Abstract
Multi-component fingerprinting and quantitation of the glucosinolates and nucleosides in samples of Radix Isatidis have been carried out using high-performance liquid chromatography with diode-array detection and electrospray ionization tandem mass spectrometry (HPLC–DAD–ESI/MS). Five nucleosides together with one glucosinolate were identified by comparing retention times, ultraviolet spectra, mass spectra and/or empirical molecular formulae of reference compounds. Quantitation of these six compounds was carried out simultaneously by HPLC on a Phenomenex Luna C18 column using gradient elution with methanol and water and detection at 254 nm. All calibration curves were linear (r>0.9994) within test ranges. Limits of detection and quantitation were 0.33 ng and 2.50 ng on column, respectively. Intra- and inter-day precision (as relative standard deviation) for all analytes was <2.19% with recoveries in the range 99.6%–101.8% at three concentration levels. The validated method was successfully applied to fingerprinting and assay of 25 batches of Radix Isatidis sourced from different geographical regions of China. The method is simple and reliable and has potential value in the quality control of Radix Isatidis.
KEY WORDS: Radix Isatidis; Glucosinolate; Nucleoside; R,S-Goitrin; Quality control; HPLC–DAD–ESI/MS
Graphical abstract
Chemical fingerprinting using HPLC-DAD-ESI/MS based on simultaneous identification and quantitation of five nucleosides and one glucosinolate in 25 samples of Radix Isatidis sourced from three pharmaceutical companies has been developed. The contents of the six compounds were subjected to similarity analysis to investigate variations relevant to quality. The method established in this paper is simple and reliable and shows potential for the quality control of Radix Isatidis.
1. Introduction
Radix Isatidis (Banlangen or Bei-Banlangen in Chinese) is a traditional Chinese medicine (TCM) consisting of the dried root of Isatis indigotica Fort (Cruciferae). It was first recorded in Shen Nong's Herbal classic and has been used in China as an antipyretic and detoxifying herb for over 2000 years1, 2. Like many TCM, Radix Isatidis displays diverse pharmacological activities and exerts antibacterial3, 4, 5, antiviral6, 7, 8, 9, 10, antiinflammatory11, 12, antitumor13, 14, antiendotoxic15, 16, 17, 18, 19, 20, 21, 22, 23 and immune regulatory effects24, 25, 26. In fact, these effects have translated into pronounced clinical success in the treatment and/or prevention of influenza, tonsillitis and malignant infectious diseases27, 28, 29, 30 particularly severe acute respiratory syndrome (SARS) and H1N1-influenza31, 32, 33. It is now an established component of many TCM preparations such as Banlangen Granules and Qingkailing Injection which is widely used as antipyretic and antiviral drug in clinical practice.
The potent antiviral activity of Radix Isatidis is attributed to the presence of nucleosides which interfere in the gene expression of virus and thereby inhibit their proliferation and protect cells from virus damage34. As a result, some water soluble compounds including glucosinolates (R,S-goitrin, progoitrin, epiprogoitrin and gluconapin) and nucleosides (hypoxanthine, adenosine, uridine and guanosine) have been investigated as potential indicators of the quality of Radix Isatidis and its finished products rather than non-polar components such as indigotin and indirubin35, 36, 37, 38, 39, 40. Recently, holistic chemical profiling of Radix Isatidis has been developed using HPLC41, 42 to determine eight bioactive constituents, including 5 glucosinolates and 3 nucleosides43.
In this paper, a powerful strategy incorporating chemical fingerprinting and simultaneous quantitation has been developed for the quality assessment of Radix Isatidis. The method involving HPLC coupled with diode-array detection and electrospray ionization tandem mass spectrometry (HPLC–DAD–ESI/MS) was applied to evaluate the holistic qualities of commercially available Radix Isatidis from the herbal market in China. Chemical fingerprints based on the detection and simultaneous assay of five nucleosides and one glucosinolate were subjected to similarity analysis and partial least squares discrimination analysis (PLS-DA) to evaluate the technique. The validated method is expected to offer a more effective strategy to ensure consistent quality of Radix Isatidis.
2. Materials and methods
2.1. Chemicals, reagents and plant materials
Reference standards (batch numbers) and suppliers were as follows: uridine (887-200001), adenine (886-200001) and adenosine (110879-200202), National Institute for Food and Drug Control (Beijing, China); cytidine (200-610-9) and guanosine (G6752), Sigma-Aldrich (St. Louis, M.O., USA); R,S-goitrin (101103), Sichuan Victory Pharmaceutical Co., Ltd. (Chengdu, China). All standards were biochemical reagent grade (purities≥98%) as confirmed by HPLC.
HPLC-grade methanol was obtained from J.T. Baker. Ultrapure water (18.2 mΩ) daily prepared with a Milli-Q water purification system (Millipore, Molsheim, France) was used in the mobile phase. Other reagents were of analytical grade and used as received.
Twenty-five batches of Radix Isatidis from different geographical regions around China were obtained from three pharmaceutical companies producing the same TCM preparations using Radix Isatidis as raw material. All samples were morphologically authenticated by the authors and voucher specimens were deposited with the Department of Chemistry, Tsinghua University, Beijing, China.
2.2. Sample preparation
The root of Isatis indigotica Fort. were pulverized and approximately 0.5 g of ground sample accurately weighed and extracted by ultrasonication (44 kHz, 250 W) with 5 mL water for 20 min at room temperature. The extracts were then filtered through 0.2 μm PTFE syringe filters and 10 μL of each filtrate subjected to HPLC–DAD–ESI/MS analysis. Stock solutions (ca. 1.0 mg/mL) of the six reference compounds viz uridine, adenine, adenosine, cytidine, guanosine and R,S-goitrin were prepared in 50% methanol and stored at 4 °C until required. Equal volumes of these six stock solutions were mixed and diluted with 50% methanol to obtain a series of seven mixed standard solutions. The mixed standards were filtered through 0.2 μm PTFE syringe filters and 10 μL injected into the HPLC system.
2.3. HPLC–DAD analysis
HPLC was performed on an Agilent 1200 series system (Agilent Series 1200, Palo Alto, CA, USA) consisting of a binary pump, autosampler and diode array detector. After scanning in the range 200–400 nm, a wavelength of 254 nm was selected for qualitative and quantitative analysis. Chromatography was performed on a Phenomenex Luna C18 analytical column (250 mm×4.6 mm, 5 μm) at 25 °C using gradient elution at 0.8 mL/min of (A) water and (B) methanol according to the following program: 0–10 min 5% B; 10–25 min 5–15% B; 25–34 min 15–35% B; 34–40 min 35–95% B. The column was re-equilibrated for 10 min between individual runs.
2.4. Mass spectrometry
Accurate mass determination to generate empirical formulae was performed on an Agilent 1100 Series LC/MSD TOF (Agilent Technologies Corp., Santa Clara, CA, USA) using electrospray ionization (ESI) in both positive (ESI+) and negative (ESI−) ion modes. HPLC effluent was introduced into the mass spectrometer with a post-column split of 3:1. Optimized ESI-MS parameters were: capillary voltage 3500 V (ESI−) or 4000 V (ESI+); drying gas 8.0 L/min; nebulizer pressure 40 psi; gas temperature 325 °C; fragmentor voltage 175 V (ESI+) and 190 V (ESI−); skimmer voltage 60 V; octopole dcl 37.5 V (ESI+) or 38.0 V (ESI−); octopole RF 250 V. Full-scan mass spectra were recorded in the range m/z 50–1500. Continuous calibration was maintained using reference masses of m/z 121.0509 and 922.0098. Analyst QS software (Applied Biosystems, Framingham, MA) was used to process mass data and to generate exact masses corresponding to particular elemental compositions. Instrument tuning was carried out daily using the tuning solution (G1969-85000, Agilent Corp, USA) to ensure a mass error <5 ppm prior to sample analysis.
Fragmentation data for structure confirmation was generated on an Agilent 1100 Series HPLC instrument coupled to a 6300 series ion trap (G2445A, Agilent Technologies, Santa Clara, USA) via an ESI interface. The following operation parameters were used: capillary voltage 3500 V (ESI−) or 4000 V (ESI+); skimmer voltage 40 V; capillary exit voltage 137 V; nebulizer pressure 30 psi; drying gas 8 L/min; gas temperature 325 °C; target mass m/z 622; compound stability 100%; trap drive level 60%; threshold 50,000 (ESI+) and 10,000 (ESI−); ion charged control (ICC) on; target 10,000; accumulation time 200 ms. The amplitude voltage was generally 0.6 V for fragmentation in MS3 experiments. Data was processed by Agilent Chemstation Rev. A. 09.01 software (Agilent, Palo Alto, CA, USA).
2.5. Data analysis
Each sample solution was analyzed in triplicate and concentrations based on peak areas determined from the appropriate calibration curve. Identification of all marker compounds was based on a comparison of HPLC retention times, MS fragmentation pattern and UV spectra of peaks with data for corresponding standards. Similarity evaluation was performed using the Similarity Evaluation System for Chromatographic Fingerprinting of Traditional Chinese Medicines (Version 2004A) published by the Chinese Pharmacopoeia Committee. The software mainly uses the correlation coefficient for evaluating the similarities of different chromatograms44. Partial least squares discrimination analysis (PLS-DA) was performed using SIMCA-P (version 12.0, Demo).
3. Results and discussion
3.1. Optimization of HPLC conditions
Column type, mobile phase composition, gradient elution program, flow rate and column temperature were optimized to achieve maximum resolution in the minimum run time. The detection wavelength for quantitative analysis was selected as 254 nm at which all marker components showed absorption. Representative HPLC chromatograms of Radix Isatidis are shown in Fig. 1.
Figure 1.
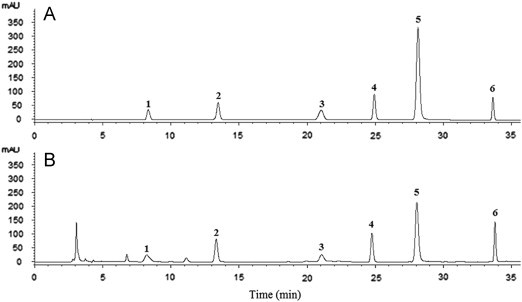
Typical chromatograms of (A) a mixed standard solution and (B) an aqueous extract of Radix Isatidis showing peaks from six bioactive compounds. 1, cytidine; 2, uridine; 3, adenine; 4, guanosine; 5, R,S-goitrin; 6, adenosine.
3.2. Optimization of extraction method
Considering the analytes of interest are relatively polar, aqueous methanol was predicted and found to be a suitable extraction solvent. Extraction conditions including extraction method (reflux or ultrasonication), number of extractions (1, 2 or 3), methanol concentration (0%, 25%, 50%, 75% and 100% v/v) and extraction time (20, 40 and 60 min) were investigated to give the maximum number of peaks in the HPLC fingerprint. Finally, the extraction method presented in Section 2.2 was selected.
3.3. Identification of marker compounds
HPLC-UV and LC–ESI/MS analysis of the aqueous extract of Radix Isatidis (Sample 1) showed six peaks (Fig. 1) which were characterized by the online UV spectra. These showed maximum absorption at 260–270 nm for nucleosides and 245–252 nm for the glucosinolate. By comparing retention times, UV spectra, m/z of characteristic molecular ions, empirical molecular formulae and low-energy collision induced dissociation fragment ions with those produced by authentic standards, the compounds corresponding to Peaks 1–6 were identified as cytidine, uridine, adenine, guanosine, R,S-goitrin and adenosine, respectively. The characteristics of these six compounds are given in Table 1 which shows that mass accuracy for all molecular ions, quasi-molecular ions and fragment ions was <30 ppm. This indicates that the empirical molecular formulae were well matched to the putative deprotonated ions, quasi-molecular ions and fragment ions.
Table 1.
Components identified in samples of Radix Isatidis.
| Compound No. | tR (min) | Identificationa | UV(λmax, nm) | MS Selected ion | Formula | Measured mass | Calculated mass | Mass accuracy (ppm) | MS (pos/neg ionization) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| MS (m/z) | MS2 (m/z) | |||||||||
| 1 | 8.3 | Cytidine | 236(sh), 270 | [M+H]+ | C9H14N3O5 | 244.0925 | 244.0927 | −1.2175 | 242.8[M−H]− | 199.7[M−H−HNCO]− |
| 2 | 13.5 | Uridine | 260 | [M−H]− | C9H11N2O6 | 243.0672 | 243.0611 | 24.8378 | 242.9[M−H]− | 199.9[M−H−HNCO]− |
| 3 | 21.0 | Adenine | 260 | [M+H]+ | C5H6N5 | 136.0625 | 136.0617 | 5.3526 | 136.0[M+H]+ | |
| 4 | 25.0 | Guanosine | 250, 280 (sh) | [M+H]+ | C10H14N5O5 | 284.0982 | 284.1046 | −2.6231 | 281.9[M−H]− | 194.8[M−H−C2N3HO]− |
| 5 | 28.1 | R,S-Goitrin | 245 | [M+H]+ | C5H7NOS | 130.0340 | 130.0151 | 14.5177 | 130.0[M+H]+ | |
| 6 | 33.6 | Adenosine | 260 | [M+H]+ | C10H14N5O4 | 268.1089 | 268.1040 | 2.1238 | 268.1[M+H]+ | 136.0[M−C4H4O3]+ |
Compared with authentic compounds.
3.4. Assay validation for quantitation
The quantitative HPLC method was validated by defining linearity, limit of quantitation (LLOQ) and limit of detection (LOD), repeatability, accuracy and precision and stability. Linearity was assessed by linear regression of calibration curves for each analyte. The regression equations, correlation coefficients and linear ranges for six marker compounds are shown in Table 2. The LOD and LLOQ, calculated as the concentrations giving signal-to-noise ratios of 3 (S/N=3) and 10 (S/N=10) respectively, are also listed in Table 2.
Table 2.
Calibration curves and detection limits (LOD and LLOQ) for the six marker compounds in Radix Isatidis.
| Compound | Calibration curvea | r | Linear range (mg/mL) | LODb(ng) | LLOQb(ng) |
|---|---|---|---|---|---|
| Cytidine (1) | Y=19573X+2.5872 | 0.9998 | 3.4×10–3–11.0×10–2 | 0.34 | 1.13 |
| Uridine (2) | Y=24259X+41.870 | 0.9994 | 4.7×10–3–15.0×10–2 | 0.44 | 1.47 |
| Adenine (3) | Y=65688X−19.286 | 0.9998 | 1.7×10–3–5.33×10–2 | 0.56 | 1.88 |
| Guanosine (4) | Y=32675X−7.3438 | 0.9998 | 4.3×10–3–13.6×10–2 | 0.34 | 1.15 |
| R,S-Goitrin (5) | Y=26506X−571.99 | 0.9996 | 23.7×10–3–75.5×10–2 | 0.75 | 2.50 |
| Adenosine (6) | Y=39284X−4.1087 | 1.000 | 2.4×10–3–7.71×10–2 | 0.33 | 1.09 |
Y is peak area; X is concentration (mg/mL) of analytes.
The LOD and LLOQ are defined as the concentrations that can be detected at S/N ratios of 3 and 10 respectively.
Intra- and inter-day precision were determined by assay of six replicate extracts of a powdered Radix Isatidis sample on one day and assay of the same six extracts on three separate days respectively. Intra- and inter-day precision (as relative standard deviation RSD) were found to be <0.76% and <2.19%, respectively. Accuracy (recovery) was determined by assay of an extract spiked in triplicate with low, medium and high concentrations of the mixed standard solution. Accuracy was found to be in the range 98.6%–101.5% with an RSD of 1.24%–2.98% ( Table 3). Stability was assessed by measuring peak areas of the six compounds initially and after 4, 8, 12, 16 and 24 h at room temperature. Variation in peak area of the six compounds was <3.0%. These results indicate that the HPLC method is precise, accurate and sensitive for quantitative determination of the nucleosides and glucosinolates in Radix Isatidis samples.
Table 3.
Accuracy of HPLC-UV method for the determination of six marker compounds.
| Compound | Original (μg) | Spiked (μg) | Found (μg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Cytidine (1) | 5.98 | 4.79 | 10.76 | 101.25 | 1.77 |
| 5.62 | 11.73 | ||||
| 7.36 | 13.47 | ||||
| Uridine (2) | 9.76 | 7.32 | 17.16 | 101.53 | 1.59 |
| 9.56 | 19.47 | ||||
| 11.91 | 21.89 | ||||
| Adenine (3) | 5.49 | 4.50 | 10.13 | 100.95 | 2.97 |
| 5.76 | 11.13 | ||||
| 6.48 | 12.07 | ||||
| Guanosine (4) | 5.80 | 4.52 | 10.28 | 99.81 | 2.94 |
| 5.60 | 11.52 | ||||
| 6.96 | 12.61 | ||||
| R,S-Goitrin (5) | 58.97 | 45.41 | 103.99 | 99.30 | 1.24 |
| 61.92 | 120.66 | ||||
| 70.18 | 128.54 | ||||
| Adenosine (6) | 2.99 | 2.33 | 5.33 | 98.98 | 2.98 |
| 3.23 | 6.11 | ||||
| 3.68 | 6.65 | ||||
3.5. Chemical profiling and quantitation
Chemical profiling and assay of 25 samples of Radix Isatidis from different geographical regions of China were carried out. Fig. 2 presents typical HPLC chromatograms of their aqueous extracts.
Figure 2.
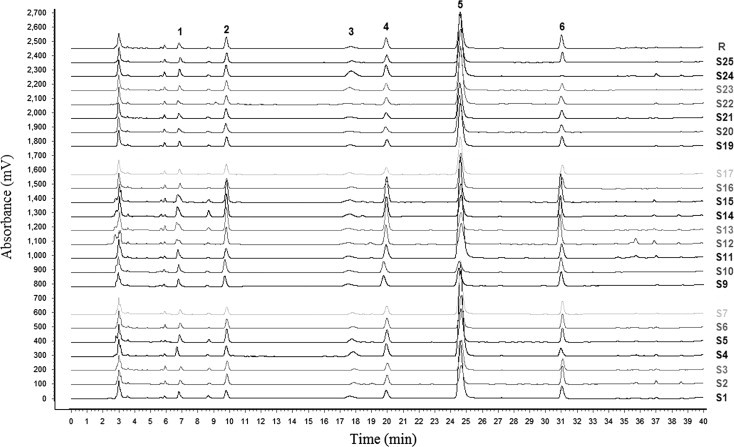
HPLC fingerprints of 25 samples of Radix Isatidis and the reference standard fingerprint generated by similarity evaluation software based on identification and quantitation of six compounds. 1, cytidine; 2, uridine; 3, adenine; 4, guanosine; 5, R,S-goitrin; 6, adenosine.
The State Food and Drug Administration (SFDA) suggests that chromatograms of herbal samples should be evaluated in terms of similarity by calculating the correlation coefficient and/or angle cosine value of the original data. A representative fingerprint of Radix Isatidis was generated for all samples using the software of the Similarity Evaluation System and the peak from R,S-goitrin as reference. As shown in Fig. 3, all samples showed average similarity of 0.928. The chemical constituents in samples from company A (samples 1–10) and B (samples 11–15) were significantly different within groups with similarities in the ranges 0.729–0.986 and 0.751–0.976, respectively, while those in samples from company C (samples 16–25) exhibited satisfactory consistency within the group with a similarity in the range 0.959–0.993. The fingerprints were also subjected to PLS-DA which showed the samples from different companies were well separated by PLS-DA with variability in the contents of the six marker components ( Fig. 4). Thus the results of PLS-DA and similarity analysis are consistent. In the scatter plot, samples from companies A and B were more discrete than those of company C (only sample 25 was shown to be an outlier) suggesting the quality of the Radix Isatidis samples sourced from company C were more stable.
Figure 3.
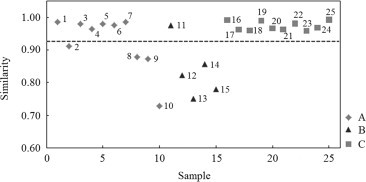
Similarity evaluation of the HPLC fingerprints of 25 Radix Isatidis samples classified into three groups (A, B and C) based on the three pharmaceutical companies from which they were sourced.
Figure 4.
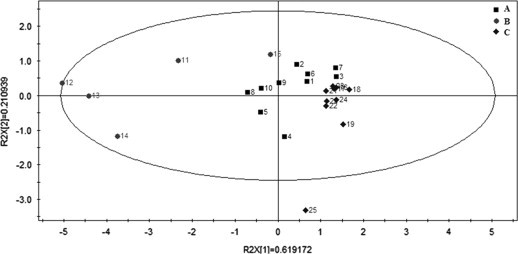
Discrimination analysis based on a PLS-DA plot of the fingerprints of 25 samples of Radix Isatidis generated by SIMCA-P (version 12.0, Demo). Samples are classified into three groups (A, B and C) based on the three pharmaceutical companies from which they were sourced.
The content (mg/g) of the six marker compounds in the 25 samples of Radix Isatidis is shown in Table 4. All samples contained all six compounds. The content of R,S-goitrin was in the range 0.36–4.50 mg/g which is higher than the defined limit of 0.2 mg/g set by the Chinese Pharmacopoeia. This means that all samples qualified as crude material. However, as shown in Fig. 5, significant differences exist in the content of the six marker compounds particularly for R,S-goitrin where the contents showed an RSD of 66%. Such significant differences in the content of chemical constituents would likely be associated with variable quality and uncertain therapeutic efficacy of this herb and the TCM which include it.
Table 4.
Content (mg/g) of six marker compounds in 25 samples of Radix Isatidis.
| Batch no. | Sample itemsa | Contentb (mg/g) |
||||||
|---|---|---|---|---|---|---|---|---|
| Cytidine | Uridine | Adenine | Guanosine | R,S-Goitrin | Adenosine | Total | ||
| 120502 | A | 0.24 | 0.27 | 0.07 | 0.27 | 2.82 | 0.32 | 3.98 |
| 120501 | A | 0.15 | 0.31 | 0.04 | 0.27 | 0.67 | 0.39 | 1.83 |
| 120516 | A | 0.12 | 0.29 | 0.03 | 0.22 | 1.20 | 0.24 | 2.10 |
| 120503 | A | 0.31 | 0.34 | 0.13 | 0.44 | 4.50 | 0.21 | 5.92 |
| 120518 | A | 0.27 | 0.42 | 0.08 | 0.37 | 1.28 | 0.30 | 2.72 |
| 120519 | A | 0.19 | 0.28 | 0.04 | 0.30 | 1.28 | 0.29 | 2.39 |
| 120514 | A | 0.16 | 0.23 | 0.04 | 0.25 | 2.32 | 0.27 | 3.27 |
| 120507 | A | 0.26 | 0.42 | 0.06 | 0.37 | 0.36 | 0.36 | 1.84 |
| 120602 | A | 0.23 | 0.35 | 0.04 | 0.33 | 0.65 | 0.31 | 1.91 |
| 120509 | A | 0.28 | 0.36 | 0.06 | 0.36 | 0.68 | 0.34 | 2.09 |
| 120401 | B | 0.25 | 0.51 | 0.06 | 0.51 | 0.52 | 0.65 | 2.50 |
| 120501 | B | 0.49 | 0.70 | 0.08 | 0.80 | 0.57 | 0.71 | 3.33 |
| 120602 | B | 0.45 | 0.67 | 0.08 | 0.75 | 0.59 | 0.63 | 3.19 |
| 120601 | B | 0.49 | 0.71 | 0.10 | 0.61 | 0.73 | 0.47 | 3.11 |
| 110503 | B | 0.25 | 0.35 | 0.04 | 0.35 | 3.93 | 0.46 | 5.38 |
| 110317 | C | 0.15 | 0.29 | 0.04 | 0.21 | 1.35 | 0.21 | 2.26 |
| 110209 | C | 0.15 | 0.30 | 0.04 | 0.21 | 1.43 | 0.22 | 2.36 |
| 110216 | C | 0.14 | 0.28 | 0.04 | 0.18 | 1.56 | 0.19 | 2.39 |
| 110304 | C | 0.17 | 0.31 | 0.09 | 0.21 | 1.90 | 0.14 | 2.81 |
| 110213 | C | 0.19 | 0.32 | 0.05 | 0.21 | 1.61 | 0.20 | 2.58 |
| 110220 | C | 0.17 | 0.32 | 0.05 | 0.22 | 1.98 | 0.23 | 2.97 |
| 110225 | C | 0.19 | 0.34 | 0.06 | 0.20 | 1.64 | 0.20 | 2.62 |
| 110228 | C | 0.17 | 0.30 | 0.04 | 0.24 | 2.69 | 0.22 | 3.67 |
| 110223 | C | 0.17 | 0.30 | 0.06 | 0.20 | 1.78 | 0.19 | 2.71 |
| 110307 | C | 0.27 | 0.39 | 0.21 | 0.40 | 3.23 | 0.02 | 4.52 |
| Min | 0.12 | 0.23 | 0.03 | 0.18 | 0.36 | 0.02 | 1.83 | |
| Max | 0.49 | 0.71 | 0.21 | 0.80 | 4.50 | 0.71 | 5.92 | |
| Average | 0.24 | 0.37 | 0.07 | 0.34 | 1.65 | 0.31 | 2.98 | |
| SD (n=25) | 0.10 | 0.13 | 0.04 | 0.17 | 1.09 | 0.16 | 1.04 | |
All samples were morphologically authenticated as Radix Isatidis according to the current standard of the Chinese Pharmacopoeia.
Sample item A, B and C refers to the three pharmaceutical companies from which the samples were sourced.
Values are in mg/g of dry raw material and expressed as mean±SD, n=3; the SD was <4%.
Figure 5.
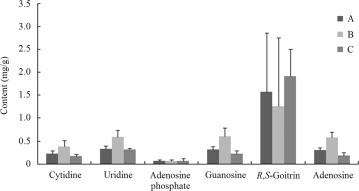
Comparison of the average content (mg/g) of the six marker compounds in 25 samples of Radix Isatidis classified into three groups (A, B and C) based on the three pharmaceutical companies from which they were sourced.
4. Conclusions
HPLC fingerprint analysis was used to evaluate the quality and consistency of 25 samples of Radix Isatidis sourced from different regions of China and used by three pharmaceutical companies to make various TCM preparation. Fingerprints were based on the simultaneous identification and quantitation of five nucleosides and one glucosinolate. Similarity analysis was used to compare the fingerprints and demonstrate the consistency of the groups of samples sourced from the three companies. The method was shown to provide an effective quality evaluation strategy to ensure the consistent quality of Radix Isatidis.
Acknowledgments
This research was financially supported by the Special Program for New Drug Innovation of the Ministry of Science and Technology, China (Nos. 2011ZX09201-201-12 and 2011ZX09201-201-07).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Yuanyuan Xie, Email: yuanyuan_sy@tsinghua.edu.cn.
Guoan Luo, Email: luoga@mail.tsinghua.edu.cn.
References
- 1.State Administration of Traditional Chinese Medicine of the People's Republic of China. Chinese Materia Medica. No. 21. Shanghai: Shanghai Scientific & Technical Publishers; 1999.
- 2.Xiao S.S., Jin Y., Sun Y.Q. Recent progress in the studies of chemical constituents, pharmacological effects and quality control methods on the roots of Isatix indigotica. J Shenyang Pharm Univ. 2003;20:455–459. [Google Scholar]
- 3.Du P., Zhu S., Lv P. Antibacterial activity of 20 kinds of Chinese medicinal materials for Helicobacter pylori in vitro. J Chin Med Mater. 2001;24:188–189. [PubMed] [Google Scholar]
- 4.Ma F., Chen Y., Li J., Qing H.P., Wang J.D., Zhang Y.L. Screening test for anti-helicobacter pylori activity of traditional Chinese herbal medicines. World J Gastroenterol. 2010;16:5629–5634. doi: 10.3748/wjg.v16.i44.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong W., Zhao Y., Shan L., Xiao X., Guo W. Thermochemical studies on the quantity-antibacterial effect relationship of four organic acids from Radix Isatidis on Escherichia coli growth. Biol Pharm Bull. 2008;31:1301–1305. doi: 10.1248/bpb.31.1301. [DOI] [PubMed] [Google Scholar]
- 6.Fu X.X. Therapeutic effect of combined treatment with Ara-A dauricine and Chinese herbs in chronic hepatitis B infection. Chin J Inter Med. 1991;30:498–501. [PubMed] [Google Scholar]
- 7.Wei Z.Y., Wang X.B., Zhang H.Y., Yang C.H., Wang Y.B., Xu D.H. Inhibitory effects of indigowoad root polysaccharides on porcine reproductive and respiratory syndrome virus replication in vitro. Antiviral Ther. 2011;16:357–363. doi: 10.3851/IMP1755. [DOI] [PubMed] [Google Scholar]
- 8.Guo H., Mao J., Qian X., Sun C., Sun H. Varicella-zoster virus prophylaxis with the traditional Chinese medicine Radix isatidis (Banlangen) in patients with multiple myeloma treated with bortezomib. J Altern Complement Med. 2011;17:985–986. doi: 10.1089/acm.2011.0386. [DOI] [PubMed] [Google Scholar]
- 9.Liao H.F., Lu M.C., Chang H.C., Wei C.C., Kao C.H., Chen Z.H. Effects of herbal medicinal formulas on suppressing viral replication and modulating immune responses. Am J Chin Med. 2010;38:173–190. doi: 10.1142/S0192415X10007749. [DOI] [PubMed] [Google Scholar]
- 10.Li H.B., Yan D., Wu Y.S., Xiao X.H., Tang H.Y., Zhang S.F. Bio-evaluation methods and optimization for Isatidis Radix quality control based on antiviral activity detection. Chin Tradit Herb Drugs. 2011;42:1560–1565. [Google Scholar]
- 11.Shin E.K., Kim D.H., Lim H., Shin H.K., Kim J.K. The anti-inflammatory effects of a methanolic extract from Radix Isatidis in murine macrophages and mice. Inflammation. 2010;33:110–118. doi: 10.1007/s10753-009-9164-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z.W., Wu L.W., Liu S.T., Cai C.P., Rao P.F., Ke L.J. Mechanism study of anti-influenza effects of Radix Isatidis water extract by red blood cells capillary electrophoresis. Chin J Chin Mater Med. 2006;31:1715–1719. [PubMed] [Google Scholar]
- 13.Chen Q. Pharmacological action and clinical application of Radix Isatidis. Chin Pharm Aff. 2009;23:607–608. [Google Scholar]
- 14.Liang Y.H., Hou H.X., Li D.R., Qing J., Qiu L., Wu H.H. Studies on in vitro anticancer activity of tryptanthrin B. Chin Tradit Herb Drug. 2000;31:531–537. [Google Scholar]
- 15.Liu Y.H., Liu Y.F., Guo X.X. Current studies on anti-endotoxic chemical components of traditional Chinese medicine in China. Acta Pharmacol Sin. 2001;22:1071–1077. [PubMed] [Google Scholar]
- 16.Liu S., Qiao C., Wang Y. Research on cause of distinction in antiendotoxic activation of Radix Isatidis from different cultivated populations. Chin J Chin Mater Med. 1999;24(398–400):446. [PubMed] [Google Scholar]
- 17.Lin A.H., Fang S.X., Fang J.G., Du G., Liu Y.H. Studies on anti-endotoxin activity of F022 from Radix Isatidis. Chin J Chin Mater Med. 2002;27:439–442. [PubMed] [Google Scholar]
- 18.Liu Y., Fang J., Lei T., Wang W., Lin A. Anti-endotoxic effects of syringic acid of Radix Isatidis. J Huazhong Univ Sci Technol Med Sci. 2003;23:206–208. doi: 10.1007/BF02859960. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Liu Y., Fang J., Chen X., Xie W. Effect of Radix Isatidis on the expression of moesin mRNA induced by LPS in the tissues of mice. J Huazhong Univ Sci Technol Med Sci. 2007;27:135–137. doi: 10.1007/s11596-007-0206-y. [DOI] [PubMed] [Google Scholar]
- 20.Wang W., Fang J., Liu Y., Xie W. Screening of anti-endotoxin components from Radix isatidis. J Huazhong Univ Sci Technol Med Sci. 2006;26:261–264. doi: 10.1007/BF02895833. [DOI] [PubMed] [Google Scholar]
- 21.Fang J., Wang W., Hu Y., Feng D., Tang J. Influence of Radix Isatidis on the endotoxin-induced release of TNF-alpha and IL-8 from HL-60 cells. J Huazhong Univ Sci Technol Med Sci. 2005;25:546–548. doi: 10.1007/BF02896013. [DOI] [PubMed] [Google Scholar]
- 22.Fang J.G., Liu Y.H., Wang W.Q., Xie W., Fang S.X., Han H.G. The anti-endotoxic effect of O-aminobenzoic acid from Radix Isatidis. Acta Pharmacol Sin. 2005;26:593–597. doi: 10.1111/j.1745-7254.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen K., Dou Y., Chen Z., Tian J.Z. Advance of Radix Isatidis pharmacological action and active substances. Chin J Exp Tradit Med Formlae. 2011;17:275–278. [Google Scholar]
- 24.Zhao Y.L., Wang J.B., Shan L.M., Jin C., Ma L., Xiao X.H. Effect of Radix Isatidis polysaccharides on immunological function and expression of immune related cytokines in mice. Chin J Integr Med. 2008;14:207–211. doi: 10.1007/s11655-008-0207-2. [DOI] [PubMed] [Google Scholar]
- 25.Xue J., Xu Y., Jin L., Liu G., Sun Y., Li S. Effects of traditional Chinese medicine on immune responses in abalone, Haliotis discus hannai Ino. Fish Shellfish Immunol. 2008;24:752–758. doi: 10.1016/j.fsi.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y.L., Xiao X.H., Liao Q.W., Wang J.B., Ma Y.G., Yan D. Effects of different extracts from Radix isatidis on lymphocytes of mice by biothermodynamics. Chin J Chin Mater Med. 2006;31:590–593. [PubMed] [Google Scholar]
- 27.Yang Y., Xiong L. Li Jiafeng's experience in treating children's acute tonsillitis. J Yunnan Univ Tradit Chin Med. 2006;29:34–36. [Google Scholar]
- 28.Lui C.J. Clinical observation on the treatment of upper respiratory tract infections with Banlangen granules. Asia Pacific Tradit Med. 2012;8:82–83. [Google Scholar]
- 29.Shen J.G., Xu Y.S., Yu X.J. Treatment for varicella with Radix Isatidis at large dose. Chin J Clin. 2003;31:63. [Google Scholar]
- 30.Jin M.Z., Ren D.X., Meng F.P., Li X.M. The effects of Radix Isatidis on immunological function and influenza virus (FM1) in Kunming mice. Lishizhen Med Mater Med Res. 2007;18:394–396. [Google Scholar]
- 31.Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsuan S.L., Chang S.C., Wang S.Y., Liao T.L., Jong T.T., Chien M.S. The cytotoxicity to leukemia cells and antiviral effects of Isatis indigotica extracts on pseudorabies virus. J Ethnopharmacol. 2009;123:61–67. doi: 10.1016/j.jep.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y.T., Yang Z.F., Zhao H.S., Qin S., Guan W.D. Screening of anti-H1N1 active constituents from Radix Isatidis. J Guangzhou Univ Tradit Chin Med. 2011;28:419–422. [Google Scholar]
- 34.Sun G.L., Hu Z.L., Meng H., Li Y., Wang X.L., Liu J.H. Study on anti-human cytomegalovirus effect of Isatis Root by MTT colorimetry. J Shandong Univ Tradit Chin Med. 2000;24:137–138. [Google Scholar]
- 35.The State Pharmacopoeia Committee of China. The pharmacopoeia of the People's Republic of China. Part 1. Beijing: China Medical and Technology press; 2010, p. 191.
- 36.An Y.Q., Jia X.B., Yuan H.J., Sun E., Xu Z.Z. Content determination of epigoitrin in Radix Isatidis and its preparation by RP-HPLC. Chin J Chin Mater Med. 2008;33:2074–2076. [PubMed] [Google Scholar]
- 37.Zhang H.Y., Luo J., Huang Y.N., Wang D.Q., Deng Q.H., Li L.F. Simultaneous determination of uridine, adenosine and epigoitrin in compound Banlangen granule by HPLC. Pharm Today. 2011;21:337–339. [Google Scholar]
- 38.Wang X., Meng X.J., Zhang Q. Determination of three nucleosides in injection of banlangen by HPLC. Mod J Integr Tradit Chin West Med. 2012;21:646–647. [Google Scholar]
- 39.Xu Y., Liu X. Determination of Indirubin in Indigowoad Root and its affecting factors. Lishizhen Med Mate Medica Res. 2008;1:1122–1123. [Google Scholar]
- 40.Yan S.K., Luo G.A., Wang Y.M., Cheng Y.Y. Simultaneous determination of nine components in Qingkailing injection by HPLC/ELSD/DAD and its application to the quality control. J Pharm Biomed Anal. 2006;40:889–895. doi: 10.1016/j.jpba.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Xiao X., Luo G.A., Wang Y.M., Liang L., Wang Y.S. Fingerprints of Isatidis Radix by HPLC. J Jiangxi Univ Tradit Chin Med. 2010;22:67–69. [Google Scholar]
- 42.Zou P., Hong Y., Koh H.L. Chemical fingerprinting of Isatis Indigatica root by RP-HPLC and Hierarchical clustering analysis. J Pharm Biomed Anal. 2005;38:514–520. doi: 10.1016/j.jpba.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Shi Y.H., Xie Z.Y., Wang R., Huang S.J., Li Y.M., Wang Z.T. Quantitative and chemical fingerprint analysis for the quality evaluation of Isatis indigotica based on ultra-performance liquid chromatography with photodiode array detector combined with chemometric methods. Int J Mol Sci. 2012;13:9035–9050. doi: 10.3390/ijms13079035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Y., Li S.P., Wang Y.T., Chen X.J., Tu P.F. Differentiation of Herba Cistanches by fingerprint with high-performance liquid chromatography-diode array detection-mass spectrometry. J Chromatogr A. 2009;1216:2156–2162. doi: 10.1016/j.chroma.2008.04.040. [DOI] [PubMed] [Google Scholar]



