Significance
Clostridioides difficile is a bacterial pathogen of global importance that is a major cause of hospital-acquired diarrhea. Antibiotic-mediated disruptions to the gut microbiota and associated metabolome promote C. difficile growth and infection through mechanisms that are poorly understood. Here, we show that intestinal bile acids, which are known to play a role in C. difficile germination and outgrowth, also directly bind and inhibit TcdB toxin, the primary virulence determinant of C. difficile. Bile acid binding induces a major conformational change in TcdB structure that prevents receptor binding and uptake into cells. In addition to suggesting a role for bile acids in protecting against C. difficile pathogenesis, these findings highlight an approach to block C. difficile virulence.
Keywords: C. difficile, toxin, bile acid, pathogenesis, structure
Abstract
Intestinal bile acids are known to modulate the germination and growth of Clostridioides difficile. Here we describe a role for intestinal bile acids in directly binding and neutralizing TcdB toxin, the primary determinant of C. difficile disease. We show that individual primary and secondary bile acids reversibly bind and inhibit TcdB to varying degrees through a mechanism that requires the combined oligopeptide repeats region to which no function has previously been ascribed. We find that bile acids induce TcdB into a compact “balled up” conformation that is no longer able to bind cell surface receptors. Lastly, through a high-throughput screen designed to identify bile acid mimetics we uncovered nonsteroidal small molecule scaffolds that bind and inhibit TcdB through a bile acid-like mechanism. In addition to suggesting a role for bile acids in C. difficile pathogenesis, these findings provide a framework for development of a mechanistic class of C. difficile antitoxins.
Clostridioides difficile is the most frequent cause of infectious diarrhea in hospitals and has emerged as a major public-health concern in recent decades (1). Antibiotic-induced disruption of the protective gut microbiota triggers C. difficile infections (CDIs) by creating an environment in the gut that enables C. difficile germination and growth. Virulent strains of C. difficile produce protein toxins that are responsible for the clinical symptoms of disease, which can range from self-limiting diarrhea to pseudomembranous colitis, and potentially death in severe cases (2). In particular, the homologous toxins TcdA and TcdB produced by pathogenic strains of C. difficile are capable of causing disease in animal models (3), with TcdB appearing to be the primary determinant of disease in humans (4). TcdA and TcdB are large homologous toxins (sharing 48% sequence identity) with similar multidomain architectures consisting of a glucosyltransferase domain (GTD), an autoprocessing domain (APD), a translocation domain, and a C-terminal domain consisting of oligopeptide repeats, known as the CROP domain (5). After binding to their cell surface receptors (6–9), TcdA and TcdB are internalized into acidified endosomes, whereupon the central translocation domain forms transmembrane pores that are thought to mediate entry of the upstream GTD and APD into the cytosol (10). Processed and released GTD enzymatically glucosylates, and thereby inactivate intracellular Rho and Ras family GTPases (11, 12), leading first to cytopathic effects (i.e., cell rounding) (13), and later cytotoxic effects (i.e., apoptosis and necrosis) (14, 15). Blocking the actions of TcdB has emerged as a promising nonantibiotic-based strategy to treat CDI in recent years. Indeed, bezlotoxumab, a monoclonal antibody against TcdB, was recently approved for recurrent CDI prevention in adults (4), and small molecules blocking TcdB action have shown efficacy in preventing CDI in preclinical animal models (16, 17).
Although toxins are responsible for symptomatic CDI, the mere presence of toxigenic C. difficile in an individual, however, does not portend disease. Indeed, asymptomatic carriage of toxigenic C. difficile has been observed, particularly in hospitals and healthcare settings (18–20). Though it is not known what factors are responsible for rendering an individual susceptible to infection and disease by C. difficile, several studies have shown that intestinal bile acids play a role in modulating various aspects of the C. difficile lifecycle. For instance, it has been established that the primary bile acid taurocholic acid and other cholic acid derivatives trigger germination of C. difficile spores into their toxin-producing vegetative state via the germinant receptor CspC (21–23), whereas chenodeoxycholate derivatives and other secondary bile acids inhibit cholate-induced germination (22, 24). Moreover, the microbial-derived secondary bile acids, including deoxycholic acid and lithocholic acid, are able to inhibit growth of C. difficile (25). In a recent study investigating the gut metabolome in mice before and after antibiotic exposure, it was shown that C. difficile bacterium can exploit specific metabolites that become more abundant in the mouse gut after antibiotics, including the primary bile acid taurocholate for spore germination (26). Further, in a landmark study, Buffie et al. were able to pinpoint the single bacterium, Clostridium scindens, which they showed enhances resistance to C. difficile infection by producing key bile acids that directly inhibit C. difficile outgrowth (27). Finally, a recent study showed differences in bile acid composition between asymptomatic carriers of C. difficile and patients with CDI (28). Taken together, these studies highlight a complex interplay between C. difficile and the host, which is dictated by the host-produced and microbiota-modified bile acid composition.
In this study, we describe an entirely unexpected role for bile acids in the C. difficile lifecycle as directly binding to and inhibiting toxin uptake into cells. This work stems from a recent high-throughput phenotypic screen that we conducted to identify small molecules that prevent TcdB-induced toxicity, where methyl cholate—a synthetic methyl ester of cholic acid—was among the handful of hits in the primary screen that protected cells from TcdB (29). Given that bile acids are abundant in the gut lumen where C. difficile and its toxins act, we hypothesized that bile acids may, in addition to playing a role in spore germination and bacterial viability, play a role in modulating virulence and therefore disease. To explore the biological and therapeutic significance of this work we set out here to uncover the effects of natural bile acids on toxin pathogenesis and define the mechanism of inhibition.
Results
Primary and Secondary Bile Acids Bind TcdB and Inhibit Cellular Intoxication.
Building on our previous findings that a synthetic bile acid derivative methyl cholate bound to and inhibited TcdB (29), we set out initially to evaluate whether and to what extent each of the individual human intestinal bile acids are able to interact with and modulate the activity of TcdA and TcdB. To account for the fact that each of the individual human primary and secondary bile acids exist across a broad range of concentrations within the gastrointestinal tract, individual bile acids and salts were evaluated over a range of concentrations that encompassed physiological levels. To minimize any potential artifacts that could arise due to nonspecific promiscuous effects at higher concentrations of bile acids, care was taken to test all bile acids well below their critical micelle concentrations (SI Appendix, Table S1). Binding of each individual human bile acid to toxins was evaluated using differential scanning fluorimetry (DSF), which quantifies the denaturation temperature (TM) of proteins. Binding of small molecule ligands to a defined site in a protein will often, but not always, stabilize the native state of a target and lead to a measurable increase in the TM (30). In parallel, we evaluated the capacity of each bile acid to inhibit TcdA- and TcdB-mediated intoxication of human IMR-90 fibroblast cells using an automated cell-rounding assay we developed previously (29). The results for bile acid binding to and inhibition of TcdA and TcdB are summarized in Fig. 1A.
Fig. 1.
Human bile acids binding and inhibition of TcdA and TcdB. (A) Structures of primary and secondary bile acids, and corresponding potency in the DSF binding and cell rounding assays. Average binding EC50s were calculated from five to seven experiments. Average cell rounding IC50s were calculated from two to six biological replicates. n.d., not done. (B) Representative images of human IMR-90 fibroblasts from at least six biological replicates. Cells were treated with DMSO or TCDCA along with buffer or 0.5 pM TcdB (Left) or 1 nM TcdA (Right) and images were collected 3.5 h later. (Scale bars, 100 µm.) (C) Titration curves of TCDCA by DSF and cell rounding protection. Bars represent SEM of three biological replicates for DSF and seven biological replicates for cell rounding. (D) Intoxication of human IMR-90 cells by TcdB in the presence of different doses of TCDCA after 3.5 h. Protection factor, PF, represents the extent to which TCDCA shifts the curve for TcdB (i.e., EC50TCDCA/EC50vehicle). Representative graph from four experiments. (E) Normalized transepithelial resistance measurements in human Caco-2 cells, 3 to 6 h posttreatment. A total of 200 μM TCDCA significantly increased resistance across Caco-2 monolayer cells compared to mock control values (n = 5). Bars represent SEM of mean. ****P = 0.000001.
The most striking observation from this analysis is the differences between TcdA and TcdB with respect to binding and inhibition by bile acids. Whereas all bile acids tested showed evidence of binding and inhibition of TcdB to varying extents, none interacted with or inhibited TcdA (Fig. 1 A and B), suggesting that the bile acid binding site is either absent or inaccessible in TcdA (vide infra). We also noted the concordance in binding EC50 (half maximal effective concentration) and inhibition of cell rounding IC50 (half maximal inhibitory concentration) values among the bile acids tested (Fig. 1A), indicating that binding and inhibition are coupled as exemplified clearly by taurochenodeoxycholic acid (TCDCA) (Fig. 1C).
An examination of the differences in binding/inhibition of TcdB by each of the individual bile acids reveals insights into the structure–activity relationship of different primary and secondary bile acids. In general, it can be seen that secondary bile acids are more potent than their corresponding primary bile acid precursors, which differ only by the presence of a hydroxyl group at the α7 position (Fig. 1A). For instance, the secondary bile acid glycolithocholic acid (GLCA) is approximately an order of magnitude more potent than its corresponding primary bile acid glycochenodeoxycholic acid (GCDCA) with respect to binding and inhibition of TcdB. Similarly, taurolithocholic acid (TLCA), glycodeoxycholic (GDCA), and taurodeoxycholic acid (TDCA) are more potent than the corresponding α7-hydroxylated primary bile acids TCDCA, glycocholic acid (GCA), and taurocholic acid (31), respectively. The binding site in TcdB can accommodate and tolerate substitutions at R2 while the degree of hydroxylation at α7 and α12, which lie on the “alpha face” of bile acids, is important for the binding interaction (SI Appendix, Fig. S1). The complete lack of binding of TcdB to dehydrocholic acid (dCA or dehydro-CA), oxidized at α3, α7, and α12, however, confirms that the oxidation state at these positions, and likely the stereochemistry of the bile acid ring system, is important for binding.
To evaluate the extent of protection by bile acids across a range of different concentrations of TcdB that might be experienced during an infection, cells were treated with a broad range of TcdB concentrations at different fixed doses of TCDCA—a highly soluble prototypic bile acid. In the absence of drug, TcdB dose-dependently rounds cells with an EC50 = 0.6 pM (Fig. 1D). With increasing concentrations of TCDCA, the amount of TcdB required to reach equivalent levels of rounding increases and progressively shifts the dose–response curve to the right in accordance with the protection offered by bile acids (Fig. 1D). Finally, we tested the ability of TCDCA to protect human colonic cells from TcdB, which compromises monolayer integrity and dissipates the transepithelial electrical resistance. TCDCA dose-dependently resulted in complete protection that is equivalent to mock-treated cells (Fig. 1E).
The C-Terminal CROP Region Is Essential for Bile Acid Binding.
In order to better understand the molecular determinants of bile acid-induced neutralization of TcdB, we focused our attention on where TcdA and TcdB diverge most in terms of sequence to narrow our search. The most striking differences are in the C-terminal combined repetitive oligopeptide repeats (CROP) domains, where TcdB has fewer repeats than TcdA and shares only 38% sequence identity in the overlapping regions (SI Appendix, Fig. S2). To indirectly test the potential role of the CROP in binding, we took advantage of the naturally “CROP-less” homolog TpeL from Clostridium perfringens (Fig. 2A). We observed no evidence of binding/stabilization of CROP-less TpeL by TCDCA (Fig. 2 B, Inset), supporting a possible role for CROP in binding bile acids; however, based on this alone, we could not exclude the possibility that other differences in the other three domains could be involved in binding. To address this more directly, we generated truncations within the TcdB CROP domain itself. Three C-terminal truncations were generated: TcdB1–2,283, with 4 terminal repeats deleted; TcdB1–2,200, with 8 terminal repeats deleted; and, TcdB1–2,101 with 12 terminal repeats deleted (Fig. 2A). Consistent with previous data showing that the terminal 15 repeats of TcdB could be removed without affecting function (7), we found all three truncations generated here to be fully functional on cells (SI Appendix, Fig. S3A).
Fig. 2.
Role of the C-terminal CROP region of TcdB in binding bile acids. (A, Top) Domain architecture of TcdB and TpeL, highlighting the naturally occurring truncation of the CROP region in TpeL. Short repeats are colored green; long repeats are colored blue. (A, Bottom) The three CROP truncations in TcdB, generated by genetic mutation in order to approximate the natural deletion in TpeL. (B) DSF as a measure of TCDCA binding. TCDCA binds to full-length TcdB in a dose-dependent manner, but does not bind to TcdB CROP truncations, or TpeL (Inset). Bars represent SEM of six experiments for TcdB, three experiments for TpeL, and three experiments for the TcdB CROP truncations. (C) Dose- dependent protection by representative bile acids against full-length TcdB challenge by cell rounding assay. Bars represent SEM of four experiments. (Inset) No protection by the same panel of bile acids against TcdB1–2,283 challenge by cell rounding assay. Bars represent SEM of three experiments. Similar results observed for TcdB1–2,101 and TcdB1–2,200.
To evaluate bile acid binding, DSF was used to determine the thermal stability of each truncation in the presence of TCDCA, initially. Whereas full-length TcdB displayed a dose-dependent increase in thermal stability induced by TCDCA, we found that removing as few as four terminal repeats rendered TcdB insensitive to TCDCA. To corroborate these findings on cells, we compared the ability of full-length TcdB and TcdB1–2,283 to be inhibited by a larger panel of inhibitory bile acids. Consistent with the binding data, none of the bile acids were able to block the action of TcdB1–2,283 (Fig. 2C). To show that TcdB1–2,283 was otherwise sensitive to inhibition by other small molecule antitoxins, we tested a panel of inhibitors of TcdB discovered previously (17, 29) that block TcdB intoxication through nonbile acid mechanisms on TcdB1–2,283 and found that these molecules retained the ability to inhibit truncated TcdB (SI Appendix, Fig. S3B). These findings reveal an unexpected role for the CROP domain in mediating the bile acid-induced neutralization of TcdB.
Bile Acid Binding Induces a Major Conformational Change in TcdB.
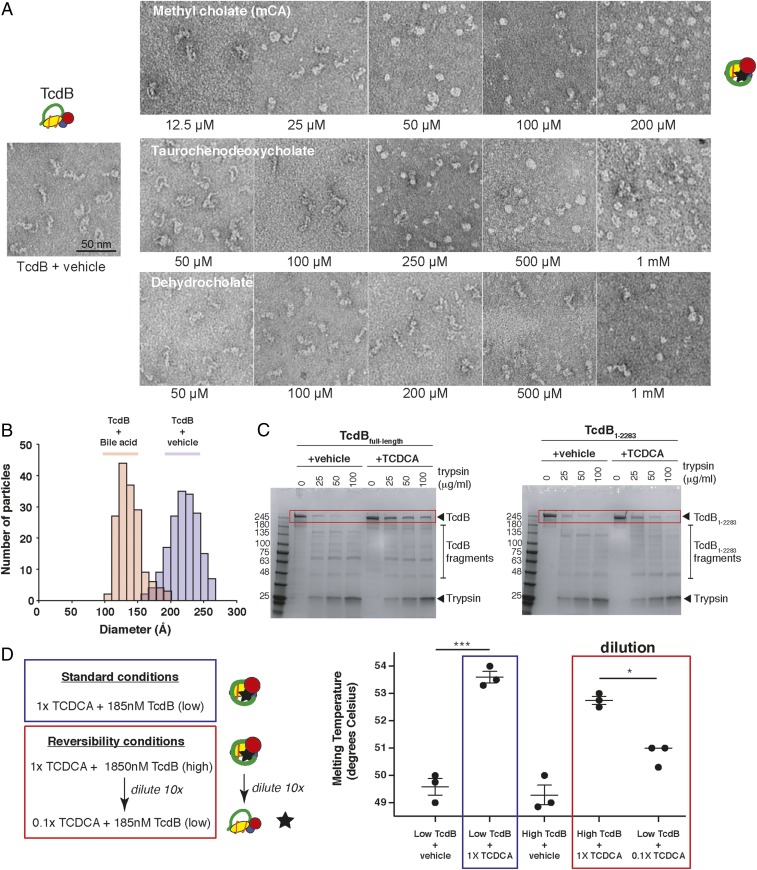
That bile acids could so dramatically affect both the thermal stability and function of TcdB was surprising and led us to hypothesize that bile acids may induce a large conformational change in TcdB upon binding that affects both its structure and function. To monitor whether any major structural changes were induced by bile acids, TcdB was incubated with increasing amounts of two inhibitory bile acids (methyl cholate and TCDCA) and a nonbinding bile acid control (dehydrocholate), and the resulting samples were imaged by negative-stain electron microscopy. In the absence of ligand, we see as reported in previous studies, that the C-terminal CROP of TcdB adopts a variety of conformations relative to the main body of the toxin made up of the three N-terminal domains (Fig. 3A). Similarly, in the presence of dehydrocholate, which does not bind or inhibit TcdB, no noticeable structural changes in the toxin were seen. With increasing concentrations of methyl cholate and TCDCA, however, TcdB adopts an increasingly “balled up” conformation, with the toxin adopting an overall more compacted conformation. Quantification of the distribution of sizes of TcdB in the absence and presence of bile acid shows an average decrease of ∼100 Å in overall size of TcdB upon binding to bile acids (Fig. 3B).
Fig. 3.
Bile acids induce major conformational changes in TcdB that are reversible. (A) Negative-stain EM images of TcdB (100 nM) in the absence and presence of increasing concentrations of methyl cholate, TCDCA, and dehdydrocholate. TcdB was incubated with increasing concentrations of the individual bile salts, and the reactions were diluted 10-fold in reaction buffer immediately before grid preparation. Samples were applied to glow-discharged, carbon-coated copper grids and stained with uranyl formate (0.75%). Micrographs were collected at 44,000 magnification on a Morgagni (100 keV; FEI) transmission electron microscope equipped with an AMT CCD camera. (B) Dual histogram illustrates the relative size distribution of TcdB alone (blue bars) compared to the round structures observed in the presence of methyl cholate (red bars). Individual particles (160 to 180 total) from representative negative-stain EM images were measured manually in Photoshop, with lengths/diameters converted from pixels to Ångstroms based on the scale bar for each image. The length of each TcdB particle was measured from end to end (GTD region to end of delivery domain), and the diameters of the methyl cholate-bound TcdB were measured through the center of the balls (C, Left), dose-dependent trypsin digestion of full-length TcdB is prevented by 1,000 μM TCDCA. (C, Right) TCDCA does not protect TcdB1–2,283 truncation from trypsin digestion. Representative gels from three experiments each. (D) Reversibility measured by DSF, showing reduction of temperature shift back toward baseline upon 10× dilution of TCDCA (500 µM diluted to 50 µM). Four biological replicate wells per condition. ***P = 0.008, *P = 0.0284.
Consistent with bile acids inducing a major conformational change in TcdB, we observed significant differences in the susceptibility of TcdB to proteolysis by trypsin when bound to the bile acid TCDCA. Incubation of TcdB with increasing amounts of trypsin, followed by visualization on SDS/PAGE shows that TcdB is progressively degraded with no full-length TcdB detectable at 100 μg/mL trypsin (Fig. 3C). When bound to TCDCA, TcdB showed increased resistance to trypsin-mediated degradation. TcdB1–2,283, which is incapable of binding bile acid, however, was not protected from proteolytic degradation in the presence of TCDCA (Fig. 3C). Consistent with these results, functional experiments show that intoxication by TcdB under the above conditions is correlated with the amount of remaining full-length toxin remaining (SI Appendix, Fig. S4).
Bile Acid Binding to TcdB Is Reversible.
The observation in the above studies that TCDCA was no longer able to protect against TcdB-induced cell rounding following the dilution by 6,000-fold from the gel-based assay to the functional assay in the above experiments (SI Appendix, Fig. S4B) prompted us to probe more directly whether the bile acid–TcdB interaction was reversible. To this end, we evaluated the reversibility of binding of TCDCA to TcdB by comparing the melting temperature of TcdB in the presence of 500 μM TCDCA at levels where TcdB is complexed with bile acid and after a 10-fold dilution to levels where TCDCA is only partially bound, using previous titrations as a guide (Fig. 2B). As expected, addition of 500 μM TCDCA (i.e., 1× TCDCA) to either 185 nM or 1,850 nM TcdB resulted in a significant increase in the melting temperature of TcdB of ∼3 °C to 4 °C (Fig. 3D). Following a 10-fold dilution of the [high TcdB + 500 μM] complex, we see a significant decrease in the melting temperature of TcdB, further demonstrating that bile acid–TcdB interaction is reversible (Fig. 3D).
Bile Acids Prevent TcdB from Binding to Cell Surface Receptors.
The major changes in TcdB stability, protease susceptibility, and structure induced by bile acids very clearly alter the toxin in a way that inhibits its ability to intoxicate cells. To demonstrate the mechanism by which bile acids protect cells from intoxication by TcdB, we investigated the ability of TcdB to bind the cell surface when bound to the prototypic bile acid TCDCA. Confluent HCT116 cells were mixed with TcdB and vehicle or TCDCA on ice for 60 min to prevent internalization, washed in ice-cold phosphate buffered saline (PBS), then harvested for quantification of bound TcdB by Western blot. In the absence of bile acids, both full-length TcdB and truncated TcdB1–2,283 were recovered from the cell surface and could be visualized by Western blot (Fig. 4A). For full-length TcdB, preincubation with TCDCA blocked cell surface binding, whereas the noninhibitory control bile acid dehydro-CA had no effect on cell surface binding (Fig. 4 A and B). By contrast, cell surface binding of TcdB1-2,283 was unaffected in the presence of either TCDCA or dehydro-CA. Thus, bile acid acts by inducing a conformation in TcdB that is no longer able to bind to cell surface receptors—the first step in the intoxication pathway of TcdB.
Fig. 4.
Cell surface binding assay. TcdB (2 nM) and either 1,000 µM TCDCA or dehydro-CA were preincubated together for 30 min on ice in serum-free media before adding to HCT116 cells. After incubating for 60 min on ice, cells were harvested and lysed. (A) Clarified material was analyzed by Western blot by probing with anti-TcdB antibody (R&D Systems, AF6246) and anti-tubulin antibody (Sigma, T6074) as a loading control. (B) Cell-associated TcdB bands were measured by densitometry using a ChemiDoc MP Imaging System (Bio-Rad). The TcdB-binding compound TCDCA, but not dehydro-CA, prevented surface binding of TcdB to cells. Bars represent SEM of three biological replicates.
Identification of Small Molecules with a Bile Acid-Like Mechanism of Action.
The remarkable ability of bile acids to drive TcdB into a stabilized, but nonfunctional state prompted us to next search for other small molecules that act through this mechanism, both to probe the binding interaction further, and to identify other potentially more “drug-like” molecules that could act as novel antitoxins. To this end, we conducted a high-throughput screen against the same chemical library consisting of approved drugs, bioactive molecules, and natural products. To bias toward compounds with a bile acid-like mechanism, we screened for compounds that increased the thermal stability of TcdB using a temperature-dependent fluorescence binding assay. Hits emerging from this screen were subsequently tested for their ability to protect cells from TcdB-induced toxicity. Only molecules that were active in both assays were characterized further to distinguish those that were binding and inhibiting through a bile acid-like mechanism.
Of the 2,401 small molecules that were screened, we identified 15 small molecules that increased the TM of TcdB by greater than three SDs of the mean (Fig. 5A and SI Appendix, Table S2). As expected, on this short list of hits was methyl cholate (ΔTM = 6 °C), and the three bile acids chenodeoxycholic acid (ΔTM = 3.5 °C), cholic acid (ΔTM = 3 °C), and lithocholic acid (ΔTM = 3 °C). Also present on this list were four compounds, betulinic acid, oleanoic acid, pregnanolone, and asiatic acid, with structures bearing significant structural similarity to the core bile acid scaffold (SI Appendix, Fig. S5). Though just below the statistical cutoff for classification as hits, three additional bile acid-like compounds, deoxycholic acid, madecassic acid, and ursolic acid increased the TM of TcdB (SI Appendix, Table S2).
Fig. 5.
Identification of nonsteroidal bile acid mimetics. (A) Results from high-throughput DSF screening of 2,400 drugs from the Microsource Spectrum collection. A statistical cutoff of ΔT = 3 °C inhibition of increase in stabilization was based on identification of molecules that were greater than 3 SDs above the mean of the data. Green dots represent hits that were bile acids or bile acid-like molecules. (B) Titration of ethaverine and parent compound papaverine against TcdB by DSF. Ethaverine dose dependently binds and thermally stabilizes TcdB with greater potency than papaverine. Bars represent SEM of four experiments. (C) Titration of ethaverine against full-length and CROP-less TcdB by DSF. Ethaverine dose dependently binds and thermally stabilizes full-length TcdB but not CROP-truncated TcdB1–2,283. (D) Cell surface binding assay. TcdB (2 nM) and either 100 µM of positive control methyl cholate, 50 µM ethaverine, or 50 µM papaverine were preincubated together for 30 min on ice in serum-free media before adding to HCT116 cells. After incubating for 60 min on ice, cells were harvested and lysed. Clarified material was analyzed by Western blot by probing with anti-TcdB antibody (R&D Systems, AF6246) and anti-tubulin antibody as a loading control. Cell-associated TcdB bands were measured by densitometry using a ChemiDoc MP Imaging System (Bio-Rad). The TcdB-binding compound ethaverine and to a lesser extent papaverine, prevented surface binding of TcdB to cells. Bars represent SEM of four biological replicates. (E) Normalized transepithelial resistance measurements in human Caco-2 cells, 3 to 6 h posttreatment. Ethaverine preserved significantly increased resistance across Caco-2 monolayer cells compared to mock control values (n = 3 biological replicates). Bars represent SEM of mean. ***P < 0.0006.
From the remaining hits with no structural similarity to bile acids, two compounds were capable of protecting cells from TcdB. The first, phenoxybenzamine, a nonselective irreversible alpha-adrenoreceptor antagonist (32), was excluded from further follow-up owing to its potentially highly reactive carbonium ion that results from cleavage of its tertiary amine ring (33). The second compound, a substituted benzylisoquinoline known as ethaverine hydrochloride—the ethyl analog of papaverine, both of which are potent peripheral coronary vasodilator drugs (34)—was characterized. Papaverine, which was in the primary screen, but was not identified as a hit, was also characterized alongside ethaverine. By DSF, ethaverine dose-dependently increased the thermal stability of TcdB, whereas papaverine increased the stability only marginally up to 100 μM (Fig. 5B). To determine whether ethaverine acted in a similar fashion to bile acids, we next tested whether it was capable of inhibiting TcdB-ΔBASR. Ethaverine displayed no affinity for truncated TcdB, consistent with its inhibiting TcdB in a bile acid fashion (Fig. 5C). Similarly, ethaverine was specific for TcdB, as it was unable to inhibit TcdA-induced cell rounding (SI Appendix, Fig. S6A). Moreover, ethaverine and to a lesser extent papaverine, shared the same mechanism of inhibition of TcdB by preventing cell surface binding of TcdB (Fig. 5D and SI Appendix, Fig. S6B). Lastly, we show that ethaverine protects human colonic epithelial cell monolayers from TcdB-mediated damage and loss of transepithelial resistance (Fig. 5E).
Discussion
In this study, we describe a role for bile acids in binding to and inhibiting the function of TcdB—the major determinant of virulence for C. difficile. The unexpected finding that the homologous toxins TcdA and TpeL were insensitive to bile acid binding and inhibition led us to uncover a role for a region within the poorly defined CROP domain in this interaction. The role of the CROP domain in the function of large clostridial toxins has been a matter of debate ever since their discovery (5), fueled in part by their demonstrated ability to bind carbohydrates (35) which were reasonably speculated to serve as receptors for toxin entry. The discovery of TpeL, which naturally lacked the CROP region, but was otherwise toxic to cells (36), along with the demonstration that removing the CROP had no impact on cytotoxicity (7, 37), however, challenged its role as the receptor-binding domain for this family of toxins. The recent discovery of cellular receptors for TcdA (9) and TcdB (6, 8, 38) that bind outside of, or at the junction of the CROP domain has further called the role of this enigmatic domain into question.
Our findings here show that the C-terminal region of the TcdB CROP domain is required for the bile acid-induced effects on TcdB structure and function. Remarkably, we demonstrate that this massively compacted inhibited state of TcdB is reversible and thus in a dynamic active–inactive equilibrium that depends on the local concentrations of bile acids. The extent to which these phenomena contribute to colonization resistance (39, 40) and disease pathogenesis, particularly in asymptomatic carriers of toxigenic C. difficile, where toxins are often present at similar levels as CDI patients (41), will be an important aim of future studies aimed at better understanding the physiological implications of this interaction. It is tempting to speculate that bile acids may help in the timing of the action of TcdB, for instance by protecting and inhibiting TcdB in the upper GI where bile acid levels are highest and later allowing “release” of active toxin in the lower GI where bile acids are lower in concentration.
From a therapeutic perspective, the demonstrated ability of bile acids to offer complete protection against TcdB—a major target in CDI drug development (4)—provides a mechanism-based approach to inhibit toxin action to prevent disease pathogenesis through development of antitoxins. The therapeutic use of bile acids themselves to this end, however, is problematic due to the potential for overloading physiological functions of bile acids, in particular with signal transduction pathways and the recycling of bile acids (42). Moreover, disruption of the host microbiota itself through bile acid-mediated antimicrobial effects (43) are not desired within the context of treating C. difficile where the goal of therapy is to prevent further dysbiosis. Our discovery of ethaverine, an alternative nonsteroidal scaffold that is an already approved drug, and that binds and inhibits TcdB through a bile acid-like mechanism offers an ideal means to exploit this mechanism for the development of therapeutic inhibitors of TcdB action to treat CDI.
Methods
Consumables, Cell Lines, and Reagents.
Plasticware used for cell culture and enzyme assays was purchased from Corning.
Cell lines HCT116, Caco-2, and IMR-90 were from ATCC. Natural and synthetic bile acids were purchased from Sigma, and Ethaverine was purchased from Onbio. Western blot reagents, including Amersham ECL Prime blocking, ECL Select Detection, and anti-mouse conjugated peroxidase antibody were from GE Healthcare. PCR and cloning reagents Q5 High Fidelity PCR polymerase (M0491) and NEBuilder HiFi DNA Assembly Master Mix (E2621) were from New England Biolabs. The Spectrum library, consisting of 2,400 individual compounds formatted as 10 mM solutions in DMSO, was purchased from Microsource.
Protein Expression and Purification.
Plasmid pHis1522 encoding his-tagged TcdB was a kind gift from Hanping Feng (University of Maryland, Baltimore, MD) and plasmid pHis1522 encoding his-tagged TcdA was a kind gift from Merck. Expression and isolation of recombinant TcdB and TcdA was as described by Yang et al. (44). Briefly, transformed Bacillus megaterium was inoculated into Luria broth (LB) containing tetracycline and grown to an A600 of 1.6, followed by overnight xylose induction at 30 °C. Bacterial pellets were collected, resuspended with 20 mM Tris pH 8/0.1 M NaCl, and passed twice through an EmulsiFlex C3 microfluidizer (Avestin) at 15,000 psi. The resulting lysate was clarified by centrifuging for 14,000 × g for 20 min. TcdB was purified by nickel affinity chromatography followed by anion exchange chromatography using HisTrap FF Crude and HiTrap Q columns (GE Healthcare), respectively. Fractions containing TcdB or TcdA were verified by SDS/PAGE, then pooled and diafiltered with a 100,000 molecular weight cutoff (MWCO) ultrafiltration device (Corning) into 20 mM Tris PH 7.5/150 mM NaCl. Finally, glycerol was added to 5% vol/vol, the protein concentration was estimated by A280, divided into single-use aliquots, and stored at −80 °C.
To generate CROP truncations, plasmid pHis1522 encoding TcdB was used as a DNA template along with the forward primer “upstreamBsrGI-Fwd” (CTTGTTCACTTAAATCAAAGGGGG), plus the respective reverse primers “TcdB-2101-RevHis” (TAGTGATGGTGATGGTGATGACCTATATATGCTTCTGCTGTATCTTC), “TcdB-2200-RevHis” (TAGTGATGGTGATGGTGATGACCACTATATTCAACTGCTTGTCC), and “TcdB-2283-RevHis” (TAGTGATGGTGATGGTGATGAAATTGCATTTCACCATTCTCATTAAAG) for the three truncated DNA products. To generate the accepting vector, the same template was used with primers “TcdB-His-Fwd” (CATCACCATCACCATCACTAAC) and “upstreamBsrGI-Rev” (CCCCCTTTGATTTAAGTGAACAAG). Q5 High Fidelity PCR polymerase (M0491) and NEBuilder HiFi DNA Assembly Master Mix (E2621) were used for PCR reactions and cloning, respectively. Protein expression and purifications of the truncations were performed as for full-length TcdB.
Arrayscan High-Content Imaging.
IMR-90 cells were grown in Eagle’s Minimum Essential Medium (EMEM) (Wisent) supplemented with 10% fetal bovine serum (FBS) and penicillin–streptomycin (complete EMEM) and were seeded in 96-well Cellbind plates (Corning) at a density of 8,000 cells/well. The next day, the media was exchanged with serum-free EMEM (SFM) containing 1 μM Celltracker Orange CMRA (Invitrogen C34551). After 60 min, excess dye was removed by media exchange with SFM. An Agilent Bravo liquid handler was used to deliver 0.4 μL of compound from the compound plate to the cell plate, immediately followed by 10 μL of 5 pM TcdB (diluted in SFM), representing a concentration of toxin previously established as ∼EC99 levels of cytopathology. The cell plates were returned to the incubator for 3.5 h before imaging. Celltracker-labeled cells were evaluated on a Cellomics ArrayScan VTI HCS reader (Thermo Scientific) using the Target Acquisition mode, a 10× objective, and a sample rate of at least 150 objects per well. After recording all image data, the cell rounding and shrinking effects of TcdB intoxication were calculated using the cell rounding index (29), a combined measure of the length to width ratio (LWR) and area parameters. The % inhibition was calculated as the ratio between the sample well and the average toxin-untreated controls after subtracting the average dimethyl sulfoxide (DMSO) control values.
Dose–response curves were created and evaluated using Prism software (Graphpad Software).
Transepithelial Electrical Resistance.
Caco-2 cells (ATCC) were plated on 12-well Transwell polyester, 0.4-μm pore size plates (Corning 3460), at a density of 100,000 cells/well. Electrical resistance was monitored with a Millicell ERS-2 V-ohm meter (Millipore). When resistance readings plateaued after 14 to 21 d, TcdB (20 pM final) and test compound were added to the basolateral side. Decline in resistance as a consequence of loss of cell barrier integrity was measured over the next 3 to 6 h and reported as percentage of the baseline value.
Differential Scanning Fluorometry.
DSF was performed in a similar manner as described previously (30). TcdB protein was diluted to 0.05 µg/µL, and TpeL was diluted to 1 µg/µL using phosphate buffer (100 mM KPO4, 150 mM NaCl, pH 7) containing 5× SYPRO Orange (Invitrogen S6650), and a serial dilution of test compound. A Bio-Rad CFX96 qRT-PCR thermocycler was used to establish a temperature gradient from 30 °C to 80 °C in 0.5 °C increments, while simultaneously recording the increase in SYPRO Orange fluorescence as a consequence of binding to hydrophobic regions exposed on unfolded proteins. The Bio-Rad CFX Manager 3.1 software was used to integrate the fluorescence curves to calculate the melting point. For the high-throughput screen, an Agilent Bravo liquid handler was used to deliver 0.3 μL from the Microsource library plate to the assay plate containing 30 µL of the TcdB and SYPRO Orange reaction mix, for a final compound concentration of 100 µM. The reaction plate was read in the CFX96 thermocycler in a range of 42 °C to 65 °C in 0.5° increments, 5-s read per increment.
Cell Surface Binding Assay.
HCT116 cells in 10-cm dishes were grown to 90% confluence; one plate was used per condition. TcdB (2 nM) and test compounds were preincubated together for 30 min on ice in serum-free media before adding to cells. After incubating for 60 min on ice, cells were washed with PBS, harvested and lysed in 300 µL of 0.5% TX100/PBS. Clarified material was analyzed by Western blot by probing with anti-TcdB antibody (R&D Systems, AF6246) first, followed by anti-tubulin antibody (Sigma, T6074) as a loading control. Secondary antibodies for detection were anti-sheep biotin (Abcam, 6746) followed by streptavidin horseradish peroxidase (HRP) (Abcam, 7403) for TcdB, and anti-mouse HRP (GE Healthcare, NA931V) for tubulin. TcdB bands were measured by densitometry using a ChemiDoc MP Imaging System (Bio-Rad).
Protease Protection Assay.
Reactions were set up on ice containing the following components: 45 µL of 50 mM Tris pH 8/150 mM NaCl, 2.5 μg TcdB (185 nM), plus/minus 1,000 μM TCDCA. Trypsin dilutions (Sigma, T1426) were added to a final reaction volume of 50 µL and incubated for 15 min at 30 °C. For the cell rounding assay, 5 µL of the reaction was retained and diluted into 300 µL complete EMEM before storage at −20 °C. A further 100-fold dilution was performed prior to adding to cells; this represented a final 6,000-fold dilution of the trypsin and TCDCA for the cell assay. The remaining reaction was stopped by adding 15 µL of 4× SDS sample buffer (Bio-Rad) and heating to 90 °C for 10 min before loading 50 µL for SDS/PAGE and Coomassie staining.
Negative-Stain Electron Microscopy.
TcdB holotoxin was expressed in B. megaterium and purified by nickel-chelating, anion exchange. Sodium dehydrocholate and sodium taurochenodeoxycholate were obtained from Sigma-Aldrich, and methyl cholate was purchased from Alfa Aesar. Stock solutions of the bile salts were made in either methanol (methyl cholate) or water (dehydrocholate and taurochenodeoxycholate), and working stocks were further diluted into buffer for the experiments. Samples for negative-stain electron microscopy were prepared in reaction buffer (20 mM Hepes pH 6.9, 50 mM NaCl). In brief, TcdB (100 nM) was incubated with increasing concentrations of the individual bile salts, and the reactions were diluted 10-fold in reaction buffer immediately before grid preparation. Samples were applied to glow-discharged, carbon-coated copper grids (400 mesh, Electron Microscopy Sciences) and stained with uranyl formate (0.75%). Micrographs were collected at 44,000 magnification on a Morgagni (100 keV; FEI) transmission electron microscope equipped with an AMT 1 k × 1 k (1024 × 1024 pixels) charge-coupled device (CCD) camera.
Supplementary Material
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916965117/-/DCSupplemental.
References
- 1.Slayton R. B., et al. , The cost-benefit of federal investment in preventing Clostridium difficile infections through the use of a multifaceted infection control and antimicrobial stewardship program. Infect. Control Hosp. Epidemiol. 36, 681–687 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smits W. K., Lyras D., Lacy D. B., Wilcox M. H., Kuijper E. J., Clostridium difficile infection. Nat. Rev. Dis. Primers 2, 16020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuehne S. A., et al. , Importance of toxin A, toxin B, and CDT in virulence of an epidemic Clostridium difficile strain. J. Infect. Dis. 209, 83–86 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilcox M. H., et al. ; MODIFY I and MODIFY II Investigators , Bezlotoxumab for Prevention of Recurrent Clostridium difficile infection. N. Engl. J. Med. 376, 305–317 (2017). [DOI] [PubMed] [Google Scholar]
- 5.von Eichel-Streiber C., Sauerborn M., Clostridium difficile toxin A carries a C-terminal repetitive structure homologous to the carbohydrate binding region of streptococcal glycosyltransferases. Gene 96, 107–113 (1990). [DOI] [PubMed] [Google Scholar]
- 6.Yuan P., et al. , Chondroitin sulfate proteoglycan 4 functions as the cellular receptor for Clostridium difficile toxin B. Cell Res. 25, 157–168 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta P., et al. , Functional defects in Clostridium difficile TcdB toxin uptake identify CSPG4 receptor-binding determinants. J. Biol. Chem. 292, 17290–17301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao L., et al. , Frizzled proteins are colonic epithelial receptors for C. Difficile toxin B. Nature 538, 350–355 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao L., et al. , Sulfated glycosaminoglycans and low-density lipoprotein receptor contribute to Clostridium difficile toxin A entry into cells. Nat. Microbiol. 4, 1760–1769 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z., et al. , Translocation domain mutations affecting cellular toxicity identify the Clostridium difficile toxin B pore. Proc. Natl. Acad. Sci. U.S.A. 111, 3721–3726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Just I., Selzer J., von Eichel-Streiber C., Aktories K., The low molecular mass GTP-binding protein Rho is affected by toxin A from Clostridium difficile. J. Clin. Invest. 95, 1026–1031 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genth H., Aktories K., Just I., Monoglucosylation of RhoA at threonine 37 blocks cytosol-membrane cycling. J. Biol. Chem. 274, 29050–29056 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Donta I., et al. , Effect of beta-adrenergic blockade on physiologic growth in the Wistar rat. Res. Commun. Chem. Pathol. Pharmacol. 37, 147–150 (1982). [PubMed] [Google Scholar]
- 14.Hippenstiel S., et al. , Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L830–L838 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Chumbler N. M., et al. , Clostridium difficile Toxin B causes epithelial cell necrosis through an autoprocessing-independent mechanism. PLoS Pathog. 8, e1003072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bender K. O., et al. , A small-molecule antivirulence agent for treating Clostridium difficile infection. Sci. Transl. Med. 7, 306ra148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam J., et al. , Host-targeted niclosamide inhibits C. difficile virulence and prevents disease in mice without disrupting the gut microbiota. Nat. Commun. 9, 5233 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crobach M. J. T., et al. , Understanding Clostridium difficile Colonization. Clin. Microbiol. Rev. 31, e00021-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyne L., Warny M., Qamar A., Kelly C. P., Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 342, 390–397 (2000). [DOI] [PubMed] [Google Scholar]
- 20.McFarland L. V., Mulligan M. E., Kwok R. Y., Stamm W. E., Nosocomial acquisition of Clostridium difficile infection. N. Engl. J. Med. 320, 204–210 (1989). [DOI] [PubMed] [Google Scholar]
- 21.Wilson K. H., Sheagren J. N., Freter R., Population dynamics of ingested Clostridium difficile in the gastrointestinal tract of the Syrian hamster. J. Infect. Dis. 151, 355–361 (1985). [DOI] [PubMed] [Google Scholar]
- 22.Francis M. B., Allen C. A., Shrestha R., Sorg J. A., Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog. 9, e1003356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorg J. A., Sonenshein A. L., Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 190, 2505–2512 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thanissery R., Winston J. A., Theriot C. M., Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe 45, 86–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thanissery R., Zeng D., Doyle R. G., Theriot C. M., A small molecule-screening pipeline to evaluate the therapeutic potential of 2-aminoimidazole molecules against Clostridium difficile. Front. Microbiol. 9, 1206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theriot C. M., et al. , Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 5, 3114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buffie C. G., et al. , Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517, 205–208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson J. I., et al. , Metabolomic networks connect host-microbiome processes to human Clostridioides difficile infections. J. Clin. Invest. 130, 3792–3806 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam J., et al. , Small molecule inhibitors of Clostridium difficile toxin B-induced cellular damage. Chem. Biol. 22, 175–185 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Niesen F. H., Berglund H., Vedadi M., The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2, 2212–2221 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Blake J. E., Mitsikosta F., Metcalfe M. A., Immunological detection and cytotoxic properties of toxins from toxin A-positive, toxin B-positive Clostridium difficile variants. J. Med. Microbiol. 53, 197–205 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Bodenstein J., Venter D. P., Brink C. B., Phenoxybenzamine and benextramine, but not 4-diphenylacetoxy-N-[2-chloroethyl]piperidine hydrochloride, display irreversible noncompetitive antagonism at G protein-coupled receptors. J. Pharmacol. Exp. Ther. 314, 891–905 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Frang H., Cockcroft V., Karskela T., Scheinin M., Marjamäki A., Phenoxybenzamine binding reveals the helical orientation of the third transmembrane domain of adrenergic receptors. J. Biol. Chem. 276, 31279–31284 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Voyles C. M., Sieber H. A., Orgain E. S., Ethaverine in the treatment of angina pectoris. J. Am. Med. Assoc. 153, 12–14 (1953). [DOI] [PubMed] [Google Scholar]
- 35.von Eichel-Streiber C., Sauerborn M., Kuramitsu H. K., Evidence for a modular structure of the homologous repetitive C-terminal carbohydrate-binding sites of Clostridium difficile toxins and Streptococcus mutans glucosyltransferases. J. Bacteriol. 174, 6707–6710 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amimoto K., Noro T., Oishi E., Shimizu M., A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology 153, 1198–1206 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Olling A., et al. , The repetitive oligopeptide sequences modulate cytopathic potency but are not crucial for cellular uptake of Clostridium difficile toxin A. PLoS One 6, e17623 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaFrance M. E., et al. , Identification of an epithelial cell receptor responsible for Clostridium difficile TcdB-induced cytotoxicity. Proc. Natl. Acad. Sci. U.S.A. 112, 7073–7078 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Britton R. A., Young V. B., Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 20, 313–319 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buffie C. G., Pamer E. G., Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 13, 790–801 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roldan G. A., Cui A. X., Pollock N. R., Assessing the Burden of Clostridium difficile infection in Low- and Middle-Income Countries. J. Clin. Microbiol. 56, e01747-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Ciaula A., et al. , Bile acid physiology. Ann. Hepatol. 16 (suppl. 1), S4–S14 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Inagaki T., et al. , Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. U.S.A. 103, 3920–3925 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang G., et al. , Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol., 10.1186/1471-2180-8-192 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.