Significance
Singlet oxygen (1O2) generated in the Arabidopsis fluorescent (flu) mutant triggers cell death in young seedlings and inhibits growth of mature plants through the EXECUTER1 (EX1)-dependent retrograde signaling pathway. Here, we show another 1O2-induced chloroplast-to-nucleus retrograde signaling pathway, the 1O2-SAFEGUARD1 (SAFE1) pathway that is independent of EX1. SAFE1 is localized in the chloroplast stroma, and release of 1O2 induces SAFE1 degradation via chloroplast-originated vesicles. Without SAFE1, grana margins (GMs) of chloroplast thylakoids are specifically damaged upon 1O2 generation and associate with plastoglobules. We suggest that the Arabidopsis SAFE1 suppresses 1O2-induced stress responses by protecting GMs. Our paper, therefore, uncovers an additional 1O2-induced pathway and a unique mechanism by which higher plants deal with oxidative stress.
Keywords: singlet oxygen, chloroplast, stress, retrograde signaling, SAFEGUARD1
Abstract
Singlet oxygen (1O2), the major reactive oxygen species (ROS) produced in chloroplasts, has been demonstrated recently to be a highly versatile signal that induces various stress responses. In the fluorescent (flu) mutant, its release causes seedling lethality and inhibits mature plant growth. However, these drastic phenotypes are suppressed when EXECUTER1 (EX1) is absent in the flu ex1 double mutant. We identified SAFEGUARD1 (SAFE1) in a screen of ethyl methanesulfonate (EMS) mutagenized flu ex1 plants for suppressor mutants with a flu-like phenotype. In flu ex1 safe1, all 1O2-induced responses, including transcriptional rewiring of nuclear gene expression, return to levels, such as, or even higher than, those in flu. Without SAFE1, grana margins (GMs) of chloroplast thylakoids (Thys) are specifically damaged upon 1O2 generation and associate with plastoglobules (PGs). SAFE1 is localized in the chloroplast stroma, and release of 1O2 induces SAFE1 degradation via chloroplast-originated vesicles. Our paper demonstrates that flu-produced 1O2 triggers an EX1-independent signaling pathway and proves that SAFE1 suppresses this signaling pathway by protecting GMs.
Plants have to cope with various reactive oxygen species (ROS) which are continuously produced in cell organelles, especially in chloroplasts (1, 2). ROS can damage lipids, DNA, proteins, and other biological components. Correspondingly, plants have evolved a variety of mechanisms to detoxify ROS or protect against its effects, including low-molecular-weight antioxidants (e.g., carotenoids, flavonoids, plastoquinones, tocopherols, ascorbate, and glutathione) and scavenging enzymes (e.g., superoxide dismutase, ascorbate peroxidase, glutathione peroxidase, and catalase) (1, 3). However, under stress conditions, such as drought, high light (L), or pathogen attack, this balanced network of ROS production and degradation is frequently disturbed, favoring the production of ROS. Recent studies have demonstrated that ROS are also beneficial for plants since they are crucial for the regulation of several important biological processes, particularly, in cell differentiation and stress tolerance (4–7).
The ROS singlet oxygen (1O2), which is responsible for most photo-oxidative damage in chloroplasts (8) and has long been recognized as a cytotoxin that inhibits photosynthesis and compromises cell function, also acts as a highly versatile signal that induces various stress responses (9–12). 1O2 was first shown to regulate the expression of the glutathione peroxidase homologous (Gpxh) gene in Chlamydomonas reinhardtii (13). Two years later, a broader significance for 1O2 as a signaling molecule was described in the study of the conditional fluorescent (flu) mutant of Arabidopsis thaliana (9). FLU encodes a negative regulator of tetrapyrrole biosynthesis, and the flu mutant lacking this regulator is unable to constrain accumulation of protochlorophyllide ([Pchlide], an intermediate of chlorophyll biosynthesis) in the dark (D) (14, 15). When D-adapted flu plants are transferred to L, the photosensitizing Pchlide molecules can transfer L energy to ground-state (triplet) molecular oxygen (3O2), leading to the generation of 1O2. A burst of 1O2 in the flu mutant upon exposure to L induces bleaching of young seedlings and growth inhibition in mature plants. However, all these phenotypic changes can be suppressed by inactivation of the EX1 gene. Upon a D–L shift, flu ex1 mutants generate similar amounts of 1O2 to parental flu plants but show no obvious 1O2-induced stress responses, indicating a signaling role for 1O2 in the latter (10, 16). In the flu mutant, 1O2 generated in thylakoids (Thys) oxidizes the Trp643 residue of EX1. Subsequent FtsH2-dependent cleavage of the oxidized EX1 protein is necessary for induction of this signaling pathway (17–19). A quite recent study using the Arabidopsis lesion simulating disease1 (lsd1) mutant points out that uncoupled expression of nuclear and plastid photosynthesis-associated genes disrupts the stoichiometry of photosynthetic proteins, resulting in the generation of 1O2 in chloroplasts and a weak cell-death phenotype. The cell-death phenotype of the lsd1 mutant relies on the presence of the EX1 protein (20). Meanwhile, more conditional mutants that selectively induce generation of 1O2 were isolated. The Arabidopsis ferrochelatase2 (fc2) mutant is defective in converting of protoporphyrin IX (ProtoIX) to heme, and the elevated level of ProtoIX acts as a photosensitizer and generates 1O2 that damages chloroplasts (11, 21). The damaged chloroplasts are then ubiquitinated on the outer envelope via PUB4 ubiquitin ligase and degraded (11, 22). Another experimental system is the Arabidopsis chlorophyll b-less chlorina (ch1) mutant that is devoid of PSII antenna complexes (23). The ch1 mutant is hypersensitive to high L due to a selective increase in 1O2 in the reaction center (RC) of PSII in the grana core (GC) (appressed regions of the grana) (23). The cell-death response of the ch1 mutant to high L can be partially rescued by inactivation of the oxidative signal inducible (OXI1) gene (12). Not only interruptions of chlorophyll biosynthesis, but also disturbances of chlorophyll catabolism favor production of 1O2. The Arabidopsis ACD2 encodes a chlorophyll catabolite reductase (RCCR) that can breakdown red chlorophyll catabolite (RCC), an intermediate in the chlorophyll breakdown process, and the mutant lacking RCCR accumulates RCC and releases 1O2, inducing programmed cell death in mature leaves (24, 25).
The flu ex1 double mutant is an ideal tool for exploring 1O2-induced signaling in plants since it can specifically generate 1O2 but shows no obvious phenotypic changes. In addition, the amount of 1O2 generated in flu ex1 is positively correlated with the duration of D treatment (26). For up to 8-h D treatment, 1O2 generated in flu ex1 after transfer to L is too low to damage the cell directly, and the signaling effect of 1O2 is suppressed by the EX1 mutation, causing no obvious phenotypic changes (27). Here, we employed flu ex1 as a starting material to explore 1O2-mediated signaling in Arabidopsis. We identified a retrograde signaling pathway that negatively regulates 1O2-mediated stress responses and proved the GMs were the first targets of 1O2. Besides, our work also demonstrated that this 1O2-induced retrograde signaling pathway was suppressed by the stroma protein SAFEGUARD1 (SAFE1)-mediated protection of GMs.
Results
Identification of flu ex1 safe1 Mutants and the SAFE1 Gene.
To further explore and identify components of 1O2-induced signaling, we mutagenized the flu ex1 mutant with EMS and screened the M2 generation for mutants that restored the 1O2-induced cell-death phenotype. A group of four recessive mutants that behaved like flu when kept under nonpermissive D–L growth conditions was isolated (Fig. 1 and SI Appendix, Fig. S1). Allelism tests revealed that these represented alleles of the same locus, which was named SAFE1 because its normal product protects the flu ex1 mutant from 1O2-induced damage. A combination of next-generation sequencing and map-based cloning revealed that the four allelic flu ex1 safe1 mutants harbored mutations in the gene At5g14260 (SI Appendix, Fig. S2 A–E). The SAFE1 mutation in At5g14260 was verified by complementation of the flu ex1 safe1 phenotype with SAFE1-YFP, SAFE1-Myc, and SAFE1-GUS fusion proteins (SI Appendix, Fig. S2F).
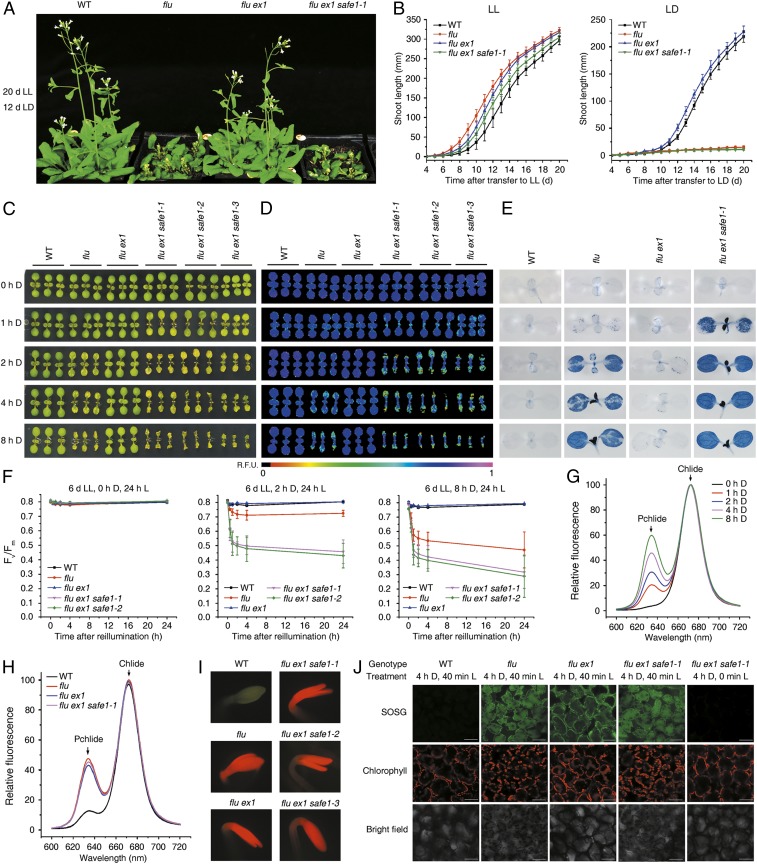
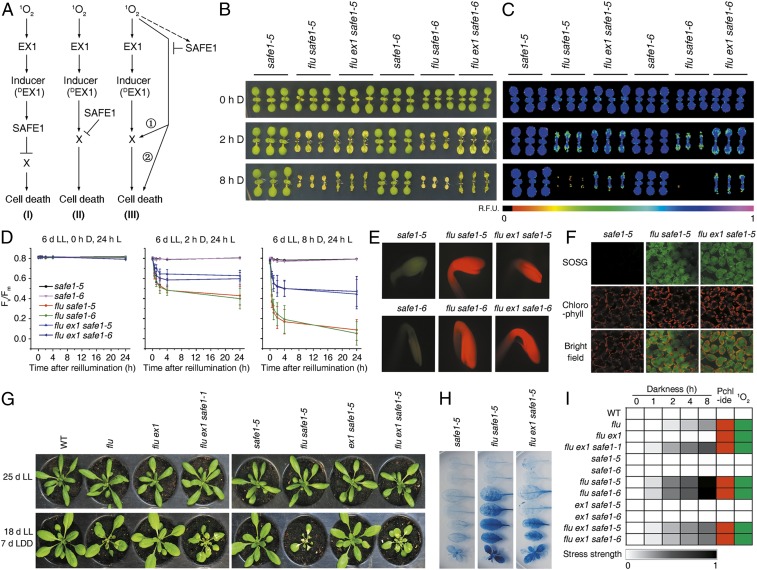
Fig. 1.
SAFE1 suppresses stress responses triggered by 1O2 signaling. (A) Mutation of SAFE1 in flu ex1 restores 1O2-induced growth inhibition. Plants were grown under continuous light (LL), then exposed to a L–D regime (LD) as indicated. (B) Growth rates of the genotypes in A under LL (Left) or LL followed by LD (Right). (C–E) Impact of enhanced 1O2 generation during extended D periods on cotyledon bleaching (C), chlorophyll autofluorescence (D), cell death (E), and maximum PSII efficiency (Fv/Fm) (F). Mean values ± SDs (n > 30) are provided. (G) Levels of Pchlide and chlorophyllide (Chlide) in flu ex1 safe1-1 following various periods of D incubation, determined on the basis of their fluorescence emission at 634 and 673 nm, respectively. (H) Pchlide and Chlide accumulation in WT, flu, flu ex1, and flu ex1 safe1-1 after 4 h of D incubation. (I) Direct visualization of Pchlide accumulation in 4-d-old etiolated seedlings, based on its characteristic red fluorescence under blue L. (J) Quantification of 1O2 in seedlings based on the 1O2 sensor green (SOSG) assay. Seedlings were first grown under LL, D incubated for 4 h, and then reexposed to L.
The flu ex1 safe1 Mutants Are Hypersensitive to flu-Generated 1O2.
Like the flu mutant, mature flu ex1 safe1 plants ceased to grow when exposed to a nonpermissive L–D regime (Fig. 1 A and B and SI Appendix, Fig. S1), and young flu ex1 safe1 seedlings displayed a cell-death phenotype (Fig. 1 C–E). The incidence of cell death revealed by trypan blue staining was correlated with the level of Pchlide accumulation in leaves (Fig. 1 C–G). In flu ex1 safe1 seedlings, Pchlide accumulation was proportional to the duration of D treatment (Fig. 1G), indicating that more 1O2 was generated after reillumination if plants had been incubated in the D for longer times. The increased 1O2 content caused enhanced cell death, in association with pronounced decreases in transient chlorophyll fluorescence and maximum photochemical efficiency (Fv/Fm) of PSII (Fig. 1 C–F). In mature flu ex1 safe1 plants, Pchlide mainly accumulated in emerging leaves when incubated in the D and caused severe cell death in these leaves after reillumination (SI Appendix, Fig. S3). In contrast, when grown under continuous L, both flu and flu ex1 safe1 behaved exactly like wild-type (WT) plants (Fig. 1 B–F and SI Appendix, Figs. S1 and S4).
During D treatment, the flu ex1 safe1 mutants produced the same amount of Pchlide as flu plants (Fig. 1 H and I) and generated similar amounts of 1O2 after reillumination (Fig. 1J). However, the flu ex1 safe1 mutants showed a more severe cell-death phenotype in both seedlings and young leaves of mature plants after release of 1O2 than did the flu mutant (Fig. 1 C–F and SI Appendix, Fig. S3). However, the SAFE1 protein apparently does not play a significant role in plants under high-L stress since the safe1 single mutant, flu safe1 double mutant, and flu ex1 safe1 triple mutant were phenotypically indistinguishable from the WT, and photosynthetic performance was not affected in these plants (SI Appendix, Fig. S5).
SAFE1 Suppresses 1O2-Induced Transcriptional Changes.
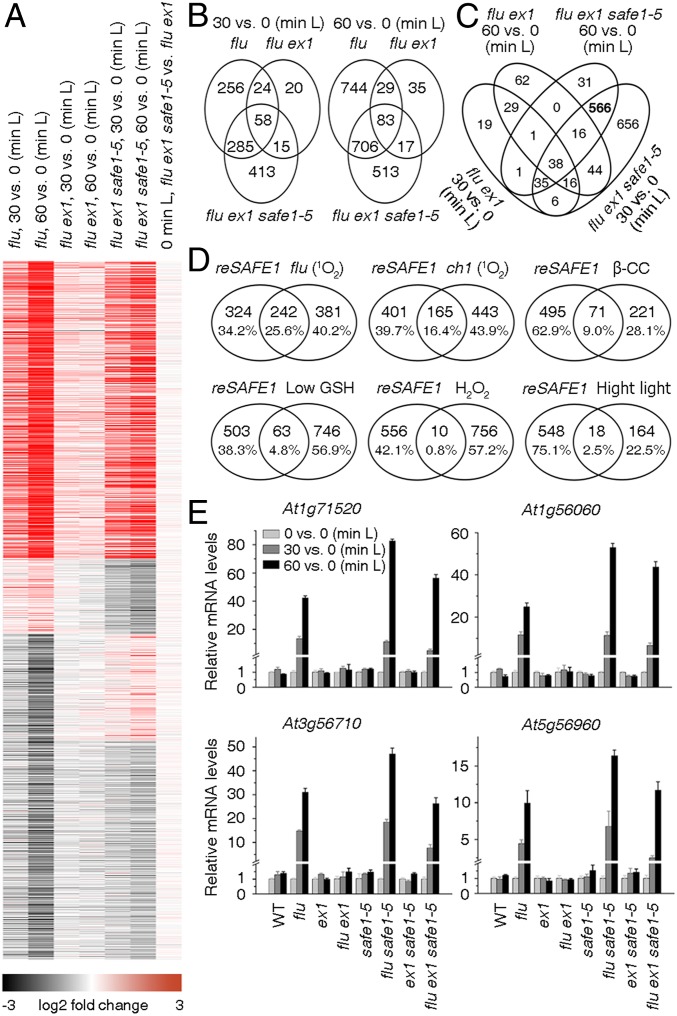
A burst of 1O2 induces widespread changes in gene expression in the flu mutant (9), and this 1O2-induced transcriptional response is largely suppressed in flu ex1 (28). However, in flu ex1 safe1, the 1O2-induced alterations at the transcriptional level were comparable to those seen in the flu mutant (Fig. 2). This was revealed by comparison of our RNA-sequencing (RNA-seq) data for 6-d-old flu ex1 and flu ex1 safe1 seedlings which were incubated in the D for 4 h and reilluminated for 30 or 60 min, respectively, with previously generated RNA-seq data for the flu mutant (18), grown under the same conditions (Fig. 2A and Datasets S1–S5). About half of the greater than twofold-induced genes in the flu mutant after release of 1O2 were also induced more than twofold in flu ex1 safe1 by 1O2 (Fig. 2B). A group of 566 genes was greater than twofold induced in flu ex1 safe1 but not in flu ex1 after 30 and 60 min of reillumination, and we designated this gene set as “induced by removal of SAFE1” or “reSAFE1” (Fig. 2C and Dataset S6). Gene ontology analysis showed that stress-, immune-, and defense-related genes were highly enriched in reSAFE1 (SI Appendix, Fig. S6A). Similarly, reSAFE1 highly overlaps not only with the sets induced by 1O2 in the flu (18) and ch1 mutants (23), but also with genes induced by pathogen attack in WT plants (Fig. 2D and SI Appendix, Fig. S6B). Moreover, reSAFE1 overlaps moderately with genes that are differentially expressed after β-CC treatment (29) or a low GSH state (30) and only to a small extent with genes differentially expressed after exposure to H2O2 or high L (31) (Fig. 2D). The RNA-seq data were confirmed by measuring the relative change in mRNA expression of representative genes (Fig. 2E). In ex1, safe1, and ex1 safe1, the expression of the four genes was not affected after a D–L shift because no 1O2 was generated under these conditions, but they were all highly induced by 1O2 in flu, flu safe1, and flu ex1 safe1 with the highest expression in flu safe1 (Fig. 2E).
Fig. 2.
1O2-induced changes in gene expression in flu ex1 safe1 are recovered to those seen in the flu mutant. (A) 1O2-induced changes in gene expression in flu, flu ex1, and flu ex1 safe1–5 plants. Before release of 1O2 (0-min L), no significant differences in gene expression were detected between flu ex1 safe1–5 and flu ex1 (last panel). Release of 1O2 was achieved by exposing D-incubated seedlings (4 h) to L for 30 or 60 min. (B) Venn diagrams showing the numbers of genes that were up-regulated greater than or equal to twofold in flu, flu ex1, and flu ex1 safe1–5 reexposed to L for 30 min or 60 min, respectively. (C) A set of 566 genes (indicated in bold and designated reSAFE1) is specifically regulated in flu ex1 safe1–5 (compared to flu ex1) after release of 1O2. (D) The reSAFE1 set displays large overlaps with genes regulated by 1O2 in flu (18) and ch1 (23) lines, moderate overlaps with genes induced by β-cyclocitral (β-CC) (29) or low glutathione (GSH) (30), and little overlap with genes induced by H2O2 (31) or high-L stress (31). (E) Quantitative RT-PCR expression analysis of selected genes. Transcript levels were normalized with respect to Actin2. Mean values ± SDs (n = 3) are provided.
GMs Are Specifically Damaged by 1O2 When SAFE1 Is Not Present.
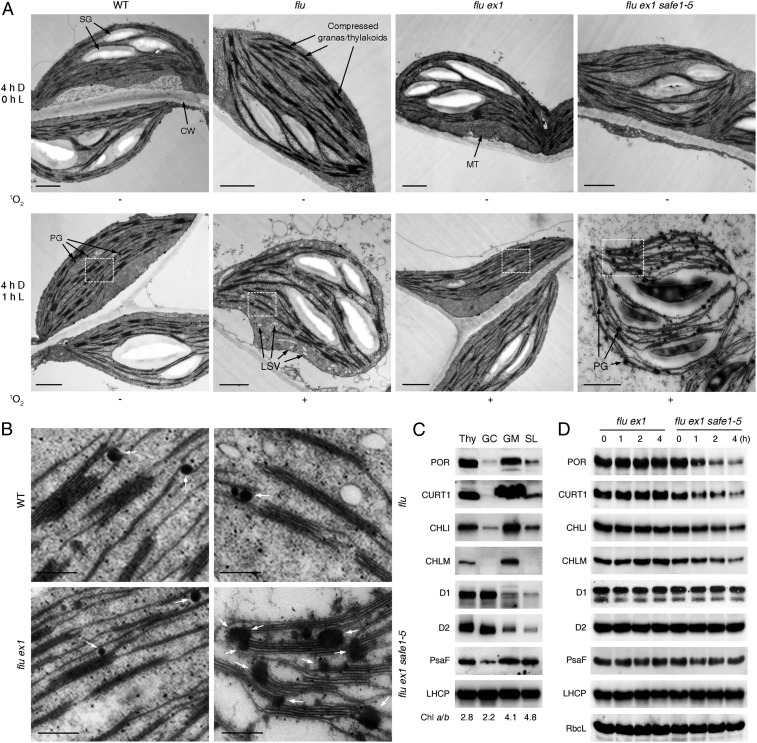
Previous studies showed that 1O2 was generated at chloroplast Thys in the flu ex1 mutant (17), but the consequence of this 1O2 generation on the structure of chloroplasts is still unclear. Thus, we analyzed the ultrastructure of chloroplasts from flu, flu ex1, and flu ex1 safe1-5 before and after 1O2 generation. Release of 1O2 in flu seedlings resulted in chloroplasts with shrunken shape and low-staining vesicles and induced degradation of chloroplasts. Whereas in flu ex1, these changes were all suppressed (Fig. 3A). The numbers and sizes of plastoglobules (PGs) in both flu and flu ex1 seedlings were not significantly affected by 1O2 (Fig. 3A). However, in flu ex1 safe1 seedlings, both numbers and sizes of PGs were dramatically increased after release of 1O2 (Fig. 3A). Enlargement of electron micrographs revealed that PGs were randomly distributed on the Thys of flu and flu ex1 seedlings (Fig. 3B). But in flu ex1 safe1 seedlings after release of 1O2, several PGs clustered and fused with each other and, together with Thy stacks, they formed dumbbell-shaped conglomerates on GMs (Fig. 3B and SI Appendix, Fig. S7). We suspected that GMs might be specifically damaged upon release of 1O2 when SAFE1 was not present in flu ex1. To test this, levels of representative proteins known to be enriched in the GC, GMs, or stroma lamellae (SL) (17) were quantified after release of 1O2. Levels of protochlorophyllide oxidoreductase (POR), curvature Thy1 (CURT1), Mg2+-chelatase subunit I (CHLI), and Mg2+-protoporphyrin IX methyl transferase (CHLM), which are representative proteins enriched in the GM (Fig. 3C), decreased drastically after release of 1O2 (Fig. 3D). In contrast, levels of PSII RC protein A (D1) and PSII RC protein D (D2)—normally enriched in the GC (Fig. 3C)—were apparently not significantly affected by 1O2 (Fig. 3D).
Fig. 3.
SAFE1 protects the GMs from 1O2-induced damages. (A) Representative electron micrographs of chloroplasts in 6-d-old WT, flu, flu ex1, and flu ex1 safe1–5 seedlings before and after release of 1O2. Release of 1O2 in flu, flu ex1, and flu ex1 safe1–5 seedlings was achieved by exposing D-incubated (4-h) seedlings to L for 1 h (4-h D, 1-h L). The status of 1O2 generation is shown below the corresponding electron micrographs. Note that chloroplasts from WT and flu ex1 were intact while those from flu and flu ex1 safe1–5 were damaged. Starch granule (SG); cell wall (CW); mitochondrion (MT); plastoglobule (PG); low staining vesicles (LSV). Bar = 1,000 nm. (B) Enlargement of the areas marked by white rectangles in A to show accumulation of PGs (indicated by white arrows) on the GMs of flu ex1 safe1–5. Bar = 250 nm. (C) Confirmation of the subcellular localization of the representative GC-, GM-, and SL-enriched proteins in purified Thy and Thy subfractions (GC, GM, and SL) by Western blot analysis. The chlorophyll a/b ratio in each fraction is indicated below the Western blot results. (D) In flu ex1 safe1, release of 1O2 induces degradation of GM-enriched (POR, CURT1, CHLI, and CHLM), but not of GC-enriched (D1 and D2) proteins, as determined by Western analysis (see Methods). Total proteins were extracted from 6-d-old flu ex1 and flu ex1 safe1–5 seedlings that were kept in the D for 4 h and exposed to L for 0–4 h as indicated.
These findings indicate that, in flu ex1 safe1, the GM is quickly and severely damaged upon release of 1O2, resulting in accumulation of PGs on damaged GMs. In flu ex1, presumably due to the protective effect of SAFE1, the GMs were not obviously affected by 1O2, PGs did not accumulate, and chloroplasts remained intact and functional, suggesting that the GMs were the first target of 1O2, and SAFE1 protects GMs from 1O2-induced damage.
1O2 Induces Degradation of the Stroma-Localized SAFE1 Protein.
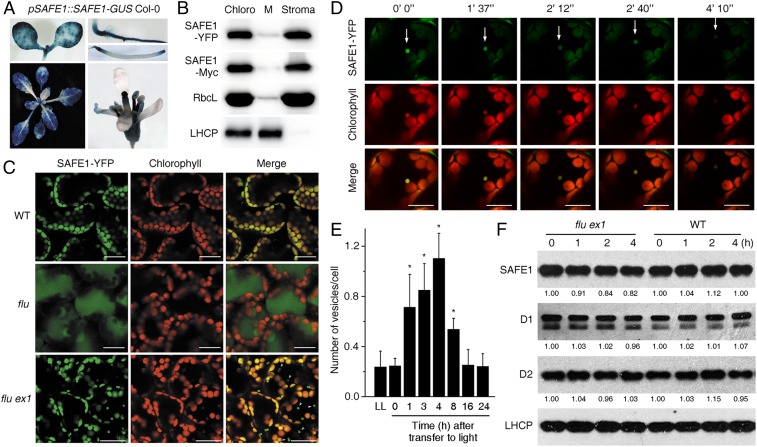
Since the localization of a protein is tightly linked with its function, we tested the localization of SAFE1 using stable pSAFE1::SAFE1-GUS, pSAFE1::SAFE1-YFP, and pSAFE1::SAFE1-Myc transgenic plants (all in the Columbia [Col-0] background). The results showed that the SAFE1-GUS fusion protein was expressed in all of the organs except the petal (Fig. 4A). Subcellular analysis revealed that SAFE1-YFP was localized in chloroplasts (Fig. 4C). Subsequently, chloroplasts from pSAFE1::SAFE1-YFP and pSAFE1::SAFE1-Myc transgenic plants were further fractionated into the membrane and stroma which were then analyzed by immunoblotting. The result showed that SAFE1 was present in the chloroplast stroma (Fig. 4B). In the WT (pSAFE1::SAFE1-YFP Col-0), SAFE1 was evenly distributed in the chloroplasts as no 1O2 was generated. A sudden release of 1O2 in the flu mutant (pSAFE1::SAFE1-YFP flu) led to chloroplast rupture. However, in the flu ex1 double mutant (pSAFE1::SAFE1-YFP flu ex1), its release did not alter the integrity of the chloroplast but induced formation of chloroplast-originated SAFE1-containing vesicles (Fig. 4 C and D). In addition to SAFE1, these vesicles also contained Rubisco (SI Appendix, Fig. S8) and Thy membranes—judged from the red chlorophyll autofluorescence (Fig. 4 C and D). The formation of SAFE1-containing vesicles occurred within 1 h, increased slightly until 4 h after 1O2 generation, and declined significantly when seedlings were exposed to L for 8 h and reached the basal level after 16 h in the L (Fig. 4E). However, only a small fraction of SAFE1 was degraded in flu ex1 after the release of 1O2 because the majority of the SAFE1-YFP protein was still retained in chloroplasts (Fig. 4 C and D), and the overall SAFE1 protein content in plants was not dramatically affected by 1O2 as evidenced by immunoblotting of SAFE1-YFP (Fig. 4F).
Fig. 4.
1O2 induces degradation of the chloroplast stroma-localized SAFE1 protein. (A) Expression analysis of SAFE1-GUS at different developmental stages. (B) Suborganellar localization of tagged SAFE1 proteins using Western analysis. Marker proteins were RbcL (stroma) and light-harvesting chlorophyll a/b binding protein (LHCP) (chloroplast membrane = M). Total chloroplasts (Chloro). (C) The fate of SAFE1 after release of 1O2. In the WT (pSAFE1::SAFE1-YFP Col-0) SAFE1-YFP was evenly distributed in the chloroplasts as no 1O2 was generated. Release of 1O2 caused chloroplast rupture in the flu (pSAFE1::SAFE1-YFP flu) mutant but induced the formation of SAFE1-containing vesicles originating from chloroplasts in flu ex1 (pSAFE1::SAFE1-YFP flu ex1). Generation of 1O2 was achieved by first keeping 6-d-old seedlings in the D for 4 h and then in the L for an additional 4 h. Bar = 20 μm. (D) The degradation process of a representative SAFE1-containing vesicle (indicated by white arrows) in pSAFE1::SAFE1-YFP flu ex1 seedlings. A series of confocal images was taken from 6-d-old seedlings that were first kept in the D for 4 h and then exposed to L for 4 h. Imaging times are indicated on top of the corresponding images. Bar = 20 μm. (E) Statistics of 1O2-induced vesicles in pSAFE1::SAFE1-YFP flu ex1. Vesicles were counted from 10 confocal images representing ∼200 cells at each time point. Asterisks indicate significant differences (P < 0.05, t test) compared to the seedlings grown under continuous light (LL). Generation of 1O2 was achieved by keeping 6-d-old seedlings in the D for 4 h and then in the L for 0–24 h as indicated. (F) The overall SAFE1 content was not dramatically reduced by 1O2. Total proteins were extracted from 6-d-old seedlings that had been incubated in the D for 4 h and reexposed to L for 0–4 h as indicated, and relative amounts of SAFE1, D1, and D2 were measured. The LHCP protein was used as the loading control.
SAFE1 Is Not Involved in Methylation of Rubisco.
SAFE1 is annotated as a Rubisco methyltransferase family protein (https://www.arabidopsis.org/). Therefore, the methylation status of Rubisco complexes from flu ex1 and flu ex1 safe1–5 were determined by mass spectrometry. Three methylation sites in two peptides were found in the RbcL subunit, but their methylation status was essentially unaffected (SI Appendix, Fig. S9). This result is compatible with previous studies in which the purified SAFE1 protein (named AtPPKMT1) showed no significant binding to Rubisco and was unable to methylate Rubisco or chloroplastic aldolases in vitro (32, 33). These findings suggest that SAFE1 is not involved in the methylation of Rubisco.
SAFE1 Is a Suppressor of the 1O2-Induced EX1-Independent Pathway.
When screening for suppressors of the flu ex1 mutant, we envisaged three possible scenarios: 1) identification of a negative regulator acting in the 1O2-induced EX1-dependent signaling pathway, 2) identification of a negative regulator that suppresses an unknown downstream component (designated as X) of EX1-dependent 1O2-induced signaling, or 3) identification of a negative component that suppresses an EX1-independent pathway (Fig. 5A). In scenario 1, the mutation of SAFE1 would abrogate its inhibitory effect and lead to constant activation of downstream signaling, causing constitutive cell death in plants lacking a functional SAFE1 protein. In scenario 2, lack of the functional SAFE1 protein would also cause constitutive cell death independent of the flu mutation. In scenario 3, the negative regulator SAFE1 would “bypass” EX1-dependent signaling, and SAFE1 action would then be dependent on the release of 1O2 (the flu mutation). The safe1 single mutant would not display a cell-death phenotype (because no 1O2 would be generated in this genetic background), and the flu safe1 double mutant would show a stronger phenotype than flu and flu ex1 safe1 because two 1O2-induced pathways would be activated in flu safe1 simultaneously.
Fig. 5.
SAFE1 is a suppressor of 1O2-induced, EX1-independent signaling. (A) Possible modes of SAFE1 function. SAFE1 might operate as a negative regulator downstream of EX1 (I), negatively regulate an unknown downstream component (II), or act in an EX1-independent pathway (III). In the latter case, flu safe1 plants should display a very strong 1O2-induced stress response, whereas safe1 plants should behave like WT. (B–D) 1O2-induced stress responses are enhanced in flu safe1 and absent in safe1 as indicated by levels of cotyledon bleaching (B), chlorophyll autofluorescence (C), and Fv/Fm values (D). Different levels of 1O2 generation were achieved by incubating seedlings in the D as indicated. Mean values ± SDs (n > 30) are provided. (E) Direct visualization of Pchlide accumulation in 4-d-old etiolated seedlings. While safe1 plants did not accumulate Pchlide, flu safe1 and flu ex1 safe1 accumulated similar amounts of Pchlide. (F) Detection of 1O2 generation by using SOSG. (G) Cell-death response after moderate 1O2 generation. Note that flu shows a weak response, while flu ex1 safe1 and flu safe1 exhibit stronger and strongest effects, respectively. LDD, light/dark/dim-light. (H) 1O2-induced cell death responses in mature leaves of safe1, flu safe1, and flu ex1 safe1. (I) A heatmap illustrates and summarizes Pchlide accumulation, 1O2 generation, and corresponding 1O2-induced stress strengths based on the decrease in Fv/Fm in all mutants examined in this study.
To ascertain which (if any) of these models applied, phenotypes of the safe1 and flu safe1 mutants were studied in comparison with the known phenotypes of flu, flu ex1, and flu ex1 safe1 mutants (Fig. 1). The safe1 single-mutant seedlings exhibited no 1O2-induced stress responses upon a D–L shift (Fig. 5 B–D), nor did the safe1 mutant accumulate Pchlide in the D (Fig. 5E), and no 1O2 was generated after a D–L shift (Fig. 5F). The flu safe1 and flu ex1 safe1 seedlings accumulated similar amounts of Pchlide in the D (Fig. 5E) and generated similar amounts of 1O2 after transfer to L (Fig. 5F). However, compared with flu ex1 safe1, the flu safe1 double mutant showed much more prominent 1O2-induced stress responses, including bleaching of cotyledons (Fig. 5B), decrease in transient chlorophyll fluorescence (Fig. 5C), and reduced maximum quantum efficiency of PSII (Fv/Fm) (Fig. 5D) after a D–L shift. To confirm these differences in the magnitude of 1O2-induced stress responses in mature plants, all three mutants were exposed to a L/D/dim-L regime (Fig. 5G) in which less 1O2 was produced. This is because the rate of 1O2 generation is dependent on both the amount of Pchlide accumulated in the D and the L intensity during reillumination (26). With reduced 1O2 levels, the mature flu mutant showed weak, the flu ex1 safe1 mutant showed stronger, and the flu safe1 mutant showed the strongest cell-death responses (Fig. 5 G and H). The levels of 1O2-induced stress responses based on the decrease in Fv/Fm values, Pchlide accumulation, and 1O2 generation in all tested mutant lines are summarized in Fig. 5I, and they agree with predictions based on scenario 3 as shown in Fig. 5A. Therefore, we postulate that SAFE1 acts as a negative regulator in a 1O2-induced EX1-independent pathway. However, it is still unclear whether the 1O2-EX1 and 1O2-SAFE1 pathways converge on same downstream component(s).
Discussion
Here, we have identified a 1O2-induced and EX1-independent retrograde signaling pathway that is suppressed by SAFE1. In flu ex1 safe1 plants, 1O2-induced responses return to the same or higher levels than those seen in the flu mutant (Fig. 1 and SI Appendix, Figs. S1 and S3), while under LL without 1O2 generation, flu ex1 safe1 plants behave like WT (Fig. 1 and SI Appendix, Figs. S1 and S4). However, a slight increase in 1O2 content suffices to initiate a cell-death response in flu ex1 safe1 (Fig. 1 C–G), although not in flu. Given that similar amounts of 1O2 are generated upon reillumination of D-adapted flu and flu ex1 safe1 seedlings (Fig. 1 H–J) and similar sets of genes are induced by it (Fig. 2), the 1O2 released in flu ex1 safe1 is apparently acting primarily as a signal rather than as a cytotoxin. Moreover, SAFE1 is not a quencher of 1O2 because levels of 1O2 in flu, flu ex1, flu safe1, and flu ex1 safe1 are comparable to each other (Figs. 1J and 5F).
Previous studies have shown that chloroplasts are the source and primary target of 1O2-mediated cell-death responses (16) and play an important role in initiating disease and defense signals (34). Our present paper indicates that, in a chloroplast, the GM is the first target of 1O2 and a damaged GM initiates a stress signaling (Fig. 3). Enzymes of tetrapyrrole biosynthesis are highly enriched in the GM (Fig. 3C) (17), indicating that Pchlide is first synthesized in the GM. However, when the flu or flu ex1 plants are incubated in the D for a longer time (8 h), Pchlide accumulates significantly not only in the GM, but also in the GC and slightly in the SL and generates 1O2 there after reillumination (17). Compared with the GC, the GM is prone to be damaged by 1O2. In flu ex1 safe1, the 1O2-induced damage on the GM is evidenced by the drastic decrease in GM proteins, especially the POR proteins (Fig. 3D). In plants, the POR proteins are responsible for the photoreduction of Pchlide to chlorophyllide (Chlide) under L (35) and, thus, might be very close to the site of 1O2 generation when the D-incubated flu ex1 safe1 plants are transferred to L. This might be why the POR proteins are those that are most severely damaged when 1O2 is released in flu ex1 safe1 (Fig. 3D). The specific and severe degradation of the GM proteins provides direct evidence that the GM is the first target of 1O2. PGs are Thy-associated droplets that function in metabolite biosynthesis, repair, and disposal, and their numbers and sizes increase upon oxidative stress and during senescence (36). Under normal or high-L stress conditions, the PGs randomly associate with Thys (37). However, in flu ex1 safe1, enlarged and clustered PGs accumulate on the GM after release of 1O2, providing additional evidence that the GM is the primary target of flu-generated 1O2 (Fig. 3) and that SAFE1 protects the GM from 1O2-induced damage. Compared to the GM, the highly compressed GC is physically more robust as evidenced by its resistance to a mild detergent treatment while the same treatment breaks down the GM from Thys (38). The relative robustness might explain why the GC is not apparently damaged by flu-generated 1O2. GMs are curved areas of Thys, and the spaces between lipid molecules are bigger than that in the GC. This curved structure might make the GM vulnerable to 1O2 and, in turn, highlights the need of a “safeguard.”
In plants, three mechanisms have been distinguished previously to cope with 1O2 stress in the chloroplast: rapid turnover of PSII RC proteins (39, 40), chloroplast rupture (16, 17), and selected degradation of entire chloroplasts (11, 41). Intriguingly, the scenario that occurs in flu ex1 seedlings after 1O2 stress is different from the three mechanisms and might represent a new strategy. In flu ex1, the content of PSII RC proteins D1 and D2 is not significantly affected (Figs. 3D and 4F), and the chloroplasts are still intact after release of 1O2 (Figs. 3A and 4C) (17). After release of 1O2, a small fraction of SAFE1 is enriched in distinctive loci of the chloroplast and degraded via formation of chloroplast-originated SAFE1-containing vesicles in flu ex1 plants (Fig. 4 C and D). For plants, this might be the most economical way to deal with 1O2 stress. In this way, only a small fraction of chloroplast proteins are expelled and degraded, and the whole chloroplast is still intact and functional. The unapparent degradation of the SAFE1 protein might be explained by the discordance between the localization of SAFE1 and the site of 1O2 production. Since 1O2 is mainly produced at Thys (17) and SAFE1 is localized in the stroma, only a small part of the SAFE1 protein is in direct contact with and can be damaged by 1O2. The 1O2-induced SAFE1-containing vesicles resemble the already described stress-induced chloroplast vesiculation-containing vesicles in their size, content, and formation/degradation process (Fig. 4 C and D and SI Appendix, Fig. S8) (42). However, more experiments are needed to fully understand this strategy that is employed by plants to cope with 1O2 stress.
Several 1O2-induced chloroplast signaling pathways have been reported (10–12, 29) (Fig. 6). In the GC region, 1O2 is mainly produced in the PSII RC under stress conditions (43) where it induces two signaling relays: the 1) β-CC-mediated (29) and 2) OXI1 kinase-mediated (12) pathways. In the GM region, 1O2 is generally produced from tetrapyrrole biosynthesis intermediates, and there, it triggers two other pathways: 1) EX1/EX2-dependent programmed cell death (10, 28) and 2) selective degradation of the entire chloroplast activated by the E3 ubiquitin ligase plant U-box 4 (PUB4) (11). Our results now allow us to define a third GM-associated and 1O2-induced pathway, the 1O2-SAFE1 pathway, which does not require EX1 and is negatively regulated by SAFE1. Activation of this pathway results in cell death of young seedlings and growth inhibition of mature plants (Fig. 1 and SI Appendix, Fig. S1), and SAFE1 suppresses this pathway by inhibiting 1O2-induced damages on GMs (Fig. 3 and SI Appendix, Fig. S7). While SAFE1 can suppress 1O2-induced signaling originating from the GM, it apparently has limited effects on GC-associated 1O2-induced signaling because 1) under high-L stress, safe1, flu safe1, and flu ex1 safe1 seedlings behave like WT (SI Appendix, Fig. S5), 2) the reSAFE1 gene set shows little overlap with high-L-induced genes (Fig. 2D), and 3) the soluble SAFE1 protein is localized in the stroma (Fig. 4B) and might not have easy access to the GC. A model summarizing the current understanding of 1O2-induced signaling pathways in chloroplasts is shown in Fig. 6.
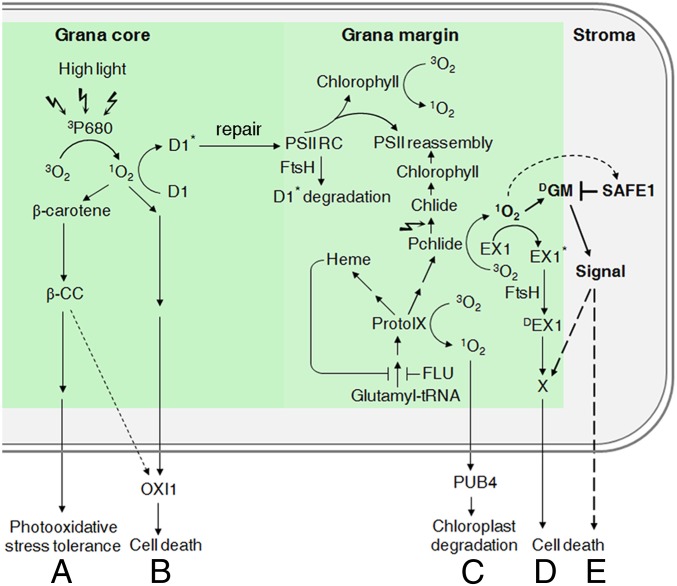
Fig. 6.
A model summarizing 1O2-induced signaling pathways in the chloroplast. (A) Under high L, 1O2 is mainly produced in the GC (PSII RC) by transfer of energy from excited triplet state P680 chlorophyll (3P680) to ground state oxygen (3O2) (43). The 1O2 produced in the PSII RC leads to rapid turnover of the D1 protein (43) and oxidizes β-carotene (29). The β-carotene oxidation product β-CC can induce a signaling cascade that confers plant tolerance to photo-oxidative stress (29). (B) 1O2 produced in the PSII RC can also lead to programmed cell death (PCD) via a pathway that involves OXI1 kinase and jasmonic acid (JA) (12). (C) In the GMs, 1O2 is mainly produced from intermediates of tetrapyrrole biosynthesis (Pchlide, ProtoIX) (11, 17) and in lesser amounts from free chlorophyll resulting from PSII RC turnover (59). 1O2 generated from ProtoIX damages the chloroplasts, which are subsequently ubiquitinated and degraded. This process involves the E3 ubiquitin ligase PUB4 and is independent of the EX1 protein (11). (D) However, when 1O2 is produced from Pchlide, EX1 is necessary for initiating another signaling transduction cascade (10). 1O2 generated from Pchlide oxidizes proteins nearby, including EX1. The oxidized EX1 proteins are then cleaved by the FtsH protease, and the proteolysis products of the EX1 protein (DEX1) probably serve as a signal to induce PCD in young seedlings and growth inhibition in mature plants (17, 19). (E) The 1O2 generated from Pchlide can induce an EX1-independent signaling pathway that is suppressed by the SAFE1 protein. The stroma-localized SAFE1 protein is a negative regulator and functions as a “protector” of GMs. Without this protector, 1O2 generated from Pchlide would damage GMs, and the damaged GMs (DGM) would initiate EX1-independent signaling and would lead to cell death in young seedlings and growth inhibition of mature plants. The signaling cascades suggested by recent studies are indicated by solid arrows, while the predicted cascades are shown by dashed arrows.
Methods
Plant Materials and Growth Conditions.
All mutants used in this study are in the Col-0 background unless otherwise stated. Seeds were surface sterilized with 0.6% (vol/vol) sodium hypochlorite solution containing 0.01% (vol/vol) Triton X-100 for 10 min and then washed four times with double distilled water. After stratification at 4 °C for 2 d, seeds of WT, flu, flu ex1, flu ex1 safe1, safe1, and complemented lines were grown either on soil or on 1/2 MS medium (with 0.5% [m/v] sucrose and 0.8% [m/v] plant agar) for 6 d under LL (100 μmol of photons m−2 s−1) or under long-day conditions (LD; 16-h L/8-h D) at 22 °C. These seedlings were further grown under LL or incubated in the D for 0–8 h and transferred to L for various lengths of time (as indicated in the figures) to generate 1O2.
Mutagenesis of Arabidopsis flu ex1 and Screening of Suppressor Mutants.
Mutagenesis of the A. thaliana flu ex1 mutant was performed using EMS according to Kim et al. (44) Approximately 100,000 M2 seeds from 5,000 M1 plants were sown on soil at a density of 10,000 seeds m−2. After stratification at 4 °C for 2 d, seeds were grown under LL for 14 d and then transferred to LD for 4 d. Plants showing cell-death responses were recognized as candidate suppressor mutants and transferred to LL and grown to maturity.
Identification of the SAFE1 Gene.
To identify the mutated SAFE1 gene, the flu ex1 safe1 (Col-0) mutant was crossed with flu ex1 (Ler). F2 plants were first grown under LL for 14 d and then under LD for 4 d. Approximately 200 from the 800 plants showing the typical cell-death responses were grown under LL for another 10 d. Leaves from these 200 plants were pooled, ground in liquid nitrogen, and suspended in 100-mL nuclear lysis buffer (0.4-M sucrose, 10-mM Tris⋅HCl [pH 7.0], 1% [vol/vol] β-mercaptoethanol, 1% [vol/vol] Triton X-100) and kept on ice for 15 min. Then, the suspension was filtered through two layers of Miracloth (Millipore, 475855–1R) into two 50-mL tubes and centrifuged at 3,000 × g for 15 min at 4 °C. The resulting pellet was resuspended in 1-mL nuclear lysis buffer, transferred to a 1.5-mL microfuge tube, and centrifuged at 3,000 × g for 15 min at 4 °C. The supernatant was discarded, and genomic DNA was extracted from the pellet using the Qiagen DNeasy Plant Mini Kit (Qiagen, 69104). About 2 μg of genomic DNA was subjected to next-generation sequencing. DNA-seq libraries were prepared, and 75-bp paired-end sequencing was conducted on an Illumina NextSeq500 instrument in the Biotechnology Resource Center at Cornell University.
To find the mutation(s) underlying the cell-death response, the sequencing data were processed with the next-generation EMS mutation mapping tool (45). Briefly, the sequencing adapters and low-quality bases were removed from raw reads using Trimmomatic v0.32 (46). The remaining cleaned reads were mapped to the Arabidopsis genome TAIR10 (https://www.arabidopsis.org/) using BWA v0.7 (47). The mapped alignment file was sorted and converted to BAM format using SAMtools (48). Next, the duplicated mapped reads were marked using Picard Tools v1.141 (http://broadinstitute.github.io/picard). Then, the single nucleotide polymorphisms were called using SAMtools (48), and the generated Variant Call Format file was converted to the “emap” format file using the BCF2NGM.pl script downloaded from the NGM website. Finally, the preprocessed emap format file was uploaded to NGM (http://bar.utoronto.ca/ngm/cgi-bin/emap.cgi) to identify the mutations associated with the phenotypes.
For the traditional map-based cloning, another 200 plants exhibiting the mutant phenotype were screened, and genomic DNAs were extracted individually. The genotypes of these plants were tested using simple sequence length polymorphism markers found on the Arabidopsis mapping platform (https://www.arabidopsis.org/portals/mutants/mapping.jsp).
Complementation of the flu ex1 safe1 Mutant.
For complementation of flu ex1 safe1, the genomic region encompassing SAFE1 (At5g14260) was amplified by PCR using the sense primer GCCTCTAAACATTTACCATAGTTTCTG and the antisense primer TTTCAAGGAAGGAGCATATGGTGC. The PCR product was inserted into the entry vector pCR8/GW/TOPO (Invitrogen) and, subsequently, cloned to the destination vectors pGWB516 (which adds a C-terminal 4×Myc tag), pGWB533 (which adds a C-terminal GUS tag), and pGWB540 (which adds a C-terminal EYFP tag) (49) via the Gateway LR cloning reaction (Invitrogen). All these constructs were transformed into Agrobacterium tumefaciens strain GV3101 and transferred to flu ex1 safe1 plants by floral dipping (50).
Isolation and Fractionation of Chloroplasts.
Chloroplasts were isolated and fractionated into membrane and stroma fractions as described by Kauss et al. (15) Fractionation of the Thy membrane into the GC, GM, and SL was performed as described by Wang et al. (17)
Measurement of Fv/Fm, PSII Operating Efficiency, Nonphotochemical Quenching, and Pulse-Amplitude Modulated Traces.
The maximum quantum efficiency of PSII (Fv/Fm), PSII operating efficiency (ΦPSII), nonphotochemical quenching, and pulse-amplitude modulated (PAM) traces (51) were recorded using an automatic PAM fluorometer (Imaging PAM, Walz) following the manual provided by the manufacturer.
Trypan Blue Staining of Dead Cells.
Trypan blue staining of dead cells was performed as described by op den Camp et al. (9).
Extraction and Determination of Pchlide and Chlide.
Pchlide and Chlide were extracted and measured as described by Yoshida et al. (52). Tetrapyrroles were extracted from D-treated WT, flu, flu ex1, and flu ex1 safe1 seedlings or mature leaves with a buffer containing 80% (vol/vol) acetone and 0.0083% (vol/vol) ammonia overnight at 4 °C in the D. An equal volume of 80% acetone-saturated hexane was added, and the solution was mixed well by vortexing. After centrifugation at 5,000 × g for 10 min at 4 °C, the acetone phase (lower layer) was transferred to a new tube and washed again with an equal volume of 80% (vol/vol) acetone-saturated hexane. The resulting acetone solution, thus obtained, contained no detectable chlorophyll. Fluorescence emission spectra (600–720 nm) excited at a wavelength of 433 nm were recorded at room temperature (RT) using a LS50 luminescence spectrophotometer (Perkin-Elmer). Acetone (80%) was used as a reference. Pchlide has its absorption peak at 634 nm and Chlide at 673 nm.
Direct Visualization of Pchlide Accumulation in Etiolated Seedlings.
To obtain etiolated seedlings, surface-sterilized seeds of WT, flu, flu ex1, flu ex1 safe1, safe1, and/or flu safe1 were sown on 1/2 Murashige and Skoog medium and grown in the D at 22 °C for 4 d. For direct visualization of Pchlide accumulation, etiolated seedlings were illuminated with blue L and examined using a fluorescence microscope (Olympus SZX-12). The bright red fluorescence emitted by the mutants lacking a functional FLU protein is caused by excitation of Pchlide.
GUS Staining.
GUS staining was performed according to Wang et al. (53).
Confocal Imaging.
Confocal imaging was performed with a Leica TCS SP5 laser scanning confocal microscope. EYFP was excited with the 514-nm line of an argon laser, and the emission was recorded by passing through the filter bandpass (BP) 535/30 and false-colored green. GFP was excited with the 488-nm line of an argon laser, and the emission from 510 to 544 nm was recorded with the BP 525/50 filter and colored green. Chlorophyll autofluorescence was excited with the 458-nm line of the argon laser, and the emission was recorded in the range of 650–700 nm.
Measurement of the Production of 1O2.
1O2 was quantified using SOSG (Invitrogen, S36002) as described by Flors et al. (54). WT, flu, flu ex1, and flu ex1 safe1 seeds were grown on soil under LL for 6 d and incubated in the D for 4 h. Hypocotyls were immersed in a solution containing 260-μM SOSG and 50-mM sodium phosphate buffer (pH 7.5). The shoots were allowed to transpire for another 3 h in the D and transferred to L for 40 min. The 1O2-activated SOSG signal was recorded using a Leica TCS SP5 laser scanning confocal microscope with excitation at 488 nm, and the emission from 510 to 600 nm was collected.
RNA-seq, Data Analysis, and Quantitative RT-PCR.
Total RNAs from Arabidopsis seedlings were isolated using TRIzol (Invitrogen) and purified using Direct-zol RNA MiniPrep Plus columns (Zymo Research) according to the manufacturer’s instructions. RNA integrity and quality were assessed with an Agilent 2100 Bioanalyzer. Ribosomal RNA depletion, generation of RNA-seq libraries, and 150-bp paired-end sequencing on an Illumina HiSeq 2500 system were conducted at Novogene Biotech (Beijing, China) with standard Illumina protocols. Two independent biological replicates were used per genotype.
RNA-seq reads were analyzed on the Galaxy platform (https://usegalaxy.org/). After grooming FASTQ files, adaptors were removed with Trimmomatic (46), and sequencing quality was accessed with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were mapped to the Arabidopsis genome (TAIR10) with the gapped-read mapper TopHat 2.1.1 (55) set for Forward Read unstranded libraries and adjusting the maximum intron length to 5,000 bp. Reads were counted with featureCounts (56) with the help of the gene annotation in Araport11 (https://www.arabidopsis.org/download/index-auto.jsp?dir=%2Fdownload_files%2FGenes%2FAraport11_genome_release). Differentially expressed genes were obtained with DESeq2 (57) applying a twofold change cutoff and an adjusted P < 0.05. Sequencing data have been deposited in NCBI's Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (58) (accession no. GSE131610).
For quantitative RT-PCR, cDNA was synthesized from 1 μg of total RNA using the iScript cDNA synthesis kit (Bio-RAD, Cat. no. 1708890) following the instructions provided with the kit. Quantitative RT-PCR was performed with an iQ5 multicolor real-time detection system (Bio-RAD). Expression of the detected genes was normalized to Actin2, and primers used in this study are listed in Dataset S7.
Protein Extraction and Western Blot Analysis.
Shoots (about 100 mg) of 6-d-old seedlings were frozen in liquid nitrogen and ground to a fine power. Proteins were extracted by adding 1 mL of protein extraction buffer (20-mM Hepes [pH 7.4], 2-mM [ethylenedinitrilo]tetraacetic acid [pH 7.4], 2-mM ethylene glycol bis[β-aminoethyl ether]-N,N,N′,N′-tetraacetic acid [pH 7.4], 25-mM NaF, 1-mM Na3VO4, 50-mM glycerophosphate, 100-mM NaCl, 0.5% [vol/vol] Triton X-100, 10% [vol/vol] glycerol, 1× SIGMAFAST Protease Inhibitor) and incubated on ice for 30 min. After centrifugation at 12,000 × g for 30 min at 4 °C, the clear supernatant was transferred to 15-mL Falcon conical centrifuge tubes. After addition of 9 mL of 100% acetone, 50 µL of 0.5-M Na2CO3, and 50 µL of 0.5-M DTT, the supernatant was mixed well and incubated at −20 °C for 30 min. Proteins were pelleted by centrifugation at 3,000 × g for 3 min. The pellet was dried for 10 min at RT and dissolved in 200 µL of 1× Laemmli buffer by heating at 75 °C for 20 min with agitation (500 rpm).
Proteins were normalized to the content of chlorophyll and then fractionated on 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, depending on the calculated molecular weight of the target protein and were then transferred to a polyvinylidene difluoride membrane (Millipore, IPVH00010). The membrane was blocked for 1 h with 5% skim milk in 1× TBS (50-mM Tris⋅HCl [pH 7.5] and 150- mM NaCl) and incubated overnight at 4 °C using antiserum against GFP (Sigma-Aldrich, G1546), Myc (Santa Cruz Biotechnology, sc-40), RbcL (Agrisera, AS03 037), LHCP (AS01 004), D1 (AS11 1786), D2 (AS06 146), PsaF (PSI subunit F; AS06 104), POR (AS05 067), CURT1A, CHLI, or CHLM. The antibody solution was decanted, and the blot was washed for 4 × 10 min with 1× TBST (50-mM Tris⋅HCl [pH 7.5], 150-mM NaCl, 0.1% Tween-20) at RT. Then the blot was incubated with either anti-rabbit IgG-HRP (Santa Cruz Biotechnology, sc-2004) or anti-mouse IgG-HRP (Santa Cruz Biotechnology, sc-2005) at RT for 1 h with slow agitation. The blot was washed for 4 × 10 min with 1× TBST and developed with an enhanced chemiluminescence substrate (Thermo Scientific, 32106). Fluorescence was recorded using a CCD camera (Peqlab, Fusion Fx7).
Supplementary Material
Acknowledgments
We thank T. Nakagawa for providing pGWB516, pGWB533, and pGWB540 vectors; B. Grimm and B. Hedtke for antisera (CHLI and CHLM); V. Dogra and C. Kim for RNA-seq data of flu; M. Srivastava, S. Schwenkert, and H. Harz for helping with confocal imaging; Z. Fei and A. Klingl for help with DNA-seq data analysis and transmission electron spectroscopy, respectively; and C. Kim and X. Xu for help in the initial phase of this work. This work was supported by National Institutes of Health Grant R01-GM085036 (to K.A.), and Grants from the Deutsche Forschungsgemeinschaft to D.L. and T.K. (KL 2362/1-1 and TRR175, Projects C01 and C05).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. C.F. is a guest editor invited by the Editorial Board.
Data deposition: Sequencing data have been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE131610).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918640117/-/DCSupplemental.
References
- 1.Apel K., Hirt H., Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Foyer C. H., Noctor G., Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 11, 861–905 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Noctor G., Reichheld J. P., Foyer C. H., ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 80, 3–12 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Mittler R., ROS are good. Trends Plant Sci. 22, 11–19 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Xia X. J., et al. , Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 66, 2839–2856 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Schmidt R., Schippers J. H. M., ROS-mediated redox signaling during cell differentiation in plants. Biochim. Biophys. Acta 1850, 1497–1508 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Gilroy S., et al. , ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 171, 1606–1615 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triantaphylidès C., et al. , Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 148, 960–968 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.op den Camp R. G. L., Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15, 2320–2332 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner D., et al. , The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306, 1183–1185 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Woodson J. D., et al. , Ubiquitin facilitates a quality-control pathway that removes damaged chloroplasts. Science 350, 450–454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shumbe L., et al. , Singlet oxygen-induced cell death in Arabidopsis under high-light stress is controlled by OXI1 Kinase. Plant Physiol. 170, 1757–1771 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leisinger U., et al. , The glutathione peroxidase homologous gene from Chlamydomonas reinhardtii is transcriptionally up-regulated by singlet oxygen. Plant Mol. Biol. 46, 395–408 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Meskauskiene R., et al. , FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 98, 12826–12831 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauss D., Bischof S., Steiner S., Apel K., Meskauskiene R., FLU, a negative feedback regulator of tetrapyrrole biosynthesis, is physically linked to the final steps of the Mg(++)-branch of this pathway. FEBS Lett. 586, 211–216 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Kim C., et al. , Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 24, 3026–3039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L., et al. , Singlet oxygen- and EXECUTER1-mediated signaling is initiated in grana margins and depends on the protease FtsH2. Proc. Natl. Acad. Sci. U.S.A. 113, E3792–E3800 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dogra V., et al. , FtsH2-dependent proteolysis of EXECUTER1 is essential in mediating singlet oxygen-triggered retrograde signaling in Arabidopsis thaliana. Front. Plant Sci. 8, 1145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dogra V., Li M., Singh S., Li M., Kim C., Oxidative post-translational modification of EXECUTER1 is required for singlet oxygen sensing in plastids. Nat. Commun. 10, 2834 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv R., et al. , Uncoupled expression of nuclear and plastid photosynthesis-associated genes contributes to cell death in a lesion mimic mutant. Plant Cell 31, 210–230 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharfenberg M., et al. , Functional characterization of the two ferrochelatases in Arabidopsis thaliana. Plant Cell Environ. 38, 280–298 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Ling Q., Jarvis P., Plant signaling: Ubiquitin pulls the trigger on chloroplast degradation. Curr. Biol. 26, R38–R40 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Ramel F., et al. , Light-induced acclimation of the Arabidopsis chlorina1 mutant to singlet oxygen. Plant Cell 25, 1445–1462 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruzinská A., et al. , In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. Plant Cell 19, 369–387 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mach J. M., Castillo A. R., Hoogstraten R., Greenberg J. T., The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc. Natl. Acad. Sci. U.S.A. 98, 771–776 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L., Apel K., Dose-dependent effects of 1O2 in chloroplasts are determined by its timing and localization of production. J. Exp. Bot. 70, 29–40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Przybyla D., et al. , Enzymatic, but not non-enzymatic, 1O2-mediated peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1-dependent stress response program in the flu mutant of Arabidopsis thaliana. Plant J. 54, 236–248 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Lee K. P., Kim C., Landgraf F., Apel K., EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 104, 10270–10275 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramel F., et al. , Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. U.S.A. 109, 5535–5540 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnaubelt D., et al. , Low glutathione regulates gene expression and the redox potentials of the nucleus and cytosol in Arabidopsis thaliana. Plant Cell Environ. 38, 266–279 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Cheng H., Zhang Q., Guo D., Genes that respond to H2O2 are also evoked under light in Arabidopsis. Mol. Plant 6, 226–228 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Mininno M., et al. , Characterization of chloroplastic fructose 1,6-bisphosphate aldolases as lysine-methylated proteins in plants. J. Biol. Chem. 287, 21034–21044 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alban C., et al. , Uncovering the protein lysine and arginine methylation network in Arabidopsis chloroplasts. PLoS One 9, e95512 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Torres Zabala M., et al. , Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat. Plants 1, 15074 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Beale S. I., Enzymes of chlorophyll biosynthesis. Photosynth. Res. 60, 43–73 (1999). [Google Scholar]
- 36.Austin J. R. 2nd, Frost E., Vidi P. A., Kessler F., Staehelin L. A., Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 18, 1693–1703 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bréhélin C., Kessler F., van Wijk K. J., Plastoglobules: Versatile lipoprotein particles in plastids. Trends Plant Sci. 12, 260–266 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Puthiyaveetil S., et al. , Compartmentalization of the protein repair machinery in photosynthetic membranes. Proc. Natl. Acad. Sci. U.S.A. 111, 15839–15844 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aro E. M., Virgin I., Andersson B., Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143, 113–134 (1993). [DOI] [PubMed] [Google Scholar]
- 40.Ohira S., Morita N., Suh H. J., Jung J., Yamamoto Y., Quality control of photosystem II under light stress–turnover of aggregates of the D1 protein in vivo. Photosynth. Res. 84, 29–33 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Izumi M., Ishida H., Nakamura S., Hidema J., Entire photodamaged chloroplasts are transported to the central vacuole by autophagy. Plant Cell 29, 377–394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S., Blumwald E., Stress-induced chloroplast degradation in Arabidopsis is regulated via a process independent of autophagy and senescence-associated vacuoles. Plant Cell 26, 4875–4888 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieger-Liszkay A., Fufezan C., Trebst A., Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 98, 551–564 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Kim Y., Schumaker K. S., Zhu J. K., EMS mutagenesis of Arabidopsis. Methods Mol. Biol. 323, 101–103 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Austin R. S., et al. , Next-generation mapping of Arabidopsis genes. Plant J. 67, 715–725 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Bolger A. M., Lohse M., Usadel B., Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H., Durbin R., Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H. et al.; 1000 Genome Project Data Processing Subgroup , The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa T., et al. , Improved Gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 71, 2095–2100 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Clough S. J., Bent A. F., Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- 51.Rühle T., Reiter B., Leister D., Chlorophyll fluorescence video imaging: A versatile tool for identifying factors related to photosynthesis. Front. Plant Sci. 9, 55 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida K., et al. , Correlated changes in the activity, amount of protein, and abundance of transcript of NADPH:protochlorophyllide oxidoreductase and chlorophyll accumulation during greening of cucumber cotyledons. Plant Physiol. 109, 231–238 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L., et al. , The Arabidopsis purple acid phosphatase AtPAP10 is predominantly associated with the root surface and plays an important role in plant tolerance to phosphate limitation. Plant Physiol. 157, 1283–1299 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flors C., et al. , Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green. J. Exp. Bot. 57, 1725–1734 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Kim D., et al. , TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao Y., Smyth G. K., Shi W., featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edgar R., Domrachev M., Lash A. E., Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer B. B., Hideg É., Krieger-Liszkay A., Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid. Redox Signal. 18, 2145–2162 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.