Abstract
Background
Head and neck cancer treatment has developed over the last decade, with improved mortality and survival rates, but the treatments often result in dysphagia (a difficulty in swallowing) as a side effect. This may be acute, resolving after treatment, or remain as a long‐term negative sequela of head and neck cancer (HNC) treatment. Interventions to counteract the problems associated with dysphagia include swallowing exercises or modification of diet (bolus texture, size), or both.
Objectives
To determine the effects of therapeutic exercises, undertaken before, during and/or immediately after HNC treatment, on swallowing, aspiration and adverse events such as chest infections, aspiration pneumonia and profound weight loss, in people treated curatively for advanced‐stage (stage III, stage IV) squamous cell carcinoma of the head and neck.
Search methods
The Cochrane ENT Information Specialist searched the ENT Trials Register; Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 6); MEDLINE; PubMed; Embase; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; Web of Science; ClinicalTrials.gov; ICTRP; speechBITE; Google Scholar; Google and additional sources for published and unpublished trials. The date of the search was 1 July 2016.
Selection criteria
We selected randomised controlled trials (RCTs) of adults with head and neck cancer (stage III, stage IV) who underwent therapeutic exercises for swallowing before, during and/or immediately after HNC treatment to help produce safe and efficient swallowing. The main comparison was therapeutic exercises versus treatment as usual (TAU). Other possible comparison pairs included: therapeutic exercises versus sham exercises and therapeutic exercises plus TAU versus TAU. TAU consisted of reactive management of a patient's dysphagia, when this occurred. When severe, this included insertion of either a percutaneous endoscopic gastroscopy or nasogastric tube for non‐oral feeding.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were: safety and efficiency of oral swallowing, as measured by reduced/no aspiration; oropharyngeal swallowing efficiency (OPSE) measures, taken from videofluoroscopy swallowing studies; and adverse events, such as chest infections, aspiration pneumonia and profound weight loss. Secondary outcomes were time to return to function (swallowing); self‐reported changes to quality of life; changes to psychological well‐being ‐ depression, anxiety and stress; patient satisfaction with the intervention; patient compliance with the intervention; and cost‐effectiveness of the intervention.
Main results
We included six studies (reported as seven papers) involving 326 participants whose ages ranged from 39 to 83 years, with a gender bias towards men (73% to 95% across studies), reflecting the characteristics of patients with HNC. The risk of bias in the studies was generally high.
We did not pool data from studies because of significant differences in the interventions and outcomes evaluated. We found a lack of standardisation and consistency in the outcomes measured and the endpoints at which they were evaluated.
We found no evidence that therapeutic exercises were better than TAU, or any other treatment, in improving the safety and efficiency of oral swallowing (our primary outcome) or in improving any of the secondary outcomes.
Using the GRADE system, we classified the overall quality of the evidence for each outcome as very low, due to the limited number of trials and their low quality. There were no adverse events reported that were directly attributable to the intervention (swallowing exercises).
Authors' conclusions
We found no evidence that undertaking therapeutic exercises before, during and/or immediately after HNC treatment leads to improvement in oral swallowing. This absence of evidence may be due to the small participant numbers in trials, resulting in insufficient power to detect any difference. Data from the identified trials could not be combined due to differences in the choice of primary outcomes and in the measurement tools used to assess them, and the differing baseline and endpoints across studies.
Designing and implementing studies with stronger methodological rigour is essential. There needs to be agreement about the key primary outcomes, the choice of validated assessment tools to measure them and the time points at which those measurements are made.
Plain language summary
Swallowing exercises for affecting swallowing after treatment in people with advanced‐stage head and neck cancers
Review question
To establish the evidence for the effects of therapeutic swallowing exercises, undertaken before, during and/or immediately after head and neck cancer treatment, on swallowing.
Background
A swallowing impairment (dysphagia) commonly occurs as a result of head and neck cancer treatment. It may be temporary, resulting from a dry mouth or irritation of the lining of the mouth during treatment, or permanent due to a narrowing (stricture) of the throat after surgery and/or radiotherapy. Undertaking swallowing exercises before, during and/or immediately after HNC treatment may prevent dysphagia occurring, or may reduce its severity.
Clinicians who are treating dysphagia in head and neck cancer patients lack evidence‐based guidelines so it is challenging to determine which interventions are suitable, but many speech and language therapists encourage patients to undertake exercises intensively throughout head and neck cancer treatment, based on a 'use it or lose it' principle.
Study characteristics
We included six studies with 326 participants who undertook therapeutic exercises before, during and/or after HNC treatment. We could not combine the results of the studies because of the variation in participants' cancers, their treatments, the outcomes measured and the tools used to assess them, as well as the differing time points for testing. Researchers have compared: (i) therapeutic exercises versus treatment as usual (TAU); (ii) therapeutic exercises versus sham therapy; (iii) therapeutic exercises plus TAU versus TAU. The therapeutic exercises varied in their design, timing and intensity. TAU involved managing patients' dysphagia when it occurred, including inserting a tube for non‐oral feeding.
Key results
The evidence is up to date to 1 July 2016.
We found no evidence that therapeutic exercises were better than TAU, or any other treatment, in improving the safety and efficiency of oral swallowing (our primary outcome) or in improving any of the secondary outcomes. However, there is insufficient evidence to draw any clear conclusion about the effects of undertaking therapeutic exercises before during and/or immediately after HNC treatment on preventing or reducing dysphagia. Studies had small participant numbers, used complex interventions and varied in the choice of outcomes measured, making it difficult to draw reliable conclusions. There were no reported adverse events directly attributable to the intervention (swallowing exercises).
Quality of evidence
The current quality of the evidence to support the use of therapeutic exercises before, during and/or immediately after HNC treatment to prevent/reduce dysphagia is very low. We need better designed, rigorous studies with larger participant numbers and agreed endpoints and outcome measurements in order to draw clear(er) conclusions.
Summary of findings
Summary of findings for the main comparison. Therapeutic swallowing exercises compared with treatment as usual (TAU) for dysphagia in head and neck cancer patients.
| Therapeutic swallowing exercises compared with treatment as usual (TAU) for dysphagia in head and neck cancer patients | |||||||
|
Patient or population: adults with advanced head and neck cancer Settings: acute/hospital departments and clinics Intervention: therapeutic swallowing exercises Comparison: treatment as usual (TAU) |
|||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Treatment as usual (TAU) | Therapeutic swallowing exercises | ||||||
|
Swallowing function/efficiency Measured using a rating of oropharyngeal swallowing efficiency (OPSE) quantifying the interaction of the speed of bolus movement and safety/efficiency in clearing material from the oropharynx Dysphagia is defined as an OPSE score of less than 39 10 weeks post‐treatment |
N/A | N/A | 10 weeks post‐treatment: MD ‐8.06 (95% CI ‐25.37 to 9.25) | 16 (TAU group 8: intervention group: 8) (1 study) Lazarus 2014 |
⊕⊝⊝⊝ very low1,2 | Swallowing function (OPSE) improved following treatment, However, the effect did not reach statistical significance. | |
|
Adverse event: Weight loss (> 10%) Change in nutritional status reflected by a patient's weight measured at 6 weeks post‐treatment |
N/A | N/A | Weight loss at 6 weeks: RR 0.62 (95% CI 0.22 to 1.71) | 27 (TAU group 13; Pharyngocise group 14) (1 study) Carnaby‐Mann 2012 |
⊕⊝⊝⊝ very low1,2 |
At 6 weeks post‐treatment, the risk of weight loss (> 10%) is around 40% lower in the Pharyngocise group compared to the TAU group. However, the estimate has a wide confidence interval and is not statistically significant. | |
|
Adverse event: Weight loss (> 10%) Change in nutritional status reflected by a patient's weight during the study period 10 weeks post‐treatment |
N/A | N/A | Not estimable | 55 (TAU 28; intervention group 27) (1 study) van der Molen 2011 |
⊕⊝⊝⊝ very low1,2 |
There was no comparison between the intervention and TAU group. Results were from the pre‐ and post‐treatment scores for 49 participants. | |
|
Adverse event: Weight change Patient's body weight 6 months before the start of treatment was retrieved from the patient's medical notes and "relative weight change" was calculated as the percentage weight change relative to the weight at week 0 6 months post‐treatment |
N/A | N/A | 6 months post‐treatment): MD 1.34 (95% CI ‐0.46 to 3.14) | 120 (intervention group 60; TAU group 60) (1 study) van den Berg 2014 |
⊕⊝⊝⊝ very low1,2 |
The weight change estimate has a wide confidence interval, therefore the effect is uncertain. | |
|
Aspiration Scored as present, trace or absent, for any consistency 5 months post‐treatment |
N/A | N/A | 5 months post‐treatment: TAU group 18.18%; intervention group 7.69% | 39 (TAU group 20; intervention group 19) (1 study) Mortensen 2015 |
⊕⊝⊝⊝ very low1,2,3 |
Results were reported as a percentage ‐ not estimable. | |
|
Penetration Scored as present, trace or absent, for any consistency 5 months post‐treatment |
N/A | N/A | 5 months post‐treatment: TAU group 45.45%; intervention group 23.08% | 39 (TAU group 20; intervention group 19) (1 study) Mortensen 2015 |
⊕⊝⊝⊝ very low1,2,3 |
Results were reported as a percentage. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; N/A: not applicable; OPSE: oropharyngeal swallowing efficiency; RR: risk ratio; TAU: treatment as usual | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1Few participants.
2Risk of performance bias is high (Carnaby‐Mann 2012; Lazarus 2014; Mortensen 2015; van den Berg 2014) or unclear (Kotz 2012; van der Molen 2011).
3Unclear if the assessor was blinded to the intervention (Mortensen 2015).
Summary of findings 2. Therapeutic swallowing exercises compared with sham intervention for dysphagia in head and neck cancer patients.
| Therapeutic swallowing exercises compared with sham intervention for dysphagia in head and neck cancer patients | ||||||
|
Patient or population: adults with advanced head and neck cancer Settings: acute/hospital departments and clinics Intervention: therapeutic swallowing exercises (Pharyngocise) Comparison: sham exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham exercises | Pharyngocise | |||||
|
Adverse events: Dysphagia‐related complications Occurrence of dysphagia‐related complications: pneumonia, dehydration, mucositis and oral yeast infection |
N/A | N/A | No data | 27 (Pharyngocise group: 14; sham group 13) (1 study) Carnaby‐Mann 2012 |
⊕⊝⊝⊝ very low1,2 | There was no comparison of outcomes between the interventions in the study. |
|
Adverse event: Weight loss (> 10%) Change in nutritional status reflected by a patient's weight at 6 weeks after treatment |
N/A | N/A | Change at 6 weeks RR 0.62 (95% CI 0.22 to 1.71) |
27 (Pharyngocise group: 14; sham group 13) (1 study) Carnaby‐Mann 2012 |
⊕⊝⊝⊝ very low1,2 | At 6 weeks post‐treatment the risk of weight loss (> 10%) is lower in the Pharyngocise group compared to the sham group. The sham group has less likelihood of weight loss than the Pharyngocise group. However, the estimate has a wide confidence interval, so it is not statistically significant. The risk of weight loss > 10% (of baseline body weight) is around 40% lower in the Pharyngocise group compared to the sham group. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Few participants.
2Risk of performance bias is high (Carnaby‐Mann 2012).
Background
Description of the condition
Head and neck cancer encompasses malignant tumours of the upper aerodigestive tract including the pharynx, larynx, oral cavity, nasal cavity, paranasal sinuses and salivary glands (Argiris 2008). Approximately 550,000 new cases are diagnosed each year throughout the world (Jemal 2011), the majority of which are mucosal squamous cell carcinoma (www.ncri.ie). Tobacco and alcohol abuse are two important risk factors associated with oral, pharyngeal and laryngeal cancer (Blot 1988; Decker 1982). Viral infection also plays a role (Marur 2010), with associations between the human papilloma virus and oropharyngeal cancer (Gillison 2000; Ragin 2007), as well as the Epstein‐Barr virus and nasopharyngeal carcinoma (Sankaranarayanan 1998).
An individual patient's prognosis is determined by the type and extent of their cancer, established during staging. Head and neck cancer staging takes into consideration anatomic subsite, tumour size, cervical lymph node involvement and the presence of distant metastasis (AJCC 2010; NCCN 2014). Up to 40% of patients have early stage I and II cancer when they first present (NCCN 2014).
Treatment options include surgery, radiation and chemotherapy. The majority of early stage I and II patients can be treated with single modality therapy using either surgery or radiation alone, and survival rates are similar for both types of treatment (Gregoire 2010; Higgins 2009; NCCN 2014). In contrast, when advanced stage III and IV cancer is treated with the aim of curing the patient, this requires multimodality therapy to include surgery with adjuvant radiotherapy or organ preservation chemoradiation (Pignon 2009). Adjuvant chemotherapy has proven beneficial for some patients with advanced disease (Forastiere 2003). Ultimately, head and neck cancer treatment is individualised to the patient and based not only on the stage of the cancer and the likely prognosis associated with that stage, but also the patient's co‐morbidities and wishes. Sometimes treatment is palliative and not intended to try and elicit a cure.
Description of the problem
Although survival rates from head and neck cancer have improved overall, this has been due to more aggressive treatment so morbidity has correspondingly increased. Morbidity includes dysphagia, which may be due to the cancer itself or may occur as a result of modern aggressive treatment(s), or both. Dysphagia ranges from a temporary problem in swallowing (due to mucositis or xerostomia during treatment) through to a more long‐term or even permanent problem (due to fibrosis or a stricture), which results in non‐oral tube feeding being needed, using a nasogastric tube or a percutaneous endoscopic gastrostomy.
The consequences of dysphagia are not only a reduction in food/fluid intake, but often a reduction in people's activity and social participation (International Classification of Functioning, ICF 2001), with corresponding negative changes to their quality of life. In countries where much socialisation revolves around preparing and eating food together, psychological well‐being can be negatively affected by dysphagia (Rappoport 2003).
Description of the intervention
Dysphagia therapy is recognised internationally as the provision of services to patients with a difficulty in swallowing, usually by a speech and language therapist (also known as a speech pathologist or speech therapist). Therapy may occur through using behavioural management procedures, principally divided into compensatory strategies and direct techniques (Logemann 1999). Direct techniques are the focus of this review.
Direct techniques (also known as therapeutic exercises) consist of either swallowing or neuromuscular exercises. Swallowing exercises are designed to change the swallow physiology by improving sensory motor integration or by gaining voluntary control over the timing or the co‐ordination of selected oropharyngeal movements during swallowing (Logemann 1999).
Neuromuscular exercises target tongue strength, endurance and/or power. Strength is achieved by exercises that use high levels of resistance (isometric) exercises. Endurance is achieved through repeated performance of exercises involving low levels of resistance. Power is achieved by using exercises that focus on the speed of muscle contraction (Clark 2003).
How the intervention might work
Swallowing exercises are designed to improve swallowing safety (i.e. to reduce penetration, in which a bolus (a ball of food, fluid) enters the larynx at/above the vocal folds, or to reduce aspiration, in which a bolus enters below the vocal folds into the trachea/upper airway). Other swallowing exercises are designed to improve efficiency (i.e. to increase the speed or amount of a bolus swallowed, or both) (Logemann 1999).
Neuromuscular exercises are designed to increase tongue range of motion and/or strength, thus indirectly improving oral bolus transit (speed and bolus clearance) as tongue force/strength is a key component of a safe swallow. Exercises to increase tongue range of motion are used to keep the tongue mobile during/after head and neck cancer treatment and to mitigate against the stiffening and fibrosis that can result from radiotherapy, surgery or both (Appleton 1994; Fujiu 1996).
Why it is important to do this review
Changes to HNC treatment mean that patient survival has improved over the last decade, but this has been at the expense of increased morbidity (speech, swallowing function).
Treatment nowadays promotes 'organ preservation', which is appealing, but preservation of structure does not always mean preservation of function and, unfortunately, some HNC treatment regimens have profound and long‐lasting negative side effects. While all HNC treatments have side effects, these are compounded when multi‐modal treatments are used, as in modern protocols (Frowen 2006).
A swallowing problem (dysphagia) is now widely accepted as both an acute and a late toxicity after radiotherapy treatment and it has been stated that, "the problem of swallowing dysfunction is probably becoming one of the most important and clinically relevant side‐effects after curative radiotherapy or chemo‐radiation." (Langendijk 2007, pp4).
Given the need to prevent or reduce treatment‐related morbidity in order to reduce the survivorship burden for patients and families, as well as the cost to healthcare systems, a review of the usefulness of therapeutic exercises for affecting post‐treatment swallowing in patients treated for advanced squamous cell carcinoma of the head and neck using Cochrane methodology was warranted. This review is based on a published protocol (Perry 2014).
Objectives
To determine the effects of therapeutic exercises, undertaken before, during and/or immediately after HNC treatment, on swallowing, aspiration and adverse events such as chest infections, aspiration pneumonia and profound weight loss, in people treated curatively for advanced‐stage (stage III, stage IV) squamous cell carcinoma of the head and neck.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We did not plan to include studies employing quasi‐randomisation.
Types of participants
Adults with a clinical and histological diagnosis of a large (stage III, stage IV) squamous cell carcinoma of the head and neck who received any type of cancer treatment (surgery and radiation therapy, surgery and chemoradiotherapy, or chemoradiotherapy without surgery), who were deemed at risk of, or presented with, dysphagia (swallowing impairment). We included people with all levels of dysphagia severity and we set no age limits.
Types of interventions
We included direct therapeutic techniques involving swallowing or neuromuscular exercises or both.
Therapy programmes might be delivered before, during and/or immediately after HNC treatment, but they must be designed to prevent/reduce dysphagia from occurring and have lasted for more than one session. Interventions may have been provided by one or more health disciplines (for example, we included studies involving only speech and language therapists (SLTs) and those involving other healthcare professionals).
The main comparison was therapeutic exercises versus treatment as usual (TAU).
Other possible comparison pairs included: therapeutic exercises versus sham exercises; sham exercises versus TAU; and therapeutic exercises plus TAU versus TAU.
Types of outcome measures
We analysed the following outcomes in the review, but they were not used as a basis for including or excluding studies.
Primary outcomes
-
Safety and efficiency of oral swallowing, as measured by:
reduced/no aspiration;
oropharyngeal swallowing efficiency (OPSE) measures, taken from videofluoroscopy swallowing studies;
adverse events, such as chest infections, aspiration pneumonia, profound weight loss.
Secondary outcomes
Time to return to function (swallowing).
Self‐reported changes to quality of life.
Changes to psychological well‐being: depression, anxiety and stress.
Patient satisfaction with the intervention.
Patient compliance with the intervention.
Cost‐effectiveness of the intervention.
We assessed both short‐term (three months or less) and long‐term (at, or more than, six months) outcomes.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 1 July 2016.
Electronic searches
The Information Specialist searched:
Cochrane ENT Trials Register, via the Cochrane Register of Studies (searched 1 July 2016);
Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 6);
-
Ovid MEDLINE (1946 to June Week 4 2016);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations);
PubMed (as a top up to searches in Ovid MEDLINE) (searched 1 July 2016);
Ovid Embase (1974 to 2016 week 26);
EBSCO CINAHL (1982 to 4 July 2016);
LILACS (searched 4 July 2016);
KoreaMed via Google Scholar (searched 4 July 2016);
IndMed (searched 4 July 2016);
PakMediNet (searched 4 July 2016);
Web of Knowledge, Web of Science (1945 to 4 July 2016);
ClinicalTrials.gov, www.clinicaltrials.gov (searched via the Cochrane Register of Studies 4 July 2016);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (searched 4 July 2016);
speechBITE (Australian speech and language therapy) (searched 4 July 2016);
Google Scholar (searched 4 July 2016);
Google (searched 4 July 2016).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched PubMed, TRIPdatabase, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
Selection of studies
Two review authors (AP and SC) independently reviewed the titles and abstracts of the records identified from electronic searches and immediately excluded irrelevant studies. We obtained the full text of the remaining studies and two review authors (AP and CK) selected studies based on the inclusion criteria of the review. Where these authors were unsure, another review author (SC) made a final decision. We contacted trial authors for further details and documented the reasons for exclusion.
Data extraction and management
Two review authors (AP and CK) independently extracted study data and recorded information on a data extraction form. We resolved any discrepancies through discussion. We entered the data into Review Manager (RevMan), version 5.3 (RevMan 2014). We extracted the following from each study:
Citation details: title, authors, source and year of publication.
Participant inclusion and exclusion criteria.
Participant details: age, gender, location/size of tumour, time since diagnosis, level of swallowing ability, setting.
Recruitment details: number of people screened, eligible, recruited and randomised, withdrawals.
Methodological quality details (for the Cochrane 'Risk of bias' tool).
Intervention details: description of intervention/exercises, personnel involved, training of personnel, duration, dosage, comparison intervention.
Outcome measures: measures chosen, by whom and when they were administered, how they were administered (in person, via other communication technologies or by mail).
Study results (positive, negative, equivocal results, withdrawal).
Assessment of risk of bias in included studies
Two review authors (SHL and CK) independently assessed risk of bias for each study, using the criteria outlined in theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), with any disagreement resolved by discussion (AP, CK and SHL). We completed a 'Risk of bias' table for each individual study using the 'Risk of bias' tool in RevMan, version 5.3 (RevMan 2014):
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting (reporting bias); and
other sources of bias.
We assessed the above domains for each trial and then assigned a judgement about the adequacy of each entry as: 'low risk', 'high risk' or 'unclear risk' of bias.
Measures of treatment effect
Three review authors (AP, CK and SHL) independently assigned outcome measures to the health domain assessed (oral swallowing; aspiration; adverse events; time to return to oral swallowing; quality of life; psychological well‐being; patient satisfaction; patient compliance; cost‐effectiveness of intervention).
Where more than one outcome measure was used for the same domain from the same study, we included the measure most frequently used across included studies.
We conducted separate analyses for short‐term (three months or less) and long‐term (at, or more than, six months) outcomes.
We calculated risk ratios (RR) and 95% confidence intervals (CI) for dichotomous outcomes and mean differences (MD) or standardised mean differences (SMD) and 95% CI for continuous outcomes, as appropriate.
Unit of analysis issues
The unit of randomisation in the included studies was the individual patient.
Dealing with missing data
We did not need to contact any trial authors for missing data. As recommended in the Cochrane Handbook for Systematic Reviews of Interventions, we conducted intention‐to‐treat analyses. Where dropouts were clearly identified, we used the denominator of participants contributing data at the relevant outcome assessment.
Assessment of heterogeneity
We planned to pool the results to present an overall estimate of the treatment effect, using a random‐effects model. We planned to assess heterogeneity by visual inspection of the forest plot along with consideration of the Chi2 test for heterogeneity and the I2 statistic (Handbook 2011).
Data synthesis
We planned to conduct a meta‐analysis using a random‐effects model with 95% CI using RevMan 5.3 (RevMan 2014). Due to the heterogeneity of the studies, we could not conduct meta‐analyses of any study data.
Subgroup analysis and investigation of heterogeneity
With a sufficient number of comparable studies (four or more), we had planned to perform subgroup analyses to determine whether outcomes varied according to:
type of therapeutic exercise (swallowing and/or neuromuscular);
when therapeutic exercises were initiated (pre‐, peri‐ or post‐cancer treatment);
frequency of therapeutic exercise;
intensity of therapeutic exercise (dosage ‐ number of hours of intervention);
intervention approach (for example, retraining with a speech and language therapist or self‐directed);
mode of delivery (face to face versus use of a brochure/DVD);
whether the intervention was provided by a healthcare professional or not;
cancer treatment modality (surgery and radiation therapy; surgery and chemoradiotherapy; chemoradiotherapy treatment without surgery);
site of tumour;
size of tumour;
HPV status.
We were not able to conduct subgroup analysis due to the insufficient number of comparable studies.
GRADE and 'Summary of findings' table
We used the GRADE approach to rate the overall quality of evidence. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high quality of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision; and
publication bias.
We included two 'Summary of findings' tables, constructed according to the recommendations described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). We included the following six outcomes in the 'Summary of findings' table: swallowing function/swallowing efficiency; weight loss; weight change; aspiration; penetration; dysphagia‐related complications.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
We retrieved a total of 714 records through database searching which, when 267 duplicates were removed, became 445 papers. We also identified four ongoing studies, so this became 449 papers. First level screening (removal of clearly non‐relevant references) left 25 references for further consideration (Figure 1). We discarded 11 records after further assessment. Four studies are still recruiting participants (Barretos Cancer Hospital 2015; Fredslund 2015; Govender 2014; van Nuffelen 2014) (see Characteristics of ongoing studies).
1.
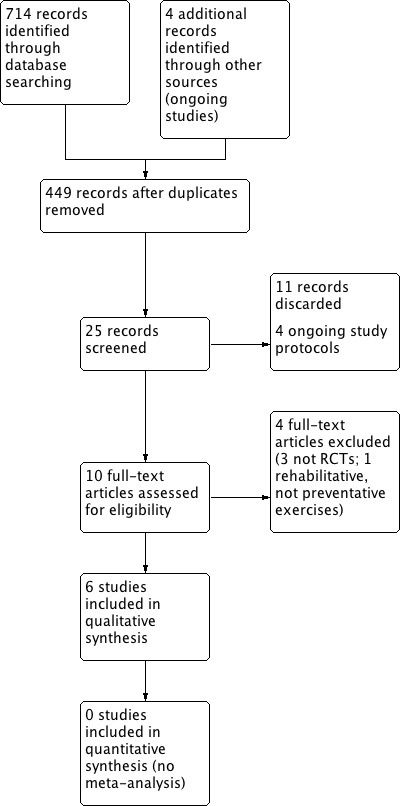
Process for sifting search results and selecting studies for inclusion.
Two review authors (SC and AP) independently assessed the 10 remaining full‐text articles and included six studies in the review. We excluded three studies that were not RCTs (see Excluded studies) and one that was an RCT but the post‐treatment exercises were rehabilitative, rather than designed to reduce/prevent dysphagia from occurring (Tang 2011). The selection was discussed with the last author (CK) who agreed with a final inclusion of six studies for this review (Figure 1).
Included studies
We include six studies, reported as seven papers, which met the inclusion criteria for this review (Carnaby‐Mann 2012; Kotz 2012; Lazarus 2014; Mortensen 2015; van den Berg 2014; van der Molen 2011). See Characteristics of included studies for detailed descriptions for each study. van der Molen 2011 also included data from their first study in a second (follow‐up) report (van der Molen 2014 ‐ see van der Molen 2011 for reference). All included studies were published in English.
Design
All six included studies were randomised controlled trials where the authors evaluated the effectiveness of a swallowing exercise protocol on swallowing outcomes in patients treated for HNC compared with either treatment as usual (TAU) and/or a 'sham' intervention.
Sample sizes
The sample sizes of the included studies ranged from 23 (Lazarus 2014) to 120 adults (van den Berg 2014). Carnaby‐Mann 2012 included 58; Kotz 2012 included 26; Mortensen 2015 included 44 and van der Molen 2011 included 55 participants, of whom 29 were followed up at two years (van der Molen 2014 ‐ see van der Molen 2011 for reference).
Settings
Three of the six studies were undertaken in the USA. The study by Carnaby‐Mann 2012 was undertaken at a university hospital cancer centre in Gainesville, Florida. Kotz 2012 took place at Mount Sinai medical centre (tertiary care) in New York. The study by Lazarus 2014 was at Mount Sinai Beth Israel Medical Centre, New York. The Mortensen 2015 study was from Aarhus university in Denmark. The study by van den Berg 2014 took place in Nijmegen in the Netherlands. The study van der Molen 2011 was from the Netherlands Cancer Institute in Amsterdam.
Participants
Age
The mean age of participants across studies did not vary widely, typically ranging from a mean of 58 years (range 39 to 77) in Mortensen 2015 to a mean of 63 years (range 33 to 83 years) (van den Berg 2014), representing the population age characteristics.
Gender
Participants were predominantly male, but the sex ratio varied across studies from 74% male (van den Berg 2014) to 95% male (Lazarus 2014).
Head and neck cancer disease characteristics
Patients with a diagnosed head and neck cancer were recruited to all studies but the site and size of tumours varied.
Carnaby‐Mann 2012, recruited patients with a head and neck cancer of the oropharyngeal region, confirmed by histopathology. Kotz 2012 recruited consecutive patients, newly diagnosed with head and neck cancer; 77% had stage IV disease. Patients in Lazarus 2014 had stage II to IV oral and oropharyngeal cancers. The primary tumour location was the tonsil (48%, 11 patients), then the base of tongue (39%; nine patients), lateral pharyngeal wall (9%; two patients) and soft palate (4%; one patient). T stages ranged from II to IVB (70% were IVA). Mortensen 2015 recruited consecutive adults (< 18 years) presenting with tumours of the larynx, pharynx, oral cavity or an unknown primary. In their control group, 75% (15) had stage IV tumours; in the intervention group 84% (16) had stage IV tumours. van den Berg 2014 recruited people with T2 to T4 tumours of the oral cavity, nasopharynx, oropharynx, hypopharynx or larynx. Primary tumour sites included the oral cavity/oropharynx. T stage included 33% (16 patients) at stage III and 67% (33 patients) at stage IV. van der Molen 2011 recruited patients with advanced squamous cell carcinoma (stage III‐IV) of the oral cavity, larynx, oropharynx hypopharynx and/or nasopharynx, with 33% at stage III and 67% at stage IV.
Primary treatments for head and neck cancer
In Carnaby‐Mann 2012, patients were all planned for external beam radiotherapy (either conventional or intensity‐modulated radiotherapy). They were able to undergo magnetic resonance imaging (MRI) and had no history of non‐oral feeding. In Kotz 2012, patients all completed seven weeks of radiation treatment. In Lazarus 2014, 21 patients (91%) underwent chemoradiotherapy and two (9%) had radiotherapy alone. In Mortensen 2015, patients were scheduled for curative radiotherapy and none had primary surgery. In the study by van den Berg 2014, patients were planned for curative treatment (primary or postoperatively with chemoradiotherapy or radiotherapy). In van der Molen 2011, patients were planned for curative treatment (primary or postoperatively) with chemoradiotherapy or radiotherapy. There were no studies in which patients were treated with surgery.
Interventions
Most researchers used protocols involving tongue exercises or swallowing exercises, or both, but with varying regimens (in terms of intensity and 'dosage').
Carnaby‐Mann 2012 divided participants across three groups: controls (TAU), sham therapy ('Valchuff') and Pharyngocise (intervention), whereas all other studies had two groups. Kotz 2012 allocated adults to control (TAU) and intervention groups (five targeted swallowing exercises). In Lazarus 2014, participants were randomised into control (traditional exercises) and intervention (traditional exercise plus specific tongue exercises) groups. Mortensen 2015 randomised participants en bloc to a control group (TAU – receipt of individual dietary advice from a dietician) and an intervention group (range of movement and resistance exercises from an occupational therapist, as well as dietary advice from a dietician). van den Berg 2014 randomised patients to a control group (dietary counselling) and an intervention group (home exercises, delivered by speech and language therapists). van der Molen 2011 randomised patients into a control (TAU) and intervention group (three times daily use of the Therabite, a tool for mouth and jaw stretching).
Primary outcomes
Safety and efficiency of oral swallowing
There was considerable heterogeneity in the assessment tools and outcome measures used across the included studies. Swallowing orally as a primary outcome was reported in all six studies (Carnaby‐Mann 2012; Kotz 2012; Lazarus 2014; Mortensen 2015; van den Berg 2014; van der Molen 2011), but swallowing safely and/or efficiently was not always documented (van den Berg 2014). The clinical criterion standard for assessing swallowing (dys)function is by use of a videofluoroscopic swallowing study (VFSS), which occurred in five studies (Carnaby‐Mann 2012; Kotz 2012; Lazarus 2014; Mortensen 2015; van der Molen 2011). By contrast, van den Berg 2014 used a (non‐validated) water swallowing velocity and volume test.
Reduced/no aspiration
In the studies by Kotz 2012, Carnaby‐Mann 2012 and van den Berg 2014 aspiration was not assessed.
Mortensen 2015 and van der Molen 2011 each used a VFSS to assess laryngeal penetration/aspiration and a validated scale was used to rate each swallow at each time point (Rosenbek 1996): baseline ‐ before treatment ‐ in both studies, and at two, five and 13 months post‐treatment in the study by Mortensen 2015 and at 10 weeks post‐treatment completion in the study by van der Molen 2011.
Lazarus 2014 used a (non‐validated) scale to rate the presence, timing and percentage of aspiration of each bolus.
Oropharyngeal swallowing efficiency
Lazarus 2014 measured the oropharyngeal swallowing efficiency (OPSE) of each swallow during a VFSS by calculating the percentage of liquid, thin and thick paste boluses swallowed (by subtracting any aspirate and/or oral residue), divided by the time taken to swallow it. Dysphagia was defined as an OPSE score of 39 or less. Swallowing function (OPSE) was assessed from a VFSS of three swallows each of 1 ml, 3 ml and 10 ml thin liquid barium, 1 ml, 3 ml and 10 ml thick liquid barium, and 3 ml thin paste and 3 ml thick paste.
van der Molen 2011 assessed swallowing function (not efficiency per se) using a surrogate measure of OPSE. From a VFSS at each time point, contrast residue was assessed (binary rating of 'yes' (present), or 'no' (not present)) and site (above or below valleculae, or both).
Adverse events
Aspiration pneumonia, chest infection, dehydration and profound weight loss were documented by Carnaby‐Mann 2012 and being tube fed was documented by Mortensen 2015 and van den Berg 2014.
Secondary outcomes
Time to return to function (swallowing)
This was not documented in any of the studies. Lazarus 2014 assessed quality of life from a patient‐completed questionnaire, the Head & Neck Cancer Inventory (HNCI) at each time point. There was no significant difference pre‐ or post‐treatment.
Self‐reported changes to quality of life
Kotz 2012 and van den Berg 2014 both assessed swallowing‐related quality of life from the 'Eating in Public' subscale of the Performance Status Scale for Head and Neck Cancer (PSS‐HN), which documents a patient's ability to share a meal with others and in what environment (which is an indicator of swallowing performance, not quality of life). Although self‐reporting was not used, quality of life was assessed by Lazarus 2014 using the HNCI, by Mortensen 2015 using the EORTC, the QLC‐C30 and H&N 36 (all validated tools) and van der Molen 2011 used a 'study‐specific' (non‐standardised) questionnaire to evaluate quality of life.
Lazarus 2014 assessed quality of life from a patient‐completed questionnaire, the HNCI, at each time point.
Changes to psychological wellbeing: depression, anxiety and stress
Changes to patients' psychological wellbeing were not measured in any study.
Patient satisfaction with intervention
Patient satisfaction was not documented in any study.
Patient compliance with intervention
Carnaby‐Mann 2012, Lazarus 2014 and Mortensen 2015 each included patients' self‐report of compliance with the protocol. Adherence to the protocol and gender differences were recorded by the study speech pathologist in van der Molen 2011.
Cost‐effectiveness of intervention
Only in the study by van der Molen 2011 (reported in van der Molen 2014) was a cost‐benefit/economic evaluation reported.
Excluded studies
We excluded three studies from the review because, despite the study titles, in none of the studies were the participants randomised (Carroll 2008; Virani 2013; Zhen 2012). We excluded one study because, although it was an RCT, the exercises were rehabilitative, rather then preventative, of dysphagia (Tang 2011). See Characteristics of excluded studies.
Risk of bias in included studies
See Figure 2 for a 'Risk of bias' graph, which illustrates our judgements about each 'Risk of bias' item, presented as percentages across all included studies and Figure 3 for a 'Risk of bias' summary of our judgements about each 'Risk of bias' item for each included study.
2.
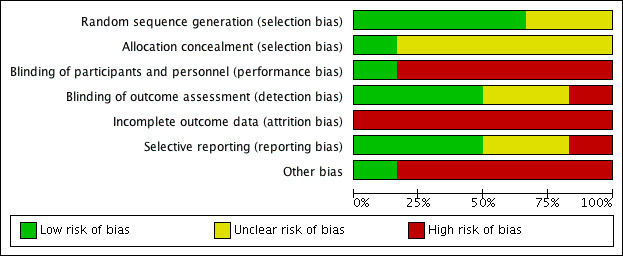
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
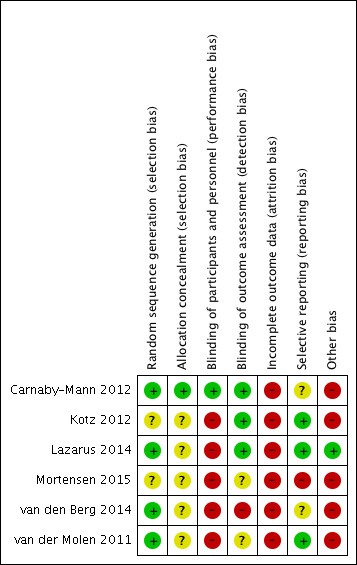
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation (selection bias)
In all included studies the authors describe randomisation, but the level of description of allocation varied. In four papers, computer‐generated randomisation was described (Carnaby‐Mann 2012; Lazarus 2014; van den Berg 2014; van der Molen 2011), which means a low risk of bias. We classified the other two studies as having an unclear risk of bias for this domain.
Allocation concealment (selection bias)
Carnaby‐Mann 2012 described the randomisation schedule being held remotely from the study environment (low risk of bias), but the other five studies gave no detail about allocation sequence concealment (unclear risk of bias).
Blinding
Blinding of participants and personnel (performance bias)
In only one study were the participants blinded to their intervention, not knowing whether they were assigned to an intervention ('Pharyngocise') or to a sham exercise ('Valchuff') group (Carnaby‐Mann 2012). We classified this as a low risk of bias. In all other studies the participants knew to which group they were assigned (high risk of bias).
Blinding of outcome assessment (detection bias)
In three studies there was single‐blinding of an assessor to the intervention (low risk of bias) (Carnaby‐Mann 2012; Kotz 2012; Lazarus 2014). In the study by Mortensen 2015 there was partial blinding, as the radiologist (scoring primary outcomes) was blinded (low risk of bias), but the occupational therapist who scored the secondary outcomes was not blinded (high risk of bias) so we judged this study as having an unclear risk of detection bias. The study by van der Molen 2011 had an unclear risk of bias and van den Berg 2014 stated that there was no concealment (high risk of bias).
Incomplete outcome data
All authors described, and gave reasons for, dropouts and for missing outcome data (Carnaby‐Mann 2012; Kotz 2012; Lazarus 2014; Mortensen 2015; van den Berg 2014; van der Molen 2011). However, in all six studies a high rate of poor compliance with treatment was reported, resulting in a high risk of attrition bias.
Selective reporting
Reported data were linked to pre‐specified outcomes in all studies (low risk of bias). However, van den Berg 2014 did not report on all endpoints, as swallowing velocity and swallowing volume tests were not reported and there was no protocol for the Carnaby‐Mann 2012 study (unclear risk of bias). We judged Mortensen 2015 to have a high risk of bias due to discrepancy between planned and actual time point reporting.
Other potential sources of bias
Five studies had small sample sizes (low power) with the number of participants ranging from 23 to 58 (high risk of bias), but van den Berg 2014 had 120 participants, based on a sound sample size calculation (low risk of bias). Timing of assessments varied. All studies had assessment of participants at baseline (pre‐treatment ‐ although it was not always specified exactly when) and then immediately after intervention, but thereafter the times of assessment ranged from three to six, nine and/or 11 or 12 months post‐intervention.
Compliance was self‐reported by participants in the studies by Carnaby‐Mann 2012, Lazarus 2014 and Mortensen 2015 (high risk of bias).
In the studies by Kotz 2012 and van den Berg 2014, the intervention group also had weekly speech and language therapy sessions (high risk of bias) and in Kotz 2012, 9 of 13 patients (69%) were unable to complete the protocol (high risk of bias).
In the study by Carnaby‐Mann 2012, the number and duration of swallowing therapy sessions for patients assigned to the two treatment arms were significantly greater than those for the usual care group (F(2.81) = 4.8, P < 0.0001) (high risk of bias). The number of sessions also differed significantly between the treatment groups (Pharyngocise, 19.9; sham, 25.8; t = ‐2.194; P <= 0.03) (high risk of bias).
van der Molen 2011 stated that patients in their control group practised significantly more than those in the intervention group (high risk of bias).
Lazarus 2014 measured tongue strength using an instrument of unknown psychometric properties and it is not clear if the assessor was blinded to the intervention (unclear risk of bias), whereas all swallows were analysed by a blinded assessor (low risk of bias).
Given that all six studies had one or more potential sources of bias (sample size, attrition and compliance with treatment), we classified them all as being at high risk of bias.
Effects of interventions
See: Table 1 for the main comparison 'Therapeutic exercises versus treatment as usual (TAU)' and Table 2 for the comparison 'Therapeutic exercises versus sham exercises'.
We were not able to pool data from the included studies because of significant differences in the protocols and interventions. Such a lack of standardisation and consistency in outcome measures is a major barrier to assessing the effects of interventions. The low power (due to small sample sizes), diversity of study designs and interventions, and the potential for bias meant that we were unable to aggregate cross‐study data. We therefore report (below) on individual study results for the primary and secondary outcomes.
Primary outcome: safety and efficiency of oral swallowing
Reduced/no aspiration
In the study by Lazarus 2014, at baseline seven participants aspirated (five in the treatment group and two in the control group) and there was no difference between the groups post‐intervention. The studies by Mortensen 2015 and van der Molen 2011 both demonstrated no significant difference between the groups on post‐treatment scores. In the study by van der Molen 2011 the intra‐observer agreement for both time points was good at 0.98 and 0.88.
Oropharyngeal swallowing efficiency: OroPharyngeal Swallowing Efficiency (OPSE) measures
Lazarus 2014 found that the OPSE was lower (worse) than for (previously reported) healthy controls for both groups, both before and after treatment, with marked data variability within and across each group at both time points. At 10 weeks post‐treatment the OPSE scores in the treatment group improved; however the difference was not statistically significant (‐8.06, 95% confidence interval (CI) ‐25.37 to 9.25) (see Analysis 1.1).
1.1. Analysis.
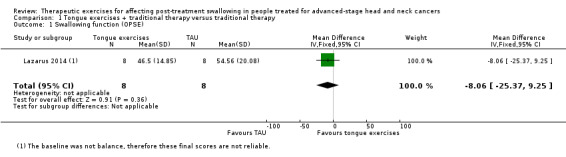
Comparison 1 Tongue exercises + traditional therapy versus traditional therapy, Outcome 1 Swallowing function (OPSE).
van der Molen 2011 achieved good agreement across two assessors (0.80 and 0.89 for pre‐ and post‐treatment group ratings respectively). At 10 weeks post‐treatment, the intervention group showed significantly less residue after swallowing a solid bolus than the control group (P < 0.02).
Adverse events
Adverse events, such as chest infections, aspiration pneumonia and profound weight loss, were recorded in only three studies. Carnaby‐Mann 2012 assessed dysphagia‐related complications (pneumonia, dehydration, mucositis and oral yeast infection) and found no significant associations between these and the treatment group. Weight loss was measured at six weeks post‐treatment. A total of 40% of patients (n = 23) lost more than 10% of their body weight by six weeks post‐treatment, but the average weight loss was not significantly different across the three treatment groups (see Analysis 4.2). Kotz 2012 had four patients discontinue exercise at week five and five more discontinued after week five, due to oral pain, throat discomfort and/or fatigue. Lazarus 2014 reported that most participants were unable to continue the protocol during their last two weeks of treatment. Participants reported mouth/throat pain, coughing and gagging.
4.2. Analysis.
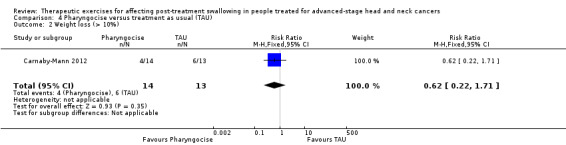
Comparison 4 Pharyngocise versus treatment as usual (TAU), Outcome 2 Weight loss (> 10%).
Secondary outcomes
Time to return to function (swallowing)
This was not measured in any of the studies.
Self‐reported changes to quality of life
Kotz 2012 found no difference between the intervention and control groups immediately post‐treatment, but patients in the intervention group were significantly better at three months (median intervention score: 100, range 75 to 100 versus median control group score: 100, range 25 to 100; P = 0.03), and at six months later (median intervention score 100, range 75 to 100 versus median control score 100, range 25 to 100; P = 0.03). van den Berg 2014 also found no significant difference between groups using the Performance Status Scale for Head and Neck Cancer (PSS‐HN) scale.
Lazarus 2014 found no significant difference between groups on the Head & Neck Cancer Inventory (HNCI) after treatment. However, the control group had a higher social disruption score and the eating domain was significantly higher for the intervention group post‐treatment.
Mortensen 2015 found no significant difference between groups, except for a lower global quality of life score at two weeks and at two months in the intervention group. At five, eight and 11 months post‐treatment scores were not significantly different across the groups. van der Molen 2011 found no differences between groups in either the pre‐ or post‐treatment answers on the subscales of their study questionnaire.
Changes to psychological well‐being: depression, anxiety and stress
Changes to patients' psychological wellbeing were not assessed in any study.
Patient satisfaction with intervention
There was no assessment of patient satisfaction in any study.
Patient compliance with intervention
In all studies the participants had self‐reported difficulties with compliance with the protocol.
In Kotz 2012, 69% of participants discontinued the protocol at five weeks (due to pain from chemoradiotherapy). In Carnaby‐Mann 2012, only 68% of participants complied with a home practice protocol. In Lazarus 2014, the treatment group had six participants with 'poor', two with 'fair' and one with 'good' compliance. Their controls had, respectively, two with 'poor', five with 'fair' and four with 'good' compliance. In the study by Kotz 2012, three patients (of 46) were excluded from analysis because of poor compliance with the protocol, four patients discontinued exercises after week four and five more discontinued after week five. van der Molen 2011 reported that 51% of participants (n = 28) in their study stopped exercising after 27 days. The low compliance rate in all trials is of concern when assessing results.
Cost‐effectiveness of the intervention
Cost‐effectiveness was reported in only one study. van der Molen 2011 examined the addition of their preventative swallowing rehabilitation programme in improving quality‐adjusted life years (QALY) and calculated an additional cost of EUR 3200/QALY. They state that the programme had "a higher probability of being cost‐effective compared to usual care" (p1268).
Ongoing studies
We identified four ongoing studies of the effects of therapeutic exercises before, during and/or after head and neck cancer treatment to improve oral swallowing (Barretos Cancer Hospital 2015; Fredslund 2015; Govender 2014; van Nuffelen 2014) (see Characteristics of ongoing studies). The studies are being undertaken in the United Kingdom (Govender 2014), Belgium (van Nuffelen 2014), Brazil (Barretos Cancer Hospital 2015), and Denmark (Fredslund 2015). The Brazilian study has been completed, however no results are yet available (Barretos Cancer Hospital 2015).
Discussion
This is the first systematic review to examine the evidence to support the use of therapeutic exercises before, during and/or immediately after head and neck cancer (HNC) treatment to improve oral swallowing.
Summary of main results
We assessed six included studies (reported as seven papers) but we were not able to conduct a meta‐analysis of their outcome data, due to the heterogeneity of the participants and the variation in study methodology.
The significant question of whether therapeutic exercises affect post‐treatment swallowing in people treated for advanced‐stage head and neck cancer cannot be answered; nor can we answer the question of whether undertaking pre‐ and/or peri‐treatment exercises results in better outcomes than delaying exercise until dysphagia becomes evident.
The lack of definitive results is mainly due to the small participant numbers in trials, the lack of consistency in the choice of primary outcome variables, the variation in the measurement tools used for assessing them, and the choice of different baseline and endpoints across studies.
Adverse events, such as weight loss/weight change, were likely due to the concurrent HNC treatment, rather than the result of undertaking swallowing exercise protocols. There were no reported adverse events that were directly attributable to the intervention (swallowing exercises).
Overall completeness and applicability of evidence
Only six studies were eligible for this review; this number was insufficient to allow firm conclusions to be drawn regarding our primary outcome measure, the safety and efficiency of oral swallowing. In all six studies, swallowing orally was a primary outcome, but in only one study was swallowing safety and efficiency assessed (Lazarus 2014).
A lack of standardisation and of consistency in reporting of outcome measures meant that comparison of results was not possible. Due to differing study outcomes, aggregation of data was also not possible, so no meta‐analyses could be performed. We noted a paucity of secondary outcomes measured; some were not reported at all in any of the included studies. These included time to return to function (swallowing), changes to psychological wellbeing and satisfaction with the intervention. Changes to patients' quality of life using a self‐report tool (clinical criterion standard) were reported by Lazarus 2014; validated clinician‐rated quality of life measures were reported by Mortensen 2015 and a non‐validated questionnaire was reported in another study (van den Berg 2014).
In terms of patient compliance, when reported, it was unclear how this had been recorded – whether by patient self‐report or by clinician evaluation. Either method raises potential issues of bias.
Quality of the evidence
Unfortunately, the overall quality of evidence available for this review was disappointing, as all trials had small participant numbers and were of variable methodological quality. The 'Summary of findings' tables indicate that all studies had low participant numbers ‐ fewer than 120 (see table footnotes). We classified none of the six studies as having a low risk of bias for all seven domains of the Cochrane 'Risk of bias' tool (see Figure 2 and Figure 3).
Using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system, we classified the overall quality of evidence as very low, due to the limited number of participants in each study and the poor methodological quality of all but one (Lazarus 2014) of the six studies reviewed (see Table 1).
Potential biases in the review process
The review protocol was thorough and included a comprehensive search strategy using multiple sources and an independent screening of trials for inclusion. Selection, data extraction and risk of bias considerations were independently performed by the first and third authors. Disagreements were resolved and confirmed by the second and the last authors. We have presented and discussed all outcomes described in the protocol for this review that were available for analysis, whether statistically significant or not.
The GRADE method of construction of 'Summary of findings' tables was undertaken by the second and last review authors, and may have been influenced by their interpretation, where ambiguous or incomplete data were supplied. The first author (an experienced head and neck cancer clinician) independently examined the 'Summary of findings' tables and resolved any uncertainty, but we acknowledge this could be a source of bias in the review.
Agreements and disagreements with other studies or reviews
This is the first systematic review on this topic. Other studies have been published in the literature where therapeutic exercises for swallowing dysfunction were applied, but these were not randomised trials and they each had very small sample sizes (Carroll 2008; Virani 2013; Zhen 2012).
Authors' conclusions
Implications for practice.
For people with dysphagia as a result of advanced head and neck cancer
Swallowing exercises undertaken before, during or immediately after head and neck cancer (HNC) treatment to reduce the possibility of dysphagia developing as a treatment side effect are often suggested or implemented. However, there is currently no evidence to support such advice.
For clinicians
Caution needs to be exercised in suggesting a benefit of pre‐ and/or peri‐treatment swallowing exercises to patients with advanced HNC as the published evidence for efficacy is lacking, patient compliance is poor and the question of whether such therapeutic exercises can positively affect post‐treatment swallowing in people treated for advanced‐stage head and neck cancer remains to be answered.
Although there has been an improvement in survival rates for people with advanced head and neck cancer, dysphagia remains as a significant negative side effect of modern treatments (radiotherapy or chemoradiotherapy) and there is a need to ameliorate this outcome. To address the problem, trials of therapeutic swallowing exercises during head and neck cancer treatment have been undertaken, but exercise protocols vary considerably in type, amount and delivery.
More research is needed to learn whether implementing swallowing exercises before and/or during HNC treatment are justified to reduce dysphagia as a side effect of treatment. However, before further controlled clinical trials are implemented, pilot studies are needed to ascertain patients' tolerance for swallowing exercises, and to design simple, effective swallowing exercise protocols that can be undertaken by HNC patients at home and incorporated into daily life, without an additional burden of attendance at a hospital or clinic.
As there is no consensus or underpinning evidence to establish the swallowing exercise protocols, they vary considerably and rely on the capacity of patients to undertake them while simultaneously undergoing intensive HNC treatment that may result in fatigue, pain and/or mucositis. From this review, there is evidence of patients' difficulty in complying with swallowing exercise protocols/poor fidelity to treatment, as the protocols themselves are often onerous to undertake. Consequently, the results are not definitive. Well‐validated tools should be used to measure key outcomes; for example, safety and efficiency of swallowing, side effects of interventions, changes to quality of life and/or to psychosocial domains, and the cost‐benefit of the intervention.
For policy makers
Recently published UK National Institute for Care and Health Excellence (NICE) guidelines include the following advice about speech and language therapy intervention, "Consider swallowing‑exercise programmes for people having radiotherapy" (NICE Guidelines 2016). Given the results of this review, more work is needed to establish which are the effective evidence‐based interventions given by healthcare professionals who manage head and neck cancer patients, to identify timing, duration and dosage, and to establish the feasibility of such 'exercise programmes'.
Rehabilitation of swallowing during and after HNC treatment remains a significant challenge to be addressed. Until dysphagia as a side effect of treatment is reduced, the benefit of improved survival from modern treatments will be tempered by the profound and debilitating side effects that many patients live with.
For funders
There is an urgent need to fund quality studies to examine the usefulness of swallowing exercises before, during and/or after HNC treatment in reducing/eliminating dysphagia as a profound and debilitating side effect of HNC treatment. Collaboration across HNC centres is needed, with agreed standards (methods and time points) for documenting dysphagia as an adverse event. To obtain the necessary sample sizes with such a heterogeneous patient cohort, multicentre (preferably international) trials need to be implemented.
Feasible exercise regimes (in terms of 'dosage' ‐ i.e. intensity and timing) need to be designed and rigorously assessed, to provide good evidence to underpin and direct quality swallowing therapy.
Implications for research.
General
The randomised controlled trials in this area need to be better planned and preceded by good vanguard studies. The dropout rate in all published trials has been unacceptably high, which has negatively impacted on the statistical power of studies to show positive outcomes. One reason for dropout has been the interventions used: each study has had an overly onerous exercise burden for patients. Good qualitative research studies to better understand patients' tolerance for undertaking swallowing exercises during treatment would likely improve the design of such exercise interventions and thereby enhance fidelity to treatment.
Design
Published studies that investigate the benefit of therapeutic swallowing exercises for reducing dysphagia in people treated for advanced head and neck cancer lack methodological rigour.
Interventions need to be better designed as simple to follow, easy to undertake exercises, and the 'dosage' (duration, timing) of exercises needs to be feasible and based on evidence of effect. To fully capture the negative effect of dysphagia on patients' health, applying the World Health Organization (WHO) International Classification of Functioning, Disability and Health (ICF) model offers a multi‐dimensional assessment to describe the effect of dysphagia on a head and neck cancer population (Frowen 2006).
Measurement (endpoints)
The primary outcomes are not always specified: tools used to measure outcomes are not always well validated and the outcomes measured are either not variables that are key (viz. safety and efficiency of swallowing) or else are measuring a surrogate, such as changes to tongue muscle bulk (Carnaby‐Mann 2012), rather than changes to swallowing.
Realistic time points for assessment need to be agreed as standard across studies for comparison and/or aggregation of data to occur. Baseline (pre‐treatment) recordings enable the effect of the initial cancer on swallowing to be examined. Post‐treatment assessment would then enable the acute effect of (chemo)radiation to be audited as an adverse event of treatment and, as data suggest that the time to optimal recovery of swallowing is six months post‐HNC treatment completion (Perry 2003), this would be a suitable endpoint for measurement.
Other
Future trials should take into account the limitations highlighted in this review, for example:
report research results following the CONSORT guidelines (CONSORT 2010);
clearly operationalise primary and secondary outcomes;
report adverse events;
make protocols available online;
use well‐validated, suitable measurement tools to assess change from therapeutic swallowing exercises in people with head and neck cancer;
choose time points that are meaningful for assessing change and ensure that baseline (pre‐treatment) measures are included;
use therapeutic swallowing exercises with manageable protocols (type, amount of exercise; dosage, timing of exercises specified).
What's new
| Date | Event | Description |
|---|---|---|
| 30 August 2016 | Amended | Author contact details updated. |
Acknowledgements
Ms Joanne MacCormack, former MSc student in Speech and Language Therapy at the University of Limerick, assisted with data extraction/completion of forms during 2014. Ms Samantha Faulkner, Information Specialist, Cochrane ENT, conducted the searches for this review. Dr Christopher Cates, Senior Clinical Research Fellow and Cochrane Co‐ordinating Editor, Cochrane Airways Review Group, provided advice on the presentation of the 'Summary of findings' table. Ms Jenny Bellorini, Managing Editor, Cochrane ENT, provided support throughout the review process.
The first three paragraphs of the Description of the condition section were written by Cochrane ENT editor Dr. Cecelia E Schmalbach as a standard introduction to Cochrane head and neck cancer reviews and are reproduced with permission.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
| CENTRAL | Ovid MEDLINE | EMBASE (Ovid) |
| #1 MeSH descriptor: [Oropharyngeal Neoplasms] explode all trees #2 MeSH descriptor: [Head and Neck Neoplasms] this term only #3 MeSH descriptor: [Otorhinolaryngologic Neoplasms] explode all trees #4 MeSH descriptor: [Neoplasms] explode all trees #5 cancer* or carcinoma* or neoplas* or tumor* or tumour* or malignan* or SCC #6 #4 or #5 #7 MeSH descriptor: [Oropharynx] explode all trees #8 oropharyn* or mesopharyn* or tonsil* or (head near/3 neck) or "tongue base" #9 #7 or #8 #10 #6 and #9 #11 HNSCC or SCCHN or OP‐SCC or OPSCC #12 #1 or #2 or #3 or #10 or #11 #13 MeSH descriptor: [Deglutition] explode all trees #14 MeSH descriptor: [Deglutition Disorders] this term only #15 swallow* or deglutit* or dysphag* #16 #13 or #14 or #15 #17 MeSH descriptor: [Exercise Therapy] explode all trees #18 MeSH descriptor: [Isometric Contraction] explode all trees #19 MeSH descriptor: [Behavior Therapy] explode all trees #20 (swallow* or deglutit* or dysphag* or neuromuscular or mendelsohn or masako or "neuro muscular") near/3 (exercis* or maneuver* or manoeuvre* or manoeuver* or technique* or treatment* or strateg* or rehab* or therap*) #21 isometric or IOPI or "iowa oral pressure instrument*" or pharyngocise or OPSE or "oropharyngeal swallowing efficiency" #22 nmt or "lingual exercis*" or "effortful swallow*" or "supraglottic swallow" or "super glottic swallow*" or "supra glottic swallow*" #23 (tongue or BOT) near/3 ("range of motion" or ROM or resistance or strength* or holding) #24 (voluntary near/3 (control or maneuver* or manoeuvre* or manoeuver*)) or "bearing down" or "progressive resistance" or "behav* management" or (exercis* near/3 (therap* or regime)) or "therapeutic techniq*" or "behav* therap*" or "larygeal elevat*" #25 #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 #26 #25 and #16 #27 MeSH descriptor: [Deglutition Disorders] explode all trees and with qualifier(s): [Prevention & control ‐ PC, Rehabilitation ‐ RH, Therapy ‐ TH] #28 #26 or #27 #29 #12 and #28 |
1 exp Oropharyngeal Neoplasms/ 2 "Head and Neck Neoplasms"/ 3 exp Otorhinolaryngologic Neoplasms/ 4 exp Neoplasms/ 5 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or malignan* or SCC).tw. 6 4 or 5 7 exp Oropharynx/ 8 (oropharyn* or mesopharyn* or tonsil* or (head adj3 neck) or "tongue base").tw. 9 7 or 8 10 6 and 9 11 (HNSCC or SCCHN or OP‐SCC or OPSCC).tw. 12 1 or 2 or 3 or 10 or 11 13 exp Deglutition/ 14 Deglutition Disorders/ 15 (swallow* or deglutit* or dysphag*).tw. 16 13 or 14 or 15 17 exp Exercise Therapy/ 18 exp Isometric Contraction/ 19 exp Behavior Therapy/ 20 ((swallow* or deglutit* or dysphag* or neuromuscular or mendelsohn or masako or "neuro muscular") adj3 (exercis* or maneuver* or manoeuvre* or manoeuver* or technique* or treatment* or strateg* or rehab* or therap*)).tw. 21 (isometric or IOPI or "iowa oral pressure instrument*" or pharyngocise or OPSE or "oropharyngeal swallowing efficiency").tw. 22 (nmt or "lingual exercis*" or "effortful swallow*" or "supraglottic swallow" or "super glottic swallow*" or "supra glottic swallow*").tw. 23 ((tongue or BOT) adj3 ("range of motion" or ROM or resistance or strength* or holding)).tw. 24 ((voluntary adj3 (control or maneuver* or manoeuvre* or manoeuver*)) or "bearing down" or "progressive resistance" or "behav* management" or (exercis* adj3 (therap* or regime)) or "therapeutic techniq*" or "behav* therap*" or "larygeal elevat*").tw. 25 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26 16 and 25 27 exp Deglutition Disorders/pc, rh, th [Prevention & Control, Rehabilitation, Therapy] 28 26 or 27 29 12 and 28 |
1 exp Oropharyngeal Neoplasms/ 2 "Head and Neck Neoplasms"/ 3 exp Otorhinolaryngologic Neoplasms/ 4 exp Neoplasms/ 5 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or malignan* or SCC).tw. 6 4 or 5 7 exp Oropharynx/ 8 (oropharyn* or mesopharyn* or tonsil* or (head adj3 neck) or "tongue base").tw. 9 7 or 8 10 6 and 9 11 (HNSCC or SCCHN or OP‐SCC or OPSCC).tw. 12 1 or 2 or 3 or 10 or 11 13 exp swallowing/ 14 dysphagia/ 15 (swallow* or deglutit* or dysphag*).tw. 16 13 or 14 or 15 17 exp Exercise Therapy/ 18 exp Isometric Contraction/ 19 exp Behavior Therapy/ 20 ((swallow* or deglutit* or dysphag* or neuromuscular or mendelsohn or masako or "neuro muscular") adj3 (exercis* or maneuver* or manoeuvre* or manoeuver* or technique* or treatment* or strateg* or rehab* or therap*)).tw. 21 (isometric or IOPI or "iowa oral pressure instrument*" or pharyngocise or OPSE or "oropharyngeal swallowing efficiency").tw. 22 (nmt or "lingual exercis*" or "effortful swallow*" or "supraglottic swallow" or "super glottic swallow*" or "supra glottic swallow*").tw. 23 ((tongue or BOT) adj3 ("range of motion" or ROM or resistance or strength* or holding)).tw. 24 ((voluntary adj3 (control or maneuver* or manoeuvre* or manoeuver*)) or "bearing down" or "progressive resistance" or "behav* management" or (exercis* adj3 (therap* or regime)) or "therapeutic techniq*" or "behav* therap*" or "larygeal elevat*").tw. 25 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26 16 and 25 27 exp dysphagia/pc, rh, th [Prevention, Rehabilitation, Therapy] 28 26 or 27 29 12 and 28 |
| CINAHL (EBSCO) | Web of Science (Web of Knowledge) | Trial registries |
| S29 S11 AND S28 S28 S25 OR S26 OR S27 S27 (MH "Swallowing Therapy") S26 (MH "Deglutition Disorders/PC/RH/TH") S25 S15 AND S24 S24 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 S23 TX (voluntary n3 (control or maneuver* or manoeuvre* or manoeuver*)) or "bearing down" or "progressive resistance" or "behav* management" or (exercis* n3 (therap* or regime)) or "therapeutic techniq*" or "behav* therap*" or "larygeal elevat*" S22 TX (tongue or BOT) n3 ("range of motion" or ROM or resistance or strength* or holding) S21 TX nmt or "lingual exercis*" or "effortful swallow*" or "supraglottic swallow" or "super glottic swallow*" or "supra glottic swallow*" S20 TX isometric or IOPI or "iowa oral pressure instrument*" or pharyngocise or OPSE or "oropharyngeal swallowing efficiency" S19 TX (swallow* or deglutit* or dysphag* or neuromuscular or mendelsohn or masako or "neuro muscular") n3 (exercis* or maneuver* or manoeuvre* or manoeuver* or technique* or treatment* or strateg* or rehab* or therap*) S18 (MH "Behavior Therapy+") S17 (MH "Isometric Contraction") S16 (MH "Therapeutic Exercise+") S15 S12 OR S13 OR S14 S14 TX swallow* or deglutit* or dysphag* S13 (MH "Deglutition Disorders") S12 (MH "Deglutition") S11 S1 OR S2 OR S9 OR S10 S10 TX HNSCC or SCCHN or OP‐SCC or OPSCC S9 S5 AND S8 S8 S6 OR S7 S7 TX oropharyn* or mesopharyn* or tonsil* or (head n3 neck) or "tongue base" S6 (MH "Oropharynx+") S5 S3 OR S4 S4 TX cancer* or carcinoma* or neoplas* or tumor* or tumour* or malignan* or SCC S3 (MH "Neoplasms+") S2 (MH "Otorhinolaryngologic Neoplasms+") S1 (MH "Head and Neck Neoplasms+") |
#1 TOPIC: (cancer* or carcinoma* or neoplas* or tumor* or tumour* or malignan* or SCC) #2 TOPIC: (oropharyn* or mesopharyn* or tonsil* or (head near/3 neck) or "tongue base") #3 #2 AND #1 #4 TOPIC: (HNSCC or SCCHN or OP‐SCC or OPSCC) #5 #4 OR #3 #6 TITLE: (swallow* or deglutit* or dysphag*) #7 TITLE: ((neuromuscular or mendelsohn or masako or "neuro muscular") and (exercis* or maneuver* or manoeuvre* or manoeuver* or technique* or treatment* or strateg* or rehab* or therap*)) #8 TITLE: (isometric or IOPI or "iowa oral pressure instrument*" or pharyngocise or OPSE or "oropharyngeal swallowing efficiency") #9 TITLE: (nmt or "lingual exercis*" or "effortful swallow*" or "supraglottic swallow" or "super glottic swallow*" or "supra glottic swallow*") #10 TITLE: ((tongue or BOT) and ("range of motion" or ROM or resistance or strength* or holding)) #11 TITLE: ((voluntary and (control or maneuver* or manoeuvre* or manoeuver*)) or "bearing down" or "progressive resistance" or "behav* management" or (exercis* and (therap* or regime)) or "therapeutic techniq*" or "behav* therap*" or "larygeal elevat*") #12 #11 OR #10 OR #9 OR #8 OR #7 OR #6 #13 #12 AND #5 |
ICTRP Title: swallow* OR deglutit* OR dysphag* ClinicaTrials.gov (swallow* OR deglutit* OR dysphag*) AND (HNSCC OR SCCHN OR OP‐SCC OR OPSCC OR oropharyn* OR mesopharyn* OR tonsil* OR (head AND neck) OR "tongue base") |
Data and analyses
Comparison 1. Tongue exercises + traditional therapy versus traditional therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Swallowing function (OPSE) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐8.06 [‐25.37, 9.25] |
Comparison 2. Swallow therapy versus treatment as usual (TAU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Swallowing velocity (ml/sec) | 1 | 120 | Mean Difference (Fixed, 95% CI) | ‐2.0 [‐5.30, 1.30] |
| 2 Swallowing volume, ml | 1 | 120 | Mean Difference (Fixed, 95% CI) | 5.9 [‐1.10, 12.90] |
| 3 Weight change | 1 | 120 | Mean Difference (Fixed, 95% CI) | 1.34 [‐0.46, 3.14] |
| 4 Dysphagia severity | 1 | 120 | Mean Difference (Fixed, 95% CI) | ‐0.1 [‐0.50, 0.30] |
2.1. Analysis.
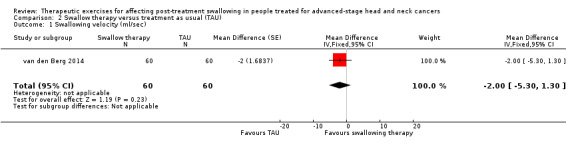
Comparison 2 Swallow therapy versus treatment as usual (TAU), Outcome 1 Swallowing velocity (ml/sec).
2.2. Analysis.
Comparison 2 Swallow therapy versus treatment as usual (TAU), Outcome 2 Swallowing volume, ml.
2.3. Analysis.
Comparison 2 Swallow therapy versus treatment as usual (TAU), Outcome 3 Weight change.
2.4. Analysis.
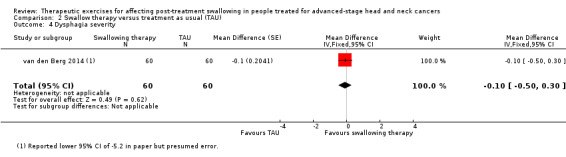
Comparison 2 Swallow therapy versus treatment as usual (TAU), Outcome 4 Dysphagia severity.
Comparison 3. Pharyngocise versus sham care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Swallowing ability (VFE) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.24, ‐0.04] |
| 2 Weight loss (> 10%) | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.22, 1.71] |
3.1. Analysis.
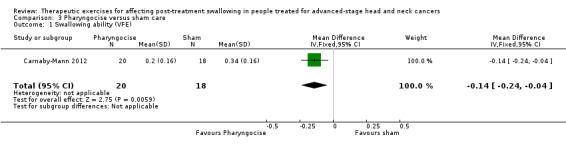
Comparison 3 Pharyngocise versus sham care, Outcome 1 Swallowing ability (VFE).
3.2. Analysis.
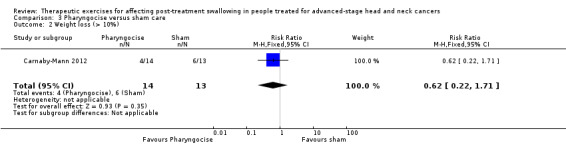
Comparison 3 Pharyngocise versus sham care, Outcome 2 Weight loss (> 10%).
Comparison 4. Pharyngocise versus treatment as usual (TAU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Swallowing ability ‐ VFE | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 2 Weight loss (> 10%) | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.22, 1.71] |
4.1. Analysis.
Comparison 4 Pharyngocise versus treatment as usual (TAU), Outcome 1 Swallowing ability ‐ VFE.
Comparison 5. Sham exercises versus treatment as usual (TAU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Swallowing ability (VFE) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.05, 0.21] |
5.1. Analysis.
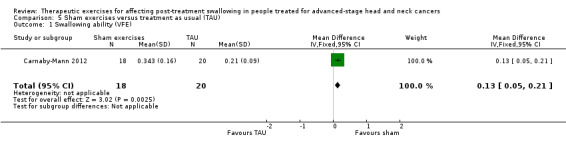
Comparison 5 Sham exercises versus treatment as usual (TAU), Outcome 1 Swallowing ability (VFE).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carnaby‐Mann 2012.
| Methods |
Allocation: randomised controlled trial Design: parallel‐group |
|
| Participants |
Number: 58 Age (mean years): sham group: 60 ± 12.2; Pharyngocise group: 59 ± 10.4; control group: 54 ± 11.3 Gender: sex ratio: sham group: 11 (61%) males, 7 (39%) females; Pharyngocise group: 18 (90%) males, 2 (10%) females; control (TAU) group: 15 (75%) males, 5 (25%) females Setting: university hospital cancer centre Eligibility criteria: Patients were included if they presented with: 1. Head and neck cancer of the oropharyngeal regions confirmed by the clinical history and examination findings, with positive cross‐sectional imaging studies and histopathologic biopsy, excluding other pathologic factors; 2. planned external beam radiotherapy; and 3. no history of non‐oral feeding for cancer‐related illness and were able to undergo MRI procedures. Exclusion criteria: nil reported Baseline characteristics: Tumour size (T grade): sham group median 2 (range 1 to 4); Pharyngocise group median 2 (range 1 to 4); TAU group median 2 (range 0 to 4) Tumour site: ‐ Base of tongue: sham group 3; Pharyngocise group 5; TAU group 3 ‐ Tonsil: sham group 4; Pharyngocise group 3; TAU group: 9 Tumour side: ‐ Left: sham group: 7; Pharyngocise group: 9; TAU group: 6 ‐ Right: sham group 5; Pharyngocise group 6; TAU group 5 ‐ Bilateral: sham group 6; Pharyngocise group 5; TAU group 9 Radiotherapy: Conventional: sham group 6; Pharyngocise group 9; TAU group 9 Intensity‐modulated radiotherapy (IMRT): sham group 12; Pharyngocise group 11; TAU 11 Plus chemotherapy: sham group 6, Pharyngocise group 6; TAU group 10 Radiotherapy dose (Gy): sham group 69.2 ± 1.4; Pharyngocise group 72.5 ± 1.18; TAU group 67.5 ± 2.5 Neck dissection (n): sham group 6; Pharyngocise group 8; TAU group 8 Left: sham group 1; Pharyngocise group 4; TAU group 3 Right: sham group 5; Pharyngocise group 4; TAU group 5 Baseline body mass index (BMI) (kg/m2): sham group 26.9 ± 1.3; Pharyngocise group 26.8 ± 1.0; TAU 28.6 ± 1.3 |
|
| Interventions |
Allocation of participants: 58 head and neck cancer patients treated with chemoradiotherapy (CRT). Interventions were conducted twice daily during CRT (up to a maximum of 6 weeks). Intervention A: TAU (control) group: ‐ Attending radiation oncologist "as usual", and treatment (if offered) consisted of supervision for feeding and precautions for safe swallowing (e.g. positioning, slowed rate of feeding) by the hospital speech pathology service n = 20 Intervention B: standardised (sham) treatment: ‐ A buccal extension maneuver ("Valchuff") and appropriate dietary modification, under the direction of the study speech pathologist. The patients assigned to this group completed the exercise for 10 repetitions over 4 cycles, each of 10 minutes' duration. The treatment sessions were 45 minutes in duration n = 18 Intervention C: active swallowing exercises (Pharyngocise): ‐ A battery of exercises (e.g. falsetto, tongue press, hard swallow and jaw resistance/strengthening using the Therabite Jaw Motion Rehabilitation System) and dietary modification, under the direction of the study speech pathologist, twice daily for the duration of the CRT (up to a maximum of 6 weeks). The patients assigned to this condition completed the 4 swallowing exercises as 10 repetitions over 4 cycles, each of 10 minutes' duration. The treatment sessions were each 45 minutes in duration n = 20 Use of additional interventions: none |
|
| Outcomes |
NB: Specific outcomes of interest to this review are bolded and marked with an asterisk Primary outcome:
Secondary outcomes:
|
|
| Funding sources | None reported | |
| Declarations of interest | Reported | |
| Notes | Single‐centre study Cross‐over between treatment groups: no Sample size calculation: based on previous reports, calculated that 60 patients would give 80% power at the 5% (two‐tailed) significance level to identify this treatment effect Language: English Participants lost to follow‐up*: TAU group (n = 5); sham group (n = 5); Pharyngocise group (n = 6) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported the use of computer‐generated blocked random number list |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule was remotely generated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants to sham or intervention arm |
| Blinding of outcome assessment (detection bias) Primary outcomes | Low risk | Blinding of 2 speech pathologists to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants who died and were lost to follow‐up accounted for by data being censored for time spent in study and included in analyses. High rate of poor compliance reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available. High attrition rates. The reported outcomes matched those planned. |
| Other bias | High risk | Discrepancies in the numbers of participants reported between table 4 and 5 (TAU group), which are not explained. 'Dosage' of intervention in the 2 treatment arms was significantly greater than in the TAU group and the number of sessions varied between the treatment groups (high risk of bias) Fidelity to protocol was self‐reported (high risk of bias) |
Kotz 2012.
| Methods |
Allocation: randomised controlled trial Design: parallel‐group |
|
| Participants |
Number: 26 Age (mean): total study participants: 59 years; intervention group: 57 years; control group: 62 years Gender: sex ratio: prophylactic swallowing exercises group 10 (77%) males, 3 (23%) females; control group: 13 (77%) males, 3 (23%) females Setting: Department of Otolaryngology ‐ Head and Neck Surgery, Mount Sinai Medical Centre, New York, USA Eligibility criteria: newly diagnosed HNC Exclusion criteria: patients were excluded if they had: ‐ a prior history of head and neck surgery including tracheostomy; ‐ previously undergone radiation treatment; ‐ a history of neurological diseases that could affect swallowing function; ‐ mental, cognitive, deficits mitigating against following instructions, answering questionnaires. Baseline characteristics: Primary site of tumour: Base of tongue: intervention group 6; control group 5 Tonsil: intervention group 4; control group 7 Glottic larynx: intervention group 0; control group 1 Nasopharynx: intervention group 1; control group 0 Oropharyngeal wall: intervention group 1; control group 0 Unknown primary: intervention group 1; control group 0 Tumour stage: Stage 2: intervention group 1; control group 0 Stage 3: intervention group 3; control group 2 Stage 4: intervention group 9; control group 11 Chemoradiation (CRT) therapy regimen: RT alone: intervention group: 0; control group: 1 RT + cisplatin: intervention group:8; control group: 6 RT + fluorouracil, hydroxyurea, cetuximab: intervention group: 5; control group: 6 Radiation dosage, cGy, median (range): intervention group 7000 (range: 6300 to 7200); control group 7200 (range 6200 to 7350) FOIS score baseline, median (range): intervention group: 7 (range 6 to 7); control group: 7 (range 7) PSS H&N ‐ eating in public subscale, median (range): intervention group: 100 (range 100); control group 100 (range 100) PSS H&N ‐ normalcy of diet subscale, median (range): intervention group: 100 (range 50 to 100); control group: 100 (range 100) |
|
| Interventions |
Allocation of participants: 26 head and neck cancer patients treated with chemoradiotherapy (CRT), consecutively recruited Intervention A: TAU (control group) ‐ The control group treatment was 'standard care' that involved referral to a head and neck speech pathologist for swallowing assessment and treatment if dysphagia symptoms were present after the completion of cancer treatment (n = 13) Intervention B: prophylactic swallowing exercises ‐ Swallowing exercises were initiated before the start of treatment and continued for the duration of the CRT This involved 5 sets of swallowing exercises counterbalanced (to avoid fatigue on the last exercise). The exercises included effortful swallow, 2 x tongue base retraction exercises, super‐supraglottic swallowing technique and the Mendelssohn manoeuvre (n = 13) Use of additional interventions: none |
|
| Outcomes |
NB: Specific outcomes of interest to this review are bolded and marked with an asterisk The efficacy of prophylactic swallowing exercises on (1) swallowing function and (2) swallowing‐related quality of life* pre‐CRT within a week after completion of CRT, at 3, 6, 9 and 12 months after CRT 1) Swallowing function scored using Functional Oral Intake Scale (FOIS): 7‐point scale of oral dietary tolerance. Ranges from 'complete PEG dependence' to 'tolerance of an oral diet with no restrictions.' 2) Performance Status Scale for Head and Neck Cancer (PSS‐H&N) has 3 discrete subscales. Each subscale ranges from 0 to 100, with a higher score indicating better function: eating in public; understandability of speech and normalcy of diet.
|
|
| Funding sources | Reported none | |
| Declarations of interest | Not reported | |
| Notes | Single‐centre study Cross‐over between treatment group: no Sample size calculation: not reported Language: English Participants lost to follow‐up: reported all patients completed the 12‐month follow‐up. Intervention group: 4 patients discontinued the swallowing exercise after week 4 of their radiation treatment and 5 additional patients discontinued the exercises after week 5 of their radiation treatment. This was due to considerable CRT‐associated oral pain, throat discomfort and fatigue. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported that the study was randomised with no further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported blinding of personnel. Participants' blinding not reported. |
| Blinding of outcome assessment (detection bias) Primary outcomes | Low risk | Reported that the clinician who performed the assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were no dropouts but a high rate of poor compliance was reported (9 of 13 (69%) patients were unable to complete intervention throughout CRT due to treatment‐related oral pain, throat discomfort and/or overall fatigue) |
| Selective reporting (reporting bias) | Low risk | Protocol is available and reported outcomes matched those planned |
| Other bias | High risk | Intervention group also had weekly speech and language therapy sessions |
Lazarus 2014.
| Methods |
Allocation: randomised clinical trial Design: parallel‐group |
|
| Participants |
Number: 23 Age: 62 years (50 to 79 years) males, 60 years female. No details of control or experimental group provided. Gender: sex ratio: 22 (96%) males, 1 (4%) female (no details of breakdown of control and experimental group); no details provided of gender breakdown of control and intervention group Setting: multiple sites Eligibility criteria: ‐ Newly diagnosed (AJCC) stage II to stage IVB oral and oropharyngeal cancer who underwent radiotherapy ± chemotherapy ‐ Patients with planned neck dissection post‐radiotherapy Exclusion criteria: patients were excluded if they had: ‐ a history of cervical spine surgery; ‐ other neurosurgical procedures that might affect swallowing. Baseline characteristics: (not subdivided into groups) Primary tumour site: Tonsil: 11 (48%) Base of tongue: 9 (39%) Lateral pharyngeal wall: 2 (9%) Soft palate: 1 (4%) American Joint Committee on Cancer (AJCC) staging: Stage II ‐ 1 (4%) Stage III ‐ 3 (13%) Stage IVA ‐ 16 (70%) Stage IVB ‐ 3 (13%) |
|
| Interventions |
Allocation of participants: 23 patients with head and neck cancer treated with radiotherapy ± chemotherapy Intervention A: traditional therapy (control group) ‐ Range of motion exercises and the Mendelsohn manoeuvre Therapy started 1 month post‐radiotherapy ± chemotherapy and was undertaken for 6 weeks (n = 11) Intervention B: isometric tongue resistance exercise plus traditional therapy ‐ Active resistance in all directions ‐ i.e. protrusion, lateralisation, elevation against tongue depressor along with traditional exercises Therapy started 1 month post‐radiotherapy ± chemotherapy, and was performed 5 days a week for 6 weeks, practising 5 times per day, 10 repetitions per practice session (n = 12) Use of additional interventions: none |
|
| Outcomes |
NB: Specific outcomes of interest to this review are bolded and marked with an asterisk
|
|
| Funding sources | NIH/NIDCD Grant 5 R03DC007497‐02 | |
| Declarations of interest | Reported | |
| Notes | Multiple sites study (hospitals, clinics, in New York, USA) Both groups underwent 6 weeks of exercise starting at 1 month post‐radiotherapy ± chemotherapy Cross‐over between treatment group: no Sample size calculation: not reported Language: English Participants lost to follow‐up: TAU group (n = 1); intervention group (n = 4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation was not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or clinical personnel |
| Blinding of outcome assessment (detection bias) Primary outcomes | Low risk | The outcomes were analysed by a speech‐language pathologist blinded to the experiment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for participants' loss to follow‐up partly reported |
| Selective reporting (reporting bias) | Low risk | Protocol is available. Reported outcomes matched those as planned |
| Other bias | Low risk | |
Mortensen 2015.
| Methods |
Allocation: randomised controlled trial Design: parallel‐group |
|
| Participants |
Number: 44 Age (mean): TAU (control) group 59 years (40 to 74 years), intervention group 58 years (39 to 77 years) Gender: sex ratio: treatment as usual or TAU (control) group: 17 male (85%), 3 female (15%); intervention group: 17 male (89%), 2 female (11%) Setting: Aarhus University Hospital, Denmark Eligibility criteria: ‐ Patients aged 21 to 79 years with no prior head and neck cancer or pre‐existing swallowing disorder ‐ T2 to T4 tumour classification (Union for International Cancer Control TNM tumour classification) ‐ Patients with squamous cell carcinoma (SCC) of the oral cavity, nasopharynx, oropharynx, hypopharynx or larynx and who received curative treatment with either primary or postoperative (chemo)radiation treatment Exclusion criteria: Patients with a history of surgery that might negatively affect swallowing Baseline characteristics: Primary site of tumour: larynx: 3 (16%) intervention, 4 (20%) control; pharynx: 11 (58%) intervention, 10 (50%) control; oral cavity: 0 intervention, 4 (20%) control; unknown primary: 5 (26%) intervention, 2 (10%) control Tumour stage: all tumours. TAU (control) group: 15 (75%) stage IV; intervention group: 16 (84%) stage IV |
|
| Interventions |
Allocation of participants: 44 participants (> 18 years) scheduled for curative chemoradiation therapy TAU (control) group: individual dietary advice from a dietician; VFSSs as needed and advice to continue oral feeding (n = 20) Intervention group: range of motion (ROM) exercises for: jaw, tongue, pharyngeal constrictors, laryngeal elevators, adductors. Also resistance (isometric) exercises for same. Overall 10 to 15 minutes daily practice; 10 repetitions, 3 x day, 7 days/week Interventions were conducted before, during and after treatment (n = 19) Use of additional interventions: none |
|
| Outcomes |
NB: Specific outcomes of interest to this review are bolded and marked with an asterisk
|
|
| Funding sources | CIRRO and the Danish Cancer Society | |
| Declarations of interest | Reported | |
| Notes | Single‐centre study Cross‐over between treatment groups Sample size calculation: not reported Ethical approval by the local ethics committee Trial registered in ClinicalTrials.gov, identifier NCT00332865 Language: English Participants lost to follow‐up: exclusion after randomisation (n = 5), TAU group ‐ lost to follow‐up (n = 8), excluded from analysis (n = 2); intervention group ‐ lost to follow‐up (n = 6), excluded from analysis (n = 3) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was done en bloc |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and occupational therapist who delivered/oversaw treatment were not blinded |
| Blinding of outcome assessment (detection bias) Primary outcomes | Unclear risk | Radiologist who did VFSS and scored primary outcomes was blinded Treating occupational therapist who scored secondary outcomes was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of dropout and loss to follow‐up (in both groups) |
| Selective reporting (reporting bias) | High risk | Protocol is available and reported outcomes matched those planned, however time points differed (table 1 planned time points does not match table 4 actual time points) |
| Other bias | High risk | Higher proportion of intervention group had concomitant chemotherapy Log kept to document fidelity to daily exercises (self‐report) |
van den Berg 2014.
| Methods |
Allocation: randomised controlled trial Design: parallel‐group |
|
| Participants |
Number: 120 Age (mean): intervention group: 63 years (33 to 83); control group 60 years (40 to 86) Gender: sex ratio: intervention group: 46 (77%) males, 14 (23%) female; control group: 43 (72%) males, 17 (28%) female Setting: Radboud University Medical Centre, Netherlands Eligibility criteria: ‐ Patients > 18 years of age with T2 to T4 classification (Union for International Cancer Control TNM tumour classification) squamous cell carcinoma of the oral cavity, nasopharynx, oropharynx, hypopharynx or larynx ‐ Patients who received curative treatment with primary or postoperative (chemo)radiation Exclusion criteria: ‐ Patients with previous head and neck cancer treated with primary or postoperative (chemo)radiation or surgery ‐ Neurological or other non‐tumour related swallowing problems ‐ An inability to comprehend and/or an inability to answer the study questionnaires Baseline characteristics: Primary site of tumour: Nasopharynx: 3 (5%) intervention group, 3 (5%) control group Oropharynx: 20 (33%) intervention group, 16 (27%) control group Hypopharynx: 5(9%) intervention group, 10(16%) control group Oral cavity: 12 (20%) intervention group, 12 (20%) control group Larynx: 20 (33%) intervention group, 19 (32%) control group Tumour stage: Stage II‐IV tumours. Intervention group: 29 (48%) stage IV; control group (TAU): 26 (44%) stage IV |
|
| Interventions |
Allocation of participants: 120 patients with head and neck cancer treated with (chemo)radiation recruited; 6 withdrew consent before baseline measures taken Intervention group: exercises delivered by speech and language therapists a. Stretching exercises for "maximising tongue, jaw and larynx mobility" b. Compensatory manoeuvres, such as taking smaller bites, longer chewing, more use of liquid swallows, swallowing with more effect c. Swallowing manoeuvres: changed head posture, supraglottic swallowing to avoid/reduce aspiration Same individual dietary counselling as TAU group ‐ Home practice: 3 times/day for at least 5 minutes ‐ Written and drawn instructions provided ‐ Intervention was weekly (for 30 weeks) during treatment; every 2 months post‐CRT, with weekly telephone monitoring (n = 60) Control group: TAU Individual dietary counselling by dietician weekly during treatment, focused on maintaining/improving patient's energy and protein intake (n = 60) Use of additional interventions: none |
|
| Outcomes |
NB: Specific outcomes of interest to this review are bolded and marked with an asterisk Primary outcome:
Secondary outcomes:
Data collected at 0 (baseline, first week of treatment); then week 6 (last week treatment), 10 (1 month post‐treatment), 18 (3 months post‐treatment) and 30 (6 months post‐treatment) |
|
| Funding sources | Fresenius Kabi Netherlands | |
| Declarations of interest | Not reported | |
| Notes | Sample size: power calculation at 80% with an estimated withdrawal rate of 20% Ethics approval by the Radboud University ethics committee (ABR: 28638.091.09) Trial protocol was registered on ClinicalTrials.gov (FOCISD: NCT0110980) Language: English Participants lost to follow‐up: TAU group (n = 20); intervention group (n = 19) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with 1:1 ratio using a computer‐controlled process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients, dieticians and speech and language pathologists were all aware of the treatment allocation |
| Blinding of outcome assessment (detection bias) Primary outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No results on BMI at follow‐up although change of weight reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol is available but detail on measurement of outcome (BMI) is unclear |
| Other bias | High risk | Intervention group also had weekly speech and language therapy sessions |
van der Molen 2011.
| Methods |
Allocation: randomised clinical trial Design: parallel‐group |
|
| Participants |
Number: 55 Age (mean): 56 years (37 to 78); control: 57 years (32 to 75) Gender: sex ratio: TheraBite jaw motion rehabilitation group: 23 (96%) males, 1 (4%) female; standard group: jaw ROM exercises group: 16 (64%) males, 9 (36%) females Setting: Netherlands Cancer Institute Eligibility criteria: ‐ Squamous cell carcinoma ‐ Oral cavity, oropharynx, hypopharynx, larynx and nasopharynx tumours ‐ Stage III‐IV head and neck cancers ‐ Primary treatment with concomitant chemoradiotherapy (CCRT) with curative intent ‐ Written informed consent Exclusion criteria: ‐ Unable to comprehend the function and use of the rehabilitation exercises and device (e.g. Alzheimer's disease, Korsakov's disease) ‐ Physically unfit to use a rehabilitation device (e.g. neurological deficit) Baseline characteristics: Primary site of tumour: Nasopharynx: 3 (13%) intervention group, (16%) control group Oral cavity/oropharynx: 12 (50%) intervention group, 12 (47%) control group Larynx/hypopharynx: 9 (37%) intervention group, 9 (37%) control group Tumour stage: Stage III‐IV tumours Stage III: intervention group: 7 (29%) control group: 9 (36%) Stage IV: intervention group: 17 (71%), control group: 16 (64%) |
|
| Interventions |
Allocation of participants: 55 head and neck cancer patients treated with concomitant chemoradiotherapy (CRT) Intervention A: standard care (control group) ‐ Jaw ROM exercise: 3 times daily; 3 repetitions each ‐ Strengthening exercises: 3 times daily; 5 repetitions each (effortful swallow, Masako manoeuvre, super‐supra‐glottic swallow) (n = 28) Intervention B: ‐ Therabite jaw motion rehabilitation system, 3 times daily, 3 repetitions (slow mouth opening, swallowing with tongue elevated to palate and 50% maximum mouth opening) (n = 27) Interventions began 2 weeks before CRT and continued throughout treatment Use of additional interventions: none |
|
| Outcomes |
NB: Specific outcomes of interest to this review are bolded and marked with an asterisk Patients were assessed 2 weeks before the onset of CCRT and approximately 10 weeks after completing same
|
|
| Funding sources | Part of study was supported by an unrestricted research grant from Atos Medical, Horby, Sweden | |
| Declarations of interest | Not reported | |
| Notes | Single‐centre study All patients received 100 mg/m2cisplatin as a 40‐minute IV infusion on days 1, 22 and 43. Intensity‐modulated radiotherapy (IMRT) of 70 Gy in 35 fractions was administered over 7 weeks starting concurrently with chemotherapy. Cross‐over between treatment groups: not reported Sample size calculation: no details Language: English Participants lost to follow‐up: TAU group (n = 3); intervention group (n = 3) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised centrally by computer using blocks of 6 and stratified by tumour site |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) Primary outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up reported and explained but poor compliance with treatment reported; only 7 patients (17%) practised daily throughout CRT |
| Selective reporting (reporting bias) | Low risk | Protocol is available and reported outcomes matched those planned |
| Other bias | High risk | Patients in control group practised significantly more days than patients in treatment group (P = 0.05; mean 59 and 41 days respectively) Compliance was assessed by use of "familiarity with exercise" score but familiarity does not necessarily equate to compliance |
AJCC: American Joint Committee on Cancer BMI: body mass index CCRT: concomitant chemoradiotherapy CRT: chemoradiotherapy EORTC: European Organisation for Research and Treatment of Cancer FOIS: Functional Oral Intake Scale HNC: head and neck cancer HNCI: Head and Neck Cancer Inventory IOPI: Iowa Oral Performance Instrument IV: intravenous LENT/SOMA scales: Late Effects Normal Tissue (LENT)/Subjective, Objective, Management, Analytic (SOMA) scales M‐DADI: MD Anderson Dysphagia Inventory MRI: magnetic resonance imaging N/A: not applicable NFIS‐HN‐F: Normalcy of Food Intake Scale for Head and Neck ‐ Dietetic Part NFIS‐HN‐L: Normalcy of Food Intake Scale for Head and Neck‐ Logopedic Part NPC: nasopharyngeal carcinoma OPSE: Oropharyngeal Swallowing Efficiency PEG: percutaneous endoscopic gastroscopy PSS‐H&N: Performance Status Scale for Head and Neck ROM: range of motion RT: radiotherapy SCC: squamous cell carcinoma SPSS: Statistical Package for the Social Sciences TAU: treatment as usual TNM: tumour, node, metastasis VAS: visual analogue scale VFSS: videofluoroscopic swallowing study
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carroll 2008 | No randomisation |
| Tang 2011 | Randomised controlled trial but exercises directed at rehabilitation, not prevention, of dysphagia |
| Virani 2013 | No randomisation |
| Zhen 2012 | No randomisation |
Characteristics of ongoing studies [ordered by study ID]
Barretos Cancer Hospital 2015.
| Trial name or title | Swallowing intervention during radiochemotherapy on head and neck cancer (Swallowing‐1) |
| Methods | Randomised clinical trial phase II; single‐centre |
| Participants |
Age for study: < 18 years Gender: both n = 80 patients with advanced oropharynx, larynx and hypopharynx cancer diagnoses from Barretos Cancer Hospital, proposed for neoadjuvant chemotherapy followed by radiotherapy combined with chemotherapy Inclusion criteria:
Exclusion criteria:
|
| Interventions | 2 groups: control group (no intervention) and speech pathology therapy (swallowing exercise) group Procedure: pre‐, during and post‐treatment swallowing exercises given by speech pathologist(s) |
| Outcomes |
Primary: swallowing function (time frame: up to 6 months after concurrent phase). Designated as safety issue: No 1 Measures: self‐reporting swallowing questionnaire, clinical swallowing examination; VFSS or modified barium swallow (MBS), and the MD Anderson Dysphagia Inventory (MDADI) |
| Starting date | July 2012 Completed: November 2014 (final data collection date for primary outcome measure) |
| Contact information | Barretos Cancer Hospital, Barretos, São Paulo, Brazil, 14780400 |
| Notes | To evaluate and compare the swallowing and quality of life of a patient group undergoing a speech pathology therapy (intervention) and a control group (no intervention) |
Fredslund 2015.
| Trial name or title | Pre‐habilitation of patients with head and neck cancer SYNK |
| Methods | RCT; efficacy study, single‐centre |
| Participants | Age: minimum: 18 years; maximum: N/A
Gender: both Inclusion criteria: ‐ Histological proven cancer in one or more of the following areas: larynx, pharynx, cavi oris, cavi nasi, salivary glands, thyroid, sinuses, head and neck cancer; metastases to the neck lymph nodes, or unknown primary tumour ‐ Set to curative radiotherapy with or without concurrent chemotherapy for treatment of cancer in the head and neck region in accordance with the Danish Head and Neck Cancer Group (DAHANCA) guidelines ‐ Fully self‐reliant ‐ Danish skills, oral and written ‐ Informed consent Exclusion criteria: ‐ Previously received treatment for head and neck cancer (radiotherapy, chemotherapy and/or surgery) ‐ Pregnancy ‐ ECOG performance status > 2 ‐ Presence of psychological, family, sociological or geographical issues that could prevent the patient from completing the intervention ‐ Simultaneous or previous illness or conditions that could prevent the patient being able to complete the intervention Sample size: 240 |
| Interventions | Behavioural: swallowing therapy and resistance training |
| Outcomes |
Primary: swallowing function measured by penetration aspiration scale (PAS) during a Fibre‐Endoscopic Evaluation of Swallowing (FEES) (Time frame: participants will be followed up until 12 months after they finalise their radiotherapy, an expected average of 58 weeks from baseline) Secondary: ‐ Anxiety by Symptom Check List (SCL‐92) Anxiety subscale ‐ Body composition by bioimpedance ‐ Depression by Major Depression Inventory (MDI) ‐ Health‐related quality of life by the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)‐30 and EORTC QLQ‐H&N35 ‐ Level of fatigue by the EORTC QLQ‐C30 3‐point fatigue subscale. ‐ Level of oral intake by Functional Oral Intake Scale (FOIS) ‐ Maximal inter‐incisal distance (MID) by Therabite range of motion (ROM) scale ‐ Pain by numerical rating scale (NRS) ‐ Performance Status by Eastern Cooperative Oncology Group (ECOG) performance scale ‐ Physical strength by 30 seconds Sit to Stand (STS) test ‐ Subjective symptoms of dysphagia by EAT‐10 (eating assessment tool) ‐ Weight loss by body weight |
| Starting date | May 2015 |
| Contact information | Sara V Fredslund, MSc Danish Cancer Society Rigshospitalet, Denmark sara.vinther.fredslund@regionh.dk Ph: 0045 51908661 |
| Notes | Efficacy study, intervention model: parallel assignment, masking: single blind (outcomes assessor) Primary purpose: supportive care Primary sponsor: Irene Wessel Secondary sponsors:
Identifiers: H‐2‐2014‐074 R108‐A6969‐14‐S31 |
Govender 2014.
| Trial name or title | SIP SMART: Swallowing intervention package ‐ self monitoring, assessment & rehabilitation training |
| Methods | RCT |
| Participants |
Inclusion criteria from qualitative interviews: 1. Patients who have completed treatment for advanced head and neck cancer 2. A minimum of 3 months post‐treatment 3. Had input from a speech and language therapist as part of their cancer care 4. Able to provide informed consent and willing to be interviewed for 40 minutes 5. Proficiency in English satisfactory for interview/participation in intervention 6. Aged 18 and above Preliminary testing: feasibility study 1. Patients with newly diagnosed stage III and stage IV cancer of the oral cavity or pharynx 2. Discussed at University College London Hospitals head and neck multidisciplinary team and planned for curative treatment via surgery and/or chemoradiotherapy or combination thereof 3. Able to provide informed consent 4. Proficiency in English satisfactory to participate/engage in the intervention 5. Aged 18 and above Exclusion criteria: 1. Patients who are mid treatment or those receiving palliation 2. Patients who have been treated solely by non‐standard treatment i.e. not surgery, radiotherapy, chemoradiotherapy or combinations thereof. Patients treated by chemotherapy, brachytherapy, photodynamic therapy alone will be ineligible. 3. Patients who are considered vulnerable or unable to provide informed consent 4. Patients with brain tumours and other primary sites not within the head and neck Sample size: 45 |
| Interventions | The study design is informed by the Medical Research Council complex intervention guidelines The development phase will be informed by literature reviews, in‐depth patient interviews and paper modelling of the intervention The preliminary testing phase ‐ feasibility using stratified block randomisation 1:1 allocation to treatment or usual care group Followed up for 6 months from date of surgery (if surgery only) or date of first radiotherapy treatment (if radiotherapy or combined modality treatment) |
| Outcomes |
Primary: 1. MDADI swallowing outcome; time point(s): pre‐treatment, post‐treatment at 1, 3, 6 months 2. Modified barium swallow (VFSS) done at 6 months Secondary: 1. FACT‐QOL; time point(s): pre‐treatment, post‐treatment 1, 3, 6 months 2. PSS‐H&N ‐ normalcy of diet, time point(s): pre‐treatment, post‐treatment at 1, 3, 6 months |
| Starting date | 6 October 2014 End date: 1 September 2016 |
| Contact information | Ms Roganie Govender Head and Neck Cancer Centre First Floor East, University College Hospital 250 Euston Road London NW1 2PQ UK roganie.govender@uclh.nhs.uk |
| Notes | Trial setting: hospitals Funders: NIHR (UK) Protocol/serial number: 17043 |
van Nuffelen 2014.
| Trial name or title | Tongue strengthening exercises in head and neck cancer patients: does level of resistance matter? |
| Methods | Single‐centre interventional RCT |
| Participants |
Participant inclusion criteria: 1. Head and neck cancer patients previously treated with chemoradiotherapy 2. Men and women older than 18, without cognitive, language, motor, hearing or visual deficits that could interfere with the correct execution of the training. 3. Chronic dysphagia (i.e. present for at least 1 month and no earlier than 6 months after the last day of radiation treatment), primarily related to reduced tongue strength 4. Score 1 or higher for the BRACS items ‘base of tongue’ and/or ‘valleculae’ at baseline judged by an experienced clinician Inclusion criteria 1. Head and neck cancer patients previously treated with chemoradiotherapy 2. Men and women older than 18, without cognitive, language, motor, hearing or visual deficits that could interfere with the correct execution of the training 3. Chronic dysphagia (i.e. present for at least 1 month and no earlier than 6 months after the last day of radiation treatment), primarily related to reduced tongue strength 4. Score 1 or higher for the BRACS items ‘base of tongue’ and/or ‘valleculae’ at baseline judged by an experienced clinician Exclusion criteria: 1. History of major oral or head and neck surgery and neurological disorders with an impact on oral function and/or swallowing (amongst others stroke, traumatic brain injury, Parkinson’s disease, amyotrophic lateral sclerosis) 2. Concurrent oral motor exercises or swallowing manoeuvres to improve swallowing are not allowed during the study period Sample size: 45 (15 per group) |
| Interventions | Tongue strengthening exercises The effect of tongue strengthening exercises will depend upon the level of resistance |
| Outcomes | Primary outcome measures: 1. Tongue strength measurements: maximum isometric pressures (expressed as kPa) anterior and posterior The primary measures are evaluated prior to (max 1 week in advance), during (after 4 weeks of therapy), after 8 weeks of therapy and at 4 and 8 weeks post‐treatment Secondary outcome measures: 1. Swallowing function: the swallowing function will be evaluated using a comprehensive fibre‐optic endoscopic evaluation of swallowing (FEES) examination; the Mann Assessment of Swallowing Ability‐Cancer (MASA‐C); the Functional Oral Intake Scale (FOIS) and a self‐evaluation. For the latter a 100 mm visual analogue scale is used with the ends defined as ‘I can't swallow’ (0) and ‘I don't have any swallowing difficulties’ (100) respectively. Both the FEES and MASA‐C are conducted with 4 different bolus types: 5 and 10 ml of thin liquid, and 5 and 10 ml of yogurt. Each bolus type is administered 3 times. Outcome measures for FEES are the Penetration‐Aspiration Scale, the Carnaby‐Videofluoroscopic Examination (C‐VFE) scales for dysphagia and aspiration; the Pooling‐Score and the Boston Residue and Clearance Scale (BRACS) 2. Quality of life: swallowing‐related quality of life will be surveyed by means of the Dutch Swallowing Quality‐of‐Life Questionnaire (DSWAL‐QoL) and the Dysphagia Handicap Index (DHI) The secondary outcome measures are evaluated prior to (max 1 week in advance), during (after 4 weeks of therapy), after 8 weeks of therapy and at 4 and 8 weeks post‐treatment |
| Starting date | March 2014 End date: December 2016 |
| Contact information | Prof Gwen Van Nuffelen Wilrijkstraat 10 Edegem (Antwerp) 2650 Belgium gwen.van.nuffelen@uza.be |
| Notes | Hospitals Protocol/serial number: NKR_2014_01 Single‐centre interventional RCT Recruiting |
BRACS: Boston Residue and Clearance Scale (BRACS) ECOG: Eastern Cooperative Oncology Group FACT: Functional Assessment of Cancer Therapy MDADI: MD Anderson Dysphagia Inventory N/A: not applicable PSS‐H&N: Performance Status Scale for Head and Neck QOL: quality of life RCT: randomised controlled trial VFSS: videofluoroscopic swallowing study
Differences between protocol and review
There were essentially no differences between the protocol and the review. However, meta‐analysis was not possible due to the heterogeneity of participants, methodological differences (e.g. in primary and secondary outcomes, measurement tools used, endpoints and variables of interest).
Contributions of authors
Alison Perry is the guarantor of the review. The protocol was written with contributions from Catriona Kennedy.
All authors (Perry, Lee, Cotton and Kennedy) were involved in searching for trials, extracting data, interpreting the analyses and drafting the final review. Specifically, Alison Perry and Sue Cotton independently reviewed the titles and abstracts of the records identified from electronic searches and excluded immediately irrelevant studies. Alison Perry and Catriona Kennedy then selected suitable studies based on the inclusion criteria for the review. If these authors were unsure, Sue Cotton made a final decision.
Alison Perry and Catriona Kennedy independently extracted study data and recorded information on a data extraction form, with Sue Cotton acting as arbitrator. Siew Hwa Lee undertook data extraction and data synthesis.
Siew Hwa Lee and Catriona Kennedy completed 'Summary of findings' and 'Risk of bias' tables and Siew Hwa Lee entered these data.
All authors contributed to the writing of the discussion and conclusion sections.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
Declarations of interest
Alison Perry: none known.
Sue Cotton: none known.
Siew Hwa Lee: none known.
Catriona Kennedy: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Carnaby‐Mann 2012 {published data only}
- Carnaby‐Mann G, Crary MA, Schmalfuss I, Amdur R. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head‐and‐neck chemoradiotherapy. International Journal of Radiation Oncology, Biology, Physics 2012;83(1):210‐9. [DOI] [PubMed] [Google Scholar]
Kotz 2012 {published data only}
- Kotz T, Federman AD, Kao J, Milman, L, Packer S, Lopez‐Prieto C, et al. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2012;138(4):376‐82. [DOI] [PubMed] [Google Scholar]
Lazarus 2014 {published data only}
- Lazarus CL, Husainib H, Falcigliac D, DeLacurec M, Branskid RC, Krause D, et al. Effects of exercise on swallowing and tongue strength in patients with oral and oropharyngeal cancer treated with primary radiotherapy with or without chemotherapy. International Journal of Oral & Maxillofacial Surgery 2014;43(5):523‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mortensen 2015 {published data only}
- Mortensen HR, Jensen J, Aksglaede K, Lambertsen K, Eriksen E, Grau C. Prophylactic swallowing exercises in head and neck cancer radiotherapy. Dysphagia 2015;30:304‐14. [DOI 10.1007/s00455‐015‐9600‐y] [DOI] [PubMed] [Google Scholar]
van den Berg 2014 {published data only}
- Berg MGA, Kalf JG, Hendriks JCM, Takes RP, Herpen CML, Wanten GJA, et al. Normalcy of food intake in patients with head and neck cancer supported by combined dietary counselling and swallowing therapy: a randomized clinical trial. Head and Neck 2014 Dec 22 [Epub ahead of print]. [DOI: 10.1002/hed.23970] [DOI] [PubMed]
van der Molen 2011 {published data only}
- Molen L, Rossum MA, Burkhead LM, Smeele LE, Rasch CRN, Hilgers FJM. A randomized preventative rehabilitation trial in advanced HN cancer patients treated with chemoradiotherapy: feasibility, compliance and short‐tem effect. Dysphagia 2011; Vol. 26:155‐70. [DOI 10 1007/s00455‐010‐9288‐y] [DOI] [PMC free article] [PubMed]
- Molen L, Rossum MA, Rasch CRN, Smeele LE, Hilgers FJM. Two‐year results of a prospective preventive swallowing rehabilitation trial in patients treated with chemoradiation for advanced head and neck cancer. European Archives of Oto‐Rhino‐Laryngology 2014;271(5):1257‐70. [DOI: 10.1007/s00405-013-2640-8] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Carroll 2008 {published data only}
- Carroll WR, Locher JL, Canon CL, Bohannon IA, McColloch NL, Magnuson JS. Pre‐treatment swallowing exercises improves swallowing function after chemotherapy. Laryngoscope 2008;118(1):39‐43. [DOI] [PubMed] [Google Scholar]
Tang 2011 {published data only}
- Tang Y, Shen Q, Wang Y, Lu K, Wang Y, Peng Y. A randomized prospective study of rehabilitation therapy in the treatment of radiation‐induced dysphagia and trismus. Strahlentherapie und Onkologie 2011;187(1):39‐44. [DOI: 10.1007/s00066-010-2151-0] [DOI] [PubMed] [Google Scholar]
Virani 2013 {published data only}
- Virani A, Kunduk M, McWhorter AJ. Effects of performing two different prophylactic swallowing exercise protocols on swallowing outcomes post‐chemoradiation therapies for head and neck cancers. Dysphagia 2013;28(4):605. [DOI: 10.1002/hed.23570] [DOI] [Google Scholar]
Zhen 2012 {published data only}
- Zhen Y, Wang J, Tao D, Wang H‐J, Chen W‐L. Efficacy survey of swallowing function and quality of life in response to therapeutic intervention following rehabilitation treatment in dysphagic tongue cancer patients. European Journal of Oncology Nursing 2012;16:54‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Barretos Cancer Hospital 2015 {published data only}
- NCT02075385. Randomized phase II trial: swallowing speech pathology intervention during radiochemotherapy on patients with head and neck cancer. https://clinicaltrials.gov/ct2/show/NCT02075385 (first received 23 January 2014).
Fredslund 2015 {published data only}
- NCT02385929. Pre‐habilitation of patients with head and neck cancer (SYNK). https://clinicaltrials.gov/ct2/show/NCT02385929 (first received 9 February 2015).
Govender 2014 {published data only}
- ISRCTN40215425. SIP SMART: swallowing intervention package ‐ self monitoring, assessment & rehabilitation training. http://www.isrctn.com/ISRCTN40215425 (first received 23 October 2014).
van Nuffelen 2014 {published data only}
- ISRCTN14447678. Tongue strengthening exercises in head and neck cancer patients: does level of resistance matter?. http://www.isrctn.com/ISRCTN14447678 (first received 12 February 2015).
Additional references
AJCC 2010
- Edge S, Byrd DR, Compton CC, Fritz, AG, Greene FL, et al. AJCC Cancer Staging Manual. 7th Edition. New York, NY: Springer, 2010. [Google Scholar]
Appleton 1994
- Appleton J, Machin J. Working with Oral Cancer. Oxon, UK: Winslow Press, 1994. [Google Scholar]
Argiris 2008
- Argiris A, Karamouzis MA, Raben D, Ferris RL. Head and neck cancer. Lancet 2008;1371(9625):1695‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blot 1988
- Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston‐Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Research 1988;48(11):3282. [PubMed] [Google Scholar]
Clark 2003
- Clark HM. Neuromuscular treatments for speech and swallowing: a tutorial. American Journal of Speech‐Language Pathology 2003;12:400‐15. [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Decker 1982
- Decker J, Goldstein JC. Risk factors in head and neck cancer. New England Journal of Medicine 1982;306(19):1151‐5. [DOI] [PubMed] [Google Scholar]
Forastiere 2003
- Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. New England Journal of Medicine 2003;349:2091‐8. [DOI] [PubMed] [Google Scholar]
Frowen 2006
- Frowen J, Perry A. Swallowing outcomes after radiotherapy for head and neck cancer: a systematic review. Head and Neck 2006;28(10):932‐44. [DOI] [PubMed] [Google Scholar]
Fujiu 1996
- Fujiu M, Logemann JA. Effect of a tongue‐holding maneuver on posterior pharyngeal wall movement during deglutition. American Journal of Speech‐Language Pathology 1996;5:23‐30. [Google Scholar]
Gillison 2000
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. Journal of the National Cancer Institute 2000;92:709‐20. [DOI] [PubMed] [Google Scholar]
Gregoire 2010
- Gregoire V, Lefebvre JL, Licitra L, Felip E. Squamous cell carcinoma of the head and neck: EHNS‐ESMO‐ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2010;21(Suppl 5):184‐6. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2009
- Higgins KM, Shah MD, Ogaick MJ, Enepekides D. Treatment of early‐stage glottic cancer: meta‐analysis comparison of laser excision versus radiotherapy. Journal of Otolaryngology ‐ Head & Neck Surgery 2009;38(6):603‐12. [PubMed] [Google Scholar]
ICF 2001
- WHO. International Classification of Functioning, Disability and Health: ICF Short Version. Geneva: World Health Organization, 2001. [Google Scholar]
Jemal 2011
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians 2011;61:69–90. [DOI: 10.3322/caac.20107] [DOI] [PubMed] [Google Scholar]
Langendijk 2007
- Langendijk JA. New developments in radiotherapy of head and neck cancer: higher precision with less patient discomfort?. Radiotherapy and Oncology 2007;85:1‐6. [DOI] [PubMed] [Google Scholar]
Logemann 1999
- Logemann JA. Behavioural management for oropharyngeal dysphagia. Folia Phoniatrica et Logopaedica 1999;51(4‐5):199‐212. [DOI] [PubMed] [Google Scholar]
Marur 2010
- Marur S, D'Souza G, Westra WH, Forestiere AA. HPV‐associated head and neck cancer: a virus‐related cancer epidemic. Lancet Oncology 2010;11(8):781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCCN 2014
- National Comprehensive Cancer Network. Head and Neck Cancers (Version 2.2014). http://www.nccn.org/professionals/physician_gls/pdf/head‐and‐neck.pdf (accessed 16 June 2014).
NICE Guidelines 2016
- National Institute for Care and Health Excellence. Cancer of the upper aerodigestive tract: assessment and management in people aged 16 and over. https://www.nice.org.uk/guidance/ng36/chapter/recommendations#information‐and‐support February 2016. [NICE guidelines [NG36]] [PubMed]
Perry 2003
- Perry A, Shaw M. The evaluation of functional outcomes (speech and swallowing) after head and neck cancer treatment: results and analysis. Journal of Laryngology and Otology 2003;117(5):368‐81. [DOI] [PubMed] [Google Scholar]
Pignon 2009
- Pignon JP, Maître A, Maillard E, Bourhis J, MACH‐NC Collaborative Group. Meta‐analysis of chemotherapy in head and neck cancer (MACH‐NC): an update on 93 randomised trials and 17,346 patients. Radiotherapy and Oncology 2009;92:4–14. [DOI] [PubMed] [Google Scholar]
Ragin 2007
- Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papilloma virus. Journal of Dental Research 2007;86(2):104‐14. [DOI] [PubMed] [Google Scholar]
Rappoport 2003
- Rappoport L. How We Eat. Ontario, Canada: ECW Press, 2003. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenbek 1996
- Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration‐aspiration scale. Dysphagia 1996;11(2):93‐8. [DOI] [PubMed] [Google Scholar]
Sankaranarayanan 1998
- Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Research 1998;18(6B):4779. [PubMed] [Google Scholar]
www.ncri.ie
- The Irish Cancer Society. Cancer Trends No 10. National Cancer Registry, 2011. Cancers of the head and neck. www.ncri.ie (accessed March 2013).
References to other published versions of this review
Perry 2014
- Perry A, Cotton S, Kennedy C. Therapeutic exercises for affecting post‐treatment swallowing in people treated for advanced‐stage head and neck cancers. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD011112] [DOI] [PMC free article] [PubMed] [Google Scholar]


