Abstract
Retinal gene therapy has shown great promise in treating retinitis pigmentosa (RP), a primary photoreceptor degeneration that leads to severe sight loss in young people1,2,3,4,5,6. Here we report the first in human Phase I/II dose escalation clinical trial for X-linked RP caused by mutations in the RP GTPase regulator (RPGR) gene7 in 18 patients up to 6 months follow-up (Clinicaltrials.gov: NCT03116113). The primary outcome of the study was safety and secondary outcomes included visual acuity, microperimetry and central retinal thickness. Apart from steroid-responsive subretinal inflammation in patients at the higher doses, there were no significant safety concerns following subretinal delivery of an adeno-associated viral vector encoding codon-optimized human RPGR (AAV8.coRPGR)8 meeting the pre-specified primary endpoint. Visual field improvements beginning at one month and maintained to the last point of follow-up were observed in six patients.
X-linked RP, caused by mutations in the RPGR gene, is the most common form of recessive RP.7 It is characterised by a degeneration of rod and cone photoreceptors in childhood, leading to visual field constriction and early, severe sight loss. Currently, there is no treatment for RPGR related RP and despite proof of principle in animal models,1–4 the effect of gene therapy targeting a photoreceptor degeneration (RP) in humans has not until now been reported. Amongst potential therapeutic approaches,1,2,3,4,5,6,9 the most promising strategy for RPGR related RP is gene therapy using adeno-associated viral vector.10 The recent approval of gene therapy for RPE65 related retinal degeneration11 represents a significant advance in clinical medicine and offers the potential to correct many other inherited diseases. The inherent instability of the RPGR coding sequence, which has a repetitive purine rich region and undergoes alternative splicing, presents challenges to translate this therapy into human trials.12–15 Codon optimisation of the sequence has increased RPGR stability and fidelity, conferred therapeutic effects in animal models of the disease8 and provided the basis for this Phase I/II dose escalation retinal gene therapy clinical trial.
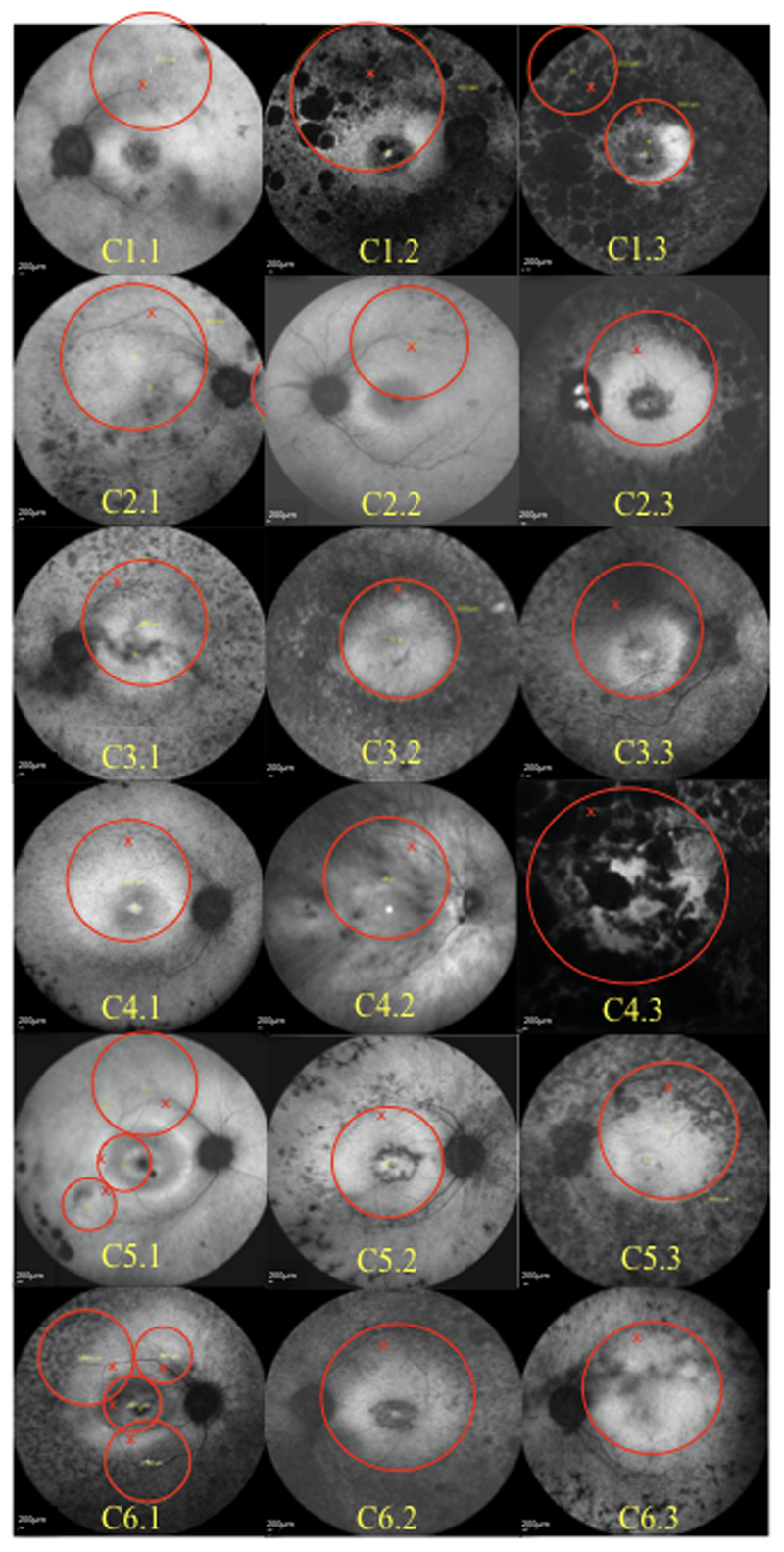
In total, eighteen patients with genetically confirmed variants in RPGR were recruited into 6 cohorts of 3 patients (C1-6.1-3) receiving increasing concentrations (from 5x1010 genomic particles (gp)/ml to 5x1012 gp/ml) of codon-optimised (co) AAV2 serotype 8 vector (AAV8.coRPGR) that was validated in pre-clinical studies8 prior to use in humans (Extended Data Fig. 1). Details of all trial participants are shown in Table 1. The vector was successfully delivered into the sub-retinal space, creating a ‘bleb’ under the retina, via a two-step injection as previously described.16 The bleb area covered the posterior pole with foveal detachment in all subjects except in C1.1 (Extended Data Fig. 2). We measured visual acuity by the Early Treatment Diabetic Retinopathy Study, ETDRS chart and retinal sensitivity and visual field by mesopic microperimetry (MAIA, CenterVue MP Systems, Padova, Italy) using a standard 68-stimulus (10-2) grid covering the central 10 degrees of the macula. As part of a standard protocol, the patients received a 21-day course of oral prednisolone starting at 1 mg per kg from 2 days prior to gene therapy.17
Table 1. Participant demographics.
The trial participants were 18 Caucasian males with X-linked retinitis pigmentosa and genetically confirmed mutations within RPGR. Patients were recruited in 6 cohorts of 3 patients receiving increasing concentration of AAV8.coRPGR vector (from 5x1010 gp/ml to 5x1012 gp/ml).
| ID | Age (years) | RPGR mutation | COHORT | Vector concentration (gp/ml) | Volume injected (ml) | Vector dose (gp) |
|---|---|---|---|---|---|---|
| C1.1 | 30 | c.2993_2997delAAGGG | 1 | 5x1010 | 0.10 | 5x109 |
| C1.2 | 41 | c.1571delA | 0.10 | 5x109 | ||
| C1.3 | 41 | c.2628_2629delGG | 0.04 | 2x109 | ||
| C2.1 | 32 | c.2426_2427delAG | 2 | 1x1011 | 0.04 | 4x109 |
| C2.2 | 25 | c.2236_2237delGA | 0.10 | 1x1010 | ||
| C2.3 | 46 | c.2586_2587delGG | 0.10 | 1x1010 | ||
| C3.1 | 27 | c.2650G>T | 3 | 5x1011 | 0.10 | 5x1010 |
| C3.2 | 32 | c.581G>A | 0.03 | 1.5x1010 | ||
| C3.3 | 31 | c.1572+1G>A | 0.10 | 5x1010 | ||
| C4.1 | 24 | c.2993_2997delAAGGG | 4 | 1x1012 | 0.06 | 6x1010 |
| C4.2 | 27 | c.2236_2237delGA | 0.10 | 1x1011 | ||
| C4.3 | 50 | c.1243_1244dupAG | 0.10 | 1x1011 | ||
| C5.1 | 26 | c.808C>T | 5 | 2.5x1012 | 0.15 | 3.75x1011 |
| C5.2 | 20 | c.2420-2435del16bp | 0.10 | 2.5x1011 | ||
| C5.3 | 30 | c.2405_2406delAG | 0.05 | 1.25x1011 | ||
| C6.1 | 25 | c.2405_2406delAG | 6 | 5x1012 | 0.08 | 4x1011 |
| C6.2 | 50 | c.2650G>T | 0.05 | 2.5x1011 | ||
| C6.3 | 22 | c.2268_2269AG | 0.03 | 1.5x1011 |
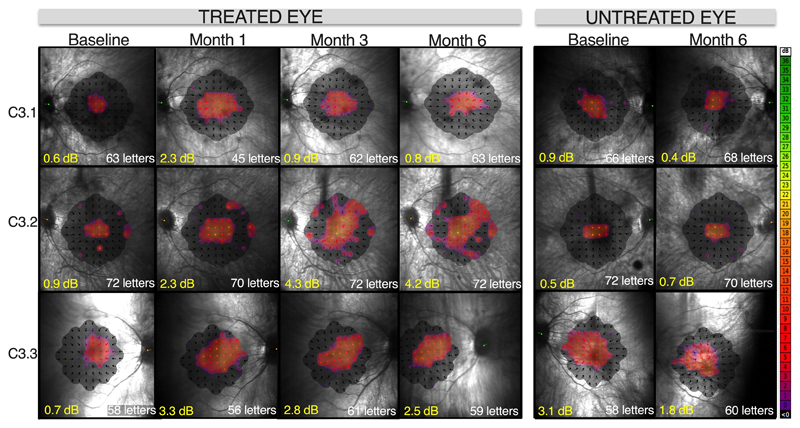
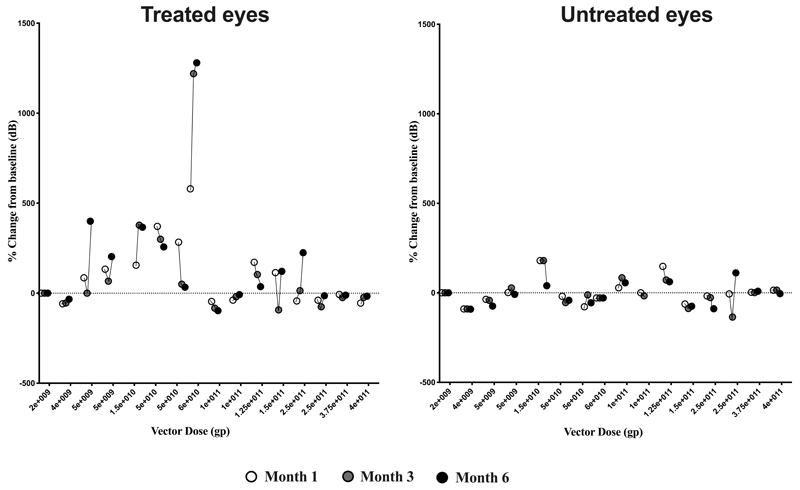
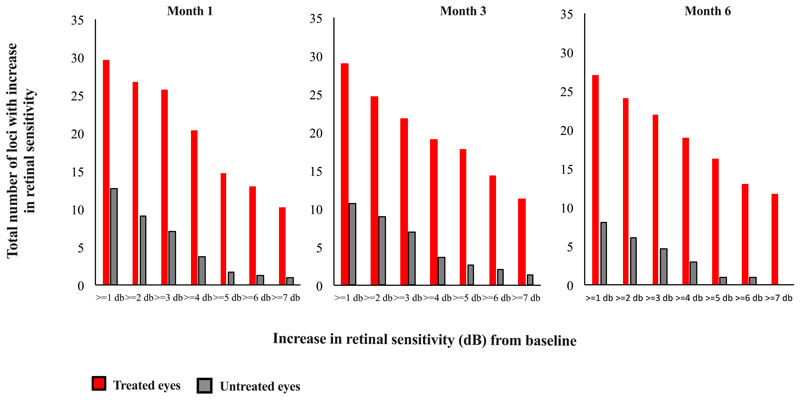
Following gene therapy, we observed a dose-response effect across the trial cohorts in terms of gains in visual function in treated eyes. As this was primarily a safety study, early cohort patients had very advanced retinal degeneration. Thus, at low vector doses (cohorts 1 and 2) there were no obvious gains in visual function in treated eyes (Extended Data Fig. 3) but in all cases visual acuity of the treated eyes returned to baseline levels by 3 months post-surgery. All three mid-dose patients (C3.1, C3.2 and C3.3) who received 5x1011 gp/ml of AAV8.coRPGR showed gains in retinal sensitivity and reversal of some of the visual field loss by month 1 in operated eyes, with treatment effects sustained through to month 6 follow-up (Fig. 1, Extended Data Fig. 3 and Extended Data Fig. 4). This was not seen in the un-operated fellow eyes. All patients described subjective improvement in visual clarity and increase in field of vision in the treated eye by 1 month of follow-up. Functional assessment showed similar visual acuity to baseline (Fig. 1).
Fig. 1. Retinal sensitivity following gene therapy for X-linked retinitis pigmentosa in cohort 3 patients.
Mean retinal sensitivity (decibels, dB) and visual field, (represented by sensitivity heat maps) measured by microperimetry, showed progressive improvement in the treated eye from baseline to 6 months post-treatment. The untreated eye showed no change. Visual acuity, measured by ETDRS chart reading (number of letters), remained stable in both eyes. To minimize learning effects, baseline microperimetry was conducted in triplicate over a 2-day period at visit 1 for all subjects. The third reading was validated as an accurate baseline. At follow-up visits, assessments were conducted once for each eye, as per trial protocol.
Each participant was carefully monitored for signs of intraocular inflammation, (previously reported in gene therapy trials, Curkas et al 2018)18 particularly those in later cohorts receiving higher concentrations of vector. All patients in cohorts 1 to 3 (concentrations up to 5x1011 gp/ml) remain inflammation free up to the most recent follow-up. In cohorts 4 to 6 receiving higher vector concentrations (up to 5x1012 gp/ml), we noted subtle signs of inflammation in 7 out of 9 patients (C4.1, C4.2, C4.3, C5.2, C5.3, C6.1 and C6.3). Moreover, the inflammation observed in this trial was mild and contained within the subretinal space where the vector was deposited during the surgery. Clinically, there were no cases of choroidal or retinal lesions, vasculitis, cystoid macular oedema or vitritis. We observed one case of anterior uveitis in cohorts 4-6 (C4.1) that was likely to be related to surgical procedure causing post-operative inflammation and which settled after 4 weeks of treatment with topical steroids. The subretinal inflammation appears to have resolved in all cases by 6 months following a course of oral corticosteroids. We describe the corticosteroid therapy regime for each patient affected by inflammation in Table S1 in Supplementary Appendix. The medical therapy was tailored individually for each patient and titrated according to the response. Notably, no patient required secondary immunosuppressive therapy such as ciclosporin that has been necessary to treat severe inflammation in other gene therapy studies.18 Despite inflammation, 3 patients (C4.1, C5.3 and C6.3) demonstrated visual gains in treated eyes at month 1 that were sustained at month 6 follow-up (although microperimetry in one cohort 5 patient, C5.3, was unreliable) (Extended Data Fig. 3).
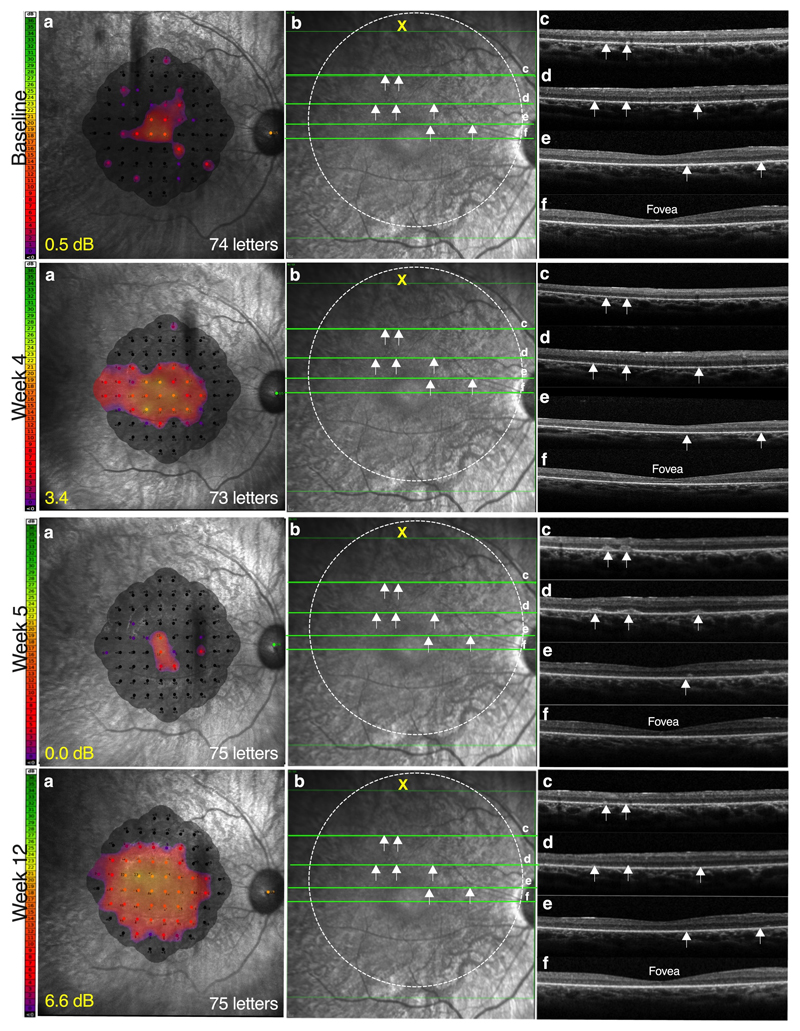
Fig. 2 demonstrates these post-operative events for one of the cohort 4 patients (C4.1). He described subjective improvement in visual clarity and visual field in the treated eye two weeks post treatment, which was corroborated by microperimetry testing of retinal sensitivity at 1-month follow-up, showing an improvement from 0.5 dB to 3.4 dB in the treated eye. However, at 5 weeks, the patient noticed partial visual regression in the treated eye with subjective paracentral scotomata. Microperimetry testing confirmed a reduction in retinal sensitivity to 0.0 dB. This was associated with the appearance of scattered subretinal lesions over the treated area on retinal imaging using optical coherence tomography (OCT, Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany). The lesions were round or oval in shape and seen as hyperreflective clumps on the OCT. Although visual acuity was unaffected, the patient was treated with oral prednisone (60 mg/day) and, after one week, the subretinal lesions resolved, associated with both subjective and objective recovery. Further sustained improvement in retinal sensitivity to 6.6 dB was demonstrated at 3 months follow-up visit by which time the prednisolone had been stopped (Fig. 2).
Fig. 2. Transient regression of retinal function in the treated eye associated with subretinal inflammation at 5 weeks post high-dose gene therapy.
At week 5 post treatment with AAV8.coRPGR (6x1010 gp), patient C4.1 noticed some regression of vision in the treated eye following improvements over the preceding week. Microperimetry showed regression of retinal sensitivity and visual field (a), which was associated with subretinal lesions (white arrows) on optical coherence tomography (OCT) (b). Subretinal lesions (white arrows) could be seen to be scattered over the treated area of the macula on the OCT cross-sections (c-e), but spared the fovea (f); visual acuity was unaffected following treatment with a course of oral corticosteroids, the inflammation resolved with corresponding gains in retinal sensitivity recorded at 3 months follow-up.
The two other patients in cohort 4 who received higher vector doses (1x1011gp) had no measurable gains in microperimetry and their post-operative course was complicated by retinal inflammation, which likely reduced any potential microperimetry gains. Two further patients, C5.3 (1.25x1011 gp) and C6.3 (1.5x1011 gp) also showed moderate functional improvements post treatment (Extended Data Fig. 3) despite retinal inflammation, although C5.3 had unreliable microperimetry. The rest of the cohort 5 and 6 patients, who received very high vector doses suffered inflammation or were very advanced at baseline and showed no functional gains, except C6.2 who showed some improvement in microperimetry.
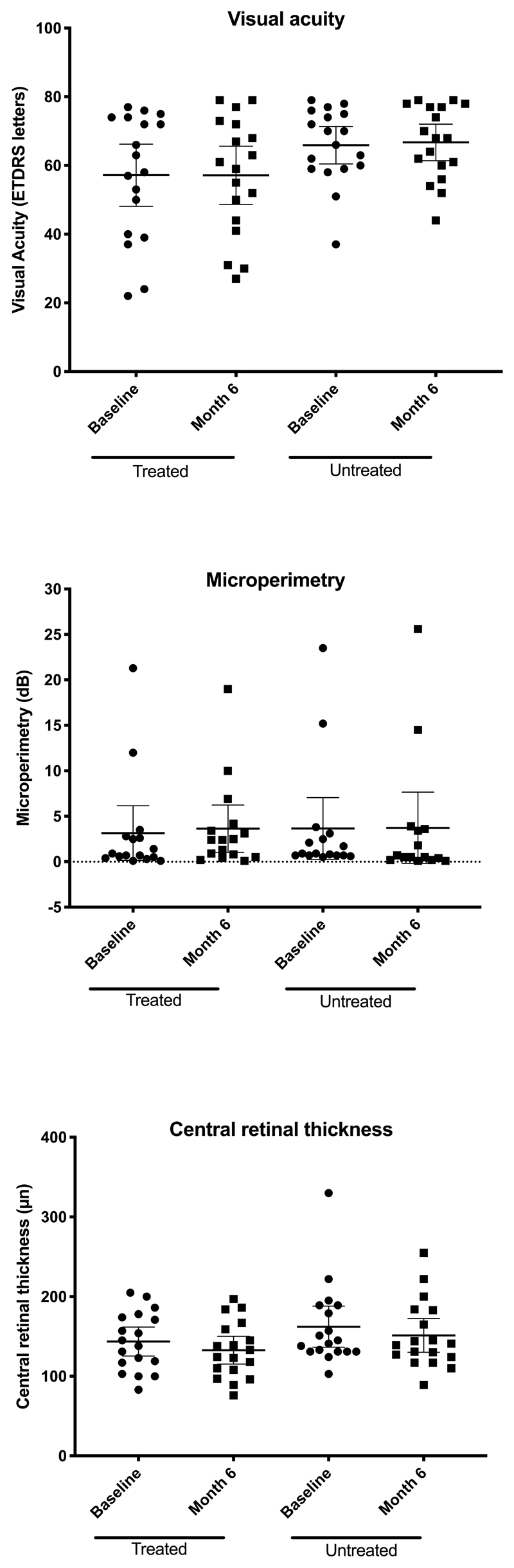
The mean change in microperimetry across all 18 trial participants in treated eyes from baseline to 6 months was +0.5 dB (95% CI: -0.7 to +1.7) and in untreated eyes was +0.1 dB (95% CI: -0.5 to +0.7) (Extended Data Fig. 5 and Table S2 in Supplementary Appendix). Visual acuity showed a mean change of -0.1 letters (95% CI: -2.8 to +2.6) in the treated eyes at 6 months with +0.8 letters (95% CI: -1.3 to + 2.9) in the untreated eyes compared to baseline. (Extended Data Fig. 5 and Table S3 in Supplementary Appendix). SD-OCT retinal structure remained morphologically similar to baseline in treated and untreated eyes across the cohort. Specifically, the foveal central retinal thickness changed by -10.8 μm (95% CI: -25.1 to + 3.5) in the treated eyes and -10.9 μm (95% CI: -19.8 to -2.1) in the untreated eyes over the 6-month period (Extended Data Fig. 5, Table S4 and Fig. S1 in Supplementary Appendix). Fundus autofluorescence images showed small localized areas of retinal pigment epithelium atrophy in treated eyes at month 6 in subjects C2.2, C3.1, C3.2, C4.1, C5.1, C5.2, C5.3, C6.1 and C6.2 that related to surgical retinotomy sites for subretinal vector delivery (Fig. S2 in Supplementary Appendix). In addition, subjects C4.1, C4.2, C5.2, C5.3 and C6.3 showed presence of hyperreflective dots/granulations in treated eyes at 6 months follow-up related to areas of subretinal blebs. The precise nature of these changes remains unclear but could be related to inflammation, viral capsids or tissue debris deposition/clearance. No obvious changes from baseline were observed in untreated eyes.
Fifty-five adverse events (35 ocular and 20 non-ocular) were reported in 18 participants by 6 months of follow-up. A detailed description of adverse events is shown in Table S5 in Supplementary Appendix. Most ocular adverse events were mild and self-limiting. There were 7 cases of intraocular inflammation in cohorts 4-6 that required additional course of oral corticosteroids. The inflammation appeared to have resolved in all cases by 6 months, when all patients had ceased oral corticosteroid treatment. We observed no serious adverse events or dose limiting toxicities during the first 6 months.
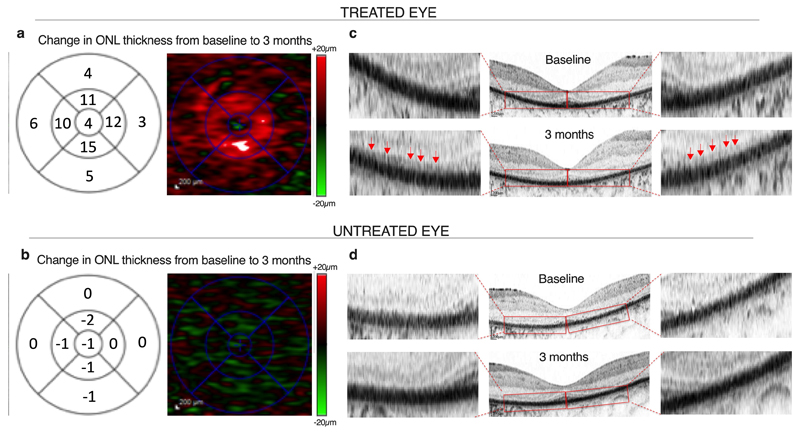
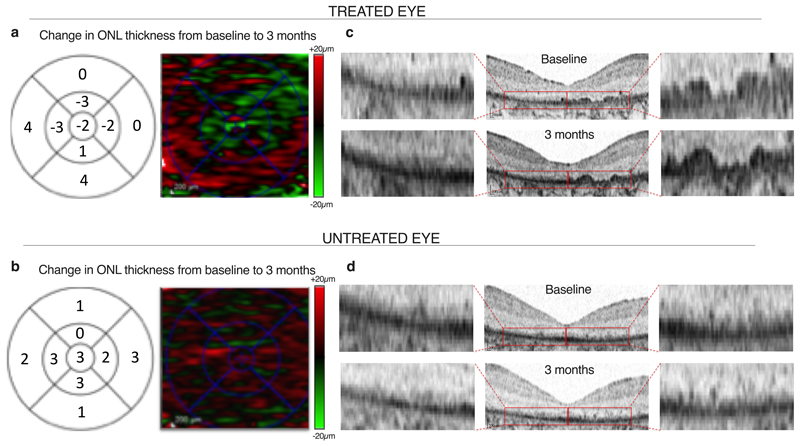
Natural history studies have previously shown that retinal degeneration in RPGR-related RP is characterized by outer nuclear layer (ONL) thinning on OCT19, associated with contraction of the ellipsoid zone20, visual field21 and foveal avascular zone on OCT angiography22. There is a characteristic macular cone display on adaptive optics scanning laser ophthalmoscopy23 in addition to functional loss of retinal sensitivity.24 Having observed greatest change from baseline in retinal sensitivity in patient C4.1, further more detailed analysis of retinal thickness by manual segmentation of each horizontal scan on OCT was performed. We purposely avoided automated OCT software to reduce any potential errors in measurements. The manual segmentation allowed us to increase the precision, clearly demarcate the ONL and avoid inaccurate measurements into any adjacent structures (e.g. the choroid). The analysis showed an increase in ONL thickness from baseline by 3 months in the treated eye (Fig. 3A). No change in retinal thickness was observed in the untreated eye (Fig. 3B), nor had this been observed in the earlier cohort patients who received low doses of the vector and had had no observed improvement in visual function (Extended Data Fig. 6). The increase in ONL thickness in the treated eye was associated with re-emergence of a new linear anatomical structure at the distal photoreceptor level (Fig. 3C). No anatomical differences were observed in untreated eyes or in low-dose controls (Fig. 3D and Extended Data Fig. 6).
Fig. 3. Outer nuclear layer changes following RPGR gene therapy.
(a) Full manual segmentation of 121-line scans of the OCT at three months of the central macula in the treated eye of patient C4.1 who received the high-dose AAV8.coRPGR vector. This shows increased thickness of the outer nuclear layer (ONL) compared with baseline. The 1, 3 and 6 mm ETDRS macula grid (right column) show mean sectoral ONL thickness changes (μm) and the heat map of ONL thickness (left column). The map shows an increase in retinal thickness (red) in a ring around the fovea of 10-15μm per quadrant, consistent with the OCT images both in terms of the magnitude and location of the retinal thickness change. (b) No change in retinal thickness was observed in the untreated eye. (c) Emergence of novel anatomical structures over areas of previously degenerate macula (red arrows) in the treated eye at 3 months following high-dose (6x1010 gp) RPGR gene therapy. This led to the OCT appearance of ‘double lines’, consisting of the new layer (red arrows) over the RPE layer (black layer). (d) No outer retinal changes were seen in the untreated eye. Scale bars = 200 μm.
To establish any potential correlation between the site of vector deposition at the subretinal bleb and the improvement in visual perception, we demarcated the bleb area for all patients across the cohorts (Extended Data Fig. 2). In all trial subjects except C1.1 the bleb covered most of the central, para-foveal area in the posterior pole including foveal detachment. The C1.1 patient was part of early cohort patients (cohorts 1 and 2) who had more advanced degeneration. Thus, retinal areas treated with gene therapy corresponded to areas of expected gains in visual function as measured by microperimetry in all patients expect C1.1. The improvement in visual function was observed in all three patients in cohort 3 treated with the mid-dose (optimal vector dose, 5x1010 gp) a cohort 4 patient, C4.1 (similar dose to cohort 3 patients, 6x1010 gp), a cohort 5 patient, C5.3 (1.25x1011 gp) and two cohort 6 patients C6.2 (2.5x1011 gp) and C6.3 (1.5x1011 gp). Detailed anatomical analysis was performed in patient C4.1 who showed maximum gain in microperimetry demonstrating, in addition, an anatomical change which might explain the functional improvement. This was not observed in the untreated eye. Moreover, since a subretinal injection could potentially interfere with the retinal architecture, we looked for similar anatomical changes in a patient whose treated eye did not have a functional improvement (C1.2), confirming that the observation was specific.
The combination of advanced degeneration and low vector dose in cohorts 1 and 2 may explain why we did not observe functional gains in these earlier cohorts. At higher doses (1x1011-4x1011 gp) which include patients in cohort 4 (C4.2 and C4.3) and cohorts 5 and 6, we believe that the observed inflammation may have reduced the potential gains in visual function. Thus, although we observed a correlation between the site of the sub-retinal bleb and the improvement in visual perception in some patients, the process is more complex with the interplay between the stage of retinal degeneration, the vector dose and any negative effects related to inflammation.
Discussion
We report early results from the first retinal gene therapy clinical trial for X-linked retinitis pigmentosa, which is a significant cause of untreatable sight loss in young men. Improved night vision has been observed following gene therapy for Leber congenital amaurosis, but had been expected, as the missing RPE65 protein has an important role in the visual cycle.24 By contrast, in XLRP the expectation has been stabilisation of visual decline by rescuing the remaining cells from cell death, because improvements in visual function above baseline were not previously detected in the animal models.12 Herein we describe the early improvement of visual field in a cohort of patients receiving mid doses of AAV8.coRPGR vector. The clinical benefit was sustained up to the last time point (minimum 6 months for the last patient).
Overall, seven patients (all cohort 3 patients, C4.1, C5.3, C6.2 and C6.3) demonstrated visual gains in treated eyes beginning at month 1 that were sustained at month 6 follow-up (although microperimetry in C5.3 was unreliable). The visual gains correlated with the site of the subretinal bleb, although some patients showed no improvements in visual perception despite the similar bleb site. Since this is a Phase 1 clinical trial, with variable doses in patients at variable stages of degeneration, the results of gene therapy will most likely involve an interplay between the stage of retinal degeneration, the vector dose and any negative effects related to inflammation.
At higher doses (cohorts 4 to 6) we observed mild retinal inflammatory responses in treated eyes (in 7 out of 9 patients) that potentially offset the visual gains observed in cohort 3 patients at lower vector doses. The inflammatory response was noted in the form of subretinal deposits that were seen as hyper-reflective clumps on the OCT. Without histological examination it is difficult to establish the nature of these lesions, but we predict them to be aggregations of inflammatory cells, including macrophages, and the associated tissue debris. Treatment with oral corticosteroids resulted in resolution of these deposits consistent with an inflammatory mechanism.
The patient C4.1 demonstrated greatest functional gains in terms of reversal of visual field loss together with an early stage degeneration in which anatomical features could be clearly identified before surgery. Hence his anatomical changes were reported in more detail. He received a higher vector dose (6x1010 gp) than patients in cohort 3 and at this dose we observed some subtle signs of intraocular inflammation, which corresponded to initial reduction in microperimetry. Once the inflammation settled post treatment with oral prednisolone, the microperimetry gains were re-confirmed. Thus, in addition to the greatest gain in microperimetry from baseline, the case demonstrates the use of microperimetry as a sensitive biomarker of inflammation.
Furthermore, a detailed anatomical analysis of the patient’s treated retina demonstrated that the functional gains could be correlated to a putative anatomical improvement, indicating a potential regeneration mechanism post gene therapy.
Whilst we cannot exclude a general increase in retinal thickness being due to inflammation, the emergence of a novel linear structure anatomically is unlikely to represent an inflammatory response, supporting our hypothesis that this may be due to regeneration of photoreceptor outer segments (~10-15μm) in response to gene therapy.
Moreover, some degree of outer segment re-organisation or rehabilitation might be taking place. The RPGR protein is localised to the photoreceptor cilium that connects the cell body to the outer segment and shortening of outer segments has been observed in early stages in RPGR deficient mice.13
In conclusion, a Phase I portion of a gene therapy trial with AAV8.coRPGR vector did not have any dose limiting toxicities. Some, but not all, of the secondary endpoints of efficacy suggest reversal of visual field loss, which may relate to regeneration of outer retinal structures following successful gene transfer.
Methods
Details of the study protocol
Ethical and regulatory considerations
The study was conducted with approval from appropriate research ethics committees, regulatory committees and host institutions. The study was conducted in full conformity with all applicable laws and regulations, including the International Conference on Harmonisation Guidelines for Good Clinical Practice (CPMP/ICH/135/95) and relevant articles of the Declaration of Helsinki (seventh revision, 2013). Written informed consent was obtained from each study participant.
Study recruitment
A total of 18 patients were enrolled in the gene therapy trial for X-linked retinitis pigmentosa (Clinicaltrials.gov ref: NCT03116113, ‘XIRIUS’ trial) using an AAV8 vector encoding codon-optimized human RPGR. Prior to use in humans, the AAV8.coRPGR vector which had been made to good manufacturing practices (GMP) standards was validated in pre-clinical studies (Extended Data Fig. 1) (for details please see methods section in Supplementary Appendix and Life Sciences Reporting Summary). The XIRIUS trial is a multicenter Phase I/II, first-in-human, dose-escalation interventional study of AAV8.coRPGR in male subjects with X-linked retinitis pigmentosa and genetically confirmed mutations in RPGR. The study used a 3+3 escalation scheme26 and involved 6 AAV8.coRPGR dose cohorts (Table 1). An independent Data Monitoring Committee reviewed safety data before confirming whether escalation to a higher dose level could occur. Study sites included Oxford, Manchester and Southampton in the UK and Miami in the USA. The XIRIUS trial started in the UK and was registered on the European Clinical Trials database on January 9 2017 (EudraCT number: 2016-003852-60) – the first patient was enrolled in the EU over two months after this date, on 16 March 2017. The Clinicaltrials.gov registration was posted on 14 April 2017 and the first US patient was treated sometime after this, on Nov 7 2018. Under the FDA AA801 regulations (42 CFR Part 11), a clinical trial approved by the FDA must be submitted for registration no later than 21 days after the first trial participant has been enrolled. The XIRIUS trial was submitted to Cinicaltrials.gov on March 29 2017 (NCT03116113). Hence notwithstanding the fact that the first patient was treated in the EU under the EU equivalent of the Clinicaltrials.gov registration, the registration of the first patient was still within the timelines stipulated by the FDA in the AA801 regulations.
Inclusion and exclusion criteria
Trial participants were recruited if they were willing and able to provide informed consent for participation in the trial. The following key inclusion criteria applied: (1) male ≥18 years of age, with a genetically confirmed mutation in RPGR, and able to comply and adequately perform all study assessments; (2) evidence of active disease clinically visible within the macular region in both eyes; (3) measurable ellipsoid zone (EZ) on spectral domain optical coherence tomography (SD-OCT) measured at screening within the nasal and temporal border of any B-scan, and not visible on the most inferior and superior B-scan; (4) best-corrected visual acuity (BCVA) in both eyes better than or equal to light perception (cohort 1), between 34-73 Early Treatment for Diabetic Retinopathy Study (ETDRS) chart letters (cohorts 2-3) and ≥34 ETDRS letters (cohorts 4-6). The exclusion criteria were as follows: (1) history of amblyopia in either eye; (2) unwilling to use barrier contraception for a period of 3 months following administration of the vector; (3) any significant ocular or non-ocular disease which, in the opinion of the investigator, may put the subject at risk or influence the study results, may influence the subject’s ability to perform study diagnostic tests, or impact the subject’s ability to participate in the study. This would include, but is not limited to: a) clinically significant cataract or b) contraindication to oral corticosteroid; (4) participation in a gene therapy trial or clinical trial with an investigational drug in the past 12 weeks.
Clinical assessments
Study outcomes
The primary safety endpoint in this Phase I/II trial was incidence of dose-limiting toxicities and treatment-emergent adverse events over a 24-month period. Secondary endpoints were changes from baseline in: (1) retinal sensitivity (MAIA microperimetry, dB); (2) BCVA (ETDRS letters) (3) SD-OCT changes and (4) autofluorescence over a 24-month period. Data were summarised for all trial patients using mean and its 95% confidence intervals. Exploratory endpoints included changes from baseline in other anatomical and functional outcomes including low luminance visual acuity, contrast sensitivity, colour vision test, speed reading test and full-field stimulus threshold (FST) test. Study outcomes were subjected to rolling / evolving study protocols and some exploratory endpoints (e.g. FST) were excluded following patient and operator feedback about the usefulness, the limited patient ability to perform the tests and validity of the test results.
Visual acuity
Trial participants had BCVA measured in each eye separately after refraction in accordance with established protocols of the ETDRS visual acuity chart.27
Microperimetry
Mesopic microperimetry (MAIA, CenterVue SpA, Padova, Italy) was conducted for both eyes at various study points using a standard 68-stimulus (10-2) grid covering the central 10 degrees of the macula. The study used the instrument’s automatic test pattern centering on the subject’s preferred retinal locus. Assuming the subject’s fovea was fairly preserved, the test pattern was centered on the fovea. To minimize learning effects, baseline microperimetry was conducted in triplicate over a 2-day period at visit 1 for all subjects. The third reading was validated as an accurate baseline28. Fig. S3 in Supplementary Appendix shows an example of baseline microperimetry assessments in one subject (C4.1).
SD-OCT
SD-OCT (Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany) of the retina was obtained using standardized settings: 30° high resolution macula volume scan consisting of 121 B-scans, using Automated Real-time Tracking (ART) mode 4. Foveal central retinal thickness was measured through the foveal OCT scan. In addition, OCT segmentation analysis (an exploratory endpoint) was performed in a subject that showed greatest improvements in microperimetry (and a control subject). This detailed analysis was performed in order to more accurately measure the retinal thickness, as the precise alignment if OCT images can be challenging after an iatrogenic retinal detachment and because any outer segment changes will by definition also change the retinal landmarks. Thus, complete segmentation of 121 OCT horizontal line-scans over the macula, with manual tracing of the outer retinal layers, was obtained by two masked independent observers. Outer retinal thickness changes from baseline were then generated using the automated topography comparison function of Heidelberg Eye Explorer with the ETDRS grid centered over the fovea, generating topographic heat maps (increase in thickness is colour-coded red and reduction in thickness green).
Statistics
This is a Phase I/II dose escalation trial including 18 patients. The primary objective of the study is to evaluate the safety profile of AAV8-coRPGR. The primary safety endpoint is incidence of dose limiting toxicities and treatment emergent adverse events during the study period. The analysis population for the safety analysis includes all patients enrolled in the study who received AAV8-coRPGR treatment. Secondary metric endpoints including visual acuity, microperimetry and central retinal thickness follow normal distribution and the data are therefore presented as change in mean from baseline at month 6 assessment time-point with corresponding 95% confidence intervals.
Concomitant therapy
Throughout the study, investigators were allowed to prescribe any concomitant medications deemed necessary to provide adequate supportive care. To minimize inflammation resulting from surgery or potential immune responses to vector/transgene, all subjects were given a 21-day course of oral prednisolone following closely the 17-day protocol established in the Phase III Vortigene naparvovec AAV gene therapy clinical trial,29 except allowing an extra 4 days for tapering the dose at the end of the course. Therefore, each subject received a total of 10 days of 1mg/kg/day of prednisolone (beginning 2 days prior to gene therapy, on the day of surgery and for 7 days afterwards) followed by 0.5mg/kg/day for 7 days, 0.25mg/kg/day for 2 days, and 0.125 mg/kg/day for 2 days.
Extended Data
Supplementary Material
For further details of the study protocols and data please see the Supplementary Appendix.
Acknowledgments
We thank all trial participants for their commitment to the trial and attending their follow-up visits and staff members of the Eye Research Group Oxford (ERGO) for their support throughout the study. The trial was sponsored by Nightstar Therapeutics, now Biogen Inc., (Clinicaltrials.gov: NCT03116113) with additional research infrastructure support provided by the NIHR Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the National Institute for Health Research, or the UK Department of Health. We thank Dr Brandon Lujan Medical Director of the Casey Eye Institute Reading Center, Portland, USA and the Doheny Image Reading Center, Doheny Eye Institute, Los Angeles, USA, both of whom independently reviewed and verified the findings from the SD-OCT data.
Footnotes
Reporting Summary
Further information on experimental design and reagents is included in Nature Research Reporting Summary which can be found here: https://www.nature.com/authors/policies/ReportingSummary.pdf
Data Availability
All requests for raw and analysed data and materials are promptly reviewed by the Oxford University, Nuffield Laboratory of Ophthalmology, and the sponsor, Biogen Inc. to verify if the request is subject to any intellectual property or confidentiality obligations. Patient-related data not included in the paper were generated as part of clinical trials and may be subject to patient confidentiality. Any data and materials that can be shared will be released via a Material Transfer Agreement upon reasonable request.
Author contributions
J.C.-K., K.X., A.N., A.D., L.J.W., A.P.S., J.K.J., G.C.M.B., N.Z.G., J.L.D., P.R.R., A.J.L., B.L.L., P.E.S., R.E.M., recruited and monitored trial participants during screening and follow-up visits; R.E.M., P.E.S., J.L.D., performed gene therapy surgeries.; J.C.-K., K.X, N.Z.G., assisted with surgeries; J.C.-K., K.X., E.L., J.W.A., B.J.L., T.O., A.G., G.C.M.B., R.E.M., collected data and performed data analysis; C.M.-F.d.I.C., M.D.F., R.E.M., performed pre-clinical studies to validate the vector; A.R.B., biological safety officer; A.G., T.O., R.E.M., designed the trial protocol; J.C.-K., K.X., R.E.M, wrote and revised the manuscript; All authors provided scientific input, read and approved the manuscript.
Competing Interest
R.E.M. scientific cofounder of Nightstar Therapeutics Inc. (now owned by Biogen Inc.); R.E.M., G.C.M.B. scientific advisors to the UK National Health Service National Institute for Health and Care Excellence (NICE) in relation to retinal gene therapy; M.D.F., B.L.L., B.J.L., R.E.M. consulting or on advisory board for Biogen Inc.; R.E.M., M.D.F. named inventors on the patent relating to codon-optimised RPGR gene therapy owned by the University of Oxford (US20180273594A1, originally filed 2015-09-10). M.D.F., G.C.M.B., R.E.M. scientific advisory board to Novartis; E.L., T.O., A.G. were employees of the sponsor of the trial, Nightstar Therapeutics (now Biogen Inc). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the UK Department of Health.
References
- 1.Bennett J. Taking stock of retinal gene therapy: looking back and moving forward. Mol Ther. 2017;25:1076–1094. doi: 10.1016/j.ymthe.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandenberghe LH. What is next for retinal gene therapy? Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a017442. a017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boye SE, Boye SL, Lewin AS, Hauswirth WW. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21:509–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahel JA, Marazova K, Audo I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb Perspect Med. 2014;5 doi: 10.1101/cshperspect.a017111. a017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan JL, et al. and the Foundation Fighting Blindness Scientific Advisory Board. Inherited Retinal Degenerations: Current Landscape and Knowledge Gaps. Transl Vis Sci Technol. 2018;7(4):6. doi: 10.1167/tvst.7.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiCarlo JE, Mahajan VB, Tsang SH. Gene therapy and genome surgery in the retina. J Clin Invest. 2018;128:2177–2188. doi: 10.1172/JCI120429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talib M, et al. Clinical and genetic characteristics of male patients with RPGR-associated retinal dystrophies: A Long-Term Follow-up Study. Retina. 2018 Mar;8 doi: 10.1097/IAE.0000000000002125. [DOI] [PubMed] [Google Scholar]
- 8.Fischer MD, et al. Codon-optimized RPGR improves stability and efficacy of AAV8 gene therapy in two mouse models of X-Linked retinitis pigmentosa. Mol Ther. 2017;25:1854–1865. doi: 10.1016/j.ymthe.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker CK, Flannery JG. Innovative Optogenetic Strategies for Vision Restoration. Front Cell Neurosci. 2018;12:316. doi: 10.3389/fncel.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Megaw RD, Soares DC, Wright AF. RPGR: Its role in photoreceptor physiology, human disease, and future therapies. Exp Eye Res. 2015;138:32–41. doi: 10.1016/j.exer.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell S, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng W-T, et al. Stability and safety of an AAV vector for treating RPGR-ORF15X-linked retinitis pigmentosa. Human Gene Therapy. 2015;26(9):593–602. doi: 10.1089/hum.2015.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z, et al. A long-term efficacy study of gene replacement therapy for RPGR-associated retinal degeneration. Hum Mol Genet. 2015;24(14):3956–3970. doi: 10.1093/hmg/ddv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawlyk BS, et al. Photoreceptor rescue by an abbreviated human RPGR gene in a murine model of X-linked retinitis pigmentosa. Gene Therapy. 2015;23(2):196–204. doi: 10.1038/gt.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, et al. Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations. Proc Natl Acad Sci U S A. 2016;113(21):E2925–34. doi: 10.1073/pnas.1523201113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue K, Groppe M, Salvetti AP, MacLaren RE. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond) 2017;31:1308–1316. doi: 10.1038/eye.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maguire AM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cukras C, et al. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol Ther. 2018;26(9):2282–2294. doi: 10.1016/j.ymthe.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hood DC, Lazow MA, Locke KG, Greenstein VC, Birch DG. The transition zone between healthy and diseased retina in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52(1):101–8. doi: 10.1167/iovs.10-5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tee JJL, Carroll J, Webster AR, Michaelides M. Quantitative Analysis of Retinal Structure Using Spectral-Domain Optical Coherence Tomography in RPGR-Associated Retinopathy. Am J Ophthalmol. 2017;178:18–26. doi: 10.1016/j.ajo.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birch DG, et al. Rates of decline in regions of the visual field defined by frequency-domain optical coherence tomography in patients with RPGR-mediated X-linked retinitis pigmentosa. Ophthalmology. 2015;122(4):833–9. doi: 10.1016/j.ophtha.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang PH, Jauregui R, Tsang SH, Bassuk AG, Mahajan VB. Optical Coherence Tomography Angiography of RPGR-Associated Retinitis Pigmentosa Suggests Foveal Avascular Zone is a Biomarker for Vision Loss. Ophthalmic Surg Lasers Imaging Retina. 2019;50(2):e44–e48. doi: 10.3928/23258160-20190129-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan JL, et al. High-resolution imaging with adaptive optics in patients with inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48(7):3283–91. doi: 10.1167/iovs.06-1422. [DOI] [PubMed] [Google Scholar]
- 24.Cideciyan AV, et al. Progression in X-linked Retinitis Pigmentosa Due to ORF15-RPGR Mutations: Assessment of Localized Vision Changes Over 2 Years. Invest Ophthalmol Vis Sci. 2018;59(11):4558–4566. doi: 10.1167/iovs.18-24931. [DOI] [PubMed] [Google Scholar]
- 25.Bennett J, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388:661–72. doi: 10.1016/S0140-6736(16)30371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45(3):925–37. [PubMed] [Google Scholar]
- 27.Klein R, Klein BE, Moss SE, DeMets D. Inter-observer variation in refraction and visual acuity measurement using a standardized protocol. Ophthalmology. 1983;90(11):1357–9. doi: 10.1016/s0161-6420(83)34382-7. [DOI] [PubMed] [Google Scholar]
- 28.MacLaren RE, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–37. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maguire AM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.