Significance
Hunter-gatherers like the Ju/’hoãnsi (!Kung) San use exchange networks to dampen subsistence and reproductive risks, but almost nothing is known of how, when, and why such practices emerged. Strontium isotope analysis of one preferred San exchange item, ostrich eggshell beads, from highland Lesotho shows that since the late Middle Stone Age ∼33 ka, such networks connected ecologically complementary regions over minimal distances of several hundred kilometers. Rapidly changing environmental conditions during Marine Isotope Stage 3 (∼59 to 25 ka) likely placed a premium on developing effective means of mitigating subsistence and demographic risks, with ostrich eggshell beads providing a uniform medium of personal decoration and exchange highly suitable for binding together extended open social networks.
Keywords: ostrich eggshell beads, strontium isotope analysis, social networks, late Quaternary, southern Africa
Abstract
Hunter-gatherer exchange networks dampen subsistence and reproductive risks by building relationships of mutual support outside local groups that are underwritten by symbolic gift exchange. Hxaro, the system of delayed reciprocity between Ju/’hoãn individuals in southern Africa’s Kalahari Desert, is the best-known such example and the basis for most analogies and models of hunter-gatherer exchange in prehistory. However, its antiquity, drivers, and development remain unclear, as they do for long-distance exchanges among African foragers more broadly. Here we show through strontium isotope analyses of ostrich eggshell beads from highland Lesotho, and associated strontium isoscape development, that such practices stretch back into the late Middle Stone Age. We argue that these exchange items originated beyond the macroband from groups occupying the more water-stressed subcontinental interior. Tracking the emergence and persistence of macroscale, transbiome social networks helps illuminate the evolution of social strategies needed to thrive in stochastic environments, strategies that in our case study show persistence over more than 33,000 y.
Exchanges of material culture characterize all living societies (1). Their pervasiveness positions them at the core of the human experience and anthropological inquiry (2, 3). Among small-scale societies, material exchanges typically take the form of mutual gift-giving. While extraordinarily diverse with respect to specific traditions, the value of such transactions lies less with the goods exchanged than with the underlying social relations they symbolize (4, 5). Reciprocal transfers are particularly vital among hunter-gatherers, kindling and cementing bonds within and between communities. The exchange of portable, symbol-laden objects like beads is widely thought to represent a major threshold in human behavioral evolution (6–8), a “release from proximity” that massively expanded the spatial and temporal scope of human interaction and information transmission (9). The social relations and networks such transfers underwrite serve both as conduits for socioecological information and as assurances of access to resources (10). Exchange is thus one of numerous strategies—including mobility, division of labor, food sharing, storage, and traditions of oral and visual art—that hunter-gatherers enact to dampen subsistence and demographic risks (11, 12).
The best-known example of hunter-gatherer exchange is hxaro, the system of delayed, balanced reciprocity practiced by the Ju/’hoãnsi of southern Africa’s Kalahari Desert. Prominent thanks to the work of Wiessner (13–17), hxaro is one of many analogous traditions observed among San cultures (18–20). Hxaro relationships are mutual gift-giving partnerships between two members of the same or different bands. Hxaro fulfills several key societal functions, but primarily provides Ju/’hoãn individuals and families with alternative residences and associated foraging possibilities when resources in their own territory become scarce (15). Indebtedness runs between but also—via affinal kin—beyond individual partnerships, enveloping participants in broader networks of mutual reliance. Risks are minimized by strategically selecting partners at diverse distances to meet diverse exigencies, from daily subsistence fluctuations (coresident and neighboring kin) to regional resource failures (distant relatives living far away). Although the former are more common, nonlocal partnerships are considered essential. Wiessner found that nearly a third of an average Ju/’hoãn’s hxaro partners live over 50 km away, and nearly all families in her sample had at least one based 150- to 200-km distant (14). These multiscalar networks of reciprocity enmesh Ju/’hoãn communities in support systems spanning many thousands of square kilometers that are simultaneously a superbly resilient adaptation to the ecological vagaries of desert life (15), a means of securing access to potential marriage partners (21), and a mechanism for reinforcing egalitarian social values (22).
The most ubiquitous, highly valued, and traditionally important hxaro gifts are those adorned or strung with ostrich eggshell beads, and so intimately are the two associated that the same word (//ri) denotes both beadwork and hxaro gifts in general (15). In the Kalahari, ostrich eggshell beads are particularly fundamental to girls’ initiation ceremonies and marriage exchanges, with the most lavish beadwork items employed for the latter and in advertising the status and connections of families (23). Although not as ancient as marine shell ornaments, ostrich eggshell beads have a deep African pedigree, first appearing ∼50 to 40 ka during mid-Marine Isotope Stage (MIS) 3 in archaeological contexts variously described as late Middle Stone Age (MSA), early Later Stone Age (LSA), and transitional MSA/LSA (24–28). Like all objects of bodily adornment, they and later archaeological specimens were clearly intended for interpersonal and intergroup signaling (7). Beyond this obvious inference, however, we currently possess no knowledge of how they functioned in African hunter-gatherer societies in any period or place beyond the ethnographic Kalahari. Did they form part of multiscalar exchange networks that helped distribute risk as among historical and contemporary Ju/’hoãnsi? Or were their uses in earlier periods or regions outside the Kalahari altogether different? How might these roles have changed through time, and when and in what socioecological contexts do we see the advent in Africa of materially mediated, geographically expansive social networks? The answers to these questions concerning the antiquity, drivers, and development of hxaro and allied practices remain unclear. This is both problematic and ironic, given hxaro’s widespread use in modeling hunter-gatherer material and information exchange in southern Africa and beyond (21, 29–34).
Here we report a large-scale archaeometric investigation into the antiquity of social networks mediated by ostrich eggshell beads. We analyze the strontium (Sr) isotope ratios (87Sr/86Sr) of archaeological ostrich eggshell beads spanning the past ∼33 ka from the Maloti-Drakensberg Mountains of highland Lesotho (Fig. 1). 87Sr/86Sr ratios of ostrich eggshell beads reflect the bioavailable Sr in local rocks that is transferred to vegetation and water consumed by ostriches during the breeding season. When 87Sr/86Sr ratios are sufficiently differentiated across the landscape, they can be used to identify the predominant lithology of where an egg was originally laid and discovered. These bead data are presented alongside—and interpreted using—a strontium isoscape that we are developing for the Karoo Supergroup, southern Africa’s most extensive geological series, which centers on the Maloti-Drakensberg, a rugged, deeply incised montane grassland that is the highest mountain system along southern Africa’s Great Escarpment. This system exacerbates the steep east-west rainfall and evaporation gradients that characterize southernmost Africa, forming a climatic interface between its high-rainfall coastal forelands and its drier, more seasonal interior plateau (Fig. 1). It also gives rise to southernmost Africa’s largest river, the Orange (or Senqu), which originates in highland Lesotho before flowing west to form a >2,000-km-long freshwater artery through that interior to the Atlantic.
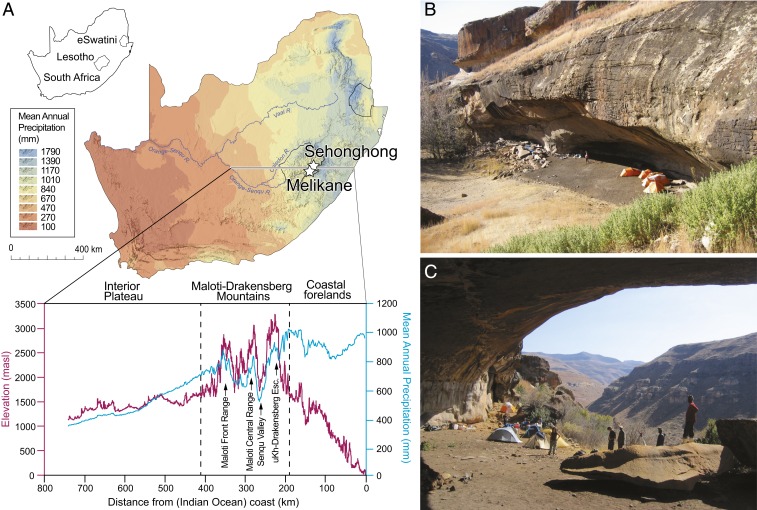
Fig. 1.
Location of Melikane and Sehonghong. (A) Map of southern Africa showing the location of Melikane and Sehonghong in relation to mean annual precipitation across the subcontinent. The call-out below shows the Maloti-Drakensberg’s position along elevation and rainfall profiles for an ∼800-km transect across southeastern southern Africa (for methods, see SI Appendix). (B and C) Site photographs of the rockshelters at (B) Sehonghong and (C) Melikane.
Archaeologists have long suspected that ostrich eggshell artifacts found in highland Lesotho and areas of South Africa below the Maloti-Drakensberg Escarpment were imported from elsewhere (21, 31). The social connections and human dispersals underwritten by these sorts of material movements may have been influenced by southeastern Africa’s steep climate gradients and resultant ecological contrasts (35, 36). Our results support both hypotheses by suggesting that archaeologically recovered ostrich eggshell beads from highland Lesotho originated from groups occupying the more water-stressed subcontinental interior. The distances involved exceed those documented ethnographically for foragers’ seasonal rounds, suggesting that social networks reaching beyond the macroband (“maximum band”) (37) were the primary means of introducing them into the highlands. Moreover, we furnish evidence that long-distance, macroscale social networking in Africa stretches back at least 33 ka. Once innovated, it continued into the ethnographic present, although appreciation of diachronic changes and spatial diversity must await further data. That these networks and their exchange-based substructures emerged during MIS 3, one of the Quaternary’s most volatile climatic phases, may suggest that they evolved to buffer subsistence and demographic risks associated with fluctuating resource availabilities. We argue that ostrich eggshell beads were innovated and harnessed as a social technology expressly to feed these emergent networks.
Results
Archaeological Sites and Geological Geometry.
The ostrich eggshell beads sampled for this study derive from archaeological excavations at Melikane (1,860 m above sealevel) and Sehonghong (1,800 m above sealevel), two rock-shelters in the Lesotho highlands (Fig. 1 and SI Appendix, Fig. S1). Melikane’s sequence runs from late MIS 5 (∼83 ka) to the late Holocene and is currently Lesotho’s oldest radiometrically dated site (35, 36) (SI Appendix, Fig. S2 and Tables S1 and S2). Ostrich eggshell beads (n = 12) were only encountered in the late Holocene levels (layers 1 to 2), dated 3.3 to 0.15 ka (SI Appendix, Fig. S3). Their absence from underlying strata is likely due to taphonomic alterations associated with the repeated passage of water through the deposit (35). The sequence at Sehonghong, ∼24 km north of Melikane, extends from early MIS 3 (∼56 ka) to the 19th century (38–41) (SI Appendix, Figs. S4 and S5 and Table S3). In contrast to Melikane, Sehonghong is relatively dry and its organic preservation excellent. Ostrich eggshell beads (n = 553) occur throughout the upper half of its ∼2.5-m-deep sequence (40), with the oldest recovered from a final MSA context (Context 170) dated to 33.7 to 31.9 ka (39) (SI Appendix, Fig. S6).
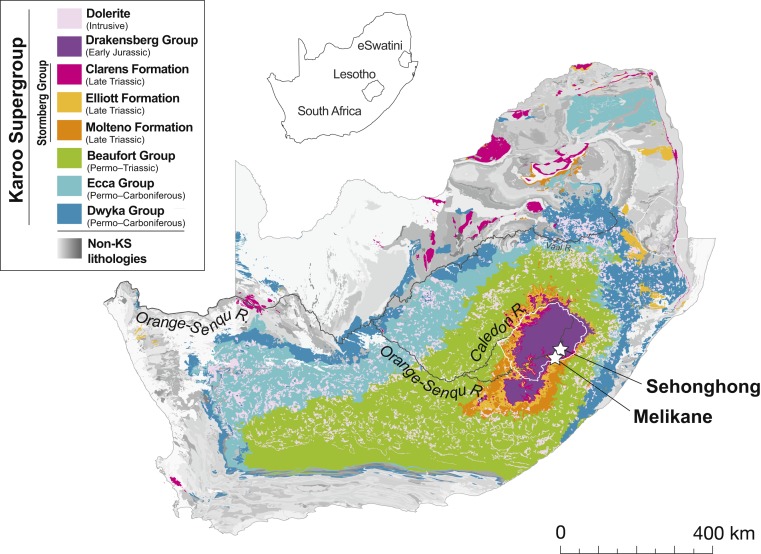
The 87Sr/86Sr ratio of an ostrich’s eggshell reflects the values of bioavailable Sr in its breeding-season territory. Bioavailable Sr derives primarily from mineral weathering of underlying bedrock, although atmospheric dust, precipitation, and sea-spray can all contribute to Sr in soils (42). Lesotho and the central two-thirds of South Africa share a common geology of mostly sedimentary and volcanic rocks. The subdivisions of this Karoo Supergroup (KS) form a broadly concentric pattern focused on highland Lesotho (Fig. 2), where the lavas of the Drakensberg Group—the youngest and highest KS unit—are thickest (43). Formed in a brief volcanic eruptive period ∼183 to 180 Ma, these ≤1,600-m-thick flood basalts once covered most of southern Africa. Subsequent erosion, however, has restricted them to the highest reaches of the Maloti-Drakensberg (44). The widespread presence of intrusive, contemporaneously formed dolerite dykes and sills across much of the interior plateau (Fig. 2) reveals the former extent of volcanic activity (45).
Fig. 2.
The KS geological succession in relation to Melikane and Sehonghong. Note that the entire succession is cross-cut by the Orange-Senqu River.
Underlying the Drakensberg lavas, the Stormberg Group (∼230 to 183 Ma) was deposited soon after the end-Permian extinction. Sandstones dominate, notably in the most recent stratum, the Clarens Formation, which outcrops along major valleys and plateaus in the southern and western Maloti-Drakensberg (Fig. 2) and contains innumerable rock-shelters (including Melikane and Sehonghong). The Stormberg’s older Molteno and Elliot Formations, whose sandstones intercalate with mudstones and shales, extend deeper into South Africa’s Free State, Eastern Cape, and KwaZulu-Natal provinces (46). Beneath the Stormberg lie the shales and mudstones of the Beaufort Group (Permo-Triassic: ∼255 to 237 Ma), which dominates the former two provinces and parts of southern KwaZulu-Natal. Finally, the outermost units of the KS are the Permo-Carboniferous tillites, shales, and mudstones of the Ecca (∼290 to 255 Ma) and Dwyka (∼300 to 290 Ma) groups. Together, these rocks are exposed along the KS’s periphery, principally in the Northern Cape, the western Free State, southern Mpumalanga, and much of northern KwaZulu-Natal (Fig. 2). Beyond the KS, South Africa’s margins mostly feature more complex and ancient geological units, including the Transvaal Supergroup (∼2,500 Ma) in the north and the Cape Supergroup (∼510 to 330 Ma) in the southwest, with younger rocks chiefly located near the coast or present as the Kalahari Group in the Northern Cape and Northwest Province (47).
The concentric geologic outcrop pattern of the KS makes it ideal for conducting geologically dependent artifact provenancing using the Sr isotope tracer method. Sr is a naturally abundant element with four stable isotopes, one of which is radiogenic (87Sr), meaning that it changes in abundance over geological timescales due to the radioactive decay of an isotope of rubidium (87Rb). Old, high Rb/Sr rocks (e.g., Precambrian granitoids and their derivatives) typically evolve the highest 87Sr/86Sr ratios with time. Conversely, 87Sr/86Sr ratios in young volcanic rocks having low Rb/Sr ratios (e.g., basalts) (48) are much lower. The concentric geographic pattern of the KS’s bedrock units means that 87Sr/86Sr ratios increase with distance from the young basalt-capped “bullseye” of highland Lesotho, where our archaeological sites and samples are located. De Villiers et al’s whole-river Sr transect of the Orange-Senqu and its largest tributary, the Vaal, shows that 87Sr/86Sr rises steadily as the Orange-Senqu flows westward across the KS’s lithological succession (49). From a low of 0.70811 near the river’s headwaters in northeastern Lesotho (Drakensberg Formation), 87Sr/86Sr reaches 0.71286 just before its confluence with the Vaal in the central Karoo (Dwyka Group) (Fig. 2). Bioavailable Sr should likewise vary positively with distance from highland Lesotho. Produced by the KS’s geological geometry, this Sr isotope gradient affords a superb opportunity for detecting and assessing the spatial flow of organic ornaments like ostrich eggshell beads, in this case into the highlands from surrounding areas. Southern Africa’s more irregular geological geometries beyond the KS are less conducive to such research, although we know of one much smaller-scale study from Zimbabwe (50).
Ostrich Eggshell Bead 87Sr/86Sr and the Highland Baseline.
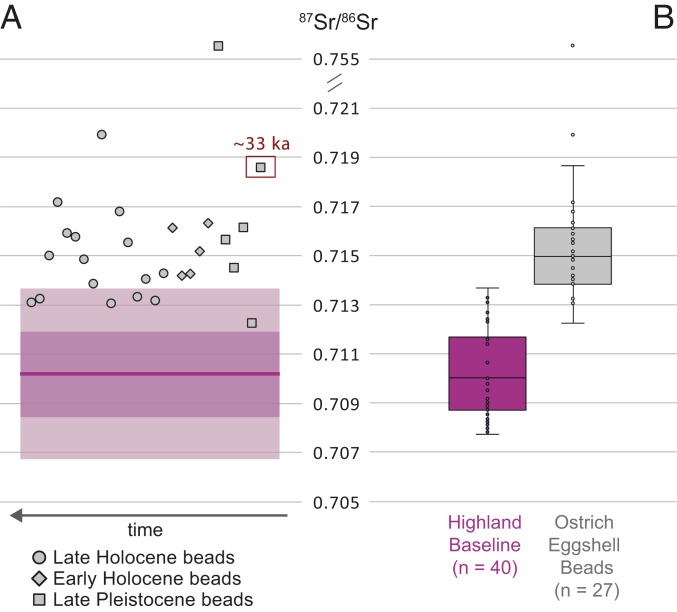
We conducted high-precision strontium isotope ratio analysis using thermal ionization mass spectrometry (TIMS), and Ca and Sr concentration analysis using inductively coupled plasma optical emission spectrometry (ICP-OES) on 27 ostrich eggshell beads from the rock-shelters of Melikane and Sehonghong. Eleven derive from Melikane’s uppermost, late Holocene layers 1 and 2 (3.3 to 0.15 ka) (35), and 16 from strata at Sehonghong that include layers dated to the late Holocene (layer DC; ≤1.2 ka; n = 5), the early Holocene (layer ALP; 8.3–7.7 ka; n = 5), and the late Pleistocene (layers BARF, RF, RBL-CLBRF, and Context 170; 33.7 to 12.6 ka; n = 6) (40, 41). Results are presented in Fig. 3 and Table 1. 87Sr/86Sr ratios for the ostrich eggshell beads range from 0.75554 to 0.71252, with a mean of 0.71652 ± 0.00783. While excluding a notable outlier (0.75554) reduces the range to 0.71989 to 0.71252 (mean = 0.71507 ± 0.00175), variation remains high. The data are nevertheless normally distributed, with the bulk of the samples (67%) having 87Sr/86Sr ratios of 0.713 to 0.716. Although understanding of diachronic change is currently inhibited by uneven subsample sizes per chronological phase, each phase includes at least one bead exceeding 87Sr/86Sr 0.716 (Fig. 3). Our sample set’s third highest 87Sr/86Sr bead (0.71865), moreover, is also the most ancient (33.7 to 31.9 ka) (Fig. 3 and Table 1).
Fig. 3.
Ostrich eggshell bead 87Sr/86Sr versus the highland baseline. (A) 87Sr/86Sr values of individual ostrich eggshell bead specimens from Melikane and Sehonghong arranged according to broad chronological phase and shown against the highland baseline (purple line = mean; purple bands = 2σ). Note that the sample’s third most 87Sr/86Sr-enriched bead (SEH I13-170) is also its oldest (∼33 ka). (B) The same data presented as boxplots.
Table 1.
Sample details and 87Sr/86Sr values of the ostrich eggshell beads from Melikane and Sehonghong rock-shelters analyzed in this study
| Sample ID | Site | Layer | Age (kcal BP) | Stage | 87Sr/86Sr | 2SE± | 2SE± (%) |
| SF-4 | Melikane | 1 | 0.3–0.15 | Late Holocene | 0.713080 | 0.000017 | 0.0023 |
| SF-10 | Melikane | 2 | 3.3–0.15 | Late Holocene | 0.713242 | 0.000016 | 0.0016 |
| SF-13 | Melikane | 2 | 3.3–0.15 | Late Holocene | 0.714967 | 0.000016 | 0.0016 |
| SF-20 | Melikane | 2 | 3.3–0.15 | Late Holocene | 0.717149 | 0.000014 | 0.0020 |
| SF-29 | Melikane | 2 | 3.3–0.15 | Late Holocene | 0.715892 | 0.000014 | 0.0020 |
| SF-43 | Melikane | 2 | 3.3–0.15 | Late Holocene | 0.715753 | 0.000014 | 0.0019 |
| SF-48 | Melikane | 2 | 3.3–0.15 | Late Holocene | 0.714822 | 0.000013 | 0.0018 |
| SF-56 | Melikane | 2 | 3.3–0.15 | Late Holocene | 0.713849 | 0.000012 | 0.0017 |
| SF-70 | Melikane | 2 | 3.3–0.15 | Late Holocene | 0.719894 | 0.000019 | 0.0026 |
| SF-87 | Melikane | 2 | 3.3–0.15 | Late Holocene | 0.713054 | 0.000015 | 0.0021 |
| SF-90 | Melikane | 2 | 3.3–0.15 | Late Holocene | 0.716775 | 0.000014 | 0.0019 |
| SF-86 | Sehonghong | DC | 1.26–0.98 | Late Holocene | 0.715509 | 0.000013 | 0.0018 |
| SF-101 | Sehonghong | DC | 1.26–0.98 | Late Holocene | 0.713296 | 0.000013 | 0.0017 |
| J12-11B | Sehonghong | DC | 1.26–0.98 | Late Holocene | 0.714032 | 0.000012 | 0.0016 |
| J12-11C | Sehonghong | DC | 1.26–0.98 | Late Holocene | 0.713137 | 0.000014 | 0.0019 |
| SF-691 | Sehonghong | DC | 1.26–0.98 | Late Holocene | 0.714276 | 0.000014 | 0.0020 |
| SF-174 | Sehonghong | ALP | 8.3–7.67 | Early Holocene | 0.716115 | 0.000012 | 0.0018 |
| SF-231 | Sehonghong | ALP | 8.3–7.67 | Early Holocene | 0.714180 | 0.000013 | 0.0019 |
| SF-265 | Sehonghong | ALP | 8.3–7.67 | Early Holocene | 0.714251 | 0.000014 | 0.0020 |
| SF-267 | Sehonghong | ALP | 8.3–7.67 | Early Holocene | 0.715159 | 0.000018 | 0.0025 |
| SF-751 | Sehonghong | ALP | 8.3–7.67 | Early Holocene | 0.716321 | 0.000014 | 0.0020 |
| SF-430 | Sehonghong | BARF | 13.4–12.6 | Late Pleistocene | 0.755539 | 0.000012 | 0.0016 |
| SF-430 replicate | Sehonghong | BARF | 13.4–12.6 | Late Pleistocene | 0.755541 | 0.000021 | 0.0028 |
| SF-1090 | Sehonghong | RF | 15–13.7 | Late Pleistocene | 0.715643 | 0.000015 | 0.0021 |
| SF-1091 | Sehonghong | RF | 15–13.7 | Late Pleistocene | 0.714490 | 0.000014 | 0.0020 |
| SF-1092 | Sehonghong | RF | 15–13.7 | Late Pleistocene | 0.716109 | 0.000012 | 0.0017 |
| SF-392 | Sehonghong | RBL-CRBLF | 15.7–15.2 | Late Pleistocene | 0.712252 | 0.000015 | 0.0021 |
| I13-170 | Sehonghong | n/a | 33.7–31.9 | Late Pleistocene | 0.718649 | 0.000013 | 0.0018 |
Mean value for the Sr standard NBS 987: 87Sr/86Sr = 0.710250 ± 0.000016 (n = 16) Finnigan MAT 262; 87Sr/86Sr = 0.710245 ± 0.000014 (n = 11) Thermo Scientific Triton Plus. Accepted value of McArthur et al. (75) = 0.710248. n/a, not applicable (layers currently unassigned as this portion of the Sehonghong sequence remains under excavation).
To assess whether these beads were made from locally collected ostrich eggshells, we generated a baseline of bioavailable Sr isotope ratios for highland Lesotho (SI Appendix, Table S4). This integrates three components, one local and two highland-wide. Locally, a series of vegetation and soil samples (n = 18) were analyzed from three 5-km transects within and paralleling the Sehonghong Valley near Sehonghong rock-shelter itself (SI Appendix, Fig. S7). These transects target the highlands’ two dominant lithologies—the Drakensberg (basalt) and uppermost Stormberg (Clarens sandstone) Groups—as well as the valley floor whose alluvial sediments incorporate both. Because virtually identical geological and geomorphological contexts characterize the Melikane Valley, this local component is valid for both our sites. The second baseline component comprises published 87Sr/86Sr ratios from de Villiers et al.’s Orange-Senqu River transect (49). Specifically, we employ their samples (n = 17) for sections of the upper Orange-Senqu and Caledon Rivers and the tributaries draining the Drakensberg and Stormberg Groups (SI Appendix, Fig. S7 and Table S4). The third component consists of modern rodent tooth enamel samples (n = 8) from museum zoological specimens collected from across highland Lesotho (SI Appendix, Fig. S7 and Table S4). Together, these datasets produce a robust multiproxy baseline for highland Lesotho values of the sort advocated by Grimstead et al. (51). The baseline’s 87Sr/86Sr values range from 0.71368 to 0.70773, with a mean (0.71035 ± 0.00173) significantly below that of the ostrich eggshell beads [t(54) = 10.67, P < 0.001] as determined by a Welch’s t test for unequal sample sizes (Fig. 3 and SI Appendix). Only six beads (22%) have 87Sr/86Sr values that overlap with the baseline, whether we use the latter’s total range or 2σ around the mean (52) (0.71376 to 0.70675) (Fig. 4). These data indicate that over three-quarters (78%; n = 21) of our beads could not have originated from the eggs of ostriches living locally or in highland Lesotho as a whole.
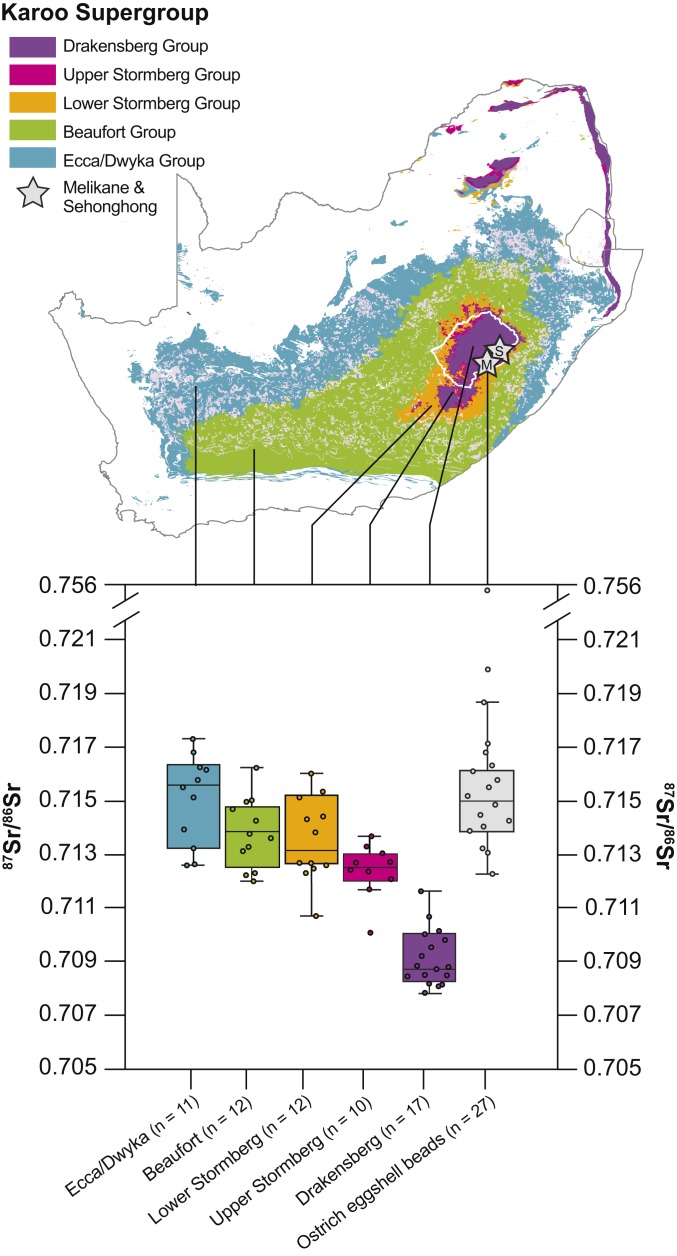
Fig. 4.
Ostrich eggshell bead 87Sr/86Sr versus KS 87Sr/86Sr. Boxplot showing Melikane and Sehonghong ostrich eggshell bead 87Sr/86Sr values against those generated for the KS’s primary lithological units. Boxes color-coded to the map above.
Isoscape Development and Implications for Long-Distance Imports.
To trace the potential sources of ostrich eggshell beads from Melikane and Sehonghong, we are developing an 87Sr/86Sr isoscape of bioavailable Sr for the KS. Because our sample distribution across this vast area is not yet sufficiently dense to map 87Sr/86Sr using geospatial interpolation, we employ the term isoscape sensu lato. To date, this working isoscape is primarily based on 87Sr/86Sr ratios of small mammal tooth enamel samples from museum zoological collections (SI Appendix, Fig. S7 and Table S5). They comprise a range of rodent, lagomorph, and hyracoid species (for further information, see SI Appendix, Table S5). We supplement these small mammal data with our local baseline plant and soil samples (for the Drakensberg and Upper Stormberg only) and, where appropriate, published water samples (49) (SI Appendix, Fig. S7 and Table S5). The latter are used judiciously by only employing samples from river sections entirely encapsulated within (Drakensberg), or having flowed entirely through (Lower Stormberg, Beaufort, Eca/Dwyka), the lithology to which they are allocated. The decision to employ small mammals in constructing this geographically expansive isoscape is driven by methodological and practical concerns. The broad diets and circumscribed ranges of most small mammal taxa make them excellent at averaging bioavailable Sr in a given location (52). Leveraging the large, taxonomically diverse, professionally curated and well-provenanced mammal collections of southern African museums is, moreover, preferable to field sampling across such a large geographic area.
Our results are summarized in Fig. 4, with 87Sr/86Sr values of small mammals grouped by the lithologies of their capture locations and color-coded to the accompanying geological map. For comparison, our highland ostrich eggshell bead 87Sr/86Sr results are also shown (Fig. 4, far right). The highest and youngest two supergroup strata—the Drakensberg basalts and upper Stormberg (Clarens) sandstones—correspond closely to our highland baseline, with which they share a number of samples (SI Appendix, Tables S4 and S5). With the data segregated by geological substrate, however, we see no overlap between the ostrich eggshell beads and the Drakensberg Group, whose basalts dominate the highland landscape around Melikane, Sehonghong, and beyond (Fig. 4). A Welch’s t test confirms that the basalts’ mean 87Sr/86Sr is highly significantly lower than that of the beads [t(40) = 13.83, P < 0.001] (SI Appendix). Instead, overlap between bead and highland geological 87Sr/86Sr is restricted to the underlying Clarens sandstones (upper Stormberg Group), whose range (0.71368 to 0.71007) encapsulates nearly a quarter (22%; n = 6) of the former’s variability. However, the sandstones have extremely limited areal exposure within the highlands and only outcrop in the deepest river valleys and as sheer cliffs. This makes it highly unlikely that they could have contributed substantially to the 87Sr/86Sr signatures of large, terrestrially wide-ranging animals like ostriches. Their mean (0.71240 ± 0.00096) is, moreover, again highly significantly lower [t(29) = 5.63, P < 0.001] (SI Appendix). Together with the fact that ostriches prefer semiarid to desert habitats with low topographic relief (53), these data strongly suggest that all the beads in our sample derive from ostriches living outside highland Lesotho.
As we move through the KS’s deeper units as they outcrop beyond highland Lesotho, overlap with bead 87Sr/86Sr values increases (Fig. 4). Small mammals inhabiting areas underlain by the Stormberg Group’s lower two units—the Elliott and Molteno Formations—returned 87Sr/86Sr values of 0.71600 to 0.71067 (mean: 0.71357 ± 0.00147). A similar if slightly higher range (0.71623 to 0.71198; mean: 0.71385 ± 0.00123) was obtained for those from the underlying (and geographically much more expansive) Beaufort Group. Together, these geological units account for the bulk (78%) of bead 87Sr/86Sr variability. Nevertheless, nearly a third (29%; n = 8) and a quarter (22%; n = 6) of the ostrich eggshell beads, respectively, yielded values that exceed the lower Stormberg’s and Beaufort’s ranges (Fig. 4). Relative to the beads, moreover, the means of each are significantly lower [lower Stormberg: t(25) = 2.66, P = 0.013; Beaufort: t(29) = 2.39, P = 0.023] (SI Appendix). In contrast, overlap with ostrich eggshell bead 87Sr/86Sr is greatest with the KS’s outmost unit—the Ecca/Dwyka Group—which accounts for 85% (n = 23) of the former’s variability (0.71732 to 0.71256; mean: 0.71498 ± 0.00155) (Fig. 4). Correspondence in data structure is supported by an insignificantly different Welch’s t test result between their mean values [t(21) = 0.15, P = 0.883] (SI Appendix). Finally, three beads (11%), including the sample’s oldest at 33 ka, yielded values higher even than the Ecca/Dwyka Group (Fig. 4) and must have originated from Precambrian geological settings beyond the KS.
Discussion
Although overhunting and subsequent reintroductions for commercial farming have fundamentally altered southern African ostrich population sizes, densities, and compositions, ostrich distribution across the modern landscape still largely reflects their habitat preferences (54) and aligns with historical observations. Ostriches do not occur in highland Lesotho today, were not observed there in the 19th century, and are unlikely to have been present before then. Ostrich eggshell beads recovered from highland archaeological sites have thus long seemed likely to reflect imports from further afield, a suspicion reinforced by the extreme scarcity of eggshell fragments and complete absence of bead preforms at those same sites (31). Our results provide firm evidence that this was indeed the case. That the beads sampled from Melikane and Sehonghong are nonlocal is demonstrated by their minimal 87Sr/86Sr overlap with our multiproxy highland baseline and total lack of overlap with its dominant component, the Drakensberg Group. Rather, our data suggest that the ostriches from whose eggs these beads derived lived in regions underlain by the KS’s lower lithologies—the lower Stormberg, Beaufort, and Ecca-Dwyka Groups—with the latter, the deepest and outermost KS unit, displaying the greatest overlap and statistical agreement.
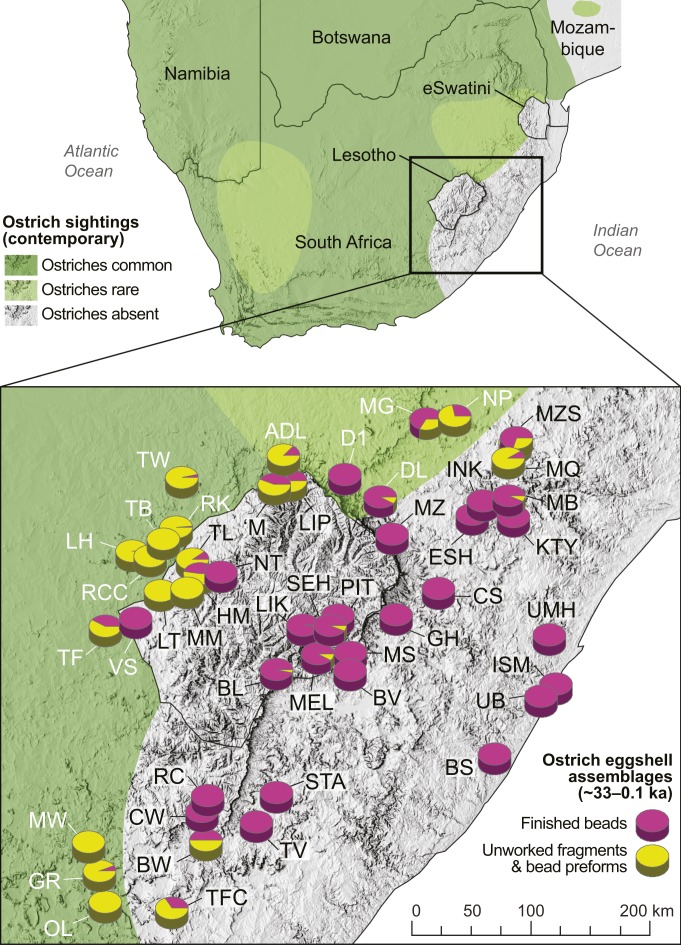
Determining from where and over what distances these beads were imported into the highlands is, however, complicated by the fact that the KS’s geometry, while concentric, is asymmetrically so. Its lateral dimension is particularly skewed, with exposures of the full lithological succession occurring much closer to highland Lesotho to the east than to the west (Fig. 2). We must therefore ask whether some of the beads could have come from eggs laid below the uKhahlamba-Drakensberg Escarpment in KwaZulu-Natal or the Eastern Cape. In northern KwaZulu-Natal, high frequencies of ostrich eggshell and evidence of on-site bead-making occur throughout the mid/late Holocene sequence at Maqonqo Shelter in an area where Ecca rocks outcrop (55). However, ornithological records indicate that historically ostriches occurred no closer than the northeastern Free State ∼150 km away, and that they were absent from the rest of KwaZulu-Natal and the former Transkei (56). As in Lesotho, the near total absence of eggshell fragments or bead-making debris from hunter-gatherer sites south of the Thukela River reinforces this picture. In fact, as is evident from Fig. 5, the spatial distribution of hunter-gatherer sites in southeasternmost Africa whose ostrich eggshell assemblages are either consistently dominated by finished beads (or lack them altogether) shows remarkable congruence with that of contemporary ostrich sightings. This strongly suggests that the humid bioregions of KwaZulu-Natal and the easternmost Eastern Cape formed a consistent barrier to ostriches over the past ∼30 ka at least.
Fig. 5.
Southern African ostrich and ostrich eggshell artifact distributions. (Upper) Map showing the distribution of ostriches in southern Africa based on contemporary sightings (54). (Lower) Detail of southeasternmost Africa (including Lesotho and the Maloti Drakensberg) showing contemporary ostrich distributions against proportions of finished ostrich eggshell beads versus unmodified fragments plus bead preforms for late MSA and LSA archaeological sites in the region (∼33 to 0.1 ka). For site abbreviations, see SI Appendix.
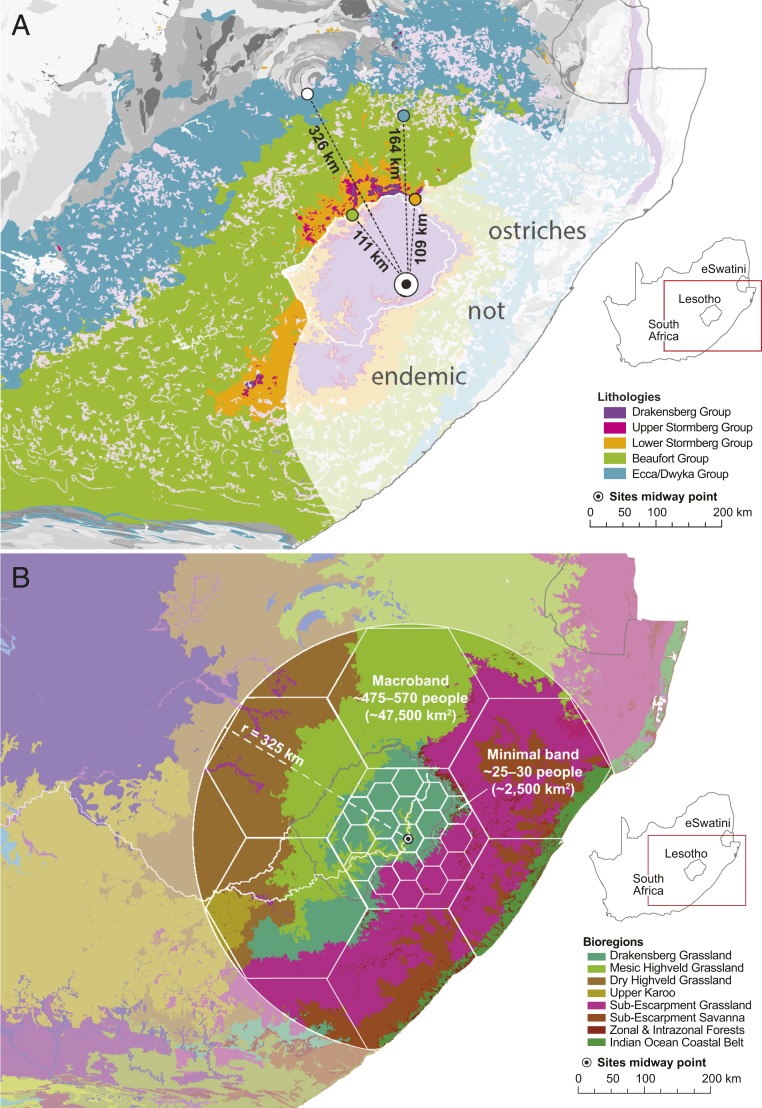
These long-term ostrich-free areas could almost certainly not, therefore, have supplied the beads comprising our highland sample, and we exclude them from consideration. Doing so allows us to ascertain the minimum distances over which our sample of beads was introduced into highland Lesotho (Fig. 6 and SI Appendix). For simplicity, we report distances from a point precisely midway between Melikane and Sehonghong rock-shelters. That the large majority of our beads (78%; n = 21) have 87Sr/86Sr values exceeding the highland baseline indicates they must derive from eggs laid beyond the upper Stormberg, and thus at least 109 km from our sites. The nearly one-third (29%; n = 8) and one-quarter (22%; n = 6) of our sample exceeding lower Stormberg and Beaufort 87Sr/86Sr ranges could not have come, respectively, from closer than 111 km and 164 km away. Finally, the three beads (11%) whose 87Sr/86Sr values surpass the Ecca/Dwyka Group, and thus the KS as a whole, originated from no nearer than 326 km (Fig. 6). While the magnitudes of movement suggested by these estimates are impressive, we emphasize that they are minima. Particularly important is the statistical agreement in 87Sr/86Sr between the highland beads and the Ecca/Dwya Group (SI Appendix), which suggests that most beads were imported from viable ostrich habitats underlain by this outermost KS unit. Such areas range up to >1,000 km away from Melikane and Sehonghong.
Fig. 6.
Minimum social network size and ecological heterogeneity. Maps of southeastern Africa showing (A) the minimal distances over which Melikane and Sehonghong’s ostrich eggshell beads were imported given the distribution of viable ostrich habitats, and (B) the diversity of ecological bioregions intersected by a hexagonally packed macroscale social network (10) of the size minimally suggested by our data. For methods used to create this figure, see SI Appendix.
Our minimum distances agree well with heuristic reconstructions of hunter-gatherer social network sizes (10). Since their long-term demographic viability requires that human populations be of a minimal size, variously estimated as ≥475 (37) to 730 to 950 (57), we can estimate how large social networks must be to ensure this. In reality, the distribution and reliability of both resources and terrain will affect how such a metapopulation manifests itself spatially. At its lower limits, however, Whallon, working with data from Upper Paleolithic Germany, estimates that a maximal band of 475 to 570 individuals consisting of 19 minimal bands of 25 to 30 people, each with a foraging radius of ∼28 km, would, on a uniform plane, have a territorial radius of ∼123 km (10). The area of such a mesoscale network (47,530 km2) is more than 50% larger than Lesotho, yet from Melikane and Sehonghong reaches no further than regions underlain by the Beaufort Group. Since, as Whallon notes, people may interact and move between adjacent mesoscale social networks to ensure access to maximally diverse resources, to obtain highly exotic items, and conceivably for other reasons as well, we can expect items to move >300 km. It is precisely such macroscale social networking that our analysis reveals.
In southeastern Africa, a macroscale network of the size minimally suggested by our data would have given participants access to high ecological diversity. No fewer than eight southern African bioregions are intersected, from semiarid Karoo scrub to subtropical coastal forest and thicket (Fig. 6) (58). That groups occupying the latter settings were tied into such networks is borne out by finds of rare marine shell beads from sites in lowland and highland Lesotho, including Sehonghong itself (31). Our data demonstrate that such social enchainments extended hundreds of kilometers further inland to interior hunter-gatherers for whom such connections would have been particularly vital. Relative to the Maloti-Drakensberg, the Karoo and highveld grasslands west of the Caledon River experience lower and more unreliable rainfall, have few natural shelters, are ecologically more uniform, and have more episodic archaeological records (59). For groups inhabiting such areas, gaining access to resource-rich, heterogeneously structured uplands (35, 36, 59) would have incentivized the establishment and maintenance of exchange relationships with highland foragers. Such transbiome connections recall key aspects of hxaro gift-exchange networks since the Ju/’hoãnsi focused their hxaro ties on individuals living in areas with resources complementary to their own and over distances of up to 200 km (13, 14). The chains created by summing the hxaro partnerships of multiple individuals stretched even further than this, providing access to desired trade goods from beyond the bounds of kinship reckoning. Moreover, because the Orange-Senqu provides comparatively easy access into highland Lesotho, the region’s physical relief may itself have helped channel these exchange chains along the prescribed courses reproduced through time described for the Kalahari (60).
Our study also reveals a hitherto unappreciated longevity for macroscale social networking using ostrich eggshell beads. In East Africa, long distance (≥150 km) movements are documented by ∼200 ka for obsidian tools (61), utilitarian artifacts not worn on the body, and so with less potential than ornaments for social signaling. While we do not expect that the specific mechanisms of ostrich eggshell transfers remained unchanged, our data do raise questions about the selective contexts in which this social technology evolved. It would be tempting to link the advent of ostrich eggshell bead-making to that of ostrich eggshell flask technology, especially as some early flasks carry incised designs with symbolic potential (62). However, such a technological knock-on scenario does not fit current evidence. Some 25 ka separate southern Africa’s earliest ostrich eggshell flasks (∼70 ka) and ostrich eggshell beads (∼45 ka) (25), and there is no evidence that East Africa’s first ostrich eggshell beads, which are even older (27), were preceded by the development of flask technology, decorated or not. These spatiotemporal incongruencies suggest that decisions to employ ostrich eggshell as a raw material for ornament manufacture were independent of innovations in subsistence technology. Instead, we hypothesize that the deliberate targeting of ostrich eggshell for beads reflects fundamental shifts in the interconnectedness of social relations at the macroband scale. The question then becomes what about these relationships may have changed to necessitate these greater, materially mediated social investments?
Kuhn and Stiner (7) argue that codified use of beads and similar ornaments (∼<100 ka) ushered in a major evolutionary shift in symbolic communication since, unlike pigment-based symbols, they are durable, easily transferred, and capable of standardization for complex messaging. They also emphasize how broad commonalities in Upper Paleolithic bead form far outweigh differences between regions or across periods (7, 8). Ostrich eggshell beads are highly suitable for producing such standardized items as they are produced, at least ethnographically, by stringing numerous preforms together and mass-grinding their peripheries on an abrasive surface to achieve the final disk-shaped form (20). Remembering also that ostriches occur across most of Africa and that their eggs are relatively easy to procure (except, of course, in humid and/or highland habitats like southeasternmost Africa), this makes ostrich eggshell far more suitable for mass producing beads than the gastropod shells used for the earliest known personal ornaments (63–65). Although they had likely consumed ostrich eggs for hundreds of millennia, and in southern Africa employed them as containers from at least early MIS 4 (62), it was not until MIS 3 that people exploited them to make beads. The novel demand for a relatively ubiquitous and plastic material from which highly standardized ornaments could be fashioned at low cost (relative to sea shells) conceivably stems from two related phenomena.
One is the extraordinary pace of climatic and resultant environmental flux operating during MIS 3 (66), which would have placed selective premiums on developing efficient and effective solutions for mitigating subsistence and demographic risks. Few search further than Weissner’s work with the Ju/’hoãnsi precisely because of its elegance in exemplifying how such systems help stabilize life in unpredictable resource configurations. The second factor is the human demographic changes that presumably accompanied rapid climatic swings, particularly cyclical shifts in the presence and sizes of viable macrobands as regional habitats fluctuated in desirability. Open and extensive social networks are precisely what we should expect in such situations, both to maximize flows of information and to socially enchain populations inhabiting newly settled or increasingly precarious landscapes to those residing where resources are more predictable.
In southernmost Africa, the densest, most varied, most predictable resources occur along and immediately inland of the coast. Especially once people started regularly consuming marine foods (67), these regions were presumably always the subcontinent’s main population centers (68). In contrast, human settlement of semiarid interior and arid western biomes was probably always more discontinuous. From early MIS 4, foragers inhabiting coastal regions began making shell ornaments from a limited range of gastropod taxa. At this time, there is no firm evidence for a substantial human presence in the deep subcontinental interior (38). The advent of ostrich eggshell ornaments in early MIS 3 coincides with an apparent (re)establishment of human populations there (69). Alongside its cost-effectiveness and scalability, ostrich eggshell may have been targeted as a symboling material by newly dispersed ornament-users because of its ubiquity in these more water-stressed and uncertain environments. The establishment and maintenance of social safety nets among such populations, as well as between them and groups in more stable coastal and upland habitats, would have been highly advantageous. Although stone tool industries signal enhanced regionalization during MIS 3 (70), it is possible that social signaling shifted to other, more effective media (e.g., ostrich eggshell beads) when the latter came into play.
Finally, our results may suggest that marine shell beads and ostrich eggshell beads ultimately functioned quite differently. Whereas the former perhaps mostly signaled differences in social roles within groups and group identity between them, it is possible that geographically more ubiquitous ostrich eggshell was harnessed, initially at least, not to differentiate individuals and groups but rather to bind them together. This may help explain why diameter sizes of southern and East Africa’s earliest ostrich eggshell beads (50 to 40 ka) overlap and then subsequently diverge (71). It may also be why our data indicate that ostrich eggshell beads, even early on, moved further than those made from marine shells (72). Conceivably, the rationale of risk reduction underlying the creation of widespread ties included the need to attract desirable mates and win their families. Unsubstantiated back-projections of ethnographic marriage practices should, however, be resisted. Indeed, our study serves as an important caution against culturally connecting those who produced the earliest ostrich eggshell beads in southern Africa with 20th century Kalahari San (24). If we are correct that this social technology was explicitly developed to gloss and downplay cultural differences, its first appearance, counter intuitively, could not be any less San.
Materials and Methods
Preparation of Plant, Soil, Ostrich Eggshell, and Small Mammal Tooth Enamel.
Vegetation was freeze-dried, crushed in a precleaned agate mortar and pestle, and 0.5 g of material digested using high-pressure hydrothermal microwave acid digestion (CEM MARS 5) in concentrated (double-distilled) HNO3-HCl acid mixture. Several HNO3-H2O2 dry down steps on temperature-controlled hotplates (all under clean laboratory conditions) were employed until residues and organics were eliminated. Soils were freeze-dried and crushed (as above) prior to sequential extraction steps (73) for isolating bioavailable soil cations and nonsilicate (mineral available) soil cations: 1) Overnight agitation on shaker table at room temperature (pH neutral 1M NH4Cl), centrifugation and transfer of leachate; and 2) same treatment (as above) in 1M HNO3 followed by filtration (0.45 μ). Aliquots of these were subjected to HNO3-H2O2 hotplate treatment (as above) prior to dilution and analysis. Ostrich eggshell bead interiors were drilled out using a (re)sterilized dental microdrill device, and ∼5 mg of material cold-digested using concentrated (double-distilled) HNO3 and (Suprapur) H2O2. Small mammal teeth were precleaned [reductive-oxidative cleaning protocols of Gleason et al. (74)], digested in concentrated (double-distilled) HNO3, and centrifuged. In both cases, hotplate HNO3-H2O2treatments were employed (as above) prior to analysis. For further details, see SI Appendix.
TIMS and ICP-OES Analysis.
Following chromatographic isolation from matrix with Eichrom Sr-spec resin (73), Sr was loaded on Re filaments and run alongside the NBS 987 Sr isotope standard using high-precision isotope ratio solid source TIMS at the University of Michigan (Thermo Scientific Triton Plus or Finnigan MAT 262). 87Sr/86Sr ratios were normalized to 86Sr/88Sr = 0.1194 using exponential law mass bias correction, and online corrections employed for collector gain efficiencies, isotope ratio outlier analysis, and 87Rb mass interference on 87Sr (85Rb monitored at < 0.1 mV). Mean 87Sr/86Sr values for the Sr isotopic standard NBS 987 during this study: 0.710250 ± 0.000016 (n = 16; Finnigan MAT 262); and 0.710245 ± 0.000014 (n = 11; Triton Plus). Total Sr process blanks were ∼50 pg. ICP-OES cation elemental analysis was conducted at the University of Michigan on a Perkin-Elmer Optima 3300DV ICP-OES. All sample solutions and calibration standards were run using 2% Trace Metal grade HNO3. Ca and Sr abundances, and Ca/Sr elemental ratios, are accurate in most cases to better than ± 5% based on repeat analysis of replicates, reference materials, bracketing (calibration check) standards, and system blanks for quantifying limits of detection. For further details see SI Appendix.
Data Availability.
All data generated in this study are included in the main text and SI Appendix.
Supplementary Material
Acknowledgments
We thank the Kingdom of Lesotho’s Ministry of Tourism, Environment, and Culture for granting permits to excavate at Sehonghong and Melikane; Beryl Wilson and David Morris of the McGregor Museum in Kimberley, South Africa, and Jurie du Plessis and Nico Avenant of the National Museum in Bloemfontein, South Africa, for access to their respective zoological collections and continued assistance and support; Caitlin Dickinson (University of Michigan LSA Technology Services) for skillful assistance with geospatial analyses, and both her and John Klausmeyer (University of Michigan Museum of Anthropological Archaeology) for helping produce several of the figures; Kyra Pazan and Rethabile Mokhachane for help with sample collection, and Sophie Harrison for sample preparation, in the project’s earliest phase; and two anonymous reviewers for thoughtful comments that helped to strengthen this paper. This research was funded by a generous award from the University of Michigan Mcubed funding program.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921037117/-/DCSupplemental.
References
- 1.Appadurai A., Ed., The Social Life of Things (Cambridge University Press, Cambridge, UK, 1986). [Google Scholar]
- 2.Lévi-Strauss C., The Elementary Structures of Kinship (Beacon Press, Boston, MA, 1969). [Google Scholar]
- 3.Malinowski B., Argonauts of the Western Pacific (Routledge, London, UK, 1922). [Google Scholar]
- 4.Mauss M. G., The Gift (Routledge, London, UK, 1925). [Google Scholar]
- 5.Sahlins M. D., Stone Age Economics (Aldine, Chicago, IL, 1972). [Google Scholar]
- 6.Bar-Yosef Mayer D., Bosch M., Humans’ earliest personal ornaments: An introduction. PaleoAnthropol 2019, 19–23 (2019). [Google Scholar]
- 7.Kuhn S. L., Stiner M. C., “Body ornamentation as information technology: Towards an understanding of the significance of early beads” in Rethinking the Human Revolution: New Behavioural and Biological Perspectives on the Origin and Dispersal of Modern Humans, Mellars P. A., Boyle K., Bar-Yosef O., Stinger C., Eds. (McDonald Institute for Archaeological Research, Cambridge, UK, 2007), pp. 45–54. [Google Scholar]
- 8.Stiner M. C., Finding a common bandwidth: Causes of convergence and diversity in Paleolithic beads. Biol. Theory 9, 51–64 (2014). [Google Scholar]
- 9.Gamble C. S., Palaeolithic society and the release from proximity: A network approach to intimate relations. World Archaeol. 29, 426–449 (1998). [Google Scholar]
- 10.Whallon R., Social networks and information; non-“utilitarian” mobility among hunter-gatherers. J. Anthropol. Archaeol. 25, 259–270 (2006). [Google Scholar]
- 11.Binford L. R., Constructing Frames of Reference: An Analytical Method for Archaeological Theory Building Using Ethnographic and Environmental Data Sets (University of California Press, Berkeley, CA, 2001). [Google Scholar]
- 12.Kelly R. L., The Lifeways of Hunter-Gatherers: The Foraging Spectrum (Cambridge University Press, Cambridge, UK, 2013). [Google Scholar]
- 13.Wiessner P., “Hxaro: A regional system of reciprocity for reducing risk among the !Kung San,” PhD dissertation, Department of Anthropology, University of Michigan, Ann Arbor, MI (1977).
- 14.Wiessner P., “Risk, reciprocity and social influences on !Kung San economics” in Politics and History in Band Societies, Leacock E., Lee R. B., Eds. (Cambridge University Press, Cambridge, UK, 1982), pp. 61–84. [Google Scholar]
- 15.Wiessner P., “!Kung San networks in a generational perspective” in The Past and Future of !Kung Ethnography: Critical Reflections and Symbolic Perspectives, Biesele M., Gordon R., Lee R. B., Eds. (Helmut Buske Verlag, Hamburg, Germany, 1986), pp. 103–129. [Google Scholar]
- 16.Wiessner P., “Pathways of the past !Kung San hxaro exchange and history” in Überlebensstrategien in Afrika, Bollig M., Klees F., Eds. (Heinrich-Barth Institut, Cologne, Germany, 1994), pp. 101–124. [Google Scholar]
- 17.Wiessner P., Hunting, healing, and hxaro exchange: A long term perspective on !Kung (Ju/’hoansi) large game hunting. Evol. Hum. Behav. 23, 407–436 (2002). [Google Scholar]
- 18.Barnard A., Hunters and Herders of Southern Africa: A Comparative Ethnography of the Khoisan Peoples (Cambridge University Press, Cambridge, UK, 1992). [Google Scholar]
- 19.Mitchell P. J., “Anyone for hxaro? Thoughts on the theory and practice of exchange in southern African Later Stone Age archaeology” in Researching Africa’s Past: New Perspectives from British Archaeology, Mitchell P. J., Haour A., Hobart J. H., Eds. (Oxbow Books, Oxford, UK, 2003), pp. 35–43. [Google Scholar]
- 20.Hitchcock R. K., Ostrich eggshell jewelry manufacturing and use of ostrich products among San and Bakgalagadi in the Kalahari. Botsw. Notes Rec. 44, 93–105 (2012). [Google Scholar]
- 21.Mazel A. D., People making history: The last 10,000 years of hunter-gatherer communities in the Thukela Basin. Natal Mus. J. Hum. 1, 1–168 (1989). [Google Scholar]
- 22.Lee R. B., The !Kung San: Men, Women and Work in a Foraging Society (Cambridge University Press, Cambridge, UK, 1979). [Google Scholar]
- 23.Wiessner P., “Parent-offspring conflict in marriage: Implications for social evolution and material culture among the Ju/’hoansi Bushmen” in Patterns and Process in Cultural Evolution (Origins of Human Behavior and Culture), Shennan S., Ed. (University of California Press, Berkeley, CA, 2009), pp. 251–264. [Google Scholar]
- 24.Ambrose S. H., Chronology of the Later Stone Age and food production in East Africa. J. Archaeol. Sci. 25, 377–392 (1998). [Google Scholar]
- 25.d’Errico F., et al. , Early evidence of San material culture represented by organic artifacts from Border Cave, South Africa. Proc. Natl. Acad. Sci. U.S.A. 109, 13214–13219 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gliganic L. A., Jacobs Z., Roberts R. G., Domínguez-Rodrigo M., Mabulla A. Z., New ages for Middle and Later Stone Age deposits at Mumba rockshelter, Tanzania: Optically stimulated luminescence dating of quartz and feldspar grains. J. Hum. Evol. 62, 533–547 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Miller J. M., Willoughby P. R., Radiometrically dated ostrich eggshell beads from the Middle and Later Stone Age of Magubike Rockshelter, southern Tanzania. J. Hum. Evol. 74, 118–122 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Tryon C. A., et al. , Middle and Later Stone Age chronology of Kisese II rockshelter (UNESCO World Heritage Kondoa rock-art sites), Tanzania. PLoS One 13, e0192029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadley L., Later Stone Age Hunters and Gatherers of the Southern Transvaal: Social and Ecological Interpretations (British Archaeological Reports, Oxford, UK, 1987). [Google Scholar]
- 30.Hall S. L., Binneman J. N. F., Later Stone Age burial variability in the Cape: A social interpretation. S. Afr. Archaeol. Bull. 42, 140–152 (1987). [Google Scholar]
- 31.Mitchell P. J., Prehistoric exchange and interaction in south-eastern southern Africa: Marine shells and ostrich eggshell. Afr. Archaeol. Rev. 13, 35–76 (1996). [Google Scholar]
- 32.Jefferies R. W., “Late Middle Archaic exchange and interaction in the North American midcontinent” in Native American Interactions: Multiscalar Analyses and Interpretations in the Eastern Woodlands, Nassaney M. S., Sassaman K. E., Eds. (University of Tennessee Press, Knoxville, TN, 1995), pp. 73–100. [Google Scholar]
- 33.Fitzhugh B., Phillips S. C., Gjesfjeld E., “Modeling hunter-gatherer information networks: An archaeological case study from the Kuril Islands” in The Role of Information in Hunter-Gatherer Bands, Whallon R., Lovis W. A., Hitchcock R. K., Eds. (Cotsen Institute of Archaeology, Los Angeles, CA, 2011), pp. 85–116. [Google Scholar]
- 34.Smith G. M., LaValley S. J., Wiggins K. M., Late Holocene lithic procurement strategies in the northwestern Great Basin: The view from Paiute Creek Shelter, Nevada. N. Am. Archaeol. 33, 399–427 (2012). [Google Scholar]
- 35.Stewart B. A., et al. , Afromontane foragers of the Late Pleistocene: Site formation, chronology and occupational pulsing at Melikane Rockshelter, Lesotho. Quat. Int. 270, 40–60 (2012). [Google Scholar]
- 36.Stewart B. A., Parker A. G., Dewar G. I., Morley M. W., Allott L. F., “Follow the Senqu: Maloti-Drakensberg paleoenvironments and implications for early human dispersals into mountain systems” in Africa from MIS 6–2, Jones S. A., Stewart B. A., Eds. (Springer, Dordrecht, Netherlands, 2016), pp. 247–272. [Google Scholar]
- 37.Wobst M., Boundary conditions for Paleolithic social systems: A simulation approach. Am. Antiq. 39, 147–178 (1974). [Google Scholar]
- 38.Jacobs Z., et al. , Ages for the Middle Stone Age of southern Africa: Implications for human behavior and dispersal. Science 322, 733–735 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Loftus E., Stewart B. A., Dewar G. I., Lee-Thorp J. A., Stable isotope evidence of late MIS 3 to middle Holocene palaeoenvironments from Sehonghong Rockshelter, eastern Lesotho. J. Quaternary Sci. 30, 805–816 (2015). [Google Scholar]
- 40.Mitchell P. J., The late Quaternary of the Lesotho highlands, southern Africa: Preliminary results and future potential of ongoing research at Sehonghong Shelter. Quat. Int. 33, 35–44 (1996). [Google Scholar]
- 41.Pargeter J., Loftus E., Mitchell P. J., New ages from Sehonghong rock shelter: Implications for the late Pleistocene occupation of highland Lesotho. J. Archaeol. Sci. Rep. 12, 307–315 (2017). [Google Scholar]
- 42.Bentley R. A., Strontium isotopes from the earth to the archaeological skeleton: A review. J. Archaeol. Method Theory 13, 135–187 (2006). [Google Scholar]
- 43.Catuneau O., et al. , The Karoo basins of south-central Africa. J. Afr. Earth Sci. 43, 211–253 (2005). [Google Scholar]
- 44.Marsh J. S., Hooper P. R., Rehacek J., Duncan A. R., “Stratigraphy and age of Karoo basalts of Lesotho and implications for correlations within the Karoo Igneous Province” in Large Igneous Provinces: Continental, Oceanic and Planetary Flood Volcanism, Mahoney J. J., Coffin M. F., Eds. (American Geophysical Union, Washington, DC, 1997), pp. 247–272. [Google Scholar]
- 45.Neumann E.-R., Svensen H., Galerne C. Y., Planke S., Multistage evolution of dolerites in the Karoo Large Igneous Province, central South Africa. J. Petrol. 52, 959–984 (2011). [Google Scholar]
- 46.Schlüter T., Geological Atlas of Africa (Springer, Dordrecht, Netherlands, 2008). [Google Scholar]
- 47.McCarthy T., Rubidge B., The Story of Earth and Life (Struik, Cape Town, South Africa, 2005). [Google Scholar]
- 48.Faure G., Mensing T., Isotopes: Principles and Applications (John Wiley & Sons, Oxford, UK, 2004). [Google Scholar]
- 49.de Villiers S., Compton J. S., Lavelle M., The strontium isotope systematics of the Orange River, southern Africa. S. Afr. J. Geol. 103, 237–248 (2000). [Google Scholar]
- 50.Wriston T. A., “The Late Stone Age to Early Iron Age in Hwange National Park, Zimbabwe: Using archaeology, soils, sediments, and stable isotopes to trace past peoples and environments.” PhD dissertation. Department of Anthropology, University of Nevada, Reno, NV (2013).
- 51.Grimstead D. N., Nugent S., Whipple J., Why a standardization of strontium isotope baseline environmental data is needed and recommendations for methodology. Adv. Archaeol. Pract. 5, 184–195 (2017). [Google Scholar]
- 52.Price T. D., Blitz J., Burton J. H., Ezzo J., Diagenesis in prehistoric bone: Problems and solutions. J. Archaeol. Sci. 19, 513–529 (1992). [Google Scholar]
- 53.Maclean G. L., Roberts’ Birds of Southern Africa (Trustees of the John Voelcker Bird Book Fund, Cape Town, South Africa, 1984). [Google Scholar]
- 54.Sinclair I., Hockey P., Tarboton W., Ryan P., SASOL Birds of Southern Africa (Struik Nature, Cape Town, South Africa, ed. 4, 2011). [Google Scholar]
- 55.Mazel A. D., Maqonqo Shelter: The excavation of Holocene deposits in the eastern Biggarsberg, Thukela Basin, South Africa. Natal Mus. J. Hum. 8, 1–39 (1996). [Google Scholar]
- 56.Dean W. R. J., “Ostriches” in The Complete Book of Southern African Birds, Ginn P. J., McIlleron W. G., Milstein P., Eds. (Struik Winchester, Cape Town, South Africa, 1982), pp. 32. [Google Scholar]
- 57.Hamilton M. J., Milne B. T., Walker R. S., Burger O., Brown J. H., The complex structure of hunter-gatherer social networks. Proc. Biol. Sci. 274, 2195–2202 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mucina L., Rutherford M. C., The Vegetation of South Africa, Lesotho and Swaziland (South African National Biodiversity Institute, Pretoria, South Africa, 2006). [Google Scholar]
- 59.Stewart B. A., Mitchell P. J., “Beyond the shadow of a desert: Aquatic resource intensification on the roof of southern Africa” in Foraging in the Past: Archaeological Studies of Hunter-Gatherer Diversity, Lemke A., Ed. (University of Colorado Press, Boulder, CO, 2018), pp. 159–208. [Google Scholar]
- 60.Wiessner P., On network analysis: The potential for understanding (and misunderstanding) !Kung hxaro. Curr. Anthropol. 39, 514–519 (1998). [Google Scholar]
- 61.Blegen N., The earliest long-distance obsidian transport: Evidence from the ∼200 ka Middle Stone Age Sibilo School Road Site, Baringo, Kenya. J. Hum. Evol. 103, 1–19 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Texier P.-J., et al. , From the Cover: A Howiesons Poort tradition of engraving ostrich eggshell containers dated to 60,000 years ago at Diepkloof Rock Shelter, South Africa. Proc. Natl. Acad. Sci. U.S.A. 107, 6180–6185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouzouggar A., et al. , 82,000-year-old shell beads from North Africa and implications for the origins of modern human behavior. Proc. Natl. Acad. Sci. U.S.A. 104, 9964–9969 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.d’Errico F., Henshilwood C., Vanhaeren M., van Niekerk K., Nassarius kraussianus shell beads from Blombos Cave: Evidence for symbolic behaviour in the Middle Stone Age. J. Hum. Evol. 48, 3–24 (2005). [DOI] [PubMed] [Google Scholar]
- 65.d’Errico F., Vanhaeren M., Wadley L., Possible shell beads from the Middle Stone Age layers of Sibudu Cave, South Africa. J. Archaeol. Sci. 35, 2675–2685 (2008). [Google Scholar]
- 66.Lim S., Chase B. M., Chevalier M., Reimer P. J., 50,000 years of vegetation and climate change in the southern Namib Desert, Pella, South Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 451, 197–209 (2016). [Google Scholar]
- 67.Marean C. W., et al. , Early human use of marine resources and pigment in South Africa during the Middle Pleistocene. Nature 449, 905–908 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Hall S. L., Burial and sequence in the Later Stone Age of the Eastern Cape Province, South Africa. S. Afr. Archaeol. Bull. 55, 137–146 (2000). [Google Scholar]
- 69.Mitchell P. J., Developing the archaeology of Marine Isotope Stage 3. South Afr. Archaeol. Soc. Goodwin Ser. 10, 52–65 (2008). [Google Scholar]
- 70.Mackay A., Stewart B. A., Chase B. M., Coalescence and fragmentation in the late Pleistocene archaeology of southernmost Africa. J. Hum. Evol. 72, 26–51 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Miller J. M., “Variability in ostrich eggshell beads from the Middle and Later Stone Age of Africa,” PhD thesis, University of Alberta, Canada (2019).
- 72.Steele T. E., Álvarez-Fernández E., Hallett-Desguez E., A review of shell as personal ornamentation during the African Middle Stone Age. PaleoAnthropology 2019, 24–51 (2019). [Google Scholar]
- 73.Blum J. D., et al. , Mycorrhizal weathering of apatite as an important calcium source in base-poor forest ecosystems. Nature 417, 729–731 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Gleason J. D., et al. , Ichthyolith strontium isotope stratigraphy of a Neogene red clay sequence: Calibrating eolian dust accumulation rates in the central North Pacific. Earth Planet. Sci. Lett. 202, 625–636 (2002). [Google Scholar]
- 75.McArthur J. M., Howarth R. J., Bailey T. R., Strontium Isotope Stratigraphy: LOWESS version 3: Best fit to the marine Sr-isotope curve for 0–509 Ma and accompanying look-up table for deriving numerical age. J. Geol. 109, 155–170 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this study are included in the main text and SI Appendix.