Significance
Behavioral responses of prey to perceived predation risk are now recognized as important components of predator−prey interactions, but have rarely been quantified in marine vertebrates. Using telemetry data from the eastern Canadian Arctic, we document pronounced and prolonged changes in bowhead whale behavior and selection for sea ice when under perceived predation threat by killer whales. Although the energetic or fitness costs of such nonconsumptive effects (NCEs) are difficult to quantify, our results strongly suggest the ecological impacts of killer whales as apex predators extend beyond consumptive/density-mediated effects (direct mortality). Killer whale-induced NCEs may compound the negative consequences of sea ice loss on Arctic endemic marine mammals as they cope with more-frequent, longer exposures to predator threat.
Keywords: nonconsumptive effects, predator−prey dynamics, state space model, risk effects, trait-mediated interactions
Abstract
The effects of predator intimidation on habitat use and behavior of prey species are rarely quantified for large marine vertebrates over ecologically relevant scales. Using state space movement models followed by a series of step selection functions, we analyzed movement data of concurrently tracked prey, bowhead whales (Balaena mysticetus; n = 7), and predator, killer whales (Orcinus orca; n = 3), in a large (63,000 km2), partially ice-covered gulf in the Canadian Arctic. Our analysis revealed pronounced predator-mediated shifts in prey habitat use and behavior over much larger spatiotemporal scales than previously documented in any marine or terrestrial ecosystem. The striking shift from use of open water (predator-free) to dense sea ice and shorelines (predators present) was exhibited gulf-wide by all tracked bowheads during the entire 3-wk period killer whales were present, constituting a nonconsumptive effect (NCE) with unknown energetic or fitness costs. Sea ice is considered quintessential habitat for bowhead whales, and ice-covered areas have frequently been interpreted as preferred bowhead foraging habitat in analyses that have not assessed predator effects. Given the NCEs of apex predators demonstrated here, however, unbiased assessment of habitat use and distribution of bowhead whales and many marine species may not be possible without explicitly incorporating spatiotemporal distribution of predation risk. The apparent use of sea ice as a predator refuge also has implications for how bowhead whales, and likely other ice-associated Arctic marine mammals, will cope with changes in Arctic sea ice dynamics as historically ice-covered areas become increasingly ice-free during summer.
Predators alter prey behavior, causing increased vigilance, reduced activity, and shifts in habitat use that reduce predation risk. These nonconsumptive effects (NCEs) or risk effects (1–4) are now widely understood to be important predator−prey interactions (5). NCEs can be costly to individuals through lost foraging or mating opportunities (6) or stress-induced reproductive failure (7), potentially impacting population dynamics and indirectly affecting community dynamics beyond a given predator−prey relationship, sometimes strongly (8–11). Both empirical and modeling studies have demonstrated that NCEs can have greater ecological and demographic impacts than direct predation (refs. 10 and 12; but see ref. 13), and can be at least as important as resource availability in shaping distribution and habitat use of prey (14, 15).
In aquatic systems, NCEs have been demonstrated primarily in small-scale experimental or natural systems such as streams, small lakes, and tide pools (e.g., refs. 16–18). These studies have shown clear shifts in habitat use or behavior in which prey balance foraging needs against perceived predation risk by selecting less profitable but safer habitat when in good condition, but expose themselves to higher levels of predatory risk when in poorer condition (1). Extrapolating these findings to larger systems, however, is difficult (13, 19), and simply demonstrating the existence of NCEs in large marine systems has been rare. Several well-known examples of predator-mediated shifts in habitat use and behavior have been documented in marine mammals and sea turtles in the presence of sharks, sometimes with cascading effects on basal resource availability (6, 4, 20–23). More often, however, data available to quantify predator intimidation effects are limited to directly observed predator−prey interactions (e.g., ref. 24), which restricts or biases inference of NCEs at larger spatiotemporal scales.
Killer whale (Orcinus orca) presence has recently been shown to strongly alter the behavior, habitat use, and distribution of belugas (Delphinapterus leucas) and narwhals (Monodon monocercos; refs. 15 and 25). The antipredator responses of both species, which include hugging shorelines and range contractions, persisted beyond discrete predation events, raising questions about how extensive NCEs induced by marine apex predators might be in the Arctic and elsewhere. Bowhead whales (Balaena mysticetus), the only Arctic endemic baleen whale, are predated by killer whales throughout much of their range (26–29). Their association with sea ice is thought to mitigate predation risk (27, 28, 30), as killer whales avoid heavy ice cover in the Arctic (26, 27, 31, 32). Heterogeneous ice cover should therefore mediate spatial variation in predation risk, and commensurately alter prey habitat selection when predators are present (33, 34).
Here, we test for such NCEs via analysis of telemetry data collected from bowhead and killer whales tracked simultaneously in a large (63,000 km2) gulf in the eastern Canadian Arctic with persistent summer sea ice. Our data provided a rare opportunity to quantify large marine vertebrate responses to predation risk, which we derived from killer whale tracking data, across spatially heterogeneous habitat over large, ecologically relevant spatiotemporal scales. In accordance with the basic prediction of the landscape of fear model (3), we expected predation risk would modify bowhead whale association with sea ice. However, we found its impact was so strong that bowhead habitat selection and behavior could not be quantified or sensibly interpreted without knowledge of predation risk distribution in space and time. Ice-covered areas have frequently been interpreted as preferred bowhead foraging habitat in analyses that did not assess effects of predators. However, if the killer whale-induced NCEs documented here are representative of NCEs elicited by apex predators in other marine systems, our results imply that a complete understanding of marine vertebrate habitat selection and distribution may not be possible without knowledge of the spatiotemporal distribution of predation risk.
Methods
Satellite Tracking.
Eight bowhead whales were tagged with MK10 satellite transmitters (Wildlife Computers) in western Foxe Basin, southwest of Baffin Island, in June 2013 (Fig. 1 and SI Appendix, Table S1). Six (two females, three males, and one sex unknown) were juveniles 9 m to 12 m in length, while two (13 m to 14 m) were likely adult females without calves. Tags were affixed near the dorsal ridge using a hand-held fiberglass pole (details in ref. 35). The tag on the juvenile of unknown sex malfunctioned and was not analyzed. In mid-August, five killer whales from a group of ∼20 were tagged with SPOT5 satellite transmitters (Wildlife Computers) in Milne Inlet and Tremblay Sound at the northern end of Baffin Island, ∼1,200 km from the bowhead tagging location (Fig. 1). Tags were affixed to the dorsal fin using a crossbow (details in ref. 32). Two tags failed and were not analyzed; the remaining three (two adult males and one adult female or immature male) transmitted for 3 to 8 wk (SI Appendix, Table S2). We assume the killer whale group remained together through the tracking period, based on highly synchronized movements of the three tracked individuals (Movie S1).
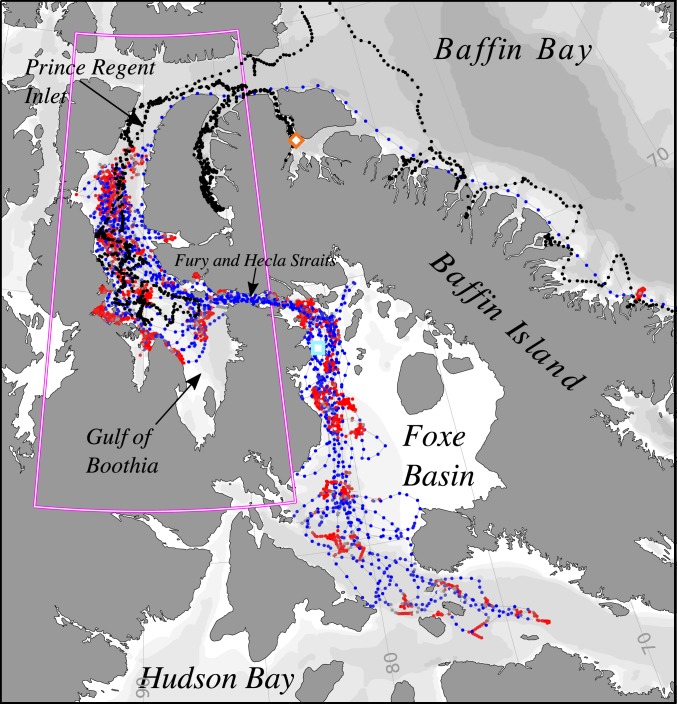
Fig. 1.
SSM fitted tracks of the three killer whales and seven bowhead whales. The fitted killer whale track (black dots) did not change states and represents the movements of the three whales, which followed nearly identical paths. The color of bowhead tracks represents SSM fitted behavioral states, with red showing inferred resident behavior, and blue showing inferred transit behavior. Lower-intensity colors indicate less certain behavioral states, with white being completely uncertain. The orange diamond indicates the location of killer whale tag deployments, and the cyan square indicates the location of bowhead whale tag deployments. See Movie S1 for a dynamic movie of predator−prey interactions and their interactions with sea ice. The magenta box surrounds the Gulf of Boothia, where interactions took place, and represents the area shown in Fig. 4.
By mid-July, all but one (a juvenile female) of the tagged bowhead whales had moved into the Gulf of Boothia, a large (∼63,000 km2) gulf with persistent summer sea ice, from the south via Fury and Hecla Strait, and remained there until October (Fig. 1). In late August, the tagged killer whales entered the Gulf of Boothia from the north via Prince Regent Inlet (Fig. 1 and Movie S1), allowing us to estimate bowhead (prey) habitat selection and behavior before (mid-July to mid-August) and during a 3-wk (late August to mid-September) period of predation threat (Fig. 2). Our general analytical and hypothesis testing approach is therefore similar to Breed et al. (15) for narwhals. However, the spatial scale is much larger, and we could directly address the effect of predation risk on prey selection for sea ice, which was not possible in that previous work.
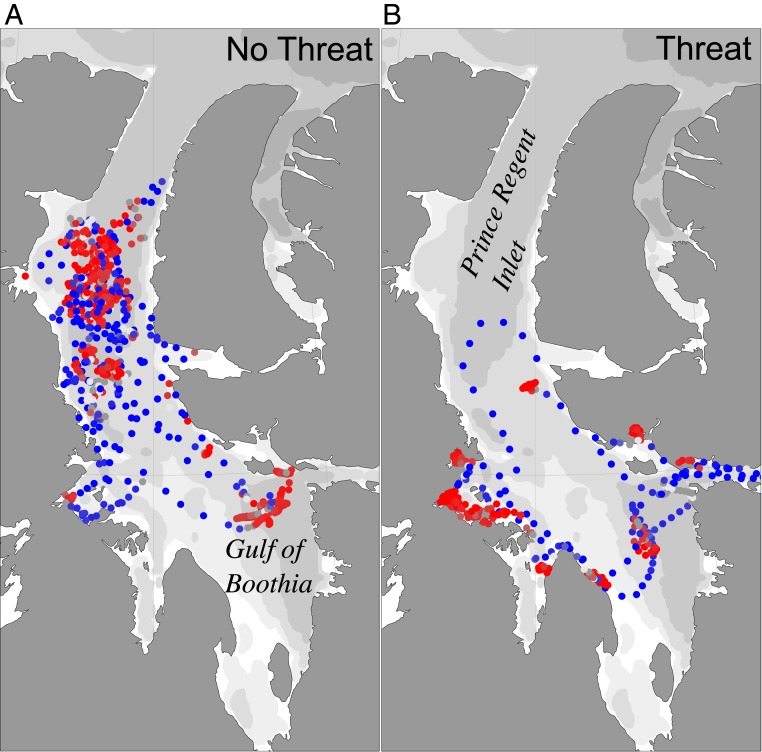
Fig. 2.
SSM fitted tracks of bowhead whales in Prince Regent Inlet and the Gulf of Boothia (A) prior to arrival of killer whales (July 23 to August 23, 2013) and (B) during the period of killer whale presence (August 24 to September 16, 2013). Red locations are SSM inferred encamped/foraging behavior, and blue points are inferred transit. Less intense colors indicate less certain state assignment, and white points are completely uncertain. See Movie S1 for a dynamic movie of interacting predator and prey, including sea ice.
Tagging procedures were approved by the Fisheries and Oceans Canada (DFO) Freshwater Institute Animal Care Committee and followed animal use protocols FWI-ACC-2013-018 and FWI-ACC-2013-022.
State Space Model Fitting.
Tracking data were first fit with a state-switching state space model (sSSM) to estimate locations from noisy Argos data and infer behavioral state (36–38). Models were run hierarchically using a 2-h time step following methods described in Breed et al. (37). We inferred, from the model, two behavioral states based on fitted movement parameters (correlation, γ, and turning angle, θ). “Resident” behavior (sometimes referred to as “foraging” or “encamped”) has fitted correlated random walk parameters with θ near 180° and γ near 0, while “transit” behavior yields fitted parameters for θ near 0° and γ near 1. After fitting, time stamps were aligned across tracks, and distances between all tracked bowhead and killer whales were calculated.
Step Selection Function.
We implemented a step selection function (SSF; refs. 14, 39, and 40) to understand how predation risk affected habitat selection of tracked bowhead whales. From each real location in an animal’s track, a set of available locations is generated by drawing a step from the fitted probability distributions describing the turning angle and step length. Depending upon the application, between 1 and 20 available steps are drawn from each real location at time t, and these potential steps are compared to the actual relocation observed at time t + 1 using a conditional logistic regression. This method was originally developed to understand how predators (wolves, Canis lupus) affected habitat selection of prey (elk, Cervus canadensis), and has since been adapted and advanced to address a wide array of behavioral hypotheses from animal telemetry data (14, 39, 40).
SSF analyses were performed on bowhead whale movement data, using the distance to the nearest tagged killer whale as an environmental covariate. Data were limited to July 25 to October 1, the open water period that included a clear no-threat period prior to killer whale arrival and a well-defined period of clear killer whale threat. Prior to July 25, the study area was largely covered by sea ice, and, after October 1, sea ice forms and bowheads begin migrating out of the system. For each real location, we generated 20 matched available locations by drawing steps from the empirical step length and turn angle distributions (39). Any steps that fell on land were redrawn so that all control locations occurred in the ocean, and then environmental covariate data at each real and control location were extracted.
The matched control cases were compared to the steps animals selected, with a series of candidate conditional logistical regression models using two different packages in R: the clogit function in the package survival (41) and the Ts.estim function in the package TwoStepCLogit (42). Both perform conditional logistic regressions but use different fitting algorithms, and TwoStepCLogit has more flexibility in random effects structures. In addition to distance to killer whales, models included the following habitat variables: sea ice concentration, distance to shore, water depth, and distance to sea ice edge (Table 1). Finally, we added a categorical variable, isice, that identifies whether a location is in front of or behind the ice edge. This allowed separate functional responses for distance to sea ice edge for locations in front of vs. behind the ice edge (see ref. 43).
Table 1.
Definitions of habitat variables used in mixed-effects and generalized linear mixed models used to assess bowhead−killer whale interactions
| Variable | Definition |
| Dsh | Distance to nearest coastline (continuous) |
| Dkw | Distance to killer whale (continuous) |
| Dedge | Distance to the sea ice edge* (continuous) |
| Depth | Water depth (continuous) |
| SIconc | Sea ice concentration (continuous) |
| Sea ice concentration squared (continuous) | |
| isice | Categorical indicating whether individuals are in front of or behind the sea ice edge* |
| B | SSM inferred behavioral state, expressed continuously between 0 and 1 |
| Bcat | SSM inferred behavioral state, expressed as two discrete categories |
| use | {0,1} categorical flag indicating if a location is a case (real location) or a conditional control location |
Sea ice edge is defined as 15% sea ice concentration; <15% concentration is in front of the edge, while >15% concentration is behind the edge.
Sea ice concentration data were collected using the Advanced Microwave Scanning Radiometer 2 (AMSR2) sensor on the Global Change Observer Mission W1 (GCOM-W1) satellite and downloaded from the Institute of Environmental Physics at the University of Bremen (44). Daily raster images at 3.125-km spatial resolution were used for our analyses. Depth data were extracted from the 1 arc-minute global relief model (ETOPO1) maintained and available for download at the US National Geophysical Data Center (45). Coastline data were global 10-m resolution vector format, downloaded from the public domain and freely available as the Natural Earth 10-m global resolution coastline version 4.0.0 (46). Sea ice, depth, and coastline raster and vector data were imported and projected using the sp package in R (47, 48). Because sea ice concentration is a proportion, it was logit-transformed. Similarly, because distances (to shore, tagged killer whales, and sea ice edge) and depths are all continuous positive, they were log-transformed. These transformations improved model fit and convergence.
The conditional logistic regression took the general form
| [1] |
| [2] |
where usei,j indicates whether a location was a true relocation (1) or a matched case-control location (0) in the conditional logistic regression. β are the linear parameter estimates on the environmental covariates (x), and νj was included as an individual random effect. Conditional logistic regressions are fit using Cox proportional hazard model to estimate relative differences within the set of matched cases (clusters); consequently, they have no intercept (β0).
Models were compared and selected using AICc (49). Note that, as our key hypothesis predicts that killer whale presence will affect bowhead habitat selection and use, the most important predictors in our model are the interaction terms between distance from bowhead to the nearest tagged killer whale (Dkw) and other habitat characteristics (sea ice, distance to shore, and depth). As our goal was biological inference and not to find the best-fitting model, our global model set included a limited set of two-way interactions to specifically assess whether killer whales affected bowhead movement and habitat selection.
Effect of Killer Whale Presence on Behavior.
The sSSM-inferred behavioral state estimates quantify the degree of directionality and persistence in movement and categorized behavior into two states. The degree to which these respective states are expressed will be affected by environmental conditions (43), including predators. Conditions affecting expressed behavior were analyzed using a linear mixed-effects model with the R package nlme (50). The model took the general form
| [3] |
where Bi,j is the sSSM inferred behavioral state at the ith location of the jth individual expressed along a continuum between 0 and 1 (see ref. 37 for discussion of using these continuous estimates). Models included a first-order autocorrelation function (φ) to correct for bias in variance estimation that occurs when observations are not temporally independent, and a random effect of individual νj. Explanatory parameters (β) were fit to the same environmental covariates (x) included in the SSF. The primary set of models included those that specifically tested hypotheses arising from the SSF results. We included a set of single-parameter models and single-parameter models plus interactions with Dkw, and also models that explored interactions between SI, Dsh, and Dkw. For completeness, we also performed a multimodel selection procedure across a wider set of possible candidate models, which is available in Dataset S1.
Results
SSM Fit and Behavioral State Estimation.
Bowhead whale movement behavior differed considerably from killer whale movement; sSSM fits easily discriminated two behavioral states in all bowhead tracks, indicating clear switches between transit (highly autocorrelated) and resident/foraging states (negatively or nonautocorrelated; see ref. 37). The sSSM fitted tracks indicated the seven bowheads moved independently and not as a social unit, although some individuals occasionally swam near (within 1 km) each other for short periods. Killer whale movement, by contrast, was not discriminated into two clear states, likely owing to a patrolling movement pattern that remained highly autocorrelated at all times (Fig. 1). The sSSM fitted tracks provided superior location estimates, and these were used in all subsequent analyses (38, 51–53).
Movement and Step Selection with and without Predation Threat.
Single covariate SSF models fitting distance to shore, depth, distance to ice edge, and sea ice concentration all improved fit compared to the null model, indicating these environmental features are important and affect bowhead whale movement (Table 2). However, adding an interaction between these covariates and distance to killer whales improved model fit greatly in all cases. For some covariates, particularly sea ice concentration, the increase in model fit when the interaction with predator distance was added (as assessed by drop in AICc score) was greater than the improvement attributable to the main effect. Importantly, distance to killer whale as a main effect was never a helpful explanatory variable; it served only as a key interaction term that modified how bowhead whales responded to environmental covariates.
Table 2.
Bowhead whale SSF model selection table for all single-variable models (italicized models) paired with a model for that variable that also includes the interaction of that variable with distance to killer whales (bolded models)
| Model | d.f. | AICc | ∆AIC | L.Ratio | p |
| use ∼ Dsh | 1 | 21,081 | 154 | — | — |
| use ∼ Dsh + Dsh:Dkw | 2 | 20,927 | 0 | 156.1 | <0.0001 |
| use ∼ Depth | 1 | 21,239 | 312 | — | — |
| use ∼ Depth + Depth:Dkw | 2 | 21,133 | 206 | 108.5 | <0.0001 |
| use ∼ Dedge*isice | 2 | 21,366 | 439 | — | — |
| use ∼ Dedge:isice + Dedge:isice:Dkw | 3 | 21,139 | 219 | 232.8 | <0.0001 |
| use ∼ SIconc + SI2conc | 2 | 21,272 | 345 | — | — |
| use ∼ SIconc + SI2conc + SIconc:Dkw + SI2conc:Dkw | 4 | 21,173 | 246 | 102.0 | <0.0001 |
| use ∼ SIconc | 1 | 21,323 | 396 | — | — |
| use ∼ SIconc + SIconc:Dkw | 2 | 21,237 | 314 | 84.0 | <0.0001 |
| use ∼ null | 1 | 21,337 | 410 | — | — |
| use ∼ Dkw | 1 | 21,348 | 421 | — | — |
In every case, adding interaction with distance to killer whales improves model fit substantially. In all cases, likelihood ratio tests indicate improvement in fit is highly significant. Also note that distance to killer whale as a single main effect fits worse than the null model. Interaction models that also include the main effect of Dkw are not shown, as they do not improve fit. See Table 1 for definition of terms. d.f., model degrees of freedom.
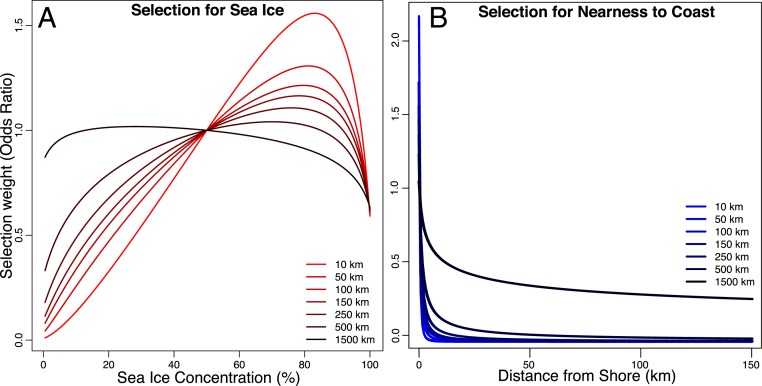
Bowhead whale use of sea ice could only be understood in the context of predation threat, as killer whale presence effectively reversed the direction of selection. Under no predation threat, the selection surface for sea ice concentration was essentially equivalent across sea ice conditions, with perhaps a slight preference for lower sea ice concentrations (Fig. 3). Distance to ice edge was an extremely important parameter, but only in the context of predator presence (it explains no more variance than the null model as a main effect). When killer whales were not present, bowheads preferred areas a short distance (10 to 50 km) in front of the sea ice edge. When killer whales were present and bowheads were already behind the ice edge, they preferred to move farther from the ice edge into areas of ∼80% sea ice concentration (Fig. 3). Similarly, there is weak selection for areas closer to shore in the absence of killer whales, but selection for nearshore areas intensifies as killer whales move increasingly closer (Fig. 3).
Fig. 3.
(A) Bowhead whale habitat selection surfaces for sea ice concentration as killer whales move closer. (B) Bowhead whale habitat selection surfaces for distance to shoreline as killer whales move closer. Surfaces shown are habitat selection predictions under different distances to killer whale predators, ranging from 10 km to 1,500 km.
The best-fitting multiparameter model included sea ice concentration, sea ice concentration squared, depth, distance to shore, distance to ice edge, and pairwise interactions between all main effects and distance to killer whales (Table 3). Parameter estimates fit with the two different methods (clogit and TwoStepCLogit) were broadly consistent, as were the uncertainty estimates around parameters, although the robust SEs around parameter estimates were somewhat smaller when fitting with clogit as compared to TwoStepCLogit, which calculates random effects more conservatively (Table 4). Still, the set of parameters whose uncertainty estimates included zero were the same in both fitting procedures, and the parameters that did not include zero differed by no more than 15% between the two fitting methods.
Table 3.
Multiparameter SSF models of bowhead whale locations relative to sea ice concentration, sea ice concentration squared, depth, distance to shore, distance to ice edge, and interactions between all main effects and distance to killer whales
| Model | d.f. | AIC | ∆AIC |
| SIconc*Dkw + SI2conc*Dkw + Depth*Dkw + Dsh*Dkw + (Dedge:isice)*Dkw | 13 | 20,787 | 0 |
| SIconc*Dkw + SI2conc*Dkw + Dsh*Dkw + (Dedge:isice)*Dkw | 11 | 20,794 | 7 |
| Depth*Dkw + Dsh*Dkw + (Dedge:isice)*Dkw | 9 | 20,821 | 34 |
| SIconc*Dkw + SI2conc*Dkw + Depth*Dkw + Dsh*Dkw | 9 | 20,842 | 55 |
| SIconc*Dkw + SI2conc*Dkw + Dsh*Dkw | 7 | 20,851 | 64 |
| Depth*Dkw + Dsh*Dkw | 5 | 20,913 | 126 |
| Dsh + Dsh: Dkw | 2 | 20,927 | 140 |
| SIconc*Dkw + SI2conc*Dkw + Depth*Dkw + (Dedge:isice)*Dkw | 11 | 20,961 | 174 |
| SIconc + SI2conc + Depth + Dsh + (Dedge:isice) + Dkw | 7 | 20,973 | 186 |
| SIconc + SI2conc + Depth + Dsh + (Dedge:isice) | 6 | 20,986 | 199 |
| SIconc*Dkw + SI2conc*Dkw + Depth*Dkw | 7 | 21,027 | 240 |
| Dkw + Dsh: Dkw | 2 | 21,079 | 292 |
| SIconc*Dkw + SI2conc*Dkw + (Dedge:isice)*Dkw | 9 | 21,082 | 295 |
| Null | 1 | 21,337 | 550 |
See Table 1 for definition of terms.
Table 4.
Selected bowhead−killer whale interaction model (shown in Table 3) parameter estimates fitted using the clogit function and the TwoStepCLogit function for comparison
| clogit function | TwoStepCLogit function | |||||
| Variable | Coefficient | Robust SE | z | p | Coefficient | SE |
| SIconc | −0.119 | 0.243 | −0.491 | 0.623 | −0.005 | 0.364 |
| Dkw | 0.365 | 1.545 | 0.237 | 0.812 | 1.866 | 2.934 |
| SI2conc | −0.201 | 0.084 | −2.385 | 0.017 | −0.171 | 0.079 |
| Dsh | −3.362 | 0.508 | −6.610 | <0.0001 | −3.446 | 0.988 |
| SIconc:Dkw | 0.014 | 0.038 | 0.385 | 0.700 | −0.001 | 0.055 |
| SI2conc:Dkw | 0.023 | 0.012 | 1.898 | 0.057 | 0.019 | 0.011 |
| Dkw:Dsh | 0.423 | 0.068 | 6.174 | <0.0001 | 0.445 | 0.138 |
| Dedge:isice = 0 | 1.223 | 0.912 | 1.340 | 0.180 | 1.055 | 0.636 |
| Dedge:isice = 1 | 2.349 | 0.574 | 4.089 | <0.0001 | 1.880 | 0.916 |
| Dkw:Dedge:isice = 0 | −0.150 | 0.128 | −1.169 | 0.242 | −0.127 | 0.089 |
| Dkw:Dedge isice = 1 | −0.321 | −0.073 | −4.393 | <0.0001 | −0.254 | 0.121 |
Parameter estimates, parameter SEs, and levels of significance are comparable for both fitting methods. See Table 1 for definition of terms. z, = z-score on a standard normal distribution. Bolded p values are statistically significant.
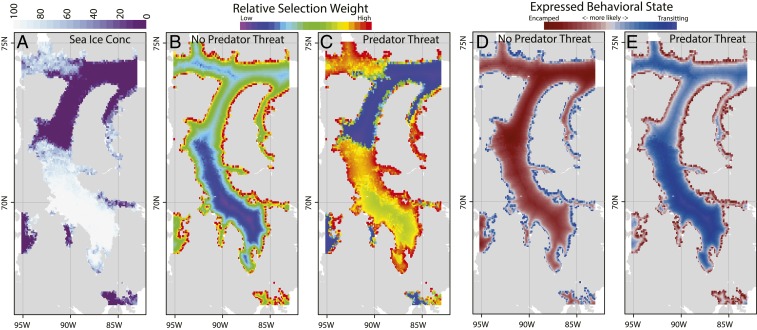
Visualizing the best-fitting model on an ice data layer from July 13, 2013, the effect of predation threat on overall habit preference is marked (Fig. 4). In the absence of threat, open water and light sea ice are preferred to dense ice, and nearshore areas are only slightly more favored than offshore. Under threat, open water, offshore areas were strongly avoided, while areas very near shore or in intermediate density sea ice well behind the ice edge were strongly selected.
Fig. 4.
Predicted SSF selection weight surface and behavioral state of bowhead whales in the presence and absence of predation risk. (A) Sea ice conditions used to make predictions were taken from July 13, 2013. (B) Predicted relative selection weight under those sea conditions with no predation threat (Dkw = 1,000 km), (C) selection weights under the same conditions but with predation threat (Dkw = 30 km), and (D and E) most likely behavioral state given across space (D) under predator-free conditions and (E) under predator threat. See Fig. 1 for geographic reference and orientation; Fig. 1 magenta box shows the boundaries of the panels of this figure.
Effect of Predator Threat on Movement Behavior.
The best-fitting mixed-effects model revealed that, similar to findings of habitat use, the main effect of killer whale presence did not affect behavior. Predator threat mediated how other environmental conditions affected behavior (SI Appendix, Tables S1 and S2 and Dataset S1). The most consequential was modification of the effect of distance to shore on behavioral state (SI Appendix, Tables S1 and S2). In the absence of killer whales, bowheads were increasingly likely to express transiting behavior as they neared the coast, and were more likely to express resident/foraging behavior in offshore areas. Predation threat reversed this effect. Under threat, bowheads were more likely to express resident-type movement near shorelines and more likely to be transiting when farther from shore (Fig. 4 and SI Appendix, Tables S1 and S2). Sea ice had a small, marginally significant effect on behavior, with resident-type movement slightly more likely as sea ice density decreased; predation threat did not modify this effect (Fig. 4 and SI Appendix, Tables S3 and S4).
Discussion
The retreat of bowhead whales into dense ice and shallow nearshore waters in the presence of killer whales has been previously observed (29, 31, 54, 55), and has long been recognized by Inuit, who call the behavior “aarlirijuk” (“fear of killer whales”; refs. 56–58). However, this study rigorously quantifies these behaviors over relevant spatiotemporal scales using telemetry data. We demonstrate that risk effects projected by marine apex predators can be intense and prolonged, acting over much larger spatiotemporal scales than previously demonstrated in any terrestrial or marine ecosystem (3, 6, 20, 21). The strong NCEs induced by killer whales demonstrated here and previously on narwhals (15) in this Arctic system, and more recently on white sharks in the temperate Pacific (23), clearly show that the predatory role of killer whales extends beyond the consumptive, or density-mediated, effects typically considered (e.g., ref. 59).
NCEs typically cause reduced net energy intake due to lost foraging opportunities or increased energy expenditure. Bowhead whales forage extensively during the open water season (60–62). In accordance with ideal free distribution theory (63), bowhead selection of ice-free areas prior to the arrival of killer whales suggests open water is more profitable foraging habitat, which would also be consistent with studies that show phytoplankton primary production exceeds ice algae production (e.g., ref. 64). Bowhead movement into heavy sea ice and shallow water, coupled with greatly reduced activity, when under predation threat therefore suggests predator avoidance occurred at the expense of foraging. Although bowhead whales have immense blubber stores that buffer periodic disruptions in foraging (65), the brief, intense pulse of productivity during the Arctic open water season may contribute disproportionately to their annual energetic requirements (66). Risk effects that disrupt foraging during this period may therefore cause nontrivial energetic costs to individuals, particularly for calves and juveniles, which have higher mass-specific energetic requirements than adults, and for lactating females, whose gross energy requirements are more than double those of other adults (see ref. 67). Quantifying these costs, which can be the dominant aspect of predator−prey interactions (12, 68), would require data on the fraction of annual caloric need met during summer foraging, the proportion of that time killer whales effectively modify bowhead behavior, and the degree to which antipredator behaviors reduce foraging efficiency. All of these are logistically difficult to assess for large, mobile marine species.
We found that NCEs were elicited when killer whales were as far as 100 km and perhaps farther away. This is too far for direct predator detection by bowheads using visual, chemosensory, or acoustic cues—mammal-hunting killer whales rarely vocalize while hunting (69–71), and the relatively high frequency of killer whale calls attenuates over shorter distances (72). We therefore speculate that bowhead whales receive cues from conspecifics or heterospecifics that project risk information much farther than direct predator cues, either by low-frequency calls that can travel long distances (e.g., ref. 73) or by individuals informed of predation risk spreading this information as they flee (Arctic cetaceans can cover well over 100 km per day; refs. 35 and 74). Advanced warning could thus afford time to move into protective sea ice, particularly given the marginal cost of moving into refuge habitat compared to that of predation or predator harassment (75).
Assuming NCEs like those demonstrated here are insignificant could lead to incorrect inference about the drivers of animal distribution, habitat selection, and demographic changes, particularly with respect to resource distribution (4, 15, 68, 76). Selection of sea ice by bowhead whales is thought to reflect both bottom-up and top-down factors, as productive marginal ice zones support high densities of zooplankton, while offering proximity to protective refuge from killer whales (30, 77). In two previous studies in this same region, the first found bowheads selected moderate to heavy ice in summer, which was believed to reduce killer whale predation risk while also providing access to enhanced foraging (30), and the second assumed bowheads foraged in moderate ice presumed to aggregate zooplankton (78). The explicit incorporation of predation risk in the analyses presented here, however, supports the hypothesis that bowhead selection of sea ice in summer is strongly predator mediated, reinforcing the need to incorporate predation risk in analyses of animal space use (4, 15). The large change in prey space use in response to a relatively small number of wide-ranging predators also illustrates that predator distribution and density, unlike resource distribution, may not be a straightforward predictor of prey distribution (79), because prey responses to predation risk are often disproportionate or nonlinear (1, 80).
Understanding how landscapes of fear influence individuals, populations, and communities is becoming increasingly important given increasing anthropogenic changes to habitat structure and predator abundances (5). Declines in sea ice extent and duration are allowing killer whales to access areas where they have had little or no historical occurrence, in both the eastern Canadian Arctic (81, 82) and Chukchi and Beaufort Seas (83–85). In the Western Arctic, there has been a commensurate increase in killer whale predation of bowheads (84, 86), and although no information is available to quantify the impacts of NCEs in the Western Arctic, these effects almost certainly occur there as well. The potential population-level impacts of killer whale range expansions on bowhead whales and other Arctic marine mammal populations via NCEs are unknown, but as ice-covered areas that have served as refugia diminish in area and duration, it seems likely that any current impacts will increase.
Protracted predator disturbance in shifting Arctic seascapes of fear (sensu 4) could drive energetically costly or stressful behavior modifications (e.g., refs. 7 and 67), or lead to large-scale redistributions (e.g., refs. 87 and 88). Lima and Bednekoff (80) predicted that prey should respond intensely to predators that spend only infrequent, brief periods in a system, but should decrease the amount of time allocated to antipredator effort with more-frequent or prolonged bouts of predation risk. As predation risk becomes more protracted with diminishing sea ice, bowheads should engage in riskier foraging behavior, potentially leading to greater direct predation mortality than currently experienced. While most predictions of the consequences of sea ice loss on bowhead whales and other ice-associated marine mammals have focused on sensitivity to bottom-up influences such as shifts in resource distribution and phenology (e.g., refs. 89–93), we suggest NCEs from emerging predator regimes are an overlooked effect of climate change that could compound the negative effects of sea ice loss on many Arctic species.
The prevalence of killer whale predation on large whales has been a contentious topic (e.g., refs. 94 and 95), and by extension, so too has the significance of killer whale predation in shaping baleen whale behavior and distribution (96, 97). Corkeron and Connor (96) contend that killer whale predation has shaped the migratory behavior of baleen whales, with pregnant females migrating to low-latitude regions where relatively low killer whale abundance confers reduced predation risk to calves. The year-round association of bowhead whales with sea ice in this population, and near−year-round association in others, is unique among baleen whales, and Corkeron and Connor (96) hypothesized that ice-seeking behavior in the presence of killer whales was an “ice-as-alternative-refuge” strategy used by female bowheads with calves to escape predation at high latitudes. Females with calves and juveniles precede adult males and nonreproductive females on the spring migration through leads in the sea ice to occupy “nursery grounds” in protective bays and inlets with persistent summer ice cover that characterize the central Canadian Arctic (56, 55, 98). Our findings support Corkeron and Connor’s (96) hypothesis, and are consistent with segregation by sex, age, and reproductive status during migration and on summering grounds as evolved behavior to mitigate predation risk (56, 55, 98–100). More broadly, our study adds to a growing body of evidence that killer whale predation, or the threat of it, has been an underrated, if not major, selective force shaping the life history, behavior, and distribution of large whales (101–104).
Data Availability.
The data reported in this paper are available in Dataset S1. Metadata have been deposited in the open access Polar Data Catalogue (accession code 12989).
Supplementary Material
Acknowledgments
The Hunters and Trappers Organisations in the Nunavut communities of Igloolik, Hall Beach, and Pond Inlet were instrumental in planning and implementing fieldwork logistics. Levi Qaunaq and Natalino Piugattak assisted with bowhead whale fieldwork, and Charlie, Enookie, and Michael Inuarak and Natalie Reinhart assisted with killer whale fieldwork. The work was permitted under DFO Licenses to Fish for Scientific Purposes S-13/14-1009-NU and S-13/14-1024-NU. This research was funded largely by the Nunavut General Monitoring Plan and the Nunavut Wildlife Management Board, as well as the Nunavut Implementation Fund through Fisheries and Oceans Canada (DFO), the Natural Sciences and Engineering Research Council of Canada, and the US NSF. Four anonymous reviewers provided constructive comments that improved this manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Metadata have been deposited in the open access Polar Data Catalogue (accession code 12989).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911761117/-/DCSupplemental.
References
- 1.Lima S. L., Dill L. M., Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 68, 619–640 (1990). [Google Scholar]
- 2.Lima S. L., Nonlethal effects in the ecology of predator-prey interactions. Bioscience 48, 25–34 (1998). [Google Scholar]
- 3.Laundré J. W., Hernández L., Altendorf K. B., Wolves, elk, and bison: Reestablishing the “landscape of fear” in Yellowstone National Park, U.S.A. Can. J. Zool. 79, 1401–1409 (2001). [Google Scholar]
- 4.Wirsing A. J., Heithaus M. R., Frid A., Dill L. M., Seascapes of fear: Evaluating sublethal predator effects experienced and generated by marine mammals. Mar. Mamm. Sci. 24, 1–15 (2008). [Google Scholar]
- 5.Gaynor K. M., Brown J. S., Middleton A. D., Power M. E., Brashares J. S., Landscapes of fear: Spatial patterns of risk perception and response. Trends Ecol. Evol. 34, 355–368 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Wirsing A. J., Heithaus M. R., Dill L. M., Fear factor: Do dugongs (Dugong dugon) trade food for safety from tiger sharks (Galeocerdo cuvier)? Oecologia 153, 1031–1040 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Boonstra R., Hik D., Singleton G. R., Tinnikov A., The impact of predator-induced stress on the snowshoe hare cycle. Ecol. Monogr. 79, 371–394 (1998). [Google Scholar]
- 8.Schmitz O. J., Beckerman A. P., O’Brien K. M., Behaviorally mediated trophic cascades: Effects of predation risk on food web interactions. Ecology 78, 1388–1399 (1997). [Google Scholar]
- 9.Dill L. M., Heithaus M. R., Walters C. J., Behaviorally mediated indirect interactions in marine communities and their conservation implications. Ecology 84, 1151–1157 (2003). [Google Scholar]
- 10.Werner E. E., Peacor S. D., A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100 (2003). [Google Scholar]
- 11.Schmitz O. J., Krivan V., Ovadia O., Trophic cascades: The primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163 (2004). [Google Scholar]
- 12.Preisser E. L., Bolnick D. I., Benar M. F., Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86, 501–509 (2005). [Google Scholar]
- 13.Peers M. J. L., et al. , Quantifying fear effects on prey demography in nature. Ecology 99, 1716–1723 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Fortin D., et al. , Wolves influence elk movements: Behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86, 1320–1330 (2005). [Google Scholar]
- 15.Breed G. A., et al. , Sustained disruption of narwhal habitat use and behavior in the presence of Arctic killer whales. Proc. Natl. Acad. Sci. U.S.A. 114, 2628–2633 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonn W. M., Paszkowski C. A., Holopainen I. J., Piscivory and recruitment: Mechanisms structuring prey populations in small lakes. Ecology 73, 951–958 (1992). [Google Scholar]
- 17.Wooster D., Sih A., A review of the drift and activity responses of stream prey to predator presence. Oikos 73, 3–8 (1995). [Google Scholar]
- 18.Trussell G. C., Ewanchuk P. J., Bertness M. D., Silliman B. R., Trophic cascades in rocky shore tide pools: Distinguishing lethal and nonlethal effects. Oecologia 139, 427–432 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Schmitz O. J., Scaling from plot experiments to landscapes: Studying grasshoppers to inform forest ecosystem management. Oecologia 145, 225–234 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Heithaus M. R., Dill L. M., Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology 83, 480–491 (2002). [Google Scholar]
- 21.Heithaus M. R., et al. , State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. J. Anim. Ecol. 76, 837–844 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Burkholder D. A., Heithaus M. R., Fourqurean J. W., Wirsing A., Dill L. M., Patterns of top-down control in a seagrass ecosystem: Could a roving apex predator induce a behaviour-mediated trophic cascade? J. Anim. Ecol. 82, 1192–1202 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Jorgensen S. J., et al. , Killer whales redistribute white shark foraging pressure on seals. Sci. Rep. 9, 6153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laidre K. L., Heide-Jørgensen M. P., Orr J. R., Reactions of narwhals, Monodon monoceros, to killer whale, Orcinus orca, attacks in the eastern Canadian Arctic. Can. Field Nat. 120, 457–465 (2006). [Google Scholar]
- 25.Westdal K. H., Davies J., MacPherson A., Orr J., Ferguson S. H., Behavioural changes in belugas (Delphinapterus leucas) during a killer whale (Orcinus orca) attack in southwest Hudson Bay. Can. Field Nat. 130, 315–319 (2016). [Google Scholar]
- 26.George J. C., et al. , Frequency of killer whale (Orcinus orca) attacks and ship collisions based on scarring on bowhead whales (Balaena mysticetus) of the Bering-Chukchi-Beaufort Seas stock. Arctic 47, 247–255 (1994). [Google Scholar]
- 27.Higdon J. W., Hauser D. D. W., Ferguson S. H., Killer whales (Orcinus orca) in the Canadian Arctic: Distribution, prey items, group sizes, and seasonality. Mar. Mamm. Sci. 28, E93–E109 (2011). [Google Scholar]
- 28.Reinhart N. R., et al. , Occurrence of killer whale Orcinus orca rake marks on Eastern Canada-West Greenland bowhead whales Balaena mysticetus. Polar Biol. 36, 1133–1146 (2013). [Google Scholar]
- 29.Shpak O. V., Paramonov A. Y., The bowhead whale, Balaena mysticetus Linnaeus, 1758, in the Western Sea of Okhotsk (2009-2016): Distribution pattern, behavior, and threats. Russ. J. Mar. Biol. 44, 210–218 (2018). [Google Scholar]
- 30.Ferguson S. H., Dueck L., Loseto L. L., Luque S. P., Bowhead whale Balaena mysticetus seasonal selection of sea ice. Mar. Ecol. Prog. Ser. 411, 285–297 (2010). [Google Scholar]
- 31.Reeves R. R., Mitchell E., Distribution and seasonality of killer whales in the eastern Canadian Arctic. Rit Fiskid. 11, 136–160 (1988). [Google Scholar]
- 32.Matthews C. J. D., Luque S. P., Petersen S. D., Andrews R. D., Ferguson S. H., Satellite tracking of a killer whale (Orcinus orca) in the eastern Canadian Arctic documents ice avoidance and rapid, long-distance movement into the North Atlantic. Polar Biol. 34, 1091–1096 (2011). [Google Scholar]
- 33.Laundré J. W., et al. , The landscape of fear: the missing link to understand top-down and bottom-up controls of prey abundance? Ecology 95, 1141–1152 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Atuo F. A., O’Connell T. J., The landscape of fear as an emergent property of heterogeneity: Contrasting patterns of predation risk in grassland ecosystems. Ecol. Evol. 7, 4782–4793 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heide-Jørgensen M. P., et al. , From Greenland to Canada in ten days: Tracks of bowhead whales, Balaena mysticetus, across Baffin Bay. Arctic 56, 21–31 (2003). [Google Scholar]
- 36.Jonsen I. D., Mills Flemming J., Myers R. A., Robust state-space modeling of animal movement data. Ecology 86, 2874–2880 (2005). [Google Scholar]
- 37.Breed G. A., Jonsen I. D., Myers R. A., Bowen W. D., Leonard M. L., Sex-specific, seasonal foraging tactics of adult grey seals (Halichoerus grypus) revealed by state-space analysis. Ecology 90, 3209–3221 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Jonsen I. D., et al. , State-space models for bio-loggers: A methodological road map. Deep Sea Res. Part II Top. Stud. Oceanogr. 88, 34–46 (2013). [Google Scholar]
- 39.Thurfjell H., Ciuti S., Boyce M. S., Applications of step-selection functions in ecology and conservation. Mov. Ecol. 2, 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avgar T., Potts J. R., Lewis M. A., Boyce M. S., Integrated step selection analysis: Bridging the gap between resource selection and animal movement. Methods Ecol. Evol. 7, 619–630 (2016). [Google Scholar]
- 41.Therneau T., A package for survival analysis in S, Version 2.38. https://cran.r-project.org/web/packages/survival/index.html. Accessed 27 August 2017.
- 42.Craiu R. V., Duchesne T., Fortin D., Baillargeon S., TwoStepCLogit: Conditional logistic regression: A two-step estimation method. R package Version 1.2.5. https://CRAN.R-project.org/package=TwoStepCLogit. Accessed 10 November 2017. [Google Scholar]
- 43.Breed G. A., et al. , Seasonal sea ice dynamics drive movement and migration of juvenile bearded seals (Erignathus barbatus). Mar. Ecol. Prog. Ser. 600, 223–237 (2018). [Google Scholar]
- 44.Spreen G., Kaleschke L., Heygster G., Sea ice remote sensing using AMSR-E 89-GHz channels. J. Geophys. Res. Oceans 113, C02S03 (2008). [Google Scholar]
- 45.Amante C., Eakins B. W., “Data from “ETOPO1 1 arc-minute global relief model: Procedures, data sources and analysis. Data sources and analysis” (NOAA Technical Memorandum NESDIS NGDC-24, National Geophysical Data Center, 2009).
- 46.Natural Earth , Natural Earth. http://www.naturalearthdata.com. Accessed 27 August 2017.
- 47.Pebesma E. J., Bivand R. S., Classes and methods for spatial data in R. R News 5, 9–13 (2005). [Google Scholar]
- 48.Bivand R. S., Pebesma E., Gómez-Rubio V., Applied Spatial Data Analysis with R (Springer, ed. 2, 2013). [Google Scholar]
- 49.Burnham K. P., Anderson D. R., Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2002). [Google Scholar]
- 50.Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team, nlme: Linear and Nonlinear Mixed Effects Models. R package Version 3.1-120. https://cran.r-project.org/web/packages/nlme/index.html. Accessed 10 November 2017.
- 51.Breed G. A., Costa D. P., Goebel M. E., Robinson P. W., Electronic tracking tag programming is critical to data collection for behavioral time‐series analysis. Ecosphere 2, 1–12 (2011). [Google Scholar]
- 52.Jonsen I., Joint estimation over multiple individuals improves behavioural state inference from animal movement data. Sci. Rep. 6, 20625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Auger-Méthé M., et al. , Spatiotemporal modelling of marine movement data using Template Model Builder (TMB). Mar. Ecol. Prog. Ser. 565, 237–249 (2017). [Google Scholar]
- 54.Mitchell E. D., Reeves R. R., Factors affecting abundance of bowhead whales Balaena mysticetus in the eastern Arctic of North America, 1915-1980. Biol. Conserv. 22, 59–78 (1982). [Google Scholar]
- 55.Finley K. J., Natural history and conservation of the Greenland whale, or bowhead, in the northwest Atlantic. Arctic 54, 55–76 (2001). [Google Scholar]
- 56.Finley K. J., Isabella Bay, Baffin Island: An important historical and present-day concentration area for the endangered bowhead whale (Balaena mysticetus) of the eastern Canadian Arctic. Arctic 43, 137–152 (1990). [Google Scholar]
- 57.NWMB , Final Report of the Inuit Bowhead Knowledge Study (Nunavut Wildlife Management Board, Iqaluit, NU, Canada, 2000), pp. 90. [Google Scholar]
- 58.Ferguson S. H., Higdon J. W., Westdal K. H., Prey items and predation behavior of killer whales (Orcinus orca) in Nunavut, Canada based on Inuit hunter interviews. Aquat. Biosyst. 8, 3 (2012a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferguson S. H., Kingsley M. C. S., Higdon J. W., Killer whale (Orcinus orca) predation in a multi-prey system. Popul. Ecol. 54, 31–41 (2012b). [Google Scholar]
- 60.Lowry L. F., “Food and feeding ecology” in The Bowhead Whale, Burns J. J., Montague J. J., Bowles C. J., Eds. (Special Publication no. 2, Society for Marine Mammalogy, 1993), pp. 201–238. [Google Scholar]
- 61.Moore S. E., George J. C., Sheffield G., Bacon J., Ashjian C. J., Bowhead whale distribution and feeding near Barrow, Alaska, in late summer 2005-06. Arctic 63, 195–205 (2010). [Google Scholar]
- 62.Pomerleau C., et al. , Prey assemblage isotopic variability as a tool for assessing diet and the spatial distribution of bowhead whale Balaena mysticetus foraging in the Canadian eastern Arctic. Mar. Ecol. Prog. Ser. 469, 161–174 (2012). [Google Scholar]
- 63.Sutherland W. J., Aggregation and the ideal free distribution. J. Anim. Ecol. 52, 821–828 (1983). [Google Scholar]
- 64.Carmack E. C., Macdonald R. W., Jasper S., Phytoplankton productivity on the Canadian Shelf of the Beaufort Sea. Mar. Ecol. Prog. Ser. 277, 37–50 (2004). [Google Scholar]
- 65.Lindstedt S. L., Boyce M. S., Seasonality, fasting endurance, and body size in mammals. Am. Nat. 125, 873–878 (1985). [Google Scholar]
- 66.Matthews C. J. D., Ferguson S. H., Seasonal foraging behaviour of Eastern Canada-West Greenland bowhead whales: An assessment of isotopic cycles along baleen. Mar. Ecol. Prog. Ser. 522, 269–286 (2015). [Google Scholar]
- 67.Fortune S. M. E., Trites A. W., Mayo C. A., Rosen D. A. S., Hamilton P. K., Energetic requirements of North Atlantic right whales and the implications for species recovery. Mar. Ecol. Prog. Ser. 478, 253–272 (2013). [Google Scholar]
- 68.Creel S., Christianson D., Relationships between direct predation and risk effects. Trends Ecol. Evol. 23, 194–201 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Barrett-Lennard L. G., Ford J. K. B., Heise K. A., The mixed blessing of echolocation: Differences in sonar use by fish-eating and mammal-eating killer whales. Anim. Behav. 51, 553–565 (1996). [Google Scholar]
- 70.Deecke V. B., Ford J. K. B., Slater P. J. B., The vocal behavior of mammal-eating killer whales: Communicating with costly calls. Anim. Behav. 69, 395–405 (2005). [Google Scholar]
- 71.Deecke V. B., Nykänen M., Foote A. D., Janik V. M., Vocal behavior and feeding ecology of killer whales Orcinus orca around Shetland, UK. Aquat. Biol. 13, 79–88 (2011). [Google Scholar]
- 72.Miller P. J., Diversity in sound pressure levels and estimated active space of resident killer whale vocalizations. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 192, 449–459 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Sirović A., Hildebrand J. A., Wiggins S. M., Blue and fin whale call source levels and propagation range in the Southern Ocean. J. Acoust. Soc. Am. 122, 1208–1215 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Dietz R., Heide-Jørgensen R. M. P., Movements and swimming speed of narwhals, Monodon monoceros, equipped with satellite transmitters in Melville Bay, northwest Greenland. Can. J. Zool. 73, 2106–2119 (1995). [Google Scholar]
- 75.Abrams P. A., Should prey overestimate the risk of predation? Am. Nat. 144, 317–328 (1994). [Google Scholar]
- 76.Creel S., Christianson D., Liley S., Winnie J. A. Jr, Predation risk affects reproductive physiology and demography of elk. Science 315, 960 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Dueck L., Ferguson S. H., “Habitat use by bowhead whales (Balaena mysticetus) of the eastern Canadian Arctic” (Research Document 2008/082, Fisheries and Oceans Canada, 2008).
- 78.Pomerleau C., et al. , Bowhead whale Balaena mysticetus diving and movement patterns in the eastern Canadian Arctic: Implications for foraging ecology. Endanger. Species Res. 15, 167–177 (2011). [Google Scholar]
- 79.Willems E. P., Hill R. A., Predator-specific landscapes of fear and resource distribution: Effects on spatial range use. Ecology 90, 546–555 (2009). [DOI] [PubMed] [Google Scholar]
- 80.Lima S. L., Bednekoff P. A., Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659 (1999). [DOI] [PubMed] [Google Scholar]
- 81.Higdon J. W., Ferguson S. H., Loss of Arctic sea ice causing punctuated change in sightings of killer whales (Orcinus orca) over the past century. Ecol. Appl. 19, 1365–1375 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Higdon J. W., Westdal K. H., Ferguson S. H., Distribution and abundance of killer whales (Orcinus orca) in Nunavut, Canada–An Inuit knowledge survey. J. Mar. Biol. Assoc. U.K. 94, 1293–1304 (2014). [Google Scholar]
- 83.O’Corry-Crowe G., et al. , Genetic profiling links changing sea-ice to shifting beluga whale migration patterns. Biol. Lett. 12, 20160404 (2016). [Google Scholar]
- 84.George J. C., et al. , Frequency of injuries from line entanglements, killer whales, and ship strikes on Bering-Chukchi-Beaufort seas bowhead whales. Arctic 70, 37–46 (2017). [Google Scholar]
- 85.Stafford K. M., Increasing detections of killer whales (Orcinus orca) in the Pacific Arctic. Mar. Mamm. Sci. 35, 696–706 (2019). [Google Scholar]
- 86.Willoughby A. L., Clarke J. T., Ferguson M. C., Stimmelmayr R., Brower A. B., “Bowhead whale carcasses in the eastern Chukchi and western Beaufort Seas, 2009-2017” (SC/67B/AWMP/02, International Whaling Commission).
- 87.Peckarsky B. L., et al. , Revisiting the classics: Considering nonconsumptive effects in textbook examples of predator-prey interactions. Ecology 89, 2416–2425 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Swain D. P., Benoît H. P., Hammill M. O., Spatial distribution of fishes in a Northwest Atlantic ecosystem in relation to risk of predation by a marine mammal. J. Anim. Ecol. 84, 1286–1298 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Tynan C. T., DeMaster D. P., Observations and predictions of Arctic climatic change: Potential effects on marine mammals. Arctic 50, 308–322 (1997). [Google Scholar]
- 90.Laidre K. L., et al. , Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecol. Appl. 18 (suppl. 2), S97–S125 (2008). [DOI] [PubMed] [Google Scholar]
- 91.George J. C., Druckenmiller M. L., Laidre K. L., Suydam R., Person B., Bowhead whale body condition and links to summer sea ice and upwelling in the Beaufort Sea. Prog. Oceanogr. 136, 250–262 (2015). [Google Scholar]
- 92.Citta J. J., et al. , Ecological characteristics of core-use areas used by Bering–Chukchi–Beaufort (BCB) bowhead whales, 2006–2012. Prog. Oceanogr. 136, 201–222 (2015). [Google Scholar]
- 93.Citta J. J., et al. , Oceanographic characteristics associated with autumn movements of bowhead whales in the Chukchi Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 152, 121–131 (2018). [Google Scholar]
- 94.Springer A. M., et al. , Sequential megafaunal collapse in the North Pacific Ocean: An ongoing legacy of industrial whaling? Proc. Natl. Acad. Sci. U.S.A. 100, 12223–12228 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wade P. R., et al. , Killer whale and marine mammal trends in the north Pacific–A re-examination of evidence for sequential megafaunal collapse and the prey-switching hypothesis. Mar. Mamm. Sci. 23, 766–802 (2007). [Google Scholar]
- 96.Corkeron P. J., Connor R. C., Why do baleen whales migrate? Mar. Mamm. Sci. 15, 1228–1245 (1999). [Google Scholar]
- 97.Clapham P., Why do baleen whales migrate? A response to Corkeron and Connor. Mar. Mamm. Sci. 17, 432–436 (2001). [Google Scholar]
- 98.Reeves R. R., Mitchell E., Mansfield A., McLaughlin M., Distribution and migration of the bowhead whale, Balaena mysticetus, in the eastern North American Arctic. Arctic 36, 5–64 (1983). [Google Scholar]
- 99.Cosens S. E., Blouw A., Size- and age-class segregation of bowhead whales summering in northern Foxe Basin: A photogrammetric analysis. Mar. Mamm. Sci. 19, 284–296 (2003). [Google Scholar]
- 100.Heide-Jørgensen M. P., et al. , Large-scale sexual segregation of bowhead whales. Endanger. Species Res. 13, 73–78 (2010). [Google Scholar]
- 101.Pitman R. L., Ballance L. T., Mesnick S. I., Chivers S. J., Killer whale predation on sperm whales: Observations and implications. Mar. Mamm. Sci. 17, 494–507 (2001). [Google Scholar]
- 102.Reeves R. R., Berger J., Clapham P. J., “Killer whales as predators of large baleen whales and sperm whales” in Whales, Whaling, and Ocean Ecosystems, Estes J., DeMaster D. P., Doak D. F., Williams T. M., Brownell R. L., Eds. (University of California Press, 2007), pp. 172−186. [Google Scholar]
- 103.Ford K. B., Reeves R. R., Fight or flight: Antipredator strategies of baleen whales. Mammal Rev. 38, 50–86 (2008). [Google Scholar]
- 104.Pitman R. L., Totterdell J. A., Fearnbach H., Ballance L. T., Durban J. W., Whale killers: Prevalence and ecological implications of killer whale predation on humpback whale calves off western Australia. Mar. Mamm. Sci. 31, 629–657 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported in this paper are available in Dataset S1. Metadata have been deposited in the open access Polar Data Catalogue (accession code 12989).