Abstract
Background:
Fracture healing in alcoholics is delayed and often associated with infections resulting in prolonged rehabilitation. It has been reported that binge drinking of alcohol increases oxidative stress and delays fracture healing in rats, which is prevented by treatment with the antioxidant n-acetyl cysteine (NAC). Oxidative stress is a significant factor in pathologies of various organs resulting from chronic alcoholism. Therefore, we hypothesize that treatment with NAC reduces oxidative stress and restores fracture healing in chronic alcoholics.
Methods:
Rats (10 months old) were pair-fed the Lieber-DeCarli ethanol (EtOH) diet or control diet for 16 weeks. A closed fracture was performed and rats allowed to recover for 72 hours. Rats were divided into 4 groups—control, control + NAC, EtOH, and EtOH + NAC—and injected intraperitoneally with 200 mg/kg of NAC daily for 3 days. Serum and bone fracture callus homogenates were collected and assayed for traditional markers of inflammation, oxidative stress, and bone regeneration.
Results:
The oxidative stress marker malondialdehyde (MDA) was increased in both serum and bone tissue in EtOH-fed animals compared to controls. NAC treatment significantly (p < 0.01) reduced MDA to near normal levels and dramatically increased the index of antioxidant efficacy (catalase/MDA ratio) (p < 0.01). Inflammatory markers tumor necrosis factor-α, interferon-γ, and interleukin-6 were significantly decreased in serum and callus following NAC treatment. NAC treatment reduced EtOH-induced bone resorption as evidenced by significant decreases in C-telopeptide of type-I-collagen levels (p < 0.05) and band-5 tartrate-resistant acid phosphatase levels in the tissue (p < 0.001).
Conclusions:
Oxidative stress and excessive inflammation are involved in the inhibition of fracture healing by EtOH. In this study, early short-term treatment of EtOH-fed animals with the antioxidant NAC reduced oxidative stress and normalized the innate immune response to fracture in the early phase of fracture healing, thereby restoring the normal onset of bone regeneration.
Keywords: N-Acetyl Cysteine, Alcoholic Liver Disease, Fracture Model, Ethanol, Rat Model, Inflammation, Antioxidants, Reactive Oxygen Species
Chronic consumption of alcohol (“alcoholism”) compromises host defense to pathogens and to traumatic injury prolonging recovery from injury (Greiffenstein and Molina, 2008; Szabo and Mandrekar, 2009). Alcoholics experience more complications during the first few weeks after fracture-fixation surgery (Faroug et al., 2014; Mathog et al., 2000; Nyquist et al., 1997; Serena-Gomez and Passeri, 2008), which often includes infections in bone (Duckworth et al., 2011; Ovaska et al., 2013; Senel et al., 2007; Tonnesen et al., 1991), surgical site infections in the soft tissue, and other (aseptic) wound-healing problems (Bonnevialle et al., 2012; Hoiness et al., 2003; Rantala et al., 1997). In fact, in fracture models of alcoholism, bone regeneration is inhibited by the tumor necrosis factor (TNF)-α and interleukin (IL)-1 signaling axis as demonstrated by the direct effect of cytokine antagonists on increased bone formation (Perrien et al., 2004; Wahl et al., 2007).
When alcohol is metabolized, reactive oxygen and nitrogen species as well as acetaldehyde are generated (Preedy et al., 1999). These reactive oxygen species (ROS) have been shown to be involved in the fracture healing process (Lippross et al., 2014; Yeler et al., 2005), which is thought to be a suppression of the Wnt signaling pathway (Lauing et al., 2014). In fact, forkhead box O, a transcription factor that regulates Wnt signaling, is partially responsible for oxidative stress resistance and cell cycle inhibition. These molecules have been shown to be increased in a mouse alcohol fracture model, which could be augmented with the addition of the antioxidant N-acetyl cysteine (NAC) (Roper et al., 2016). To counteract the effects of oxidative stress following ethanol (EtOH) administration, antioxidants have been demonstrated to play a key role in suppression of the ROS system. Studies have shown that the antioxidant NAC can reduce bone loss by decreasing the number of osteoclast and increasing osteoblastic activity (Chen et al., 2010, 2011), thereby reducing the effects of EtOH on fracture healing (Roper et al., 2016). NAC has been shown to reduce oxidative stress following alcohol and fracture in a rat model of binge exposure (Volkmer et al., 2011). However, the effects of long-term alcohol consumption were not looked at in this model system.
In the Veterans Affairs (VA) healthcare system, patients suffer from alcohol and other dependencies at an alarming rate (Fuehrlein et al., 2014). It is well established that these patients have increased skeletal pathologies (Harris et al., 2009) and are therefore more susceptible to fracture healing problems. In a preliminary retrospective study at the Omaha VA Medical Center, fracture-healing time in heavy drinkers was twice that in nonalcoholics (Askew et al., 2001), which was consistent with a previous finding in Swedish population showing 37% longer fracture-healing time in heavy drinkers (Nyquist et al., 1997). To begin looking at treatment strategies in chronic alcoholics following fracture, we developed a rat fracture model mimicking both age and long-term effects of EtOH consumption in the older veteran population.
The hypothesis of this study is that treatment with the antioxidant NAC reduces the oxidative stress and restores the normal onset of bone regeneration in the fracture site. The specific objectives were to determine the effects of alcoholism and NAC treatment on (i) oxidative stress and antioxidant defense in the fractured bone, (ii) inflammatory response and immunomodulation, and (iii) onset of bone regeneration in the fracture site in a rat model.
MATERIALS AND METHODS
Animals
Male Wistar rats (Charles River, Spencerville, OH) of ages specified below were used in this study. The experimental protocol was approved by the IACUC at Omaha VA Medical Center. Animals were pair-fed using Lieber-DeCarli liquid control diet and Lieber-DeCarli liquid EtOH diet purchased from Dyets, Inc. (Bethlehem, PA) as previously described (Lieber and DeCarli, 1989). NAC (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% saline (Hospira, Lake Forest, IL) and adjusted to pH 7.4 by 10 N NaOH for intraperitoneal (i.p.) injections (Wrotek et al., 2011).
Animal Age Consideration
For these studies, 270-day-old (NAC dosing study) or 305-day-old (NAC fracture study) rats were used to represent the age range of chronic human alcoholics in the veteran population (ages 19 to 75). It has been shown that femur growth in male Wistar rats slows down significantly by 9 months (270 days) of age (Iida and Fukuda, 2002), which suggests that, skeletal maturity of the femur in male Wistar rats at 9 months of age is comparable to human skeletal maturity at 20 years of age. Therefore, the age of the animals was chosen latter in the rat’s life span.
NAC Dosing in Chronic EtOH-Fed Rats
To determine the NAC dosing effects on EtOH-fed rats, 9-month (270 day)-old male Wistar rats were pair-fed control and EtOH diet daily for 7 weeks. Rats were fed control diet for 1 week to acclimate them to the liquid diet prior to weight matching at the start of the second week. Previous groups have treated rats with NAC doses of 225 or 250 mg/kg (Nurulain et al., 2015; Wu et al., 2005), demonstrating an antioxidant effect, while treatment of alcoholic hepatitis patients with 150 mg/kg NAC has shown improvement in the disease severity (Thursz and Morgan, 2016). Therefore, EtOH-fed rats were treated with NAC at concentrations of 800, 400, and 200 mg/kg of body weight (b.w.) to determine whether increased levels would be beneficial. Test groups included saline-treated, EtOH-fed, control group, and an untreated pair-fed control group. Starting at week 5, EtOH-fed rats were injected i.p. with 10 ml of NAC or saline for 5 d/wk for 3 weeks. The high volume of the vehicle was needed to generate the higher concentrations of NAC in this dosing experiment. At week 7, rats were anesthetized with isoflurane, blood collected, and both the right and left femurs were harvested.
NAC Blood Concentration
High-pressure liquid chromatography (HPLC) was used to detect NAC in the serum of rats using methods adapted from Ercal and colleagues (1996) and Wu and colleagues (2006). In humans, an intravenous (i.v.) dose of 150 mg/kg NAC, resulted in plasma levels of 3.4 mM in 15 minutes with a half-life of 5.7 hours (Sadowska et al., 2007). To begin looking at levels following the dose response, serum from rats injected with NAC doses of 0, 200, 400, and 800 mg/kg were collected and derivatized with N-(1-pyrenyl) maleimide (NPM) (Sigma, St. Louis, MO), diluted in acetonitrile, and incubated for 5 minutes at room temperature. Acetic acid was added to stop the reaction and the solution was filtered through a 0.2-micron Acrodisc (Pall Corporation, Port Washington, NY). Samples were then injected into a Varian HPLC (Agilent Technologies, Santa Clara, CA) through a Phenomenex Luna C18 column from Agilent and detected using a fluorescent detection system. A standard curve was prepared using known concentrations of NAC and the data extrapolated. Standard curves were prepared in control rat serum to eliminate background noise from the NPM reaction.
Four-Point Bend Testing
Both femurs were excised from the rat after euthanasia, wrapped in gauze soaked with phosphate-buffered saline (PBS), and stored frozen at −70°C. Prior to performing the bend test, they were thawed at 4°C for 24 hours. Bend testing was performed using the Instron Model 5943 Biomedical Test System (Instron Corporation, Norwood, MA). Femurs were supported on 2 rollers 15 mm apart with 2 points of force application 5 mm apart and centered over the 15 mm span. Force was applied at the rate of 1 mm/min until the femurs broke. Instron’s Bluehill 3 Software was used to determine force applied at the point of break as a measure of the flexural strength of the femur. Data are expressed as maximal force.
Serum Assays for Liver Integrity
Animals were tested for liver toxicity following NAC dose administration using the Clinical Chemistry Laboratory (VA NWIHCS, Omaha, NE). Blood alcohol level (BAL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALKphos) were tested using a VITROS 4600 Chemistry System (Ortho Clinical Diagnostics, Raritan, NJ).
Closed Fracture Model
Three-hundred and five-day-old rats were pair-fed the Lieber-DeCarli EtOH diet or control diet as described above for 21 weeks. At the end of week 21, a closed fracture was produced in the right femur of the rats according to the weight-drop procedure of Bonnarens and Einhorn (1984). Briefly, a 1.4-mm k-wire was inserted surgically as an intramedullary implant, followed by creation of a closed fracture of the femur using a 500 gram weight. Following surgery, rats were given an antibiotic (Covenia, 1.6 mg/kg; Covenia Pfizer Animal Health, New York, NY), anti-inflammatory (Rimadyl, 5 mg/kg; Rimadyl Zoetis, Parsippany, NJ), and pain medications (Buprenex, 0.05 mg/kg; Buprenex Reckitt Benckiser Healthcare (UK) Ltd., Hull, England) for 3 days. At the end of day 3, animals were treated i.p with 200 mg/kg of NAC or 10 ml of saline daily for 3 days. During this 6-day period, rats were continued on the control and EtOH diet. The start of the NAC treatment was delayed intentionally until after the 72-hour acute inflammatory phase to avoid the additional anti-inflammatory effects of NAC interfering with the normal cellular and biomolecular activities that support bone regeneration (Ai-Aql et al., 2008; Andrew et al., 1994; Thomas and Puleo, 2011). All rats were euthanized 8 days after fracture so that we could determine the changes in markers of oxidative activity, antioxidant defense, immune status, inflammatory response, and osteogenesis associated with chronic EtOH consumption and NAC treatment. Following NAC injections, animals were anesthetized, blood was collected, and both femurs were harvested for testing.
Oxidative Stress and Bone Regeneration Markers
Serum and bone homogenates were assayed for markers of oxidative stress and bone regeneration using commercially available test kits. Fractured and nonfractured femurs were homogenized by freezing with LN2 and then fracturing into small pieces with a hammer. Bone chips were then placed in PBS and homogenized using a disperser and further broken up using sonication. Thiobarbituric Acid Reactive Substances assay kit and Catalase assay kit from Cayman (Ann Arbor, MI) were used to detect malondialdehyde (MDA) and catalase, respectively. Bone formation markers rat C-terminal peptide of bone-specific alkaline phosphatase (BAP) (Cusabio Biotech, Houston, TX), osteocalcin (OCN) (MyBioSource, San Diego,CA) were tested using commercial ELISA kits. Cross- linked C-telopeptide of type-I-collagen (CTX) and band-5 tartrate-resistant acid phosphatase (TRAP5b) assay kits were purchased from Immunodiagnostic Systems (Gaithersburg, MD). Soluble receptor activator of nuclear factor-kB ligand and osteoprotegerin (OPG) assay kits were purchased from TSZ ELISA (Waltham, MA). Reactivity was detected using an Epoch ELISA reader with Gen5 software (Biotek, Winooski, VT).
Inflammatory Cytokines
In order to evaluate the inflammatory process involved in the actions of EtOH and treatment of fractures with NAC, both serum and bone homogenates were tested. As described above, supernatant and serum were collected and tested for IL-6, interferon (IFN)-γ, TNF-α, IL-1beta, and IL-2 using kits from BD Biosciences (San Diego, CA). Reactivity was detected using an Epoch ELISA reader with Gen5 software (Biotek, Winooski, VT). The left nonfractured femur homogenate was used as a negative control, and data were subtracted from the fractured femur homogenate.
Statistical Analysis
Statistical analysis was carried out using 2-way analysis of variance (ANOVA) and multiple comparison procedures (Sidak’s multiple comparisons test). Differences were determine whether statistically significance reached (p < 0.05).
RESULTS
NAC Dosing in EtOH-Fed Rats
Initial steps were taken to determine what concentrations of NAC were achieved in the blood following EtOH feeding and NAC administration. Rats that were injected with NAC at concentrations of 200, 400, and 800 mg/kg body weight (b.w.) for 3 days had NAC blood levels of 286, 453, and 547 nM, respectively (Fig. 1A). When injections were carried out for 21 days, these numbers increased to 433, 579, and 780 nM (Fig. 1A). These data demonstrate that NAC blood concentrations in our rats were less than the 3.4 mM level achieved in humans dosed with 150 mg/kg i.v. In fact with a half-live of 5.7 hours, and adjustments for weight in the rat, approximately 1,320 nm of NAC in the rat serum would be equivalent to the human concentration of 3.4 nM. Statistical analysis of these data indicated that increasing the amount of time NAC was given did not significantly increase the blood concentration. Furthermore, the blood levels of NAC achieved at the 400 and 800 mg/kg b.w. doses were not significantly higher than that at 200 mg/kg b.w. dose.
Fig. 1.
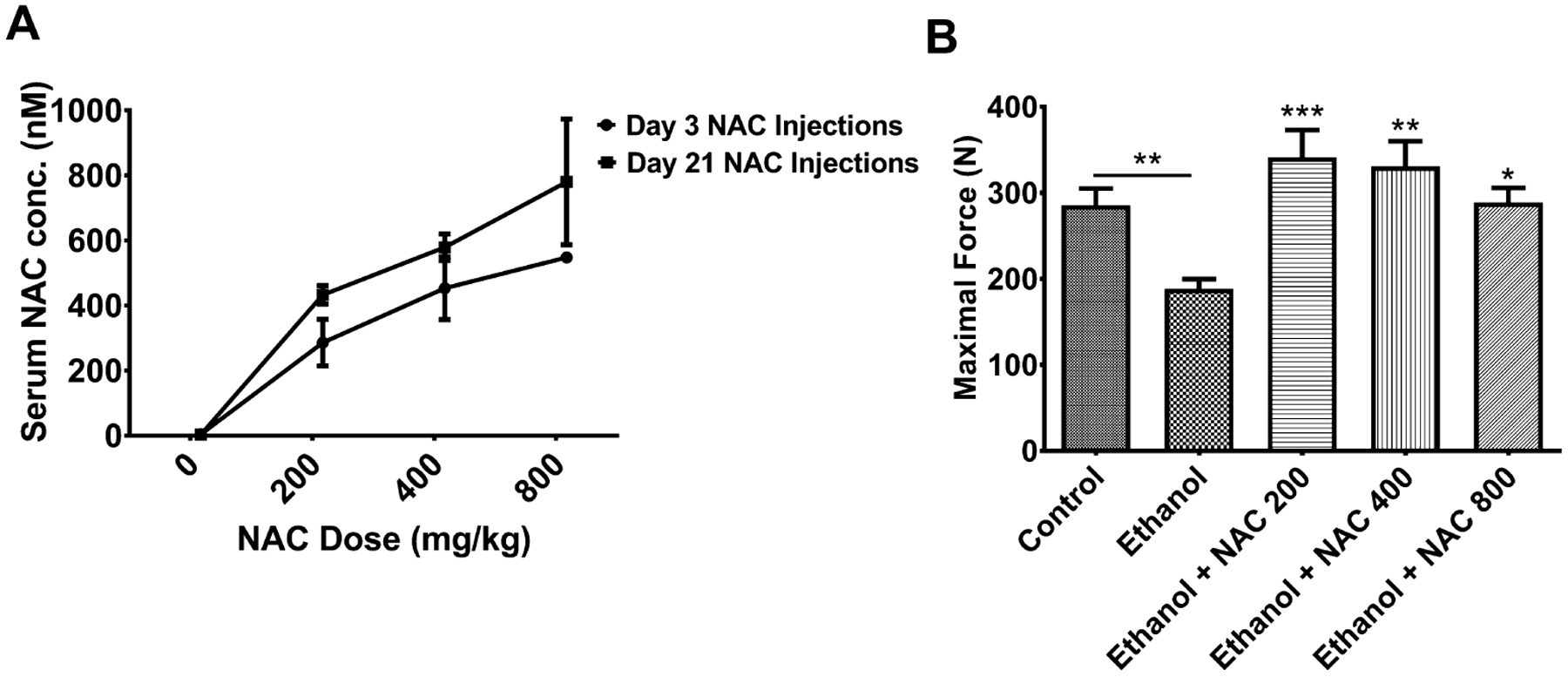
Serum n-acetyl cysteine (NAC) concentrations and 4-point bend testing in ethanol (EtOH)-fed rats following treatment with NAC. EtOH-fed rats were injected with NAC at 200, 400, and 800 mg/kg body weight (b.w.) for 3 days. Serum and femur bones were collected and tested for (A) serum NAC concentrations determined by HPLC following 3 or 21 days of injections. (B) Four-point bend testing on femurs following EtOH and NAC administration. Significantly different compare to EtOH alone NAC 200 (***p < 0.001), NAC 400 (**p < 0.002), and NAC 800 (*p < 0.03), respectively. N = 6 for control and EtOH-fed rats. N = 3 for 200, 400, and 800 mg/kg b.w. NAC-treated EtOH-fed rats.
Skeletal Homeostasis Following NAC Administration
We next determined whether NAC treatment of EtOH-fed rats restores the normal skeletal homeostasis in the rats by measuring bending strength by 4-point bend test, serum OCN as a bone formation marker, and serum TRAP5b as a bone resorption marker. Both femurs in rats in this experiment were intact (i.e., not fractured), and they were subjected to 4-point bend testing following EtOH and NAC administration to confirm that EtOH decreased bone strength and to determine whether NAC treatment increased it. Bend testing demonstrated that the femurs in EtOH-fed rats failed at a force of 188 N, significantly reduced compared with control-fed rats at 286 N (p < 0.01, Fig. 1B). Increasing doses of NAC (200, 400, 800 mg/kg b.w.) given to the EtOH-fed animals showed an increase in failure force from 188 N to 341 N (p < 0.001), 330 N (p < 0.002), and 288 N (p < 0.03), respectively (Fig. 1B). Treatment of EtOH-fed rats with 200 and 400 mg/kg doses increased bone strength 2-fold over the untreated animals. But the differences between the 3 doses of NAC were not significant. Numerically, the highest mean strength was achieved with the 200 mg/kg b.w. dose. All doses of NAC reduced OCN to control level (data not shown). EtOH did not affect serum TRAP5b, but the group treated with 800 mg/kg b.w. dose of NAC had a significant 69% increase in TRAP5b compared with all other groups (data not shown). These data indicate there is no additional therapeutic benefit in using the higher doses.
Liver Function Following NAC Administration
To address the concerns that giving NAC at high concentrations may affect liver function, studies were performed to determine whether NAC at the concentrations used was toxic to the animal. BALs were measured to ensure the animals were obtaining high enough EtOH concentrations and that NAC had no effects on these levels. As shown in Fig. 2A, BALs in the EtOH-fed rats increased to approximately 60 mg/dl over control-fed rats. This concentration remained constant with increasing doses of NAC demonstrating that NAC has no effect on the alcohol in the blood. There was a moderate but significant increase (116 to 200 U/l, p < 0.01) in ALKphos levels due to EtOH feeding, but this was reduced (p < 0.05) by NAC treatment of EtOH-fed rats (Fig. 2B). AST levels were significantly increased from 139 to 735 U/l in EtOH-fed rats compared with control-fed animals (p < 0.001), but were decreased to control levels (p < 0.01) by NAC treatment of EtOH-fed animals (Fig. 2C). Similarly, the elevated levels of ALT (Fig. 2D) in EtOH-fed rats (259 U/l) compared with controls (48 U/l) (p = 0.001) were lowered to control levels by NAC treatment (p < 0.02). The differences in the restoration of the increased levels of ALKphos, AST, and ALT to control levels by the 3 NAC doses were not statistically significant. Thus, the results of the liver function tests revealed that co-administration of NAC with EtOH feeding has no toxicity effects and actually restore normal liver function. The results in Figs 1 and 2, as described above also indicate that the 200 mg/kg b.w. dose of NAC is the safest and most effective of the 3 doses in maintaining liver integrity and skeletal homeostasis in EtOH-fed rats without fracture. Therefore, we selected this dose for subsequent experiments.
Fig. 2.
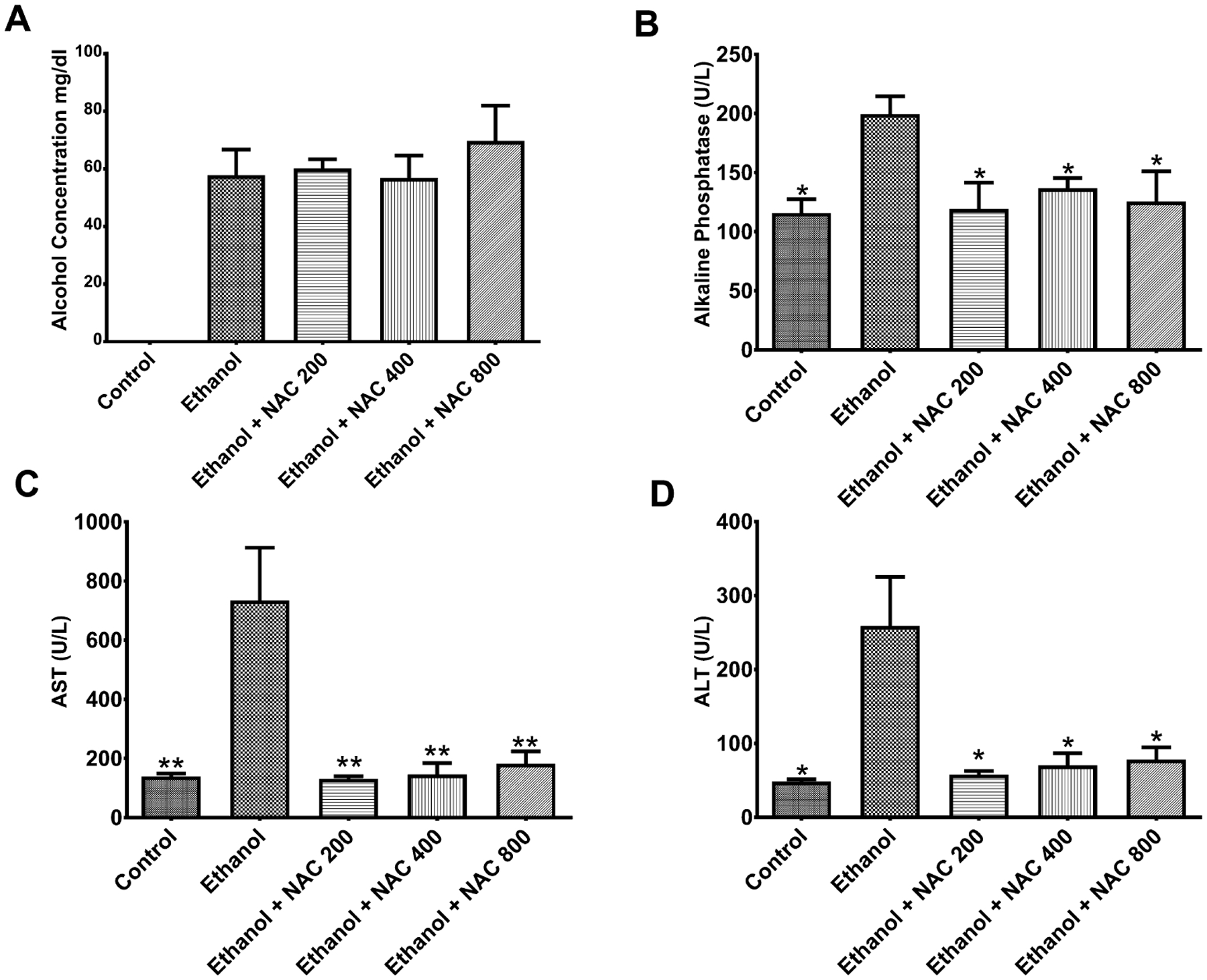
Blood alcohol levels (BALs) and liver functionality tests following n-acetyl cysteine (NAC) treatment. Ethanol (EtOH)-fed rats were injected with NAC at 200, 400, and 800 mg/kg body weight (b.w.) for 3 days. (A) BAL following NAC treatment in EtOH-fed rats. Serum tested by GC for alcohol levels did not significantly change concentrations with the addition of NAC. (B) Alkaline phosphatase levels in the serum of EtOH-fed rats following treatment with NAC (*p < 0.05). (C) AST levels in the serum of EtOH-fed rats following treatment with NAC (**p < 0.01) (D) ALT levels in the serum of EtOH-fed rats following treatment with NAC (*p < 0.02). N = 6 for control and EtOH-fed rats. N = 3 for 200, 400, and 800 mg/kg b.w. NAC-treated EtOH-fed rats.
Oxidative Stress and NAC Treatment
Chronic alcohol consumption increases oxidative stress, which may limit the ability of the bone to heal following fracture. Therefore, studies were performed to investigate whether NAC decreases oxidative stress in our model system. Mean serum MDA levels in the EtOH-fed animals (44 μM/mg) were significantly (p = 0.0002) higher than those in controls (16 μM/mg). NAC treatment decreased this to 32 μM/mg but was not significant by 2-way ANOVA. However, there was a significant interaction between diet and treatment (p = 0.0326) (Fig. 3A). A significant reduction in MDA was observed in the bone homogenate following treatment. EtOH increased MDA levels 4-fold over controls (1.7 to 6.9 μM/mg, p = 0.05), and NAC treatment restored it to control levels (1.4 μM/mg, p < 0.04). The interaction between diet and treatment was significant at (p < 0.05) (Fig. 3B). We also measured levels of the antioxidant catalase, a key scavenger of peroxide, in bone homogenate (Fig. 3C). Although EtOH only slightly decreased catalase (40.7 to 36.0 nmol/min/mg, NS), NAC treatment of EtOH-fed rats increased it almost 3-fold (p < 0.001) compared to the level in untreated EtOH-fed rats. There was a significant interaction between diet and treatment (p = 0.0012). The efficacy of NAC in overcoming EtOH-induced oxidative stress is more clearly demonstrated by determining the ratio of catalase to MDA, which is an index of antioxidant efficacy (Fig. 3D). This index was decreased from 22.7 (control) to 6.67 by EtOH, but was increased dramatically to 72.8 by NAC treatment of EtOH-fed rats (p < 0.01). Thus, NAC treatment not only abolished the oxidative stress caused by EtOH, but also achieved an antioxidant status significantly higher than controls.
Fig. 3.
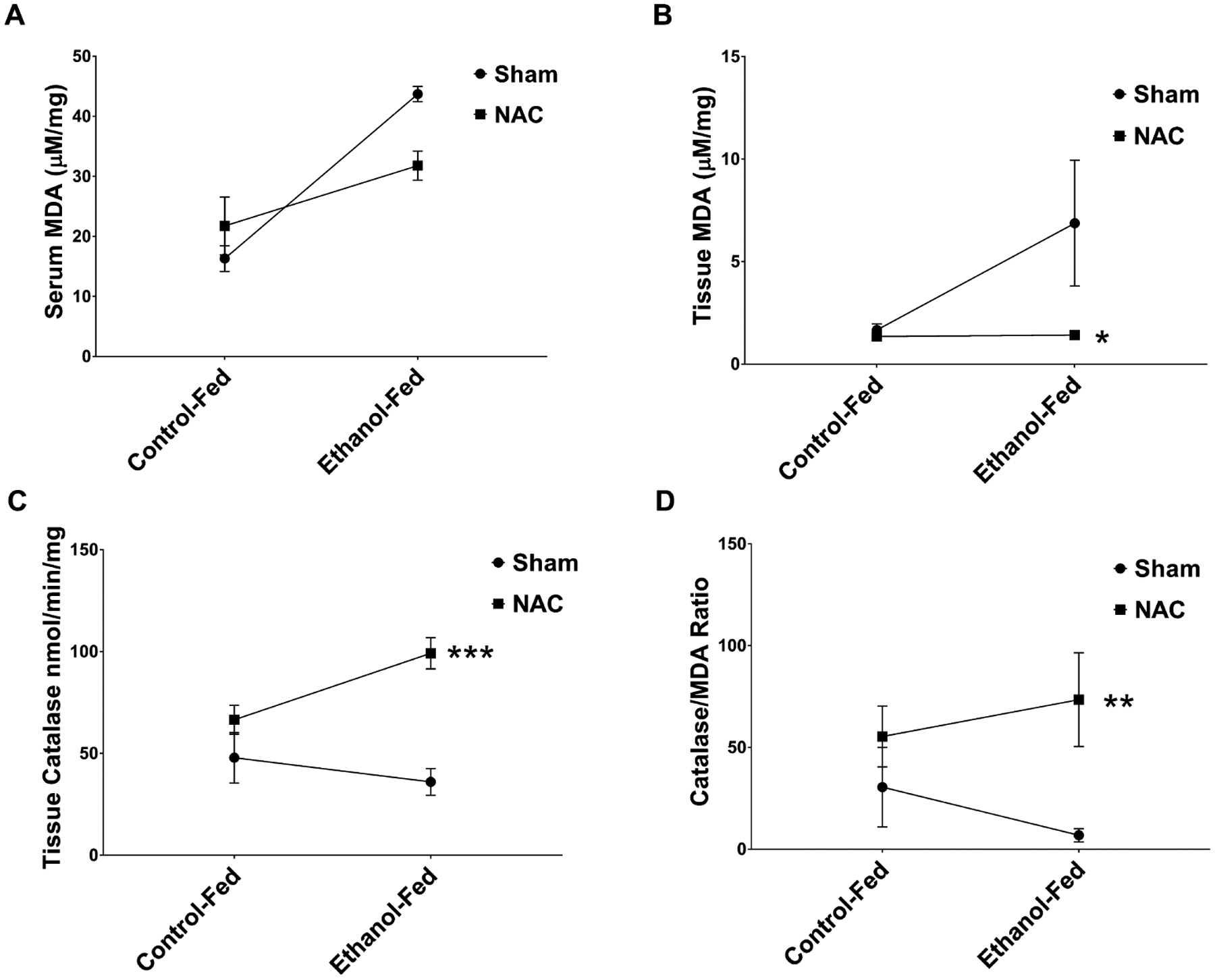
Oxidative stress markers in ethanol (EtOH)-fed rats following fracture and treatment with n-acetyl cysteine (NAC). (A) Serum MDA levels in control-fed, control-fed NAC-treated, EtOH-fed, and EtOH-fed NAC-treated rats. Mean serum MDA levels in the EtOH-fed animals were significantly (p = 0.002) higher than in controls. NAC treatment decreased MDA levels (NS), but there was a significant (p = 0.033) interaction. (B) Tissue callus homogenate MDA levels. EtOH increased MDA levels 4-fold over controls (p = 0.05) and NAC treatment restored it to control levels (*p < 0.04). No significant interaction was observed. (C) Tissue callus homogenate catalase levels. EtOH slightly decreased catalase activity compared to controls. NAC treatment of EtOH-fed rats increased it almost 3-fold (***p < 0.001) compared to the level in untreated EtOH-fed rats, with a significant (**p < 0.001) interaction with diet. (D) The efficacy of NAC demonstrated by determining the ratio of catalase to MDA, which is an index of antioxidant efficacy. Catalase/MDA ratio was significantly decreased in the EtOH-treated animals. This ratio was significantly (**p < 0.002) restored higher than control levels with NAC injection. N = 4 animals per group.
Inflammatory Markers and Fracture Healing
Chronic alcohol consumption also alters the innate immune response in the fracture. Therefore, we investigated the systemic and local effects of EtOH feeding and NAC treatment on fracture healing in our animal model by measuring cytokines in serum and fracture callus tissue.
In these studies, EtOH feeding increased serum TNF-α (Fig. 4A) more than 12-fold (p < 0.001). NAC treatment of EtOH-fed rats lowered TNF-α, but it remained higher than the control level. However, there was a significant interaction between diet and treatment (p = 0.023). There was a more dramatic effect in the fracture callus tissue from EtOH-fed rats (Fig.4B). TNF-α was approximately 5 times that of control animals (p < 0.0001), which was reduced significantly (p = 0.0002) by NAC treatment and an interaction of (p = 0.0004) between diet and treatment. The serum IFN-γ level in EtOH-fed animals (Fig. 4C) was also higher (≈ 6 times, p < 0.0001) than that in controls. This was reduced significantly (p = 0.0005) by NAC treatment, but remained almost 2 times higher than control levels. There was a significant interaction (p = 0.0005) between diet and treatment for IFN-γ. In the fracture callus tissue (Fig. 4D) of EtOH-fed rats there was almost 3 times more IFN-γ production, and NAC treatment reduced it to control levels (p < 0.05) with a significant interaction (p = 0.0487) between diet and treatment. EtOH feeding increased IL-6 level in serum (Fig. 4E) by 2 orders of magnitude (p = 0.009). NAC treatment of EtOH-fed animals restored IL-6 almost to control level (p < 0.02) with a significant interaction (p < 0.02) between diet and treatment. In the fracture callus tissue of EtOH-fed animals, IL-6 was approximately twice as that of the controls (Fig. 4F). Although NAC treatment reduced it toward control level, this change was not significant. In contrast to the results for serum cytokines described above, serum IL-2 was not affected by EtOH feeding or NAC treatment (Fig. 4G). However, EtOH feeding increased mean IL-2 level in callus more than 2-fold from controls, which was restored to control level (p = 0.03) by NAC treatment of EtOH-fed animals, interaction was significant between diet and treatment (p < 0.02) (Fig. 4H). Taken together, the data reported above demonstrate that the EtOH and fracture increase pro-inflammatory cytokines in serum and bone tissue, which were significantly reduced by NAC treatment.
Fig. 4.

Inflammatory markers in ethanol (EtOH)-fed rats following fracture and treatment with n-acetyl cysteine (NAC). (A) Serum TNF-α levels were increased in response to EtOH compared to controls (p < 0.0001). NAC significantly (NS) reduced these levels in the EtOH-fed rats, with a significant (p < 0.02) interaction to diet. (B) Fracture callus tissue TNF-α levels in the EtOH-fed animals was approximately 5 times that of control animals (p < 0.0001), which was reduced significantly (**p < 0.001) by NAC treatment, with an interaction to diet (p < 0.001). (C) Serum IFN-γ levels were higher in the EtOH-fed rats compared to controls (p < 0.0001). These levels were significantly (***p < 0.005) reduced to near control levels by NAC treatment with a significant (p < 0.005) interaction with diet. (D) Fracture callus tissue IFN-γ levels increased 3 times more in the EtOH-fed rats compared to controls (NS), and NAC treatment reduced it to control levels (NS). There was a significant (p < 0.05) interaction. (E) Serum IL-6 levels were increased in the serum of EtOH fed compared to controls (p < 0.01) and NAC treatment of EtOH-fed animals restored IL-6 almost to control level (*p < 0.02). (F) Fracture callus tissue IL-6 levels in the tissue callus were increased over control-fed animals and decreased in the EtOH fed with NAC, yet significance was not reached between the groups. (G) Serum IL-2 levels were not affected by EtOH feeding or NAC treatment. However, there was a significant (p < 0.04) interaction with diet. (H) Fracture callus tissue IL-2 level in the callus was more than 2-fold from controls, which was restored to control level (*p < 0.03) by NAC treatment of EtOH-fed animals, with a significant (p < 0.02) interaction with diet. N = 4 animals per group.
Bone Markers and NAC Treatment
Healing of bone fractures is accomplished by bone regeneration via 2 complementary pathways, namely, direct (or, “intramembranous”) bone formation by osteoblasts and indirect (or, endochondral) bone formation, which begins with cartilage formation by chondroblasts. We evaluated the effects of EtOH and NAC on markers of bone formation and bone resorption in serum and bone fracture callus tissue to understand their effects on bone regeneration and hence the outcome of fracture healing.
Osteoblastic activity was measured in the fracture callus for BAP and OCN. BAP levels (Fig. 5A) in the fracture callus showed a significant (p = 0.04) decrease with EtOH treatment compared to control. Treatment with NAC significantly (p < 0.001) increased BAP levels for both control and EtOH-fed animals. OCN levels (Fig. 5C) were increased in response to EtOH with significance not being reached. NAC treatment decreased these levels slightly (NS). There was a significant (p = 0.014) interaction between diet and treatment for this bone marker. Our results for bone resorption markers were more robust than for bone formation markers. We measured markers for osteoclastogenesis which included RANKL and OPG, and for bone resorptive action of mature osteoclasts, CTX and TRAP5b were tested. EtOH increased tissue RANKL, but significance was not reached compared to control. NAC treatment decreased RANKL in the EtOH-fed animals, yet was not significant. However, there was a significant interaction between diet and treatment (p = 0.046) (Fig. 6A). Interestingly, serum RANKL was significantly increased in the EtOH-fed animals (p < 0.0001), decreased by treatment (p = 0.004), and there was a significant (p < 0.0001) interaction between treatment and diet. OPG, a marker of osteoclastogenesis downregulation, was looked at following treatment with NAC’s. Tissue from the fracture callus showed a small in significant decrease in OPG in the EtOH-fed animals compared to controls (Fig. 6B). There was no interaction between diet and treatment. However, there was a significant (p < 0.005) increase in OPG following treatment with NAC. When the RANKL/OPG ratio was calculated, there was a significant (p = 0.004) difference between the EtOH-fed group compared to animals that received NAC, (Fig. 6C), demonstrating the overall effects of the treatment. In EtOH-fed animal tissue, levels of cross-linked CTX (Fig. 6D) were higher (NS) than those in controls, which was restored to control level by NAC treatment (NS). On the other hand, there was a significant (p < 0.01) interaction between diet and treatment. In contrast, EtOH feeding increased TRAP5b levels in fracture callus tissue (Fig. 6E) 3.5-fold compared with controls (p < 0.001), indicating increased bone resorption activity of mature osteoclasts in the fracture callus caused by EtOH. NAC treatment of EtOH-fed rats reduced this significantly (p < 0.0001) to 1.56 U/ml with a significant interaction (p < 0.0001) with diet.
Fig. 5.
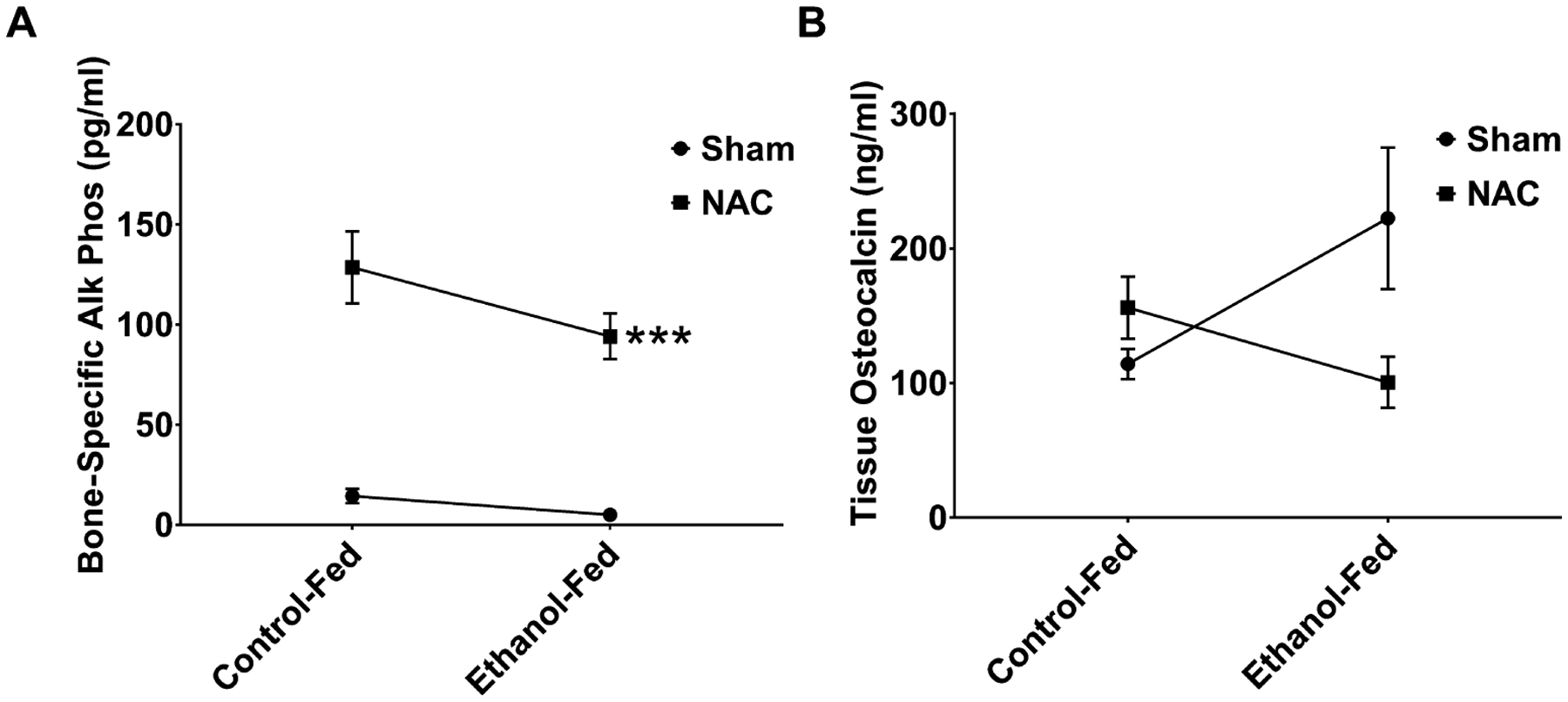
Bone regeneration markers in ethanol (EtOH)-fed rats following fracture and treatment with n-acetyl cysteine (NAC). (A) Bone-specific alkaline phosphatase (BAP), levels in the fracture callus of NAC-treated EtOH-fed rats. BAP levels were decreased by EtOH (p = 0.04), while NAC treatment increased both control and EtOH levels significantly (***p < 0.001). No significant interaction was observed for BAP. (B) Osteocalcin (OCN) levels in the fracture callus of NAC-treated EtOH-fed rats. EtOH increased OCN levels were increased (NS) and NAC reduced levels in the EtOH-fed rats comparable to controls, yet significance was not achieved. There was a significant interaction (p = 0.014) with diet and treatment. N = 4 animals per group.
Fig. 6.
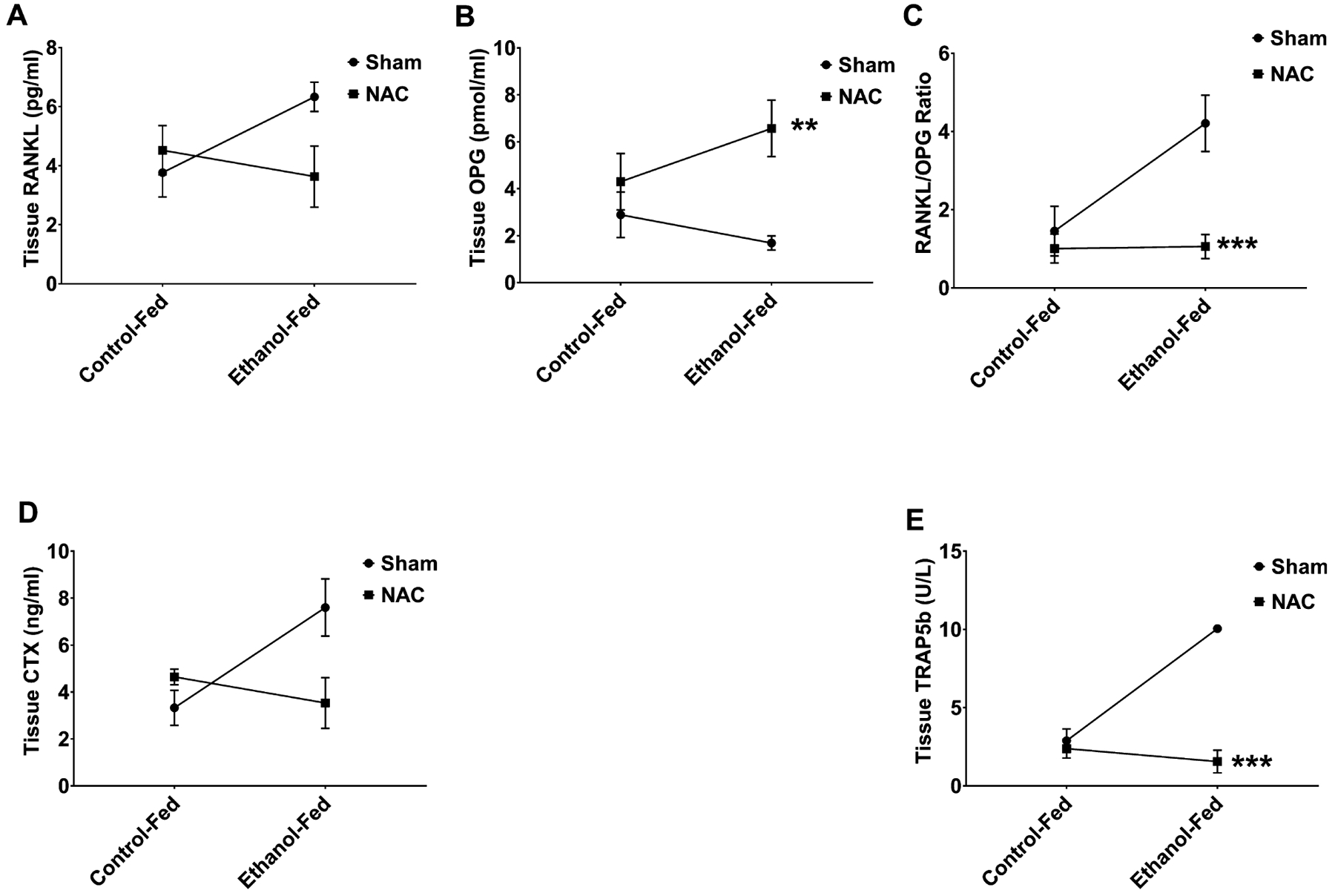
Bone resorption markers in ethanol (EtOH)-fed rats following fracture and treatment with n-acetyl cysteine (NAC). (A) RANKL in the callus of NAC-treated EtOH-fed rats. EtOH had increased RANKL (NS) compared to controls. NAC treatment decreased this RANKL in the EtOH-fed rats (NS), with a significant (p < 0.05) interaction with diet. (B) Osteoprotegerin (OPG) in the tissue callus of NAC-treated EtOH-fed rats. EtOH feeding decreased (NS) the OPG in fracture callus, while NAC treatment of EtOH-fed animals significantly (**p < 0.005) increased OPG to restore it beyond control levels. However, there was no interaction with diet and treatment. (C) The RANKL/OPG ratio was significantly (**p = 0.004) decreased in the EtOH NAC-treated rats compared to EtOH alone. (D) CTX in the serum of NAC-treated EtOH-fed rats. EtOH-fed serum levels of (CTX) were higher (NS) than in controls, which was restored to control levels by NAC treatment (NS), with a significant (p < 0.01) interaction with the diet. (E) TRAP levels in the tissue callus of NAC-treated EtOH-fed rats. EtOH feeding increased (p < 0.001) TRAP5b levels in the fracture callus tissue 3.5-fold compared to controls. NAC treatment of EtOH-fed rats reduced this significantly (***p < 0.001), with a significant (p < 0.0001) interaction. N = 4 animals per group.
DISCUSSION
Oxidative stress causes many deleterious effects on fracture healing following EtOH consumption. Previous work carried out by Ronis and colleagues has shown that EtOH increases the RANKL/RANK signaling between osteoblasts and osteoclasts, thereby increasing bone resorption, making bone fracture healing difficult (Alund et al., 2017; Mercer et al., 2014). Treatment with the antioxidant NAC has been shown by a number of groups to diminish the effects of EtOH and oxidative stress on fracture healing (Alund et al., 2017; Chen et al., 2011; Roper et al., 2016; Volkmer et al., 2011). To determine the efficacy of using NAC in treatment for fractures in the veteran population, we developed a rat model that mimics the age and chronicity of EtOH abused seen in these individuals. This study is based on the premise that alcoholism is associated with oxidative stress that overrides cellular defenses and disrupts the onset of bone regeneration in the fracture site. We used a clinically adapted animal model of chronic alcoholism to obtain data on biomarkers that characterize the cellular responses to alcohol consumption and NAC treatment. We found that in fractured bone NAC not only restored the oxidative stress marker MDA to control levels but also increased the index of antioxidant efficacy (catalase/MDA ratio) to more than control levels, supporting our hypothesis that NAC abolishes the EtOH-induced oxidative stress in the fractured bone.
Oxidative stress alters the innate immune response to injury. Delay and failure of fracture healing in alcoholics are frequently associated with infections in bone and soft tissues in the wound site occurring in the early stages of healing (Serena-Gomez and Passeri, 2008). We measured INF-c in bone and serum as a marker for the immune status. EtOH caused 2-and 6-fold increases of INF-c concentrations in bone and serum, respectively, indicating immune suppression, which may be responsible for the increased incidence of infections associated with delayed fracture healing in alcoholics. NAC treatment abrogated the increase of INF-γ in bone and partially restored these values to control levels in serum.
The coordinated actions of several molecular factors regulate the onset and orderly progression of bone regeneration in the fracture site. A normal inflammatory response, characterized by appropriate time-dependent levels of TNF-α and IL-6, is required for prevention of delays in this process. TNF-α has a biphasic response, with high serum levels expressed immediately following injury that become undetectable within 72 hours (Thomas and Puleo, 2011). Thus, the high serum levels of these cytokines persisting 8 days after fracture in EtOH-fed rats indicate that normal onset of osteogenesis and chondrogenesis that are required for bone regeneration might not have occurred. NAC treatment of EtOH-fed rats abolished this excess in IL-6, whereas TNF-α was reduced significantly, but not restored to control level. These actions of NAC to normalize the inflammatory response in EtOH-treated animals promote the normal onset of bone regeneration in the fracture site of these animals. The increased production of IL-2 in the EtOH-fed animal fracture callus indicates the infiltration of T cells into the site. The reduction in this cytokine back to normal levels with NAC treatment also contributes to restoration of normal bone regeneration by reducing the increased T-cell infiltration in the fracture site of NAC-treated EtOH-fed animals.
The cascade of EtOH-induced changes described above, namely oxidative stress, immunodeficiency, and abnormal inflammatory response, leads to inhibition of bone regeneration in the fracture site that is required for normal healing of the fractured bone. Therefore, the balance between osteoclast and osteoblast is crucial in regulating bone loss and bone formation following fracture. The increase in bone resorption by EtOH was evident on day 8 after fracture when the mean level of TRAP5b, a marker for mature osteoclast activity, in the fracture callus tissue in EtOH-fed animals, was 3.5-fold higher than in control-fed animals. NAC treatment of EtOH-fed animals completely restored TRAP5b to control level. The molecule, RANKL promotes osteoclastogenesis, whereas OPG has the ability to inactivate RANKL and promote osteoblastogenesis. Accumulation of ROS in osteoblast following EtOH consumption has been shown to stimulate NFκB to initiate the increase in RANKL, resulting in osteoclastogenesis and bone loss (Mercer et al., 2014). In this study, RANKL in the fracture callus was increased in response to EtOH consumption and decreased with NAC treatment. More interesting was the ability of NAC to increase OPG, which shuts down osteoclast functionality. CTX, another marker of collagen breakdown, was decreased in response to NAC, suggesting remodeling of bone.
Bone formation markers demonstrated variable results. There was no change in bone morphogenetic protein with EtOH or NAC treatment. However, BAP levels were significantly increased in both control and EtOH-fed rat fracture sites following NAC treatment. Interestingly, OCN levels were increased in response to EtOH, which was unexpected. There was not a significant difference between controls, yet NAC normalized the levels back to control. A similar trend was observed in the serum of these animals.
This is the first animal study that evaluated the efficacy of NAC treatment to overcome alcohol-induced inhibition of fracture healing in the context of chronic alcoholism. In a previous study, Volkmer and colleagues (2011) demonstrated in a rat model that binge alcohol consumption impairs fracture healing in closed femur fractures and that treatment with NAC reverses this effect. The similarities in methods in the 2 studies were the use of closed femur fractures and i.p. administration of NAC after fracture. But there were several methodological differences. They injected EtOH daily for 3 days per week during 2 weeks starting at 16 weeks of age, followed by unilateral closed fracture and NAC treatment for 1 and 2 weeks. The male Wistar rats in our study were approximately 10 months old at the start of EtOH feeding and 15 months old when the fracture was created. We injected NAC on postfracture days 5 to 7 and euthanized the rats on day 8. The BAL in our EtOH-fed rats at the time of euthanasia was significantly lower than the BAL in the binge EtOH consumption model, which was expected by the delivery of the EtOH. We also note that the observed effects of NAC treatment in our study were not mediated by any reduction in BAL, but due to the direct action of NAC. In spite of these methodological differences, the biomarker data appear to be consistent with the histological findings of Volkmer and colleagues (2011) for the deleterious effects of EtOH on fracture healing and its reversal by NAC treatment. In NAC-treated rats, they observed a qualitative normalization of callus histological structure including recovery of endochondral bone formation activity to levels indistinguishable from normal fracture healing observed in saline-treated EtOH-free control rats. This suggests that NAC treatment reduces EtOH-induced oxidative stress and restores the normal balance of oxidant–antioxidant activities, which is what we observed in the present study. Our observations in this study are unique, in that we wanted to mimic the older chronic alcoholic, in an effort to show efficacy in treatment strategies with NAC in patients following fracture. In fact, patients chronically consuming alcohol tend to have hip fractures at an earlier age (64) compared to nonalcoholics (80) (Johnston and Parker, 2014). These younger patients experience increased postop wound infections and fracture healing compared to the older nondrinkers. While binge drinking definitely has numerous effects on fracture healing, this study was aimed at the long-term effects of alcoholism and the antioxidant abilities of NAC.
In conclusion, in this rat model of human chronic alcoholism, NAC treatment reduced oxidative stress, restored normal immune status and pro-inflammatory cytokine expressions, and abolished the excessive bone resorption in the process of maturation of the newly formed bone, thus restoring the normal onset of bone regeneration in the early phase of fracture healing. NAC treatment of chronic alcoholics following fracture may increase the healing process when abstinence is not possible.
FUNDING
This work was supported by The U.S. Department of Veterans Affairs Rehabilitation Research and Development Service VA Merritt Application #839867 Project # 1I21RX000353-01A2 “Prevention of Fracture Healing Failure in Alcohol Abusers.”
Footnotes
CONFLICTS OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA (2008) Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res 87:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alund AW, Mercer KE, Pulliam CF, Suva LJ, Chen JR, Badger TM, Ronis MJ (2017) Partial protection by dietary antioxidants against ethanol-induced osteopenia and changes in bone morphology in female mice. Alcohol Clin Exp Res 41:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew JG, Andrew SM, Freemont AJ, Marsh DR (1994) Inflammatory cells in normal human fracture healing. Acta Orthop Scand 65:462–466. [DOI] [PubMed] [Google Scholar]
- Askew A, Chakkalakal DA, Fang X, McGuire MH (2001) Delayed fracture healing in alcohol abusers – a preliminary retrospective study. Open Bone J 3:1–5. [Google Scholar]
- Bonnarens F, Einhorn TA (1984) Production of a standard closed fracture in laboratory animal bone. J Orthop Res 2:97–101. [DOI] [PubMed] [Google Scholar]
- Bonnevialle P, Bonnomet F, Philippe R, Loubignac F, Rubens-Duval B, Talbi A, Le Gall C, Adam P, SOFCOT (2012) Early surgical site infection in adult appendicular skeleton trauma surgery: a multicenter prospective series. Orthop Traumatol Surg Res 98:684–689. [DOI] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ (2010) A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. J Bone Miner Res 25:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Lumpkin CK, Badger TM, Ronis MJ (2011) Inhibition of NADPH oxidases prevents chronic ethanol-induced bone loss in female rats. J Pharmacol Exp Ther 336: 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AD, Bennet SJ, Aderinto J, Keating JF (2011) Fixation of intracapsular fractures of the femoral neck in young patients: risk factors for failure. J Bone Joint Surg Br 93:811–816. [DOI] [PubMed] [Google Scholar]
- Ercal N, Oztezcan S, Hammond TC, Matthews RH, Spitz DR (1996) High-performance liquid chromatography assay for N-acetylcysteine in biological samples following derivatization with N-(1-pyrenyl)maleimide. J Chromatogr B Biomed Appl 685:329–334. [DOI] [PubMed] [Google Scholar]
- Faroug R, Amanat S, Ockendon M, Shah SV, Gregory JJ (2014) The outcome of patients sustaining a proximal femur fracture who suffer from alcohol dependency. Injury 45:1076–1079. [DOI] [PubMed] [Google Scholar]
- Fuehrlein B, Ralevski E, O’Brien E, Jane JS, Arias AJ, Petrakis IL (2014) Characteristics and drinking patterns of veterans with alcohol dependence with and without post-traumatic stress disorder. Addict Behav 39: 374–378. [DOI] [PubMed] [Google Scholar]
- Greiffenstein P, Molina PE (2008) Alcohol-induced alterations on host defense after traumatic injury. J Trauma 64:230–240. [DOI] [PubMed] [Google Scholar]
- Harris AH, Bryson CL, Sun H, Blough D, Bradley KA (2009) Alcohol screening scores predict risk of subsequent fractures. Subst Use Misuse 44:1055–1069. [DOI] [PubMed] [Google Scholar]
- Hoiness P, Engebretsen L, Stromsoe K (2003) Soft tissue problems in ankle fractures treated surgically. A prospective study of 154 consecutive closed ankle fractures. Injury 34:928–931. [DOI] [PubMed] [Google Scholar]
- Iida H, Fukuda S (2002) Age-related changes in bone mineral density, cross-sectional area and strength at different skeletal sites in male rats. J Vet Med Sci 64:29–34. [DOI] [PubMed] [Google Scholar]
- Johnston LE, Parker MJ (2014) Hip fractures and chronic alcohol excess: a series of 7,023 cases. Hip Int 24:644–649. [DOI] [PubMed] [Google Scholar]
- Lauing KL, Sundaramurthy S, Nauer RK, Callaci JJ (2014) Exogenous activation of Wnt/beta-catenin signaling attenuates binge alcohol-induced deficient bone fracture healing. Alcohol Alcohol 49:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM (1989) Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24:197–211. [PubMed] [Google Scholar]
- Lippross S, Beckmann R, Streubesand N, Ayub F, Tohidnezhad M, Campbell G, Kan YW, Horst F, Sonmez TT, Varoga D, Lichte P, Jahr H, Pufe T, Wruck CJ (2014) Nrf2 deficiency impairs fracture healing in mice. Calcif Tissue Int 95:349–361. [DOI] [PubMed] [Google Scholar]
- Mathog RH, Toma V, Clayman L, Wolf S (2000) Nonunion of the mandible: an analysis of contributing factors. J Oral Maxillofac Surg 58:746–752; discussion 752–3. [DOI] [PubMed] [Google Scholar]
- Mercer KE, Sims CR, Yang CS, Wynne RA, Moutos C, Hogue WR, Lumpkin CK, Suva LJ, Chen JR, Badger TM, Ronis MJ (2014) Loss of functional NADPH oxidase 2 protects against alcohol-induced bone resorption in female p47phox−/− mice. Alcohol Clin Exp Res 38:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurulain SM, Ojha S, Tekes K, Shafiullah M, Kalasz H, Adem A (2015) Efficacy of n-acetylcysteine, glutathione, and ascorbic acid in acute toxicity of Paraoxon to Wistar rats: survival study. Oxid Med Cell Longev 2015:329306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist F, Berglund M, Nilsson BE, Obrant KJ (1997) Nature and healing of tibial shaft fractures in alcohol abusers. Alcohol Alcohol 32:91–95. [DOI] [PubMed] [Google Scholar]
- Ovaska MT, Makinen TJ, Madanat R, Vahlberg T, Hirvensalo E, Lindahl J (2013) Predictors of poor outcomes following deep infection after internal fixation of ankle fractures. Injury 44:1002–1006. [DOI] [PubMed] [Google Scholar]
- Perrien DS, Wahl EC, Hogue WR, Feige U, Aronson J, Ronis MJ, Badger TM, Lumpkin JR CK (2004) IL-1 and TNF antagonists prevent inhibition of fracture healing by ethanol in rats. Toxicol Sci 82:656–660. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Reilly ME, Patel VB, Richardson PJ, Peters TJ (1999) Protein metabolism in alcoholism: effects on specific tissues and the whole body. Nutrition 15:604–608. [DOI] [PubMed] [Google Scholar]
- Rantala A, Lehtonen OP, Niinikoski J (1997) Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control 25:381–386. [DOI] [PubMed] [Google Scholar]
- Roper PM, Abbasnia P, Vuchkovska A, Natoli RM, Callaci JJ (2016) Alcohol-related deficient fracture healing is associated with activation of FoxO transcription factors in mice. J Orthop Res 34:2106–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska AM, Manuel YKB, de Backer WA (2007) Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther 20:9–22. [DOI] [PubMed] [Google Scholar]
- Senel FC, Jessen GS, Melo MD, Obeid G (2007) Infection following treatment of mandible fractures: the role of immunosuppression and polysubstance abuse. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103: 38–42. [DOI] [PubMed] [Google Scholar]
- Serena-Gomez E, Passeri LA (2008) Complications of mandible fractures related to substance abuse. J Oral Maxillofac Surg 66:2028–2034. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P (2009) A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res 33:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MV, Puleo DA (2011) Infection, inflammation, and bone regeneration: a paradoxical relationship. J Dent Res 90:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thursz M, Morgan TR (2016) Treatment of severe alcoholic hepatitis. Gastroenterology 150:1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen H, Pedersen A, Jensen MR, Moller A, Madsen JC (1991) Ankle fractures and alcoholism. The influence of alcoholism on morbidity after malleolar fractures. J Bone Joint Surg Br 73:511–513. [DOI] [PubMed] [Google Scholar]
- Volkmer DL, Sears B, Lauing KL, Nauer RK, Roper PM, Yong S, Stover M, Callaci JJ (2011) Antioxidant therapy attenuates deficient bone fracture repair associated with binge alcohol exposure. J Orthop Trauma 25:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl EC, Aronson J, Liu L, Liu Z, Perrien DS, Skinner RA, Badger TM, Ronis MJ, Lumpkin JR CK (2007) Chronic ethanol exposure inhibits distraction osteogenesis in a mouse model: role of the TNF signaling axis. Toxicol Appl Pharmacol 220:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrotek S, Jedrzejewski T, Potera-Kram E, Kozak W (2011) Antipyretic activity of N-acetylcysteine. J Physiol Pharmacol 62:669–675. [PubMed] [Google Scholar]
- Wu W, Goldstein G, Adams C, Matthews RH, Ercal N (2006) Separation and quantification of N-acetyl-l-cysteine and N-acetyl-cysteine-amide by HPLC with fluorescence detection. Biomed Chromatogr 20:415–422. [DOI] [PubMed] [Google Scholar]
- Wu YJ, Muldoon LL, Neuwelt EA (2005) The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J Pharmacol Exp Ther 312:424–431. [DOI] [PubMed] [Google Scholar]
- Yeler H, Tahtabas F, Candan F (2005) Investigation of oxidative stress during fracture healing in the rats. Cell Biochem Funct 23:137–139. [DOI] [PubMed] [Google Scholar]


