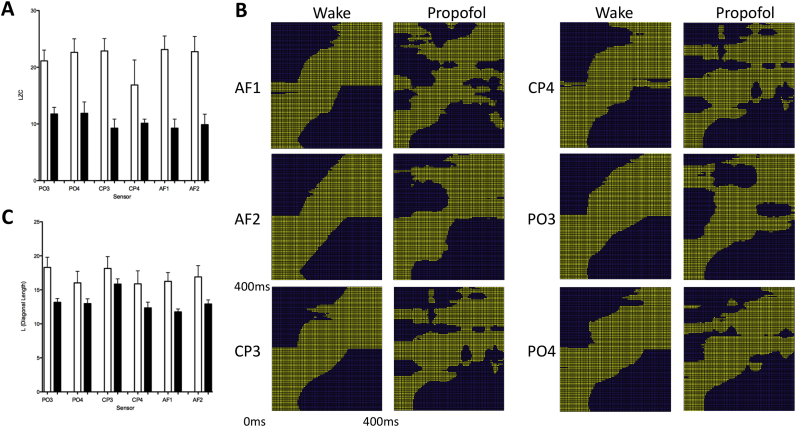
Editor—We showed recently that propofol induces changes in the evoked EEG power spectral response after transcranial magnetic stimulation (TMS) of the occipital cortex.1 As an extension of these analyses, we further analysed these data to investigate whether propofol reduced Lempil–Ziv complexity (LZC), or increased stability, of the evoked EEG power spectrum after TMS. The evoked power spectrum was calculated using time–frequency decomposition using a seventh-order Morlet wavelet transform to provide good time resolution.1 Initially we calculated LZC, as described previously for the evoked response potential,2 using a 10% threshold for evoked power changes to binarise the results. A three-way analysis of variance (anova) was used to identify the effects of propofol, sensor, or laterality after left-sided occipital TMS. Similar to changes in absolute power,1 propofol reduced the complexity of the power spectrum in sensor space with three-way anova showing a significant effect of propofol (F=71.2; P<0.0001) but not sensor or laterality (P>0.05; Fig. 1a). These data indicate a consistent effect of propofol in reducing the complexity of the evoked power spectrum across sensors. This adds to accumulating evidence suggesting that during unconsciousness the complexity of the spontaneous EEG, and evoked responses, is reduced.
Fig 1.
Propofol reduces the complexity and increases the stability of the EEG power spectral responses to occipital transcranial magnetic stimulation (TMS. (a) Lempil–Ziv complexity (LZC) of evoked power spectral response from 0 to 400 ms after TMS is reduced by propofol (black bars) compared with awake [white bars; p<0.05 on three-way analysis of variance (anova)]. (b) Grand mean recurrence plots of the evoked power spectrum across subjects for each electrode. X and Y axes are time in ms after TMS. Each yellow or blue dot represents a 4 ms sample. Yellow dots are the 50 nearest neighbours in Euclidean distance (between power values) to that time point. Propofol increases the length of yellow vertical and horizontal lines, while decreasing the length of diagonal lines, L (as shown in panel c), showing increased spectral recurrence (the spectral pattern has recurred in time) and therefore increased neuronal stability. (c) Quantitative analysis shows that propofol (black bars) decreases L across electrodes compared with the awake state (white bars; P<0.05 on three-way anova).
Next, inspired by recent demonstrations that neuronal dynamics become more stable under anaesthesia,3 we quantified temporal complexity (or stability) of the evoked time–frequency responses using recurrence quantification analysis (RQA).4 This form of non-linear dynamical systems analysis can specifically be used in short epochs of data obtained from non-stationary systems.5, 6 Here, we used a state space based on frequencies as quasi-dimensions (mathematically each frequency is not orthogonal and hence not a true dimension). Thus, we are able to describe the time evolution of the spectral power through a multidimensional frequency space. We calculated the Euclidean distance (ED) between power responses across time for the 0.5–40 Hz frequency range, and then performed recurrence plot analysis using the 50 nearest neighbours in state space. In the resulting recurrence plots, diagonal lines represent a trajectory in which the evoked spectral power evolves progressively with time (‘spectral progression’), whereas vertical and horizontal lines represent periods of neuronal stability such that evoked power has recurred to the same point in spectral space (Fig. 1b). We calculated a standard recurrence analysis statistic, the average length of diagonal lines, L. Greater values of L imply greater spectral progression, whereas lower values imply greater spectral stasis or recurrence. Hence, L provides a description of the dynamic changes (or stability) in spectral power over time as captured in the time–frequency decomposition of the cortical TMS-evoked responses. We conducted a three-way anova showing a significant effect of propofol (F=26.7; P<0.0001) but not of sensor or laterality (P>0.05; Fig. 1c). These data suggest that propofol reduces complexity in the power spectrum with stabilisation of neuronal dynamics in time after TMS. This supports recent notions that general anaesthesia is associated with stabilisation of neuronal dynamics3 with a reduction in the repertoire of available neuronal responses to a stimulus.2
Acknowledgements
The authors thank S. Laureys, M. Boly, and M. Massimini for providing TMS data and advice on the analyses.
Declaration of interest
The authors declare that they have no conflict of interest. RDS is a member of the BJA editorial board.
References
- 1.Sanders R.D., Banks M.I., Darracq M. Propofol-induced unresponsiveness is associated with impaired feedforward connectivity in the cortical hierarchy. Br J Anaesth. 2018;121:1084–1096. doi: 10.1016/j.bja.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casali A.G., Gosseries O., Rosanova M. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med. 2013;5:198ra05. doi: 10.1126/scitranslmed.3006294. [DOI] [PubMed] [Google Scholar]
- 3.Solovey G., Alonso L.M., Yanagawa T. Loss of consciousness is associated with stabilization of cortical activity. J Neurosci. 2015;35:10866–10877. doi: 10.1523/JNEUROSCI.4895-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouyang G., Li X., Dang C., Richards D.A. Using recurrence plot for determinism analysis of EEG recordings in genetic absence epilepsy rats. Clin Neurophysiol. 2008;119:1747–1755. doi: 10.1016/j.clinph.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Tosic T., Sellers K.K., Frohlich F., Fedotenkova M., Beim Graben P., Hutt A. Statistical frequency-dependent analysis of trial-to-trial variability in single time series by recurrence plots. Front Syst Neurosci. 2015;9:184. doi: 10.3389/fnsys.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Sleigh J.W., Voss L.J., Ouyang G. Measure of the electroencephalographic effects of sevoflurane using recurrence dynamics. Neurosci Lett. 2007;424:47–50. doi: 10.1016/j.neulet.2007.07.041. [DOI] [PubMed] [Google Scholar]