF | IAV (H1N1) | MDCK | [34] |
| Seph 4 FF | AdV-5 | HEK-293 | [35] | |
| AFC | HEP Seph HP | PRRSV | MARC-145 | [36] |
| HEP Seph HP | HPV VLPs | S. cerevisiae | [27,28] | |
| AVB Seph HP | rAAV | Sf9 | [37] | |
| ConA Seph 4B | rAcMNPV | HeLa | [38] | |
| pAFC | Cellufine™ sulfate | MVA | CEF | [39] |
| WNV and WNV VLPs | Vero CHO (VLPs) |
[40] | ||
| Alphavirus VRPs for IAV (H3N2) | Vero | [41] | ||
| IAV and IBV | Embryonated chicken eggs | [42] | ||
| IAV and IBV | MDCK | [43] | ||
| Dengue virus | C6/36 | [44] | ||
| Alphavirus VRPs for CMV | Vero | [45] | ||
| IMAC | Ni-NTA AG | rAAV | AAV-293 | [46] |
| Ni-NTA AG | FMDV | BHK-21 | [47] | |
| Ni Seph 6FF | NiV VLPs | E. coli | [48] | |
| Ni Seph HP | NiV VLPs | Pichia pastoris | [49] | |
| Ni Seph HP | HBV VLPs | E. coli | [50] | |
| IMAC (EBAC) | Streamline chelating | HBV VLPs | E. coli | [51] |
| HIC | Butyl Seph 4FF | FMDV | BHK-21 | [52] |
| Phenyl Seph 6FF | NiV VLPs | E. coli | [53] | |
| HIC (EBAC) | Streamline phenyl | NiV VLPs | E. coli | [54] |
| AEC + SEC | Q Seph XL + Seph 4 FF | AdV-5 | HEK-293 | [55] |
| SEC + AEC | Seph 4 FF + Q seph XL | IAV (H1N1) | MDCK | [56] |
| Seph4 FF + Q Seph HP | PRRSV | MARC-145 | [57] | |
| Seph Cl–6B + DEAE Seph FF | Poliovirus | Vero | [58] | |
| WorkBeads™ 40/10000 + Macro-Prep® High Qe | AdV VLPs | Sf21 | [59] | |
| SEC + CEC | Sephacryl S-1000 + P11 phosphocellulose | HPV VLPs | S. cerevisiae | [60,61] |
| CEC + AEC + SEC | Mustang® SXT/QXTf + Superdex 200 | rAAV | HEK-293 | [62] |
| HIC + SEC | Butyl Seph 4FF + Superdex 200 | FMDV | BHK-21 | [52] |
| AEC + HIC | Capto Q + Toyopearl PPG-600Mg | IAV and IBV | MDCK | [63] |
| AEC + pAFC + SEC | DEAE Seph FF + Cellufine sulfate + Seph 6 FF | IAV (H1N1) | Vero | [64] |
| SEC + AEC + CEC + HIC | Q + SP Seph Big Beads | IAV and IBV | MDCK | [65] |
| MMC: SEC + AEC | Q SepFast™ InertShell and InertLayer 1000 SQ |
HBV VLPs | E. coli | |66] |
| MMC: SEC + AEC + HIC (CC700-MMC) | Capto™ Core 700 (CC700) | IAV (H3N2) | Embryonated chicken eggs | [67] |
| Reovirus | L929 | [68] | ||
| Respiratory syncytial virus | Vero | [69] | ||
| AEC + CC700-MMC | Capto Q + CC700 | IAV and IBV | MDCK | [70] |
| IAV (H1N1) | Vero | |71] | ||
| IAV (H5N1, H7N9) | MDCK, Vero | [72] | ||
| CC700-MMC + AEC | CC700 + Capto Q ImpRes | HPV VLPs | Sf9 | [73] |
| CC700-MMC + SEC | CC700 + Superdex 200 | IAV VLPs | E.coli | [74] |
| CC700-MMC + AFC | CC700 + Capto HEP | HIV-1 VLPs | HEK-293 | [75] |
| CC700-MMC + AEC + HIC | CC700 + Capto Adhere + Capto Butyl | EV71 VLPs | Sf9 | [76] |
AEC, anion-exchange chromatography; AFC, affinity chromatography; CEC, cation-exchange chromatography; EBAC, expanded-bed adsorption chromatography; HIC, hydrophobic interaction chromatography; IMAC, immobilized metal affinity chromatography; MMC, mixed mode (multimodal) chromatography; pAFC, pseudo-affinity chromatography; SEC, size exclusion chromatography; SMBC, simulated moving bed chromatography.
See Table 3 for a brief description.
rAAV, (recombinant) adeno-associated virus; rAcMNPV, (recombinant) Autographa californica multicapsid nucleopolyhedrovirus (baculovirus); AdV-5, adenovirus type 5; rBmNPV, (recombinant) Bombyx mori nucleopolyhedrovirus; rBV, (recombinant) baculovirus (derived from AcMNPV); CMV, cytomegalovirus; CSFV, classical swine fever virus; EV71, enterovirus 71; FMDV, foot-and-mouth disease virus; HBV, hepatitis B virus; HIV-1, human immunodeficiency virus type 1; HPV, human papillomavirus; IAV/IBV, influenza A/influenza B virus; (Mo)MLV, (Moloney) murine leukaemia virus; MVA, modified vaccinia Ankara virus; NiV, Nipah virus; PRRSV, porcine reproductive and respiratory syndrome virus; VRP, virus-like replicon particle.
AAV-293, HEK-293 optimized for the packaging of AAV virions; BHK-21, baby hamster kidney; C6/36, Aedes albopictus (tiger mosquito); CEF, chicken embryo fibroblast; CHO, Chinese hamster ovary; HEK-293, human embryonic kidney (transformed with sheared adenovirus type 5 DNA); HeLa, (Henrietta Lacks) human cervical cancer; L929, mouse fibroblast; MARC-145, (Meat Animal Research Center 145) monkey kidney; MDCK, Madin Darby canine kidney; PK-15, porcine kidney; Sf21, Spodoptera frugiperda (fall armyworm) ovaries (IPLB-SF21-AE); Sf9, Spodoptera frugiperda (derived from the parental Sf21 cell line); TE fly A7, drosophila cell line optimized for retroviral vector packaging (derived from the TE-671 human rhabdomyosarcoma cell line); Vero, African green monkey kidney.
Methacrylate-based Q beads (Bio-Rad, Hercules, Calif.).
PES-based strong CE/AE membrane adsorbers (Pall Corporation, Port Washington, NY, USA).
Polymethacrylate beads bonded with polypropylene glycol groups (Tosoh, Tokyo, Japan).
Table 3.
PS-based stationary phases used in column chromatography for virus purification or viral clearance [[77], [78], [79], [80], [81]].
| Adsorbents sorted by chromatography mode | Commercial names | Brief description |
|---|---|---|
| Base/SEC matrices | Sepharose® (Seph) | Purified AG with very few residual charged PS (GE Healthcare, Chicago, Ill.) |
| Seph CL-4B/-6B | 4%/6% cross-linked AG (CLA) | |
| Seph 4 FF/6 FF (Fast Flow) | 4%/6% CLA with improved pressure/flow characteristics | |
| Seph HP (High Performance) | High CLA (6% AG) | |
| Seph XL | High CLA (6% AG) with dextran surface extenders | |
| Capto™ | Very rigid, high CLA with an optimized pore structure improving pressure/flow properties (GE Healthcare) | |
| Superdex™ 200 | Composite matrix of dextran covalently bounded to high CLA (GE Healthcare) | |
| Sephacryl™ S-500 HR (high resolution) Sephacryl™ S-1000 SF (superfine) |
Beads of allyl dextran cross-linked with N,N′-methylene-bis-acrylamide {poly([allyl dextran]-co-N,N′-methylenebisacrylamide)} (GE Healthcare) | |
| Streamline™ | 6% CLA containing a quartz core (to provide the required high density for stable bed expansion) (GE Healthcare) | |
| WorkBeads™ 40/10000 | 5% AG beads (10,000 kD exclusion limit) manufactured using a proprietary method (Bio-Works, Uppsala, Sweden) [77] | |
| CEC adsorbents | CM Seph FF | Seph 6 FF with carboxymethyl (CM) weak CE groups |
| SP Seph FF | Seph 6 FF with sulphopropyl (SP) strong CE groups | |
| SP Seph Big Beads | Large 6% CLA beads (100–300 μm) modified with SP groups | |
| Capto S | Capto matrix with dextran surface extenders bearing sulfonate (S: SO3-) strong CE groups | |
| P11 phosphocellulose | Ammonium CEL phosphate bifunctional cation exchanger with ester-linked orthophosphate groups (Whatman/GE Healthcare) | |
| AEC adsorbents | DEAE Seph FF | Seph 6 FF with diethylaminoethyl [DEAE: N+(C2H5)2H] weak AE groups |
| Streamline DEAE | Streamline matrix with DEAE groups | |
| Q Seph FF | Seph 6 FF with quaternary ammonium [Q: –N+(CH3)3] strong AE group | |
| Q Seph HP | Seph HP modified with Q groups | |
| Q Seph XL | Seph XL modified with Q groups | |
| Q Seph Big Beads | Large 6% CLA beads (100–300 μm) modified with Q groups | |
| Capto Q | Capto matrix with dextran surface extenders bearing Q groups | |
| Capto Q ImpRes | High-flow AG matrix functionalized with Q groups | |
| Streamline Q XL | Streamline matrix with dextran surface extenders bearing Q groups | |
| Q SepFast™ InertShell Q SepFast™ Inert Layer 1000 |
Cationized (Q ligand) AG particles coated with an inert (ligand free) AG layer, differing by the thickness of the inert shell (BioToolomics Ltd, Consett, UK) [66] | |
| SQ | 6% CLA cationized with glycidyltrimethylammonium chloride (GTMAC) and further grafted with inert, non-crosslinked short polymer chains [poly(oligo(ethylene glycol) methacrylate)] [78] | |
| AFC adsorbents | AVB Seph HP | Seph HP to which the ligand (a 14 kD recombinant protein derived from heavy chain camelid antibodies and expressed in Saccharomyces cerevisiae) is attached covalently via an amide linkage |
| ConA Seph 4B | 4% AG (Seph 4B) with concanavalin A (a tetrameric metalloprotein isolated from Canavalia ensiformis) ligand | |
| HEP Seph HP | Seph HP with covalently bound heparin (HEP) | |
| MabSelect SuRe™ | Rigid, highly CLA with alkali-tolerant, protein A-derived ligand (epoxy coupling) (GE Healthcare) | |
| Protein A Seph 4 FF | Seph 4 FF matrix with protein A immobilized by the CNBr method | |
| Protein G Seph (4 FF) | Seph (4 FF) matrix with protein G immobilized by the CNBr method | |
| Capto HEP | Capto matrix with HEP ligand | |
| VIIISelect | Capto matrix to which a factor VIII-selective, camelid-antibody-derived ligand is attached via a hydrophobic spacer arm [79] | |
| pAFC ligands | Cellufine® sulfate | CEL beads functionalized with a low concentration of sulfate esters (JNC Corporation, Tokyo, Japan) [80] |
| IMAC adsorbents | Ni Seph 6 FF | Seph 6 FF to which a proprietary metal-chelating group has been coupled and charged with Ni2+ ions |
| Ni Seph HP | Seph HP to which a proprietary metal-chelating group has been coupled and charged with Ni2+ ions | |
| Ni-NTA AG | Seph 6 FF derivatized with nitrilotriacetic acid (NTA) (metal-chelating agent) and loaded with Ni2+ ions | |
| Chelating Seph FF | Seph 6 FF derivatized with iminodiacetic acid (metal-chelating agent) | |
| Streamline chelating | Streamline matrix conjugated to iminodiacetic acid groups through ether linkages and hydrophilic spacer arm | |
| HIC adsorbents | Butyl Seph 4 FF | Seph 4 FF with hydrophobic butyl ligand |
| Capto butyl | Capto matrix with butyl ligand | |
| Phenyl Seph 6 FF | Seph 6 FF with hydrophobic phenyl ligand | |
| Phenyl Seph Big Beads | Large 6% CLA beads (100–300 μm) modified with phenyl ligand | |
| Capto phenyl | Capto matrix with phenyl ligand | |
| Streamline phenyl | Streamline matrix with phenyl ligand | |
| MMC adsorbents | Capto MMC | Capto matrix with a multimodal, weak CE ligand containing in particular negatively charged (carboxylic) and hydrophobic (phenyl) groups [81] |
| Capto adhere | High CLA with N-benzyl-N-methylethanolamine as a strong AE ligand exhibiting various functionalities for interaction (ionic, hydrophobic, hydrogen bonding) [81] | |
| Capto Core 700 (CC700) | Porous beads of highly CLA whose core is activated with octylamine ligand (both hydrophobic and positively charged) and shell is inactive [81] |
2.1.1. Ion-exchange chromatography (IEC)
Anion-exchange chromatography (AEC) involves the adsorption of viral particles, whose surface is negatively charged at near-neutral pH, to positively charged chromatographic supports, i.e., strong or weak anion exchangers carrying positively charged functional groups (quaternary ammonium (Q) or diethylaminoethyl groups (DEAE)). Adsorbed viral particles are usually eluted in high salt buffer (around 1 M NaCl) contributing to elution via charge screening. In the studies quoted in Table 2, AEC based on Seph anion exchangers provides high purity (>95%, based on SDS-PAGE analysis) VLPs as vaccine candidates [24] and viral vectors designed for gene delivery with high infectious particle yields [23]. The use of expanded-bed adsorption chromatography (EBAC) with AG-based anion exchangers is also illustrated. EBAC was proposed initially to simplify downstream purification processes of target products from particulate-containing biological feedstocks by combining clarification, concentration and product capture chromatography in a single step [83]. EBAC has been applied with some success to the initial purification of adenoviral vectors [25] and hepatitis B core antigen (HBcAg) protein [26] from crude virus suspensions with Q and DEAE AG ligands, respectively. Adenovirus (AdV)-based vectors are widely used as vaccine vectors against infectious (bacterial and viral) diseases and cancer [84]. EBAC of unclarified culture bulk supplemented with Benzonase yields 45% recovery and 5-fold concentration of adenoviral vectors whose purity compared to that obtained with the classical density gradient ultracentrifugation method [25]. HBcAg self-assembles into icosahedral VLPs which have also promising applications as vaccine platform [85], in particular as therapeutic vaccine for chronic hepatitis B [86]. The recovery yield of HBcAg from heat-treated, unclarified E. coli homogenate is about 50% with a purity (ratio of HBcAg content to total protein content) of c. 0.5 and a purification factor (ratio of HBcAg purity in EBAC eluate to that in the feedstock) of c. 2 [26]. The antigenicity of recovered HBcAg is preserved. So far, EBAC has limited commercial development [83]. Note, however, that an AE-EBAC method for capturing influenza (containing hemagglutinin (HA)) VLPs from tobacco plant cells using particularly DEAE PS (CEL, AG, dextran) beads with an inert core has been patented by Philip Morris Products S.A. (Neuchatel, Switzerland)– a shareholder of Medicago Inc. (Québec, Canada), a company developing plant-based technologies to produce antiviral vaccines [87].
Cation exchange (CE) chromatography (CEC) resins retain viral particles through ionic interactions between negatively charged functional groups of the stationary phase (e.g., sulfonate and phosphate groups) and positively charged (amino) groups of viral surface proteins – generally under weakly acidic conditions. Since, however, most viruses, including human viruses, have isoelectric points below 6 and are sensitive to low pH [13], CEC is seldom applied to the purification of viral particles. Some isolated studies by Kim et al. [27,28] using PS-based chromatography media concern the purification of the major capsid protein L1 of HPV type 16 that self-assembles into VLPs (Ref. [88] for a review on papillomavirus assembly). HPV (16 and 18) L1 VLPs, highly immunogenic, are the main component of manufactured prophylactic vaccines against HPV infection [89] – a major risk factor in the development of cervical and other anogenital cancers [90]. The CEC-based purification process ensures 60% recovery of L1 protein while removing most contaminating proteins – note that c. 80% of those were eliminated by precipitation prior to the chromatography step [27]. L1 protein purity was increased by more than 300-fold compared to the cell lysate. Viral clearance for biopharmaceutical safety is actually a more investigated application field of CEC, as will be illustrated further on (Section 3.1.1).
2.1.2. Size exclusion chromatography (SEC)
Size exclusion chromatography (SEC) exploits the large size of viruses (20–30 nm size for parvoviruses and picornaviruses, the smallest ones) compared to cellular and medium contaminants. Viral particles are excluded from the internal pores of the non-adsorbing packing material in the column and migrate faster than smaller contaminants, retained by matrix pores. Some years ago, dextran-based adsorbents from GE Healthcare were commonly applied to the SEC purification of a variety of viruses or VLPs: Sephacryl™ resins with varying fractionation ranges [[91], [92], [93], [94], [95]], Superdex™ [[96], [97], [98], [99]]. More recent works quoted in Table 2 also make use of these dextran-based resins for SEC, often associated with other chromatography steps. However, AG-based media have been most frequently used.
Like AEC, SEC has been successfully applied to the purification of gene delivery vectors [30,31] and VLPs for vaccine use [29]. In the pilot-scale evaluation of a manufacturing process for a vaccine candidate against Enterovirus 71 (EV71) infection, Chang et al. [32] used SEC for downstream purification of crude EV71 bulks derived from infected Vero cell cultures. The chromatographic step removed most (>99%) protein contaminants from four viral stocks with some inconsistencies in virus recovery yield among the four tests, however. In addition, the residual DNA level in vaccine bulk complied with the current guidelines for human vaccines. After chemical inactivation, purified viruses were found immunogenic in animal models, inducing strong virus neutralizing antibody responses in mice and rabbits. Lately, Wang et al. [33] applied the same process, i.e., SEC preceded by tangential-flow ultrafiltration (UF), to the purification of cell culture-derived classical swine fever virus, with similarly positive results in terms of virus recovery, purity and infectivity.
Some purification procedures have exploited the complementarity between SEC and AEC, i.e., SEC efficiency at removing small-sized impurities (but less at separating viral particles from larger impurities such as genomic HC DNA fragments) and AEC ability to discriminate between negatively charged species (e.g., viruses, HC DNA and proteins) by acting on the pH/ionic strength of the mobile phase (elution buffer essentially). For instance, a two-step chromatographic procedure, i.e., SEC followed by AEC, is currently applied for poliovirus purification in the routine downstream processing of inactivated polio vaccine [58]. Kalbfuss et al. [56] and, more recently, Bohua et al. [57] followed the same chromatographic scheme to purify cell culture-derived IAV and porcine reproductive and respiratory syndrome virus (PRRSV), respectively. In the former work, however, the AEC column was used in flow-through mode to remove HC DNA from SEC-purified IAV concentrate: DNA adsorbed to the Q Seph XL stationary phase while viruses were collected in flow through. Purification resulted in significant reduction in total protein (>19-fold) and HC DNA (>500-fold) contents of the treated viral suspension, which is yet insufficient to fulfill the current purity standards for whole virus human influenza vaccines. Szurgot et al. [59] replaced sucrose density gradient ultracentrifugation with SEC as the first purification step of adenoviral dodecahedron VLPs from insect cell lysates, which improved significantly the recovery yield of VLPs. Purification was completed by AEC using a synthetic Q adsorbant, providing the basis for a shorter and more cost-effective, and fully chromatographic two-step procedure.
Eglon et al. [55] have reported the production of high-quality AdV vectors, suitable for clinical development, combining AEC and SEC purifications. Benzonase treatment was applied before chromatography. They obtained better purity with AEC-SEC than with SEC-AEC. Before focusing on the single-step chromatographic purification of HPV L1 VLPs via CEC (or AFC: see below) [27,28], Kim et al.[60] and Park et al. [61] have implemented a two-step purification process where SEC was followed by CEC [60,61]. HPV 16 L1 VLPs purified in this way were immunogenic in mice [60]. Purification was effective but the recovery yield was very low when ultracentrifugation of the yeast lysate was used as a first purification step preceding SEC [60,61]. Substituting ultracentrifugation with ammonium sulfate precipitation strongly decreased the loss in VLPs during cell lysate pre-treatment [61]. In these conditions, the whole process ensured 30% recovery of approximatively 200-fold purified VLP-forming protein [61] – which is lower than what was obtained later using single-step CEC [27]. Another means to avoid the density gradient ultracentrifugation step needed by conventional cell lysate pre-treatment is to produce and purify viral particles from cell culture supernatant. This strategy was followed by Tomono et al. [62] who implemented an ultracentrifugation-free, purely chromatography-based technique for purification of rAAV. Applying a dual CEC/AEC step (using poly(ethersulfone) (PES)-based MA) followed by SEC on the dextran-based Superdex 200 led to highly purified gene delivery vectors.
Countercurrent simulated moving bed (SMB) technology has been implemented to improve the performance of chromatographic separations in the downstream processing of biopharmaceutical products, replacing single-column discontinuous steps with continuous multicolumn processes [100,101]. To date, biopharmaceuticals targeted by SMB chromatography (SMBC) are mainly proteins and mAbs. Nevertheless, SEC-SMBC has been recently applied to purification of cell culture-derived IAV [34] and human AdV type 5 (AdV-5) [35] using columns packed with Seph 4 FF resin. Particularly promising results were obtained by Nestola et al. [35] with a two-column setup yielding higher virus recovery and purity (HC DNA and protein clearance) than the batch (single-column) SEC process with the same amount of stationary phase. Both studies claimed an increased productivity compared to the batch mode – a key feature in the development of continuous downstream bioprocessing [101].
2.1.3. Affinity chromatography (AFC)
Affinity chromatography (AFC) is based on the bioselective adsorption of a compound to an immobilized ligand, generally bound to the packing material via a spacer arm. The adsorbed compound is eluted either by pH and/or ionic strength shift or by competitive displacement. Heparin (HEP) is widely used in AFC as ligand for protein isolation and purification. HEP is structurally related to heparan sulfate (a highly sulfated glycosaminoglycan), which is ubiquitously present on mammalian cell surfaces and serves as receptor for virus attachment and entry into HCs [102]. Hence, HEP can bind to viral particles. Displaying antiviral activity in solution – in particular against HIV [103], HEP can also serve as AFC ligand in a viral targeting approach. HEP-Seph AFC columns, in which HEP is covalently coupled to cross-linked AG beads, have been applied to the purification of whole viruses from cell culture [36] and VLPs from yeast culture [27,28]. HEP AFC was associated with an UF concentration step to recover PRRSV from clarified cell lysate [36]. The chromatography step removed 95% of proteins and captured 53% of viruses present in the UF retentate. By elution at two different ionic strengths, 27.5% and 25.4% of PRRSV loaded onto the column could be recovered with an increase in purity of respectively c. 6 and 120 compared to the retentate. HEP AFC is comparable to CEC in their ability to purify the VLP-forming HPV 16 L1 protein [27]. However, HEP AFC-purified VLPs show higher immunogenicity in mice than CEC-purified ones [28]. Other affinity ligands such as camelid antibody fragments [37] or concanavalin A [38] have been used to purify recombinant viral vectors intended for cellular and gene therapy, i.e., with no antiviral objective, sensu lato.
2.1.4. Pseudo-affinity chromatography (pAFC)
Like HEP, CEL sulfate (a semi-synthetic product) has well-documented – but controversial – antiviral activity against HIV [104], mimicking HEP affinity for a wide range of proteins. “Pseudo-affinity” (“pseudobiospecific ligand”) chromatography (pAFC) [105] media based on CEL sulfate was patented by Kaketsuken (the Chemo-Sero Therapeutic Research Institute, Kumamoto, Japan) at the end of the 1980s to purify influenza virus [106], Japanese encephalitis virus [107], and rabies virus [108]. Since then, CEL sulfate beads have been extensively implemented as packing material for pAFC in a number of virus purification processes. The commercial product Cellufine™ Sulfate (JNC Corporation), which is produced by the functionalization of CEL beads [109] with a low concentration of sulfate esters [80], has been shown to be effective for virus purification with a view to improving production processes of vaccines, in particular against flavivirus infections [110]. Thus, cellufine sulfate chromatography is the polishing step of Vero cell-derived Japanese encephalitis virus in the production scheme of inactivated Japanese encephalitis vaccine [111], commercialized as Encevac® (Kaketsuken, Kumamoto, Japan) and tested in recent years in phase III clinical trial [112,113]. A final cellufine sulfate chromatographic step was also applied in the purification of XRX-001, a whole-virus, inactivated yellow fever vaccine candidate produced in Vero cell cultures and adjuvanted by alum (aluminum hydroxide) [114]. Originally manufactured by Xcellerex (Marlborough, Mass.), XRX-001 has been tested in phase I clinical trial [115] and is now developed by Pnuvax (Montreal, Canada). In Table 2 are quoted two studies that detail the performance of cellufine sulfate column chromatography in purifying West Nile (VLPs and virions) [40] and dengue [44] viruses, two other members of the Flaviviridae family. Ohtaki et al. [40] captured infectious West Nile virions from a (Vero cell) culture supernatant with high recovery yields (up to 93%). The concentration of contaminant proteins in the purified dengue virus suspension obtained by Kanlaya and Thongboonkerd [44] was 6 times lower than in the virus stock but infectivity (PFU number/mg protein) was only doubled due to poor virus recovery. In addition to flavivirus vaccines, cellufine sulfate-based pAFC has been used to purify (a) cytomegalovirus, (b) smallpox and (c) influenza vaccines.
(a) AVX601 is an alphavirus-based vectored vaccine against human cytomegalovirus (CMV), developed originally by Alphavax (Research Triangle Park, NC, USA) and currently by GlaxoSmithKline (Brentford, UK). This two-component vaccine consists of alphavirus (Venezuelan equine encephalitis virus) virus-like replicon particles (VRPs) [116] expressing CMV glycoprotein B (gB) or a pp65/IE1 (major tegument protein/immediate-early 1 protein) fusion protein. Reap et al. [45] have purified by cellufine sulfate chromatography the two types of alphavirus VRPs grown in Vero cells. After pAFC, VRP preparations have low residual protein (<1 μg/108 IU (infective unit) dose) and DNA (<1 ng/108 IU dose) concentrations with overall process recovery yields in the range 30%–40%. The AVX601 vaccine purified in this way has undergone promising phase I clinical trial [117], but plans for phase II studies are unknown at this time [118].
(b) MVA-BN® is the third-generation smallpox vaccine of Bavarian Nordic (Kvistgaard, Denmark) based on modified vaccinia Ankara virus (MVA, an attenuated, non-replicating strain of the poxvirus chorioallantois vaccinia virus Ankara) [119]. MVA-BN® is commercialized under the trade names IMVAMUNE® (Canada) and IMVANEX® (EU). Wolff et al. [39] investigated the performance of cellufine sulfate resin in capturing CEF cell-derived MVA-BN virus particles from homogenized and clarified suspensions, provided by the manufacturer as liquid-frozen samples. Bead-based pAFC yielded overall virus recovery (59%) and contaminant depletion (7% double stranded (ds) DNA recovery in the product fraction and 102% protein recovery in the flow-through fraction) comparable to those of a sulfated CEL MA (SCMA) (see details in Section 2.2). The immunogenicity and safety of IMVAMUNE have been widely tested in phase I ([119] and references therein) and, more recently, phase II (e.g., Refs. [[120], [121], [122]]) clinical trials. Liquid-frozen (most frequently) or freeze-dried formulations of IMVAMUNE were tested, with, however, no mention of an additional pAFC-based purification step of vaccine samples.
(c) The production of safe and effective influenza vaccines, either egg-based or cell-based, is a major goal of the pharmaceutical industry [[123], [124], [125]]. While most currently licensed influenza vaccines are derived from embryonated eggs [124,125], cell culture technology [19] is an emerging approach in influenza vaccine manufacturing [[123], [124], [125]]. MDCK and, to a lesser extent, Vero are the main cell lines used for influenza vaccine production [123]. The work by Kalbfuss et al. [56] coupling Seph-based SEC and AEC (in flow-through mode) to purify IAV propagated in MDCK cells has been detailed earlier. Not surprisingly, cellufine sulfate pAFC has also been used to purify influenza viruses designed for vaccine formulation from allantoic fluid (derived from fertilized eggs) or cell culture media. Column pAFC with CEL sulfate ester as the affinity medium was initially patent protected for the purification of influenza viruses from allantoic fluid [106]. More recently, the chromatographic step using commercial cellufine sulfate has showed efficiency in decreasing the total amount of proteins in samples of egg-grown human and avian influenza viruses (diverse IAV and influenza B (IBV) strains) [42]. Mainly egg proteins were eliminated since most proteins co-eluted with viral particles had viral origins. However, R&D issues are principally related to the purification of cell culture-derived viruses. Some twenty years ago, Palache et al. [126], from Solvay Pharmaceuticals B.V. (Weesp, the Netherlands), produced an influenza subunit vaccine in MDCK cells. Intact H1N1 viruses were isolated from the cell culture medium by cellufine sulfate pAFC before processed into an inactivated surface antigen vaccine. Tested in clinical trials, the resulting preparation showed safety and efficiency equivalent to the existing egg-based commercial vaccine (Influvac®). This cell-based vaccine was licensed in 2001 in the Netherlands under the name Influvac® TC. The R&D pipeline of Solvay Pharmaceutical (acquired by Abbott Labs [North Chicago, Ill, USA] in 2009) still contained Influvac® TC in 2008 [127]. However, the vaccine was not commercially distributed later on. To develop a cell-based live attenuated influenza vaccine (LAIV) manufacturing process, scientists from MedImmune (Gaithersburg, Md.) (a subsidiary of AstraZeneca, Cambridge, UK) showed that MDCK cells are a suitable production substrate [128] that can be grown on microcarriers in disposable bioreactors allowing the large-scale production of seasonal flu vaccine [129]. MedImmune patented a purification method of MDCK cell-grown influenza viruses using a cellufine sulfate column (together with Benzonase treatment) that removed efficiently MDCK cell DNA from clarified and concentrated virus harvests [130]. The resulting cell-based LAIV would be in a preclinical phase of development [124]. An example of cellufine sulfate utilization to purify Vero cell culture-derived influenza vaccine is given by He et al. [64]. They associated three chromatographic separation modes in the downstream processing of (inactivated) IAV H1N1 virus from Vero cells. pAFC was preceded by AEC (flow-through mode) and followed by SEC. The overall scheme resulted in high recovery of viral (HA) activity with efficient removal of contaminating DNA and proteins. HC DNA was mainly removed by AEC-pAFC (reduction factors of 40 and 223, respectively) while most HC proteins were eliminated by pAFC-SEC (reduction factor of c. 13 for each chromatographic step). The total protein and DNA contents in the final product, i.e., 89 μg protein and 33 pg DNA per normal vaccine dose (15 μg HA), complied with the World Health Organization (WHO) recommendations for inactivated, single strain influenza vaccines [131].
Apart from whole-cell vaccines, researchers at Alphavax produced VRPs expressing HA and neuraminidase from IAV (H3N2) [41]. Like CMV-targeted alphavirus VRPs [45], these VRPs expressing influenza antigens, produced in Vero cells, were efficiently purified by pAFC using a cellufine sulfate column. The vaccine was tested for immunogenicity in animals, more particularly in swine [132] (see also [116,133] and references therein), but not in humans. In fact, most human clinical trials of virus-vectored influenza vaccines use poxvirus and AdV vectors [134].
2.1.5. Immobilized metal affinity chromatography (IMAC)
Immobilized metal (pseudo)affinity chromatography (IMAC) is essentially a protein purification technique based on the affinity of transition metal ions such as Zn2+, Cu2+, Ni2+, and Co2+ towards certain amino acid residues, e.g., histidine (His) and cysteine (see Refs. [135,136] for details). IMAC has been used to purify His-tagged nucleocapsid (N) [48] and matrix (M) [49] proteins of Nipah virus (NiV) expressed in E. coli and Pichia pastoris, respectively. N and M proteins of NiV spontaneously assemble into VLPs with potential applications in the serological diagnosis of NiV infection. In the same way, Yap et al. [50,51] used IMAC to purify the His-tagged VLP-forming HBcAg protein. IMAC was operated in a classical fixed-bed [50] or an expanded bed [51] mode to recover HBcAg from clarified or unclarified E. coli cell lysates, respectively. Scarce examples of IMAC application to whole virus purification concern His-tagged recombinant viral vectors for gene delivery [46,137] and foot-and-mouth disease virus (FMDV) for vaccine formulation [47].
2.1.6. Hydrophobic interaction chromatography (HIC)
Hydrophobic interaction chromatography (HIC) is based on the interaction between the chromatographic support functionalized with moderately hydrophobic ligands such as propyl, butyl, octyl or phenyl groups and hydrophobic regions of adsorbates. HIC has been mainly operated in the negative (flow-through) mode as a polishing step in the purification process of proteins and antibodies, ensuring the removal of both product related impurities such as aggregates, and process contaminants such as HC proteins [138]. Column HIC with AG-based resins (Seph) has been used as virus reduction step in biomolecule purification processes, as detailed hereinafter. HIC has been occasionally applied to virus purification in the bind-and-elute mode, using columns packed with hydrophobic polymethacrylate beads [139,140] or, as concerns PS chromatography media, butyl Seph beads [52]. In this work, HIC ensured 89% FMDV recovery from the crude virus preparation and increased by 7.2-fold the virus/protein ratio in eluted samples compared to the load. An additional polishing step by SEC, applied to HIC-purified, UF-concentrated virus samples, improved the purification performance in terms of residual HC proteins and DNA with no significant loss in virus recovery. Chong et al. [53,54] also used HIC with Seph-based hydrophobic resins to purify the VLP-forming N protein of NiV from clarified [53] or unclarified [54] E. coli homogenates – similar to what the same team did for HBcAg purification by IMAC [50,51]. Iyer et al. [65] associated phenyl Seph beads (HIC) with Q (AEC) and SP (CEC) Seph beads in the same column to improve flow-through purification of influenza virus from clarified cell culture supernatant. To improve the flow-through recovery of viruses, they used beads with increased mean size (“Big Beads”, 200-μm diameter) which minimized the external surface area available for virus adsorption. Hence, their process – patented [141] – was a combination of SEC and binding chromatography operated in different modes. High virus recovery (70%–80%) and total protein removal (80%–85%) were achieved, but the flow-through fraction still contained residual DNA, a virus-sized contaminant – a well-identified limitation of negative chromatography in its application to viral particle purification [14].
2.1.7. Mixed mode chromatography (MMC)
In mixed mode (multimodal) chromatography (MMC), compounds of interest in the mobile phase interact with the stationary phase materials through a combination of binding modes including ion exchange, hydrogen bonding, and hydrophobic interactions [142]. MMC methods have existed since the 1950s with hydroxyapatite chromatography combining CE and metal affinity [143]. Although not a new idea, MMC is being increasingly developed for pharmaceutical/biopharmaceutical applications owing to the new generation of mixed-mode stationary phases and a better understanding of multimodal interactions [142]. Most particle-based MMC media reported over recent years are based on silica gels [144] implemented for separation purposes. However, several beaded AG supports have been recently applied to the purification of viruses or VLPs in the negative mode. The Q SepFast™InertShell and InertLayer 1000 (BioToolomics Ltd, Consett, UK) adsorbents consist of highly cross-linked AG beads which are quaternary ammonium cationized with glycidyltrimethylammonium chloride (GTMAC) and coated with an inert (ligand free) AG layer, differing by the thickness of the inert shell (30 μm and 5 μm, respectively) [66]. In the same way, Capto™ Core 700 (CC700) (GE Healthcare) particles are composed of a ligand-activated AG core and an inactive AG shell. Here, the ligand is octylamine, which is both hydrophobic and positively charged [81]. In both materials, the inert shell excludes large molecules and nanoparticles such as viruses and VLPs while smaller impurities bind to the internalized ligands in the funnel-shaped pores of the core (Fig. 2). Target viruses/VLPs are collected in the column flow through. Thus, the purification step combines size separation (SEC) and binding chromatography (IEC or IEC/HIC).
Fig. 2.
Schematic cross-sectional view of a Capto Core 700 bead showing the inactive (nonfunctionalized) shell and the ligand-activated core with its porous network. Small protein impurities can enter the resin pores and bind to the ligand. The inactive shell excludes large molecules and molecular entities such as virus particles that are collected in the flow through (cut-off c. 700 kDa). Extrated from Ref. [142] with permission from Elsevier.
Lee et al. [66] have investigated the purification of HBV VLPs (hepatitis B core antigen particles) from clarified E. coli feedstock by negative chromatography using the core-shell Q adsorbents from BioToolomics. Increasing the thickness of the inert shell (Q SepFast InertShell vs. InertLayer 1000) improved the recovery but decreased the purity of VLPs in the flow-through fraction, as more VLPs and impurities (HC proteins) were excluded from large-shell beads. A better compromise between recovery and purity was obtained using GTMAC-cationized AG beads grafted with poly(oligo(ethyleneglycol) methacrylate) [78] – the (non-crosslinked) polymer chains acting as the size exclusion layer.
CC700 chromatography has been shown effective in purifying different viruses, first IAV [67] as illustrated also by the manufacturer in application notes [145,146], then reoviruses and AdVs [68] and respiratory syncytial virus (RSV) [69]. Reovirus is an oncolytic virus with potential applications in the targeted treatment of cancer. AdVs are widely applied as vectors for gene delivery. RSV causes severe infections of the lungs and respiratory tract in infants. The development of a safe and effective prophylactic vaccine against RSV remains a challenge. In this context, Mundle et al. [69] have purified a live-attenuated RSV vaccine strain by a four-step purification process centered on CC700 chromatography. The chromatographic step was preceded by Benzonase treatment and clarification, and followed by virus concentration using UF/diafiltration (DF) (hollow fiber tangential flow filtration). MMC was found to be highly efficient in removing Vero HC proteins and DNA from the virus-containing column flow through (87- and 190-fold purification factors, respectively), with no noticeable reduction in virus titer. Tested in vivo in rats, the resulting vaccine displayed immunogenicity and protectiveness against RSV challenge.
Typical downstream purification processes of viral particles – whole viruses and VLPs – for biomedical applications include several chromatography unit operations separated or not by other concentration/purification procedures such as UF/DF [14,20,21]. Various examples of two-step chromatography procedures associating SEC and AEC [[55], [56], [57], [58]], CEC [60,61] or HIC [52] for virus purification have already been mentioned. In the same way, CC700 based MMC has been frequently coupled with another chromatographic step to purify whole viruses or VLPs, SEC also [74], AFC [75], but more particularly AEC [[70], [71], [72],146]. Thus, Capto Q AEC was associated with CC700 MMC in a two-step flow-through process to purify cell-based influenza viruses for vaccine manufacturing [[70], [71], [72]]. In these works, the AEC column captured HC DNA and the DNA-purified flow through containing viruses was applied onto the MMC column that retained HC proteins and hydrophobic compounds. According to the application note published by GE Healthcare [71], running the two chromatography columns in series gave similar process yield (HA recovery) and purity as when running the columns separately – an arrangement minimizing handling of virus suspensions and being likely to improve productivity. However, both Weigel et al. [70] and Tseng et al. [72] used the two columns separately. While the former added Benzonase in the AEC flow through, the latter proposed a DNase-free process yielding 82% HA recovery, 86% protein removal and 96% DNA removal by the two columns. The overall purification process, including UF/DF steps before and after chromatography, ensured 33% HA recovery, 18.7% protein removal and 99.9% DNA removal referring to the crude virus harvest. Recently, however, Weigel et al. [63] replaced MMC flow through with bind-and-elute HIC to purify IAV/IBV from clarified/concentrated cell culture broth, i.e., they used HIC as a virus capture step from DNAse-free AEC flow through. Losing the advantages of the flow-through mode but avoiding Benzonase treatment, the process yielded similar/slightly improved virus recovery and purification performance compared with two-step flow-through process. Therefore, HIC only was found more efficient than MMC combining both binding and size exclusion properties. As concerns VLPs, GE Healthcare [73] has described the purification of HPV VLPs by CC700 MMC followed by Capto Q AEC in the positive mode. HPV VLPs consisted of self-assembled pentamers of recombinant HPV capsid protein L1 (expressed in insect Sf9 cells infected with baculovirus containing the L1 protein gene). VLPs present in the clarified Sf9 cell lysate were collected in the MMC flow through that was next provided with dithiothreitol (DTT) to disassemble VLPs into L1 protein monomers. L1 proteins were then captured by the AEC column and eluted with a stepwise gradient of NaCl. VLPs could be reassembled by removal of DTT from the eluate. The purity of L1 protein (ratio of L1 protein to total protein) recovered by this two-step procedure was high (>99%), but DNA and endotoxin removal were not evaluated. Lagoutte et al. [74] have associated CC700 MMC with Superdex 200 SEC in their purification process of a VLP-based influenza A vaccine produced in E. coli. They introduced a detergent extraction step between the two chromatographic steps to remove residual E. coli protein contaminant from the MMC flow-through. The process efficiency was discussed in terms of VLP purity and recovery yield: MMC resulted in a 2.4-fold increase of the VLP purity (60%) compared to the clarified bacterial lysate, with 90% recovery yield. The VLP purity reached 89% (×3.56) with only 19% recovery yield after detergent extraction and SEC polishing. Here again, the removal of contaminant DNA and endotoxins – that may be packaged in cell-assembled VLPs [147] – was not assessed. Nevertheless, the quality monitoring of VLP-based vaccine bulks should include the control of residual DNA, protein and endotoxin contents in addition to the determination of protein purity [21]. A recent example of VLP purification from Benzonase-treated cell culture supernatant associates CC700 MMC with AFC [75]. Here, the HEP AFC step was used to separate HIV-1 VLPs from other extracellular vesicles such as exosomes and microvesicles present in the MMC flow through. Only 15% of the MMC flow-through particles were recovered in the AFC elution peak, but the particle mixture was enriched in HIV-1 VLPs.
2.2. Membrane adsorbers
As illustrated above by many conclusive results obtained using PS-based stationary phases, conventional packed-bed column chromatography has proved efficient in capture of viral particles. However, this technology faces many technical limitations [14,148], among which limited accessibility of ligands within resins, hindered pore diffusion of viral particles (Fig. 3A) and limited flow rates due to high column back pressure – leading to sub-optimal production rates. Alternative chromatography media, i.e., monolithic columns and MAs, have been developed to overcome these technical limitations. A monolith is a continuous stationary phase that is cast in a single block and inserted into a chromatography housing [150]. Most monoliths are made of inorganic polymers based on silica or synthetic organic polymers such as polyacrylamide and polymethacrylate [150,151]. Membrane chromatography is a “relatively new and immature bioseparation technology based on the integration of membrane filtration and liquid chromatography into a single-stage operation” [148]. MAs, which represent a fast-growing segment of the biopharmaceutical manufacturing market [152], are based on microporous polymer membranes, generally used in multiple layers. Membrane materials include synthetic organic polymers, such as PES, polyvinylidene fluoride (PVDF) and polypropylene (PP), and natural polymers – essentially (regenerated) CEL [148]. Monolithic and membrane media can be functionalized by the same ligands as those used in conventional chromatography resins, e.g., weak (DEAE) or strong (Q) anion exchangers. Owing to the porous architecture of these adsorptive media, the mass transfer of the adsorbate to the binding sites is governed predominantly by convection rather than by diffusion [149,153] (Fig. 3B). The dynamic binding capacity is therefore largely independent of the mobile phase flow rate and the processing time is greatly reduced.
Fig. 3.
Solute transport in packed bed chromatography (A) and membrane chromatography (B). Extracted from Ref. [149] with permission from Elsevier.
Both monolithic columns and MAs have shown promising virus capture efficiency. For example, quaternary amine-functionalized polymethacrylate monoliths (CIM® QA, Bia Separations, Villach, Austria) have been extensively applied to virus [[154], [155], [156], [157], [158]] and VLP [159,160] purification in recent years. Some monoliths based on PS, in particular AG, AG-chitosan composites or AG/chitosan-hybrids, have been implemented in AFC columns for purification of biological targets [161] with no reference to viral contaminants. In the work by Fernandes et al. [162] – an exception, AdV-5 was successfully bound to and eluted from a chitosan-PVA monolith functionalized with a Q ligand. Conversely, the implementation of MAs as chromatography tools for the capture of viral particles has been well-documented over the past ten years. Table 4 summarizes the main characteristics [43,148] that control the virus purification performance of MAs, compared to those of packed-bed resins, indicating a gain in productivity (virus production rate) and yield, a reduction in process time, but also a potential loss in virus purity (excess levels of DNA and protein impurities) when replacing a column with an MA. Therefore, a number of virus purification processes have associated the two chromatographic processes that are complementary. Table 5 focuses on CEL-based adsorptive membranes [[163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183]], widely tested for virus purification together with PES membrane-based adsorbers [148]. Most works quoted in Table 5 use flat sheets of Sartobind® (Sart) membranes (Sartorius AG, Göttingen, Germany), i.e., cross-linked, regenerated CEL membranes with large pores containing homogeneously grafted binding sites, that mainly include strong and weak anion exchangers, but also affinity ligands. Pseudo-affinity sulfated CEL membranes are also increasingly developed under patent protection (e.g., Refs. [182,183]). These studies are keeping pace with the search for virus/VLP purification methods allowing large scale production of clinical-grade human vaccines (e.g., against influenza) or viral vectors (e.g., recombinant AdVs and baculoviruses) for gene delivery to mammalian cells.
Table 4.
Some characteristics of column resins and membrane adsorbers towards large biomolecules and viruses [43,148].
| Characteristics | Column resin | Membrane adsorber |
|---|---|---|
| Mass transfer resistance | High | Low |
| Pressure drop | High | Low |
| Flow rate | Low | High |
| Dynamic binding capacity | High | Low |
| Resolution | High | Moderate |
Table 5.
Chromatographic purification of viral particles by CEL-based MA [[163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183]].
| Chromatographic purification step(s)a | Stationary phaseb | Target viral particlesc | Cell substrated | Ref. |
|---|---|---|---|---|
| AEC | Sart Q | IAV (H1N1, H3N8) | MDCK | [163] |
| rAcMNPV | Sf21 | [164] | ||
| Sart Q, Sart Direct Q | AdV-5 | HEK-293 | [165] | |
| Sart D | rBV vector | Sf9 | [166] | |
| Sart Q, Sart D | AeDNV | C6/36 | [167] | |
| MVA | CEF | [39] | ||
| CEC | Sart S, Sart C | IAV and IBV | MDCK | [43] |
| AEC/CEC | Sart Q/Sart S | Influenza VLPs | High Five | [168] |
| AFC | Sart Epoxy-HEP | MVA | CEF | [ [39] |
| Sart Epoxy-lectin | IAV (H1N1) | MDCK | [169] | |
| IMAC | Sart Zn-IDA | IAV (H1N1) | MDCK | [170] |
| pAFC | SCMA(a) | IAV and IBV | MDCK | [43] |
| MVA | CEF | [39] | ||
| Influenza VLPs | High Five | [168] | ||
| IAV (H1N1) | MDCK | [168] | ||
| SCMA(b) | IAV (H1N1) | MDCK | [171] | |
| IAV (H1N1, H3N2) and IBV | MDCK | [172] | ||
| SEX | RC membrane | IAV (H1N1) | MDCK | [173] |
| AEC + IMAC | Q Seph XLe + Sart Zn-IDA | AdV-5 | HEK-293 | [174] |
| AEC + SEC + AEC | Sart D + Sephacryl™ S-500e + Sart D | Rotavirus VLPs | Sf9 | [175] |
| AEC + SEC/MMC | Sart Q + Superdex 200e/CC700e | CAdV-2 | MDCK | [176] |
| AEC + MMC | Sart STIC PA + CC700 | Retrovirus VLPs | HEK-293 | [177] |
| pAFC + AEC | SCMA(a) + Sart STIC PA | IAV (H1N1) | MDCK | [178] |
| pAFC/AFC + AEC | SCMA(a)/Sart Epoxy-HEP + Sart Q | MVA | CEF | [39] |
| pAFC/AFC + SEC | SCMA(a)/Sart Epoxy-HEP + HIC-phenylf | MVA | CEF | [139] |
AEC, anion-exchange chromatography; AFC, affinity chromatography; CEC, cation-exchange chromatography; IMAC, immobilized metal affinity chromatography; MMC, mixed mode (multimodal) chromatography; pAFC, pseudo-affinity chromatography; SEC, size exclusion chromatography; SEX, steric exclusion chromatography.
Sart stands for Sartobind® (Sartorius AG, Goettingen, Germany). RC membrane, regenerated CEL acetate membrane, pore size 1 μm (GE Healthcare/Whatman); Sart D/Sart Q, AE MA, formed by cross-linked CEL support and a hydrogel layer on its pore surface [179]. Forming a tentacle-like coating, the gel layer carries diethylamine (weak basic anion exchanger) (D) or trimethylammonium (strong basic anion exchanger) (Q) groups; Sart C/Sart S, CE MA, consisting of a Sart membrane with weak (carboxylic acid, C) or strong (sulfonic acid, S) acidic CE groups; Sart Direct Q, membrane chromatography module where the mobile phase is passed over the adsorptive membrane (Sart Q) in a tangential flow mode; Sart Epoxy-HEP/Sart Epoxy-lectin, epoxy-activated Sart membrane bearing covalently attached HEP/lectin (from Euonymus europaeus spindle tree); Sart STIC PA, i.e., Sart STIC (salt-tolerant interaction chromatography) PA (primary amine), salt tolerant AEC membrane consisting of a polyallylamine ligand covalently coupled to a cross-linked, regenerated CEL membrane with ultrapores, providing a novel double-porous structure [180,181]; Sart Zn-IDA, Sart membrane to which IDA (iminodiacetic acid) is bound covalently as chelating functional group, charged with Zn2+ ions; SCMA, sulfated CEL MA: (a) reinforced, non-cross-linked CEL membrane sulfated by reaction with sulfurochloridic acid in pyridine [182]; (b) CEL hydrate membrane crosslinked before sulfation [183]. The RC membrane and most Sart membranes were used in syringe filter-type units packed with 15 membrane layers (75 cm2 total membrane area), except in the works by Peixoto et al. [165] (total membrane areas: 90 cm2 and 900 cm2), Grein et al. [164] (3 membrane layers, 15 cm2 area), Nestola [177] and Weigel et al. [178] where reduced membrane areas were used for small-scale experiments. For SCMA, 10 (50 cm2 area) [43,178] or 15 (75 cm2 area) [39] membrane layers were stacked in a stainless steel holder, while Carvalho et al. [168] and Fortuna et al. [171,172] used smaller membrane surfaces, i.e., 5.65 cm2 (5 layers) [168] and 2.1 cm2 (6 layers) [171] or 2.9 cm2 (15 layers) [172].
rAcMNPV, (recombinant) Autographa californica multicapsid nucleopolyhedrovirus (baculovirus); AdV-5, adenovirus type 5; rBV, (recombinant) baculovirus (derived from AcMNPV); AeDNV, Aedes aegypti densonucleosis virus; CAdV-2, canine adenovirus type 2; IAV/IBV, influenza A/influenza B virus; MVA, modified vaccinia Ankara virus.
C6/36, Aedes albopictus (tiger mosquito); CEF, chicken embryo fibroblast; HEK-293, human embryonic kidney (transformed with sheared adenovirus type 5 DNA); High Five™, moth (Trichoplasiani) ovaries; MDCK, Madin Darby canine kidney; Sf21, Spodoptera frugiperda (fall armyworm) ovaries (IPLB-SF21-AE); Sf9, Spodoptera frugiperda (derived from the parental Sf21 cell line).
See Table 3 for abbreviations.
Toyopearl® phenyl-650M (Tosoh Bioscience, Tokyo, Japan), a polymethacrylate resin functionalized with phenyl ligand groups.
2.2.1. Sartobind® IEC/AFC MAs
Thoroughly supported and promoted by the manufacturer [184], virus purification processes making use of commercial Sart MAs were developed some years ago, with particular attention to AE MAs. In agreement with the above trends, some general results from these works can be underlined. MAs gave improved virus yields and/or productivities compared to conventional packed beads [39,43,166,169,174]. Strong membrane ion exchangers (Q, S) were more efficient than weak ones (D, C) [43,163,167]. Whatever the chromatography separation mode, ion exchange or affinity, the purification efficiency of Sart MAs was quasi-systematically affected by the presence of HC DNA in the (eluted) product fraction – the level of these DNA impurities exceeding the current regulatory requirements for clinical applications. For instance, HC DNA was recovered completely in the HA (i.e., IAV) enriched product fraction eluted from Sart Q MA [163]. After IMAC treatment in the best adsorption/elution buffer conditions, the amount of HC DNA in IAV desorbate was reduced but remained equal to 7% of that in the untreated virus pool [170]. Mustang® Q (Pall Corporation, Port Washington, NY, USA), a PES-based strong AE membrane, appeared more promising than Sart Q for baculovirus purification, yielding lower levels of HC DNA and proteins in the viral eluate [164]. The Sart Q membrane was also less efficient than CIM® QA monolith in AdV vector purification [176]. Residual DNA impurities were not eliminated, however [164,176]. The amount of contaminating DNA could be lowered by nuclease pre-treatment, which was applied in some studies [165,174,177] and suggested in others [39,43,166,176], and/or by the combination of chromatographic steps. Thus, Vicente et al. [175] used SEC with Sephacryl™ S-500 resin to polish the pool of rotavirus-like particles captured by Sart D AEC. Most of the contaminant DNA (98%) was eliminated after this polishing step. However, an additional AEC step was necessary to complete DNA removal, yielding about 1.4 μg DNA/mg VLP in the final product. Sart IMAC was found insufficient to reduce the level of DNA contaminants co-eluting with AdV particles in packed-bed AEC [174]. Treatment of the AdV pool with Benzonase before the second chromatographic step remained necessary to lower significantly the DNA concentration in the final MA pool. In the same way, Wolff et al. [39] associated heparin-MA pAFC and Sart Q AEC to reduce DNA contamination in vaccinia virus eluates. The level of double stranded DNA (dsDNA) was reduced by pAFC to 23% of total dsDNA present in the starting viral material and further depleted to 5% by AEC, which, however, was still not sufficient to evade nuclease treatment. Submitting pAFC virus eluates to column HIC led to similar conclusions [139]. More recently, Nestola et al. [177] proposed a flow-through purification process for retrovirus VLPs in which a Sart STIC (salt tolerant interaction chromatography) AE MA and a CC700 (MMC) packed bead column were connected in series. The chromatography train was preceded by UF/DF of the Benzonase-treated VLP bulk. A 2-log reduction in DNA content was achieved together with a 3.5-log reduction in HC protein content and a VLP recovery of 45%.
2.2.2. Sulfated cellulose pAFC MAs
However, the latest studies have been performed using sulfated CEL MAs [168,171,172,178]. These pseudo-affinity MAs are made of regenerated CEL crosslinked [183] or not [182] before sulfation. Allowing like commercial Sart MAs higher volumetric flow rates during adsorption, SCMAs led to enhanced virus productivities compared to conventional beaded adsorbents, in particular cellufine sulfate [39,43]. The purification performance of SCMAs has also been compared to those of AFC [39,139] and ion-exchange [39,43,168] Sart MAs in terms of contaminant depletion. Contaminant removal by SCMA was equivalent to (total proteins) or slightly higher than (DNA) that obtained using heparin-functionalized MA [39,139]. Also, SCMAs ensured significantly improved DNA depletion compared with CE [43] and AE [39] exchange MAs. However, DNA elimination still remained incomplete, even when adding a second chromatography step in the purification process [39,139]. Using a statistical optimization methodology, i.e., “design of experiments” (DoE) [185], Carvalho et al. [168] reported better VLP yield and total protein removal by SCMAs than by Sart AEC/CEC MAs. The DNA level in both Sart and SCMA product fractions was low, below the limit of detection of the quantification methods used. However, the cell culture was treated with Benzonase before VLP harvest. Carvalho et al. [168] also assessed the purification of whole IAV particles with no preliminary nuclease treatment. A virus yield of 64% was reached, with a residual DNA level of 3.8 ng DNA/μg HA that remained well above the regulatory recommendations for influenza vaccines (i.e., <10 ng DNA/dose for parenteral administration – knowing that a normal vaccine dose should contain 15 μg HA per IAV strain [131]. In a parallel study [171], the same group applied this DoE method specifically to the optimization of IAV purification using an industrial SCMA prototype [183]. They obtained comparable results regarding the virus yield and the removal of HC-related DNA and protein impurities. MA displayed higher productivity than commercial sulfated resins [172]. Weigel et al. [178] tested a two-step membrane chromatography approach to improve the purification level of IAV preparations while avoiding nuclease treatment – contrary to the two-step packed-bed chromatography process they had reported previously [70] (see Section 2.2.5). Sart STIC AEC was operated in the flow-through mode as a polishing step to remove residual DNA from SCMA elution product. This arrangement showed higher purification efficiency than the former MA-based 2-step process associating SCMA and Sart Q AEC operated in the bind-and-elute mode [39], whose purification performance was impeded by the co-elution of virus particles and DNA impurities from the Sart Q MA. Thus, the purified IAV preparation (75% virus yield) contained 1.2 ng DNA per monovalent vaccine dose (15 μg HA), which complies with the contamination limit recommended by regulatory authorities.
2.2.3. Cellulose steric exclusion chromatography MA
In the so-called steric exclusion chromatography (SEX) [186], large-sized target species such as proteins and viruses are adsorbed at the surface of a hydrophilic, nonreactive membrane by mutual steric exclusion of poly(ethylene glycol). Lee et al [186] illustrated the separation efficiency of this technique by purifying immunoglobulin G (IgG) and bacteriophage M13K07, using hydroxyl-substituted CIM® polymethacrylate monoliths as a hydrophilic surface. SEX has been implemented recently in the purification of cell culture-derived IAV using simple regenerated CEL MA (1 μm pore size membrane filters) as an alternative to monoliths [173]. High virus recoveries (>95%) were reached. Applying nuclease treatment prior to chromatography, HC (ds)DNA depletion was as high as 99.7%–99.9%, but the lowest residual DNA level in the product eluate was c. 30 mg/15 μg HA.
3. Viral clearance
In viral clearance applications, viral particles are no more the product of interest, but impurities that must be reduced to levels acceptable for human use. Virus elimination (inactivation/removal) is critical in a number of biopharmaceutical and clinical applications. Validation of virus clearance (see Refs. [187,188] for a comprehensive overview) is essential in the manufacture of biopharmaceuticals – a prerequisite to clinical trials and commercial launch of blood- and mammalian cell culture-derived therapeutic proteins [189] such as Igs [190] and mAbs [12]. Virus inactivation technologies include physical (e.g., heat application, ultraviolet- and gamma irradiation) and chemical methods (e.g., low pH and solvent/detergent treatments) [3,188,190]. Virus removal is ensured by a combination of filtration and chromatography steps [3,12,191] (Fig. 4). The quantitative assessment of the virus reduction capacity of each unit operation (e.g., chromatography) in a manufacturing process is performed by viral clearance studies which are submitted to a number of regulatory considerations [187]. In these experiments, a product intermediate is artificially contaminated (“spiked”) with selected viruses – generally representative of potential viral contaminants in source materials (Table 6 [[192], [193], [194]]). The virus reduction capacity of the tested step is expressed as log reduction value (LRV), i.e., log ratio of the viral load in the spiked product intermediate to that recovered in the product after processing. Steps yielding LRV ≥4 are considered effective for viral clearance.
Fig. 4.
Overview of integrated adventitious agent control strategy. Adapted from Ref. [191] with permission from Elsevier.
Table 6.
| Name | Family | Genome | Envelope | Size (nm) |
|---|---|---|---|---|
| Murine leukaemia virus (MLV), xenotropic MLV (XMLV) | Retroviridae | ssRNA | Yes | 80–120 |
| Reovirus (respiratory enteric orphan virus) type 3 (Reo-3) | Reoviridae | dsRNA | No | 60–80 |
| Minute virus of mice (MVM), bovine/canine parvovirus (BPV/CPV) | Parvoviridae | ssDNA | No | 18–25 |
| Parainfluenza 3 virus (PI-3) | Paramyxoviridae | ssRNA | Yes | 150–350 |
| Herpes simplex virus 1 (HSV-1) | Herpesviridae | dsDNA | Yes | 120–300 |
| Pseudorabies virus (PRV)a | Herpesviridae | dsDNA | Yes | 150–200 |
| Sindbis virus (SINV) | Togaviridae | ssRNA | Yes | 60–70 |
| Simian (vacuolating) virus 40 (SV40) | Polyomaviridae | dsDNA | No | 40–50 |
| Poliovirus 1 (PV-1) | Picornaviridae | ssRNA | No | 25–30 |
Alias suid herpesvirus 1 (SuHV-1).
Allied with chemical inactivation, nanofiltration (NF) is the main process step dedicated to viral removal by most manufacturers. The performance of CEL-based virus-retentive filters (typically Planova™ filters from Asahi Kasei Medical, Tokyo, Japan) in the downstream purification processes of biopharmaceuticals has been detailed in review [10]. For instance, the purification process for Nuwiq®, a recombinant coagulation factor VIII (a blood-clotting protein whose deficiency is associated with hemophilia A) patented by Octapharma AG (Lachen, Switzerland) [195], includes solvent/detergent (S/D) treatment, Planova NF, and five chromatography steps using PS-based stationary phases, i.e., MMC (Capto MMC), CEC (SP Seph FF), AFC (VIIISelect, a Capto matrix with factor VIII-selective ligand), AEC (Q Seph FF) and SEC (Superdex 200). Of these, however, only S/D treatment and NF have been evaluated for viral clearance [196]. Nevertheless, these chromatography steps provide a significant contribution to the overall virus removal efficiency of purification processes and have been well-documented over the past 25 years [3,193,194,197].
3.1. Packed bed column chromatography
3.1.1. Ion-exchange chromatography (IEC)
In early studies (see Ref. [193] for a detailed review on chromatography for plasma product manufacturing), IEC processes associating weak AEC (DEAE Seph FF) and CEC (carboxymethyl (CM) Seph FF) were tested for their capacity to remove poliovirus type 1 (PV-1) and canine parvovirus (CPV) [198] or hepatitis A virus [199] during human albumin purification. The latter process involved an additional SEC step (using a Sephacryl column), yielding a cumulative LRV of 11 across the three chromatographic steps [199]. In these works, as well as in studies cited below, CEC columns were operated in the bind-and-elute mode where the desired product was retained by the CEC resin while most viral particles remained in the flow through. AEC columns were operated in the flow-through mode, i.e., negatively charged viral impurities were captured by the adsorbent mainly through electrostatic interactions while the desired product remained in the flow through.
More recently, CEC columns, packed with CM Seph FF, were used successfully to clear viruses from Ig samples spiked with enveloped viruses such as dengue virus [200] or herpes simplex virus type 1 (HSV-1) and Sindbis virus (SINV) [201]. Virus removal from antibody solutions was also achieved using CEC columns packed with beads of Capto S [202] and SP Seph FF resin [203]. In the latter work, however, model retroviruses (xenotropic MLV (XMLV)) bound tightly to the resin and remained attached while antibodies eluted in salt gradient.
Despite these positive results published some years ago, CEC has been considered to be less robust than AEC in removal of viral contaminants from therapeutic protein preparations [194,204]. AEC robustness has been validated by several studies in which AEC columns were packed with the strong AE resin Q Seph FF [[205], [206], [207], [208], [209]]. In these works, mAb samples were spiked with model viruses such as XMLV, simian virus 40 (SV40) and minute virus of mice (MVM), and viral clearance was assessed under varied operating conditions including obviously the pH and conductivity (salt concentration) of the mobile phase, but also the column load density (mg mAb/ml resin) |208], the impurity level (aggregated mAb, HC DNA and proteins) of the mAb feedstock [209], or the pooling criteria of the pass-through fractions [206]. The viral clearance efficiency of the chromatography step was highlighted by LRVs greater than 4 in proper operating conditions. Q Seph FF chromatography was also shown to remove effectively porcine circovirus type 1 (PCV1), an adventitious contaminant of oral vaccines against rotavirus gastroenteritis in children which is small-sized and highly resistant to physical inactivation procedures [210]. A LRV value of 4.12 was achieved while NF through Planova filters was inefficient or moderate (LRV≈1.5 for a 15-nm pore size filter). Here, however, the column load consisted of a viral suspension in buffer with no biopharmaceutical or other contaminants added. Now virus-spiked product intermediates are used to determine the virus reduction capacity of different process steps in order to assess the viral safety of manufactured biopharmaceuticals. As already stated, AEC is not considered as an effective virus reduction step in the manufacturing processes, contrary to NF. In addition to the already mentioned study by Winge et al. [196], another recent example is given by Nowak et al. [211] evaluating the viral safety of human prothrombin complex Beriplex® P/N (CSL Behring, King of Prussia, PA, USA). They reported efficient removal of test viruses by serial filtrations through Planova filters whereas column AEC with DEAE Sephadex™ A-50 resin (a cross-linked dextran matrix with DEAE groups attached to glucose units) was only tested for prion removal (LRV≈1).
3.1.2. Affinity chromatography (AFC)
AFC is a well-established primary isolation step in the purification processes of biopharmaceuticals, in particular antibodies [212,213]. Here, the product of interest is selectively adsorbed by the matrix-immobilized ligand while non-interacting impurities remain in the flow through. This “capture” step may achieve efficient viral clearance provided that, contrary to HEP-based ligands used for virus purification (see Section 2.1.3), the ligand does not adsorb viruses or adsorbed viruses are not eluted with the product in proper operating conditions. Therefore, many manufacturing processes of biopharmaceuticals, e.g., antihemophilic factors, incorporate an AFC step that contributes to the overall virus removal efficiency in addition to the dedicated NF step (Table 7). Most PS-based AFC resins evaluated for viral clearance consist of an AG (Seph) matrix to which an antibody binding protein or an antibody/antibody fragment is attached.
Table 7.
Virus removal efficiency (LRV) of affinity chromatography steps included in the manufacturing processes of some antihemophilic agents compared to well-established nanofiltration using Planova viral filters [[214], [215], [216], [217]].
| Product (brand name) | Virus removal stepa | Model viruses |
Ref | |||||
|---|---|---|---|---|---|---|---|---|
| BEV | MLVb | MVM | PI-3 | PRV | Reo-3 | |||
| rFIXFc (Alprolix®) | AFC (MabSelect SuRe) NF (Planova 15N) |
4.4 >5.9 |
2.9 4.5 |
3.7 >5.1 |
3.3 >6.6 |
[214] | ||
| rFVIIIFc (Elocta®/Eloctate®) | AFC (VIIISelect) NF (Planova 15N) |
2.4 ≥5.6 |
>4.6 ≥5.7 |
3.1 ≥4.0 |
2.8 ≥5.5 |
[215] | ||
| Xyntha™/ReFacto AF | AFC (TN8.2 Seph) NF (Planova 35 N) |
>2.99 >5.18 |
2.52 0.63 |
1.51 >4.95 |
3.13 >6.00 |
4.40 >5.93 |
[216] | |
| Turoctocog alfa (NovoEight®) | IAC (rF25 Seph) NF (×2) (Planova 20N) |
4.6 >7.4 |
>4.9 5.3 |
2.0 6.5 |
3.9 – |
[217] | ||
3.1.2.1. Antibody binding protein ligands: protein A/Protein G AFC
Staphylococcal protein A and streptococcal protein G are common affinity ligands for antibody capture [218]. Protein A is a surface protein from the Gram-positive bacterium Staphylococcus aureus that has high affinity to Igs from various species, in particular IgGs [218,219]. Most commercial mAb processes incorporate protein A AFC steps for antibody purification [12,[219], [220], [221]]. Protein A media specifically capture antibodies but may bind modest levels of adventitious/endogenous viruses via nonspecific interactions of viruses both with the media itself (resin backbone and protein A ligand) and the mAb product [222]. Hence, protein A media have been frequently subjected to viral clearance validation studies [194,222], yielding low and high variable LRV values [189], e.g., between 0.8 and 2.9 for XMLV, depending on the mAb product [222]. This is the case of PS materials based on a cross-linked AG matrix, coupled to natural staphylococcal protein A (Protein A Seph 4 FF) [223] or alkali-stabilized recombinant protein A (MabSelect SuRe™, GE Healthcare) [224]. These two studies reported acceptable (c. 4) [223] or moderate (2–3) [224] LRV levels for endogenous retroviral particles present in antibody feedstocks (cell culture harvests), LRVs remaining stable over several hundred cycles of purification/cleaning. The robustness of viral clearance with respect to media age was confirmed using multi-spiked (SV40, XMLV, and MVM) feedstock [224]. Bach et al. [225] investigated the mechanism of XMLV clearance by the MabSelect SuRe resin. They showed that low clearance was due mainly to virus binding to and eluting with the mAb. There was no noticeable interaction of XMLV with the ligand or the AG matrix since the virus alone (with no mAb added to the column load) was retained neither on the protein A resin nor on underivatized Seph (LRV>5.5). Protein A AFC using the MabSelect SuRe resin has also been used as the capture step in the manufacturing process of the recombinant factor IX Fc fusion protein rFIXFc (Alprolix®, Biogen, Cambridge, Mass., and Sobi AB, Stockholm, Sweden), an approved blood-clotting medicine for hemophilia B [214]. AFC was followed by two AEC steps and final NF, all of which were tested for clearance of four model viruses. In addition to Planova filtration, both AFC and Q Seph FF AEC contributed significantly to virus removal, with LRVs ranging from 2.9 (MVM) to 4.4 (XMLV) for AFC (Table 7) and 3.5 (MVM and XMLV) to 6.1 (reovirus type 3 (Reo-3)) for AEC.
Protein G is extracted from group C and G streptococci. Though widely used in antibody purification processes [212,218], protein G chromatography resins have been scarcely evaluated for viral clearance. An example is given by Roberts [226] who investigated virus elimination during the purification of immunoglubulins G1 and G3 produced from human hybridoma cell-lines. The purification process involved three sequential column chromatography steps using PS-based resins, i.e., protein G AFC (protein G Seph), CEC (SP Seph FF), and SEC (Superdex 200), completed by two specific virus elimination steps (S/D treatment and NF). Protein G AFC was proved effective in eliminating both enveloped (HSV-1, SINV) and non-enveloped (PV-1) model viruses, showing even better removal efficiency than NF (using PVDF filters) for the small-sized PV-1. However, IgG acid elution probably contributed to the high LRV values obtained for enveloped, pH-sensitive viruses. Virus removal by CEC was lower (HSV-1, LRV = 3–5; PV-1, LRV = 1–2), and SEC had a limited impact on viral clearance.
3.1.2.2. mAb ligands: anti-FVIII immunoaffinity chromatography (IAC)
In addition to Nuwiq® [196], a wealth of antihemophilic FVIII products is currently available on the market, ranging from early plasma-derived to the fourth-generation recombinant products [227]. MAb-based immunoaffinity chromatography (IAC) is a key purification step in most manufacturing processes of these commercial FVIII compounds [228,229], with chemical inactivation and NF devoted to viral clearance – like for mAb products. Nevertheless, a few studies have investigated the virus removal efficiency of the IAC step where a Seph matrix with an anti-FVIII mAb ligand was used as adsorbent: murine mAb (IgG) coupled to N-hydroxysuccinimide (NHS)-activated Seph 4FF [230] or Seph CL-2B [231], recombinantly produced anti-FVIII mAb (rF25) coupled to cyanogen bromide (CNBr)-activated Seph FF [217].
MAb IAC was associated with SEC (Superdex 200) and S/D treatment (but not NF) to ensure the viral safety of B-domain deleted recombinant factor VIII BDDrFVIII (ReFacto®, Wyeth Ltd., now Pfizer, New York, NY, USA) produced in CHO cells – a second generation product [227] in which no human-derived protein was added to the final formulation, reducing the risk of viral transmission [230]. The mAb-Seph column poorly retained model viruses, with LRVs ranging from 3.8 for PV to ≥7.9 for infectious bovine rhinotracheitis virus (bovine herpesvirus 1). The manufacturing process included three other chromatography steps using Seph-based resins (CEC, AEC, HIC) [232], not tested for viral clearance but expected to provide additional virus removal. Note, however, that the anti-FVIII mouse mAb (8A4) ligand was replaced later by a synthetic peptide (TN8.2) to avoid the use of animal-derived materials in the manufacturing process of ReFacto – leading to the third-generation product Xyntha™/ReFacto AF (albumin free) (Pfizer). The TN8.2-Seph AFC step also significantly contributed to the viral clearance capacity of the modified process [216] (Table 7).
In the production process of plasma-derived Replenate® (Bio Products Laboratory, Elstree, UK), the IAC column is loaded with the S/D treated FVIII intermediate and the eluate applied to an AEC column for additional purification, similar to the method used to make Hemofil M® (Baxter, Deerfield, Ill., USA) [233,234] – the two products differing in the plasma source [235]. Roberts [231] evaluated the effectiveness of IAC for eliminating viruses during the manufacturing process of Replenate®. The IAC step was proved effective in eliminating model viruses, with LRVs of 4.4 that of (SINV), 4.0 (PV-1) and >5.6 (bovine parvovirus (BPV)). However, only clearance of PV-1, contrary to that of SINV and BPV, was poorly affected by S/D presence, which could be attributed in part to the chromatography step by itself. Turoctocog alfa (NovoEight®, Novo Nordisk A/S, Bagsværd, Denmark), a B-domain truncated recombinant FVIII molecule, is manufactured in CHO cells with no use of human or animal-derived materials [236] to minimize viral risk (third-generation rFVIII product [227]). The manufacturing process consists of several successive chromatography steps for capture of the FVIII product and its purification whose virus clearance capacity has been evaluated by Ellgaard et al. [217]. In particular, MMC with Capto MMC resin was used for FVIII capture and anti-FVIII IAC using rF25 mAb [237] coupled to Seph was used as purification step. In the initial capture step, the MMC resin with adsorbed FVIII was washed with detergent and virus inactivation significantly contributed to the resulting viral clearance. However, anti-FVIII IAC ensured efficient virus elimination from the detergent-free MMC eluate, with LRVs ranging from 2.0 (MVM) to >4.9 (ecotropic MLV) (Table 7).
3.1.2.3. Camelid-antibody-derived ligands
Recombinant protein ligands derived from heavy-chain-only antibodies that are found in Camelidae [238] have been developed under the trademark CaptureSelect® (now owned by Thermo Fisher Scientific, Waltham, Mass., USA) to ensure the AFC primary capture step in the purification processes of therapeutic proteins, including nonantibody targets – an equivalent to protein A for mAb purification [239]. Incorporating this proprietary ligand technology, the VIIISelect resin [79], designed for the purification of factor VIII compounds, provided good viral clearance (XMLV, LRV≈4; MVM, LRV>5) and improved HC DNA and protein clearance from cell culture samples containing factor VIII compounds [240]. Together with other chromatography steps (AEC and HIC), a VIIISelect AFC purification step was then included in the manufacturing process of recombinant factor VIII Fc fusion protein rFVIIIFc (Elocta®/Eloctate®, Sobi and Biogen), a long-acting coagulation factor approved for the treatment of hemophilia A [215]. The process also comprised detergent inactivation and NF to ensure viral clearance. Contrary to Winge et al. [196] assessing the pathogen safety of Nuwiq® purification process, virus removal studies involved the AFC and AEC steps. They demonstrated significant contribution of AFC – and, to a much lesser extent, AEC – to viral clearance for the overall process, even though NF (Planova 15N filter) achieved the most substantial removal of model viruses (Table 7).
3.1.3. Other modes (IMAC, HIC, MMC)
IMAC and HIC have been seldom applied to the purification of viral/subviral particles and VLPs (see Sections 2.1.5, 2.1.6, respectively). IMAC has also been used occasionally to purify plasma coagulation factors: factor VIII [241] and, particularly factor IX [231,242]. Roberts [231,242] assessed the virus elimination efficiency of the chromatographic process during the manufacture of Replenine®-VF (Bio Products Laboratory), a human plasma-derived concentrate of coagulation factor IX [243]. IMAC was performed using a copper-charged Chelating Seph FF column. Substantial virus removal was demonstrated for all viruses tested (PV-1, SINV, vaccinia virus) independently of S/D presence, including the enveloped SINV – susceptible to S/D treatment.
As mentioned in the last section, HIC column chromatography using hydrophobic Seph beads has been involved in the purification schemes of plasma coagulation factors [215,216]. Butyl Seph HIC showed some ability to clear the small-sized (not retained by NF) and non-enveloped (resistant to S/D treatment) MVM from MVM-spiked Xyntha/ReFacto AF samples [216]. Octyl Seph HIC was not tested for viral clearance in the manufacturing process of Elocta/Eloctate [215]. Nevertheless, HIC has been considered at times as a virus reduction step in protein manufacturing [244,245]. Butyl Seph HIC was the second of the three chromatographic steps – between AFC (using Cellufine sulfate) and tandem AEC/CEC (using Q/SP Seph) – in the purification process of recombinant bone morphogenetic protein 2 (dibotermin alfa), the active ingredient of InductOs® bone regeneration formulation [244]. Phenyl Seph HIC was the second of three successive virus reduction steps – following pasteurization and preceding filtration with 15N/20N Planova filters – in the manufacturing process of human C1 esterase inhibitor (a plasma-derived product) [245]. In both studies, the viral clearance efficiency of the different steps was assessed by spiking studies using a panel of model viruses. Virus reduction factors of HIC compared to those of the other, are more frequently used in virus reduction steps.
In the manufacturing process of turoctogog alfa [217], the purification step by anti-FVIII IAC (Section 3.2.1.2) is preceded by a product capture step by MMC using the Capto MMC resin [81] which contains in particular negatively charged (carboxylic) and hydrophobic (phenyl) groups – thus combining weak CEC and HIC. Ellgaard et al. [217] evaluated the virus clearance capacity of this capture step during which most viruses were eliminated in the column flow through. Since viral clearance was evaluated after washing of the resin with detergent, inactivation of viruses co-adsorbed with rFVIII significantly contributed to the resulting viral clearance data, as concerns more particularly enveloped model viruses that were cleared completely. Combining SEC, AEC and HIC, CC700 MMC operated in the negative mode has been shown effective in purifying different viruses, recovered in the column flow through while smaller-sized impurities were retained by the column packing (see Section 2.1.7). The mixed-mode resin Capto adhere, whose ligand also contains positively charged (quaternary amine), hydrophobic (phenyl), and hydrogen-bonding (hydroxy) groups but is devoid of size exclusion layer, has shown a promising ability to retain viruses in suitable operating conditions, even in the presence of mAb products and process impurities [209]. Zhao et al. [76] exploited this property by introducing a Capto adhere MMC step in the multistep chromatography purification process of EV71 VLPs produced from insect cells, following negative CC700 MMC. The Capto adhere-based AEC-HIC step may also contribute to viral clearance by removing adventitious viruses from product streams in downstream purification processes of mAbs and other biopharmaceuticals [209,246,247]. In recent studies, Brown et al. [246,247] investigated the binding mechanisms of model virus surrogates (i.e., bacteriophages displaying different surface charge characteristics and hydrophobicities) to the Capto adhere resin. They first compared the efficiencies of the MMC resin and purely anionic (Capto Q) or hydrophobic (Capto Phenyl) Seph-based resins to remove phages from spiked buffers with varying pH and salt concentrations [246]. The most hydrophobic and negatively charged phages were adsorbed to Capto adhere by synergistic binding to the quaternary amine and phenyl groups, yielding a better phage removal efficiency of the MMC resin (LRV > 3in all tested buffer conditions) compared to single mode ones. However, the presence of in-process impurities in spiked mAb pools affected the virus removal performance of the multi-modal resin [247]. The Capto adhere MMC step as a contributor to viral clearance remains to be implemented practically in downstream purification processes of mAbs or other biomolecules intended for human use.
3.2. Membrane adsorbers
Membrane chromatography devices, in particular commercial AEC (Sart Q) and pAFC (SCMA) MAs, have shown efficacy in capture of viral particles (see Section 2.2). Operated in the bind-and-elute mode, they have been widely implemented to purify viruses and VLPs from complex culture media (Table 5). Since their emergence in the downstream processing technology of biopharmaceuticals, MAs have also been frequently used in flow-through mode to remove trace impurities – including viral contaminants – from product streams (i.e., polishing) [152]. In this application field, most clearance data refer to HC proteins, DNA and endotoxins. However, a few works published over the last 15 years have investigated virus removal by Q membrane-based polishing steps in antibody purification [12,[248], [249], [250]].
As concerns CEL-based MAs, the first viral clearance experiments conducted with the commercial Sart Q membrane (10-layer format) in flow-through mode showed only moderate-to-low capacity of the Q membrane for removing XMLV from a purified, high-titer IgG load, LRVs decreasing with increasing antibody load [251]. The Sart Q membrane, operated in a spirally winding format where the membrane was rolled on a core to form a cylinder of 125-cm2area, was then tested by Zhou et al. [252] for viral clearance of antibody feed stock spiked with model viral particles of different sizes (MVM, Reo-3, MLV, pseudorabies virus). The MA capsule displayed efficient removal of both viruses (LRVs>5) and trace amounts of process-related impurities at varying process capacities (i.e., mass of antibody processed per unit area of membrane). Zhou et al. [253] detailed the influence of several operational parameters on the viral clearance power of syringe filter-type Sart Q MA capsules (stacked membrane configuration) with varying membrane areas. They showed in particular that the removal efficiency of MVM decreased by decreasing the pH and increasing the conductivity (salt concentration) of the virus-spiked mAb feed.
The negative effect of high salt concentrations – that screen the electrostatic interactions between the positively charged quaternary ammonium-based ligands and the negatively charged impurities –on the binding capacity of Q products is well-documented, referring in particular to conventional Q resins [207,209]. In the overall purification process of mAbs and recombinant proteins, however, final flow-through AEC-based polishing is usually preceded by chromatographic steps in the bind-and-elute mode, e.g., AFC (protein A) and CEC. Elution of bound materials generates high salt eluates containing the target product and trace impurities. Feed stream dilution prior to flow-through polishing is therefore necessary. This drawback of Q ligands has led to the development of MAs functionalized with alternative AE ligands showing extended salt tolerance. It has been shown that primary/secondary amine-based ligands display high ability to bind negatively charged species at high salt concentrations, owing to secondary hydrogen bonding interactions [254]. Using regenerated CEL discs as the base membrane material, Riordan et al. [255] have tested various amine-containing ligands for virus removal from a high salt challenge virus suspension. Four alternative ligands, including polycations (polyhexamethylene biguanide and polyethyleneimine), achieved >5 LRV of bacteriophage FX174 (Microviridae) at 150 mM NaCl, compared to 0 LRV for commercial (Mustang) Q membranes. The authors concluded that the virus removal efficiency of the cationic MA was dependent on the net (positive) charge of the ligand, its molecular structure, and the ligand density of the membrane. Using surface-initiated atom transfer radical polymerization (ATRP), Bhut et al. [256] grafted polyelectrolyte Q chains {poly([2-(methacryloyloxy)ethyl]trimethylammonium chloride)} from the surface of regenerated CEL macroporous membranes to produce strong AE membranes with variable polymer chain graft densities, yielding MAs with high protein-binding capacities. Then the virus clearance performance of these Q-based MAs was evaluated using an MVM spiked loading buffer [257]. LRVs increased with the degree of polymer grafting (i.e., polymer chain density) reaching a value > 4.5 for the given MVM load. Here, however, virus clearance experiments were performed in a protein-free, low conductivity (0.5 mS/cm) buffer. The recently developed Sart STIC (PA) MA uses a polyallylamine ligand – a polycation with multiple primary amine (PA) groups – to improve adsorption performance at high salt concentrations [180]. Sart STIC MA operated in the flow-through mode has been included in the purification scheme of viruses [178] and VLPs [177] to remove DNA and protein impurities from viral preparations (see Table 5). Sart STIC displayed significantly higher virus binding capacity than Sart Q in the presence of 150 mM NaCl (16.8 mS/cm conductivity), achieving an LRV>4.96 for MVM compared to 1.81 for the Q membrane [180]. Here also, these virus capture assays were carried out in simple buffer provided with various salt amounts. Results of experiments assessing the viral clearance efficiency of Sart STIC processes have not been published so far. The efficiency of virus removal by Q MAs (Sartobind and Mustang) under different buffer conductivities/salt concentrations also compared unfavourably with that of ChromaSorb™ (EMD Millipore, Billerica, MA, USA), another commercially available salt-tolerant MA consisting of a polyethylene microporous membrane coated with a chemically cross-linked hydrogel of allylamine polymer [258]. The Chromasorb adsorber actually displayed higher resistance to salt concentration than Q membranes, ensuring robust virus retention from MVM-spiked mAb feed at high conductivity – contrary to Q products. Weaver et al. [259,260] further compared the performance of MVM clearance by the three commercial AE MAs over a range of feed conditions, including the absence [259] or presence [260] of HC DNA/protein impurities and mAbs. The reduction of MVM removal by Q membranes at high salt concentration and low pH was confirmed, as was the tolerance of the primary amine-based ChromaSorb adsorber to ionic strength/conductivity of the feed solution. However, the Chromasorb capacity for binding MVM was severely compromised by the presence of ions capable of forming hydrogen bonds with the primary amine groups, such as hydrogen phosphate ions. Furthermore, the primary amine-based ChromaSorb showed lower mAb recovery (i.e., higher mAb binding) than Q membranes [260].
4. Conclusion and future trends
Chromatography currently remains a basic tool in the manufacturing of biopharmaceuticals, more particularly downstream purification processes [261], despite an increasing interest in nonchromatographic techniques [262]. This prevalence is reflected in a number of recent reviews, covering for instance well-established AFC [263,264] and fast developing MMC [265,266], emerging materials – including AFC and MMC ligands and cellulose-based IEC media – [267], or continuous chromatography [268].
From an industrial viewpoint, considering the time course of published patents/patent applications on chromatography techniques over the last twenty years (Fig. 5A), column chromatography sensu lato remains the dominant concern of research and development in the field, far above other formats of chromatography (monolith, membrane, expanded bed), and continuous chromatography – in particular SMBC. Restricting to innovations implying viruses, column processes associated with emergence of MAs still dominate (Fig. 5A). Affinity and ion-exchange are the main chromatography modes in published patents associated with chromatography processes and viruses (Fig. 5B). The present review highlights the major part played by chromatography steps using commercially available, PS-based chromatography media in ongoing purification processes of viral particles, whole-cell viruses or VLPs, designed for vaccine formulations or drug/gene delivery vectors. These PS-based adsorbents are also currently implemented in the chromatographic reduction of viral contaminants from biopharmaceutical products. What perspectives for PS-based chromatography materials in this background of technological development are? R&D efforts still focus on CEL- and AG-based particulate adsorbents. A few technological innovations also concern dextran-containing resins.
Fig. 5.
(A) Time evolution of published patents/patent applications on chromatography techniques over the last twenties. Citation counts were obtained from the Lens patent database (www.lens.org/lens/search) applying the following queries in title or abstract (from left to right): “column chromatography”; chromatography AND “simulated moving bed”; chromatography AND monolith; “membrane chromatography” OR “membrane adsorber”; chromatography AND “expanded bed”; “continuous chromatography”. For scaling reasons, citation counts for “column chromatography” are divided by 20. Date range: from origins (1917-01-01) to the X-axis year (included) (latest date: 2018-12-31). Insert shows the number of granted patents/patent applications published between 2000 and latest date associating chromatography techniques and viruses. The above queries were completed by AND (virus OR viral) (AND V). (B) Time evolution of patents/patent applications associating main chromatography modes and viruses published over the last twenties. Citation counts were obtained from the Lens patent database applying the following queries in title or abstract (from left to right): “affinity chromatography” AND V; “ion exchange chromatography” AND V; “size exclusion chromatography” AND V; “hydrophobic interaction chromatography” AND V; “mixed mode chromatography” AND V. Data range as in (A).
The JNC Corporation has developed porous CEL particles [269] functionalized with a sulfated PS (CEL, dextran or pullulan), designed for influenza virus purification [270]. The PS is sulfated before (CEL) or after (dextran, pullulan) binding to CEL beads. The virus purification performance of these new pAFC adsorbents was compared to that of commercial Cellufine® sulfate (see Section 2.1.4), where sulfate groups were introduced into CEL particles via sulfate esterification treatment: they showed higher IAV adsorption capacity allied with reduced adsorption capability of nucleic acid contaminants. The commercial chromatography medium Cellufine™ MAX DexS-VirS uses dextran sulfate polymer as ligand [271]. Academic studies illustrating the potential of this new pAFC resin for virus and VLP capture and purification have not been published so far.
The manufacturing method of monolithic structures made of esterified CEL was patented some years ago [272], but applications in purification bioprocesses are still lacking. CEL-based MAs consisting of non-woven electrospun CEL nanofibers functionalized with DEAE groups for AEC are developed as “FibroSelect” by Puridify Ltd. (Stevenage, UK) (now part of GE Healthcare). They displayed higher dynamic binding capacity for bovine serum albumin (model protein) and protein productivity than Sartobind® membranes when tested in an SMB system [273]. These MAs might be used for viral vaccine isolation, as shown very recently for AdV-5 viral vector [274].
A variety of new ligands to AG beads have been patented by GE Healthcare, including CE ligands (copolymer chains of vinyl sulfonate and N-vinyl pyrrolidone) [275], affinity ligands comprising Ig-binding domains of protein A or protein Z [276,277], and multimodal ligands comprising Q groups and hydrophobic ring structures – as an alternative to N-methyl, N-benzyl ethanolamine ligand of commercial Capto adhere [278]. Note, however, that no reference is made to viruses in examples illustrating biomolecule separation/purification using these new CEC, AFC or MMC adsorbents. Another trend is the manufacture of core bead resins that ally size separation and binding chemistry in a single matrix, as exemplified by the commercial Capto™ Core 700 resin [81] (Table 2). AG bead cores have been functionalized with hydrophobic interaction ligands, e.g., C4–C16 ligands (more particularly octyl ligands) [279], and proteinaceous affinity ligands, e.g., protein A or protein L [280]. Here, these novel core-shell adsorbents were tested for virus (IAV) purification from concentrated cell culture lysates [279] or removal of viral contaminants from mAb samples [280]. It was shown that a protein A core bead prototype had better virus (Φx174 bacteriophage) reduction performance than MabSelect SuRe™ chosen as reference resin [280]. Another type of core bead adsorbent is under development at Upfront Chromatography A/S (Copenhagen, Denmark). The Rhobust® adsorbent consists of spherical beads with a tungsten carbide core and a functionalizable, cross-linked AG outer shell [281], allowing high process flow rates in EBAC for protein capture but not yet applied to viral particle processing.
Dextran is used as a surface extender for binding Q groups to cross-linked AG (CLA) beads in AEC media from GE Healthcare (Q Sepharose® XL, Streamline™ Q XL, Capto™ Q), and for binding weak/strong IE groups to cross-linked CEL beads in Cellufine MAX IEC resins manufactured by JNC [282]. CLA-based AEC media have been widely applied to viral particle purification (Table 2) but Cellufine MAX-based media (IEC and pAFC: see above) have not, so far. Diversifying towards the manufacturing of biopharmaceuticals, the Fujifilm Corporation (Tokyo, Japan) has patented an MMC carrier consisting of a commercial crosslinked AG-based matrix (Seph beads) with a dextran coating functionalized by protein A and C (weak CE) groups [283]. The MMC resin showed better IgG purification efficiency (HCP elimination) than commercial MabSelect Sure (CLA with protein A ligand) and Cellufine MAX S-r bearing S (strong CE) groups. Viral clearance studies on this new CEC/AFC resin have not yet been performed. SEC matrices from GE Healthcare also contain dextran, cross-linked with epichlorohydrin (Sephadex™) or (as allyl dextran) N,N′-methylene-bis-acrylamide (Sephacryl™), and together with CLA (Superdex™) in composite matrices. These CLA-based, dextran-containing media, except the early Sephadex SEC resin, have been frequently implemented in viral particle purification processes (Table 2). The flow properties of composite dextran-CLA SEC medium have been improved with maintained resolution by applying a dextranase treatment [284]. The dextranase-treated resin displayed improved pressure flow compared to a Superdex reference resin. An innovative manufacturing process for cross-linked dextran beads that can be employed in particulate chromatography for purification of biomolecules has been patented [285]. The process advantageously avoided the use of halogenated or aromatic solvents, commonly used in prior art processes. Dextran sulfate is used as ligand coupled to CLA substrate in the Capto™ DeVirS pAFC resin launched some years ago by GE Healthcare [286]. Capto DeVirS may potentially replace the proven Cellufine sulfate in the purification process of yellow fever virus from Vero cell cultures – the pAFC step being followed by Capto Core 700 MMC [287].
In addition to efforts to improve column chromatography media, R&D is also directed towards new methods for purifying viruses/VLPs using commercial chromatographic materials, as illustrated by the latter patent. Other examples are the use of DEAE Seph FF AEC for purifying human caliciviruses such as noroviruses and sapoviruses [288] and Capto Core 700 core bead MMC for purification of respiratory syncytial virus [289].
As a general conclusion of this review and the two preceding ones [10,11], materials based on natural PS, either crude or chemically modified, are essential, widely implemented tools for human struggle against viruses. It appears from these surveys that three widespread PS, namely, agarose, cellulose and chitosan, are prevalently used in the manufacturing of materials with antiviral applications sensu lato. Agarose is derived from agar, which is the third major seaweed PS produced worldwide – behind carrageenan and alginate [290]. AG is the basic component of an arrow of particulate resins for column chromatography that are used for viral vaccine purification or to ensure the viral safety of biopharmaceuticals in industrial bioprocesses and in both academic and R&D studies. In the same way, cellulose, the most widespread PS in the vegetable kingdom, is used in chromatography resins but also in MAs with antiviral applications. Furthermore, CEL-based materials are widely present in viral filters currently used for virus filtration from biopharmaceutical and environmental water samples and in individual protection equipment against airborne viral pathogens [10]. Chitosan is obtained by deacetylation of chitin, which is extracted from the shells of marine crustaceans and is the most widespread PS in the animal kingdom – the second most abundant natural polymer after CEL [291]. Having only limited utilization in chromatography media, e.g., in composite monoliths, chitosan is extensively used as a micro/nanocarrier for the delivery of antiviral drugs and vaccines [11]. Currently, most antiviral applications of PS-based materials involve one of these “big three” natural biopolymers.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Gibson K.E. Viral pathogens in water: occurrence, public health impact, and available control strategies. Curr. Opin. Virol. 2014;4:50–57. doi: 10.1016/j.coviro.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ijaz M.K., Zargar B., Wright K.E. Generic aspects of the airborne spread of human pathogens indoors and emerging air decontamination technologies. Am. J. Infect. Contr. 2016;44:S109–S120. doi: 10.1016/j.ajic.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klamroth R., Gröner A., Simon T.L. Pathogen inactivation and removal methods for plasma-derived clotting factor concentrates. Transfusion. 2014;54:1406–1417. doi: 10.1111/trf.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medlock J.M., Hansford K.M., Schaffner F. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12:435–447. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues A.F., Soares H.R., Guerreiro M.R. Viral vaccines and their manufacturing cell substrates: new trends and designs in modern vaccinology. Biotechnol. J. 2015;10:1329–1344. doi: 10.1002/biot.201400387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guy B., Lang J., Saville M. Vaccination against dengue: challenges and current developments. Annu. Rev. Med. 2016;67:387–404. doi: 10.1146/annurev-med-091014-090848. [DOI] [PubMed] [Google Scholar]
- 7.Pavot V. Ebola virus vaccines: where do we stand? Clin. Immunol. 2016;173:44–49. doi: 10.1016/j.clim.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Esparza J. A brief history of the global effort to develop a preventive HIV vaccine. Vaccine. 2013;31:3502–3518. doi: 10.1016/j.vaccine.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Baumert T.F., Fauvelle C., Chen D.Y. A prophylactic hepatitis C virus vaccine: a distant peak still worth climbing. J. Hepatol. 2014;61:S34–S44. doi: 10.1016/j.jhep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Junter G.A., Lebrun L. Cellulose-based virus-retentive filters: a review. Rev. Environ. Sci. Biotechnol. 2017;16:455–489. doi: 10.1007/s11157-017-9434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junter G.A., Karakasyan C. Polysaccharides against viruses: immunostimulatory properties and the delivery of antiviral vaccines and drugs. Crit. Rev. Ther. Drug. Carrier. Syst. 2020;37:1–64. doi: 10.1615/CritRevTherDrugCarrierSyst.2019027229. [DOI] [PubMed] [Google Scholar]
- 12.Liu H.F., Ma J., Winter C. Recovery and purification process development for monoclonal antibody production. mAbs. 2010;2:480–499. doi: 10.4161/mabs.2.5.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramberger P., Urbas L., Štrancar A. Downstream processing and chromatography based analytical methods for production of vaccines, gene therapy vectors, and bacteriophages. Hum. Vaccines Immunother. 2015;11:1010–1021. doi: 10.1080/21645515.2015.1009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nestola P., Peixoto C., Silva R.R.J.S. Improved virus purification processes for vaccines and gene therapy. Biotechnol. Bioeng. 2015;112:843–857. doi: 10.1002/bit.25545. [DOI] [PubMed] [Google Scholar]
- 15.Josefsberg J.O., Buckland B. Vaccine process technology. Biotechnol. Bioeng. 2012;109:1443–1460. doi: 10.1002/bit.24493. [DOI] [PubMed] [Google Scholar]
- 16.Kushnir N., Streatfield S.J., Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31:58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirbaghaee Z., Bolhassani A. Different applications of virus-like particles in biology and medicine: vaccination and delivery systems. Biopolymers. 2015;105:113–132. doi: 10.1002/bip.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X., Wei M., Pan H. Robust manufacturing and comprehensive characterization of recombinant hepatitis E virus-like particles in Hecolin®. Vaccine. 2014;32:4039–4050. doi: 10.1016/j.vaccine.2014.05.064. [DOI] [PubMed] [Google Scholar]
- 19.Aubrit F., Perugi F., Léon A. Cell substrates for the production of viral vaccines. Vaccine. 2015;33:5905–5912. doi: 10.1016/j.vaccine.2015.06.110. [DOI] [PubMed] [Google Scholar]
- 20.Vicente T., Mota J.P.B., Peixoto C. Rational design and optimization of downstream processes of virus particles for biopharmaceutical applications: current advances. Biotechnol. Adv. 2011;29:869–878. doi: 10.1016/j.biotechadv.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 21.L Effio C., Hubbuch J. Next generation vaccines and vectors: designing downstream processes for recombinant protein-based virus-like particles. Biotechnol. J. 2015;10:715–727. doi: 10.1002/biot.201400392. [DOI] [PubMed] [Google Scholar]
- 22.US D.H.H.S., FDA C.B.E.R. U.S. Department of Health and Human Services; 2010. Guidance for Industry: Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications.https://www.fda.gov/media/78428/download Food and Drug Administration, Center for Biologics Evaluation and Research. [Google Scholar]
- 23.Rodrigues T., Carvalho A., Roldão A. Screening anion-exchange chromatographic matrices for isolation of onco-retroviral vectors. J. Chromatogr. B. 2006;837:59–68. doi: 10.1016/j.jchromb.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 24.Koho T., Mäntylä T., Laurinmäki P. Purification of norovirus-like particles (VLPs) by ion exchange chromatography. J. Virol. Methods. 2012;181:6–11. doi: 10.1016/j.jviromet.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Peixoto C., Ferreira T.B., Carrondo M.J.T. Purification of adenoviral vectors using expanded bed chromatography. J. Virol. Methods. 2006;132:121–126. doi: 10.1016/j.jviromet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Ng M.Y.T., Tan W.S., Abdullah N. Direct purification of recombinant hepatitis B core antigen from two different pre-conditioned unclarified Escherichia coli feedstocks via expanded bed adsorption chromatography. J. Chromatogr. A. 2007;1172:47–56. doi: 10.1016/j.chroma.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 27.Kim H.J., Kim S.Y., Lim S.J. One-step chromatographic purification of human papillomavirus type 16 L1 protein from Saccharomyces cerevisiae. Protein Expr. Purif. 2010;70:68–74. doi: 10.1016/j.pep.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.J., Lim S.J., Kwag H.L. The choice of resin-bound ligand affects the structure and immunogenicity of column-purified human papillomavirus type 16 virus-like particles. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peixoto C., Sousa M.F.Q., Silva A.C. Downstream processing of triple layered rotavirus like particles. J. Biotechnol. 2007;127:452–461. doi: 10.1016/j.jbiotec.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Transfiguracion J., Jorio H., Meghrous J. High yield purification of functional baculovirus vectors by size exclusion chromatography. J. Virol. Methods. 2007;142:21–28. doi: 10.1016/j.jviromet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Kato T., Manoha S.L., Tanaka S. High-titer preparation of Bombyx mori nucleopolyhedrovirus (BmNPV) displaying recombinant protein in silkworm larvae by size exclusion chromatography and its characterization. BMC Biotechnol. 2009;9:55. doi: 10.1186/1472-6750-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang J.Y., Chang C.P., Tsai H.H.P. Selection and characterization of vaccine strain for Enterovirus 71 vaccine development. Vaccine. 2012;30:703–711. doi: 10.1016/j.vaccine.2011.11.087. [DOI] [PubMed] [Google Scholar]
- 33.Wang R., Zhi Y., Guo J. Efficient purification of cell culture-derived classical swine fever virus by ultrafiltration and size-exclusion chromatography. Front. Agr. Sci. Eng. 2015;2:230–236. [Google Scholar]
- 34.Kröber T., Wolff M.W., Hundt B. Continuous purification of influenza virus using simulated moving bed chromatography. J. Chromatogr. A. 2013;1307:99–110. doi: 10.1016/j.chroma.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 35.Nestola P., Silva R.J.S., Peixoto C. Adenovirus purification by two-column, size-exclusion, simulated countercurrent chromatography. J. Chromatogr. A. 2014;1347:111–121. doi: 10.1016/j.chroma.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 36.Hu J., Ni Y., Dryman B.A. Purification of porcine reproductive and respiratory syndrome virus from cell culture using ultrafiltration and heparin affinity chromatography. J. Chromatogr. A. 2010;1217:3489–3493. doi: 10.1016/j.chroma.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Smith R.H., Levy J.R., Kotin R.M. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009;17:1888–1896. doi: 10.1038/mt.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen G.Y., Chen C.Y., Chang M.D.T. Concanavalin A affinity chromatography for efficient baculovirus purification. Biotechnol. Prog. 2009;25:1669–1677. doi: 10.1002/btpr.253. [DOI] [PubMed] [Google Scholar]
- 39.Wolff M.W., Siewert C., Lehmann S. Capturing of cell culture-derived modified vaccinia Ankara virus by ion exchange and pseudo-affinity membrane adsorbers. Biotechnol. Bioeng. 2010;105:761–769. doi: 10.1002/bit.22595. [DOI] [PubMed] [Google Scholar]
- 40.Ohtaki N., Takahashi H., Kaneko K. Purification and concentration of non-infectious West Nile virus-like particles and infectious virions using a pseudo-affinity Cellufine Sulfate column. J. Virol. Methods. 2011;174:131–135. doi: 10.1016/j.jviromet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Hubby B., Talarico T., Maughan M. Development and preclinical evaluation of an alphavirus replicon vaccine for influenza. Vaccine. 2007;25:8180–8189. doi: 10.1016/j.vaccine.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakoda Y., Okamatsu M., Isoda N. Purification of human and avian influenza viruses using cellulose sulfate ester (Cellufine Sulfate) in the process of vaccine production. Microbiol. Immunol. 2012;56:490–495. doi: 10.1111/j.1348-0421.2012.00468.x. [DOI] [PubMed] [Google Scholar]
- 43.Opitz L., Lehmann S., Reichl U. Sulfated membrane adsorbers for economic pseudo-affinity capture of influenza virus particles. Biotechnol. Bioeng. 2009;103:1144–1154. doi: 10.1002/bit.22345. [DOI] [PubMed] [Google Scholar]
- 44.Kanlaya R., Thongboonkerd V. Cellufine sulfate column chromatography as a simple, rapid, and effective method to purify dengue virus. J. Virol. Methods. 2016;234:174–177. doi: 10.1016/j.jviromet.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 45.Reap E.A., Morris J., Dryga S.A. Development and preclinical evaluation of an alphavirus replicon particle vaccine for cytomegalovirus. Vaccine. 2007;25:7441–7449. doi: 10.1016/j.vaccine.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koerber J.T., Jang J.H., Yu J.H. Engineering adeno-associated virus for one-step purification via immobilized metal affinity chromatography. Hum. Gene Ther. 2007;18:367–378. doi: 10.1089/hum.2006.139. [DOI] [PubMed] [Google Scholar]
- 47.Biswal J.K., Bisht P., Subramaniam S. Engineering foot-and-mouth disease virus serotype O IND R2/1975 for one-step purification by immobilized metal affinity chromatography. Biologicals. 2015;43:390–398. doi: 10.1016/j.biologicals.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Chong F.C., Tan W.S., Biak D.R.A. Purification of histidine-tagged nucleocapsid protein of Nipah virus using immobilized metal affinity chromatography. J. Chromatogr. B. 2009;877:1561–1567. doi: 10.1016/j.jchromb.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 49.Joseph N.M.S., Ho K.L., Tey B.T. Production of the virus-like particles of Nipah virus matrix protein in Pichia pastoris as diagnostic reagents. Biotechnol. Prog. 2016;32:1038–1045. doi: 10.1002/btpr.2279. [DOI] [PubMed] [Google Scholar]
- 50.Yap W.B., Tey B.T., Ng M.Y.T. N-terminally His-tagged hepatitis B core antigens: construction, expression, purification and antigenicity. J. Virol. Methods. 2009;160:125–131. doi: 10.1016/j.jviromet.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 51.Yap W.B., Tey B.T., Alitheen N.B.M. Purification of His-tagged hepatitis B core antigen from unclarified bacterial homogenate using immobilized metal affinity-expanded bed adsorption chromatography. J. Chromatogr. A. 2010;1217:3473–3480. doi: 10.1016/j.chroma.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 52.Li H., Yang Y., Zhang Y. A hydrophobic interaction chromatography strategy for purification of inactivated foot-and-mouth disease virus. Protein Expr. Purif. 2015;113:23–29. doi: 10.1016/j.pep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Chong F.C., Tan W.S., Biak D.R.A. A preparative hydrophobic interaction chromatography for purification of recombinant nucleocapsid protein of Nipah virus from clarified Escherichia coli homogenate. Separ. Purif. Technol. 2010;71:97–101. [Google Scholar]
- 54.Chong F.C., Tan W.S., Biak D.R.A. Direct recovery of recombinant nucleocapsid protein of Nipah virus from unclarified Escherichia coli homogenate using hydrophobic interaction expanded bed adsorption chromatography. J. Chromatogr. A. 2010;1217:1293–1297. doi: 10.1016/j.chroma.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 55.Eglon M.N., Duffy A.M., O’Brien T. Purification of adenoviral vectors by combined anion exchange and gel filtration chromatography. J. Gene Med. 2009;11:978–989. doi: 10.1002/jgm.1383. [DOI] [PubMed] [Google Scholar]
- 56.Kalbfuss B., Wolff M., Morenweiser R. Purification of cell culture-derived human influenza A virus by size-exclusion and anion exchange chromatography. Biotechnol. Bioeng. 2007;96:932–944. doi: 10.1002/bit.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bohua L., Ming S., Lu Y. Purification of porcine reproductive and respiratory syndrome virus using ultrafiltration and liquid chromatography. J. Chromatogr. B. 2016;1017:182–186. doi: 10.1016/j.jchromb.2016.01.060. [DOI] [PubMed] [Google Scholar]
- 58.Thomassen Y.E., van ’t Oever A.G., Vinke M. Scale-down of the inactivated polio vaccine production process. Biotechnol. Bioeng. 2013;110:1354–1365. doi: 10.1002/bit.24798. [DOI] [PubMed] [Google Scholar]
- 59.Szurgot I., Jedynak M., Podsiadla-Bialoskorska M. Adenovirus dodecahedron, a VLP, can be purified by size exclusion chromatography instead of time-consuming sucrose density gradient centrifugation. Mol. Biotechnol. 2015;57:565–573. doi: 10.1007/s12033-015-9850-9. [DOI] [PubMed] [Google Scholar]
- 60.Kim S.N., Jeong H.S., Park S.N. Purification and immunogenicity study of human papillomavirus type 16 L1 protein in Saccharomyces cerevisiae. J. Virol. Methods. 2007;139:24–30. doi: 10.1016/j.jviromet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Park M.A., Kim H.J., Kim H.J. Optimum conditions for production and purification of human papillomavirus type 16 L1 protein from Saccharomyces cerevisiae. Protein Expr. Purif. 2008;59:175–181. doi: 10.1016/j.pep.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 62.Tomono T., Hirai Y., Okada H. Ultracentrifugation-free chromatography-mediated large-scale purification of recombinant adeno-associated virus serotype 1 (rAAV1) Mol. Ther.-Meth. Clin. D. 2016;3:15058. doi: 10.1038/mtm.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weigel T., Soliman R., Wolff M.W. Hydrophobic-interaction chromatography for purification of influenza A and B virus. J. Chromatogr. B. 2019;1117:103–117. doi: 10.1016/j.jchromb.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 64.He C., Yang Z., Tong K. Downstream processing of Vero cell-derived human influenza A virus (H1N1) grown in serum-free medium. J. Chromatogr. A. 2011;1218:5279–5285. doi: 10.1016/j.chroma.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 65.Iyer G., Ramaswamy S., Asher D. Reduced surface area chromatography for flow-through purification of viruses and virus like particles. J. Chromatogr. A. 2011;1218:3973–3981. doi: 10.1016/j.chroma.2011.04.086. [DOI] [PubMed] [Google Scholar]
- 66.Lee M.F.X., Chan E.S., Tan W.S. Negative chromatography of hepatitis B virus-like particle : comparative study of different adsorbent designs. J. Chromatogr. A. 2016;1445:1–9. doi: 10.1016/j.chroma.2016.03.066. [DOI] [PubMed] [Google Scholar]
- 67.Blom H., Åkerblom A., Kon T. Efficient chromatographic reduction of ovalbumin for egg-based influenza virus purification. Vaccine. 2014;32:3721–3724. doi: 10.1016/j.vaccine.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 68.James K.T., Cooney B., Agopsowicz K. Novel high-throughput approach for purification of infectious virions. Sci. Rep. 2016;6:36826. doi: 10.1038/srep36826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mundle S.T., Kishko M., Groppo R. Core bead chromatography for preparation of highly pure, infectious respiratory syncytial virus in the negative purification mode. Vaccine. 2016;34:3690–3696. doi: 10.1016/j.vaccine.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 70.Weigel T., Solomaier T., Peuker A. A flow-through chromatography process for influenza A and B virus purification. J. Virol. Methods. 2014;207:45–53. doi: 10.1016/j.jviromet.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 71.GE Healthcare Downstream scale-up purification of influenza virus using single-use bioprocessing equipment, Application note. 2013;29–0435:49. AA. [Google Scholar]
- 72.Tseng Y.F., Weng T.C., Lai C.C. A fast and efficient purification platform for cell-based influenza viruses by flow-through chromatography. Vaccine. 2018;36:3146–3152. doi: 10.1016/j.vaccine.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 73.GE Healthcare . 2014. The use of Capto™ Core 700 and Capto Q ImpRes in the purification of human papilloma virus like particles. Application note 29-0983-01. AA. [Google Scholar]
- 74.Lagoutte P., Mignon C., Donnat S. Scalable chromatography-based purification of virus-like particle carrier for epitope based influenza A vaccine produced in Escherichia coli. J. Virol. Methods. 2016;232:8–11. doi: 10.1016/j.jviromet.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 75.Reiter K., Aguilar P.P., Wetter V. Separation of virus-like particles and extracellular vesicles by flow-through and heparin affinity chromatography. J. Chromatogr. A. 2019;1588:77–84. doi: 10.1016/j.chroma.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 76.Zhao D., Sun B., Jiang H. Enterovirus 71 virus-like particles produced from insect cells and purified by multistep chromatography elicit strong humoral immune responses in mice. J. Appl. Microbiol. 2015;119:1196–1205. doi: 10.1111/jam.12922. [DOI] [PubMed] [Google Scholar]
- 77.Lindgren G., Method for the manufacture of agarose gels, U.S. Pat. 9045566 B2, 2015.
- 78.Lee M.F.X., Chan E.S., Tan W.S. Negative chromatography purification of hepatitis B virus-like particles using poly (oligo (ethylene glycol) methacrylate) grafted cationic adsorbent. J. Chromatogr. A. 2015;1415:161–165. doi: 10.1016/j.chroma.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 79.GE Healthcare . AA; 2008. VIIISelect. Data file 28-9662-37. [Google Scholar]
- 80.Yoshida N., Ishida K., Spherical Sulfated Cellulose and production process for the same, U.S. Pat. Appl. US 20070049746 A1, 2007.
- 81.GE Healthcare Life Sciences . 2013. Multimodal Chromatography, Handbook 29-0548-08. AA. [Google Scholar]
- 82.Trilisky E.I., Lenhoff A.M. Sorption processes in ion-exchange chromatography of viruses. J. Chromatogr. A. 2007;1142:2–12. doi: 10.1016/j.chroma.2006.12.094. [DOI] [PubMed] [Google Scholar]
- 83.Jin Z. Expanded bed adsorption–Challenges and advances in column and process design. Pharmeuropa Bio. 2015;35:66–78. [Google Scholar]
- 84.Zhang C., Zhou D. Adenoviral vector-based strategies against infectious disease and cancer. Hum. Vaccines Immunother. 2016;12:2064–2074. doi: 10.1080/21645515.2016.1165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frietze K.M., Peabody D.S., Chackerian B. Engineering virus-like particles as vaccine platforms. Curr. Opin. Virol. 2016;18:44–49. doi: 10.1016/j.coviro.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kosinska A.D., Bauer T., Protzer U. Therapeutic vaccination for chronic hepatitis B. Curr. Opin. Virol. 2017;23:75–81. doi: 10.1016/j.coviro.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 87.Lihme A., Oishi K., Vaarst I. 2012. Methods for Capturing Virus like Particles from Plants Using Expanded Chromatography. PCT Pat. Appl. WO 2012055986 Al. [Google Scholar]
- 88.Cerqueira C., Schiller J.T. Papillomavirus assembly: an overview and perspectives. Virus Res. 2017;231:103–107. doi: 10.1016/j.virusres.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schiller J.T., Castellsagué X., Garland S.M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30S:F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forman D., de Martel C., Lacey C.J. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30S:F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 91.Crooks A.J., Lee J.M., Stephenson J.R. The purification of alphavirus virions and subviral particles using ultrafiltration and gel exclusion chromatography. Anal. Biochem. 1986;152:295–303. doi: 10.1016/0003-2697(86)90412-4. [DOI] [PubMed] [Google Scholar]
- 92.Nagano H., Yagyu K., Ohta S. Purification of infectious bronchitis coronavirus by Sephacryl S-1000 gel chromatography. Vet. Microbiol. 1989;21:115–123. doi: 10.1016/0378-1135(89)90023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Persson R.H., Cao S.X., Cates G. Modifications of HIV-1 retrovirus-like particles to enhance safety and immunogenicity. Biologicals. 1998;26:255–265. doi: 10.1006/biol.1998.0142. [DOI] [PubMed] [Google Scholar]
- 94.Loa C.C., Lin T.L., Wu C.C. Purification of Turkey coronavirus by Sephacryl size-exclusion chromatography. J. Virol. Methods. 2002;104:187–194. doi: 10.1016/S0166-0934(02)00069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamen A., Henry O. Development and optimization of an adenovirus production process. J. Gene Med. 2004;6(S1):184–192. doi: 10.1002/jgm.503. [DOI] [PubMed] [Google Scholar]
- 96.Cruz P.E., Peixoto C.C., Devos K. Characterization and downstream processing of HIV-1 core and virus-like-particles produced in serum free medium. Enzym. Microb. Technol. 2000;26:61–70. [Google Scholar]
- 97.Dormitzer P.R., Greenberg H.B., Harrison S.C. Purified recombinant rotavirus VP7 forms soluble, calcium-dependent trimers. Virology. 2000;277:420–428. doi: 10.1006/viro.2000.0625. [DOI] [PubMed] [Google Scholar]
- 98.Vellekamp G., Porter F.W., Sutjipto S. Empty capsids in column-purified recombinant adenovirus preparations. Hum. Gene Ther. 2001;12:1923–1936. doi: 10.1089/104303401753153974. [DOI] [PubMed] [Google Scholar]
- 99.Smith R.H., Ding C., Kotin R.M. Serum-free production and column purification of adeno-associated virus type 5. J. Virol. Methods. 2003;114:115–124. doi: 10.1016/j.jviromet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 100.Steinebach F., Müller-Späth T., Morbidelli M. Continuous counter-current chromatography for capture and polishing steps in biopharmaceutical production. Biotechnol. J. 2016;11:1126–1141. doi: 10.1002/biot.201500354. [DOI] [PubMed] [Google Scholar]
- 101.Zydney A.L. Continuous downstream processing for high value biological products: a review. Biotechnol. Bioeng. 2016;113:465–475. doi: 10.1002/bit.25695. [DOI] [PubMed] [Google Scholar]
- 102.Liu J., Thorp S.C. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 2002;22:1–25. doi: 10.1002/med.1026. [DOI] [PubMed] [Google Scholar]
- 103.Baba M., Pauwels R., Balzarini J. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Q., Lin D., Yao S. Review on biomedical and bioengineering applications of cellulose sulfate. Carbohydr. Polym. 2015;132:311–322. doi: 10.1016/j.carbpol.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 105.Vijayalakshmi M.A. Pseudobiospecific ligand affinity chromatography. Trends Biotechnol. 1989;7:71–76. [Google Scholar]
- 106.Oka T., Ohkuma K., Kawahara T. Method for purification of influenza virus. US Pat. 1988;4724210 [Google Scholar]
- 107.Sakamoto K., Gotoh I., Kawahara T. Method for purification of Japanese encephalitis virus. US Pat. 1988;4725546 [Google Scholar]
- 108.Sakamoto K., Ohkuma K., Kawahara T. Method for Purification of Rabic Virus. U.S. Pat. 1988;4725547 [Google Scholar]
- 109.Gericke M., Trygg J., Fardim P. Functional cellulose beads: preparation, characterization, and applications. Chem. Rev. 2013;113:4812–4836. doi: 10.1021/cr300242j. [DOI] [PubMed] [Google Scholar]
- 110.Vicalvi J.J., Hayman E.G., Makowiecki J. Virus purification and formulation process. U.S. Pat. Appl. US 20160264943. 2016;A1 [Google Scholar]
- 111.Sugawara K., Nishiyama K., Ishikawa Y. Development of Vero cell-derived inactivated Japanese encephalitis vaccine. Biologicals. 2002;30:303–314. doi: 10.1006/biol.2002.0345. [DOI] [PubMed] [Google Scholar]
- 112.Miyazaki C., Okada K., Ozaki T. Phase III clinical trials comparing the immunogenicity and safety of the vero cell-derived Japanese encephalitis vaccine Encevac with those of mouse brain-derived vaccine by using the Beijing-1 strain. Clin. Vaccine Immunol. 2014;21:188–195. doi: 10.1128/CVI.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yun K.W., Lee H.J., Kang J.H. Safety and immunogenicity of a freeze-dried, Vero cell culture-derived, inactivated Japanese encephalitis vaccine (KD-287, ENCEVAC®) versus a mouse brain-derived inactivated Japanese encephalitis vaccine in children: a phase III, multicenter, double-blinded, randomized trial. BMC Infect. Dis. 2015;15:7. doi: 10.1186/s12879-014-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Monath T.P., Lee C.K., Julander J.G. Inactivated yellow fever 17D vaccine: development and nonclinical safety, immunogenicity and protective activity. Vaccine. 2010;28:3827–3840. doi: 10.1016/j.vaccine.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 115.Monath T.P., Fowler E., Johnson C.T. An inactivated cell-culture vaccine against yellow fever. N. Engl. J. Med. 2011;364:1326–1333. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]
- 116.Vander Veen R.L., Harris D.L.H., Kamrud K.I. Alphavirus replicon vaccines. Anim. Health Res. Rev. 2012;13:1–9. doi: 10.1017/S1466252312000011. [DOI] [PubMed] [Google Scholar]
- 117.Bernstein D.I., Reap E.A., Katen K. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine. 2010;28:484–493. doi: 10.1016/j.vaccine.2009.09.135. [DOI] [PubMed] [Google Scholar]
- 118.Schleiss M.R. Cytomegalovirus vaccines under clinical development. J. Virus Erad. 2016;2:198–207. doi: 10.1016/S2055-6640(20)30872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kennedy J.S., Greenberg R.N. IMVAMUNE®: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev. Vaccines. 2009;8:13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Von Krempelhuber A., Vollmar J., Pokorny R. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE®. Vaccine. 2010;28:1209–1216. doi: 10.1016/j.vaccine.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frey S.E., Winokur P.L., Hill H. Phase II randomized, double-blinded comparison of a single high dose (5× 10 8 TCID 50) of modified vaccinia Ankara compared to a standard dose (1× 10 8 TCID 50) in healthy vaccinia-naïve individuals. Vaccine. 2014;32:2732–2739. doi: 10.1016/j.vaccine.2014.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Greenberg R.N., Hay C.M., Stapleton J.T. A randomized, double-blind, placebo-controlled Phase II trial investigating the safety and immunogenicity of modified vaccinia Ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hegde N.R. Cell culture-based influenza vaccines: a necessary and indispensable investment for the future. Hum. Vaccines Immunother. 2015;11:1223–1234. doi: 10.1080/21645515.2015.1016666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Milián E., Kamen A.A. Current and emerging cell culture manufacturing technologies for influenza vaccines. BioMed Res. Int. 2015:1–11. doi: 10.1155/2015/504831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Song J.M. Advances in novel influenza vaccines: a patent review. J. Microbiol. 2016;54:403–412. doi: 10.1007/s12275-016-6176-7. [DOI] [PubMed] [Google Scholar]
- 126.Palache A.M., Brands R., M van Scharrenburg G.J. Immunogenicity and reactogenicity of influenza subunit vaccines produced in MDCK cells or fertilized chicken eggs. J. Infect. Dis. 1997;176(S1):20–23. doi: 10.1086/514169. [DOI] [PubMed] [Google Scholar]
- 127.Steinborn C. Solvay Investors Days; London, UK: 2008. Solvay Pharmaceuticals: R&D pipeline overview and key developments.https://www.solvay.com/sites/g/files/srpend221/files/tridion/documents/20080930_Pharma.pdf [Google Scholar]
- 128.Liu J., Shi X., Schwartz R. Use of MDCK cells for production of live attenuated influenza vaccine. Vaccine. 2009;27:6460–6463. doi: 10.1016/j.vaccine.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 129.George M., Farooq M., Dang T. Production of cell culture (MDCK) derived live attenuated influenza vaccine (LAIV) in a fully disposable platform process. Biotechnol. Bioeng. 2010;106:906–917. doi: 10.1002/bit.22753. [DOI] [PubMed] [Google Scholar]
- 130.Liu J., Schwartz R., Thompson M. US Pat. 8357376 B2; 2013. Method of purifying influenza virus and removing MDCK cell DNA contaminants. [Google Scholar]
- 131.World Health Organization (WHO) WHO; Geneva: 2005. Expert Committee on Biological Standardization, Recommendations for the Production and Control of Influenza Vaccine (Inactivated) pp. 99–134. [Google Scholar]
- 132.Erdman M.M., Kamrud K.I., Harris D.L. Alphavirus replicon particle vaccines developed for use in humans induce high levels of antibodies to influenza virus hemagglutinin in swine: proof of concept. Vaccine. 2010;28:594–596. doi: 10.1016/j.vaccine.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 133.de Vries R.D., Rimmelzwaan G.F. Viral vector-based influenza vaccines. Hum. Vaccines Immunother. 2016;12:2881–2901. doi: 10.1080/21645515.2016.1210729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tripp R.A., Tompkins S.M. Virus-vectored influenza virus vaccines. Viruses. 2014;6:3055–3079. doi: 10.3390/v6083055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Block H., Maertens B., Spriestersbach A. Immobilized-metal affinity chromatography (IMAC): a review. Methods Enzymol. 2009;463:439–473. doi: 10.1016/S0076-6879(09)63027-5. [DOI] [PubMed] [Google Scholar]
- 136.Cheung R.C.F., Wong J.H., Ng T.B. Immobilized metal ion affinity chromatography: a review on its applications. Appl. Microbiol. Biotechnol. 2012;96:1411–1420. doi: 10.1007/s00253-012-4507-0. [DOI] [PubMed] [Google Scholar]
- 137.Ye K., Jin S., Ataai M.M. Tagging retrovirus vectors with a metal binding peptide and one-step purification by immobilized metal affinity chromatography. J. Virol. 2004;78:9820–9827. doi: 10.1128/JVI.78.18.9820-9827.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lu Y., Williamson B., Gillespie R. Recent advancement in application of hydrophobic interaction chromatography for aggregate removal in industrial purification process. Curr. Pharmaceut. Biotechnol. 2009;10:427–433. doi: 10.2174/138920109788488897. [DOI] [PubMed] [Google Scholar]
- 139.Wolff M.W., Siewert C., Hansen S.P. Purification of cell culture-derived modified vaccinia Ankara virus by pseudo-affinity membrane adsorbers and hydrophobic interaction chromatography. Biotechnol. Bioeng. 2010;107:312–320. doi: 10.1002/bit.22797. [DOI] [PubMed] [Google Scholar]
- 140.Segura M.M., Puig M., Monfar M. Chromatography purification of canine adenoviral vectors. Hum. Gene Ther. Methods. 2012;23:182–197. doi: 10.1089/hgtb.2012.058. [DOI] [PubMed] [Google Scholar]
- 141.Iyer G., Ramaswamy S., Cheng K.S. Flow through purification processes for large biomolecules. U.S. Pat. Appl. US 2011012863. 2011;A1 [Google Scholar]
- 142.Zhang K., Liu X. Mixed-mode chromatography in pharmaceutical and biopharmaceutical applications. J. Pharm. Biomed. Anal. 2016;128:73–88. doi: 10.1016/j.jpba.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 143.Gagnon P., Cheung C.W., Lepin E.J. Minibodies and multimodal chromatography methods: a convergence of challenge and opportunity. Bioprocess Int. 2010;8:26–35. [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang L., Dai Q., Qiao X. Mixed-mode chromatographic stationary phases: recent advancements and its applications for high-performance liquid chromatography. Trends Anal. Chem. 2016;82:143–163. [Google Scholar]
- 145.Healthcare GE. Purification of influenza A/H1N1 using Capto™ core 700. Application note. 2012;29–0003-34 AA. [Google Scholar]
- 146.Healthcare GE. Ovalbumin removal in egg-based influenza vaccine production using Capto™ Core 700. Application note. 2014;29–1037–62 AA. [Google Scholar]
- 147.Zeltins A. Construction and characterization of virus-like particles: a review. Mol. Biotechnol. 2013;53:92–107. doi: 10.1007/s12033-012-9598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Orr V., Zhong L., Moo-Young M. Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 2013;31:450–465. doi: 10.1016/j.biotechadv.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 149.Ghosh R. Protein separation using membrane chromatography: opportunities and challenges. J. Chromatogr. A. 2002;952:13–27. doi: 10.1016/s0021-9673(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 150.Jungbauer A., Hahn R. Polymethacrylate monoliths for preparative and industrial separation of biomolecular assemblies. J. Chromatogr. A. 2008;1184:62–79. doi: 10.1016/j.chroma.2007.12.087. [DOI] [PubMed] [Google Scholar]
- 151.Svec F., Lv Y. Advances and recent trends in the field of monolithic columns for chromatography. Anal. Chem. 2014;87:250–273. doi: 10.1021/ac504059c. [DOI] [PubMed] [Google Scholar]
- 152.Langer E.S. Industry adoption of membrane adsorbers. Bioprocess Int. 2013;11:16–19. [Google Scholar]
- 153.Gagnon P. The emerging generation of chromatography tools for virus purification. Bioprocess Int. 2008;6(S6):24–30. [Google Scholar]
- 154.Forcic D., Brgles M., Ivancic-Jelecki J. Concentration and purification of rubella virus using monolithic chromatographic support. J. Chromatogr. B. 2011;879:981–986. doi: 10.1016/j.jchromb.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 155.Gerster P., Kopecky E.M., Hammerschmidt N. Purification of infective baculoviruses by monoliths. J. Chromatogr. A. 2013;1290:36–45. doi: 10.1016/j.chroma.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 156.Banjac M., Roethl E., Gelhart F. Purification of Vero cell derived live replication deficient influenza A and B virus by ion exchange monolith chromatography. Vaccine. 2014;32:2487–2492. doi: 10.1016/j.vaccine.2014.02.086. [DOI] [PubMed] [Google Scholar]
- 157.Lucero A.T., Mercado S.A., Sánchez A.C. Purification of adenoviral vector serotype 5 for gene therapy against alcoholism using anion exchange chromatography. J. Chem. Technol. Biotechnol. 2017;92:2445–2452. [Google Scholar]
- 158.Fischer L.M., Wolff M.W., Reichl U. Purification of cell culture-derived influenza A virus via continuous anion exchange chromatography on monoliths. Vaccine. 2018;36:3153–3160. doi: 10.1016/j.vaccine.2017.06.086. [DOI] [PubMed] [Google Scholar]
- 159.Burden C.S., Jin J., Podgornik A. A monolith purification process for virus-like particles from yeast homogenate. J. Chromatogr. B. 2012;880:82–89. doi: 10.1016/j.jchromb.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 160.Steppert P., Burgstaller D., Klausberger M. Purification of HIV-1 gag virus-like particles and separation of other extracellular particles. J. Chromatogr. A. 2016;1455:93–101. doi: 10.1016/j.chroma.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 161.Li Z., Rodriguez E., Azaria S. Affinity monolith chromatography: a review of general principles and applications. Electrophoresis. 2017;38:2837–2850. doi: 10.1002/elps.201700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fernandes C.S.M., Gonçalves B., Sousa M. Biobased monoliths for adenovirus purification. ACS Appl. Mater. Interfaces. 2015;7:6605–6612. doi: 10.1021/am508907b. [DOI] [PubMed] [Google Scholar]
- 163.Kalbfuss B., Wolff M., Geisler L. Direct capture of influenza A virus from cell culture supernatant with Sartobind anion-exchange membrane adsorbers. J. Membr. Sci. 2007;299:251–260. [Google Scholar]
- 164.Grein T.A., Michalsky R., López M.V. Purification of a recombinant baculovirus of Autographa californica M nucleopolyhedrovirus by ion-exchange membrane chromatography. J. Virol. Methods. 2012;183:117–124. doi: 10.1016/j.jviromet.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 165.Peixoto C., Ferreira T.B., Sousa M.F.Q. Towards purification of adenoviral vectors based on membrane technology. Biotechnol. Prog. 2008;24:1290–1296. doi: 10.1002/btpr.25. [DOI] [PubMed] [Google Scholar]
- 166.Vicente T., Peixoto C., Carrondo M.J.T. Purification of recombinant baculoviruses for gene therapy using membrane processes. Gene Ther. 2009;16:766–775. doi: 10.1038/gt.2009.33. [DOI] [PubMed] [Google Scholar]
- 167.Czermak P., Grzenia D.L., Wolf A. Purification of the densonucleosis virus by tangential flow ultrafiltration and by ion exchange membranes. Desalination. 2008;224:23–27. [Google Scholar]
- 168.Carvalho S.B., Fortuna A.R., Wolff M. Purification of influenza virus-like particles using sulfated cellulose membrane adsorbers. J. Chem. Technol. Biotechnol. 2017;93:1988–1996. doi: 10.1002/jctb.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Opitz L., Lehmann S., Zimmermann A. Impact of adsorbents selection on capture efficiency of cell culture derived human influenza viruses. J. Biotechnol. 2007;131:309–317. doi: 10.1016/j.jbiotec.2007.07.723. [DOI] [PubMed] [Google Scholar]
- 170.Opitz L., Hohlweg J., Reichl U. Purification of cell culture-derived influenza virus A/Puerto Rico/8/34 by membrane-based immobilized metal affinity chromatography. J. Virol. Methods. 2009;161:312–316. doi: 10.1016/j.jviromet.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 171.Fortuna A.R., Taft F., Villain L. Optimization of cell culture-derived influenza A virus particles purification using sulfated cellulose membrane adsorbers. Eng. Life Sci. 2018;18:29–39. doi: 10.1002/elsc.201700108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fortuna A.R., van Teeffelen S., Ley A. Use of sulfated cellulose membrane adsorbers for chromatographic purification of cell cultured-derived influenza A and B viruses. Separ. Purif. Technol. 2019;226:350–358. [Google Scholar]
- 173.Marichal-Gallardo P., Pieler M.M., Wolff M.W. Steric exclusion chromatography for purification of cell culture-derived influenza A virus using regenerated cellulose membranes and polyethylene glycol. J. Chromatogr. A. 2017;1483:110–119. doi: 10.1016/j.chroma.2016.12.076. [DOI] [PubMed] [Google Scholar]
- 174.Lee D.S., Kim B.M., Seol D.W. Improved purification of recombinant adenoviral vector by metal affinity membrane chromatography. Biochem. Biophys. Res. Commun. 2009;378:640–644. doi: 10.1016/j.bbrc.2008.11.096. [DOI] [PubMed] [Google Scholar]
- 175.Vicente T., Sousa M.F.Q., Peixoto C. Anion-exchange membrane chromatography for purification of rotavirus-like particles. J. Membr. Sci. 2008;311:270–283. [Google Scholar]
- 176.Fernandes P., Peixoto C., Santiago V.M. Bioprocess development for canine adenovirus type 2 vectors. Gene Ther. 2013;20:353–360. doi: 10.1038/gt.2012.52. [DOI] [PubMed] [Google Scholar]
- 177.Nestola P., Peixoto C., Villain L. Rational development of two flow through purification strategies for adenovirus type 5 and retro virus-like particles. J. Chromatogr. A. 2015;1426:91–101. doi: 10.1016/j.chroma.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 178.Weigel T., Solomaier T., Wehmeyer S. A membrane-based purification process for cell culture-derived influenza A virus. J. Biotechnol. 2016;220:12–20. doi: 10.1016/j.jbiotec.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 179.Tatárová I., Fáber R., Denoyel R. Characterization of pore structure of a strong anion-exchange membrane adsorbent under different buffer and salt concentration conditions. J. Chromatogr. A. 2009;1216:941–947. doi: 10.1016/j.chroma.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 180.Faber R., Yang Y., Gottschalk U. Salt tolerant interaction chromatography for large-scale polishing with convective media. BioPharm Int. Suppl. October. 2009:11–14. [Google Scholar]
- 181.Fröhlich H., Villian L., Melzner D. Membrane technology in bioprocess science. Chem. Ing. Tech. 2012;84:905–917. [Google Scholar]
- 182.M. Wolff, U. Reichl, L. Opitz, Method for the preparation of sulfated cellulose membranes and sulfated cellulose membranes, U.S. Pat. US 8173021 B2, 2012.
- 183.Villain L., Hörl H.H., Brumm C. Sulfated cellulose hydrate membrane, method for producing same, and use of the membrane as an adsorption membrane for a virus purification process. U.S. Pat. Appl. US 20160257941 A1. 2016 [Google Scholar]
- 184.Fischer-Frühholz S., Chirica L., Hirai M. 1st Workshop European Network of Viral Vaccines Processes; Frankfurt am Main, Germany: 2010. Virus Purification with Membrane Chromatography. [Google Scholar]
- 185.Mandenius C.F., Brundin A. Bioprocess optimization using design-of-experiments methodology. Biotechnol. Prog. 2008;24:1191–1203. doi: 10.1002/btpr.67. [DOI] [PubMed] [Google Scholar]
- 186.Lee J., Gan H.T., Latiff S.M.A. Principles and applications of steric exclusion chromatography. J. Chromatogr. A. 2012;1270:162–170. doi: 10.1016/j.chroma.2012.10.062. [DOI] [PubMed] [Google Scholar]
- 187.Remington K.M. Fundamental strategies for viral clearance. Part 1: exploring the regulatory implications. Bioprocess Int. 2015;13:10–16. [Google Scholar]
- 188.Remington K.M. Fundamental strategies for viral clearance. Part 2: technical approaches. Bioprocess Int. 2015;13:10–17. [Google Scholar]
- 189.Shukla A.A., Aranha H. Viral clearance for biopharmaceutical downstream processes. Pharm. Bioprocess. 2015;3:127–138. [Google Scholar]
- 190.Radosevich M., Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control and quality assurance. Vox Sanguinis. 2010;98:12–28. doi: 10.1111/j.1423-0410.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- 191.Roush D.J. Integrated viral clearance strategies—reflecting on the present, projecting to the future. Curr. Opin. Biotechnol. 2018;53:137–143. doi: 10.1016/j.copbio.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 192.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland. 1999. ICH harmonised tripartite guideline, Viral safety evaluation of biotechnology products derived from cell lines of human or animal origin Q5A(R1), Current Step 4 version.https://database.ich.org/sites/default/files/Q5A_R1_Guideline.pdf [Google Scholar]
- 193.Johnston A., Adcock W. The use of chromatography to manufacture purer and safer plasma products. Biotechnol. Genet. Eng. Rev. 2000;17:37–70. doi: 10.1080/02648725.2000.10647987. [DOI] [PubMed] [Google Scholar]
- 194.Miesegaes G., Lute S., Brorson K. Analysis of viral clearance unit operations for monoclonal antibodies. Biotechnol. Bioeng. 2010;106:238–246. doi: 10.1002/bit.22662. [DOI] [PubMed] [Google Scholar]
- 195.Borgvall C., Ericsson U., Gilljam G. 2012. process of purifying coagulation Factor VIII. U.S. Pat. 8329871 B2. [Google Scholar]
- 196.Winge S., Yderland L., Kannicht C. Development, upscaling and validation of the purification process for human-cl rhFVIII (Nuwiq®), a new generation recombinant factor VIII produced in a human cell-line, Protein Expr. Purif. 2015;115:165–175. doi: 10.1016/j.pep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 197.Burnouf T. Chromatography in plasma fractionation: benefits and future trends. J. Chromatogr. B. 1995;664:3–15. doi: 10.1016/0378-4347(94)00532-a. [DOI] [PubMed] [Google Scholar]
- 198.Cameron R., Davies J., Adcock W. The removal of model viruses, poliovirus type 1 and canine parvovirus, during the purification of human albumin using ion-exchange chromatographic procedures. Biologicals. 1997;25:391–401. doi: 10.1006/biol.1997.0115. [DOI] [PubMed] [Google Scholar]
- 199.Adcock W.L., MacGregor A., Davies J.R. Chromatographic removal and heat inactivation of hepatitis A virus during manufacture of human albumin. Biotechnol. Appl. Biochem. 1998;28:85–94. [PubMed] [Google Scholar]
- 200.Xie Y.W., Chan P.K.S., Szeto C.K. Clearance of dengue virus in the plasma-derived therapeutic proteins. Transfusion. 2008;48:1342–1347. doi: 10.1111/j.1537-2995.2008.01647.x. [DOI] [PubMed] [Google Scholar]
- 201.Roberts P.L., Dunkerley C., Walker C. Virus reduction in an intravenous immunoglobulin by solvent/detergent treatment, ion-exchange chromatography and terminal low pH incubation. Biologicals. 2012;40:345–352. doi: 10.1016/j.biologicals.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 202.Miesegaes G.R., Lute S., Strauss D.M. Monoclonal antibody capture and viral clearance by cation exchange chromatography. Biotechnol. Bioeng. 2012;109:2048–2058. doi: 10.1002/bit.24480. [DOI] [PubMed] [Google Scholar]
- 203.Connell-Crowley L., Nguyen T., Bach J. Cation exchange chromatography provides effective retrovirus clearance for antibody purification processes. Biotechnol. Bioeng. 2012;109:157–165. doi: 10.1002/bit.23300. [DOI] [PubMed] [Google Scholar]
- 204.Cipriano D., Burnham M., Hughes J.V. Therapeutic Proteins, Methods in Molecular Biology (Methods and Protocols) vol. 899. Humana Press; Totowa, NJ: 2012. Effectiveness of various processing steps for viral clearance of therapeutic proteins: database analyses of commonly used steps; pp. 277–292. [DOI] [PubMed] [Google Scholar]
- 205.Norling L., Lute S., Emery R. Impact of multiple re-use of anion-exchange chromatography media on virus removal. J. Chromatogr. A. 2005;1069:79–89. doi: 10.1016/j.chroma.2004.09.072. [DOI] [PubMed] [Google Scholar]
- 206.Strauss D.M., Gorrell J., Plancarte M. Anion exchange chromatography provides a robust, predictable process to ensure viral safety of biotechnology products. Biotechnol. Bioeng. 2009;102:168–175. doi: 10.1002/bit.22051. [DOI] [PubMed] [Google Scholar]
- 207.Strauss D.M., Lute S., Tebaykina Z. Understanding the mechanism of virus removal by Q sepharose fast flow chromatography during the purification of CHO-cell derived biotherapeutics. Biotechnol. Bioeng. 2009;104:371–380. doi: 10.1002/bit.22416. [DOI] [PubMed] [Google Scholar]
- 208.Strauss D.M., Cano T., Cai N. Strategies for developing design spaces for viral clearance by anion exchange chromatography during monoclonal antibody production. Biotechnol. Prog. 2010;26:750–755. doi: 10.1002/btpr.385. [DOI] [PubMed] [Google Scholar]
- 209.Connell-Crowley L., Larimore E.A., Gillespie R. Using high throughput screening to define virus clearance by chromatography resins. Biotechnol. Bioeng. 2013;110:1984–1994. doi: 10.1002/bit.24869. [DOI] [PubMed] [Google Scholar]
- 210.Yang B., Wang H., Ho C. Porcine circovirus (PCV) removal by Q sepharose fast flow chromatography. Biotechnol. Prog. 2013;29:1464–1471. doi: 10.1002/btpr.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nowak T., Popp B., Gröner A. Pathogen safety of a pasteurized four-factor human prothrombin complex concentrate preparation using serial 20N virus filtration. Transfusion. 2017;57:1184–1191. doi: 10.1111/trf.14010. [DOI] [PubMed] [Google Scholar]
- 212.Ayyar B.V., Arora S., Murphy C. Affinity chromatography as a tool for antibody purification. Methods. 2012;56:116–129. doi: 10.1016/j.ymeth.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 213.Arora S., Saxena V., Ayyar B.V. Affinity chromatography: a versatile technique for antibody purification. Methods. 2017;116:84–94. doi: 10.1016/j.ymeth.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 214.McCue J., Osborne D., Dumont J. Validation of the manufacturing process used to produce long-acting recombinant factor IX Fc fusion protein. Haemophilia. 2014;20:e327–e335. doi: 10.1111/hae.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McCue J., Kshirsagar R., Selvitelli K. Manufacturing process used to produce long-acting recombinant factor VIII Fc fusion protein. Biologicals. 2015;43:213–219. doi: 10.1016/j.biologicals.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 216.Kelley B., Jankowski M., Booth J. An improved manufacturing process for Xyntha/ReFacto AF. Haemophilia. 2010;16:717–725. doi: 10.1111/j.1365-2516.2009.02160.x. [DOI] [PubMed] [Google Scholar]
- 217.Ellgaard T.W., Bindslev L., Kamstrup S. Evaluation of the virus clearance capacity and robustness of the manufacturing process for the recombinant factor VIII protein, turoctocog alfa, Protein Expr. Purif. 2017;129:94–100. doi: 10.1016/j.pep.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 218.Kruljec N., Bratkovič T. Alternative affinity ligands for immunoglobulins. Bioconjug. Chem. 2017;28:2009–2030. doi: 10.1021/acs.bioconjchem.7b00335. [DOI] [PubMed] [Google Scholar]
- 219.Hober S., Nord K., Linhult M. Protein A chromatography for antibody purification. J. Chromatogr. B. 2007;848:40–47. doi: 10.1016/j.jchromb.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 220.Gronemeyer P., Ditz R., Strube J. Trends in upstream and downstream process development for antibody manufacturing. Bioengineering. 2014:188–212. doi: 10.3390/bioengineering1040188. [DOI] [PubMed] [Google Scholar]
- 221.Bolton G.R., Mehta K.K. The role of more than 40 years of improvement in protein A chromatography in the growth of the therapeutic antibody industry, Biotechnol. Prog. 2016;32:1193–1202. doi: 10.1002/btpr.2324. [DOI] [PubMed] [Google Scholar]
- 222.Zhang M., Miesegaes G.R., Lee M. Quality by design approach for viral clearance by protein A chromatography. Biotechnol. Bioeng. 2014;111:95–103. doi: 10.1002/bit.24999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Brorson K., Brown J., Hamilton E. Identification of protein A media performance attributes that can be monitored as surrogates for retrovirus clearance during extended re-use. J. Chromatogr. A. 2003;989:155–163. doi: 10.1016/s0021-9673(02)01697-7. [DOI] [PubMed] [Google Scholar]
- 224.Lute S., Norling L., Hanson M. Robustness of virus removal by protein A chromatography is independent of media lifetime. J. Chromatogr. A. 2008;1205:17–25. doi: 10.1016/j.chroma.2008.07.094. [DOI] [PubMed] [Google Scholar]
- 225.Bach J., Connell-Crowley L. Clearance of the rodent retrovirus, XMuLV, by protein A chromatography. Biotechnol. Bioeng. 2015;112:743–750. doi: 10.1002/bit.25484. [DOI] [PubMed] [Google Scholar]
- 226.Roberts P.L. Virus elimination during the purification of monoclonal antibodies by column chromatography and additional steps. Biotechnol. Prog. 2014;30:1341–1347. doi: 10.1002/btpr.1984. [DOI] [PubMed] [Google Scholar]
- 227.Lieuw K. Many factor VIII products available in the treatment of hemophilia A: an embarrassment of riches? J. Blood Med. 2017;8:67–73. doi: 10.2147/JBM.S103796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Garger S., Severs J., Regan L. BAY 81-8973, a full-length recombinant factor VIII: manufacturing processes and product characteristics. Haemophilia. 2017;23:e67–e78. doi: 10.1111/hae.13148. [DOI] [PubMed] [Google Scholar]
- 229.Swiech K., Picanço-Castro V., Covas D.T. Production of recombinant coagulation factors: are humans the best host cells? Bioengineered. 2017;8:462–470. doi: 10.1080/21655979.2017.1279767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Charlebois T.S., O’Connell B.D., Adamson S.R. Viral safety of B-domain deleted recombinant factor VIII, Semin. Hematologia. 2001;38:32–39. doi: 10.1016/s0037-1963(01)90106-4. [DOI] [PubMed] [Google Scholar]
- 231.Roberts P.L. Virus elimination during the recycling of chromatographic columns used during the manufacture of coagulation factors. Biologicals. 2014;42:184–190. doi: 10.1016/j.biologicals.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 232.Eriksson R.K., Fenge C., Lindner-Olsson E. The manufacturing process for B-domain deleted recombinant factor VIII, Semin. Hematologia. 2001;38:24–31. doi: 10.1016/s0037-1963(01)90105-2. [DOI] [PubMed] [Google Scholar]
- 233.Griffith M. Ultrapure plasma factor VIII produced by anti-FVIIIc immunoaffinity chromatography and solvent/detergent viral inactivation. Characterization of the Method M process and Hemofil M antihemophilic factor (human) Ann. Hematol. 1991;63:131–137. doi: 10.1007/BF01703243. [DOI] [PubMed] [Google Scholar]
- 234.Neslund G.G., Liu S.L., Griffith M.J. 1995. Ultrapurification process for factor VIII. U.S. Pat. 5470954. [Google Scholar]
- 235.United Kingdom Haemophilia Centre Doctors’ Organisation (UKHCDO) Guidelines on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. Haemophilia. 2003;9:1–23. [Google Scholar]
- 236.Ezban M., Vad K., Kjalke M. Turoctocog alfa (NovoEight®)–from design to clinical proof of concept. Eur. J. Haematol. 2014;93:369–376. doi: 10.1111/ejh.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ahmadian H., Hansen E.B., Faber J.H. Molecular design and downstream processing of turoctocog alfa (NovoEight), a B-domain truncated factor VIII molecule, Blood Coagul. Fibrinolysis. 2016;27:568–575. doi: 10.1097/MBC.0000000000000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hassanzadeh-Ghassabeh G., Devoogdt N., De Pauw P. Nanobodies and their potential applications. Nanomedicine. 2013;8:1013–1026. doi: 10.2217/nnm.13.86. [DOI] [PubMed] [Google Scholar]
- 239.Detmers F., Hermans P., Jiao J.A. Novel affinity ligands provide for highly selective primary capture. Bioprocess Int. 2010;8:50–54. [Google Scholar]
- 240.McCue J.T., Selvitelli K., Walker J. Application of a novel affinity adsorbent for the capture and purification of recombinant factor VIII compounds. J. Chromatogr. A. 2009;1216:7824–7830. doi: 10.1016/j.chroma.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 241.Rodrigues E.S., Verinaud C.I., Oliveira D.S. Purification of coagulation factor VIII by immobilized metal affinity chromatography. Biotechnol. Appl. Biochem. 2015;62:343–348. doi: 10.1002/bab.1276. [DOI] [PubMed] [Google Scholar]
- 242.Roberts P. Virological safety of the purified factor IX concentrate, Replenine. Haemophilia. 1995;1:19–22. doi: 10.1111/j.1365-2516.1995.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 243.Feldman P.A. Development and characterization of the high-purity factor IX, Replenine. Haemophilia. 1995;2:12–15. doi: 10.1111/j.1365-2516.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 244.Kelley B.D., Jakubik J., Vicik S. Viral clearance studies on new and used chromatography resins: critical review of a large dataset. Biologicals. 2008;36:88–98. doi: 10.1016/j.biologicals.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 245.Gröner A., Nowak T., Schäfer W. Pathogen safety of human C1 esterase inhibitor concentrate. Transfusion. 2012;52:2104–2112. doi: 10.1111/j.1537-2995.2012.03590.x. [DOI] [PubMed] [Google Scholar]
- 246.Brown M.R., Johnson S.A., Brorson K.A. A step-wise approach to define binding mechanisms of surrogate viral particles to multi-modal anion exchange resin in a single solute system. Biotechnol. Bioeng. 2017;114:1487–1494. doi: 10.1002/bit.26251. [DOI] [PubMed] [Google Scholar]
- 247.Brown M.R., Burnham M.S., Johnson S.A. Evaluating the effect of in-process material on the binding mechanisms of surrogate viral particles to a multi-modal anion exchange resin. J. Biotechnol. 2018;267:29–35. doi: 10.1016/j.jbiotec.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 248.Zhou J.X., Tressel T. Membrane chromatography as a robust purification system for large-scale antibody production. BioProcess Int. 2005;3:32–37. [Google Scholar]
- 249.Miesegaes G.R., Lute S.C., Read E.K. Viral clearance by flow-through mode ion exchange columns and membrane adsorbers. Biotechnol. Prog. 2014;30:124–131. doi: 10.1002/btpr.1832. [DOI] [PubMed] [Google Scholar]
- 250.Liu Z., Wickramasinghe S.R., Qian X. Membrane chromatography for protein purifications from ligand design to functionalization. Separ. Sci. Technol. 2017;52:299–319. [Google Scholar]
- 251.Knudsen H.L., Fahrner R.L., Xu Y. Membrane ion-exchange chromatography for process-scale antibody purification. J. Chromatogr. A. 2001;907:145–154. doi: 10.1016/s0021-9673(00)01041-4. [DOI] [PubMed] [Google Scholar]
- 252.Zhou J.X., Tressel T., Gottschalk U. New Q membrane scale-down model for process-scale antibody purification. J. Chromatogr. A. 2006;1134:66–73. doi: 10.1016/j.chroma.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 253.Zhou J.X., Solamo F., Hong T. Viral clearance using disposable systems in monoclonal antibody commercial downstream processing. Biotechnol. Bioeng. 2008;100:488–496. doi: 10.1002/bit.21781. [DOI] [PubMed] [Google Scholar]
- 254.Johansson B.L., Belew M., Eriksson S. Preparation and characterization of prototypes for multi-modal separation media aimed for capture of negatively charged biomolecules at high salt conditions. J. Chromatogr. A. 2003;1016:21–33. doi: 10.1016/s0021-9673(03)01140-3. [DOI] [PubMed] [Google Scholar]
- 255.Riordan W., Heilmann S., Brorson K. Design of salt-tolerant membrane adsorbers for viral clearance. Biotechnol. Bioeng. 2009;103:920–929. doi: 10.1002/bit.22314. [DOI] [PubMed] [Google Scholar]
- 256.Bhut B.V., Weaver J., Carter A.R. The role of polymer nanolayer architecture on the separation performance of anion-exchange membrane adsorbers: I. Protein separations. Biotechnol. Bioeng. 2011;108:2645–2653. doi: 10.1002/bit.23221. [DOI] [PubMed] [Google Scholar]
- 257.Bhut B.V., Weaver J., Carter A.R. The role of polymer nanolayer architecture on the separation performance of anion-exchange membrane adsorbers: Part II. DNA and virus separations. Biotechnol. Bioeng. 2011;108:2654–2660. doi: 10.1002/bit.23222. [DOI] [PubMed] [Google Scholar]
- 258.Woo M., Khan N.Z., Royce J. A novel primary amine-based anion exchange membrane adsorber. J. Chromatogr. A. 2011;1218:5386–5392. doi: 10.1016/j.chroma.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 259.Weaver J., Husson S.M., Murphy L. Anion exchange membrane adsorbers for flow-through polishing steps: Part I. clearance of minute virus of mice. Biotechnol. Bioeng. 2013;110:491–499. doi: 10.1002/bit.24720. [DOI] [PubMed] [Google Scholar]
- 260.Weaver J., Husson S.M., Murphy L. Anion exchange membrane adsorbers for flow-through polishing steps: Part II. Virus, host cell protein, DNA clearance, and antibody recovery. Biotechnol. Bioeng. 2013;110:500–510. doi: 10.1002/bit.24724. [DOI] [PubMed] [Google Scholar]
- 261.Rathore A.S., Kumar D., Kateja N. Recent developments in chromatographic purification of biopharmaceuticals. Biotechnol. Lett. 2018;40:895–905. doi: 10.1007/s10529-018-2552-1. [DOI] [PubMed] [Google Scholar]
- 262.Shukla A.A., Wolfe L.S., Mostafa S.S. Evolving trends in mAb production processes. Bioeng. Transl. Med. 2017;2:58–69. doi: 10.1002/btm2.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhao M., Vandersluis M., Stout J. Affinity chromatography for vaccines manufacturing: finally ready for prime time? Vaccine. 2019;37:5491–5503. doi: 10.1016/j.vaccine.2018.02.090. [DOI] [PubMed] [Google Scholar]
- 264.Ramos-de-la-Peña A.M., González-Valdez J., Aguilar O. Protein A chromatography: challenges and progress in the purification of monoclonal antibodies. J. Sep. Sci. 2019;42:1816–1827. doi: 10.1002/jssc.201800963. [DOI] [PubMed] [Google Scholar]
- 265.Halan V., Maity S., Bhambure R. Multimodal chromatography for purification of biotherapeutics – a review. Curr. Protein Pept. Sci. 2019;20:4–13. doi: 10.2174/1389203718666171020103559. [DOI] [PubMed] [Google Scholar]
- 266.Sýkora D., Řezanka P., Záruba K. Recent advances in mixed-mode chromatographic stationary phases. J. Sep. Sci. 2019;42:84–129. doi: 10.1002/jssc.201801048. [DOI] [PubMed] [Google Scholar]
- 267.Li Y., Stern D., Lock L.L. Emerging biomaterials for downstream manufacturing of therapeutic proteins. Acta Biomater. 2019;95:73–90. doi: 10.1016/j.actbio.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 268.Vogg S., Müller-Späth T., Morbidelli M. Current status and future challenges in continuous biochromatography. Curr. Opin. Chem. Eng. 2018;22:138–144. [Google Scholar]
- 269.Kurabayashi T., Aoyama S., Uchida A. 2016. Method for producing porous cellulose particles, and porous cellulose particles. U.S. Pat. Appl. US 20160200835 A1. [Google Scholar]
- 270.Yamamoto Y., Yamamoto Y., Umeda Y. 2018. Preparation method of chromatography medium. U.S. Pat. 10010863 B2. [Google Scholar]
- 271.Corporation J.N.C. 2018. Cellufine™ MAX DexS-VirS, Technical Note TN_MAX DexS-VirS_Ver 1.0_20180615. [Google Scholar]
- 272.Nakama T., Todokoro M., Iwamoto E. A1, 2014. Polysaccharide monolithic structure and manufacturing method therefor. U.S. Pat. Appl. US 20140311984. [Google Scholar]
- 273.D.G. Bracewell, R. Stevens, O. Hardick, Chromatography Medium, U.S. Pat. 9802979 B2, 2017.
- 274.Turnbull J., Wright B., Green N.K. Adenovirus 5 recovery using nanofiber ion exchange adsorbents. Biotechnol. Bioeng. 2019;116:1698–1709. doi: 10.1002/bit.26972. [DOI] [PubMed] [Google Scholar]
- 275.Hansson J., Rodrigo G., Soderman T.E. 2018. Separation method and separation matrix. U.S. Pat. 10124328 B2. [Google Scholar]
- 276.Johansson H.J., Palmgren R. 2017. Affinity chromatography matrix. U.S. Pat. 9657055 B2. [Google Scholar]
- 277.Ander M., Bauren G., Bjorkman T. 2019. Affinity chromatography matrix. U.S. Pat. 10189891 B2. [Google Scholar]
- 278.Maloisel J.L., Rodrigo G., Noren B. 2019. Multimodal anion exchange matrices. U.S. Pat. Appl. US 20190023735 A1. [Google Scholar]
- 279.Bergstrom J., Glad G., Johansson B.L. 2017. Chromatography medium. U.S. Pat. Appl. US 20170050121 A1. [Google Scholar]
- 280.Maloisel J.L., Lind O., Noren B. Chromatography method. U.S. Pat. Appl. US 20170051010. 2017;A1 [Google Scholar]
- 281.Cheng K.S., Ramaswamy S., Bian N. Method and apparatus for making porous agarose beads. Eur. Pat. EP. 2016;1764151 B1 [Google Scholar]
- 282.Corporation J.N.C. vol 3. 2015. Ion Exchange Chromatography Media: Cellufine MAX S, Q, CM, DEAE, Technical Data Sheet DF-MAX IEX-EN Ver. 1. [Google Scholar]
- 283.Kihara S. Affinity chromatography carrier and method for purifying biological substance. 2018;A1 U.S. Pat. Appl. US 20180229215. [Google Scholar]
- 284.Norrman N., Algotsson M., Eriksson S.D. Method and chromatography medium. 2018;A1 US Pat. Appl. US 20180304231. [Google Scholar]
- 285.Emilsson P.E., Lindberg S.K.M., Wernersson J. 2018. Manufacturing process for polysaccharide beads. U.S. Pat. Appl. US 20180291184 A1. [Google Scholar]
- 286.Healthcare GE, DeVirS Capto™. AA; 2009. Data file 28-9616-49. [Google Scholar]
- 287.Vicalvi J.J., Hayman E.G., Makowiecki J. 2019. Virus purification and formulation process. U.S. Pat. 10196615 B2. [Google Scholar]
- 288.Vedvick T.S., Steadman B., Richardson C. 2019. Virus like particle purification. U.S. Pat. 10172930 B2. [Google Scholar]
- 289.Mundle S.T. Purification of respiratory syncytial virus. U.S. Pat. Appl. US 20180273909. 2018;A1 [Google Scholar]
- 290.Bixler H.J., Porse H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011;23:321–335. [Google Scholar]
- 291.Kurita K. Chitin and chitosan: functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006;8:203–226. doi: 10.1007/s10126-005-0097-5. [DOI] [PubMed] [Google Scholar]








