Abstract
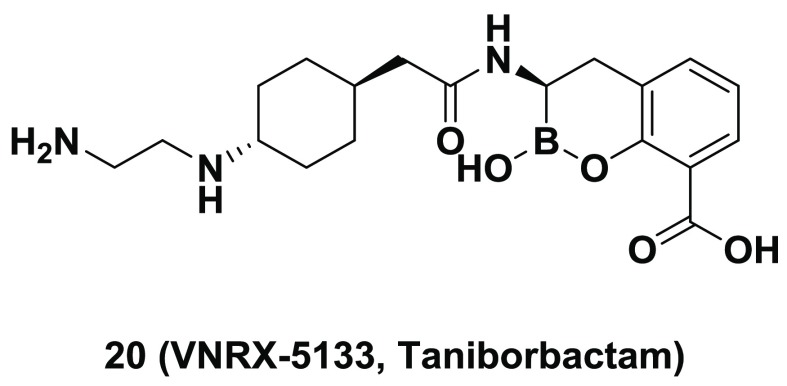
A major resistance mechanism in Gram-negative bacteria is the production of β-lactamase enzymes. Originally recognized for their ability to hydrolyze penicillins, emergent β-lactamases can now confer resistance to other β-lactam drugs, including both cephalosporins and carbapenems. The emergence and global spread of β-lactamase-producing multi-drug-resistant “superbugs” has caused increased alarm within the medical community due to the high mortality rate associated with these difficult-to-treat bacterial infections. To address this unmet medical need, we initiated an iterative program combining medicinal chemistry, structural biology, biochemical testing, and microbiological profiling to identify broad-spectrum inhibitors of both serine- and metallo-β-lactamase enzymes. Lead optimization, beginning with narrower-spectrum, weakly active compounds, provided 20 (VNRX-5133, taniborbactam), a boronic-acid-containing pan-spectrum β-lactamase inhibitor. In vitro and in vivo studies demonstrated that 20 restored the activity of β-lactam antibiotics against carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Enterobacteriaceae. Taniborbactam is the first pan-spectrum β-lactamase inhibitor to enter clinical development.
Introduction
β-Lactam antibiotics (BLs), including penicillins, cephalosporins, monobactams, and carbapenems, represent the most important group of antibiotics, accounting for >50% of all antibiotic prescriptions.1 BLs inhibit bacterial cell-wall biosynthesis by binding to penicillin-binding proteins (PBPs), thus preventing peptidoglycan cross-linking and ultimately causing bacterial cell death. Although BLs are safe and demonstrate high efficacy in the clinical setting, the overuse of BLs has resulted in the emergence and spread of BL-resistant bacteria, which has progressively eroded their clinical utility. Mechanisms of resistance to BLs include the overproduction of efflux pumps, the modification or down-regulation of outer-membrane porins (in Gram-negative bacteria), the modification of PBPs (especially in Gram-positive bacteria), and the evolution of β-lactamase enzymes that catalytically inactivate BLs by hydrolyzing the β-lactam ring. Over eight decades of BL use, bacteria have evolved an extraordinary number of β-lactamase variants (>2000 reported to date) exhibiting significant structural and functional heterogeneity resulting from the introduction of different BLs approved for medical use.2 On the basis of amino acid sequence homology, β-lactamases are divided into four classes (Ambler classification): A, B, C, and D.3 Classes A, C, and D are serine β-lactamases (SBLs), including the clinically relevant TEM-, SHV-, CTX-M-, and KPC-type variants (class A), the AmpC- and the plasmid-encoded CMY-type cephalosporinases (class C), and the OXA-type enzymes (oxacillinases, class D). Class B β-lactamases are zinc-dependent metallo-β-lactamases (MBLs) characterized by an exceedingly broad substrate profile and strong carbapenemase activity. MBLs are classified into three subclasses (B1, B2, and B3) based on the primary amino acid sequence.4 Subclass B1 includes clinically important NDM-, IMP-, and VIM-type variants. KPC-, OXA-, NDM-, VIM-, and IMP-type β-lactamases are mostly encoded by plasmids in clinical isolates of Enterobacteriaceae, Pseudomonas spp., and Acinetobacter spp., thus giving rise to the emergence and spread of multi-drug-resistant (MDR) Gram-negative opportunistic pathogens, which threaten the ability to efficiently treat hospital-acquired infections.5 Global antibiotic resistance has reached critical levels, with major bacterial pathogens (including Gram-negative organisms such as Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa) quickly evolving toward pan-drug-resistant phenotypes. There is an urgent medical need for strategies that address both serine- and metallo-based β-lactamase-mediated bacterial resistance.
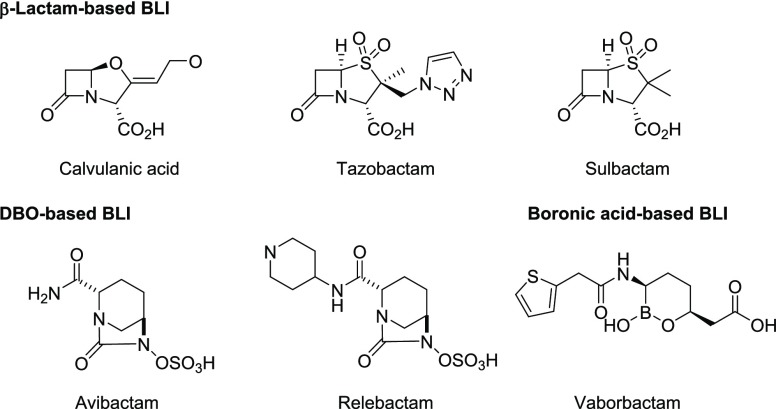
One successful strategy for overcoming β-lactamase-mediated resistance is to coadminister the BL with an appropriate β-lactamase inhibitor (BLI). Under these combinations, the BLI forms a covalent adduct with the active SBL enzyme, thereby preventing it from hydrolyzing the BL antibiotic. Three therapeutically important BLIs, that is, clavulanic acid, tazobactam (TZB), and sulbactam (Figure 1), are effective against certain class A SBLs but are mostly ineffective against class C cephalosporinases, carbapenem-hydrolyzing serine enzymes (KPC- and OXA-type), and class B MBLs (including NDM- and VIM-type MBLs). In addition, these first-generation BLIs are themselves β-lactams that have driven the rapid evolution of resistance to these structurally similar compounds (e.g., inhibitor-resistant variants). A next wave of BLI discovery led to the approval of the diazabicyclooctane (DBO) avibactam (AVI) (NXL-104) in 2015 and relebactam in 2019 (Figure 1). The DBOs display a broad spectrum of β-lactamase inhibition, which includes KPC-type and some of the class D OXA-type enzymes.6 Studies have shown that AVI is a covalent and slowly reversible inhibitor.7 Another reversible BLI vaborbactam (VAB) (RPX7009) was the first approved boron-based BLI that also efficiently inhibits KPC-type carbapenemases.8 However, the DBOs and VAB lack activity against MBLs. There is a clear, unmet medical need for the development of broad-spectrum BLIs that would exhibit inhibitory activity against class A, B, C, and D enzymes, especially considering that β-lactamases belonging to multiple classes are now commonly produced by MDR Gram-negative pathogens.
Figure 1.
Structures of the approved BLIs.
Boronic acids and esters are electrophilic moieties that covalently bind the active-site serine residue of certain enzymes, including SBLs. Thus boronic acids or esters have been successfully developed as enzyme inhibitors.9 The covalent tetrahedral adduct formed between the boron atom of the inhibitor and the catalytic serine residue of the β-lactamase mimics either the acylation or deacylation step of the enzyme-mediated hydrolysis. Although the boron covalently binds to the enzyme, the inhibitors are reversible in nature.10 Because boron-based BLIs are not β-lactams, they have the added benefit of not being inactivated by β-lactam-recognizing enzymes. The preliminary discovery of boron-based BLIs emanated from academic laboratories;10−15 however, over the past decade, the pharmaceutical industry has shown increased interest in this field. The bicyclic boronates were first discovered and disclosed as pan-spectrum BLIs (i.e., able to inhibit both SBLs and MBLs) by Burns et al. at Protez Pharmaceuticals (a subsidiary of Novartis) in 2010.16 The class of bicyclic boronate BLIs was then further developed at Venatorx Pharmaceuticals.17,18 Subsequently, additional structural and mechanistic studies of bicyclic boronate BLIs have appeared in the literature.19−22 In parallel, Rempex Pharmaceuticals has discovered and developed the monocyclic boron-based SBL inhibitor, RPX7009 (VAB), which has been approved for use in combination with meropenem.8 Herein we describe the iterative program including medicinal chemistry, structural biology, biochemical testing, and microbiological profiling that led to the discovery of VNRX-5133 (taniborbactam), a highly potent pan-spectrum BLI that inhibits all four Ambler classes of β-lactamase enzymes with excellent Gram-negative outer membrane penetration.17,23 The combination of VNRX-5133 with the fourth-generation cephalosporin, cefepime, is currently in phase 3 clinical studies.
Results and Discussion
Design of BLIs for Both Serine- and Metallo-β-lactamases (“Pan-Spectrum BLIs”)
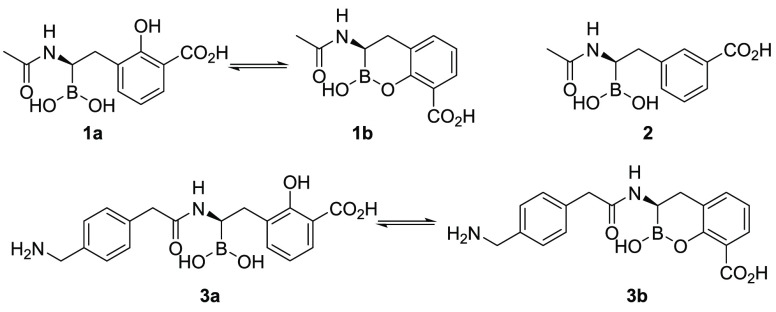
Ness et al. described the design of boronic acids 1 as inhibitors of TEM-1, a narrow-spectrum SBL (Figure 2).24 Using structure-based design, the authors reasoned that the introduction of a hydroxyl group onto the aromatic ring of 2 might facilitate intramolecular cyclization (i.e., 1b) or provide an additional hydrogen-bonding interaction with the enzyme. Indeed, compound 1 showed a significant increase in affinity to the TEM-1 enzyme compared with 2. However, X-ray crystallography revealed that 1 was binding in the acyclic binding mode (i.e., 1a). Burns et al. expanded this concept by introducing a further substituted acetamide group.16 Extensive structure–activity relationship (SAR) studies and microbiological profiling identified a series of compounds exemplified as 3 with potent activity against a broad panel of SBLs and weak activity against certain class B metallo-enzymes. This study also suggested the cyclic boronate 3b as a possible β-lactamase binding mode.16 In our hands, the enzymatic activity of 1 and 3 against both class A KPC-type and class C β-lactamases appeared suboptimal, as small structural changes resulted in vastly different IC50 values. In addition, 1 and 3 only inhibited the MBL VIM-2 at micromolar levels. On the basis of the published X-ray cocrystal structures of 1 with TEM-1 β-lactamase,24 we understood that the boron atoms of 1 and 3 were interacting covalently with the active-site serine residue. For MBL binding, we hypothesized that the carboxylic acid group, cyclic boronate oxygen, and hydroxyl groups might be interacting with the active-site zinc atoms. Thus we recognized that the cyclic boronate scaffold might serve for the development of a pan-spectrum BLI.
Figure 2.
Chemical structures and proposed equilibria of known cyclic boronates 1–3.
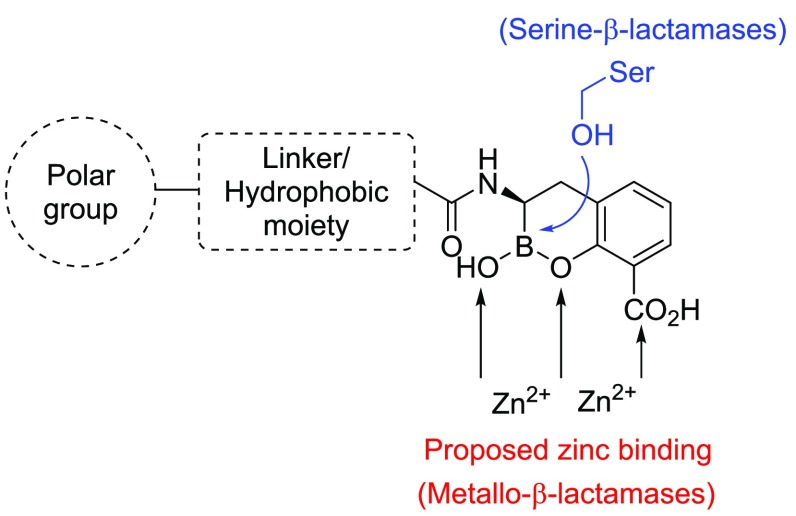
Our design strategy was to append the cyclic boronate scaffold with a hydrophobic group to enhance van der Waals interactions between the ligand and hydrophobic residues within the β-lactamase active site (Figure 3). Notably, both SBL and MBL enzymes commonly contain hydrophobic residues in the loops defining this portion of the active site pocket.25
Figure 3.
Strategy for designing a cyclic boronate-based pan-BLI showing putative active-site serine- or zinc-binding interactions.
Because of the vast diversity of clinically relevant β-lactamase-producing bacteria, broad-spectrum BLs and BLIs must be able to cross the outer membrane (OM) of multiple Gram-negative pathogens to be classified as an effective therapeutic agent. The entry of nutrients and xenobiotics through the OM is highly controlled and often facilitated by the OM porins—a family of transmembrane hydrophilic channels that allow for the passive transport of molecules into the periplasm.26 O’Shea et al. have observed that high-molecular-weight BLs with clogP < 1 and polar surface area (PSA) > 150 Å2 have preferred access into Gram-negative bacteria.27 Thus, to bias entry into Gram-negative bacteria, polar group(s) would be ideally included in the amide group substituent of the designed BLIs (Figure 3).
Synthesis of the Cyclic Boronate-based BLIs
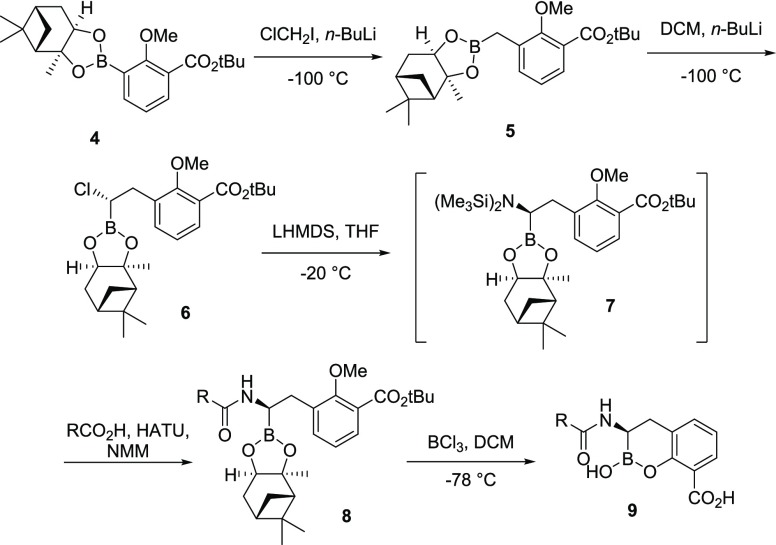
The cyclic boronate-based BLIs were prepared using modifications of previously reported procedures (Scheme 1).16 Compound 4 was synthesized in two steps from commercially available 3-borono-2-methoxybenzoic acid by a reaction with isobutylene, followed by the esterification of the boronic acid with (+)-pinanediol. Following Matteson’s protocol,28 the aryl boronate (4) was reacted with chloromethyllithium at −100 °C to provide 5 in excellent yield. A second homologation reaction with the anion derived from dichloromethane (−100 °C) selectively provided the (S)-α-chloro-boronate (6). Stereospecific displacement of the chlorine atom of 6 with lithium bis(trimethylsilyl)amide at −20 °C afforded the intermediate α-silylaminoboronate (7), which was coupled in situ (HATU, NMM) to the requisite carboxylic acid. The resultant amides (8) were deprotected and cyclized by treatment with BCl3 (1 M in DCM) at −78 °C to afford the crude boronates (9), which were purified by reverse-phase high-performance liquid chromatography (HPLC) then lyophilized to dryness.
Scheme 1. Generalized Synthesis of Cyclic Boronate-based BLIs.
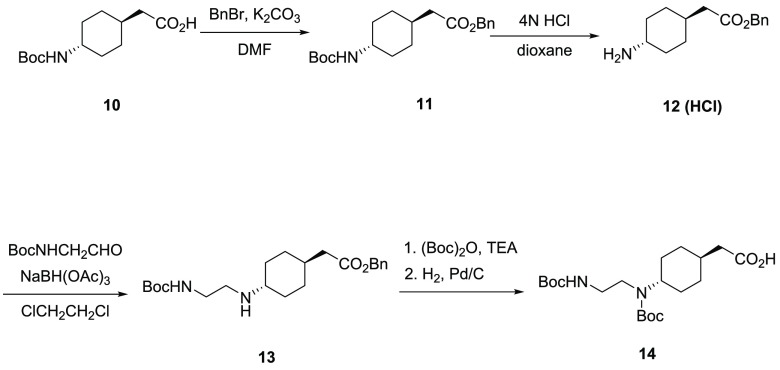
The carboxylic acids (RCO2H) used for SAR studies were either commercially available or synthesized from readily available starting materials. A representative synthesis of carboxylic acid side chain 14 is provided in Scheme 2. In this case, the alkylation of acid 10 with benzyl bromide gave ester 11. The removal of the Boc protective group afforded the amine HCl salt (12), which was converted to intermediate 13 via reductive amination. The protection of the secondary amine and the removal of the benzyl group provided 14 in a ca. 30% yield over five steps.
Scheme 2. Synthesis of Carboxylic Acid 14.
Structure–Activity Relationship
The cyclic boronates were evaluated for both inhibition of selected β-lactamase enzymes and rescue of meropenem activity in selected meropenem-resistant bacterial strains. Our primary in-house biochemical screening panel was composed of four β-lactamases belonging to each of the four unique enzyme classes. Cellular assays were performed using meropenem-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa isolates and the minimum inhibitory concentrations (MICs) of the BL/BLI combinations were determined using a fixed concentration of meropenem (4 μg/mL). Importantly, three K. pneumoniae strains had acquired meropenem resistance, in part, due to the production of certain β-lactamase enzymes, that is, KPC-2, OXA-48, and VIM-4, whereas the nonfermenting P. aeruginosa strain predominantly produced VIM-2. The MIC values of meropenem alone against these strains are ≥32 μg/mL.
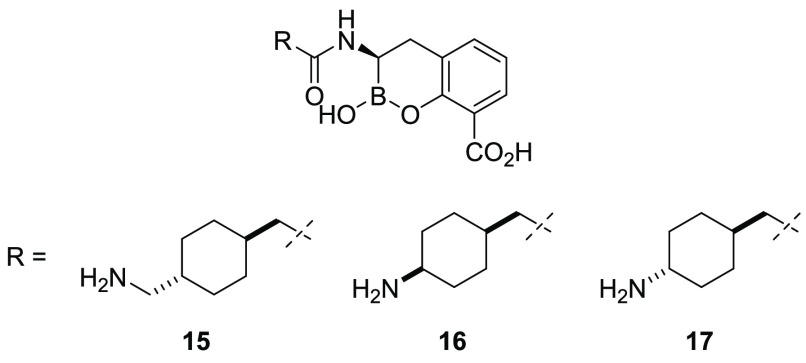
Our initial efforts focused on the replacement of the aryl group of 3 with a cyclohexyl moiety. We reasoned that a cyclohexyl group might offer a different trajectory to possibly place the attached polar group in a productive interaction with the amino acids of β-lactamases and improve the physicochemical properties owing to the increased sp3 character of the inhibitor.29 When evaluated against our primary panel of β-lactamase enzymes and bacterial isolates, 3 displayed potent inhibition of the class A KPC-2 carbapenemase and AmpC-type enzymes but only modest inhibition of the class D OXA-48 carbapenemase. The excellent MIC of 3 plus meropenem (fixed at 4 μg/mL) against the KPC-2-producing K. pneumoniae strain (MIC = 0.5 μg/mL) suggested a reasonable accumulation of the drug in this Gram-negative pathogen (Table 1). However, despite the modest inhibition of the VIM-2 MBL, 3 was less effective at rescuing meropenem activity in VIM-producing strains. The cyclohexyl analogue (15) showed comparable inhibition of KPC-2, AmpC, and OXA-48 to 3 but possessed reduced activity against VIM-2. The truncation of the terminal alkyl amine afforded the 1,4-cis- and 1,4-trans-cyclohexylamines 16 and 17, respectively. Relative to 15, analogue 16 had reduced inhibitory activity against OXA-48 and comparable activity against VIM-2. The higher MIC values of 16 relative to 15 for the VIM-producing isolates, however, suggested that this truncated 1,4-cis-cyclohexylamine was less able to accumulate in the periplasm. Gratifyingly, the isomeric 1,4-trans-17 demonstrated significant inhibitory activity against all four classes of β-lactamases, with a striking low-nanomolar inhibition of the VIM-2 MBL and a potent rescue of meropenem (4 μg/mL) observed in the presence of 2 μg/mL of BLI in the meropenem-resistant isolates.
Table 1. Biochemical and Microbiological Activity of BLIs 3 and 15–17.
| microbiological assay, MIC (μg/mL)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| biochemical assay, IC50(μM)b |
K. pneumoniae |
P. aeruginosa | ||||||||
| entry | clogP | tPSA | KPC-2 (class A) | AmpC (class C) | OXA-48 (class D) | VIM-2 (class B) | KPC-2 | OXA-48 | VIM-4 | VIM-2 |
| 3 | –1.67 | 122 | 0.04 | 0.012 | 1.8 | 0.27 | 0.5 | 1 | 4 | 8 |
| 15 | –1.04 | 122 | 0.10 | 0.025 | 1.8 | 1.6 | 1 | 2 | 8 | 4 |
| 16 | –1.66 | 122 | 0.12 | 0.005 | 18.0 | 1.67 | 2 | 16 | 32 | 32 |
| 17 | –1.66 | 122 | 0.03 | 0.03 | 2.24 | 0.15 | 2 | 2 | 2 | 2 |
Reported as the BLI concentration able to restore meropenem (fixed at 4 μg/mL) antibacterial activity.
Results are expressed as the average from duplicates unless otherwise indicated.
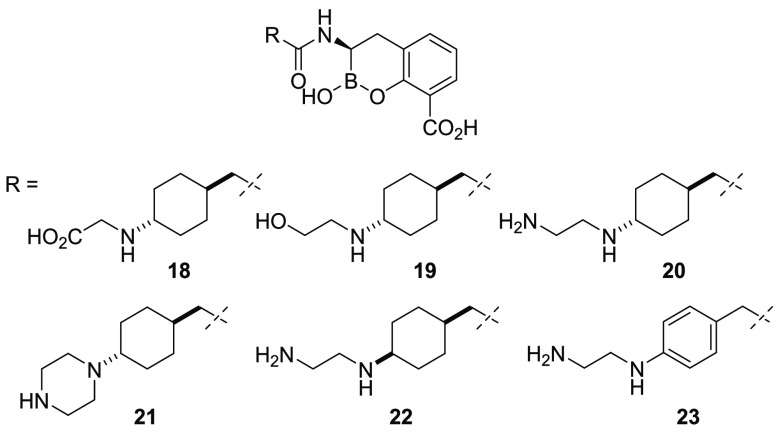
In an effort to modify the molecular properties of the inhibitors (i.e., MW, clogP, PSA, and net charge at physiological pH), we substituted the terminal amine of 17 with a variety of polar and nonpolar groups and observed how these factors affected the enzyme inhibition and Gram-negative bacteria OM penetration (Table 2). Acetic acid analogue 18 and ethanolamine analogue 19 only poorly inhibited the VIM-2 MBL. However, when an additional amine group was added, as in 20, we observed a dramatic improvement in the enzyme inhibition against both OXA-48 and VIM-2 β-lactamases. Importantly, 20 demonstrated excellent potentiation of meropenem activity in the whole-cell assay, which suggested excellent penetration across the Gram-negative OM. Comparing 20 with 19 and 18, we observed that replacing the neutral hydroxyl group or negatively charged carboxylate with a primary amine could dramatically increase the inhibitory activity toward VIM-2 and OXA-48 and, most importantly, improve the BLI permeability toward the Gram-negative OM. Conversely, constrained diamine 21 had reduced activity in the biochemical and cell-based assays. Notably, relative to 20, both the 1,4-cis-22 and aryl analogue 23 displayed reduced biochemical activity against VIM-2.
Table 2. Biochemical and Microbiological Activity of BLIs 18–23.
| microbiological assay, MIC (μg/mL)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| biochemical assay, IC50 (μM)b |
K. pneumoniae |
P. aeruginosa | ||||||||
| entry | clogP | tPSA | KPC-2 (class A) | AmpC (class C) | OXA-48 (class D) | VIM-2 (class B) | KPC-2 | OXA-48 | VIM-4 | VIM-2 |
| 18 | –1.85 | 145 | 0.019 | 0.006 | 3.14 | 2.4 | 4 | 8 | 32 | 16 |
| 19 | –1.99 | 128 | 0.017 | 0.01 | 7.55 | 1.4 | 2 | 2 | 0.5 | 4 |
| 20 | –2.39 | 134 | 0.03 | 0.032 | 0.42 | 0.02 | 0.5 | 2 | 0.5 | 0.06 |
| 21 | –1.32 | 111 | 0.02 | 0.003 | 1.52 | 1.5 | 4 | 16 | 32 | 16 |
| 22 | –2.39 | 134 | 0.03 | 0.035 | 0.67 | 0.13 | 8 | 8 | 4 | 0.5 |
| 23 | –1.81 | 134 | 0.022 | 0.005 | 0.288 | 0.491 | 1 | 1 | 8 | 0.5 |
Reported as the BLI concentration able to restore meropenem (fixed at 4 μg/mL) antibacterial activity.
Results are expressed as the average from duplicates unless otherwise indicated.
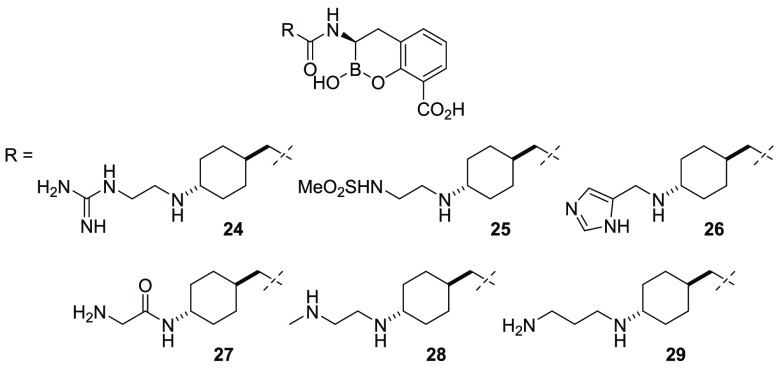
Additional analogues were prepared to vary the basicity, the distance from the cyclohexylamine moiety, the steric environment, and the geometry of the attached distal substituent. The replacement of the distal amino group with guanidine (24) or methylsulfonamide (25) did not significantly alter in vitro enzyme inhibition, albeit with modest loss of activity on OXA-48 and VIM-2, respectively (Table 3). Additionally, 25 exhibited a significant loss in whole-cell activity possibly due to poor penetration. Imidazole replacement (26) or the introduction of a carbonyl group (27) were detrimental to pan-β-lactamase activity compared with 20. The introduction of a methyl group to the distal nitrogen atom (28) resulted in improved inhibition with VIM-2 in the biochemical assays and was well tolerated in the cell-based assay. Furthermore, increasing the distance between the two nitrogen atoms (29) was also well tolerated in the biochemical assays.
Table 3. Biochemical and Microbiological Activity of BLIs 24–29.
| microbiological assay, MIC (μg/mL)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| biochemical assay, IC50 (μM)b |
K. pneumoniae |
P. aeruginosa | ||||||||
| entry | clogP | tPSA | KPC-2 (class A) | AmpC (class C) | OXA-48 (class D) | VIM-2 (class B) | KPC-2 | OXA-48 | VIM-4 | VIM-2 |
| 24 | –2.53 | 170 | 0.027 | 0.006 | 1.59 | 0.086 | 1 | 2 | 0.06 | 0.5 |
| 25 | –1.97 | 154 | 0.017 | 0.006 | 0.499 | 0.11 | 4 | 8 | 8 | 16 |
| 26 | –1.66 | 132 | 0.036 | 0.014 | 0.454 | 0.477 | 1 | 8 | 8 | 1 |
| 27 | –2.60 | 151 | 0.034 | 0.006 | 0.355 | 0.594 | 2 | 8 | 8 | 8 |
| 28 | –1.61 | 119 | 0.012 | 0.011 | 0.579 | 0.002 | 0.5 | 2 | 0.5 | N.D.c |
| 29 | –1.97 | 134 | 0.07 | 0.005 | 0.664 | 0.082 | 0.5 | 8 | 4 | 0.25 |
Reported as the BLI concentration able to restore meropenem (fixed at 4 μg/mL) antibacterial activity.
Results are expressed as the average from duplicates unless otherwise indicated.
Not determined.
Taken together, these data suggest that cyclic boronates have the potential to potently inhibit both SBLs and MBLs with a variety of substituents occupying the β-lactamase active site(s). Because of the permeability barrier of Gram-negative bacteria, however, lead optimization focused on identifying the desired amide-based side chain that would partially mediate OM permeability. We noticed that the preferred analogues to achieve better Gram-negative OM penetration possessed a clogP value between −1 and −2.5 and a tPSA between 110 and 170 Å2. The addition of a second amino or guanidino group, which is protonated at physiological pH, appeared to be beneficial for the Gram-negative OM permeability. In addition, the steric environment surrounding the distal amine was critical, with a primary amine or a secondary amine bearing a small alkyl group being preferred for increased permeability. Richter et al. recently showed that a primary amino group (together with high rigidity and low globularity) could predict compound accumulation within Gram-negative bacteria.30 We propose that in addition to the polarity of the compound, the net charge of the molecule may have an important role. The inclusion of an amine moiety can alter the net charge of a compound at physiological pH and might be a contributing factor for improved Gram-negative OM penetration. Following this preliminary investigation, compound 20 (VNRX-5133) was selected for further evaluation as a potential development candidate owing to its potency against the four classes of β-lactamases as well as its broad range of cellular activity in combination with meropenem.
Compound 20 Inhibited both Serine- and Metallo-β-lactamases
Cyclic boronate 20 and four known BLIs were tested against a panel consisting of the four classes of β-lactamase enzymes (A–D). As described in Table 4, 20 exhibited a broad spectrum of activity, inhibiting all of the class A, C, and D SBLs tested as well as two of the class B MBLs. In contrast, the two β-lactam-derived BLIs, clavulanic acid and TZB, showed reduced activity on KPC-2, CMY-2, and AmpC. The recently approved boronic-acid-based BLI, VAB, showed good activity against KPC-2 but was only weakly active against the SHV-5 and CTX-M-15 extended-spectrum β-lactamases (ESBLs) and the OXA-type variants. Importantly, unlike AVI, VAB, clavulanic acid, and TZB, 20 displayed significant inhibition of the clinically relevant NDM-1 and VIM-2 MBLs. However, 20 was only weakly active against IMP-1, another clinically relevant subclass B1 MBL. A recent study also reported a similar spectrum of activity for VNRX-5133 against β-lactamases.22 In addition, the authors observed that VNRX-5133 was weakly active against the subclass B2 MBL CphA (IC50 ≈ 2.51 μM) and did not inhibit subclass B3 MBL L1.22
Table 4. Inhibitory Activity of 20 and Known BLIs Measured with Clinically Relevant β-Lactamases of Classes A to D.
| IC50 (μM)a,b |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| class A |
class C |
class D |
class B |
|||||||
| entry | SHV-5 | CTX-M-15 | KPC-2 | CMY-2 | p99 AmpC | OXA-1 | OXA-48 | NDM-1 | VIM-2 | IMP-1 |
| 20 | 0.0004 | 0.01 | 0.03 | 0.007 | 0.03 | 0.16 | 0.42 (0.54)22 | 0.19 | 0.026 | 39.8 (2.51)22 |
| avibactam | 0.013 | 0.003 | 0.06 | 0.007 | 0.016 | 0.04 | 0.55 | >100 | >100 | >100 |
| vaborbactam | 0.44 | 0.42 | 0.1 | 0.22 | 0.09 | 7.9 | 38.8 (25)31 | >100 (631)31 | >100 (316)31 | >100 (126)31 |
| clavulanic acid | 0.012 | 0.04 | 1.8 | >100 | >100 | 0.12 | 30 | >100 | >100 | >100 |
| tazobactam | 0.015 | 0.001 | 1.7 | 0.41 | 0.73 | 0.43 | 0.55 | >100 | >100 | >100 |
Results are expressed as the average from duplicates unless otherwise indicated.
IC50 values reported in the literature are shown in parentheses.
Crystallography
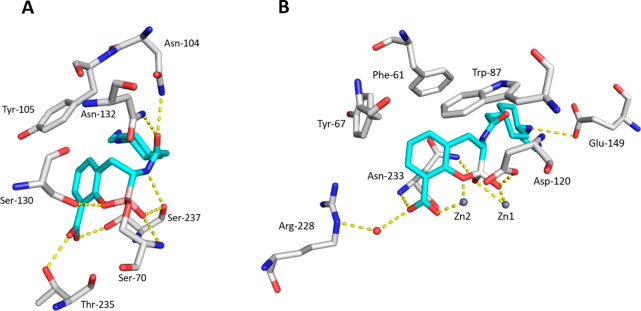
To explore the structural basis for the pan-β-lactamase inhibition of 20, we determined the structures of the class A ESBL CTX-M-15 and the VIM-2 MBL in complex with 20 at 1.1 and 1.8 Å resolution.32 Notably, several cocrystal structures of cyclic boronates including 20 in complex with the four classes of β-lactamases including CTX-M-15,20 AmpC,33 OXA-10,19,22 VIM-2,19 BcII,19 and NDM-122 have been reported. Together, these crystallographic studies reveal the structural basis for the dual inhibition of both SBLs and MBLs. As shown in Figure 4A, the structure of 20/CTX-M-15 shows that the boron-based BLI interacts with the β-lactamase in the closed or cyclic form. The catalytic Ser70 of the enzyme is covalently bound to the boron atom of 20. One hydroxyl group on the boron atom is located in the oxyanion hole, thus mimicking the acylation tetrahedral intermediate. The amide moiety of the inhibitor forms important interactions with the enzyme, donating a hydrogen bond to the Ser237 backbone carbonyl and accepting hydrogen bonds from the side chains of both Asn132 and Asn104, residues that are largely conserved in class A and class C enzymes. The carboxylate of 20 forms hydrogen bonds with the Thr235 (which belong to the K234TG conserved motif) and Ser237 side chains. The cyclohexyl moiety of 20 occupies the same space where the aryl group of several penicillins and cephalosporins resides. The ethyl diamine appendage is directed toward a crystallographic water molecule, with no apparent direct interaction with the enzyme. Overall, the binding mode of 20/CTX-M-15 is very similar to that observed by Cahill et al. in the cocrystal structure of a different cyclic boronate with CTX-M-15 (PDB code 5T66).20 In addition, for class C SBL (AmpC) and class D SBL (OXA-10), crystallography studies also revealed that the cyclic boronate mimics the tetrahedral oxyanion with an overall similar conformation.19,22,33
Figure 4.
Co-crystal structures of CTX-M-15/20 (A, PDB ID: 6SP6) and VIM-2/20 (B, PDB ID: 6SP7).
The structure of 20/VIM-2 (Figure 4B) revealed that the tetrahedral boron of 20 was bound to the active-site zinc atom (Zn1), thus mimicking the tetrahedral intermediate formed following the nucleophilic attack of the BL carbonyl by the hydroxide anion. The second active-site zinc atom (Zn2) was extensively coordinated by the carboxylate and phenolic oxygen atoms. In contrast with the class A enzyme, in which the amido group substituent does not interact with any of the enzyme residues, the proximal amine of 20 interacted with Glu149 in the 20/VIM-2 structure. This additional hydrogen-bonding interaction between the side chain and the enzyme in the 20/VIM-2 structure is also noticed in the binding mode of another cyclic boronate in complex with VIM-2 reported by Brem et al. (PDB code 5FQC).19 Interestingly, a cocrystal structure of 20 complexed within NDM-1 showed that in addition to the anticipated bicyclic form, a tricyclic structure that resulted from the cyclization of the amide oxygen onto the boron atom was also observed (PDB code 6RMF).22 This observation suggests that boronate-based BLIs are able to adapt multiple forms when interacting with different enzymes. The same study revealed that despite a variety of differing side-chain orientations, the binding mode of the fused bicyclic boronate motif was strikingly conserved in both SBLs and MBLs.22 Taken together, the scaffold with a cyclic boronate fused to a benzoic acid is demonstrated to be the key binding motif for pan-β-lactamase inhibition.
On the basis of these structural studies, we conclude that 20 and its analogues act as pan-BLIs by mimicking the common tetrahedral intermediates proposed for substrate hydrolysis by both SBL and MBL enzymes and that the N-(2-aminoethyl) cyclohexylamine moiety creates further stabilizing interactions within the active site of MBL by targeting conserved and negatively charged residues (such as Glu149 in VIM-2 and NDM-1).
Compound 20 Potentiated β-Lactam-Mediated Antibiotic Activity
The ability of 20 to rescue the activity of the three major subclasses of BLs—piperacillin (PIP) (a ureido-penicillin), cefepime (a fourth-generation oxyimino-cephalosporin), or meropenem (a 1-β-methyl-carbapenem)—when provided at the prescribed fixed concentration was evaluated using MDR β-lactamase-producing Gram-negative clinical isolates (Tables 5–7). For comparison, we also included the known BLIs VAB, TZB, and AVI as well as cyclic boronates 1 and 3 and the truncated trans-cyclohexylamine analogue (17). Compared with 1 and 3, 20 demonstrated superior potentiation of the antibacterial activity of all three tested BL antibiotics. Specifically, significant improvements were observed in terms of rescuing BL activity on bacterial strains producing class D or class B β-lactamases. In addition, the critical role of the appended aminoethyl group was revealed by a comparison of 20 with truncated 17, which exhibited inferior performance against many of the tested strains. Finally, the combination of a BL with 20 demonstrated excellent antibacterial activity in BL-resistant strains, which appeared greater than that of the VAB- or TZB-containing BL combinations and better or comparable to that of the AVI/BL combinations. The strong correlation observed between the in vitro inhibitory activity on purified β-lactamases and the potentiation effect in whole-cell assays of 20 appeared particularly encouraging. Although 20 rescued the activity of all relevant anti-Gram-negative BLs including meropenem (vide supra), we selected the fourth-generation cephalosporin cefepime as the preferred development partner for 20. This selection was driven by the following considerations: (i) Cefepime is considered the most advanced “broad-spectrum” cephalosporin, with demonstrated safety and efficacy for infections caused by major Gram-positive organisms including Staphylococcus aureus and Streptococcus pneumoniae as well as important Gram-negative pathogens, including B. mallei and B. pseudomallei, Salmonella spp., E. coli, K. pneumoniae, Enterobacter spp., and P. aeruginosa. (ii) Pharmacokinetic (PK) analysis suggested that cefepime and 20 would be compatible. (iii) Cefepime plus 20 would avoid the resistance-selective pressure derived by the further usage of carbapenems, as would be the case for meropenem plus 20.
Table 5. MICs against Selected Gram-Negative Pathogens for Piperacillin (PIP) and Piperacillin Plus 20.
| MIC
with piperacillin fixed at 16 μg/mL (μg/mL) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| species | strain ID | β-lactamase content | PIP MIC (μg/mL) | 20 | 17 | 1 | 3 | VAB | TZB | AVI |
| K. pneumoniae | ATCC BAA 1705 | KPC-2 | >128 | 4 | 4 | 2 | 2 | >16 | >16 | 4 |
| K. pneumoniae | CDC-0040 | VIM-27, CTX-M-15, SHV-11, OXA-1 | >128 | 16 | >16 | >16 | >16 | >16 | >16 | >16 |
| E. coli | CDC-0055 | NDM-1, CMY-6, OXA-1 | >128 | 16 | >16 | >16 | 16 | >16 | >16 | 16 |
| K. pneumoniae | CDC-0066 | OXA-232, OXA-9, TEM-1A, CTX-M-15, OXA-1 | >128 | >16 | >16 | >16 | >16 | >16 | >16 | 8 |
| P. aeruginosa | CDC-0356 | KPC-2, PDC-42 | >128 | 1 | 4 | 2 | 2 | >16 | >16 | 1 |
| K. pneumoniae | CDC-0361 | KPC-2, SHV-12, TEM-1 | >128 | 1 | 2 | 2 | 0.5 | >16 | >16 | 2 |
| E. coli | CDC-0452 | NDM | >128 | 2 | >16 | >16 | 8 | >16 | >16 | 8 |
| P. aeruginosa | CDC-0457 | VIM | 64 | 16 | >16 | >16 | >16 | >16 | >16 | >16 |
Table 7. MICs against Selected Gram-Negative Pathogens for Meropenem (MEM) and Meropenem Plus 20.
| MIC with meropenem fixed at 4 μg/mL (μg/mL) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| species | strain ID | β-lactamase content | MEM MIC (μg/mL) | 20 | 17 | 1 | 3 | VAB | TZB | AVI |
| K. pneumoniae | ATCC BAA 1705 | KPC-2 | 16 | 0.015 | 0.015 | 0.0079 | 0.0079 | 0.03 | 8 | 0.015 |
| K. pneumoniae | CDC-0040 | VIM-27, CTX-M-15, SHV-11, OXA-1 | >128 | 8 | >16 | >16 | >16 | >16 | >16 | >16 |
| E. coli | CDC-0055 | NDM-1, CMY-6, OXA-1 | 32 | 0.25 | 8 | 16 | 1 | >16 | >16 | 8 |
| K. pneumoniae | CDC-0066 | OXA-232, OXA-9, TEM-1A, CTX-M-15, OXA-1 | 32 | 4 | 8 | 2 | 4 | >16 | >16 | 2 |
| P. aeruginosa | CDC-0356 | KPC-2, PDC-42 | 32 | 1 | 4 | 2 | 1 | 8 | >16 | 0.5 |
| K. pneumoniae | CDC-0361 | KPC-2, SHV-12, TEM-1 | 16 | 0.015 | 0.015 | 0.0079 | 0.0079 | 0.06 | >16 | 0.015 |
| E. coli | CDC-0452 | NDM | 32 | 0.25 | 8 | 8 | 2 | >16 | >16 | 8 |
| P. aeruginosa | CDC-0457 | VIM | 128 | >16 | >16 | >16 | >16 | >16 | >16 | >16 |
Table 6. MICs against Selected Gram-Negative Pathogens for Cefepime (FEP) and Cefepime Plus 20.
| MIC with cefepime fixed at 8 μg/mL (μg/mL) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| species | strain ID | β-lactamase content | FEP MIC (μg/mL) | 20 | 17 | 1 | 3 | VAB | TZB | AVI |
| K. pneumoniae | ATCC BAA 1705 | KPC-2 | 16 | 0.06 | 0.125 | 0.0079 | 0.06 | 0.25 | >16 | 0.06 |
| K. pneumoniae | CDC-0040 | VIM-27, CTX-M-15, SHV-11, OXA-1 | >128 | 4 | >16 | >16 | >16 | >16 | >16 | >16 |
| E. coli | CDC-0055 | NDM-1, CMY-6, OXA-1 | 128 | 2 | >16 | >16 | 4 | >16 | >16 | 16 |
| K. pneumoniae | CDC-0066 | OXA-232, OXA-9, TEM-1A, CTX-M-15, OXA-1 | >128 | 2 | 4 | 1 | 4 | >16 | >16 | 1 |
| P. aeruginosa | CDC-0356 | KPC-2, PDC-42 | >128 | 1 | 4 | 2 | 2 | >16 | >16 | 1 |
| K. pneumoniae | CDC-0361 | KPC-2, SHV-12, TEM-1 | 32 | 0.015 | 0.015 | 0.0079 | 0.0079 | >16 | 16 | 0.0079 |
| E. coli | CDC-0452 | NDM | 64 | 0.125 | 4 | 8 | 2 | >16 | >16 | 8 |
| P. aeruginosa | CDC-0457 | VIM | 16 | 0.125 | 2 | >16 | 8 | >16 | >16 | >16 |
Compound 20 Reduced Cefepime MIC in Serine-β-lactamase-Expressing Enterobacteriaceae
To evaluate the activity of cefepime plus 20 against SBL-producing Enterobacteriaceae, cefepime MICs were determined alone or with a fixed concentration of 20 or TZB (4 μg/mL) against a panel of Gram-negative isolates producing class A ESBLs or KPCs, class C cephalosporinases, or class D oxacillinases. As described in Table 8, these cefepime-resistant strains were susceptible to cefepime/20 at MIC ranges below the Clinical and Laboratory Standards Institute’s (CLSI) susceptible-dose dependent (SDD) breakpoint of 8 μg/mL. By comparison, cefepime/TZB demonstrated only modest activity in three of the eight strains, which is likely due to the presence of class C or KPC-type β-lactamases.
Table 8. Rescue of Cefepime Activity by BLI in Serine-β-lactamase-Expressing Enterobacteriaceae.
| MIC (μg/mL)b |
||||
|---|---|---|---|---|
| strain | β-lactamase contenta | cefepime | cefepime/TZB | cefepime/20 |
| E. coli EC1552 | ESBL/C | 16 | 4 | 0.5 |
| E. coli CH1 | ESBL/C | >128 | 16 | <0.125 |
| E. cloacae CH23 | ESBL/C | >128 | >16 | 0.5 |
| K. pneumoniae 9032 | ESBL | 64 | 4 | <0.125 |
| K. pneumoniae 664-1 | KPC/C | >128 | >16 | 0.25 |
| K. pneumoniae 964-2 | ESBL/KPC | >128 | >16 | <0.125 |
| K. oxytoca 971 | ESBL/D | >128 | >16 | 0.5 |
ESBL includes TEM, CTX-M, and SHV types.
MIC of cefepime alone or cefepime plus BLI fixed at 4 μg/mL.
Compound 20 Rescued Cefepime Activity against Metallo-β-lactamase-Producing Gram-Negative Pathogens
Fourteen clinical isolates expressing Ambler class B VIM- or NDM-type MBLs were tested for susceptibility to cefepime alone or cefepime in combination with 20, TZB, or AVI. The test set included 10 cefepime-resistant strains of Enterobacteriaceae, one strain of P. aeruginosa, and three strains of A. baumannii. Not surprisingly, on the basis of their lack of enzyme inhibition, neither TZB nor AVI had any effect on the susceptibility of these organisms to cefepime (Table 9). In contrast, cefepime plus 20 displayed potent antibacterial activity on these difficult-to-treat organisms. Notably, the addition of 20 elicited a ≥512-fold decrease in the MIC of cefepime in these resistant organisms. In these 14 MBL-producing strains, cefepime plus 20 resulted in MIC50/MIC90 values of 1 and 4 μg/mL, respectively.
Table 9. Rescue of Cefepime Activity by BLI (Fixed at 4 μg/mL) in 14 Gram-Negative Pathogens Expressing Class B Enzymesa.
| entry | MIC range (μg/mL)b | MIC50 (μg/mL) | MIC90 (μg/mL) | % of strains with MICs ≤8c |
|---|---|---|---|---|
| cefepime | 32 to >512 | >512 | >512 | 0 |
| cefepime/20 | 0.125–4 | 1 | 4 | 100 |
| cefepime/TZB | 16 to >32 | >32 | >32 | 0 |
| cefepime/AVI | 16 to >32 | >32 | >32 | 0 |
Strains expressed VIM-1 (6), VIM-2 (4), VIM-4 (3), or NDM-1 (1). Strains tested included E. coli (4), E. cloacae (2), K. pneumoniae (4), P. aeruginosa (1), and A. baumannii (3).
MIC testing conducted using CLSI broth microdilution assay with BLI fixed at 4 μg/mL and cefepime titrated.
CLSI SDD breakpoint, M100, 2019.
Compound 20 Displayed No Intrinsic Antibacterial Activity
DBO BLIs are reported to possess stand-alone antibacterial activity owing to the inhibition of penicillin-binding protein 2 (PBP2), which can lead to rapid resistance development.34,35 To ascertain whether 20 possessed any intrinsic antibacterial activity, meropenem, oxacillin, and 20 were independently tested against a panel of both Gram-positive and Gram-negative bacteria. Unlike meropenem and oxacillin, 20 was devoid of intrinsic antibacterial activity and thus was acting solely as a BLI (Table 10).
Table 10. Antibacterial Activity of 20, Meropenem, and Oxacillin.
| MIC (μg/mL) |
|||||
|---|---|---|---|---|---|
| species | strain ID | β-lactamase content | 20 | meropenem | oxacillin |
| S. aureus | 29213 | W/T | >128 | 0.06 | 0.125 |
| S. aureus | 33591 | MRSA | >128 | 32 | >128 |
| E. coli | 25922 | W/T | >128 | 0.03 | >128 |
| E. coli | J53 | SHV5, AmpC, TEM-1 | >128 | 0.06 | >128 |
| K. pneumoniae | CI 09 | W/T | >128 | 0.06 | >128 |
| K. pneumoniae | UMM | SHV5, KPC-2, TEM-1 | >128 | 16 | >128 |
| K. pneumoniae | A-1797 | KPC-2, VIM-4, CMY-4 | >128 | 128 | >128 |
| K. pneumoniae | 11978 | OXA-48 | >128 | 64 | >128 |
| E. cloacae | 144200 | p99 AmpC | >128 | 0.25 | >128 |
| E. aerogenes | 188548 | AmpC | >128 | 2 | >128 |
| P. aeruginosa | 27853 | W/T | >128 | 1 | >128 |
| P. aeruginosa | Ps296 | AmpC, VIM-2, TEM-1 | >128 | 64 | >128 |
Compound 20 Selectively Inhibited β-Lactamase Enzymes
BLI 20 displayed no cytotoxicity when tested up to 256 μg/mL (i.e., <20% inhibition of growth observed) against three mammalian cell lines (i.e., HeLa, MRC-5, and 3T3). Because compounds possessing low clogP and high aqueous solubility are typically excreted via the kidney, we included an assessment of toxicity in human primary renal proximal tubule cells. No toxicity was observed in human primary proximal tubule cells when 20 was tested up to 1000 μg/mL.
Compound 20 had no significant activity when tested against a panel of 50 human enzymes and receptors at a concentration of 100 μM. The panel included serine proteases (i.e., cathepsin G, chymotrypsin, Factor Xa, trypsin, and neutrophil elastase 2), metalloproteinases (MMP-1, -2, -3, and -9), cytochrome P450s (1A2, 2C9, 2C19, 2D6, and 3A4), and the hERG potassium channel.
Combination of Cefepime Plus 20 Displayed Antibacterial Efficacy in Vivo
Compound 20 demonstrated a PK profile in mice that is typical of highly polar, ionizable compounds and is similar to the PK profile of known β-lactams (i.e., Vss consistent with good diffusion to tissue and low-to-moderate plasma clearance; Table 11). In comparison with AVI, 20 exhibited higher exposure (AUC) and lower clearance.
Table 11. Pharmacokinetic Parameters of 20, Cefepime, and AVI in Mice Following Intravenous Administration.
| entry | t1/2 (h) | AUCinf (h·ng/mL) | Vss (mL/kg) | CL (mL/h/kg) |
|---|---|---|---|---|
| 20 | 0.16 | 16 189 | 143 | 618 |
| cefepime | 0.39 | 15 800 | 330 | 648 |
| AVI | 0.1 | 8075 | 210 | 1238 |
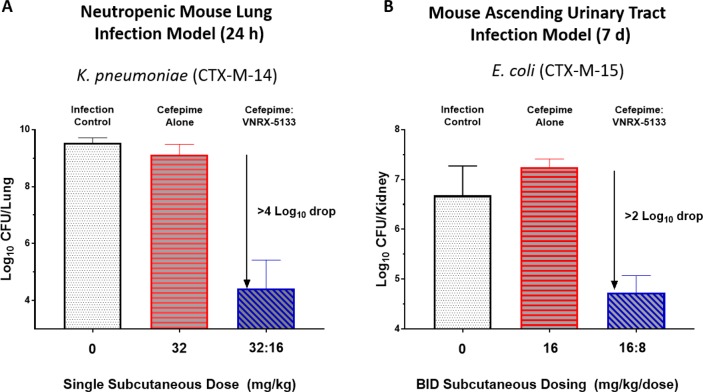
The in vivo efficacy of cefepime/20 was assessed in both the neutropenic mouse lung infection model against a CTX-M-14-producing strain of K. pneumoniae and in the ascending urinary tract infection model against a CTX-M-15-producing strain of E. coli (Figure 5). In the former, cefepime alone, when dosed subcutaneously at 32 mg/kg, was not effective at reducing viable bacterial counts in lung tissue. A single subcutaneous dose of cefepime/20 (32 and 16 mg/kg, respectively), however, achieved >4 log10 reduction in viable bacterial counts. In the latter model, twice-a-day cefepime/20 (16 and 8 mg/kg, respectively) demonstrated >2 log10 reductions in viable bacterial counts in the kidney.
Figure 5.
Results of cefepime/VNRX-5133 in the neutropenic mouse lung infection model and ascending urinary tract infection model. (A) Neutropenic mouse lung infection model experiment. The MICs for cefepime alone and cefepime/VNRX-5133 against K. pneumoniae were 64 and ≤0.125 μg/mL, respectively. At 24 h, log10 CFU/lung values for cefepime alone, cefepime/VNRX-5133 (32:16), and positive control Caz/Avi (32:8) were 9.27 ± 0.21, 4.51 ± 0.12, and 5.13 ± 0.54, respectively. (B) Mouse ascending urinary tract infection model. The MICs for cefepime alone and cefepime/VNRX-5133 against E. coli were 128 and 0.5 μg/mL, respectively. At day 7, log10 CFU/kidney values for cefepime alone, cefepime/VNRX-5133 (16:8), and positive control Caz/Avi (16:4) were 7.25 ± 0.16, 4.87 ± 0.43, and 5.58 ± 0.67, respectively.
Boronate 20 also demonstrated a clean safety profile in the preclinical toxicity evaluation. A phase-1 clinical trial of 20 including SAD, MAD, and DDI studies has been completed.36 A phase 3 efficacy trial of cefepime plus 20 is in progress (under registration no. NCT03840148 at ClinicalTrials.gov).
Conclusions
Starting from a cyclic boronate template, we have discovered a highly potent inhibitor of all four Ambler classes of β-lactamase enzymes that is also correspondingly highly active in a wide range of Gram-negative bacteria. The N-(2-aminoethyl)cyclohexylamine side chain of 20 (VNRX-5133) proved to be critical for broad-spectrum β-lactamase inhibition and enhanced Gram-negative outer membrane permeability and periplasmic accumulation. Structural studies revealed that the pan-β-lactamase inhibition is due to 20 mimicking the tetrahedral intermediates of both the serine-based and zinc-based enzymatic hydrolysis processes. The synthetic chemistry to 20 relied on two successive Matteson homologations, the latter of which installed an α-chloroboronate with high stereoselectivity.
Boronate 20 is selective for bacterial enzymes and is nontoxic to mammalian cells. Boronate 20 demonstrated the potent in vivo rescue of cefepime activity in both the murine neutropenic lung infection model and the murine ascending urinary tract infection model. Compound 20 is the first pan-spectrum BLI to enter clinical development.
Experimental Section
General Methods
All solvents and reagents were purchased from commercial vendors and were used without further purification unless otherwise mentioned. Column chromatography was conducted using prepacked silica gel cartridges (Biotage) on a Biotage Isolera Prime system. LC–MS was conducted on an Agilent 1100 series HPLC apparatus and a 6120 Quadrupole LC–MS apparatus in API-ES mode using a Waters Xbridge C18 column (4.6 mm × 50 mm, 3.5 μm). Mobile phase A was 0.1% trifluoroacetic acid in water, and mobile phase B was 0.1% trifluoroacetic acid in acetonitrile. 1H NMR spectra were obtained using a Varian Mercury 300 MHz spectrometer. The final products were purified by preparative HPLC on a Waters XBridge C18 column (19 mm × 100 mm, 3.5 μm). The purity of all tested compounds was ≥95%, as determined by HPLC with UV detection at 220 nm and 1H NMR analyses.
All animal experiments performed in the manuscript were conducted in compliance with institutional guidelines, as defined by the Institutional Animal Care and Use Committee (IACUC).
2-Methoxy-3-(2,9,9-trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.02,6]dec4-ylmethyl)-benzoic Acid tert-Butyl Ester (5)
A solution of 2-methoxy-3-(2,9,9-trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.02,6]dec4-yl)-benzoic acid tert-butyl ester (9.78 g, 25.3 mmol) and chloroiodomethane (2.9 mL, 39.8 mmol) in THF (72 mL) under argon was cooled to −100 °C (MeOH/liquid N2 bath). n-BuLi (15.0 mL, 2.5 M in hexanes, 37.5 mmol) was added dropwise over 25 min. The reaction was allowed to slowly warm to room temperature and was stirred for a total of 17.5 h. The reaction mixture was quenched with water and extracted three times with EtOAc. The combined organic layer was washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. Purification by flash silica gel chromatography (0–7% EtOAc/hexane) provided 8.92 g (88%) of 5 as a clear oil. ESI-MS m/z: 401.1 (M + H)+.
tert-Butyl 3-((S)-2-Chloro-2-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)ethyl)-2-methoxybenzoate (6)
To a solution of DCM (19.4 mL, 303 mmol) in THF (276 mL) at −100 °C (MeOH/liquid N2 bath) was added dropwise n-BuLi (77.4 mL, 193 mmol, 2.5 M in hexanes) over a period of 40 min. After the addition was completed, the resulting white milky mixture was stirred for 30 min; then, a solution of 2-methoxy-3-(2,9,9-trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.02,6]dec-4-ylmethyl)-benzoic acid tert-butyl ester 5 (55.3 g, 138 mmol) in THF (100 mL) was added dropwise over 30 min. After the addition was completed, the mixture was stirred for 45 min; then, a solution of ZnCl2 (182 mL, 182 mmol, 1.0 M in ether) was added slowly over 30 min, and stirring continued for an additional 5 min. The reaction mixture was stirred for 80 min at −10 °C (dry ice in acetone). The resulting yellow solution was diluted with ether (1000 mL) and washed with HCl (0.05M, 800 mL). The organic layer was separated, washed with brine, dried over anhydrous Na2SO4, concentrated, and chromatographed on a silica gel (500 g) column eluted with hexanes/EtOAc (2–5%) to give 6 as a colorless oil (56.5 g, 91.2% yield), which was used directly in the next step. ESI-MS m/z: 471.2 (M + Na)+.
General Method for the Preparation of Compound 8
To a solution of tert-butyl 3-((S)-2-chloro-2-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)ethyl)-2-methoxybenzoate 6 (1.35g, 3 mmol) in THF (9 mL) at −20 °C was added dropwise a solution of lithium bistrimethylsilylamide (3.0 mL, 1 M in THF, 3 mmol). Following the addition, the bath was removed, and stirring continued for 17 h. The resulting solution, containing ∼0.25 M of intermediate 7 in THF, was used without further purification.
To a mixture of carboxylic acid (1 mmol) and HATU (418 mg, 1.1 mmol) was added DMA (3 mL), followed by N-methyl-morpholine (120 μL, 1.1 mmol). The resulting solution was stirred for 90 min. To this solution was added a solution of 7 (4 mL, ∼0.25 M in THF, 1 mmol). The resulting mixture was stirred for 2.5 h, diluted with EtOAc, washed with water and brine, dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified by silica gel chromatography (10 g silica; eluted with 20–100% EtOAc/hexanes) to provide amide 8.
General Method for the Preparation of Compound 9
To a solution of amide 8 (0.30 mmol) in anhydrous DCM (5 mL) at −78 °C was added BCl3 (2.1 mL, 1 M in DCM, 2.1 mmol). After 1 h, the reaction mixture was warmed to 0 °C. After 1 h, the reaction mixture was quenched by the addition of water (5 mL) at 0 °C. The aqueous phase was washed with DCM and then purified by reverse-phase preparative HPLC. The product-containing fractions were combined and lyophilized to dryness.
2-(trans-4-((tert-Butoxycarbonyl)(2-((tert-butoxycarbonyl) amino) ethyl) amino) cyclohexyl) Acetic Acid (14)
To a solution of Boc-trans-4-aminocyclohexane acetic acid (10,5.14 g, 20 mmol) in DMF (60 mL) was added K2CO3 (3.32 g, 24 mmol), followed by benzyl bromide (2.62 mL, 22 mmol). The reaction mixture was stirred at ambient temperature overnight, quenched by the addition of water (250 mL), and extracted with diethyl ether (2 × 200 mL). The organic extracts were combined, washed successively with water, saturated aqueous NaHCO3, and brine, dried over anhydrous Na2SO4, and concentrated in vacuo to yield 11 (6.94 g, quant. yield) which was used without further purification.
Compound 11 was dissolved in DCM (20 mL), treated with 4 M HCl in dioxane (100 mL, 400 mmol) at ambient temperature for 1 h, then concentrated in vacuo to give amine 12, which was used for the next step without further purification.
To a solution of 12 (3.78 g, 13.4 mmol) in DCE (220 mL) was added triethylamine (1.9 mL, 13.6 mmol), followed by HOAc (0.8 mL, 13.4 mmol), N-Boc-2-aminoacetaldehyde (3.05 g, 19.32 mmol), and NaBH(OAc)3 (4.15 g, 19.6 mmol). The reaction mixture was stirred at ambient temperature for 3 h then quenched with saturated aqueous NaHCO3. The organic layer was separated, and the aqueous phase was extracted with DCM. The combined organic extracts were dried over anhydrous Na2SO4 then concentrated in vacuo to provide crude 13.
Crude 13 was dissolved in DCM (200 mL) containing triethylamine (2.8 mL, 20 mmol) and Boc2O (3.5 g, 16 mmol). The reaction mixture was stirred at ambient temperature overnight, concentrated to dryness, then purified by flash silica gel column chromatography (hexane/EtOAc 40:1–2:1) to afford the crude benzyl ester (3.3 g, 62% yield over three steps), which was dissolved in EtOAc (250 mL) and hydrogenated in the presence of 10% Pd/C (700 mg) at ambient temperature overnight. The reaction mixture was filtered through a pad of celite. The filtrate was concentrated and purified by flash silica gel column chromatography (hexane/acetone 10:1 to 1:1) to afford the acid 14 (2.35 g, 44% yield). 1H NMR (CD3OD) δ 3.8–3.6 (m, 1H) 3.15 (br s, 4H), 2.17 (d, 2H, J = 7.2 Hz), 1.92–1.80 (m, 2H), 1.78–1.50 (m, 5H), 1.46 (s, 9H), 1.43 (s, 9H), 1.14–1.07 (m, 2H). ESI-MS m/z: 423.3 (M + Na)+.
(3R)-3-(2-{trans-4-[(2-Aminoethyl)amino]cyclohexyl}acetamido)-2-hydroxy-3,4-dihydro-2H-1,2-benzoxaborinine-8-carboxylic Acid (20/VNRX-5133)
Compound 20 was synthesized following the general procedure for the synthesis of 8 and 9. 1H NMR (D2O) δ 7.84 (d, 1H, J = 6.9 Hz), 7.49 (d, 1H, J = 6.9 Hz), 7.13 (t, 1H, J = 7.5 Hz), 3.41–3.38 (m, 4H), 3.32 (s, 1H), 3.02–2.99 (m, 2H), 2.85–2.81 (m, 1H), 2.38–2.35 (m, 1H), 2.18–2.13 (m, 1H), 1.93–1.90 (m, 1H), 1.88–1.85 (m, 1H), 1.47–1.44 (m, 1H), 1.41–1.37 (m, 1H), 1.24–1.20 (m, 1H), 1.10–1.05 (m, 1H), 0.88–0.84 (m, 1H), 0.71–0.67 (m, 1H), 0.54–0.50 (m, 1H). ESI-MS m/z: 390.2 (M + H)+. The final enantiomeric purity was measured by chiral HPLC to be >98% (column: Daicel Crownpak CR (−), 150 mm × 4.0 mm, 5 μm; mobile phase: 1% (v/v) HClO4 in H2O).
X-ray Crystallography
The CTX-M-15 and VIM-2 β-lactamases were purified and crystallized using the sitting drop method, as previously described.37−39 Crystals of the native enzymes were soaked for up to 60 min with a 5 mM inhibitor (20) solution in the crystallization buffer prior to flash-freezing in the presence of ethylene glycol as the cryoprotectant. X-ray diffraction data were collected at the European Radiation Synchrotron Facility (ESRF, Grenoble, France) or the Diamond Light Source (Didcot, UK). Data were processed as described elsewhere,40,41 and the structures obtained by molecular replacement using the native structure of the corresponding enzymes (CTX-M-15, PDB code 4HBT; VIM-2, PDB code 1KO3), excluding solvent and other ligands. Refinement was performed with REFMAC542 from the CCP4 suite using an iterative manual rebuilding and modeling of missing atoms in the electron density using COOT.43 Water molecules were added using the standard procedures implemented in the ARP/wARP suite.44 Data collection and model refinement statistics are shown in Table S1.
Acknowledgments
This project has been funded in whole or in part from the National Institute for Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272201300019C and the Wellcome Trust under award no. 360G-Wellcome-101999/Z/13/Z. We thank Cassandra Chatwin and Kaitlyn John for generating MIC data. We thank Dr. Stephen M. Condon for helpful discussions and the review of this manuscript.
Glossary
Abbreviations Used
- BLI
β-lactamase inhibitor
- BL
β-lactam antibiotic
- PBP
penicillin-binding protein
- MDR
multi-drug-resistant
- SBL
serine β-lactamase
- MBL
metallo-β-lactamase
- NDM
New Delhi metallo-β-lactamase
- IMP
imipenemase
- VIM
Verona integron metallo β-lactamase
- KPC
Klebsiella pneumoniae carbapenemase
- ESBL
extended spectrum β-lactamase
- MIC
minimum inhibitory concentration
- SAR
structure–activity relationship
- TZB
tazobactam
- AVI
avibactam
- AUC
area under the curve
- CL
clearance
- Vss
steady-state volume of distribution
- PK
pharmacokinetic
- SAD
single ascending dose
- MAD
multiple ascending dose
- DDI
drug–drug interaction
- OM
outer membrane
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.9b01518.
Accession Codes
PDB codes 6SP6 (CTX-M-15/20) and 6SP7 (VIM-2/20).
The authors declare the following competing financial interest(s): B.L., R.T., G.C., D.M., R.J., J.H., D.D., S.C., D.P., L.X., and C.B. are employees of Venatorx Pharmaceuticals.
Supplementary Material
References
- Elander R. P. Industrial production of beta-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. 10.1007/s00253-003-1274-y. [DOI] [PubMed] [Google Scholar]
- Queenan A. M.; Bush K. Carbapenemases: the versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440. 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K.; Jacoby J. A. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969. 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palzkill T. Metallo-β-lactamase structure and function. Ann. N. Y. Acad. Sci. 2013, 1277, 91–104. 10.1111/j.1749-6632.2012.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins R. R.; Bonomo R. A. Increasing prevalence of carbapenem-resistant Enterobacteriaceae and strategies to avert a looming crisis. Expert Rev. Anti-Infect. Ther. 2013, 11, 543–545. 10.1586/eri.13.46. [DOI] [PubMed] [Google Scholar]
- Papp-Wallace K. M.; Bonomo R. A. New β-lactamase inhibitors in the clinic. Infect. Dis. Clin. North Am. 2016, 30, 441–464. 10.1016/j.idc.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann D. E.; Jahić H.; Ross P. L.; Gu R. F.; Hu J.; Kern G.; Walkup G. K.; Fisher S. L. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 11663–11668. 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker S. J.; Reddy K. R.; Totrov M.; Hirst G. C.; Lomovskaya O.; Griffith D. C.; King P.; Tsivkovski R.; Sun D.; Sabet M.; Tarazi Z.; Clifton M. C.; Atkins K.; Raymond A.; Potts K. T.; Abendroth J.; Boyer S. H.; Loutit J. S.; Morgan E. E.; Durso S.; Dudley M. N. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class A serine carbapenemases. J. Med. Chem. 2015, 58, 3682–3692. 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- Smoum R.; Rubinstein A.; Dembitsky V. M.; Srebnik M. Boron containing compounds as protease inhibitors. Chem. Rev. 2012, 112, 4156–4220. 10.1021/cr608202m. [DOI] [PubMed] [Google Scholar]
- Kiener P. A.; Waley S. G. Reversible inhibitors of penicillinases. Biochem. J. 1978, 169, 197–204. 10.1042/bj1690197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley T.; Gascoyne N.; Knott-Hunziker V.; Petursson S.; Waley S. G.; Jaurin B.; Grundstrom T. The inhibition of class C β-lactamases by boronic acids. Biochem. J. 1983, 209, 229–233. 10.1042/bj2090229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynadka N. C.; Adachi H.; Jensen S. E.; Johns K.; Sielecki A.; Betzel C.; Sutoh K.; James M. N. Molecular structure of the acylenzyme intermediate in beta-lactam hydrolysis at 1.7 A resolution. Nature 1992, 359, 700–705. 10.1038/359700a0. [DOI] [PubMed] [Google Scholar]
- Strynadka N. C.; Martin R.; Jensen S. E.; Gold M.; Jones J. B. Structure-based design of a potent transition state analogue for TEM-1 beta-lactamase. Nat. Struct. Mol. Biol. 1996, 3, 688–695. 10.1038/nsb0896-688. [DOI] [PubMed] [Google Scholar]
- Wang X.; Minasov G.; Blazquez J.; Caselli E.; Prati F.; Shoichet B. K. Recognition and resistance in TEM beta-lactamase. Biochemistry 2003, 42, 8434–8444. 10.1021/bi034242y. [DOI] [PubMed] [Google Scholar]
- Morandi F.; Caselli E.; Morandi S.; Focia P. J.; Blazquez J.; Shoichet B. K.; Prati F. Nanomolar inhibitors of AmpC beta-lactamase. J. Am. Chem. Soc. 2003, 125, 685–695. 10.1021/ja0288338. [DOI] [PubMed] [Google Scholar]
- Burns C. J.; Goswami R.; Jackson R. W.; Lessen T.; Li W.; Pevear D.; Tirunahari P. K.; Xu H.. Beta-Lactamase Inhibitors. WO 2010130708 A1, 2010.
- Burns C. J.; Daigle D.; Liu B.; McGarry D.; Pevear D. C.; Trout R. E. L.. Beta-Lactamase Inhibitors. WO 2014089365 A1, 2014.
- Burns C. J.; Daigle D.; Liu B.; Jackson R. W.; Hamrick J.; McGarry D.; Pevear D. C.; Trout R. E. L.. Beta-Lactamase Inhibitors. WO 2015191907 A1, 2015.
- Brem J.; Cain R.; Cahill S.; McDonough M. A.; Clifton I. J.; Jiménez-Castellanos J. C.; Avison M. B.; Spencer J.; Fishwick C. W.; Schofield C. J. Structural basis of metallo-β-lactamase, serine-β-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nat. Commun. 2016, 7, 12406–12415. 10.1038/ncomms12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill S. T.; Cain R.; Wang D. Y.; Lohans C. T.; Wareham D. W.; Oswin H. P.; Mohammed J.; Spencer J.; Fishwick C. W. G.; McDonough M. A.; Schofield C. J.; Brem J. Cyclic boronates inhibit all classes of β-Lactamases. Antimicrob. Agents Chemother. 2017, 61, e02260-e02216 10.1128/AAC.02260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnc A.; Lang P. A.; Panduwawala T. D.; Brem J.; Schofield C. J. Will morphing boron-based inhibitors beat the beta-lactamases?. Curr. Opin. Chem. Biol. 2019, 50, 101–110. 10.1016/j.cbpa.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnc A.; Brem J.; Hinchliffe K.; Calvopiña K.; Panduwawala T. D.; Lang P. A.; Kamps J. J. A. G.; Tyrrell J. M.; Widlake E.; Saward B. G.; Walsh T. R.; Spencer J.; Schofield C. J. Bicyclic boronate VNRX-5133 inhibits metallo- and serine-β-lactamases. J. Med. Chem. 2019, 62, 8544–8556. 10.1021/acs.jmedchem.9b00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns C. J.; Liu B.; Chu G.; Trout R.; Jackson R.; McGarry D.; Hamrick J.; Daigle D.; Cusick S.; Pevear D.; Xerri L. Discovery of VNRX-5133: A Broad-Spectrum Serine- and Metallo-β-lactamase Inhibitor (BLI) for Carbapenem-Resistant Bacterial Infections (“Superbugs”). Proceedings of the 255th National Meeting of the American Chemical Society, New Orleans, LA, 2018; American Chemical Society: Washington, DC, 2018; Abstract MEDI-309.
- Ness S.; Martin R.; Kindler A. M.; Paetzel M.; Gold M.; Jensen S. E.; Jones J. B.; Strynadka N. C. Structure-based design guides the improved efficacy of deacylation transition state analogue inhibitors of TEM-1 beta-lactamase. Biochemistry 2000, 39, 5312–5321. 10.1021/bi992505b. [DOI] [PubMed] [Google Scholar]
- Linciano P.; Cendron L.; Gianquinto E.; Spyrakis F.; Tondi D. Ten Years with New Delhi Metallo-β-lactamase-1 (NDM-1): From structural insights to inhibitor design. ACS Infect. Dis. 2019, 5, 9–34. 10.1021/acsinfecdis.8b00247. [DOI] [PubMed] [Google Scholar]
- Delcour A. H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta, Proteins Proteomics 2009, 1794, 808–816. 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea R.; Moser H. E. Physicochemical properties of antibacterial compounds: implications for drug discovery. J. Med. Chem. 2008, 51, 2871–2878. 10.1021/jm700967e. [DOI] [PubMed] [Google Scholar]
- Matteson D. S. α-Halo boronic esters in asymmetric synthesis. Tetrahedron 1998, 54, 10555–10607. 10.1016/S0040-4020(98)00321-4. [DOI] [Google Scholar]
- Gunaydin H.; Bartberger M. D. Stacking with no planarity. ACS Med. Chem. Lett. 2016, 7, 341–344. 10.1021/acsmedchemlett.6b00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M. F.; Drown B. S.; Riley A. P.; Garcia A.; Shirai T.; Svec R. L.; Hergenrother P. J. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 2017, 545, 299–304. 10.1038/nature22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooke C. L.; Cain R.; Tyrrell J. M.; Hinchliffe P.; Calvopiña K.; Langley G. W.; Widlake E.; Dowson C. G.; Spencer J.; Walsh T. R.; Schofield C. J.; Brem J. Profiling interactions of vaborbactam with metallo-β-lactamases. Bioorg. Med. Chem. Lett. 2019, 29, 1981–1984. 10.1016/j.bmcl.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docquier J.-D.; De Luca F.; Benvenuti M.; Pozzi C.; Daigle D. M.; Pevear D. C.; Burns C. J.; Mangani S.. Structural Basis for Serine- and Metallo-β-lactamase Inhibition by VNRX-5133, a New β-Lactamase Inhibitor (BLI) in Clinical Development; European Congress of Clinical Microbiology and Infectious Diseases (ECCMID): Madrid, Spain, 2018. [Google Scholar]
- Cahill S. T.; Tyrrell J. M.; Navratilova I. H.; Calvopina K.; Robinson S. W.; Lohans C. T.; McDonough M. A.; Cain R.; Fishwick C. W. G.; Avison M. B.; Walsh T. R.; Schofield C. J.; Brem J. Studie on the inhibition of AmpC and other beta-lactamases by cyclic boronates. Biochim. Biophys. Acta, Gen. Subj. 2019, 1863, 742–748. 10.1016/j.bbagen.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Morinaka A.; Tsutsumi Y.; Yamada M.; Suzuki K.; Watanabe T.; Abe T.; Furuuchi T.; Inamura S.; Sakamaki Y.; Mitsuhashi N.; Ida T.; Livermore D. M. OP0595, a new diazabicyclooctane: mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam ‘enhancer’. J. Antimicrob. Chemother. 2015, 70, 2779–2786. 10.1093/jac/dkv166. [DOI] [PubMed] [Google Scholar]
- Livermore D. M.; Mushtaq S.; Warner M.; Vickers A.; Woodford N. In vitro activity of cefepime/zidebactam (WCK 5222) against gram-negative bacteria. J. Antimicrob. Chemother. 2017, 72, 1373–1385. 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- Geibel B.; Dowell J.; Dickerson D.; Henkel T. 1401. A Randomized, Double-Blind, Placebo-Controlled Study of the Safety and Pharmacokinetics of Single and Repeat Doses of VNRX-5133 in Healthy Subjects. Open Forum Infect. Dis. 2018, 5, S431. 10.1093/ofid/ofy210.1232. [DOI] [Google Scholar]
- Lahiri S. D.; Mangani S.; Durand-Reville T.; Benvenuti M.; De Luca F.; Sanyal G.; Docquier J.-D. Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC β-lactamases. Antimicrob. Agents Chemother. 2013, 57, 2496–2505. 10.1128/AAC.02247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docquier J.-D.; Lamotte-Brasseur J.; Galleni M.; Amicosante G.; Frère J.-M.; Rossolini G. M. On functional and structural heterogeneity of VIM-type metallo-β-lactamases. J. Antimicrob. Chemother. 2003, 51, 257–266. 10.1093/jac/dkg067. [DOI] [PubMed] [Google Scholar]
- Garcia-Saez I.; Docquier J.-D.; Rossolini G. M.; Dideberg O. The three-dimensional structure of VIM-2, a Zn-β-lactamase from Pseudomonas aeruginosa in its reduced and oxidised form. J. Mol. Biol. 2008, 375, 604–611. 10.1016/j.jmb.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Docquier J.-D.; Benvenuti M.; Calderone V.; Stoczko M.; Menciassi N.; Rossolini G. M.; Mangani S. High-resolution crystal structure of the subclass B3 metallo-β-lactamase BJP-1: rational basis for substrate specificity and interaction with sulfonamides. Antimicrob. Agents Chemother. 2010, 54, 4343–4351. 10.1128/AAC.00409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi C.; Di Pisa F.; De Luca F.; Benvenuti M.; Docquier J.-D.; Mangani S. Atomic-resolution structure of a class C β-lactamase and its complex with avibactam. ChemMedChem 2018, 13, 1437–1446. 10.1002/cmdc.201800213. [DOI] [PubMed] [Google Scholar]
- Murshudov G. N.; Skubák P.; Lebedev A. A.; Pannu N. S.; Steiner R. A.; Nicholls R. A.; Winn M. D.; Long F.; Vagin A. A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2011, 67, 355–367. 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P.; Lohkamp B.; Scott W. G.; Cowtan K. Features and development of Coot. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010, 66, 486–501. 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G.; Cohen S. X.; Lamzin V. S.; Perrakis A. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat. Protoc. 2008, 3, 1171–1179. 10.1038/nprot.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.