Significance
This study indicates that a subpopulation of tumor cells expresses both PD-1 and PD-L1, which decreases the tumor growth by suppressing the canonical signaling pathways, i.e. the AKT and ERK1/2 pathways. In the absence of adaptive immune system, tumor cell-intrinsic PD-1/PD-L1 mediates the resistance to the treatment with FDA-approved anti-PD-1/PD-L1 antibodies by activating AKT and ERK1/2. These findings provide an additional explanation for resistance to cancer immunotherapy.
Keywords: tumor cell-intrinsic PD-1, tumor cell-intrinsic PD-L1, biomarker, tumor suppressor, drug resistance
Abstract
The programmed cell death 1 (PD-1) receptor on the surface of immune cells is an immune checkpoint molecule that mediates the immune escape of tumor cells. Consequently, antibodies targeting PD-1 have shown efficacy in enhancing the antitumor activity of T cells in some types of cancers. However, the potential effects of PD-1 on tumor cells remain largely unknown. Here, we show that PD-1 is expressed across a broad range of tumor cells. The silencing of PD-1 or its ligand, PD-1 ligand 1 (PD-L1), promotes cell proliferation and colony formation in vitro and tumor growth in vivo. Conversely, overexpression of PD-1 or PD-L1 inhibits tumor cell proliferation and colony formation. Moreover, blocking antibodies targeting PD-1 or PD-L1 promote tumor growth in cell cultures and xenografts. Mechanistically, the coordination of PD-1 and PD-L1 activates its major downstream signaling pathways including the AKT and ERK1/2 pathways, thus enhancing tumor cell growth. This study demonstrates that PD-1/PD-L1 is a potential tumor suppressor and potentially regulates the response to anti-PD-1/PD-L1 treatments, thus representing a potential biomarker for the optimal cancer immunotherapeutic treatment.
Cancer is one of the leading causes of death worldwide (1). The pathogenesis of tumors is complex and at least in part mediated by driver somatic mutations, which form the distinct characteristics of cancer cells. It is also able to escape immune surveillance, which is defined as a hallmark of cancer (2). Immune checkpoints are crucial regulatory pathways that mediate the escape of tumor cells from immune-mediated destruction (3, 4). The programmed cell death 1 (PD-1) receptor is one of the crucial immune checkpoint molecules and is mainly expressed on mature cytotoxic T lymphocytes in peripheral tissues and the tumor microenvironment (TME) (5, 6). PD-1 signaling is mediated via engagements of its two ligands, PD-L1 and PD-L2, which are mainly expressed by cancer cells, thus leading to immune tolerance (7). Consequently, treatment strategies based on these molecules have been developed and are known as immune checkpoint therapy (ICT) (8–10).
A number of antibodies targeting PD-1 or PD-L1 have demonstrated benefits in the treatment of several tumor types, including melanoma (11, 12), Hodgkin’s lymphoma (13), renal cell carcinoma (14) and nonsmall cell lung cancer (NSCLC) (15), compared to standard chemotherapy or molecular targeted therapy. However, effectiveness in only a small fraction of patients and resistance after initial response are commonly observed (16, 17). Moreover, like other therapies, PD-1/PD-L1-targeted antibody therapies may lead to side effects and toxicities, which mainly include immune-related adverse events associated with inflammatory conditions (18) and cardiac toxicity (19). Notably, two atypical responses, i.e., hyperprogressive disease (HPD) and pseudoprogressive disease (PPD) have been observed after the ICT (20, 21). This evidence demonstrates that the mechanism underlying ICT targeting PD-1/PD-L1 remains incompletely understood.
Clearly, the molecular understanding of PD-1 is mainly confined to the interaction between the immune system and tumor cells (5). Recent studies have revealed that PD-1 plays important roles in cancers lacking adaptive immunity (22–24). However, the potential function and mechanism of PD-1 expressed on tumor cells remain largely unknown. Here, we found that tumor cells express both PD-1 and PD-L1. In the absence of adaptive immunity, the PD-1/PD-L1 signaling axis suppresses the tumor growth via canonical signaling pathways, including protein kinase B (AKT) and extracellular regulated protein kinases1/2 (ERK1/2). Our study highlights the molecular function and mechanism of the PD-1 signaling pathway and expands the understanding of the effects of ICT antibodies on tumor cells. Furthermore, these findings shed light on tumor-cell intrinsic PD-1 as a potential biomarker for ICT selection in patients.
Results
PD-1 Is Expressed by a Subpopulation of Tumor Cells.
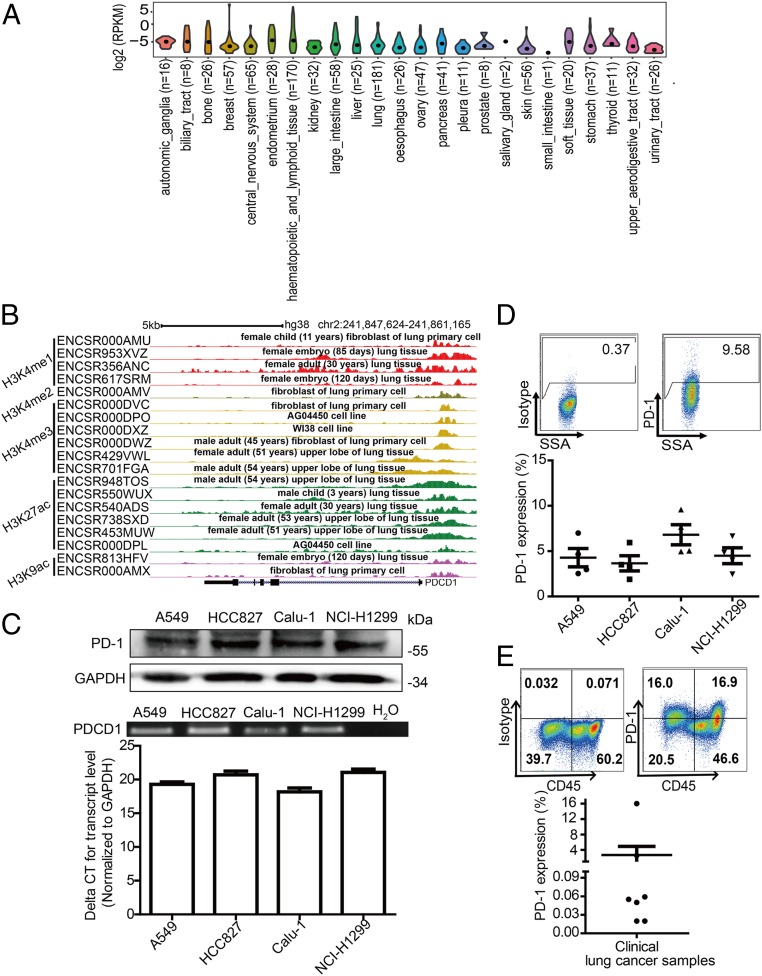
To investigate whether PD-1 is expressed on tumor cells, we analyzed the expression profile of the PDCD1 gene encoding PD-1 in data from The Cancer Genome Atlas (TCGA) database and found that human PDCD1 was widely transcribed in 32 cancer tissue types (SI Appendix, Fig. S1A). However, these isolated tissues may include infiltrated lymphocytes. Therefore, we further examined PDCD1 transcription in data from the Cancer Cell Line Encyclopedia (CCLE) database, which includes pure cancer cell lines. These established cancer cell lines also exhibited PDCD1 transcription (Fig. 1A). We also observed that five epigenetic signatures commonly associated with the activation of transcription were enriched around the transcriptional start sites of PDCD1 in data from the Encyclopedia of DNA Elements (ENCODE) database (Fig. 1B). Together, these data indicate that PDCD1 is transcribed by cancer cells.
Fig. 1.
PD-1 expression by tumor cells. (A) Violin plots showing the expression levels of PDCD1 in various kinds of cancer cells based on data from Cancer Cell Line Encyclopedia (CCLE). The black dot indicates the median. (B) The relative binding enrichments of H3K4me1, H3K4me2, H3K4me3, H3K27ac, and H3K9ac around the PDCD1 gene determined from the indicated datasets of the Encyclopedia of DNA Elements (ENCODE) database. (C) Immunoblot for PD-1 protein expression (Top), and RT-PCR (Middle) and qRT-PCR (Bottom) expression analysis of PDCD1 mRNA levels in lung cancer cell lines. (D) Representative flow cytometry plots (Top) and percentages (mean ± SDs [SD], Bottom) of PD-1 surface protein expression on human lung cancer cell lines (n = 4 independent experiments, respectively). Different shapes represent different cancer cell lines. (E) Representative flow cytometry plots (Top) and percentages (mean ± SD, Bottom) of PD-1 surface protein expression on clinical tumor biopsy-derived lung cancer cells from n = 7 distinct lung cancer patients.
To verify this observation, we next evaluated PDCD1 expression in a range of cancer cell lines that are used in our laboratory, including 40 cell lines representing 13 kinds of cancers (SI Appendix, Fig. S1B). RT-PCR, qRT-PCR, and sequencing all confirmed that PDCD1 messenger RNA (mRNA) was expressed in all examined cancer cell lines (Fig. 1C and SI Appendix, Fig. S1 B and C). Immunoblot analysis revealed that PD-1 was expressed by NSCLC cell lines and that the expressed PD-1 was ∼55 KDa in size, which is similar to the size of T cell-expressed PD-1 (Fig. 1C) (25). Moreover, flow cytometry revealed that PD-1 was expressed in a subpopulation of all examined cancer cells (PD-1 positively ranged from 2.48 to 68.14%) (Fig. 1D and SI Appendix, Fig. S2 and Table S1). PD-L1 has been demonstrated to be a major ligand that binds to PD-1 expressed on T cells to promote immune surveillance escape (26). We also examined whether PD-L1 is expressed on these cancer cells. Indeed, PD-L1 was expressed on these cancer cell lines with a range of PD-L1 positivity from 1.02 to 97.2% (SI Appendix, Table S1 and Fig. S2). It is widely known that interferon-γ (IFN-γ) stimulation up-regulates PD-L1 expression on tumor cells (27), so we further examined the effects of IFN-γ on the expression of PD-1 and PD-L1 in tumor cells. Indeed, IFN-γ induced elevated expression of PD-L1 but not PD-1 at either the mRNA or cell surface protein levels (SI Appendix, Fig. S3 A–D). Next, we explored whether PD-1 is expressed in clinical samples. Flow cytometry analysis of single-cell suspensions derived from clinical tumor specimens (seven patients) revealed PD-1 was expressed in two of the seven clinical samples, with PD-1 positive cells accounting for more than 2.5% subpopulation of lung cancer cells, which were negative for the pan-lymphocyte marker CD45, and the endothelial marker CD31 (Fig. 1E and SI Appendix, Fig. S4). Overall, these data demonstrate that PD-1 is expressed in a subpopulation of cancer cells.
PD-1 Inhibits Tumor Cell Growth and Activation of AKT and ERK1/2.
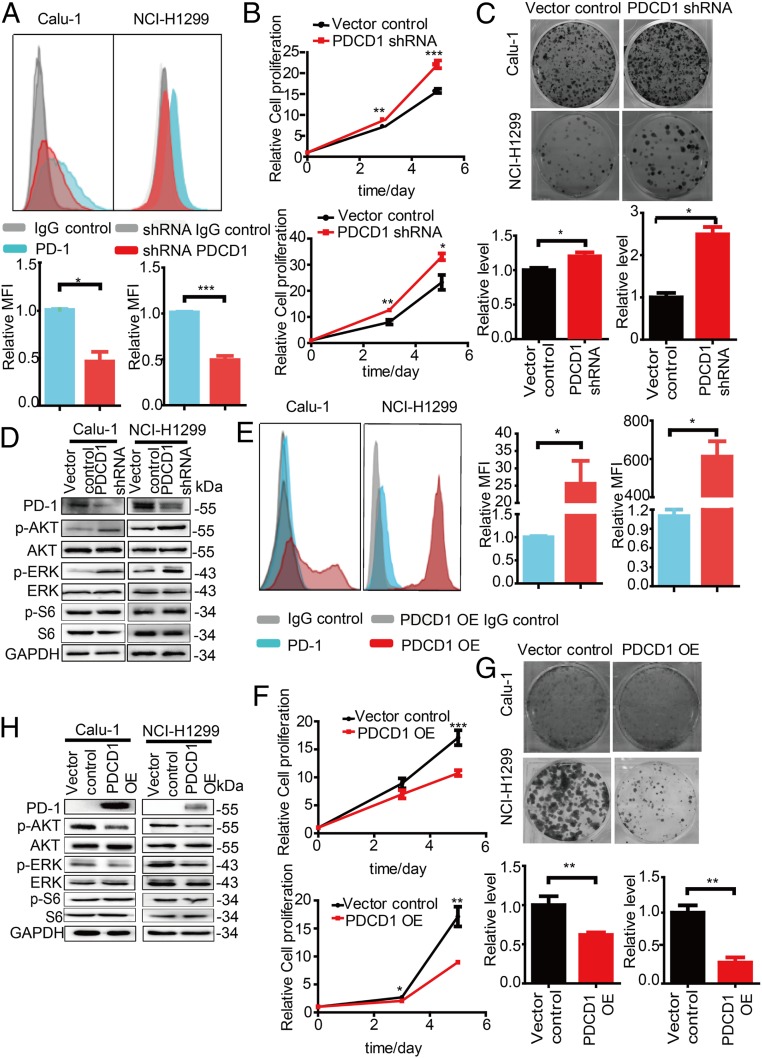
To explore the potentially underlying role of tumor cell-intrinsic PD-1 in tumor cells, we used RNA interference (RNAi) with one short hairpin RNA (shRNA) and one short interfering RNA (siRNA) that target the different sequence of PDCD1 to knockdown PDCD1 expression in NCI-H1299 and Calu-1 cells. Correspondingly, the mRNA and protein levels were significantly reduced in PD-1-depleted cells compared to control cells (Fig. 2 A and D and SI Appendix, Figs. S5A, S6 A and B, and S7 A, B, and E). PDCD1 silencing resulted in the increased cell proliferation and colony formation (Fig. 2 B and C and SI Appendix, Fig. S6 A and C). PD-1 regulates several downstream signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)/AKT, MAPK/ERK1/2, and mammalian target of rapamycin (mTOR) pathways, in T cells (25), so we further examined whether these major signaling pathways are regulated by PD-1 expressed on tumor cells. After PDCD1 knockdown, phospho(p)-AKT and p-ERK1/2 levels were increased in the PDCD1 knockdown cells compared to control cells, but the level of p-S6, an indicator of mTOR activity, was unchanged (Fig. 2D). Conversely, we overexpressed PDCD1 in both cell lines, as indicated by the observed increases in the mRNA and protein levels (Fig. 2E and SI Appendix, Figs. S5B and S7 C, D, and F) and found inhibition of cell proliferation and colony formation (Fig. 2 F and G). Correspondingly, cells with overexpression of PDCD1 showed decreased p-AKT and p-ERK1/2 levels but not p-S6 level compared to control cells (Fig. 2H). Overall, these data demonstrate that tumor cell-intrinsic PD-1 is a potential tumor suppressor that deregulates the AKT and ERK1/2 signaling pathways.
Fig. 2.
Inhibition of tumor growth by tumor cell-intrinsic PD-1. (A) Representative flow cytometry plots showing changes in the PD-1 levels on the cell surface of Calu-1 and NCI-H1299 cells 72 h after transfection with the indicated plasmids (Top). Quantitation of PD-1 surface levels is shown as the mean fluoresce intensity (MFI) (Bottom). (B) A Cell Titer-Glo Luminescent Cell Viability (CTG) assay for the cell proliferation of Calu-1 (Top) and NCI-H1299 (Bottom) cells transfected with the indicated shRNAs. (C) Representative images of a colony formation assay (Top) and quantification data (down) for Calu-1 (Left) and NCI-H1299 (Right) cells transfected with the indicated shRNAs. (D) Immunoblot analysis of the indicated proteins in cells transfected with the indicated plasmids. (E) Representative flow cytometry plots showing changes in the PD-1 levels on the cell surface of Calu-1 and NCI-H1299 cells 72 h after transfection with the indicated plasmids (Left). Quantitation of PD-1 surface levels is shown as MFI (Right). (F) The CTG assay assessing the cell proliferation of Calu-1 (Top) and NCI-H1299 (Bottom) cells transfected with the indicated shRNAs. (G) Representative images of a colony formation assay (Top) and quantification data (down) for Calu-1 (Left) and NCI-H1299 (Right) cells transfected with the indicated shRNAs. (H) Immunoblot analysis of the indicated proteins in cells transfected with the indicated plasmids. Data are presented as the mean ± SD from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.01.
PD-L1 Inhibits Tumor Cell Growth and Activation of AKT and ERK1/2.
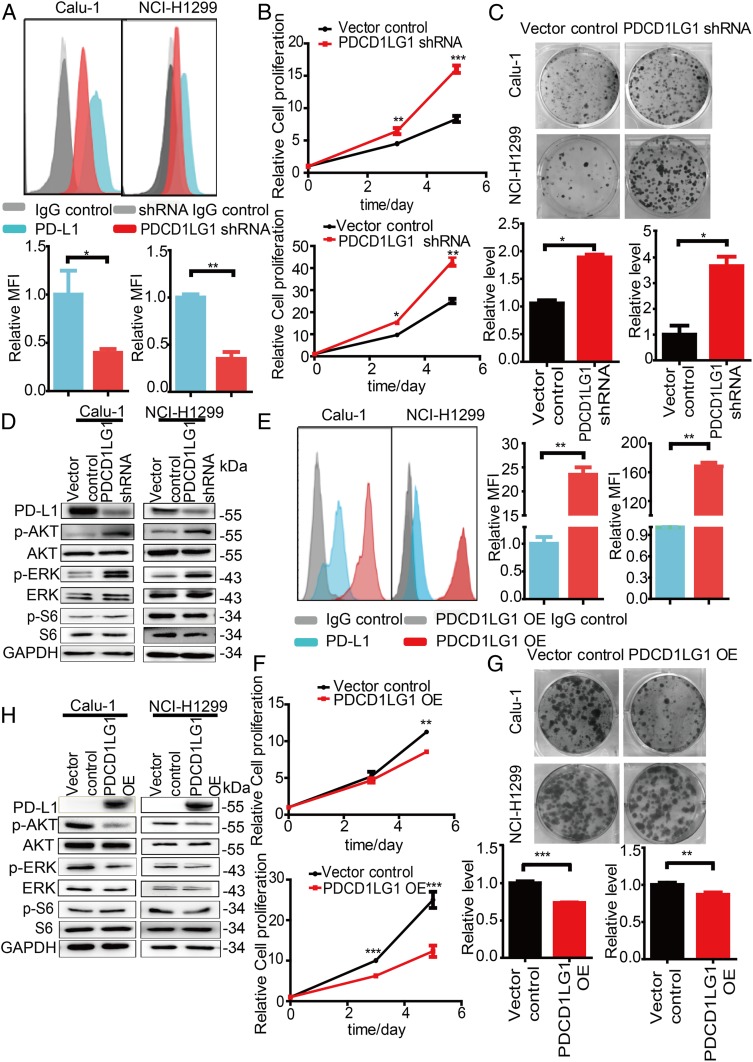
PD-L1 is a predominant ligand that engages PD-1 to inhibit the activation of T cells and is expressed on lung cancer cells to mediate cancer cell escape from immune-mediated destruction (5). As shown above, PD-L1 is expressed on NCI-H1299 and Calu-1 cells (SI Appendix, Table S1 and Fig. S2); thus, we hypothesized that PD-L1 may have effects similar to those of PD-1 on tumor cells. We also used the RNAi (one shRNA and one siRNA that target the different sequence of PDCD1LG1) to knockdown endogenous PDCD1LG1, which encodes PD-L1, in both tumor cell lines. The mRNA and protein levels were correspondingly reduced in PD-L1-depleted cells compared to control cells (Fig. 3 A and D and SI Appendix, Figs. S5C, S6 D and E, and S8 A, B, and E). The PDCD1LG1 knockdown cells showed enhanced proliferation and colony formation in comparison with the control cells (Fig. 3 B and C and SI Appendix, Fig. S6 D and F). Consistent with the results for the PD-1-depleted tumor cells, we observed increased p-AKT and p-ERK1/2 expression and unchanged p-S6 expression in the PD-L1-depleted cells compared to the control cells (Fig. 3D and SI Appendix, Fig. S6E). We further overexpressed PDCD1LG1 in both cell lines, as indicated by the observed increases in the mRNA and protein levels (Fig. 3 E and H and SI Appendix, Figs. S5D and S8 C, D, and F). Notably, PD-L1 significantly inhibited cell proliferation and colony formation in the cells overexpressing PDCD1LG1 compared to the control cells (Fig. 3 F and G). Indeed, we detected decreased AKT and ERK1/2 phosphorylation and unchanged S6 phosphorylation in the PDCD1LG1-overexpressing tumor cells compared to the control cells (Fig. 3H). Together, these data demonstrate that PD-L1 inhibits tumor cell growth and inactivates AKT and ERK1/2.
Fig. 3.
Inhibition of tumor growth by tumor cell-intrinsic PD-L1. (A) Representative flow cytometry plots showing changes in the PD-L1 levels on the cell surface of Calu-1 and NCI-H1299 cells 72 h after transfection with the indicated plasmids (Top). Quantitation of PD-L1 surface levels is shown as the MFI (Bottom). (B) The CTG assay assessing the cell proliferation of Calu-1 (Top) and NCI-H1299 (Bottom) cells transfected with the indicated shRNAs. (C) Representative images from a colony formation assay (Top) and quantification data (Bottom) for Calu-1 (Left) and NCI-H1299 (Right) cells transfected with the indicated shRNAs. (D) Immunoblot analysis of the indicated proteins for cells transfected with the indicated plasmids. (E) Representative flow cytometry plots showing changes in the PD-L1 levels on the cell surface of Calu-1 and NCI-H1299 cells 72 h after transfection with the indicated plasmids (Left). Quantitation of PD-L1 surface levels is shown as the MFI (Right). (F) The CTG assay assessing the cell proliferation of Calu-1 (Top) and NCI-H1299 (Bottom) cells transfected with the indicated shRNAs. (G) Representative images of a colony formation assay (Top) and quantification data (Bottom) for Calu-1 (Left) and NCI-H1299 (Right) cells transfected with the indicated shRNAs. (H) Immunoblot analysis of the indicated proteins in cells transfected with the indicated plasmids. Data are presented as mean ± SD from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
PD-1 and PD-L1 Depletion Enhances Tumorigenicity In Vivo.
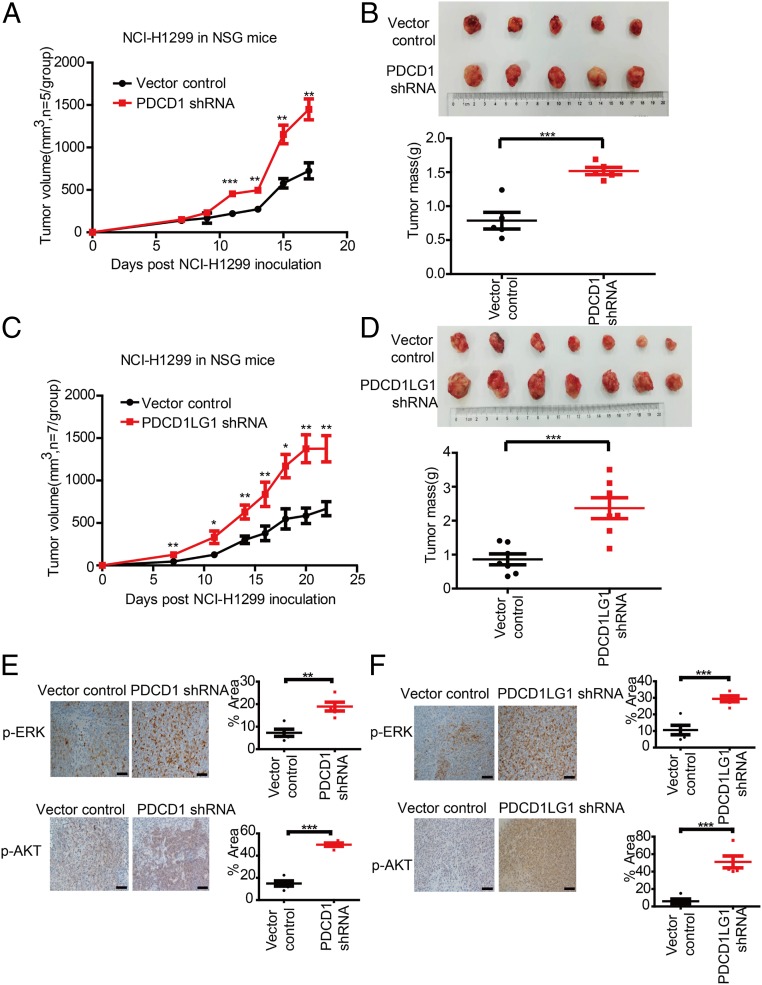
To study the roles of PD-1 and PD-L1 in tumorigenicity in vivo, we inoculated NCI-H1299 cells transfected with a control or PDCD1-specific shRNA into nonobese diabetic (NOD)/severe combined immunodeficiency disease (SCID)/interleukin (IL)-2Rγnull (NSG) mice. Compared to control expression, knockdown of PDCD1 expression significantly enhanced the tumor growth (Fig. 4A). Moreover, the mean tumor weight was markedly higher in the PDCD1-knockdown group than in the control group at the end of the experiment (Fig. 4B). We further examined p-AKT and p-ERK levels by immunohistochemistry (IHC). We performed IHC after the mice were killed and found that the tumors formed by the PDCD1 shRNA-transfected cells exhibited increases in p-AKT and p-ERK levels compared to those formed by the control cells (Fig. 4E). Consistently, compared to control expression, PDCD1LG1 knockdown strongly enhanced the tumor growth of in vivo xenograft NCI-H1299 cells in either size or weight (Fig. 4 C and D). IHC also revealed that p-AKT and p-ERK levels in the PDCD1LG1-knockdown group were significantly increased compared to those in the control group (Fig. 4F). Collectively, these data indicate that PD-1 and PD-L1 negatively regulate tumor cell growth in vivo and therefore complement the results of the in vitro functional studies.
Fig. 4.
Knockdown of PDCD1/PDCD1LG1 promotes in vivo tumor growth and enhances AKT and ERK1/2 activities. (A and C) Effects of the indicated plasmids transfected into NCI-H1299 cells on tumor growth in s.c. implanted NSG mice. (B and D) Tumor size (Top) and mass (Bottom) in NSG mice s.c. implanted with NCI-H1299 cells transfected with PDCD1 (n = 5) and PDCD1LG1 (n = 7) knockdown versus those s.c. implanted with control NCI-H1299 cells at the end point. (E and F) Representative images of p-ERK and p-AKT IHC (Left) and quantification of the signal intensities (Right) of tumor biospecimens from the indicated xenografts. Data represent the mean ± SD. (Scale bars, 20 μm.) *P < 0.05, **P < 0.01, ***P < 0.001.
PD-1/PD-L1 Axis Functions on Growth of Cancer Cells and Signaling Pathway.
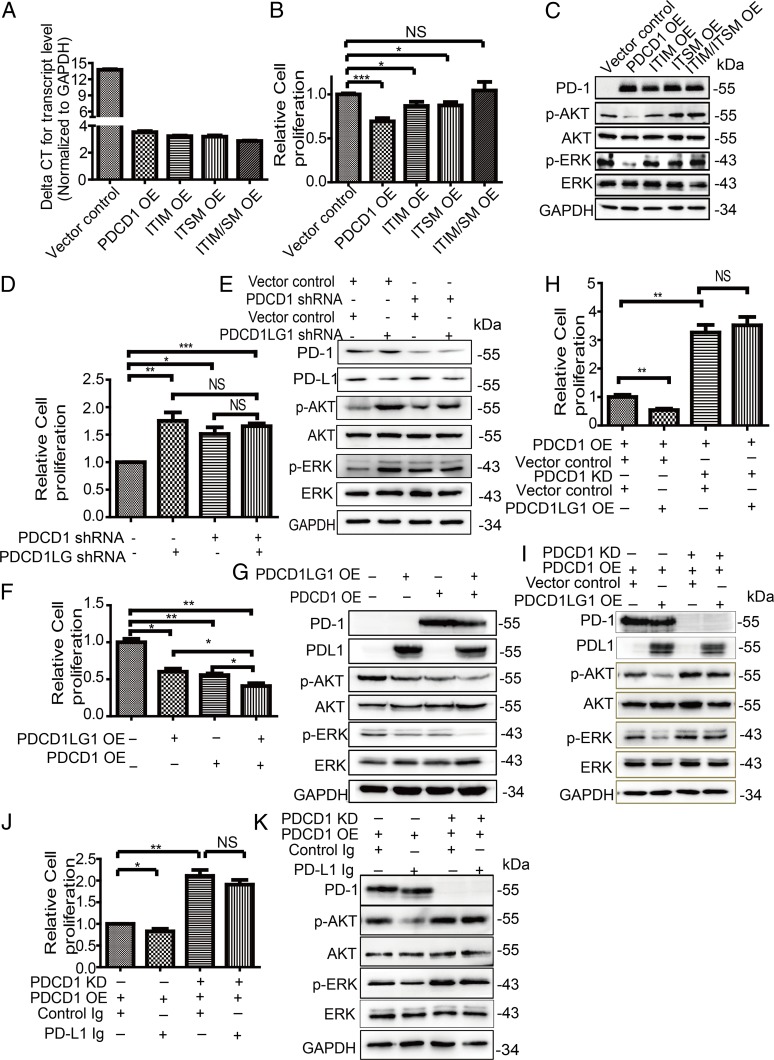
As we showed that PD-1 and PD-L1 had similar effects on tumor cell growth both in vitro and in vivo, we wondered whether tumor cell-intrinsic PD-1 is engaged by PD-L1 to regulate tumor cell growth and the corresponding signaling pathway. We first determined whether tumor cell-intrinsic PD-1 signaling is required to efficiently inhibit tumor cell growth. As PD-1 signaling transduction depends on the immunoreceptor tyrosine-based inhibitory motif (ITIM) and the immunoreceptor tyrosine-based switch motif (ITSM) of the PD-1 cytoplasmic tail in immune cells, we generated three mutants including tyrosine 225 mutated into a phenylalanine (Y225F) in the ITIM, Y248F in the ITSM, and both mutations (Y225F/Y248F) in the ITIM and ITSM (25, 28). Then, these mutants were overexpressed in tumor cells to reach similar levels as detected by qRT-PCR and immunoblot (Fig. 5 A and C). Overexpression of either single-point mutant resulted in slightly increased cell proliferation compared to the wild-type PDCD1 overexpression, but the double mutant completely abrogated the effects of overexpressing wild-type PDCD1 on cell proliferation (Fig. 5B). Consistently, the effects of wild-type PDCD1 overexpression on p-AKT and p-ERK levels were completely reversed by overexpression of the double mutant and partially rescued by overexpression of either single mutant (Fig. 5C). It has been shown that Src homology domain-containing tyrosine phosphatase (SHP2) is a main driver of PD-1 function and signaling in T cells (29), so we further examined whether SHP2 is involved in effects of PD-1 in tumor cells. SHP2 knockdown was not able to abrogate effects of PDCD-1 overexpression on cell proliferation and signaling in cancer cells (SI Appendix, Fig. S9 A and B). Overall, these data suggest that tumor cell-intrinsic PD-1 initiates the signaling pathways similar to those activated in T cells, but SHP2 is dispensable for PD-1 function of cancer cells.
Fig. 5.
Effects of PD-1 depends on PD-L1. (A–C) Relative PDCD1 mRNA expression (A), relative cell proliferation (B), and immunoblot analysis of the indicated proteins (C) of cells expressing the indicated plasmids. (D–I) Relative cell proliferation (D, F, and H) and immunoblot analysis of the indicated proteins (E, G, and I) of cells expressing the indicated plasmids. (J and K) Relative cell proliferation (J) and immunoblot analysis of the indicated proteins (K) of cells expressing the indicated plasmids and/or treated with control Ig and/or PD-L1 Ig. Three independent experiments were performed for each analysis. Data represent the mean ± SEM (SEM). NS, no significance. *P < 0.05, **P < 0.01, ***P < 0.001.
As the effects of PD-1 and PD-L1 on cell proliferation and signaling pathway were found, we next explored the effects of the simultaneous knockdown of PDCD1 and PDCD1LG1 expression. Immunoblot verified knockdown efficiencies and found that all knockdown cells exhibited increased cell proliferation compared to control cells, but the simultaneous double knockdown of both PDCD1 and PDCD1LG1 expression did not further increase cell proliferation compared to either PDCD1 or PDCD1LG1 knockdown alone (Fig. 5 D and E). Consistently, knockdown of either PDCD1 or PDCD1LG1 expression enhanced p-AKT and p-ERK levels, but the simultaneous double knockdown of both did not further enhance p-AKT and p-ERK levels (Fig. 5E). As we showed that PD-1 and PD-L1 were expressed in a subpopulation of cancer cells, we hypothesized that the simultaneous overexpression of PDCD1 and PDCD1LG1 may further inhibit cell proliferation and signaling transduction. Indeed, cells simultaneously transfected with both PDCD1 and PDCD1LG1 showed significantly decreased proliferation compared to cells transfected with PDCD1, PDCD1LG1, or the control (Fig. 5F). Consistently, simultaneous overexpression of both PDCD1 and PDCD1LG1 markedly suppressed the p-AKT and p-ERK levels compared with the overexpression of either PDCD1 or PDCD1LG1 alone (Fig. 5G).
Next, we generated NCI-H1299 cells stably overexpressing PDCD1, which were silenced by control- or PDCD1-specific shRNA and then overexpressed PDCD1LG1 or treated cells with a recombinant PD-L1 Fc-fusion protein (PD-L1 immunoglobulin [Ig]), known to elicit PD-1 signaling in T cells (30). Neither PDCD1 overexpression nor PD-L1 Ig treatment suppressed the effects of PD-1 knockdown on cell proliferation or signaling activity (Fig. 5 H–K), suggesting that PD-1 transduces signaling via PD-L1. Taken together, these data demonstrate that the PD-1 and PD-L1 axis coordinate to regulate cell growth and signaling pathways in tumor cells.
Anti-PD-1/PD-L1 Antibodies Mimic the Functions of PD-1/PD-L1 in Tumor Cells.
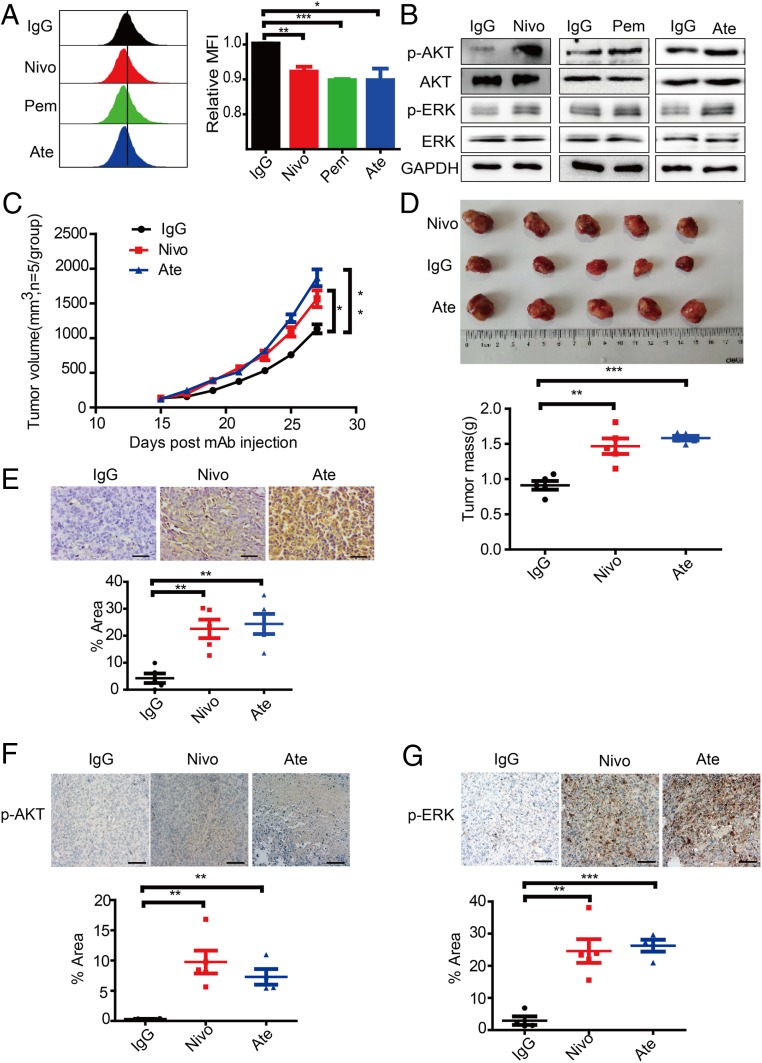
As we found that the tumor cell-intrinsic PD-1/PD-L1 interaction inhibits tumor cell growth and PD-1 signal transduction, we then hypothesized that drugs targeting the PD-1/PD-L1 axis may recapitulate the effects of PDCD1 and PDCD1LG1 knockdown. To evaluate whether clinical drugs targeting PD-1/PD-L1 are able to phenocopy the genetic manipulation of PD-1/PD-L1, we used the US Food and Drug Administration (FDA)-approved antibodies targeting PD-1/PD-L1 to block the tumor cell-intrinsic PD-1/PD-L1 signaling pathway. We treated Calu-1, SW480, HT-29, BxPC-3, SK-BR-3, and U-2 OS cells with PD-1-targeted nivolumab or pembrolizumab, PD-L1-targeted atezolizumab or isotype control antibodies for 48 h. Both 5 (6)-carboxyflurescein N-hydrxysuccinimidyl ester (CFSE) assay and xCELLigence real-time cell analysis (RTCA) assay revealed that anti-PD-1/PD-L1 antibody-treated cells exhibited increased proliferation compared to isotype control antibody-treated cells (Fig. 6A and SI Appendix, Fig. S10 A–C). Consistently, these cells treated with nivolumab, pembrolizumab, or atezolizumab had higher p-AKT and p-ERK levels than the cells treated with the isotype control antibody (Fig. 6B and SI Appendix, Fig. S10A).
Fig. 6.
Effects of clinical antibodies targeting PD-1/PD-L1. (A) Representative CFSE assay assessing the relative proliferation of Calu-1 cells after treatment with the indicated antibodies (100 µg/mL) for 48 h (Left). Quantification data are shown as MFI (Right) (n = 3). (B) Immunoblot analysis (Right) of cells after treatment with the indicated antibody (100 µg/mL) for 6 h. (C) Treatment effects of the indicated antibodies on s.c. NCI-H1299 tumor growth (n = 5). (D) Tumor size (Top) and quantification of tumor weight mass (Bottom) of the experiments in C at the end point. (E) Representative PD-1 and PD-L1 IHC (Top) and quantification of the signal intensities (Bottom) of tumor biospecimens obtained from the above-mentioned experiments. (Scale bars, 50 μm.) (F and G) Representative images of p-AKT (E) and p-ERK (F) IHC (Top) and quantification of the signal intensities (Bottom) of tumor biospecimens obtained from the above experiments. (Scale bars, 200 μm.) Data represent the mean ± SD or SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
To expand the observation of the effects of clinical antibodies on tumor cells and signaling, we inoculated NCI-H1299 cells into immunocompromised NSG mice. When the tumors recached a volume of 150 to 200 mm3, we administered nivolumab, atezolizumab, or IgG control antibodies respectively via subcutaneous (s.c.) injection every 2 d for 4 wk. Consistent with the in vitro study, we found that compared to isotype control treatment, antibody-mediated PD-1 or PD-L1 blockade significantly promoted tumor cell growth and increased tumor mass (Fig. 6 C and D). IHC showed significantly increased staining levels in the nivolumab- and atezolizumab-treated groups compared with the isotype-treated group, suggesting that these antibodies efficiently bind to the xenografted cells (Fig. 6E). Furthermore, IHC also revealed that the targeted antibody-treated groups had the higher p-AKT and p-ERK1/2 levels than the groups treated with the isotype control (Fig. 6 F and G). Together, these findings show that antibody-mediated PD-1/PD-L1 blockade directly affects tumor cells to promote tumor cell growth in the absence of adaptive immunity in vivo.
Discussion
PD-1 is mainly expressed on the activated T cells, B cells, and monocytes (31). Recent studies have shown that PD-1 is expressed in a subpopulation of various cancer cells, including melanoma (23), hepatocellular carcinoma (HCC) (32), and NSCLC (22). The expression spectrum of PD-1 on tumor cells has been expanded based on the transcriptomic and proteomic data (24). PD-1 expression on ovarian cancer cells is induced by interferon-α (IFN-α) or IFN-γ (33). Furthermore, a melanoma cell subpopulation expressing PD-1 is capable of tumor initiation (34, 35). In this study, we found that IFN-γ could induce PD-L1 expression, but not PD-1 expression in NSCLC. Consistently, the expression of PD-L1 induced by IFN-γ has been well established by other studies of tumor cells (36–38). We also demonstrated that PDCD1 is transcribed in various cancer cell lines, and that PD-1 protein expression is further confirmed by immunoblot and flow cytometry. We further revealed that PD-L1 is expressed on a subpopulation of cancer cells, which is the same or a distinct subpopulation expressing PD-1. Thus, our work clearly establishes that tumor cells contain PD-1- and PD-L1-positive subpopulations.
PD-1 expression is inducible upon the activation of T cells, and PD-1 acts as a coinhibitory receptor that functions as an immune checkpoint to maintain the peripheral immune tolerance and prevents autoimmunity (31). PD-1 ligation by PD-L1 expressed on tumor cells transduces signaling via the ITSM and ITIM of the PD-1 cytoplasmic tail, which further inhibits the PI3K/AKT, MAPK/ERK1/2, and/or mTOR, thus suppressing tumor cell growth (25). The functional role of PD-1 is currently being extended into nonimmune cell types. Tumor cell-intrinsic PD-1 has a protumor effect on melanoma and HCC via activation of the mTOR signaling pathway independently of adaptive immunity (23, 32). PD-1-targeted antibody treatment reduces the cell growth of ovarian and bladder cancer cells in the absence of adaptive immunity (33). These data suggest that tumor cell-intrinsic PD-1 is a potential oncogene. Tumor cell-intrinsic PD-L1 has also been shown to confer cancer cell resistance to proapoptotic stimuli, regulate cancer cell proliferation, or promote tumor-initiation cell generation (39–42). These data suggest that tumor cell-intrinsic PD-L1 plays a protumor effect. However, a functional study shows that murine tumor cells expressing PD-1 exhibit increased growth under PD-1-targeted antibody treatment both in vitro and in vivo, suggesting that tumor cell-intrinsic PD-1 plays an antitumor role in NSCLC (22). Consistently, our study reveals that PD-1 is a tumor suppressor that suppresses the canonical signaling pathways, such as the AKT and ERK1/2 pathways, in NSCLC in vitro and in vivo systems. However, we do not observe the activation of mTOR after PD-1/PD-L1 dysfunction. PD-1 blockade instead promotes cell proliferation and activates the AKT and ERK1/2 signaling pathways in both NSCLC and colon cancer cells. These data suggest that the antitumor function of the PD-1 is not limited to NSCLC and may function across a broad range of tumor types. These studies demonstrate that tumor cell-intrinsic PD-1 plays an antagonist function in different tumor types/cell lines. This antagonist switching in tumor cell function may be defined as the “tumor cell-intrinsic PD-1/L1 paradox,” which is mediated by the different types of tumor cells or by selective signaling pathways. The precise molecular and cellular mechanisms that mediate this paradox of PD-1 axis blockade will be further investigated in depth in future clinical studies. These findings will benefit cancer patients through the development of optimal ICT strategies.
In the TME, tumor cell-expressed PD-L1 binding to PD-1 expressed on T cells leads to T cell exhaustion, which enables the tumor cells to escape from immune-mediated destruction. Thus, the blockade of PD-1/PD-L1 unleashes antitumor T cell responses (43). Recently, the complex structures of FDA-approved antibodies targeting PD-1 or PD-L1 have provided critical information for our understanding of antibody-based PD-1/PD-L1 blockade for ICT (44–46). Antibodies targeting PD-1/PD-L1 are widely exploited to treat a broad range of tumor types by activating T cell immunity in the clinic. Amounting evidence supports the conclusion that blockade of the PD-1 signaling axis exerts antitumor activity in a subset of patients across a broad range of cancers. The response of cancer patients is associated with increased PD-L1 expression on tumor cells, elevated numbers of tumor-infiltrating lymphocytes in the TME, tumor cells with microsatellite instability, mismatch-repair deficiency or an increased mutational burden, and the existence of neoantigens (43, 47). However, a substantial proportion of patients have failed to respond or have relapsed after responding to blockade of the PD-1 axis, which can result from any defects in any of the steps mentioned above. Furthermore, oncogenic activation of tumor cells through MAPK and/or PI3K resulting from phosphatase and tension homology (PTEN) loss also contributes to resistance to PD-1/PD-L1 blockade (17). We found that antibodies targeting PD-1/PD-L1 are able to activate tumor cell-intrinsic ERK1/2 and AKT, which may confer to tumor cell resistance to antibody treatments. Moreover, HPD and PPD are increasingly recognized phenomena in clinical patients (20). PPD involves the transient enlargement of a tumor or metastatic sites before regression occurs (48, 49), and patients treated with ICT may experience a rapid paradoxical progression of their tumor with a worse clinical status, which appears to negatively impact survival, which is termed as HPD (50, 51). The underlying mechanisms or possible explanations of PPD and HPD under ICT remains completely elusive. No predictive biomarkers have been identified other than possibly advanced age (20, 51) and regulatory T cell activation (52). In this study, we identified tumor-cell intrinsic PD-1/PD-L1 function as having antitumor effect in the absence of adaptive immunity. This study may provide a possible explanation for PPD and HPD. When antibodies efficiently activate T cells, tumor cells are destroyed by the activated T cells (SI Appendix, Fig. S11A). However, if antibody-activated T cell levels are not sufficient at the starting point, tumors grow more rapidly via activation of tumor-intrinsic PD-1/PD-L1 functions and subsequently undergo regression after T cell overactivation, termed PPD. Comparably, in the presence of poor immunity or insufficiently antibody-activated T cells and/or appropriately elevated expression of PD-1/PD/L1 on tumor cells, antibody-mediated treatments would enhance tumor cell growth and overwhelm antitumor immunity, and consequently HPD occurs (SI Appendix, Fig. S11B). Indeed, an NSCLC clinical patient with substantial cancer cell-intrinsic PD-1 expression progresses rapidly after pembrolizumab treatment (22). Our study further supports this notion that the balance between T cell activation and tumor cell growth upon PD-1/PD-L1 blockade is critical in the clinical outcome of ICT (SI Appendix, Fig. S11).
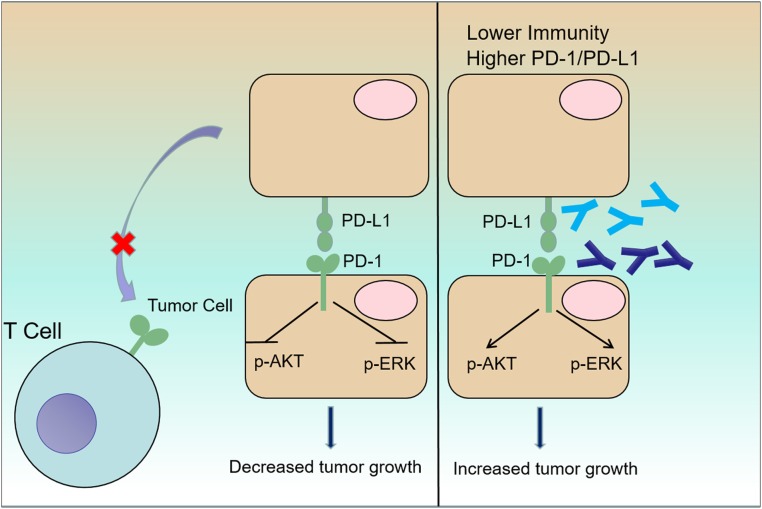
In conclusion, our data show the comprehensive characterization of PDCD1 transcription and protein expression in tumor cells in the established cancer cell lines and clinical tumor biopsies. The tumor cell-intrinsic PD-1/PD-L1 axis suppresses the tumor growth and inhibits the AKT and ERK1/2 signaling pathways, and also maybe prevents the interaction with PD-1-expressing T cells (Fig. 7). Tumor cells expressing PD-1/PD-L1 are resistant to antibodies targeting PD-1/PD-L1 treatments (Fig. 7). This study provides potential explanation for antibody-mediated resistance and/or HPD after ICT (SI Appendix, Fig. S11). The timing and dosing of PD-1/PD-L1-targeted antibodies may be important factors in clinical ICT.
Fig. 7.
A proposed model of the mechanism underlying the impact of the PD-1/PD-L1 axis on tumor growth. Tumor cells express PD-1/PD-L1, which inhibit tumor cell growth through deregulation of canonical signaling pathways, including the AKT and ERK1/2 pathways, and prevent the interaction with PD-1-expressing T cells. Clinically available antibodies targeting PD-1 (blue) or PD-L1 (cyan) enhance tumor cell growth via activation of AKT and ERK1/2 in the absence of adaptive immunity, which may be associated with HPD and PPD in the clinic.
Materials and Methods.
Clinical Specimens.
Clinical tumor specimens were obtained from Peking University Cancer Hospital. All of the patients were enrolled with written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Ethical Review Board of the Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences.
Flow Cytometric Analysis.
Cell surface expression of PD-1 and PD-L1 was detected by flow cytometry. Cells were collected and suspended in phosphate-buffered saline (PBS) containing 0.5% albumin bovine V (bovine serum albumin [BSA]). The cell density was adjusted to 1 × 106 cells/vial. Cells were incubated with allophycocyanin (APC) Goat anti-mouse IgG and Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, respectively, at room temperature for 1 h protected from light after incubated with primary PD-1 antibody and PD-L1 antibody at 4 °C for 1 h. Cells were washed with PBS for analysis. A total of 30,000 cells were analyzed on BD FACSCelesta (BD Biosciences) using the CELLQuest software. Images were analyzed by FlowJo software (FlowJo, LLC).
CellTrace CFSE and xCELLigence RTCA Cell Proliferation Assay.
For PD-1 and PD-L1 treated experiments in vitro, cancer cells were labeled with CellTrace CFSE Cell Proliferation Kit according to the manufacturer’s protocol. Cells were treated with human IgG protein (100 ug/mL), nivolumab (100 µg/mL), pembrolizumab (100 µg/mL), and atezolizumab (100 µg/mL), respectively, for 48 h prior to being analyzed using a BD FACSCelesta (BD Biosciences). The data were analyzed by FlowJo software (FlowJo, LLC). xCELLigence RTCA is used according to the manufacturer’s protocol and data were analyzed by RTCA software version 2.0 (Roche).
CellTiter-Glo Luminescent Cell Viability Assay.
Cells were plated in 96-well plates with a density of 1 × 103 or 2 × 103 cells per well. Cell proliferation was determined with a CellTiter-Glo Luminescent Cell Viability Assay kit measured with a multimode microplate reader (Synergy HTX; BioTek) every other day (53). All experiments were performed in three independent biological experiments.
Statistical Analysis.
Data are all presented as the mean ± SD or SEM. Comparisons for gene and protein levels and colony formation were performed using two-tailed unpaired Student’s t tests. Comparison of two groups are used nonparametric Mann–Whitney U test. Comparisons for three or more groups were performed using two-way ANOVA. All statistical analyses and graph plotting were performed with GraphPad Prism 8.
Data Availability.
All data in this study are available within this paper and SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by grants from Strategic Pilot Science and Technology Project (XDB29040103) and Frontier Research Program (QYZDB-SSW-SMC038) of the Chinese Academy of Sciences, National Natural Science Foundation of China (NSFC, 81773023 and 81802526), the National Key Research and Development (R&D) Program of China (2016YFC1302103), and the Technological Innovation Project of Shanxi Transformation and Comprehensive Reform Demonstration Zone (2017KJCX01). S.G. is supported in part by One-Hundred Talent of Shanxi Province.
Footnotes
Competing interest statement: G.F.G. and C.X. are both members of the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS). G.F.G. and C.X. have not collaborated directly in this position.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921445117/-/DCSupplemental.
References
- 1.Bray F., et al. , Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Vinay D. S., et al. , Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35 (suppl.), S185–S198 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Sharma P., Allison J. P., Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 161, 205–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll D. M., The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou W., Wolchok J. D., Chen L., PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8, 328rv4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L., Kroemer G., Targeting PD-1/PD-L1 interactions for cancer immunotherapy. OncoImmunology 1, 1223–1225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan S., Gao G. F., New hope for cancer treatment: Cancer immunotherapy [in Chinese]. Chin. Sci. Bull. 60, 3155–3157 (2015). [Google Scholar]
- 9.Tan S., et al. , Crystal clear: Visualizing the intervention mechanism of the PD-1/PD-L1 interaction by two cancer therapeutic monoclonal antibodies. Protein Cell 7, 866–877 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L., Han X., Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. J. Clin. Invest. 125, 3384–3391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert C., et al. , Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Schachter J., et al. , Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival analysis of KEYNOTE-006. J. Clin. Oncol. 34 (suppl. 15), 9504 (2016). [Google Scholar]
- 13.Ansell S. M., et al. , PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 372, 311–319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kammerer-Jacquet S. F., et al. , Targeting the PD-1/PD-L1 pathway in renal cell carcinoma. Int. J. Mol. Sci. 20, E1692 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi N. A., et al. , Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 16, 257–265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins R. W., Barbie D. A., Flaherty K. T., Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 118, 9–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P., Hu-Lieskovan S., Wargo J. A., Ribas A., Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puzanov I. et al.; Society for Immunotherapy of Cancer Toxicity Management Working Group , Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 5, 95 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varricchi G., et al. , Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2, e000247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurman J. S., Murgu S. D., Hyperprogressive disease in patients with non-small cell lung cancer on immunotherapy. J. Thorac. Dis. 10, 1124–1128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara R., et al. , Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 4, 1543–1552 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du S., et al. , Blockade of Tumor-Expressed PD-1 promotes lung cancer growth. OncoImmunology 7, e1408747 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleffel S., et al. , Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell 162, 1242–1256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao H., Wang H., Li C., Fang J. Y., Xu J., Cancer cell-intrinsic PD-1 and implications in combinatorial immunotherapy. Front. Immunol. 9, 1774 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia Y., Jeffrey Medeiros L., Young K. H., Signaling pathway and dysregulation of PD1 and its ligands in lymphoid malignancies. Biochim. Biophys. Acta 1865, 58–71 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topalian S. L., Drake C. G., Pardoll D. M., Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keir M. E., Butte M. J., Freeman G. J., Sharpe A. H., PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman G. J., et al. , Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arasanz H., et al. , PD1 signal transduction pathways in T cells. Oncotarget 8, 51936–51945 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francisco L. M., et al. , PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boussiotis V. A., Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 375, 1767–1778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H., et al. , Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology 66, 1920–1933 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Osta E., et al. , Tumor cell-intrinsic programmed death protein 1 expression and induction in human cancer cell lines. J. Immunol. 200 (suppl. 1), 178.33, (2018). [Google Scholar]
- 34.Schatton T., et al. , Identification of cells initiating human melanomas. Nature 451, 345–349 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schatton T., et al. , Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 70, 697–708 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mimura K., et al. , PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 109, 43–53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandai M., et al. , Dual faces of IFNγ in cancer progression: A role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin. Cancer Res. 22, 2329–2334 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Abiko K., et al. , IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 112, 1501–1509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azuma T., et al. , B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 111, 3635–3643 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gato-Cañas M., et al. , PDL1 signals through conserved sequence motifs to overcome interferon-mediated cytotoxicity. Cell Rep. 20, 1818–1829 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Clark C. A., et al. , Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 76, 6964–6974 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta H. B., et al. , Tumor cell-intrinsic PD-L1 promotes tumor-initiating cell generation and functions in melanoma and ovarian cancer. Signal Transduct. Target. Ther. 1, 16030 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribas A., Wolchok J. D., Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan S., et al. , An unexpected N-terminal loop in PD-1 dominates binding by nivolumab. Nat. Commun. 8, 14369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan S., et al. , Distinct PD-L1 binding characteristics of therapeutic monoclonal antibody durvalumab. Protein Cell 9, 135–139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu K., et al. , Structural basis of anti-PD-L1 monoclonal antibody avelumab for tumor therapy. Cell Res. 27, 151–153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelderman S., Schumacher T. N. M., Haanen J. B. A. G., Acquired and intrinsic resistance in cancer immunotherapy. Mol. Oncol. 8, 1132–1139 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiou V. L., Burotto M., Pseudoprogression and immune-related response in solid tumors. J. Clin. Oncol. 33, 3541–3543 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurra V., et al. , Pseudoprogression in cancer immunotherapy: Rates, time course and patient outcomes. J. Clin. Oncol. 34 (suppl. 15), 6580, (2016). [Google Scholar]
- 50.Ferrara R., et al. , 1306PDHyperprogressive disease (HPD) is frequent in non-small cell lung cancer (NSCLC) patients (pts) treated with anti PD1/PD-L1 monoclonal antibodies (IO). Ann. Oncol. 28, mdx380.009 (2017). [Google Scholar]
- 51.Champiat S., et al. , Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin. Cancer Res. 23, 1920–1928 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Kamada T., et al. , PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. U.S.A. 116, 9999–10008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X., et al. , The long non-coding RNA PCSEAT exhibits an oncogenic property in prostate cancer and functions as a competing endogenous RNA that associates with EZH2. Biochem. Biophys. Res. Commun. 502, 262–268 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in this study are available within this paper and SI Appendix.