Abstract
In 1929 Kurt von Neergaard performed experiments suggesting the presence of pulmonary surfactant and its relevance to the newborn's first breath. Almost 25 years later, Richard Pattle, John Clements and Chris Macklin, each working on the effects of nerve gases on the lungs, contributed to the understanding of the physiology of pulmonary surfactant. About 5 years later Mary Ellen Avery and Jere Mead published convincing evidence that preterm neonates dying of hyaline membrane disease (respiratory distress syndrome, RDS) had a deficiency of pulmonary surfactant. The first trials of nebulized synthetic (protein-free) surfactant to prevent RDS were published soon after Patrick Bouvier Kennedy (son of President John F Kennedy) died of this disorder after treatment in Boston. These trials were unsuccessful; however, Goran Enhorning and Bengt Robertson in the early 1970s demonstrated that natural surfactants (containing proteins) were effective in an immature rabbit model of RDS. Soon after this Forrest Adams showed that a natural surfactant was also effective in an immature lamb model. Working with him was Tetsuro Fujiwara who 2 years later, after returning to Japan, published the seminal article reporting the responses of 10 preterm infants with RDS to a bolus of modified bovine surfactant. During the 1980s there were numerous randomized controlled trials of many different natural and synthetic surfactants, demonstrating reductions in pulmonary air leaks and neonatal mortality. Subsequently natural surfactants were shown to be superior to the protein-free synthetic products. Recently there have been a number of randomized trials comparing different natural surfactant preparations. Commercially available bovine surfactants may have similar efficacy but there is some evidence that a porcine surfactant used to treat RDS with an initial dose of 200 mg per kg is more effective than a bovine surfactant used in an initial dose of 100 mg per kg. Bovine and porcine surfactants have not been compared in trials of prophylaxis. Very recently a new synthetic surfactant with a surfactant protein mimic has been compared with other commercially available natural and synthetic surfactants in two trials. The new surfactant may be superior to one of the older protein-free synthetic surfactants but there is no evidence of its superiority over established natural products and it is currently not approved for clinical use. A number of other new synthetic surfactants have been tested in animal models or in treatment of adults with ARDS, but so far there have been no reports of treatment of neonatal RDS. Natural surfactants work best if given by a rapid bolus into the lungs but less invasive methods such as a laryngeal mask, pharyngeal deposition or rapid extubation to CPAP have showed promise. Unfortunately, delivery of surfactant by nebulization has so far been ineffective. Surfactant treatment has been tried in a number of other neonatal respiratory disorders but only infants with meconium aspiration seem to benefit although larger and more frequent doses are probably needed to demonstrate improved lung function. A surfactant protocol based upon early treatment and CPAP is suggested for very preterm infants. Earlier treatment may improve survival rates for these infants; however, there is a risk of increasing the prevalence of milder forms of chronic lung disease. Nevertheless, surfactant therapy has been a major contribution to care of the preterm newborn during the past 25 years.
Keywords: surfactant, respiratory distress syndrome, preterm infant, hyaline membrane disease, history, chronic lung disease
Pioneers of surfactant research—Von Neergaard, Pattle, Clements and Macklin
I have chosen 1929 as the start date for this presentation on the history of surfactant administration in the treatment of neonatal respiratory distress syndrome (RDS). In 1929 Kurt von Neergaard, a German-born physiologist working in Switzerland, evacuated air from an isolated porcine lung which he then filled with an isotonic gum solution ‘to eliminate surface tension of the air tissue interfaces.’1 Von Neergaard then performed pressure-volume curves during expansion of the lungs with air and liquid. From these experiments he was able to conclude three things:
‘Surface tension is responsible for the greater part of total lung recoil compared to tissue elasticity.’
‘A lower surface tension would be useful for the respiratory mechanism because without it pulmonary retraction might become so great as to interfere with adequate expansion’ and
‘Surface tension as a force counteracting the first breath of the newly born should be investigated further.’
Unfortunately, von Neergaard did not follow his own advice so it was left to Peter Gruenwald, a pathologist in New York, to repeat these experiments with the lungs of stillborn infants some 18 years later.2 In 1947 Gruenwald stated ‘the resistance to aeration is due to surface tension which counteracts the entrance of air’ and he also showed that surface active substances reduced the pressure needed for lung aeration. It is perhaps noteworthy that Peter Gruenwald later taught Mary Ellen Avery.
In the 1950s Richard Pattle (Figure 1) was working with nerve gases in England when he made an unexpected discovery. Pattle was a physicist working at a Ministry of Defence laboratory at Porton Down when he noticed that nerve gases caused pulmonary edema foam in the rabbits he was studying. These bubbles remained stable for many hours. The usual antifoaming agents were ineffective and Pattle surmised that ‘the air bubbles must be covered with a unique substance from the lining layers of the alveoli which made them so stable.’3 He also speculated that ‘absence of the lining substance may sometimes be one of the difficulties with which a premature baby has to contend.’ At almost the same time John Clements (Figure 2) working at the US Army Chemical Center in Edgewood, Maryland was performing quantitative studies using a modified Wilhelmy balance.4 Clements was a physiologist with no particular interest in the respiratory system until he was assigned the task of finding out how nerve gases damaged the lungs. In 1957, he published an article reporting the surface tension of films from the lungs of rats, cats and dogs.5 Together with Chris Macklin, a pathologist working with phosgene in Canadian chemical warfare laboratories in 19546, 7 it is remarkable how they and Pattle came to the same conclusions independently and within a few months of each other. Clearly some good came from studies in three countries of the adverse effects of nerve gases on the lungs.
Figure 1.

Richard Pattle and Mary Ellen Avery CIBA Foundation meeting, 1964.
Figure 2.

John Clements.
Avery and Mead
The next step was to show that hyaline membrane disease (HMD) of the newborn was caused by abnormal surface tension in the lungs. In the late 1950s Mary Ellen Avery (Figure 1) was a research fellow in Jere Mead's laboratory in Boston with Clement Smith as her clinical supervisor. Mel Avery was so impressed with Clements’ 1957 article that she visited him in Edgewood to learn more about the surface film balance and adapt it to study extracts from the lungs of infants who had died soon after birth. In 1959, along with Jere Mead, she published a seminal article demonstrating that HMD, later known as RDS, was due to lack of surfactant.8 The lungs of babies dying of HMD had a mean surface tension of about 30 dynes cm−1 compared to about 8 dynes cm−1 for those who died of other causes. Avery and Mead concluded ‘hyaline membrane disease is associated with the absence or the late appearance of some substances which in the normal subject renders the internal surface capable of attaining a low surface tension when the lung volume is decreased.’ Despite knowledge of the cause of RDS as early as 1959, progress in developing a cure was proceeding slowly until a significant event took place.
Patrick Bouvier Kennedy, the son of President John F Kennedy and Jacqueline Bouvier Kennedy was born at 34 to 35 weeks’ gestation on 7 August, 1963. His birth took place in Otis Air Force Base Hospital following an emergency Cesarean section and he weighed about 1860 g. Soon after birth he was transferred to Boston Children's Hospital where he died 2 days later of HMD (Figure 3). An obituary in the New York Times noted that at that time all that could be done ‘for a victim of hyaline membrane disease is to monitor the infant's blood chemistry and to try to keep it near normal levels. Thus, the battle for the Kennedy baby was lost only because medical science has not yet advanced far enough to accomplish as quickly as necessary what the body could do by itself in its own time.’ Patrick Kennedy's death from HMD increased public awareness of the disease and stimulated further research into its treatment. Within a few years two trials reporting the use of synthetic surfactants to treat RDS had been published.9, 10
Figure 3.

Patrick Kennedy's death in 1963 placed the spotlight on respiratory distress syndrome (RDS).
First synthetic surfactant trials
Unfortunately, the results of these studies were largely negative; both had used nebulized dipalmitoylphosphatidylcholine (DPPC), and there were no discernible beneficial clinical effects.9, 10 The results of the study by Jacqueline Chu et al.,10 conducted in Singapore, left the authors so disillusioned that they entitled their article ‘Neonatal Pulmonary Ischemia’ implying that the underlying cause of RDS was low pulmonary blood flow rather than a primary surfactant deficiency. We know now that phospholipids lower surface tension on their own in vitro, but they need proteins to allow the rapid spreading and adsorption that are necessary for efficacy in vivo. Furthermore, nebulization is not an effective method of delivering surfactant to the airways.
Animal studies with natural surfactants
A few years later in Stockholm, Goran Enhorning, an obstetrician, and Bengt Robertson, a pediatric pathologist (Figure 4), showed that preterm rabbits treated with natural surfactant did not die as expected soon after birth.11 After one year later, in 1973, they showed that pharyngeal deposition rather than tracheal instillation of natural surfactant was also effective12 and more than 30 years later, this is still not an established method of surfactant administration in the newborn. After 5 years Forrest Adams and his colleagues in California demonstrated the beneficial effects of a natural bovine surfactant on the lungs of preterm lambs.13 One of his co-authors was Tetsuro Fujiwara from Japan who was then working in Adams’ laboratory in California.
Figure 4.
(a) Goran Enhorning (obstetrician). (b) Bengt Robertson (perinatal pathologist).
First clinical trials with a natural surfactant
Before Adams could begin studies with human babies, Fujiwara had returned to Japan and in 1980 published a seminal article in the Lancet giving the results of administration of a modified bovine surfactant (Surfactant-TA) to 10 preterm infants.14
Fujiwara's infants were relatively mature with a mean gestation of about 30 weeks and a mean birth weight of over 1500 g. Within a short time the mean arterial oxygen tension had increased from about 45 torr to 210 torr (Figure 5) and chest radiographs also improved.14 Nine of the 10 infants developed patent ductus arteriosus, and two died, but the authors clearly demonstrated the acute beneficial effects of natural surfactant in the treatment of RDS despite the absence of untreated controls.
Figure 5.
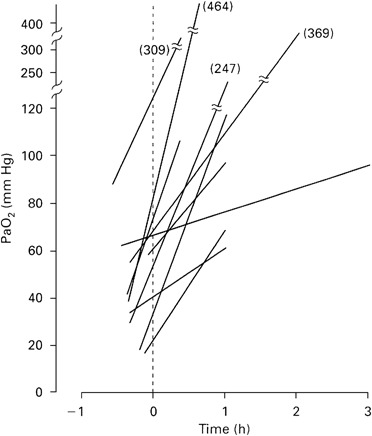
Changes in arterial oxygen tension after surfactant instillation. Fujiwara. Lancet, 1980; i: 55–59.
Meanwhile back in Stockholm, Bengt Robertson teamed up with Tore Curstedt (Figure 6), a clinical chemist with an interest in phospholipids and proteins. Together they produced a porcine surfactant that they named after themselves—the Curstedt–Robertson surfactant or Curosurf for short. This surfactant was unique in that, apart from being produced from pig lungs rather than cow lungs, it went through an additional preparation step of liquid gel chromatography, leaving only polar lipids and SP-B and SP-C with a phospholipid concentration of 80 mg ml−1.15
Figure 6.

Bengt Robertson and Tore Curstedt. Curstedt–Robertson surfactant,Curosurf.
Randomized trials with many surfactants
The 1980s were the era of the randomized controlled trials (RCTs) during which many different synthetic and natural surfactant preparations (Figure 7) were assessed in treatment or prevention of RDS in preterm infants.16 These surfactant preparations can be classified into one of five groups as follows: (1) old synthetic or protein free, (2) natural minced lung extracts, (3) natural lung lavage extracts, (4) natural amniotic fluid extract and (5) new synthetic protein analogues (Figure 7). Human amniotic fluid-derived natural surfactant initially showed promising results17 but had to be withdrawn from clinical use, without ever being licensed, when the risk of human immunodeficiency virus contamination became apparent. The old synthetic and the other natural surfactants were studied in many RCTs and both groups have been shown to reduce pulmonary air leaks and mortality.18 The results have been summarized in three systematic reviews published in the Cochrane Library by Roger Soll and his colleagues.19, 20, 21
Figure 7.
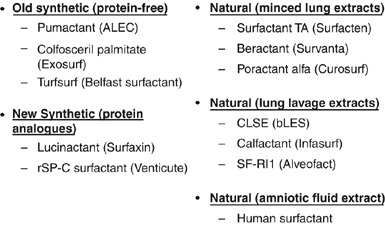
Surfactants used in clinical trials.
The Cochrane Library also contains systematic reviews demonstrating the benefits of multiple doses over a single dose,22 early versus delayed selective treatment,23 prophylaxis versus selective use24 and natural versus old synthetic surfactant.25 All of these interventions have survival benefits (Table 1) and help us to develop guidelines for surfactant treatment in preterm infants. The numbers needed to treat (NNT) to obtain one less death vary from 14 for multiple doses versus a single dose to 50 for natural surfactants versus synthetic surfactants (Table 1). The technique of intubation to administer surfactant before extubation to CPAP known as INSURE also appears to have benefits over surfactant administration followed by continued mechanical ventilation although the reduction in mortality is not statistically significant.26
Table 1.
Results from systematic reviews
| Mortality | RR | 95% CI | NNT | 95% CI |
|---|---|---|---|---|
| Multiple doses | 0.63 | 0.39–1.02 | 14 | 7–1000 |
| Natural surfactant | 0.86 | 0.76–0.98 | 50 | 20–1000 |
| Prophylaxis | 0.61 | 0.48–0.77 | 20 | 14–50 |
| Early | 0.87 | 0.77–0.99 | 33 | 17–1000 |
| Early INSURE | 0.38 | 0.08–1.81 | — | — |
Comparison of natural surfactants
Natural surfactants differ from one another; all but one is bovine and some are derived from minced lung extracts and some from lung lavage extracts (Figure 7). Beractant has added DPPC, tripalmitin and palmitic acid, whereas poractant alfa contains only polar lipids and is more concentrated than the other surfactants. There have been a number of comparative natural surfactant trials (Table 2). Five trials compared poractant alfa and beractant with Ramanathan et al.27 reporting results of two different doses of poractant alfa (200 mg per kg and 100 mg per kg), Bloom et al.28, 29 reported three studies comparing calfactant and beractant, and in Europe there have been at least two studies comparing bovactant and beractant.30, 31 The studies comparing poractant alfa and beractant were all of rescue treatment and used initial doses of either 200 mg per kg or 100 mg per kg in the poractant alfa arm compared to 100 mg per kg in the beractant arms. (Table 3) In the study by Ramanathan et al.27 both doses of poractant alfa (100 mg per kg and 200 mg per kg) were compared with one dose of beractant (100 mg per kg) and neonatal mortality was lowest in the higher dose poractant alfa group (3%) versus lower dose poractant alfa (6%) and beractant (8%). Similar reductions in mortality have been found in other trials that used a 200 mg per kg initial dose.32, 33 (Table 3) If the Ramanathan et al. study is taken to report two separate trials, beractant versus two groups of poractant alfa-treated infants, then six comparative trials of these two natural surfactants can be considered with a total of 20 deaths in the poractant alfa group and 33 in the beractant group. (Table 3) A meta-analysis of these six comparisons using neonatal mortality as an outcome shows a reduction favoring poractant alfa (RR 0.57; 0.34 to 0.96) with NNT 20 (11 to 1000).18 (Table 4) When the three comparisons using an initial dose of poractant alfa of 200 mg per kg are examined the RR is reduced to 0.29 (0.10 to 0.79) with NNT of 14 (8 to 50). However, when the 100 mg per kg doses of each surfactant are compared the reduction in neonatal mortality is no longer significant. (Table 4).
Table 2.
Comparative trials of natural surfactants
| Reference | Surfactants | Number |
|---|---|---|
| Speer et al.32 | Curosurf versus Survanta | 73 |
| Halahakoon37 | Curosurf versus Survanta | 27 |
| Baroutis et al.38 | Curosurf versus Survanta versus Alveofact | 80 |
| Ramanathan et al.27 | Curosurf (2) versus Survanta | 293 |
| Malloy et al.33 | Curosurf versus Survanta | 58 |
| Total | 531 | |
| Bloom et al.28 | Infasurf versus Survanta | 608 |
| Infasurf versus Survanta (2) | 2100 | |
| Van overmeire et al.30 | Alveofact versus Survanta | 131 |
| Griese et al.31 | Alveofact versus Survanta | 14 |
Table 3.
Poractant versus beractant: neonatal mortality
| References | Dose of poractant (mg per kg) | Poractant (n=301) | Beractant (n=301) |
|---|---|---|---|
| Speer et al32 | 200 | 1/33 | 5/40 |
| Halahakoon37 | 100 | 5/17 | 3/10 |
| Baroutis et al.38 | 100 | 5/27 | 6/26 |
| Ramanathan et al.27 | 200 | 3/99 | 8/98 |
| Ramanathan et al.27 | 100 | 6/96 | 8/98 |
| Malloy et al.33 | 200 | 0/29 | 3/29 |
Halliday.18
Table 4.
Poractant versus beractant: relative risks and numbers needed to treat for neonatal mortality
| N | RR | 95% CI | NNT | 95% CI | |
|---|---|---|---|---|---|
| All studies | 602 | 0.57 | 0.34–0.96 | 20 | 11–1000 |
| 100 mg per kg | 274 | 0.82 | 0.44–1.55 | — | — |
| 200 mg per kg | 328 | 0.29 | 0.10–0.79 | 14 | 8–50 |
Abbreviation: NNT, numbers needed to treat.
Halliday.18
Babies treated with poractant alfa are more likely to need only one dose of surfactant, especially when the initial dose is 200 mg per kg, which may have economic advantages. A recent study by Bhatia and colleagues assessed outcomes of surfactant-treated babies in the United States using the Premier's Perspective Clinical Database.34 The three surfactants compared were poractant alfa, beractant and calfactant and the database of RDS-treated infants totalled just under 25 000 (Table 5). For this cohort, the unadjusted mortality rates were 6.25, 8.15 and 8.31%, respectively. After adjustment for birth weight, gestational age, race, gender and transfer status odds ratios (ORs) for mortality of 1.28 for beractant and 1.47 for calfactant were obtained compared to poractant alfa. When a subset of over 10 000 infants with no missing data was evaluated the adjusted ORs for mortality increased to 1.52 for beractant and 1.60 for calfactant compared to poractant alfa. (Table 5) Also, these authors showed using the same database that lengths of hospital stay were reduced with poractant alfa compared to beractant and calfactant consistent with significant cost savings when the porcine surfactant is used to treat RDS.34
Table 5.
Premier's perspective clinical database—mortality
| Outcome | Poractant | Beractant | Calfactant |
|---|---|---|---|
| RDS-treated (n) | 4956 | 12 674 | 7277 |
| Mortalitya (%) | 6.25 | 8.15 | 8.31 |
| Adjusted OR (95% CI) | 1.00 | 1.28 (1.20–1.36) | 1.47 (1.37–1.58) |
| RDS data (n) | 2191 | 5248 | 2798 |
| Adjusted OR (95% CI) | 1.00 | 1.52 (1.32–1.70) | 1.60 (1.37–1.58) |
In 2005, Bloom et al.29 reported two prospective randomized clinical trials comparing calfactant and beractant. The prophylaxis trial enrolled 749 infants of 23 to 29 weeks’ gestation, whereas the treatment trial enrolled 1361 infants weighing between 401 and 2000 g who needed mechanical ventilation with more than 40% oxygen for RDS within 36 h of birth. The primary outcome for both studies was survival to 36 weeks’ corrected age without need for supplemental oxygen. The sample size estimates for both studies were about 2000 and an interim analysis was planned after 1000 babies had been enrolled in the treatment trial.29 The reduction in inspired oxygen concentration was significantly faster for the calfactant-treated group compared to beractant during the first 24 h after treatment. Unfortunately, both trials were halted after 32 months because of poor enrollment. The primary outcome data were almost identical for both groups in both studies: 52% in both groups for prophylaxis and 57 and 59%, respectively, for calfactant and beractant in the treatment trial. The conclusions of the authors were clear and unambiguous: ‘because of inadequate sample sizes, early trial closures prevent us from either accepting or rejecting our null hypotheses because of substantial risks of type-2 errors. Questions about relative safety and efficacy for surfactant preparations will remain unanswered until clinical investigators are willing to complete the difficult tasks involved in the participation of a large-scale, randomized, clinical trial’.29
Comparison trials with a new synthetic surfactant
Also in 2005, two trials comparing the new synthetic surfactant, lucinactant with other synthetic35 and natural36 surfactants were reported. Lucinactant was previously called KL4 surfactant and is a gel at both room and body temperature. In a prophylaxis trial, defined as treatment in the delivery suite within 30 min of birth, lucinactant was compared with colfosceril palmitate in a randomized trial conducted mainly in South America.35 Over 1000 infants were enrolled in this comparison, which included a reference arm containing half the number of beractant-treated infants. The inclusion criteria were birth weight between 600 and 1250 g, successful intubation soon after birth and informed parental consent. The major primary outcome was RDS-related mortality through 14 days, which is an unusual endpoint for neonatal trials. The results for beractant and colfosceril palmitate were similar and significantly higher than for lucinactant (nearly 10% versus about 5%).35 However, there were no significant differences among the three groups for mortality at 28 days, corrected age 36 weeks or survival to discharge; all more established endpoints for neonatal clinical trials.
The second study compared lucinactant with poractant alfa and it was conducted largely in Europe with many babies recruited in Poland.36 The entry criteria were as follows: estimated gestational age 24 to 28 weeks, rupture of the membranes for less than 2 weeks, birth weight between 600 and 1250 g, successful intubation at birth and signed informed parental consent. Randomization was after birth to either lucinactant or poractant alfa treatment and babies were stratified by both birth weight (600 to 1000 and 1001 to 1250 g) and center. The surfactants were given within 30 min of birth so it was not conventional prophylaxis (within 10 to 15 min of birth) but it was in the delivery suite. The initial dose of each surfactant was 175 mg per kg, and this is also unusual, as poractant alfa has only been administered in doses of either 100 or 200 mg per kg in previous studies.27, 32, 33, 37, 38 The volumes of surfactant needed to deliver the doses of 175 mg per kg were 5.8 and 2.2 ml kg−1, respectively, for lucinactant and poractant alfa.36 In addition, lucinactant is a gel that needs to be warmed to 44 °C for 15 min in a water bath and later shaken to form a liquid before it can be instilled into the lungs. This would explain why it was not possible to administer this surfactant within 10 to 15 min of birth in these studies.35, 36
The study design was also unusual in that it was a non-inferiority trial,36 perhaps the first surfactant study to use this design. The primary outcome was survival without bronchopulmonary dysplasia (BPD) at 28 days and to estimate a sample size a 20-year-old trial of treatment of severe RDS in babies using poractant alfa39 was used. This older trial had a 50% mortality rate in the control arm and was probably an inappropriate comparator for this so-called prophylaxis study.36 As a result the estimated sample size was only 496, much less than the 2000 estimated for the beractant versus calfactant trials.29 The trial was designed to run for 12 months but had to be extended to 24 months because of slow recruitment. It was finally stopped early when less than half the estimated sample size had been attained; the results from 243 babies were presented in the article.36 The reasons given for terminating the study were slow recruitment and lack of financial support in that resources were to be diverted to the South American trial.35 For the primary outcome of survival without BPD at 28 days the figures were 37.8% in the lucinactant group and 33.1% in the poractant alfa group but at 36 weeks’ corrected age the results were in the opposite direction with figures of 64.7% for lucinactant and 66.9% for poractant alfa. Nevertheless none of these differences was statistically significant.36 The readers must decide if this study truly demonstrated equivalence or non-superiority of these two surfactant preparations as stated by the authors36 or represented a substantial risk of a type-2 error as stated in the comparative trials of beractant and calfactant.29
Basic science of the new synthetic surfactants
Lucinactant is only one of many new synthetic surfactant preparations in various stages of development.40 Lucinactant contains 2.7% KL4, DPPC/POPG in a ratio of 3:1 and 13.5% palmitic acid. It contains 30 mg phospholipids per ml and is suspended in buffer at pH 7.6 forming a gel until warmed to 44 °C.35, 36 Another synthetic surfactant with SP-B fragments such as Mini-B has been tested in animal models but not yet studied in humans.41 At least four different peptides have been shown to have clear effects both in vivo and in vitro but it is believed that a commercially produced surfactant is some time away.42 To date it has not been possible to synthesize SP-B as it has a complex structure and although KL4 has been described as an SP-B mimic it seems to form a transmembrane α-helix making it more likely to function as an SP-C mimic.40 SP-C itself is very hydrophobic and especially in pure form is structurally very unstable.40 Recombinant SP-C has been used in a commercially produced surfactant called Venticute.43 This has 2% rSP-C, DPPC/POPG in a ratio of 7:3 and 5% palmitic acid. It contains 50 mg phospholipids per ml and is a liquid suspension.40 rSP-C surfactant has been studied in adults with ARDS43 but not in neonatal RDS. Another surfactant containing SP-C33 is currently being studied in Stockholm but no clinical trials have yet been reported.40 This synthetic surfactant contains 2% SP-C33, DPPC/POPG in a 7:3 ratio but no palmitic acid. It is a liquid suspension and can be concentrated to 80 mg phospholipids per ml.40 In summary, it is possible to prepare physiologically active surfactants from mixtures of phospholipids and peptides but PEEP seems necessary for adequate alveolar stability in animal models of RDS and finally synthetic surfactant is probably not yet ready for the market.40, 44
Methods of administration of surfactant
Surfactants generally have been administered by intratracheal instillation. Colfosceril palmitate, one of the first synthetic surfactants approved for use, has been administered by both rapid instillation45 and slow instillation through a side port adapter attached to the endotracheal tube.46 However, clinical experience and the results of animal studies show that rapid instillation is more effective than slow instillation, at least for natural surfactants. Valls-i-Soler and colleagues showed that use of a dual lumen tube rather than disconnection from the ventilator led to fewer dosing problems.47 To date, it has not been possible to demonstrate that surfactant can be successfully administered by nebulization,48 although this remains an attractive route as it avoids intubation of the trachea. Four methods of administration that aim to reduce the duration of endotracheal intubation or avoid it altogether have been described.49, 50, 51, 52 The INSURE method involves a short intubation to administer surfactant followed by extubation to continuous positive airway pressure (CPAP).49 Pharyngeal deposition of surfactant soon after birth has been reported by Kattwinkel and colleagues,50 and in Italy Trevisanuto et al.51 used a laryngeal mask to deliver surfactant, both with some success although the results of randomized trials are awaited. At a recent meeting in Ancona, Herting et al. presented a poster on surfactant administration in spontaneously breathing infants using a fine gastric tube. However, intubation for rapid instillation is still the method of choice for administration of surfactant. Early extubation to CPAP seems to be a plausible ideal but has not been adequately tested in clinical trials to date.
Other potential indications for surfactant therapy
Apart from RDS there are a number of other potential indications for surfactant therapy. Infants with meconium aspiration syndrome show some response to surfactant treatment but larger and more frequent doses are needed for these effects52 and thus far no studies show improved survival. Similarly, infants with congenital pneumonia, especially those due to group B streptococcal infection, improve with surfactant treatment; however, the response is less marked than in infants with RDS.53 Whether surfactant really alters the course of infants with congenital diaphragmatic hernia or not is still a question,54 but infants with respiratory failure on extracorporeal membrane oxygenation (ECMO) can be decannulated earlier if treated with a natural surfactant.55 To date, there have only been reports of relatively small case series of infants with acute respiratory distress syndrome RDS (ARDS),56 early chronic lung disease (CLD)57 and pulmonary hemorrhage58 treated with surfactant. Some acute improvement in oxygenation usually occurs, but long-term benefits have not been reported to date. Recently Yeh et al.59 from Taiwan reported, in a RCT of infants with RDS, delivery of budesonide to the lung by instilling it with beractant. They were able to demonstrate some acute responses and CLD was reduced in the steroid-treated group but further larger studies are needed before this becomes an accepted indication for surfactant treatment.
Developing a protocol for surfactant treatment
The timing of surfactant treatment for babies of <31 weeks’ gestation at risk of developing RDS was assessed during the early 1990s in three European randomized clinical trials using poractant alfa. A meta-analysis of these prophylaxis versus rescue treatment trials was published by Egberts et al.60 in 1997. Prophylaxis was defined as administration of surfactant in the delivery suite within 10 to 15 min of birth. Rescue treatment was generally undertaken when intermittent positive pressure ventilation (IPPV) was needed with more than 40 to 50% oxygen. Prenatal steroid use was only about 20% and there were significant advantages for prophylaxis (Table 6). The adjusted ORs demonstrate an approximate halving the odds of developing severe RDS, neonatal mortality and BPD in 28-day survivors favoring prophylaxis. In 2002 Walti and colleagues using the same database of three trials demonstrated significant reductions in intraventricular hemorrhage (IVH) in the prophylaxis group.61 (Table 6) The OR for all grades of IVH was 0.65 (95% CI 0.47 to 0.90) and for severe IVH this was 0.56 (0.35 to 0.89). For the subgroup of outborn infants the reduction in severe IVH was even more impressive (OR 0.11; 0.02 to 0.49).
Table 6.
Timing of surfactant: meta-analysis of three trials of poractant alfa (Curosurf)
| Outcome | OR | 95% CI | Adjusted OR a | 95% CI |
|---|---|---|---|---|
| Severe RDS | 0.55 | 0.38–0.79 | 0.50 | 0.33–0.74 |
| Air leaks | 0.54 | 0.35–0.82 | — | — |
| Neonatal mortality | 0.52 | 0.35–0.76 | 0.47 | 0.30–0.73 |
| BPD in 28-day survivors | 0.67 | 0.45–1.00 | 0.54 | 0.34–0.86 |
| Meta-analysis of prophylaxis versus rescue (n=671) | ||||
| Overall IVH | 0.65 | 0.47–0.90 | ||
| Severe IVH | 0.56 | 0.35–0.89 | ||
| Severe IVH (outborn) | 0.11 | 0.02–0.49 | ||
Three criteria can be used to help develop a protocol for surfactant treatment: type of surfactant, timing of treatment and the dose of phospholipids required. Current evidence favors the use of natural surfactants rather than the old protein-free synthetic surfactants, and it is too soon to say whether the new synthetic surfactants have a role to play and none is currently approved for treatment of the newborn.40, 62 In regard to timing, the evidence favors prophylaxis for infants of less than 31 weeks’ gestation and early treatment for others who develop signs of RDS. The dose of surfactant needed may depend upon timing, severity of illness and whether or not prenatal steroids were given. A recent study from the Vermont-Oxford Neonatal Network highlighted the advantages of early or prophylactic surfactant treatment for infants of 23 to 29 weeks’ gestation.63 This was a cluster randomized trial involving 114 neonatal intensive care units assessing the utility of a multifaceted intervention in promoting good practice as regards surfactant treatment. Neonatal units in the intervention group gave surfactant at a mean of 21 min after birth compared to 78 min in the control hospitals. Prophylactic surfactant use in the delivery suite increased from 18 to 55%. These researchers also found reductions in rates of IVH—all grades 33 to 28% and severe IVH 14 to 10%—in the hospital group, giving surfactant earlier,63 results in keeping with the meta-analysis of poractant alfa trials reported by Walti et al.61
The suggested protocol for surfactant treatment is based upon early treatment and CPAP (Figure 8). For gestational ages less than 27 or 28 weeks prophylaxis in the delivery suite would seem to be indicated based upon the currently available evidence. A dose of 100 mg per kg is recommended (although this is not approved in the United States) as surfactant inactivation is unlikely at this time. For these immature infants an attempt to extubate to CPAP should be made soon after transfer to the neonatal unit and this is usually possible in infants with gestational ages over 24 weeks. For infants of 28 to 31 weeks’ gestation early CPAP and surfactant treatment—if intubation is needed for resuscitation—seem sensible recommendations. Early rescue treatment should be used with either 100 or 200 mg per kg (only 200 mg per kg is approved in the United States) when inspired oxygen concentrations are above 30%. The presence of chest X-ray appearances of RDS help to determine the need for early treatment. For infants of greater than 31 weeks’ gestation initial observation and early CPAP—if respiratory distress appears—seem sensible. These infants can be given rescue surfactant treatment with 200 mg per kg when their inspired oxygen concentrations are over 40% (Figure 8).
Figure 8.
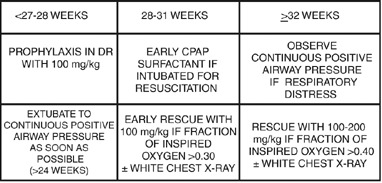
Protocol for surfactant treatment of respiratory distress syndrome (RDS) and early CPAP.
Effects of introducing a protocol for earlier surfactant treatment
Are there benefits of introducing a policy of early or prophylactic surfactant treatment into a neonatal unit? An observational study from Belfast addressed this question.64 (Table 7). This study compared outcomes of extremely low gestational age (23 weeks to 27 weeks) neonates between 1994 and 2004 at the Regional Neonatal Unit, Royal Maternity Hospital, Belfast. Surfactant use increased from 44 to 90% and the first dose was given much earlier (median 9 versus 180 min) in the second era. This was associated with an improved survival rate from 67 to 80% but indicators of CLD were also increased—oxygen at 28 days (49 to 66%) and oxygen at corrected age 36 weeks (14 to 31%). At the same time dexamethasone use decreased from 21 to 1%. This may have accounted for some of the need for increased oxygen supplementation. Any true increase in CLD is likely to have been of the milder variety as length of stay in hospital remained similar in the two eras (median 47 versus 44 days) (Table 7). However, with improved early neonatal care and increased survival neonatologists have to care for more babies with CLD and, therefore, this remains one of the biggest problems to be solved in neonatal practice.
Table 7.
Management of ELGANS in Belfast
| 1994 (n=87) | 2004 (n=156) | P | |
|---|---|---|---|
| Surfactant given | 38 (44%) | 141 (90%) | 0.0001 |
| First surfactant (min) | 180 (122–275) | 9 (5–15) | 0.0001 |
| Survival | 58 (67%) | 123 (80%) | 0.04 |
| Oxygen at 28 days | 28/57 (49%) | 75/113 (66%) | 0.05 |
| Oxygen at 36 weeks | 8/57 (14%) | 31/100 (31%) | 0.05 |
| Dexamethasone | 18 (21%) | 2 (1%) | 0.0001 |
| Length of stay (days) | 47 (10–73) | 44 (27–70) | NS |
Data presented at 2006 Pediatric Academic Societies’ Annual Meeting (O’Neill et al.64).
Conclusions
Surfactant was the first drug developed solely for treatment of neonates. Its use has been a major advance in neonatology during the past 25 years. Surfactant therapy reduces both neonatal mortality and pulmonary air leaks by about 50%. The introduction of surfactant therapy was associated with an overall reduction of about 6% in infant mortality in the United States. Prophylactic or very early treatment with a natural surfactant seems to give the best results for very preterm infants at risk of developing RDS. Long-term follow-up studies have not identified any increases in major neurodevelopmental or pulmonary sequelae in surviving infants.65
Footnotes
Disclosure
This paper was supported from a grant from Dey, L.P. Dr. Halliday has received consultancy fees from Chiesi Farmaceutici for giving advice and has received honoraria for lecturing from Abbott, Burroughs-Wellcome, Dey and Chiesi. He also holds equity.
References
- 1.Von Neergaard K. Neue auffassungen uber einen grundbegriff der atemmechanik. Die retraktionskraft der lunge, abhangig von der oberflachenspannung in den alveolen. Z Gesamt Exp Med. 1929;66:373–394. doi: 10.1007/BF02621963. [DOI] [Google Scholar]
- 2.Gruenwald P. Surface tension as a factor in the resistance of neonatal lungs to aeration. Am J Obstet Gynecol. 1947;53:996–1007. doi: 10.1016/S0002-9378(16)39775-7. [DOI] [PubMed] [Google Scholar]
- 3.Pattle RE. Properties, function and origin of the alveolar lining layer. Nature. 1955;175:1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- 4.Clements JA. Dependence of pressure-volume characteristics of lungs on intrinsic surface active material. Am J Physiol. 1956;187:592. [Google Scholar]
- 5.Clements JA. Surface tension of lung extracts. Proc Soc Exp Biol Med. 1957;95:170–172. doi: 10.3181/00379727-95-23156. [DOI] [PubMed] [Google Scholar]
- 6.Macklin CC. The pulmonary alveolar mucoid film and the pneumonocytes. Lancet. 1954;i:1099–1104. doi: 10.1016/S0140-6736(54)92154-6. [DOI] [PubMed] [Google Scholar]
- 7.Obladen M. History of surfactant up to 1980. Biol Neonate. 2005;87:308–316. doi: 10.1159/000084878. [DOI] [PubMed] [Google Scholar]
- 8.Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. Am J Dis Child. 1959;97:517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 9.Robillard E, Alarie Y, Dagenais-Perusse P, Baril E, Guilbeault A. Microaerosol administration of synthetic beta-gamma-dipalmitoyl-L-alpha-lecithin in the respiratory distress syndrome. A preliminary report. Can Med Assoc J. 1964;90:55–57. [PMC free article] [PubMed] [Google Scholar]
- 10.Chu J, Clements JA, Cotton EK, Klaus MH, Sweet AY, Tooley WH. Neonatal pulmonary ischemia: clinical and physiologic studies. Pediatrics. 1967;40:709–782. [PubMed] [Google Scholar]
- 11.Enhorning G, Robertson B. Lung expansion in the premature rabbit fetus after tracheal deposition of surfactant. Pediatrics. 1972;50:58–66. [PubMed] [Google Scholar]
- 12.Enhorning G, Grossmann G, Robertson B. Pharyngeal deposition of surfactant in the premature rabbit fetus. Biol Neonate. 1973;22:126–132. doi: 10.1159/000240546. [DOI] [PubMed] [Google Scholar]
- 13.Adams FH, Towers B, Osher AB, Ikegami M, Fujiwara T, Nozaki M. Effects of tracheal instillation of natural surfactant in premature lambs. I. Clinical and autopsy findings. Pediatr Res. 1978;12:841–848. doi: 10.1203/00006450-197808000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara T, Chida S, Watabe YJ, Maeta H, Morita T, Abe T. Artificial surfactant therapy in hyaline membrane disease. Lancet. 1980;i:55–59. doi: 10.1016/S0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 15.Robertson B, Curstedt T, Johansson J, Jornvall H, Kobayashi T. Structural and functional characterization of porcine surfactant isolated by liquid-gel chromatography. Prog Resp Res. 1990;25:237–246. doi: 10.1159/000417829. [DOI] [Google Scholar]
- 16.Halliday HL. Overview of clinical trials comparing natural and synthetic surfactants. Biol Neonate. 1995;67(suppl 1):32–47. doi: 10.1159/000244205. [DOI] [PubMed] [Google Scholar]
- 17.Merritt TA, Hallman M, Bloom BT, Berry C, Benirschke K, Sahn D. Prophylactic treatment of very premature infants with human surfactant. N Engl J Med. 1986;315:785–790. doi: 10.1056/NEJM198609253151301. [DOI] [PubMed] [Google Scholar]
- 18.Halliday HL. History of surfactant from 1980. Biol Neonate. 2005;87:317–322. doi: 10.1159/000084879. [DOI] [PubMed] [Google Scholar]
- 19.Soll RF. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 1997;4:CD000511. doi: 10.1002/14651858.CD000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soll RF. Prophylactic synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 1998;2:CD001079. doi: 10.1002/14651858.CD001079. [DOI] [PubMed] [Google Scholar]
- 21.Soll RF. Synthetic surfactant for respiratory distress syndrome in preterm infants. Cochrane database. Syst Rev. 1998;3:CD001149. doi: 10.1002/14651858.CD001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soll RF. Multiple versus single dose natural surfactant extract for severe neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 1999;2:CD000141. doi: 10.1002/14651858.CD000141. [DOI] [PubMed] [Google Scholar]
- 23.Yost CC, Soll RF. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 1999;4:CD001456. doi: 10.1002/14651858.CD001456. [DOI] [PubMed] [Google Scholar]
- 24.Soll RF, Morley CJ. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2001;2:CD000510. doi: 10.1002/14651858.CD000510. [DOI] [PubMed] [Google Scholar]
- 25.Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2001;2:CD0014444. doi: 10.1002/14651858.CD000144. [DOI] [PubMed] [Google Scholar]
- 26.Stevens TP, Blennow M, Soll RF. Early surfactant treatment with brief ventilation versus selective surfactant and continued mechanical ventilation for preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. 2004;3:CD003063. doi: 10.1002/14651858.CD003063.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Ramanathan R, Rasmussen MR, Gerstman DR, Finer N, Sekar K, North American Study Group A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol. 2004;21:109–119. doi: 10.1055/s-2004-823779. [DOI] [PubMed] [Google Scholar]
- 28.Bloom BT, Kattwinkel J, Hall RT, Delmore PM, Egan EA, Trout JR. Comparison of Infasurf (calf lung surfactant extract) to Survanta (beractant) in the treatment and prevention of respiratory distress syndrome. Pediatrics. 1997;100:31–38. doi: 10.1542/peds.100.1.31. [DOI] [PubMed] [Google Scholar]
- 29.Bloom BT, Clark RH, Infasurf Survanta Clinical Trial Group Comparison of Infasurf (calfactant) and Survanta (beractant) in the prevention and treatment of respiratory distress syndrome. Pediatrics. 2005;116:392–399. doi: 10.1542/peds.2004-2783. [DOI] [PubMed] [Google Scholar]
- 30.Van Overmeire B, Jansens J, van Reempts PJ. Comparative evaluation of the respiratory and circulatory responses after instillation of two bovine surfactant preparations. Pediatr Res. 1999;45:324A. doi: 10.1203/00006450-199904020-01928. [DOI] [Google Scholar]
- 31.Griese M, Dietrich P, Reinhardt D. Pharmacokinetics of bovine surfactant in neonatal respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152:1050–1054. doi: 10.1164/ajrccm.152.3.7663782. [DOI] [PubMed] [Google Scholar]
- 32.Speer CP, Gefeller O, Groneck P, Laufkötter E, Roll C, Hanssler L. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 1995;72:F8–F13. doi: 10.1136/fn.72.1.F8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malloy CA, Nicoski P, Muraskas JK. A randomised trial comparing beractant and poractant treatment in neonatal respiratory distress syndrome. Acta Paediatr. 2005;94:779–784. doi: 10.1111/j.1651-2227.2005.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia J, Saunders WB, Friedlich P, Lavin PT, Sekar KC, Ramanathan R. Differences in mortality among infants treated with three different natural surfactants for respiratory distress syndrome. Neonatology. 2007;91:324. [Google Scholar]
- 35.Moya FR, Gadzinowski J, Bancalari E, Salinas V, Kopelman B, Bancalari A. A multicenter, randomised, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics. 2005;115:1018–1029. doi: 10.1542/peds.2004-2183. [DOI] [PubMed] [Google Scholar]
- 36.Sinha SK, Lacaze-Masmonteil T, Valls-I-Soler A, Wiswell TE, Gadzinowski J, Hajdu J, Surfaxin Therapy Against Respiratory Distress Syndrome Collaborative Group A multicenter, randomised, controlled trial of lucinactant versus poractant alfa among very premature infants at high risk for respiratory distress syndrome. Pediatrics. 2005;115:1030–1038. doi: 10.1542/peds.2004-2231. [DOI] [PubMed] [Google Scholar]
- 37.Halahakoon CW . A study of cerebral function following surfactant treatment for respiratory distress syndrome MD thesis, Queen's University, Belfast, 1999.
- 38.Baroutis G, Kaleyias J, Liarou T, Papathoma E, Hatzistmatiou Z, Costalos C. Comparison of three treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Eur J Pediatr. 2003;162:476–480. doi: 10.1007/s00431-002-1144-0. [DOI] [PubMed] [Google Scholar]
- 39.Collaborative European Multicenter Study Group Surfactant replacement therapy for severe neonatal respiratory distress syndrome: an international randomized clinical trial. Pediatrics. 1988;82:683–691. [PubMed] [Google Scholar]
- 40.Curstedt T, Johannson J. New synthetic surfactant—how and when? Biol Neonate. 2006;89:336–339. doi: 10.1159/000092871. [DOI] [PubMed] [Google Scholar]
- 41.Walther FJ, Hernandez-Juviel JM, Gordon LM, Waring AJ, Stenger P, Zasadzinski JA. Comparison of three lipid formulations for synthetic surfactant with a surfactant protein B analog. Exp Lung Res. 2005;31:563–579. doi: 10.1080/019021490951531. [DOI] [PubMed] [Google Scholar]
- 42.Walther FJ, Waring AJ, Sherman MA, Zasadzinski JA, Gordon LM. Hydrophobic surfactant proteins and their analogues. Neonatology. 2007;91:303–310. doi: 10.1159/000101346. [DOI] [PubMed] [Google Scholar]
- 43.Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, Laterre PF. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351:884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 44.Kattwinkel J. Synthetic surfactants: the search goes on. Pediatrics. 2005;115:1075–1076. doi: 10.1542/peds.2005-0202. [DOI] [PubMed] [Google Scholar]
- 45.Phibbs RH, Ballard RA, Clements JA, Heilbron DC, Phibbs CS, Schlueter MA. Initial clinical trial of Exosurf, protein-free synthetic surfactant, for the prophylaxis and early treatment of hyaline membrane disease. Pediatrics. 1991;88:1–9. [PubMed] [Google Scholar]
- 46.Long W, Thompson T, Sundell H, Schumacher R, Volberg F, Guthrie R. Effects of two rescue doses of a synthetic surfactant on mortality rate and survival without bronchopulmonary dysplasia in 700–1350-gram infants with respiratory distress syndrome. J Pediatr. 1991;118:595–605. doi: 10.1016/S0022-3476(05)83388-8. [DOI] [PubMed] [Google Scholar]
- 47.Valls-i-Soler A, Fernandez-Ruanova B, Lopez-Heredia Y, Goya J, Roman-Etxebarral L, Rodriguez-Soriano J. A randomised comparison of surfactant dosing via a dual-lumen endotracheal tube in respiratory distress syndrome. Pediatrics. 1998;101:E4. doi: 10.1542/peds.101.4.e4. [DOI] [PubMed] [Google Scholar]
- 48.Berggren P, Liljedahl M, Winblath B, Andreasson B, Curstedt T, Robertson B. Pilot study of nebulized surfactant therapy for neonatal respiratory distress syndrome. Acta Paediatr. 2000;89:460–464. doi: 10.1111/j.1651-2227.2000.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 49.Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of <30 weeks’ gestation. Pediatrics. 1999;103:E24. doi: 10.1542/peds.103.2.e24. [DOI] [PubMed] [Google Scholar]
- 50.Kattwinkel J, Robinson M, Bloom BT, Delmore P, Ferguson JE. Technique for intrapartum administration of surfactant without requirement for an endotracheal tube. J Perinatol. 2004;24:360–365. doi: 10.1038/sj.jp.7211103. [DOI] [PubMed] [Google Scholar]
- 51.Trevisanuto D, Grazzina N, Ferrarese P, Micaglio M, Verghese C, Zanardo V. Laryngeal mask airway used as a delivery conduit for the administration of surfactant to preterm infants with respiratory distress syndrome. Biol Neonate. 2005;87:217–220. doi: 10.1159/000083370. [DOI] [PubMed] [Google Scholar]
- 52.Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics. 1996;97:48–52. [PubMed] [Google Scholar]
- 53.Herting E, Gefeller O, Land M, van Sonderen L, Harms K, Robertson B. Surfactant treatment of neonates with respiratory failure and group B streptococcal infection. Pediatrics. 2000;106:957–964. doi: 10.1542/peds.106.5.957. [DOI] [PubMed] [Google Scholar]
- 54.Bos AP, Tibboel D, Hazelrock FW, Malenaar JC, Lachmann B, Gommes D. Surfactant replacement therapy in high-risk congenital diaphragmatic hernia. Lancet. 1991;338:1279. doi: 10.1016/0140-6736(91)92151-Q. [DOI] [PubMed] [Google Scholar]
- 55.Lotze A, Knight GR, Martin GR, Bulas DI, Hull WM, O’Donnell RM. Improved pulmonary outcome after exogenous surfactant therapy for respiratory failure in term infants requiring extracorporeal membrane oxygenation. J Pediatr. 1993;122:261–268. doi: 10.1016/S0022-3476(06)80131-9. [DOI] [PubMed] [Google Scholar]
- 56.Khammash H, Perlman M, Wojtulewicz J, Dunn M. Surfactant therapy in full-term neonates with severe respiratory failure. Pediatrics. 1993;92:135–139. [PubMed] [Google Scholar]
- 57.Pandit PB, Dunn MS, Kelly EN, Perlman M. Surfactant replacement in neonates with early chronic lung disease. Pediatrics. 1995;96:851–854. [PubMed] [Google Scholar]
- 58.Pandit PB, O’Brien K, Asztalos E, Colucci E, Dunn MS. Outcome following pulmonary haemorrhage in very low birthweight neonates treated with surfactant. Arch Dis Child Fetal Neonatal Ed. 1999;81:F40–F44. doi: 10.1136/fn.81.1.F40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeh TF, Su BH, Chang CH, Lin HC, Tsai CH, Pyati S et al. Early intratracheal instillation of budesonide by using surfactant (Survanta) as a vehicle to preterm infants at risk for chronic lung disease. PAS Meeting, San Francisco (3724. 1). 2006.
- 60.Egberts J, Brand R, Walti H, Bevilacqua G, Breart G, Gardini F. Mortality, severe respiratory distress syndrome and chronic lung disease of the newborn are reduced more after prophylactic than therapeutic administration of the surfactant Curosurf. Pediatrics. 1997;100:E4. doi: 10.1542/peds.100.1.e4. [DOI] [PubMed] [Google Scholar]
- 61.Walti H, Paris-Llado J, Egberts J, Brand R, Bevilacqua G, Gardini F. Prophylactic administration of porcine-derived lung surfactant is a significant factor in reducing the odds for peri-intraventricular haemorrhage in premature infants. Biol Neonate. 2002;81:182–187. doi: 10.1159/000051532. [DOI] [PubMed] [Google Scholar]
- 62.Sweet D, Bevilacqua G, Carnielli V, Greisen G, Plavka R, Saugstad OD. European consensus guidelines on the management of neonatal respiratory distress syndrome. J Perinat Med. 2007;35:175–186. doi: 10.1515/JPM.2007.048. [DOI] [PubMed] [Google Scholar]
- 63.Horbar JD, Carpenter JH, Buzas J, Soll RF, Suresh G, Bracken MB. Collaborative quality improvement to promote evidence based surfactant for preterm infants: a cluster randomised trial. BMJ. 2004;329:1004–1007. doi: 10.1136/bmj.329.7473.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Neill CP, Sweet DG, Halliday HL. Changing pattern of surfactant use for extremely preterm infants over the past decade in Belfast. Biol Neonate. 2005;87:357. [Google Scholar]
- 65.Sinn JKH, Ward MC, Henderson-Smart DJ. Developmental outcome of preterm infants after surfactant therapy: systematic review of randomised controlled trials. J Paediatr Child Health. 2002;38:597–600. doi: 10.1046/j.1440-1754.2002.00061.x. [DOI] [PubMed] [Google Scholar]


