Abstract
Objective
The aim of this systematic review was to report pregnancy and perinatal outcomes of coronavirus spectrum infections, and particularly coronavirus 2019 (COVID-19) disease because of severe acute respiratory syndrome–coronavirus-2 infection during pregnancy.
Data Sources
Medline, Embase, Cinahl, and Clinicaltrials.gov databases were searched electronically utilizing combinations of word variants for coronavirus or severe acute respiratory syndrome or SARS or Middle East respiratory syndrome or MERS or COVID-19 and pregnancy. The search and selection criteria were restricted to English language.
Study Eligibility Criteria
Inclusion criteria were hospitalized pregnant women with a confirmed coronavirus related–illness, defined as severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), or COVID-19.
Study Appraisal and Synthesis Methods
We used meta-analyses of proportions to combine data and reported pooled proportions, so that a pooled proportion may not coincide with the actual raw proportion in the results. The pregnancy outcomes observed included miscarriage, preterm birth, preeclampsia, preterm prelabor rupture of membranes, fetal growth restriction, and mode of delivery. The perinatal outcomes observed were fetal distress, Apgar score <7 at 5 minutes, neonatal asphyxia, admission to a neonatal intensive care unit, perinatal death, and evidence of vertical transmission.
Results
Nineteen studies including 79 hospitalized women were eligible for this systematic review: 41 pregnancies (51.9%) affected by COVID-19, 12 (15.2%) by MERS, and 26 (32.9%) by SARS. An overt diagnosis of pneumonia was made in 91.8%, and the most common symptoms were fever (82.6%), cough (57.1%), and dyspnea (27.0%). For all coronavirus infections, the pooled proportion of miscarriage was 64.7% (8/12; 95% confidence interval, 37.9-87.3), although reported only for women affected by SARS in two studies with no control group; the pooled proportion of preterm birth <37 weeks was 24.3% (14/56; 95% confidence interval, 12.5–38.6); premature prelabor rupture of membranes occurred in 20.7% (6/34; 95% confidence interval, 9.5–34.9), preeclampsia in 16.2% (2/19; 95% confidence interval, 4.2–34.1), and fetal growth restriction in 11.7% (2/29; 95% confidence interval, 3.2–24.4), although reported only for women affected by SARS; 84% (50/58) were delivered by cesarean; the pooled proportion of perinatal death was 11.1% (5/60; 95% confidence interval, 84.8–19.6), and 57.2% of newborns (3/12; 95% confidence interval, 3.6–99.8) were admitted to the neonatal intensive care unit. When focusing on COVID-19, the most common adverse pregnancy outcome was preterm birth <37 weeks, occurring in 41.1% of cases (14/32; 95% confidence interval, 25.6–57.6), while the pooled proportion of perinatal death was 7.0% (2/41; 95% confidence interval, 1.4–16.3). None of the 41 newborns assessed showed clinical signs of vertical transmission.
Conclusion
In hospitalized mothers infected with coronavirus infections, including COVID-19, >90% of whom also had pneumonia, preterm birth is the most common adverse pregnancy outcome. COVID-19 infection was associated with higher rate (and pooled proportions) of preterm birth, preeclampsia, cesarean, and perinatal death. There have been no published cases of clinical evidence of vertical transmission. Evidence is accumulating rapidly, so these data may need to be updated soon. The findings from this study can guide and enhance prenatal counseling of women with COVID-19 infection occurring during pregnancy, although they should be interpreted with caution in view of the very small number of included cases.
Key words: coronavirus, coronavirus 2019, infection, Middle East respiratory syndrome, pregnancy, severe acute respiratory syndrome, severe acute respiratory syndrome–coronavirus-2
Coronavirus (CoV) is an enveloped, positive-stranded ribonucleic acid (RNA) virus of the family of Coronaviridae and belonging to the Nidovirales order,1 generally causing respiratory and gastrointestinal infections that might range from mild, self-limiting conditions to more serious disorders, such as viral pneumonia with systemic impairment.2
AJOG MFM at a Glance.
Why was this study published?
Coronavirus 2019 (COVID-19) disease secondary to severe acute respiratory syndrome–coronavirus-2 infection is a worldwide pandemic with an increasing number of confirmed cases every day. Little is known about the effect of coronavirus (CoV)-related infections during pregnancy.
Key findings
Hospitalized pregnant women with COVID-19 infection had higher rates (and pooled proportions) of preterm birth, preeclampsia, cesarean delivery, and perinatal death. These findings should be interpreted with caution in view of the very small number of included cases.
What does this add to what is known?
This is the first systematic review exploring pregnancy and perinatal outcomes of CoV infections occurring during pregnancy. Although limited, these data can guide and enhance prenatal counseling of women with COVID-19 infection occurring during pregnancy. Evidence is accumulating rapidly, so these data may need to be updated soon.
In the last 2 decades, CoV has been responsible for 2 large epidemics: the severe acute respiratory syndrome (SARS) that infected 8098 people with a case-fatality rate of about 10.5%3 and the Middle East respiratory syndrome (MERS) with a total of 2519 laboratory-confirmed cases and a case fatality rate of 34.4%.4
Towards the end of 2019, a novel mutation of CoV (labeled as SARS–coronavirus-2) was identified as the cause of a severe respiratory illness, called coronavirus 2019 (COVID-19), that typically presents with fever and cough.5 Infected people show abnormal findings at diagnostic imaging, suggestive for pneumonia.
After beginning as an epidemic in China, COVID-19 infection has rapidly spread in many other countries, and the number of affected cases continues to increase significantly on a daily basis. The overall mortality rate ranges from 3% to 4% according to the World Health Organization reports,6 but a higher rate of patients require admission to the intensive care unit (ICU).7
It is well known that physiologic maternal adaptations to pregnancy predispose pregnant women to a more severe course of pneumonia, with subsequent higher maternal and fetal morbidity and mortality,1 , 8 but there is a lack of data in the literature about the effect of CoV infections during pregnancy, thus limiting both counseling and management of these patients.
Objective
The aim of this systematic review was to report pregnancy and perinatal outcomes of CoV spectrum infections and particularly COVID-19 during pregnancy.
Materials and Methods
Search strategy and selection criteria
This review was performed according to a priori–designed protocol recommended for systematic reviews and meta-analysis.9, 10, 11 Medline, Embase, Cinahl, and Clinicaltrials.gov databases were searched electronically on March 13, 2020, utilizing combinations of the relevant medical subject heading (MeSH) terms, key words, and word variants for coronavirus or severe acute respiratory syndrome or SARS or Middle East respiratory syndrome or MERS or COVID-19 and pregnancy. The search and selection criteria were restricted to English language. Reference lists of relevant articles and reviews were hand searched for additional reports. Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-Analysis of Observational Studies in Epidemiology guidelines were followed.12, 13, 14
Inclusion criteria were hospitalized pregnant women with a confirmed coronavirus-spectrum illness, defined as SARS, MERS or COVID-19 infection.
The pregnancy outcomes observed were as follows:
-
•
Preterm birth (PTB; either before 37 or 34 weeks of gestation).
-
•
Preeclampsia (PE).
-
•
Preterm prelabor rupture of membranes (pPROM).
-
•
Fetal growth restriction (FGR).
-
•
Miscarriage, as defined by the authors.
-
•
Cesarean mode of delivery.
The perinatal outcomes observed were as follows:
-
•
Fetal distress (as defined by original authors).
-
•
Apgar score <7 at 5 minutes.
-
•
Neonatal asphyxia (as defined by original authors).
-
•
Admission to neonatal intensive care unit (NICU).
-
•
Perinatal death, including both stillbirth and neonatal death.
-
•
Evidence of vertical transmission, defined as the presence of clinical signs of mother-to-child transmission in the antenatal or perinatal period.
Furthermore, we aimed to perform a subgroup analysis according to the trimester of pregnancy at infection and the type of coronavirus.
Data from studies reporting the incidence of these outcomes in pregnancies with CoV-spectrum infections were considered eligible for analysis. For the purpose of the analysis, we included only full-text articles with data of pregnant women who already delivered; we excluded data regarding ongoing pregnancies. Furthermore, because these are relatively rare infections occurring during pregnancy with the majority of data coming from studies with small sample sizes, case reports and case series were also included in the analysis.
Studies reporting cases of infective pneumonia or other respiratory disorders during pregnancy caused by other viral agents were excluded. We also excluded studies pediatric series on newborns and children from which maternal and pregnancy information could not be extrapolated.
Two authors (D.D.M., G.S.) reviewed all abstracts independently. Agreement regarding potential relevance or inconsistencies was reached by consensus or resolved by discussion with a third reviewer (F.D.A.). Full-text copies of applicable papers were obtained, and the same reviewers independently extracted relevant data regarding study characteristics and pregnancy outcome. If more than 1 study was published on the same cohort with identical endpoints, the report containing the most comprehensive information on the population was included to avoid overlapping populations.
Data analysis
We used meta-analyses of proportions to combine data and reported pooled proportions. Funnel plots (displaying the outcome rate from individual studies vs their precision [1 per SE]) were carried out with an exploratory aim. Tests for funnel plot asymmetry were not used when the total number of publications included for each outcome was <10. In this case, the power of the tests is too low to distinguish chance from real asymmetry.
Between-study heterogeneity was explored using the I2 statistic, which represents the percentage of between-study variation that is due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, whereas I2 values ≥50% indicate a substantial level of heterogeneity. In view of the clinical heterogeneity, a random-effect model was used to compute the pooled data analyses. Therefore, polled proportions were used, also given small numbers, but should not be confused with raw proportions (rates). A pooled proportion may not coincide with the actual raw proportion. All proportion meta-analyses were carried out by using StatsDirect version 2.7.9 (StatsDirect, Ltd, Altrincham, Cheshire, United Kingdom).
Quality assessment of the included studies was assessed using the methodological quality and synthesis of case series and case reports described by Murad et al.15 According to this tool, each study is judged on 4 broad perspectives: the selection of the study groups, the ascertainment and the causality of the outcome observed, and the reporting of the case. A study can be awarded a maximum of 1 star for each numbered item within the selection and reporting categories, 2 stars for ascertainment, and 4 stars for comparability.15 Given emergency need for this guidance, Prospective Register of Systematic Reviews, registration was not sought.
Results
Study selection and characteristics
Five hundred thirty-eight articles were identified, 27 were assessed with respect to their eligibility for inclusion, and 19 studies were included in the systematic review (Table 1 , Figure , and Supplemental Table 1).
Table 1.
General characteristics of the included studies
| Author | Year | Study location | Study period | Study design | Pregnancies, n | Type of coronavirus | Mean maternal age, y |
|---|---|---|---|---|---|---|---|
| Chen et al16 | 2020 | China | 2020 | Retrospective | 9 | SARS-CoV-2 | 29.9 |
| Wang et al7 | 2020 | China | 2020 | Case report | 1 | SARS-CoV-2 | 28.0 |
| Zhu et al18 | 2020 | China | 2020 | Retrospective | 9 | SARS-CoV-2 | 30.9 |
| Li et al19 | 2020 | China | 2020 | Case report | 1 | SARS-CoV-2 | 30.0 |
| Liu et al20a | 2020 | Hubei, China | 2020 | Retrospective | 11 | SARS-CoV-2 | 32.5 |
| Liu et al21 | 2020 | Guangdong, China | 2020 | Retrospective | 10 | SARS-CoV-2 | 30.5 |
| Alfaraj et al22 | 2019 | Saudi Arabia | 2015 | Case series | 2 | MERS-CoV | 34.0 |
| Jeong et al23 | 2017 | South Korea | 2015 | Case report | 1 | MERS-CoV | 39.0 |
| Alserehi et al24 | 2016 | Saudi Arabia | NR | Case report | 1 | MERS-CoV | 33.0 |
| Assiri et al25 | 2016 | Saudi Arabia | 2012–2016 | Case series | 5 | MERS-CoV | 30.8 |
| Malik et al26 | 2016 | United Arab Emirates | 2013 | Case report | 1 | MERS-CoV | 32.0 |
| Park et al27 | 2016 | South Korea | 2015 | Case report | 1 | MERS-CoV | 39.0 |
| Payne et al28 | 2014 | Jordan | 2012 | Case report | 1 | MERS-CoV | 39.0 |
| Yudin et al29 | 2005 | Canada | NR | Case report | 1 | SARS-CoV | 33.0 |
| Wong et al30 | 2004 | Hong Kong, China | 2003 | Retrospective | 12 | SARS-CoV | 30.6 |
| Lam et al31 | 2004 | China | 2003 | Retrospective | 10 | SARS-CoV | 31.6 |
| Robertson et al32 | 2004 | USA | 2003 | Case report | 1 | SARS-CoV | 36.0 |
| Schneider et al33 | 2004 | United States | 2003 | Case report | 1 | SARS-CoV | NR |
| Stockman et al34 | 2004 | United States | 2003 | Case report | 1 | SARS-CoV | 38.0 |
CoV, coronavirus; CoV-2, novel mutation of CoV; MERS, Middle East respiratory syndrome; NR, not reported; SARS, severe acute respiratory syndrome.
Di Mascio et al. Outcome of coronavirus spectrum infections during pregnancy. AJOG MFM 2020.
Preliminary data, before peer review version.
Figure.
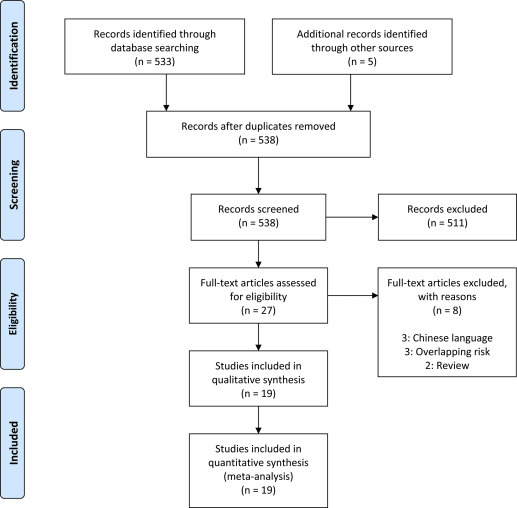
Systematic review flowchart
Di Mascio et al. Outcome of coronavirus spectrum infections during pregnancy. AJOG MFM 2020.
These 19 studies16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 included 79 pregnancies affected by CoV infections. The mean maternal age was 34.6 years. Of the 79 pregnancies affected by CoV infections: 41 (51.9%) were affected by COVID-19, 12 (15.2%) by MERS, and 26 (32.9%) by SARS.
Clinical symptoms and laboratory parameters in the overall population of pregnant with CoV infections are reported in Table 2 . An overt diagnosis of pneumonia was made in 91.8% of cases (54 of 57) (when available, radiological findings suggestive for pneumonia are reported in Supplemental Table 2). The most common symptom was fever that affected 82.6% (64 of 76) of women, followed by cough (57.1%, 44 of 77) and dyspnea (27%, 21 of 77). Lymphopenia and elevated liver enzymes were found in 79.8% (40 of 48) and 36.6% (9 of 26) of cases, respectively.
Table 2.
Pooled proportions of the different clinical symptoms and laboratory parameters in the overall population of pregnancies infected with CoV infection
| Outcome | Studies, n | Pregnancies, n/N (%)∗ | I2, % | Pooled proportions (95% CI)∗∗ |
|---|---|---|---|---|
| Fever | 17 | 64/76 (84.2%) | 8.2 | 82.57 (74.4–90.2) |
| Cough | 18 | 44/77 (57.1%) | 7.3 | 57.10 (45.8–68.0) |
| Dyspnea | 18 | 21/77 (27.3%) | 53.2 | 26.98 (18.2–36.8) |
| Chest pain | 17 | 3/66 (4.5%) | 0 | 8.61 (3.4–16.0) |
| Pneumonia | 16 | 54/57 (94.7%) | 0 | 91.84 (84.0–97.2) |
| Lymphopenia | 10 | 40/48 (83.3%) | 49.1 | 79.87 (60.4–93.9) |
| Elevated liver enzymes | 7 | 9/26 (34.6%) | 0 | 36.59 (20.4–54.5) |
| Admission to ICU | 18 | 22/70 (31.4%) | 58.1 | 34.10 (17.5–53.0) |
| Need for mechanical ventilation | 17 | 16/69 (23.2%) | 42.9 | 26.29 (13.3–41.9) |
| Maternal death | 19 | 9/79 (11.4%) | 0 | 12.30 (6.3–19.9) |
CI, confidence interval; CoV, coronavirus; ICU, intensive care unit; n/N, number of cases per total number of included pregnancies.
Di Mascio et al. Outcome of coronavirus spectrum infections during pregnancy. AJOG MFM 2020.
Raw proportions
A pooled proportion is calculated by using a random-effect model, and therefore it may not refect the actual raw proportion.
A total of 34.1% of pregnant women affected by CoV infections (22 of 70) were admitted to the ICU, and 26.3% (16 of 69) required mechanical ventilation. Maternal death occurred in 12.3% of all reported CoV-related diseases cases (9 of 79). Of note, the pooled proportions of admission to the ICU (9.3% vs 44.6% vs 53.3%), need for mechanical ventilation (5.4% vs 40.9% vs 40%), and maternal death (0% vs 28.6% vs 25.8%) were significantly lower in pregnancies affected by COVID-19, compared with MERS and SARS, respectively (Supplemental Table 3).
The majority of women affected by CoV infections were usually treated first with broad-spectrum antibiotics in 89.3% of cases (49 of 52) and then with antiviral therapy and steroids in 67.7% (37 of 51) and 29.8% (12 of 31) of cases (Table 3 and Supplemental Table 4).
Table 3.
Pooled proportions of treatment used in the overall population of pregnancies infected with coronavirus infection
| Outcome | Studies, n | Pregnancies, n/N (%)∗ | I2, % | Pooled proportions (95% CI)∗∗ |
|---|---|---|---|---|
| Antiviral therapya | 14 | 37/51 (72.5%) | 50.0 | 67.66 (47.2–85.1) |
| Antibiotic therapy | 14 | 49/52 (94.2%) | 27.9 | 89.26 (76.8–97.3) |
| Steroidsb | 12 | 12/31 (38.7%) | 58.6 | 29.81 8.2–57.9) |
CI, confidence interval; n/N, number of cases per total number of included pregnancies.
Di Mascio et al. Outcome of coronavirus spectrum infections during pregnancy. AJOG MFM 2020.
Lopinavir/ritonavir or oseltamivir were the most common antiviral agents. Ribavirin was used by Wong et al.30
Maternal (not fetal) indications
Raw proportions
A pooled proportion is calculated by using a random-effect model, and therefore it may not refect the actual raw proportion.
The results of the quality assessment of the included studies are presented in Supplemental Table 5.
Synthesis of the results
In the overall population of pregnancies infected with CoV, the pooled proportion of miscarriage for CoV infections was 64.7% (8 of 12, 95% confidence interval [CI], 37.9-87.3), although reported only for women affected by SARS in two studies with no control group. The pooled proportions of PTB <37 and 34 weeks of gestation were 24.3% (14 of 56, 95% CI, 12.5–38.6) and 21.8% (11 of 56, 95% CI, 12.5–32.9), respectively; pPROM occurred in 20.7% (6 of 34, 95% CI 9.5–34.9), while the pooled proportions of pregnancies experiencing PE and FGR was 16.2% (2 of 19, 95% CI, 4.2–34.1) and 11.7% (2 of 29, 95% CI, 3.2–24.4), respectively, although data on FGR come only from women affected by SARS.
The pooled proportion of cesarean delivery (CD) was 83.9% (50 of 58, 95% CI, 73.8–91.9) (Tables 4 and 5 ). The pooled proportion of perinatal death was 11.1% (5 of 60, 95% CI, 84.8–19.6) including 3 stillbirths and 2 neonatal deaths (further details are provided in Supplemental Table 6). A total of 34.2% (15 of 44, 95% CI, 20.3–49.5) of fetuses suffered from fetal distress and 57.2% (3 of 12, 95% CI, 3.6–99.8) of newborns were admitted to the NICU. The pooled proportion of Apgar score <7 at 5 minutes was 6.1% (1 of 48, 95% CI, 1.3–13.9), but no cases of neonatal asphyxia were reported.
Table 4.
Pooled proportions of the different pregnancy outcomes in the overall population of pregnancies infected with coronavirus infection
| Outcome | Studies (n) | Pregnancies (n/N) (%)∗ | I2 (%) | Pooled proportions (95% CI)∗∗ |
|---|---|---|---|---|
| PTB <37 wks | 16 | 14/56 (25%) | 25.5 | 24.30 (12.5–38.6) |
| PTB <34 wks | 16 | 11/56 (19.6%) | 1.9 | 21.79 (12.5–32.9) |
| PE | 6 | 2/19 (10.5%) | 0 | 16.21 (4.2–34.1) |
| pPROM | 8 | 6/34 (17.6%) | 0 | 20.72 (9.5–34.9) |
| FGR | 10 | 2/29 (6.9%) | 0 | 11.66 (3.2–24.4) |
| Miscarriage | 2 | 8/12 (66.6%) | 0 | 64.74 (39.90-87.32) |
| Cesarean delivery | 17 | 50/58 (86.2%) | 4.0 | 83.91 (73.8–91.9) |
CI, confidence interval; FGR, fetal growth restriction; n/N, number of cases per total number of included pregnancies; PE, preeclampsia; pPROM, preterm prelabor rupture of membranes; PTB, preterm birth.
Di Mascio et al. Outcome of coronavirus spectrum infections during pregnancy. AJOG MFM 2020.
Raw proportions
A pooled proportion is calculated by using a random-effect model, and therefore it may not refect the actual raw proportion.
Table 5.
Pooled proportions of the different pregnancy outcomes explored in the present systematic review according to the type of viral infection
| Outcome | SARS-CoV |
MERS-CoV |
SARS-CoV-2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies | Pregnancies (n/N) (%)∗ | Pooled % (95% CI)∗∗ | I2 (%) | Studies | Pregnancies (n/N) (%)∗ | Pooled % (95% CI)∗∗ | I2 (%) | Studies | Pregnancies (n/N) (%)∗ | Pooled % (95% CI)∗∗ | I2 (%) | |
| PTB <37 wks | 5 | 1/15 (6.6%) | 15.03 (0.3–45.6) | 31.8 | 6 | 0/11 (0%) | 0 (0–28.9) | 0 | 6 | 14/32 (43.8%) | 41.11 (25.6–57.6) | 0 |
| PTB <34 wks | 5 | 4/15 (26.6%) | 28.89 (10.7–51.6) | 0 | 6 | 3/11 (27.3%) | 32.11 (10.0–59.8) | 9.5 | 6 | 4/32 (12.5%) | 15.03 (3.9–31.7) | 22.6 |
| Preeclampsia | 2 | 0/2 (0%) | 0 (0–67.0) | 0 | 2 | 1/7 (14.3%) | 19.10 (1.1–51.3) | 0 | 2 | 1/10 (10%) | 14.64 (0.94–40.34) | 0 |
| pPROM | 2 | 1/2 (50%) | 50.0 (0.5–95.3) | 46.0 | 2 | 0/2 (0%) | 0 (0–54.4) | 0 | 5 | 5/31 (16.1%) | 18.78 (0.8–33.5) | 0 |
| FGR | 5 | 2/15 (13.3%) | 18.52 (4.4–39.5) | 0 | 3 | 0/4 (0%) | 0 (0–48.7) | 0 | 3 | 0/10 (0%) | 0 (0–22) | 0 |
| Miscarriage | 2 | 8/12 (66.6%) | 64.74 (37.90–87.32) | 0 | — | — | — | — | — | — | — | — |
| Cesarean delivery | 5 | 7/9 (77.7%) | 72.23 (44.1–93.1) | 0 | 6 | 5/8 (62.5%) | 61.79 (32.7–86.9) | 0 | 6 | 38/41 (92.7%) | 91.04 (81.0–97.6) | 0 |
CI, confidence interval; CoV, coronavirus; CoV-2, novel mutation of CoV; FGR, fetal growth restriction; n/N, number of cases per total number of included pregnancies; MERS, Middle East respiratory syndrome; pPROM, preterm premature rupture of membranes; PTB, preterm birth; SARS, severe acute respiratory syndrome.
Di Mascio et al. Outcome of coronavirus spectrum infections during pregnancy. AJOG MFM 2020.
Raw proportions
A pooled proportion is calculated by using a random-effect model, and therefore it may not refect the actual raw proportion.
Finally, none of the newborns showed signs of vertical transmission during the follow-up period (Tables 6 and 7 ).
Table 6.
Pooled proportions of the different perinatal outcomes in the overall population of pregnancies infected with coronavirus infection
| Outcome | Studies (n) | Fetuses/Newborns (n/N) (%)∗ | I2 (%) | Pooled proportions (95% CI)∗∗ |
|---|---|---|---|---|
| Fetal distress | 13 | 15/44 (34.1%) | 13.6 | 34.15 (20.3–49.5) |
| Apgar score <7 | 12 | 1/48 (2.1%) | 0 | 6.08 (1.3–13.9) |
| Neonatal asphyxia | 9 | 0/27 (0%) | 0 | 0 (0–15.7) |
| Admission to NICU | 4 | 3/12 (25%) | 76.3 | 57.16 (3.6–99.8) |
| Perinatal death | 16 | 5/60 (8.3%) | 0 | 11.11 (84.8–19.6) |
| Vertical transmission | 16 | 0/60 (0%) | 0 | 1 (0–10.7) |
CI, confidence interval; NICU, neonatal intensive care unit; n/N, number of cases per total number of included pregnancies.
Di Mascio et al. Outcome of coronavirus spectrum infections during pregnancy. AJOG MFM 2020.
Raw proportions
A pooled proportion is calculated by using a random-effect model, and therefore it may not refect the actual raw proportion.
Table 7.
Pooled proportions of the different perinatal outcomes explored in the present systematic review according to the type of viral infection
| Outcome | SARS-CoV |
MERS-CoV |
SARS-CoV-2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies | Fetuses/newborns (n/N) (%)∗ | Pooled % (95% CI)∗∗ | I2 (%) | Studies | Fetuses/newborns (n/N) (%)∗ | Pooled % (95% CI)∗∗ | I2 (%) | Studies | Fetuses/newborns (n/N) (%)∗ | Pooled % (95% CI)∗∗ | I2 (%) | |
| Fetal distress | 5 | 3/9 (33.3%) | 35.89 (12.0–64.4) | 0 | 4 | 0/5 (0%) | 0 (0–44.5) | 0 | 4 | 12/30 (40%) | 43.02 (15.3–73.4) | 64.7 |
| Apgar score <7 | 4 | 0/4 (0%) | 0 (0–60.2) | 0 | 3 | 0/3 (0%) | 0 (0–56.9) | 0 | 5 | 1/41 (2.4%) | 4.53 (0.4–12.6) | 0 |
| Neonatal asphyxia | 4 | 0/4 (0%) | 0 (0–60.2) | 0 | 2 | 0/2 (0%) | 0 (0–67.0) | 0 | 3 | 0/21 (0%) | 0 (0–13.5) | 0 |
| Admission to NICU | — | — | — | — | 2 | 2/2 (100%) | 100 (33.03–100) | 0 | 2 | 1/10 (1%) | 8.71 (0.01–31.4) | 81.3 |
| Perinatal death | 5 | 0/9 (0%) | 0 (0–31.4) | 0 | 6 | 3/10 (3%) | 33.15 (11.2–59.9) | 0 | 5 | 2/41 (4.9%) | 7.00 (1.4–16.3) | 0 |
| Vertical transmission | 6 | 0/14 (0%) | 0 (0–24–0) | 0 | 4 | 0/4 (0%) | 0 (0–60.2) | 0 | 6 | 0/42 (0%) | 0 (0–9.6) | 0 |
CI, confidence interval; CoV, coronavirus; CoV-2, novel mutation of CoV; MERS, Middle East respiratory syndrome; NICU, neonatal intensive care unit; n/N, number of cases per total number of included pregnancies; SARS, severe acute respiratory syndrome.
Di Mascio et al. Outcome of coronavirus spectrum infections during pregnancy. AJOG MFM 2020.
Raw proportions
A pooled proportion is calculated by using a random-effect model, and therefore it may not refect the actual raw proportion.
COVID-19
Six studies16, 17, 18, 19, 20, 21 reported information on COVID-19 infection during pregnancy. There were no data on miscarriage for COVID-19 infection occurring during the first trimester. The pooled proportions of PTB <37 and 34 weeks of gestation were 41.1% (14 of 32, 95% CI, 25.6–57.6) and 15% (4 of 32, 95% CI, 3.9–31.7), respectively. pPROM occurred in 18.8% (5 of 31, 95% CI, 0.8–33.5), while the pooled proportion of pregnancies experiencing PE was 14.6% (1 of 10, 95% CI, 0.94–40.34), with no reported cases of FGR.
The pooled proportion of CD was 91% (38 of 41, 95% CI, 81.0–97.6) (Table 5). The pooled proportion of perinatal death was 7% (2 of 41, 95% CI, 1.4–16.3) including 1 stillbirth (2.4%) and 1 neonatal death (2.4%); 43% of fetuses (12 of 30, 95% CI, 15.3–73.4) had fetal distress, and 8.7% of newborns (1 of 10, 95% CI, 0.01–31.4) were admitted to the NICU. The pooled proportion of Apgar score <7 at 5 minutes was 4.5% (1 of 41, 95% CI, 0.4–12.6), and no case of neonatal asphyxia was reported. Finally, none of the newborns showed signs of vertical transmission during the follow-up period (Table 7).
MERS
Seven studies22, 23, 24, 25, 26, 27, 28 reported information on MERS infection during pregnancy. There were no data on miscarriage for MERS infection occurring during the first trimester. The pooled proportion of PTB was 32.1% (3 of 11, 95% CI, 10.0–59.8), all occurring before 34 weeks of gestation. Preeclampsia occurred in 19.1% (1 of 7, 95% CI, 1.1–51.3), respectively, while no case of pPROM or FGR was reported in these studies. The pooled proportion of CD was 61.8% (5 of 8, 95% CI, 32.7–86.9) (Table 5). The pooled proportion of perinatal death was 33.2% (3 of 10, 95%, CI 11.2–59.9) including 2 stillbirths and 1 neonatal death (4 hours after birth of an extremely preterm infant). There were no cases of fetal distress, Apgar score <7 at 5 minutes and neonatal asphyxia. Two case reports described two infants admitted to NICU. Finally, none of the newborns showed signs of vertical transmission during the follow-up period (Table 7).
SARS
Six studies29, 30, 31, 32, 33, 34 reported information on SARS infection during pregnancy. The pooled proportion of first trimester miscarriage for SARS infection was 64.7% (8 of 12, 95% CI, 37.9–87.3). The pooled proportions of PTB <37 and 34 weeks of gestation was 15% (1 of 15, 95% CI, 0.3–45.6) and 28.9% (4 of 15, 95% CI, 10.7–51.6), respectively. pPROM and FGR occurred in 50% (1 of 2, 95% CI, 0.5–95.3) and 18.5% (2 of 15, 95% CI, 4.4–39.5), respectively, while no cases of preeclampsia were reported. The pooled proportion of CD was 72.2% (7 of 9, 95% CI, 44.1–93.1) (Table 5). Fetal distress occurred in 35.9% of pregnancies (3 of 9, 95% CI, 12.0–64.4), while no case of perinatal death, Apgar score <7 at 5 minutes, and neonatal asphyxia was reported. There were no data on pooled proportions of admission to the NICU of infants born to infected mothers. Finally, none of the newborns showed signs of vertical transmission during the follow-up period (Table 7).
It was not possible to perform a comprehensive pooled data synthesis on the incidence of pregnancy and perinatal outcomes according to the trimester of pregnancy at infection because of the very small number of included studies for each trimester of pregnancy.
Comment
Main findings
The findings from this systematic review show that more than 90% of hospitalized pregnant women affected by CoV infections present radiological signs suggestive for pneumonia, detected either at chest x-ray or computerized tomography and the most common symptoms are fever, cough, and lymphopenia. Pregnancies affected by CoV infections have high rates (and pooled proportions) of PTB before 37 and 34 weeks. Preeclampsia and cesarean delivery are also more common than in the general population. The pooled proportion of perinatal mortality is about 10%, while the most common adverse perinatal outcome is fetal distress, with more than half of the newborns admitted in the NICU. Importantly, clinical evidence of vertical transmission was found in none of the newborns included. However, these findings should be interpreted with caution in view of the very small number of included cases and heterogeneity in clinical presentation and perinatal management among the included cases.
Strengths and limitations
To the best of our knowledge, this is the first systematic review exploring pregnancy and perinatal outcomes of CoV infections occurring during pregnancy. This comprehensive meta-analysis included all series published so far on this topic.
The small number of cases in some of the included studies, their retrospective nonrandomized design, and the lack of standardized criteria for the antenatal surveillance, management, and timing of delivery of pregnancies affected by CoV infections represent the major limitations of this systematic review, thus making it difficult to draw any convincing evidence on this clinical management strategies. Furthermore, there is a possibility that some patients were included in more than 1 report, although 2 authors independently reviewed all the included studies, carefully focusing on the different Institutions reporting outcomes.
Moreover, when focusing on the outcomes of COVID-19 infection, and particularly perinatal outcomes, reported data are intuitively limited to a very short-term follow-up period and thus infectious that occurred proximate to the delivery. This has the potential to overestimate the magnitude of risks such as PTB and underestimate more longitudinal risks such as FGR.
Additionally, it was not possible to extrapolate data about the pooled proportions of both spontaneous and iatrogenic PTB and indications for CD that was performed in the majority of cases; furthermore, few outcomes (ie, fetal distress) were not clearly defined, thus leading to some discrepancies in the results, like the pooled proportion of PTB <34 weeks (15%) and the pooled proportion of newborns admitted to the NICU (9%), particularly in COVID-19 infection.
Another limitation of the present review was the lack of stratification of the analysis according to the gestational age at CoV infection because of the very small number of included studies for each trimester of pregnancy. We cannot assume that the pooled proportions of miscarriage (only reported for SARS infection) and PTB should be attributed solely to the virus/infection because there are no comparable control groups of uninfected women from the same time. It may be that the stress of the situation in the community contributed to some of these outcomes.
Finally, we also included case reports and case series, thus facing a higher risk of publication bias and decreasing the level of the evidence of our findings.
Implications
COVID-19 is the last CoV infection identified at the end of 2019 in Wuhan, a city in the Hubei Province of China.5 Currently, Europe has become the epicenter of the COVID-19 pandemic,6 but the infection has spread in more than 150 countries, leading governments to adopt rigorous mitigation measures to reduce both the viral spread and its detrimental effects on health care systems and therefore on the whole economy of the countries.35
Despite the relatively low mortality, one of the main concerns related to COVID-19 infection is the development of an acute respiratory distress syndrome, often requiring invasive ventilation, that is the clinical epiphenomenon of the viral pneumonia.6 , 7
The lack of knowledge about COVID-19 infection has raised urgent questions among physicians regarding clinical management and expected outcomes of the affected patients, and therefore, there is currently a compelling need of data to guide clinical decisions.
Regarding pregnancy, the findings from this study found that radiological features suggestive for pneumonia can be found in almost all of the hospitalized pregnant women, usually presenting with fever, cough, and lymphopenia similar to the nonpregnant population. Of note, serious conditions requiring admission to the ICU and mechanical ventilation are significantly less common when compared with the 2 previous CoV infections (MERS and SARS). Similarly, we found no case of maternal death related to COVID-19 infection, while MERS and SARS infections caused a mortality pooled proportion in pregnant women ranging from 25% to 30%.
In this systematic review, women affected by COVID-19 disease had higher rates of preterm birth, and preeclampsia, while the babies had a 2.4% rate of stillbirth, a 2.4% rate of neonatal death, and higher rate of admission to the NICU.
Furthermore, because all the included studies reported data on hospitalized women, the reported rates and pooled proportions of infection-related adverse outcomes, including pregnancy and perinatal outcomes, might not reflect the overall population of pregnant when who got infected with SARS–coronavirus-2, and there may be a cohort of patients with no or mild symptoms whose pregnancy outcome is, as of yet, unknown.36
More importantly, it should be emphasized that there are no known neonatal symptoms and therefore no clinical evidence suggestive for vertical transmission, particularly when COVID-19 infection occurs later in pregnancy. Unfortunately, the lack of data of first- and early second-trimester infection does not allow to determine whether in this case the infection may cause more severe perinatal outcomes and how to monitor the pregnancy once the infection has passed.1
Based on the limited information from this study, COVID-19 cannot be considered as an indication for delivery, and therefore, the timing and mode of delivery should be individualized according to maternal clinical conditions or obstetric factors as usual (and not COVID-19 status alone), and the decision should involve a multidisciplinary team including maternal-fetal doctors, neonatologists, anesthesiologists, and infective disease specialists.
Conclusions
In summary, with the limited data reported to date, hospitalized mothers infected with coronavirus infections, including COVID-19, >90% of whom also had pneumonia, are at increased risks of adverse obstetrical outcomes, compared with the general population and in particular, COVID-19 infection was associated with a relatively higher rates of preterm birth, preeclampsia, cesarean delivery, and perinatal death. There have been no published cases of clinical evidence of vertical transmission. Evidence is accumulating rapidly, so these data may need to be updated soon.
Footnotes
The authors report no conflict of interest.
Supplementary Material
References
- 1.Poon L.C., Yang H., Lee J.C.S., et al. ISUOG interim guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol. 2020 doi: 10.1002/uog.22013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su S., Wong G., Shi W., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva: 2004. Guidelines for the global surveillance of severe acute respiratory syndrome (SARS) Accessed March 15, 2020. [Google Scholar]
- 4.World Health Organization . World Health Organization; Geneva: 2020. Regional Office for Eastern Mediterranean: MERS situation update. Accessed March 15, 2020. [Google Scholar]
- 5.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet 2020 Jan 30] Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronavirus disease 2019 (COVID-2019). Situation report 54. World Health Organization; Geneva: 2020. Accessed March 15, 2020. [Google Scholar]
- 7.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [published online ahead of print, Feb. 7, 2020] JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y.H., Keller J., Wang I.T., Lin C.C., Lin H.C. Pneumonia and pregnancy outcomes: a nationwide population-based study. Am J Obstet Gynecol. 2012;207:288.e1–288.e7. doi: 10.1016/j.ajog.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson L.K., Craig J.C., Willis N.S., Tovey D., Webster A.C. How to write a Cochrane systematic review. Nephrology (Carlton) 2010;15:617–624. doi: 10.1111/j.1440-1797.2010.01380.x. [DOI] [PubMed] [Google Scholar]
- 10.NHS Center for Reviews and Dissemination Systematic reviews: CRD’s guidance for undertaking reviews in health care. York, United Kingdom: University of York; 2009. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf Available at:
- 11.Welch V., Petticrew M., Petkovic J., et al. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration. J Clin Epidemiol. 2016;70:68–89. doi: 10.1016/j.jclinepi.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.Zorzela L., Loke Y.K., Ioannidis J.P., et al. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ. 2016;352:i157. doi: 10.1136/bmj.i157. [DOI] [PubMed] [Google Scholar]
- 14.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H., Guo J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery [published online ahead of print, Feb. 28, 2020] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa200. ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu H., Wang L., Fang C., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Zhao R., Zheng S., et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China [published online ahead of print, June 17, 2020] Emerg Infect Dis. 2020;26 doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D., Li L., Wu X., et al. Pregnancy and perinatal outcomes of women with COVID-19 pneumonia: a preliminary analysis (Feb. 29, 2020) https://ssrn.com/abstract=3548758 Available at SSRN: [DOI] [PubMed]
- 21.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS–CoV-2 infection during pregnancy [published online ahead of print, March 4, 2020] J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. S0163-4453(20)30109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alfaraj S.H., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases and review of the literature. J Microbiol Immunol Infect. 2019;52:501–503. doi: 10.1016/j.jmii.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong S.Y., Sung S.I., Sung J.H., et al. MERS-CoV infection in a pregnant woman in Korea. J Korean Med Sci. 2017;32:1717–1720. doi: 10.3346/jkms.2017.32.10.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alserehi H., Wali G., Alshukairi A., Alraddadi B. Impact of Middle East respiratory syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect Dis. 2016;16:105. doi: 10.1186/s12879-016-1437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assiri A., Abedi G.R., Al Masri M., Bin Saeed A., Gerber S.I., Watson J.T. Middle East respiratory syndrome coronavirus infection during pregnancy: a report of 5 cases from Saudi Arabia. Clin Infect Dis. 2016;63:951–953. doi: 10.1093/cid/ciw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malik A., El Masry K.M., Ravi M., Sayed F. Middle East respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerg Infect Dis. 2016;22:515–517. doi: 10.3201/eid2203.151049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park M.H., Kim H.R., Choi D.H., Sung J.H., Kim J.H. Emergency cesarean section in an epidemic of the middle east respiratory syndrome: a case report. Korean J Anesthesiol. 2016;69:287–291. doi: 10.4097/kjae.2016.69.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne D.C., Iblan I., Alqasrawi S., et al. Stillbirth during infection with Middle East respiratory syndrome coronavirus. J Infect Dis. 2014;209:1870–1872. doi: 10.1093/infdis/jiu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yudin M.H., Steele D.M., Sgro M.D., Read S.E., Kopplin P., Gough K.A. Severe acute respiratory syndrome in pregnancy. Obstet Gynecol. 2005;105:124–127. doi: 10.1097/01.AOG.0000151598.49129.de. [DOI] [PubMed] [Google Scholar]
- 30.Wong S.F., Chow K.M., Leung T.N., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam C.M., Wong S.F., Leung T.N., et al. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. 2004;111:771–774. doi: 10.1111/j.1471-0528.2004.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson C.A., Lowther S.A., Birch T., et al. SARS and pregnancy: a case report. Emerg Infect Dis. 2004;10:345–348. doi: 10.3201/eid1002.030736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider E., Duncan D., Reiken M., et al. SARS in pregnancy: this case study explores the first documented infection in the U.S.A. WHONN Lifelines. 2004;8:122–128. doi: 10.1177/1091592304265557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockman L.J., Lowther S.A., Coy K., Saw J., Parashar U.D. SARS during pregnancy, United States. Emerg Infect Dis. 2004;10:1689–1690. doi: 10.3201/eid1009.040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? [published online ahead of print, March 9, 2020] Lancet. 2020 doi: 10.1016/S0140-6736(20)30567-5. S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Royal College of Obstetricians and Gynaecologists Coronavirus (COVID-19) infection in pregnancy—information for healthcare professionals. March 2020. https://www.rcog.org.uk/globalassets/documents/guidelines/coronavirus-covid-19-infection-in-pregnancy-v2-20-03-13.pdf Availabel at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


