Abstract
Many different surfactant preparations derived from animal sources, as well as synthetic surfactants, are available for the treatment of preterm infants with respiratory distress syndrome (RDS). Natural, modified surfactants containing surfactant-associated proteins appear to be more effective than non-protein-containing synthetic surfactants. Comparative trials with poractant alfa at a higher initial dose of 200 mg/kg appear to be associated with rapid weaning of FiO2, less need for additional doses, and decreased mortality in infants <32 weeks gestation when compared with beractant. Early rescue (<30 min of age) surfactant therapy is an effective method to minimize over treatment of some preterm infants who may not develop RDS. Surfactant therapy followed by rapid extubation to nasal ventilation appears to be more beneficial than continued mechanical ventilation. In near-term or term newborns with acute RDS, surfactant therapy has been shown to be 70% effective in improving respiratory failure.
Keywords: poractant, beractant, preterm infants, nasal continuous positive airway pressure
Introduction
Surfactant therapy has become the standard of care in preterm infants with respiratory distress syndrome (RDS), and is used increasingly in near-term and term newborns with acute respiratory distress syndrome (ARDS). Incidence of prematurity is increasing in the United States.1 Respiratory distress syndrome remains a major cause of morbidity and mortality in preterm infants, especially in the extremely low birth weight infants <1000 g.2 The incidence of RDS is inversely proportional to gestational age. With the increasing use of prenatal steroids, the incidence as well as the severity of RDS has decreased by nearly 50% over the last few years.3, 4 Respiratory distress syndrome occurs in approximately 50% of preterm infants born at <30 weeks gestation, but only in about 25% of those born ⩾30 weeks. Surfactant therapy has been shown to reduce the combined outcomes of death and bronchopulmonary dysplasia (BPD) in preterm infants with RDS.5, 6
Surfactant therapy for respiratory distress syndrome
Natural, modified surfactants derived from animal sources, and synthetic surfactants that are protein free, have been evaluated extensively. Exogenous synthetic surfactants studied include colfosceril palmitate (Exosurf®, (GlaxoSmithKlein, Research Triangle, NC, USA, no longer in market)), pumactant, turfsurf, and lucinactant (Surfaxin®, Discovery Laboratories, Warrington, PA, USA). Of these four synthetic surfactants, the first three are no longer available for clinical use and lucinactant is pending approval from the FDA as of 2005. Several different natural, modified surfactant preparations have been studied. They differ in composition, onset of response, and duration of action, dosing volume, and the need for additional doses. Fifteen trials7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 comparing natural vs synthetic surfactants (Table 1),21 and seven studies comparing different natural surfactants have been published (Table 2).21 Multiple, randomized, controlled trials have consistently shown better clinical outcomes during the acute phase of RDS, and improved survival with natural surfactants than with synthetic surfactants that lack surfactant-associated proteins, especially, surfactant protein-B (SP-B).
Table 1.
Comparison of natural vs synthetic surfactants in the treatment of respiratory distress syndrome
| Trials (15) | Surfactant | N | Prophylaxis (P) or rescue (Tx) | Patients | Results |
|---|---|---|---|---|---|
| Horbar7 | Survanta. vs Exosurf. | 617 | Tx | 500–1500 g | Survanta: lower 0–72 h FiO2 and MAP |
| Sehgal8 | Survanta. vs Exosurf. | 41 | Tx | 600–1750 g | No differences in any variables |
| Vermont-Oxford Network, 1996 | Survanta. vs Exosurf. | 1296 | Tx | 501–1500 g | Survanta: lower FiO2 at 72 h, lower 0–72 h MAP, fewer air leaks |
| Hudak et al.10 | Infasurf. vs Exosurf. | 1126 | Tx | All with RDS | Infasurf: lower 0–72 h FiO2 and MAP, fewer air leaks |
| Hudak et al.11 | Infasurf. vs Exosurf. | 846 | P | <29 weeks | Infasurf: less RDS, lower 0–72 h FiO2 and MAP, fewer air leaks, more cystic PVL |
| Rollins et al.12 | Curosurf. vs Exosurf. | 66 | Tx | All with RDS | Curosurf: lower FiO2 and improved a/A PO2 ratio |
| Alvarado et al.13 | Survanta. vs Exosurf. | 66 | Tx | <1500 g | Survanta: decreased duration of PPV, O2 and LOS |
| Pearlman et al.14 | Survanta. vs Exosurf. | 121 | Tx | All with RDS | No differences in any variables |
| Modanlou et al.15 | Survanta. vs Exosurf. | 122 | Tx | < 1500 g | Survanta: lower FiO2, MAP and oxygenation index |
| da Costa16 | Survanta. vs Exosurf. | 89 | Tx | <37 weeks >1000 g | No difference |
| Kukkonen et al.17 | Curosurf. vs Exosurf. | 228 | Tx | All with RDS | Curosurf: lower FiO2, and MAP |
| Ainsworth et al.18 | Curosurf. vs pumactant. | 212 | Tx | <30 weeks | Curosurf: decreased mortality (trial stopped after interim analysis) |
| Sinha et al.19 | Curosurf. vs Surfaxin. | 252 of 496a | P | 600–1250 g | Primary outcome: Alive and not on O2 at 28 days: Curosurf vs Surfaxin: 33.1 vs 37.8%. Noninferiority was set at −14.5% |
| Moya et al.20 | Surfaxin. vs Exosurf. vs Survanta. | 1294 | P | 600–1250 g | Surfaxin more effective than Exosurf; similar to Survanta |
Abbreviations: RDS, respiratory distress syndrome; MAP, mean airway pressure; LOS, length of stay; PVL, periventricular leukomalacia; PPV, positive pressure ventilation.
aTrial stopped due to slow recruitment.
Copyright 2000 from Lung Surfactants: Basic Science and Clinical Applications by Notter RH. Adapted by permission of Routledge/Taylor & Francis Group, LLC.21
Table 2.
Comparative trials of natural surfactants for respiratory distress syndrome
| Trials (7) | Surfactant | N | Prophylaxis (P) or Rescue (Tx) | Patients | Results |
|---|---|---|---|---|---|
| Bloom et al.22 | Survanta. vs Infasurf. | 374 | P | <1250 g | No difference in any variables; Infasurf: increased mortality in infants <600 g |
| Bloom et al.22 | Survanta. vs Infasurf. | 608 | Tx | <2000 g | Infasurf: lower average 0–72 h FiO2 and MAP |
| Speer et al.23 | Survanta. vs Curosurf. | 73 | Tx | 700–1500 g | Curosurf: lower FiO2, PIP, MAP at 12–24 h |
| Baroutis et al.24 | Alveofact. vs Survanta. vs Curosurf. | 80 | Tx | <2000 g | Curosurf: fewer days on O2 & mechanical ventilation, decreased length of stay |
| Ramanathan et al.25 | Survanta. vs Curosurf. | 293 | Tx | Curosurf: faster weaning, fewer doses, decreased mortality, cost effective | |
| Malloy et al.26 | Survanta. vs Curosurf. | 58 | Tx | Curosurf: lower FiO2 up to 48 h, fewer doses | |
| Bloom and Clark27 | Survanta. vs Infasurf. | 749a | P | <30 weeks | No definite conclusions |
| Bloom and Clark27 | Survanta. vs Infasurf. | 1361a | Tx | 401–2000 g | No definite conclusions |
Abbreviations: MAP, mean airway pressure; PIP, peak inspiratory pressure.
aTrial stopped due to slow enrollment.
Copyright 2000 from Lung Surfactants: Basic Science and Clinical Applications by Notter RH. Adapted by permission of Routledge/Taylor & Francis Group, LLC.21
Bloom et al.22 compared beractant vs calfactant in the prophylactic treatment of RDS in infants <1250 g. They showed no difference between these two surfactants in mortality or BPD. However, mortality in a subgroup of infants <600 g was significantly lower in the beractant treated group compared to calfactant (26 vs 63%, respectively). The authors also compared these two surfactants in the rescue treatment of RDS in 608 preterm infants. They demonstrated lower FiO2 and mean airway pressure (MAP) at 72 h of age in the group treated with calfactant as compared with beractant. However, there were no significant differences in death or BPD between these two surfactants in this rescue trial.
In a pilot trial comparing beractant and poractant alfa, Speer et al.23 showed a significant improvement in oxygenation, and a decrease in peak inspiratory pressure and MAP, which persisted up to 24 h after poractant alfa. They noted no significant differences in mortality or BPD between these two surfactants.
In another study, Baroutis et al.24 compared natural bovine surfactant (Alveofact®) vs beractant vs poractant alfa. They demonstrated that treatment with poractant alfa resulted in significantly less days on mechanical ventilation and supplemental oxygen, and shorter length of hospital stay.
Ramanathan et al.25 compared poractant alfa with beractant in a multicenter, randomized, controlled trial in the United States. Treatment with poractant alfa was associated with faster weaning of oxygen, fewer additional doses, and decreased mortality in preterm infants <32 weeks gestation when compared with beractant. Cumulatively, 36% of infants randomized to poractant alfa received two or more doses vs 68% in the beractant treated group (P<0.05). In a meta-analysis of the two studies23, 25 comparing beractant vs poractant alfa, neonatal mortality was significantly lower with poractant alfa (odds ratio 0.35, 95% CI 0.13, 0.92). In a recent study comparing these two surfactants, Malloy et al.26 extended the observations of Ramanathan et al.25 They showed improvement in oxygenation to persist up to 48 h (Figure 1)26 after treatment with poractant alfa, and a significantly lower number of additional doses with poractant alfa compared to beractant.
Figure 1.
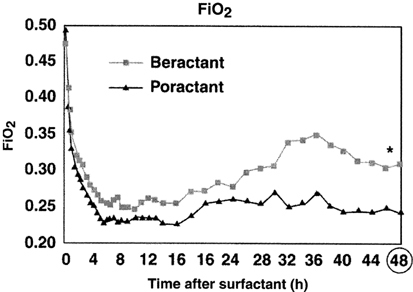
Changes in FiO2 during 48 h after poractant alfa versus beractant treatment. Reprinted with permission, Acta Paediatrica.25
It is also interesting to note that the use of a higher initial dose of poractant alfa at 200 mg/kg vs 100 mg/kg of beractant in three comparison trials have consistently shown a lower mortality favoring poractant alfa (Figure 2).23, 25, 26 This observation may have been due to a larger dose of a more effective surfactant moderating disease severity during the acute phase of RDS in these ill preterm infants, thus resulting in a better survival. However, none of these trials was powered to evaluate mortality as a primary outcome.
Figure 2.
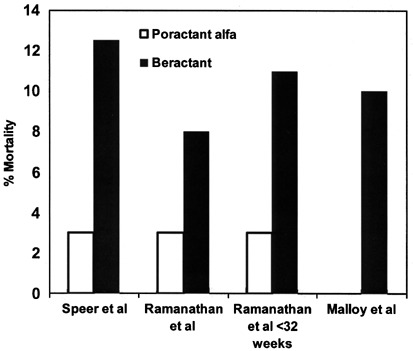
Mortality in comparison trials between poractant alfa and beractant.
More recently, Bloom and Clark27 published results from two large but incomplete, prospective, randomized, and masked clinical trials comparing calfactant and beractant. Both these trials were stopped for not meeting enrollment targets after a 32-month recruitment period. Primary outcome of infants alive without BPD were not different between calfactant and beractant in both of these trials. In addition, there were no differences in any of the secondary outcomes between these two surfactants. Investigators from these trials caution about making any definite conclusions due to premature closure of the trials and their inability to accept or reject their null hypothesis.
In a pharmacoeconomic analysis of poractant alfa vs beractant using the data from two randomized studies, Marsh et al.28 showed a 20–53% reduction in cost with poractant alfa compared with beractant.
Surfactant therapy and nasal continuous positive airway pressure (NCPAP)
Clinicians are increasingly attempting to extubate preterm infants following surfactant therapy to decrease the risk of barotrauma and/or volutrauma, and ultimately decrease the incidence of BPD. Since 1999, there have been six trials29, 30, 31, 32, 33, 34 evaluating the outcome of early surfactant therapy followed by extubation to NCPAP (Table 3). Overall, these reports demonstrated decreased duration of mechanical ventilation and length of stay, and decreased need for additional doses of surfactant. In a recent study by the Texas Neonatal Research Group,35 rescue surfactant therapy in the more, mature preterm population (birth weight ⩾1250 g) with mild to moderate RDS not requiring mechanical ventilation showed no benefits following routine elective intubation for surfactant administration when compared to expectant management with intubation and surfactant treatment as clinically indicated. Further studies are needed to evaluate the effects of early surfactant therapy followed by extubation to NCPAP or nasal ventilation on the incidence of BPD. Use of NCPAP with or without surfactant therapy was shown to be consistently associated with a lower incidence of classical as well as the newly defined ‘physiological’ BPD.36
Table 3.
Outcome of early surfactant therapy followed by extubation to nasal CPAP
| Trials (6) | Surf+CPAP/Surf+MV | GA (weeks)/age at surf Rx | Key findings |
|---|---|---|---|
| Verder29 | 35/33a | 25–35 weeks | Curosurf+CPAP: ↓ need for MV |
| Verder30 | 33/27 (early vs late Curosurf+CPAP) | <30 weeks/median age at Rx 5.2 vs 9.9 h | Early Curosurf+CPAP: ↓ need for MV; improved oxygenation |
| Haberman31 | 32/29 | 1250–2000 g/<12 h | ↓ Days on MV; early termination of study |
| D’Angio32 | 52/53 | 25–36 weeks/<24 h | ↓ Days on MV; early termination of study |
| Soll33 | 138/132 (early surf+CPAP vs CPAP with later rescue surf+MV) | 1501–2000 g/2–24 h | ↓ Days on MV |
| Dani34 | 13/14 | <30 weeks;/mean age at Rx 2.7 vs 3.5 h | ↓ Days on MV, O2, NICU LOS and second dose of Curosurf |
Abbreviations: MV, mechanical ventilation; LOS, length of stay; CPAP, continuous positive airway pressure; Surf, surfactant.
aCPAP alone.
Surfactant therapy for acute respiratory distress syndrome
Acute respiratory distress syndrome (ARDS) is not an uncommon cause of respiratory failure in near-term and term newborns admitted to neonatal intensive care units. Acute respiratory distress syndrome is often secondary to meconium aspiration syndrome (MAS), congenital pneumonia, sepsis-induced ARDS, viral pneumonia, pulmonary hemorrhage and partial or complete deficiency of SP-B. In these conditions, surfactant inactivation or dysfunction has been shown to be a major factor. Major mechanisms of surfactant inactivation in ARDS include decreased synthesis and secretion of surfactant by the type II pneumocytes, decrease in surface active small aggregates of surfactant in the alveoli, and direct inhibition of surfactant function by substances like meconium, blood, serum proteins or proteinaceous edema fluid.37
Exogenous surfactant therapy has been shown to be beneficial 70% of the time in patients with ARDS.38 In a randomized, controlled trial using beractant in forty newborns with MAS, Findley et al.39 demonstrated a significant reduction in the need for extracorporeal membrane oxygenation (ECMO) therapy. Lotze et al.40 performed a large, multicenter study in 328 term newborns with ARDS secondary to MAS, sepsis-induced ARDS or persistent pulmonary hypertension. They showed a significant reduction in the need for ECMO in patients with MAS and sepsis-related ARDS. They also showed that surfactant therapy was more beneficial when used early and in patients with an oxygenation index of <23. In a meta-analysis of data from these two studies, relative risk for ECMO therapy was significantly reduced to 0.64 (95% confidence intervals 0.46, 0.91).41
Eight, non-randomized studies using different surfactants have also been reported. Most of these studies used bolus surfactant therapy in an attempt to overcome the inactivation of endogenous surfactant. Three of the studies used either saline lavage followed by surfactant therapy or lavage using a dilute surfactant solution. In all these studies, there was improvement in oxygenation following surfactant therapy. No adverse effects were reported. In a multicenter study, Wiswell et al.42 examined the use of surfactant lavage for MAS using lucinactant, a synthetic surfactant. Although it was potentially safe and effective, lucinactant failed to show a significant advantage with surfactant lavage. The researchers were able to recruit only 15 patients in the surfactant group and seven patients served as controls.
In preterm infants with group B streptococcal pneumonia, different surfactant preparations have been shown to be effective.38 Newborns with congenital diaphragmatic hernia (CDH) have been shown to have surfactant abnormalities. In a multicenter, randomized study, Anderson et al.43 demonstrated significant adverse outcomes among infants treated with surfactant. They concluded that surfactant therapy offers no benefits in newborns with CDH. In preterm infants with evolving BPD, surfactant dysfunction has been reported. In an observational study, surfactant therapy resulted in improvement in lung function. Recently, poractant alfa treatment in infants with respiratory syncytial virus pneumonia has been shown to improve gas exchange and lung compliance.44
Conclusion
In summary, when published data from different surfactant comparison studies in preterm infants with RDS are evaluated, treatment with poractant alfa at a higher initial dose of 200 mg/kg has been shown to be associated with faster response, fewer additional doses, and a decrease in mortality, in addition to be cost effective. Furthermore, early rescue (<30 min of age) surfactant therapy, followed by rapid extubation to NCPAP or nasal ventilation should be considered to minimize lung injury and BPD. In near-term or term newborns with ARDS secondary to MAS, sepsis induced ARDS, aspiration pneumonia, bacterial or viral pneumonia, and in patients with pulmonary hemorrhage, surfactant therapy appears to be beneficial. A dose of 50 to 100 mg/kg administered at 6 to 12 h intervals may be appropriate. Surfactant containing higher amounts of saturated phosphatidyl choline and SP-B, such as poractant alfa, may be preferable over other surfactants. However, no randomized trials comparing different surfactants in ARDS have been reported. Bolus instillation of surfactant or mini-saline lavage using 3 to 5 ml/kg, followed by bolus surfactant therapy appears to be well tolerated and effective in near-term and term newborns with ARDS.
References
- 1.Martin JA, Hamilton BE, Sutton PD, Menacker F, Munson ML Births: Final Data for 2002. National Center for Health Statistics. Vital Health Stat Series vol. 52, no. 10, 2003. [PubMed]
- 2.Lemons JA, Bauer CR, Oh W, Korones S, Papile LA, Stoll BJ. Very low-birth weight infant (VLBW) outcomes of the NICHD neonatal research network. Pediatrics. 2001;107:1–8. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 3.Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials. Am J Obstet Gynecol. 1995;173:322–335. doi: 10.1016/0002-9378(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 4.Gilstrap Larry C. Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273(5):413. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 5.Soll RF. The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd; 2004. Synthetic surfactant for respiratory distress syndrome in preterm infants (Cochrane Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soll RF, Blanco F. The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd; 2004. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. (Cochrane Review) [DOI] [PubMed] [Google Scholar]
- 7.Horbar JD, Wright LL, Soll RF, Wright EC, Fanaroff AA, Korones SB. A multicenter randomized trial comparing two surfactants for the treatment of neonatal respiratory distress syndrome. J Pediatr. 1993;123:757–766. doi: 10.1016/S0022-3476(05)80856-X. [DOI] [PubMed] [Google Scholar]
- 8.Sehgal SS, Ewing CK, Richards T, Taeusch W. Modified bovine surfactant (survanta) versus a protein free surfactant (exosurf) in the treatment of respiratory distress syndrome in preterm infants: a pilot study. J Natl Med Assoc. 1994;86:46–52. [PMC free article] [PubMed] [Google Scholar]
- 9.The Vermont Oxford Neonatal Network. A multicenter randomized trial comparing synthetic surfactant with modified bovine surfactant extract in the treatment of neonatal respiratory distress syndrome. Pediatrics 1996; 97: 1–6. [PubMed]
- 10.Hudak ML, Farrell EE, Rosenberg AA, Jung AL, Auten RL, Durand DJ. A multicenter randomized masked comparison trial of natural versus synthetic surfactant for the treatment of respiratory distress syndrome. J Pediatr. 1996;128:396–406. doi: 10.1016/S0022-3476(96)70291-3. [DOI] [PubMed] [Google Scholar]
- 11.Hudak ML, Martin DJ, Egan EA, Matteson EJ, Cummings J, Jung AL. A multicenter randomized masked comparison trial of synthetic surfactant versus calf lung surfactant extract in the prevention of neonatal respiratory distress syndrome. Pediatrics. 1997;100:39–50. doi: 10.1542/peds.100.1.39. [DOI] [PubMed] [Google Scholar]
- 12.Rollins M, Jenkins J, Tubman R, Corkey C, Wilson D. Comparison of clinical responses to natural and synthetic surfactants. J Perinat Med. 1993;21:341–347. doi: 10.1515/jpme.1993.21.5.341. [DOI] [PubMed] [Google Scholar]
- 13.Alvarado M, Hingre R, Hakason D, Gross S. Clinical trial of survanta versus exosurf in infants <1500 g with respiratory distress syndrome. Pediatr Res. 1993;33:314A. [Google Scholar]
- 14.Pearlman SA, Leef KH, Stefano JL, Spear ML, Esterly KL. A randomized trial comparing exosurf versus survanta in the treatment of neonatal RDS. Pediatr Res. 1993;33:340A. [Google Scholar]
- 15.Modanlou HD, Beharry K, Padilla G, Norris K, Safvati S, Aranda JV. Comparative efficacy of exosurf and survanta surfactants on early clinical course of respiratory distress syndrome and complications of prematurity. J Perinatol. 1997;17:455–460. [PubMed] [Google Scholar]
- 16.da Costa DE, Pai MG, Al Khusabiby SM. Comparative trial of artificial and natural surfactants in the treatment of respiratory distress syndrome of prematurity: experience in a developing country. Pediatr Pulmonol. 1999;27:303–304. doi: 10.1002/(SICI)1099-0496(199905)27:5<312::AID-PPUL3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Kukkonen AK, Virtanen M, Jarvenpaa AL, Pokela ML, Ikonen S, Fellman V. Randomized trial comparing natural and synthetic surfactant: increased infection rate after natural surfactant? Acta Pediatr. 2000;89:556–561. doi: 10.1111/j.1651-2227.2000.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth SB, Beresford MW, Milligan DWA, Shaw NJ, Matthews JNS, Fenton AC. Pumactant and poractant alfa for treatment of respiratory distress syndrome in neonates born at 25–29 weeks’ gestation: a randomised trial. Lancet. 2000;355:1387–1392. doi: 10.1016/S0140-6736(00)02136-X. [DOI] [PubMed] [Google Scholar]
- 19.Sinha SK, Lacaze-Masmonteil T, Soler A, Wiswell TE, Gadzinowski J, Hajdu J. A multicenter, randomized, controlled trial of lucinactant versus poractant alfa among very premature infants at high risk for respiratory distress syndrome. Pediatrics. 2005;115:1030–1038. doi: 10.1542/peds.2004-2231. [DOI] [PubMed] [Google Scholar]
- 20.Moya FR, Gadzinowski J, Bancalari E, Salinas V, Kopelman B, Bancalari A. A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics. 2005;115:1018–1029. doi: 10.1542/peds.2004-2183. [DOI] [PubMed] [Google Scholar]
- 21.Notter RH. Lung Surfactants: Basic Science and Clinical Applications. New York: Marcel Dekker Inc.; 2000. Lung biology in health and disease; pp. 281–298. [Google Scholar]
- 22.Bloom BT, Kattwinkel J, Hall RT, Delmore PM, Egan EA, Trout JR. Comparison of infasurf (calf lung surfactant extract) to survanta in the treatment and prevention of respiratory distress syndrome. Pediatrics. 1997;100:31–38. doi: 10.1542/peds.100.1.31. [DOI] [PubMed] [Google Scholar]
- 23.Speer CP, Gefeller O, Groneck P, Laufkotter E, Roll C, Hanssler L. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 1995;72:F8–F13. doi: 10.1136/fn.72.1.F8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baroutis G, Kaleyias J, Liarou T, Papathoma E, Hatzistamatiou Z, Costalos C. Comparison of three treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Eur J Pediatr. 2003;162:476–480. doi: 10.1007/s00431-002-1144-0. [DOI] [PubMed] [Google Scholar]
- 25.Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K, North American Study Group A randomized, multicenter masked comparison trial of poractant alfa (curosurf) versus beractant (survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol. 2004;21:109–119. doi: 10.1055/s-2004-823779. [DOI] [PubMed] [Google Scholar]
- 26.Malloy CA, Nicoski P, Muraskas JM. A randomized trial comparing beractant and poractant treatment in neonatal respiratory distress syndrome. Acta Paediatr. 2005;94:779–784. doi: 10.1080/08035250510028740. [DOI] [PubMed] [Google Scholar]
- 27.Bloom BT, Clark RH. Comparison of infasurf (calfactant) and survanta (beractant) in the prevention and treatment of respiratory distress syndrome. Pediatrics. 2005;116:392–399. doi: 10.1542/peds.2004-2783. [DOI] [PubMed] [Google Scholar]
- 28.Marsh W, Smeeding J, York JM, Ramanathan R, Sekar K. A cost minimization comparison of two surfactants, beractant and poractant alfa, based upon prospectively designed, comparative clinical trial data. J Pediatr Pharmacol Ther. 2004;9:113–121. doi: 10.5863/1551-6776-9.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrom K. Surfcatant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. N Engl J Med. 1994;331:1051–1055. doi: 10.1056/NEJM199410203311603. [DOI] [PubMed] [Google Scholar]
- 30.Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks’ gestation. Pediatrics. 1999;103:e24. doi: 10.1542/peds.103.2.e24. [DOI] [PubMed] [Google Scholar]
- 31.Haberman B, Shankaran S, Stevenson DK, Papile LA, Stark A, Korones S. Does surfactant and immediate extubation to nasal continuous positive airway pressure reduce use of mechanical ventilation? Pediatr Res. 2002;51:349A. [Google Scholar]
- 32.D’Angio CT, Khalak R, Stevens TP, Reininger A, Reubens L, Kendig JW. Intratracheal surfactant administration by transient intubation in infants 29–35 weeks’ gestation with RDS requiring nasal CPAP decreases the likelihood of later mechanical ventilation: a randomized controlled trial. Pediatr Res. 2003;53:367A. doi: 10.1038/sj.jp.7211381. [DOI] [PubMed] [Google Scholar]
- 33.Soll RF, Conner JM, Howard D, the investigators of the early surfactant replacement study. Early surfactant replacement in spontaneously breathing premature infants with RDS. Pediatr Res 2003; Late breaker abstract 12, PAS 2003 meeting.
- 34.Dani C, Bertini G, Pezzati M, Cecchi A, Caviglioli C, Rubaltelli FF. Early extubation and nasal continuous positive airway pressure after surfactant treatment for respiratory distress syndrome among preterm infants <30 weeks’ gestation. Pediatrics. 2004;113:e560–e563. doi: 10.1542/peds.113.6.e560. [DOI] [PubMed] [Google Scholar]
- 35.The Texas Neonatal Research Group. Early surfactant for neonates with mild to moderate respiratory distress syndrome: a multicenter, randomized trial. J Pediatr 2004; 144: 804–808. [DOI] [PubMed]
- 36.Sahni R, Ammari A, Suri MS, Milisavljevic V, Ohira-Kist K, Wung JT. Is the new definition of bronchopulmonary dysplasia more useful? J Perinatol. 2005;25:41–46. doi: 10.1038/sj.jp.7211210. [DOI] [PubMed] [Google Scholar]
- 37.Lewis JF, Brackenbury A. Role of exogenous surfactant in acute lung injury. Crit Care Med. 2003;31:S324–S328. doi: 10.1097/01.CCM.0000057911.19145.9F. [DOI] [PubMed] [Google Scholar]
- 38.Herting E, Gefeller O, Land M, Sonderen L, Harms K, Robertson B, Members of the Collaborative European Multicenter Study Group Surfactant treatment of neonates with respiratory failure and Group B streptoloccal infection. Pediatrics. 2000;106:957–964. doi: 10.1542/peds.106.5.957. [DOI] [PubMed] [Google Scholar]
- 39.Findley RD, Taeusch W, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics. 1996;97:48–52. [PubMed] [Google Scholar]
- 40.Lotze A, Mitchell BR, Bulas DI. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. J Pediatr. 1998;132:40–47. doi: 10.1016/S0022-3476(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 41.Marks SD, Nicholl RM. The reduction in the need for ECMO by using surfactant for meconium aspiration syndrome. J Pediatr. 1999;135:267–268. doi: 10.1016/S0022-3476(99)70040-5. [DOI] [PubMed] [Google Scholar]
- 42.Wiswell TE, Knight GR, Finer NN, Donn SM, Desai H, Walsh WF. A multicenter, randomized controlled trial comparing surfaxin (lucinactant) lavage with standard care for treatment of meconium aspiration syndrome. Pediatrics. 2002;109:1081–1087. doi: 10.1542/peds.109.6.1081. [DOI] [PubMed] [Google Scholar]
- 43.Anderson JM and the Congenital Diaphragmatic Hernia Study Group. Is surfactant therapy beneficial in the management of congenital diaphragmatic hernia? Pediatr Res 2003; Late breaker abstract 13, PAS 2003 meeting.
- 44.Luchetti M, Ferraro F, Gallini C, Natale A, Pigna A, Tortorolo L. Multicenter, randomized, controlled study of porcine surfactant in severe respiratory syncytial virus-induced respiratory failure. Pediatr Crit Care Med. 2002;3:261–268. doi: 10.1097/00130478-200207000-00011. [DOI] [PubMed] [Google Scholar]


