Significance
The mitochondria, organelles that produce the largest amounts of ATP and reactive oxygen species (mROS) in living cells, are equipped with a universal mechanism that can completely prevent mROS production. This mechanism consists of mild depolarization of the inner mitochondrial membrane to decrease the membrane potential to a level sufficient to form ATP but insufficient to generate mROS. In short-lived mice, aging is accompanied by inactivation of the mild depolarization mechanism, resulting in chronic poisoning of the organism with mROS. However, mild depolarization still functions for many years in long-lived naked mole rats and bats.
Keywords: naked mole rat, aging, mitochondria, mild depolarization, antioxidant
Abstract
The mitochondria of various tissues from mice, naked mole rats (NMRs), and bats possess two mechanistically similar systems to prevent the generation of mitochondrial reactive oxygen species (mROS): hexokinases I and II and creatine kinase bound to mitochondrial membranes. Both systems operate in a manner such that one of the kinase substrates (mitochondrial ATP) is electrophoretically transported by the ATP/ADP antiporter to the catalytic site of bound hexokinase or bound creatine kinase without ATP dilution in the cytosol. One of the kinase reaction products, ADP, is transported back to the mitochondrial matrix via the antiporter, again through an electrophoretic process without cytosol dilution. The system in question continuously supports H+-ATP synthase with ADP until glucose or creatine is available. Under these conditions, the membrane potential, ∆ψ, is maintained at a lower than maximal level (i.e., mild depolarization of mitochondria). This ∆ψ decrease is sufficient to completely inhibit mROS generation. In 2.5-y-old mice, mild depolarization disappears in the skeletal muscles, diaphragm, heart, spleen, and brain and partially in the lung and kidney. This age-dependent decrease in the levels of bound kinases is not observed in NMRs and bats for many years. As a result, ROS-mediated protein damage, which is substantial during the aging of short-lived mice, is stabilized at low levels during the aging of long-lived NMRs and bats. It is suggested that this mitochondrial mild depolarization is a crucial component of the mitochondrial anti-aging system.
In 1997, one of the authors of this paper (V.P.S.) and his coworkers S. Korshunov and A. Starkov reported that a rather small decrease in the mitochondrial electric potential results in the complete arrest of the generation of mitochondrial reactive oxygen species (mROS) (1). In particular, the activation of oxidative phosphorylation by the addition of ADP to isolated mitochondria decreased the membrane potential by ∼20%, which is sufficient to prevent H2O2 formation by these organelles. In 2004 to 2008, this effect was investigated by A. Galina and colleagues in Rio de Janeiro (2–4). The authors described a novel antioxidant mechanism in mitochondria from the rat brain. This mechanism consists of the cyclic movement of ADP produced by hexokinase or creatine kinase bound to the outer surface of the outer or inner mitochondrial membrane, respectively. Researchers proposed that ATP (formed inside mitochondria from ADP and phosphate Pi at the expense of the respiratory chain-produced protonic potential, ), is used to regenerate ADP in the kinase active sites. As a result, glucose-6-phosphate (G6P) or creatine phosphate are formed. ADP produced by these kinases returns to the matrix (without dilution by the cytosol) to be converted again into ATP (schemes shown in Fig. 1 and SI Appendix, Fig. S2).
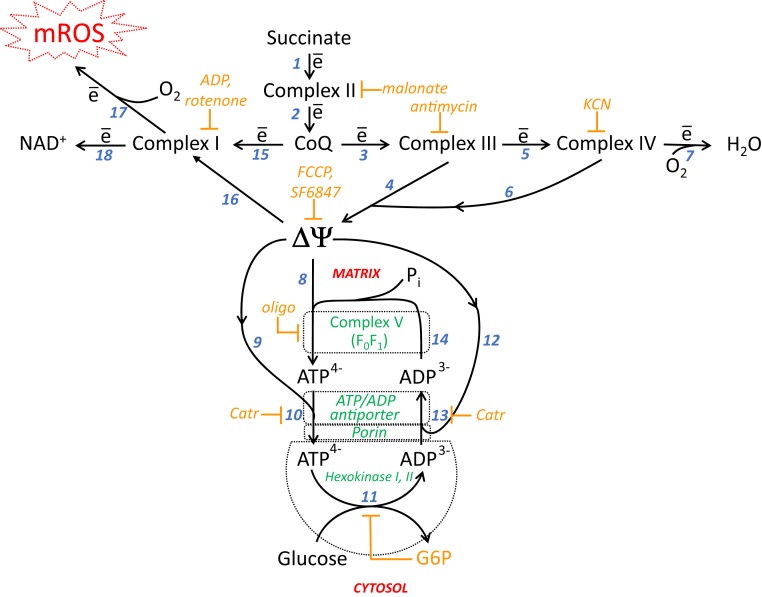
Fig. 1.
Interrelations of respiratory chain Complexes I, II, III, and IV; H+-ATP-synthase (Complex V); ∆ψ; ATP/ADP-antiporter; porin; mitochondrion-bound hexokinase I or II; and mROS. Complex I, NADH-CoQ oxidoreductase; Complex II, succinate dehydrogenase; Complex III, CoQH2-cytochrome c oxidoreductase; Complex IV, cytochrome c oxidase; Pi, inorganic phosphate; Catr, carboxyatractylate.
The difference in the electrical potentials of the mitochondrial membrane, Δψ, is the main component of mitochondrial . The activation of two kinase-mediated cycles results in a decrease in Δψ. This depolarization is rather small, however, since ATP synthesis from ADP and Pi becomes impossible if Δψ (initially ∼190 mV) decreases to ∼140 mV (1). Therefore, the kinase-induced effect is termed mild depolarization. The situation resembles the function of uncoupling protein 1 (UCP1), which serves as a protonophore if Δψ is greater than a critical value (5). The kinase-linked mild depolarization appears to be a more sophisticated mechanism than UCP1, since it is accompanied by some useful bioenergetic functions (rather than the complete dissipation of respiration energy as heat by UCP1). In the case of hexokinase or creatine kinase, the mobilization of carbohydrate catabolism (glucose phosphorylation to G6P) or the accumulation of a high-energy buffer (creatine phosphate) occurs. G6P is the first intermediate in a complicated metabolic pathway resulting in the formation of pyruvate, which is used in respiration during the Krebs cycle or, alternatively, converted to lactate, the final product of glycolysis.
In tissues other than the brain, the mild depolarization mechanism, according to Galina et al. (2–4), is much less active (kidney), almost inactive (heart), or absent (liver). In this paper, we summarize our analysis of the role of mitochondria-bound kinases in preventing mROS generation. Adult mice and mouse embryos, naked mole rats (NMRs) and their embryos, and bats of different ages were investigated. We found that in adult mice, all tissues but the liver are equipped with bound kinases that can completely prevent mROS formation. In mouse embryos, mild depolarization was observed in all tissues studied, including the liver. In our experiments, all tissues in NMRs exhibited a mild depolarization mechanism.
We also observed that aging in mice up to age 2.5 y is accompanied by partial or even complete arrest of the mild depolarization phenomenon in different tissues. This arrest does not occur in NMRs and bats at least before age 12 to 13 y.
We assume that the slow permanent attenuation of mild depolarization in mice represents a result of the operation of the aging program, an effect that increases mROS production by the respiratory chain. Regarding mild depolarization, it seems to be a novel, universal anti-aging mechanism that specifically prevents the pro-aging effect of mROS.
Results
Mild Depolarization Is Inherent in Different Tissues.
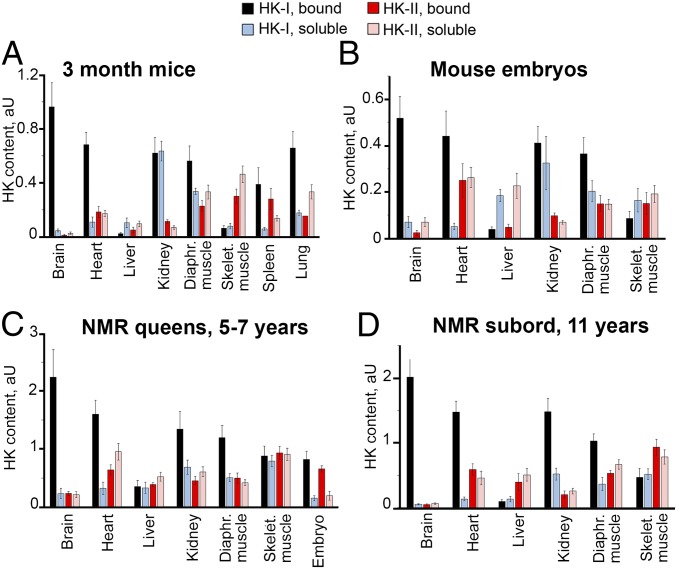
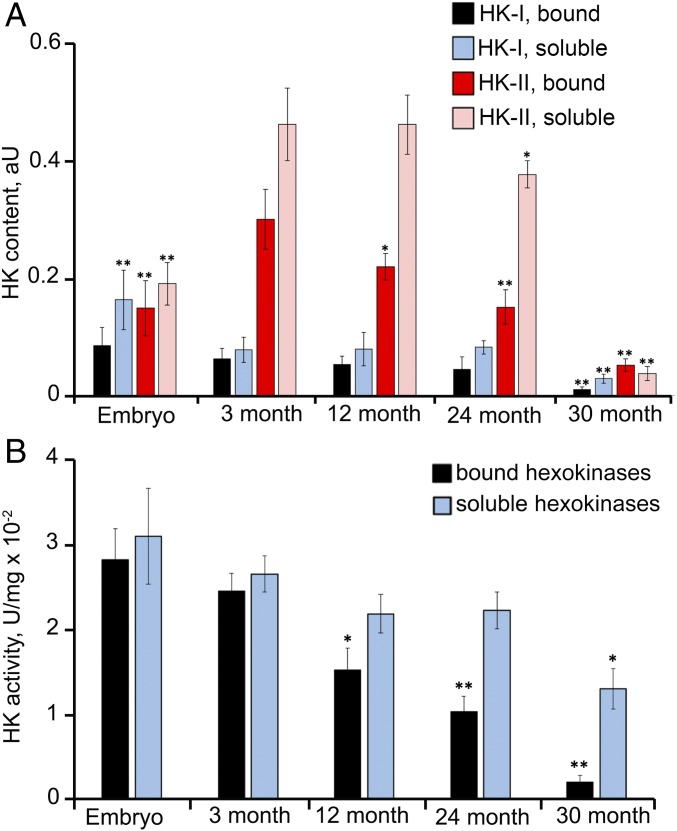
In the first series of experiments, we measured the amounts of soluble and mitochondria-bound hexokinases I and II in eight tissues from adult mice and NMRs of different ages, as well as in six tissues from mouse embryos (Fig. 2 and SI Appendix, Fig. S3). In adult mice, consistent with the data reported by Galina et al. (2–4), very high concentrations of bound hexokinases were detected in the brain and very low levels were detected in the liver. However, surprisingly, rather high hexokinase levels were also observed in the heart, kidney, diaphragm muscle, skeletal muscle, lung, and spleen. In mouse embryos and adult NMRs, measurable amounts of bound hexokinases were present in all tissues studied. Significant differences between NMR queens and NMR subordinates were not observed for most tissues (Fig. 2 C and D); moreover, in all tissues from adult mice except skeletal muscle, bound hexokinase I dominated over bound hexokinase II which is known to have much lower affinity to glucose. In skeletal muscle tissue from 3-mo-old mice, hexokinase II strongly dominated over hexokinase I (Fig. 2A). Levels of the two hexokinases were equal in skeletal muscle tissue from 3-mo-old NMRs, but hexokinase II was higher in 3-y-old and older animals (SI Appendix, Fig. S11). The late appearance of hexokinase II dominance in skeletal muscles is apparently a trait of neoteny (prolongation of youth), explaining the unique longevity of NMRs (6–8).
Fig. 2.
Contents of mitochondria-bound and soluble hexokinases (HK) I and II in different tissues from mice and NMRs. Western blot analyses were performed with the appropriate monoclonal antibodies. Mean values for three to four repeats are presented. (A) 3-mo-old mice (n = 7). (B) Mouse embryos (n = 21). (C) NMR queens (n = 5). (D) NMR subordinates (n = 3).
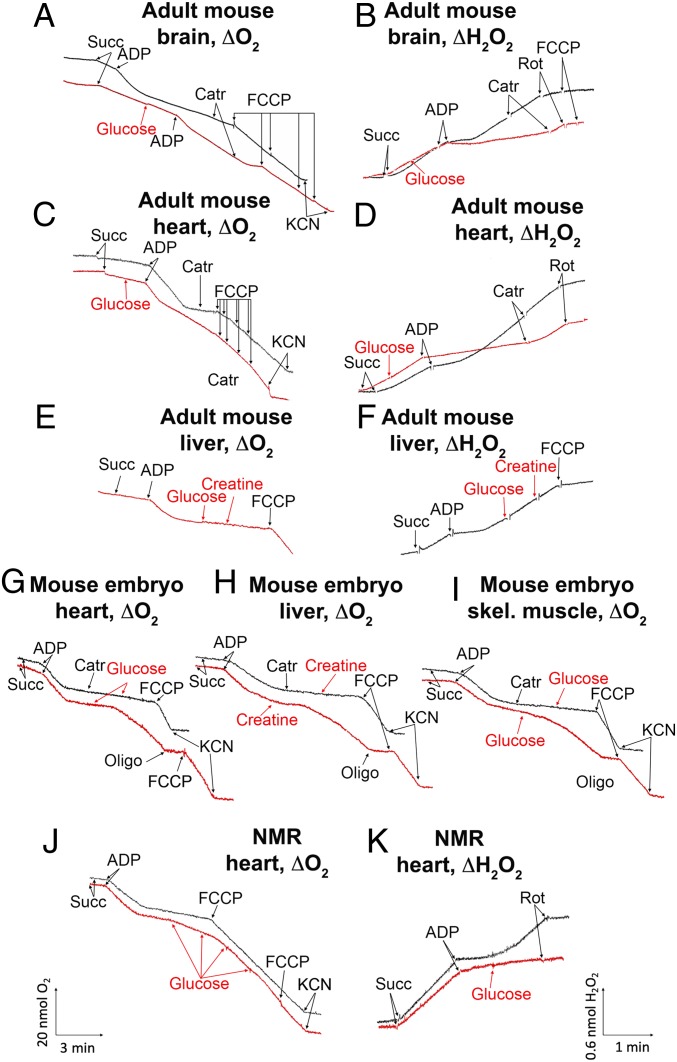
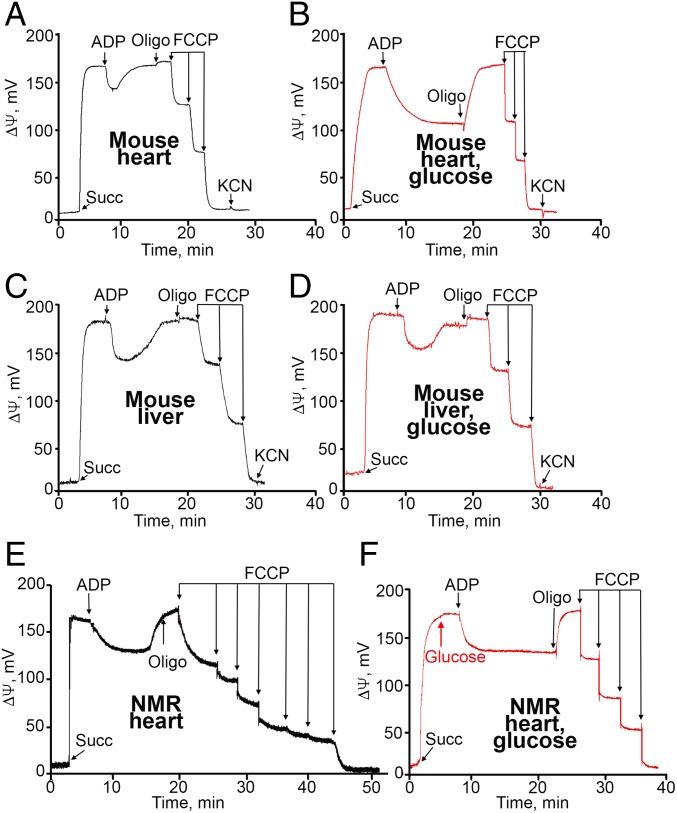
Confocal microscopy of cultures of mouse and NMR liver fibroblasts revealed that hexokinase is bound to mitochondria in intact cells (SI Appendix, Fig. S4). A subsequent series of experiments (Fig. 3) was carried out in mitochondria isolated from various tissues of mice and NMRs. Respiration and H2O2 generation were measured (Fig. 3 A, C, E, and G–J and Fig. 3 B, D, F, and K, respectively). The respiration rate was followed polarographically when [O2] was estimated as a function of time. Respiration was initiated by the addition of a respiratory substrate, succinate (succ). Before succinate addition (e.g., black curve in Fig. 3A), O2 consumption was negligible, since respiration is limited by the absence of its substrate (State 1). Succinate slightly stimulated respiration; now it was limited by the absence of ADP (energy acceptor, State 2). The addition of ADP increased the respiration rate to the maximal level (State 3); however, within a few minutes, O2 consumption was lowered to the level before ADP addition, since ADP was exhausted while being phosphorylated to ATP (State 4). Carboxyatractylate (CAtr), the ATP/ADP antiporter inhibitor, and oligomycin, a H+-ATP synthase inhibitor, decreased O2 consumption when added before ADP. The maximal O2 consumption rate was achieved by adding an uncoupler, carbonylcyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), a protonophorous uncoupler of respiration and phosphorylation (9). The red curves in Fig. 3 show mitochondrial respiration when glucose or creatine was added in State 2. It can be seen that this addition does not influence States 2 and 3, but State 4 does not exist now. In fact, State 3 continues as if the added ADP was not exhausted. The novel state was inhibited by CAtr, and the inhibition was abolished by FCCP. Fig. 3B shows the formation of mitochondrial H2O2 measured by fluorescent spectrophotometry (using Amplex Red reagent and horseradish peroxidase). The inhibition of respiration at the State 3 to State 4 transition due to ADP exhaustion was accompanied by a strong increase in H2O2 formation (black curve). This increase was completely abolished by adding glucose (red curve).
Fig. 3.
Respiration and H2O2 generation by mitochondria isolated from different tissues from adult mice (A–F), mouse embryos (G–I), and adult NMRs (J and K). Additions to the incubation mixture: 50 μM CArt, 3 mM creatine, 1 μg oligomycin (Oligo), 1 mM glucose, 10−7 M FCCP, 2 μM rotenone, and 0.5 mM KCN. Mitochondria were isolated in 300 mM mannitol, 0.5 mM EDTA, and 20 mM Hepes-KOH, pH 7.6. Mitochondria were incubated in medium containing 220 mM mannitol, 10 mM potassium lactobionate, 5 mM potassium phosphate, 2 mM MgCl2, 10 μM EGTA, and 20 mM Hepes-KOH, pH 7.6. State 2 respiration was initiated by adding succinate (10 mM). State 3 or 3u was induced by the addition of 100 µM ADP or 10 nM FCCP, respectively. The concentration of mitochondria in the incubation vessel was 0.1 mg protein/mL. The temperature was 25 °C.
The data in Fig. 3 A and B were obtained using isolated mouse brain mitochondria, confirming the results of Galina et al. (2, 4). Further experiments revealed that many mouse tissues other than the brain demonstrate complete arrest of State 4 H2O2 formation by adding glucose (Figs. 3 C and D and SI Appendix, Figs. S6 and S12). On the other hand, in line with the observations of Galina et al., mouse liver mitochondria do not respond to the glucose addition with an increase in respiration (Fig. 3 E and F). However, the mouse embryo liver showed an effect of glucose similar to that seen in other tissues (Fig. 3H).
Consistent with observations in the brain reported by Galina et al. (3), creatine replaced sugars in stimulating the State 4 respiration of the mouse mitochondria (Fig. 3H). Similar to glucose, the effect of creatine was completely blocked by oligomycin and CAtr (Fig. 3H). Creatine-induced respiration was specifically inhibited by the creatine kinase inhibitor guanidopropionic acid (SI Appendix, Fig. S5D). The glucose effect was strongly suppressed by the hexokinase inhibitor glucose 6-phosphate (G6P) (Figs. 7 and 10 and SI Appendix, Figs. S5, S9, and S10) but not by fructose 6-phosphate (F6P) and 2-deoxyglucose 6-phosphate (DOG6P), which cannot inhibit hexokinase (SI Appendix, Fig. S5).
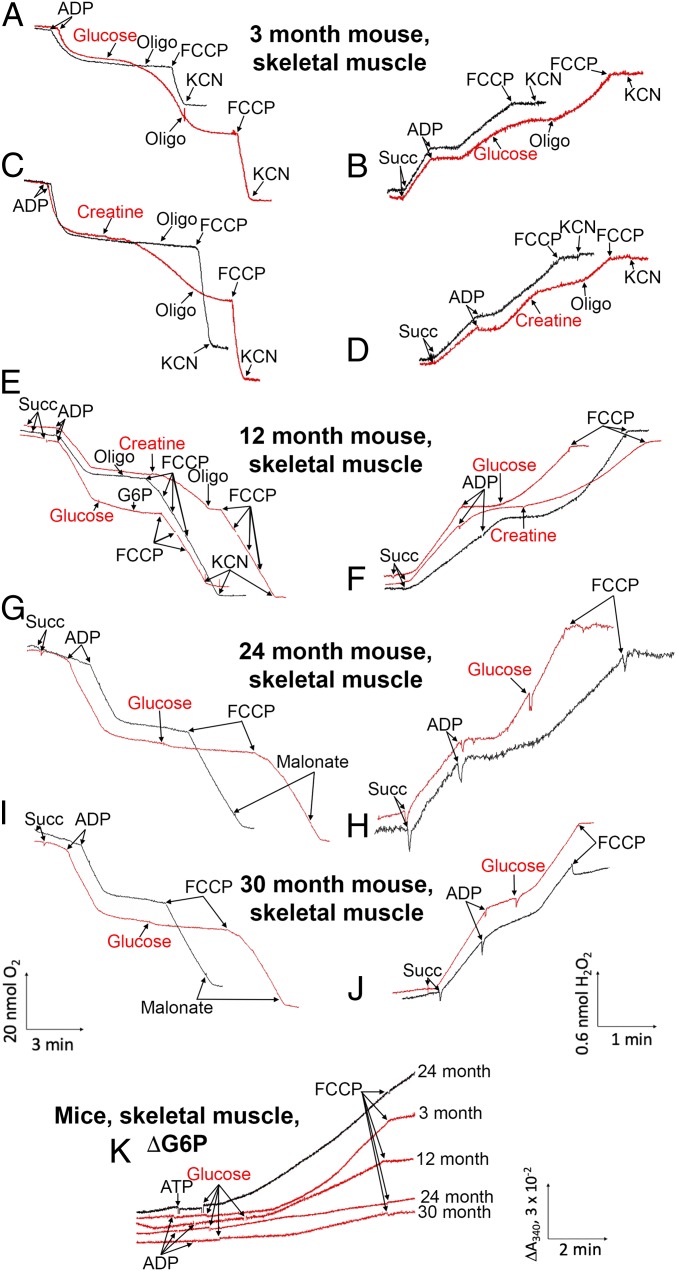
Fig. 7.
(A–J) Aging inhibits glucose- and creatine-stimulated State 4 respiration, as well as the antioxidant activity of these two compounds in mouse skeletal muscle mitochondria. (K) Aging-induced inhibition of glucose phosphorylation in skeletal muscle mitochondria from mice of different ages; comparison of added ADP and ATP. Conditions are as described in Fig. 3.
Fig. 10.
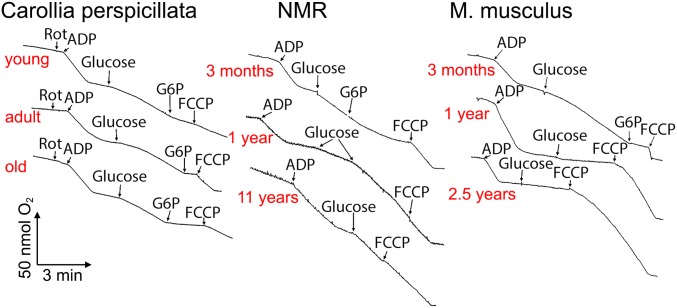
Bats (C. perspicillata), similar to NMRs and in contrast to mice, maintain mild polarization for many years. Heart mitochondria were analyzed. Conditions are as detailed in Fig. 3.
Fig. 4 shows the generation of Δψ by heart or liver mitochondria from adult mice and NMRs. The addition of ADP to heart mitochondria induced a decrease in Δψ (mild depolarization of the mitochondrial membrane due to the State 2 to State 3 transition). When ADP was exhausted (the State 3 to State 4 transition), Δψ increased. This increase disappeared on the addition of 1 mM glucose. Subsequent additions of the uncoupler FCCP completely depolarized the mitochondria. In the adult mouse liver, glucose did not affect Δψ. In mouse embryos and adult NMRs, a glucose effect was observed in both the liver and the heart.
Fig. 4.
Effect of glucose on the membrane potential of the heart (A and B) and liver (C and D) mitochondria from adult mice or of heart mitochondria from adult NMRs (E and F). In B, D, and F, 1 mM glucose was added before succinate (red curves). Black curves represent absence of glucose. The conditions were the same as described in Fig. 3.
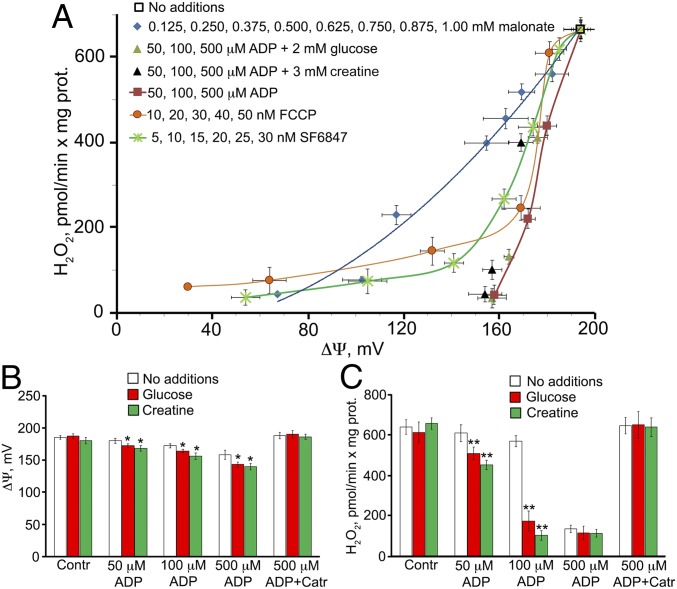
Fig. 5A shows some quantitative relationships among Δψ, ADP, and the generation of H2O2 are presented. A rather small decrease in Δψ resulted in substantial inhibition of H2O2 generation by mitochondria. Malonate (an inhibitor of succinate dehydrogenase), two protonophores (FCCP and 3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile [SF6847]), ADP, glucose, and creatine have been used as Δψ-lowering agents. Of note, some of the compounds listed above may decrease H2O2 levels through mechanism(s) other than a decrease in Δψ. Unfortunately, the molecular details of mROS generation remain obscure. However, this process was strongly suppressed by rotenone and other Complex I inhibitors, even when succinate was used as the respiratory substrate. Interestingly, high ADP concentrations partially inhibited both reverse electron transfer from succinate to NAD+ and H2O2 generation. This effect was resistant to oligomycin but sensitive to CAtr (SI Appendix, Fig. S7A). The mechanism of this phenomenon might be competition between ADP and NAD+ for binding to Complex I (10) or the occupation by ADP of the predicted ADP-specific binding site in subunit NDUFA10 of this complex (11). ATP inhibited Complex I to a lesser extent than ADP did; however, the effect of ATP was observed at a lower nucleotide concentration than seen with ADP (SI Appendix, Fig. S7B).
Fig. 5.
(A) H2O2 generation by heart mitochondria from adult mice as a function of the membrane potential. The conditions and the effects of various depolarizing agents (malonate, uncouplers [FCCP and SF6847], ADP, glucose, and creatine) are as described in Fig. 3A. (B and C) Membrane potential (B) and H2O2 generation (C) as a function of ADP concentration. *P < 0.01; **P < 0.001, for the effects of glucose or creatine.
Hydrophobic protonophorous uncouplers such as FCCP and SF6847 inhibit electron transport in Complex I and some other steps in the respiratory chain (12). Therefore, similar to ADP, they exert two inhibitory effects on mROS generation: a decrease in ∆ψ due to an increase in H+ conductance and inhibition of Complex I. On the other hand, hydrophilic malonate only exerts a decrease in Δψ due to a decreased rate of succinate oxidation, without any direct influence on Complex I.
Fig. 5 B and C illustrate two requirements for mild depolarization as an antioxidant mechanism: a decrease in Δψ and the addition of glucose and/or creatine in the presence of 100 μM ADP. In the absence of glucose or creatine, the same antioxidant effect requires 500 μM ADP. The decrease in Δψ induced by glucose, creatine, and ADP (Fig. 4B) is actually much smaller than the decrease in H2O2 production induced by the aforementioned compounds (Fig. 5C). A possible explanation is that the Δψ ordinate, but not the ΔH2O2 ordinate, is presented on the logarithmic scale. According to the Nernst equation, a decrease in mitochondrial Δψ from 180 mV to 120 mV (i.e., by 33%) results in a 10-fold decrease in the transmembrane gradient of a univalent penetrating cation.
Importantly, the addition of glucose and creatine was not necessary if mitochondria were isolated and incubated with sucrose instead of mannitol. An explanation for this observation is the spontaneous formation of glucose from sucrose, which does not occur with mannitol. Consistent with this hypothesis, glucose decomposition (mediated by the addition of glucose oxidase to a sucrose-containing medium) inhibited State 4 respiration (SI Appendix, Fig. S8A). As shown in SI Appendix, Fig. S8C, the addition of glucose in the presence of mannitol (SI Appendix, Fig. S8B) may be substituted by fructose or 2-deoxyglucose (2DOG). In SI Appendix, Fig. S10, the fast rate of State 4 respiration observed in mouse embryos and adult NMRs was decreased to State 2 after the mitochondria were washed with 1 mM G6P or 300 mM KCl, which are known to remove bound hexokinases from the surface of the outer mitochondrial membrane (2–4).
Aging Is Accompanied by the Disappearance of Mild Depolarization in Mice but Not in NMRs or Bats.
In the next series of experiments, we studied the effects of aging on the mild depolarization mechanism. Therefore, we measured the number of mitochondria-bound hexokinases, assessed their ability to phosphorylate glucose, and evaluated the antioxidant activities of kinases in mouse embryos as well as in adult mice, NMRs, and bats of different ages. The results are presented in Figs. 6–10 and SI Appendix, Figs. S11 to S20. The levels and activities of mitochondria-bound hexokinases I and II were substantially decreased in aging mice beginning at age 1 y. By age 2.5 y, these parameters were very low in skeletal muscle (Fig. 6 and SI Appendix, Fig. S13). Not surprisingly, mild depolarization induced by hexokinases was not observed in the muscles of 2.5-y-old mice (Fig. 7). In the lung, kidney, and brain, the levels and activities of hexokinases also decreased with age, but the effect was less than that in skeletal muscle. In the lungs and kidneys of 2.5-y-old mice, the addition of glucose still produced some antioxidant effect, but to a lesser degree than the changes observed in 3- and 12-mo-old mice (SI Appendix, Fig. S12). Mild depolarization was not observed in the brains of 2.5-y-old mice despite the clearly incomplete decreases in levels of bound hexokinases (SI Appendix, Fig. S13). Remarkably, hexokinase activity was decreased to a greater extent than the content of the bound enzyme. In fact, the amount of hexokinases in the brains of 2.5-y-old mice was twofold lower than that in 3-mo-old mice, and the rate of G6P formation by the mitochondria was threefold lower, whereas mild depolarization completely disappeared (SI Appendix, Figs. S12 and S13). These relationships might be related to possible alterations in the oligomeric structures of bound hexokinases, which can exist as tetramers, dimers, and monomers. Mitochondria-bound hexokinase is a tetramer that displays greater enzymatic activity compared with monomers (13, 14).
Fig. 6.
Disappearance of hexokinases bound to skeletal muscle mitochondria during aging of mice. (A) Contents of hexokinases I and II in mitochondria and cytosol. (B) Activities of hexokinases I and II in the mitochondria and cytosol. *P < 0.01; **P < 0.001 (mouse embryos, 12-, 24-, or 30-mo-old mice compared with 3-mo-old mice).
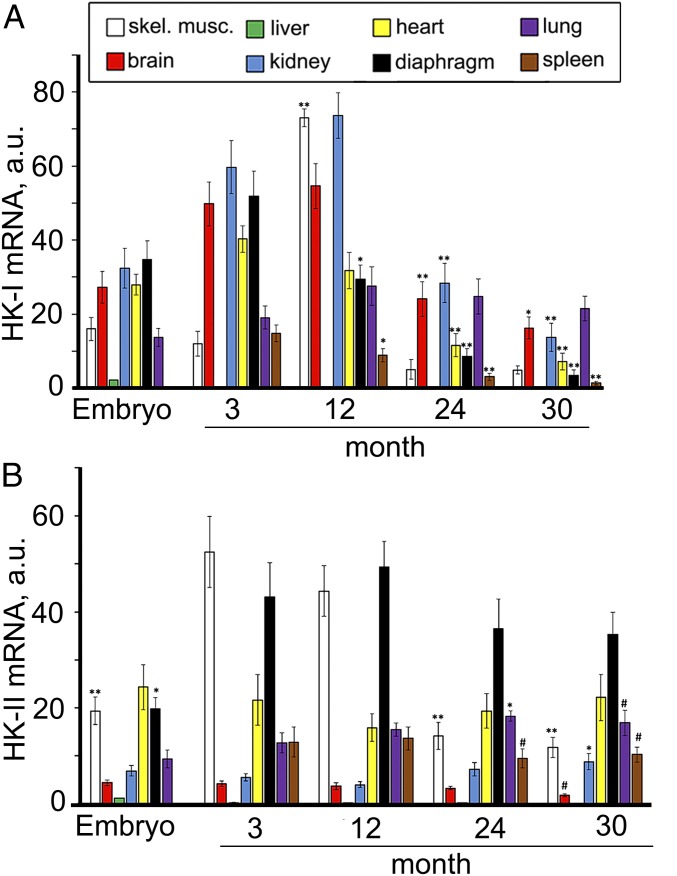
In Fig. 8A and SI Appendix, Fig. S22 A and B, glucose stimulation of State 4 respiration in mitochondria from different tissues of mice and NMRs is shown as a function of age. In fact, the curves represent the values of the ratio of the respiration rate after exhaustion of ADP in the presence of glucose to the respiration rate without glucose. In the majority of mouse tissues, this ratio decreased to 1 (no glucose stimulation) at age 30 mo (Fig. 8A and SI Appendix, Fig. S22A). In NMRs, the curves proved to be parallel to the abscissa at least up to 7 y and slightly decreases at 11 y (Fig. 8A and SI Appendix, Fig. S22B). Fig. 9 shows the expression of hexokinase I and II mRNA in mice of various ages. Hexokinase I mRNA expression was strongly decreased at age 24 and 30 mo. Hexokinase II mRNA expression was increased in skeletal muscle at 3 and 12 mo and strongly decreased thereafter. Among other tissues, only the lung showed a stable increase in hexokinase II mRNA up to 30 mo.
Fig. 8.
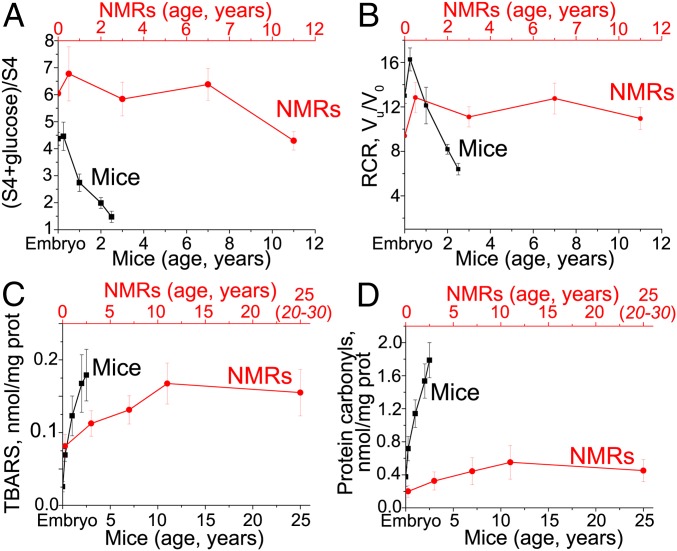
(A and B) Average values of glucose stimulation or the RCR for all tissues in mice and NMRs of various ages. Stimulation of State 4 respiration by glucose disappears with age in mice but not in NMRs. In mice, RCR decreases with age, but this effect is lower than that of glucose stimulation of respiration (A). In NMRs, the RCR does not depend on age (B). RCR was measured as the ratio of the State 3u respiration rate in the presence of uncoupler FCCP to State 4o (respiration rate after oligomycin addition). (C and D) Age-dependent oxidation of lipids and proteins in mouse and NMR tissues. The average values in all tissues of the malondialdehyde level (TBARS probe, lipid peroxidation) (C) and protein carbonylation level (D) are shown. Black, mice; red, NMRs. The data measured for individual tissues are shown in SI Appendix, Figs. S22 and S23.
Fig. 9.
Effect of aging on the expression of hexokinase I (A) and II (B) mRNAs in eight mouse tissues. #P < 0.05; *P < 0.01; **P < 0.001 (12-, 24-, or 30-mo-old mice compared with 3-mo-old mice).
To at least partially compensate for the dramatic decrease of the hexokinase-linked antioxidant effect, liver and lung stimulate the expression of mRNA of two specific antioxidant enzymes, catalase and glutathione peroxidase 1 (SI Appendix, Fig. S20 A and C). Another compensatory mechanism appears to be partial uncoupling of respiration and oxidative phosphorylation, which, like mild depolarization, can lower Δψ and inhibit mROS generation. Such an effect was measured using the respiratory control ratio (RCR). This parameter was decreased in aging mice but not in NMRs (Fig. 8B and SI Appendix, Fig. S22 C and D).
In the majority of tissues, the levels of catalase, GPx1, and some other antioxidant enzymes, as well as glutathione, decrease with age in mice but not in NMRs. However, in certain mouse tissues, a temporary stabilization or even an increase in these levels was seen (SI Appendix, Figs. S15–S18). Nevertheless, total antioxidant activity was lower in older mice, as demonstrated by the growing peroxidation of lipids (TBARS probe for malondialdehyde) and proteins (their carbonylation) in old mouse tissues (Fig. 8 C and D and SI Appendix, Figs. S19 and S23). In the case of lipids, dramatic peroxidation is observed in the mouse skeletal muscles, diaphragm, spleen and heart (SI Appendix, Fig. S23A). In NMRs, the only tissue studied that showed strong lipid peroxidation was spleen (SI Appendix, Fig. S23C).
The difference in protein carbonylation between the two rodents is even more obvious than the difference in lipid peroxidation. Here all the mouse tissues demonstrated a very strong effect, whereas in NMRs, six of eight tissues showed no or very small age-dependent increases in carbonylation. Fig. 8D is especially demonstrative. In this figure, we calculated the average carbonylation level for eight tissues of mice and NMRs of different ages. A qualitative difference between the two animals is obvious in a strong permanent increase in carbonylation up to 30 mo in mice and very low carbonylation in the 30-mo-old NMRs, which increased slightly up to 11 y and was then stabilized for up to 20 to 30 y (see below).
The tissue-specific differences within an animal might be due in part to the activity of anti-ROS defense mechanisms other than mild depolarization. As shown in SI Appendix, Table S1, three types of defense mechanisms are seen in vertebrates: a decrease in intracellular [O2], mild depolarization of the inner mitochondrial membrane, and antioxidant enzymes and some low molecular weight antioxidant compounds, mainly glutathione. The first mechanism is the simplest. The generation of mROS is a linear function of [O2] (15). A decrease in O2 transport from the lungs to other tissues is sufficient to attenuate this process. Normally, the [O2] in tissues is approximately one order of magnitude lower than the [O2] in the air; however, in one tissue, the lung this anti-ROS mechanism is impossible. Therefore, unsurprisingly, a smaller decrease in age-related mild depolarization was observed in the lung than that in most of the other tissues (SI Appendix, Table S2 and Figs. S12 and S13). Moreover, the levels of some anti-ROS enzymes, such as GR (SI Appendix, Fig. S16A), SOD (SI Appendix, Fig. S18A), and GPx1 (SI Appendix, Fig. S20C), increase with age in the lung compared with many other tissues, in which levels of catalase, GR, glutathione, and mild depolarization are substantially decreased in older mice.
Regarding mild depolarization, we should consider that one mouse tissue, the liver, is deprived of this mechanism immediately after birth. In our opinion, the explanation for this finding is the Cori cycle (SI Appendix, Fig. S21; discussion below). In this case, catalase, a potent antioxidant enzyme, plays a leading role in anti-ROS defense. Its activity increases with age in the mouse liver. This enzyme at least partially compensates for the severe oxidative stress observed in older organisms.
Summarizing our studies on mice from embryos to very old (2.5 y) adults clearly shows that aging is accompanied by a strong decrease in mild depolarization (Fig. 7 and SI Appendix, Fig. S12), the activity of antioxidant enzymes (GR, catalase, and GPx1; SI Appendix, Figs. S16A and S20 B and D), and a low molecular weight antioxidant (reduced glutathione) (SI Appendix, Fig. S17A).
Experiments on long-lived NMRs yielded quite different results from those in mice. Here the enzymes mediating mild depolarization (bound hexokinases I and II) were observed in the mitochondria of all tissues studied (Figs. 2 and 8 and SI Appendix, Figs. S5, S9, and S11). Glucose and creatine exerted similar effects on State 4 respiration in embryos, very young, and adult NMRs. Moreover, liver mitochondria of NMRs contained hexokinases, similar to other NMR tissues (SI Appendix, Fig. S11B). These findings might indicate the neoteny inherent in NMRs (6, 8); adult NMRs resemble mouse embryos with respect to mild depolarization in the liver. Remarkably, high catalase levels were detected in NMR livers (SI Appendix, Fig. S15A), although the mild depolarization enzymes were not decreased for at least 11 y (Fig. 2).
In the final series of experiments, we decided to compare the short-lived mouse and long-lived NMR with the long-lived bat Carollia perspicillata (lifespan, 17 y). As shown in Fig. 10, the addition of glucose to heart mitochondria from the old bat substantially increased respiration, just as occurred with mitochondria from the 11-y-old NMR but not with mitochondria from the 2.5-y-old mouse. Thus, the glucose probe in mice, NMRs, and bats indicates that the rapid disappearance of mild depolarization with age is inherent to short-lived, but not long-lived, mammals.
Discussion
Mild Depolarization Represents an Antioxidant Mechanism to Inhibit mROS Generation in the Majority of Tissues; the Effect Disappears with Age in Mice but Not in NMRs.
The results of the experiments described in this paper can be summarized as follows:
1. A moderate decrease in mitochondrial membrane potential, coupled with the respiratory synthesis of ATP consumed by mitochondria-bound hexokinases and creatine kinase (mild depolarization), prevents mROS generation. This antioxidant mechanism is present in any organ of the mouse, with the exception of the liver.
2. In mouse embryos, mild depolarization occurs in all studied tissues, including the liver.
3. Aging in mice inhibits mild depolarization mechanisms. In 2.5-y-old mice, the inhibition is complete in the skeletal muscles, heart, brain, diaphragm, and spleen and partial in the lung and kidney. The aging effects are due to the substantial decrease in the number of mitochondria-bound hexokinases and/or the loss of the ability of bound hexokinases to activate the depolarization mechanism.
4. The aforementioned decrease in mild depolarization does not occur in old NMRs or bats.
5. Bound hexokinases I and II dominate over their soluble forms in all adult mouse tissues except the liver. Bound hexokinase I is present at higher levels than hexokinase II in the brain, heart, kidney, diaphragm, and lung, whereas hexokinase II, which has a 10-fold lower affinity for glucose than hexokinase I (16), is present at higher levels in skeletal muscle. Approximately equal amounts of the two bound hexokinases are detected in the spleen.
6. In all tissues in which sugars or creatine stimulate State 4 respiration and inhibit mitochondrial H2O2 production, this increase in O2 consumption is accompanied by a small but measurable and highly reproducible decrease in Δψ, which is sufficient to prevent mROS generation.
Thus, the antioxidant mechanism based on the mild depolarization of mitochondria, resulting in the inhibition of mROS generation, is present in the majority of the main tissues of mammals and is not specific to the brain, as was originally suggested (2, 4). Aging arrests depolarization in short-lived mice but not in long-lived NMRs and bats.
Molecular Mechanism of Mild Depolarization.
Our data are consistent with the conclusion reported by Galina et al. that the tightly bound hexokinases I and II and creatine kinase act to maintain a high ADP concentration inside mitochondria. The ATP/ADP antiporter is the only protein that translocates ATP and ADP through the inner mitochondrial membrane. The antiporter is responsible for the exchange of one molecule of ATP4− for one molecule of ADP3−. Thus, the protein mediates the electrophoretic (∆ψ-driven) transmembrane flux of one negative charge from the negatively charged matrix to the positively charged cytosol. In the matrix, ADP binds to factor F1 of H+-ATP synthase, an enzyme that saturates the inner surface of the inner mitochondrial membrane. In the synthase catalytic site, ADP is converted to ATP at the expense of , which is presented mainly as ∆ψ. At this stage, as well as during the ATP4−/ADP3− antiport, a decrease in ∆ψ occurs, which is defined here as mild depolarization. ATP is released from F1 to the matrix and binds the ATP/ADP antiporter, which, like H+-ATP synthase, is located in the inner membrane. ATP4− is translocated (in exchange for ADP3−) to the opposite (outer) surface of the inner membrane. The antiporter forms a complex with porin, a protein located in the outer mitochondrial membrane. ATP is transferred from the antiporter to porin, which is bound to hexokinase. From porin, the nucleotide is transferred to the hexokinase catalytic site, forming G6P and ADP from glucose and ATP, respectively. The cycle is completed by the transport of ADP to the matrix. Importantly, neither ATP nor ADP can be released to the cytosol from the bound hexokinase catalytic site. For ADP, the only way to escape from the bound hexokinase is the porin-antiporter complex (Fig. 1 and SI Appendix, Fig. S2).
The translocation of ATP and ADP inside the ATP/ADP antiporter, porin, and hexokinase allows nucleotides to move from the matrix to the hexokinase active site without release to the intermembrane space and cytosol. This movement resembles the transport of oil by a pipeline on the bottom of an ocean. The role of the pump is performed by the ATP/ADP antiporter, which performs electrophoretic ATP4−/ADP3− exchange.
Mystery of Complex I.
As shown in Fig. 1, two complexes are present in the respiratory chain that reduce O2: Complex I and Complex IV. In these complexes, O2 is reduced to superoxide and H2O, respectively. The second reaction represents the main mechanism of mitochondrial respiration. The first reaction is traditionally considered an inevitable side effect of electron transfer at the start of the respiratory chain, namely the premature leakage of some electrons from the chain due to the direct attack of electron carrier(s) by O2. This reaction consists of one electron reduction of O2. The process is characterized by a negative redox potential, in contrast to the four electron O2 reduction by Complex IV, which has a very positive redox potential (9). All respiratory chain electron carriers with negative redox potential are localized in Complex I (9); therefore, superoxide production by the respiratory chain is assumed to be catalyzed mainly by Complex I. Apparently, the attack of O2 by a Complex I electron carrier requires a high Δψ value for the reduction of such a carrier. This process might be mediated by the Complex I cluster FeS1a, which has a more negative redox potential than NAD (9). Based on these relationships, FeS1a is not involved in electron transfer from NAD to CoQ, the main function of Complex I. If this hypothesis is true, FeS1a appears to be a dangerous mistake of evolution, as mitochondria are equipped with a mROS-producing redox group that is unnecessary for respiration per se. However, the conclusion of an evolutionary mistake should not be touted! Francis Crick stated that each biologist should follow a good rule that evolution is always cleverer than he.
The mystery of Complex I can hardly be explained by assuming that mROS are involved in the defense against pathogenic microorganisms. Such a function is first of all inherent in the NADPH oxidase of the outer cell membrane, which produces O2•– released in the tissue intercellular space. More likely, mROS induce programmed death of the cell (apoptosis) or even the macroorganism (phenoptosis) (17–19). In the latter case, mROS may eliminate a badly infected individual from a lineage or population, thereby preventing an epidemic (septic shock; ref. 20). The mROS-generating machinery should be tightly controlled by the macroorganism to operate as a suicide inducer. Occasional leakage of electrons from the respiratory chain would not be an optimal mechanism. Rather, the mechanism in question must be very specialized in its unusual function that is crucial for the choice between life and death. Remarkably, the maximal production of mROS by Complex I does not result from mitochondrial damage. It requires a maximal Δψ level and the optimal state of all other mitochondrial parameters.
An additional function of the Complex I mROS-producing machinery may consist of maintaining stable and regulated levels of mROS in the cell. Its control must be organized by a special regulatory mechanism. Here the situation may resemble that of aquaporin. Biomembranes are permeable to water molecules; nevertheless, evolution led to the development of aquaporin, a special protein with one function, to increase the water permeability of membranes by a factor of ∼10. Thus, the actual water permeability of the cell is determined by the activity of aquaporin (which is regulated) rather than by the spontaneous (and thus uncontrolled) H2O permeability of a membrane.
mROS and Aging.
The participation of mROS and Complex I in aging has received increasing attention in recent years (e.g., ref. 21). Our concept of the aging program is based on the assumption that mROS (the levels of which increase with age) function as a poison that causes chronic phenoptosis (9). Originally, the idea of mROS as endogenous poisons initiating aging was put forward by Harman (22) as a version of his concept connecting aging with ROS (23). Later, we used this idea to develop a scheme of aging as a program invented by evolution to increase its rate (24–26). The data presented in this paper are in fact in line with such a scheme, which is already supported by several other pieces of evidence (reviewed in refs. 6, 9, 27, and 28). Among the data in question, the most obvious observations are that in short-lived mice, such a potent mechanism preventing mROS generation as mild depolarization is switched off with age, whereas in long-lived NMRs and bats, it operates for many years. We can explain how NMRs prevent this switching off. This is neoteny, a strong deceleration of the total ontogenesis program, which includes aging as its subprogram (8).
An interesting possibility is that the mild depolarization is retained during a the long lifetime of NMRs because of the high iron level in the African red soil where NMRs live (29). This should inevitably result in a high concentration of nonheme iron in NMR tissues. Due to the Fenton reaction (OH• formation from H2O2, catalyzed by Fe2+), NMRs are always under some conditions of oxidative stress, which gives rise to rather high levels of lipid peroxidation products in the blood, urine, and tissues. These levels are higher than those in young mice. In fact, NMRs stabilize mROS and thus lipid peroxidation at rather high levels even under laboratory conditions (Fig. 8C). However, this is not the case for protein peroxidation, which obviously may be even more dangerous than the peroxidation of lipids. Remarkably, the peroxidation of lipids and proteins increased with age very slowly in NMRs but very strongly in mice (Fig. 8 C and D). At any age, the amount of peroxidized (carbonylated) proteins is significantly lower in adult NMRs than in mice (Fig. 8D). This is a consequence of the age-dependent decrease in proteasome levels in mice and other animals (30–34) and their high and stable level in NMRs at any age (35, 36). Demonstrative results were obtained by Buffenstein et al. (37), who compared the activities and contents of various cytosolic proteasomes and immunoproteasomes in the livers of NMRs and mice with age. These parameters in NMRs were increased compared with those in mice from 1.5-fold to many-fold. Moreover, the percentage of polyunsaturated fatty acids (i.e., the peroxidation index) increases in aging mice and other animals (38) but not in aging NMRs (29, 39). Buffenstein et al. also identified specific protein-stimulating activity of the proteasome in the NMR cytosol that is absent in other rodents (35).
According to Lassing et al. (40), cysteine 272 in β-actin is one of the most ROS-sensitive amino acid residues among mammalian proteins. Replacement of this residue by the ROS-resistant serine specifically in NMRs was recently reported by Gladyshev et al. (41). Certainly, mitochondrial proteins should be one of the first targets for attack by mROS. To decrease this risk, the NMR cells contain much larger amounts of autophagosomes than the cells of other mammals, indicating a high probability of purification of the cytoplasm from damaged mitochondria (30). Perhaps there are also other, yet unknown mechanisms preventing the accumulation of peroxidated proteins in old NMRs.
Our knowledge of NMR aging remains limited. The age of the oldest animal living in a laboratory is 31 y (42). Perhaps in older animals, some traits of mROS-related aging will be found. One of the aging traits has been described in this paper. We found replacement of hexokinase I by hexokinase II in skeletal muscle. Our experiments showed that mouse embryo mitochondria have higher levels of hexokinase I than of the less-active hexokinase II. The muscle mitochondria of the adult mouse, in contrast to the embryo, mainly bind hexokinase II (Fig. 2 A and B); therefore, initiation of mild depolarization requires much higher glucose concentrations in adult mouse skeletal muscle mitochondria compared with mitochondria from mouse embryos. These relationships could be predicted if it is assumed that aging program is activated in adult mice but not in embryos.
An interesting observation was obtained when skeletal muscles of NMRs were studied. Here, in 3-mo-old NMR subordinates, similar levels of hexokinases I and II were observed. The hexokinase II levels were higher than the hexokinase I levels at age 3 y (SI Appendix, Fig. S11, NMR subordinates). In NMR queens, two hexokinases showed the same levels even at age 5 to 7 y (Fig. 2C). Most likely, the mROS-initiated aging of muscles occurs much later in NMRs than in mice, which correlates with the much longer life span of NMRs compared with mice.
As early as 1967, Il’in et al. (43) reported that mitochondria-bound hexokinase in skeletal muscle from the adult rabbit is inhibited by G6P at lower concentrations than in rabbit embryos. The sensitization of hexokinase to G6P-mediated inhibition is completely blocked by the denervation of the muscle. This suggests that inhibition of hexokinase does not result from age-related damage but rather is controlled by the master biological clock responsible for the timing of ontogenesis (8). A tempting speculation is that sarcopenia, the age-dependent decrease in skeletal muscle cellularity (44), results from mROS-induced apoptosis of muscle cells caused by two synergetic events: substitution of hexokinase II for hexokinase I and stronger inhibition of hexokinases by G6P. Interestingly, according to our data (45), the mitochondria-targeted antioxidant SkQ1 almost completely prevents the substantial decrease in the number of mitochondria in myocytes from old Wistar rats and particularly OXYS rats. This decrease usually accompanies sarcopenia. Similar to mild depolarization, SkQ1 decreases the ROS level specifically in the mitochondria (45).
According to Galina et al. (4), antioxidant deficiency in the liver due to the absence of bound kinases may be compensated for to some degree by high levels of catalase and GPx1 in this tissue. This observation was confirmed in our experiments (SI Appendix, Fig. S20 A and B). The levels of catalase and Gpx1 mRNAs were substantially increased after the transition from embryonic to adult mouse liver. This effect should not be considered an equivalent substitution for the mild depolarization mechanism, however. In fact, catalase quenches already-generated ROS, whereas the kinases prevent ROS generation (SI Appendix, Table S1). Moreover, mild depolarization is an antioxidant mechanism specific for mROS, whereas catalase and GPx1 quench H2O2 regardless of the origin of this molecule. Quenching all the cytosolic H2O2 may inhibit important functions of H2O2 as a messenger for some intracellular and intercellular signaling pathways.
There are observations showing that an age-dependent decrease in hexokinases bound to mitochondria increases the vulnerability of cells to apoptosis. Hexokinases I and II are able to interact with porin (46). Importantly, hexokinases bound to mitochondria inhibit apoptosis by blocking the binding of porin with proapoptotic BAX protein (47, 48) and preventing opening of the mitochondrial permeability transition pores and subsequent cytochrome c release (49). Thus, the observed age-dependent decrease in hexokinases bound to mitochondria can be an important factor leading to the loss of the tissue cellularity observed in aged organisms.
The Cori Cycle.
The outstanding position of the liver among other mammalian tissues is most likely due to the specific role of this tissue in carbohydrate metabolism. During intensive muscular work, oxygen is exhausted in myocytes, which attempt to substitute respiration with anaerobic glycolysis as an energy source. Thus, large amounts of lactate accumulate in myocytes. Lactate is transported by the blood to the liver, where it is used to form carbohydrates through the reversal of glycolysis in the Cori cycle (17, 50, 51) (SI Appendix, Fig. S21). In fact, the accumulation of carbohydrates, rather than their utilization in the hexokinase reaction, represents the main direction of carbohydrate metabolism in the liver after intensive work by muscles. Thus apparently explains the very low levels of hexokinases I and II in the livers of adult mice and why the mild depolarization mechanism is not functioning in this tissue. In the mouse embryo, where intensive muscle work does not occur and the Cori cycle does not function, the amount of liver hexokinases is much higher than that in adult mice, and the mild depolarization mechanism functions.
A similar situation is observed in adult NMRs, potentially due to the eusocial organization of their community, where only royal family members produce progeny. Because of the presence of numerous subordinates (up to 300 individuals) and the huge underground labyrinth used for habitation, the queen and her mate(s) are rarely required to perform intense muscular work. It should be mentioned that the difference between the energetics of the mouse and NMR cannot be explained by a lower level of oxygen in the NMR labyrinths, since the authors of this paper (S.H. and T.B.H.) and their coworkers recently reported normal [O2] in natural NMR burrows in Africa (52).
Thus, hepatocytes do not contain the bound hexokinase-mediated mild depolarization system, which might compete with the carbohydrate-synthesizing branch of the Cori cycle. However, this feature is not inherent in highly glycolytic hepatoma cells, which are not controlled by the hormonal regulation required for the cooperation of various organs. As reported by the Pedersen group (53–55), these cancer cells contain large amounts of mitochondria-bound hexokinases interacting with porin. Remarkably, liver fibroblasts from young mice, in contrast to hepatocytes, possess large amounts of mitochondria-bound hexokinases and rather low levels of cytosolic hexokinases. In older animals, the bound enzymes disappeared, but the levels of the cytosolic enzymes increased (SI Appendix, Fig. S4). Thus, the absence of mild depolarization is inherent to hepatocytes but not to any other cell types present in liver tissue.
An open question is why the adult mouse liver does not use the second mechanism of mild depolarization, creatine kinase. In contrast to G6P, the product (creatine phosphate) is not involved in a metabolic reaction other than ADP phosphorylation. Apparently, the physiological concentration of creatine in mitochondria is not so high that it can effectively replace glucose in mild depolarization for long periods. More likely, creatine kinase is able to decrease the ∆ψ by cooperating with hexokinase rather than operating as a single mild depolarization mechanism (56). This does not mean that addition of creatine at high concentrations in vitro cannot stimulate respiration and inhibit H2O2 formation at State 4 like glucose over several minutes. However, creatine should be exhausted in vivo rather quickly as a source of mild depolarization if sugars do not support such a source.
Opposite Effects of Glucose on Mouse Mortality Induced by Bacterial and Viral Sepsis.
Numerous studies have indicated that many pathophysiological conditions are characterized by impaired energy metabolism, including abnormal glucose levels and mitochondrial dysfunction. In particular, the change in metabolic processes in immune cells (recently referred to as “immunometabolism”) is now believed to orchestrate the entire immune response (57). Hexokinases are at the core of many metabolic processes, since they catalyze a rate-limiting step in respiration and glycolysis. It is necessary to investigate the interconnections among the metabolic activity of cells and the mitochondrial localization of hexokinases and downstream effects such as mROS production, opening of the mitochondrial permeability transition pores, and apoptosis. Interestingly, hexokinases may directly function in macrophages as innate immune receptors for bacterial damage-associated molecular patterns (58).
Medzhitov et al. (59) recently found that glucose supplementation in food increased the mortality of mice either infected by bacteria or challenged by injection of lipopolysaccharides. This enhanced lethality was likely mediated through increased ROS and neuronal dysfunction. Importantly, hexokinase I bound to neuronal mitochondria has been shown to be neuroprotective, preventing ROS production through mitochondrial mild depolarization (2).
It is well known that aging is associated with in increased mortality from bacterial infections (60). This fact is usually explained by the senescence of the immune system. However, the results obtained by Medzhitov et al., along with our data, suggest that increased lethality in aged animals may also be specifically mediated via the loss of mitochondria-bound hexokinases in neurons, leading to their exacerbated sensitivity to oxidative damage, dysfunction, and cell death.
One more intriguing observation of Medzhitov et al. (59) is that carbohydrate supplementation of food increases the mortality of mice infected with bacteria (Listeria) and decreases the mortality of mice infected with influenza virus. When analyzing this paradox, we should take into account that bacteria- and virus-induced inflammation initially kills the pathogens to save the organism, but if such an attempt fails, a further increase in inflammation kills the organism to prevent epidemics in the population. In both cases, death seems to require mROS-mediated apoptosis in vitally important tissues. Glucose can decrease mROS levels by stimulating mild depolarization (virus infection) or, alternatively, can increase mROS levels by inhibiting mild depolarization due to the accumulation of G6P produced from glucose by hexokinases (bacterial infection). Consistent with this reasoning, 2DOG converted by hexokinase to inactive 2DOG6P was found to inhibit food toxicity in subjects with bacterial sepsis (59). To solve this problem, it would be desirable to measure G6P in neurons and other tissues in mice infected by bacteria or viruses and test fructose instead of 2DOG. Like 2DOG, fructose cannot desorb hexokinase from mitochondria, but in contrast to 2DOG, fructose initiates (rather than inhibits) glycolysis (SI Appendix, section S3). In any case, investigation of the opposite effects of 2DOG vs. glucose or fructose in bacterial and virus infections seems promising in further studies of Medzhitov’s paradox.
Materials and Methods
Mitochondria from different tissues were isolated using standard differential centrifugation methods reported by Hogeboom (61) with slight modifications described in SI Appendix, section S4. Mitochondria were isolated from tissues of 18-d mouse embryos as described previously (62). The respiratory activity of isolated mitochondria was measured polarographically with a Clark-type electrode as described previously (45). The mitochondrial membrane potential, Δψ, was analyzed in a mitochondrial suspension by monitoring changes in the fluorescence of the lipophilic cationic dye safranin O, using a previously described method (63). The protein concentrations of the samples were measured using the Lowry method. Western blot analyses of protein extracts of fractionated tissues were performed as described previously (64). Hydrogen peroxide production by the mitochondria was estimated using the method reported by Zhou et al. (65), with modifications described in our previous study (66). Total RNA was extracted using Extract RNA Reagent (Evrogen). RT-PCR was performed on individual cDNAs using 5× SYBR Green Mix (Evrogen) and a DT-96 thermocycler (DNA Technology). The hexokinase assay was based on the method reported by Scheer et al. (67). Catalase, SOD, GPx1, and GR activities; lipid peroxidation and protein carbonylation assays; and the levels of total glutathione and the ratio of reduced to oxidized glutathione were estimated using special kits according to protocols provided by Abcam. Primary cultures of hepatic fibroblasts and confocal microscopy were performed as described previously (68) and in SI Appendix, section S5. Data are presented as mean ± SD or SEM as applicable. All calculations were performed using GraphPad Prism 7.0. Detailed protocols are provided in SI Appendix, Materials and Methods.
Data Availability Statement.
All relevant data are provided in the paper and supporting information file.
Supplementary Material
Acknowledgments
We thank Stockholm Zoo Skansen and Ann-Marie Appel for providing tissue samples of very old NMRs and Papillorama and its staff for providing tissue samples of bats; and Prof. Janine Kirstein and Manuel Iburg for providing access to fluorimetry at the Max Delbrück Center for Molecular Medicine and generous support. We also thank Louis-Felix Bersier and Laurence Charrier (University of Fribourg) for supplying liquid nitrogen; A. Vinogradov, B. Chernyak, and G. Shilovsky for providing useful advice; and R. A. Simonyan for participating in some experiments. This study was supported by grants from the Russian Foundation for Basic Research (17-04-02112A) and the Leibniz Institute for Zoo and Wildlife Research.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916414117/-/DCSupplemental.
References
- 1.Korshunov S. S., Skulachev V. P., Starkov A. A., High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 416, 15–18 (1997). [DOI] [PubMed] [Google Scholar]
- 2.da-Silva W. S., et al. , Mitochondrial bound hexokinase activity as a preventive antioxidant defense: Steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J. Biol. Chem. 279, 39846–39855 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Meyer L. E., et al. , Mitochondrial creatine kinase activity prevents reactive oxygen species generation: Antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J. Biol. Chem. 281, 37361–37371 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Santiago A. P., Chaves E. A., Oliveira M. F., Galina A., Reactive oxygen species generation is modulated by mitochondrial kinases: Correlation with mitochondrial antioxidant peroxidases in rat tissues. Biochimie 90, 1566–1577 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Rupprecht A., et al. , Role of the transmembrane potential in the membrane proton leak. Biophys. J. 98, 1503–1511 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skulachev M. V., Skulachev V. P., Programmed aging of mammals: Proof of concept and prospects of biochemical approaches for anti-aging therapy. Biochemistry (Mosc.) 82, 1403–1422 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Penz O. K., et al. , Protracted brain development in a rodent model of extreme longevity. Sci. Rep. 5, 11592 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skulachev V. P., et al. , Neoteny, prolongation of youth: From naked mole rats to “naked apes” (Humans). Physiol. Rev. 97, 699–720 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Skulachev V. P., Bogachev A. V., Kasparinsky F. O., Principles of Bioenergetics (Springer, 2013). [Google Scholar]
- 10.Birrell J. A., Hirst J., Investigation of NADH binding, hydride transfer, and NAD(+) dissociation during NADH oxidation by mitochondrial complex I using modified nicotinamide nucleotides. Biochemistry 52, 4048–4055 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agip A. A., et al. , Cryo-EM structures of complex I from mouse heart mitochondria in two biochemically defined states. Nat. Struct. Mol. Biol. 25, 548–556 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skulachev V. P., et al. , The effect of uncouplers on mitochondria, respiratory enzyme complexes and artificial phospholipid membranes. Curr. Mod. Biol. 2, 98–105 (1968). [DOI] [PubMed] [Google Scholar]
- 13.Vyssokikh M. Y., Brdiczka D., The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim. Pol. 50, 389–404 (2003). [PubMed] [Google Scholar]
- 14.Parra J., Brdiczka D., Cusso R., Pette D., Enhanced catalytic activity of hexokinase by work-induced mitochondrial binding in fast-twitch muscle of rat. FEBS Lett. 403, 279–282 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Skulachev V. P., Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 29, 169–202 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Kabir F., Wilson J. E., Mitochondrial hexokinase in brain of various species: Differences in sensitivity to solubilization by glucose 6-phosphate. Arch. Biochem. Biophys. 300, 641–650 (1993). [DOI] [PubMed] [Google Scholar]
- 17.Nuttall F. Q., Ngo A., Gannon M. C., Regulation of hepatic glucose production and the role of gluconeogenesis in humans: Is the rate of gluconeogenesis constant? Diabetes Metab. Res. Rev. 24, 438–458 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Skulachev V. P., Uncoupling: New approaches to an old problem of bioenergetics. Biochim. Biophys. Acta 1363, 100–124 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Skulachev V. P., Lyamzaev K. G., “Aging as phenoptotic phenomenon” in Encyclopedia of Gerontology and Population Aging, Gu D., Dupre M. E., Eds. (Springer, 2019), pp. 1–4. [Google Scholar]
- 20.Skulachev V. P., Programmed death phenomena: From organelle to organism. Ann. N. Y. Acad. Sci. 959, 214–237 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Timmons J. A., et al. , Longevity-related molecular pathways are subject to midlife “switch” in humans. Aging Cell 18, e12970 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraupp O., Adler-Kastner L., Niessner H., Plank B., The effects of starvation and of acute and chronic alloxan diabetes on myocardial substrate levels and on liver glycogen in the rat in vivo. Eur. J. Biochem. 2, 197–214 (1967). [DOI] [PubMed] [Google Scholar]
- 23.Harman D., The biologic clock: The mitochondria? J. Am. Geriatr. Soc. 20, 145–147 (1972). [DOI] [PubMed] [Google Scholar]
- 24.Skulachev V. P., Aging is a specific biological function rather than the result of a disorder in complex living systems: Biochemical evidence in support of Weismann’s hypothesis. Biochemistry (Mosc.) 62, 1191–1195 (1997). [PubMed] [Google Scholar]
- 25.Skulachev V. P., “Aging and the programmed death phenomena” in Topics Curr Genet, Model Systems in Aging, Nystrom T., Osiewacz H. D., Eds. (Springer, 2003), vol. 3, pp. 192–237. [Google Scholar]
- 26.Skulachev V. P., Lyamzaev K. G., “Mitochondrial reactive oxygen species aging theory” in Encyclopedia of Gerontology and Population Aging, Gu D., Dupre M. E., Eds. (Springer, 2019), pp. 1–8. [Google Scholar]
- 27.Skulachev V. P., SkQ1 treatment and food restriction—two ways to retard an aging program of organisms. Aging (Albany N.Y.) 3, 1045–1050 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skulachev V. P., What is “phenoptosis” and how to fight it? Biochemistry (Mosc.) 77, 689–706 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Andziak B., Buffenstein R., Disparate patterns of age-related changes in lipid peroxidation in long-lived naked mole-rats and shorter-lived mice. Aging Cell 5, 525–532 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Fredriksson Å., et al. , Effects of aging and reproduction on protein quality control in soma and gametes of Drosophila melanogaster. Aging Cell 11, 634–643 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Friguet B., Bulteau A. L., Chondrogianni N., Conconi M., Petropoulos I., Protein degradation by the proteasome and its implications in aging. Ann. N. Y. Acad. Sci. 908, 143–154 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Koga H., Kaushik S., Cuervo A. M., Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res. Rev. 10, 205–215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyström T., Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 24, 1311–1317 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shringarpure R., Davies K. J., Protein turnover by the proteasome in aging and disease. Free Radic. Biol. Med. 32, 1084–1089 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez K. A., et al. , A cytosolic protein factor from the naked mole rat activates proteasomes of other species and protects these from inhibition. Biochim. Biophys. Acta 1842, 2060–2072 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez V. I., et al. , Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole rat. Proc. Natl. Acad. Sci. U.S.A. 106, 3059–3064 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez K. A., Edrey Y. H., Osmulski P., Gaczynska M., Buffenstein R., Altered composition of liver proteasome assemblies contributes to enhanced proteasome activity in the exceptionally long-lived naked mole rat. PLoS One 7, e35890 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hulbert A. J., Pamplona R., Buffenstein R., Buttemer W. A., Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 87, 1175–1213 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Edrey Y. H., Park T. J., Kang H., Biney A., Buffenstein R., Endocrine function and neurobiology of the longest-living rodent, the naked mole rat. Exp. Gerontol. 46, 116–123 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Lassing I., et al. , Molecular and structural basis for redox regulation of beta-actin. J. Mol. Biol. 370, 331–348 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Fang X., et al. , Adaptations to a subterranean environment and longevity revealed by the analysis of mole rat genomes. Cell Rep. 8, 1354–1364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis K. N., et al. , Unraveling the message: Insights into comparative genomics of the naked mole rat. Mamm. Genome 27, 259–278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Il’in V. S., Pleskov V. M., Razumovskaia N. I., Hexokinase from the soluble fraction and mitochondria of skeletal muscles, embryo, intact and denervated muscles from adult rabbits. Biokhimiya 32, 807–811 (1967). [PubMed] [Google Scholar]
- 44.Larsson L., et al. , Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 99, 427–511 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vays V. B., et al. , Antioxidant SkQ1 delays sarcopenia-associated damage of mitochondrial ultrastructure. Aging (Albany N.Y.) 6, 140–148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiek C., Benz R., Roos N., Brdiczka D., Evidence for identity between the hexokinase-binding protein and the mitochondrial porin in the outer membrane of rat liver mitochondria. Biochim. Biophys. Acta 688, 429–440 (1982). [DOI] [PubMed] [Google Scholar]
- 47.Abu-Hamad S., Zaid H., Israelson A., Nahon E., Shoshan-Barmatz V., Hexokinase-I protection against apoptotic cell death is mediated via interaction with the voltage-dependent anion channel 1: Mapping the site of binding. J. Biol. Chem. 283, 13482–13490 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Pastorino J. G., Hoek J. B., Regulation of hexokinase binding to VDAC. J. Bioenerg. Biomembr. 40, 171–182 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiara F., et al. , Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS One 3, e1852 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cori C. F., Cori G. T., Glycogen formation in the liver from D- and L-lactic acid. J. Biol. Chem. 81, 389–403 (1929). [Google Scholar]
- 51.Waterhouse C., Keilson J., Cori cycle activity in man. J. Clin. Invest. 48, 2359–2366 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holtze S., et al. , The microenvironment of naked mole rat burrows in East Africa. Afr. J. Ecol. 56, 279–289 (2018). [Google Scholar]
- 53.Parry D. M., Pedersen P. L., Intracellular localization and properties of particulate hexokinase in the Novikoff ascites tumor: Evidence for an outer mitochondrial membrane location. J. Biol. Chem. 258, 10904–10912 (1983). [PubMed] [Google Scholar]
- 54.Nakashima R. A., Mangan P. S., Colombini M., Pedersen P. L., Hexokinase receptor complex in hepatoma mitochondria: Evidence from N,N′-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry 25, 1015–1021 (1986). [DOI] [PubMed] [Google Scholar]
- 55.Arora K. K., Pedersen P. L., Functional significance of mitochondrial bound hexokinase in tumor cell metabolism: Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J. Biol. Chem. 263, 17422–17428 (1988). [PubMed] [Google Scholar]
- 56.Vyssokikh M. Y., et al. , Proteinaceous complexes from mitochondrial contact sites. Biochemistry (Mosc.) 64, 390–398 (1999). [PubMed] [Google Scholar]
- 57.Wang A., Luan H. H., Medzhitov R., An evolutionary perspective on immunometabolism. Science 363, eaar3932 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf A. J., et al. , Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166, 624–636 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang A., et al. , Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 166, 1512–1525.e12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schütze S., et al. , Higher mortality and impaired elimination of bacteria in aged mice after intracerebral infection with E. coli are associated with an age-related decline of microglia and macrophage functions. Oncotarget 5, 12573–12592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hogeboom G. H., Fractionation of cell components of animal tissues. Methods Enzymol. 1, 16–19 (1955). [Google Scholar]
- 62.Nyquist-Battie C., Freter M., Cardiac mitochondrial abnormalities in a mouse model of the fetal alcohol syndrome. Alcohol Clin. Exp. Res. 12, 264–267 (1988). [DOI] [PubMed] [Google Scholar]
- 63.Figueira T. R., Melo D. R., Vercesi A. E., Castilho R. F., Safranine as a fluorescent probe for the evaluation of mitochondrial membrane potential in isolated organelles and permeabilized cells. Methods Mol. Biol. 810, 103–117 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Vyssokikh M. Y., et al. , Adenine nucleotide translocator isoforms 1 and 2 are differently distributed in the mitochondrial inner membrane and have distinct affinities to cyclophilin D. Biochem. J. 358, 349–358 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou M., Diwu Z., Panchuk-Voloshina N., Haugland R. P., A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: Applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 253, 162–168 (1997). [DOI] [PubMed] [Google Scholar]
- 66.Antonenko Y. N., et al. , Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: Synthesis and in vitro studies. Biochemistry (Mosc.) 73, 1273–1287 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Scheer W. D., Lehmann H. P., Beeler M. F., An improved assay for hexokinase activity in human tissue homogenates. Anal. Biochem. 91, 451–463 (1978). [DOI] [PubMed] [Google Scholar]
- 68.Chugunova A., et al. , LINC00116 codes for a mitochondrial peptide linking respiration and lipid metabolism. Proc. Natl. Acad. Sci. U.S.A. 116, 4940–4945 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are provided in the paper and supporting information file.