Significance
Synucleinopathies such as Parkinson’s disease feature deposition of misfolded α-synuclein. It is likely that cellular proteostasis compensates for misfolded α-synuclein to some extent, but, once exhausted, α-synuclein can form seeds for a prion-like spread in the brain. Here, we demonstrate that, in human dopaminergic neurons and in mouse brain, H1N1 influenza virus induces aggregation of α-synuclein by blocking protein degradation pathways. Following intranasal instillation, H1N1 spreading along the olfactory route into brain areas mimics α-synuclein deposits in synucleinopathies. H1N1 may therefore be considered a risk factor for synucleinopathies that could potentially be minimized by regular vaccination. On the contrary, H1N1 tropism for olfactory epithelium suggests that live attenuated virus vaccines should be investigated for possible long-term effects on protein misfolding.
Keywords: influenza, α-synuclein, DISC1, protein misfolding, Parkinson’s disease
Abstract
Neurodegenerative diseases feature specific misfolded or misassembled proteins associated with neurotoxicity. The precise mechanisms by which protein aggregates first arise in the majority of sporadic cases have remained unclear. Likely, a first critical mass of misfolded proteins starts a vicious cycle of a prion-like expansion. We hypothesize that viruses, having evolved to hijack the host cellular machinery for catalyzing their replication, lead to profound disturbances of cellular proteostasis, resulting in such a critical mass of protein aggregates. Here, we investigated the effect of influenza virus (H1N1) strains on proteostasis of proteins associated with neurodegenerative diseases in Lund human mesencephalic dopaminergic cells in vitro and infection of Rag knockout mice in vivo. We demonstrate that acute H1N1 infection leads to the formation of α-synuclein and Disrupted-in-Schizophrenia 1 (DISC1) aggregates, but not of tau or TDP-43 aggregates, indicating a selective effect on proteostasis. Oseltamivir phosphate, an antiinfluenza drug, prevented H1N1-induced α-synuclein aggregation. As a cell pathobiological mechanism, we identified H1N1-induced blocking of autophagosome formation and inhibition of autophagic flux. In addition, α-synuclein aggregates appeared in infected cell populations connected to the olfactory bulbs following intranasal instillation of H1N1 in Rag knockout mice. We propose that H1N1 virus replication in neuronal cells can induce seeds of aggregated α-synuclein or DISC1 that may be able to initiate further detrimental downstream events and should thus be considered a risk factor in the pathogenesis of synucleinopathies or a subset of mental disorders. More generally, aberrant proteostasis induced by viruses may be an underappreciated factor in initiating protein misfolding.
A hallmark of neurodegenerative diseases is the occurrence of misfolded proteins (1), each specific for a clinical disease: α-synuclein–containing Lewy bodies in synucleinopathies such as Parkinson’s disease (PD), PD with dementia (PDD), multiple systems atrophy (MSA), and dementia with Lewy bodies (DLB); extracellular beta-amyloid plaques and intraneuronal tangles of hyperphosphorylated tau in Alzheimer’s disease (AD); or TAR-DNA–binding protein 43 (TDP-43) aggregates in amyotrophic lateral sclerosis (ALS). The fact that the same proteins form aggregates in familial cases of neurodegenerative disease where a mutant protein is involved, and in sporadic cases where aggregates are caused independent of a mutation (2), has led to the notion that aggregates are a manifestation of a step common to both familial and sporadic disease that is critical for the disease process ultimately leading to neuronal death.
The synucleinopathies are characterized by cytosolic Lewy bodies, consisting of fibrillar α-synuclein associated with neuronal degeneration (3). In PD, degeneration is most prominent in dopaminergic neurons of the substantia nigra (SN), which leads to a loss of dopaminergic innervation of basal ganglia and the characteristic motor symptoms of PD. In PDD, limbic structures are also involved, while, in DLB, the changes do not extend widely out of the olfactory system connectome as described and reviewed (4).
Aggregate formation may also occur in at least a subset of chronic mental illnesses (CMI) such as schizophrenia or the recurrent affective disorders (5). Insoluble Disrupted-in-schizophrenia 1 (DISC1) protein has been identified in 15% of postmortem brains from patients with schizophrenia, bipolar disease, or major depression (6), and, in a transgenic rat model modestly overexpressing the DISC1 protein, modeling this CMI subset features aberrant dopaminergic homeostasis, as seen in behavioral, neurochemical, and biochemical changes, including the induction of perinuclear DISC1 aggregates mainly in dopamine-rich regions (7).
An increasing set of data suggests that misfolded proteins, including α-synuclein and DISC1, amplify their pathogenic signaling similar to prion replication, i.e., misfolded conformers accelerate conversion of correctly folded physiological conformers (8–11). In this case, once a critical threshold of aggregated proteins is present, a cascade of events is triggered that ends with neuronal death in affected anatomical areas (11). Similar to prions, misfolded α-synuclein can also propagate transneuronally following anatomical connections (4). The prion hypothesis of α-synuclein as the sole cause for PD or other synucleinopathies in humans is currently controversial, with valuable arguments on both sides (12). Experimental transmission experiments in animals, though, have yielded good support for the prion-like nature of α-synuclein (9, 11).
We currently lack an understanding for the initial phases of the formation of the critical mass of misfolded proteins (“first prions”) that can initiate prion replication and trigger signaling events leading to acceleration of the process and neuronal death. One hypothesis is that a disturbance in protein homeostasis (proteostasis), i.e., a cellular imbalance between genesis of misfolded proteins and their degradation, can contribute to the steady accumulation of aggregated proteins (13). Reasons for disturbed proteostasis can be manifold and involve toxins, inflammation, oxidative stress, and infections with viruses (14).
Viruses have much shorter replication cycles than their host cells (15), and thus they have evolved to take advantage of a host cell’s machinery for an efficient viral replication. We have previously demonstrated that this catalysis is not limited to the viral genome but also comprises its capsid assembly that is catalyzed by host multiprotein complexes (16). During viral replication, host cell proteostasis is disturbed by affecting quality control mechanisms (17) and depleting energy resources (reviewed in ref. 18). Viral infections could thus lead to aberrant proteostasis and misassembly or aggregation of susceptible proteins instrumental in fatal signaling cascades in neurodegenerative diseases (19).
An overt influenza-induced encephalitis is rare and concerns mostly children or immunocompromised individuals, since the immune system is usually able to prevent CNS invasion (20–22). In immunodefective mice, H1N1 virus persists in the midbrain over prolonged periods of time (23) and can enter via olfactory or trigeminal routes (24, 25). In immunodeficient patients, influenza virus may enter the CNS via the olfactory tract (26). In ferrets, an asymptomatic neuroinvasion with H5N1 has been reported (25). Remarkably, two mutations in the viral NS1 protein are sufficient to change virus tropism from lung to brain (27), and a variety of host genetic factors affect tropism and virulence of influenza H1N1 that is subject to constant mutation (28). Thus, several viral and/or host factors may play a role in neuroinvasion of influenza and do not necessarily have to exhibit overt symptoms of classical encephalitis.
Suggestions for a pathogenic role of influenza in nervous system diseases such as postencephalitic parkinsonism appeared following the “Spanish flu” of 1918 to 1920 that overlapped with the lethargic encephalitis epidemic of 1916 to 1926, but are controversial (29–31). Also, evidence from epidemiological studies demonstrating a relation between influenza and PD is ambiguous, with some studies supporting a role of influenza in PD (32, 33) and some not (34). However, such studies are methodologically very difficult since (i) up to 60% of influenza-infected individuals are asymptomatic or have mild symptoms, i.e., there is no correlation between clinical symptoms and serology (35); and (ii) variations in genetic factors determine clinical outcome (36). In order to perform well-controlled studies, extensive diagnostic measures would have to be taken in order to achieve meaningful epidemiological results, which has not been done so far.
In this study, we used the H1N1 influenza A/WS/33 strain for infections in vitro (37) and the mouse-neuroadapted H1N1 influenza A/WSN/33 in vivo (38). This strain has been used for studies on molecular and neurophysiological interactions with neurons (39). Here, we demonstrate that the H1N1 influenza A/WS/33 strain (37) leads to aggregation of endogenous α-synuclein and DISC1 in differentiated, dopaminergic, neuron-like Lund human mesencephalic (LUHMES) cells. In addition, α-synuclein aggregates were seen in populations of infected neurons connected to the olfactory bulb following intranasal viral instillation of the neuroadapted A/WSN/33 strain in mice. We also show mechanistically that the proteostatic disturbance of the autophagic flux in LUHMES cells caused by H1N1 infection, which impairs protein degradation, could initiate a critical mass of aggregated proteins.
Results
Influenza A (H1/N1) Infection Disturbs Protein Homeostasis in Human Dopaminergic Neurons.
In order to understand whether influenza A (H1N1) viral infection leads to a specific impairment in protein homeostasis relevant for PD, LUHMES cells were differentiated into human dopaminergic neurons (40). At postdifferentiation day 5, the morphology (SI Appendix, Fig. S1A) as well as the expression of dopaminergic cellular markers (dopamine transporter and tyrosine hydroxylase; SI Appendix, Fig. S1 B and C) indicated neuronal maturity. In a candidate protein approach, several endogenously expressed proteins related to protein misfolding diseases were investigated in differentiated LUHMES cells. In undifferentiated, proliferating LUHMES cells, human DISC1 and α-synuclein protein expression was barely detected (SI Appendix, Fig. S1D). A small amount of TDP-43 and a noticeable expression of total tau was already detected in undifferentiated cells (SI Appendix, Fig. S1D). At differentiation day 5, a significant increase in the expression of all proteins was observed (P < 0.05; SI Appendix, Fig. S1D). All subsequent experiments were therefore done in 5-d-differentiated LUHMES cells.
LUHMES cells were infected with a low multiplicity of infection (MOI) of H1N1 influenza A virus strain A/WS/33 (41) (MOI = 1), which resulted in an infection rate of 30 to 40% in LUHMES cells 24 h after viral infection (33.3 ± 3.92% infected cells; SI Appendix, Fig. S2A). The replication kinetics of H1N1 influenza A virus from 0 to 24 h p.i. were analyzed. The viral concentration in the medium increased substantially 12 h p.i. (0.6 × 104 PFU/mL ± 0.46 × 104 PFU/mL), being stable at 24 h p.i. (1.7 × 104 PFU/mL ± 1.32 × 104 PFU/mL; SI Appendix, Fig. S2B). Since no morphological changes in the cells 24 h p.i. were observed, all following analyses in the present study were done at 24 h p.i.
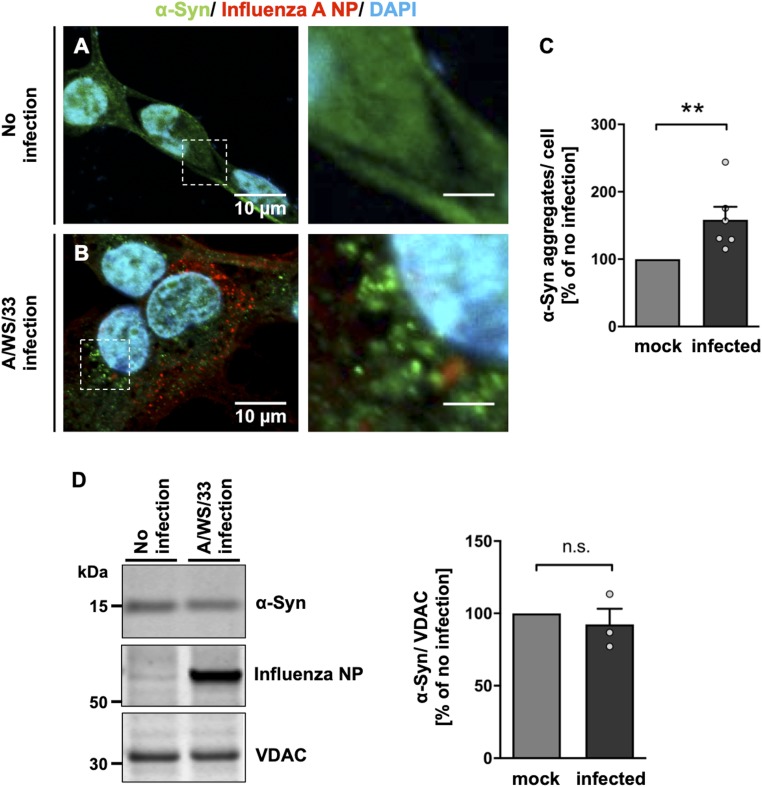
Immunofluorescence analysis revealed α-synuclein aggregates in LUHMES cells infected with H1N1 influenza A virus (Fig. 1 A and B). Quantification of aggregated α-synuclein showed a significant increase in aggregate number after viral infection (59 ± 19.20%; P = 0.002) in comparison with the noninfected condition, where α-synuclein aggregates were rarely detectable (Fig. 1C). An immunoblot analysis indicated that total protein levels of α-synuclein were not affected by H1N1 influenza A infection (Fig. 1D). There was, in part, colocalization of α-synuclein with thioflavin S (ThS; S2D), a marker for amyloid fibrils. In order to perform a functional assay to test the potential α-synuclein seeding activity of H1N1-induced aggregates in LUHMES cells, we performed a real-time quaking-induced conversion (RT-QuIC) assay (42). With the reservation that this method cannot be considered fully established for α-synuclein (in contrast to the pathological prion protein), it nevertheless indicated that the influenza-infected LUHMES cells displayed increased seeding potential (SI Appendix, Fig. S3A) as compared to uninfected control.
Fig. 1.
A/WS/33 (H1N1) infection induces α-synuclein aggregation in human dopaminergic neurons. (A and B) An increase in aggregated α-synuclein was detected in the cytoplasm of LUHMES cells 24 h after influenza infection (B; MOI of 1) compared to noninfected cells (A; green, α-synuclein; red, influenza A NP; blue, DAPI). (Scale bars: Left, 10 μm; Right, 3 μm; dotted boxes show regions of higher magnification.) (C) Quantification of influenza-induced α-synuclein aggregates per cell of the infected (black bar) versus the noninfected condition (gray bar) in percent showed an increase of α-synuclein aggregates. Mann–Whitney U test with one-tailed Dunn’s post hoc test was used as a statistical test (**P < 0.01; n = 6; means ± SEM). (D) Immunoblot of total protein levels of α-synuclein in lysates of infected and noninfected LUHMES cells. VDAC-normalized α-synuclein levels were comparable between infected (black bar) and noninfected (gray bar) cells (n = 3; means ± SEM). Circles in bar graphs represent results of single experiments. n.s., statistically not significant.
To demonstrate the effect of H1N1 influenza A viral infection on α-synuclein in an independent cell line, human NLF (nondopaminergic) neuroblastoma cells (43) transiently transfected with human α-synuclein were also infected with H1N1 (for 24 h; MOI of 1), and likewise led to the induction of α-synuclein aggregates (SI Appendix, Fig. S3 B and C).
We were also interested to understand whether H1N1 influenza A replication leads to misassembly of other disease-specific proteins such as DISC1, which we had previously identified to aggregate in vitro (44) and in vivo in a subpopulation of CMI patients (6, 7), tau protein, or TDP-43. As for α-synuclein, H1N1 influenza A/WS/33 infection induced aggregation of endogenously expressed DISC1 as observed by immunofluorescence in differentiated LUHMES cells (SI Appendix, Fig. S4 A and B). DISC1 aggregates were quantified, and a significant increase in the percentage of aggregates per cell was observed after viral infection (93 ± 29.20%; P = 0.01) compared to the noninfected condition (SI Appendix, Fig. S4C). We thus clearly demonstrate that endogenous human DISC1 protein aggregates if stressed by H1N1 infection. Induction of aggregated DISC1 by H1N1 infection was corroborated in human NLF neuroblastoma cells transiently transfected with human mRFP-DISC1 (SI Appendix, Fig. S4D). Of note, α-synuclein and DISC1 did not coaggregate, excluding cross-seeding mechanisms (SI Appendix, Fig. S4E). Protein half-life time of both α-synuclein (SI Appendix, Fig. S5 A and B) and DISC1 (SI Appendix, Fig. S5 C and D) was increased by H1N1 infection.
H1N1 influenza A infection in LUHMES cells did not lead to abnormal accumulation of cytoplasmic TDP-43 aggregates (SI Appendix, Fig. S6A). Neither tau protein changes such as aggregation or hyperphosphorylation were seen after H1N1 infection (SI Appendix, Fig. S6B). Neither DISC1 nor tau or TDP-43 costained with amyloid fibril marker ThS (SI Appendix, Fig. S7 A and B).
These data demonstrate that influenza A/WS/33 infection induces aggregation of α-synuclein and DISC1, but not tau or TDP-43, and suggest a protein-selective effect of the viral replication on molecular circuitry of proteostasis.
Influenza A Infection Dramatically Increases α-Synuclein Protein Levels In Vivo.
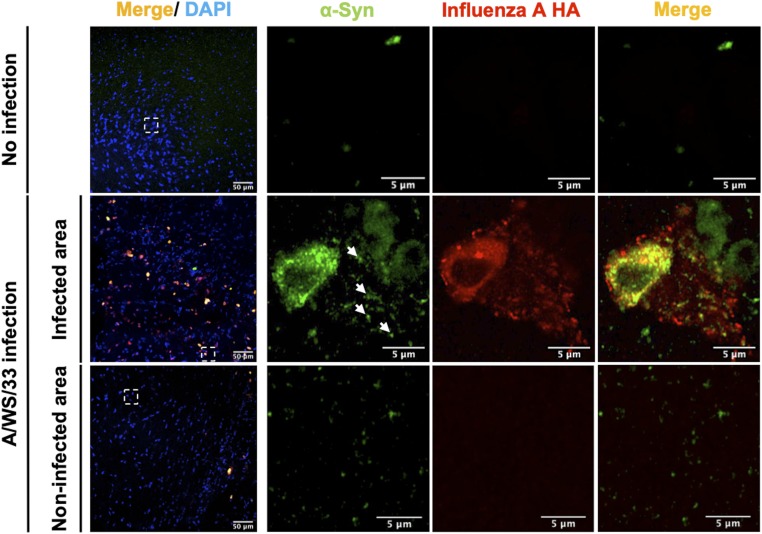
Next, we wanted to understand the direct effect of H1N1 influenza A virus infection on neuronal proteostasis of α-synuclein in vivo. In order to prevent any interference of an influenza infection with the adaptive immune system, genetically modified mice lacking B and T cells due to a deletion of the recombinant activating gene 1 (Rag−/−), necessary for an MHC-dependent adaptive immune response, were used (38). Three- to 5-mo-old female knockout mice were intranasally instilled with 3.6 × 105 PFU/µL of A/WSN/33 (H1N1) strain (38) or with PBS and euthanized 28 d later. Notably, in these animals, H1N1 viral antigens were mainly detected in brain areas connected to the olfactory and trigeminal pathway projections (38). Sections of the lateral hypothalamus from the study by Tesoriero and coworkers (38) taken at 28 d p.i. showed cells with punctuated and cytoplasmic immunolabeling for α-synuclein only in those sections also immunolabeled for viral antigens. In neighboring noninfected areas of infected animals or in noninfected animals, α-synuclein was not detected (Fig. 2). Previous research demonstrated the infection to affect mainly neurons, few microglia, and many astroglia (38). Even though there was basal immunoreactivity of α-synuclein, levels were dramatically increased after H1N1 infection (Fig. 2). To assess whether the different α-synuclein protein expression levels observed in the H1N1-infected brain area were due to an effect in the promoter activity of α-synuclein gene (SNCA), a luciferase reporter assay was performed. A pGL3 luciferase reporter vector with the canonical transcriptional start site 5′ of exon 1 of the SNCA gene was transiently transfected into NLF neuroblastoma cells, and the cells were subsequently infected with H1N1 influenza A/WS/33. Changes in the expression of an SNCA 5′-promoter construct upon influenza infection were not detected (SI Appendix, Fig. S5E), suggesting that the increase in α-synuclein levels was not due to increased expression but decreased degradation.
Fig. 2.
Twenty-eight days of A/WSN/33 (H1N1) influenza virus infection affects mouse α-synuclein protein levels in vivo. Mouse α-synuclein protein levels were highly up-regulated and exclusively detected in influenza A (hemagglutinin [HA] protein)-positive cells of Rag1 knockout mice (green, α-synuclein; red, influenza A HA; blue, DAPI). In noninfected areas and in noninfected mice, there was no detectable α-synuclein signal. Arrows mark α-synuclein aggregates colocalized with influenza A HA protein. (Scale bars as labeled on the images; dotted boxes show regions of higher magnification.)
Next, we investigated whether H1N1 influenza A/WSN/33 infection in mouse brain affected Disc1 protein levels as well as tau and Tdp-43. For Disc1, an increase in immunoreactivity was observed in brain areas infected with H1N1 influenza A (SI Appendix, Fig. S8A) compared to PBS-inoculated mice, where a low background Disc1 expression level was observed (SI Appendix, Fig. S8A). In a control experiment in vitro, promoter activity of the DISC1 gene after H1N1 infection was probed using a luciferase reporter assay. NLF neuroblastoma cells transiently transfected with a construct comprising the DISC1 promoter, from −2,300 to +45 bp relative to the transcription start site (45), in front of the luciferase gene were infected with H1N1 influenza A/WS/33. No change in DISC1 promoter activity in infected vs. noninfected controls was observed (SI Appendix, Fig. S5F), likewise suggesting that the increase in Disc1 protein levels was not due to an effect of increased transcription or translation but likely decreased degradation. For tau and Tdp-43, we observed protein expression patterns that were independent of H1N1 influenza A infection and without signs of aggregation (SI Appendix, Fig. S9 A–D) in contrast to what we observed for α-synuclein and Disc1. These data suggest that H1N1 influenza A infection increased aggregation propensity of α-synuclein and Disc1 but not of tau and Tdp-43 in mouse brains, and thus that H1N1 infection modulates a particular proteostatic molecular circuitry.
Complete Influenza A Replication Is Required to Disrupt α-Synuclein Protein Homeostasis.
Influenza virus is an RNA coding virus with a helical nucleocapsid that comprises the viral genome and four viral proteins with the nucleocapsid protein (NP) as the quantitatively major component. During the viral replication cycle, NP plays a central role in transcription, replication, and packaging. Each viral RNA segment is associated with NP molecules in order to protect the viral genome from nuclease degradation by the host cell (46).
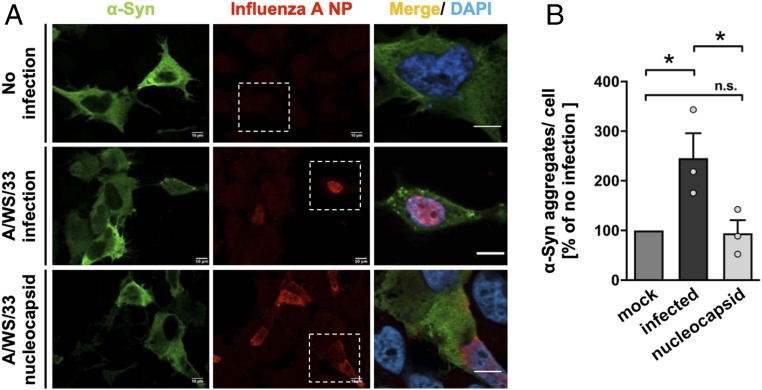
To understand whether a complete influenza A virus replication with assembly of all polypeptides was required for proteostasis disruption or whether NP assembly alone was sufficient to induce α-synuclein proteostatic changes, NLF neuroblastoma cells were transiently cotransfected with influenza A virus (A/WS/33) segment 5 NP and human α-synuclein. Forty-eight hours after transfection, changes in the α-synuclein proteostasis were analyzed by immunocytochemistry (Fig. 3A). In NLF neuroblastoma cells, expression of the viral NP alone was not able to trigger α-synuclein aggregates to the same extent as a complete H1N1 influenza A infection (147 ± 50.40% increase; P = 0.04; Fig. 3B). Viral antigen detection in the immunolabeling confirmed that the helical capsid protein was expressed by the host cell (Fig. 3A).
Fig. 3.
Influenza A virus (A/WS/33) segment 5 nucleocapsid protein (NP) is not sufficient to induce α-synuclein aggregates. (A) Influenza-induced α-synuclein aggregation was only detected in NLF neuroblastoma cells transiently transfected with human α-synuclein and infected with A/WS/33 virus. In cells double-transfected with α-synuclein and influenza virus segment 5 nucleocapsid, α-synuclein aggregates were not visible. Noninfected cells did not show aggregated α-synuclein (green, α-synuclein; red, influenza A NP; blue, DAPI). (Scale bars, 10 μm; dotted boxes frame the area magnified in the picture on Right.) (B) Quantification of percentage of aggregated α-synuclein per cell infected for 24 h with A/WS/33 virus (MOI of 1; black bar) or transfected with A/WS/33 nucleocapsid (light gray bar) relative to the noninfected condition (dark gray bar). Infection led to an increase in α-synuclein aggregates per cell, whereas transfection of nucleocapsid did not (n = 3; means ± SEM). One-way ANOVA was used as a statistical test [Dunnett’s multiple comparisons test, F(2,4) = 10.12; *P < 0.05; noninfected vs. infected P = 0.0401; infected vs. capsid only P = 0.0356; *P < 0.05; n.s. not significant]. Circles in bar graphs represent results of single experiments.
NLF neuroblastoma cells were also cotransfected with H1N1 NP and mRFP-DISC1. Forty-eight hours after transfection, immunocytochemistry was performed (SI Appendix, Fig. S10A). A slight but not significant increase in DISC1 aggregate number was detected in cells cotransfected with NP (14 ± 21.70% increase) in comparison with the noninfected condition (SI Appendix, Fig. S10B). As expected, the number of DISC1 aggregates after H1N1 influenza A infection (63 ± 31.30% increase; P = 0.05) was increased. We conclude that replication of a complete viral genome of H1N1 influenza virus is required to trigger aberrant proteostasis of α-synuclein and DISC1.
An Antiviral Compound Can Prevent the Alterations in α-Synuclein Protein Homeostasis Caused by Influenza A Infection.
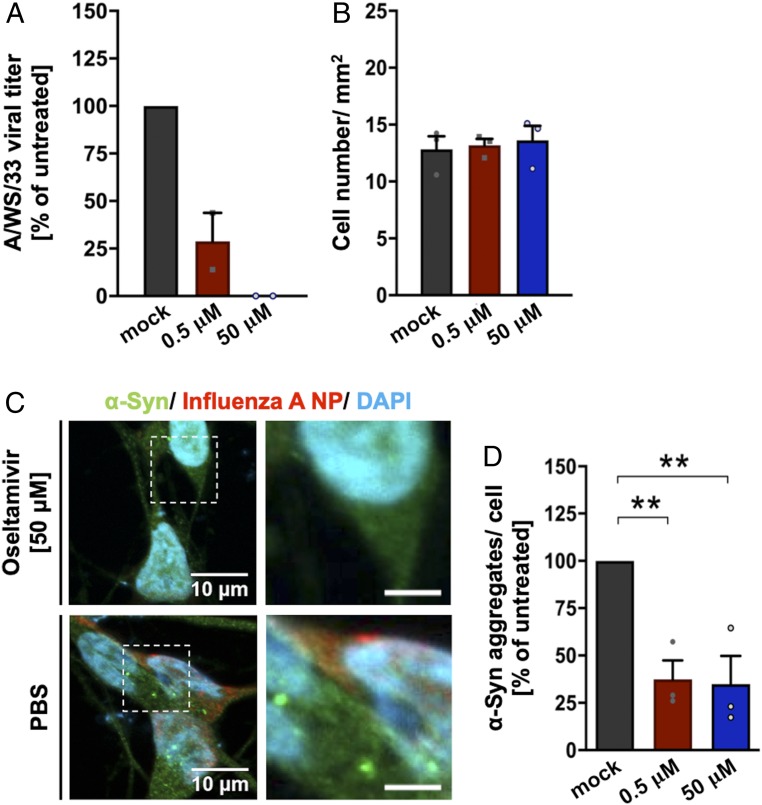
Next, we wanted to understand whether a pharmacological compound that interferes with H1N1 viral life cycle would inhibit α-synuclein aggregation. Oseltamivir phosphate inhibits the neuraminidase enzyme of influenza A and B, preventing the efficient release of newly replicated influenza virus (47). Differentiated LUHMES cells were used to assess the effect of the antiviral compound on H1N1-induced α-synuclein aggregates. Cells were treated with two different concentrations of oseltamivir phosphate, 0.5 or 50 µM. Oseltamivir phosphate concentrations were selected according to their known EC50 against influenza virus (EC50, 0.0008 to 35 μM) (48). After 8 h of compound treatment, cells were infected with H1N1 influenza A virus (MOI of 1 for 24 h). The antiviral activity of oseltamivir phosphate was assessed by TCID50 assay, and a reduction in influenza infectivity was observed in a concentration-dependent manner. In the supernatant of LUHMES cells treated with oseltamivir phosphate, the viral infectivity decreased by 75% for 0.5 µM and 99.9% for 50 µM (Fig. 4A).
Fig. 4.
Oseltamivir phosphate prevents α-synuclein aggregate formation in human dopaminergic neurons. The effect of the antiviral drug oseltamivir phosphate on α-synuclein aggregate formation in infected LUHMES cells (MOI of 1, 24 h) was tested. Black bar represents PBS-treated condition; red bar, 0.5 µM oseltamivir; and blue bar, 50 µM oseltamivir. (A) Compound antiviral activity was measured by reduction in the percentage of PFU/mL of A/WS/33 virus. TCID50 assay was performed in MDCK.2 cells. Treatment of LUHMES cells with oseltamivir phosphate led to a decrease in viral infectivity (n = 2; means ± SEM). (B) Quantification of LUHMES cell number per square millimeter did not show a compound-dependent effect in cell viability. (C) Representative image of LUHMES cells virally infected and treated with 50 µM oseltamivir phosphate or the respective PBS control (green, α-synuclein; red, influenza A NP; blue, DAPI). (Scale bars: Left, 10 μm; Right, 3 μm; dotted boxes show regions of higher magnification.) (D) Calculation of aggregated α-synuclein per cell in infected LUHMES cells treated with low and high compound concentrations. Oseltamivir phosphate treatment showed a reduction in α-synuclein aggregate formation. Data presented as percentage of aggregated α-synuclein in infected condition normalized to noninfected condition (n = 3; means ± SEM). One-way ANOVA was used as a statistical test [Dunnett’s multiple comparisons test, F(2,4) = 23.69; **P < 0.01; PBS vs. 0.5 µM P = 0.0074; PBS vs. 50 µM P = 0.0064; **P < 0.01]. Circles in bar graphs represent results of single experiments.
The compound concentrations used were nontoxic for the cells, not demonstrating any effect on cell viability in comparison with the PBS control condition (PBS, 12.8 ± 1.14 cells/mm2; 50 µM, 13.6 ± 1.26 cells/mm2; 0.5 µM, 13.18 ± 0.56 cells/mm2; Fig. 4B). The effect of oseltamivir phosphate on H1N1-induced α-synuclein aggregates was measured 30 h after compound treatment by immunofluorescence (Fig. 4C). We observed a significant decrease in the percentage of α-synuclein aggregates per cell with both concentrations used (0.5 µM, 63 ± 10.00% reduction; P = 0.007; 50 µM, 65 ± 14.90% reduction; P = 0.006) relative to PBS control conditions (Fig. 4D). These data suggest that pharmacological modulation of H1N1 influenza A/WS/33 replication also affects α-synuclein aggregation levels.
Influenza A Infection Affects the Autophagic Machinery Leading to Changes of α-Synuclein Protein Homeostasis.
A disturbance in the later macroautophagy stage (hereafter autophagy) in GFP-LC3–expressing epithelial cells due to the inhibition of autophagosome fusion with lysosome was previously described (49). Through modulation of c-Jun N-terminal protein kinase 1 and PI3K-Akt-mTOR pathways, an inhibition in the autophagosome formation was also reported (50). Early autophagy disruption was assumed to affect the clearance of presynaptic α-synuclein. In 20-mo-old mice with a deletion in an essential gene involved in autophagosome formation (Atg7), α-synuclein aggregates in striatal neuritic swellings were detected. The same inclusions were also seen in cerebellar Purkinje axons from younger animals (1.5 mo old) (51). Therefore, we hypothesized that the disturbance of autophagy by an external stressor such as an influenza infection could lead to the accumulation of influenza-induced α-synuclein aggregates.
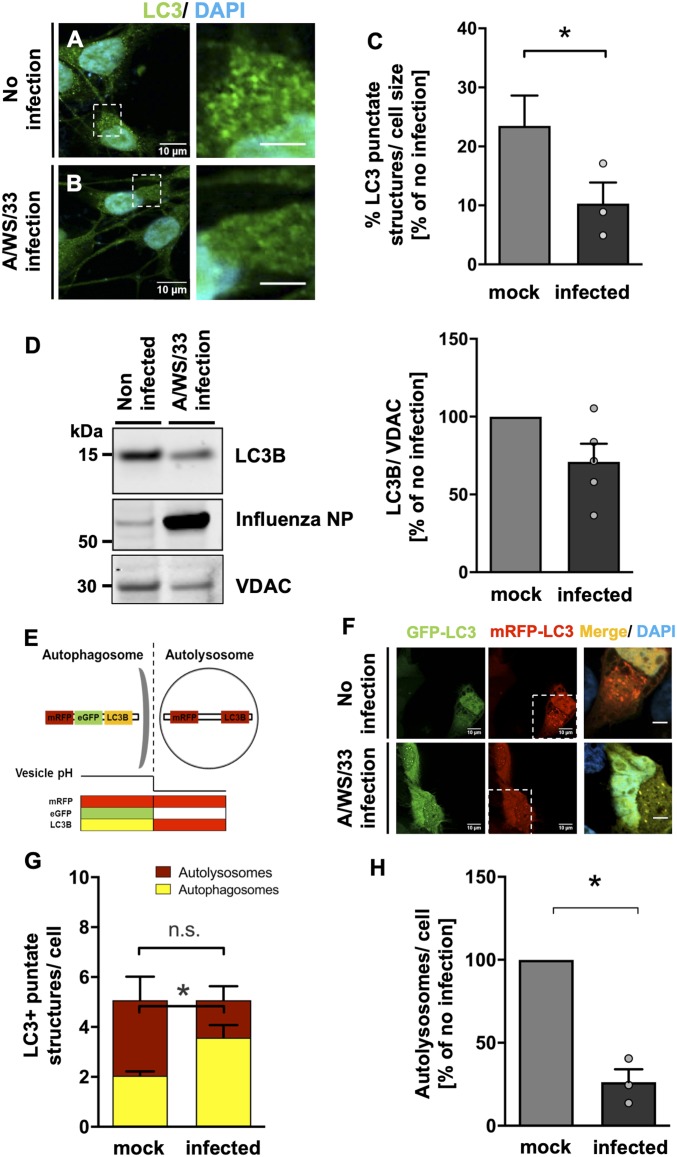
In order to investigate the specific consequences of H1N1 influenza A infection for cellular autophagy in a model as close to primary human dopaminergic neurons as possible, differentiated LUHMES cells were again used. Cells were infected with H1N1 influenza A (MOI of 1 for 24 h), and, by immunolabeling a decrease in cytoplasmic LC3 punctate structures, a specific protein involved in the autophagosome formation was observed (Fig. 5 A and B). Quantification of LC3 punctate structures showed a significant reduction of autophagosomes after H1N1 influenza A infection (62 ± 22.70% reduction; P < 0.05) in comparison with noninfected cells (Fig. 5C). A tendency in reduction of total LC3 levels was observed by immunoblot (Fig. 5D). Immunolabeling of infected LUHMES cells did not show an overlap between α-synuclein aggregates and LC3 punctate structures, indicating an inefficient packing of these aggregates in autophagosomes or an interruption in the maturation of autophagosomes to autolysosomes due to impairment of the fusion with lysosomes.
Fig. 5.
Twenty-four hours of influenza A (A/WS/33) infection decreases autophagosome number and impairs autophagic flux in human dopaminergic neurons. (A and B) Visualization of the decrease in LC3 structures in influenza-infected LUHMES cells (MOI of 1, 24 h) in comparison with noninfected condition (green, LC3; blue, DAPI). (Scale bars: Left, 10 μm; Right, 3 μm; dotted boxes show regions of higher magnification.) (C) Quantification of percentage of LC3 punctate structures in infected condition (black bar) relative to the noninfected condition (gray bar). After infection, the LC3 punctate structures per cell were decreased (n = 3; means ± SEM). Mann–Whitney U with two-tailed Dunn’s post hoc test was used as a statistical test (*P < 0.05). (D) Immunoblot for LC3B in lysates of infected and noninfected LUHMES cells. VDAC-normalized LC3B signal in infected (black bar) as percent of noninfected condition (gray bar) is shown. A tendency in reduction of total LC3 levels after influenza infection was observed (n = 5; means ± SEM). Mann–Whitney U with one-tailed Dunn’s post hoc test was used as a statistical test. (E) Schematic representation of the tandem reporter construct mRFP-GFP-LC3 used to assess the changes in autophagic flux under influenza infection. (F) NLF neuroblastoma cells transiently transfected with the mRFP-GFP-LC3 construct and subsequently infected with influenza (MOI of 1, 24 h) or control. GFP and mRFP fluorescent signals were analyzed. (Scale bars, 10 μm; dotted boxes show regions of higher magnification.) (G) Quantification of yellow LC3 structures (merged mRFP and GFP signals, yellow bars) labeling autophagosomes and the red LC3 structures (mRFP, red bars) labeling autolysosomes. Under viral infection, an increased number of autophagosomes was detected (n = 3; means ± SEM). Mann–Whitney U with one-tailed Dunn’s post hoc test was used as a statistical test (*P < 0.05; n.s. not significant). (H) A decrease in the percentage of autolysosomes formed per cell was visible in infected condition (black bar) relative to the noninfected control (gray bar; n = 3; means ± SEM). Mann–Whitney U with one-tailed Dunn’s post hoc test was used as a statistical test (*P < 0.05; n.s. not significant). Circles in bar graphs represent results of single experiments.
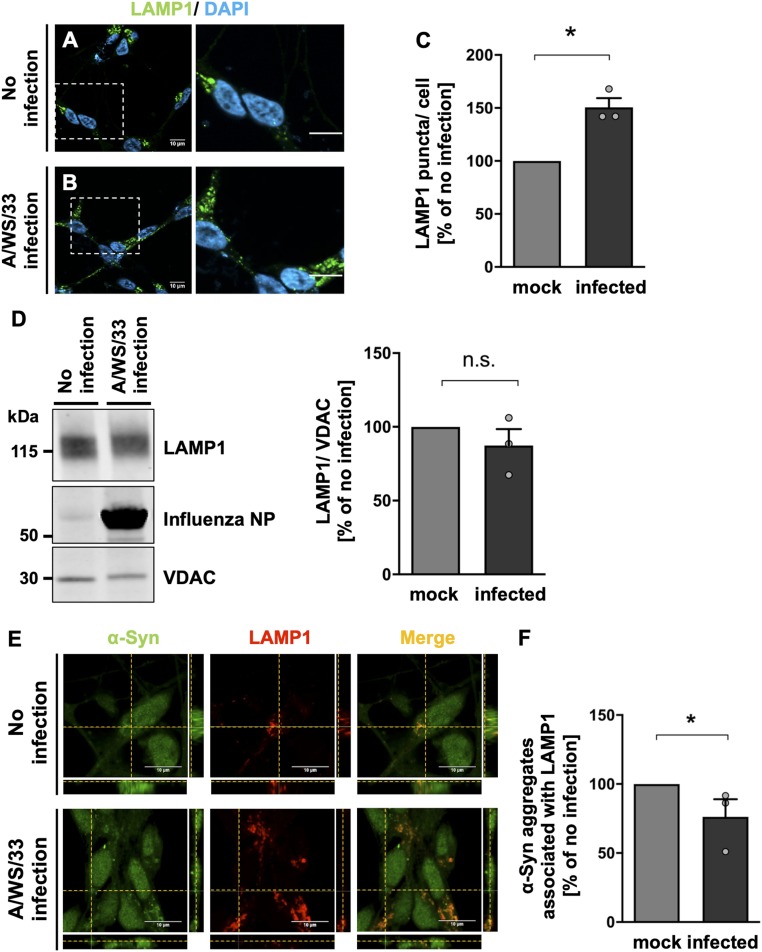
Next, we wanted to analyze whether H1N1 infection leads to impairment of the lysosomal turnover of the autophagosomes. For that, NLF neuroblastoma cells were transiently transfected with a tandem-reporter-construct mRFP-GFP-LC3 (52) and infected for 24 h with H1N1 influenza A/WS/33 virus (MOI of 1). The GFP component is pH-sensitive, thus not emitting light in the acidic conditions of the lysosome. However, this pH sensitivity does not apply to mRFP. Therefore, the green (GFP) and red (mRFP) moieties of this construct are both active when LC3 is localized in autophagosomes (neutral pH), being visible as yellow color in the images when merged. Upon fusion of autophagosomes and lysosomes to acidic autolysosomes, the majority of LC3 is visible in the red channel (Fig. 5E). These changes in fluorescent signal from yellow to red were used to analyze the effect of H1N1 influenza A virus on the autophagic flux and to determine LC3 location (Fig. 5F). In the infected cells, a nonsignificant decrease in mRFP-LC3 punctate structures (1.5 ± 0.56 autolysosomes per cell) was seen in comparison with noninfected condition (3.0 ± 0.94 autolysosomes per cell; Fig. 5G). Concomitantly, influenza A infection led to a significant increase in yellow-LC3 punctate structures (3.6 ± 0.50 autophagosomes per cell; P < 0.05) in comparison to the noninfected controls (2.0 ± 0.17 autophagosomes per cell; Fig. 5G). H1N1 influenza A infection led to a 73 ± 7.8% reduction (P = 0.05) in the autolysosomes formed compared to noninfected cells, suggesting an impairment in the autophagosome fusion with lysosomes (Fig. 5H). The effects of influenza infection on colocalization of LC3 with lysosome-associated membrane protein 1 (LAMP1) were also investigated in LUHMES cells. While, in noninfected cells, a considerable colocalization was detected, 24 h after influenza A infection, a reduction in the colocalization of autophagosomes with the lysosomal marker LAMP1 was observed (SI Appendix, Fig. S10 C and D), again indicating impaired autophagosome–lysosome fusion.
Next, we wanted to understand the effect of H1N1 influenza A infection on the number of lysosomal structures. LUHMES cells were infected with H1N1 influenza A for 24 h (MOI of 1), and immunolabeling of LAMP1 was performed (Fig. 6 A and B). A significant increase in number of lysosomes was observed in infected cells (51 ± 8.60% increase; P = 0.05) in comparison with noninfected cells (Fig. 6C). Immunoblot analysis of total lysates of infected LUHMES cells did not show a significant effect of H1N1 influenza A on LAMP1 protein levels (Fig. 6D). When we investigated the presence of α-synuclein aggregates in the acidified proteolytic lysosomes by immunolabeling of infected LUHMES cells, costaining between α-synuclein aggregates and LAMP1 had decreased 24 h after viral infection (Fig. 6E). Quantification of α-synuclein aggregates colocalizing with LAMP1 structures confirmed the significant reduction in the aggregate number present in the lysosomes in the influenza-infected condition (24 ± 12.70% reduction; P < 0.05) in comparison with noninfected cells (Fig. 6F).
Fig. 6.
Twenty-four hours of influenza A (A/WS/33) infection leads to an accumulation of lysosomal structures and a reduction in α-synuclein aggregate clearance by autolysosomes in human dopaminergic neurons. (A and B) Immunostaining of LAMP1 showed an accumulation of lysosomes in influenza-infected LUHMES cells (MOI of 1, 24 h) in comparison with noninfected cells (green, LAMP1; blue, DAPI). (Scale bars: 10 μm; dotted boxes show regions of higher magnification.) (C) Quantification of the percentage of acidified lysosomes in infected LUHMES cells (black bar) relative to the noninfected condition (gray bar). Infection led to an increase in LAMP1 puncta per cell (n = 3; means ± SEM). Mann–Whitney U with one-tailed Dunn’s post hoc test was used as a statistical test (*P < 0.05). (D) Immunoblot for LAMP1 in lysates of infected and noninfected LUHMES cells. VDAC-normalized LAMP1 signal in infected (black bar) as percent of noninfected condition (gray bar) did not reveal differences (n = 3; means ± SEM). (E) Orthogonal projections demonstrated a decrease in the colocalization of aggregated α-synuclein with lysosomal structures in infected LUHMES cells in comparison with noninfected cells (green, α-synuclein; red, LAMP1). (Scale bars, 10 μm.) (F) Quantification of percentage of aggregated α-synuclein associated with LAMP1 per cell in infected condition (black bar) relative to the noninfected condition (gray bar). After viral infection, less costaining between α-synuclein aggregates and LAMP1 was observed (n = 3; means ± SEM). Mann–Whitney U with one-tailed Dunn’s post hoc test was used as a statistical test (*P < 0.05). Circles in bar graphs represent results of single experiments.
These data suggest that H1N1 influenza A virus disrupts (i) the number of autophagosomes and (ii) the autophagic flux in neurons, leading to an impairment in autophagy that might explain the accumulation of α-synuclein aggregates observed after viral infection.
Discussion
In this study, we demonstrated that H1N1 influenza A viral infection and replication could represent a significant initiating event in the genesis of a critical mass of misfolded α-synuclein and DISC1 species that could trigger disease. We showed that, in human dopaminergic neuron-like cells, the H1N1 infection disrupts proteostasis at the level of protein degradation by inhibiting autophagosome–lysosome fusion that could explain the observed increase in endogenous α-synuclein and DISC1 aggregation levels. These findings are mirrored in Rag1 knockout mice in vivo, where, 4 wk after intranasal H1N1 infection, increased protein levels of α-synuclein and Disc1 could be detected in neurons, which was likely due to decreased degradation rather than up-regulated expression. Our advances are important for understanding how a critical, initiating concentration of misfolded α-synuclein or DISC1 is built up to trigger fatal downstream cascades of cellular events.
In our study, we provide a mechanistic link as to how a direct cellular H1N1 infection leads to α-synuclein and DISC1 aggregates. This makes a scenario possible in which H1N1 infection initiates α-synuclein seeds by disturbing proteostasis via inhibition of autophagosomes and autophagic flux. It is noteworthy that α-synuclein aggregates themselves inhibit autophagy (53), thereby leading to a self-reinforcing loop of autophagy inhibition that promotes α-synuclein aggregation. Inhibition of autophagy, in turn, facilitates the secretion of α-synuclein containing exosomes (54) and promotes α-synuclein prion spread in the CNS (55).
A similar effect of H1N1 infection was seen on the DISC1 protein, the product of a gene linked to familial cases of CMI (6) that has been identified to be insoluble in a subset of cases with CMI but not controls (6, 44). In continuation to our findings of insoluble DISC1 in the brains of CMI patients (6), we demonstrate conditions under which endogenous DISC1 protein aggregates are induced by aberrant proteostasis (SI Appendix, Fig. S4 A–C). This could mean that, in addition to the suggested effects of influenza infections on prenatal immune activation (56), influenza virus might affect DISC1 protein functions directly during neurodevelopment (57). Furthermore, our data suggest that DISC1 aggregation induced by H1N1 infection may also affect the adult brain and influence homeostatic processes related to CMI. Interestingly, the induction of protein aggregation is dramatically stronger for α-synuclein and DISC1 than for tau and TDP-43, two proteins misfolded in different neurodegenerative diseases. Hyperphosphorylated and aggregated tau protein can be found in AD and frontotemporal dementia (FTD) brains, and TDP-43 protein in cytoplasmic aggregates in neurons of patients with ALS or FTD. This suggests that the effects of H1N1 on proteostasis are selective. The increase in α-synuclein and DISC1 protein levels in vivo was remarkable (Fig. 2 and SI Appendix, Fig. S8). In contrast, the weak increase in protein half-life time of α-synuclein after H1N1 infection in vitro in neuroblastoma cells (SI Appendix, Fig. S5 C and D) could be due to a complex regulation of alternative degradation pathways well known for α-synuclein (58, 59), but which still led to increased α-synuclein aggregation. In vivo, it has been shown that increased expression of the nonmutant form of α-synuclein is genetically linked to PD (60) and is sufficient to induce pathology (61).
One of the control mechanisms that cells use to maintain cellular homeostasis in order to avoid accumulation of protein aggregates is autophagy (62). Indeed, many pathogens have been found to interfere with this catabolic process in order to reduce host cell death during the infection process (63, 64). Previous publications described a disruption in the autophagy process in epithelial cells and in human lung samples by influenza A virus (49, 50). In line with this, our findings showed that a 24-h acute infection with H1N1 influenza virus (A/WS/33) led to a decrease in autophagosome formation (Fig. 5 A–C) and an increase in lysosomes (Fig. 6 A–C) in human dopaminergic neurons. Interestingly, we observed a reduction in the number of α-synuclein aggregates in the acidified proteolytic lysosomes (Fig. 6 E and F). This might be the effect of a decreased degradation of the cargo that is targeted to the autolysosomes by the reduced fusion with autophagosomes. This finding could be a consequence of the impairment in the autophagic flux in neuronal cells seen after influenza infection (Fig. 5 F–H and SI Appendix, Fig. S10). A similar effect was previously reported by Gannagé et al. in epithelial cells (49).
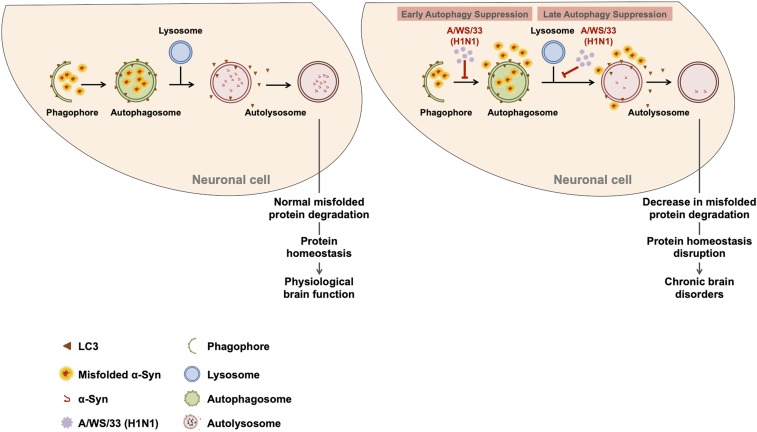
Tanik and coworkers reported that seeded α-synuclein aggregates, per se, in an in vitro cellular model, were able to impair autophagy due to a reduction in autophagosome clearance (53). However, since we already showed a low background of existing α-synuclein aggregates in noninfected cells (Fig. 1 A–C), the dramatic increase in α-synuclein aggregates must have been the consequence of H1N1 infection and cannot have resulted from an interaction of α-synuclein with autophagosomes alone. Taking this into account, we propose a possible scenario for the increase in α-synuclein aggregates: first, influenza A infection suppresses the autophagy process at an early stage, leading to less available autophagosomes to transport the misfolded proteins for lysosomal degradation. Subsequently, infection also affects autophagy at a later stage, with a blockage of autophagosome fusion with lysosomes. These effects will consequently lead to a vicious circle of increase in misfolded α-synuclein in dopaminergic neurons, protein homeostasis impairment, and a concomitant loss of dopaminergic neurons, one of the neuropathological characteristics of PD brains (Fig. 7). This scenario is corroborated by reports that impairments in the autophagy system result in α-synuclein accumulation (51, 65).
Fig. 7.
Potential mechanism of protein homeostasis disruption in neuronal cells due to H1N1 influenza A infection. Schematic representation of influenza A infection effects on autophagy. Autophagy is one of the clearance mechanisms in the cell responsible for removing misfolded proteins and dysfunctional organelles in order to prevent accumulation of toxic species. Under physiological conditions, cell components selected for autophagy degradation are engulfed into a phagophore to form the autophagosome. Then, the autolysosome is created by the fusion of autophagosome with lysosome, where the cargo, such as α-synuclein, is delivered for degradation. Regular autophagy allows a proper cellular protein homeostasis and a normal cell function. Influenza A infection impairs autophagy at two stages: the early-stage autophagosome formation and the late-stage autolysosome formation.
Considering the fact that misassembled DISC1 protein located in aggresomes has been shown to be degraded by autophagy (66), it is straightforward to attribute the accumulation of DISC1 aggregates also to impairments in autophagy (SI Appendix, Fig. S4). It is still not clear what the main degradation pathways for clearing aggregated tau (67, 68) and TDP-43 protein (69) are. An equal involvement of ubiquitin–proteasome system (UPS) and the autophagy system has been proposed. Links between UPS and autophagy have being extensively demonstrated (70, 71). Korolchuk and coworkers showed that autophagy inhibition compromises the UPS due to accumulation of p62 that inhibits the clearance of soluble ubiquitinated proteins, leading to an increase in the levels of UPS-destined proteins (72). For example, aggregation levels of a mutant, disease-associated polyglutamine (polyQ)-expanded huntingtin protein fragment were cleared by both UPS and autophagy systems (72, 73). Thus, if we take into account that, for different proteins, the clearance mechanisms used by the cell for degradation of misfolded forms are different, we cannot exclude a UPS compensatory mechanism as the reason for the absence of influenza A infection effect in tau and TDP-43 protein homeostasis.
We observed a >50% reduction of α-synuclein aggregates in H1N1-infected human dopaminergic neurons treated with oseltamivir phosphate (Fig. 4 C and D). This compound is commonly used in the prophylaxis of influenza A and B and inhibits neuraminidase, i.e., the exosomal release of the virus from the host cell. Indirectly, this affects the reinfection rate of the same cell and neighboring cells through decrease in viral titers (74) and leads, eventually, to a higher rate of defective viral assembly (75) and consequently less disturbed proteostasis in the overall cell population. The effect of oseltamivir phosphate on α-synuclein aggregates was concentration-dependent, and our data also confirmed a parallel antiviral activity with a decrease in the viral titer for both concentrations tested (0.5 µM and 50 μM; Fig. 4A). These data demonstrate that pharmacological treatment of H1N1-infected dopaminergic neurons can prevent α-synuclein aggregation.
In our in vivo study, the changes in α-synuclein were detected strictly in conjunction with the presence of viral antigens after viral replication. Our data therefore suggest that influenza A replication was able to disturb protein homeostasis directly and not only as a consequence of the activation of the innate or adaptive immune system. The infection was restricted to areas connected to the olfactory and trigeminal systems, and there was no further spread into the neocortex or hippocampus except in rare instances. From the infection in the olfactory bulbs, the retrograde axonal spread of the virus has been followed to nerve cell groups that send projections to the olfactory bulbs (76). These included the diagonal bands, piriform cortex, cortical amygdaloid area, lateral hypothalamic areas, and locus coeruleus, but not, or only the most medial aspect of, the substantia nigra (38). The lack of involvement of the substantia nigra does not reflect a default neurotropism for the virus, since this nucleus is heavily infected following intracerebral viral injections (77), probably through retrograde spread in axonal projections to the striatum. Following injections of fibrillary α-synuclein into the olfactory bulbs, thread-like aggregates were initially seen only in areas that send or receive projections from olfactory regions with a later slow (months) and secondary spread to limbic structures (4). This distribution of Lewy bodies in neurons tallies well with that described in DLB disease (reviewed in ref. 78) and has been proposed as a trajectory for spreading α-synuclein aggregates (79).
Notably, various strains of the highly pathogenic H5N1 influenza can, without prior adaptation, distribute within the olfactory pathway only, or in combination with the brainstem, in ferrets following intranasal infection (25). A systemic infection with the H5N1 strain, which spread widely in the brain, including substantia nigra, was followed by appearance of α-synuclein granules, which were localized to the nuclei of the cells (24). Whether our present findings of cytoplasmic α-synuclein aggregates could link influenza infections to the postencephalitic parkinsonism following encephalitis lethargica is not clear, since neither Lewy bodies nor α-synuclein aggregates have been found in the brainstem of patients deceased from postencephalitic parkinsonism. This disease is instead characterized by neurofibrillary degeneration and tau aggregates (reviewed by Jellinger [80]). The initiation of α-synuclein seeding activity in CNS is also conceivable from a peripheral route through uptake by the gut (81) followed by retrograde transport to brain nuclei as hypothesized by Braak et al. (79).
In summary, we show that direct effects of H1N1 replication may lead to a critical mass of α-synuclein aggregates that subsequently have the potential to trigger a synucleinopathy. In light of the efficient pharmacotherapy presented, our findings may inspire further research, e.g., on the effects of antiinfluenza vaccines on synucleinopathy incidence. It should also be carefully evaluated whether live vaccines taken intranasally could cause aggregates in the olfactory epithelium, and, if so, whether they will be cleared by the turnover of neurons in the epithelia or olfactory bulbs or slowly propagate over months or years as α-synuclein injected into the bulb does. Since the olfactory epithelium is the only site where neurons are in direct contact with the environment, effects of vaccine on the recruitment of resident memory T (TREM) cells (82) to prevent viral-induced misfolding in the olfactory domain, burdened by DLB and PDD, appears to be an urgent research topic.
Materials and Methods
The A/WS/33 H1N1 influenza A strain, with 1 × 108 PFU/µL, purchased from ATCC (ATCC VR-1520), was used to infect LUHMES cells (ATCC CRL-2927). Mouse neuroadapted influenza A virus (A/WSN/33, 1.4 × 105 PFU/mL; provided by S. Nakajima, The Institute of Public Health, Tokyo, Japan) was used to infect mice (38). For all immunocytochemistry data, randomized confocal images were taken in an average of 30 images from two independent wells per biological replicate, in a total number of at least three independent biological experiments. For quantification of protein aggregates in LUHMES and NLF neuroblastoma cell lines, a systematic, manual, blinded counting procedure was used.
Extensive, detailed information on the methods and materials used in the study are provided in the SI Appendix.
Data Availability.
All data are published in this publication or the SI Appendix. Any further questions can be addressed to the corresponding author.
Supplementary Material
Acknowledgments
The authors thank Krister Kristensson from the Karolinska Institute, Sweden, for providing sections of influenza H1N1 WSN/33-infected Rag−/− mice and for critical discussion of results and the manuscript; as well as Wolfgang Hoyer from the Heinrich Heine University Düsseldorf for providing preformed α-synuclein fibrils and technical advice, Leonidas Stefanis, University of Athens, Greece, and Kathryn Evans, University of Edinburgh, United Kingdom, for kindly providing α-synuclein and DISC1 promoter constructs, respectively. This research was funded by grants from European Union 7th Framework Programme IN-SENS (no. 60671), a grant from the Forschungskommission of the Medical Faculty of the Heinrich Heine University Düsseldorf (no. 9772651), and a grant from the BMBF (01GQ1422A; REMOVAGE).
Footnotes
Competing interest statement: Co-authors A.R.M., S. Sahu, I.S., S. Selvarajah, and V.R.L. are full-time employees of Prosetta Biosciences Inc., located in San Francisco, CA; however, no intellectual property, products, or other commercial interests were pursued during the studies presented here.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1906466117/-/DCSupplemental.
References
- 1.Taylor J. P., Hardy J., Fischbeck K. H., Toxic proteins in neurodegenerative disease. Science 296, 1991–1995 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Prusiner S. B., Shattuck lecture–Neurodegenerative diseases and prions. N. Engl. J. Med. 344, 1516–1526 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Spillantini M. G., Crowther R. A., Jakes R., Hasegawa M., Goedert M., alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rey N. L., et al. , Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J. Exp. Med. 213, 1759–1778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradshaw N. J., Korth C., Protein misassembly and aggregation as potential convergence points for non-genetic causes of chronic mental illness. Mol. Psychiatry 24, 936–951 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Leliveld S. R., et al. , Insolubility of disrupted-in-schizophrenia 1 disrupts oligomer-dependent interactions with nuclear distribution element 1 and is associated with sporadic mental disease. J. Neurosci. 28, 3839–3845 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trossbach S. V., et al. , Misassembly of full-length Disrupted-in-Schizophrenia 1 protein is linked to altered dopamine homeostasis and behavioral deficits. Mol. Psychiatry 21, 1561–1572 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desplats P., et al. , Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. U.S.A. 106, 13010–13015 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woerman A. L., et al. , α-Synuclein: Multiple system Atrophy prions. Cold Spring Harb. Perspect. Med. 8, a024588 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu S., Abounit S., Korth C., Zurzolo C., Transfer of disrupted-in-schizophrenia 1 aggregates between neuronal-like cells occurs in tunnelling nanotubes and is promoted by dopamine. Open Biol. 7, 160328 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luk K. C., et al. , Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 209, 975–986 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leak R. K., Frosch M. P., Beach T. G., Halliday G. M., Alpha-synuclein: Prion or prion-like? Acta Neuropathol. 138, 509–514 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balchin D., Hayer-Hartl M., Hartl F. U., In vivo aspects of protein folding and quality control. Science 353, aac4354 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Aviner R., Frydman J., Proteostasis in viral infection: Unfolding the complex virus-chaperone interplay. Cold Spring Harb. Perspect. Biol., a034090 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baccam P., Beauchemin C., Macken C. A., Hayden F. G., Perelson A. S., Kinetics of influenza A virus infection in humans. J. Virol. 80, 7590–7599 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lingappa U. F., et al. , Host-rabies virus protein-protein interactions as druggable antiviral targets. Proc. Natl. Acad. Sci. U.S.A. 110, E861–E868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips A. M., et al. , Host proteostasis modulates influenza evolution. eLife 6, e28652 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravindran M. S., Bagchi P., Cunningham C. N., Tsai B., Opportunistic intruders: How viruses orchestrate ER functions to infect cells. Nat. Rev. Microbiol. 14, 407–420 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marreiros R., et al. , Viral capsid assembly as a model for protein aggregation diseases: Active processes catalyzed by cellular assembly machines comprising novel drug targets. Virus Res. 207, 155–164 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Yokomichi H., et al. , Incidence of hospitalisation for severe complications of influenza virus infection in Japanese patients between 2012 and 2016: A cross-sectional study using routinely collected administrative data. BMJ Open 9, e024687 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Britton P. N., et al. ; Australian Childhood Encephalitis (ACE) Study Investigators, Influenza Complications Alert Network (FluCAN) Investigators, and Paediatric Active Enhanced Disease Surveillance (PAEDS) Network , The spectrum and burden of influenza-associated neurological disease in children: Combined encephalitis and influenza sentinel site surveillance from Australia, 2013-2015. Clin. Infect. Dis. 65, 653–660 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Glaser C. A., et al. , A population-based study of neurologic manifestations of severe influenza A(H1N1)pdm09 in California. Clin. Infect. Dis. 55, 514–520 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Aronsson F., Robertson B., Ljunggren H.-G., Kristensson K., Invasion and persistence of the neuroadapted influenza virus A/WSN/33 in the mouse olfactory system. Viral Immunol. 16, 415–423 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Jang H., et al. , Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 106, 14063–14068 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinya K., et al. , Subclinical brain injury caused by H5N1 influenza virus infection. J. Virol. 85, 5202–5207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Riel D., Verdijk R., Kuiken T., The olfactory nerve: A shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 235, 277–287 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Spesock A., et al. , The virulence of 1997 H5N1 influenza viruses in the mouse model is increased by correcting a defect in their NS1 proteins. J. Virol. 85, 7048–7058 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horby P., Nguyen N. Y., Dunstan S. J., Baillie J. K., An updated systematic review of the role of host genetics in susceptibility to influenza. Influenza Other Respir. Viruses 7 (suppl. 2), 37–41 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCall S., Henry J. M., Reid A. H., Taubenberger J. K., Influenza RNA not detected in archival brain tissues from acute encephalitis lethargica cases or in postencephalitic Parkinson cases. J. Neuropathol. Exp. Neurol. 60, 696–704 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Sheng Z. M., et al. , Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc. Natl. Acad. Sci. U.S.A. 108, 16416–16421 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilensky J. A., Gilman S., McCall S., A historical analysis of the relationship between encephalitis lethargica and postencephalitic parkinsonism: A complex rather than a direct relationship. Mov. Disord. 25, 1116–1123 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Vlajinac H., et al. , Infections as a risk factor for Parkinson’s disease: A case-control study. Int. J. Neurosci. 123, 329–332 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Harris M. A., Tsui J. K., Marion S. A., Shen H., Teschke K., Association of Parkinson’s disease with infections and occupational exposure to possible vectors. Mov. Disord. 27, 1111–1117 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Toovey S., Jick S. S., Meier C. R., Parkinson’s disease or Parkinson symptoms following seasonal influenza. Influenza Other Respir. Viruses 5, 328–333 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuya-Kanamori L., Yakob L., Heterogeneous and dynamic prevalence of asymptomatic influenza virus infections. Emerg. Infect. Dis. 24, 951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wellington D., Laurenson-Schafer H., Abdel-Haq A., Dong T., IFITM3: How genetics influence influenza infection demographically. Biomed. J. 42, 19–26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith W., Manch M. D., Andrewas C. H., Lond M. D., A virus obtained from influenza patients. Lancet 225, 66–68 (1933). [Google Scholar]
- 38.Tesoriero C., et al. , H1N1 influenza virus induces narcolepsy-like sleep disruption and targets sleep-wake regulatory neurons in mice. Proc. Natl. Acad. Sci. U.S.A. 113, E368–E377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kristensson K., Avian influenza and the brain–Comments on the occasion of resurrection of the Spanish flu virus. Brain Res. Bull. 68, 406–413 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Lotharius J., et al. , Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J. Biol. Chem. 277, 38884–38894 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Burnet F. M., A genetic approach to variation in influenza viruses; the characters of three substrains of influenza virus A (WS). J. Gen. Microbiol. 5, 46–53 (1951). [DOI] [PubMed] [Google Scholar]
- 42.Candelise N., et al. , Seeding variability of different alpha synuclein strains in synucleinopathies. Ann. Neurol. 85, 691–703 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Schwab M., et al. , Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature 305, 245–248 (1983). [DOI] [PubMed] [Google Scholar]
- 44.Ottis P., et al. , Convergence of two independent mental disease genes on the protein level: Recruitment of dysbindin to cell-invasive disrupted-in-schizophrenia 1 aggresomes. Biol. Psychiatry 70, 604–610 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Walker R. M., et al. , The DISC1 promoter: Characterization and regulation by FOXP2. Hum. Mol. Genet. 21, 2862–2872 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Lo C.-Y., Tang Y.-S., Shaw P.-C., Structure and function of influenza virus ribonucleoprotein. Subcell. Biochem. 88, 95–128 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Mendel D. B., et al. , Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob. Agents Chemother. 42, 640–646 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roche Laboratories , Tamiflu (oseltamivir phosphate) information, NDA 21-087/S-033, NDA 21-246/S-021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021087s033ppi.pdf. Assessed 19 February 2020.
- 49.Gannagé M., et al. , Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe 6, 367–380 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuroki T., Osari S., Nagata K., Kawaguchi A., Influenza A virus NS1 protein suppresses JNK1-dependent autophagosome formation mediated by Rab11a recycling endosomes. Front. Microbiol. 9, 3120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman L. G., et al. , Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of α-synuclein and LRRK2 in the brain. J. Neurosci. 32, 7585–7593 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimura S., Noda T., Yoshimori T., Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452–460 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Tanik S. A., Schultheiss C. E., Volpicelli-Daley L. A., Brunden K. R., Lee V. M., Lewy body-like α-synuclein aggregates resist degradation and impair macroautophagy. J. Biol. Chem. 288, 15194–15210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee H. J., et al. , Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp. Mol. Med. 45, e22 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karpowicz R. J. Jr, Trojanowski J. Q., Lee V. M., Transmission of α-synuclein seeds in neurodegenerative disease: Recent developments. Lab. Invest. 99, 971–981 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi L., Fatemi S. H., Sidwell R. W., Patterson P. H., Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 23, 297–302 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamiya A., et al. , A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat. Cell Biol. 7, 1167–1178 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Cuervo A. M., Stefanis L., Fredenburg R., Lansbury P. T., Sulzer D., Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Stefanis L., et al. , How is alpha-synuclein cleared from the cell? J. Neurochem. 150, 577–590 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Chartier-Harlin M. C., et al. , Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364, 1167–1169 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Burré J., Sharma M., Südhof T. C., Systematic mutagenesis of α-synuclein reveals distinct sequence requirements for physiological and pathological activities. J. Neurosci. 32, 15227–15242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamark T., Johansen T., Aggrephagy: Selective disposal of protein aggregates by macroautophagy. Int. J. Cell Biol. 2012, 736905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson W. T., et al. , Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 3, e156 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirkegaard K., Taylor M. P., Jackson W. T., Cellular autophagy: Surrender, avoidance and subversion by microorganisms. Nat. Rev. Microbiol. 2, 301–314 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xilouri M., Vogiatzi T., Vekrellis K., Stefanis L., alpha-synuclein degradation by autophagic pathways: A potential key to Parkinson’s disease pathogenesis. Autophagy 4, 917–919 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Atkin T. A., Brandon N. J., Kittler J. T., Disrupted in Schizophrenia 1 forms pathological aggresomes that disrupt its function in intracellular transport. Hum. Mol. Genet. 21, 2017–2028 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Tai H.-C., et al. , The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am. J. Pathol. 181, 1426–1435 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo J. L., et al. , The dynamics and turnover of tau aggregates in cultured cells: Insights into therapies for tauopathies. J. Biol. Chem. 291, 13175–13193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scotter E. L., et al. , Differential roles of the ubiquitin proteasome system and autophagy in the clearance of soluble and aggregated TDP-43 species. J. Cell Sci. 127, 1263–1278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pandey U. B., et al. , HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447, 859–863 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Ding W. X., et al. , Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am. J. Pathol. 171, 513–524 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Korolchuk V. I., Mansilla A., Menzies F. M., Rubinsztein D. C., Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol. Cell 33, 517–527 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ravikumar B., Duden R., Rubinsztein D. C., Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 11, 1107–1117 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Dou D., et al. , Analysis of IAV replication and Co-infection dynamics by a versatile RNA viral genome labeling method. Cell Rep. 20, 251–263 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Heldt F. S., Kupke S. Y., Dorl S., Reichl U., Frensing T., Single-cell analysis and stochastic modelling unveil large cell-to-cell variability in influenza A virus infection. Nat. Commun. 6, 8938 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shipley M. T., Adamek G. D., The connections of the mouse olfactory bulb: A study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res. Bull. 12, 669–688 (1984). [DOI] [PubMed] [Google Scholar]
- 77.Takahashi M., et al. , The substantia nigra is a major target for neurovirulent influenza A virus. J. Exp. Med. 181, 2161–2169 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cersosimo M. G., Propagation of alpha-synuclein pathology from the olfactory bulb: Possible role in the pathogenesis of dementia with lewy bodies. Cell Tissue Res. 373, 233–243 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Braak H., et al. , Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Jellinger K. A., Absence of alpha-synuclein pathology in postencephalitic parkinsonism. Acta Neuropathol. 118, 371–379 (2009). [DOI] [PubMed] [Google Scholar]
- 81.Kim S., et al. , Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103, 627–641.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schøller A. S., Fonnes M., Nazerai L., Christensen J. P., Thomsen A. R., Local antigen encounter is essential for establishing persistent CD8+ T-cell memory in the CNS. Front. Immunol. 10, 351 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are published in this publication or the SI Appendix. Any further questions can be addressed to the corresponding author.