Abstract
Background
The incidence of chorioamnionitis occurs in between eight and 12 women for every 1000 live births and 96% of cases of chorioamnionitis are due to ascending infection. Following spontaneous vaginal delivery, 1% to 4% of women develop postpartum endometritis. The incidence of neonatal sepsis is 0.5% to 1% of all infants born. Maternal vaginal bacteria are the main agents for these infections. It is reasonable to speculate that prevention of maternal and neonatal infections might be possible by washing the vagina and cervix with an antibacterial agent for all women during labour. Chlorhexidine belongs to the class of compounds known as the bis‐biguanides. Chlorhexidine has antibacterial action against a wide range of aerobic and anaerobic bacteria, including those implicated in peripartal infections.
Objectives
To evaluate the effectiveness and side effects of chlorhexidine vaginal douching during labour in reducing maternal and neonatal infections (excluding group B streptococcal and HIV).
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 June 2014), reference lists of retrieved reports and journal letters and editorials.
Selection criteria
Randomized or quasi‐randomized trials comparing chlorhexidine vaginal douching during labour with placebo or other vaginal disinfectant to prevent (reduce) maternal and neonatal infections (excluding group B streptococcal and HIV).
Data collection and analysis
Two review authors independently assessed trial eligibility and quality, extracted and interpreted the data. A third review author analyzed and interpreted the data. The fourth author also interpreted the data.
Main results
We included three studies (3012 participants). There was no evidence of an effect of vaginal chlorhexidine during labour in preventing maternal and neonatal infections. Although the data suggest a trend in reducing postpartum endometritis, the difference was not statistically significant (three trials, 3012 women, risk ratio 0.83; 95% confidence interval 0.61 to 1.13).
Assessment of the quality of the evidence using GRADE indicated that the levels of evidence for all primary outcomes and one important secondary outcome were low to moderate.
Authors' conclusions
There is no evidence to support the use of vaginal chlorhexidine during labour in preventing maternal and neonatal infections. There is a need for a well‐designed randomized controlled trial using appropriate concentration and volume of vaginal chlorhexidine irrigation solution and with adequate sample size.
Keywords: Adult; Female; Humans; Infant, Newborn; Pregnancy; Labor, Obstetric; Anti‐Infective Agents, Local; Anti‐Infective Agents, Local/administration & dosage; Bacterial Infections; Bacterial Infections/prevention & control; Chlorhexidine; Chlorhexidine/administration & dosage; Chorioamnionitis; Chorioamnionitis/prevention & control; Endometritis; Endometritis/prevention & control; Randomized Controlled Trials as Topic; Vaginal Douching; Vaginal Douching/methods
Plain language summary
Vaginal chlorhexidine during labour for preventing maternal and neonatal infections (excluding group B streptococcal and HIV)
Bacteria live in women's vaginas and generally cause no problems. Very occasionally they infect the placenta during labour and can pass to the baby, causing an infection. These infections can occasionally make the baby very ill and very occasionally the baby might die.
The review of three trials (3012 participants) found there was not enough information to say whether the use of chlorhexidine washing of the vagina during labour led to fewer infections for mothers and babies. More research is needed.
Summary of findings
Summary of findings for the main comparison. Chlorhexidine vaginal wash versus placebo for preventing maternal and neonatal infections (excluding Group B Streptococcal and HIV).
| Chlorhexidine vaginal wash versus placebo for preventing maternal and neonatal infections (excluding Group B Streptococcal and HIV) | ||||||
| Population: Pregnant women with a gestational age greater than 28 weeks, considered to be in labour receiving chlorhexidine vaginal douching during labour versus placebo to prevent maternal and neonatal infections (excluding Group B Streptococcal and HIV). Settings: Hospitals in the United States. Intervention: Chlorhexidine vaginal douching during labour versus placebo or other vaginal disinfectant. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Chlorhexidine vaginal wash versus placebo | |||||
| Chorioamnionitis Clinical findings | Study population | RR 1.1 (0.86 to 1.42) | 3012 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 69 per 1000 | 76 per 1000 (59 to 98) | |||||
| Moderate | ||||||
| 62 per 1000 | 68 per 1000 (53 to 88) | |||||
| Postpartum endometritis Clinical findings | Study population | RR 0.83 (0.61 to 1.13) | 3012 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 57 per 1000 | 47 per 1000 (35 to 64) | |||||
| Moderate | ||||||
| 72 per 1000 | 60 per 1000 (44 to 81) | |||||
| Neonatal sepsis Clinical findings | Study population | RR 0.75 (0.17 to 3.35) | 2987 (3 studies) | ⊕⊕⊝⊝ low2 |
||
| 3 per 1000 | 2 per 1000 (0 to 9) | |||||
| Moderate | ||||||
| 4 per 1000 | 3 per 1000 (1 to 13) | |||||
| Blood culture confirming neonatal sepsis Clinical findings and confirmation by laboratory | Study population | RR 0.75 (0.17 to 3.35) | 2077 (2 studies) | ⊕⊕⊝⊝ low2 |
||
| 4 per 1000 | 3 per 1000 (1 to 13) | |||||
| Moderate | ||||||
| 4 per 1000 | 3 per 1000 (1 to 13) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Confidence interval crossing the line of no effect. 2 Wide confidence interval crossing the line of no effect & few events.
Background
Description of the condition
Chlorioamnionitis is an inflammatory reaction of the placental tissues in response to organism invasion. The incidence of chorioamnionitis occurs in eight to 12 women for every 1000 live births and 96% of the cases of chorioamnionitis are due to an ascending infection (Monif 1993). Following spontaneous vaginal delivery, 1% to 4% of women develop postpartum endometritis (Monif 1993). Although uterine infections are relatively uncommon following uncomplicated vaginal delivery, they continue to be a major problem in women delivered by caesarean section. Vaginal examinations increase the risk of inoculation and colonization of lower uterine incisions and laceration and therefore increase the risk of postpartum endometritis in women delivered by caesarean section (Cunningham 2001).
Bacterial infection is an important cause of neonatal morbidity and mortality. A prospective study from Pakistan reported a prevalence of blood culture proven bacterial sepsis to be 5.6 per 1000 live births (Bhutta 1997). Septicemia accounted for 11% to 30.4% of all neonatal deaths (Boo 1994). Other forms of infection include ophthalmia neonatorum; neonatal pneumonia and neonatal meningitis. Maternal vaginal bacteria are the main agents for these infections.
Description of the intervention
It is reasonable to speculate that prevention of maternal and neonatal infections might be possible by washing the vagina and cervix with an antibacterial agent in women during labour. Vaginal and cervical washing is usually performed by gently introducing a catheter attached to a 50 to 60 mL syringe up to the cervix. The cervical area is flushed with 50 to 60 mL of the solution. The syringe is then refilled without removing the catheter, and a second flushing with the same amount of the solution is performed while slowly withdrawing the catheter (Gaillard 2001). This procedure can be performed within a few minutes and would not interfere with women's labour when they wish to move and adopt a position which they feel is right for them. To be clinically useful, such an agent would need to possess antimicrobial activity against a broad range of bacteria that have been implicated in peripartal infection, be non‐toxic and non‐irritating for mother and fetus/neonate. Ideally, the agent would be inexpensive and commercially available. Chlorhexidine, a widely used medical disinfectant, satisfies these requirements.
How the intervention might work
Chlorhexidine belongs to the class of compounds known as the bis‐biguanides. Because of its high cationic nature, it has a strong affinity for the cell wall of micro‐organisms, to which it binds, disrupting osmotic equilibrium. The disrupted cytoplasmic membrane precipitates intracellularly, preventing repair of the cell wall and eventually resulting in cell death (Davies 1973). These actions endow chlorhexidine with antibacterial action against a wide range of aerobic and anaerobic bacteria, including those implicated in peripartal infections (Emilson 1977; Hennessey 1973). This antibacterial action is achieved at a very low concentration: typical minimum inhibitory concentrations are in microgram (mcg) per millilitre (mL), whereas clinically used concentrations are in milligram (mg) per mL. A randomized controlled trial demonstrated that vaginal douching with chlorhexidine during labour can significantly reduce maternal and early neonatal (including group B streptococcal) infection (Stray‐Pedersen 1999). Finally, complete resistance to chlorhexidine rarely emerges even after long‐term use (Ferretti 1990).
The allergic and toxic potential of chlorhexidine is very low. For many decades, chlorhexidine has been the major medical skin and the mucous membrane disinfectant in use. Despite its widespread use, only individual cases of anaphylactic or even mild allergic reactions in exposed medical personnel have been reported (Bergqvist‐Karlsson 1988). Chlorhexidine tends not to be absorbed by human skin and mucous membrane barrier (Johnsson 1987; Nilsson 1989). A long‐term human oral safety trial did not show any systemic or serious local side effects after two years of continuous use (Johnsson 1987). In contrast to povidone‐iodine, vaginally applied chlorhexidine was not absorbed in measurable amounts into the blood stream (Vorherr 1984).
Why it is important to do this review
Maternal and neonatal infections occur commonly and have serious ramifications for both mothers and newborns. Vaginal chlorhexidine douching may offer a safe, inexpensive, and theoretically sound approach to prevent maternal and neonatal infections. Vaginal disinfection during labour for reducing the risk of mother‐to‐child transmission (MTCT) of HIV infection is addressed in one Cochrane review which indicated that there was no evidence of an effect of vaginal disinfection on MTCT of HIV (Wiysonge 2005). Another Cochrane review also does not support the use of vaginal chlorhexidine during labour to prevent early‐onset neonatal group B streptococcal infection (Stade 2008).
Objectives
To evaluate the effectiveness and side effects of chlorhexidine vaginal douching during labour in reducing maternal and neonatal infections (excluding group B streptococcal and HIV).
Methods
Criteria for considering studies for this review
Types of studies
Randomized, quasi‐randomized or cluster‐randomized trials comparing chlorhexidine vaginal douching during labour with placebo or other vaginal disinfectant to prevent (reduce) maternal and neonatal infections (excluding group B streptococcal and HIV).
Types of participants
All pregnant women with a gestational age greater than 28 weeks, considered to be in labour.
Types of interventions
Chlorhexidine vaginal douching during labour versus placebo or other vaginal disinfectant.
Types of outcome measures
Primary outcomes
1. Maternal outcomes
(a) Chorioamnionitis (variously defined by the authors); (b) postpartum endometritis (variously defined by the authors).
2. Neonatal outcomes
(a) Neonatal sepsis (variously defined by the authors).
Secondary outcomes
1. Maternal outcomes
(a) Intrapartum fever; (b) intrapartum treatment with antibiotics; (c) maternal side effects (vaginal irritation, thrush, antimicrobial resistance); (d) serious maternal complication of treatment (e.g. anaphylaxis); (e) laparotomy for infection; (f) hysterectomy; (g) maternal death; (h) satisfaction with care; (i) length of hospital stay; (j) postnatal depression; (k) successful breastfeeding (variously defined by the authors); (l) costs of care; (m) antimicrobial resistance.
2. Neonatal outcomes
(a) Ophthalmia neonatorum; (b) neonatal pneumonia by clinical assessment and/or chest X‐ray; (c) neonatal meningitis by clinical assessment and/or culture; (d) blood culture confirming sepsis; (e) admission to neonatal intensive care unit; (f) length of hospital stay; (g) perinatal mortality; (h) abnormal neurodevelopmental assessment at follow‐up.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 June 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
[For details of additional author searching carried out the previous version of the review (Lumbiganon 2004), please see Appendix 1.]
Searching other resources
We searched cited references from retrieved articles for additional studies. We reviewed abstracts and letters to the editor to identify randomized controlled trials that had not been published and reviewed editorials, indicating expert opinion, to identify and ensure that no key studies were missed for consideration for inclusion in this review.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeLumbiganon 2004.
For this update, two new studies were identified and excluded (Pereira 2011; Saleem 2006). We will use the methods outlined in Appendix 2 for new trials identified at the next update.
We used the following updated methods to assess the risk of bias and assess the quality of already included studies using the GRADE approach. The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Two review authors (PL and JT) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving the third review author (JET).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
We classified blinding as 'high risk of bias' if the blinding status of a trial was unclear or the trial was open.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
We used a cut‐off point of less than 20% of missing data to assess that a study is adequate.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update, the quality of the evidence was re‐assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following key outcomes for the main comparison between intervention (chlorhexidine vaginal wash) and control groups.
Chorioamnionitis
Postpartum endometritis
Neonatal sepsis
Blood culture confirming sepsis
The GRADEprofiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
Two new studies (Pereira 2011; Saleem 2010) (both excluded) were identified from an updated search of the PCG Trials Register.
Included studies
We included three studies (Eriksen 1997; Rouse 1997; Rouse 2003) involving 3012 women in this review.
Eriksen 1997, from the USA, reported the effectiveness of chlorhexidine vaginal wash during labour to prevent neonatal infection. The data on peripartum infections of this study were reported in the other paper (Sweeten 1997). As they are both reports of the same trial we listed them under Eriksen 1997. Participants at 36 or more weeks' gestation in labour, excluding preterm labour, fetal distress, malpresentation, intra‐amniotic infection, cervical dilatation greater than 6 cm and known allergy to chlorhexidine were eligible for the study. Informed consent was obtained from 1024 women who were eligible. Of these, 77 were excluded from the analysis because of incomplete records (71, 38 in the control and 33 in the study group); three participants were enrolled and subsequently discharged home; the vaginal wash was not given to two women; and there was one infant with anencephaly. Of the remaining 947 participants, 481 were randomized to the study arm and 466 served as controls. A computer software program was used to generate a random block allocation sequence to assign participants to either group. The randomization assignments were contained in sequentially numbered, opaque, sealed packets that were made up independent of the physicians managing the participants. The authors chose not to blind the study because the syringe containing chlorhexidine solution was pink and the investigators could not reproduce the colour artificially in the syringe containing the sterile water. For maternal outcomes, it is not very clear whether the intention‐to‐treat analysis was performed because it was not stated that 77 women were excluded before or after randomization. For neonatal outcomes, intention‐to‐treat analysis was not done because 24 neonatal charts in the chlorhexidine group and 13 in the control group were unavailable for review.
Rouse 1997, from the USA, reported a double‐blinded clinical trial to determine whether chlorhexidine vaginal irrigation prevents maternal peripartal infection. Participants were eligible if they were admitted for delivery at or beyond 24 weeks' gestation. Exclusion criteria included a contra‐indication for cervical digital examination, active genital herpes, chorioamnionitis and known or suspected allergy to chlorhexidine. The chlorhexidine and placebo bottles were randomly ordered with a computer‐generated list and sequentially numbered with a peel‐off study label. The active and placebo solutions were clinically indistinguishable. Among 3234 eligible participants, 1024 were randomized, 508 in chlorhexidine and 516 in placebo groups respectively. Because of incomplete or contradictory data, treatment allocation could not be determined for additional 10 women and these women were not included in the analysis. Trial analysis was restricted to 1024 women and 1030 infants (six sets of twins).
Rouse 2003, from the USA, reported a clinical trial of chlorhexidine vaginal irrigation to prevent peripartal infection in nulliparas. The study was conducted in two hospitals serving predominantly publicly funded patients. Women were eligible if they were nulliparous and admitted for delivery at or beyond 32 weeks' gestation. Exclusion criteria included a contra‐indication to digital cervical examination, active genital herpes, chorioamnionitis and allergy to chlorhexidine. The chlorhexidine and placebo bottles were sequentially numbered (in groups of four) and randomly ordered based on a computer‐generated list (one for each hospital). Each study bottle contained a peel‐off label which, after use, was used to link participants to the correct study group. The chlorhexidine and placebo preparations were clinically indistinguishable. Four women (two women in each group) were enrolled but actually did not undergo irrigation. They are included in the intention‐to‐treat analysis.
Excluded studies
We excluded five studies from an updated search in October 2010 (Cutland 2009; Mushangwe 2006; Pereira 2006; Saleem 2007; Saleem 2010) and two additional studies (Pereira 2011; Saleem 2010) from the most recent updated search. In total, there are nine excluded studies. See the Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 1; Figure 2 for a summary of all 'Risk of bias' assessments.
1.
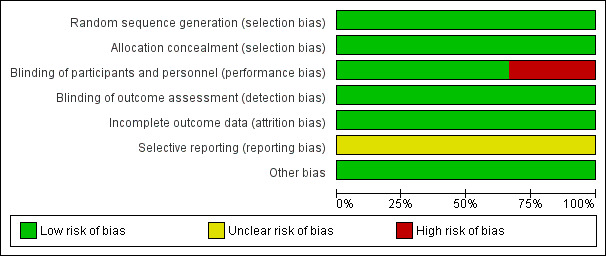
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Details for each trial are in the Characteristics of included studies table.
All included trials have a low risk of bias.
Allocation
All three included trials used computer‐generated allocation sequences and used appropriate random allocation concealment.
Blinding
Two trials (Rouse 1997; Rouse 2003) used indistinguishable placebo controls while one trial (Eriksen 1997) chose not to blind participants and personnel but the physicians managing infants in the nursery were unaware to which arm of the study each participant was randomized.
Incomplete outcome data
Two trials (Rouse 1997; Rouse 2003) obtained the outcomes of all recruited mothers while the other (Eriksen 1997) excluded 7.5% (77/1024) and 11.1% (114/1024) of mothers and neonates from the analysis respectively.
Selective reporting
We did not have the protocols for all three trials and therefore could not evaluate selective reporting.
Other potential sources of bias
There were no other obvious potential sources of biases.
Effects of interventions
See: Table 1
We included three studies involving 3012 women in this review.
Chlorhexidine vaginal wash versus placebo
Primary outcomes
Maternal outcomes
Three trials (3012 women) (Eriksen 1997; Rouse 1997; Rouse 2003) reported the incidence of chorioamnionitis, including 1514 and 1498 participants in the chlorhexidine and placebo groups respectively. There was no statistically significant difference between the two groups (risk ratio (RR) 1.10; 95% confidence interval (CI) 0.86 to 1.42) (Analysis 1.1). The same three trials also reported the incidence of postpartum endometritis. Although the data suggest a small reduction in the risk of postpartum endometritis with the use of the chlorhexidine vaginal wash, the difference was not statistically significant (RR 0.83; 95% CI 0.61 to 1.13) (Analysis 1.2).
1.1. Analysis.
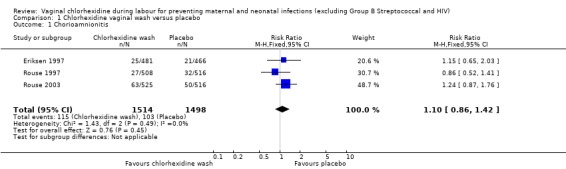
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 1 Chorioamnionitis.
1.2. Analysis.
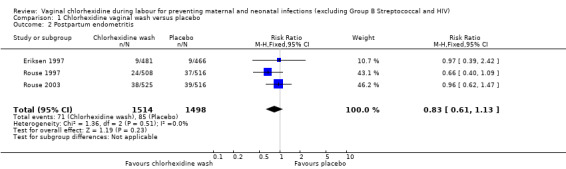
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 2 Postpartum endometritis.
Neonatal outcomes
Three trials (2987 infants) (Eriksen 1997; Rouse 1997; Rouse 2003) reported on neonatal outcomes, involving 1495 and 1492 neonates in the chlorhexidine and placebo groups respectively. For neonatal sepsis, which was evaluated in the three trials (Eriksen 1997; Rouse 1997; Rouse 2003) involving 1495 and 1492 neonates in the chlorhexidine and placebo groups respectively, there was no significant difference (RR 0.75; 95% CI 0.17 to 3.35) (Analysis 1.7).
1.7. Analysis.
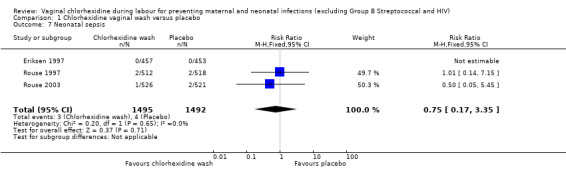
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 7 Neonatal sepsis.
Secondary outcomes
Maternal outcomes
There was no report about the other maternal outcomes and side effects of chlorhexidine in these three trials.
Neonatal outcomes
Two trials involving 1038 and 1039 (2077) neonates in the intervention and control groups respectively (Rouse 1997; Rouse 2003) did not find significant difference in blood culture confirming sepsis (RR 0.75; 95% CI 0.17 to 3.35) (Analysis 1.6) and perinatal mortality (RR 1.00, 95% CI 0.17 to 5.79) (2071 infants, Analysis 1.8). One trial with 457 and 453 neonates in the intervention and control group respectively (Eriksen 1997), indicated that there was no significant difference in neonatal pneumonia (RR 0.33; 95% CI 0.01 to 8.09) (910 infants, Analysis 1.4). For neonatal meningitis, one trial with 508 and 516 neonates in the intervention and control groups respectively (Rouse 1997) did not show significant difference (RR 0.34; 95% CI 0.01 to 8.29) (1024 infants, Analysis 1.5). There was a trend that vaginal chlorhexidine during labour might lead to a higher tendency for newborns to receive antibiotics but this association is not statistically significant (RR 1.65; 95% CI 0.73 to 3.74) (one trial, 910 infants, Analysis 1.9). There was no report about the other neonatal outcomes and side effects of chlorhexidine in these three trials.
1.6. Analysis.
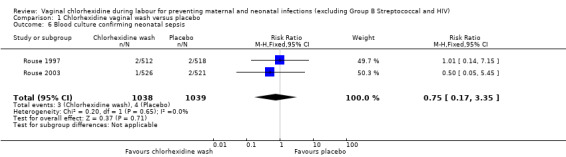
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 6 Blood culture confirming neonatal sepsis.
1.8. Analysis.
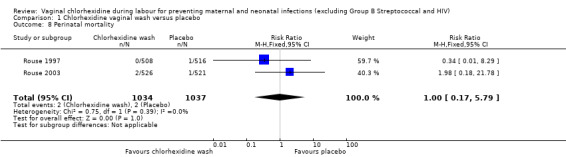
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 8 Perinatal mortality.
1.4. Analysis.
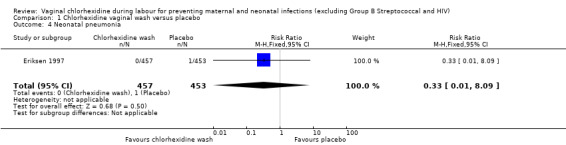
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 4 Neonatal pneumonia.
1.5. Analysis.
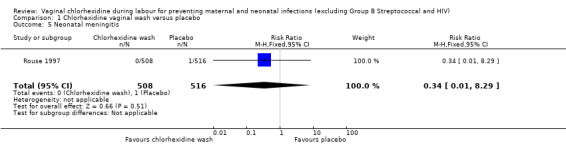
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 5 Neonatal meningitis.
1.9. Analysis.
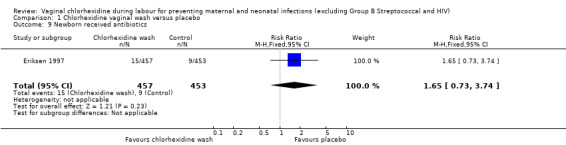
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 9 Newborn received antibiotics.
Discussion
Although all three included trials are of high quality, one trial (Eriksen 1997) used only 20 mL of chlorhexidine or sterile water for vaginal irrigation, while the other two trials (Rouse 1997; Rouse 2003) used 200 mL of chlorhexidine or sterile saline solution. The effectiveness of vaginal chlorhexidine might also depend on the volume of the solution used for irrigation. Since chlorhexidine solution is quite safe, not expensive and vaginal irrigation is not difficult to perform, there is a need for a well‐designed randomized controlled trial with adequate sample size to evaluate this simple intervention. However, the investigators of future trials must use the appropriate concentration and volume of vaginal chlorhexidine irrigation solution. We have identified two ongoing studies from the trial registration website www.clinicaltrials.gov (Madhi 2006; Moss 2006) since the review was first published.
Summary of main results
There was no evidence of an effect of vaginal chlorhexidine during labour in preventing maternal and neonatal infections.
Overall completeness and applicability of evidence
All three included studies were conducted in the USA. This limits the completeness and applicability of evidence to other parts of the world.
Quality of the evidence
All three included studies were of overall low risk of bias; however, only 3012 women were included in total. This review might not have enough power to detect small effect size. Assessment of the quality of the evidence using GRADE indicated that the levels of evidence for all primary outcomes and one important secondary outcome were low to moderate due to wide confidence interval crossing the line of no effect with few events (Table 1).
Potential biases in the review process
We carefully conducted this review following all steps recommended by the Cochrane Pregnancy and Childbirth Review Group. All review authors have no conflict of interest. There should not be any bias in the review process.
Agreements and disagreements with other studies or reviews
One Cochrane review (Stade 2008) has evaluated the effectiveness of vaginal chlorhexidine during labour for preventing early‐onset group B streptococcal infection in newborn infants. Although there was a significant reduction in group B streptococcal colonization in neonates, there were no significant reductions in early‐onset group B streptococcal sepsis, pneumonia, meningitis and mortality. One randomized controlled trial, involving 8011 women and their 8129 newborns, evaluated the effectiveness of chlorhexidine intravaginally and neonatal wipes for preventing early‐onset neonatal sepsis (Cutland 2009). There was no significant reductions in either early‐onset neonatal sepsis nor colonization of group B streptococcal. Another trial, involving 5008 women and their neonates, reported that chlorhexidine vaginal wipes during labour together with neonatal chlorhexidine wipes did not prevent neonatal sepsis, maternal and perinatal mortality (Saleem 2010).
Authors' conclusions
Implications for practice.
There is no evidence to support the use of vaginal chlorhexidine douching during labour in preventing maternal and neonatal infections.
Implications for research.
There is a need for a well‐designed randomized controlled trial with appropriate concentration and volume of irrigation solution and adequate sample size.
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2014 | New citation required but conclusions have not changed | Review updated. |
| 30 June 2014 | New search has been performed | Search updated. Two new trials identified and excluded (Pereira 2011; Saleem 2006). |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 9 April 2011 | New search has been performed | We reviewed five more studies from the updated search and excluded them all (Cutland 2009; Mushangwe 2006; Pereira 2006; Saleem 2007; Saleem 2010). |
| 30 June 2009 | Amended | Search updated. Three new reports identified. |
| 20 September 2008 | Amended | Converted to new review format. |
| 27 April 2006 | New search has been performed | We have identified two ongoing studies from the trial registration web site (Madhi 2006; Moss 2006) and another report for Rouse 2003. There are no relevant data available yet. |
Notes
The ongoing reports identified through the updated search are preliminary reports of trials that may be relevant to this review. They will be assessed by the review team once the results of the main trials are available, at which time the review team will update this review.
Acknowledgements
We would like to thank Erika Ota for her support in the creation of the 'Summary of findings' table for this update. Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication. Khon Kaen University, Thailand and Thomas Jefferson University, USA.
Appendices
Appendix 1. Search strategy for CENTRAL and MEDLINE
Authors searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2010, Issue 10), MEDLINE (from 1966 to 31 October 2010), LILACS (from 1982 to 31 October 2010 using the following keywords and free text terms: "chlorhexidine" or "vaginal‐creams‐foams‐and‐jellies" or "vaginal gel" or "vaginal wash" or "vaginal disinfection" and "peripartum" or "maternal" or "neonatal" or "labour" or "labor" or "infant‐newborn".
Appendix 2. Methods to be used in future updates
Data collection and analysis
Selection of studies
Two review authors will independently assess for inclusion all the potential studies we identify as a result of the search strategy. We will resolve any disagreement through discussion or, if required, we will consult a third author.
We will create a study flow diagram to map out the number of records identified, included and excluded.
Data extraction and management
We will design a form to extract data. For eligible studies, at least two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult a third author. We will enter data into Review Manager software (RevMan 2014) and check for accuracy.
When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We will describe for each included study any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardized mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomized trials
We will include cluster‐randomized trials in the analyses along with individually‐randomized trials. We will adjust their sample sizes using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomized trials and individually‐randomized trials, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
Cross‐over trials
We determined that it was not possible to include cross‐over trials in this review.
Other unit of analysis issues
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomized to each group in the analyses, and all participants will be analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomized minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30% and either a Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We plan to carry out the following subgroup analyses for primary outcomes:
concentration of chlorhexidine (less than 0.4% versus 0.4% or higher);
volume of chlorhexidine solution (less than 200 mL versus 200 mL or more).
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We plan to perform sensitivity analyses to explore the effect of study quality relating to the 'Risk of bias' items including allocation concealment, incomplete data collection to assess for any substantive difference to the overall result. If cluster‐randomized trials are included in the review, we aim to apply other sensitivity analysis incorporating an estimate of the ICC taken from a different study, to see what the effect of different values of the ICC on the results of the analysis would be.
Data and analyses
Comparison 1. Chlorhexidine vaginal wash versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chorioamnionitis | 3 | 3012 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.86, 1.42] |
| 2 Postpartum endometritis | 3 | 3012 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.13] |
| 3 Side effects | 2 | 2065 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Neonatal pneumonia | 1 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Neonatal meningitis | 1 | 1024 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.29] |
| 6 Blood culture confirming neonatal sepsis | 2 | 2077 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.35] |
| 7 Neonatal sepsis | 3 | 2987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.35] |
| 8 Perinatal mortality | 2 | 2071 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.17, 5.79] |
| 9 Newborn received antibiotics | 1 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.73, 3.74] |
1.3. Analysis.
Comparison 1 Chlorhexidine vaginal wash versus placebo, Outcome 3 Side effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eriksen 1997.
| Methods | Randomized controlled trial, unblinded. | |
| Participants | 1024 term pregnant women presenting to labour and delivery. Included: 947 women (481 in chlorhexidine group (intervention) and 466 in sterile water group (control). Excluded after randomization: 77 women (71 incomplete records, 3 discharged home, 2 vaginal wash was not given, 1 anencephalic). |
|
| Interventions | Vaginal wash with 20 mL of 0.4% chlorhexidine solution versus sterile water. | |
| Outcomes | Neonatal infection (pneumonia or sepsis) and use of antibiotics in neonates. Peripartal infection (intra‐amniotic infection and endometritis). |
|
| Notes | The report by Eriksen 1997 provided details of the neonatal outcomes. The report by Sweeten 1997 provided details of the maternal outcomes. This study was conducted in Houston, Texas, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer software program (True Epistat, Richardson, TX) was used to generate a random block allocation sequence to assign women to either group. |
| Allocation concealment (selection bias) | Low risk | The randomization assignments were contained in sequentially numbered, opaque, sealed packets that were made up independent of the physicians managing the women. The randomization was known only after the woman had been enrolled in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors chose not to blind the study because the syringe containing the chlorhexidine solution was pink and "we could not reproduce the colour artificially in the syringe containing the sterile water". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Additionally, the physicians managing infants in the nursery were unaware to which arm of the study each participant was randomized. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1024 women were eligible for the study. Of these, 77 were excluded from the analysis for the following reasons: incomplete records (71), the patient was enrolled and subsequently discharged home (3), vaginal wash was not given (2), and a patient whose infant had anencephaly was inadvertently enrolled. Of the remaining 947 women, 481 were randomized to the study arm and 466 served as controls. 24 neonatal charts in the chlorhexidine group and 13 in the control group were unavailable for review. This left 457 neonates in the study group and 453 in a control group for analysis. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available for review. |
| Other bias | Low risk | No other obvious biases. |
Rouse 1997.
| Methods | Double‐blinded, placebo‐controlled randomized clinical trial. | |
| Participants | 1024 women (508 in the chlorhexidine group and 516 in the placebo group). | |
| Interventions | Vaginal irrigation with 200 mL of 0.2% chlorhexidine or sterile water placebo. | |
| Outcomes | Peripartal infection (chorioamnionitis and endometritis (mutually exclusive) diagnosed) and neonatal infections. | |
| Notes | The study was conducted in Birmingham, Alabama, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | The chlorhexidine and placebo bottles were randomly ordered with a computer‐generated list and sequentially numbered with peel‐off study labels. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The active and placebo solutions were clinically indistinguishable. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The active and placebo solutions were clinically indistinguishable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 1024 women were enrolled in the trial and underwent irrigation: 508 in the chlorhexidine group and 516 in the placebo group (Figure 1). Trial analysis was restricted to these 1024 women and their 1030 infants (6 sets of twins). |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available for review. |
| Other bias | Low risk | No other obvious biases. |
Rouse 2003.
| Methods | Double‐blinded, placebo‐controlled randomized trial. | |
| Participants | 1041 women (525 in the chlorhexidine group and 516 in the placebo group). | |
| Interventions | Vaginal irrigation with 200 mL of 0.2% chlorhexidine solution or sterile water every 6 hours during labour. | |
| Outcomes | Peripartal infection (chorioamnionitis and endometritis), neonatal sepsis and perinatal death. | |
| Notes | The study was conducted in BIrmingham, Alabama, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list (1 for each hospital). |
| Allocation concealment (selection bias) | Low risk | Both solutions were dispensed into sterile 225 mL flexible, high‐density polyethylene bottles (W Braun, Miami, Fla), capped, and sealed. The chlorhexidine and placebo bottles were numbered sequentially (in groups of 4) and ordered randomly on the basis of a computer‐generated list (1 for each hospital). The bottles were then placed into a study packet along with four sterile 12 cm douche nozzles (Massengill, SmithKline Beecham, Pittsburgh, Pa). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each study bottle contained a peel‐off label which, after use was used to link participants to the correct study group. The chlorhexidine and placebo preparations were clinically indistinguishable. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Each study bottle contained a peel‐off label which, after use, was used to link participants to the correct study group. The chlorhexidine and placebo preparations were clinically indistinguishable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 1041 women were enrolled in the trial: 525 women in the chlorhexidine group and 516 women in the placebo group. 4 women (2 women in each group) were enrolled but actually did not undergo irrigation. They are included in the intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available for review. |
| Other bias | Low risk | No other obvious biases. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Calkin 1996 | The intervention in this study was vulva swabbing, not vaginal douching or washing during labour. |
| Cutland 2009 | This is a randomized placebo‐controlled trial assessing the effect of vaginal and neonatal wipe with 0.6% chlorhexidine. The intervention of the study is not compatible with this intervention of interest in this review, which is vaginal douching only not including neonatal cleansing. |
| Henrichsen 1994 | Participants in the control group received chlorhexidine gel during vaginal exploration. Inadequate allocation concealment ‐ randomization was performed by changing the regimen on a weekly basis, every Monday morning at 0800. |
| Mushangwe 2006 | Not a randomized controlled trial. |
| Pereira 2006 | This is a randomized controlled trial to determine the safety and acceptability of 1% chlorhexidine vaginal washing of the vagina in labour and of the neonate after delivery. The intervention of the study is not compatible with this intervention of interest in this review, which is vaginal douching only not including neonatal cleansing. |
| Pereira 2011 | This is a randomized controlled trial conducted in Zimbabwe to determine the safety, acceptability, and antimicrobial effect of 1% chlorhexidine (CHX) vaginal washing of women in labour and their neonates. The intervention of the study is not compatible with the intervention of interest in this review, which is vaginal douching only not including neonatal cleansing. |
| Saleem 2006 | This is a randomized controlled trial to evaluate the feasibility of using vaginal/neonatal chlorhexidine (CHX) to reduce perinatal infections, morbidity and mortality in Pakistan. The intervention of the study is not compatible with the intervention of interest in this review, which is vaginal douching only not including neonatal cleansing. |
| Saleem 2007 | This is a randomized placebo‐controlled trial assessing tolerance and safety of vaginal and neonatal wipe with 0.6% chlorhexidine. The intervention of the study is not compatible with the intervention of interest in this review, which is vaginal douching only not including neonatal cleansing. |
| Saleem 2010 | This is a randomized placebo‐controlled trial assessing the effects of 0.6% chlorhexidine vaginal and baby wipes on fetal and neonatal mortality and infection‐related morbidity. The intervention of the study is not compatible with the intervention of interest in this review, which is vaginal douching only, not including neonatal cleansing. |
Characteristics of ongoing studies [ordered by study ID]
Madhi 2006.
| Trial name or title | Preventing serious neonatal and maternal peripartum infections in developing country settings with a high prevalence of HIV infection: assessment of the disease burden and evaluation of an affordable intervention in Soweto, South Africa. |
| Methods | |
| Participants | Healthy female volunteers aged 15 years and above. Expected enrolment: 8000. |
| Interventions | 0.5% chlorhexidine wipes of the birth canal during labour and of the infant at birth compared with external genitalia sterile water wipes. |
| Outcomes | Primary outcomes: rates of culture‐confirmed or clinical neonatal sepsis, < 3 days of life; rate of vertical transmission of colonization with group B streptococcus. Secondary outcomes: rates of culture‐confirmed or clinical neonatal sepsis (non‐nosocomial), 3 to 28 days of life; rates of serious maternal per partum infections including: endometritis, culture‐confirmed postpartum sepsis, and postpartum perineal wound infection; rates of neonatal hospitalization, < 3 days of life; rates of neonatal hospitalization, < 28 days of life; rates of neonatal hospitalization, suspected sepsis; rate of vertical transmission of colonization with E. coli or Klebsiella species. |
| Starting date | April 2004. |
| Contact information | Clare Cutland: +27 11 9899894; cutlandc@hivsa.com Shabir Madhi: +27 11 9899894; madhis@hivsa.com |
| Notes | Expected completion date: May 2008. ClinicalTrials.gov identifier NCT00136370 |
Moss 2006.
| Trial name or title | Randomized pilot trial of chlorhexidine vaginal and infant wash to reduce neonatal mortality. |
| Methods | |
| Participants | Healthy female volunteers aged 16 years and above. Expected total enrolment: 1000. Setting: civil hospital in Karachi, Pakistan. |
| Interventions | 0.6% chlorhexidine solution every 4 hours until delivery (4 washes maximum) and 1 neonatal wash with the same solution compared with 200 mL of sterile physiologic saline solution. |
| Outcomes | Primary outcomes: neonatal death or severe sepsis at 7 days. Secondary outcomes: maternal: clinical chorioamnionitis, clinical endometritis, urinary tract infection, sepsis, length of hospitalization, readmission to hospital, death; neonatals: receipt of antibiotics, duration of hospitalization, readmission to hospital |
| Starting date | June 2005. |
| Contact information | Nancy Moss: mossn@mail.nih.gov |
| Notes | Expected completion date: June 2006. ClinicalTrials.gov identifier NCT00121394 |
Differences between protocol and review
Used Background sub‐headings to structure the Background.
Categorized outcomes into primary and secondary outcomes.
Created a 'Summary of findings' table using GRADEprofiler.
Contributions of authors
Pisake Lumbiganon (PL) wrote the protocol. Jadsada Thinkhamrop (JT), Bandit Thinkhamrop (BT) and Jorge Tolosa (JET) commented on the early drafts and approved the published version. PL and JT conducted the review. BT assisted in the data analysis. PL drafted the review. JT, BT and JET gave significant intellectual comments on the review and approved the final version.
For the 2011 update, PL and JT independently reviewed five more studies from the updated search. PL drafted the updated review, which was then revised and approved by all review authors.
For the 2014 update, PL updated the search and PL and JT assessed the eligibility of the two studies identified from the updated search. PL created the 'Summary of findings' table using GRADEprofiler and drafted the text of the updated review, which was then revised and approved by all review authors.
Sources of support
Internal sources
Khon Kaen University, Thailand.
Thomas Jefferson University, USA.
External sources
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Thailand Research Fund, Distinguished Professor Award, Thailand.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Eriksen 1997 {published data only}
- Eriksen NL, Sweeten KM, Blanco JD. Chlorhexidine versus sterile vaginal wash during labor to prevent neonatal infection. Infectious Diseases in Obstetrics and Gynecology 1997;5:286‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen NL, Sweeten KM, Blanco JD. Chlorhexidine versus sterile water vaginal wash during labor to prevent neonatal infection. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S57. [DOI] [PubMed] [Google Scholar]
- Sweeten KM, Eriksen NL, Blanco JD. Chlorhexidine versus sterile water vaginal wash during labor to prevent peripartal infection. American Journal of Obstetrics and Gynecology 1995;172:304. [DOI] [PubMed] [Google Scholar]
- Sweeten KM, Eriksen NL, Blanco JD. Chlorhexidine versus sterile water vaginal washing during labor to prevent peripartal infection. American Journal of Obstetrics and Gynecology 1997;176:426‐30. [DOI] [PubMed] [Google Scholar]
Rouse 1997 {published data only}
- Rouse D, Hauth J, Andrews W, Mills B, Maher J. Chlorhexidine vaginal irrigation to prevent puerperal infection. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S57. [DOI] [PubMed] [Google Scholar]
- Rouse DJ, Hauth JC, Andrews WW, Mills BB, Maher JE. Chlorhexidine vaginal irrigation for the prevention of peripartal infection: a placebo‐controlled randomized clinical trial. American Journal of Obstetrics and Gynecology 1997;176:617‐22. [DOI] [PubMed] [Google Scholar]
Rouse 2003 {published data only}
- Rouse DJ, Cliver S, Lincoln TL, Andrews WW, Hauth JC. Clinical trial of chlorhexidine vaginal irrigation to prevention peripartal infection in nulliparous women. American Journal of Obstetrics and Gynecology 2003;189(1):166‐70. [DOI] [PubMed] [Google Scholar]
- Rouse DJ, Cliver S, Lincoln TL, Andrews WW, Hauth JC. Intrapartum chlorhexidine vaginal irrigation for the prevention of peripartal infection. American Journal of Obstetrics and Gynecology 2002;187:S220. [DOI] [PubMed] [Google Scholar]
- Rouse DJ, Lincoln T, Cliver S, Lyon MD, Andrews WW, Hauth JC. Intrapartum chlorhexidine vaginal irrigation and chorioamnion and placental microbial colonization. International Journal of Gynecology & Obstetrics 2003;83:165‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Calkin 1996 {published data only}
- Calkin S. Chlorhexidine swabbing in labour. Modern Midwife 1996;6(1):28‐33. [PubMed] [Google Scholar]
Cutland 2009 {published data only}
- Cutland CL, Madhi SA, Zell ER, Kuwanda L, Laque M, Groome M, et al. Chlorhexidine maternal‐vaginal and neonate body wipes in sepsis and vertical transmission of pathogenic bacteria in South Africa: a randomised, controlled trial. Lancet 2009;374(9705):1909‐16. [DOI] [PubMed] [Google Scholar]
Henrichsen 1994 {published data only}
- Henrichsen T, Lindemann R, Svenningsen L, Hjelle K. Prevention of neonatal infection by vaginal chlorhexidine disinfection during labour. Acta Paediatrica 1994;83:923‐6. [DOI] [PubMed] [Google Scholar]
Mushangwe 2006 {published data only}
- Mushangwe V, Tolosa JE, Pereira L, Mashu A, Bangdiwala S, Rusakaniko S, et al. Chlorhexidine washing of the vagina in labor effectively reduces bacterial colonization: a study by the global network for perinatal & reproductive health. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S66. [Google Scholar]
Pereira 2006 {published data only}
- Pereira L, Chipato T, Mashu A, Mushangwe V, Rusakaniko S, Bangdiwala S, et al. Chlorhexidine washing of the vagina in labor and neonate after birth: an RCT by the global network for perinatal & reproductive health. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S70. [Google Scholar]
Pereira 2011 {published data only}
- Pereira L, Chipato T, Mashu A, Mushangwe V, Rusakaniko S, Bangdiwala SI, et al. Randomized study of vaginal and neonatal cleansing with 1% chlorhexidine. International Journal of Gynecology & Obstetrics 2011;112(3):234‐8. [DOI] [PubMed] [Google Scholar]
Saleem 2006 {published data only}
- Saleem S, Goldenberg RL, McClure EM, Wright LL, Pasha O, Moss N, et al. The feasibility of using vaginal/neonatal chlorhexidine (CHX) to reduce perinatal infections, morbidity and mortality in Pakistan. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco, California, USA. 2006.
Saleem 2007 {published data only}
- Saleem S, Reza T, McClure EM, Pasha O, Moss N, Rouse DJ, et al. Chlorhexidine vaginal and neonatal wipes in home births in Pakistan: a randomized controlled trial. Obstetrics & Gynecology 2007;110(5):977‐85. [DOI] [PubMed] [Google Scholar]
Saleem 2010 {published data only}
- Saleem S, Rouse DJ, McClure EM, Zaidi A, Reza T, Yahya Y, et al. Chlorhexidine vaginal and infant wipes to reduce perinatal mortality and morbidity: a randomized controlled trial. Obstetrics & Gynecology 2010;115(6):1225‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Madhi 2006 {published data only}
- Madhi S. Prevention of perinatal sepsis (PoPS): evaluation of chlorhexidine wipes of birth canal and newborn. www.clinicaltrials.gov (accessed 21 March 2006).
Moss 2006 {published data only}
- Moss N. Chlorhexidine vaginal and infant wash in Pakistan. www.clinicaltrials.gov (accessed 21 March 2006).
Additional references
Bergqvist‐Karlsson 1988
- Bergqvist‐Karlsson A. Delayed and immediate type hypersensitivity to chlorhexidine. Contact Dermatitis 1988;18:84‐8. [DOI] [PubMed] [Google Scholar]
Bhutta 1997
- Bhutta ZA, Yusuf K. Early onset neonatal sepsis in Pakistan: a case control study of risk factors in a birth cohort. American Journal of Perinatology 1997;14:577‐81. [DOI] [PubMed] [Google Scholar]
Boo 1994
- Boo NY, Chor CY. Six year trend of neonatal septicemia in a large Malaysian maternity hospital. Journal of Paediatrics and Child Health 1994;30:23‐7. [DOI] [PubMed] [Google Scholar]
Cunningham 2001
- Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC III, Hauth JC, Wenstrom KD. Williams Obstetrics. 21st Edition. London: McGraw‐Hill, 2001:675. [Google Scholar]
Davies 1973
- Davies A. The mode of action of chlorhexidine. Journal of Periodontal Research 1973;12(8 Suppl):68‐75. [DOI] [PubMed] [Google Scholar]
Emilson 1977
- Emilson CG. Susceptibility of various microorganisms to chlorhexidine. Scandinavian Journal of Dental Research 1977;85:255‐65. [DOI] [PubMed] [Google Scholar]
Ferretti 1990
- Ferretti GA, Brown AT, Raybould TP Lillich TT. Oral antimicrobial agents ‐ chlorhexidine. National Cancer Institute Monograph 1990; Vol. 9:51‐5. [PubMed]
Gaillard 2001
- Gaillard P, Mwanyumba F, Verhofstede C, Claeys P, Chohan V, Goerghebeur E, et al. Vaginal lavage with chlorhexidine during labour to reduce mother‐to‐child transmission: clinical trial in Mombasa, Kenya. AIDS 2001;15:389‐96. [DOI] [PubMed] [Google Scholar]
GRADE 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.6. The Cochrane Collaboration, 2008.
Hennessey 1973
- Hennessey TS. Some antibacterial properties of chlorhexidine. Journal of Periodontal Research 1973;12(12 Suppl):28‐35. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnsson 1987
- Johnsson J, Seeberg S, Kjellmer I. Blood concentration of chlorhexidine in neonates undergoing routine cord care with 4% chlorhexidine gluconate solution. Acta Paediatrica Scandinavica 1987;76:675‐6. [DOI] [PubMed] [Google Scholar]
Monif 1993
- Monif GRG. Infectious Diseases in Obstetrics and Gynecology. 3rd Edition. Omaha: IDI Publication, 1993. [Google Scholar]
Nilsson 1989
- Nilsson G, Larsson L, Christensen KK, Dykes A. Chlorhexidine for prevention of neonatal colonization with group B streptococci. V. Chlorhexidine concentration in blood following vaginal washing during delivery. European Journal of Obstetrics & Gynecology and Reproductive Biology 1989;31:221‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PubMed: 19839216] [DOI] [PubMed] [Google Scholar]
Stade 2008
- Stade BC, Shah V, Ohlsson A. Vaginal chlorhexidine to prevent early onset neonatal group B streptococcal infection. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD003520] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stray‐Pedersen 1999
- Stray‐Pedersen B, Bergan T, Hafstad A, Normann E, Grogaad J, Vangdal M. Vaginal disinfection with chlorhexidine during childbirth. International Journal of Antimicrobial Agents 1999;12(3):245‐51. [DOI] [PubMed] [Google Scholar]
Sweeten 1997
- Sweeten KM, Eriksen NL, Blanco JD. Chlorhexidine versus sterile water vaginal washing during labor to prevent peripartal infection. American Journal of Obstetrics and Gynecology 1997;176:426‐30. [DOI] [PubMed] [Google Scholar]
Vorherr 1984
- Vorherr H, Vorherr UF, Mehta P, Ulrich JA, Messer RH. Antimicrobial effect of chlorhexidine and povidone‐iodine on vaginal bacteria. Journal of Infection 1984;8(3):195‐9. [DOI] [PubMed] [Google Scholar]
Wiysonge 2005
- Wiysonge CS, Shey MS, Shang JD, Sterne JAC, Brocklehurst P. Vaginal disinfection for preventing mother‐to‐child transmission of HIV infection. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003651.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lumbiganon 2004
- Lumbiganon P, Thinkhamrop J, Thinkhamrop B, Tolosa JE. Vaginal chlorhexidine during labour for preventing maternal and neonatal infections (excluding Group B Streptococcal and HIV). Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004070.pub2] [DOI] [PubMed] [Google Scholar]


