Abstract
Synthetic cathinones (SCs) are designer, psychostimulant drugs of abuse that primarily act on monoamine transporters; little is known about their off-target liability. Abuse of pyrrolidine-containing SCs, such as α-PHP, has been linked to clinical features, including tachycardia and hypertension, and psychiatric events, including delusions and memory impairments—effects mimicking deliriant hallucinogens that are acetylcholine muscarinic receptor (MR) antagonists. α-PHP and nine analogs with modifications in the α-carbon side chain length and/or containing a methylenedioxy moiety were screened for activity at each of the five human MRs. Increasing the length of the α-carbon side chain of 1-phenyl-2-(pyrrolidin-1-yl)ethan-1-one analogs from a methyl (α-PPP) to a propyl (α-PVP) group caused a steep increase in affinity at all MR subtypes, and one extra carbon (α-PHP) further enhanced MR affinity; the presence of a methylenedioxy moiety generally hindered this effect. Highest MR affinity was observed with α-PHP at M2Rs—its M2R affinity (Ki = 251 nM) was 302-fold higher than α-PPP’s. M2R-cAMP inhibition and β-arrestin recruitment assays showed that α-PHP is an M2R antagonist (Kb = 120 and 502 nM, respectively). Additional experiments showed α-PHP is also an antagonist of M1R-inositol phosphate production (Kb = 1.4 μM). Human toxicology studies report blood concentrations of pyrrolidine-containing SCs, including α-PHP, that reach micromolar levels during intoxication, indicating α-PHP’s MR activity might have physiological relevance. As M2Rs and M1Rs are widely expressed in the autonomic and central nervous systems, α-PHP’s anticholinergic activity might be relevant to adverse events associated with α-PHP intoxication.
Keywords: synthetic cathinones, α-PHP, muscarinic receptor antagonist
Graphical Abstract
INTRODUCTION
Synthetic cathinone (SC) drugs of abuse are derivatives of the psychostimulant, cathinone, an alkaloid found in the plant Catha edulis.1 Although many SCs originated from pharmaceutical industry research in the 1960s,2,3 they have been circulating in the United States clandestine drug market since about 2010. By 2017, there were more than 100 SCs identified worldwide.4 The pyrrolidine-containing SCs (PSCs) α-pyrrolidinopentiophenone (α-PVP, known colloquially as “flakka” or “gravel”) and 3,4-methylenedioxypyrovalerone (MDPV) are two of the most notorious.5 Nonhuman animal studies and human reports suggest they have high abuse and dependence liability, and they have been linked to numerous deaths worldwide.6−9 After the Drug Enforcement Administration (DEA) classified MDPV and α-PVP as U.S. Schedule I substances in 2011 and 2014, respectively, α-pyrrolidinohexiophenone (α-PHP), which was unscheduled, grew in popularity. In July, 2019 α-PHP was classified as a Schedule 1 substance via a temporary scheduling order. α-PHP, however, remains a popular drug of abuse. According to a recent DEA report, α-PHP is one of the ten most frequently reported SCs in the United States.10
PSCs chiefly produce effects by directly inhibiting dopamine and norepinephrine transporters (DAT and NET, respectively), which increases extracellular dopamine and norepinephrine levels.4 Euphoria, increased empathy, sociability, and energy are desired effects produced by PSCs, but adverse effects abound, especially at high doses. These include clinical features such as tachycardia, hypertension, and urinary retention but also psychiatric symptoms such as anxiety, paranoia, and delirium, e.g., psychosis and memory impairments.11−13 Although these symptoms can be attributed to sympathomimetic actions or dopaminergic toxicity,14 we found it unusual that the prevalence of adverse events, such as tachycardia, urinary retention, and delirium, appears to vary across psychostimulants. For example, tachycardia caused by α-PHP is reportedly stronger compared to other PSCs, including α-pyrrolidinobutiophenone (α-PBP) and MDPV.12,15 Also, symptoms of delirium are more associated with α-PVP and α-PHP than α-pyrrolidinopropiophenone (α-PPP), amphetamine, or cathinone, according to searches of online forums (e.g., Bluelight and Erowid) and Assi et al.13 Though delirium can occur after prolonged (decades) use of cathinone,8 we could not find any reports of delirium associated with acute cathinone or α-PPP intoxication searching PubMed or Web of Science databases. We are aware, however, that the absence of reports does not prove an association is lacking.
One common, distinguishing chemical feature of α-PVP and α-PHP compared to α-PPP, amphetamine, and cathinone is the lipophilic, α-carbon chain length. Cathinone, amphetamine, and α-PPP have a single methyl group attached, whereas α-PVP has three (propyl group) and α-PHP has four (butyl group) hydrogen-saturated carbons in the chain (see Table 1). These observations suggest the α-carbon chain may contribute to adverse side effects of unsubstituted PSCs. Indeed, increasing the chain length from one carbon to four causes a stepwise increase in DAT affinity and dopamine reuptake inhibition potency.16,17 The differences in DAT potency, however, between α-PVP and α-PHP are minor and not significant,16 yet α-PHP reportedly produces more pronounced tachycardia than α-PVP.15 There is a paucity of clinical data, however, directly comparing effects of individual PSCs, and pharmacokinetic factors might account for differences in subjective effects. Nevertheless, preclinical cytotoxicity studies using dopaminergic SH-SY5Y cells show that α-PPP and α-PBP are less potent than α-PVP, despite all drugs possessing LC50 values at millimolar concentrations that far exceed their IC50 potencies at DAT and NET,18 i.e., DAT and NET are saturated by PSCs at high micromolar concentrations.16 These data suggest pharmacodynamic effects beyond DAT and NET.
Table 1.
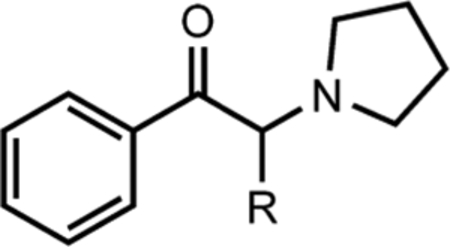
Structures, Abbreviated Names and Affinities (pKis (±SEM)) of Unsubstituted PSCs at MRs Determined from [3H]Scopolamine Competition Binding (N = 3)a
 |
||||||
|---|---|---|---|---|---|---|
| R | name | M1R | M2R | M3Rb | M4Rb | M5Rb |
| −CH3 | α-PPP | 3.71 (0.05) | 4.12 (0.05) | NC | NC | NC |
| −CH2CH3 | α-PBP | 4.12 (0.17) | 4.40 (0.06) | NC | NC | NC |
| −CH2CH2CH3 | α-PVP | 5.29 (0.04) | 6.08 (0.10) | 5.22 | 5.09 | 5.38 |
| −CH2(CH2)2CH3 | α-PHP | 5.80 (0.03) | 6.60 (0.04) | 5.78 | 5.71 | 5.82 |
| −CH2(CH2)3CH3 | α-PHPP | 5.71 (0.06) | 6.18 (0.01) | 5.95 | 5.86 | 5.94 |
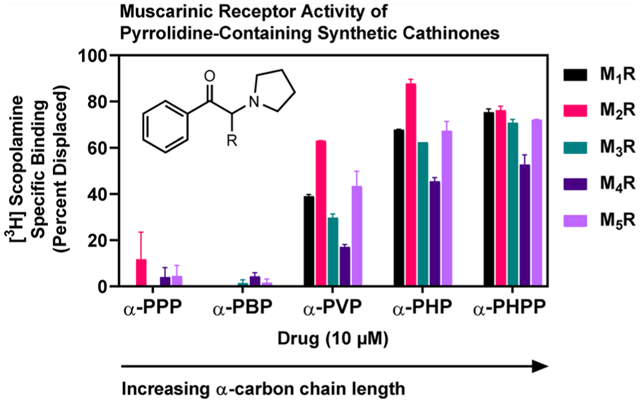
Notice that affinities of PSCs at M1Rs and M2Rs improve with increasing α-carbon side chain length up to α-PHP (emboldened). Affinities of α-PPP and α-PBP at M1Rs and M2Rs had to be interpolated (see Methods), as neither drug completely displaced [3H]scopolamine at 100 μM (see Figure 1).
Indicates that M3R, M4R, and M5R affinities are estimated pKis derived from results of [3H]scopolamine competition binding assays using 1 and 10 μM concentrations of PSCs (N = 2); NC, not calculated; these drugs displaced <5% of radioligand at 10 μM. Estimated M1R and M2R affinities from the 1 and 10 μM spot tests reasonably matched the obtained values from the full dose-response studies. For example, the estimated pKi values of α-PHP at M1Rs and M2Rs were 5.59 and 6.33, respectively, i.e., less than 2-fold different than values obtained from full-dose response studies. It is therefore believed that affinity values we report at M3Rs, M4Rs, and M5Rs are fair estimates.
Muscarinic receptors (MRs) are G protein-coupled receptors (GPCRs) expressed ubiquitously in mammalian tissue and serve numerous, critical physiological functions, from regulating cognition and memory to heart rate and bladder detrusor muscle contraction.19 MR antagonists, such as tropicamide, atropine, and scopolamine, when insufflated, taken orally, or intravenously, are well-known deliriant hallucinogenic drugs that also cause tachycardia and urinary retention,20−22 which led us to hypothesize that certain SCs are MR antagonists. Our initial experiments showed that SCs and PSCs with a single methyl group at the α-carbon, e.g., 4-bromomethcathinone, α-PPP, and 3,4-methylenedioxy-α-pyrrolidinopropiophenone (MDPPP), have nil affinity at M1Rs.23 In the current structure-activity relationship (SAR) study, we conducted pharmacological assays assessing the activity of 10 PSCs at human M1Rs, M2Rs, M3Rs, M4Rs, and M5Rs, systematically evaluating the effect of increasing the α-carbon side chain of unsubstituted PSCs and methylenedioxy-containing PSCs. The purpose of this study was to provide new information regarding the pharmacodynamic properties of PSCs. Our primary finding suggests that, in addition to its well-reported sympathomimetic properties,12 α-PHP is also a parasympatholytic drug; it blocks M2Rs and M1Rs at physiological concentrations.
RESULTS AND DISCUSSION
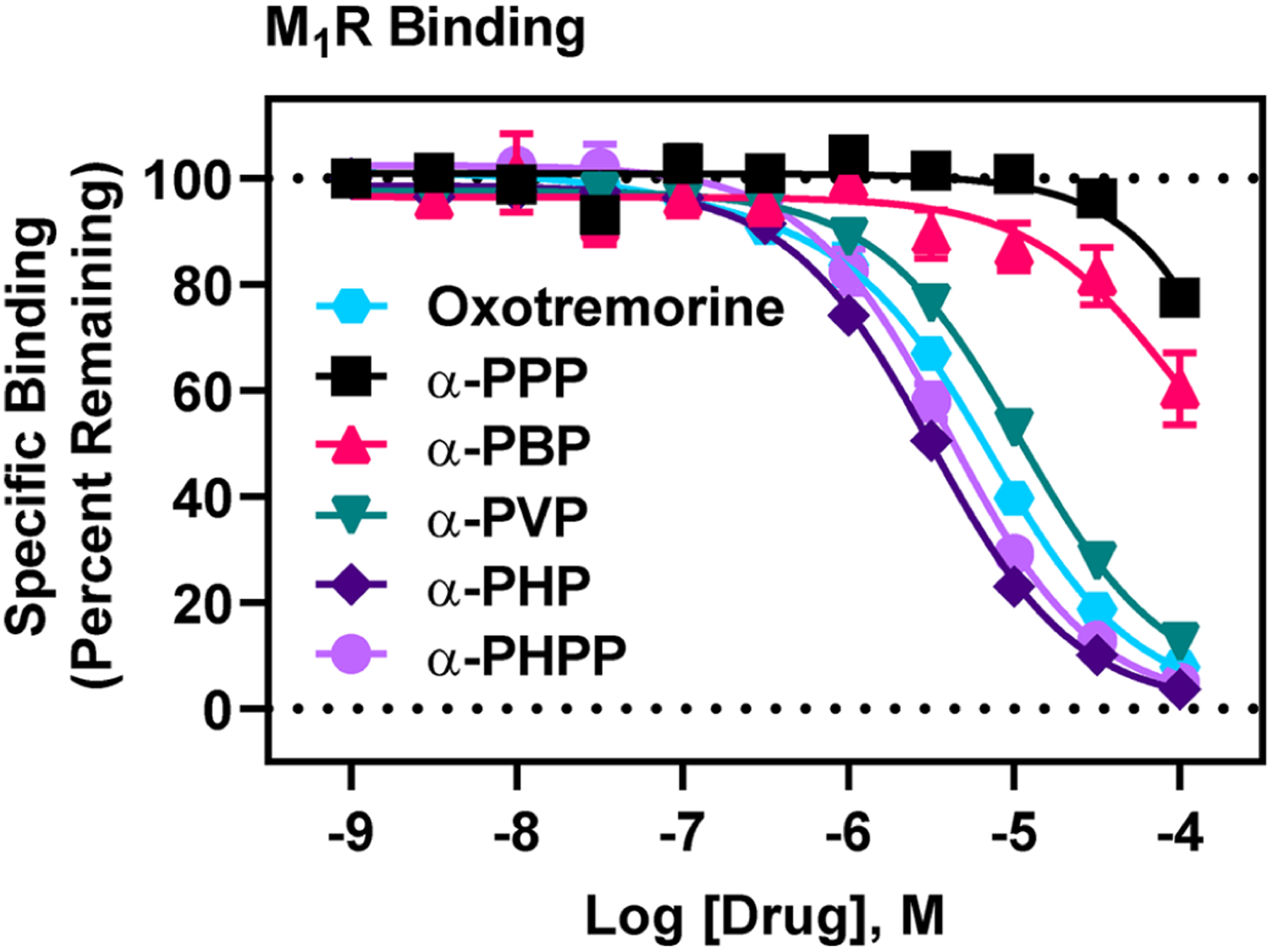
The motivation to evaluate the activity of PSCs at MRs emanated from reports that adverse events caused by certain PSCs appear to mimic anticholinergic deliriants. Ten SCs, including five unsubstituted and five methylenedioxy-containing PSCs (Tables 1 and 2, respectively), were initially screened at 1 and 10 μM concentrations in [3H]scopolamine radioligand competition binding assays to obtain estimated affinities at each of the five MRs subtypes. [Determination of estimated affinities is described in Methods.] In these experiments, α-PPP and α-PBP displaced <5% of 1 nM [3H]scopolamine from each of the MR subtypes; however, increasing the lipophilic, α-carbon side chain length to butyl, α-PHP, caused a precipitous increase in MR binding, providing the initial observations of a SAR for unsubstituted PSCs, which we further explored and discuss in detail in the following paragraphs.
Table 2.
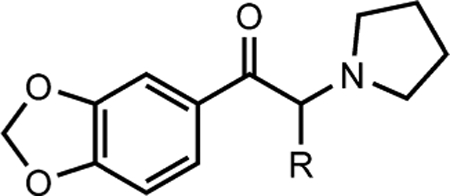
Structures, Abbreviated Names, and Estimated Affinities (pKis) of Methylenedioxy-Containing PSCs at MRs
 |
||||||
|---|---|---|---|---|---|---|
| R | name | M1Ra | M2Ra | M3Ra | M4Ra | M5Ra |
| −CH3 | MDPPP | NC | NC | NC | NC | NC |
| −CH2CH3 | MDPBP | NC | 4.90 | 4.84 | 4.99 | 4.74 |
| −CH2CH2CH3 | MDPV | 5.06 | 4.86 | 4.15 | 5.05 | 5.20 |
| −CH2(CH2)2CH3 | MDPHP | 5.44 | 5.30 | 4.59 | 4.89 | 5.10 |
| −CH2(CH2)3CH3 | MDPHPP | 5.22 | 4.92 | 4.44 | 4.48 | 5.25 |
Note that all MR affinities are estimates derived from results of [3H]scopolamine competition binding assays using 1 and 10 μM concentrations of PSCs (N = 2); NC, not calculated, is defined here as minimal (<5%) to no displacement of radioligand at 10 μM.
Affinity screens at 1 and 10 μM concentrations showed no consistent SAR for the methylenedioxy-containing analogs (Table 2, Figure S-1), except at M1Rs, where, like the unsubstituted PSCs, increasing the lipophilic side chain to butyl, 3,4-methylenedioxy-α-pyrrolidinohexiophenone (MDPHP), led to increased affinity. The estimated Ki of MDPHP at M1Rs was 3.63 μM—the highest estimated affinity of all methylenedioxy-containing PSCs at MRs. Thus, the bulky, methylenedioxy moiety hinders interaction with MRs.
M2Rs are the highest expressed MR in the heart and urinary bladder, and M1Rs are the highest expressed MR in the brain,24,25 organs impacted by PSCs, such as α-PHP. Thus, we focused our attention on the activity of unsubstituted PSCs at M2Rs and M1Rs. We performed full dose-response [3H]scopolamine competition binding experiments with the five unsubstituted PSCs at M2Rs and M1Rs; the MR agonist, oxotremorine, and the MR antagonist, atropine, were included as controls. Figure 1 shows competition binding curves at M2Rs for the unsubstituted PSCs and oxotremorine. As shown in Table 1 (and confirming initial estimates), the affinity of unsubstituted PSCs at M2Rs and M1Rs increased stepwise with increasing side chain length up to butyl.
Figure 1.
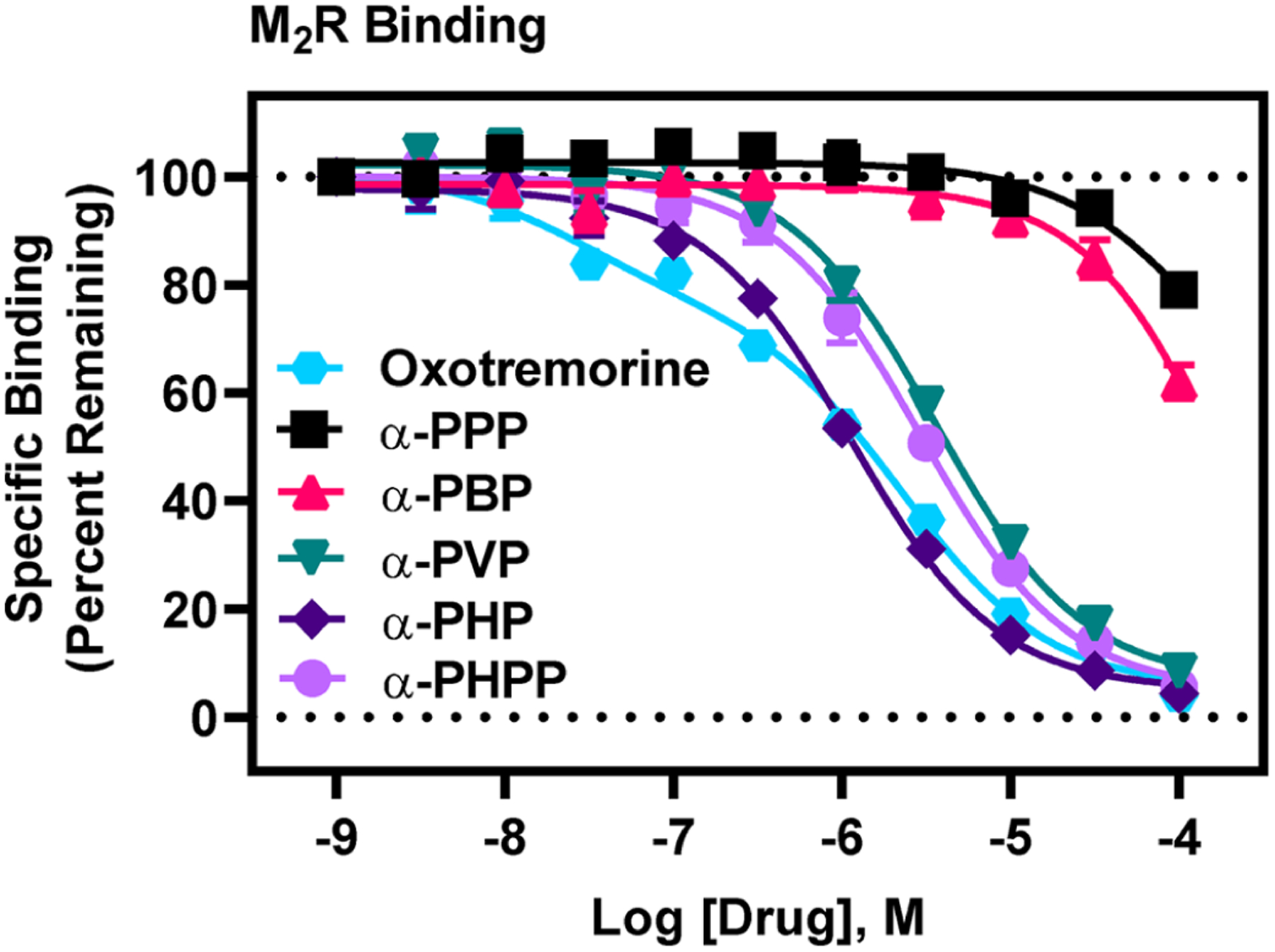
[3H]Scopolamine competition binding at M2Rs reveals nanomolar affinity of α-PHP. Data are expressed as normalized means (±SEM) of three, independent determinations, with drugs tested at each concentration in triplicate, except for oxotremorine, which was tested in duplicate. Curves for PSCs were best fit with a one-site model, whereas the oxotremorine curve best fit to a two-site model, suggestive of oxotremorine’s agonist activity, recognizing agonist high and low affinity M2R conformations. Note that α-PHP exhibited the highest affinity at M2Rs of all PSCs tested.
There was, first, a drastic leap in affinity from α-PPP to α-PVP. For example, the affinities (Ki values) of α-PPP and α-PBP at M2Rs were 73 μM and 40 μM, respectively, whereas the Ki of α-PVP at M2Rs was 824 nM—an 89-fold and 49-fold increase in affinity compared to α-PPP and α-PBP, respectively. The Ki of α-PHP at M2Rs was 251 nM—a striking 302-fold increase in M2R affinity compared to α-PPP. At M1Rs, from α-PPP to α-PHP, there was a similarly striking 121-fold increase in affinity. With an additional carbon, α-pyrrolidinoheptaphenone (α-PHPP), there was a modest decrease in affinity at M2Rs and M1Rs. α-PVP, α-PHP, and α-PHPP exhibited modestly higher affinity for M2Rs compared to M1Rs, and like M2Rs, α-PHP possessed highest M1R affinity relative to all other unsubstituted PSCs. Considering estimated affinities at M3Rs, M4Rs, and M5Rs, all unsubstituted PSCs had lowest affinity at M4Rs. Based on these results, we conclude that a butyl side chain on unsubstituted PSCs is optimal for binding M2Rs and M1Rs.
The massive impact of the α-carbon chain length of unsubstituted PSCs on M2R and M1R potency indicates it is a critical determinant for interaction with MRs. Moreover, the impact is remarkably greater than what has been reported for binding of unsubstituted PSCs at DAT and NET—the primary targets of PSCs; the affinity of α-PHP at DAT and NET is 81-fold and 6-fold higher than α-PPP, respectively.16 Nevertheless, the fact that increasing the lipophilic, α-carbon chain length of unsubstituted PSCs, but not methylenedioxy-substituted PSCs, causes substantial increases in affinities at two disparate targets—MRs and monoamine transporters—begs the question of whether this phenomenon extends to other proteins, and whether these other putative targets further contribute to toxicity caused by unsubstituted PSCs. Unsubstituted PSCs with extended alkyl chains appear to be more cytotoxic than methylenedioxy-substituted PSCs, including the extremely potent DAT inhibitor, MDPV.26 For example, Wojcieszak et al.26 reported that α-pyrrolidinooctanophenone (α-PV9) potently decreased mitochondrial activity and severely damaged cellular membranes; this effect was not observed with MDPV. Though this could be attributed to the shorter α-carbon chain in MDPV, a recent clinical report notes that the frequency of tachycardia and agitation is higher in α-PVP compared to MDPV cases.30 Additional studies are needed to determine whether aliphatic nitrogen substituents with increased lipophilic side chains increase toxicity. For example, tests of 2-(methylamino)-1-phenylpropan-1-one (methcathi-none) versus 2-(methylamino)-1-phenylhexan-1-one (hexe-drone) are warranted. Also warranted are studies evaluating effects of electronics and increased lipophilicity that arise from heterocyclic nitrogen substituents; for example, tests of differences between PSCs and piperidine-containing SCs. In conclusion, our results suggest two features of SCs impart MR off-target activity: 1) alkyl chains extending to at least propyl; and 2) the absence of a methylenedioxy moiety.
To test whether α-PHP displays antimuscarinic properties, we assessed, first, its functional capability to interfere with the agonist response of oxotremorine at M2Rs. We conducted cell-based, M2R-cAMP and M2R-β-arrestin recruitment experiments, with EC80 concentrations of oxotremorine [80% of the maximum effective concentration—a concentration chosen to maximize signal-to-noise without reducing sensitivity to detect antagonist effects] and a range of concentrations of α-PHP, spanning its affinity obtained from radioligand competition binding experiments. As shown in Figure 2, the positive control agonist, oxotremorine, decreased forskolin-stimulated cAMP production with an EC50 of 29 nM (pEC50 = 7.54 ± 0.12) and an EC80 of 130 nM (Figure 2A); analysis of [3H]scopolamine M2R competition binding results showed that oxotremorine data best fit to a two-site model (R2 = 0.98; F (2, 60) = 23.02, P < 0.0001, relative to a one-site model; pKi High = 7.92 ±0.40; pKi Low = 6.15 ± 0.10), consistent with its agonist activity. α-PHP completely inhibited M2R stimulation by oxotremorine, with a potency, Kb, of 120 nM (pKb = 6.92 ±0.03) (Figure 2B). The maximal effect of α-PHP on cAMP production was above the effect of forskolin alone, suggesting basal (or constitutive) M2R activity that was blocked by α-PHP.27 Thus, α-PHP is likely an inverse agonist of the M2R-cAMP signaling pathway. α-PHP exhibited similar pharmacology, but had lower potency, to block oxotremorine-stimulated β-arrestin recruitment to M2Rs (Figure 3). Oxotremorine increased β-arrestin recruitment with an EC50 of 1.95 μM (pEC50 of 5.71 ± 0.11). α-PHP completely inhibited oxotremorine-stimulated β-arrestin recruitment with a Kb of 501 nM (pKb = 6.30 ± 0.12), and α-PHP’s maximal effect extended below basal signaling, suggesting α-PHP is also an inverse agonist of M2R-β-arrestin recruitment. We also tested atropine as a control in this assay (Figure S-2). Its Kb at M2Rs was 2.57 nM (pKb = 8.59 ± 0.00), and it appeared to act as a neutral antagonist. The Kb for atropine is consistent with the Ki we obtained, 1.26 nM (pKi = 8.90 ± 0.07; best fit to a one-site model) from [3H]scopolamine competition binding (data not shown)—a value close to other observations (pKi = 8.91), i.e., as reported on the Psychoactive Drug Screening Program Ki database.
Figure 2.
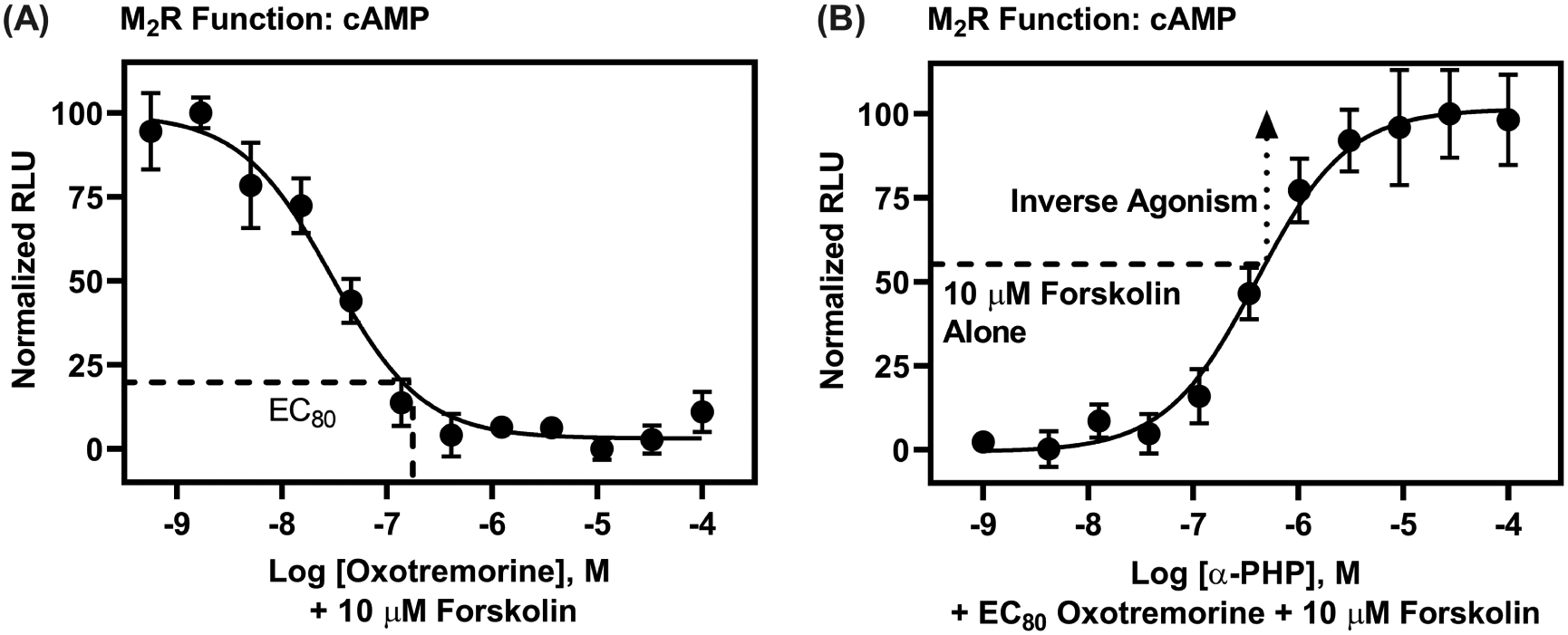
α-PHP is an antagonist/inverse agonist of M2R-cAMP signaling with nanomolar potency. (A) Oxotremorine dose-dependently decreased 10 μM forskolin-stimulated cAMP production; data are reported as percentages of the maximal, normalized relative luminescence units (RLUs) produced by forskolin alone. (B) α-PHP blocked effects of oxotremorine (EC80 concentration) at M2Rs and, moreover, increased cAMP levels beyond levels stimulated by forskolin alone, suggesting inverse agonist activity. Data are shown as normalized means (±SEM) from two, independent determinations, with oxotremorine tested at each concentration in duplicate and α-PHP tested at each concentration in quadruplicate.
Figure 3.
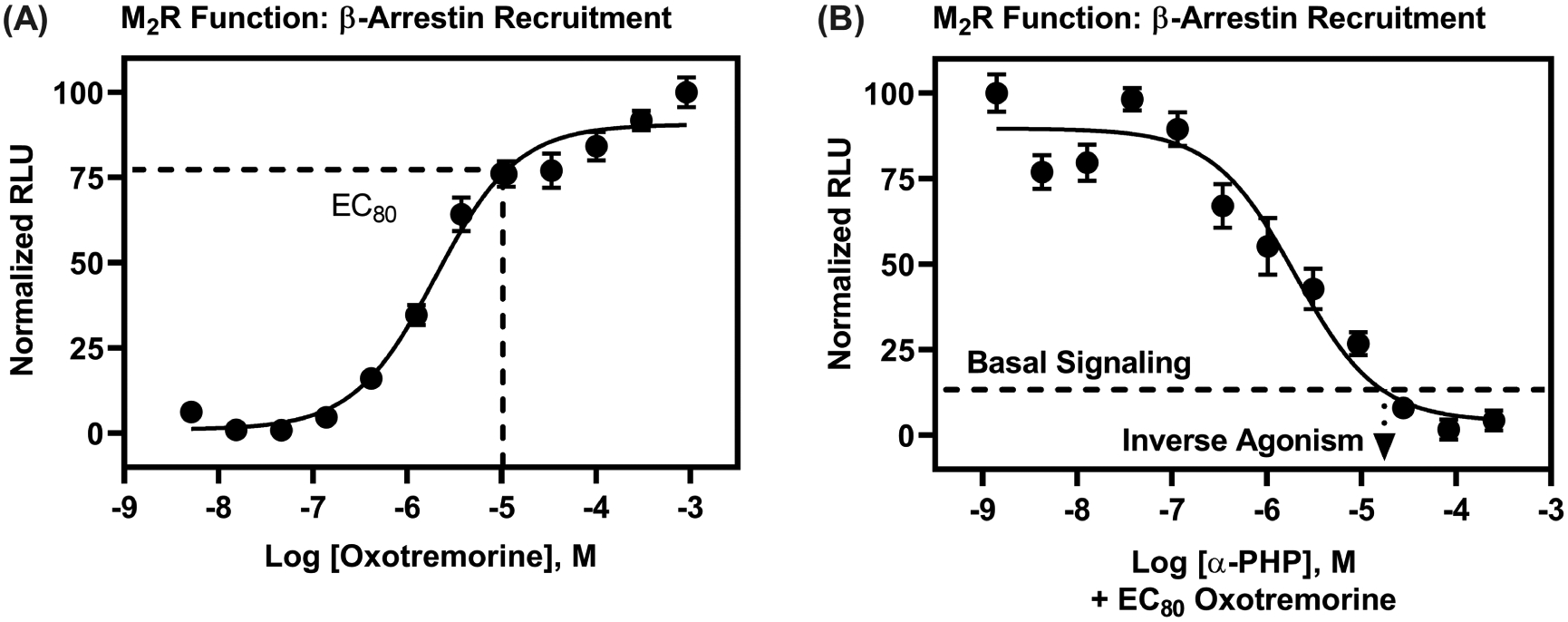
α-PHP is an antagonist/inverse agonist of M2R-β-arrestin recruitment with nanomolar potency. (A) Oxotremorine dose-dependently stimulated β-arrestin recruitment. (B) α-PHP blocked effects of oxotremorine (EC80 concentration) at M2Rs and, moreover, decreased β-arrestin recruitment below basal* levels, suggesting inverse agonist activity. Data are shown as normalized means (±SEM) of two, independent determinations, with oxotremorine tested at each concentration in duplicate and α-PHP tested in quadruplicate. [*Basal signaling is defined as the normalized RLUs emitted by untreated cells.]
M2Rs in the heart are essential for controlling chronotropic activity and contribute to inotropic activity.28 Parasympathetic, autonomic activation of cardiac M2Rs lowers heart rate to maintain normal physiological rhythms. Blocking these receptors causes tachycardia and can cause acute hypertension. The MR antagonist, atropine, for example, is used in hospitals to treat bradycardia, increasing heart rate, and tachycardia is a well-characterized side effect of atropine used medicinally for other conditions. Cardiovascular events are a primary cause of death associated with PSCs.29 Our observations demonstrating that α-PHP acts as a potent antagonist at M2R suggest this pharmacodynamic effect may contribute to the reportedly higher tachycardia associated with α-PHP; according to the World Health Organization’s Critical Review Report on α-PHP, tachycardia is an extremely common side effect, and some consumers report that it is stronger than other PSCs;15 we are mindful, however, of the inherent subjectivity and perhaps inaccuracies associated with self-reports.
Owing to its relatively short history as a drug of abuse, the clinical toxicology data on α-PHP are scant relative to other PSCs, like α-PVP, but given the high structural similarity between the two, we conjecture that they have similar pharmacological actions at MRs. In one study where α-PVP was confirmed analytically to be the only stimulant responsible for intoxication, 80% of the patients presented with tachycardia.30 While this is attributed to sympathomimetic activity, e.g., increased norepinephrine release, the cardiotoxic mechanism of α-PVP could also involve activity at M2Rs; we found that α-PVP binds M2R with a Ki of 824 nM, well within the range of postmortem blood concentrations detected in humans.6,31 M2Rs are also the highest expressed MR in the urinary bladder, where they contribute to detrusor smooth muscle contraction, resulting in urination.32 Side effects of α-PHP include suppression of urinary urgency,7 which we speculate could also be associated with its M2R antagonist activity. However, we acknowledge that pharmacology and gene knockout studies show that M3Rs are the predominant MR subtype mediating bladder contraction33,34 and that sympathomimetic actions also can cause urinary retention.
Because PSCs, including α-PHP, can produce cognitive side effects, including symptoms of delirium, such as memory impairments, we also determined the activity of α-PHP at M1Rs (Figures 4 and 5) that are highly expressed in brain regions implicated in symptoms of delirium, including the hippocampus, cortex, and striatum.24,35 Figure 4 shows results from [3H]scopolamine M1R competition binding experiments and illustrates that, amongst the unsubstituted PSCs, α-PHP has the highest M1R affinity (Ki = 1.6 μM). We conducted cell-based, M1R-phosphoinositide hydrolysis assays, with EC80 concentrations of oxotremorine, and a range of concentrations of α-PHP, spanning its affinity obtained from radioligand competition binding experiments. As shown in Figure 5, oxotremorine increased inositol phosphate 1 (IP1) production with an EC50 of 62 nM (pEC = 7.21 ± 0.20) and an EC80 of 500 nM (Figure 5A); in M1R competition binding assays, oxotremorine data best fit to a two-site model (R2 = 0.98, F (2,94) = 4.01, P = 0.0214, relative to a one-site model; pKi High = 7.62 ± 0.36; pKi Low = 5.41 ± 0.04) consistent with its M1R agonist activity. The affinity of atropine at M1Rs was 1.55 nM (pKi = 8.81 ± 0.05), determined from [3H] scopolamine competition binding (data not shown; we did not evaluate atropine in M1R functional assays). α-PHP completely inhibited M1R-IP1 production caused by oxotremorine, with a potency, Kb, of 1.38 μM (pKb = 5.86 ± 0.35) (Figure 5B). The maximal effect of α-PHP was lower than basal signaling, suggesting M1R constitutive activity that was blocked by α-PHP.27 Thus, like its activity at M2Rs, α-PHP appears to possess inverse agonist activity at the M1R-inositol phosphate signaling pathway.
Figure 4.
[3H]Scopolamine competition binding at M1Rs reveals low micromolar affinity of α-PHP. Data are expressed as normalized means (±SEM) of three, independent determinations, with drugs tested at each concentration in triplicate. Curves for PSCs were best fit with a one-site model, whereas the oxotremorine curve best fit to a two-site model, suggestive of oxotremorine’s agonist activity, recognizing agonist high and low affinity M1R conformations. Note that α-PHP exhibited the highest affinity at M1Rs of all PSCs tested.
Figure 5.
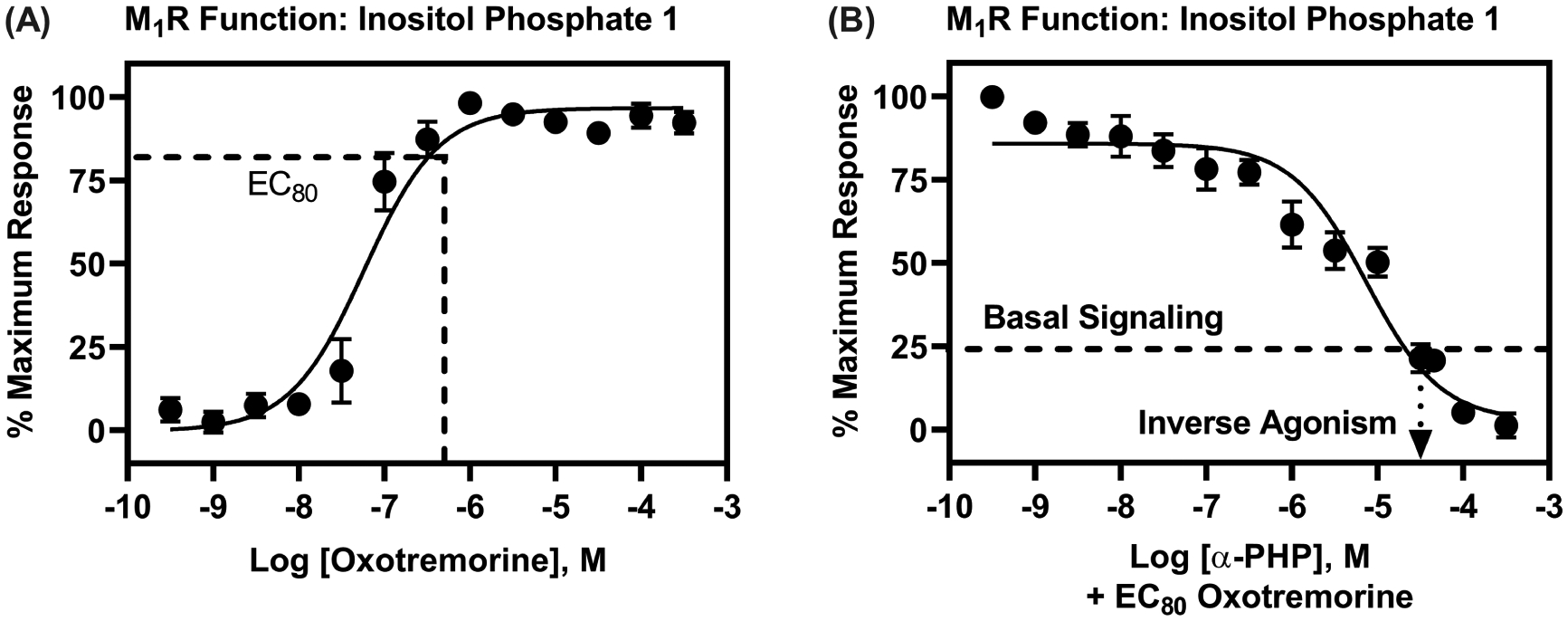
α-PHP is an antagonist/inverse agonist of M1R-inositol phosphate signaling with low micromolar potency. (A) Oxotremorine dose-dependently stimulated inositol phosphate 1 (IP1) production. (B) α-PHP blocked effects of oxotremorine (EC80 concentration) at M1Rs and, moreover, decreased IP1 production below basal levels, suggesting inverse agonist activity. Data are expressed as normalized means (±SEM) of three, independent determinations, with oxotremorine tested at each concentration in duplicate and α-PHP tested at each concentration in triplicate.
We note here that since α-PHP exhibited inverse agonist activity at M2Rs and M1Rs while employing experimental methods to determine functional affinity as described by ref 27, our reported α-PHP Kb values may be higher/less potent than actuality. α-PHP’s IC50 includes concentrations that extend beyond M2R and M1R constitutive activity, i.e., beyond complete interference of oxotremorine-elicited M2R and M1R signaling; if we were to determine α-PHP’s IC50 using concentrations where it fully blocked oxotremorine signaling but did not reduce basal activity, then its IC50 would be shifted to the left; its calculated Kb would then be lower. We are unaware that this is an acceptable practice, so we stuck with convention. Future experiments could be designed to explicitly examine inverse agonist activity, comparing α-PHP to other reported MR inverse agonists (e.g., scopolamine); results would clarify α-PHP’s inverse agonist potency and efficacy. Specifically, we could evaluate α-PHP’s functional activity at MRs without an agonist ligand present.
Although clinical toxicology data on α-PHP are scant relative to other PSCs, what is reported illustrates that, in intoxicated non-fatal cases where α-PHP was the only drug detected, and in postmortem cases involving only α-PHP, blood or serum concentrations can exceed its Kb at M2Rs and M1Rs; these data indicate α-PHP’s MR activity is physiologically relevant. A male admitted to the hospital after inhaling only α-PHP had blood concentrations of 1.28 μM.15 Another patient who was admitted to the hospital after taking α-PHP had serum concentrations of 0.71 μM.36 In fatal cases that have been reported, blood concentrations were as high as 2.86 μM.15 More knowledge can be gleaned by reviewing toxicology reports from closely related PSCs. In non-fatal cases, serum concentrations of α-PVP have been reported to be as high as 2.6 μM,30 and in postmortem cases, levels as high as 86 μM have been reported.6 MDPV concentrations in blood from intoxicated individuals exceed 1 μM and reach 30 μM,37,38 and yet MDPV is reportedly active at doses lower than α-PHP. According to psychonautwiki.org, the common to strong dose of MDPV is 8–25 mg orally, whereas the common to strong dose of α-PHP is 10–40 mg orally.
α-PHP is highly lipophilic (cLogP = 4.13), suggesting it likely reaches significantly greater concentrations in fatty tissues, such as the brain, than in the blood.26,39,40 Thus, α-PHP might reach concentrations in the brain that interfere with M1Rs. Individuals who abuse psychostimulants typically redose multiple times in a session, and as tolerance develops with repeated administration, individuals often increase their doses.15,41 Thus, reported blood concentrations from acute intoxications may not accurately reflect levels in individuals who are binging. We realize, however, that it is difficult to determine if blood or serum concentrations reported in the literature are from acute (single, high-dose administration) or binge intoxications, especially in cases of overdose resulting in death.
In conclusion, this study indicates that, in addition to its well-known actions as a sympathomimetic and psychostimulant drug, α-PHP is an M2R and M1R antagonist, suggesting it is a parasympatholytic drug and likely has effects on M1Rs expressed in the central nervous system.
METHODS
Drugs.
All PSCs were made as hydrochloride salts. α-PPP and MDPPP were synthesized and provided by Dr. Bruce Blough at Research Triangle Institute. α-PHP (catalog #9001934), α-PHPP (catalog #14762), 3,4-methylenedioxy-α-pyrrolidinobutiophenone (MDPBP, catalog #10437), MDPHP (catalog #16361), and 3,4-methylenedioxy-α-pyrrolidinoheptaphenone (MDPHPP, catalog #16358) analytical standards were purchased from Cayman Chemical (Ann Arbor, MI, USA). α-PBP (catalog # P-110, 1 mg of free base per mL methanol), α-PVP (catalog # P-090, 1 mg of free base per mL methanol), and MDPV (catalog # M-146, 1 mg of free base per mL methanol) analytical standards were purchased from MilliporeSigma (Darmstadt, Germany). Atropine sulfate monohydrate was purchased from Alfa Aesar (Tewksbury, MA, USA). Oxotremorine sesquifuma-rate was purchased from Tocris Bioscience (Minneapolis, MN, USA). All solid compounds were dissolved in dimethyl sulfoxide to 10 mM concentrations for receptor binding assays and in Milli-Q water (MilliporeSigma) to 10 mM concentrations for receptor function assays, prior to diluting in assay buffer. All compounds that were procured as methanol solutions were diluted in assay buffer for receptor binding assays. [No drugs procured as methanol solutions were tested in functional assays.] [3H]Scopolamine (scopolamine methyl chloride, N-methyl-[3H]scopolamine; specific activity 80.1 Ci/mmol) was purchased from PerkinElmer (Waltham, MA, USA).
Radioligand Competition Binding.
Radioligand competition binding assays were conducted using membranes collected from transiently transfected HEK293 cells (P < 20 from a procured stock, CRL-1573, ATCC, Manassas, VA, USA) as previously described.23 Briefly, cells ~90% confluent in 10 cm plates were transfected with 5 μg of human M1R, M2R, M3R, M4R or M5R cDNA (cDNA resource center, Bloomsburg, PA, USA) using a lipid-based method (LipoD293 reagent, SignaGen Laboratories, Rockville, MD, USA). Membranes were collected by centrifugation ~48 h later and stored at −80 °C until used. For initial affinity estimations, membranes were incubated in 96-well plates with 1 nM [3H]scopolamine and test compounds in assay buffer at final concentrations of 1 and 10 μM. Atropine (10 μM) was used to define nonspecific binding. After a 90 min incubation at room temperature on a shaker, contents from the plates were rapidly filtered through GF/B fiberglass filter mats, presoaked with ice cold 50 mM Tris buffer (pH 7.4), using a Microbeta Filtermat-96 cell harvester (PerkinElmer, Waltham, MA, USA). Approximately 200 mL of ice cold, 50 mM Tris buffer was then vacuumed through filter mats to wash away unbound radioligand. Filter mats were dried on a hot plate and soaked in Betaplate (PerkinElmer, Waltham, MA, USA) scintillation fluid in plastic bags. Bags were sealed and placed in cassettes. Radioactivity was measured in a Microbeta2 Microplate Counter (PerkinElmer, Waltham, MA, USA), and counts per minute were recorded. For full dose-response competition binding assays, increasing half-log unit concentrations of five unsubstituted PSCs were tested at M2Rs and M1Rs, with the same procedures described above.
M2R cAMP Signaling.
cAMP measurements were performed with the cAMP Hunter eXpress CHRM2 CHO-K1 GPCR assay (Eurofins DiscoveRx, Fremont, CA, USA) according to the manufacturer’s protocol. Briefly, in the provided 96-well plate, 100 μL of human M2R expressing CHO-K1 cells were seeded in cell plating reagent and incubated at 37 °C, 5% CO2, 95% humidity overnight. The next day, cell plating reagent was replaced with 30 μL of cell assay buffer. For agonist stimulation, cells were treated with increasing half-log unit concentrations of oxotremorine in the presence of 10 μM forskolin, followed by incubation, as above, for 30 min. A cAMP antibody, cAMP working detection solution and enzyme acceptor solution were then added according to the protocol. Chemiluminescence was measured by a Mithras LB 940 microplate reader (Berthold Technologies, Bad Wildbad, Germany). Antagonist tests were run by pretreating cells with increasing half-log unit concentrations of α-PHP, followed by incubation, as above, for one hour. Cells were then treated with EC80 concentration of oxotremorine (130 nM) and 10 μM forskolin. The same incubation and detection procedure used for agonist assays was used for the remaining steps.
M2R β-Arrestin Recruitment.
M2R-elicited β-arrestin recruitment was assessed using the PathHunter β-Arrestin GPCR assay (Eurofins DiscoveRx, Fremont, CA, USA) according to the manufacturer’s protocol. Briefly, in the provided 96-well plate, 100 μL of human M2R expressing CHO-K1 cells were seeded in cell plating reagent and incubated for ~48 h. For agonist stimulation, cells were treated with increasing half-log unit concentrations of oxotremorine and incubated, as performed in the M2R cAMP assays, for 1.5 h, followed by addition of working detection solution. After further incubation for 1 h at room temperature in the dark, the plate was read for chemiluminescent detection in the Mithras LB 940 microplate reader. Antagonist tests were run by pretreating cells with increasing half-log unit concentrations of atropine or α-PHP, followed by incubation, as performed in the M2R cAMP assays, for 1.5 h. Cells were then treated with EC80 concentration of oxotremorine (10 μM). The same incubation and detection procedures used for agonist assays were used for the remaining steps.
M1R Phosphoinositide Hydrolysis.
The activity of α-PHP at M1Rs was measured using the IP-One homogeneous time-resolved fluorescence (HTRF) kit (Cisbio, Bedford, MA, USA). The assay was performed per the manufacturer’s protocol with optimization based on our published methods.23,42 Transfection of HEK293 cells with human M1Rs using LipoD293 reagent was performed as described for radioligand competition binding assays with one exception: The transfection reagent was replaced with normal growth media (Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum) after 8 h to improve cell viability. Twenty-four hours after transfection, cells were serum starved in an incubator (37 °C, 5% CO2, 95% humidity) for 2 h and then were plated at 2500 cells per well in 384-well plates. For agonist stimulation, cells were treated with oxotremorine at increasing half-log unit concentrations and then placed in the incubator for 2 h. Antagonist tests were run by pretreating cells with increasing half-log unit concentrations of α-PHP and then incubating for 1 h. Cells were then treated with 500 nM (EC80) oxotremorine for 1 h in the incubator. The reaction was terminated with lysis buffer containing d2-labeled IP1 and anti-IP1-Cryptate. HTRF resonance energy transfer was measured by a Mithras LB 940 microplate reader.
Curve Fitting and Statistical Analyses.
All data were analyzed by GraphPad 8.3.0 (San Diego, CA, USA). Ki values were determined from data fit to both one- and two-site models. Kd values of [3H]scopolamine at each of the MRs used in the determination of Ki values were based on ref 43 and in nM were as follows: M1R = 1.00; M2R = 0.34; M3R = 0.36; M4R = 0.19; M5R = 0.45. Since both α-PPP and α-PBP displaced less than 50% of radioligand when tested at the highest concentration (100 μM) in full dose-response competition binding assays, we interpolated the data by adding a 10 mM data point, which we set as nonspecific binding. This is also the approach we used to determine estimated Ki values for all compounds tested at 1 and 10 μM concentrations only. We acknowledge the reliability of our Ki estimation likely decreases as numbers deviate beyond 10 μM, e.g., for methylenedioxy-containing PSCs at certain MRs (Table 2). EC50 and IC50 values from functional assays were determined from data fit to the log(agonist) vs response (three parameters) or log(inhibitor) vs response (three parameters) model, respectively. Kb values were calculated from IC50 values using the Cheng-Prusoff equation with modification44
where [A] is the EC80 concentration of agonist; [A50] is the agonist EC50; and nH is the Hill slope of the agonist, which was constrained to 1.
Supplementary Material
ACKNOWLEDGMENTS
The authors owe a debt of gratitude to Dr. Bruce Blough for providing α-PPP and MDPPP used in the studies and acknowledge Professor Kevin S. Murnane who was MPI on R21DA040907, which provided the foundation for conceiving this project. The authors also thank Ms. Rachel A. Lukavsky who conducted, under the direction of Y.C., some of the 1 and 10 μM MR affinity screening tests of PSCs while engaging in Mercer University’s Undergraduate Biomedical Scholar Training Initiative.
Funding
This work was funded by a grant from the National Institute on Drug Abuse, R21DA040907, and by start-up funds from Mercer University, College of Pharmacy.
ABBREVIATIONS
- α-PPP
α-pyrrolidinopropiophenone
- α-PBP
α-pyrrolidinobutiophenone
- α-PVP
α-pyrrolidinopentiophenone
- α-PHP
α-pyrrolidinohexiophenone
- α-PHPP
α-pyrrolidinoheptaphenone
- MDPPP
3,4-methylenedioxy-α-pyrrolidinopropiophenone
- MDPBP
3,4-methylenedioxy-α-pyrrolidinobutiophenone
- MDPV
3,4-methylenedioxypyrovalerone
- MDPHP
3,4-methylenedioxy-α-pyrrolidinohexiophenone
- MDPHPP
3,4-methylenedioxy-α-pyrrolidinoheptaphenone
- M1R
muscarinic 1 receptor
- M2R
muscarinic 2 receptor
- M3R
muscarinic 3 receptor
- M4R
muscarinic 4 receptor
- M5R
muscarinic 5 receptor
- MR
muscarinic receptor
- DEA
Drug Enforcement Administration
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acschemneuro.0c00008
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.0c00008.
Figure S-1, MR affinity screening of methylenedioxy-containing PSCs to investigate a potential SAR; and Figure S-2, atropine antagonism of oxotremorine-stimulated M2R-β-arrestin recruitment (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Katz DP, Bhattacharya D, Bhattacharya S, Deruiter J, Clark CR, Suppiramaniam V, and Dhanasekaran M (2014) Synthetic cathinones: ″a khat and mouse game″. Toxicol. Lett 229, 349–356. [DOI] [PubMed] [Google Scholar]
- (2).Seeger E (1967) α-Pyrrolidino ketones, In US3314970A (States, U., Ed.). [Google Scholar]
- (3).Seeger E (1966) Compositions and methods for stimulating the central nervous system and increasing the blood pressure, In US3287217A (States, U., Ed.). [Google Scholar]
- (4).Baumann MH, Walters HM, Niello M, and Sitte HH (2018) Neuropharmacology of Synthetic Cathinones In New Psychoactive Substances: Pharmacology, Clinical, Forensic and Analytical Toxicology (Maurer HH, and Brandt SD, Eds.), pp 113–142, Springer International Publishing, Cham, DOI: 10.1007/164_2018_178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Karila L, Megarbane B, Cottencin O, and Lejoyeux M (2015) Synthetic cathinones: a new public health problem. Curr. Neuropharmacol 13, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kubo SI, Waters B, Hara K, Fukunaga T, and Ikematsu K (2017) A report of novel psychoactive substances in forensic autopsy cases and a review of fatal cases in the literature. Leg. Med 26, 79–85. [DOI] [PubMed] [Google Scholar]
- (7).Kraemer M, Boehmer A, Madea B, and Maas A (2019) Death cases involving certain new psychoactive substances: A review of the literature. Forensic Sci. Int 298, 186–267. [DOI] [PubMed] [Google Scholar]
- (8).Simmons SJ, Leyrer-Jackson JM, Oliver CF, Hicks C, Muschamp JW, Rawls SM, and Olive MF (2018) DARK Classics in Chemical Neuroscience: Cathinone-Derived Psychostimulants. ACS Chem. Neurosci 9, 2379–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Cunningham A, Evans-Brown M, Gallegos A, Sedefov R, Almeida A, and Dudek D (2014) EMCDDA-Europol Joint Report on a new psychoactive substance: MDPV (3,4-methylenedioxypyrovalerone), In European Monitoring Centre for Drugs and Drug Addiction, http://www.emcdda.europa.eu/system/files/publications/819/TDAS14001ENN_466653.pdf (accessed Feb 23, 2020). [Google Scholar]
- (10).DEA. (2019) National Drug Threat Assessment, In U.S. Department of Justice, Drug Enforcement Administration (DEADCT-DIR-007–20, Ed.). [Google Scholar]
- (11).Nobrega L, and Dinis-Oliveira RJ (2018) The synthetic cathinone alpha-pyrrolidinovalerophenone (alpha-PVP): pharmacokinetic and pharmacodynamic clinical and forensic aspects. Drug Metab. Rev 50, 125–139. [DOI] [PubMed] [Google Scholar]
- (12).Beck O, Backberg M, Signell P, and Helander A (2018) Intoxications in the STRIDA project involving a panorama of psychostimulant pyrovalerone derivatives, MDPV copycats. Clin. Toxicol 56, 256–263. [DOI] [PubMed] [Google Scholar]
- (13).Assi S, Gulyamova N, Kneller P, and Osselton D (2017) The effects and toxicity of cathinones from the users’ perspectives: A qualitative study. Hum. Psychopharmacol 32, e2610. [DOI] [PubMed] [Google Scholar]
- (14).Mash DC, Duque L, Pablo J, Qin Y, Adi N, Hearn WL, Hyma BA, Karch SB, Druid H, and Wetli CV (2009) Brain biomarkers for identifying excited delirium as a cause of sudden death. Forensic Sci. Int 190, e13–19. [DOI] [PubMed] [Google Scholar]
- (15).Spanagel R, Poovendran D, and Forte G (2019) World Health Organization. Critical Review Report. alpha-PHP (α-pyrrolidinohexanophenone) or PV-7 In Expert Committee on Drug Dependence, Forty-second Meeting, Geneva, Switzerland, 21–25 October 2019, https://www.who.int/medicines/access/controlled-substances/Final_alphaPHP.PDF?ua=1 (accessed Feb 23, 2020). [Google Scholar]
- (16).Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson T, Johnson RA, and Janowsky A (2017) Structure-Activity Relationships of Substituted Cathinones, with Transporter Binding, Uptake, and Release. J. Pharmacol. Exp. Ther 360, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kolanos R, Sakloth F, Jain AD, Partilla JS, Baumann MH, and Glennon RA (2015) Structural Modification of the Designer Stimulant alpha-Pyrrolidinovalerophenone (alpha-PVP) Influences Potency at Dopamine Transporters. ACS Chem. Neurosci 6, 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Soares J, Costa VM, Gaspar H, Santos S, de Lourdes Bastos M, Carvalho F, and Capela JP (2019) Structure-cytotoxicity relationship profile of 13 synthetic cathinones in differentiated human SH-SY5Y neuronal cells. NeuroToxicology 75, 158–173. [DOI] [PubMed] [Google Scholar]
- (19).Fryer AD, Christopoulos A, and Nathanson NM (2012) Muscarinic receptors; Springer, Heidelberg, DOI: 10.1007/978-3-642-23274-9. [DOI] [Google Scholar]
- (20).Lakstygal AM, Kolesnikova TO, Khatsko SL, Zabegalov KN, Volgin AD, Demin KA, Shevyrin VA, Wappler-Guzzetta EA, and Kalueff AV (2019) DARK Classics in Chemical Neuroscience: Atropine, Scopolamine, and Other Anticholinergic Deliriant Hallucinogens. ACS Chem. Neurosci 10, 2144–2159. [DOI] [PubMed] [Google Scholar]
- (21).Bersani FS, Corazza O, Simonato P, Mylokosta A, Levari E, Lovaste R, and Schifano F (2013) Drops of madness? Recreational misuse of tropicamide collyrium; early warning alerts from Russia and Italy. Gen Hosp Psychiatry 35, 571–573. [DOI] [PubMed] [Google Scholar]
- (22).FDA. (2018) Atropine sulfate - atropine sulfate injection full prescribing information. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4a4fa6e8-0e0a-0e76-e054-00144ff8d46c (accessed Feb 23, 2020).
- (23).Chen Y, Blough BE, Murnane KS, and Canal CE (2019) The synthetic cathinone psychostimulant alpha-PPP antagonizes serotonin 5-HT2A receptors: In vitro and in vivo evidence. Drug Test. Anal 11, 990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lebois EP, Thorn C, Edgerton JR, Popiolek M, and Xi S (2018) Muscarinic receptor subtype distribution in the central nervous system and relevance to aging and Alzheimer’s disease. Neuropharmacology 136, 362–373. [DOI] [PubMed] [Google Scholar]
- (25).Frazier EP, Peters SL, Braverman AS, Ruggieri MR Sr., and Michel MC (2008) Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and beta-adrenoceptors. Naunyn-Schmiedeberg’s Arch. Pharmacol 377, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wojcieszak J, Andrzejczak D, Woldan-Tambor A, and Zawilska JB (2016) Cytotoxic Activity of Pyrovalerone Derivatives, an Emerging Group of Psychostimulant Designer Cathinones. Neurotoxic. Res 30, 239–250. [DOI] [PubMed] [Google Scholar]
- (27).Kenakin TP (2014) A pharmacology primer: techniques for more effective and strategic drug discovery, 4th ed., Academic Press, Amsterdam; Boston. [Google Scholar]
- (28).Gordan R, Gwathmey JK, and Xie LH (2015) Autonomic and endocrine control of cardiovascular function. World J. Cardiol 7, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kronstrand R, Guerrieri D, Vikingsson S, Wohlfarth A, and Green H (2018) Fatal Poisonings Associated with New Psychoactive Substances. Handb. Exp. Pharmacol 252, 495–541. [DOI] [PubMed] [Google Scholar]
- (30).Beck O, Franzen L, Backberg M, Signell P, and Helander A (2016) Toxicity evaluation of alpha-pyrrolidinovalerophenone (alpha-PVP): results from intoxication cases within the STRIDA project. Clin. Toxicol 54, 568–575. [DOI] [PubMed] [Google Scholar]
- (31).Adamowicz P, Gieron J, Gil D, Lechowicz W, Skulska A, Tokarczyk B, and Zuba D (2016) Blood concentrations of alpha-pyrrolidinovalerophenone (alpha-PVP) determined in 66 forensic samples. Forensic Toxicol. 34, 227–234. [Google Scholar]
- (32).Hegde SS (2006) Muscarinic receptors in the bladder: from basic research to therapeutics. Br. J. Pharmacol 147 (Suppl 2), S80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Fetscher C, Fleichman M, Schmidt M, Krege S, and Michel MC (2002) M-3 muscarinic receptors mediate contraction of human urinary bladder. Br. J. Pharmacol 136, 641–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Matsui M, Motomura D, Fujiwara T, Jiang J, Takahashi S, Manabe T, and Taketo MM (2002) Mice lacking M-2 and M-3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J. Neurosci 22, 10627–10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).van Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, and de Rooij SEJA (2011) Neuroinflammation in Delirium: A Postmortem Case-Control Study. Rejuvenation Res. 14, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Fujita Y, Mita T, Usui K, Kamijo Y, Kikuchi S, Onodera M, Fujino Y, and Inoue Y (2018) Toxicokinetics of the Synthetic Cathinone alpha-Pyrrolidinohexanophenone. J. Anal. Toxicol 42, e1–e5. [DOI] [PubMed] [Google Scholar]
- (37).Adamowicz P, Gil D, Skulska A, and Tokarczyk B (2013) Analysis of MDPV in Blood-Determination and Interpretation. J. Anal. Toxicol 37, 308–312. [DOI] [PubMed] [Google Scholar]
- (38).Kriikku P, Wilhelm L, Schwarz O, and Rintatalo J (2011) New designer drug of abuse: 3,4-Methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci. Int 210, 195–200. [DOI] [PubMed] [Google Scholar]
- (39).Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, and Liechti ME (2013) Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol 168, 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Canal CE, Felsing DE, Liu Y, Zhu W, Wood JT, Perry CK, Vemula R, and Booth RG (2015) An Orally Active Phenylaminotetralin-Chemotype Serotonin 5-HT7 and 5-HT1A Receptor Partial Agonist that Corrects Motor Stereotypy in Mouse Models. ACS Chem. Neurosci 6, 1259–1270. [DOI] [PubMed] [Google Scholar]
- (41).Prosser JM, and Nelson LS (2012) The toxicology of bath salts: a review of synthetic cathinones. J. Med. Toxicol 8, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Liu Y, Canal CE, Cordova-Sintjago TC, Zhu W, and Booth RG (2017) Mutagenesis Analysis Reveals Distinct Amino Acids of the Human Serotonin 5-HT(2C) Receptor Underlying the Pharmacology of Distinct Ligands. ACS Chem. Neurosci 8, 28–39. [DOI] [PubMed] [Google Scholar]
- (43).Roth BL (2013) NIMH PDSP Assay Protocol Book, Version II. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=2ahUKEwih4r6Azc_nAhUnx1kKHYzyCvEQFjABegQIBxAB&url=https%3A%2F%2Fpdspdb.unc.edu%2FpdspWeb%2Fcontent%2FPDSP%2520Protocols%2520II%25202013-03-28.old.pdf&usg=AOvVaw0Dnr1VTnOJHxfa7Ig2SDld (accessed Feb 23, 2020).
- (44).Leff P, and Dougall IG (1993) Further Concerns over Cheng-Prusoff Analysis. Trends Pharmacol. Sci 14, 110–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


