Abstract
Background
With over two billion airline passengers annually, in-flight transmission of infectious diseases is an important global health concern. Many instances of in-flight transmission have been documented, but the relative influence of the many factors (see below) affecting in-flight transmission has not been quantified. Long-standing guidance by public health agencies is that the primary transmission risk associated with air travel for most respiratory infectious diseases is associated with sitting within two rows of an infectious passenger. The effect of proximity may be one of these factors.
Objective
The aim of this study was to determine the risk of infection within and beyond the 2-row rule given by public health guidance.
Methods
We searched the literature for reports of in-flight transmission of infection which included seat maps indicating where the infectious and infected passengers were seated.
Findings
There is a ∼ 6% risk to passengers seated within the 2-rows of infected individual(s) and there is ∼ 2% risk to passengers seated beyond 2-rows from the infectious individual.
Discussion
Contact tracing limited to passengers within 2-rows of the infectious individual(s) could fail to detect other cases of infections. This has important consequences for assessing the spread of infectious diseases.
Conclusions
Infection at a distance from the index case indicates other factors, such as airflow, movement of passenger/crew members, fomites and contacts between passengers in the departure gate before boarding, or after deplaning, are involved.
Key Words: Airplane cabin, infectious disease transmission, disease risk, SARS, influenza
Background
With more than 2 billion airline passengers annually, in-flight transmission of infectious diseases is an important global health concern.1, 2 Many instances of in-flight transmission have been documented, including cases of cholera,3 influenza,4, 5, 6, 7, 8 measles,9, 10 meningococcal infections,11 norovirus,12 severe acute respiratory syndrome (SARS),13, 14 shigellosis,15 and tuberculosis.16, 17, 18 However, the risks of in-flight transmission are largely unknown.14
Cabin transmission of infectious diseases can occur through several routes. In this paper we concentrate on droplet transmission, which occurs via respiratory droplets (≥5 microns) propelled short distances (mostly ≤ 1 meter) when an infectious traveler sneezes, coughs, talks, or breathes.19, 20, 21 Droplets are sufficiently large to be largely impervious to cabin airflow. Direct transmission occurs when pathogen-containing droplets fall onto a susceptible traveler’s conjunctiva or mucosa or are inhaled. Indirect transmission occurs when droplets are deposited onto fomites (surfaces such as tray tables, seat belts, or lavatory door handles) or an infected traveler’s hand. A susceptible traveler who touches these surfaces and then touches her or his conjunctiva or mucosa allows the pathogen to enter the body.
The 2-Row Transmission Zone Guideline
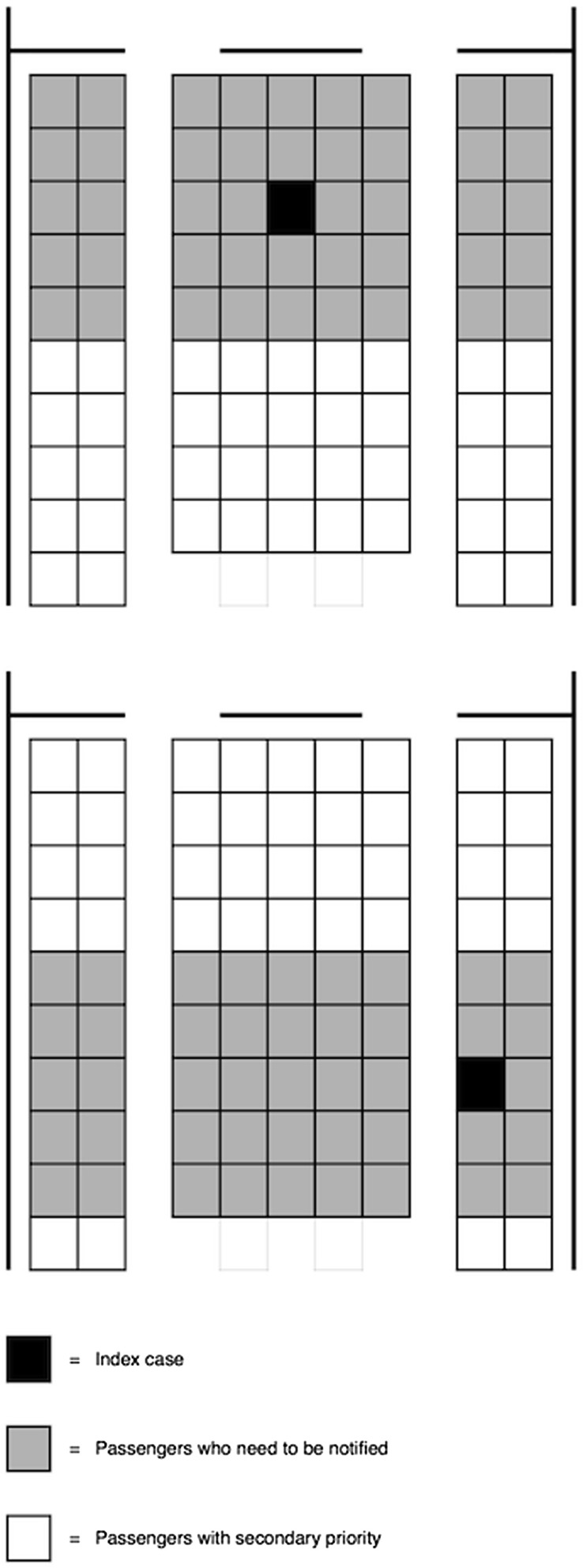
Long-standing guidance by public health agencies is that the primary transmission risk associated with air travel for most respiratory infectious diseases is associated with sitting within 2 rows of an infectious passenger. This transmission zone, which actually comprises 5 rows—2 in front of the index case, 2 behind the index case, and the row in which the index case is seated—has been based on investigations of in-flight transmission of tuberculosis but is believed to have wide applicability.1 This rule is empirical and does not directly take into account the physical and biological bases for droplet transmission—that is, ≤1 meter of contact.
Figure 1 appears in Centers for Disease Control and Prevention (CDC) guidelines to public health officers needing to find and alert travelers who may have been exposed to an ill passenger during flight. Implicit in this guideline is that cabin airflow and passenger and crew movements play negligible roles in disease transmission. Thus there are serious questions about this guideline.
Figure 1.
The 2-row transmission zone diagram provided in the Centers for Disease Control and Prevention guidelines to public health officers.
(From Protecting travelers' health from airport to community: investigating contagious diseases on flights. Atlanta: Centers for Disease Control and Prevention; 2014. Available at: http://www.cdc.gov/quarantine/contact-investigation.html. Accessed 25 July, 2014.)
Case Study: SARS
SARS is a viral respiratory illness likely transmitted through both droplets and aerosols. SARS was first reported in the Guangdong province of southern China in November 2002. The illness quickly spread by air travel to 25 countries, infecting more than 8000 people, with a case fatality rate of nearly 10%.
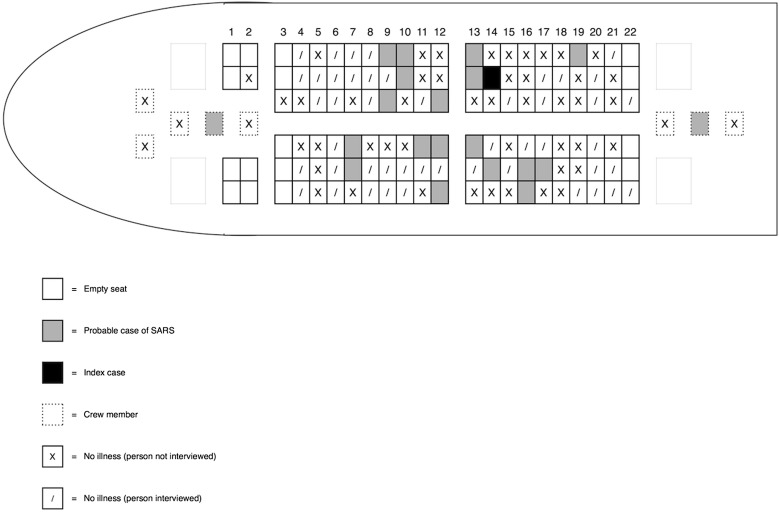
In March 2003, a 72-year-old passenger with SARS infected 18 passengers and 2 flight attendants on a 3-hour flight from Hong Kong to Beijing.14 The infectious passenger died 3 days later. Only 9 (50%) of the infected passengers were seated within 2 rows of the index case (Fig. 2 ). Indeed, more transmissions occurred to passengers sitting across the center aisle than on the infectious passenger’s side of the plane.
Figure 2.
SARS transmissions on a flight from Hong Kong to Beijing.14
Case Study: Novel H1N1 Influenza
The 2009 outbreak of novel swine-origin influenza A (H1N1) pdm09 virus began in Veracruz, Mexico. Several months later, Mexican health officials acknowledged the severity of the epidemic after the first cases began to appear in Mexico City in mid-March. This was about the same time as the first cases appeared in the United States. Within days Mexico City was effectively shut down in an attempt to contain the spread of the epidemic. However, it continued to spread within Mexico and globally. In June, the World Health Organization and CDC declared the outbreak a pandemic. Individuals in 214 countries were infected, with 14,000 confirmed deaths worldwide. The disease was particularly severe in individuals younger than 50 years of age.
In April 2009, on a 9.5-hour flight from Mexico to Birmingham, UK, a passenger contagious with novel H1N1 virus infected 6 passengers.22 Only 2 of the infected passengers were seated within 2 rows of the infectious passenger.
In this paper we document reports of in-flight transmission of respiratory infectious diseases by large droplets for which seat plans are given. We summarize these reports and estimate the risks for passengers seated within and outside the 2-row risk zone.
Methods
In addition to those described earlier, we identified 8 reports of respiratory infectious disease transmission on airplanes for which enough information was available to calculate post-flight attack rates inside and outside the 2-row transmission zone. Five reports concerned diseases transmitted by droplets, specifically SARS, influenza, and measles.
Results
Table 1 summarizes the reported literature. There are 39 cases of infection transmission within 2 rows of an index case. There were 37 cases of infection transmission to passengers seated outside of this risk zone on these same flights. Thus, although there is an elevated relative risk for passengers inside the ±2 row zone (Mantel-Haenszel relative risk estimate [95% confidence interval = 2.4 (1.6, 3.6)]), there is still a non-negligible chance of cross infection beyond this zone.
Table 1.
Reports of in-flight transmission of infection with seat maps indicating infectious and infected passengers
| Disease | Aircraft | Origin | Destination | Flight Time (Hours:Minutes) | No. of Cases Within ±2 Rows/No. at Risk | No. of Cases Beyond ±2 Rows/No. at Risk |
|---|---|---|---|---|---|---|
| SARS14 | Boeing 737 | Hong Kong | Beijing | 3:00 | 9/29 | 9/75 |
| SARS13 | ∗ | Hanoi† | Paris | 14:50 | 1/9 | 1/60 |
| Influenza A/H1N1/p094 | Boeing 747 | Los Angeles | Auckland | 12:40 | 4/67 | 0/52 |
| Influenza A/H1N1/p0922 | Boeing 767 | ‡ | Birmingham, UK | 9:30 | 2/39 | 4/242 |
| Influenza A/H1N1/p09 | Boeing 767 | Cancun | Birmingham, UK | 9:30 | 5/128 | 4/43 |
| Influenza-like illness23 | British Aerospace 146 | § | § | 3:20 | 9/24 | 8/50 |
| Measles9 | ‖ | ‖ | ‖ | ‖ | 9/343¶ | 11/750¶ |
SARS, severe acute respiratory syndrome.
Aircraft was not given. The flight was Air France 171, so it presumably was on an Airbus aircraft.
Flight was direct with 1 stop in Bangkok, where the passenger deplaned and then reboarded.
Flight was from Mexico to Birmingham, UK. Neither city nor airport of origin was given. The plane had 282 seats.
Reported in a letter to the editor. Origin and destination airports were not given. The flight was to a remote mining community in northwestern Australia.
Authors reported data on 7 flights on which 9 passengers who were seated within ±2 rows of an infectious passenger became infected. Aircraft types were not given. The average flight time was 6 hours, 5 minutes.
Conservatively assumed that all 7 flights were on large long-haul carriers with 300-passenger capacity and estimated 10 seats per row.
Conclusions
These inflight transmissions highlight (1) how air travel serves as a conduit for rapid spread of newly emerging infections with potential to start pandemics,1 and (2) how there must be 1 or more other factors affecting transmission other than seating. Contact tracing limited to passengers within 2 rows of the infectious individual may lead to failure to determine other cases, which may have important, potentially dire consequences for spread of infectious diseases. We speculate that infection at a distance from the index case is due to factors such as cabin airflow and movements of passengers and flight attendants. Public health officers investigating suspected disease transmission on an airplane should prioritize passengers seated within 2 rows of the index case for surveillance but should not neglect other passengers for follow-up.
Footnotes
The authors received support from The Boeing Company (HNW) by way of a subcontract to the Georgia Institute of Technology (VSH).
Both authors had access to the sources used to develop the manuscript.
References
- 1.Mangili A., Gendreau M.A. Transmission of infectious diseases during commercial air travel. Lancet. 2005;365:989–996. doi: 10.1016/S0140-6736(05)71089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson M.E. Travel and the emergence of infectious diseases. Emerg Infect Dis. 1995;1:39–46. doi: 10.3201/eid0102.950201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberhart-Phillips J., Besser R.E., Tormey M.P. An outbreak of cholera from food served on an international aircraft. Epidemiol Infect. 1996;116:9–13. doi: 10.1017/s0950268800058891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker M.G., Thornley C.N., Mills C. Transmission of pandemic A/H1N1 2009 influenza on passenger aircraft: retrospective cohort study. BMJ. 2010;340:c2424. doi: 10.1136/bmj.c2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foxwell A.R., Roberts L., Lokuge K., Kelly P.M. Transmission of influenza on international flights, May 2009. Emerg Infect Dis. 2011;17:1188–1194. doi: 10.3201/eid1707.101135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J.H., Lee D.-H., Shin S.-S. In-flight transmission of novel influenza A (H1N1) Epidemiol Health. 2010;32:e2010006. doi: 10.4178/epih/e2010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ooi P.L., Lai F.Y.L., Low C.L. Clinical and molecular evidence for transmission of novel influenza A(H1N1/2009) on a commercial airplane. Arch Intern Med. 2010;170:913–915. doi: 10.1001/archinternmed.2010.127. [DOI] [PubMed] [Google Scholar]
- 8.Young N., Pebody R., Smith G. International flight-related transmission of pandemic influenza A(H1N1) pdm09: an historical cohort study of the first identified cases in the United Kingdom. Influenza Other Respir Viruses. 2014;8:66–73. doi: 10.1111/irv.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoad V.C., O'Connor B.A., Langley A.J., Dowse G.K. Risk of measles transmission on aeroplanes: Australian experience 2007-2011. Med J Aust. 2013;198:320–323. doi: 10.5694/mja12.11752. [DOI] [PubMed] [Google Scholar]
- 10.Slater P., Anis E., Bashary A. An outbreak of measles associated with a New York / Tel Aviv flight. Travel Med Int. 1995;199:92–95. [Google Scholar]
- 11.O'Connor B.A., Chang K.G., Binotto E., Maidment C.A., Maywood P., McAnulty J.M. Meningococcal disease—probable transmission during an international flight. Commun Dis Intell Q Rep. 2005;29:312–314. doi: 10.33321/cdi.2005.29.35. [DOI] [PubMed] [Google Scholar]
- 12.Kirking H.L., Cortes J., Burrer S. Likely transmission of norovirus on an airplane, October 2008. Clin Infect Dis. 2010;50:1216–1221. doi: 10.1086/651597. [DOI] [PubMed] [Google Scholar]
- 13.Desenclos J.C., van der Werf S., Bonmarin I. Introduction of SARS in France, March-April, 2003. Emerg Infect Dis. 2004;10:195–200. doi: 10.3201/eid1002.030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen S.J., Chang H., Cheung T.Y. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349:2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 15.Hedberg C.W., Levine W.C., White K.E. An international foodborne outbreak of shigellosis associated with a commercial airline. JAMA. 1992;268:3208–3212. [PubMed] [Google Scholar]
- 16.Kenyon T.A., Valway S.E., Ihle W.W., Onorato I.M., Castro K.G. Transmission of multidrug-resistant Mycobacterium tuberculosis during a long airplane flight. N Engl J Med. 1996;334:933–938. doi: 10.1056/NEJM199604113341501. [DOI] [PubMed] [Google Scholar]
- 17.Miller M.A., Valway S., Onorato I.M. Tuberculosis risk after exposure on airplanes. Tuber Lung Dis. 1996;77:414–419. doi: 10.1016/s0962-8479(96)90113-6. [DOI] [PubMed] [Google Scholar]
- 18.Wang P.D. Two-step tuberculin testing of passengers and crew on a commercial airplane. Am J Infect Control. 2000;28:233–238. doi: 10.1067/mic.2000.103555. [DOI] [PubMed] [Google Scholar]
- 19.FIore A.E., Shay D.K., Broder K. Prevention and control of influenza: recommendations of the advisory committee on immunization practices. Morb Mortal Wkly Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- 20.Garner J.S., Simmons B.P. CDC guideline for isolation precautions in hospitals. Infect Control. 1983;4:247–325. [PubMed] [Google Scholar]
- 21.Hagen D.L. Diseases of air travel. Environmental Medicine. 2010:244. [Google Scholar]
- 22.Shankar A.G., Janmohamed K., Olowokure B. Contact tracing for influenza A(H1N1) pdm09 virus–infected passenger on international flight. Emerg Infect Dis. 2014;20:118–120. doi: 10.3201/eid2001.120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsden A.G. Influenza outbreak related to air travel. Med J Aust. 2003;179:172–173. doi: 10.5694/j.1326-5377.2007.tb01296.x. Erratum in Med J Aust 2007;187:374. [DOI] [PubMed] [Google Scholar]