Abstract
Background
Schizophrenia is a psychiatric disorder which involves distortions in thought and perception, blunted affect, and behavioural disturbances. The longer psychosis goes unnoticed and untreated, the more severe the repercussions for relapse and recovery. There is some evidence that early intervention services can help, and diagnostic techniques that could contribute to early intervention may offer clinical utility in these situations. The index test being evaluated in this review is the structural magnetic resonance imaging (MRI) analysis technique known as voxel‐based morphometry (VBM) that estimates the distribution of grey matter tissue volume across several brain regions. This review is an exploratory examination of the diagnostic ‘potential’ of VBM for use as an additional tool in the clinical examination of patients with first episode psychosis to establish whether an individual will progress on to developing schizophrenia as opposed to other types of psychosis.
Objectives
To determine whether VBM applied to the brain can be used to differentiate schizophrenia from other types of psychosis in participants who have received a clinical diagnosis of first episode psychosis.
Search methods
In December 2013, we updated a previous search (May 2012) of MEDLINE, EMBASE, and PsycInfo using OvidSP.
Selection criteria
We included retrospective and prospective studies that consecutively or randomly selected adolescent and adult participants (< 45 years) with a first episode of psychosis; and that evaluated the diagnostic accuracy of VBM for differentiating schizophrenia from other psychoses compared with a clinical diagnosis made by a qualified mental health professional, with or without the use of standard operational criteria or symptom checklists. We excluded studies in children, and in adult participants with organic brain disorders or who were at high risk for schizophrenia, such as people with a genetic predisposition.
Data collection and analysis
Two review authors screened all references for inclusion. We assessed the quality of studies using the QUADAS‐2 instrument. Due to a lack of data, we were not able to extract 2 x 2 data tables for each study nor undertake any meta‐analysis.
Main results
We included four studies with a total of 275 participants with first episode psychosis. VBM was not used to diagnose schizophrenia in any of the studies, instead VBM was used to quantify the magnitude of differences in grey matter volume. Therefore, none of the included studies reported data that could be used in the analysis, and we summarised the findings narratively for each study.
Authors' conclusions
There is no evidence to currently support diagnosing schizophrenia (as opposed to other psychotic disorders) using the pattern of brain changes seen in VBM studies in patients with first episode psychosis. VBM has the potential to discriminate between diagnostic categories but the methods to do this reliably are currently in evolution. In addition, the lack of applicability of the use of VBM to clinical practice in the studies to date limits the usefulness of VBM as a diagnostic aid to differentiate schizophrenia from other types of psychotic presentations in people with first episode of psychosis.
Plain language summary
Brain imaging for diagnosing schizophrenia in people with first episode psychosis
Background
Schizophrenia is a psychiatric disorder which involves psychotic symptoms such as distortions in thought and perception, blunted affect, and behavioural disturbances. It is important for patients who have a first episode of psychosis to be correctly diagnosed as soon as possible. The earlier schizophrenia is diagnosed the better the treatment outcome. However, other diseases sometimes have similar psychotic symptoms as schizophrenia, for example bipolar disorder. This review looks at how accurate a type of brain imaging technique called voxel‐based morphology (VBM) is at diagnosing schizophrenia in people who have a first episode of psychosis. VBM is used to measure differences in the structure of the brain in people with different types of psychosis. These differences could be used to make a diagnosis.
Study characteristics
The evidence is current to December 2013. We found four studies that used VBM in 275 adolescents and adults with first episode psychosis. One study recruited participants from a hospital, one from an outpatient clinic and one from inpatient and outpatient psychiatric services, and the fourth study did not report setting. Participants' mean age ranged from 18.6 to 27.1 years. Only two studies reported on participants' gender and included both males and females.
Quality of the evidence
Study quality was found to be fairly good overall. In some instances it was unclear, mainly due to three of the studies that did not sufficiently describe method of VBM image processing. We were concerned about all of the studies' applicability because VBM was not used to diagnose schizophrenia in any of the studies, instead VBM was used to characterise differences in the brain's grey matter.
Key results
The four studies we identified used VBM on adolescents and adults with first episode psychosis, but VBM was used to describe brain structure and not to make a diagnosis. There is no evidence to support the use of VBM to diagnose schizophrenia in patients with first episode psychosis.
Summary of findings
Summary of findings'. 'Summary of findings table.
| What is the diagnostic accuracy of VBM for schizophrenia? | |||||
| Patients/population | People with first episode psychosis | ||||
| Prior testing | Participants presenting for the first time at mental health services | ||||
| Settings | Inpatients and outpatients | ||||
| Index test | VBM | ||||
| Importance | Exploratory examination of the diagnostic ‘potential’ of VBM for use as an additional tool in the clinical examination of patients | ||||
| Reference standard | There is no gold standard for diagnosing schizophrenia. Reference standard used: clinical diagnosis using clinical interview and/or medical records, and based on DSM‐III‐R, DSM‐IV and ICD‐10 diagnoses. | ||||
| Studies | Prospective studies including people with first episode psychosis (n = 4) | ||||
| Test / subgroup | Summary accuracy % (95% CI) | No. of participants (studies) | Prevalence median (range) | Implications | Quality and comments |
| VBM | Not applicable, see Quality and comments | 275 (4) | See Quality and comments | See Quality and comments | No studies provided 2 x 2 data. There were high applicability concerns for all studies on the index test domain as VBM was not used to diagnose schizophrenia, instead VBM was used to characterise grey matter. |
| CAUTION: The results on this table should not be interpreted in isolation from the results of the individual included studies. These are reported in the main body of the text of the review. | |||||
ICD: International Statistical Classification of Diseases and Related Health Problems DSM: Diagnostic and Statistical Manual of Mental Disorders VBM: voxel‐based morphometry
Background
Target condition being diagnosed
Schizophrenia is a psychiatric disorder described in the Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM‐5) (APA 2013) and the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD‐10) (WHO 1992), and is characterised by the presence of delusions, hallucinations, disorganised speech and behaviour, and negative symptoms. To warrant a diagnosis, an individual must have been exhibiting at least two of the preceding symptoms for six months, with at least one symptom active during the prior month (APA 2013). Schizophrenia can occur as a single episode of illness, although the majority of sufferers have remissions and relapses, and for many sufferers the condition becomes chronic and disabling (Bustillo 2001). The most effective method of treatment is antipsychotic medication (first‐ and second‐generation antipsychotics), which has been shown to reduce the risk of relapse and psychotic symptoms (Kane 2001). However, these medications produce various side effects (Kane 2001), so low doses, used in as timely a fashion as possible, are indicated. It has been suggested that the longer psychosis goes unnoticed and untreated, the more severe the repercussions for relapse and recovery (Bottlender 2003; Malla 2005). There is some evidence to suggest that early intervention for people with schizophrenia can be beneficial, helping avoid or postpone damaging relapses and the need for prolonged use of medications (Marshall 2011). Thus, accurate diagnostic techniques that could contribute to early intervention may offer clinical utility in these situations.
Index test(s)
The index test being evaluated in this review is the structural magnetic resonance imaging (MRI) analysis technique known as voxel‐based morphometry (VBM) that estimates the distribution of grey matter tissue volume across several brain regions (Good 2001). As a ‘whole brain’‐based method, VBM has often been used to locate the brain regions showing maximal tissue reduction in one group relative to the other (Mechelli 2005; McCarley 1999; Whitwell 2009). It is believed that VBM provides a means of measuring brain abnormalities in first episode psychosis that could potentially be used in the differential diagnosis of schizophrenia and other psychotic disorders (McCarley 1999; Shenton 2001).
Radiological inspection of brain scans is not currently used as a diagnostic tool in schizophrenia, and there is also a lack of evidence to support the routine use of standard radiological reporting of MRI to detect clinically relevant structural abnormalities in first episode psychosis (NICE 2008; Sommer 2013). Despite this, it is important to evaluate whether specific techniques, such as VBM, could be applied in order to help differentiate first episode schizophrenia from other forms of psychosis. At present there are no tools that are available in routine practice to complement the prolonged period of clinical observation required to diagnose schizophrenia. Neuroimaging has been highlighted as a promising tool in this regard, due to its non‐invasive nature and consistency of findings from research studies investigating the pathophysiology of schizophrenia (Gur 2007).
VBM studies have been primarily employed to detect the anatomical patterns of group differences in grey matter volume that are characteristic of schizophrenia compared to non‐schizophrenia groups (healthy controls or other psychotic disorders). VBM studies generally report group differences in terms of effect size metrics such as T statistics rather than absolute volume measures, as the measurement is based on a probabilistic estimate of image intensity and not the actual volume of brain tissue. Meta‐analyses estimating the likelihood of anatomical distribution of VBM changes in schizophrenia compared to controls implicate several brain regions such as the fronto‐insular cortex, anterior cingulate cortex, superior temporal gyrus, thalamus, hippocampus and parahippocampal region (Chan 2011; Ellison‐Wright 2008; Glahn 2008; Honea 2005).
An appraisal of the current VBM meta‐analytic literature in schizophrenia highlights several important issues. Firstly, most VBM studies are conducted in patients with chronic, established illness. Secondly, in most cases the control groups include healthy individuals rather than a clinically ill patients group with psychosis other than schizophrenia. Thirdly, most studies have been conducted on patients exposed to various doses and length of antipsychotic medications, which can have confounding effects on the findings. Finally, the identification (a ‘positive’ result) of grey matter changes in VBM when comparing two groups is generally based on diverse statistical approaches that vary in terms of their choice of multiple‐test correction, number of voxels (spatial extent or cluster size) and the effect size (T values or peak height threshold) for including voxels in a reported finding (Henley 2010; Whitwell 2009). As a result, there are currently no generally accepted standards for VBM positivity threshold.
In practice, most studies have considered regional grey matter changes to be significant if the group differences survive the test of a pre‐specified statistical threshold on parametric T maps (or z score maps) of the brain. Grey matter changes that are picked up by VBM technique occur in a wide variety of disorders including other psychiatric and neurological disorders (Goodkind 2015). As a result, a diagnostic VBM test result should be based on both the spatial distribution and the extent of changes that define the pattern of abnormalities in schizophrenia. While no distinct patterns of grey matter changes have shown to be specific to schizophrenia to date, a reduction in grey matter in the aforementioned brain regions appears to be a consistent and robust finding that is reliably demonstrated in schizophrenia. In addition, statistical discriminatory approaches aimed at the detection of diagnostic patterns to estimate the test accuracy of VBM are also becoming increasingly popular, including the use of machine‐learning algorithms to study the pattern of multivariate changes that best discriminate between two groups and allow test accuracy to be quantified using permutation approaches (Schnack 2014).
Clinical pathway
Schizophrenia typically develops in adolescence or early adulthood, though several premorbid indicators are associated with the onset of the illness. At present, a clinical diagnosis is considered only after a full‐blown psychotic episode. For someone with psychotic symptoms, if it is the first time they have experienced delusions or hallucinations, they would be considered to have 'first episode psychosis'. Patients typically present to primary care or emergency services from where they are referred to 'Early Intervention Teams' in the UK and similar secondary care services elsewhere. At present, diagnosis of schizophrenia is made only after several months of longitudinal observation using widely accepted nosological criteria (ICD or DSM). Once someone has received such a diagnosis this has major treatment, psychological and social implications. People may be treated with antipsychotic medications, which carry risk of serious adverse effects, may be treated for long periods, and a person's life course may alter. A diagnosis of schizophrenia is thought to be useful ‐ swiftly communicating much information about the person's condition ‐ but it carries with it a stigma.
While radiological investigations are not currently used in the diagnosis of schizophrenia, it is envisaged that a diagnostic tool (such as VBM) will be available either at the primary or secondary care setting to reduce the lead‐time for diagnosis, thus reducing the duration of the current prolonged assessment pathway (Gur 2007; Lawrie 2011).
Rationale
It has long been thought that abnormalities in the brain might be involved in the aetiology of schizophrenia. To date, brain imaging studies have found grey matter deficits, ventricular enlargement, and reduced overall brain volume in first episode psychosis (Fannon 2000). However, as brain abnormalities in schizophrenia have been found to be small and indistinct, it is important to use techniques that might be able to detect these (McCarley 1999). Therefore, this review will focus on a fine‐tuned measurement of brain structure known as voxel‐based morphometry (VBM), which examines the whole brain and can detect small regional differences in grey or white matter concentration and volume (Mechelli 2005).
This review is an exploratory examination of the diagnostic ‘potential’ of VBM for use as an additional tool in the clinical examination of patients with first episode psychosis to establish whether an individual has schizophrenia as opposed to other types of psychosis, specifically bipolar disorder and drug‐induced psychosis. It has been suggested that the longer psychosis goes unnoticed and untreated, the more severe the repercussions for relapse and recovery (Bottlender 2003; Malla 2005). There is some evidence to suggest that early intervention for people with schizophrenia can be beneficial, helping avoid or postpone damaging relapses and the need for prolonged use of medications (Marshall 2011). Thus, accurate diagnostic techniques that could contribute to early intervention may offer clinical utility in these situations. For instance, first episode psychosis in bipolar disorder can be prone to initially receiving a misdiagnosis of schizophrenia which is only resolved at a later stage (Gonzalez‐Pinto 1998). Furthermore, a first episode of psychosis that results from toxic effect substances may mimic psychosis due to schizophrenia. Thus, an objective test to identify schizophrenia, will be most useful in first episode psychosis.
In this review we have not included VBM studies that focus on individuals who do not exhibit frank psychosis but are deemed to be at a high risk for later development of schizophrenia. This so called 'at‐risk population' is highly heterogenous, comprised of genetically high‐risk (having first degree relatives with schizophrenia), clinically high‐risk (experiencing variously defined prodromal symptoms) and both genetic and clinical high‐risk groups (Fusar‐Poli 2013). To diagnose later development of schizophrenia, these samples must be followed up for long periods to observe transition to psychosis (36% conversion to psychosis in three years (Fusar‐Poli 2012). Owing to the poor conversion rates, lack of defined prevention strategies and the heterogeneity of identification criteria, the clinical utility and predictive value of developing a diagnostic test for schizophrenia for this sample is questionable (Lawrie 2011). Consequently, we have not considered VBM studies in high‐risk samples in the current review.
This review is part of a series of Cochrane reviews using the same methodology to assess the diagnostic accuracy of tests for schizophrenia, such as first‐rank symptoms (Soares‐Weiser 2013) and the Operational Criteria Checklist for Psychotic Illness and Affective Illness (OPCRIT+) (Bergman 2014).
Objectives
To determine the test accuracy of voxel‐based morphometry (VBM) in diagnosis of schizophrenia compared with other types of psychosis in adults and adolescents who have experienced a first episode psychosis.
Methods
Criteria for considering studies for this review
Types of studies
We included cross‐sectional and delayed verification (cohort) accuracy studies that evaluated the diagnostic accuracy of VBM for differentiating schizophrenia from other psychoses in participants with first episode psychosis. We included both retrospective and prospective studies that consecutively or randomly selected participants. We excluded case‐control studies that compared confirmed target disease‐positive individuals with healthy controls. Studies were included irrespective of publication status and language.
Participants
We included studies with adolescents and adults presenting with symptoms thought indicative of first episode psychosis, which are likely to include psychotic symptoms such as, hallucinations, delusions, disordered thinking, and speech. We did not exclude on grounds of co‐morbidities. We excluded studies involving participants with organic brain disorders, such as that triggered by an existent physical disease or alcohol and drug abuse. We also excluded studies that included participants with childhood psychosis, with early onset in childhood, as well as late onset psychosis, with onset at or above the age of 45 (DSM‐III), and studies with participants at high risk for schizophrenia. In addition, we collected data on whether participants were given some specific prior test or treatment before entering the study.
Index tests
The index test is voxel‐based morphometry (VBM), which is a structural magnetic resonance imaging (MRI) technique that is able to identify neuroanatomical differences between brains by making voxel‐wise comparisons (Mechelli 2005, McCarley 1999, Whitwell 2009). We specifically investigated grey matter deficits in individuals presenting with first episode psychosis. There is no accepted threshold for a diagnosis of schizophrenia, as this test is at an early stage of development and interpretation is likely to be subjective and qualitative. Therefore, in line with diagnostic test accuracy review protocols of other similar neuroimaging techniques (for example, Vacante 2013), we used the criteria which were applied in each included primary study to classify participants as either VBM positive or VBM negative.
VBM tests can be applied in different ways (see Investigations of heterogeneity), and we noted how each study employed VBM. The most important aspect of the index test evaluated here is the application of VBM technique to the entire brain in a voxel‐wise fashion, rather than selecting regions‐of‐interest. As the pre‐test probability, image processing methodology, statistical approaches and posterior probability of group differences are likely to be very different when selected regions are studied as opposed to a whole brain voxel‐wise search, we defined the fidelity of VBM on the basis of whole brain voxel‐wise application.
The quality of VBM analysis of MRI data can mainly be affected by two sources of variation, image movement artefacts and uniformity of image pre‐processing (Ridgway 2008). As a result, we planned to explore these sources of variation in Investigations of heterogeneity and considered them in the Assessment of methodological quality.
Target conditions
All types of schizophrenia disorder, regardless of descriptive subcategory (e.g. paranoid, disorganised, catatonic, undifferentiated and residual). We included studies that reported results combined for diagnoses related to schizophrenia (e.g. schizoaffective and schizophreniform disorder) in which data could not be separated, but considered these studies a potential source of heterogeneity.
Reference standards
The reference standard is diagnosis through history and clinical examination collected by a qualified professional (e.g. psychiatrist, nurse or social worker), which may or may not involve the use of operational criteria or checklists of symptoms such as International Statistical Classification of Diseases (ICD‐9 or ICD‐10; WHO 1992) or Diagnostic and Statistical Manual of Mental Disorder (DSM‐III, DSM‐IV or DSM‐5; APA 2013). This type of clinical diagnosis requires the presence of symptoms for at least six months to confirm the diagnosis. We considered differences in reference standard a potential source of heterogeneity.
Search methods for identification of studies
Electronic searches
We conducted searches in MEDLINE, EMBASE, and PsycInfo using OvidSP in May 2012 and December 2013, see Appendix 1 for details of the search strategies.
We did not apply any restrictions based on language or type of document in the search. We used the multipurpose search command for the OvidSP interface (.tw.) to search both database abstract and title fields. To capture variations in suffix endings, the truncation operator ‘$’ was used.
Searching other resources
We identified additional references by manually searching the reference lists of included studies.
Data collection and analysis
We followed the guidelines provided in the Cochrane Diagnostic Reviewer’s Handbook (DTA Handbook 2013).
Selection of studies
Atoosa Khodabakhsh (AK) and Nicola Mayaan (NM) independently screened all titles and abstracts for eligibility. Full papers of potentially relevant studies were retrieved, as well as review articles, if they were relevant, for manual reference search. AK and NM independently reviewed the full papers for eligibility according to the inclusion criteria detailed above. We included abstracts in the absence of a full publication if sufficient data were provided for analysis. Any disagreements were resolved by discussion between AK and NM and all decisions documented. Lena Palaniyappan (LP) made a final decision regarding inclusion or exclusion, if a consensus could not be reached.
Data extraction and management
We developed data extraction forms using web‐based software and piloted these on a small selection of studies. NM and KSW, again working independently, completed the data extraction form for each included study. Agreements and disagreements were recorded and resolved by discussion between Karla Soares‐Weiser (KSW) and NM. LP made a final decision if a consensus could not be reached.
We extracted the following information on study characteristics: number, age, gender and ethnicity of participants, co‐morbid disorders, duration of symptoms, use of medication, study aim, clinical setting and country, who conducted the index test, cut‐off points used, type of scanner, MRI parameters, pre‐processing steps, covariates used in the General Linear Model, description of the reference standard, whether any operational criteria or checklists were used, target condition, who conducted the reference standard, description of the study process, and duration of follow‐up. We had also planned to record the number of true positive (TP), true negative (TN), false positive (FP), and false negative (FN) test results. If such data were not available, we planned to derive them from summary statistics such as sensitivity, specificity, and/or likelihood ratios, in order to construct a 2 x 2 table for each study. However, none of the included studies had test accuracy data that we could extract, so instead we have reported their findings narratively (see Findings).
Assessment of methodological quality
We used QUADAS‐2 (Quality Assessment of Diagnostic Accuracy Studies), an updated version of the original QUADAS tool for the assessment of quality in systematic reviews of diagnostic accuracy studies (Whiting 2011). The QUADAS‐2 tool is made up of four domains: patient selection, index test, reference standard, and flow and timing. We tailored the tool to our review, and which was used to judge the risk of bias and applicability of included studies. Review authors NM and KSW, working independently assessed the included studies using a form that we piloted on a small selection of studies. The inter‐rater agreement was then measured and the form adapted (see Appendix 2). It was then applied to the other included studies. Any disagreements were resolved by consensus with Clive E Adams (CEA) and Clare Davenport (CD).
We used the results of the quality assessment to describe the internal validity and external validity (applicability) of the included studies. The results were also used to make recommendations for the design of future studies. The use of overall quality scores for individual studies is not recommended as the relative importance of each quality domain is specific to each review topic. We, therefore, did not use QUADAS‐2 other than to help the qualitative commentary.
Statistical analysis and data synthesis
The primary objective for statistical analysis and data synthesis for this review was to derive summary sensitivities and specificities and corresponding 95% confidence regions from included studies. This would have been undertaken using either the hierarchical summary ROC curve (HSROC) method (Rutter 2001), where there was evidence of explicit or implicit threshold, or the hierarchical bivariate model (Reitsma 2005), if included studies had used the same threshold to classify participants as either test positive or test negative. However as we were unable to extract or derive test accuracy data from included studies (TP, FP, TN, FN), meta‐analysis was not possible.
Investigations of heterogeneity
Planned investigations of heterogeneity using either hierarchical modelling or subgroup analysis were not possible due to lack of test accuracy data. The following variables are considered important potential causes of heterogeneity that could not be investigated as part of this review, but which may be relevant for updates of this review or other reviews concerned with VBM for the diagnosis of schizophrenia.
-
Participants
Age of onset: adolescents compared to adults. Adolescence is a time where dynamic changes in the brain occur. The interaction between the stage of brain development and timing of first episode psychosis could confer heterogeneity when comparing adolescent versus adult first episode psychosis (Douaud 2007). Earlier onset of first episode psychosis has been linked to a greater likelihood of exacerbated psychotic symptoms following first episode (Gonzalez‐Pinto 1998). Schizophrenia has been shown to have an earlier onset of first episode psychosis (adolescence and early adulthood) (McGrath 2008) in comparison to first episode psychosis in bipolar disorder, which typically occurs at a later stage (adulthood) (Gonzalez‐Pinto 1998).
Duration of untreated psychosis prior to first episode diagnosis. It is thought that the longer the period of untreated psychosis, the more progressed pathological changes in the brain may be (Wyatt 1997).
Medication: previously received, currently receiving, and medication naive.
Gender of participants. The mean age of onset for first episode schizophrenia has been shown to be younger for men in comparison to women (Larson 1996; Ochoa 2012).
-
Index test
MRI parameters: type of scanner, magnetic field strength, image slice thickness‐orientation‐sequence, bandwidth, and voxel dimensions.
Pre‐processing steps (spatial normalisation, smoothing, modulation) (See Appendix 3).
Use of covariates in the General Linear Model: covariates relating to head or cranial size can be significant, due to neurodevelopmental aberrations associated with schizophrenia that can affect overall brain size and intracranial volume.
-
Reference standard
Reference standard used, e.g. ICD‐9 or DSM‐IV.
Application of the reference standard, e.g. type of medical professional, and individual medical professional, algorithm or consensus decision.
Duration of follow‐up between undergoing VBM and schizophrenia diagnosis.
Sensitivity analyses
Had data been available for meta‐analysis, we planned to use sensitivity analyses to investigate the impact of study quality on overall diagnostic accuracy of VBM diagnoses. This strategy may be relevant for updates of this review or other reviews concerned with VBM for the diagnosis of schizophrenia.
Assessment of reporting bias
Standard funnel plots and tests for publication bias are likely to be misleading for meta‐analysis of test accuracy studies (Deeks 2005). We did not plan to assess publication bias.
Results
Results of the search
We initially identified 405 potentially relevant references. After removing 34 duplicate references, we excluded 280 references through title and abstract screening. We assessed the full text of the remaining 91 references, which resulted in 87 references being excluded, mainly because they did not include participants with first episode psychosis, did not include relevant comparisons for our review, or the comparisons were with healthy controls (see Characteristics of excluded studies for details of reasons for exclusion). We included four studies (with five references), however none of the studies provided the numbers or percentages of true positives, false positives, true negatives and false negatives. See Figure 1 for an overview of the selection process.
1.
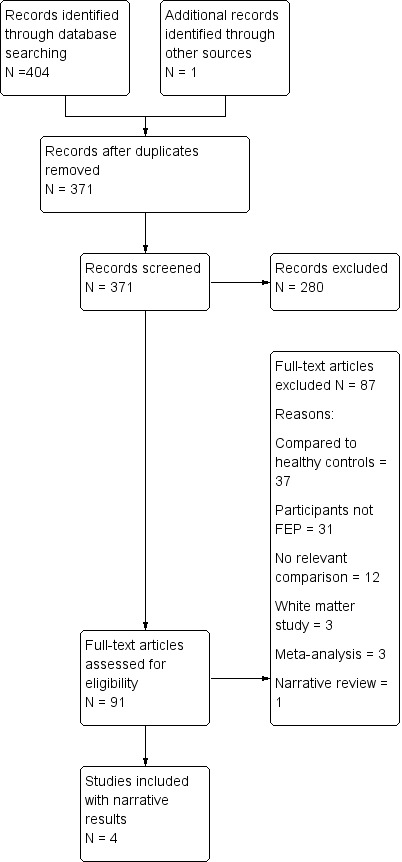
Prisma Study flow diagram.
Included studies
Study Design
Two studies were cross‐sectional (Kubicki 2002; Morgan 2007) and two were delayed verification (cohort) studies (de Castro‐Manglano 2011; Tordesillas‐Gutierrez 2010). Two studies (de Castro‐Manglano 2011; Morgan 2007) recruited a consecutive series of patients, and in the other two studies (Kubicki 2002; Tordesillas‐Gutierrez 2010), the method of enrolment was not reported.
Setting
Kubicki 2002 was based in a hospital, de Castro‐Manglano 2011 at an outpatient clinic, Morgan 2007 inpatient and outpatient psychiatric services, and Tordesillas‐Gutierrez 2010 did not report the setting. Two studies were conducted in Spain (de Castro‐Manglano 2011; Tordesillas‐Gutierrez 2010), one study in the UK (Morgan 2007), and one in the USA (Kubicki 2002).
Participants
The included studies had a total of 275 participants (range from 28 to 118 participants), although only 251 were analysed in the studies. All studies included participants with first episode psychosis, however Morgan 2007 excluded participants with schizoaffective disorder. Three studies reported the mean age of participants, which ranged from 18.6 (standard deviation (SD) 4.9) years to 27.1 (SD 7.6) years old; Tordesillas‐Gutierrez 2010 did not report on age. Two of the studies reported on the gender of participants and included both males and females (de Castro‐Manglano 2011; Kubicki 2002). Duration of psychotic symptoms was reported in two studies (de Castro‐Manglano 2011; Morgan 2007), which ranged from 9.8 (SD 18.2) weeks to 67.1 (SD 124.9) weeks.
In addition to participants with first episode psychosis, all four studies also included a healthy control group, which we did not include for the purposes of this review.
Index Test
All studies used a magnetic field strength of 1.5 T. Two studies (de Castro‐Manglano 2011; Kubicki 2002) smoothed images using 12 mm isotropic Gaussian kernal and Tordesillas‐Gutierrez 2010 used 5 mm FWHM. Pre‐processing steps were clearly described in de Castro‐Manglano 2011 and Kubicki 2002; it was unclear whether Morgan 2007 carried out smoothing and normalisation; Tordesillas‐Gutierrez 2010 used a specific pre‐processing algorithm (DARTEL), but the various optional processes chosen to be employed are not reported. See Characteristics of included studies for more details.
Reference standard
The reference standard in all studies was clinical diagnosis. In Kubicki 2002, Morgan 2007 and Tordesillas‐Gutierrez 2010 this included the use of a clinical interview; medical records were also used in de Castro‐Manglano 2011 and Morgan 2007. Diagnoses were based on operational criteria in all studies (de Castro‐Manglano 2011, DSM‐IV and ICD‐10; Kubicki 2002, DSM‐III‐R; Morgan 2007, ICD‐10; Tordesillas‐Gutierrez 2010, DSM‐IV).
Target Condition
The target condition was first episode schizophrenia in all studies. Morgan 2007 excluded patients with schizoaffective disorder, and Tordesillas‐Gutierrez 2010 separated participants with schizophreniform disorder from those with schizophrenia. The other two studies did not report what was included in the definition of schizophrenia.
Excluded studies
We excluded 87 studies, the majority for more than one reason: 12 studies included participants with first episode psychosis, but only compared them as a group with healthy controls, and a further six included first episode schizophrenia compared with healthy controls; 15 studies included participants with schizophrenia and/or bipolar disorder, and again, compared these only with health controls; 33 studies included participants with chronic psychosis, or at‐risk of psychosis; 12 studies did not make a relevant comparison as they were correlational studies, mostly with cognition; five studies compared white matter only; three were meta‐analyses and one was a narrative review.
Awaiting assessment studies
There are no studies awaiting assessment.
Ongoing studies
We did not find any ongoing studies.
Methodological quality of included studies
See also Risk of bias and applicability concerns in Characteristics of included studies, Figure 2 and Figure 3, for an overview of the assessment of risk of bias and applicability concerns for each of the four studies included in the review.
2.
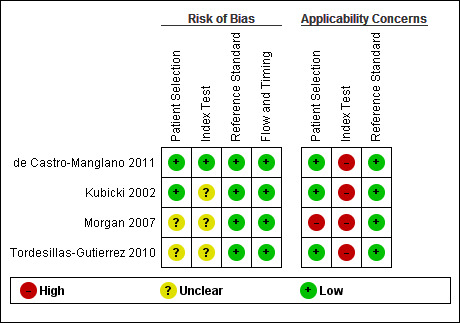
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
3.
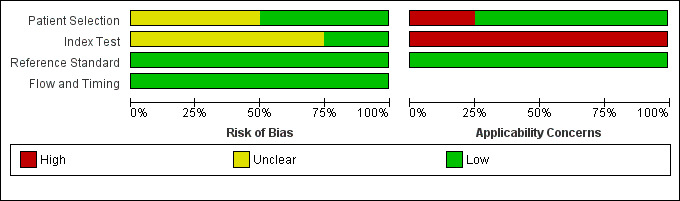
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
Patient Selection
Two studies (de Castro‐Manglano 2011; Morgan 2007), consecutively enrolled participants, and the other two studies were unclear about how participants were selected. In all studies participants were undiagnosed at entry as they all included participants with first episode psychosis. Kubicki 2002 and de Castro‐Manglano 2011 avoided inappropriate exclusions, it was unclear in Tordesillas‐Gutierrez 2010, and Morgan 2007 excluded participants with schizoaffective disorder. Overall for patient selection, two studies (de Castro‐Manglano 2011; Kubicki 2002), were considered to be at low risk of bias and the other studies two were considered to be at unclear risk of bias. In terms of applicability concerns regarding patient characteristics and setting, we judged Morgan 2007 to be of high concern as they included in the schizophrenia group only patients meeting a narrow definition of schizophrenia; the remaining studies were judged as low concern.
Index test
The index test results were interpreted without previous knowledge of the reference standard results in de Castro‐Manglano 2011 and Tordesillas‐Gutierrez 2010; it was unclear in the other studies. The major focus of the four included studies was to locate the brain regions with maximum diagnostic differences. None of these studies used a feature discrimination (or pattern classification) approach; consequently, there were no statistical cut‐offs used to separate the patient groups. Pre‐processing was clearly described in both de Castro‐Manglano 2011 and Kubicki 2002; it was unclear in the other studies. It was unclear in all studies whether images were inspected for motion artefacts. As a result, one study was considered to be low risk of bias (de Castro‐Manglano 2011) and three studies at unclear risk of bias. All four studies were judged to be of high applicability concern as VBM was not used to diagnose schizophrenia, instead VBM was used to characterise grey matter.
Reference standard
In all studies the reference standard was described and would correctly classify schizophrenia, although it was unclear if the reference standard was interpreted without knowledge of the index test result. As a result, for the reference standard domain, all studies were considered to be of low risk of bias. In terms of applicability concerns, all studies were judged as low concern.
Flow and timing
We considered all four studies to be of low concern for risk of bias as in most studies all participants received a reference standard, received the same reference standard, had an appropriate interval between tests, and accounted for all of the participants in the analysis.
Findings
The four included studies did not report data that we could use in 2 x 2 tables and meta‐analysis. Instead we have summarised their findings narratively:
de Castro‐Manglano 2011
This study included 28 outpatients with first presentation of psychosis who underwent a clinical assessment and scanning on entry to the study. Patients were diagnosed according to the DSM‐IV and ICD‐10 criteria after three years using all available clinical information: 10 received a diagnosis of first episode schizophrenia and 18 a diagnosis of first episode affective psychosis.
The study found no significant differences at the pre‐specified statistical threshold, but a trend for the first episode affective psychosis group to have less grey matter volume in the cerebellar vermis than the first episode schizophrenia group in the VBM analysis (Talairach coordinates (x = ‐1, y = ‐45, z = ‐14); Z = 3.59; P < 0.001 uncorrected).
The study also found that participants with a poor outcome after three years, compared to those with a good outcome at three years, had less grey matter volume in the right hippocampus (Talairach coordinates x = 38, y = ‐11, z = ‐18; Z = 4.42; P < 0.001 uncorrected).
Kubicki 2002
This study recruited inpatients with first episode psychosis, who were diagnosed based on their medical records and the Structured Clinical Interview for DSM‐III‐R (SCID). Sixteen patients were diagnosed with schizophrenia and 16 with affective disorder. The majority of patients were scanned within three weeks of their admission to hospital, but four patients were scanned between nine and 22 months after admission.
The same pattern of differences compared to healthy controls was found bilaterally in the insula for patients with schizophrenia and affective disorder, however, statistical differences were found between the groups of patients in the left superior temporal gyrus and the left hippocampus, with patients with schizophrenia having reduced grey matter density in these areas (Talairach coordinates, Z score and P value not reported).
Morgan 2007
Ninety‐seven inpatients with first episode psychosis were included in this study, but only 73 were analysed as some participants refused consent and others were excluded by study investigators due to participant motion, congenital hydrocephalus and subarachnoid cyst. Participants were interviewed using the WHO Schedules for Clinical Assessment in Neuropsychiatry (WHO–SCAN) and received diagnoses based on the ICD‐10. There were 44 patients with schizophrenia and 29 with bipolar disorder or psychotic depression.
When schizophrenia was compared with affective psychosis, controlled for age and gender, no differences in total grey matter, cerebrospinal fluid volume or ventricular volumes were found. When total grey matter was added as a covariate, a deficit in regional grey matter was found in the anterior cingulate gyrus, centred on Brodmann’s Area in the affective psychosis group (Talairach coordinates x = 1, y = ‐2, z = 36; Z score and P value not reported). However, when type of antipsychotic was also added as a covariate, no differences in regional grey matter were found.
Tordesillas‐Gutierrez 2010
This study included 118 patients with first episode psychosis. They were diagnosed with the SCID‐I (Structured Clinical Interview for Diagnosis) by an independent psychiatrist. Sixty‐five patients were diagnosed with schizophrenia, 32 with schizophreniform disorder and 21 with non‐schizophrenic non‐affective psychosis.
No differences in grey matter were found between the schizophrenic and schizophreniform patients. However, they did find grey matter reductions in patients when compared to healthy controls (Talairach coordinates and Z score not reported; P < 0.001 corrected), and these reductions were greater with greater severity of disease.
Discussion
Summary of main results
This systematic review includes four studies that measure differences in grey matter using voxel‐based morphometry (VBM) in patients with psychosis. The studies included participants with first episode psychosis, but did not use VBM to diagnose the participants. The included studies had a total of 275 participants and 251 were analysed. The reference standard in all studies was clinical diagnosis including the use of operational criteria.
The quality assessments of the studies were mostly rated as having low and unclear risk of bias. There were high applicability concerns for all studies on the index test domain as VBM was not used to diagnose schizophrenia, rather VBM was used to quantify the magnitude of differences in grey matter volume. None of the included studies reported data that could be used in the analysis. There are no data on the diagnostic accuracy of VBM for diagnosing schizophrenia.
Table 1 gives information on the quality and applicability of evidence.
Strengths and weaknesses of included studies
We did not find any studies that assessed the diagnostic accuracy of VBM for schizophrenia in participants with first episode psychosis. The four studies we were able to identify included people with first episode psychosis, who were scanned and were assessed with a relevant reference standard, but the studies were not designed to use VBM to diagnose participants, instead they identified differences in grey matter distribution in brain regions.
Patient selection was unclear in Morgan 2007 and Tordesillas‐Gutierrez 2010 as the study authors did not report the methods used to select participants. The risk of bias in the index test domain was unclear in all studies except de Castro‐Manglano 2011 as blinding of the reference standard results was not reported and the pre‐processing steps used were not always fully reported. The reference standard was well described in most studies and matched the inclusion criteria of the review. All studies were conducted in a research setting, rather than clinical setting.
Strengths and weaknesses of the review
We tried to identify all relevant studies in our search strategy, and we did not apply any restrictions based on language or type of document in the search. However, it is possible that we were not able to identify some studies.
We found that the completion of QUADAS 2 involved much discussion between review authors, and needed to adapt the signalling questions multiple times before we reached agreement. We see this process as a strength of the review, designed to mitigate against bias.
Previous research
We know of no other reviews testing the diagnostic accuracy of VBM for schizophrenia.
Applicability of findings to the review question
An important issue with respect to the applicability of VBM studies to the question of this review is that almost all studies were principally aimed at ‘localising’ grey matter changes, rather than establishing the accuracy of VBM per se in separating the diagnostic groups. As a result, the statistical approaches applied were aligned to the goal of discovering continuous differences in grey matter distribution, rather than establishing the utility of distributed, albeit low effect‐size differences, in diagnosing schizophrenia during first presentation.
In recent times, reports on explicit attempts to test the usefulness of VBM approach to diagnose schizophrenia are emerging (for example, Schnack 2014 and Iwabuchi 2013). Unfortunately, to date most of these studies focus on separating people with schizophrenia from healthy individuals, rather than from those with non‐schizophreniform but psychotic disorders. Also, none of these studies have tackled the issue of diagnostic utility for first episode psychosis. Our present review highlights the gap in this area of research.
Schnack 2014, however, did measure the diagnostic accuracy of VBM with the addition of support vector machine modelling to separate participants with schizophrenia and bipolar disorder, although this was in a chronically ill sample of patients. In this study they used scans from a discovery sample of participants with schizophrenia and bipolar disorder to train support vector machine to build models to separate patients with schizophrenia from bipolar patients, based on their grey matter densities. These models were then tested by applying them to the data of the validation sample of participants. A post hoc analysis was done, recalculating the diagnostic accuracy with the participants' gender weighted according to the discovery sample. When the participants’ scans were applied to the discovery sample’s model, the test sensitivity was 48% and the specificity was 79%. When the classification thresholds were adjusted after the ROC curve analysis, the sensitivity was 55% and the specificity was 63%. When the analysis was re‐weighted by gender, the sensitivity was 71% and the specificity was 65%. The model was thus developed in the same sample it was then tested on. Further research of this kind in investigating the potential of VBM for diagnosing schizophrenia needs to be undertaken mindful of this limitation.
Authors' conclusions
Implications for practice.
There is no evidence to currently support diagnosing schizophrenia (as opposed to other psychotic disorders) using the pattern of brain changes seen in voxel‐based morphometry (VBM) studies in patients with first episode psychosis. The lack of clinical applicability of VBM studies conducted so far significantly limits their utility for diagnostic purposes.
Implications for research.
VBM studies are largely focused on locating the distribution of the most prominent structural changes in schizophrenia, rather than estimating how the presence of structural changes can assist in clinical diagnostic decisions per se. Studies with an emphasis on statistical discrimination approaches, using all of the brain‐wide information collected from VBM studies, especially in first episode populations with prospective diagnostic validation, need to be conducted to realise the diagnostic potential of VBM. We also note a wide variation in the reporting of the image processing approaches, use of multiple test corrections and quality of clinical case ascertainment.
Acknowledgements
We would like to thank Atoosa Khodabakhsh for help with screening and data extraction, Danielle Phoenix for help with background research, Amanda J Kirkham for statistical support, and the editorial base of the Diagnostic Test Accuracy Working Group for their support throughout the process of writing this review.
Appendices
Appendix 1. Search strategies
| Database | Phase and date | Search strategy |
| MEDLINE (OvidSP) Dates:1946 to 2013 |
Phase I Date: 08‐05‐12 Phase II Date: 06‐12‐13 |
1 voxel‐based morphometry.tw. (1593) 2 VBM.tw. (883) 3 1 or 2 (1785) 4 exp Schizophrenia/ (77987) 5 schizophren$.tw. (76507) 6 4 or 5 (95672) 7 3 and 6 (220) |
| EMBASE (OvidSP) Dates: 1980 to 2013 |
Phase I Date: 08‐05‐12 Phase II Date: 06‐12‐13 |
1 voxel‐based morphometry.tw. (2318) 2 VBM.tw. (1419) 3 1 or 2 (2635) 4 exp Schizophrenia/ (119513) 5 schizophren$.tw. (99363) 6 4 or 5 (131921) 7 3 and 6 (345) |
| PsycINFO (OvidSP) Dates:1806 to 2013 |
Phase I Date: 08‐05‐12 Phase II Date: 06‐12‐13 |
1 voxel‐based morphometry.tw. (1167) 2 VBM.tw. (528) 3 1 or 2 (1223) 4 exp Schizophrenia/ (65558) 5 schizophren$.tw. (89948) 6 4 or 5 (90931) 7 3 and 6 (199) |
| All databases searched from inception. All searches were undertaken, added to a common database and duplicates deleted. | ||
Appendix 2. QUADAS‐2
| DOMAIN 1: PATIENT SELECTION | |
| Risk of bias: Could the selection of patients have introduced bias? | |
| Signalling question | 1. Was a consecutive or random sample of patients enrolled? ‘Yes’ if a random sample of patients with suspected psychotic symptoms were included, or consecutive patients were enrolled ‘No’ if the patients were specifically selected (not random sample) to be included in the study ‘Unclear’ if insufficient information is provided |
| 2. Were participants undiagnosed at study entry? ‘Yes’ if participants did not have a specific diagnosis at entry to the study even if they had psychotic symptoms. ‘No’ if participants had a specific diagnosis at entry to the study. ‘Unclear’ if insufficient information is provided | |
| 3. Did the study avoid inappropriate exclusions? ‘Yes’ if the study explicitly states that there were no exclusions or there were no inappropriate exclusions ‘No’ if some patients were inappropriately excluded e.g. if they were deemed “difficult‐to‐diagnose” patients ‘Unclear’ if exclusions were not explicitly reported in the study | |
| Applicability | |
| Are there concerns that the included patients and setting do not match the review question? ‘No’ if included patients were those with psychosis but not a specific diagnosis ‘Yes’ if patients already had a specific diagnosis upon entry to study (e.g. inclusion criteria lists specific diagnoses) 'Unclear' Not enough information to decide | |
| DOMAIN 2: INDEX TEST | |
| Risk of bias: Could the conduct or interpretation of the index test have introduced bias? | |
| Signalling question | 1. Were the index test results interpreted without knowledge of the results of the reference standard? ‘Yes’ if the index test was conducted before the reference standard, or if the person applying the index test was blinded to the results of the reference standard ‘No’ if the index test operator knew the results of the reference standard ‘Unclear’ if insufficient information is provided |
| 2. Did the study pre‐specify cut‐offs for VBM? 'Yes’ if the study stated the VBM threshold needed to be present to diagnose schizophrenia ‘No’ if the study does not state the VBM threshold they considered necessary to diagnose schizophrenia | |
| 3. Was image pre‐processing carried out? 'Yes’ if the method of image pre‐processing was clearly stated and the same for all participants ‘No’ if image pre‐processing was not uniformly carried out for all participants ‘Unclear’ if insufficient information is provided | |
| 4. Were images inspected for motion artefacts? 'Yes’ if images were inspected for motion artefacts for all participants using prespecified criteria ‘No’ if motion artefact inspection was not uniformly carried out for all participants ‘Unclear’ if insufficient information is provided | |
| Applicability | |
| Are there concerns that the index test, its conduct, or interpretation, differ from the review question? 'Yes' VBM is not being applied to differentiate early stage schizophrenia disorder from other forms of psychosis 'No' VBM is being applied to differentiate early stage schizophrenia from other forms of psychosis | |
| DOMAIN 3: REFERENCE STANDARD | |
| Risk of bias: Could the reference standard, its conduct or interpretation have introduced bias? | |
| Signalling question | 1. Is the reference standard likely to correctly classify the target condition? ‘Yes’ if the history and clinical examination is conducted by a qualified professional (psychiatrist, nurse, social worker) ‘No’ if the history and clinical examination is conducted by insufficiently qualified individuals ‘Unclear’ if insufficient information is provided |
| 2. Were the reference standard results interpreted without knowledge of the results of the index test? ‘Yes’ if the reference standard was conducted before the index test, or if the person applying the reference standard was blinded to the results of the index test ‘No’ if the reference standard operator knew the results of the index test ‘Unclear’ if insufficient information is provided | |
| Applicability | |
| Are there concerns that the target condition as defined by the reference standard does not match the question? ‘No’ the paper specifically looks at diagnosing early stage schizophrenia ‘Yes’ if the paper also includes schizophrenia‐like illnesses ‘Unclear’ if insufficient information is provided | |
| DOMAIN 4: FLOW AND TIMING | |
| Risk of Bias: Could the patient flow have introduced bias? | |
| Signalling question | 1. Was there an appropriate interval between index test and reference standard? 'Yes' if reference standard and index text were applied within 4 weeks 'No' if reference standard or index test were applied beyond 4 weeks between them 'Unclear' if not enough information is given to assess whether there was an appropriate interval |
| 2. Did all patients receive a reference standard? 'Yes’ if all patients had details of history and clinical examination, with or without operational criteria ‘No’ if not all patients had a description of history and clinical examination with or without operational criteria ‘Unclear’ if insufficient information is provided | |
| 3. Did all patients receive the same reference standard? ‘Yes’ if all patients were diagnosed with the same operational criteria that were applied to all patients, and that received the same clinical follow up ‘No’ if all patients received history and clinical examination but only some received operational criteria, or different operational criteria ‘Unclear’ if insufficient information is provided | |
| 4. Were all patients included in the analysis? 'Yes' if there are no patients excluded from the analysis 'No' if there are patients excluded from the analysis 'Unclear' if not enough information is given to assess whether any patients were excluded from the analysis | |
| 5. Were missing or uninterpretable results reported? 'Yes' if there were no missing or uninterpretable results, or they were adequately reported 'No' if missing and uninterpretable results were not adequately reported 'Unclear' if there was no information about missing or uninterpretable results | |
Appendix 3. Pre‐processing steps (spatial normalisation, smoothing, modulation)
| Normalisation | Images are spatially normalized so that they are in the same stereotactic space (a precise mapping system using 3‐D coordinates) and registered to the same template image. This allows for the combination of data across individuals and comparison of different studies. Heterogeneity will arise if studies do not all report results in the form of coordinates within a common stereotactic space (of which 'Talairach space' is most commonly used) (Huettel 2009). |
| Optimised versus standardised voxel‐based morphometry | ‘Optimised voxel‐based morphometry’ (where images are first separated into grey matter, white matter and cerebrospinal fluid before being registered to a template) can reduce the misinterpretation of significant differences in comparison to ‘standard voxel‐based morphometry (where images are first registered to the template and then separated) (Mechelli 2005). Comparisons of studies that use different methods can distort results. |
| Smoothing | This makes the data more normally distributed and reduces the variance across subjects (Whitwell 2009). However, the Gaussian smoothing kernels that are used can vary in size from 0‐16 mm which can produce heterogeneity (Jones 2005). |
| Modulation | Spatial normalization requires the warping of brain regions to fit the same sterotactic space. Modulation can be used to compensate for this warping process by providing a relative volume for each voxel in contrast to unmodulated parameters which give data about concentration (Segall 2009). Heterogeneity can arise if some studies use modulation and others do not. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
de Castro‐Manglano 2011.
| Study characteristics | |||
| Patient sampling | Prospective and consecutive: "consecutive series of patients...presenting for the first time with a psychotic episode at the outpatient clinic". | ||
| Patient characteristics and setting |
N of participants: 28.
N analysed: 28. Age: mean age 18.6 years (SD 4.9). Gender: 17 M, 11 F. Ethnicity: Caucasian. Comorbid disorders: not reported. Duration of symptoms: 9.8 weeks (SD 18.2). Concurrent medications used: 22/28 of the participants (olanzapine, n = 11; risperidone, n = 8; ziprasidone, n = 4; aripiprazole, n = 1; haloperidol plus clozapine and olanzapine, n = 2) mood stabilisers (n = 9) or antidepressants (n = 15). Inclusion criteria: first presentation of symptoms meeting criteria for a non‐medical, non‐toxic psychosis [International Classification of Diseases (ICD)‐10: F20 schizophrenia and F30–39 affective disorders‐psychotic coding; World Health Organization and DSM‐IV classification]; age at first evaluation between 11 and 29 years old. Exclusions criteria: presence of a concomitant Axis I disorder; alcohol or other substance abuse; a significant neurological or medical illness, or a history of head trauma resulting in loss of consciousness for over one hour; previous psychotic episode; substance‐induced psychotic symptoms (ICD‐10: F10) (25); being unable to complete or to perform some of the different evaluations included in the protocol. Study aim: "to examine regional GM volume in adolescents and young adults with a first episode of psychosis, and, within this sample, to compare patients with affective psychoses and schizophrenia. A second objective was to assess the extent to which volumetric MRI measures at first presentation (in the whole sample) predicted subsequent clinical outcome". Clinical setting: outpatients. Country: Spain. |
||
| Index tests |
Test conducted by: not reported.
Cut‐off points used: not reported.
Type of scanner: 1.5 Tesla Siemens Symphony Maestro Class Imaging System (Erlangen, Germany) MRI parameters "The voxel dimensions were 1.0 x 1.0 x 1.5 mm3; matrix size 256 x 256; field of view (FOV): 256 x 256 mm2; band width 230 Hz ⁄ pixel; time‐to‐repetition (TR): 1,810 msec; time‐to‐echo (TE): 2.39 msec, inversion time 1.1 msec; flip angle 20; echo time 4.6 msec; slice thickness 2 mm." Pre‐processing steps
Use of covariates in the General Linear Model: age, gender and total grey matter. |
||
| Target condition and reference standard(s) | Reference standard: "Diagnoses were made on the basis of all the clinical information available at follow‐up, according to the ICD‐10 and DSM‐IV criteria." Operational criteria used: DSM‐IV and ICD‐10. Target condition: schizophrenia, first episode. Conducted by: not reported. | ||
| Flow and timing |
Study process: At baseline, all subjects were scanned and had a clinical assessment. The clinical assessment was repeated three years later, and included a clinical interview using various scales (K‐SADS‐PL, PANSS, HDRS, CGI, DSM‐IV (GAF). Diagnoses were made on the basis of all the clinical information available at follow‐up, according to the ICD‐10 and DSM‐IV criteria. Follow up: 3 years, follow‐up data were obtained from 22 (79%) of the 28 patients: two had died from suicide during the follow‐up period, and 4 declined to be re‐assessed. |
||
| Comparative | |||
| Notes | Also included a healthy control group, which we did not consider for this review. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Were participants undiagnosed at entry to study? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Did the study pre‐specify cut‐offs for VBM? | No | ||
| Was imaging pre‐processing carried out? | Yes | ||
| Were images inspected for motion artefacts? | Unclear | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were missing or uninterpretable results reported? | Yes | ||
| Low | |||
Kubicki 2002.
| Study characteristics | |||
| Patient sampling | Prospective, not reported if consecutive: "Patients who were entering the hospital for the first time with psychosis or were being transferred or readmitted within 8 months of the first hospitalisation for psychosis were recruited." | ||
| Patient characteristics and setting |
N of participants: 32.
N analysed: 32. Age: 26 years (SD 7.5) schizophrenic group; 23.7 (SD 4) years affective psychosis group. Gender: 27 M, 5 F. Ethnicity: not reported. Comorbid disorders: not reported. Duration of symptoms: not reported. Concurrent medications used: not reported. The median duration of treatment with any psychotropic medication before scanning was 1.7 months for the schizophrenic patients and 0 months for the patients with affective disorder. Inclusion criteria: age 18 to 55 years; INQ above 75; right‐handedness; no lifetime history of alcohol or drug dependence. Exclusions criteria: history of seizures, head trauma with loss of consciousness, and neurological disorder. Study aim: To evaluate VBM as a more efficient and complete method than ROI (regions of interest) for characterisation of grey matter abnormalities in patients with first‐episode schizophrenia. Secondary aim was to compare and validate VBM with ROI analysis by using patients who had been previously investigated using ROI methodology. Clinical setting: inpatients. Country: USA. |
||
| Index tests |
Test conducted by: not reported.
Cut‐off points used: not reported.
Type of scanner: 1.5‐T General Electric scanner (GE Medical Systems, Milwaukee). MRI parameters "A series of 124 contiguous coronal images were acquired using an SPGR sequence with the following parameters: TR, 35 ms; TE, 5 ms; 45° flip angle; 24‐cm field of view; NEX, 1.0 (number of excitations); matrix, 256 x 256 (192 phase‐encoding steps). The voxel (volume of pixel) dimensions were 0.9375 x 0.9375 x 1.5 mm." Pre‐processing steps
Use of covariates in the General Linear Model: global grey matter intensity (global normalisation). |
||
| Target condition and reference standard(s) | Reference standard: "Diagnoses were based on the Structured Clinical Interview for DSM‐III‐R (SCID), review of hospital course, and medical records." Operational criteria used: DSM‐III‐R. Target condition: schizophrenia, first episode. Conducted by: not reported. | ||
| Flow and timing |
Study process: Diagnoses were based on the Structured Clinical Interview for DSM‐III‐R (SCID), review of hospital course, and medical records. Patients were scanned using MRI, these scans were analysed previously using ROI and were re‐analysed using VBM. "Of the 33 patients scanned, 29 received an MRI within 3 weeks of entering McLean Hospital, and the remaining four (two with schizophrenia, two with affective disorder) returned after discharge for imaging (15 and 22 months for schizophrenia, respectively, and 9 and 18 months for affective disorder)." Follow up: three weeks to 22 months. |
||
| Comparative | |||
| Notes | Also included a healthy control group, which we did not consider for this review. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Were participants undiagnosed at entry to study? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Did the study pre‐specify cut‐offs for VBM? | No | ||
| Was imaging pre‐processing carried out? | Yes | ||
| Were images inspected for motion artefacts? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were missing or uninterpretable results reported? | Yes | ||
| Low | |||
Morgan 2007.
| Study characteristics | |||
| Patient sampling | Prospective and consecutive: patients "presenting consecutively for the first time to local psychiatric services (in‐patient and outpatient) between 1997 and 2000 with symptoms meeting functional psychosis". "Only patients meeting criteria for a narrow definition of schizophrenia (ICD–10 F20) were included in the ‘schizophrenia’ group. Patients diagnosed with bipolar disorder or depressive psychosis were allocated to the ‘affective psychosis’ group (ICD–10 F30–39). To ensure the diagnostic homogeneity of the two groups, patients with schizoaffective disorder were excluded from either group." | ||
| Patient characteristics and setting |
N of participants: 97.
N analysed: 73. Age: mean age 27.1 years (SD 7.6). Gender: not reported. Ethnicity: white British patients 27 (37%), other ethnicities not reported. Comorbid disorders: not reported. Duration of symptoms: see notes. Concurrent medications used: Not reported. Inclusion criteria: age 16–65 years; resident in defined area; presenting consecutively for the first time to local psychiatric services (in‐patient and outpatient) between 1997 and 2000 with symptoms meeting functional psychosis criteria (ICD–10: F20 Schizophrenia and F30–39 Affective disorders – psychotic codings; World Health Organization, 1992). Exclusions criteria: head trauma history with 41‐hour unconsciousness; central nervous system disease; poor English fluency; transient psychotic symptoms resulting from acute intoxication (ICD–10) following consumption of psychoactive substance. Study aim: To determine which brain abnormalities are specific to schizophrenia and affective psychosis. Clinical setting: inpatients and outpatients. Country: UK. |
||
| Index tests |
Test conducted by: not reported.
Cut‐off points used: not reported.
Type of scanner: GE Signa 1.5–T system MRI parameters "Contiguous, interleaved proton‐density and T2‐weighted 3mm thick coronal plane dual‐echo images were acquired, providing whole brain coverage. A repetition time of 4000ms and effective echo times of 20ms and 85ms were used with 8‐echo train length. Matrix size was 256x192, collected from a rectangular field‐of‐view of 22 cm x 616.5 cm, giving an in‐plane resolution of 0.859mm. Total acquisition time was 10min, 12s." Pre‐processing steps
Use of covariates in the General Linear Model: not reported. |
||
| Target condition and reference standard(s) | Reference standard: "Patients were interviewed using the WHO Schedules for Clinical Assessment in Neuropsychiatry (WHO–SCAN). ICD–10 diagnoses were made in consensus meetings with senior clinicians, using WHO–SCAN information and clinical notes." Operational criteria used: ICD‐10. Target condition: schizophrenia, first episode. Conducted by: senior clinicians. | ||
| Flow and timing |
Study process: "Patients were interviewed using the WHO Schedules for Clinical Assessment in Neuropsychiatry (WHO–SCAN) (World Health Organization, 1994). ICD–10 diagnoses were made in consensus meetings with senior clinicians using WHO–SCAN information and notes." Scans were acquired, timing not reported.
"9 [patients] did not complete the full scanning procedure and therefore were not included in the analysis. Fifteen further scans were excluded due to (a) subject motion n=13; (b) congenital hydrocephalus n=1; (c) subarachnoid cyst n=1." Follow up: not reported. |
||
| Comparative | |||
| Notes | Also included a healthy control group, which we did not consider for this review. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Were participants undiagnosed at entry to study? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| Did the study pre‐specify cut‐offs for VBM? | No | ||
| Was imaging pre‐processing carried out? | Unclear | ||
| Were images inspected for motion artefacts? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Were missing or uninterpretable results reported? | Yes | ||
| Low | |||
Tordesillas‐Gutierrez 2010.
| Study characteristics | |||
| Patient sampling | Prospective, not reported if consecutive: "sample (118) of first episode psychosis patients". | ||
| Patient characteristics and setting |
N of participants: 118.
N analysed: 118. Age: not reported. Gender: not reported. Ethnicity: not reported. Comorbid disorders: not reported. Duration of symptoms: not reported. Concurrent medications used: not reported. Inclusion criteria: first episode psychosis patients. Exclusions criteria: not reported. Study aim: to study whether there is a tendency towards reduction in grey matter in schizophrenia patients, and to analyse the differences between schizophrenia and schizophreniform disorders. Clinical setting: not reported. Country: Spain. |
||
| Index tests |
Test conducted by: not reported.
Cut‐off points used: not reported.
Type of scanner: 1.5 T GE scanner MRI parameters "All MRI were acquired in a 1.5 T GE scanner; SPGR sequence (TE=5 ms, TR=24 ms, slice thickness=1.5 mm)." Pre‐processing steps
Use of covariates in the General Linear Model: age, gender and intracranial volume. |
||
| Target condition and reference standard(s) | Reference standard: "an independent psychiatrist confirmed diagnosis using the SCID‐I." Operational criteria used: DSM‐IV. Target condition: schizophrenia, first episode. Conducted by: psychiatrist. | ||
| Flow and timing |
Study process: 118 patients with a first episode psychosis underwent an MRI scan in the first weeks of inclusion in the PAFIP program. At 6 months of inclusion an independent psychiatrist confirmed diagnosis using the SCID‐I. Follow up: 6 months. |
||
| Comparative | |||
| Notes | Also included a healthy control group, which we did not consider for this review. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Were participants undiagnosed at entry to study? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Did the study pre‐specify cut‐offs for VBM? | No | ||
| Was imaging pre‐processing carried out? | Unclear | ||
| Were images inspected for motion artefacts? | Unclear | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Were missing or uninterpretable results reported? | Yes | ||
| Low | |||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ananth 2002 | Participants with schizophrenia compared to healthy controls. |
| Bangalore 2008 | Participants with first episode schizophrenia compared to healthy controls. |
| Bangalore 2009 | Participants with first episode psychosis, no comparison between diagnostic groups (correlation study with cognition). |
| Benedetti 2010 | Participants with chronic schizophrenia. |
| Berge 2011 | Participants with first episode psychosis compared to healthy controls. |
| Bodnar 2012 | Participants with first episode schizophrenia only. |
| Borgwardt 2010 | Participants with first episode psychosis compared to people with an at‐risk mental state and healthy controls. |
| Borgwardt 2010a | Participants with first episode psychosis compared to people with an at‐risk mental state and healthy controls. |
| Brown 2011 | Participants with chronic schizophrenia and bipolar disorder. |
| Cascella 2010 | Participants with schizophrenia compared to healthy controls. |
| Chaim 2010 | Participants with first episode psychosis compared to healthy controls. |
| Chow 2011 | Participants with 22q11.2 deletion syndrome, psychotic and non‐psychotic subgroups compared. |
| Chua 2007 | Participants with first episode psychosis compared to healthy controls. |
| Colombo 2012 | Participants with first episode psychosis. Only white matter structure compared. |
| Cui 2010 | Participants with chronic schizophrenia and bipolar disorder. |
| Cui 2011 | Participants with chronic schizophrenia and bipolar disorder. |
| Dazzan 2012 | Participants at ultra‐high risk of schizophrenia. |
| de Castro‐Manglano 2011b | Participants with first episode psychosis. Measured longitudinal changes, not cross‐sectional group comparisons. |
| Deng 2011 | Participants with first episode psychosis compared to healthy controls. |
| Douaud 2009 | Participants with schizophrenia compared to healthy controls. |
| Eack 2010 | Participants with early stage schizophrenia or schizoaffective disorder, no comparison between diagnostic groups (randomised control trial testing cognitive enhancement therapy versus enriched support therapy). |
| Ellison‐Wright 2010 | Meta‐analysis. |
| Fornito 2009 | Meta‐analysis. |
| Heuser 2011 | Participants with first episode psychosis, no comparison between diagnostic groups (correlation study with cognition). |
| Horacek 2010 | Participants with schizophrenia compared to healthy controls. |
| Huang 2009 | Participants with first episode psychosis compared to healthy controls. |
| Hulshoff 2001 | Participants with schizophrenia compared to healthy controls. |
| Ivleva 2012 | Participants with chronic psychosis. |
| James 2011 | Participants with adolescent onset schizophrenia compared to healthy controls. |
| Janssen 2008 | Participants with first episode psychosis compared with healthy controls. |
| Janssen 2009 | Participants with first episode psychosis, no comparison between diagnostic groups (correlation study with cognition). |
| Job 2003 | Participants with first episode schizophrenia compared to people at high risk of schizophrenia and healthy controls. |
| Job 2005 | Participants at high risk of schizophrenia compared to healthy controls. |
| Kawasaki 2004 | Participants with chronic schizophrenia or schizotypal disorder. |
| Lebedev 2010 | Participants with depression. |
| Lu 2011 | Participants with first episode psychosis. Only white matter structure compared using diffusion tensor imaging. |
| Lui 2009 | Participants with first episode schizophrenia compared with healthy controls. |
| Lui 2009a | Participants with first episode schizophrenia compared with healthy controls. |
| Malchow 2010 | Participants with first episode schizophrenia only. |
| Malla 2011 | Participants with first episode psychosis, no comparison with between diagnostic groups (compares length of duration of undiagnosed psychosis). |
| Mane 2009 | Participants with first episode psychosis compared with healthy controls. |
| Maric 2003 | Participants with schizophrenia compared to healthy controls. |
| McIntosh 2004 | Participants with schizophrenia, bipolar disorder and at high risk compared to healthy controls. |
| McIntosh 2006 | Participants with schizophrenia, bipolar disorder and at high risk compared to healthy controls. |
| McIntosh 2009 | Participants with schizophrenia, bipolar disorder and at high risk compared to healthy controls. |
| Meda 2008 | Participants with schizophrenia (first episode and chronic) compared to healthy controls. |
| Meijer 2009 | Participants at ultra‐high risk for psychosis. |
| Meijer 2010 | Participants at ultra‐high risk for psychosis. |
| Meijer 2011 | Participants at ultra‐high risk for psychosis. |
| Melonakos 2011 | Meta‐analysis. |
| Minatogawa‐Chang 2009 | Participants with first episode psychosis, no comparison between diagnostic groups (correlation study with cognition). |
| Modinos 2009 | Participants with schizophrenia only. |
| Molina 2010 | Participants with schizophrenia only. |
| Molina 2010a | Participants with first episode psychosis compared to healthy controls. |
| Molina 2010b | Participants with chronic schizophrenia and bipolar disorder. |
| Molina 2011 | Participants with chronic schizophrenia and bipolar disorder. |
| Moorhead 2004 | Participants with schizophrenia (with and without comorbid learning disability), people with learning disability and health controls. |
| Mouchet‐Mages 2011 | Participants with first episode psychosis, no comparison between diagnostic groups (correlation study with cognition). |
| Nenadic 2010 | Participants with schizophrenia only. |
| Palaniyappan 2011 | Participants with chronic psychosis. |
| Perez‐Iglesias 2010 | Participants with first episode psychosis. Only white matter structure compared. |
| Potvin 2007 | Participants with schizophrenia (with and without substance abuse disorders) compared to healthy controls. |
| Roman‐Urrestarazu 2011 | Participants at clinical risk, family risk and healthy controls. |
| Rosa 2012 | Participants with first episode psychosis, no comparison between diagnostic groups (correlation study with cognition). |
| Ruef 2012 | Participants with first episode psychosis compared to healthy controls |
| Rund 2004 | Participants with chronic schizophrenia and depression. |
| Salgado‐Pineda 2004 | Narrative review. |
| Salgado‐Pineda 2011 | Schizophrenia compared to healthy controls only. |
| Schiffer 2010 | Participants with chronic schizophrenia. |
| Schiffer 2010a | Participants with schizophrenia, with and without comorbid substance abuse. |
| Schiffer 2010b | Participants with schizophrenia compared to healthy controls. |
| Schnack 2014 | Participants with chronic schizophrenia, bipolar disorder and healthy controls. |
| Segall 2009 | Participants with schizophrenia (first episode and chronic) compared to healthy controls. |
| Situ 2010 | Participants with schizophrenia compared to healthy controls. |
| Stanfield 2009 | Participants with bipolar disorder compared to healthy controls. |
| Sussmann 2009 | Participants with schizophrenia and bipolar disorder. Only white matter structure compared. |
| Suzuki 2010 | Participants with first episode schizophrenia compared to healthy controls. |
| Tandon 2012 | Adolescent relatives of schizophrenic patients and healthy controls. |
| Trost 2011 | Participans with schizophrenia, bipolar disorder, obsessive compulsive disorder and healthy controls. |
| Walter 2012 | Participants with an at‐risk mental state. |
| Wang 2009 | Participants with schizophrenia compared to healthy controls. |
| Watson 2012 | Participants with first episode psychosis compared to healthy controls. |
| Witthaus 2008 | Participants with first episode schizophrenia, ultra‐high risk for schizophrenia and healthy controls. Only white matter structure compared. |
| Witthaus 2009 | Participants with first episode schizophrenia, ultra‐high risk for schizophrenia and healthy controls. |
| Wojtalik 2013 | Participants with early course schizophrenia, no comparison between diagnostic groups (correlation study with emotional intelligence). |
| Yüksel 2010 | Participants with schizophrenia, bipolar disorder and healthy controls. Not first episode. |
| Yüksel 2012 | Participants with schizophrenia, bipolar disorder and healthy controls. Not first episode. |
Contributions of authors
Lena Palaniyappan ‐ wrote protocol, screening, data extraction, wrote review.
Nicola Maayan ‐ helped draft protocol, screening, data extraction, wrote review.
Hanna Bergman ‐ helped write review, coordinated final stages of review.
Clare Davenport ‐ helped guide and draft protocol, commented on final review.
Clive E Adams ‐ gained funding, helped draft protocol and review.
Karla Soares‐Weiser ‐ led project, helped draft protocol, data extraction and commented on review.
Sources of support
Internal sources
-
University of Nottingham, UK.
Salary and logistics support for Dr. Palaniyappan and Prof. Adams.
-
University of Birmingham, UK.
Salary and logistics support for Dr. Davenport.
External sources
-
NIHR Cochrane Programme Grant 2011, Reference number: 10/4001/15, UK.
Salary support for Nicola Maayan, Hanna Bergman and Karla Soares‐Weiser.
Declarations of interest
LP received a Young Investigator Award sponsored by Eli Lilly in 2010 at the 9th Bipolar Disorder Meeting, Pittsburgh. No other conflict of interest declared.
NM, HB and KSW currently work for Enhance Reviews Ltd, a company that carries out systematic reviews mostly for the public sector, it currently does not provide services for the pharmaceutical industry.
CD and CEA ‐ none known.
New
References
References to studies included in this review
de Castro‐Manglano 2011 {published data only}
- Castro‐Manglano P, Mechelli A, Soutullo C, Landecho I, Gimenez‐Amaya JM, Ortuno F, et al. Structural brain abnormalities in first‐episode psychosis: differences between affective psychoses and schizophrenia and relationship to clinical outcome. Bipolar Disorders 2011;13:545‐55. [DOI] [PubMed] [Google Scholar]
Kubicki 2002 {published data only}
- Hirayasu Y, Shenton M, Salisbury D. Lower left temporal lobe MRI volumes in patients with first‐episode schizophrenia compared with psychotic patients with first‐episode affective disorder and normal subjects. American Journal of Psychiatry 1998;155(10):1384‐91. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, et al. Voxel‐based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 2002;17(4):1711‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2007 {published data only}
- Morgan KD, Dazzan P, Orr KG, Hutchinson G, Chitnis X, Suckling J, et al. Grey matter abnormalities in first‐episode schizophrenia and affective psychosis. British Journal of Psychiatry 2007;191:s111‐6. [DOI] [PubMed] [Google Scholar]
Tordesillas‐Gutierrez 2010 {published data only}
- Tordesillas‐Gutierrez D, Koutsouleris N, Roiz‐Santianez R, Perez‐Iglesias R, Meisenzahl E, Lucas EM, et al. Grey matter reduction in first‐episode psychosis: Relationship to diagnosis. Schizophrenia Research 2010;Conference: 2nd Schizophrenia International Research Society Conference, SIRS 2010 Florence Italy. Conference Start: 20100410 Conference End: 20100414. Conference Publication:346‐7. [Google Scholar]
References to studies excluded from this review
Ananth 2002 {published data only}
- Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RSJ, Dolan RJ. Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel‐based morphometry. American Journal of Psychiatry 2002;159:1497‐505. [DOI] [PubMed] [Google Scholar]
Bangalore 2008 {published data only}
- Bangalore SS, Prasad KM, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia‐‐A region of interest, voxel based morphometric study. Schizophrenia Research 2008;99:1‐6. [DOI] [PubMed] [Google Scholar]
Bangalore 2009 {published data only}
- Bangalore Srihari S, Goradia Dhruman D, Nutche Jeffrey, Diwadkar Vaibhav A, Prasad Konasale M R, Keshavan Matcheri S. Untreated illness duration correlates with gray matter loss in first‐episode psychoses. Neuroreport 2009;20:729‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benedetti 2010 {published data only}
- Benedetti F, Poletti S, Radaelli D, Bernasconi A, Cavallaro R, Falini A, et al. Temporal lobe grey matter volume in schizophrenia is associated with a genetic polymorphism influencing glycogen synthase kinase 3‐beta activity. Genes, Brain, & Behavior 2010;9:365‐71. [DOI] [PubMed] [Google Scholar]
Berge 2011 {published data only}
- Berge D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O. Gray matter volume deficits and correlation with insight and negative symptoms in first‐psychotic‐episode subjects. Acta Psychiatrica Scandinavica 2011;123:431‐9. [DOI] [PubMed] [Google Scholar]
Bodnar 2012 {published data only}
- Bodnar M, Malla AK, Joober R, Lord C, Smith E, Pruessner J, et al. Neural markers of early remission in first‐episode schizophrenia: A volumetric neuroimaging study of the parahippocampus. Psychiatry Research ‐ Neuroimaging 2012;201:40‐7. [DOI] [PubMed] [Google Scholar]
Borgwardt 2010 {published data only}
- Borgwardt SJ, Smieskova R, Aston J, Gschwandtner U, Pflueger M, Stieglitz RD, et al. Structural and neural abnormalities in individuals with an at‐risk mental state of psychosis. Early Intervention in Psychiatry 2010;Conference: 7th International Conference on Early Psychosis ‐ Early Psychoses: A Lifetime Perspective Amsterdam Netherlands. Conference Start: 20101129 Conference End: 20101201. Conference Publication: (var.pagings). 4:31. [Google Scholar]
Borgwardt 2010a {published data only}
- Borgwardt S. Structural and neurofunctional abnormalities in the At‐Risk Mental State (ARMS) of psychosis. Schizophrenia Research 2010;Conference: 2nd Schizophrenia International Research Society Conference, SIRS 2010 Florence Italy. Conference Start: 20100410 Conference End: 20100414. Conference Publication:112‐3. [Google Scholar]
Brown 2011 {published data only}
- Brown GG, Lee JS, Strigo IA, Caligiuri MP, Meloy MJ, Lohr J. Voxel‐based morphometry of patients with schizophrenia or bipolar I disorder: a matched control study. Psychiatry Research 2011;194(2):149‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cascella 2010 {published data only}
- Cascella NG, Fieldstone SC, Rao VA, Pearlson GD, Sawa A, Schretlen DJ. Gray‐matter abnormalities in deficit schizophrenia. Schizophrenia Research 2010;120(1‐3):63‐70. [DOI] [PubMed] [Google Scholar]
Chaim 2010 {published data only}
- Chaim TM, Schaufelberger MS, Ferreira LK, Duran FL, Ayres AM, Scazufca M, et al. Volume reduction of the corpus callosum and its relationship with deficits in interhemispheric transfer of information in recent‐onset psychosis. Psychiatry Research 2010;184(1):1‐9. [DOI] [PubMed] [Google Scholar]
Chow 2011 {published data only}
- Chow EW1, Ho A, Wei C, Voormolen EH, Crawley AP, Bassett AS. Association of schizophrenia in 22q11.2 deletion syndrome and gray matter volumetric deficits in the superior temporal gyrus.[Erratum appears in Am J Psychiatry. 2011 May;168(5):553]. American Journal of Psychiatry 2011;168(5):522‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chua 2007 {published data only}
- Chua SE, Cheung C, Cheung V, Tsang JT, Chen EY, Wong JC, et al. Cerebral grey, white matter and csf in never‐medicated, first‐episode schizophrenia. Schizophrenia Research 2007;89(1‐3):12‐21. [DOI] [PubMed] [Google Scholar]
Colombo 2012 {published data only}
- Colombo RR, Schaufelberger MS, Santos LC, Duran FL, Menezes PR, Scazufca M, et al. Voxelwise evaluation of white matter volumes in first‐episode psychosis. Psychiatry Research 2012;202(3):198‐205. [DOI] [PubMed] [Google Scholar]
Cui 2010 {published data only}
- Cui LQ, Deng W, Jiang LJ, Huang CH, Chen ZF, Li ML, et al. [A comparative study of voxel‐based morphometry in patients with paranoid schizophrenia and bipolar mania]. Sichuan da Xue Xue Bao. Yi Xue Ban/Journal of Sichuan University. Medical Science Edition 2010;41(1):5‐9. [PubMed] [Google Scholar]
Cui 2011 {published data only}
- Cui L, Li M, Deng W, Guo W, Ma X, Huang C, et al. Overlapping clusters of gray matter deficits in paranoid schizophrenia and psychotic bipolar mania with family history. Neuroscience Letters 2011;489(2):94‐8. [DOI] [PubMed] [Google Scholar]
Dazzan 2012 {published data only}
- Dazzan P, Soulsby B, Mechelli A, Wood SJ, Velakoulis D, Phillips LJ, et al. Volumetric abnormalities predating the onset of schizophrenia and affective psychoses: An MRI study in subjects at ultrahigh risk of psychosis. Schizophrenia Bulletin 2012;38:1083‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Castro‐Manglano 2011b {published data only}
- Castro‐Manglano P, Mechelli A, Soutullo C, Gimenez‐Amaya J, Ortuño F, McGuire P. Longitudinal changes in brain structure following the first episode of psychosis. Psychiatry Research 2011;191(3):166‐73. [DOI] [PubMed] [Google Scholar]
Deng 2011 {published data only}
- Deng W, Zou L, Cui L, Huang C, Chen Z, Li M, et al. Study of brain asymmetries in sporadic and familial first episode patients with schizophrenia. European Psychiatry. Conference: 19th European Congress of Psychiatry, EPA. 2011; Vol. 20110312.
Douaud 2009 {published data only}
- Douaud G, Mackay C, Andersson J, James S, Quested D, Ray MK, et al. Schizophrenia delays and alters maturation of the brain in adolescence. Brain: A Journal of Neurology 2009;132:2437‐48. [DOI] [PubMed] [Google Scholar]
Eack 2010 {published data only}
- Eack SM, Hogarty GE, Cho RY, Prasad KMR, Greenwald DP, Hogarty SS, et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2‐year randomized controlled trial. Archives of General Psychiatry 2010;67:674‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellison‐Wright 2010 {published data only}
- Ellison‐Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta‐analysis. Schizophrenia Research 2010;117:1‐12. [DOI] [PubMed] [Google Scholar]
Fornito 2009 {published data only}
- Fornito A, Yucel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel‐based morphometry studies. Schizophrenia Research 2009;108:104‐13. [DOI] [PubMed] [Google Scholar]
Heuser 2011 {published data only}
- Heuser M, Thomann PA, Essig M, Bachmann S, Schroder J. Neurological signs and morphological cerebral changes in schizophrenia: An analysis of NSS subscales in patients with first episode psychosis. Psychiatry Research 2011;192:69‐76. [DOI] [PubMed] [Google Scholar]
Horacek 2010 {published data only}
- Horacek J, Flegr J, Tintera J, Novak T, Brunovsky M, Bubenikova‐Valesova V, et al. Latent toxoplasmosis reduces gray matter density in schizophrenia but not in controls. Voxel‐Based‐Morphometry (VBM) study. Neuropsychopharmacology 2010;35:S138. [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang CH, Deng W, Chen ZF, Li ML, Lu S, Jiang LJ, et al. [Brain structure abnormality as genetic endophenotype of schizophrenia]. Chung‐Hua i Hsueh i Chuan Hsueh Tsa Chih 2009;26(5):490‐4. [DOI] [PubMed] [Google Scholar]
Hulshoff 2001 {published data only}
- Hulshoff Pol HE, Schnack HG, Mandl RC, Haren NE, Koning H, Collins DL, et al. Focal gray matter density changes in schizophrenia. Archives of General Psychiatry 2001;58(12):1118‐25. [DOI] [PubMed] [Google Scholar]
Ivleva 2012 {published data only}
- Ivleva EI, Bidesi AS, Thomas BP, Meda SA, Francis A, Moates AF, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Research ‐ Neuroimaging 2012;204:13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2011 {published data only}
- James A, Hough M, James S, Winmill L, Burge L, Nijhawan S, et al. Greater white and grey matter changes associated with early cannabis use in adolescent‐onset schizophrenia (AOS). Schizophrenia Research 2011;128:91‐7. [DOI] [PubMed] [Google Scholar]
Janssen 2008 {published data only}
- Janssen J, Reig S, Parellada M, Moreno D, Graell M, Fraguas D, et al. Regional gray matter volume deficits in adolescents with first‐episode psychosis. Journal of the American Academy of Child & Adolescent Psychiatry 2008;47:1311‐20. [DOI] [PubMed] [Google Scholar]
Janssen 2009 {published data only}
- Janssen J, Diaz‐Caneja A, Reig S, Bombin I, Mayoral M, Parellada M, et al. Brain morphology and neurological soft signs in adolescents with first‐episode psychosis. British Journal of Psychiatry 2009;195:227‐33. [DOI] [PubMed] [Google Scholar]
Job 2003 {published data only}
- Job DE, Whalley C, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel‐based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophrenia Research 2003;64:1‐13. [DOI] [PubMed] [Google Scholar]
Job 2005 {published data only}
- Job DE, Whalley HC, Johnstone Eve C, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage 2005;25:1023‐30. [DOI] [PubMed] [Google Scholar]
Kawasaki 2004 {published data only}
- Kawasaki Y, Suzuki M, Nohara S, Hagino H, Takahashi T, Matsui M, et al. Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel‐based morphometry. European Archives of Psychiatry & Clinical Neuroscience 2004;254:406‐14. [DOI] [PubMed] [Google Scholar]
Lebedev 2010 {published data only}
- Lebedev A, Korzenev A, Abritalin E, Tarumov D, Fokin V, Sokolov A. Perspectives of using neuroimaging methods in differential diagnosis of depression. European Psychiatry. Conference: 18th European Congress of Psychiatry Munich Germany. Conference Start. 2010; Vol. 20100227.
Lu 2011 {published data only}
- Lu LH, Zhou XJ, Keedy SK, Reilly JL, Sweeney JA. White matter microstructure in untreated first episode bipolar disorder with psychosis: comparison with schizophrenia. Bipolar Disorders 2011;13:604‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lui 2009 {published data only}
- Lui S, Deng W, Huang X, Jiang L, Ouyang L, Borgwardt SJ, et al. Neuroanatomical differences between familial and sporadic schizophrenia and their parents: an optimized voxel‐based morphometry study. Psychiatry Research 2009;171(2):71‐81. [DOI] [PubMed] [Google Scholar]
Lui 2009a {published data only}
- Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, et al. Association of cerebral deficits with clinical symptoms in antipsychotic‐naive first‐episode schizophrenia: an optimized voxel‐based morphometry and resting state functional connectivity study. [Erratum appears in Am J Psychiatry. 2009 Feb;166(2):237]. American Journal of Psychiatry 2009;166(2):196‐205. [DOI] [PubMed] [Google Scholar]
Malchow 2010 {published data only}
- Malchow B, Falkai P, Gruber O, Reith W, Wobrock T. No volumetric brain differences between first‐episode schizophrenia patients with and without cannabis abuse. European Archives of Psychiatry and Clinical Neuroscience 2010;Conference: 3rd Meeting of West European Societies of Biological Psychiatry Berlin Germany. Conference Start: 20100602 Conference End: 20100604. Conference Publication: (var.pagings). 260:S66. [Google Scholar]
Malla 2011 {published data only}
- Malla AK, Bodnar M, Joober R, Lepage M. Duration of untreated psychosis is associated with orbital‐frontal grey matter volume reductions in first episode psychosis. Schizophrenia Research 2011;125:13‐20. [DOI] [PubMed] [Google Scholar]
Mane 2009 {published data only}
- Mane A, Falcon C, Mateos JJ, Fernandez‐Egea E, Horga G, Lomena F, et al. Progressive gray matter changes in first episode schizophrenia: a 4‐year longitudinal magnetic resonance study using VBM. Schizophrenia Research 2009;114:136‐43. [DOI] [PubMed] [Google Scholar]
Maric 2003 {published data only}
- Maric N, Kamer T, Schneider AT, Dani I, Jasovic GM, Paunovic VR, et al. [Volumetric analysis of gray matter, white matter and cerebrospinal fluid space in schizophrenia]. Srpski Arhiv Za Celokupno Lekarstvo 2003;131:26‐30. [DOI] [PubMed] [Google Scholar]
McIntosh 2004 {published data only}
- McIntosh AM, Job DE, Moorhead TWJ, Harrison LK, Forrester K, Lawrie SM, et al. Voxel‐based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biological Psychiatry 2004;56(8):544‐52. [DOI] [PubMed] [Google Scholar]
McIntosh 2006 {published data only}
- McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, et al. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics 2006;141B:76‐83. [DOI] [PubMed] [Google Scholar]
McIntosh 2009 {published data only}
- McIntosh AM. Susceptibility gene variants for schizophrenia and bipolar disorder associated with disrupted functional and structural connectivity. Biological Psychiatry 2009;Conference: 64th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry Vancouver, BC Canada. Conference Start: 20090514 Conference End: 20090516. Conference Publication:14S. [Google Scholar]
Meda 2008 {published data only}
- Meda SA, Giuliani NR, Calhoun VD, Jagannathan K, Schretlen DJ, Pulver A, et al. A large scale (N=400) investigation of gray matter differences in schizophrenia using optimized voxel‐based morphometry. Schizophrenia Research 2008;101:95‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meijer 2009 {published data only}
- Meijer JH, Schmitz N, Nieman DH, Amelsvoort TAMJ, Becker HE, Haan L, et al. The neuroanatomy of semantic verbal fluency deficits in individuals at ultra high risk for psychosis: implications for prediction. Biological Psychiatry 2009;Conference: 64th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry Vancouver, BC Canada. Conference Start: 20090514 Conference End: 20090516. Conference Publication:5S. [Google Scholar]
Meijer 2010 {published data only}
- Meijer JH, Schmitz N, Nieman DH, Haan L, Becker HE, Linszen DH. Semantic fluency deficits and grey matter differences before transition to psychosis: A voxelwise correlational analysis. Schizophrenia Research 2010;Conference: 2nd Schizophrenia International Research Society Conference, SIRS 2010 Florence Italy. Conference Start: 20100410 Conference End: 20100414. Conference Publication:340. [Google Scholar]
Meijer 2011 {published data only}
- Meijer JH, Schmitz N, Nieman DH, Becker HE, Amelsvoort TA, Dingemans PM, et al. Semantic fluency deficits and reduced grey matter before transition to psychosis: A voxelwise correlational analysis. Psychiatry Research: Neuroimaging 2011;194:1‐6. [DOI] [PubMed] [Google Scholar]
Melonakos 2011 {published data only}
- Melonakos ED, Shenton ME, Rathi Y, Terry DP, Bouix S, Kubicki M. Voxel‐based morphometry (VBM) studies in schizophrenia‐can white matter changes be reliably detected with VBM?. Psychiatry Research 2011;193:65‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Minatogawa‐Chang 2009 {published data only}
- Minatogawa‐Chang TM, Schaufelberger MS, Ayres AM, Duran FLS, Gutt EK, Murray RM, et al. Cognitive performance is related to cortical grey matter volumes in early stages of schizophrenia: a population‐based study of first‐episode psychosis. Schizophrenia Research 2009;113:200‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Modinos 2009 {published data only}
- Modinos G, Vercammen A, Mechelli A, Knegtering H, McGuire PK, Aleman A. Structural covariance in the hallucinating brain: a voxel‐based morphometry study. Journal of Psychiatry & Neuroscience 2009;34:465‐9. [PMC free article] [PubMed] [Google Scholar]
Molina 2010 {published data only}
- Molina V, Hernandez JA, Sanz J, Paniagua JC, Hernandez AI, Martin C, et al. Subcortical and cortical gray matter differences between Kraepelinian and non‐Kraepelinian schizophrenia patients identified using voxel‐based morphometry. Psychiatry Research 2010;184:16‐22. [DOI] [PubMed] [Google Scholar]
Molina 2010a {published data only}
- Molina V, Sanz J, Villa R, Perez J, Gonzalez D, Sarramea F, et al. Voxel‐based morphometry comparison between first episodes of psychosis with and without evolution to schizophrenia. Psychiatry Research 2010;181:204‐10. [DOI] [PubMed] [Google Scholar]
Molina 2010b {published data only}
- Molina V, Galindo G, Cortes B, Hernandez JA. Voxel‐based morphometry comparison between chronic schizophrenia and bipolar patients and healthy controls. Schizophrenia Research 2010;Conference: 2nd Schizophrenia International Research Society Conference, SIRS 2010 Florence Italy. Conference Start: 20100410 Conference End: 20100414. Conference Publication:341. [Google Scholar]
Molina 2011 {published data only}
- Molina V, Galindo G, Cortés B, Herrera AG, Ledo A, Sanz J, et al. Different gray matter patterns in chronic schizophrenia and chronic bipolar disorder patients identified using voxel‐based morphometry. European Archives of Psychiatry & Clinical Neuroscience 2011;261(5):313‐22. [DOI] [PubMed] [Google Scholar]
Moorhead 2004 {published data only}
- Moorhead TW, Job DE, Whalley HC, Sanderson TL, Johnstone EC, Lawrie SM. Voxel‐based morphometry of comorbid schizophrenia and learning disability: analyses in normalized and native spaces using parametric and nonparametric statistical methods. Neuroimage 2004;22(1):188‐202. [DOI] [PubMed] [Google Scholar]
Mouchet‐Mages 2011 {published data only}
- Mouchet‐Mages S, Rodrigo S, Cachia A, Mouaffak F, Olie JP, Meder JF, et al. Correlations of cerebello‐thalamo‐prefrontal structure and neurological soft signs in patients with first‐episode psychosis. Acta Psychiatrica Scandinavica 2011;123:451‐8. [DOI] [PubMed] [Google Scholar]
Nenadic 2010 {published data only}
- Nenadic I, Smesny S, Schlosser RGM, Sauer H, Gaser C. Auditory hallucinations and brain structure in schizophrenia: voxel‐based morphometric study. [Erratum appears in British Journal of Psychiatry. 2011 Jan;198:75]. British Journal of Psychiatry 2010;196:412‐3. [DOI] [PubMed] [Google Scholar]
Palaniyappan 2011 {published data only}
- Palaniyappan L, Balain V, White TP, Jansen M, Liddle PF. Structural abnormalities in the Salience Network are common to both bipolar disorder with psychosis and schizophrenia. Bipolar Disorders 2011;Conference: 9th International Conference on Bipolar Disorder Pittsburgh, PA United States. Conference Start: 20110609 Conference End: 20110611. Conference Publication: (var.pagings). 13:76. [Google Scholar]
Perez‐Iglesias 2010 {published data only}
- Perez‐Iglesias R, Tordesillas‐Gutierrez D, Barker GJ, McGuire PK, Roiz‐Santianez R, Mata I, et al. White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. Neuroimage 2010;49:199‐204. [DOI] [PubMed] [Google Scholar]
Potvin 2007 {published data only}
- Potvin S, Mancini‐Marie A, Fahim C, Mensour B, Levesque J, Karama S, et al. Increased striatal gray matter densities in patients with schizophrenia and substance use disorder: a voxel‐based morphometry study. Psychiatry Research 2007;154:275‐9. [DOI] [PubMed] [Google Scholar]
Roman‐Urrestarazu 2011 {published data only}
- Roman‐Urrestarazu AE, Murray GK, Barnes A, Miettunen J, Jaaskelainen E, Nikkinen J, et al. Structural MRI in the 1986 northern finland birth cohort (1986‐nfbc): Findings among different psychosis risk profiles in a cross sectional study. Schizophrenia Bulletin 2011;Conference: 13th International Congress on Schizophrenia Research, ICOSR Colorado Springs, CO United States. Conference Start: 20110402 Conference End: 20110406. Conference Publication: (var.pagings). 37:174‐5. [Google Scholar]
Rosa 2012 {published data only}
- Rosa PG, Schalfelberger MS, Duran LS, Santos LC, Menezes P Ft, Scazufca M, et al. Brain structure and the prediction of outcome in first‐episode schizophrenia‐spectrum disorders and affective psychosis: A population‐based study. Biological Psychiatry 2012;Conference: 67th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry Philadelphia, PA United States. Conference Start: 20120503 Conference End: 20120505. Conference Publication:258S‐9S. [Google Scholar]
Ruef 2012 {published data only}
- Ruef A, Curtis L, Moy G, Bessero S, Ba MB, Lazeyras F, et al. Magnetic resonance imaging correlates of first‐episode psychosis in young adult male patients: Combined analysis of grey and white matter. Journal of Psychiatry and Neuroscience 2012;37:305‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rund 2004 {published data only}
- Rund BR, Egeland J, Sundet K, Asbjornsen A, Hugdahl K, Landro NI, et al. Early visual information processing in schizophrenia compared to recurrent depression. Schizophrenia Research 2004;68:111‐8. [DOI] [PubMed] [Google Scholar]
Salgado‐Pineda 2004 {published data only}
- Salgado‐Pineda P, Vendrell P. Magnetic resonance imaging in the study of schizophrenia. Anales de Psicologia 2004;20:261‐72. [Google Scholar]
Salgado‐Pineda 2011 {published data only}
- Salgado‐Pineda P, Fakra E, Delaveau P, McKenna PJ, Pomarol‐Clotet E, Blin O. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophrenia Research 2011;125:101‐9. [DOI] [PubMed] [Google Scholar]
Schiffer 2010 {published data only}
- Schiffer B, Muller BW, Scherbaum N, Forsting M, Wiltfang J, Leygraf N, et al. Impulsivity‐related brain volume deficits in schizophrenia‐addiction comorbidity. Brain 2010;133:3093‐103. [DOI] [PubMed] [Google Scholar]
Schiffer 2010a {published data only}
- Schiffer B. Enhanced non‐planning impulsivity in schizophrenia‐addiction comorbidity is related to anterior cingulate and frontopolar gray matter volumes. European Psychiatry. Conference: 18th European Congress of Psychiatry Munich Germany. Conference Start. 2010; Vol. 20100227.
Schiffer 2010b {published data only}
- Schiffer B, Muller BW, Gizewski ER. Cognition and impulsivity related brain volume changes in schizophrenia‐addiction co‐morbidity. Schizophrenia Research 2010;Conference: 2nd Schizophrenia International Research Society Conference, SIRS 2010 Florence Italy. Conference Start: 20100410 Conference End: 20100414. Conference Publication:425. [Google Scholar]
Schnack 2014 {published data only}
- Schnack H, Nieuwenhuis M, Haren NEM, Abramovic L, Scheewe TW, Brouwer R, Hulshoff Pol HE, Kahn RS. Can structural MRI aid in clinical classification? A machine learning study in two independent samples of patients with schizophrenia, bipolar disorder and healthy subjects. NeuroImage 2014;84:299‐306. [DOI] [PubMed] [Google Scholar]
Segall 2009 {published data only}
- Segall JM, Turner JA, Erp TGM, White T, Bockholt H J, Gollub RL, et al. Voxel‐based morphometric multisite collaborative study on schizophrenia. Schizophrenia Bulletin 2009;35:82‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Situ 2010 {published data only}
- Situ Wei‐jun, Zhu Xiong‐zhao, Tan Chang‐lian. Three dimensional MRI of male schizophrenia patients and the relationship between their symptoms and brain structure. Chinese Journal of Clinical Psychology 2010;18:149‐51. [Google Scholar]
Stanfield 2009 {published data only}
- Stanfield C, Moorhead TWJ, Job DE, McKirdy J, Sussmann JED, Hall J, et al. Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disorders 2009;11:135‐44. [DOI] [PubMed] [Google Scholar]
Sussmann 2009 {published data only}
- Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Munoz MS, Job D, et alussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Munoz MS, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disorders 2009;11:11‐8. [DOI] [PubMed] [Google Scholar]
Suzuki 2010 {published data only}
- Suzuki M, Takayanagi Y, Kawasaki Y, Takahashi T, Nakamur K. Structural MRI‐based classification of patients with first‐episode schizophrenia and healthy subjects. Early Intervention in Psychiatry 2010;Conference: 7th International Conference on Early Psychosis ‐ Early Psychoses: A Lifetime Perspective Amsterdam Netherlands. Conference Start: 20101129 Conference End: 20101201. Conference Publication: (var.pagings). 4:43. [Google Scholar]
Tandon 2012 {published data only}
- Tandon N, Mermon D, Montrose DM, Francis AN, Keshavan MS. Structural brain correlates of transition to psychosis in a genetic high risk sample. Biological Psychiatry 2012;Conference: 67th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry Philadelphia, PA United States. Conference Start: 20120503 Conference End: 20120505. Conference Publication:256S. [Google Scholar]
Trost 2011 {published data only}
- Trost S, Falkai P, Wobrock T, Scherk H, Focke N, Zilles D, et al. Effects of the bipolar disorder susceptibility gene CACNA1C on regional grey matter volumes: A voxel‐based morphometry (VBM) study. Bipolar Disorders 2011;Conference: 9th International Conference on Bipolar Disorder Pittsburgh, PA United States. Conference Start: 20110609 Conference End: 20110611. Conference Publication: (var.pagings). 13:101‐2. [Google Scholar]
Walter 2012 {published data only}
- Walter A, Riecher‐Rossler A, Studerus E, Smieskova R, Aston J, Borgwardt S. Hippocampal volume in individuals with an at‐risk mental state: A longitudinal magnetic resonance imaging analysis. European Neuropsychopharmacology 2012;22:S200. [Google Scholar]
Wang 2009 {published data only}
- Wang X, Wang Xiao‐sheng, Yan Li‐rong, Tan Chang‐lian, Li Ya‐jun, Situ Wei‐jun, et al. White matter volume differences in deficit and nondeficit schizophrenia: A voxel‐based morphometric study. Chinese Journal of Clinical Psychology 2009;17:387‐9. [Google Scholar]
Watson 2012 {published data only}
- Watson DR, Anderson JME, Bai F, Barrett SL, McGinnity TM, Mulholland CC, et al. A voxel based morphometry study investigating brain structural changes in first episode psychosis. Behavioural Brain Research 2012;227:91‐9. [DOI] [PubMed] [Google Scholar]
Witthaus 2008 {published data only}
- Witthaus H, Brune M, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, et al. White matter abnormalities in subjects at ultra high‐risk for schizophrenia and first‐episode schizophrenic patients. Schizophrenia Research 2008;102:141‐9. [DOI] [PubMed] [Google Scholar]
Witthaus 2009 {published data only}
- Witthaus H, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, Gallinat J, et al. Gray matter abnormalities in subjects at ultra‐high risk for schizophrenia and first‐episode schizophrenic patients compared to healthy controls. Psychiatry Research 2009;173:163‐9. [DOI] [PubMed] [Google Scholar]
Wojtalik 2013 {published data only}
- Wojtalik JA, Eack M, Keshavan MS. Structural neurobiological correlates of Mayer‐Salovery‐Caruso Emotional Intelligence Test performance in early course schizophrenia. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2013;40:207‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yüksel 2010 {published data only}
- Yüksel AC. A voxel‐based morphometry study of shared and distinct abnormalities in grey matter density in psychotic disorders. Biological Psychiatry. 2010; Vol. 65th Annual Meeting of the Society of Biological Psychiatry New Orleans, LA United States. Conference Start: 20100520 Conference End: 20100522:260S.
Yüksel 2012 {published data only}
- Yüksel C, McCarthy J, Shinn A, Pfaff DL, Baker JT, Heckers S, Renshaw P, Ongür D. Gray matter volume in schizophrenia and bipolar disorder with psychotic features. Schizophrenia Research 2012 Jul;138(2‐3):177‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
Bergman 2014
- Bergman H, Khodabakhsh A, Maayan N, Kirkham AJ, Adams CE, Soares‐Weiser K. Operational Criteria Checklist for Psychotic Illness and Affective Illness (OPCRIT+) for diagnosing schizophrenia in people with psychotic symptoms. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD011104] [DOI] [Google Scholar]
Bottlender 2003
- Bottlender R, Sato T, Jager M, Wegener U, Wittmann J, Strauss A, et al. The impact of the duration of untreated psychosis prior to first psychiatric admission on the 15‐year outcome in schizophrenia. Schizophrenia Research 2003;62(1‐2):37‐44. [PUBMED: 12765741] [DOI] [PubMed] [Google Scholar]
Bustillo 2001
- Bustillo J, Lauriello J, Horan W, Keith S. The psychosocial treatment of schizophrenia: an update. American Journal of Psychiatry 2001;158(2):163‐75. [DOI] [PubMed] [Google Scholar]
Chan 2011
- Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high‐risk individuals, first‐episode, and chronic schizophrenia: an activation likelihood estimation meta‐analysis of illness progression. Schizophrenia Bulletin 2011;37(1):177‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58:882‐93. [DOI] [PubMed] [Google Scholar]
Douaud 2007
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen‐Berg H, Vickers J, et al. Anatomically related grey and white matter abnormalities in adolescent‐onset schizophrenia. Brain 2007;130(9):2375‐86. [DOI] [PubMed] [Google Scholar]
DTA Handbook 2013
- Deeks JJ, Bossuyt PM, Gatsonis C (editors). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 0.9. The Cochrane Collaboration, 2013. Available from: http://srdta.cochrane.org/ (searched on 22 Dec 2014). The Cochrane Collaboration.
Ellison‐Wright 2008
- Ellison‐Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first‐episode and chronic schizophrenia: an anatomical likelihood estimation meta‐analysis. American Journal of Psychiatry 2008;165(8):1015‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fannon 2000
- Fannon D, Chitnis X, Doku V, Tennakoon L, Ó’Ceallaigh S, Soni W, et al. Features of structural brain abnormality detected in first‐episode psychosis. American Journal of Psychiatry 2000;157(11):1829‐34. [DOI] [PubMed] [Google Scholar]
Fusar‐Poli 2012
- Fusar‐Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta‐analysis of transition outcomes in individuals at high clinical risk. Archives of General Psychiatry 2012 Mar;69(3):220‐9. [DOI] [PubMed] [Google Scholar]
Fusar‐Poli 2013
- Fusar‐Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher‐Rössler A, Schultze‐Lutter F, et al. The psychosis high‐risk state: a comprehensive state‐of‐the‐art review. JAMA Psychiatry 2013 Jan;70(1):107‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glahn 2008
- Glahn DC, Laird AR, Ellison‐Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. Meta‐analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biological Psychiatry 2008;64(9):774‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez‐Pinto 1998
- Gonzalez‐Pinto A, Gutierreza M, Mosqueraa F, Ballesterosa J, Lopeza P, Ezcurraa J, et al. First episode in bipolar disorder: misdiagnosis and psychotic symptoms. Journal of Affective Disorders (1998) 1998;50(1):41‐4. [DOI] [PubMed] [Google Scholar]
Good 2001
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14(1 Pt 1):21‐36. [DOI] [PubMed] [Google Scholar]
Goodkind 2015
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones‐Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 2015;72(4):305‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gur 2007
- Gur RE, Keshavan MS, Lawrie SM. Deconstructing psychosis with human brain imaging. Schizophrenia Bulletin 2007;33(4):921‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henley 2010
- Henley SM, Ridgway GR, Scahill RI, Klöppel S, Tabrizi SJ, Fox NC, et al. EHDN Imaging Working Group. Pitfalls in the use of voxel‐based morphometry as a biomarker: examples from huntington disease. AJNR: American Journal of Neuroradiology 2010 Apr;31(4):711‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Honea 2005
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta‐analysis of voxel‐based morphometry studies. American Journal of Psychiatry 2005 Dec;162(12):2233‐45. [DOI] [PubMed] [Google Scholar]
Huettel 2009
- Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. 2nd Edition. Sunderland, Mass: Sinauer Associates, 2009. [Google Scholar]
Iwabuchi 2013
- Iwabuchi SJ, Liddle PF, Palaniyappan L. Clinical utility of machine‐learning approaches in schizophrenia: improving diagnostic confidence for translational neuroimaging. Frontiers in Psychiatry 2013;4:95. [DOI: 10.3389/fpsyt.2013.00095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005
- Jones DK, Mark T, Symms R, Cercignani M, Howarde RJ. The effect of filter size on VBM analyses of DT‐MRI data. Neuroimage 2005;26:546‐54. [DOI] [PubMed] [Google Scholar]
Kane 2001
- Kane JM, Marder SR, Schooler NR, Wirshing WC, Umbricht D, Baker RW, et al. Clozapine and haloperidol in moderately refractory schizophrenia: a 6‐month randomized and double‐blind comparison. Archives of General Psychiatry 2001;58(10):965‐72. [DOI] [PubMed] [Google Scholar]
Larson 1996
- Larson TK, McGlashan TH, Moe LC. First‐episode schizophrenia: I. early course parameters. Schizophenia Bulletin 1996;22(2):241‐56. [DOI] [PubMed] [Google Scholar]
Lawrie 2011
- Lawrie SM, Olabi B, Hall J, McIntosh AM. Do we have any solid evidence of clinical utility about the pathophysiology of schizophrenia?. World Psychiatry 2011 Feb;10(1):19‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malla 2005
- Malla AK, Norman RMG, Joober R. First‐episode psychosis, early intervention, and outcome: what have we learned?. Canadian Journal of Psychiatry 2005;50(14):881‐91. [DOI] [PubMed] [Google Scholar]
Marshall 2011
- Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD004718.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarley 1999
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, et al. MRI anatomy of schizophrenia. Biological Psychiatry 1999;45(9):1099‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGrath 2008
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiologic Reviews 2008;30:67‐76. [DOI] [PubMed] [Google Scholar]
Mechelli 2005
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel‐based morphometry of the human brain: methods and applications. Current Medical Imaging Reviews 2005;1(1):1‐9. [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. Structural neuroimaging in first‐episode psychosis. NICE Clinical Guideline No 136. London: National Institute for Health and Clinical Excellence, 2008. [Google Scholar]
Ochoa 2012
- Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first‐episode psychosis: a comprehensive literature review. Schizophrenia Research and Treatment 2012;2012:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58:982‐90. [DOI] [PubMed] [Google Scholar]
Ridgway 2008
- Ridgway GR, Henley SM, Rohrer JD, Scahill RI, Warren JD, Fox NC. Ten simple rules for reporting voxel‐based morphometry studies. Neuroimage 2008;40(4):1429‐35. [DOI] [PubMed] [Google Scholar]
Rutter 2001
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001 Oct 15;20(19):2865‐84. [DOI] [PubMed] [Google Scholar]
Shenton 2001
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research 2001;49(1‐2):1‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soares‐Weiser 2013
- Soares‐Weiser K, Maayan N, Bergman H, Davenport C, Kirkham AJ, Grabowski S, et al. First‐rank symptoms for schizophrenia. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010653.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sommer 2013
- Sommer IE, Kort GA, Meijering AL, Dazzan P, Hulshoff Pol HE, Kahn RS, et al. How frequent are radiological abnormalities in patients with psychosis? A review of 1379 MRI scans. Schizophrenia Bulletin 2013;39(4):815‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vacante 2013
- Vacante M, Smailagic N, Sachpekidis C, Hyde C, Martin S, Ukoumunne O. The accuracy of FDG‐PET in the early diagnosis of Alzheimer’s disease, dementia and other dementias in people with MCI. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Whitwell 2009
- Whitwell JL. Voxel‐based morphometry: an automated technique for assessing structural changes in the brain. Journal of Neuroscience 2009;29(31):9661‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. ICD‐10: The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization, 1992. [Google Scholar]
Wyatt 1997
- Wyatt RJ, Green MF, Tuma AH. Long‐term morbidity associated with delayed treatment of first admission schizophrenic patients. Psychological Medicine 1997;27:261‐8. [DOI] [PubMed] [Google Scholar]


