Abstract
Introduction
Retinitis pigmentosa (RP) is the most common form of inherited retinal degenerations with an estimated prevalence of 1 in 4,000 and more than 1 million individuals affected worldwide. With the introduction of the first retinal gene therapy in 2017 the importance of understanding the mechanisms of retinal degeneration and its natural progression has shifted from being of academic interest to being of pivotal for the development of new therapies.
Areas covered
This review covers standard and innovative diagnostic techniques and complementary examinations needed for the evaluation and treatment of RP. It includes chapters on the assessment of visual function, retinal morphology, and genotyping.
Expert Opinion
Monitoring the progression of RP can best be achieved by combining assessments of both visual function and morphology. Visual acuity testing using ETDRS charts should be complemented by low-luminance visual acuity and colour vision tests. Assessment of the visual field can also be useful in less advanced cases. In those with central RP involvement measuring retinal sensitivity using microperimetry is recommended. Retinal morphology is best assessed by OCT and autofluorescence. Genetic testing is pivotal as it contributes to the pathophysiological understanding and can guide clinical management as well as identify individuals that could benefit from retinal gene therapy.
Keywords: Autofluorescence, ETDRS letters, genotyping, inherited retinal degeneration, IRD, microperimetry, optical coherence tomography, OCT, retinitis pigmentosa, RP
1.0. Introduction
Inherited retinal degenerations (IRDs) are neurodegenerative diseases that lead to dysfunction of the retinal metabolism and/or cell death of various outer retinal cells (generally photoreceptors), and affect about 1 in 3,000 people worldwide.[1] Retinitis pigmentosa (RP), which describes a group of genetically heterogenous rod-cone dystrophies, is the most common form of IRD with an estimated prevalence of 1 in 4,000 and more than 1 million individuals affected worldwide.[2–4] More than 250 genes have been identified to cause IRD of which 80 genes have been linked to RP (Leiden Open Variation Database www.databases.lovd.nl; RetNet www.sph.uth.edu). The genetic trait of RP is usually inherited as autosomal recessive (50-60%), autosomal dominant (30-40%) or X-linked (5-15%).[4–7] The diagnosis is established clinically through a variety of examinations, which include both assessment of function and morphology that is then confirmed genetically. In addition to addressing any potential complications of the disease, the monitoring of the progression of RP was generally limited to re-assessments of visual function and provision of low vision aids as well as patient and family counselling. The recent approval of Luxturna® (voretigene nepavorec) for the RPE65 associated Leber congenital amaurosis (LCA) both by the FDA and the EMA has heralded the start of a new era, and boosted the research and development of many other retinal gene therapies of which some have already entered clinical trial phase 3 (choroideremia: NCT03496012, RPGR X-linked RP: NCT03116113). The development of retinal gene therapies requires an understanding of the mechanisms of retinal degeneration and a way to monitor response to treatment. Developing standards for clinical trials in ophthalmology are of paramount importance and help with obtaining timely approval of new therapies arising from clinical research. This review covers standard and innovative diagnostic techniques and complementary examinations needed for the evaluation and treatment of RP. The focus will be put on highlighting methods that can serve as surrogate endpoints in clinical trials. A surrogate endpoint, as defined by the FDA is a biomarker that is “reasonably likely, based on epidemiologic, therapeutic, pathophysiologic, or other evidence to predict clinical benefit”.[8] It shall also highlight the importance of genotyping not only in establishing the correct diagnosis, but also to understand the pathophysiology and ultimately the phenotype.
2.0. Retinitis Pigmentosa Diagnostics and Monitoring
2.1. Assessment of Visual Function
2.1.1. ETDRS letters
Visual acuity (VA) is a reliable marker of visual function, and is the primary measure of visual function both in clinical and research settings. It is used as an entry criterion, outcome or efficacy measure as well as safety endpoint. VA is measured as minutes of arc and calculated as the reciprocal of the minimal angle of resolution (MAR). The ETDRS (Early Treatment Diabetic Retinopathy Study) letter charts with a retro-illuminated box were developed in the 1980s by Ferris et al.[9,10] with the goal of standardization of VA measurements, and have become the standard logMAR chart used in many multicentre clinical trials.[11] The characteristic of ETDRS letter charts is that a three-line worsening of visual acuity is equivalent to a doubling of the visual angle regardless of the baseline visual acuity used, which allows for correct interpretation of changes in visual acuity over time.[9] Each ETDRS letter chart line has five letters created from the ten Sloan letters[12] with similar optotype difficulty scores with a geometric progression of letter height from line to line of 0.1 log unit. The 0.1 logMAR unit between the five letter lines allows to assign a 0.02 logMAR unit to each letter, which leads to a high repeatability and accuracy of the EDTRS letter charts.[13] Sensitivity and specificity analyses of the EDTRS charts in normal subjects however show that only a difference of 0.2 logMAR units (2 lines on the chart) reliably indicates a meaningful clinical change.[13] In patients with RP the reliability of EDTRS letter charts can vary even to a larger amount, which means that the precision to determine whether a true change in visual acuity has occurred may be reduced. The assessment of patients with visual acuities <20/200 (legal blindness) and visual field diameter of <20° showed twice the variability reported for normal sighted individuals.[14,15] Factors like glare and sensitivity to light as well as motivation and the typically higher rate of depression in the RP population increase the variability of VA assessment even in patients with good central vision, and have to be kept in mind when evaluating VA changes. The ETDRS letter charts are internally illuminated, which allow for consistent testing conditions. However, room illumination levels also must be considered since changes in illuminance levels have been found to give rise to greater changes in VA in the presence of myopic refractive errors, which are found more commonly in RP patients.[16] Another possible explanation for the reduced precision in patients with RP is the impact of the visual field loss on the ability to track letters on the chart. Increased VA test precision can be achieved by using electronic visual acuity (EVA) systems, in particular when the visual field loss affects the fovea.[17] It is certainly impossible to control fully for all psychophysical factors involved in VA testing, but efforts should be made to maximise standardisation of the procedure. In monitoring the progression of RP both in clinical routine and for trials we recommend using the standardized ETDRS charts (Precision Vision Inc., Woodstock, IL, USA) with consistent room illuminance to assure uniform contrast and luminance of the optotypes. The ETDRS-Fast method can be employed in the clinical routine setting to reduce test time, while maintaining reasonable accuracy.[18]
2.1.2. Low-luminance visual acuity, colour vision and contrast sensitivity
Visual acuity decreases significantly with luminance.[19] Low luminance visual acuity (LLVA) is performed by using 2.0 log unit neutral density filters (reduction in luminance by 102 times) while reading a normally illuminated ETDRS chart. The low luminance deficit is defined as the difference between the LLVA and the standard VA level in logMAR units. It is a well-established fact that patients with RP show a more pronounced visual acuity deficit at low luminance.[20] In patients with geographic atrophy associated with age-related macular degeneration (AMD) the low luminance visual dysfunction was strongly predictive for future visual acuity loss.[21] However, care must be taken when interpreting LLVA changes since it might not only indicate a reduction in foveal cone function, but also a shift to eccentric fixation.[22]
Many patients with RP can retain good visual acuity until late in the disease course, while colour vision deficits can be present before the onset of degeneration. The physiologic substrate of the trichromatic colour vision is the cone photoreceptor, of which there are three classes: the short- (S-), the medium- (M-), and the long- (L-) wavelength sensitive cones.[23,24] Colour discrimination can be tested using the Farnsworth-Munsell 100-Hue arrangement test under optimum lighting conditions. Patients with RP generally present with acquired S-mechanism deficiency (Verriest type III).[24] In choroideremia colour vision defects were detected prior to loss of central visual function indicating a general retinal functional impairment occurring earlier than cell loss.[25] Similarly, in a cohort of RP patients carrying the same homozygous LRAT mutation severe deficiency in all colour vision axes was found in a majority of patients despite non impaired visual acuity.[26] While colour vision testing is suitable to detect early subtle functional changes before deterioration in visual acuity, tracking of the colour discrimination is not a useful measure of disease progression.
Contrast sensitivity testing, over a range of spatial frequencies, is an alternate method of testing central visual function. Its usefulness lies in understanding the impact of visual impairment on the functional ability, and patients with IRDs often complain of reduced central vision, despite having normal visual acuity. There is no pattern of contrast sensitivity function that is unique to any particular disorder, although it could be a useful tool in monitor progression in Stargardt disease.[27]
2.1.3. Visual Field
Visual acuity tends to be an insensitive measure of disease severity, and other tests of visual function are required to diagnose and monitor RP. In static perimetry (i.e., automated perimetry), a stationary target is changed in size and brightness until seen, while in kinetic perimetry (i.e., Goldmann visual field) a target of predefined size and luminance is moved from a non-seeing to a potentially seeing area. The Goldmann visual field (GVF) is particularly useful for monitoring peripheral visual field defects, large scotomata, and the progression of visual field loss over time (Fig. 1).[28] The reliability of the GVF is however highly operator dependent, and prone to erroneous results from variable target speeds, particularly for small and dim targets.[29,30] The original Goldmann perimeter is no longer produced, and has been replaced by semi-automated kinetic perimetry (SKP) devices with standardization of target velocity and test pattern. The SKP Octopus 900 (Haag Streit AG, Koeniz, Switzerland) has shown strong agreement with the GVF, and is considered a viable alternative to the GVF in clinical trials and for clinical care.[30,31] Studies testing the quantitative analysis of OCT as a structural measure for disease progression have shown that perception of the Goldmann I4e isopter correlates with retinal areas showing an intact inner segment ellipsoid zone.[32] The usefulness of the Goldmann visual field as a clinical trial outcome measure however is limited by its high test-retest variability and its operator dependence.[28,33,34] Computerized wide-field static perimetry may however be a more reliable approach to assess the absolute sensitivity across the retina and ultimately enable an estimation of the functional change over time.[35] Assessment of the paracentral retinal sensitivity and the scotoma size can be achieved by static automated perimetry either using the Octopus 900® M pattern or the Humphrey® visual field 10-2 (Zeiss Medical Technology, Jena, Germany). Macular perimetry has also been shown to correlate well with loss of the ellipsoid zone in patients with RP[36], however does not allow accurate co-registration between the sensitivity map and the fundus image. The correlation between retinal sensitivity and fundus changes is achieved with microperimetry. The two current generation devices are the CenterVue MAIA (Macular Integrity Assessment) and the Nidek MP-3 microperimeters. The devices are essentially similar and enable tracking of eye movement and assessment of fixation stability along with quantification of retinal sensitivity. Inter-device comparison on healthy subjects showed however differing retinal sensitivity values.[37] Robust normative database that would allow inter-relating both devices are still lacking. Currently, the most widely used microperimeter in clinical trials is the CenterVue MAIA (CenterVue, Padova, Italy), which is especially useful in the evaluation of central involvement of RP (Fig.2). The Nidek MP-3 has however also proven to detect significant changes in central retinal sensitivity over time.[37,38] Both devices are excellent clinical tools to monitor the progression of RP. Microperimetry may prove to be a valuable outcome measure in assessing retinal gene therapy responses in ciliopathies in which regeneration of outer segments have been linked to improvements in central retinal sensitivity.[39]
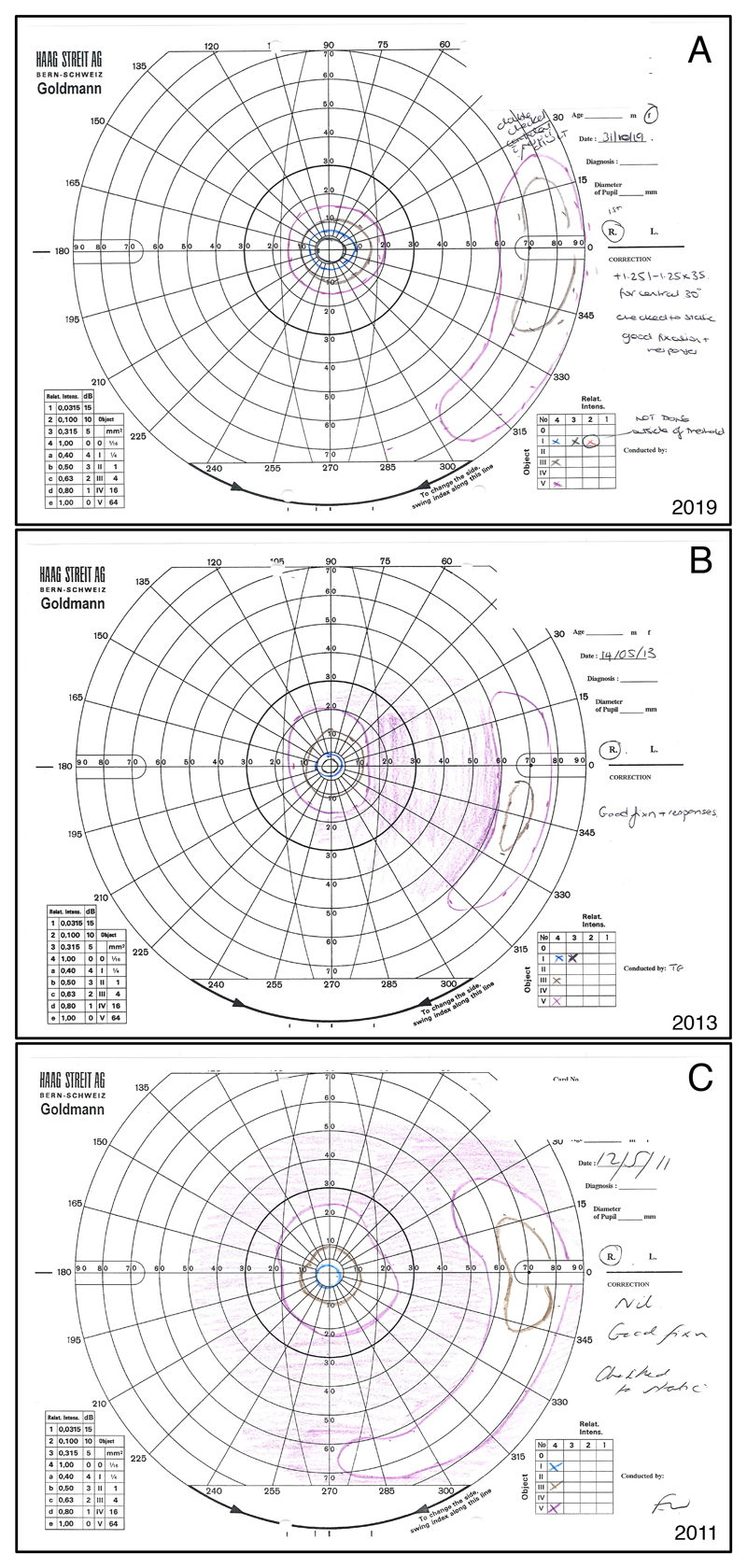
Figure 1.
Goldmann visual fields of the right eye of a 37 year-old female with PDE6B associated autosomal recessive RP. Figure 1A shows the most recent visual field from 2019, while 1B is from 2013, and 1C from 2011. The normal Goldmann visual field usually reaches about 90° temporally, 50° nasally, and 50-60° superiorly and inferiorly for the largest and brightest stimulus. All fields show a concentric constriction with a peripheral remnant of visual field, which is typical for RP. Significantly increased constriction and decrease in the size of the peripheral remnant can be noted from 2011 to 2013. However, the visual field from 2019 seems to not have worsened in comparison to the previous exams. Indeed, both the central island and the peripheral remnant appear larger. A test-retest variability of up to 20% even when using a single experienced operator has been known for Goldmann visual fields, and might explain the apparent improvement in visual field seen in this patient. The clinical improvement seen in the cystoid macular oedema between 2013 and 2019 (not shown) could however have contributed to a real improvement in visual field sensitivity in this case.
The purple lines represent the Goldmann stimulus V4e. The Roman number indicates the stimulus size of 64mm2 for V. The Arabic number and the lower case letter indicate the light intensity of 1000 apostilb (315 cd/m2). Brown = III4e (4mm2, 1000 asb); blue = I4e (1mm2, 1000 asb); black = I3e (1mm2, 315 asb).
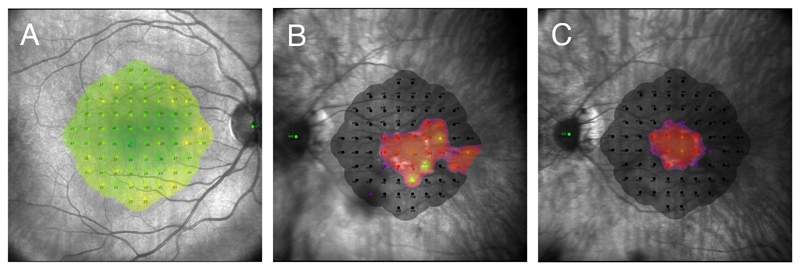
Figure 2.
Standard 10-2 grid consisting of 68 retinal points arranged in a Cartesian pattern covering the central 20°. The threshold sensitivity value at each retinal location is colour coded and shown as an overlay on the near-infrared image. A) Macular sensitivity heat map of a healthy 27 year-old male. Green indicates normal sensitivity (maximum 36dB). B and C) Macular sensitivity heat map of a 38 year-old male with RPGR X-linked RP taken two years apart. In 2017 (B) the patient still showed few retinal points of nearly normal sensitivity, while two years later (C) both the central visual field constriction, and sensitivity threshold had worsened.
2.1.4. Electrophysiology
The full-field electroretinogram (ERG) enables a global assessment of photoreceptor function, and has traditionally played an important role both in the diagnosis and characterization of RP. The ERG represents a mass electrical response generated by light-induced changes in extracellular electrolytes (mainly Na+ and K+) at the level of the photoreceptor outer segments.[40] Comprehensive information on ERG is given by the International Society for Clinical Electrophysiology of Vision.[41] According to the ERG responses, retinal degenerations may be characterized as causing rod-cone, cone-rod, or second-order neuron dysfunction. RP is a typical example of rod-cone degeneration. The amplitude of the responses is proportional to the area of functioning retina.[42] It is estimated that on average, RP patients lose about 16-18.5% per year of remaining ERG amplitudes.[28] The ERG has its greatest value in the diagnosis of RP and other IRDs, however is less useful for monitoring the disease progression. Figure 3 shows an example of a 10 year-old asymptomatic boy with a strong family history of PRPF31 associated RP, and a marked dysfunction both in rod and cone photoreceptors. In addition, ERG can be very useful in confirming the pathogenicity of novel mutations of uncertain pathogenicity, such as in a case of nuclear hormone receptor gene (NR2E3) associated with enhanced S-cone syndrome.[43] However, as an objective measure of retinal function in response to new therapies such as gene replacement, the ERG is insensitive to changes in macular function and subtle changes in photoreceptor survival because it is a pan-retinal response. The multifocal ERG (mfERG) permits assessment of cone diseases affecting local regions of the retina, and represents retinal function from the central 40-50 degrees of the macula corresponding with automated perimetry tests of the central macula. mfERG is a useful tool in screening for toxic maculopathies or diseases characterized by interocular and intraretinal variation, such as white dot syndromes, however to monitor changes in function over time, macular visual field sensitivity or microperimetry are much more readily accessible and accurate.
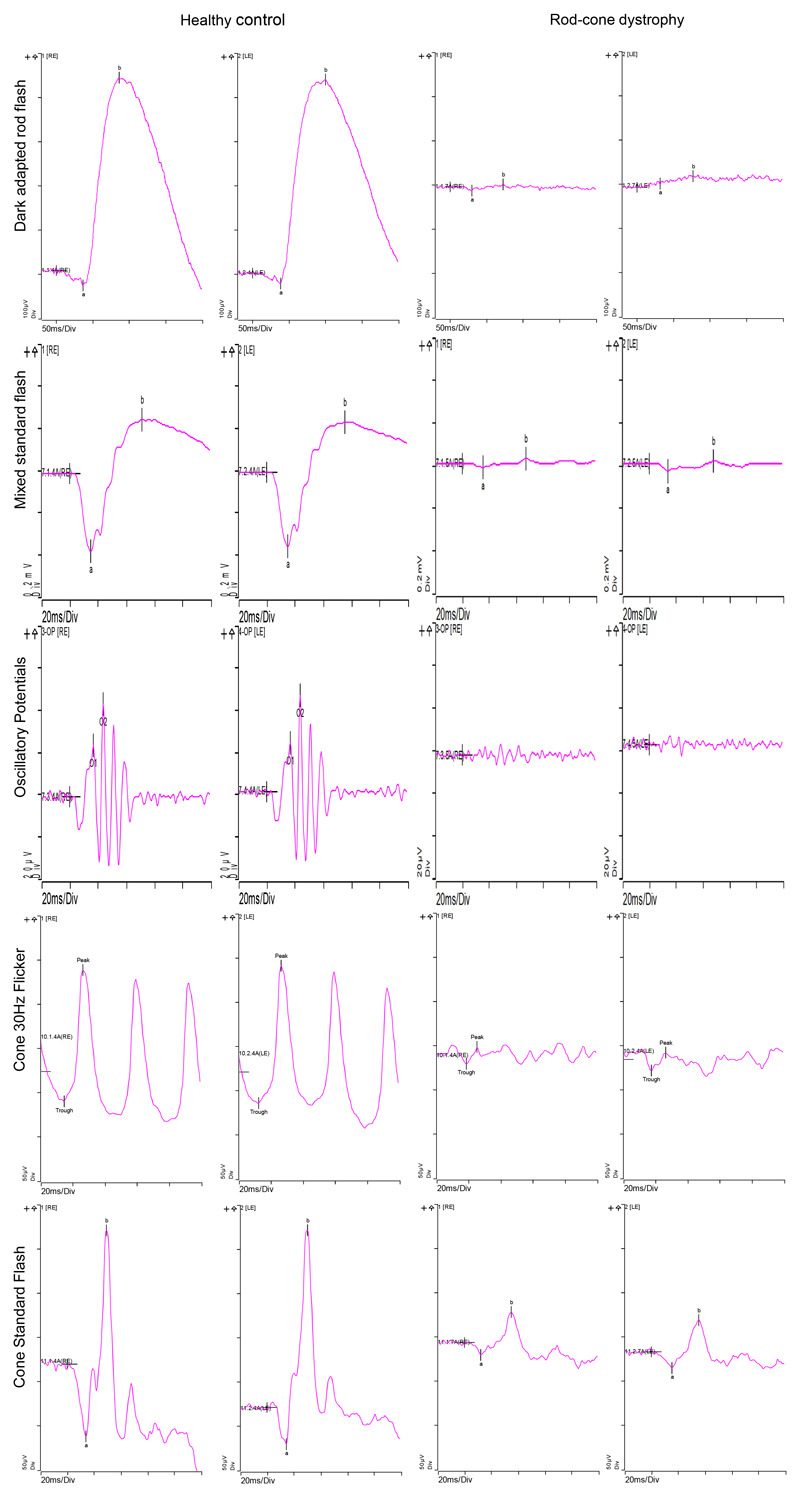
Figure 3.
ISCEV Standard ERG of a 10 year-old boy with a strong family history of PRPF31 associated RP. Normal values of a healthy control are shown on the left columns. The right columns show the ERG of the 10 year-old boy. The dark-adapted rod flash (0.01 cd*s/m2) shows barely measurable components. The mixed standard flash (3 cd*s/m2) reveals reduced amplitudes, whilst peak times are normal. The third row shows barely recordable dark-adapted (3 cd*s/m2) oscillatory potentials. The light-adapted 30 Hz flicker ERG and the cone standard flash (3 cd*s/m2) both show markedly reduced responses. In summary, the ERG confirms rod and cone dysfunction in both eyes. Overall rods seem to be more affected than cones at this stage.
The measurement of the sensitivity threshold during the adaptation to darkness is known to show a characteristic biphasic function with an initial cone response, followed by the rod-cone break after about 5 to 10 minutes, and the rod response that reaches a plateau after 40 to 50 minutes.[44] The dark adaptation function reflects the ability of the rod and cone photoreceptors to regenerate photopigment, and thus sensitivity after exposure to light.[44] Dark adaptometry is particularly useful characterizing patients with congenital stationary night blindness (CSNB) and normal fundi. In patients without a scotopic ERG, an absent rod-cone break on the dark adaptation curve is indicative for a molecular defect at the rod photoreceptor level.[45] Prolonged dark adaptation is furthermore the most characteristic feature of the RHO gene Pro23His genotype.[46]
2.1.5. Multi-luminance mobility test (MLMT), Full-field stimulus threshold (FST)
The multi-luminance mobility test (MLMT) was designed to allow quantifiable measurement of functional vision in severely affected RP patients unable to perform other forms of functional visual assessments. It tests the ability of a subject to navigate a course accurately and at a reasonable pace at different levels of illumination.[47]
The full-field stimulus threshold is an alternative to measure the dark adapted light-sensitivity in RP patients unable to perform visual field tests or with undetectable ERG. The FST is fast to perform and determines the luminance threshold for detection of a single stimulus flash without requiring patients’ fixation.[48]
Both functional tests have been used as outcome measures for the voretigene neparvovec phase III and IV pivotal trial, and in fact the MLMT represented a novel functional primary outcome.[49] Despite being FDA approved new clinical trial end-points, it would however be difficult for to adopt these as practical, widely available clinical tests, and thus neither tests are essential for monitoring.
2.2. Assessment of Retinal Structure
2.2.1. Optical Coherence Tomography
Optical coherence tomography (OCT) has revolutionized the way ophthalmologists evaluate retinal diseases and treat patients, and about two decades after its introduction it has become indispensable both for research and clinic. The introduction of spectral domain OCT (SD-OCT) has further enhanced the visualization of retinal structures with an axial resolution of approximately 5 µm for commercial devices and 2 µm for research devices, permitting correlation with histological features of the retina.[50] In high quality, high resolution SD-OCT scans at least 13 different retinal layers can be identified. The first hyper-reflective band at the vitreoretinal interface is the internal limiting membrane (ILM), followed by the hyper-reflective nerve fiber layer (NFL) and by a less hyper-reflective band composed of the ganglion cell layer (GCL) and inner plexiform layer (IPL). Both nuclear layers (inner and outer) are hypo-reflective and separated by the hyper-reflective outer plexiform layer (OPL). With variation of the incident light beam, Henle’s fiber layer (HFL) can further be visualized adjacent to the OPL either as a hyper-reflective or hypo-reflective band, depending on the recording angle.[51] In the outer retina, the SD-OCT resolves four distinct bands. The innermost band is thought to be the external limiting membrane (ELM), which is composed of junctional complexes between Müller cells and photoreceptors.[50] The origin of the second and third bands has been a source for debate in recent years. The terminology suggested by an international nomenclature panel is ellipsoid zone (EZ) for the second, and interdigitation zone (IZ) for the third band.[52] However, the nomenclature remains controversial since the inner segment ellipsoid is around 16 to 20 µm and thus too thick and also too proximally located to produce the second band.[53] The origin of the third band has similarly been challenged, and attributed to the cone outer segment tips.[54,55] The fourth hyper-reflective outer retinal band is attributed to the RPE with possible contributions from Bruch’s membrane and the choriocapillaris.[56]
The OCT has been used to aide with the diagnosis of common RP features such as cystoid macular oedema and epiretinal membrane since its introduction. More recently, detailed structural information, such as the integrity and the extent of the ELM and EZ as well as ONL thickness, have been shown to correlate well with function, and are thus used to monitor disease progression and serve as outcome measures in therapeutic trials.[32,36,57–62] Figure 3A shows a progressive decrease in the lateral extend of the ELM and EZ in a left eye of a 28 year-old male patient with autosomal recessive RP during a follow up period of six years. Figure 3B is an example of a 26 year-old female patient with CRB1 associated RP that showed clinically meaningful improvement of her right eye cystoid macular oedema upon systemic acetazolamide therapy.
2.2.2. Autofluorescence
Fundus autofluorescence, classically using blue-light excitation, is a non-invasive imaging technique that can give indirect information on the level of metabolic activity of the RPE through visualizing fluorophores, such as lipofuscin granules, which accumulate in the RPE as a byproduct of constant phagocytosis of shed photoreceptor outer segments.[63–65] Extracellular ocular fluorophores from shed photoreceptor outer segment debris may accumulate subretinally due to RPE dysfunction and loss of apposition between the photoreceptor tips and the RPE, and clinically be seen as vitelliform material.[66] The normal fundus autofluorescence pattern is characterized by a physiological foveal hypo-autofluorescence from absorption of the short-wave length excitation light by lutein and zeaxanthin. Increasing accumulation of lipofuscin granules in the RPE cytoplasm occurs naturally with aging.[67] In IRDs changes in the autofluorescence pattern can be appreciated prior to any functional deficit and can be diagnostic.[64,68] In Stargardt disease with bi-allelic mutations in the ATP-binding cassette transporter (ABCA4), the excessive accumulation of bisretinoids leads to a quantifiable early increased lipofuscin-related autofluorescence, and later to characteristic hyperautofluorescent fundus flecks.[69] In patients with RP a central ring of increased autofluorescence intensity has been shown to surround regions of preserved central photopic function, visual field sensitivity, and preservation of the inner segment EZ, while visual sensitivity was reduced across the ring and markedly decreased outside the ring of hyperautofluorescence with associated loss of the EZ.[70–72] In choroideremia the preserved central retinal island reveals a unique autofluorescence pattern of sharply demarcated edges.[73] In RPGR X-linked RP it has been recently shown that the hyperautofluorescent ring correlates nicely with retinal regions that have lost the EZ, but still retain the ELM.[74] The decrease in the extent of the ELM and EZ, observed in the patient featured in Fig. 3A, correlates nicely with the concentric constriction of the physiological foveal hypoautofluorescence. Fig. 3C features autofluorescence and OCT images of the right eye of a 61 year-old male with PRPF8 associated autosomal dominant RP. The inner hyperautofluorescent circle shows good correlation with the loss in ELM, while the outer larger hyperautofluorescent ring correlates with retinal area of absent ELM and highly thinned ONL. Absent or severely reduced autofluorescence can be observed in patients with RPE65-associated retinal dystrophy (Fig. 4D).[75] Melanin is another naturally occurring ocular fluorophore that is located at the apical end of the RPE cell compared to the basolateral accumulation of lipofuscin.[76] In contrast to lipofuscin with a peak excitation wavelength of 470nm, melanin has a peak excitation wavelength of 787nm, and is the primary fluorophore seen in near-infrared fundus autofluorescence.[77] The most striking feature in near-infrared autofluorescence imaging is increased autofluorescence in the fovea as opposed to the short-wavelength autofluorescence, which reveals a dark area at the fovea.[77] Melanin in the RPE cell is thought to have anti-oxidative properties and to protect against lipofuscin accumulation.[78,79] Having been introduced to overcome the potential phototoxic properties of short-wavelength autofluorescence, near-infrared autofluorescence does not have the same clinical applicability and diagnostic usefulness as the classical short-wavelength autofluorescence.
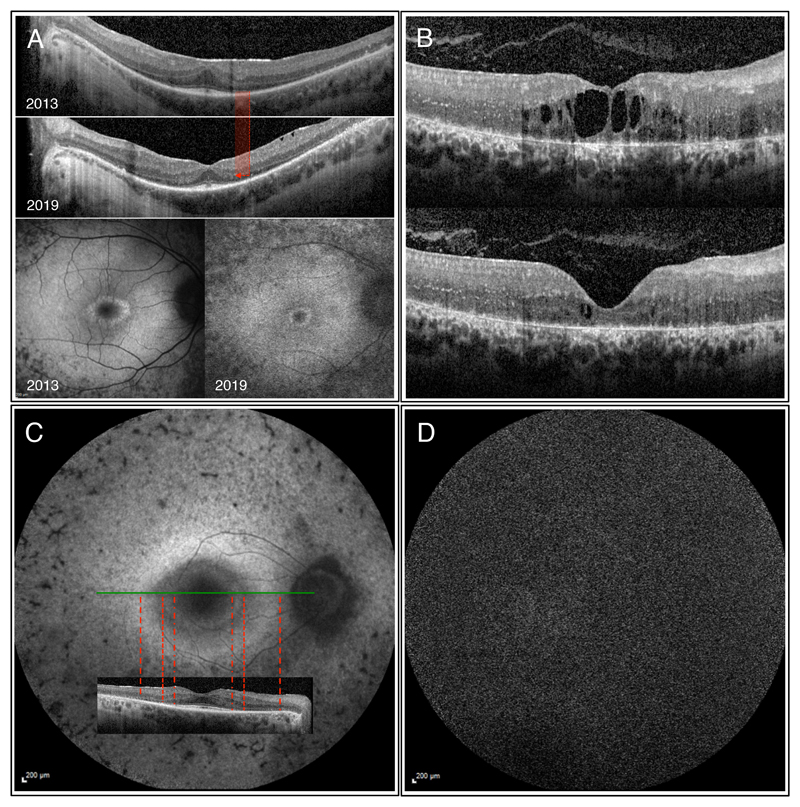
Figure 4.
OCT and autofluorescence images of various patients with RP. A) Images of the right eye of a 28 year-old male with autosomal recessive RP (compound heterozygous mutations in DFNB31 and USH2A found). Significant decrease in the width of both the ELM and EZ paralleled by diminishing foveal hypoautofluorescence can be observed between 2013 and 2019. Visual acuity in 2019 was 6/7.5, which translates to 20/25 (20 ft) or 0.8 (decimal) Snellen equivalent. B) OCT images of the right eye of a 26 year-old female patient with CRB1 associated RP showing clinically meaningful improvement of the cystoid macular oedema upon systemic acetazolamide therapy with an increase in visual acuity from 3/60 (20/400, 0.05) to 6/38 (20/125, 0.16). C) Autofluorescence and OCT images of the right eye of a 61 year-old male with PRPF8 associated autosomal dominant RP. The inner hyperautofluorescent circle shows good correlation with the loss in ELM, while the outer larger hyperautofluorescent ring correlates with retinal area of absent ELM and highly thinned ONL. D) Short-wavelength autofluorescence image of a 48 y/o male patient with biallelic RPE65 (c.11+5G>A, c.1543C>T) showing the characteristic absent autofluorescence.
Fundus autofluorescence cannot only be characterized by the spatial distribution of the autofluorescence signal, and by the emission spectrum, but also by quantifying the average time between excitation and reaching the fluorescent ground state. Fluorescence lifetime imaging ophthalmoscopy (FLIO) is an emerging imaging modality that measures the lifetime of retinal fluorophores independent of concentration and intensity, and can thus be used to detect retinal fluorophores other than lipofuscin.[80] In choroideremia FLIO helps identify retinal areas that retain photoreceptors in the absence of RPE by showing shorter fluorescence lifetimes in comparison to areas with combined RPE and photoreceptor loss.[81] In ABCA4 associated Stargardt disease FLIO has been shown to change over time thus possibly being a promising imaging modality to both monitor natural progression and response to treatments.[82]
2.2.3. Adaptive Optics Scanning Laser Ophthalmoscopy
Adaptive Optics uses a wavefront sensor to measure the ocular aberrations and compensates for them with a deformable mirror, generating non-invasive, high-resolution images of the retina.[83] The transverse resolution is approximately 2µm, which permits visualization of photoreceptors. Visualization of a single photoreceptor with the conventional confocal AOSLO (Adaptive Optics Scanning Laser Ophthalmoscopy) is enabled by the high refractive index of the photoreceptor relative to the surrounding interphotoreceptor matrix, but requires intact photoreceptor outer segments.[84] As reflectivity from the RPE can be confounded as having a photoreceptor origin, the source of photoreceptor signal continues to be debated.[85,86] In non-confocal methods, such as split-detector AOSLO, the signal is believed to arise from the photoreceptor inner segment, which can be acquired simultaneously with confocal AOSLO, and help guide image analysis.[87] By enabling direct visualization of single photoreceptors AOLSO has the potential to directly quantify the extent of photoreceptor degeneration in RP, and to monitor therapeutic interventions. However, the lack of validation of photoreceptor-based biomarkers (i.e. density, size, reflectivity) and the difficulty of resolving single cones in the fovea as well as the absence of normative database have limited the clinical applicability of AOSLO and its usefulness in monitoring the progression of RP. Furthermore, AOSLO requires significant time resources, and commercial machines that enable equal quality to the investigational devices are still missing.
2.3. Genotyping
A striking feature of RP is its genetic heterogeneity, which makes attempts to identify the causative genetic variant often challenging. Genetic testing can however direct clinical management, yield prognosis and most importantly help identify individuals that could benefit from retinal gene therapy. Next generation sequencing (NGS) has significantly reduced time and cost needed to analyse the DNA, and thus more patients with RP are being genotyped. Historically the diagnosis of RP has been a clinical one. Genotyping has helped us understand that a same phenotype can be associated with variants in different genes, and that one single gene can give rise to a multitude of phenotypes. A genetic variant is a permanent change in the nucleotide sequence of the DNA (deoxyribonucleic acid), which can be mutated either by substitution, deletion or insertion of base pairs. Single base variants are called point mutations. Depending on the impact the single base substitution has on the codon and thus on the amino acid it codes for, a point mutation can be silent, missense, or nonsense. Deletions or insertions may lead to the translational frame being altered, which typically leads to a non-functional product. A clinical example where variants in the same gene can give rise to different phenotypes is the BEST1 gene mutation. Missense mutations give rise to the less severe phenotype of autosomal dominant Best macular dystrophy, while splicing defect can cause the more generalized ADVIRC (autosomal dominant vitreoretinochoroidopathy) syndrome.[88] Variants in ABCA4, a gene encoding a retina specific ATP-binding cassette (ABC) transporter protein (subfamily A, member 4), give rise to retinal dystrophies that are genotypically and phenotypically very heterogeneous.[89] The disease phenotype, macular dystrophy (Stargardt disease) or cone-rod dystrophy, correlates with the severity of the variant.[89–91] Knowing which genetic variant gives rise to which disease phenotype is important both for genetic counselling and identification of patients that might qualify for therapies. Variants in the PRPH2 gene can lead to a remarkable variation of retinal phenotypes ranging from macular dystrophies to RP.[92] It has been recently highlighted that PRPH2 point mutations can affect mRNA splicing with a different effect on rods and cones, respectively.[93] The PROM1 gene, may have dominant or recessive phenotypes that influence disease onset and severity. Thus, the dominant cases associated with a specific missense variant (c.1117C>T) show milder, cone-driven phenotype, suggesting that the dominant disease is preferentially associated with cone photoreceptors.[94] The M- and L-cone opsin genes situated on the long arm of the X chromosome are another example of highly variable severity of the phenotype, which can vary from mild colour deficiency to blue cone monochromacy (BCM) depending on the variant.[95] USH2A is a large gene located on chromosome 1 that causes about 30% of all Usher type 2 cases, but also about 20% of autosomal recessive RP.[2] Initially believed to be always associated with hearing impairment and visual loss, in 2000 Rivolta et al. identified a missense mutation c.2276G>T (p.Cys759Phe) which was thought to cause non-syndromic RP only.[96] RHO mutations, affecting the amino acid sequence of the rod specific protein rhodopsin, account for up to 40% of all autosomal dominant RP cases (source RetNet www.sph.uth.edu). The severity and progression rate of RHO associated autosomal dominant RP can vary depending on the affected portion of the rhodopsin molecule, where the most severe disease is thought to be associated with the cytoplasmatic edge.[97] Iannaccone et al. showed that distinct point mutations at codon position 135 can cause different disease severity and progression.[97] The arginine to lysine (R135L) missense change showed a less severe disease with a slower progression rate than the arginine to tryptophan (R135W) substitution.
Since the introduction of the first retinal gene therapy for biallelic RPE65 associated retinal dystrophy (voretigene neparvovec) in 2017, genotyping is essential to confirm the mutation and thus the eligibility for treatment. With retinal gene therapies for choroideremia (NCT03496012) and RPGR X-linked RP (NCT03116113) having reached phase 3, and other emerging exciting therapeutic fields such as optogenetics, genotyping plays a key role in the management of all patients with IRDs where early and accurate diagnosis are crucial.
3.0. Conclusion
The new era launched by the clinical approval of Luxturna® (voretigene neparvovec) and the multitude of emerging therapies for IRDs demands accurate clinical characterization and genetic testing of all patients with RP. Precise phenotyping includes assessment of both function and morphology. When assessing visual acuity, it is of paramount importance to be aware of the impact low vision and advanced visual field loss have on the ability to track letters on the chart, especially when the visual field loss affects the fovea. When choosing visual acuity as a clinical trial outcome measure, ETDRS letter charts should be used along with standardization of room illumination to achieve uniform contrast and luminance of the optotypes. A minimum of at least a three-line worsening of visual acuity over time to allow for correct interpretation of changes in visual acuity should be employed in any trials with RP patients. In addition, assessment of LLVA should be included since RP patients show a more pronounced visual acuity deficit at low luminance and the test result could be predictive for future visual acuity loss. Colour vision testing should also be included in the clinical assessment of patients with RP as good visual acuity can be retained until late in the disease course, while colour vision deficits can be present before the onset of degeneration. As a clinical trial outcome measure however colour vision performance should be handled with care as little information is known on the specificity and sensitivity in RP patients, and also both low vision and advanced field loss can have a significant impact on the results, because the Farnsworth-Munsell 100 test requires comparing and selecting coloured discs spaced across a relatively wide visual field.
The Goldmann visual field remains very useful in characterizing the extent of gross visual field loss (e.g. as a marker of toxicity), which seems to correlate well with morphological OCT markers. The central visual field is best assessed by MAIA microperimetry, which allows the simultaneous observation of the retina during the perimetric testing. MAIA microperimetry has been shown to be reliable in monitoring the central retinal sensitivity over time and is thus an important surrogate endpoint in clinical trials.
The ERG plays an important role both in the diagnosis and characterization of RP through enabling global assessment of photoreceptor function. As a clinical trial endpoint however to monitor changes in function over time, visual field sensitivity is much more readily accessible and accurate.
Multimodal imaging with OCT and fundus autofluorescence used routinely in the clinical evaluation in retinal clinics plays a pivotal role in the accurate characterization of RP patients as well. The morphological information that can be extracted from OCT images is unmatched by any other imaging tool and is best described as in vivo histological dissection of the retinal anatomy. Fundus autofluorescence gives indirect information on the level of metabolic activity of the RPE through visualizing fluorophores, and has also proven to be indispensable in the management of retinal degenerations. Detailed structural information seen in OCT images, such as the integrity and the extent of the EZ, has been shown to correlate well with function, and is thus used as outcome measure in therapeutic trials, while the lack of readily available quantification of the autofluorescence signal still limits its applicability as trial biomarker.
AOLSO has the potential to directly quantify the extent of photoreceptor degeneration in RP, and as such be of highest value to monitor therapeutic interventions. However, the lack of validation and the absence of normative database have limited the clinical applicability of AOSLO and its usefulness in monitoring the progression of RP.
Genetic testing has become absolutely essential in the clinical characterization of RP patients and is vital to determine subjects suitable for retinal gene therapy. Matching the detailed genetic information with the clinical phenotype has triggered a vast expansion of the molecular mechanisms behind RP and allows the quest for tailored, even mutation specific, therapies. The information acquired through genotyping should however always be meticulously scrutinized, and its validity be matched with the clinical picture.
4.0. Expert Opinion
State-of-the-art management of patients with RP requires a super-specialized centre with a readily available visual function assessment unit and up to date multimodal imaging. Medical supporting staff, such as optometrist, nurses and technicians should all be acquainted with the specific challenges and requirements of assessing RP patients. Initial clinical characterization must include standardized visual acuity testing using ETDRS charts as well as OCT and autofluorescence imaging. Assessment of visual field should also be part of any initial assessment, and ideally include both kinetic and static measures. While genotyping is also crucial wherever available, we believe that the importance of the ERG has diminished. Certainly, electrophysiological testing still is very valuable whenever there is diagnostic uncertainty.[43] Monitoring the progression of RP can best be achieved by adhering to the same standardized assessments of both visual function and retinal morphology. Standardized visual acuity testing using ETDRS charts and multimodal imaging with OCT and autofluorescence reassures accurate monitoring of the disease course. Assessment of the visual field can also be useful in less advanced cases. In very advanced subjects visual field testing is of low clinical utility, and has the potential to induce significant psychological stress in a patient. Similarly, we also do not advise using ERG routinely to monitor disease progression.
In the setting of a clinical trial the choice of accurate and repeatable biomarkers is essential. We recommend always using standardized ETDRS charts to test visual acuity, and to consider LLVA as well. Functional assessment should be complemented with visual field tests, ideally using MAIA microperimetry. Colour vision testing should be chosen judiciously in a clinical trial situation. Morphology must be assessed by OCT imaging where detailed structural information such as the integrity and the extent of the EZ can serve as surrogate endpoint. Fundus autofluorescence can provide additional information, however quantification still remains challenging. The usefulness of AOSLO as a surrogate marker also warrants critical judgement as it still lacks validation despite being able to directly visualize photoreceptors.
In summary, the armamentarium of clinical assessment tools with recent advances seen in multimodal imaging as well as in the efficacy of genotyping allows for most accurate characterization of patients with RP and fine monitoring of the disease course. Most importantly the detailed audit of all currently emerging therapies in the field of RP is not only driving innovation, but puts all retinal specialists at a point where realistic hope can be offered.
Article highlights box.
Comprehensive discussion of standard and innovative techniques to diagnose and monitor RP
Article discusses both functional and morphological parameters
Excellent guideline for the retinal specialist interested in the clinical management of RP patients
Excellent overview of outcome measures pertinent to the design of clinical trials in retinal degenerative diseases
Contributor Information
Moreno Menghini, Email: moreno.menghini@ndcn.ox.ac.uk, Oxford Eye Hospital and Nuffield Department of Clinical Neurosciences, Oxford University, The John Radcliffe Hospital, West Wing, Oxford OX3 9DU, United Kingdom, +41 79 704 52 58.
Jasmina Cehajic-Kapetanovic, Email: jasmina.kapetanovic@eye.ox.ac.uk, Oxford Eye Hospital and Nuffield Department of Clinical Neurosciences, Oxford University, The John Radcliffe Hospital, West Wing, Oxford OX3 9DU, United Kingdom, +44 7725 197054.
Robert E MacLaren, Email: Robert.maclaren@eye.ox.ac.uk, Oxford Eye Hospital and Nuffield Department of Clinical Neurosciences, Oxford University, The John Radcliffe Hospital, West Wing, Oxford OX3 9DU, United Kingdom, +44 1865 228974.
References
- [1].Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. [cited 2019 Sep 25];Prog Retin Eye Res. 2010 29:335–375. doi: 10.1016/j.preteyeres.2010.03.004. [Internet]. Available from: https://www.sciencedirect.com/science/article/pii/S135094621000025X. [DOI] [PubMed] [Google Scholar]
- [2].Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. [cited 2019 Sep 25];Lancet. 2006 368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [Internet]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673606697407. [DOI] [PubMed] [Google Scholar]
- [3].Pagon RA. Retinitis pigmentosa. [cited 2019 Sep 25];Surv Ophthalmol. 1988 33:137–177. doi: 10.1016/0039-6257(88)90085-9. [Internet]. Available from: https://linkinghub.elsevier.com/retrieve/pii/0039625788900859. [DOI] [PubMed] [Google Scholar]
- [4].Verbakel SK, Van Huet RAC, Boon CJF, et al. Progress in Retinal and Eye Research Non-syndromic retinitis pigmentosa. Prog Retin Eye Res. 2018;66:1–30. doi: 10.1016/j.preteyeres.2018.03.005. [Internet]. [DOI] [PubMed] [Google Scholar]
- [5].Prokisch H, Hartig M, Hellinger R, et al. A Population-Based Epidemiological and Genetic Study of X-Linked Retinitis Pigmentosa. [cited 2019 Sep 25];Investig Opthalmology Vis Sci. 2007 48:4012. doi: 10.1167/iovs.07-0071. [Internet]. [DOI] [PubMed] [Google Scholar]
- [6].Fishman GA. Retinitis Pigmentosa. [cited 2019 Sep 25]; Arch Ophthalmol. 1978 96:822. doi: 10.1001/archopht.1978.03910050428005. [Internet]. [DOI] [PubMed] [Google Scholar]
- [7].Anasagasti A, Irigoyen C, Barandika O, et al. Current mutation discovery approaches in Retinitis Pigmentosa. [cited 2019 Sep 25];Vision Res. 2012 75:117–129. doi: 10.1016/j.visres.2012.09.012. [Internet]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0042698912003033. [DOI] [PubMed] [Google Scholar]
- [8].Csaky KG, Richman EA, Ferris FL. Report from the NEI/FDA ophthalmic clinical trial design and endpoints symposium. Investig Ophthalmol Vis Sci. 2008;49:479–489. doi: 10.1167/iovs.07-1132. [DOI] [PubMed] [Google Scholar]
- [9].Ferris FL, Kassoff A, Bresnick GH, et al. New Visual Acuity Charts for Clinical Research. [cited 2019 Oct 7];Am J Ophthalmol. 1982 94:91–96. [Internet]. Available from: https://www.sciencedirect.com/science/article/pii/0002939482901970?via%3Dihub. [PubMed] [Google Scholar]
- [10].Ferris FL, Sperduto RD. Standardized illumination for visual acuity testing in clinical research. Am J Ophthalmol. 1982;94:97–98. doi: 10.1016/0002-9394(82)90198-2. [Internet]. [DOI] [PubMed] [Google Scholar]
- [11].Dong LM, Marsh MJ, Hawkins BS. Measurement and analysis of visual acuity in multicenter randomized clinical trials in the United States: findings from a survey. [cited 2019 Oct 7];Ophthalmic Epidemiol. 2003 10:149–165. doi: 10.1076/opep.10.3.149.15080. [Internet]. [DOI] [PubMed] [Google Scholar]
- [12].Sloan L, Rowland WM, Altman A. Comparison of three types of test target for the measurement of visual acuity. Q Rev Ophthalmol. 1952;8 [Google Scholar]
- [13].Rosser DA, Cousens SN, Murdoch IE, et al. How Sensitive to Clinical Change are ETDRS logMAR Visual Acuity Measurements? [cited 2019 Oct 7];Investig Opthalmology Vis Sci. 2003 44:3278. doi: 10.1167/iovs.02-1100. [Internet]. [DOI] [PubMed] [Google Scholar]
- [14].Kiser AK, Mladenovich D, Eshraghi F, et al. Reliability and consistency of visual acuity and contrast sensitivity measures in advanced eye disease. Optom Vis Sci. 2005;82:946–954. doi: 10.1097/01.opx.0000187863.12609.7b. [DOI] [PubMed] [Google Scholar]
- [15].Bittner AK, Ibrahim MA, Haythornthwaite JA, et al. Vision Test Variability in Retinitis Pigmentosa and Psychosocial Factors. [cited 2019 Oct 7]; doi: 10.1097/OPX.0b013e3182348d0b. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3223543/pdf/nihms315637.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tidbury LP, Czanner G, Newsham D. Fiat Lux: the effect of illuminance on acuity testing. [cited 2019 Oct 7];Graefe’s Arch Clin Exp Ophthalmol. 2016 254:1091. doi: 10.1007/s00417-016-3329-7. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jolly JK, Juenemann K, Boagey H, et al. Validation of electronic visual acuity (EVA) measurement against standardised ETDRS charts in patients with visual field loss from inherited retinal degenerations. [cited 2019 Oct 8];Br J Ophthalmol. 2019 0:1–8. doi: 10.1136/bjophthalmol-2019-315124. [Internet]. Available from: http://bjo.bmj.com/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Camparini M, Cassinari P, Ferrigno L, et al. ETDRS-fast: Implementing psychophysical adaptive methods to standardized visual acuity measurement with ETDRS charts. Investig Ophthalmol Vis Sci. 2001;42:1226–1231. [PubMed] [Google Scholar]
- [19].Lin RJ, Ng JS, Nguyen AL. Determinants and Standardization of Mesopic Visual Acuity. [cited 2019 Oct 8];Optom Vis Sci. 2015 92:559–565. doi: 10.1097/OPX.0000000000000584. [Internet]. [DOI] [PubMed] [Google Scholar]
- [20].Alexander KR, Derlacki DJ, Fishman GA, et al. Acuity-luminance and foveal increment threshold functions in retinitis pigmentosa. Investig Ophthalmol Vis Sci. 1991;32:1446–1454. [PubMed] [Google Scholar]
- [21].Sunness Janet S, Rubin Gary S, Broman Aimee, Applegate Carol A, Bressler Neil M, H BS. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. [cited 2019 Oct 8];Ophthalmology. 2008 443:1480–1488. doi: 10.1016/j.ophtha.2008.03.009. [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2730515/pdf/nihms125011.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lei H, Schuchard RA. Using two preferred retinal loci for different lighting conditions in patients with central scotomas. [cited 2019 Oct 8];Invest Ophthalmol Vis Sci. 1997 38:1812–1818. [Internet]. [PubMed] [Google Scholar]
- [23].Mollon JD. Color vision: Opsins and options. [cited 2019 Oct 9];Proc Natl Acad Sci. 1999 96:4743–4745. doi: 10.1073/pnas.96.9.4743. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Simunovic MP. Acquired color vision deficiency. Surv Ophthalmol. 2016;61:132–155. doi: 10.1016/j.survophthal.2015.11.004. [Internet]. [DOI] [PubMed] [Google Scholar]
- [25].Jolly JK, Groppe M, Birks J, et al. Functional Defects in Color Vision in Patients with Choroideremia. Am J Ophthalmol. 2015;160:822–831.e3. doi: 10.1016/j.ajo.2015.06.018. [Internet] [DOI] [PubMed] [Google Scholar]
- [26].Talib M, van Schooneveld MJ, van Duuren RJG, et al. Long-Term Follow-Up of Retinal Degenerations Associated With LRAT Mutations and Their Comparability to Phenotypes Associated With RPE65 Mutations. [cited 2019 Nov 24];Transl Vis Sci Technol. 2019 8:24. doi: 10.1167/tvst.8.4.24. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alahmadi BO, Omari AA, Abalem MF, et al. Contrast sensitivity deficits in patients with mutation-proven inherited retinal degenerations 11 Medical and Health Sciences 1109 Neurosciences. BMC Ophthalmol. 2018;18:1–6. doi: 10.1186/s12886-018-0982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Berson EL, Sandberg MA, Rosner B, et al. Natural course of retinitis pigmentosa over a three-year interval. [cited 2019 Sep 25];Am J Ophthalmol. 1985 99:240–251. doi: 10.1016/0002-9394(85)90351-4. [Internet]. Available from: https://linkinghub.elsevier.com/retrieve/pii/0002939485903514. [DOI] [PubMed] [Google Scholar]
- [29].Nowomiejska K, Vonthein R, Paetzold J, et al. Comparison between semiautomated kinetic perimetry and conventional goldmann manual kinetic perimetry in advanced visual field loss. Ophthalmology. 2005;112:1343–1354. doi: 10.1016/j.ophtha.2004.12.047. [DOI] [PubMed] [Google Scholar]
- [30].Barnes CS, Schuchard RA, Birch DG, et al. Reliability of semiautomated kinetic perimetry (SKP) and goldmann kinetic perimetry in children and adults with retinal dystrophies. Transl Vis Sci Technol. 2019;8 doi: 10.1167/tvst.8.3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rowe FJ, Rowlands A. Comparison of diagnostic accuracy between octopus 900 and goldmann kinetic visual fields. Biomed Res Int. 2014;2014 doi: 10.1155/2014/214829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fischer MD, Fleischhauer JC, Gillies MC, et al. A new method to monitor visual field defects caused by photoreceptor degeneration by quantitative optical coherence tomography. [cited 2019 Sep 25];Invest Ophthalmol Vis Sci. 2008 49:3617–3621. doi: 10.1167/iovs.08-2003. [Internet] [DOI] [PubMed] [Google Scholar]
- [33].Ross DF, Fishman GA, Gilbert LD, et al. Variability of Visual Field Measurements in Normal Subjects and Patients With Retinitis Pigmentosa. [cited 2019 Nov 20];Arch Ophthalmol. 1984 102:1004–1010. doi: 10.1001/archopht.1984.01040030806021. [Internet]. [DOI] [PubMed] [Google Scholar]
- [34].Bittner AK, Iftikhar MH, Dagnelie G. Test-retest, within-visit variability of Goldmann visual fields in retinitis pigmentosa. [cited 2019 Nov 20];Invest Ophthalmol Vis Sci. 2011 52:8042–8046. doi: 10.1167/iovs.11-8321. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cideciyan AV, Charng J, Roman AJ, et al. Progression in X-linked retinitis pigmentosa due to ORF15-RPGR mutations: Assessment of localized vision changes over 2 years. Investig Ophthalmol Vis Sci. 2018;59:4558–4566. doi: 10.1167/iovs.18-24931. [DOI] [PubMed] [Google Scholar]
- [36].Hood DC, Ramachandran R, Holopigian K, et al. Method for deriving visual field boundaries from OCT scans of patients with retinitis pigmentosa. [cited 2019 Oct 22];Biomed Opt Express. 2011 2:1106–1114. doi: 10.1364/BOE.2.001106. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Balasubramanian S, Uji A, Lei J, et al. Interdevice comparison of retinal sensitivity assessments in a healthy population: The CenterVue MAIA and the Nidek MP-3 microperimeters. Br J Ophthalmol. 2017:109–113. doi: 10.1136/bjophthalmol-2017-310258. [DOI] [PubMed] [Google Scholar]
- [38].Igarashi N, Matsuura M, Hashimoto Y, et al. Assessing visual fields in patients with retinitis pigmentosa using a novel microperimeter with eye tracking: The MP-3. PLoS One. 2016;11:1–10. doi: 10.1371/journal.pone.0166666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cehajic-Kapetanovic J, Xue K, Martinez-Fernandez de la Camara C, et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat Med. 2020 doi: 10.1038/s41591-020-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fishman GA, Birch DG, Holder GE, M B. Electrophysiologic testing in disorders of the retina, optic nerve and visual pathway. 2nd ed. Oxford University Press; 2001. [Google Scholar]
- [41].Marmor MF, Fulton AB, Holder GE, et al. ISCEV Standard for full-field clinical electroretinography (2008 update) [cited 2019 Oct 7];Doc Ophthalmol. 2009 118:69–77. doi: 10.1007/s10633-008-9155-4. [Internet]. [DOI] [PubMed] [Google Scholar]
- [42].Miyake YSK. In: Clinical Electrophysiology. 5th ed. Sadda SR, editor. Retina, Elsevier; 2013. pp. 202–226. [Google Scholar]
- [43].Cehajic-Kapetanovic J, Cottriall CL, Jolly JK, et al. Electrophysiological verification of enhanced S-cone syndrome caused by a novel c.755T>C NR2E3 missense variant. Ophthalmic Genet. 2019;40:29–33. doi: 10.1080/13816810.2018.1547912. [Internet] [DOI] [PubMed] [Google Scholar]
- [44].Cao D. In: Color Vision and Night Vision. 5th ed. Ryan SJ, Sadda S, Hinton DR, editors. Retina, Elsevier; 2013. pp. 285–299. [Google Scholar]
- [45].Wu DM, Fawzi AA. In: Abnormalities of Cone and Rod Function. 5th ed. Ryan SJ, Schachat AP, Sadda SR, editors. Retina, Elsevier; 2013. pp. 899–906. [Google Scholar]
- [46].Kemp CM, Jacobson SG, Roman AJ, et al. Abnormal Rod Dark Adaptation in Autosomal Dominant Retinitis Pigmentosa With Proline-23-Histidine Rhodopsin Mutation. [cited 2020 Jan 30];Am J Ophthalmol. 1992 113:165–174. doi: 10.1016/s0002-9394(14)71529-6. [Internet]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002939414715296. [DOI] [PubMed] [Google Scholar]
- [47].Chung DC, McCague S, Yu Z-F, et al. Novel mobility test to assess functional vision in patients with inherited retinal dystrophies. [cited 2020 Feb 6];Clin Experiment Ophthalmol. 2018 46:247–259. doi: 10.1111/ceo.13022. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Klein M, Birch DG. Psychophysical assessment of low visual function in patients with retinal degenerative diseases (RDDs) with the Diagnosys full-field stimulus threshold (D-FST) [cited 2020 Feb 6];Doc Ophthalmol. 2009 119:217. doi: 10.1007/s10633-009-9204-7. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. [cited 2020 Feb 6];Lancet (London, England) 2017 390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. [cited 2019 Oct 9];Arch Ophthalmol (Chicago, Ill. 1960) 2003 121:695–706. doi: 10.1001/archopht.121.5.695. [Internet] [DOI] [PubMed] [Google Scholar]
- [51].Lujan BJ, Roorda A, Knighton RW, et al. Revealing Henle’s Fiber Layer Using Spectral Domain Optical Coherence Tomography. [cited 2019 Oct 21];Investig Opthalmology Vis Sci. 2011 52:1486. doi: 10.1167/iovs.10-5946. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Staurenghi G, Sadda S, Chakravarthy U, et al. Proposed Lexicon for Anatomic Landmarks in Normal Posterior Segment Spectral-Domain Optical Coherence Tomography. [cited 2019 Oct 21];Ophthalmology. 2014 121:1572–1578. doi: 10.1016/j.ophtha.2014.02.023. [Internet]. [DOI] [PubMed] [Google Scholar]
- [53].Jonnal RS, Kocaoglu OP, Zawadzki RJ, et al. The cellular origins of the outer retinal bands in optical coherence tomography images. Investig Ophthalmol Vis Sci. 2014;55:7904–7918. doi: 10.1167/iovs.14-14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang Y, Cense B, Rha J, et al. High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography. [cited 2019 Oct 21];];Opt Express. 2006 14:4380. doi: 10.1364/OE.14.004380. [Internet]. Available from: https://www.osapublishing.org/abstract.cfm?URI=oe-14-10-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jonnal RS, Gorczynska I, Migacz JV, et al. The properties of outer retinal band three investigated with adaptive-optics optical coherence tomography. Investig Ophthalmol Vis Sci. 2017;58:4559–4568. doi: 10.1167/iovs.16-21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Srinivasan VJ, Monson BK, Wojtkowski M, et al. Characterization of outer retinal morphology with high-speed, ultrahigh-resolution optical coherence tomography. [cited 2019 Aug 14];Invest Ophthalmol Vis Sci. 2008 49:1571–1579. doi: 10.1167/iovs.07-0838. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hariri AH, Yang Zhang H, Ho A, et al. Quantification of Ellipsoid Zone Changes in Retinitis Pigmentosa Using en Face Spectral Domain-Optical Coherence Tomography Group Information: The principal investigators for the Trial of Oral Valproic Acid for Retinitis Pigmentosa Group include Paul HHS Public Access Author manuscript. [cited 2019 Oct 22];Retin Found Southwest. 2016 134:628–635. doi: 10.1001/jamaophthalmol.2016.0502. [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5317200/pdf/nihms850209.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Takahashi VKL, Takiuti JT, Jauregui R, et al. Structural disease progression in PDE6 -associated autosomal recessive retinitis pigmentosa. [cited 2019 Oct 22];Ophthalmic Genet. 2018 39:610–614. doi: 10.1080/13816810.2018.1509354. [Internet]. [DOI] [PubMed] [Google Scholar]
- [59].Cai CX, Locke KG, Ramachandran R, et al. A Comparison of Progressive Loss of the Ellipsoid Zone (EZ) Band in Autosomal Dominant and X-Linked Retinitis Pigmentosa. [cited 2019 Oct 22];2014 doi: 10.1167/iovs.14-15013. Available from: www.iovs.org. [DOI] [PMC free article] [PubMed]
- [60].Battaglia Parodi M, La Spina C, Triolo G, et al. Correlation of SD-OCT findings and visual function in patients with retinitis pigmentosa. [cited 2019 Oct 22];Graefe’s Arch Clin Exp Ophthalmol. 2016 254:1275–1279. doi: 10.1007/s00417-015-3185-x. [Internet]. [DOI] [PubMed] [Google Scholar]
- [61].Menghini M, Lujan BJ, Zayit-Soudry S, et al. Correlation of outer nuclear layer thickness with cone density values in patients with retinitis pigmentosa and healthy subjects. Investig Ophthalmol Vis Sci. 2015;56:372–381. doi: 10.1167/iovs.14-15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sousa K, Fernandes T, Gentil R, et al. Outer retinal layers as predictors of visual acuity in retinitis pigmentosa: a cross-sectional study. [cited 2019 Oct 22];Graefe’s Arch Clin Exp Ophthalmol. 2019 257:265–271. doi: 10.1007/s00417-018-4185-4. [Internet] [DOI] [PubMed] [Google Scholar]
- [63].Delori FC, Dorey CK, Staurenghi G, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. [cited 2019 Oct 23];Invest Ophthalmol Vis Sci. 1995 36:718–729. [Internet] [PubMed] [Google Scholar]
- [64].Schmitz-valckenberg S, Holz FG, Bird AC, et al. FUNDUS AUTOFLUORESCENCE IMAGING Review and Perspectives. Retina. 2008;28:385–409. doi: 10.1097/IAE.0b013e318164a907. [Internet] Available from: http://journals.lww.com/retinajournal/Abstract/2008/03000/FUNDUS_AUTOFLUORESCENCE_IMAGING__Review_and.2.aspx. [DOI] [PubMed] [Google Scholar]
- [65].Yung M, Klufas MA, Sarraf D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retin Vitr. 2016;2:1–25. doi: 10.1186/s40942-016-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Freund KB, Laud K, Lima LH, et al. ACQUIRED VITELLIFORM LESIONS. [cited 2019 Oct 23];Retina. 2011 31:13–25. doi: 10.1097/IAE.0b013e3181ea48ba. [Internet] [DOI] [PubMed] [Google Scholar]
- [67].Wing GL, Blanchard GC, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. [cited 2019 Oct 23];Invest Ophthalmol Vis Sci. 1978 17:601–607. [Internet] [PubMed] [Google Scholar]
- [68].Fahim AT, Khan NW, Zahid S, et al. Diagnostic fundus autofluorescence patterns in achromatopsia. Am J Ophthalmol. 2013 doi: 10.1016/j.ajo.2013.06.033. [DOI] [PubMed] [Google Scholar]
- [69].Burke TR, Duncker T, Woods RL, et al. Quantitative Fundus Autofluorescence in Recessive Stargardt Disease. [cited 2019 Oct 23];Investig Opthalmology Vis Sci. 2014 55:2841. doi: 10.1167/iovs.13-13624. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Robson AG, Michaelides M, Saihan Z, et al. Functional characteristics of patients with retinal dystrophy that manifest abnormal parafoveal annuli of high density fundus autofluorescence; a review and update. [cited 2019 Oct 23];Doc Ophthalmol. 2008 116:79–89. doi: 10.1007/s10633-007-9087-4. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Duncker T, Tabacaru MR, Lee W, et al. Comparison of near-infrared and short-wavelength autofluorescence in retinitis pigmentosa. [cited 2019 Oct 23];Invest Ophthalmol Vis Sci. 2013 54:585–591. doi: 10.1167/iovs.12-11176. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lima LH, Burke T, Greenstein VC, et al. Progressive Constriction of the Hyperautofluorescent Ring in Retinitis Pigmentosa. [cited 2019 Oct 23];Am J Ophthalmol. 2012 153:718–727.e2. doi: 10.1016/j.ajo.2011.08.043. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cehajic Kapetanovic J, Barnard AR, MacLaren RE. Molecular Therapies for Choroideremia. Genes (Basel) 2019;10 doi: 10.3390/genes10100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Song WK, Nanda A, JCehajic-Kapetanovic JMR. Enhanced autofluorescence ring findings in RPGR-associated retinitis pigmentosa. 2019 [Google Scholar]
- [75].Lorenz B, Wabbels B, Wegscheider E, et al. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. [cited 2019 Oct 23];Ophthalmology. 2004 111:1585–1594. doi: 10.1016/j.ophtha.2004.01.033. [Internet] [DOI] [PubMed] [Google Scholar]
- [76].Lynette F. Lipofuscin and melanin of human retinal pigment epithelium. Investig. Ophthalmol Vis Sci. 1978;17:2–3. [PubMed] [Google Scholar]
- [77].Keilhauer CN, Delori FC. Near-Infrared Autofluorescence Imaging of the Fundus: Visualization of Ocular Melanin. [cited 2019 Oct 23];Investig Opthalmology Vis Sci. 2006 47:3556. doi: 10.1167/iovs.06-0122. [Internet] [DOI] [PubMed] [Google Scholar]
- [78].Wang Z, Dillon J, Gaillard ER. Antioxidant Properties of Melanin in Retinal Pigment Epithelial Cells. [cited 2019 Oct 23];Photochem Photobiol. 2006 82:474. doi: 10.1562/2005-10-21-RA-725. [Internet] [DOI] [PubMed] [Google Scholar]
- [79].Sundelin SP, Nilsson SE, Brunk UT. Lipofuscin-formation in cultured retinal pigment epithelial cells is related to their melanin content. [cited 2019 Oct 23];Free Radic Biol Med. 2001 30:74–81. doi: 10.1016/s0891-5849(00)00444-5. [Internet] [DOI] [PubMed] [Google Scholar]
- [80].Dysli C, Wolf S, Berezin MY, et al. Fluorescence lifetime imaging ophthalmoscopy. [cited 2019 Oct 30];Prog Retin Eye Res. 2017 60:120–143. doi: 10.1016/j.preteyeres.2017.06.005. [Internet] Available from: https://linkinghub.elsevier.com/retrieve/pii/S1350946217300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dysli C, Wolf S, Tran HV, et al. Autofluorescence Lifetimes in Patients With Choroideremia Identify Photoreceptors in Areas With Retinal Pigment Epithelium Atrophy. [cited 2019 Oct 30];Investig Opthalmology Vis Sci. 2016 57:6714. doi: 10.1167/iovs.16-20392. [Internet] [DOI] [PubMed] [Google Scholar]
- [82].Dysli C, Wolf S, Hatz K, et al. Fluorescence Lifetime Imaging in Stargardt Disease: Potential Marker for Disease Progression. [cited 2019 Oct 30];Investig Opthalmology Vis Sci. 2016 57:832. doi: 10.1167/iovs.15-18033. [Internet] [DOI] [PubMed] [Google Scholar]
- [83].Roorda A, Romero-Borja F, Donnelly Iii W, et al. Adaptive optics scanning laser ophthalmoscopy. [cited 2019 Oct 23];Opt Express. 2002 10:405–412. doi: 10.1364/oe.10.000405. [Internet] [DOI] [PubMed] [Google Scholar]
- [84].Litts KM, Cooper RF, Duncan JL, et al. Photoreceptor-Based Biomarkers in AOSLO Retinal Imaging. Invest Ophthalmol Vis Sci. 2017;58:BIO255–BIO267. doi: 10.1167/iovs.17-21868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Roorda A, Zhang Y, Duncan JL. High-Resolution In Vivo Imaging of the RPE Mosaic in Eyes with Retinal Disease. [cited 2019 Oct 23];Investig Opthalmology Vis Sci. 2007 48:2297. doi: 10.1167/iovs.06-1450. [Internet] [DOI] [PubMed] [Google Scholar]
- [86].Vohnsen B. Directional sensitivity of the retina: A layered scattering model of outer-segment photoreceptor pigments. [cited 2019 Oct 23];Biomed Opt Express. 2014 5:1569–1587. doi: 10.1364/BOE.5.001569. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Scoles D, Sulai YN, Langlo CS, et al. In Vivo Imaging of Human Cone Photoreceptor Inner Segments. [cited 2019 Oct 23];Investig Opthalmology Vis Sci. 2014 55:4244. doi: 10.1167/iovs.14-14542. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mullins RF, Kuehn MH, Faidley EA, et al. Differential macular and peripheral expression of bestrophin in human eyes and its implication for Best disease. Investig Ophthalmol Vis Sci. 2007;48:3372–3380. doi: 10.1167/iovs.06-0868. [DOI] [PubMed] [Google Scholar]
- [89].Cornelis SS, Bax NM, Zernant J, et al. In Silico Functional Meta-Analysis of 5,962 ABCA4 Variants in 3,928 Retinal Dystrophy Cases. Hum Mutat. 2017;38:400–408. doi: 10.1002/humu.23165. [DOI] [PubMed] [Google Scholar]
- [90].Kjellström U. Association between genotype and phenotype in families with mutations in the ABCA4 gene. [cited 2019 Oct 30];Mol Vis. 2014 20:89–104. [Internet] [PMC free article] [PubMed] [Google Scholar]
- [91].Simonelli F, Testa F, Zernant J, et al. Genotype-Phenotype Correlation in Italian Families with Stargardt Disease. [cited 2019 Oct 30];Ophthalmic Res. 2005 37:159–167. doi: 10.1159/000086073. [Internet] [DOI] [PubMed] [Google Scholar]
- [92].Boon CJF, den Hollander AI, Hoyng CB, et al. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog Retin Eye Res. 2008;27:213–235. doi: 10.1016/j.preteyeres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- [93].Becirovic E, Böhm S, Nguyen ONP, et al. In Vivo Analysis of Disease-Associated Point Mutations Unveils Profound Differences in mRNA Splicing of Peripherin-2 in Rod and Cone Photoreceptors. In: Swaroop A, editor. PLOS Genet. Vol. 12. 2016. [cited 2019 May 31]. p. e1005811. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Cehajic-Kapetanovic J, Birtel J, McClements ME, et al. Clinical and Molecular Characterization of PROM1-Related Retinal Degeneration. JAMA Netw open. 2019;2:e195752. doi: 10.1001/jamanetworkopen.2019.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mizrahi-Meissonnier L, Merin S, Banin E, et al. Variable Retinal Phenotypes Caused by Mutations in the X-Linked Photopigment Gene Array. [cited 2019 Oct 30];Investig Opthalmology Vis Sci. 2010 51:3884. doi: 10.1167/iovs.09-4592. [Internet] [DOI] [PubMed] [Google Scholar]
- [96].Rivolta C, Sweklo EA, Berson EL, et al. Missense mutation in the USH2A gene: association with recessive retinitis pigmentosa without hearing loss. [cited 2019 Oct 30];Am J Hum Genet. 2000 66:1975–1978. doi: 10.1086/302926. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Iannaccone A, Man D, Waseem N, et al. Retinitis pigmentosa associated with rhodopsin mutations: Correlation between phenotypic variability and molecular effects. Vision Res. 2006;46:4556–4567. doi: 10.1016/j.visres.2006.08.018. [DOI] [PubMed] [Google Scholar]