Significance
Adipose tissue performs multiple functions in mammals, including insulation, mechanical tissue protection, and energy balance. These functions of adipose tissue are performed by three distinct cell types: white, beige, and brown adipocytes. While white adipocytes store energy, beige and brown adipocytes dissipate energy through mitochondrial respiration. Previous studies have elucidated how environmental cold stimulates recruitment of beige adipocytes in adult animals. However, beige adipogenesis also occurs during the peri-weaning period in mice, a process that is poorly understood. Here, we demonstrate that distinct mechanisms regulate development and recruitment of beige adipocytes. We find that B cell leukemia/lymphoma 6 (BCL6) controls development of beige adipocytes during the peri-weaning period, whereas sympathetic nerves regulate the recruitment of beige adipocytes in cold environments.
Keywords: BCL6, sympathetic nervous system, thermogenesis, development, metabolism
Abstract
Adipose tissue provides a defense against starvation and environmental cold. These dichotomous functions are performed by three distinct cell types: energy-storing white adipocytes, and thermogenic beige and brown adipocytes. Previous studies have demonstrated that exposure to environmental cold stimulates the recruitment of beige adipocytes in the white adipose tissue (WAT) of mice and humans, a process that has been extensively investigated. However, beige adipose tissue also develops during the peri-weaning period in mice, a developmental program that remains poorly understood. Here, we address this gap in our knowledge using genetic, imaging, physiologic, and genomic approaches. We find that, unlike cold-induced recruitment in adult animals, peri-weaning development of beige adipocytes occurs in a temperature- and sympathetic nerve-independent manner. Instead, the transcription factor B cell leukemia/lymphoma 6 (BCL6) acts in a cell-autonomous manner to regulate the commitment but not the maintenance phase of beige adipogenesis. Genome-wide RNA-sequencing (seq) studies reveal that BCL6 regulates a core set of genes involved in fatty acid oxidation and mitochondrial uncoupling, which are necessary for development of functional beige adipocytes. Together, our findings demonstrate that distinct transcriptional and signaling mechanisms control peri-weaning development and cold-induced recruitment of beige adipocytes in mammals.
In mammals, adipose tissue provides a defense against starvation and environmental cold (1). These distinct functions of adipose tissue are performed by specialized adipocytes that can store or dissipate energy. For example, white adipocytes, which contain a single large lipid droplet, are a primary site of energy storage in mammals, whereas multilocular beige and brown adipocytes dissipate energy through uncoupled respiration (1–4). Consistent with their distinct functions in energy metabolism, loss of white adipocytes, as in lipodystrophy, leads to ectopic deposition of lipids in liver and other organs, whereas loss of beige and brown adipocytes compromises organismal defense against environmental cold (1).
Beige and brown adipocytes are considered to be two distinct cell types based on their developmental origin, transcriptome, and epigenome (1, 4, 5). Myogenic precursors give rise to brown adipocytes during embryogenesis in a temperature-independent manner, whereas environmental cold is the primary stimulus for the recruitment of mesenchyme-derived beige adipocytes in the white adipose tissue (WAT) of adult animals. A focus on cold-induced recruitment of beige adipocytes has identified a large number of signaling and transcriptional pathways that control its biogenesis in adult animals (4). In nearly all of these cases, beige-promoting factors directly or indirectly modulate the adrenergic activity of sympathetic nerves, which densely innervate the inguinal WAT (iWAT) of mice (6).
Although less well appreciated, iWAT also undergoes beige adipogenesis during the peri-weaning period (7–12). However, the importance of sympathetic stimulation in acquisition of this phenotype remains controversial. For example, Contreras et al. demonstrated that denervation of iWAT impairs commitment to the beige adipocyte lineage, whereas Chabowska-Kita et al. reported presence of UCP1+ cells in the iWAT of thermoneutral mice that presumably lacked sympathetic stimulation (7, 8). These findings raise the possibility that a combination of cell autonomous and nonautonomous mechanisms might regulate beige adipocyte development during the peri-weaning period, a gap in our knowledge that we address here.
Results and Discussion
Beige Fat Develops during the Peri-Weaning Period in a Temperature-Independent Manner.
To identify mechanisms that control beige adipogenesis during the peri-weaning period, we employed Adipo-Clear together with light sheet fluorescence microscopy to investigate how the ambient temperature (Ta) regulates developmental expression of UCP1 in the iWAT (13). In C57BL/6J mice that were bred at Ta = 22 °C, UCP1 expression began in the inguinal region (left) of iWAT as early as postnatal day (P)14, spread to the dorsolumbar region (right) by P21, peaked around P28, and began to regress by P35 (Fig. 1A, SI Appendix, Fig. S1 A and B, and Movie S1). To our surprise, this spatiotemporal pattern of UCP1 expression was preserved in iWAT of C57BL/6J mice that were bred at thermoneutrality (Ta = 30 °C), suggesting that the commitment to the beige adipocyte lineage occurs in a temperature-independent manner (Fig. 1B and Movie S2). In support of this hypothesis, quantitation of UCP1 protein expression during the peri-weaning period (P14–P35) revealed that while beige adipocyte development occurred in a temperature-independent manner, the amplitude of this response was gated by environmental temperature (Fig. 1C). For example, the UCP1+ volume was comparable at P14 between mice bred at 22 °C and 30 °C but differed in older (P21–P35) animals (Fig. 1C). Hematoxylin and eosin staining of tissue sections independently confirmed the presence of multilocular beige adipocytes in P28 iWAT of thermoneutral mice (Fig. 1D). Taken together with previous observations that neonatal mice are poikilothermic (body temperature mirrors ambient temperature) and only develop full thermoregulatory capacity around P19 to P28 (14), our data suggest that beige adipogenesis is initiated prior to acquisition of homeothermy in a temperature-independent manner.
Fig. 1.
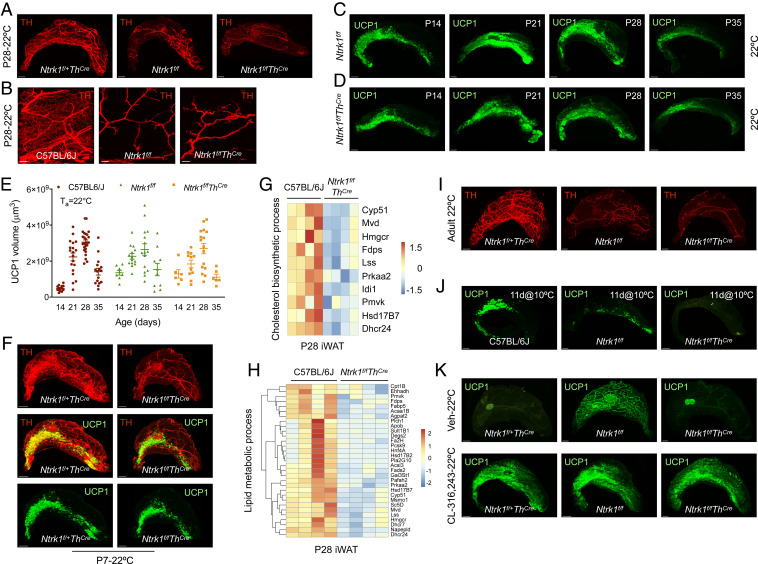
Beige fat develops during the peri-weaning period in a temperature-independent manner. (A and B) Immunostaining for uncoupling protein 1 (UCP1) in iWAT of C57BL/6J mice at various postnatal ages that were bred and housed at 22 °C (A) or 30 °C (B). (C) Quantification of UCP1 in iWAT of mice housed at 22 °C (A) or 30 °C (B) using Imaris. Data pooled from 2 to 4 litters per time point and housing temperature (n = 10–26 per group) and analyzed by Student’s t test. (D) Representative hematoxylin and eosin staining of iWAT from P28 mice housed at 22 °C or 30 °C. (E) Immunostaining for UCP1 in iWAT of 14- to 15-wk-old C57BL/6J mice housed at 22 °C or 8 °C for 2–6 d. (F) Immunostaining for RFP in iWAT of 20-wk-old Ai14 x Ucp1Cre mice housed at 22 °C. (G) Comparison of differentially expressed genes in iWAT of P28 mice housed at 22 °C with beige- and white-specific adipocyte signatures. (H) GO enrichment analysis and corresponding P values of up-regulated genes in iWAT of P28 mice housed at 22 °C. (I) Heatmap of up-regulated genes in the “Lipid metabolic process” GO category in iWAT of P28 C57BL/6J mice housed at 22 °C (n = 4 per genotype). (J) GO enrichment analysis and corresponding P values of down-regulated genes in iWAT of P28 mice housed at 22 °C. (K) Heatmap of down-regulated genes in the “Muscle contraction” GO category in iWAT of P28 C57BL/6J mice housed at 22 °C (n = 4 per genotype). Data are presented as mean ± SEM. (Scale bars: A, B, and F, 1 mm; D and E, 50 μm.)
We next asked whether the pattern of UCP1 expression in iWAT of cold-exposed adult animals was similar to its developmental expression during the peri-weaning period. We found that cold-induced beige fat recruitment in adult animals followed a similar spatiotemporal pattern as young mice; i.e., it began in the inguinal region and progressively spread to the dorsolumbar region of iWAT, as previously reported (Fig. 1 A and E) (13). This suggested that cold-induced beige fat in adult animals might result from reactivation of dormant beige adipocytes (8, 12, 15). To test this hypothesis, we examined the iWAT of adult Ai14 × Ucp1Cre mice, which allows for irreversible marking of all UCP1+ cells and their descendants with membrane-targeted tdTomato fluorescent protein. We found that the entire iWAT of 20-wk-old mice that had not been exposed to environmental cold contained cells with a history of UCP1 protein expression (Fig. 1F and Movies S3 and S4), suggesting that commitment to the beige fat lineage in iWAT occurs during the peri-weaning period. In agreement with these findings, a recent report demonstrated that depletion of UCP1+ cells during the peri-weaning period was sufficient to impair recruitment of beige fat in adult animals by β3-adrenergic agonists or environmental cold (12).
To examine similarities and differences between developmental and cold-induced beige fat, we performed RNA-sequencing (seq) on P28 iWAT isolated from C57BL/6J mice bred at 22 °C or 30 °C and compared it to signatures of adult beige and white adipocytes identified by the Rosen laboratory (5). We found that genes up-regulated by cold in iWAT of P28 mice (22 °C vs. 30 °C) were preferentially expressed by adult beige adipocytes, whereas genes that were down-regulated in iWAT of P28 mice housed at 22 °C were enriched for genes found in adult white adipocytes (Fig. 1G). Gene ontology (GO) enrichment analysis of up-regulated genes revealed that metabolic programs associated with catabolism of fatty acids and carbohydrates, as well as biosynthesis of cholesterol and sterols, were enriched in P28 iWAT of mice housed at 22 °C (Fig. 1 H and I and SI Appendix, Fig. S1 C–E), processes that are known to be important for development of beige adipocytes in adult animals (3, 16, 17). In contrast, GO enrichment analysis of down-regulated genes identified biological processes associated with muscle contraction and sarcomere organization (Fig. 1 J and K). Because myogenic precursors can contribute to glycolytic beige adipocytes in the absence of adrenergic stimuli (18), our findings suggest environmental cold suppresses the developmental expression of this myogenic program in P28 iWAT.
Distinct Requirements for the SNS in Development and Recruitment of Beige Fat.
The sympathetic nervous system (SNS) regulates cold-induced recruitment of beige adipocytes in iWAT (19), prompting us to ask whether it might also be important for physiologic development of beige adipocytes in young animals. To test this hypothesis, we generated mice in which TrkA, the high-affinity receptor for nerve growth factor (20), was selectively deleted in sympathetic nerves (designated Ntrk1f/fThCre) (21). We found that iWAT of Ntrk1f/fThCre mice lacked the dense network of sympathetic neurites that was present in iWAT of control (Ntrk1f/+ThCre) or C57BL/6J mice (Fig. 2 A and B and Movies S5–S7), as assessed by immunostaining for the SNS marker tyrosine hydroxylase (TH). However, because Ntrk1f/f is a hypomorphic allele (20), the development of TH+ neurites was also reduced in iWAT of Ntrk1f/f mice (Fig. 2 A and B and Movies S5–S7). Based on these observations, we used Ntrk1f/+ThCre or C57BL/6J mice as controls for our subsequent experiments.
Fig. 2.
Distinct requirements for the SNS in development and recruitment of beige fat. (A) Immunostaining for tyrosine hydroxylase (TH) in iWAT of P28 Ntrk1f/+ThCre, Ntrk1f/f, and Ntrk1f/fThCre mice housed at 22 °C. (B) Higher-magnification images of TH immunostained iWAT of P28 C57BL/6J, Ntrk1f/f, and Ntrk1f/fThCre mice housed at 22 °C. (C and D) Immunostaining for UCP1 in iWAT of Ntrk1f/f (C) or Ntrk1f/fThCre (D) mice at various postnatal ages that were bred and housed at 22 °C. (E) Quantification of UCP1 in iWAT of C57BL/6J (same as in Fig. 1C), Ntrk1f/f and Ntrk1f/fThCre mice using Imaris. Data pooled from 1 to 4 litters per time point and genotype (n = 8–26 samples per group) and analyzed by Student’s t test. (F) Immunostaining for TH and UCP1 in iWAT of P7 Ntrk1f/+ThCre and Ntrk1f/fThCre mice housed at 22 °C. (G and H) Heatmaps of down-regulated genes in the “Cholesterol biosynthetic process” (G) and “Lipid metabolic process” (H) GO categories in iWAT of P28 Ntrk1f/fThCre mice housed at 22 °C (n = 4 per genotype). (I) Immunostaining for TH in iWAT of 8- to 10-wk-old Ntrk1f/+ThCre, Ntrk1f/f, and Ntrk1f/fThCre mice housed at 22 °C. (J) Immunostaining for UCP1 in iWAT of 14- to 15-wk-old C57BL/6J, Ntrk1f/f, and Ntrk1f/fThCre mice housed at 10 °C for 11 d. (K) Immunostaining for UCP1 in iWAT of 10- to 11-wk-old Ntrk1f/+ThCre, Ntrk1f/f, and Ntrk1f/fThCre mice treated daily with vehicle or CL-316,243 (1 mg/kg intraperitoneally) for 7 d at 22 °C. The mesh-like structures in the Veh-22 °C- Ntrk1f/f image are autofluorescent mammary ducts. Data are presented as mean ± SEM. (Scale bars: A, C, D, and I–K, 1 mm; B, 200 μm; F, 500 μm.)
We next asked whether sympathetic innervation of iWAT was necessary for the commitment to the beige fat lineage during the peri-weaning period. To our surprise, we found that both the spatiotemporal pattern and magnitude of UCP1 expression were preserved in iWAT of Ntrk1f/f and Ntrk1f/fThCre mice housed at 22 °C or 30 °C (Fig. 2 C–E and SI Appendix, Fig. S2 A and B), indicating that the SNS is dispensable for the development of beige adipocytes during the peri-weaning period. This was independently confirmed by costaining for UCP1 and TH in P7 iWAT of Ntrk1f/+ThCre and Ntrk1f/fThCre mice, which showed emergence of UCP1+ cells in regions lacking TH+ neurites (Fig. 2F and Movies S8–S11). In agreement with these observations, histological examination revealed the presence of multilocular beige adipocytes in iWAT of Ntrk1f/f and Ntrk1f/fThCre mice housed at 22 °C (SI Appendix, Fig. S2C).
To understand the role of sympathetic nerves in regulation of iWAT gene expression during the peri-weaning period, we performed RNA-seq on iWAT of P28 Ntrk1f/fThCre mice that were housed at 22 °C. We found that only a small subset of genes was affected by the loss of sympathetic innervation in iWAT of P28 Ntrk1f/fThCre mice, which did not correlate strongly with adult beige and white adipocyte signatures (SI Appendix, Fig. S3A). GO enrichment analysis of differentially expressed genes revealed that biological processes associated with sterol, cholesterol, and fatty acid biosynthesis were decreased, whereas programs associated with inflammatory response, phagocytosis, apoptosis, and muscle contraction were increased in iWAT of P28 Ntrk1f/fThCre mice (Fig. 2 G and H and SI Appendix, Fig. S3 B–J). Although the cholesterol biosynthetic pathway has been implicated in cold-induced recruitment of beige adipocytes in adult animals (17), our results suggest that its induction is dispensable for biogenesis of beige adipocytes during the peri-weaning period.
We next asked whether the SNS was required for recruitment of beige adipocytes in adult animals. Consistent with a previous report (19), we found that cold-induced recruitment of beige adipocytes was severely impaired in adult Ntrk1f/f and Ntrk1f/fThCre mice (Fig. 2 I and J and Movies S12–S14). Because recruitment of beige adipocytes could be rescued by β3-adrenergic agonist CL-316,243 (Fig. 2K), our data suggest that the commitment to the beige adipocyte lineage occurs normally in Ntrk1f/f and Ntrk1f/fThCre mice. Taken together, these results suggest that sympathetic innervation is not necessary for the development of beige adipocytes during the peri-weaning period but is absolutely required for their recall after cold exposure in adult animals.
BCL6 Is Required for Development of Beige Fat during the Peri-Weaning Period.
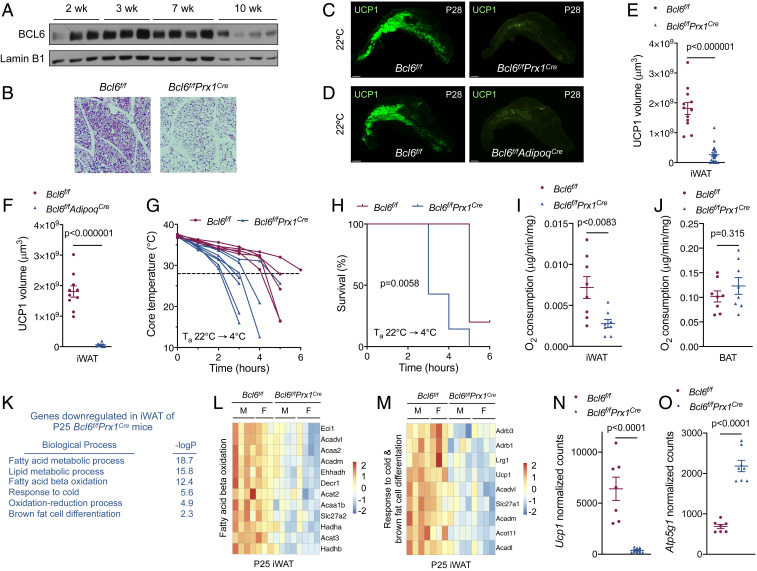
We recently reported that BCL6 is required for maintenance of thermogenic competence in dormant brown adipocytes when adrenergic input is minimal (22), prompting us to ask whether it might function in a similar manner to regulate commitment to the beige adipocyte lineage during the peri-weaning period. In support of this hypothesis, we observed that BCL6 protein was expressed in iWAT during the peri-weaning period and its expression declined as animals progressed through puberty (Fig. 3A and SI Appendix, Fig. S4A). To investigate whether BCL6 was required for the development of beige adipocytes, we generated Bcl6f/fPrx1Cre and Bcl6f/fAdipoqCre mice to selectively delete Bcl6 in subcutaneous white adipocytes of the iWAT (SI Appendix, Fig. S4B) or all adipocytes, respectively (2, 23, 24). We found that iWAT of P28 Bcl6f/fPrx1Cre and Bcl6f/fAdipoqCre mice had a near complete absence of multilocular UCP1+ beige adipocytes (Fig. 3 B–F and Movies S15 and S16). In contrast, when Bcl6 was deleted after adipocytes expressed UCP1, as in Bcl6f/fUcp1Cre mice, development and subsequent cold-induced recruitment of beige adipocytes were not significantly affected (SI Appendix, Fig. S4 C–E). These findings together suggest that BCL6 is specifically required during the commitment but not the maintenance stage of beige adipogenesis.
Fig. 3.
BCL6 is required for development of beige fat during the peri-weaning period. (A) Immunoblotting for BCL6 in iWAT of C57BL/6J male mice housed at 22 °C. (B) Representative hematoxylin and eosin staining of iWAT from P28 Bcl6f/f and Bcl6f/fPrx1Cre mice housed at 22 °C. (C and D) Immunostaining for UCP1 in iWAT of P28 Bcl6f/f, Bcl6f/fPrx1Cre (C) and Bcl6f/fAdipoqCre (D) mice housed at 22 °C. (E and F) Quantification of UCP1 in iWAT of P28 Bcl6f/f, Bcl6f/fPrx1Cre (E), and Bcl6f/fAdipoqCre (F) mice housed at 22 °C using Imaris. Data analyzed by Student’s t test (n = 12–20 per genotype). (G and H) Core temperature measurements (G) and survival curves (H) of P28 Bcl6f/f and Bcl6f/fPrx1Cre mice housed at 4 °C for up to 6 h (n = 6–7 per genotype). Kaplan–Meier survival curve analyzed by Log-Rank (Mantel–Cox) test; humane endpoint = 28 °C. (I and J) Oxygen consumption rate of iWAT (I) and BAT (J) from P28 Bcl6f/f and Bcl6f/fPrx1Cre mice housed at 22 °C. Data analyzed by Student’s t test (n = 8 per genotype). (K) GO enrichment analysis and corresponding P values of down-regulated genes in iWAT of P25 Bcl6f/fPrx1Cre mice housed at 22 °C. (L and M) Heatmaps of down-regulated genes in the “Fatty acid beta oxidation” and “Response to cold & brown fat cell differentiation” GO categories in iWAT of P25 Bcl6f/fPrx1Cre mice housed at 22 °C (n = 7–8 per genotype; M, male; F, female). (N and O) Normalized counts of Ucp1 and Atp5g1 transcripts in iWAT of P25 Bcl6f/f and Bcl6f/fPrx1Cre mice housed at 22 °C. Data analyzed by Student’s t test (n = 7–8 per genotype). (Scale bars: B, 50 μm; C and D, 1 mm.)
To determine whether loss of beige adipocytes impacted thermogenic fitness, we subjected 4-wk-old (P28) Bcl6f/f and Bcl6f/fPrx1Cre mice to an acute cold challenge at 4 °C. We found that core temperature declined at a faster rate in Bcl6f/fPrx1Cre mice, which shortened their survival during the cold challenge (Fig. 3 G and H). Measurement of oxygen consumption revealed significant reduction in the respiratory rate of iWAT, but not BAT, of P28 Bcl6f/fPrx1Cre mice (Fig. 3 I and J), suggesting that a defect in beige fat thermogenesis likely compromised survival of these young animals during the cold challenge.
We next performed RNA-seq on iWAT from P25 Bcl6f/f and Bcl6f/fPrx1Cre mice to understand how BCL6 regulates development of beige adipocytes. Analysis of 207 down-regulated and 174 up-regulated genes in iWAT of Bcl6f/fPrx1Cre mice revealed that they largely clustered with signatures of beige and white adipocytes (SI Appendix, Fig. S4F). GOs associated with β-oxidation of fatty acids, response to cold, and brown fat cell differentiation were enriched among the down-regulated genes (Fig. 3 K–M and SI Appendix, Fig. S4 G–I), whereas pathways associated with cell adhesion, osteoblast differentiation, and mammary gland development were represented among the up-regulated genes in iWAT of Bcl6f/fPrx1Cre mice (SI Appendix, Fig. S4 J and K). Of note, we observed that loss of BCL6 shifted iWAT from uncoupled to coupled respiration, as evidenced by reduction in Ucp1 and concomitant increase in Atp5g1 expression (Fig. 3 N and O), the latter encoding for a protein regulating coupled respiration via ATP synthase (22). Together, these data demonstrate that dysregulation of only a subset of beige adipocyte genes (such as Ucp1, Elovl3, Crat, Hadhb, Echdc1, Slc25a20, Acadl, and Acadm) is sufficient to impair development of beige fat during the peri-weaning period, leading us to propose that they might constitute a core set necessary for establishing uncoupled respiration in iWAT (SI Appendix, Table S1).
BCL6 Regulates the Core Program Necessary for Development and Recruitment of Beige Fat.
We next investigated the requirement of BCL6 in cold-induced recruitment of beige fat in adult animals. Compared to Bcl6f/f mice, cold-induced recruitment of UCP1+ adipocytes was nearly absent in iWAT of 14-wk-old Bcl6f/fPrx1Cre mice (Fig. 4 A and B and Movies S17 and S18). As a consequence, ex vivo oxygen consumption was reduced in iWAT, but not BAT, of Bcl6f/fPrx1Cre mice (Fig. 4 C and D). Because immunostaining for TH demonstrated similar density of sympathetic nerves in iWAT of Bcl6f/f and Bcl6f/fPrx1Cre mice (Fig. 4E and Movies S19 and S20), it suggests that BCL6 acts in a cell autonomous and SNS-independent manner to support the commitment of precursor cells to the beige adipocyte lineage. In support of this hypothesis, cold-induced recruitment of beige adipocytes was unaffected in Bcl6f/fUcp1Cre mice (Fig. 4 F and G), which had normal development of beige adipocytes during the peri-weaning period (SI Appendix, Fig. S4 C–E). These data together suggest a model in which BCL6 acts primarily at the commitment phase of beige adipogenesis, whereas cold exposure leads to the recall of committed cells in a SNS-dependent and BCL6-independent manner.
Fig. 4.
BCL6 regulates the core program necessary for development and recruitment of beige fat. (A) Immunostaining for UCP1 in iWAT of 14-wk-old Bcl6f/f and Bcl6f/fPrx1Cre mice housed at 4 °C for 2 d. (B) Quantification of UCP1 immunostaining in A using Imaris (n = 10–14 per genotype). Data analyzed by Student’s t test. (C and D) Oxygen consumption rate of iWAT (C) and BAT (D) from 9- to 10-wk-old Bcl6f/f and Bcl6f/fPrx1Cre mice housed at 4 °C for 2 d (n = 11–12 per genotype). Data analyzed by Student’s t test. (E) Immunostaining for TH in iWAT of 8-wk-old Bcl6f/f and Bcl6f/fPrx1Cre mice housed at 22 °C. (F) Immunostaining for UCP1 in iWAT from 9-wk-old Bcl6f/f and Bcl6f/fUcp1Cre mice housed at 4 °C for 2 d. (G) Quantification of UCP1 in iWAT of Bcl6f/f and Bcl6f/fUcp1Cre mice that were housed at 4 °C for 2 d (n = 8–9 per genotype). Data analyzed by Student’s t test. (H) GO enrichment analysis and corresponding P values of down-regulated genes in iWAT of 9-wk-old Bcl6f/fPrx1Cre mice housed at 4 °C for 2 d (n = 4 per genotype). (I) Heatmap of down-regulated genes in the “Fatty acid beta oxidation” GO category in iWAT of 9-wk-old Bcl6f/fPrx1Cre mice housed at 4 °C for 2 d (n = 4 per genotype). (J) Venn diagram showing overlap of genes down-regulated in iWAT of 9-wk-old Bcl6f/fPrx1Cre mice housed at 4 °C for 2 d (versus Bcl6f/f) and genes up-regulated in 9-wk-old C57BL/6J mice housed at 4 °C for 2 d (versus 22 °C). Fold change > 1.5, adjusted P value <0.05. (K) Venn diagram showing overlap of genes up-regulated in iWAT of 9-wk-old Bcl6f/fPrx1Cre mice housed at 4 °C for 2 d (versus Bcl6f/f) and genes down-regulated in 9-wk-old C57BL/6J mice housed at 4 °C for 2 d (versus 22 °C). Fold change > 1.5, adjusted P value < 0.05. (L) Comparison of cold- and BCL6-dependent genes in J and K with beige and white adipocyte signatures. (M and N) GO enrichment analysis and corresponding P values of cold- and BCL6-induced (M), and cold- and BCL6-repressed (N) genes in iWAT of 9-wk-old mice. (Scale bars: A, E, and F, 1 mm.)
To gain insights into how signals downstream of environmental cold interact with BCL6 to regulate recruitment of beige adipocytes in adult animals, we performed RNA-seq on iWAT of Bcl6f/f and Bcl6f/fPrx1Cre mice that were subjected to a cold challenge at 4 °C for 2 d. We found that 493 genes were down-regulated and 902 genes were up-regulated in iWAT of 9-wk-old Bcl6f/fPrx1Cre mice after exposure to environmental cold, which were enriched in the signature genes for beige and white adipocytes, respectively (SI Appendix, Fig. S5A). GO terms associated with lipid metabolic process, fatty acid β-oxidation, and heat generation were enriched in the down-regulated genes (Fig. 4 H and I and SI Appendix, Fig. S5 B and C), whereas processes associated with cell adhesion, inflammatory response, and angiogenesis were found among the up-regulated genes in Bcl6f/fPrx1Cre mice (SI Appendix, Fig. S5 D–F).
We next compared these BCL6-dependent genes with those regulated by environmental cold in WT C57BL/6J mice. We found that BCL6-dependent genes only partially overlapped with those regulated by cold challenge at 4 °C in C57BL/6J adult mice. For example, among the cold-induced genes in C57BL/6J mice, only 122 genes (∼21%) exhibited dependence on BCL6 (Fig. 4J). In contrast, cold exposure led to down-regulation of 780 genes in iWAT of C57BL/6J mice, but only ∼17% (135 genes) required BCL6 for their suppression (Fig. 4K). This suggests that the interactome between BCL6 and environmental cold represents a core set of genes necessary for the recruitment of beige adipocytes in adult animals. Indeed, comparison of cold- and BCL6-dependent differentially expressed genes with the signature genes of beige and white adipocytes revealed that the induced genes were highly enriched in beige adipocytes, whereas the repressed genes were abundant in white adipocytes (Fig. 4L and SI Appendix, Table S2). GO enrichment analysis confirmed that lipid and fatty oxidation processes were highly represented in the induced genes, whereas processes of skeletal muscle development, cell adhesion, and wound healing were enriched among the repressed genes (Fig. 4 M and N).
Discussion
We present here evidence that BCL6 and the SNS differentially regulate biogenesis and recruitment, respectively, of beige adipocytes in the iWAT. While previous studies had suggested that continuous sympathetic stimulation was required for commitment to the beige adipocyte lineage (8), we found that genetic absence of sympathetic nerves (as in Ntrk1f/fThCre mice) did not significantly impact development of beige adipocytes during the peri-weaning period. In contrast, we observed that cold-induced recruitment of beige adipocytes was completely dependent on the sympathetic nerves. Because this recruitment defect in Ntrk1f/fThCre mice could be rescued by administration of the β3-selective adrenergic agonist CL-316,243, it suggests that cell-autonomous mechanisms, rather than adrenergic signaling, regulate commitment of mesenchyme-derived precursor cells to the beige adipocyte lineage.
We investigated the cell-autonomous requirement of BCL6 in beige adipocyte development for two reasons. First, we had recently reported that BCL6 was required for maintenance of thermogenic fitness in dormant brown adipocytes (22), an adipocyte cell type that shares molecular and functional features with beige adipocytes. Second, we and others have demonstrated that BCL6 reinforces a cell’s identity by repressing alternative cell fate programs, including in immune cells and brown adipocytes (22, 25, 26). This observation led us to ask whether BCL6 might function as a molecular switch between beige and white adipocytes that reside in the iWAT. Indeed, using three different knockout mouse lines (Bcl6f/fPrx1Cre, Bcl6f/fAdipoqCre, and Bcl6f/fUcp1Cre), we found that BCL6 was specifically required at the commitment, but not the maintenance, phase of beige adipogenesis. For example, in Bcl6f/fPrx1Cre mice, we found that expression of genes involved in β-oxidation of fatty acids (Acaa2, Acadm, Acadl, Acadvl, Acaa2b, Decr1, Slc27a2, Hadha, Hadhb, and Ehhadh), thermogenesis (Ucp1, Elovl3, Cox7a1, Cox8b, and Cidea), and the beige adipocyte markers Tbx1 and Tmem26 was reduced in the iWAT (SI Appendix, Tables S1 and S2). Furthermore, the near absence of UCP1 protein in iWAT of Bcl6f/fPrx1Cre mice was accompanied by induction of Atp5g1, which switches mitochondria from uncoupled to coupled respiration (22). Together, these data suggest that BCL6 regulates a core set of genes that are required for full expression of the beige adipocyte phenotype during the peri-weaning period of development.
The peri-weaning period is an important transition point in mammalian physiology, as neonates switch from mother’s milk to adult food. During this critical period, a number of physiological systems undergo maturation and remodeling. For example, the absorptive intestinal epithelium remodels from its neonatal to adult state to support nutrient absorption, a process regulated by the transcriptional repressor B lymphocyte-induced maturation protein 1 (Blimp1). Deletion of Blimp1 in the intestinal epithelial cells compromises nutrient intake in neonates, resulting in growth retardation and mortality (27, 28). In a similar manner, the microbiome, which is seeded at birth, remodels during the peri-weaning period. This stabilization of the microbiome induces a weaning reaction in the immune system, which is necessary for induction of protective immunity in the gut (29). Our data extends this paradigm by demonstrating that BCL6 is required for peri-weaning remodeling of iWAT into thermogenic beige adipose tissue. Thus, in the future, it will be important to determine how these tissue remodeling responses are coordinated during the peri-weaning period to enable physiological adaptations in the adult animal.
Materials and Methods
All experiments involving mice were conducted under an approved Institutional Animal Care and Use Committee (IACUC) protocol at University of California, San Francisco (UCSF). Animal, tissue clearing and immunostaining, three-dimensional imaging, image processing and quantification, histology, immunoblotting, tissue oxygen consumption, RNA isolation and qRT-PCR, sequencing library preparation, next generation sequencing, RNA-seq, and statistical analyses for this study are described in detail in SI Appendix, Materials and Methods.
Data Availability.
Data generated or analyzed during this study are included in this published article and its SI Appendix files. The RNA-seq data has been deposited at the Gene Expression Omnibus (GEO), https://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE140259).
Acknowledgments
We thank members of the A.C. laboratory and A. Loh for comments on the manuscript, and X. Cui for assistance with mouse husbandry. This work was supported by NIH Grants DK094641 and DK101064 (to A.C.); Agency for Science, Technology, and Research (A*STAR, Singapore) National Science Scholarship (PhD to Y.W.); Dermatology Foundation Dermatologist Investigator Research Fellowship (to M.A.K.); and American Heart Association (AHA) Predoctoral Fellowships 18PRE34080250 and 16PRE26960008 (to V.I.K.). Histology support was provided by the UCSF Liver Center (NIH P30DK026743).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. C.W. is a guest editor invited by the Editorial Board.
Data deposition: All RNA-seq data are available at the Gene Expression Omnibus (GEO), https://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE140259).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920419117/-/DCSupplemental.
Change History
November 15, 2021: The Acknowledgments have been updated.
References
- 1.Rosen E. D., Spiegelman B. M., What we talk about when we talk about fat. Cell 156, 20–44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez-Gurmaches J., Hung C. M., Guertin D. A., Emerging complexities in adipocyte origins and identity. Trends Cell Biol. 26, 313–326 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harms M., Seale P., Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Wang W., Seale P., Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691–702 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roh H. C., et al., Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metab. 27, 1121–1137 e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nedergaard J., Cannon B., The browning of white adipose tissue: Some burning issues. Cell Metab. 20, 396–407 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Chabowska-Kita A., Trabczynska A., Korytko A., Kaczmarek M. M., Kozak L. P., Low ambient temperature during early postnatal development fails to cause a permanent induction of brown adipocytes. FASEB J. 29, 3238–3252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contreras G. A., Lee Y. H., Mottillo E. P., Granneman J. G., Inducible brown adipocytes in subcutaneous inguinal white fat: The role of continuous sympathetic stimulation. Am. J. Physiol. Endocrinol. Metab. 307, E793–E799 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouon-Evans V., Pollard J. W., Unexpected deposition of brown fat in mammary gland during postnatal development. Mol. Endocrinol. 16, 2618–2627 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Master S. R., et al., Functional microarray analysis of mammary organogenesis reveals a developmental role in adaptive thermogenesis. Mol. Endocrinol. 16, 1185–1203 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Odegaard J. I., et al., Perinatal licensing of thermogenesis by IL-33 and ST2. Cell 166, 841–854 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., et al., Adipocyte liver kinase b1 suppresses beige adipocyte renaissance through class IIa histone deacetylase 4. Diabetes 66, 2952–2963 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi J., Crane A., Wu Z., Cohen P., Adipo-clear: A tissue clearing method for three-dimensional imaging of adipose tissue. J. Vis. Exp., e58271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagerspetz K., The postnatal development of homoiothermy and cold resistance in mice. Experientia 18, 282–284 (1962). [DOI] [PubMed] [Google Scholar]
- 15.Rosenwald M., Perdikari A., Rülicke T., Wolfrum C., Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Kajimura S., Spiegelman B. M., Seale P., Brown and beige fat: Physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balaz M., et al., Inhibition of mevalonate pathway prevents adipocyte browning in mice and men by affecting protein prenylation. Cell Metab. 29, 901–916.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Chen Y., et al., Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565, 180–185 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang H., Ding X., Cao Y., Wang H., Zeng W., Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 26, 686–692.e3 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Chen X., et al., A chemical-genetic approach to studying neurotrophin signaling. Neuron 46, 13–21 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Borden P., Houtz J., Leach S. D., Kuruvilla R., Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep. 4, 287–301 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kutyavin V. I., Chawla A., BCL6 regulates brown adipocyte dormancy to maintain thermogenic reserve and fitness. Proc. Natl. Acad. Sci. U.S.A. 116, 17071–17080 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Gurmaches J., Hsiao W. Y., Guertin D. A., Highly selective in vivo labeling of subcutaneous white adipocyte precursors with Prx1-Cre. Stem Cell Reports 4, 541–550 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krueger K. C., Costa M. J., Du H., Feldman B. J., Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Reports 3, 1147–1158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crotty S., T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basso K., Dalla-Favera R., BCL6: Master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv. Immunol. 105, 193–210 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Harper J., Mould A., Andrews R. M., Bikoff E. K., Robertson E. J., The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc. Natl. Acad. Sci. U.S.A. 108, 10585–10590 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muncan V., et al., Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat. Commun. 2, 452 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Nabhani Z., et al., A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity 50, 1276–1288.e5 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated or analyzed during this study are included in this published article and its SI Appendix files. The RNA-seq data has been deposited at the Gene Expression Omnibus (GEO), https://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE140259).