Abstract
Introduction
Currently, there are three Phase I/II clinical trials based on gene therapy ongoing to test different AAV.RPGR or deleted RPGR vectors on patients affected by X-linked retinitis pigmentosa. These three vectors differ in the adeno-associated viral (AAV) vector capsid used, and the coding sequences: two contain codon optimized versions of RPGR which give the full-length protein, whilst the third uses a wild-type sequence that contains a large deletion encoding part of the functional domain of the RPGR protein.
Areas covered
This review approaches the different studies that have led to the initiation of three different clinical trials for RPGR related X-linked retinitis pigmentosa.
Expert opinion
The development of a gene therapy vector to deliver a normal copy of the RPGR gene into the photoreceptors has presented a challenge for the scientific community. The instability of its sequence and the fact that its function is not well understood can lead to the production of a nonfunctional or deleterious protein for the human retina. Since the RPGR protein undergoes post-translational glutamylation in the protein domain that may be particularly affected by gene instability, a functional assay of glutamylation is essential to verify the correct coding sequence.
Keywords: Adeno-associated virus, clinical trials, codon optimization, gene therapy, retinitis pigmentosa GTPase regulator (RPGR)
1. Introduction
Gene therapy is a powerful therapeutic approach to correct a pathological phenotype caused by genetic mutations, either by replacing, silencing or editing the affected gene. The potential of gene therapy to treat a broad range of diseases is reflected in the increasing number of clinical trials worldwide: over 2,335 clinical trials completed, in progress or approved in the last 30 years, with a prominent increase since 2012 [1]. The eye has several features that make it an excellent target for gene therapy: the accessibility allows relatively noninvasive procedures, the effect of the treatment can be easily monitored using different assessments performed in the clinic routinely, and the blood-retinal barrier limits the immunological response to the treatment by reducing systemic spread [2,3]. In December 2017, for the first time, a gene therapy product for a hereditary retinal degeneration came to market. Luxturna (Voretigene Neparvovec), developed by Spark Therapeutics Inc., was approved by the US Food and Drug Administration (FDA) to treat a severe inherited retinal degeneration (IRD), Leber congenital amaurosis (LCA), by delivering a normal copy of the RPE65 gene to the retinal pigment epithelium (RPE) cells [4,5].
IRDs are a group of diseases that result in a progressive degeneration of the outer retina with consequent visual impairment. Currently, mutations in over 260 genes have been discovered to be involved in IRDs [6]. The high genetic diversity and overlapping phenotypes of IRDs pose significant challenges in the genetic elucidation of these disorders. Recessive single gene retinal degenerations are the most amenable to gene therapy because there is a lack of functional protein, rather than the presence of a toxic protein. Thus, the delivery of the healthy copy of the gene should revert the phenotype without having to knock down a genetic mutation that creates a toxic product.
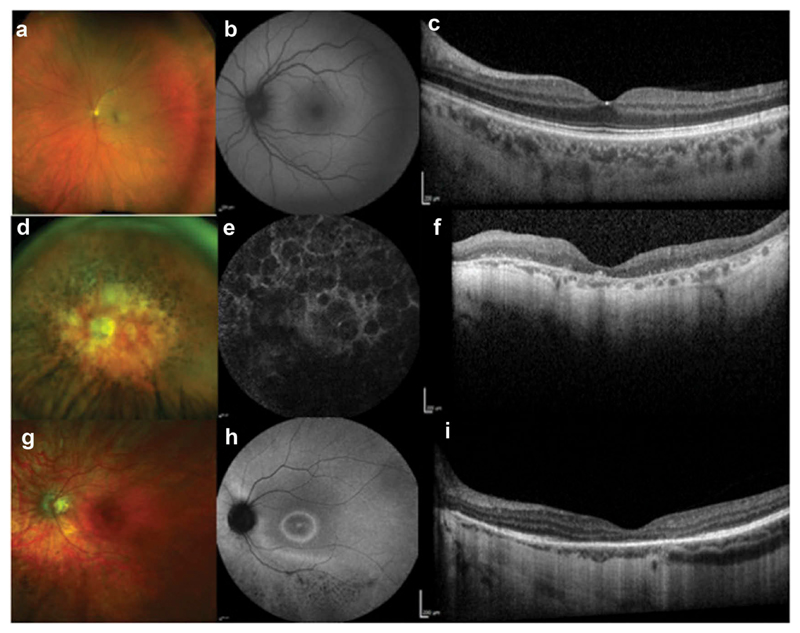
X-linked retinitis pigmentosa (XLRP) is particularly severe, with an early onset and fast progression to legal blindness by the third to fourth decade [7]. The most common form of XLRP is caused by mutations in the retinitis pigmentosa GTPase regulator (RPGR) gene, whilst less frequent forms of XLRP may be caused by mutations in RP2 or OFD1 genes [8,9]. The RPGR gene encodes different RPGR transcripts differentially spliced among tissues. RPGRORF15, the second major transcript, encodes an 1152 amino acid protein only expressed in retinal photoreceptors where it is located in the connecting cilia of both rods and cones. Although mutations in RPGR can be associated with a rod-cone or cone-rod dystrophy phenotype (Figure 1), the most common presentation is as a rod-cone dystrophy, initially identified with difficulties in scotopic visual function, where there is a predominant loss of rod photoreceptors [10]. Simultaneously, peripheral vision deteriorates, resulting in visual field constriction on perimetry findings. Sufferers usually maintain reasonably good visual acuity until the end stages of the disease wherein the fovea becomes affected by subsequent cone photoreceptor degeneration [11]. Cone-rod dystrophy, a less commonly described clinical presentation of XLRP, primarily affects cone function. Patients complain of poor visual acuity, myopia, photophobia, and abnormal color vision. Cone-rich central regions are initially affected but significant variability exists in the involvement of rod photoreceptors [12,13].
Figure 1.
Multimodal retinal imaging showing normal phenotype (A-C) and two patients with RPGR-retinal degeneration: rod-cone phenotype (D-F) and cone-rod phenotype (G-I). Optos widefield (A,D, G). Fundus autofluorescence (B,E, H). Optical coherence tomography (C,F, I).
RPGR mutations represent a major contribution to the RP disease, accounting for 70-80% of XLRP-affected families and 10-20% of all RP cases [8,14]. Its high prevalence and the severity of the disease caused by mutations in this gene make the development of genetic therapies a high impact approach for RP patients. However, the RPGR gene has an unusual genetic code that presents challenges for manufacturing gene therapy vectors able to express the full-length protein with biological activity. Different RPGR vectors, currently in clinical trials, have been developed following different approaches with greater or lesser efficiency in producing the full-length protein.
2. Body
2.1. The singularity of the RPGRORF15 sequence
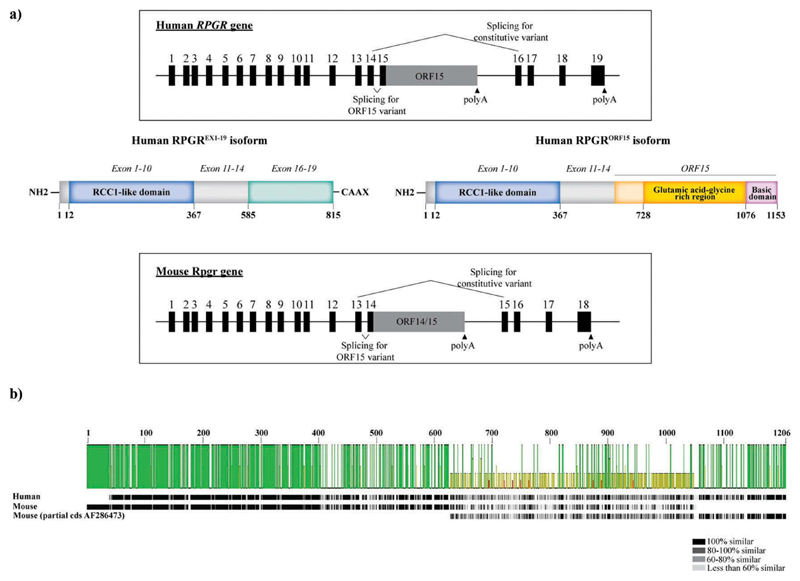
The RPGR gene occupies the X-linked ‘RP3’ locus located in the short arm of the X chromosome (Xp11.4). The constitutive sequence is composed of 19 exons that encode a protein of 815 amino acids which is widely expressed in different tissues (RPGREx1–19 isoform, also termed the constitutive variant). As mentioned previously, the second major transcript, RPGRORF15, is a unique splice variant that encodes a protein only expressed in retinal photoreceptors. Both transcripts share exons 1–14, a region that harbors the disease-causing variants responsible for around 25% of cases. Unlike the constitutive isoform, RPGRORF15 has a large alternatively spliced exon, ORF15, derived by reading through the splice donor site of exon 15, that leads to the extension of the canonical exon 15 into intron 15 (Figure 2). This ORF15 region contains a highly purine-rich repetitive region, comprising mostly adenine (A) and guanine (G) nucleotides and is terminated by its own stop codon at the end of this region. Hence, exons 16–19 are not encoded in the photoreceptor-specific ORF15 splice variant. Furthermore, since the sequence ‘AG’ is specific of splice acceptor sites, the high frequency of AG in the ORF15 region may activate cryptic splice sites in regions that usually are not spliced, resulting in truncated forms of the protein. This highly purine-rich repetitive sequence makes the RPGR gene very unstable and prone to mutations. Indeed, exon ORF15 is considered a mutation hot spot since 80% of the RPGR mutations have been identified in this region [15]. The repetitive nature of the sequence can also contribute to unusual non-standard double helix DNA conformations, affecting DNA replication and transcription, and contributing to genome instability [16]. The mutations in ORF15 comprise mostly deletions/insertions, duplications, and nonsense mutations, thus resulting in the truncation of RPGRORF15 protein. Regardless of the mutation type, it has been proposed that there may be a correlation between the location of the mutations within ORF15 and the severity of the disease; the longer the encoded wild-type amino acid sequence, the milder the disease and vice versa [17–22].
Figure 2.
RPGR gene structure and the main isoforms. (a) Schematic representation of the human and mouse RPGR gene structure and the two major protein isoforms (constitutive RPGREX1-19 and RPGRORF15 isoforms). (b) Protein sequence comparison of human and mouse RPGRORF15. The protein sequences for human RPGRORF15 (NP_001030025) and mouse RPGRORF15 (NP_001171421 and the partial coding sequence AF286473) were extracted from NCBI database and aligned using Geneious Prime 2019.1.3. The pairwise identity, represented by the green bars, is 42.4% with a variable level of identity along the sequence. The lowest identity corresponds to the ORF15 region, whilst the conserved regions (RCC1-like domain and basic domain) show the highest identity. The similarity between residues, represented by the grayscale bar, also shows a lesser degree of similarity between the human and the mouse protein sequence in the ORF15 region.
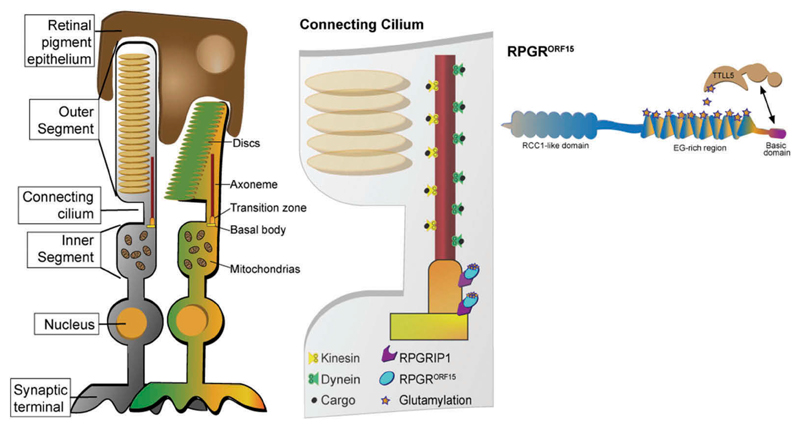
Exon ORF15 is translated into a glutamic acid-glycine rich domain comprising glutamic acid and glycine residues as repeat-like sequence such as ‘EEEGEGEGE’. Due to the repetitive nature of the glutamic acid-glycine rich region and the fact that its length varies among species, it is challenging to decipher the function of this domain. This repetitive region contains 11 glutamate-rich consensus motifs. Indeed, RPGRORF15 has been found to be a glutamylation substrate and post-translational modification appears to be critical for its function [23,24]. The addition of negatively charged glutamates to this region is likely to affect the stabilization and the folding of the RPGRORF15 protein and its interaction with other proteins in the connecting cilia, particularly with regard to trafficking [25–27]. The connecting cilium represents a critical junction between the inner and the outer segments, allowing the bidirectional transport of opsins and other phototransduction proteins and contributing to the viability of the photoreceptors. Although RPGRORF15 function is not well understood, its interaction with other ciliary proteins to form protein complexes involved in cilia regulatory signaling pathways and the glutamylation of the ORF15 region in the C-terminus suggest that it plays an important role in microtubule-based transport to and from the basal bodies and within photoreceptor axonemes, contributing to the intracellular cargo movement between inner and outer segments (Figure 3) [23,28–30]. In humans, a lack of glutamylation caused by Tubulin Tyrosine Ligase Like 5 (TTLL5) enzyme deficiency causes an inherited retinal degeneration, similar to RPGR deficiency, hence indicating that the glutamylated protein is essential for normal RPGRORF15 function [23,31,32]. The acidic, repetitive glutamic acid-glycine rich domain is followed by a non-repetitive, basic domain in the carboxy-terminus. The basic domain is known to be evolutionarily conserved with a high sequence identity across species [33], suggesting that it too constitutes a functional domain.
Figure 3.
Structure of retinal photoreceptors. RPGRORF15 is localized to the connecting cilium of the photoreceptors, tethered to the axoneme and the basal bodies by its interaction with other proteins such as RPGRIP1. Evidence suggests that RPGRORF15 regulates cargo trafficking between the inner and the outer segment, and for this function, its glutamylation by the enzyme TTLL5 is essential.
2.2. Optimization of the RPGR gene therapy vector
XLRP caused by mutations in RPGR is an ideal disease to be treated with gene therapy because (1) the size of the gene is small enough to fit within the packaging capacity of adeno-associated viral (AAV) vectors, which is limited to 4.7 kilobases, (2) the delivery of the normal gene can correct the lost function without toxicity associated to its overexpression, (3) the high prevalence, up to 20% of all RP cases are caused by mutations in RPGR gene [8,14], and (4) mutations in RPGR cause a severe phenotype with a rapid progression of the disease, facilitating the clinical trial endpoints assessment within a relatively short period of time. However, the development of the therapeutic vector for gene therapy constitutes a challenge because the purine-rich, repetitive sequence of RPGRORF15 makes it less stable and more prone to spontaneous mutations during the cloning of recombinant viral vectors.
Several groups have been working in recent years in the development of AAV vectors that could deliver a normal copy of the RPGR gene into the retinal photoreceptors. Doing this successfully depends not only on the molecular approach followed to design and produce the vector but also on carrying out the appropriate preclinical assessments to confirm that the vector will express the correct protein in humans, in terms of structure and function. In 2015, Li and collaborators proposed a shortened version of RPGRORF15 as viable for gene replacement therapy [34]. In this study, two human RPGRORF15 replacement genes, named as short and long forms, were injected subretinally in the Rpgr null mouse model for expression, localization and functional assessment. Both forms have a mutation within the ORF15 region – the short form has an in-frame deletion of 314 codons and the long form contains an in-frame deletion of 126 codons. The longer truncated protein was expressed in the retinal photoreceptors of the injected Rpgr null mice. This form reduced the rhodopsin and cone opsin mislocalization, the inner/outer segment length was greater compared to untreated eyes and seemed to improve rod and cone photoreceptor function. However, with the purpose of treating human patients as the main objective, careful consideration needs to be taken. The amino acid residues deleted in the longer mutated construct (residues 862–987) are not present in the wild-type orthologous isoform in mouse (NCBI reference sequence NP_001171421) and hence the deleted RPGR variant tested is actually more mouse-like than human. The absence of this region in mice suggests that it might not be playing an essential role in the mouse retina, and that the delivery of the other protein domains such as the RCC1-like domain at the N-terminus and the basic domain at the C-terminus are providing the photoreceptors with enough activity to rescue the phenotype and delay the degeneration in the Rpgr null mouse, which has a very mild phenotype compared to humans. This success in mice is therefore likely to be far more challenging to replicate in humans. The human ORF15 region contains 11 consensus motifs for glutamylation, modification that is essential for protein trafficking in the connecting cilia of the retinal photoreceptors, as we discussed above. The long mutated construct lacks more than one third of the Glu-Gly repeat region and 6 of the 11 consensus motifs, and its level of glutamylation is reduced by more than 70% [23]. The reduction in the level of RPGR glutamylation would affect its function in the human retina and, furthermore, the replacement with a construct missing this region could have unpredictable detrimental effects on the human retina [23,24,35]. This vector is currently being tested in a Phase I/II clinical trial.
An alternative approach to avoid the synthesis of mutated constructs is codon optimization. In the context of gene therapy with recombinant AAV as a vector system with its limited packaging capacity, codon optimization offers the potential to increase transgene expression without additional regulatory elements such as the woodchuck hepatitis virus post-translational regulatory element (WPRE) which can also increase transgene expression [36]. Moreover, changing the nucleotide sequence without altering the translated amino acid sequence (silent substitutions) of the transgene allows variation in the CG content, removing unwanted repeat sequences, cryptic splice sites and/or restriction sites that may interfere with cloning. Cloning the full-length human RPGRORF15 without random mutations being introduced is difficult – as is direct sequencing of the adenine/guanine rich regions since polymerases may often stall at guanine repeats. This underlying problem of genetic instability is most likely explained by properties of the mutational hotspot, the ORF15 region. It contains far fewer T and C nucleotides than would be predicted in the genome, for instance, the 750 base pair sequence between positions 2410 and 3160 contains no C nucleotides at all in the wild-type sequence. This leads to many repeating sequences that may recombine incorrectly during cloning and vector production.
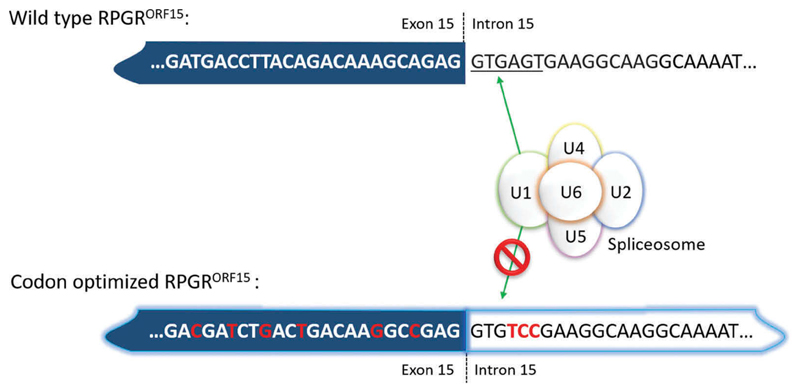
Codon optimization is extensively modeled in silico to determine the optimal modification to reduce the GA repeats and also to reduce the risk of anomalous splicing and the creation of premature polyA signals. The codon optimized gene is, therefore, more stable than the wild-type cDNA sequence, which may generate alternatively spliced variants and truncated proteins when reintroduced into the transcriptional machinery through gene therapy. Indeed, the greatest benefit of codon optimizing RPGRORF15 lies in its increased sequence fidelity or genetic stability. In 2017, two preclinical studies were published using codon optimized versions of the human RPGRORF15 sequence [37,38]. In such codon optimized vectors the splice donor site at the exon 15 boundary was disabled in order to prevent aberrant splicing, which might occur with the addition of pyrimidine bases in the ORF15 region where there are numerous potential ‘AG’ splice acceptor sites (Figure 4). This modification prevents the synthesis of truncated RPGR proteins, or RPGR proteins with large in-frame deletions.
Figure 4.
Codon optimization allows the removal of cryptic splice sites by replacing nucleotides that can be recognized by the spliceosome by other nucleotides without affecting the protein sequence. Hence, this approach makes the nucleotide sequence more stable and less prone to mutations. Note, that in this case, the canonical splice donor site ‘GT … ’ cannot easily be codon optimized because it is in-frame for valine in the ORF15 variant and all four valine amino acid codons have GT in positions 1 and 2. Positions 4–6 in the splice site, however, can be changed completely because they encode serine, which is AGT in the wild-type sequence (fulfilling the splice donor sequence) but TCC in the codon-optimized sequence. Serine has six different codons which provide a wider range of alternate nucleotide sequences to completely disrupt the splice donor site signal, whilst preserving the amino acid sequence in the protein.
The higher stability and absence of mutations in a codon-optimized RPGR vector were confirmed by the detection of the full length glutamylated protein in a human cell line in a preclinical study performed by Fischer and colleagues [37]. This vector rescued the electroretinogram (ERG) measurements in two mouse models of the disease (Rpgr knock out and Rd9 mice, which have an ORF15 mutation) and showed good safety profile in wild-type mice.
The codon optimized vector synthetized by Aguirre and collaborators (patent publication US20150353938A1) is very similar to the codon optimized sequence produced by Fischer and colleagues. This vector was validated in a large animal model of RPGR-XLRP [38]. The subretinal injection of codon optimized vector in RPGR mutant dogs provided a long-term rescue of both rod- and cone-mediated vision as shown in ERG measurements and visual behavioral studies. The treatment with this gene therapy vector also rescued photoreceptor morphology and reduced the mislocalization of the rod and the medium/long wavelength (M/L) cone opsin. These results together support the use of codon optimized RPGR sequences in clinical trials and it is difficult to see how they would not have a good chance of long-term success in humans. Both vectors are currently being tested in Phase I/II clinical trials.
2.3. Gene therapy clinical trials for RPGR
Currently, there are three human clinical trials ongoing (Table 1). The first clinical trial was initiated in March 2017 and is sponsored by Nightstar Therapeutics (NCT03116113). In this study, known as XIRIUS, a recombinant AAV2/8 vector containing the codon-optimized human RPGRORF15 under the control of the GRK1 promoter [37] was injected subretinally in male subjects with a genetically confirmed RPGR mutation. The second clinical trial, started in July 2017 and sponsored by MeiraGTx UK Ltd (NCT03252847), the safety and efficacy of the subretinal administration of the therapeutic vector is being evaluated in adults and children with XLRP for 18 months. The RPGR gene therapy vector, AAV2/5.hRKp.RPGR is based on the AAV2/5 serotype but the RPGR sequence is mutated in the ORF15 region and carries a random deletion of 126 codons – about one third of the human ORF15 coding sequence is missing [34]. Finally, the trial sponsored by Applied Genetic Technologies Corp (AGTC) (NCT03316560) was initiated in April 2018. The vector used in this study expresses a codon optimized version of the human RPGRORF15 driven by the GRK1 promoter and packaged in AAV2 capsids with tyrosine to phenylalanine (YF) mutations, described to enhance the efficiency of transduction. This trial arises from a proof-of-concept gene therapy study in dogs in which a newly developed codon-optimized human RPGR cDNA vector was able to transduce both rods and cones preserving the outer nuclear layer structure [38,39].
Table 1. Ongoing human gene therapy trials for X-linked retinitis pigmentosa.
| Gene therapy vector | RPGR coding sequence | Preclinical findings (reference) | Route of administration | Clinicaltrials.gov Number | Sponsor | Study phase |
|---|---|---|---|---|---|---|
| AAV8.GRK1.coRPGR | Codon optimized | Full length, glutamylated protein expressed in the connecting cilia of Rpgr KO mouse model (37) | Subretinal | NCT03116113 | Nightstar Therapeutics (now Biogen Inc) | Phase 1/2 |
| AAV5.GRK1.RPGR | Deleted wild type | Truncated protein: one third of the human ORF15 coding sequence is mutated (34) | Subretinal | NCT03252847 | MeiraGTx UK II Ltd | Phase 1/2 |
| AAV2tYF.GRK1.coRPGR | Codon optimized | Long-term rescue of photoreceptors degeneration in a large animal model (38) | Subretinal | NCT03316560 | Applied Genetic Technologies Corp (AGTC) | Phase 1/2 |
The promoter of choice in these three clinical trials for RPGR is the rhodopsin kinase (RK or GRK1), well known for its high efficiency in driving transgene expression in rod and cone photoreceptors. The two main differences in the three gene therapy vectors are the coding sequence and the viral capsid. As discussed above, the version of the coding sequence being tested in these trials differs mainly on its ability to produce the full-length protein that is fully glutamylated (hence, functional).
In addition to the differences in the coding sequences, the three vectors have different AAV capsids. The adeno-associated virus (AAV) is the best-characterized viral vector in ophthalmology and one of the main advantages is being a non-enveloped virus; thus, it is less likely to induce an inflammatory response. The exceptional safety profile of AAVs is also supported by the observation that AAVs have been used for more than 20 years in ocular gene transfer studies in animal models and more than 10 years in human clinical trials with no sign of malignant transformation [40]. But despite the evidence showing the safety of AAV vectors, the best AAV serotype and route of administration must be determined to maximize the safety and the efficiency of gene delivery. The use of recombinant AAV vectors is widely used in retinal gene therapy. These vectors contain the inverted terminal repeats (ITR) from AAV2 flanking the transgene cassette and packaged into the capsid from a different AAV serotype. In the retina, AAV2 is highly efficient at transducing retinal pigment epithelium (RPE) but less efficient at transducing photoreceptors, for which high doses are required [41]. The use of rAAV2/8 has a great potential in those diseases in which the photoreceptors are the target cells, since AAV8 is highly efficient transducing photoreceptors. Indeed, rAAV2/8, used in the clinical trial sponsored by Nightstar Therapeutics, has been shown to be more efficient and has a faster onset of gene expression in photoreceptors and RPE cells than AAV2/2 and AAV2/5 following subretinal injection into mice and non-human primate eyes [41,42]. It should also be noted that AAV5 is also efficient at transducing all subclasses of cones (foveal and parafoveal), as demonstrated in non-human primate retinas where vector was delivered subretinally [43].
One of the mechanisms that can affect transduction efficiency is the ubiquitination of AAV particles that can lead to their degradation by the proteasome. Since the ubiquitination mainly targets the surface-exposed tyrosine residues present on the AAV capsids, an alternative to enhance the transduction efficiency is to modify these residues, for example, by introducing tyrosine-to-phenylalanine (YF) mutations, to reduce the probability of being ubiquitin-tagged. An AAV2 capsid variant with three tyrosine to phenylalanine mutations (AAV2tYF), being used in the clinical trial sponsored by AGTC, was shown to be efficient at transducing primate cone photoreceptors after subretinal injection [44].
3. Conclusion
Gene therapy to treat inherited retinal diseases has come a long way and since the first gene therapy product approved (Luxturna®), several clinical trials have shown promising early data. XLRP caused by mutations in RPGR has been in the spotlight of different research groups in the last two years, due to its great suitability to be treated by gene therapy. However, this suitability does not imply that the path toward the development of an appropriate therapeutic product is straightforward. The repetitive nature of RPGR sequence and alternative splicing makes the synthesis of the gene therapy vector a challenge, since the introduction of mutations is highly likely. As can be anticipated, the synthesis of mutated constructs would lead to the synthesis of mutated proteins that lack functionality and/or can cause deleterious and unpredictable effects in the human retina. The codon optimization approach has facilitated the development of gene therapy constructs with stable and non-mutated coding sequence that lead to the synthesis of the full-length RPGR protein. This approach has resulted in a great leap forward to find an effective cure for these patients, and currently three clinical trials are ongoing, with the Phase I/II results awaited with great expectation.
4. Expert opinion
Gene therapy has the potential to treat several genetic and acquired diseases with a clinical benefit demonstrated in multiple fields of medicine in recent years. In ophthalmology, the potential application of gene therapy is wide due to several advantages that the eye presents. Some of these advantages are the accessibility that allows relatively noninvasive procedures, the effect of the treatment can be easily monitored and the blood-retinal barrier limits the immunological response to the treatment by reducing systemic spread. Inherited retinal diseases have an estimated incidence of 1:4,000 and are the leading cause of vision loss in persons between 15 and 45 years of age in developed countries [45]. This high prevalence and the fact that young people are the main affected group have a negative impact not only on public health but also from the social and economic point of view. Scientists and clinicians are making huge efforts to find treatment for these diseases, using gene therapy as the most promising strategy. X-linked retinitis pigmentosa caused by mutations in RPGR is an ideal target for gene therapy for several reasons: its high prevalence at 10-20% of all retinitis pigmentosa cases; its severity and rapid progression, with affected patients becoming legally blind by the third to fourth decade. This represents a highly favorable risk/benefit ratio and allows the assessment of the clinical trial endpoints within a relatively short period of time; RPGR is the single gene responsible for the pathological phenotype and, therefore, the correction of this defect by introducing a normal copy of the gene producing the functional protein would be sufficient to ameliorate the phenotype.
The difficulty here lies in the strategy to design and produce the gene therapy vector. The aim of this therapy must be to restore retinal photoreceptors with the function lost due to mutations in RPGR by introducing a normal copy of the gene able to synthetize the normal protein with biological activity, hence with therapeutic benefit. In order to do this, the entire and correct nucleotide sequence of the gene to be delivered by the AAV vector into the retinal cells where it will produce the full length and functional protein must be ensured. The instability characteristic of RPGR gene is given by the ORF15 region, which comprises mostly adenines and guanines. Indeed, 80% of the RPGR mutations have been identified in this region. Thus, it can be expected that the deletion of part of this ORF15 sequence would make the vector more stable during the cloning process. Indeed, an improvement in its stability has been shown in preclinical studies using a mouse model of the disease with an AAV vector containing a random deletion of 126 codons (vector currently being used in the clinical trial sponsored by MeiraGTx UK II Ltd). However, and although the RPGR function has not been deciphered with great detail, evidence has shown that the glutamylation of the glutamic acid-glycine region encoded by the ORF15 sequence is essential for RPGR function [23,24]. The glutamylation, that participates directly in the binding of many structural and motor tubulin-associated proteins, would facilitate the interaction between RPGR and other proteins in the connecting cilium of the photoreceptors allowing the transport of molecules required to maintain the cellular homeostasis and functionality. With codon optimization, it has been possible to maintain the full integrity of the RPGR amino acid sequence by introducing synonymous changes that do not affect the protein identity. In fact, this strategy has allowed not only to produce a gene therapy vector that keeps stable during the manufacturing process but also to produce the full length and glutamylated protein after delivery into human cell lines and animal models [37–39].
To date, there is no approved treatment for X-linked retinitis pigmentosa and efforts are focused on the demonstration of safety and efficacy of three different AAV.RPGR vectors in Phase I/II clinical trials. With the first results yet to be published, the initiation of these trials represents a step further on the way to success in finding a potential treatment to slow or even stop the degeneration in these patients.
Article highlights.
X-linked retinitis pigmentosa has a high prevalence and a severe phenotype, and there is no approved treatment to date.
Gene therapy strategies have been widely applied in preclinical and clinical studies to treat inherited retinal diseases due to its great potential to correct genetic defects, and to the advantages that the retina as a target organ presents.
The development of a gene therapy vector that provides a correctly coded copy of the RPGR gene to retinal photoreceptors is not straightforward, since the repetitive nature of its sequence and natural splice donor site makes the introduction of mutations highly likely.
Alternative approaches, such as codon optimization, need to be undertaken in order to produce a full-length stable protein without mutations that could affect post-translational modification, such as glutamylation which is required for its function.
The safety and efficacy of two different AAV.RPGR vectors and one AAV vector containing a deleted RPGR are being investigated in three clinical trials.
This box summarizes key points contained in the article.
Funding
This paper was funded by the Medical Research Council (UK) MR/K003690/1 award to RE MacLaren, Oxford NIHR Biomedical Research Center and Royal College of Surgeons of Edinburgh.
Footnotes
Declaration of interest
RE MacLaren is a consultant to Biogen, Spark Therapeutics and Novartis, which are developing AAV gene therapies for retinal disease. RE MacLaren is named inventor on a patent filed on behalf of the University of Oxford, relating to the expression cassette and codon optimization of RPGR coding sequence in general. RE MacLaren is the scientific advisor to The National Institute for Health and Care Excellence in the UK (NICE) retinal gene therapy committee. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
One of the reviewers on this paper is a on the Scientific Advisory Board of AGTC Inc. Other peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Hanna E, Rémuzat C, Auquier P, et al. Gene therapies development: slow progress and promising prospect. J Mark Access Health Policy. 2017;5(1) doi: 10.1080/20016689.2017.1265293. 1265293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore NA, Morral N, Ciulla TA, et al. Gene therapy for inherited retinal and optic nerve degenerations. Expert Opin Biol Ther. 2018;18(1):37–49. doi: 10.1080/14712598.2018.1389886. [DOI] [PubMed] [Google Scholar]
- 3.Petrs-Silva H, Linden R. Advances in gene therapy technologies to treat retinitis pigmentosa. Clin Ophthalmol. 2014;8:127–136. doi: 10.2147/OPTH.S38041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voretigene neparvovec-rzyl (Luxturna) for inherited retinal dystrophy. Med Lett Drugs Ther. 2018;60(1543):53–55. [PubMed] [Google Scholar]
- 5.Darrow JJ. Luxturna: FDA documents reveal the value of a costly gene therapy. Drug Discov Today. 2019;24(4):949–954. doi: 10.1016/j.drudis.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Daiger SP. Retinal information network. 1996-2017 https://sph.uth.edu/RetNet/home.htm. [Google Scholar]
- 7.Flaxel CJ, Jay M, Thiselton DL, et al. Difference between RP2 and RP3 phenotypes in X linked retinitis pigmentosa. Br J Ophthalmol. 1999;83(10):1144–1148. doi: 10.1136/bjo.83.10.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghupathy RK, Gautier P, Soares DC, et al. Evolutionary characterization of the retinitis pigmentosa GTPase regulator gene. Invest Ophthalmol Vis Sci. 2015;56(11):6255–6264. doi: 10.1167/iovs.15-17726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb TR, Parfitt DA, Gardner JC, et al. Deep intronic mutation in OFD1, identified by targeted genomic next-generation sequencing, causes a severe form of X-linked retinitis pigmentosa (RP23) Hum Mol Genet. 2012;21(16):3647–3654. doi: 10.1093/hmg/dds194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tee JJ, Smith AJ, Hardcastle AJ, et al. RPGR-associated retinopathy: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2016;100(8):1022–1027. doi: 10.1136/bjophthalmol-2015-307698. [DOI] [PubMed] [Google Scholar]
- 11.Campochiaro PA, Mir TA. The mechanism of cone cell death in Retinitis Pigmentosa. Prog Retin Eye Res. 2017;62:24–37. doi: 10.1016/j.preteyeres.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Demirci FY, Rigatti BW, Wen G, et al. X-linked cone-rod dystrophy (locus COD1): identification of mutations in RPGR exon ORF15. Am J Hum Genet. 2002;70(4):1049–1053. doi: 10.1086/339620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 14.Chiang JPW, Lamey TM, Wang NK, et al. Development of high-throughput clinical testing of RPGR ORF15 using a large inherited retinal dystrophy cohort. Invest Ophthalmol Vis Sci. 2018;59(11):4434–4440. doi: 10.1167/iovs.18-24555. [DOI] [PubMed] [Google Scholar]
- 15.Vervoort R, Lennon A, Bird AC, et al. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat Genet. 2000;25(4):462–466. doi: 10.1038/78182. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Bacolla A, Wang G, et al. Non-B DNA structure-induced genetic instability and evolution. Cell Mol Life Sci. 2010;67(1):43–62. doi: 10.1007/s00018-009-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharon D, Sandberg MA, Rabe VW, et al. RP2 and RPGR mutations and clinical correlations in patients with X-linked retinitis pigmentosa. Am J Hum Genet. 2003;73(5):1131–1146. doi: 10.1086/379379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira PA. Insights into X-linked retinitis pigmentosa type 3, allied diseases and underlying pathomechanisms. Hum Mol Genet. 2005;14(2):R259–67. doi: 10.1093/hmg/ddi272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Megaw RD, Soares DC, Wright AF. RPGR: its role in photoreceptor physiology, human disease, and future therapies. Exp Eye Res. 2015;138:32–41. doi: 10.1016/j.exer.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiadens AAHJ, Soerjoesing GG, Florijn RJ, et al. Clinical course of cone dystrophy caused by mutations in the RPGR gene. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1527–1535. doi: 10.1007/s00417-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Yin X, Feng L, et al. Novel mutations of RPGR in Chinese retinitis pigmentosa patients and the genotype-phenotype correlation. PLoS One. 2014;9(1):e85752. doi: 10.1371/journal.pone.0085752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahim AT, Bowne SJ, Sullivan LS, et al. Allelic heterogeneity and genetic modifier loci contribute to clinical variation in males with X-linked retinitis pigmentosa due to RPGR mutations. PLoS One. 2011;6(8):e23021. doi: 10.1371/journal.pone.0023021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X, Park JH, Gumerson J, et al. Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations. Proc Natl Acad Sci U S A. 2016;113(21):E2925–34. doi: 10.1073/pnas.1523201113. [• Glutamylation of RPGRORF15 is critical for its function in photoreceptors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao KN, Anand M, Khanna H. The carboxyl terminal mutational hotspot of the ciliary disease protein RPGRORF15 (retinitis pigmentosa GTPase regulator) is glutamylated in vivo. Biol Open. 2016;5(4):424–428. doi: 10.1242/bio.016816. [• RPGRORF15 is glutamylated in the Glu-Gly domain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regnard C, Desbruyères E, Huet JC, et al. Polyglutamylation of nucleosome assembly proteins. J Biol Chem. 2000;275(21):15969–15976. doi: 10.1074/jbc.M000045200. [DOI] [PubMed] [Google Scholar]
- 26.Jiang J, Wu X, Shen D, et al. Analysis of RP2 and RPGR mutations in five X-linked Chinese families with Retinitis Pigmentosa. Sci Rep. 2017;7 doi: 10.1038/srep44465. 44465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y, Brady ST. Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol. 2015;25(3):125–136. doi: 10.1016/j.tcb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright AF, Shu X. Focus on molecules: RPGR. Exp Eye Res. 2007;85(1):1–2. doi: 10.1016/j.exer.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Patnaik SR, Raghupathy RK, Zhang X, et al. The role of RPGR and its interacting Proteins in Ciliopathies. J Ophthalmol. 2015;2015 doi: 10.1155/2015/414781. 414781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natarajan K, Gadadhar S, Souphron J, et al. Molecular interactions between tubulin tails and glutamylases reveal determinants of glutamylation patterns. EMBO Rep. 2017;18(6):1013–1026. doi: 10.15252/embr.201643751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sergouniotis PI, Chakarova C, Murphy C, et al. Biallelic Variants in TTLL5, encoding a tubulin glutamylase, cause retinal dystrophy. Am J Hum Genet. 2014;94(5):760–769. doi: 10.1016/j.ajhg.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedoni N, Haer-Wigman L, Vaclavik V, et al. Mutations in the polyglutamylase gene TTLL5, expressed in photoreceptor cells and spermatozoa, are associated with cone-rod degeneration and reduced male fertility. Hum Mol Genet. 2016;25(20):4546–4555. doi: 10.1093/hmg/ddw282. [DOI] [PubMed] [Google Scholar]
- 33.Shu X, Fry AM, Tulloch B, et al. RPGR ORF15 isoform co-localizes with RPGRIP1 at centrioles and basal bodies and interacts with nucleophosmin. Hum Mol Genet. 2005;14(9):1183–1197. doi: 10.1093/hmg/ddi129. [DOI] [PubMed] [Google Scholar]
- 34.Pawlyk BS, Bulgakov OV, Sun X, et al. Photoreceptor rescue by an abbreviated human RPGR gene in a murine model of X-linked retinitis pigmentosa. Gene Ther. 2015;23(2):196–204. doi: 10.1038/gt.2015.93. [•• Preclinical study that led to the initiation of XLRP gene therapy trial (clinical trial NCT03252847).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khanna H. More Than Meets the Eye: current Understanding of RPGR Function. Adv Exp Med Biol. 2018;1074:521–538. doi: 10.1007/978-3-319-75402-4_64. [DOI] [PubMed] [Google Scholar]
- 36.Patrício MI, Barnard AR, Orlans HO, et al. Inclusion of the woodchuck hepatitis virus posttranscriptional regulatory element enhances AAV2-Driven transduction of mouse and human retina. Mol Ther Nucleic Acids. 2017;6:198–208. doi: 10.1016/j.omtn.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer MD, McClements ME, Martinez-Fernandez de la Camara C, et al. Codon-optimized RPGR improves stability and efficacy of AAV8 Gene therapy in two mouse models of x-linked retinitis pigmentosa. Mol Ther. 2017;25(8):1854–1865. doi: 10.1016/j.ymthe.2017.05.005. [•• Preclinical study that led to the initiation of XLRP gene therapy trial (clinical trial NCT03116113).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beltran WA, Cideciyan AV, Boye SE, et al. Optimization of retinal gene therapy for X-linked retinitis pigmentosa due to RPGR mutations. Mol Ther. 2017;25(8):1866–1880. doi: 10.1016/j.ymthe.2017.05.004. [•• Preclinical study that led to the initiation of XLRP gene therapy trial (clinical trial NCT03316560).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song C, Conlon TJ, Deng W-T, et al. Toxicology and pharmacology of an AAV vector expressing codon-optimized RPGR in RPGR-deficient Rd9 mice. Hum Gene Ther Clin Dev. 2018;29(4):188–197. doi: 10.1089/humc.2018.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balaggan KS, Duran Y, Georgiadis A, et al. Absence of ocular malignant transformation after sub-retinal delivery of rAAV2/2 or integrating lentiviral vectors in p53-deficient mice. Gene Ther. 2012;19(2):182–188. doi: 10.1038/gt.2011.194. [DOI] [PubMed] [Google Scholar]
- 41.Vandenberghe LH, Bell P, Maguire AM, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011;3(88):88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natkunarajah M, Trittibach P, McIntosh J, et al. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8. Gene Ther. 2008;15(6):463–467. doi: 10.1038/sj.gt.3303074. [DOI] [PubMed] [Google Scholar]
- 43.Boye SE, Alexander JJ, Boye SL, et al. The human rhodopsin kinase promoter in an AAV5 vector confers rod- and cone-specific expression in the primate retina. Hum Gene Ther. 2012;23(10):1101–1115. doi: 10.1089/hum.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye G-J, Budzynski E, Sonnentag P, et al. Safety and Biodistribution Evaluation in Cynomolgus Macaques of rAAV2tYF-PR1.7-hCNGB3, a Recombinant AAV Vector for Treatment of Achromatopsia. Hum Gene Ther Clin Dev. 2016;27(1):37–48. doi: 10.1089/humc.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cremers FPM, Boon C, Bujakowska K, et al. Special issue introduction: inherited retinal disease: novel candidate genes, genotype-phenotype correlations, and inheritance models. Genes (Basel) 2018;9(4):215. doi: 10.3390/genes9040215. [DOI] [PMC free article] [PubMed] [Google Scholar]