Abstract
Background
The use of conventional cardiotocographic (CTG) monitoring of fetal well‐being during labour is associated with an increased caesarean section rate, compared with intermittent auscultation of the fetal heart rate, resulting in a reduction in neonatal seizures, although no differences in other neonatal outcomes. To improve the sensitivity of this test and therefore reduce the number of caesarean sections performed for nonreassuring fetal status, several additional measures of evaluating fetal well‐being have been considered. These have demonstrated some effect on reducing caesarean section rates, for example, fetal scalp blood sampling for pH estimation/lactate measurement. The adaptation of pulse oximetry for use in the unborn fetus could potentially contribute to improved evaluation during labour and therefore lead to a reduction in caesarean sections for nonreassuring fetal status, without any change in neonatal outcomes.
Objectives
To compare the effectiveness and safety of fetal intrapartum pulse oximetry with other surveillance techniques.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 May 2014), contacted experts in the field and searched reference lists of retrieved studies. In previous versions of this review, we performed additional searches of MEDLINE, Embase and Current Contents. These searches were discontinued for this review update, as they consistently failed to identify any trials that were not shown in the Cochrane Pregnancy and Childbirth Group's Trials Register.
Selection criteria
All published and unpublished randomised controlled trials that compared maternal and fetal outcomes when fetal pulse oximetry was used in labour, (i) with or without concurrent use of conventional fetal surveillance, that is, cardiotocography (CTG), compared with using CTG alone or (ii) with or without concurrent use of both CTG and other method(s) of fetal surveillance, such as fetal electrocardiography (ECG) plus CTG.
Data collection and analysis
At least two independent review authors performed data extraction. We sought additional information from the investigators of three of the reported trials.
Main results
We included seven published trials: six comparing fetal pulse oximetry and CTG with CTG alone (or when fetal pulse oximetry values were blinded) and one comparing fetal pulse oximetry plus CTG with fetal ECG plus CTG. The published trials, with some unpublished data, were at high risk of bias in terms of the impractical nature of blinding participants and clinicians, as well as high risk or unclear risk of bias for outcome assessor for all but one report. Selection bias, attrition bias, reporting bias and other sources of bias were of low or unclear risk. The trials reported on a total of 8013 pregnancies. Differing entry criteria necessitated separate analyses, rather than meta‐analysis of all trials.
Systematic review of four trials from 34 weeks not requiring fetal blood sampling (FBS) prior to study entry showed no evidence of differences in the overall caesarean section rate between those monitored with fetal oximetry and those not monitored with fetal pulse oximetry or for whom the fetal pulse oximetry results were masked (average risk ratio (RR) 0.99 using random‐effects, 95% confidence intervals (CI) 0.86 to 1.13, n = 4008, I² = 45%). There was evidence of a higher risk of caesarean section in the group with fetal oximetry plus CTG than in the group with fetal ECG plus CTG (one study, n = 180, RR 1.56, 95% CI 1.06 to 2.29). Neonatal seizures and neonatal encephalopathy were rare in both groups. No studies reported details of long‐term disability.
There was evidence of a decrease in caesarean section for nonreassuring fetal status in the fetal pulse oximetry plus CTG group compared to the CTG group, gestation from 34 weeks (average RR (random‐effects) 0.65, 95% CI 0.46 to 0.90, n = 4008, I² = 63%). There was no evidence of differences between groups in caesarean section for dystocia, although the overall incidence rates varied between the trials.
Authors' conclusions
The addition of fetal pulse oximetry does not reduce overall caesarean section rates. One study found a higher caesarean section rate in the group monitored with fetal pulse oximetry plus CTG, compared with fetal ECG plus CTG. The data provide limited support for the use of fetal pulse oximetry when used in the presence of a nonreassuring CTG, to reduce caesarean section for nonreassuring fetal status. A better method than pulse oximetry is required to enhance the overall evaluation of fetal well‐being in labour.
Plain language summary
Fetal pulse oximetry for fetal assessment in labour
Using fetal pulse oximetry to assess the baby's well‐being during labour does not change overall caesarean section rates.
During labour, the well‐being of the baby can be assessed intermittently using a Pinard stethoscope or hand‐held monitor to listen to the heart rate, or continuously using cardiotocography (CTG), sometimes called electronic fetal monitoring (EFM). There are also additional tests that can be used if the baby is thought to be getting short of oxygen, like testing the baby's blood in a sample taken from the baby's head or bottom, or through the recording of the electrical activity of the heart using an electrocardiogram (ECG). Fetal pulse oximetry measures how much oxygen the baby's blood is carrying. It uses a probe that sits on the baby's head whilst in the uterus and vagina during labour. The probe is said not to interfere with the woman's mobility during labour. This review looked at fetal pulse oximetry and found trials that used it in conjunction with a CTG. We compared the outcomes for this combined oximetry and CTG, with outcomes where only the CTG had been used, or a combination of CTG and fetal ECG had been used.
The review identified seven trials involving 8013 women. Fetal pulse oximetry plus CTG showed no difference in caesarean section rates overall, nor any difference in the mother's or newborn's health, compared with CTG alone. If there was concern about the baby's well‐being before the fetal pulse oximetry probe was placed, the use of fetal pulse oximetry reduced caesarean sections performed for the baby's well‐being. The one trial of oximetry with CTG compared with CTG and fetal ECG showed an increase in the caesarean rate in the oximetry group. In two of the trials, the company making the fetal pulse oximetry machines provided some funding. A better method than fetal pulse oximetry is needed for checking on the well‐being of the baby during labour.
Background
Description of the condition
Cardiotocography (CTG) was introduced in the 1960s with the aim of improving neonatal outcomes by improving intrapartum fetal surveillance. The uterine contractions and the fetal heart rate, variability, decelerations and accelerations influence the way these patterns are classified. Terms in use include normal, reassuring, nonreassuring, indeterminate, suspicious, abnormal, pathological and preterminal (ACOG 2001; FIGO 1987; NICE 2007; RANZCOG 2014). In this review, we refer to the terms reassuring, nonreassuring or abnormal. Reassuring patterns require no specific action. Nonreassuring patterns occur in about 15% to 19% of labours monitored by CTG (East 2006; Umstad 1993) and may prompt clinical actions ranging from simple manoeuvres, such as a change of maternal position, through to expedited birth of the baby (vacuum, forceps, caesarean section). Abnormal patterns usually prompt expedited birth with the aim of preventing or minimising hypoxia in the fetus. The positive predictive value of CTG for adverse outcome is low and the negative predictive value high (Nonnenmacher 2010), although this is improving with computerised interpretation of CTGs (Costa 2010). Thus, while a normal CTG usually indicates reassuring fetal status, a nonreassuring or abnormal CTG does not necessarily equate with 'fetal distress'. These features, combined with marked inter‐observer variation in CTG interpretation by midwives (Devane 2005) and doctors (Palomaki 2006), result in variable but inappropriately high operative birth rates for nonreassuring fetal status in many hospitals. Electronic fetal monitoring rapidly gained widespread acceptance for monitoring the fetal heart rate during labour, but it was not until the 1970s that randomised controlled trials were conducted to assess the benefits of this technology. A Cochrane systematic review found that the use of electronic monitoring increased the odds of having a caesarean section, compared to intermittent auscultation of the fetal heart (Alfirevic 2013). Despite these shortcomings, cardiotocography remains a widely used means of assessing fetal well‐being during labour. One conclusion of the systematic review of CTG monitoring was to consider how best to convey the uncertainty of the benefits of such monitoring to enable women to make an informed choice, while not compromising labour normality (Alfirevic 2013). The National Institute for Health and Clinical Excellence (NICE 2007) suggested that, as for all aspects of care, the woman herself should be involved in decision‐making for choice of fetal monitoring, with adequate access to evidence‐based information; and recommended that electronic monitoring be offered where there is an increased risk of perinatal death, neonatal encephalopathy or cerebral palsy, and during labours induced or augmented by oxytocin.
Once a nonreassuring fetal heart rate pattern has been identified, a number of additional assessments of fetal well‐being may be considered. These do not replace the CTG, but are usually used as complementary to it, either intermittently or continuously. One example is fetal scalp blood sampling for pH or lactate analysis. A low pH (for example, less than 7.20) or a high lactate (for example greater than 4.8 mmol/L) may be considered abnormal (Kruger 1999). The addition of fetal scalp blood sampling to standard electronic monitoring reduces the odds for caesarean section, although the odds are not significantly different compared to intermittent auscultation of the fetal heart (Alfirevic 2013). Another example is fetal electrocardiogram (ECG), which measures fetal ST interval and the changes in the T/QRS ratio. An elevation of the ST segment and the ratio between the T wave and QRS amplitudes (T/QRS), identifies fetal anaerobic myocardial metabolism (Rosėn 2004). A Cochrane systematic review of the addition of fetal electrocardiogram monitoring reported no evidence of a difference in overall caesarean section rate when compared to electronic monitoring only (Neilson 2013). Dokus 2013 considered the potential impact of the declining clinical use of fetal ECG and fetal pulse oximetry (described below), noting that the overall caesarean section rate increased when either fetal ECG or fetal pulse oximetry were no longer available for use (total n = 13,413). Cochrane systematic reviews of vibroacoustic stimulation (VAS) or fetal scalp blood sampling for lactate measurement as additional fetal assessments in labour were unable to identify randomised controlled trials that compared these interventions with no intervention (East 2010; East 2013).
Description of the intervention
Fetal pulse oximetry aims to improve the accuracy of the evaluation of fetal well‐being during labour (Colditz 1999; Coldtiz 2013; East 2007a). It is generally reserved for use when a nonreassuring CTG has been recorded, to assist in identifying those fetuses that may benefit from further intervention (East 2002; East 2008) and as an adjunct to, rather than replacement of, the CTG monitor. This method has two potential advantages over conventional fetal heart rate monitoring: (i) it directly measures the proportion of haemoglobin that is carrying oxygen: thus, oxygenation, the primary variable underlying the tissue damaging effects of hypoxia/ischaemia is being monitored; and (ii) it relies on an established, safe, noninvasive, widely‐used technology found in every modern intensive care unit and operating theatre. Inaccurate oxygen saturation readings can occur with conditions that decrease arterial blood perfusion, for instance, they can occur with venous pulsations, excessive movement, intravenous pigmented dyes, and abnormal haemoglobin (Chan 2013). A variety of fetal pulse oximetry sensors has been studied. These are placed during a vaginal examination to attach to the top of the fetal head by suction (Arikan 2000) or clip (Knitza 2004), lie against the fetal temple or cheek (Mallinckrodt 2000; Nellcor 2004), or to lie along the fetal back (Prothia 2014). The sensor remains in situ and fetal pulse oximetry values are recorded for approximately 81% of the monitoring time (East 1997). Women have rated their experience with fetal oximetry during observational studies. One survey included questions about the woman's perceived level of comfort during sensor placement, mobility with the sensor in place and ongoing comfort with the sensor in place: these factors were all rated favourably by the women (East 1996). Arikan 1998 reported that the majority of women did not consider that a fetal oximetry sensor restricted their movement during labour.
How the intervention might work
Results of animal and human research suggest that when using sensors calibrated for the fetal environment, fetal oximetry values greater than or equal to 30% are considered reassuring, even when the CTG is nonreassuring, while values less than 30% warrant consideration of interventions, ranging from maternal position change, through to urgent birth via caesarean section (Kuhnert 1998; Nijland 1995; Seelbach‐Gobel 1999). A prospective, observational cohort study investigating relationship between oxygen saturation using pulse oximetry and umbilical cord arterial pH values in healthy newborns during the first 15 minutes of life found a significant correlation between both preductal and postductal oxygen saturation levels and umbilical arterial blood pH values (Uslu 2012). A prospective observational study found a low pulse oximetry oxygen saturation < 30% for at least 10 minutes correlates highly with fetal acidosis in cases of nonreassuring fetal heart rate (Nonnenmacher 2010). A novel fetal phantom based on actual fetal parameters showed that the wireless oximeter was capable of identifying 4% and 2% changes in diameter between the diastolic and systolic point in arteries of over 0.2 and 0.4 mm inner diameter, respectively (Stubán 2009). One manufacturer recommends this technology for singleton pregnancies only (Nellcor 2004). Consideration for monitoring multiple pregnancies by monitoring the first fetus during labour, then the second or subsequent fetuses following birth of the preceding fetus may be possible.
Why it is important to do this review
The value of any fetal monitoring system during labour, including the CTG or any additional surveillance, is usually expressed by its ability to predict which fetuses are hypoxic or acidotic. Measures of this may include umbilical cord blood gases (including base excess values less than or equal to 12 mmol/L and pH values less than 7.00 (Sehdev 1997), or less than 7.10 (Arikan 2000) or lactate values > 6.1 mmol/L (White 2010); or clinical outcomes including Apgar scores (an assessment of neonatal condition shortly after birth, usually at one and five minutes: Apgar scores of less than seven at five minutes or later are nonspecific but may be associated with hypoxia (MacLennan 1999; Sehdev 1997)); or abnormal neurological status of the baby, possibly caused by lack of oxygen or blood supply (hypoxic‐ischaemic encephalopathy), or both. Other outcomes of interest may include fetal/maternal infections, for example of the membranes (chorioamnionitis), or the uterine lining (endometritis). Interventions resulting from such tests are also important. For example, it is important to note not only overall modes of birth following different forms of monitoring, but also specific interventions, such as operative birth (vacuum, forceps and caesarean section) for the indication of nonreassuring fetal status, since nonreassuring fetal status is what the monitoring is intended to discern. In the longer term, such interventions may also impact on future pregnancies. For example, the likelihood of a successful vaginal birth after caesarean (VBAC) in a subsequent pregnancy is improved for women whose previous caesarean was performed for the indication of nonreassuring fetal status, compared with those where the previous caesarean was performed for dystocia (Grinstead 2004; Shipp 2000). Successful VBAC in a subsequent pregnancy will also have economic benefits, with vaginal births costing the health system considerably less than caesarean sections (Henderson 2001; Petrou 2002).
This review was undertaken to evaluate the clinical effectiveness and safety of fetal pulse oximetry to assess fetal well‐being in labour.
Objectives
To compare the effectiveness and safety of fetal intrapartum pulse oximetry with conventional fetal surveillance techniques, using the results of randomised controlled trials.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished individual‐ or cluster‐randomised and quasi‐randomised trials with reported data that compared maternal and fetal/neonatal/infant outcomes when fetal pulse oximetry was used in labour, with or without concurrent use of conventional fetal surveillance, compared with the use of conventional fetal surveillance techniques alone.
Cross‐over studies are unlikely to be appropriate for testing this intervention and therefore would be excluded if identified. We also excluded studies that are only available in abstract form.
Types of participants
Women in labour with a live baby where fetal monitoring is clinically indicated.
Types of interventions
Use of fetal pulse oximetry compared with not using fetal pulse oximetry, with or without concurrent use of conventional fetal monitoring (fetal heart rate monitoring by intermittent auscultation, intermittent/continuous cardiotocography, fetal electrocardiography [added in this review update], or fetal blood sampling (FBS) for blood gas analysis).
Types of outcome measures
Primary outcomes
(1) Caesarean section (2) Hypoxic‐ischaemic encephalopathy (3) Neonatal seizures (4) Long‐term neurodevelopmental outcome
Secondary outcomes
Maternal
(5) Caesarean section for nonreassuring fetal status (6) Caesarean section for dystocia (added since the protocol and original review were first published) (7) Overall operative birth (caesarean section, forceps, vacuum extraction) for all indications (8) Overall operative birth (caesarean section, forceps, vacuum extraction) for nonreassuring fetal status (9) Use of intrapartum antibiotics (10) Overall antibiotic use (11) Intrapartum haemorrhage (12) Postpartum haemorrhage (13) Chorioamnionitis (14) Endometritis (added since the protocol was first published) (15) Uterine rupture (16) Length of hospital stay (17) Satisfaction with labour (18) Satisfaction with fetal monitoring in labour (19) Death
Fetal/neonatal
(20) Skin trauma (21) Apgar scores less than four at five minutes (22) Apgar scores less than seven at five minutes (23) Umbilical arterial pH less than 7.10 (24) Umbilical arterial base excess less than ‐12 (25) Admission to neonatal intensive care unit (26) Length of hospital stay (27) Death (28) Death, hypoxic‐ischaemic encephalopathy, or both (29) Death, seizures, or both (30) Death, long‐term neurodevelopmental problem, or both
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (31 May 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In previous editions of this review, we searched MEDLINE, Embase and Current Contents. The Cochrane Pregnancy and Childbirth Group’s Trials Register reliably records all trials that would have been identified in these additional databases. We have therefore only searched the Cochrane Pregnancy and Childbirth Group’s Trials Register for this update. See:Appendix 1 for the search strategy used in previous editions of this review.
Searching other resources
We also sought ongoing and unpublished trials by contacting experts in the field.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeEast 2007.
For this update we used the following methods when assessing the reports identified by the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy ‐ all authors participated in these assessments for the range of studies identified, with two allocated per study. We resolved any disagreements through discussion. If it had been required, we would have consulted a third person.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form ‐ all authors participated in data extraction for the range of included studies, with two allocated per study. We resolved discrepancies through discussion. If required, we planned to consult a third person. We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). All authors participated in assessment of risk of bias of the range of included studies, with two allocated per study. We resolved any disagreement by discussion. Had it been required, we would have involved a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it produced comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
As per the original protocol, we made an a priori decision to exclude trials where outcome data were unavailable for more than 20% of participants.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis. Overall findings from our assessment of risk of bias in the included studies are provided in Figure 1 and Figure 2.
1.
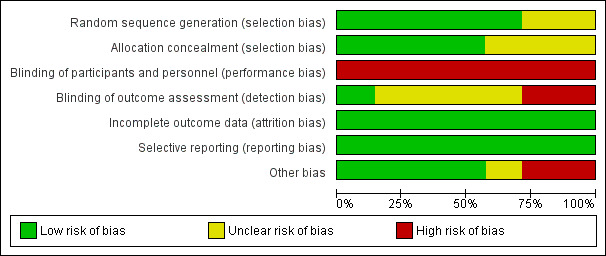
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
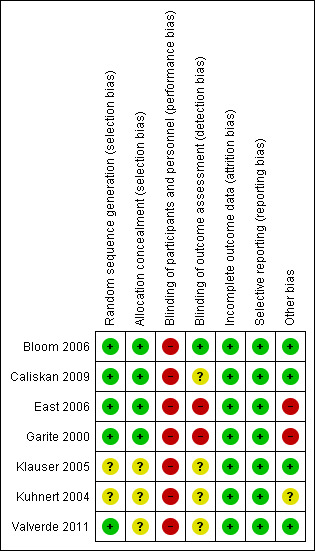
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference with 95% confidence intervals. If necessary, we planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials identified in the searches in the analyses along with individually‐randomised trials. If cluster‐randomised trials are included in future updates, we will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledged heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
It is unlikely that cross‐over designs will be a valid study design for Pregnancy and Childbirth reviews, and so will be excluded if they are identified in future updates of this review.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by considering using sensitivity analysis, although this was not ultimately necessary.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
We made an a priori decision in the original protocol to exclude trials where outcome data were unavailable for more than 20% of participants.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Had there been 10 or more studies in the meta‐analysis, we planned to investigate reporting biases (such as publication bias) using funnel plots. In future review updates where 10 or more studies are included, we will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
1. Fetal heart rate monitoring by:
intermittent auscultation;
intermittent cardiotocography;
continuous cardiotocography and fetal scalp stimulation;
continuous cardiotocography and fetal ECG analysis (ST segment);
continuous cardiotocography and fetal ECG analysis (PR interval).
2. Fetal scalp blood sampling for blood gas analysis or lactate measurement (performed after randomisation).
The primary outcomes were used in subgroup analysis.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2012). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We performed sensitivity analyses on the primary outcomes where we considered that an aspect of the review, such as risk of bias associated with the quality of some of the included trials, could have affected the results, in particular where there was a high level of statistical heterogeneity. This was applied by creating subgroups based on the different study entry criteria (see Data analysis considerations in the Results section).
Results
Description of studies
The search identified seven published randomised controlled trials (Bloom 2006; Caliskan 2009; East 2006; Garite 2000; Klauser 2005; Kuhnert 2004; Valverde 2011 (the latter added in the 2014 review update)), and two observational studies (Andres 2004; Golaszewski 1993). The trial by Garite 2000 had also been published in a number of forms and sub analyses addressing issues that were not considered in this review. Similarly, the trials by East 2006, Bloom 2006 and Valverde 2011 had several related publications (one of which had only been available as a conference abstract in the 2010 update of this review), some of which were considered in this review and were added with this update.
We found no unpublished studies.
Trials with nonreassuring fetal status not required prior to study entry
Bloom 2006 reported a multicentre trial conducted in the USA (n = 5341), which enrolled nulliparous women with CTG monitoring in labour. All participants had a fetal pulse oximetry sensor placed and were then randomly allocated to the 'open' arm with fetal pulse oximetry values displayed or the 'masked' arm with fetal pulse oximetry values stored to computer disk and not displayed to the woman or clinician. These results were analysed separately from the other studies, as the study population, labouring women with a CTG, could not be considered in the same manner as those with a nonreassuring CTG (see below). The report included limited outcomes for a separate analysis of those with a nonreassuring CTG prior to study entry.The study reported by Caliskan 2009 enrolled women from 34 weeks' gestation undergoing induction of labour by oral misoprostol in Turkey. All participants had misoprostol administered and were then randomised to either intermittent fetal pulse oximetry + electronic fetal monitoring, or electronic fetal monitoring only.
Trials with nonreassuring fetal status required prior to study entry
The trial published by Garite 2000 was conducted in the United States of America (USA) and compared caesarean section rates for nonreassuring fetal status when conventional fetal monitoring (CTG) was used, versus when fetal pulse oximetry was used in addition to CTG, with reported data on 1010 cases. An unpublished report included some pilot data for a total of 1189 cases.
Kuhnert 2004 reported a trial from Germany that compared operative birth and fetal scalp blood sampling for nonreassuring fetal status in two groups: those with CTG monitoring and those with fetal pulse oximetry added to the CTG, for a total of 146 cases. Fetal blood sampling (FBS) was required prior to study entry. Whilst not stated in the report, it is appropriate to consider that if the scalp pH was nonreassuring, intervention would have been undertaken to correct this or to deliver the baby prior to enrolment in the study. It can therefore be considered that this represents, at least in part, a different study population to that of the other studies.
A single‐centre trial from the USA, reported by Klauser 2005, included 327 women with gestation from 28 weeks onward. This study compared caesarean birth for nonreassuring fetal status in women with and without fetal pulse oximetry added to CTG monitoring (Klauser 2005). Interpretation of fetal heart rate monitoring is different in premature babies, compared with term babies. The report did not allow the reader to distinguish outcomes by gestational age. It may therefore be appropriate to consider that this represents a heterogenous population. This would make subsequent combination with other trials inappropriate. We were unable to contact the authors to consider analysis by gestation.
An Australian multicentre trial compared operative birth for nonreassuring fetal status in those with and without fetal pulse oximetry added to CTG monitoring (East 2006) on 600 pregnancies.
The trial reported by Bloom 2006 included 2168 women with a nonreassuring CTG at the time of study entry, of the 5341 enrolled in the study overall (see above).
Valverde 2011 reported a single‐centre trial from Spain that compared operative birth and fetal status in 180 women with nonreassuring CTG. Women were randomised to either fetal pulse oximetry plus CTG, or fetal ECG plus CTG.
SeeCharacteristics of included studies table.
Data analysis considerations
The trial by Bloom 2006 involved fetal pulse oximetry and cardiotocography in both of the study groups, with one group having the oximetry results displayed for clinical use and the other group having the oximetry results masked. For the purposes of this meta‐analysis, the 'masked' group of this trial has been treated in this review as 'cardiotocography‐only', since the fetal pulse oximetry values did not influence clinical decisions.
All trials were included, where outcome data were reported, in the meta‐analysis to allow a comprehensive representation of the findings. The use of a summary measure of effect for some combinations of trials was appropriate. However, we did not use a summary measure of effect for combining all trials, as the appropriateness of combining studies with differing entry criteria and significant heterogeneity if separate analyses were not used, remained uncertain. For example, we created subcategories within analyses based on differing study entry characteristics/requirements: (i) gestation from 34 weeks, FBS not required prior to study entry; (ii) gestation from 36 weeks, FBS prior to study entry; and (iii) gestation from 28 weeks, FBS not required prior to study entry. We then reported the summary effect for each subgroup, rather than a combined summary effect for all studies. The subcategories could have been considered in terms of subgroup analyses, although the different study entry characteristics were not specified as subgroups a priori in the original protocol. The findings as presented in subcategories provide a good measure of clinical realities, although the possibility of converting these to subgroups may be considered in a future update of this review.
Inclusion of the trial reported by Valverde 2011 in the 2014 review update prompted careful deliberations related to whether or not to consider the fetal ECG + CTG group in the same manner as the CTG‐only group used for the remaining studies.The latter may be a reasonable judgement, given that there is a lack of evidence of effect of adding fetal ECG, on caesarean section rates (Neilson 2013). Adding the Valverde 2011 findings to the main analysis of the primary outcome, caesarean section, did not change the overall direction of the summary effect, although the I2 test result did increase from 45% to 61%, making it likely that this heterogeneity was of some importance. In support of treating fetal ECG separately, withdrawal of fetal pulse oximetry and fetal ECG from a clinical service, as compared with their use in a research setting, has been reported to influence a rise in caesarean sections (Dokus 2013). On the balance of these considerations, a decision was made to create distinct comparison analyses for fetal ECG, rather than include the findings from Valverde 2011 in the initial meta‐analysis and then attempt subgroup analysis.
Excluded studies
The two observational studies identified in the search were excluded (Andres 2004; Golaszewski 1993). See Characteristics of excluded studies table.
Risk of bias in included studies
In all studies, the fetal oximetry values in the intervention group were used to guide clinical practice, thus making it impractical to blind either the participant or the clinician. Two studies (East 2006; Garite 2000) disclosed funding support from the manufacturers of the fetal oximetry system used in those studies (high risk). Only one study (Bloom 2006) reported that outcome assessors were blinded to group allocation, while the remainder included sufficient detail to determine that outcome assessment was unblinded (East 2006; Garite 2000) and therefore high risk, or did not specify this information (unclear risk, Caliskan 2009; Klauser 2005; Kuhnert 2004; Valverde 2011). These elements of risk may suggest the need for caution in over‐interpretation of the findings (Figure 1; Figure 2).
Allocation
Sufficient evidence of random sequence generation and allocation concealment were provided in the reports by Bloom 2006; Caliskan 2009; East 2006; and Garite 2000 to rate the risk of bias as low. The report by Valverde 2011 indicated that sealed, opaque envelopes were used, but we were unable to confirm whether or not these were sequentially numbered, thus rating this as unclear risk of bias. Klauser 2005 and Kuhnert 2004 did not report methods of randomisation and allocation concealment (unclear risk).
Blinding
In all studies (Bloom 2006; Caliskan 2009; East 2006; Garite 2000; Klauser 2005; Kuhnert 2004; Valverde 2011), blinding of the participants or clinicians (performance bias) was not feasible given that FSpO2 values were used for clinical judgement. The 'masked' group in the study by Bloom 2006 meant that the labouring woman and clinicians were blinded to fetal oximetry values, although both the women and clinicians were aware of group allocation. The overall risk of bias for these studies was therefore high for blinding of participants and clinicians.
Outcome assessment (detection bias) of the study reported by Bloom 2006 was conducted by staff who were unaware of group allocation, giving it a rating of low risk. All remaining studies and their outcomes were assessed either unblinded (and therefore high risk, East 2006; Garite 2000) or blinding was not specified (and therefore unclear risk, Caliskan 2009; Klauser 2005; Kuhnert 2004; Valverde 2011). A blinded outcome assessor analysis was performed for a post hoc analysis of partograms in the study by Garite 2000, conducted to demonstrate progress in labour for all cases of dystocia (defined) and failed induction of labour (defined).
Incomplete outcome data
All participants acknowledged to have been enrolled in the seven published studies were accounted for, suggesting that there was a low risk of bias for outcome data. This could be confirmed for three studies that had protocols available prior to or during the trial conduct (Bloom 2006; East 2006; Garite 2000).
Selective reporting
The availability of the trial protocols prior to or during the studies by Bloom 2006; East 2006; Garite 2000 provided evidence of a low risk of bias for selective reporting. There is no evidence to support any concerns of reporting bias in the remainder of the studies (Caliskan 2009; Klauser 2005; Kuhnert 2004; Valverde 2011).
Other potential sources of bias
Two studies had elements of high risk of other sources of bias, in so far as the study by Garite 2000 was funded by the manufacturer of the fetal pulse oximetry used in the trial and funding from this manufacturer also contributed to overall funds for the trial reported by East 2006. The large difference in findings from the study reported by Kuhnert 2004 to those reported in the remaining studies raises the unconfirmed possibility of unclear risk of bias. There was no evidence to suggest other potential sources of bias in the remaining studies.
Effects of interventions
We included seven trials involving 8013 participants in this review. Findings from one new trial, comparing fetal pulse oximetry plus CTG and fetal ECG plus CTG and involving 180 participants, was included in this 2014 update of the review ( Valverde 2011).
Primary outcomes
Where meta‐analysis was possible, findings from five of the seven trials resulted in no significant differences in the overall caesarean section rate between those monitored with fetal oximetry and those not monitored with fetal pulse oximetry or for whom the fetal pulse oximetry results were masked (four studies, n = 4008, summary risk ratio (RR) using random‐effects, 0.99, 95% confidence intervals (CI) 0.86 to 1.13, I² = 45, Analysis 1.1). A smaller study for which FBS was required prior to study entry (n = 146) reported a significant decrease in caesarean section in the fetal oximetry group, compared with the control group (Analysis 1.1; Kuhnert 2004). The risk of overall caesarean section rate for those monitored with fetal pulse oximetry and CTG was higher than for those monitored with fetal ECG and CTG (one study, n = 180, RR 1.56, 95% CI 1.06 to 2.29, Analysis 5.1; Valverde 2011). Hypoxic ischaemic encephalopathy was reported in only one case, in the masked group of the study by Bloom 2006 and generally not reported at all in other studies (Analysis 1.2). Few studies reported on neonatal seizures, with only one case reported in the control group of the trial by Garite 2000 and one clinical case in the intervention group of the trial by East 2006 (Analysis 1.3). No studies reported details of assessment of long‐term disability.
1.1. Analysis.
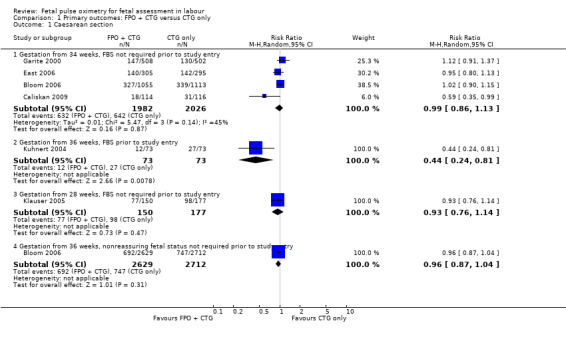
Comparison 1 Primary outcomes: FPO + CTG versus CTG only, Outcome 1 Caesarean section.
5.1. Analysis.
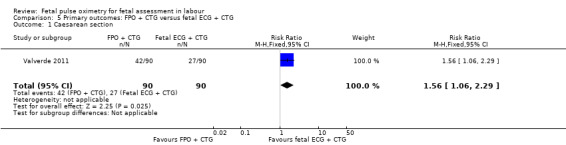
Comparison 5 Primary outcomes: FPO + CTG versus fetal ECG + CTG, Outcome 1 Caesarean section.
1.2. Analysis.
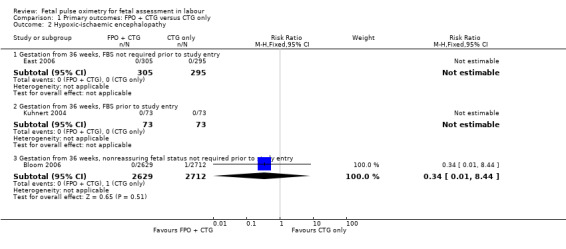
Comparison 1 Primary outcomes: FPO + CTG versus CTG only, Outcome 2 Hypoxic‐ischaemic encephalopathy.
1.3. Analysis.
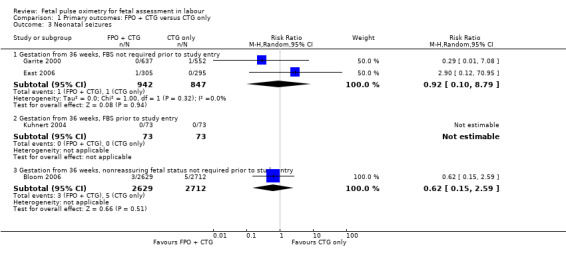
Comparison 1 Primary outcomes: FPO + CTG versus CTG only, Outcome 3 Neonatal seizures.
Secondary outcomes: maternal
There was evidence of a significant decrease in caesarean section for nonreassuring fetal status in the fetal pulse oximetry plus CTG group compared to the CTG group in two of the four analyses: (i) gestation from 34 weeks with FBS not required prior to study entry (four studies, n = 4008, average RR 0.65 using random‐effects, 95% CI 0.46 to 0.90, I² = 63%); and (ii) when FBS was required prior to study entry (one study, n = 146, average RR 0.03, 95% CI 0.00 to 0.44, Analysis 2.1). There was a statistically significant decrease in operative birth (caesarean section, forceps or vacuum birth) for nonreassuring fetal status when fetal pulse oximetry was added to CTG monitoring, compared with CTG alone (FBS not required prior to study entry, two studies, n = 1610, summary RR 0.74, 95% CI 0.62 to 0.89, (Analysis 2.4). There was a large decrease in the oximetry group for this outcome in the one study (n = 146) where FBS was required prior to study entry (RR 0.05, 95% CI 0.01 to 0.22), (Kuhnert 2004).
2.1. Analysis.
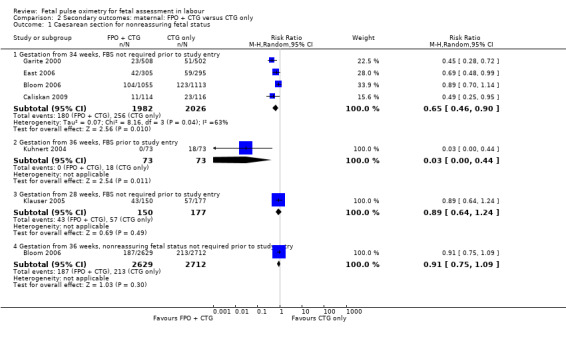
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 1 Caesarean section for nonreassuring fetal status.
2.4. Analysis.
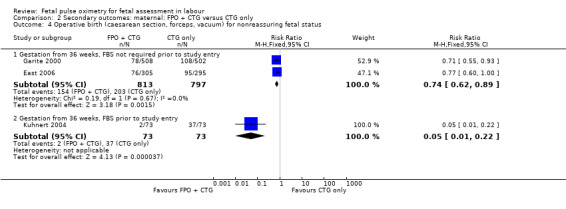
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 4 Operative birth (caesarean section, forceps, vacuum) for nonreassuring fetal status.
There was no evidence of a difference in caesarean section for dystocia when fetal pulse oximetry (fetal pulse oximetry) was added to CTG monitoring, compared with CTG monitoring alone (Analysis 2.2).
2.2. Analysis.
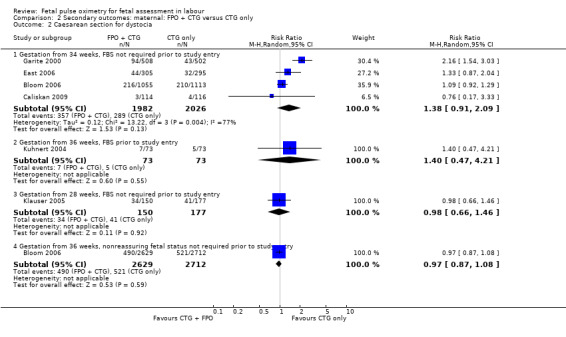
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 2 Caesarean section for dystocia.
The addition of fetal pulse oximetry to CTG monitoring resulted in no evidence of differences for overall operative birth rates (with the exception of the smaller study reported by Kuhnert 2004), endometritis, intrapartum haemorrhage, postpartum haemorrhage, chorioamnionitis, endometritis, uterine rupture, length of hospital stay, satisfaction with labour or satisfaction with fetal monitoring in labour, compared to CTG only. No maternal deaths occurred. The small study by Kuhnert 2004 reported less antibiotic use in the fetal pulse oximetry group, compared with the CTG group.
Women reported similar levels of satisfaction with their labour and fetal monitoring when fetal pulse oximetry was added to CTG monitoring, compared to CTG monitoring alone (East 2006, Analysis 2.13; Analysis 2.14).
2.13. Analysis.
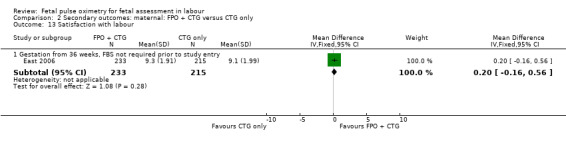
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 13 Satisfaction with labour.
2.14. Analysis.
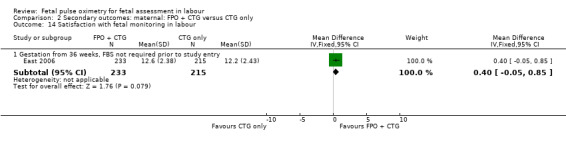
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 14 Satisfaction with fetal monitoring in labour.
The study by Valverde 2011 (n = 180), demonstrated a statistically significant increase in caesarean section for nonreassuring fetal status comparing fetal pulse oximetry plus CTG with fetal ECG plus CTG (RR 1.71, 95% CI 1.01 to 2.88, Analysis 6.1), but not in caesarean section performed for dystocia (RR 1.30, 95% CI 0.60 to 2.81, Analysis 6.2). There was also a statistically significant increase comparing fetal pulse oximetry plus CTG with fetal ECG plus CTG in overall operative birth (RR 1.20, 95% CI 1.00 to 1.45, Analysis 6.3) but not in overall operative birth for nonreassuring fetal status (RR 1.22, 0.88 to 1.70, Analysis 6.4).
6.1. Analysis.
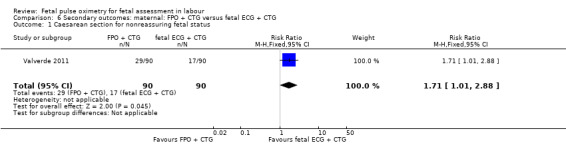
Comparison 6 Secondary outcomes: maternal: FPO + CTG versus fetal ECG + CTG, Outcome 1 Caesarean section for nonreassuring fetal status.
6.2. Analysis.
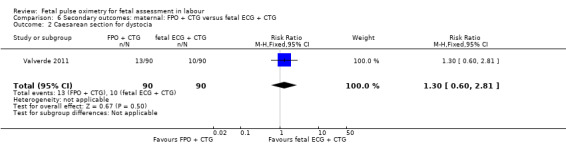
Comparison 6 Secondary outcomes: maternal: FPO + CTG versus fetal ECG + CTG, Outcome 2 Caesarean section for dystocia.
6.3. Analysis.
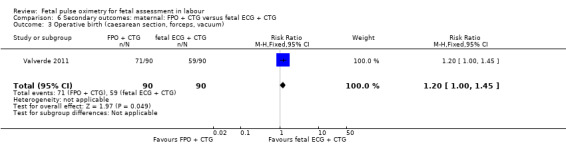
Comparison 6 Secondary outcomes: maternal: FPO + CTG versus fetal ECG + CTG, Outcome 3 Operative birth (caesarean section, forceps, vacuum).
6.4. Analysis.
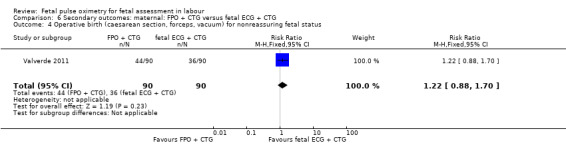
Comparison 6 Secondary outcomes: maternal: FPO + CTG versus fetal ECG + CTG, Outcome 4 Operative birth (caesarean section, forceps, vacuum) for nonreassuring fetal status.
Secondary outcomes: fetal/neonatal
No evidence of significant differences was noted for Apgar scores less than four at five minutes or less than seven at five minutes, umbilical arterial pH less than 7.10, umbilical arterial base excess less than ‐12, admission to the neonatal intensive care unit, length of hospital stay, death, or skin trauma. Transient skin markings attributable to the fetal oximetry sensor were noted in 11 of 638 babies (2%) Garite 2000; in 30 of 305 babies (10%) East 2006; and for 152 of 2629 babies (6%) in the open oximetry values group and 155 of 2712 babies (6%) in the masked group Bloom 2006.
The fetal oximetry plus CTG versus fetal ECG plus CTG study by Valverde 2011 did not demonstrate any evidence of between‐group differences in admission to neonatal intensive care unit (n = 180, RR 1.00, 95% CI 0.06 to 15.74), (Analysis 7.1). Umbilical arterial pH data from one study (Valverde 2011) were not in a suitable format for inclusion in the RevMan software: the mean (range) of pH in fetal pulse oximetry group was 7.23 (7.17 to 7.28) and in the fetal ECG group was 7.26 (7.20 to 7.29), which the study authors noted to be a non‐significant difference.
7.1. Analysis.
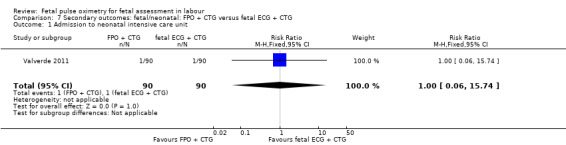
Comparison 7 Secondary outcomes: fetal/neonatal: FPO + CTG versus fetal ECG + CTG, Outcome 1 Admission to neonatal intensive care unit.
Subgroup analyses
Data were available from one trial (East 2006) to allow the planned subgroup analyses of fetal scalp blood sampling post randomisation. There were no significant differences in the primary outcome of caesarean section and no seizures were reported for any of the babies in this subgroup. Data were not available to allow the remaining subgroup analyses to be conducted.
Discussion
Summary of main results
When systematically reviewed, five of the seven published trials (with some unpublished data available), comparing fetal intrapartum pulse oximetry with CTG or fetal electrocardiography or masked fetal pulse oximetry, reported no difference in the overall caesarean section rate between the fetal pulse oximetry group and the CTG group. One smaller study did note a significant difference in favour of the fetal pulse oximetry plus CTG group. A study comparing fetal pulse oximetry plus CTG and fetal electrocardiography plus CTG reported less caesarean section births in the fetal electrocardiography plus CTG group.
Meta‐analysis of the four studies with nonreassuring fetal status from 34 weeks' gestation prior to randomisation demonstrated a reduction in caesarean section for nonreassuring fetal status, with no differences in neonatal outcomes. That is, a decision not to perform a caesarean section for nonreassuring fetal status in the fetal pulse oximetry group did not result in worse outcomes for those babies (but a larger sample would be required to demonstrate a difference in such low‐prevalence outcomes). There were no between‐group differences in caesarean section for nonreassuring fetal status when all participants in the largest study were considered, when analysed without consideration of fetal status at study entry.
The findings from more than 8000 participants provide substantial evidence to suggest that knowledge of fetal pulse oximetry values does not reduce overall caesarean section rates. However, several issues warrant consideration. Firstly, does the indication for caesarean section matter if the overall incidence of caesarean section is the same, given than there is limited support from the findings of this review, for the use of fetal pulse oximetry when used in the presence of a nonreassuring CTG, to reduce caesarean section for nonreassuring fetal status? An additional area of importance is whether or not the presence of a fetal oximetry sensor contributes to dystocia.
Overall completeness and applicability of evidence
The decision pathway leading to performing a caesarean section may be important. The additional information that fetal pulse oximetry can provide, when a nonreassuring fetal heart rate trace has been identified, may translate to avoidance of a caesarean section for nonreassuring fetal status, with its associated stress levels for the mother and resource implications for the health service providers. An 'inevitable' caesarean section may still be performed for other indications, when the woman has had more time to consider her options. Staffing levels can also be adjusted over a number of hours, rather than the immediate and potentially costly provision of staff for an emergency operation. One trial reported that the addition of fetal pulse oximetry to CTG monitoring was cost effective in reducing operative birth for nonreassuring fetal status (East 2006).
Women's reports of satisfaction with their labour and with fetal monitoring were similar when fetal pulse oximetry was added to CTG monitoring, compared to CTG monitoring alone. This is an important consideration, given that the use of technology may impact on women's perceived control over their labour experience (Wagner 2001). Although an ideal study would compare women's satisfaction with fetal pulse oximetry and without any technology, such a study is not feasible. It can be considered, however, that once continuous CTG monitoring is in use during labour, the addition of fetal pulse oximetry technology does not adversely affect women's perceptions of their labour experience or of fetal monitoring overall. Long‐term neurodevelopmental outcome has not been measured.
Quality of the evidence
Overall, the evidence (for the primary outcomes) was of moderate to high quality. The impractical nature of blinding participants and clinicians in the intervention arm of each study was not viewed as something that could be overcome and was consistent across studies, meaning that any impact of this potential bias was essentially the same for each study. The findings from the smallest of the included studies (n = 146, Kuhnert 2004) were considerably more positive for the primary outcome of caesarean section than was noted in any of the remaining studies. This inconsistency in findings is worthy of consideration when interpreting overall results. The addition in this update of another small study (n = 180, Valverde 2011) resulted in an increase in caesarean section rates for those in the fetal pulse oximetry group, compared with CTG plus fetal ECG. Where meta‐analysis was appropriate, there was considerable heterogeneity, even when using random‐effects, meaning that larger sample sizes may be necessary to address the outcomes of interest.
Potential biases in the review process
The authors are not aware of potential biases that have not already been addressed through the rigorous methods adopted in this review in line with those of the Cochrane Pregnancy and Childbirth Group. The search strategy is believed to be robust in its ability to identify all trials. The evaluation of the study that two of the review authors had conducted (CE and PB: East 2006) by an independent author (LB) in the 2007 update of this review aimed to minimise any potential reporting bias for that trial.
Agreements and disagreements with other studies or reviews
European clinicians published guidelines for fetal pulse oximetry use (Kuhnert 1998; Saling 1996) that were consistent with the management of fetal pulse oximetry in Garite 2000 and prior to its results being known. Only two small randomised controlled trials of fetal pulse oximetry have since been reported from Europe to test these guidelines (Kuhnert 2004; Valverde 2011). These trials reported divergent findings (an increase in one and decrease in the other) for overall caesarean section rates.
The American College of Obstetrics and Gynecology (ACOG) reviewed the results of the trial reported by Garite 2000 and recommended further trials before the introduction of fetal pulse oximetry into clinical practice (ACOG 2001). Their recommendation was based mainly on the increase in dystocia reported with the use of fetal pulse oximetry and the potential to increase fetal monitoring costs without improving clinical outcomes (ACOG 2001). One trial reported that the addition of fetal pulse oximetry to cardiotocography was cost effective in reducing operative birth for nonreassuring fetal status (East 2006).
When the findings of the first trials of fetal pulse oximetry became available, there was debate about why the incidence of caesarean section for dystocia more than doubled from 9% in the CTG‐only group to 19% when fetal pulse oximetry was added. The investigators explored several possible causes for the increase in dystocia in the fetal pulse oximetry group, including potential mislabelling of dystocia and the presence of the oximetry sensor slowing the labour (Garite 2000). The authors concluded that mislabelling of the indication for caesarean section had not occurred and the presence of the sensor did not result in a longer labour. They suggested that the nonreassuring CTG may indicate an underlying risk for dystocia (Garite 2000). To test this hypothesis, Porreco 2004 conducted a multicentre, prospective, observational cohort study of fetal pulse oximetry in nulliparous labouring women, with a standardised labour management protocol and a specific focus on the management of dystocia (defined). The investigators concluded that the presence of persistent, progressive and moderate to severely nonreassuring CTGs may predict the need for birth by caesarean section for dystocia, despite adequate fetal oxygenation (Porreco 2004). No other trials in this systematic review demonstrated a difference in caesarean section for dystocia. However, the incidence of dystocia in each trial varied: from 11% in the fetal pulse oximetry group and 14% in the CTG‐only group (East 2006) to 19% for all women in both the open and masked groups, where all participants had a fetal oximetry sensor placed (Bloom 2006), which was similar to that of the fetal pulse oximetry group of Garite 2000.The incidence of dystocia was much lower in the study reported by Caliskan 2009 (2.6% in the fetal oximetry group and 3.4% in the CTG‐only group). These researchers considered that the intermittent use of the fetal oximetry probe may have avoided an over representation of dystocia in the oximetry group. It remains possible that the presence of a fetal oximetry sensor alongside the fetal head contributes to dystocia.
The use of CTG has some parallels. Current clinical practice recommendations are that the clinician and the individual woman should consider the appropriateness of CTG to enable an informed choice for each case (Alfirevic 2013; NICE 2007). Given the high quality of evidence from several of the reported fetal pulse oximetry trials and the reduction in caesarean section for nonreassuring fetal status (but not for overall caesarean section rates) in those for which a nonreassuring CTG was required prior to study entry, it may be prudent when developing recommendations to encourage the individual woman and her clinicians to make the decision to use or not use fetal pulse oximetry. Unlike CTG, however, the randomised controlled trials of fetal pulse oximetry have been conducted prior to widespread clinical acceptance and medico‐legal expectation of fetal pulse oximetry usage where there is concern about fetal well‐being.
Commercial availability of the fetal pulse oximetry system used in the studies was discontinued during 2006. Other systems that have not yet been subject to trials may still remain available commercially.
Authors' conclusions
Implications for practice.
This systematic review, comparing fetal intrapartum pulse oximetry with CTG or fetal electrocardiography or masked fetal pulse oximetry, provided evidence of no effect on the overall caesarean section rate between the fetal pulse oximetry group and the CTG group. There was evidence of some effect in reducing caesarean section rates for the indication of nonreassuring CTG when fetal pulse oximetry was added to CTG. Therefore, the evidence suggests that fetal pulse oximetry does not contribute to overall clinical practice. A better method to evaluate fetal well‐being in labour is required.
Implications for research.
Further trials could address: entry criteria related to the severity of nonreassuring CTG patterns; action levels for fetal pulse oximetry values, such as a decline by 10% or 20%, rather than an absolute cut‐off value; and the endpoint of long‐term neurodevelopmental outcomes. The ideal study to address the issue of dystocia when a fetal pulse oximetry sensor is placed alongside the fetal head would compare caesarean section for dystocia in three groups: those with fetal oximetry displayed, those with fetal pulse oximetry masked and those without fetal pulse oximetry. Further studies using fetal oximetry sensors attached to the fetal scalp, rather than placed alongside the fetal head, could also be considered.
Feedback
Thornton, July 2006
Summary
The abstract states 'Use of fetal pulse oximetry with CTG decreased operative delivery (caesarean section, forceps, vacuum) for nonreassuring fetal status (RR 0.71, 95% CI 0.55 to 0.93) compared with CTG alone.'
The results text also states 'There was a statistically significant decrease in operative delivery (caesarean section, forceps or vacuum birth) for nonreassuring fetal status (RR 0.71, 95% CI 0.55 to 0.93).
But the results tables show a Relative Risk (Fixed) 95% CI 1.07 [0.95, 1.21]. Am I missing something, or has there been a mistake?
(Summary of comment from Jim Thornton, July 2006)
Reply
The data in the text are correct. The data quoted from the results table refer to the outcome 'operative delivery (caesarean section, forceps or vacuum birth)', which is for all indications; the data quoted in the text are for 'operative delivery (caesarean section, forceps or vacuum birth) for nonreassuring fetal status' and are correct.
To help clarify this, the outcome in the review now includes the wording 'for all indications'.
(Summary of response from Christine East, November 2006)
Contributors
Feedback: Jim Thornton Reply: Christine East
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2014 | New citation required but conclusions have not changed | The addition of the trial to this updated review did not change the conclusions. |
| 31 May 2014 | New search has been performed | Search updated and one additional trial included in the review (Valverde 2011). Methods and literature review updated. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 11 September 2012 | Amended | Contact details updated. |
| 31 May 2010 | New search has been performed | One new trial added to the review. This did not change the conclusions of the review. Prof FY Chan removed from authorship (deceased 2007) although previous input gratefully recognised. |
| 1 October 2009 | Amended | Search updated. Five reports added to Studies awaiting classification (Caliskan 2009a; East 2006b; Prieto 2008a; Rouse 2008; Rouse 2009). |
| 10 November 2008 | Amended | Contact details updated. |
| 18 February 2008 | Amended | Converted to new review format. |
| 17 January 2007 | New citation required and conclusions have changed | Search updated in November 2006. We identified and included four new trials (Bloom 2006; East 2006; Klauser 2005; Kuhnert 2004). The original version of this review concluded that the addition of fetal pulse oximetry to cardiotocography decreased the caesarean section and operative delivery rates for nonreassuring fetal status, with no difference in overall caesarean section rates. The addition of the four new trials confirmed these conclusions when nonreassuring fetal status was identified prior to study entry. When nonreassuring fetal status was not present prior to study entry, knowledge of fetal pulse oximetry values made no difference to caesarean section rates for nonreassuring fetal status or for all indications. |
Acknowledgements
Our thanks are extended to the Cochrane Perinatal Team, Brisbane, and Philippa Middleton, Co‐ordinator of the Australian review authors' group, for valuable assistance in preparing this review.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search strategies
Authors searched MEDLINE (1994 to May 2010), EMBASE (1994 to May 2010) and Current Contents (1994 to May 2010): searches were conducted from 1994 onwards as pulse oximetry technology calibrated for the fetal environment has only been available since 1994. Searches were conducted using search terms: (labour OR labor OR intrapartum) AND (oximetry OR pulse oximetry OR oxygen saturation) AND (clinical trial phase 1 OR clinical trial phase II OR clinical trial phase III OR controlled clinical trial OR randomized controlled trial OR randomised controlled trial) AND (fetal distress OR fetal heart OR fetal monitoring OR nonreassuring OR non‐reassuring).
Data and analyses
Comparison 1. Primary outcomes: FPO + CTG versus CTG only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Gestation from 34 weeks, FBS not required prior to study entry | 4 | 4008 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.13] |
| 1.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.24, 0.81] |
| 1.3 Gestation from 28 weeks, FBS not required prior to study entry | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.14] |
| 1.4 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.04] |
| 2 Hypoxic‐ischaemic encephalopathy | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Gestation from 36 weeks, FBS not required prior to study entry | 1 | 600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.44] |
| 3 Neonatal seizures | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1789 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.10, 8.79] |
| 3.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.15, 2.59] |
Comparison 2. Secondary outcomes: maternal: FPO + CTG versus CTG only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section for nonreassuring fetal status | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Gestation from 34 weeks, FBS not required prior to study entry | 4 | 4008 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.90] |
| 1.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.44] |
| 1.3 Gestation from 28 weeks, FBS not required prior to study entry | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.24] |
| 1.4 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.09] |
| 2 Caesarean section for dystocia | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Gestation from 34 weeks, FBS not required prior to study entry | 4 | 4008 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.91, 2.09] |
| 2.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [0.47, 4.21] |
| 2.3 Gestation from 28 weeks, FBS not required prior to study entry | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.46] |
| 2.4 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.08] |
| 3 Operative birth (caesarean section, forceps, vacuum extraction) for all indications | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Gestation from 34 weeks, FBS not required prior to study entry | 3 | 1840 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.15] |
| 3.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.36, 0.73] |
| 3.3 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.90, 1.03] |
| 4 Operative birth (caesarean section, forceps, vacuum) for nonreassuring fetal status | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.62, 0.89] |
| 4.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.22] |
| 5 Use of intrapartum antibiotics | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Gestation from 36 weeks, FBS not required prior to study entry | 1 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.87, 1.35] |
| 5.2 Gestation from 36 weeks, FBS required prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.88] |
| 6 Overall antibiotic use | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.88] |
| 7 Intrapartum haemorrhage | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.52, 4.34] |
| 7.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.69] |
| 8 Postpartum haemorrhage | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1789 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.53, 4.39] |
| 8.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Chorioamnionitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.87] |
| 9.2 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.17] |
| 10 Endometritis | 4 | 7276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.26] |
| 10.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1789 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.61, 2.12] |
| 10.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.26] |
| 11 Uterine rupture | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1789 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.12, 6.13] |
| 11.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Length of hospital stay (days) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Gestation from 36 weeks, FBS not required prior to study entry | 1 | 600 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.36, 0.24] |
| 12.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.65, 0.65] |
| 13 Satisfaction with labour | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Gestation from 36 weeks, FBS not required prior to study entry | 1 | 448 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.16, 0.56] |
| 14 Satisfaction with fetal monitoring in labour | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Gestation from 36 weeks, FBS not required prior to study entry | 1 | 448 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.05, 0.85] |
| 15 Death | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1789 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.3. Analysis.
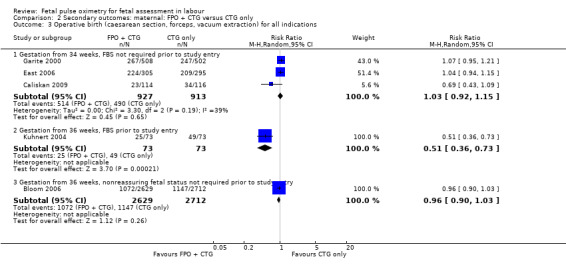
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 3 Operative birth (caesarean section, forceps, vacuum extraction) for all indications.
2.5. Analysis.
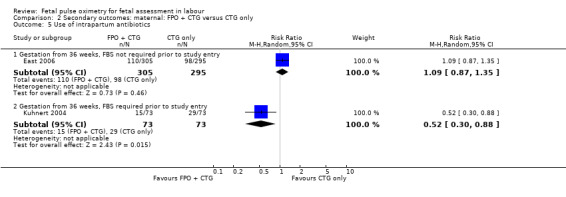
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 5 Use of intrapartum antibiotics.
2.6. Analysis.
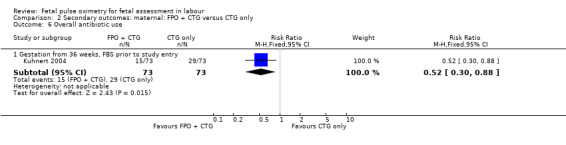
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 6 Overall antibiotic use.
2.7. Analysis.
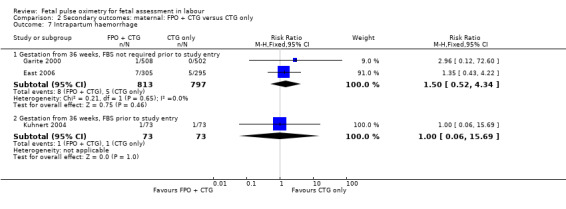
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 7 Intrapartum haemorrhage.
2.8. Analysis.
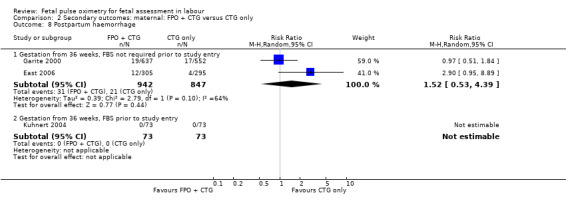
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 8 Postpartum haemorrhage.
2.9. Analysis.
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 9 Chorioamnionitis.
2.10. Analysis.
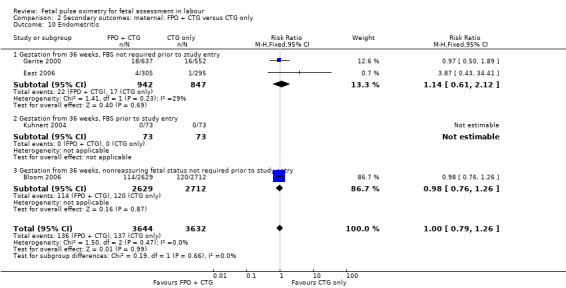
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 10 Endometritis.
2.11. Analysis.
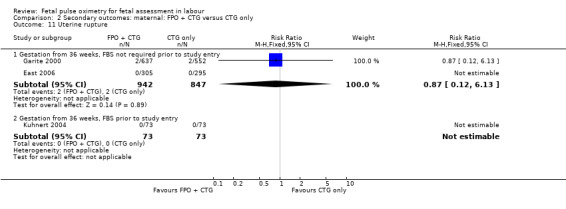
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 11 Uterine rupture.
2.12. Analysis.
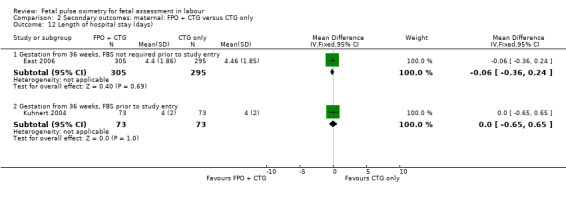
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 12 Length of hospital stay (days).
2.15. Analysis.
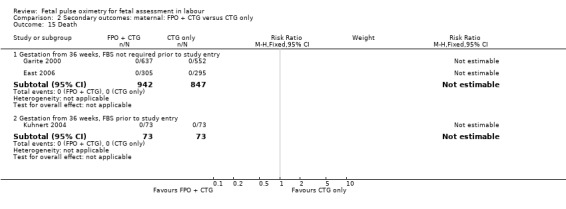
Comparison 2 Secondary outcomes: maternal: FPO + CTG versus CTG only, Outcome 15 Death.
Comparison 3. Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Apgar score less than 4 at 5 minutes | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1789 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.11, 63.70] |
| 1.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.52, 8.24] |
| 2 Apgar score less than 7 at 5 minutes | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Gestation from 34 weeks, FBS not required prior to study entry | 3 | 2019 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.38, 1.18] |
| 2.2 Gestation from 36 weeks, FBS required prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.45] |
| 2.3 Gestation from 28 weeks, FBS not required prior to study entry | 1 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.17, 2.91] |
| 3 Length of hospital stay (days) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Gestation from 36 weeks, FBS not required prior to study entry | 1 | 600 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.33, 0.33] |
| 3.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.32, 0.32] |
| 4 Umbilical arterial pH less than 7.10 | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.53] |
| 4.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.35] |
| 4.3 Gestation from 28 weeks, FBS not required prior to study entry | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.17, 1.24] |
| 5 Umbilical arterial base excess less than ‐12 | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1670 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.58, 1.92] |
| 5.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
| 6 Admission to neonatal intensive care unit | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Gestation from 34 weeks, FBS not required prior to study entry | 3 | 2019 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.84, 1.39] |
| 6.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.31] |
| 6.3 Gestation from 28 weeks, FBS not required prior to study entry | 1 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.55, 1.63] |
| 6.4 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 7 Death | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1789 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.20, 3.97] |
| 7.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.44] |
| 8 Death, hypoxic‐ischaemic encephalopathy, or both | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1789 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.20, 4.44] |
| 8.2 Gestation from 36 weeks, FBS required prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Gestation from 36 weeks, nonreassuring fetal status not required prior to study entry | 1 | 5341 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.30] |
| 9 Death, seizures, or both | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1789 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.22, 3.55] |
| 9.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Death, long‐term neurodevelopmental problem, or both | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Gestation from 36 weeks, FBS not required prior to study entry | 2 | 1789 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.20, 4.44] |
| 10.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Skin trauma | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Gestation from 36 weeks, FBS not required prior to study entry | 1 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.16, 3.21] |
| 11.2 Gestation from 36 weeks, FBS prior to study entry | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
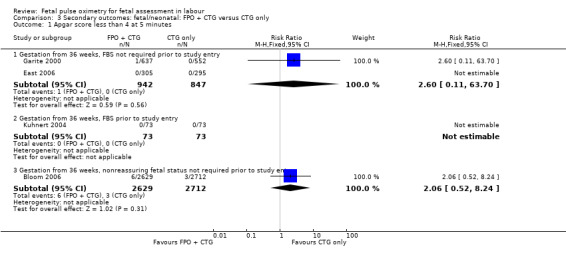
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 1 Apgar score less than 4 at 5 minutes.
3.2. Analysis.
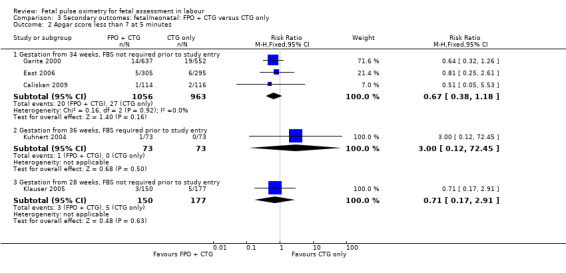
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 2 Apgar score less than 7 at 5 minutes.
3.3. Analysis.
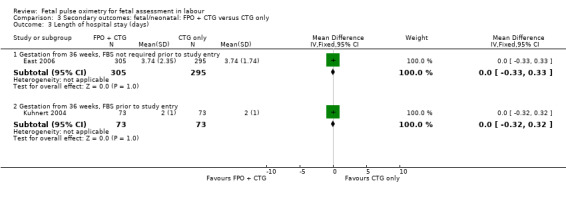
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 3 Length of hospital stay (days).
3.4. Analysis.
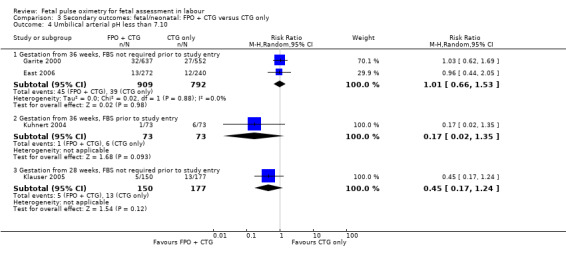
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 4 Umbilical arterial pH less than 7.10.
3.5. Analysis.
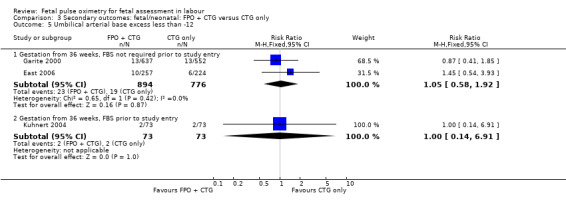
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 5 Umbilical arterial base excess less than ‐12.
3.6. Analysis.
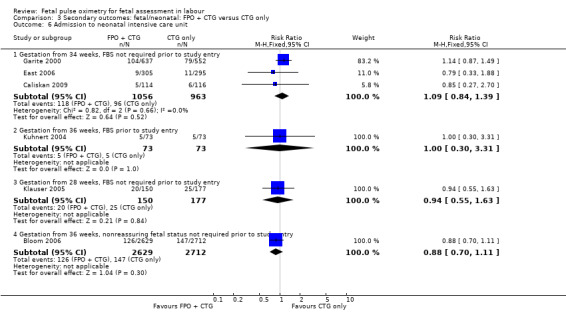
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 6 Admission to neonatal intensive care unit.
3.7. Analysis.
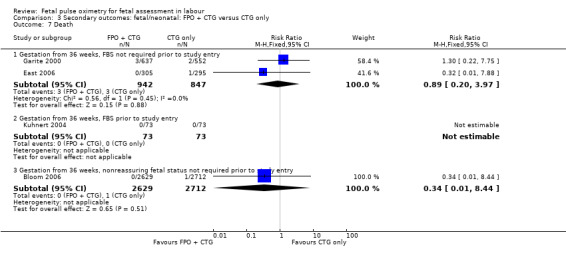
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 7 Death.
3.8. Analysis.
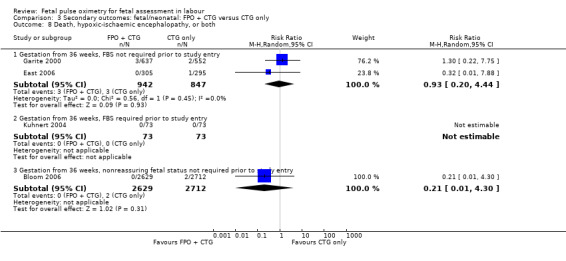
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 8 Death, hypoxic‐ischaemic encephalopathy, or both.
3.9. Analysis.
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 9 Death, seizures, or both.
3.10. Analysis.
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 10 Death, long‐term neurodevelopmental problem, or both.
3.11. Analysis.
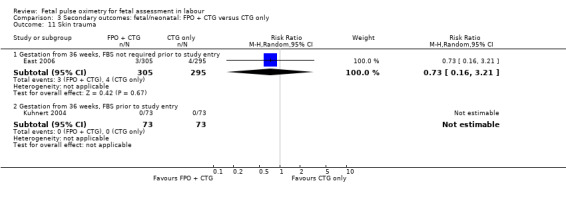
Comparison 3 Secondary outcomes: fetal/neonatal: FPO + CTG versus CTG only, Outcome 11 Skin trauma.
Comparison 4. Subgroup: fetal blood sampling: primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Neonatal seizures | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
4.1. Analysis.
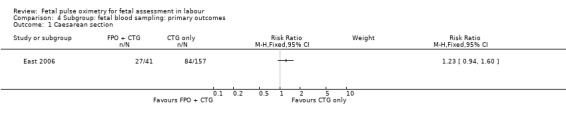
Comparison 4 Subgroup: fetal blood sampling: primary outcomes, Outcome 1 Caesarean section.
4.2. Analysis.
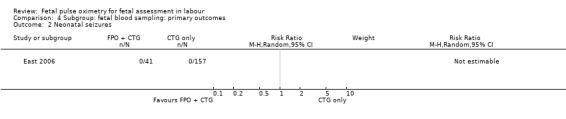
Comparison 4 Subgroup: fetal blood sampling: primary outcomes, Outcome 2 Neonatal seizures.
Comparison 5. Primary outcomes: FPO + CTG versus fetal ECG + CTG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.06, 2.29] |
Comparison 6. Secondary outcomes: maternal: FPO + CTG versus fetal ECG + CTG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section for nonreassuring fetal status | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.01, 2.88] |
| 2 Caesarean section for dystocia | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.60, 2.81] |
| 3 Operative birth (caesarean section, forceps, vacuum) | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.00, 1.45] |
| 4 Operative birth (caesarean section, forceps, vacuum) for nonreassuring fetal status | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.88, 1.70] |
Comparison 7. Secondary outcomes: fetal/neonatal: FPO + CTG versus fetal ECG + CTG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admission to neonatal intensive care unit | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.74] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bloom 2006.
| Methods | RCT. | |
| Participants | Nulliparous women from 36 weeks' gestation with a singleton pregnancy and cephalic presentation, in early labour (2‐5 cm cervical dilatation) with ruptured amniotic membranes who gave informed consent. | |
| Interventions | 'Open' group: FPO sensor placed and FPO values displayed. 'Masked' group: FPO sensor placed and FPO values not displayed (FPO values recorded on computer). Both groups: standard fetal heart rate monitoring; labour management at the clinician's discretion. |
|
| Outcomes | Primary: caesarean section (any indication). Secondary: caesarean section for nonreassuring fetal status or dystocia; "fetal vulnerability index" (stillbirth, neonatal death, 5‐min Apgar score less than 3, umbilical pH less than or equal to 7, seizures, admission to NICU for greater than or equal to 24 hours); other neonatal morbidity. |
|
| Notes | Fetal oximetry system used: Nellcor OxiFirst. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After the fetal oximetry sensor was placed, randomisation was performed by a research nurse using an encrypted computer program. |
| Allocation concealment (selection bias) | Low risk | After the fetal oximetry sensor was placed, randomisation was performed by a research nurse using an encrypted computer program. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: women and clinicians were blinded to FPO values in the 'masked' group: however, they were not actually blinded to intervention. It would not have been feasible to fully blind the clinician or participant, given that FSpO2 values were used for clinical judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research nurses obtained data from the maternal and infant charts. Adverse maternal outcomes (placental abruption or prolonged fetal heart rate deceleration at the time of sensor placement) and the composite neonatal outcome were further verified by investigators. "Chart reviewers had no knowledge of the randomization assignment" (p2198). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The published protocol noted the aim of recruiting 10,000 women. When 5017 women had been recruited and their outcomes examined at the third interim analysis. the Data Safety and Moniritoring Committee advised that sufficient recruitment had been undertaken to detect the 15% difference in the primary outcome of caesarean section rate with 90% power (as the higher than expected caesarean section rate once the trial was underway). Recruitment ceased once this decision was agreed upon, with a total of 5341 women randomised and their outcomes analysed. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. The report aligns with the limited details available in the protocol published when the RCT was in progress (see attrition bias). |
Caliskan 2009.
| Methods | RCT, single centre (Turkey). | |
| Participants | Women from 34 weeks' gestation undergoing induction of labour with oral misoprostol. Inclusion: singleton live pregnancy with vertex presentation and maternal and/or fetal indications for induction of labour; gestational age from 34 weeks; Bishop score less than or equal to 5; absence of spontaneous uterine contractions; estimated fetal body weight less than 4250 g; reactive non‐stress test. Exclusion: fetal demise; gestational age less than 34 weeks; known hypersensitivity to prostaglandin; previous caesarean section or other uterine surgery; contraindication to vaginal birth. |
|
| Interventions | Group 1: electronic fetal monitoring by CTG only. If the CTG was reassuring, labour continued unless otherwise indicated. If the CTG was nonreassuring (defined), simple measures, including lateral positioning, were instigated, with escalation to operative birth if simple measures were not effective. Group 2: CTG plus FSpO2 monitoring ‐ intermittently for 15 minutes every 2 hours. If reassuring it was removed. If nonreassuring, remained in situ. If the CTG was reassuring and FSpO2 values were greater than or equal to 30%, labour continued unless otherwise indicated. If the CTG was nonreassuring (defined) and FSpO2 values were less than 30% for 3 minutes, simple measures, including lateral positioning, were instigated. If FSpO2 values remained < 30% for 10 minutes, then operative birth was performed. |
|
| Outcomes | Primary outcome: caesarean birth rates. Secondary outcomes: induction to birth interval, caesarean section for nonreassuring CTG, neonatal outcomes, including umbilical arterial pH < 7.16, admission to neonatal intensive care. |
|
| Notes | Fetal oximetry system used: Nellcor OxiFirst. 37 weeks used as 'restriction point' to randomly allocate preterm and term fetuses to the 2 groups. This is interpreted as stratification by term/preterm, however, no further details provided of outcomes within these groups. Data were not available to allow subgroup analysis in this review by term/preterm. Similar numbers of term (total n = 195)/preterm (total n = 35) were randomised to the control and intervention groups, with the larger proportion being term in each group. There were similar neonatal outcomes (including birthweight and admission to NICU), both between the groups and compared with other studies enrolling over 36 weeks. We have therefore included these participants in the analyses of later gestations, renaming the analyses that include participants from this study as "... gestation from 34 weeks ...". Attempts at establishing contact details to clarify this were unsuccessful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation reported to be "Directed by a physician". |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded. It would not have been feasible to blind the clinician or participant, given that FSpO2 values were used for clinical judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors did not state whether or not outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A flowchart of the eligible and enrolled participants was included in the publication and outcomes were reported for all these participants. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias, although there is no evidence of trial registration or study protocol publication. |
East 2006.
| Methods | Multicentre RCT. Survey of women's perceptions: identical surveys to participants in each group within a few days of giving birth and 3 months later. Women were asked to rate their experience in 3 domains: labour (maximum score 12), fetal monitoring (maximum score 16) and participation in research (maximum score 12). Cost‐effectiveness analysis the RCT. Costs included diagnosis‐related group costs, FBS, medications, use of oxygen or intravenous fluid, or both, FPO. Effect was the primary outcome of the RCT (operative birth for nonreassuring fetal status). | |
| Participants | 601 women in labour. 1 exclusion, leaving 600 analysed. Inclusion criteria: nonreassuring CTG (defined), ≧ 36 weeks' gestation, early or active labour, ruptured amniotic membranes or eligible for artificial rupture of membranes. Exclusion criteria: multiple gestations, non vertex presentation, placenta praevia, abruptio placentae, uterine anomaly, antepartum haemorrhage, fetal anomaly, known significant viral infections (e.g. HIV), any other contraindications to invasive monitoring such as thrombocytopenia. |
|
| Interventions | Control group: fetal heart rate monitoring (CTG) (doppler/fetal scalp electrode). Intervention group: CTG plus fetal pulse oximetry. Protocol for action with reassuring (≧ 30%) and nonreassuring fetal oximetry values (< 30% for 10 minutes, or not recording). |
|
| Outcomes | Primary outcome: operative birth (caesarean section, vacuum, forceps) for nonreassuring fetal status. Maternal outcomes including: caesarean section and assisted vaginal birth for nonreassuring fetal status; caesarean and assisted vaginal birth section for dystocia/failure to progress; caesarean or assisted vaginal birth for combined indication of nonreassuring fetal status and dystocia; caesarean section; assisted vaginal birth; spontaneous vaginal birth; labour interventions and fetal evaluations (e.g. scalp pH); endometritis; postpartum haemorrhage; length of stay. Women's perceptions: satisfaction measured in 3 domains: labour, fetal monitoring and participation in research. Neonatal outcomes including: Apgar scores; umbilical cord blood gases; resuscitation; admission to NICU; length of hospital stay. Economic analysis: cost‐effectiveness of FPO to prevent operative birth for nonreassuring fetal status. |
|
| Notes | Sample size calculation: yes, based on reduction in caesarean section rate for nonreassuring fetal status. Fetal oximetry system used: Nellcor OxiFirst. Women's perceptions: results from the first survey are used in this report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Developed by a research associate not involved in recruitment. |
| Allocation concealment (selection bias) | Low risk | Adequate, through use of password protected computer randomisation system. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded. It would not have been feasible to blind the clinician or participant, given that FSpO2 values were used for clinical judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Initial analyses were done with blinded group allocation, followed by unblinded analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data from all participants were accounted for. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | Authors declared commercial funding in all publications. Limited protocol details were available online through a perinatal trials registry. The unpublished ethics‐approved study protocol was available to those assessing these risks of bias. |
Garite 2000.
| Methods | Random allocation: telephone randomisation. | |
| Participants | 1189 women in labour. This consisted of 1010 in the published trial and 179 in a pilot of the trial conducted using the same protocol, where unpublished data were accessible. Inclusion criteria: nonreassuring CTG, ≧ 36 weeks' gestation, active labour, single fetus, cephalic presentation, cervical dilatation of at least 2 cm and at station ‐2 or below, ruptured amniotic membranes (or have amniotomy). Exclusion criteria: planned caesarean section, placenta praevia, need for immediate birth, active genital herpes or known HIV infection, participation in other studies. |
|
| Interventions | Control group: fetal heart rate monitoring (CTG) (doppler/fetal scalp electrode). Study group: CTG plus fetal pulse oximetry. Protocol for action with reassuring and nonreassuring fetal oximetry values. |
|
| Outcomes | Caesarean section for nonreassuring status; caesarean section for all indications; caesarean section for fetal intolerance to labour with dystocia, mixed indication; caesarean dystocia, single indication; spontaneous vaginal birth; assisted vaginal birth for nonreassuring fetal status or for all other indications; fetal heart rate patterns; labour interventions and fetal evaluations (e.g. scalp pH). Neonatal outcomes including: Apgar scores; umbilical cord blood gases; resuscitation; admission to NICU; length of hospital stay. Maternal outcomes including: endometritis; length of stay; bleeding; uterine rupture; intrapartum fever. |
|
| Notes | Some additional unpublished data from a pilot of the trial, using the same protocol, were available. Further data were requested but were unable to be accessed. Sample size calculation: yes, based on reduction in caesarean section rate for nonreassuring fetal status. Fetal oximetry system used: Nellcor OxiFirst. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation. |
| Allocation concealment (selection bias) | Low risk | Adequate, with computer randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were unblinded. It would not have been feasible to blind, given that the FSpO2 values were used in decision making. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only some outcome analysis was blinded, e.g. retrospective examination of partograms to determine diagnosis of dystocia. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. "All analyses ... included all randomized patients" (p1053). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Available data for this review included a report to the Food and Drug Administration, which include comprehensive and otherwise unpublished results that were consistent with published findings. |
| Other bias | High risk | Commercially funded study, which was acknowledged by report authors. The study protocol was publicly available during the trial. |
Klauser 2005.
| Methods | Single‐centre RCT. | |
| Participants | 360 women in labour. Control group: 1 post randomisation exclusion as no consent. Intervention group: 30 post randomisation exclusions where FPO sensor not placed and 2 additional exclusions due to randomisation issues. Inclusion criteria: nonreassuring CTG, ≧ 28 weeks' gestation, single fetus, cephalic presentation, cervical dilatation of at least 2 cm and at station ‐5 or below, ruptured amniotic membranes (spontaneous or artificial). Exclusion criteria: planned caesarean section, contraindication to vaginal birth (including genital herpes, transverse lie), unexplained vaginal bleeding, placenta praevia, ominous CTG requiring immediate birth, known HIV infection, hepatitis B or C, unable to give consent due to intrapartum parenteral analgesia. |
|
| Interventions | Control group: fetal heart rate monitoring (CTG) (Doppler/fetal scalp electrode). Study group: CTG plus fetal pulse oximetry (Nellcor OxiFirst). Protocol for action with reassuring fetal oximetry (≧ ≥30%) and nonreassuring values (< 30% for 3 minutes). |
|
| Outcomes | Primary outcome: caesarean section for nonreassuring fetal status. Maternal outcomes: caesarean section for all indications; caesarean section for dystocia; amnioinfusion and length of labour. Neonatal outcomes including: Apgar scores; umbilical cord blood gases; resuscitation; admission to NICU. |
|
| Notes | Further data were requested, no response. Sample size calculation: yes, based on reduction in caesarean section rate for nonreassuring fetal status. This was revised following the interim analysis due to a higher than anticipated caesarean section rate in the control group, meaning that a 50% reduction in caesareans would require less participants than originally though. The study ceased at that time. Fetal oximetry system used: Nellcor OxiFirst. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation not stated. No response from request to the authors for clarification. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No mention in the report, although two participants were excluded on the basis of "randomization issues". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded. It would not have been feasible to blind the clinician or participant, given that FSpO2 values were used for clinical judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear if the outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data ‐ the flowchart in the published report accounts for all those enrolled. The trial was ceased following an interim analysis, at which time it was determined that a total of 300 of the original planned 400 would have adequate power to detect a 50% reduction in the primary outcome, caesarean section. Some recruitment occurred while the interim analysis was in progress, meaning that a total of 327 women were randomised. Of these, there were 32 postrandomisation exclusions in the fetal oximetry group and 1 in the control group, |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. The flowchart in the published report accounts for all those enrolled. |
| Other bias | Low risk | No evidence of other bias, although there is no evidence of trial registration or study protocol publication. |
Kuhnert 2004.
| Methods | Single‐centre, RCT. | |
| Participants | 146 women in labour. Inclusion criteria: CTG with International Federation of Gynecology and Obstetrics (FIGO) score ≦ 8, gestational age ≧ 36 weeks, active labour, single fetus, cephalic presentation, cervical dilatation of at least 2 cm and at station ‐2 or below, ruptured amniotic membranes (or have amniotomy). All cases had FBS prior to randomisation. Exclusion criteria: planned caesarean section, placenta praevia, need for immediate birth, active genital herpes or known HIV infection. |
|
| Interventions | Control group: fetal heart rate monitoring (CTG) and FBS. Protocol for action with reassuring, suspicious and pathologic CTG and FBS pH values. Intervention group: CTG plus FBS plus FPO. Protocol for action with reassuring (≧ 30%) and nonreassuring FPO values (< 30% for ≧ 10 mins or repeatedly ('summation effect')), and for reassuring and nonreassuring CTG and FBS pH. |
|
| Outcomes | Caesarean section or vacuum extraction for pathologic CTG; caesarean section or vacuum extraction for all indications; caesarean section or vacuum for arrest of labour; caesarean section for pelvic malformation or amnioinfection; vacuum extraction for maternal exhaustion; spontaneous vaginal birth; fetal heart rate patterns; FBS (including pH). Neonatal outcomes including: umbilical cord blood gases; resuscitation; admission to NICU. Maternal outcomes: 'adverse maternal events'. |
|
| Notes | Some additional unpublished data were provided by the authors (use of antibiotics, haemorrhage, chorioamnionitis, endometritis, uterine rupture, length of hospital stay, satisfaction with labour and fetal monitoring, death, neonatal skin trauma, Apgar score, umbilical arterial base excess, admission to neonatal intensive care, hypoxic‐ischaemic encephalopathy, seizures, long‐term disability). No details of the assessment of long‐term disability were provided (e.g. age of the infant, assessments made). Sample size calculation: no. Fetal oximetry system used: Nellcor OxiFirst. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation: method not stated and not provided on request. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No details provided in the report. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded. It would not have been feasible to blind the clinician or participant, given that FSpO2 values were used for clinical judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The report states "data acquisition was done anonymously for both groups". It is unclear whether this related to de‐identifying the data (likely) or that the data were collected without knowledge of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | The results are very different to those of the other studies in this review. No evidence of other bias, although there is no evidence of trial registration or study protocol publication. |
Valverde 2011.
| Methods | Prospective RCT. | |
| Participants | Pregnant women with a full‐term singleton fetus in cephalic presentation admitted to the dilatation and birth sections of the Department of Obstetrics and Gynecology at Virgen de las Nieves University Hospital in Granada, Spain. NRFHR patterns were recorded during the second stage of labour as per Garite et al. 2000. N = 90 in each group. |
|
| Interventions | Group 1: pulse oximetry and intrapartum CTG. Group 2: fetal ECG (spiral electrode on the scalp) and intrapartum CTG. |
|
| Outcomes | Maternal: outcome of labour, rate of caesarean birth, rate of intervention due to NRFHR, reason for the intervention, duration of each stage of labour. Neonatal: cord blood acid base (arterial and venous), Apgar score, type of resuscitation, rate of admission to the NICU. |
|
| Notes | After informed consent was obtained, an examination was performed to determine fetal well‐being with the scalp stimulation test and membranes were ruptured, if they had not already ruptured. All participants were offered epidural anaesthesia. Fetal oximetry: FS14 sensor and Nellcor 400 Fetal Oxygen Saturation Monitoring System. Normal values FSpO2 were defined as between 30% and 70%, with 30% as the cut‐off value. If FSpO2 below 10%, labour was terminated and between 10% and 30% additional information to determine the fetus’s acid‐base was sought. Fetal ECG: : Electrode Cetro AB, Neoventa, Molndal, Sweden. We have had no response from the authors to our request for the study protocol or whether or not the sealed opaque randomisation envelopes were sequentially numbered. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes. It is unclear whether or not these were sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded. It would not have been feasible to blind the clinician or participant, given that FSpO2 values were used for clinical judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The report does not state whether or not outcome assessors were blinded to group allocation. Clarification from authors was sought, with no response. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition was reported and relevant results were reported for all 180 participants. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias, although there is no evidence of trial registration or study protocol publication. |
CTG: cardiotocography ECG: fetal electrocardiography FBS: fetal blood sampling (scalp) FPO: fetal pulse oximetry FSpO2: fetal oxygen saturation value
HIV: human immunodeficiency virus NICU: neonatal intensive care unit NRFHR: nonreassuring fetal heart rate min: minute RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andres 2004 | This study was conducted in Spain. It compared caesarean section rates for pathological or nonreassuring CTG when FPO was added to CTG monitoring or when FPO was not used. The groups were not randomised. |
| Golaszewski 1993 | This was an observational study of fetal pulse oximetry, where participants were randomised to be monitored with 1 of 2 oximeters. |
CTG: cardiotocography FPO: fetal pulse oximetry
Differences between protocol and review
The 2010 and 2014 updates have incorporated the current standard methods used by the Pregnancy and Childbirth Group at the time, which have been modified since the original protocol was published (East 2003). In the 2014 update, the use of additional searching was discontinued, as the comprehensive search through the Cochrane Pregnancy and Childbirth Group has identified every publication used in the successive updates of this review, with no additional studies identified in the additional searches.
For the planned subgroup analyses, we added lactate measurement as a parameter for fetal scalp blood sampling (following randomisation), given that this is often used instead of blood gas analysis in contemporary clinical practice.
Contributions of authors
C East compiled the protocol and original review with input from all co‐authors. L Begg joined the authorship in 2006 for the 2007 update. FY Chan died in 2007. R Lau joined the authorship for the 2014 update.
C East, FY Chan (to 2007), P Colditz, R Lau and/or L Begg reviewed the articles for consideration of inclusion/exclusion and abstracted data for the 2007 review. In particular, L Begg, who was not a co‐investigator on the trial by the other three authors, reviewed that trial for quality and suitability for inclusion in this review.
C East and R Lau updated this review in 2014, with input from the remaining authors (PC, LB).
Sources of support
Internal sources
Perinatal Research Centre, The University of Queensland, Royal Brisbane and Women's Hospital, Herston, Queensland, Australia.
Department of Obstetrics and Gynaecology, University of Melbourne, Australia.
External sources
No sources of support supplied
Declarations of interest
Three authors (C East, FY Chan, P Colditz) were chief investigators in the Australian multicentre randomised controlled trial of fetal intrapartum pulse oximetry (East 2006). That trial was supported in part by a research grant and equipment loan from Nellcor Inc, manufacturers of a fetal pulse oximetry system. An additional co‐author who was not an investigator in that trial, L Begg, joined the review team to evaluate that trial for incorporation in the 2007 update of the review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bloom 2006 {published data only}
- Bloom SL, Spong CY, Thom E, Varner MW, Rouse DJ, Weininger S, et al. Fetal pulse oximetry and cesarean delivery. New England Journal of Medicine 2006;355(21):2195‐202. [DOI] [PubMed] [Google Scholar]
- Bloom SL, for the NICHD MFMU Network. The MFMU Network randomized trial of fetal pulse oximetry [abstract]. American Journal of Obstetrics and Gynecology 2006;196(6 Suppl):S2. [Google Scholar]
- Maternal Fetal Medicine Units Network. The FOX trial: a randomized clinical trial of fetal pulse oximetry. http://www.bsc.gwu.edu/mfmu/Projects/fox.cgi (accessed 7 August 2006).
- Rouse D, Shriver EK. Second stage of labour duration: relationship to maternal and perinatal outcomes. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse DJ, Weiner SJ, Bloom SL, Varner MW, Spong CY, Ramin SM, et al. Second‐stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. American Journal of Obstetrics and Gynecology 2009;201(4):357.e1‐357.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caliskan 2009 {published data only}
East 2006 {published and unpublished data}
- Colditz PB, East CE. PTO360. Fetal intrapartum pulse oximetry: a multicentre randomised controlled trial. Perinatal Trials Report. www.ctc.usyd.edu.au/6registry/PTO360.htm (accessed 3 Feb 2004).
- East C, Chan FY, Brennecke S, King J, Colditz P, the Foremost Study Group. Women's evaluations of their experience in a multicenter randomized controlled trial of intrapartum fetal pulse oximetry (The Foremost Trial). American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S102. [Google Scholar]
- East C, Gascoine M, Doran C, Brennecke S, King J, Colditz P, et al. A cost‐effectiveness analysis of the intrapartum fetal pulse oximetry multicentre randomized controlled trial (The Foremost Trial). American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S101. [DOI] [PubMed] [Google Scholar]
- East CE, Brennecke SP, Chan FY, King JF, Beller EM, Colditz PB. Clinicians' evaluations of fetal oximetry sensor placement in a multicentre randomised trial (the foremost trial). Australian & New Zealand Journal of Obstetrics & Gynaecology 2006;46(3):234‐9. [DOI] [PubMed] [Google Scholar]
- East CE, Brennecke SP, King JF, Chan FY, Colditz PB, on behalf of the FOREMOST Study Group. The effect of intrapartum fetal pulse oximetry, in the presence of a nonreassuring fetal heart rate pattern, on operative delivery rates: a multicenter, randomized, controlled trial (the FOREMOST trial). American Journal of Obstetrics and Gynecology 2006;194(3):606.e1‐16. [DOI] [PubMed] [Google Scholar]
- East CE, Brennecke SP, King JF, Chan FY, Colditz PB, on behalf of the FOREMOST study group. Fetal pulse oximetry clinical trial (the FOREMOST trial). Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:166.
- East CE, Brennecke SP, King JF, Chan FY, Colditz PB, the FOREMOST Study Group. The effect of intrapartum fetal pulse oximetry, in the presence of a non‐reassuring fetal heart rate pattern, on operative delivery rates: a multicenter randomized controlled trial (The FOREMOST Trial). American Journal of Obstetrics and Gynecology. 2005; Vol. 193, issue 6 Suppl:S33. [DOI] [PubMed]
- East CE, Chan FY, Brennecke SP, King JF, Colditz PB, on behalf of the FOREMOST Study Group. Women's evaluations of their experience in a multicenter randomized controlled trial of intrapartum fetal pulse oximetry (the FOREMOST trial). Birth 2006;33(2):101‐9. [DOI] [PubMed] [Google Scholar]
- East CE, Chan FY, Brennecke SP, King JF, Colditz PB, on behalf of the FOREMOST study group. Childbearing women's experience in the FOREMOST trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:365.
- East CE, Gascoigne MB, Doran CM, Brennecke SP, King JF, Colditz PB, et al. A cost‐effectiveness analysis of the intrapartum fetal pulse oximetry multicentre randomised controlled trial (the FOREMOST trial). BJOG: an international journal of obstetrics and gynaecology 2006;113:1080‐7. [DOI] [PubMed] [Google Scholar]
- East CE, Gasgoine MB, Doran CM, Brennecke SP, King JF, Colditz PB, et al. Cost‐effectiveness of fetal pulse oximetry (FOREMOST). Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:202.
Garite 2000 {published and unpublished data}
- Dildy G, Garite T, McNamara H, Swedlow D, Mallinckrodt (Nellcor) Fetal Oximetry Research Group. A multicenter randomized trial of fetal pulse oximetry [abstract]. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S12. [Google Scholar]
- Dildy GA, Clark SL, Garite TJ, Porter RF, Swedlow DB, Varner MW. Current status of the multicenter randomized clinical trial on fetal oxygen saturation monitoring in the United States. European Journal of Obstetrics & Gynecology and Reproductive Biology 1997;72 Suppl 1:S43‐S50. [DOI] [PubMed] [Google Scholar]
- Garite TJ, Dildy GA, McNamara H, Nageotte MP, Boehm FH, Dellinger EH, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. American Journal of Obstetrics and Gynecology 2000;183(5):1049‐58. [DOI] [PubMed] [Google Scholar]
- Gorenberg D, Pattillo C, Hendi P, Rumney P, Garite T. Fetal pulse oximetry: correlation between oxygen desaturation, duration, and frequency and neonatal outcomes [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S129. [DOI] [PubMed] [Google Scholar]
- Gorenberg DM, Pattillo C, Hendi P, Rumney PJ, Garite TJ. Fetal pulse oximetry: correlation between oxygen desaturation, duration, and frequency and neonatal outcomes. American Journal of Obstetrics and Gynecology 2003;189(1):136‐8. [DOI] [PubMed] [Google Scholar]
- Henigsman S, Garite T, Pattillo C, Brewster W. Fetal pulse oximetry: defining which variable decelerations with slow return to baseline are associated with hypoxia [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S131. [Google Scholar]
- Lee R, Moore M, Brewster W, Hendi P, Pattillo C, Ziogas A, et al. All non‐reactive fetal heart rate tracings are not equal [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S131. [Google Scholar]
- Mallinckrodt, Inc. Summary of safety and effectiveness information data. OxiFirst® Fetal Oxygen Saturation Monitoring System 2000.
- Miller H. Differences in cervical dilatation and induction in patients delivered because of dystocia during a multicenter randomized clinical trial of fetal pulse oximetry [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S124. [Google Scholar]
- Porreco R, Garite J, Swedlow D, Thomas S. The occurrence of dystocia among patients monitored in labor with fetal pulse oximetry [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S19. [Google Scholar]
Klauser 2005 {published data only}
- Klauser CK, Christensen EE, Chauhan SP, Bufkin L, Magann EF, Bofill JA, et al. Use of fetal pulse oximetry among high‐risk women in labour: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2005;192:1810‐7. [DOI] [PubMed] [Google Scholar]
Kuhnert 2004 {published and unpublished data}
- Kuhnert M, Schmidt S. Intrapartum management of nonreassuring fetal heart rate patterns: a randomized controlled trial of fetal pulse oximetry. American Journal of Obstetrics and Gynecology 2004;191:1989‐95. [DOI] [PubMed] [Google Scholar]
Valverde 2011 {published data only}
- Pareja MV, Prieto AP, Badillo MPC, Herrezuelo IP, Ventoso FM. Comparison of the effectiveness between the fetal pulse oximetry and fetal electrocardiogram during intrapartum fetal well‐being loss risks. Progresos de Obstetricia y Ginecologia 2010;53(4):141‐7. [Google Scholar]
- Prieto AP, Pareja MV, Zuniga IV, Romero TA, Leon MDR, Ventoso FM. Pulse oximetry compared with fetal electrocardiogram to control intrapartum fetal wellbeing. Journal of Maternal‐Fetal and Neonatal Medicine 2008; Vol. 21, issue Suppl 1:141.
- Valverde M, Puertas AM, Lopez‐Gallego MF, Carrillo MP, Aguilar MT, Montoya F. Effectiveness of pulse oximetry versus fetal electrocardiography for the intrapartum evaluation of nonreassuring fetal heart rate. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011;159(2):333‐7. [DOI] [PubMed] [Google Scholar]
- Valverde Pareja M, Martinez MDM, Guadix BR, Ventoso FM, Prieto AP, Criado MSL. Comparison between the pulse oximetry's effectiveness and electrocardiogram's effectiveness; two methods of fetal intrapartum monitoring. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):627. [Google Scholar]
- Valverde Pareja M, Romero Guadix B, Moreno Martinez MD, Puertas Prieto AA, Montoya Ventoso F. Influence of two methods of fetal intrapartum monitoring; pulse oximetry and fetal electrocardiogram for the duration of the labor, in the presence of alterations in the fetal heart rate. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):243. [Google Scholar]
References to studies excluded from this review
Andres 2004 {published data only}
- Andres IF, Montero IM. Fetal pulse oximetry. Intrapartum foetal hypoxia evaluation. Comparative study with invasive techniques concerning foetal welfare [Pulsioximetria fetal. Nuevo metodo de control fetal intraparto. Estudio comparativo con tecnicas invasivas acerca del bienestar fetal]. Anales del Sistema Sanitario de Navarra 2004;27(2):179‐89. [DOI] [PubMed] [Google Scholar]
Golaszewski 1993 {published data only}
- Golaszewski T, Frigo P, Ulm M, Lee A, Gruber W, Rafolt D, et al. Non‐invasive fetal pulse oximetry sub partu. Gynakologisch‐Geburtshilfliche Rundschau 1993;33:246‐50. [DOI] [PubMed] [Google Scholar]
Additional references
ACOG 2001
- ACOG Committee on Obstetric Practice. Fetal pulse oximetry. ACOG Committee Opinion No 258. Obstetrics & Gynecology 2001;98:523‐4. [DOI] [PubMed] [Google Scholar]
Alfirevic 2013
- Alfirevic Z, Devane D, Gyte GML. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD006066.pub2] [DOI] [PubMed] [Google Scholar]
Arikan 1998
- Arikan GM, Haeusler MCH, Deutsch MR, Greimel ER, Dorfer M. Maternal perceptions of labor with fetal monitoring by pulse oximetry in a research setting. Birth 1998;25(3):182‐9. [DOI] [PubMed] [Google Scholar]
Arikan 2000
- Arikan GM, Scholz HS, Haeusler MCH, Giuliani A, Haas J, Weiss PAM. Low fetal oxygen saturation at birth and acidosis. Obstetrics & Gynecology 2000;95:565‐71. [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan ED, Chan MM, Chan MM. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respiratory Medicine 2013;107:789‐99. [DOI] [PubMed] [Google Scholar]
Colditz 1999
- Colditz PB, Begg LM, East CE. Fetal pulse oximetry: instrumentation and recent clinical experience. Clinics in Perinatology 1999;26:869‐80. [PubMed] [Google Scholar]
Coldtiz 2013
- Coldtiz PB, East CE. Fetal pulse oximetry. In: Ginosar Y, Reynolds F, Halpern S, Weiner CP editor(s). Anesthesia and the Fetus. Oxford: Wiley‐Blackwell, 2013:123‐8. [DOI: 10.1002/9781118477076] [DOI] [Google Scholar]
Costa 2010
- Costa A, Santos C, Ayres‐de‐Campos D, Costa C, Bernardes J. Access to computerised analysis of intrapartum cardiotocographs improves clinicians' prediction of newborn umbilical artery blood pH. BJOG: an international journal of obstetrics and gynaecology 2010;117(10):1288‐93. [DOI] [PubMed] [Google Scholar]
Devane 2005
- Devane D, Lalor J. Midwives' visual interpretation of intrapartum cardiotocographs: intra‐ and inter‐observer agreement. Journal of Advanced Nursing 2005;52(2):133‐41. [DOI] [PubMed] [Google Scholar]
Dokus 2013
- Dokus K, Zubor P, Matasova K, Visnovsky J, Danko J. Impact of fetal pulse oximetry and ST analysis surveillance withdrawal on rates of obstetric surgery and frequency of low birth umbilical artery pH: A cause of rising caesarean rates?. Journal of Obstetrics and Gynaecology 2013;33(7):685‐8. [DOI] [PubMed] [Google Scholar]
East 1996
- East CE, Colditz PB. Women's evaluations of their experience with fetal intrapartum oxygen saturation monitoring and participation in a research project. Midwifery 1996;12:93‐7. [DOI] [PubMed] [Google Scholar]
East 1997
- East CE, Dunster KR, Colditz PB, Nath CE, Earl JW. Fetal oxygen saturation monitoring in labour: an analysis of 118 cases. Australian and New Zealand Journal of Obstetrics and Gynaecology 1997;37(4):397‐401. [DOI] [PubMed] [Google Scholar]
East 2002
- East CE, Colditz PB, Begg LM, Brennecke SP. Update on intrapartum fetal pulse oximetry. Australian and New Zealand Journal of Obstetrics and Gynaecology 2002;42(2):119‐23. [DOI] [PubMed] [Google Scholar]
East 2003
- East CE, Chan FY, Colditz PB. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004075] [DOI] [PubMed] [Google Scholar]
East 2007a
- East CE, Colditz PB. Intrapartum oximetry of the fetus. Anesthesia & Analgesia 2007;105(6):S59‐S65. [DOI] [PubMed] [Google Scholar]
East 2008
- East CE. Fetal pulse oximetry during labor: theory, clinical use and the future. Pediatric Health 2008;2(6):787‐95. [Google Scholar]
East 2010
- East CE, Leader LR, Sheehan P, Henshall NE, Colditz PB. Intrapartum fetal scalp lactate sampling for fetal assessment in the presence of a non‐reassuring fetal heart rate trace. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD006174.pub2] [DOI] [PubMed] [Google Scholar]
East 2013
- East CE, Smyth RMD, Leader LR, Henshall NE, Colditz PB, Lau R, et al. Vibroacoustic stimulation for fetal assessment in labour in the presence of a nonreassuring fetal heart rate trace. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD004664.pub3] [DOI] [Google Scholar]
FIGO 1987
- FIGO subcommittee on Standards in Perinatal Medicine. Guidelines for the use of fetal monitoring. International Journal of Gynecology and Obstetrics 1987;25(2):159‐67. [Google Scholar]
Grinstead 2004
- Grinstead J, Grobman WA. Induction of labor after one prior cesarean: predictors of vaginal delivery. Obstetrics & Gynecology 2004;103(3):534‐8. [DOI] [PubMed] [Google Scholar]
Henderson 2001
- Henderson J, McCandlish R, Kumiega L, Petrou S. Systematic review of economic aspects of alternative modes of delivery. BJOG: an international journal of obstetrics and gynaecology 2001;108(2):149. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Knitza 2004
- Knitza R, Rall G, Schaller N, Kolben M. First results of a pilot study with the FetalSAT fetal pulse oximetry system [Erste ergebnisse einer pilostudie mit dem FetalSAT pulsoximetriesystem]. Geburtshilfe und Frauenheilkunde 2004;64(6):600‐5. [Google Scholar]
Kruger 1999
- Kruger K, Hallberg B, Blennow M, Kublickas M, Westgren M. Predictive value of fetal scalp blood lactate concentration and pH as markers of neurological disability. American Journal of Obstetrics and Gynecology 1999;181(5 Pt 1):1072‐8. [DOI] [PubMed] [Google Scholar]
Kuhnert 1998
- Kuhnert M, Seelbach‐Goebel B, Renzo GC, Howarth E, Butterwegge M, Muray JM. Guidelines for the use of fetal pulse oximetry during labour and delivery. Prenatal and Neonatal Medicine 1998;3(4):432‐3. [Google Scholar]
MacLennan 1999
- MacLennan A, for the International Cerebral Palsy Task Force. A template for defining a causal relationship between acute intrapartum events and cerebral palsy: an international consensus statement. BMJ 1999;319:1054‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mallinckrodt 2000
- Mallinckrodt Inc. OxiFirst (TM) Fetal Oxygen Saturation Monitoring System. Operator's Manual. N‐400 Fetal Pulse Oximeter. Pleasanton, CA USA: Mallinckrodt Inc, 2000. [Google Scholar]
Neilson 2013
- Neilson JP. Fetal electrocardiogram (ECG) for fetal monitoring during labour. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000116.pub4] [DOI] [PubMed] [Google Scholar]
Nellcor 2004
- Nellcor Puritan Bennett Inc (A division of TYCO Healthcare). OxiFirst® Fetal Pulse Oximetry System. http://www.nellcor.com (accessed 2004).
NICE 2007
- National Institute for Health and Clinical Excellence. Intrapartum Care: Care of Healthy Women and their Babies During Childbirth. NICE Clinical Guideline 55. Manchester: National Collaborating Centre for Women's and Children's Health, 2007. [Google Scholar]
Nijland 1995
- Nijland R, Jongsma HW, Nijhuis JG, Berg PP, Oeseburg B. Arterial oxygen saturation in relation to metabolic acidosis in fetal lambs. American Journal of Obstetrics and Gynecology 1995;172:810‐9. [DOI] [PubMed] [Google Scholar]
Nonnenmacher 2010
- Nonnenmacher A, Hopp H, Dudenhausen J. Predictive value of pulse oximetry for the development of fetal acidosis. Journal of Perinatal Medicine 2010;38:83‐6. [DOI: 10.1515/JPM.2010.006] [DOI] [PubMed] [Google Scholar]
Palomaki 2006
- Palomaki O, Luukkaala T, Luoto R, Tuimal R. Intrapartum cardiotocography ‐ the dilemma of interpretational variation. Journal of Perinatal Medicine 2006;34(4):298‐302. [DOI] [PubMed] [Google Scholar]
Petrou 2002
- Petrou S, Glazener C. The economic costs of alternative modes of delivery during the first two months postpartum: results from a Scottish observational study. BJOG: an international journal of obstetrics and gynaecology 2002;109(2):214‐7. [DOI] [PubMed] [Google Scholar]
Porreco 2004
- Porreco RP, Boehm FH, Dildy GA, Miller HS, Wickstrom EA, Garite TJ, et al. Dystocia in nulliparous patients monitored with fetal pulse oximetry. American Journal of Obstetrics and Gynecology 2004;190(1):113‐7. [DOI] [PubMed] [Google Scholar]
Prothia 2014
- OB Scientific. OBS‐500 Fetal pulse oximeter. Correspondence from Prothia (accessed April 3 2014).
RANZCOG 2014
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Clinical Guidelines. Intrapartum Fetal Surveillance. Third Edition. Melbourne: RANZCOG, 2014. [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rosėn 2004
- Rosėn KG, Amer‐Wåhlin I, Luzietti R, Norėn H. Fetal ECG waveform analysis. Clinical Obstetrics and Gynaecology 2004;18(3):485‐514. [DOI] [PubMed] [Google Scholar]
Saling 1996
- Saling E. Fetal pulse oximetry during labor: issues and recommendations for clinical use. Journal of Perinatal Medicine 1996;24:467‐78. [DOI] [PubMed] [Google Scholar]
Seelbach‐Gobel 1999
- Seelbach‐Gobel B, Heupel M, Kuhnert M, Butterwegge M. The prediction of fetal acidosis by means of intrapartum fetal pulse oximetry. American Journal of Obstetrics and Gynecology 1999;180:73‐81. [DOI] [PubMed] [Google Scholar]
Sehdev 1997
- Sehdev HM, Stamilio DM, Macones GA, Graham E, Morgan MA. Predictive factors for neonatal morbidity in neonates with an umbilical arterial cord pH less than 7.00. American Journal of Obstetrics and Gynecology 1997;177(5):1030‐4. [DOI] [PubMed] [Google Scholar]
Shipp 2000
- Shipp TD, Zelop CM, Repke JT, Cohen A, Caughey A, Lieberman E. Labor after previous cesarean: influence of prior indication and parity. Obstetrics & Gynecology 2000;95:913‐6. [DOI] [PubMed] [Google Scholar]
Stubán 2009
- Stubán N, Niwayama M. Adjustable fetal phantom for pulse oximetry. Review of Scientific Instruments 2009;80(5):054301. [DOI: 10.1063/1.3131632] [DOI] [PubMed] [Google Scholar]
Umstad 1993
- Umstad MP. The predictive value of abnormal fetal heart rate patterns in early labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1993;33:145‐9. [DOI] [PubMed] [Google Scholar]
Uslu 2012
- Uslu S, Bulbul A, Can E, Zubarioglu U, Salihoglu O, Nuhoglu, A. Relationship between oxygen saturation and umbilical cord pH immediately after birth. Pediatrics and Neonatology 2012;53:340‐5. [DOI] [PubMed] [Google Scholar]
Wagner 2001
- Wagner M. Fish can't see water: the need to humanize birth. International Journal of Gynecology & Obstetrics 2001;75 Suppl 1:S25‐S37. [DOI] [PubMed] [Google Scholar]
White 2010
- White CRH, Doherty DA, Henderson JJ, Kohan R, Newham JP, Pennell CE. Benefits of introducing universal umbilical cord blood gas and lactate analysis into an obstetric unit. Australian and New Zealand Journal of Obstetrics and Gynaecology 2010;50:318‐28. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
East 2000
- East CE, Chan FY, Colditz PB. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004075] [DOI] [PubMed] [Google Scholar]
East 2004
- East CE, Chan FY, Colditz PB. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004075.pub2] [DOI] [PubMed] [Google Scholar]
East 2007
- East CE, Begg L, Colditz PB. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004075.pub3] [DOI] [PubMed] [Google Scholar]


