Abstract
Background
Heavy menstrual bleeding significantly impairs the quality of life of many otherwise healthy women. Perception of heavy menstrual bleeding is subjective and management usually depends upon what symptoms are acceptable to the individual. Surgical options include conservative surgery (uterine resection or ablation) and hysterectomy. Medical treatment options include oral medication and a hormone‐releasing intrauterine device (LNG‐IUS).
Objectives
To compare the effectiveness, safety and acceptability of surgery versus medical therapy for heavy menstrual bleeding.
Search methods
We searched the following databases from inception to January 2016: Cochrane Gynaecology and Fertility Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO and clinical trials registers (clinical trials.gov and ICTRP). We also searched the reference lists of retrieved articles.
Selection criteria
Randomised controlled trials (RCTs) comparing conservative surgery or hysterectomy versus medical therapy (oral or intrauterine) for heavy menstrual bleeding.
Data collection and analysis
Two review authors independently selected the studies, assessed their risk of bias and extracted the data. Our primary outcomes were menstrual bleeding, satisfaction rate and adverse events. Where appropriate we pooled the data to calculate pooled risk ratios (RRs) or mean differences, with 95% confidence intervals (CIs), using a fixed‐effect model. We assessed heterogeneity with the I2 statistic and evaluated the quality of the evidence using GRADE methods.
Main results
We included 15 parallel‐group RCTs (1289 women). Surgical interventions included hysterectomy and endometrial resection or ablation. Medical interventions included oral medication and the levonorgestrel‐releasing intrauterine device (LNG‐IUS). The overall quality of the evidence for different comparisons ranged from very low to moderate. The main limitations were lack of blinding, attrition and imprecision. Moreover, it was difficult to interpret long‐term study findings as many women randomised to medical interventions subsequently underwent surgery.
Surgery versus oral medication
Surgery (endometrial resection) was more effective in controlling bleeding at four months (RR 2.66, 95% CI 1.94 to 3.64, one RCT, 186 women, moderate quality evidence) and also at two years (RR 1.29, 95% CI 1.06 to 1.57, one RCT, 173 women, low quality evidence). There was no evidence of a difference between the groups at five years (RR 1.14, 95% CI 0.97 to 1.34, one RCT, 140 women, very low quality evidence).
Satisfaction with treatment was higher in the surgical group at two years (RR 1.40, 95% CI 1.13 to 1.74, one RCT, 173 women, moderate quality evidence), but there was no evidence of a difference between the groups at five years (RR 1.13, 95% CI 0.94 to 1.37, one RCT, 114 women, very low quality evidence). There were fewer adverse events in the surgical group at four months (RR 0.26, 95 CI 0.15 to 0.46, one RCT, 186 women). These findings require cautious interpretation, as 59% of women randomised to the oral medication group had had surgery within two years and 77% within five years.
Surgery versus LNG‐IUS
When hysterectomy was compared with LNG‐IUS, the hysterectomy group were more likely to have objective control of bleeding at one year (RR 1.11, 95% CI 1.05 to 1.19, one RCT, 223 women, moderate quality evidence). There was no evidence of a difference in quality of life between the groups at five or 10 years, but by 10 years 46% of women originally assigned to LNG‐IUS had undergone hysterectomy. Adverse effects associated with hysterectomy included surgical complications such as bladder or bowel perforation and vesicovaginal fistula. Adverse effects associated with LNG‐IUS were ongoing bleeding and hormonal symptoms.
When conservative surgery was compared with LNG‐IUS, at one year the surgical group were more likely to have subjective control of bleeding (RR 1.19, 95% CI 1.07 to 1.32, five RCTs, 281 women, low quality evidence, I2 = 15%). Satisfaction rates were higher in the surgical group at one year (RR 1.16, 95% CI 1.04, to 1.28, six RCTs, 442 women, I2 = 27%), but this finding was sensitive to the choice of statistical model and use of a random‐effects model showed no conclusive evidence of a difference between the groups. There was no evidence of a difference between the groups in satisfaction rates at two years (RR 0.93, 95% CI 0.81 to 1.08, two RCTs, 117 women, I2 = 1%).
At one year there were fewer adverse events (such as bleeding and spotting) in the surgical group (RR 0.36, 95% CI 0.15 to 0.82, three RCTs, moderate quality evidence). It was unclear what proportion of women assigned to LNG‐IUS underwent surgery over long‐term follow‐up, as there were few data beyond one year.
Authors' conclusions
Surgery, especially hysterectomy, reduces menstrual bleeding more than medical treatment at one year. There is no conclusive evidence of a difference in satisfaction rates between surgery and LNG‐IUS, though adverse effects such as bleeding and spotting are more likely to occur with LNG‐IUS. Oral medication suits a minority of women in the long term, and the LNG‐IUS device provides a better alternative to surgery in most cases. Although hysterectomy is a definitive treatment for heavy menstrual bleeding, it can cause serious complications for a minority of women. Most women may be well advised to try a less radical treatment as first‐line therapy. Both LNG‐IUS and conservative surgery appear to be safe, acceptable and effective.
Plain language summary
Surgery versus medical therapy for heavy menstrual bleeding
Review question
Cochrane review authors compared the effectiveness, safety and acceptability of surgery versus medical therapy for heavy menstrual bleeding.
Background
Heavy menstrual bleeding is a common problem, which can impair a woman's quality of life. Surgical treatment includes hysterectomy and various methods of endometrial ablation or resection (cutting out or destroying the lining of the uterus). Medical treatment includes various oral medications and a hormone‐releasing device that is implanted in the uterus (levonorgestrel‐releasing intrauterine device, LNG‐IUS).
Study characteristics
We included 15 randomised controlled trials that compared surgery versus oral medication or LNG‐IUS. Participants were 1289 women with self reported heavy menstrual bleeding. The evidence is current to January 2016.
Key results
Hysterectomy, endometrial surgery and the LNG‐IUS were all effective in reducing heavy menstrual bleeding, though surgery was most effective, at least over the short term. These treatments suited most women better than oral medication. Although hysterectomy will stop heavy menstrual bleeding, it is associated with serious complications and most women should probably try a less radical treatment as first‐line therapy. Both conservative surgery and LNG‐IUS appear to be safe, acceptable and effective.
Quality of the evidence
The quality of the evidence ranged from very low to moderate. The main limitations were lack of blinding, attrition and imprecision. It was difficult to interpret study findings over long‐term follow‐up because a large number of women randomised to medical treatment subsequently underwent surgery.
Summary of findings
Summary of findings for the main comparison. Surgery versus oral medication for women with heavy menstrual bleeding.
| Surgery versus oral medication for women with heavy menstrual bleeding | ||||||
| Population: women with heavy menstrual bleeding Intervention: surgery Comparison: oral medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with oral medication | Risk with Surgery | |||||
| Control of bleeding (cure or improvement to acceptable level) At 4 months |
312 per 1000 | 829 per 1000 (605 to 1000) |
RR 2.66 (1.94 to 3.64) |
186 (1 RCT) |
⊕⊕⊕⊝ MODERATE1 |
— |
| Control of bleeding (cure or improvement to acceptable level) At 2 years |
616 per 1000 | 795 per 1000 (653 to 968) | RR 1.29 (1.06 to 1.57) | 173 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | — |
| Control of bleeding (cure or improvement to acceptable level) At 5 years |
754 per 1000 | 859 per 1000 (731 to 1000) | RR 1.14 (0.97 to 1.34) | 140 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | — |
| Overall satisfaction with treatment At 2 years |
558 per 1000 | 781 per 1000 (631 to 971) | RR 1.40 (1.13 to 1.74) | 173 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | — |
| Overall satisfaction with treatment At 5 years |
710 per 1000 | 802 per 1000 (667 to 973) | RR 1.13 (0.94 to 1.37) | 141 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | — |
| Adverse events at 4 months | 495 per 1000 | 129 per 1000 (74 to 228) | RR 0.26 (0.15 to 0.46) | 186 (1 RCT) | ⊕⊕⊕⊝ MODERATE 5 | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the median risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1No blinding. High rate of cross‐over: 59% of women in the medical group had undergone surgery by two years and 77% by five years. 2Confidence intervals compatible with advantage in the surgical group or no clinically meaningful difference between the groups. 3High attrition by five years ‐ 23% attrition rate. 4Confidence intervals compatible with advantage in either group or no clinically meaningful difference between the groups. 5No blinding.
Summary of findings 2. Surgery versus LNG‐IUS for women with heavy menstrual bleeding.
| Surgery versus LNG‐IUS for women with heavy menstrual bleeding | ||||||
| Population: women with heavy menstrual bleeding Intervention: surgery Comparison: LNG‐IUS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with LNG‐IUS | Risk with Surgery | |||||
| Objective control of bleeding: menstrual loss under 80 ml per cycle LNG‐IUS versus hysterectomy At 1 year |
897 per 1000 | 995 per 1000 (941 to 1000) | RR 1.11 (1.05 to 1.19) | 223 (1 RCT) | ⨁⨁⨁◯ MODERATE 1 | — |
| Subjective control of bleeding: PBAC no more than 75 per cycle Endometrial resection or ablation versus LNG‐IUS At 1 year |
767 per 1000 | 912 per 1000 (820 to 1000) | RR 1.19 (1.07 to 1.32) | 281 (5 RCTs) | ⨁⨁◯◯ LOW 1 2 | — |
| Satisfaction rate: surgery versus LNG‐IUS Endometrial ablation versus LNG‐IUS At 1 year |
630 per 1000 | 693 per 1000 (617 to 781) | RR 1.10 (0.98 to 1.24) | 332 (5 RCTs) | ⨁⨁◯◯ LOW 1 2 | — |
| Satisfaction rate: surgery versus LNG‐IUS Endometrial ablation versus LNG‐IUS At 2 years |
894 per 1000 | 832 per 1000 (724 to 966) | RR 0.93 (0.81 to 1.08) | 117 (2 RCTs) | ⨁⨁◯◯ LOW 1 2 | — |
| Proportion of women with adverse events Endometrial ablation versus LNG‐IUS. At one year |
559 per 1000 | 285 per 1000 (201 to 414) | RR 0.51 (0.36 to 0.74) | 201 (3 RCTs) | ⨁⨁⨁◯ MODERATE 2 | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the median risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LNG‐IUS: levonorgestrel‐releasing intrauterine device; PBAC: pictorial blood loss assessment chart; RCT: randomised controlled trial; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Confidence intervals compatible with benefit in the surgical arm or no clinically meaningful difference between the groups. 2Studies unblinded.
Background
Description of the condition
Heavy menstrual bleeding, also known as menorrhagia, is a common gynaecological problem that creates a major burden in terms of quality of life and financial costs for many women (Frick 2009). Heavy menstrual bleeding also uses substantial healthcare resources (Liu 2007). A general practice (GP) survey of menstruating women conducted in the UK found that the 12‐month incidence of self reported menorrhagia was 25%, without significant variation by age (Shapley 2004). A comparable prevalence rate is likely in other western countries. In New Zealand, a GP database indicated that about 2.3% of GP consultations for women aged under 50 years were for heavy menstrual bleeding (RNZCGP 2002). Rates in non‐western countries are unknown.
Heavy menstrual bleeding has been defined as a blood loss of 80 ml or more per menstrual cycle (Hallberg 1966), which is unrelated to pregnancy or known pelvic or systemic disease. However, perception of heavy bleeding is highly subjective and the actual blood loss of women seeking medical care is often less than 80 ml. When menstrual blood loss is measured, only about half of the women attending gynaecology clinics with a complaint of menorrhagia have a loss of 80 ml per cycle or more (Chimbira 1980; Higham 1999).
An objective measure of blood loss has been devised, which involves soaking used sanitary pads and tampons and calculating the optical density of the resulting solution (Hallberg 1964). This method is accurate but is also complicated and time consuming. A simpler measure is the pictorial blood loss assessment chart (PBAC), whereby a woman assesses the blood loss on her used sanitary pads or tampons and assigns a numerical score accordingly (Higham 1990). Though popular, the PBAC system has not proved to be reliable and appears to have little advantage over a woman's subjective report of her blood loss, which in practice is usually the primary consideration (Reid 2000).
More recently it has been suggested that total menstrual fluid loss may be used as an assessment of menorrhagia (Reid 2005). Measurement is determined by the difference in weight of tampons or pads before and after use. Total menstrual fluid loss has been found to correlate well with changes in objective menstrual blood loss and may be of more relevance to women concerned mainly about flooding (rather than the composition of the loss).
Description of the intervention
A minority of women reporting heavy menstrual bleeding may simply require reassurance that their blood loss is within the normal range.
Medical interventions
Where active management is preferred, first‐line treatment is generally medical. Several alternatives are available, including:
the levonorgestrel‐intrauterine device (LNG‐IUS);
anti‐fibrinolytic drugs;
non‐steroidal anti‐inflammatory drugs (NSAIDs);
progestogens (short or long course);
the combined oral contraceptive pill;
danazol;
a combination of drugs (e.g. tranexamic acid plus a NSAID).
All the medical therapies mentioned above have been shown to be at least partially effective in reducing menstrual blood loss. A decision analysis comparing the efficacy, side effects and consumer acceptability of these treatments ranked them in the order shown above, with the LNG‐IUS coming top (NZ Guidelines 1998).
Surgical interventions
Surgery may be indicated for women who have completed childbearing and for whom medical treatment is ineffective or intolerable, or it may be chosen as first‐line therapy. Again a wide variety of options is available.
Hysterectomy has traditionally been regarded as the definitive surgical treatment for heavy menstrual bleeding and has been one of the most commonly performed operations, with menstrual disorders being a leading indication (Farquhar 2002). The surgery can be performed abdominally, vaginally or laparoscopically but there is good evidence that the vaginal route is associated with shorter recovery time and fewer complications than the abdominal route (Nieboer 2009). However, hysterectomy by any route has a relatively high incidence of short‐term complications such as haemorrhage (serious blood loss), infection and wound healing problems and it also requires a lengthy postoperative recovery period. Moreover, a relationship has been found between hysterectomy and early ovarian failure (Farquhar 2005), while long‐term effects on cardiovascular (heart) function are unclear. Nevertheless, hysterectomy is 100% successful in treating heavy menstrual bleeding and for most women any problems are relatively short‐term. Satisfaction rates after hysterectomy are very high, at over 95% up to three years after surgery (Fergusson 2013).
Given that hysterectomy is a major surgical procedure with significant adverse effects and heavy menstrual bleeding is a benign condition, many women prefer one of the less invasive surgical options that are now available and which conserve the uterus. These procedures are known as endometrial resection or ablation and involve the destruction of the endometrium (the inner lining of the uterus) and the underlying basal glands by various means. Surgery may be preceded by a course of hormonal medication to thin the walls of the endometrium in order to facilitate its removal. Hormones used include goserelin (a gonadotrophin‐releasing hormone analogue, or GnRHa) and danazol (Tan 2013).
'First‐generation' techniques for endometrial destruction utilise a surgical telescope (hysteroscope) to aid viewing of the uterus along with a variety of electrosurgical or laser tools. These techniques require a general or regional anaesthetic, specialised surgical skill and often a short hospital admission. They are significantly safer than hysterectomy but still involve a small risk of uterine perforation, haemorrhage, fluid overload and infection; the short‐term complication rate is around 4% (Overton 1997). There is no guarantee that bleeding will be reduced to acceptable levels in the long term. In one study at around four‐year follow‐up, 38% of those who had had endometrial ablation went on to receive further surgical treatment of some kind for continued excessive bleeding (Aberdeen 1999).
'Second‐generation' techniques utilise the controlled application to the surface of the endometrium of heat, cold, microwave or other forms of energy with sufficient power to produce necrosis (cell death) of the full thickness of the tissue. Most of these techniques are 'non‐hysteroscopic', meaning that they can be performed without direct visualisation through a hysteroscope (Lethaby 2013a). They include microwave ablation, a fluid‐filled thermal balloon system, free fluid thermal ablation and bipolar electrocautery (NICE 2007). Such methods require sophisticated equipment but less specialised surgical skill than hysteroscopic methods and thus can usually be done as day or outpatient surgery with a local anaesthetic (Jack 2005). Economic modelling suggests that second‐generation techniques may be more cost‐effective than first‐generation methods (Garside 2004). A Cochrane systematic review found that success rates and complication profiles of newer techniques of ablation appear to compare favourably with hysteroscopic techniques (Lethaby 2013a).
Compared to hysterectomy, endometrial destruction techniques have a shorter operation time and hospital stay, quicker recovery, fewer postoperative complications and comparable satisfaction rates. Ongoing contraception is essential for sexually active women after conservative surgery even though fertility is usually not retained (NICE 2007; Opperman 1998). Moreover, women are likely to continue to experience some degree of menstrual bleeding and may need further surgery if menorrhagia persists. This potential need for re‐treatment over the long term has narrowed the cost gap between hysterectomy and conservative surgery (Fergusson 2013).
How the intervention might work
Medical interventions vary in their mode of action, as follows:
The levonorgestrel‐intrauterine device (LNG‐IUS) is a device that is implanted in the uterus for up to five years. It releases a low dose of a progestogenic hormone, which acts locally to suppress endometrial activity. The device also provides contraception, with fertility returning when the device is removed. It has been reported to reduce menstrual blood loss by 94% after three months and to be well accepted by most women (Irvine 1998). However, a LNG‐IUS frequently causes irregular vaginal bleeding or spotting, especially during the first few months of use (Irvine 1998; Luukkainen 2001). Moreover, some women experience hormonal side effects such as weight gain, breast tenderness and bloating and occasionally the device is expelled spontaneously (Lethaby 2015). Other less common but more severe side effects include the increased risk of pelvic inflammatory disease, should a sexually transmitted infection occur, the increased risk of ectopic pregnancy should pregnancy occur, and the risk of uterine perforation, which is a rare but serious event (NICE 2005). An increased incidence of ovarian cysts has been reported in women using intrauterine progestogenic implants; these are a transient phenomenon, which resolves spontaneously (Brache 2002).
Anti‐fibrinolytic drugs such as tranexamic acid work by inhibiting the breakdown of blood clots (fibrinolysis). These drugs reduce bleeding by about 40% to 50% (Lethaby 2009b), but do not generally alleviate menstrual cramping (Preston 1995). They are taken only during menstruation and are usually well tolerated but can cause mild nausea and diarrhoea (Dunn 1999).
Non‐steroidal anti‐inflammatory drugs (NSAIDs) such as mefenamic acid and naproxen reduce blood loss by 33% to 55% and also relieve menstrual cramps. They act by inhibiting the production of prostaglandin (a fatty acid that is typically over‐produced in women with heavy menstrual bleeding). They can cause headaches and gastrointestinal disturbances though significant side effects are unlikely as NSAIDs need to be taken only during menstruation (Lethaby 2013).
Progestogens such as norethisterone and medroxyprogesterone acetate are hormones which suppress endometrial growth and activity. Given as a 21‐day course, from day 5 to day 26 of the menstrual cycle, they reduce blood flow substantially (Lethaby 2008). However, they are considered unacceptable for long‐term use by many women due to the prevalence of side effects such as breast tenderness, bloating and headaches; they may also precipitate breakthrough bleeding (Irvine 1998). Progestogens have been shown to be less effective if taken as a short course, i.e. only during the luteal phase of the menstrual cycle (between ovulation and menstruation) (Lethaby 2008).
There is some indication that the combined oral contraceptive pill significantly reduces menstrual blood loss and relieves cramping; in addition it provides contraception (Fraser 1991). However, randomised evidence is very scanty (Farquhar 2009). This treatment may work by inhibiting the growth and development of the endometrium. Side effects include irregular bleeding, especially on treatment initiation. Some women report other hormonal effects such as headaches, nausea, dizziness and breast tenderness, though these are less prevalent with currently available low‐dose combined oral contraceptives than with older preparations. Weight gain is often perceived as a side effect of combined oral contraceptive use, but no causal association has been found. The risk of arterial thrombotic events is increased threefold by combined oral contraceptive use, and the risk of venous thromboembolism is doubled; however these serious events remain very rare among healthy women of reproductive age (Dragoman 2014).
Danazol is a synthetic hormone that causes the endometrium to shrink and is usually highly effective in reducing blood loss. It is generally only used for short‐term treatment due to the prevalence and severity of side effects such as weight gain, headache, nausea, tiredness and acne (Beaumont 2007). Barrier contraception is recommended to prevent possible fetal damage (New Ethicals 2000). When treatment is discontinued, the effects of danazol persist for two to three cycles before blood loss returns to pre‐treatment levels (Chimbira 1979).
Surgical interventions work by removing or destroying the endometrium, as described in the section above.
Why it is important to do this review
There are several Cochrane systematic reviews on the effectiveness and acceptability of individual surgical and medical treatments for heavy menstrual bleeding (Beaumont 2007; Farquhar 2009; Fergusson 2013; Lethaby 2008; Lethaby 2013a; Lethaby 2015; Lethaby 2009b). The aim of the present review is to assess the efficacy, safety and acceptability of all forms of medical treatment against all forms of surgery for heavy menstrual bleeding.
Objectives
To compare the effectiveness, safety and acceptability of surgery versus medical therapy for heavy menstrual bleeding.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing surgery (conservative surgery or hysterectomy) versus medical therapy (oral, intramuscular or intrauterine) for the treatment of heavy menstrual bleeding.
Types of participants
Inclusion criteria
Women of reproductive age with regular heavy menstrual periods measured either objectively (e.g. via the alkaline haematin test) or subjectively (e.g. via the pictorial blood loss assessment chart (PBAC), a menstrual blood loss diary or according to a woman's personal judgement).
Exclusion criteria
Postmenopausal bleeding (over one year since the last menstrual period).
Irregular menses or intermenstrual bleeding.
Pathological causes of heavy menstrual bleeding (e.g. uterine cancer).
Iatrogenic (treatment‐related) causes of heavy menstrual bleeding (e.g. non‐progestogen‐releasing intrauterine contraceptive device).
Types of interventions
Hysterectomy (including abdominal, laparoscopic, vaginal and laparoscopically assisted vaginal), endometrial resection or ablation (including both first and second‐generation techniques such as transcervical resection of the endometrium, transcervical rollerball, laser, cryosurgery and balloon therapies).
Medical treatments for heavy menstrual bleeding, including a levonorgestrel‐intrauterine device (LNG‐IUS), non‐steroidal anti‐inflammatory agents, tranexamic acid, the oral contraceptive pill, progestogen in short or long courses, and danazol.
Types of outcome measures
Primary outcomes
1. Menstrual bleeding, measured at one year*, two years, five years and 10 years.
*If studies did not report this outcome at one year we included measures at less than one year and have highlighted where this is the case.
a) Objective assessment of menstrual blood loss, e.g. measured by the modified alkaline haematin method (Hallberg 1964).
b) Subjective assessment of menstrual blood loss, e.g. measured by pictorial blood assessment chart (PBAC) (Higham 1990), or a woman's perception of improvement recorded in a reproducible and validated format.
2. Satisfaction rate.
3. Adverse effects.
Secondary outcomes
4. Quality of life: self reported change in quality of life, recorded in a reproducible and validated format.
5. Requirement for additional surgical or medical treatment for heavy menstrual bleeding.
6. Cost and resource use.
Search methods for identification of studies
We searched for all published and unpublished RCTs comparing surgery versus medical therapy for heavy menstrual bleeding, without language restriction and in consultation with the Cochrane Gynaecology and Fertility (CGF) Group Trials Search Co‐ordinator. We searched all databases from inception to January 14th 2016.
Electronic searches
We searched the Specialised Register of the Cochrane Menstrual Disorders and Subfertility Group (Appendix 1), which is based on regular searches of MEDLINE, EMBASE, CINAHL and PsycINFO (from inception), handsearching of 20 relevant journals and conference proceedings, and searches of several key grey literature sources. A full description is given in the Group's module on The Cochrane Library.
In addition, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 2), MEDLINE (Appendix 3), EMBASE (Appendix 4), PsycINFO (Appendix 5), CINAHL (Appendix 6) and clinical trials registers (clinical trials.gov and ICTRP).
Searching other resources
We also searched citation lists of relevant publications.
Data collection and analysis
Selection of studies
One author (JM) scanned the titles and abstracts of articles retrieved by the search and removed those that were very clearly irrelevant. We retrieved the full texts of all potentially eligible studies. Two review authors (AL, JM) independently examined the full‐text articles for compliance with the inclusion criteria and selected the studies that were eligible for inclusion in the review. The review authors attempted to contact study investigators, as required, to clarify study eligibility (for example, with respect to randomisation and blinding). We resolved disagreements as to study eligibility by consensus.
Data extraction and management
Two authors (JM and either AL or CM) independently extracted data using a standardised form. For each study, we extracted data on study design, participants, interventions used and outcomes measured. These are presented in the Characteristics of included studies table. We also extracted data on study findings. These are presented in the Results and the Data and analyses sections of the review. We sought additional data from the principal or corresponding author of studies, if necessary. Where studies had multiple publications, we used the most recent report. We resolved discrepancies by discussion.
Assessment of risk of bias in included studies
Two review authors (JM and AL) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool to assess risk of selection bias (random sequence generation and allocation concealment); performance and detection bias (blinding of participants and personnel and outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other bias (Higgins 2011). We resolved disagreements by discussion or by referral to the third review author. We presented the conclusions in the 'Risk of bias' tables, which are incorporated into the interpretation of review findings by means of sensitivity analyses (see below).
Measures of treatment effect
For dichotomous data (for example, the proportion of women reporting control of bleeding) we used the numbers of events in the control and intervention groups of each study to calculate risk ratios (RRs). For continuous data (for example PBAC scores) we calculated mean differences between treatment groups. We treated ordinal data (for example, quality of life scores) as continuous data. For all outcomes we calculated 95% confidence intervals (CIs).
We used data in meta‐analysis only if the underlying distribution of the measurements appeared normal. The ratio of the mean to its standard deviation gives a crude method of assessing skew; if this ratio was less than 2.0 for any group in a trial, or if results were reported in the publication as median and range, we reported the data in text in 'Additional tables'. Where trial results were incomplete (for example, measures of variance were not extractable) or presented only as graphs, we reported the results descriptively in the text.
Unit of analysis issues
We identified no unit of analysis issues.
Dealing with missing data
We analysed data on an intention‐to‐treat basis, as far as possible. Where data were missing, we made attempts to obtain them from the original investigators. Where they were unobtainable, we analysed the available data using the numerator and denominator reported in study results or calculated from reported percentages.
Assessment of heterogeneity
The authors considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. Where pooling was conducted, we examined heterogeneity (variation) between the results of different studies by inspecting the scatter in the data points and the overlap in their CIs and more formally by checking the results of the I2 statistic (Higgins 2003). This quantity describes the percentage of total variation across studies that is due to heterogeneity rather than chance. We planned to tentatively assign low, moderate and high heterogeneity to I2 statistics of up to 25%, 26% to 74% and over 75%, respectively. We planned to look at the possible contribution of differences in trial design to any meta‐analyses with an I2 > 50%. Wherever possible, we statistically pooled outcomes.
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. We planned to use a funnel plot to assess the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies) if there were a sufficient number of studies for the same outcome (10 or more).
Data synthesis
We combined the data from primary studies if they were sufficiently homogeneous. We pooled dichotomous data to calculate pooled RRs with 95% CIs using a fixed‐effect model. We displayed graphically an increase in the risk of a particular outcome, which may be beneficial (for example, pain relief) or detrimental (for example, adverse effects), in the forest plots to the right of the centre line; a decrease in the risk of an outcome is shown to the left of the centre line. We combined data for continuous outcomes to calculate means differences (MDs) and 95% CIs.
Subgroup analysis and investigation of heterogeneity
Where a visual scan of the forest plots indicated heterogeneity, we planned to consider methodological and clinical differences that might account for the outliers. We planned to consider subgroup analyses to investigate significant heterogeneity found during the review process, but to interpret the results of any such analyses with caution as they were not prespecified.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcomes to determine whether the conclusions are robust to arbitrary decisions made regarding the eligibility and analysis. These analyses would include consideration of whether the review conclusions would have differed if:
eligibility were restricted to studies without high risk of bias (those with a high risk of bias in any of the domains assessed);
a random effects‐model had been adopted;
the summary effect measure had been odds ratio rather than a risk ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using Guideline Development Tool software. This table evaluated the overall quality of the body of evidence for the main review outcomes (menstrual bleeding, satisfaction, adverse events), using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We justified, documented and incorporated into the reporting of results our judgements about evidence quality (high, moderate or low) for each outcome.
Results
Description of studies
Results of the search
Our search in January 2016 retrieved 551 records, of which we checked 19 in full text. We found three new studies (six articles) eligible for inclusion (Ergun 2012 (three articles); Ghazizdeh 2014 (two articles); Sesti 2012), one new ongoing study (Herman 2013), and nine articles pertaining to three studies already included in the review (de Souza 2010 (two articles); Hurskainen 2001 (six articles); Tam 2006). We excluded four studies (Ergun 2012a; Ghazizadeh 2011; Shabaan 2011; Shokeir 2013).
The review update includes 15 studies (three new in 2016 plus 12 already in the previous version of the review). The final search date in the previous version was May 2010). Altogether we have excluded 11 studies from the review (four newly excluded in 2016 plus seven already excluded from the previous version of the review). See study flowchart: Figure 1.
1.
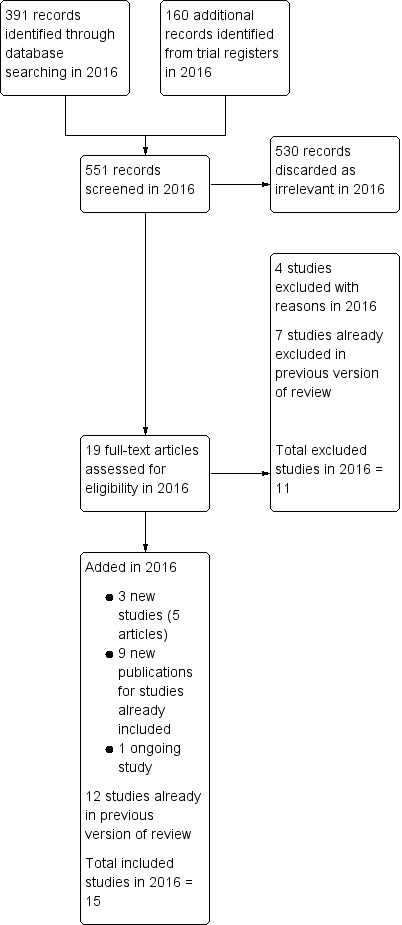
Study flow diagram.
Included studies
Design
Fifteen parallel‐group RCTs met the inclusion criteria. They included a total of 1289 women. The trials were conducted at gynaecology outpatient departments of hospitals in Brazil, Egypt, England, Finland, Hong Kong, Iran, Italy, New Zealand, Norway, Scotland, Turkey and the USA. For seven studies the primary outcome was menstrual bleeding, measured as a bleeding score (Barrington 2003; Crosignani 1997; Ergun 2012; Istre 1998; Sesti 2012; Shaw 2007; Soysal 2002; Talis 2006), or by treatment success, a composite measure based on menstrual bleeding rate, removal of the device or need for repeat surgery (Malak 2006). The primary outcome was unclear in one study (Ghazizdeh 2014). Primary outcomes in other studies were health‐related quality of life (Hurskainen 2001; Kupperman 2004), and satisfaction with treatment (Cooper 1997). Two studies reported a range of measures of menstrual health and quality of life and did not specify a primary outcome (de Souza 2010; Tam 2006). Duration of follow‐up ranged from six months to 10 years.
Participants
Study participants were women aged 30 to 50 years seeking treatment for heavy menstrual bleeding. None of the studies required objective evidence of menorrhagia, though some excluded women with a PBAC score below a minimum level (which varied from 75 ml to 150 ml).
The expectations of participants varied. Three studies required that women were equally willing to accept medical or surgical management (Cooper 1997; Hurskainen 2001; Kupperman 2004), whereas in four studies the participants were initially prepared to have a hysterectomy (Crosignani 1997; Ghazizdeh 2014; Istre 1998; Malak 2006). Inclusion criteria in seven studies required that women had unsuccessfully tried oral medical treatment (Barrington 2003; de Souza 2010; Ergun 2012; Kupperman 2004; Sesti 2012; Shaw 2007; Tam 2006), which in one had to include cyclical medroxyprogesterone (Kupperman 2004). Many of the participants in other studies had also tried oral medical therapy unsuccessfully.
Ten of the studies specifically stated that women should be premenopausal (Crosignani 1997; Istre 1998, Kupperman 2004, Malak 2006; Tam 2006), should not be postmenopausal (Sesti 2012), should have regular menstrual cycles (de Souza 2010; Ergun 2012; Talis 2006), or should "be menstruating" (Hurskainen 2001). Other studies required women to have heavy menstrual bleeding, but did not have menopausal status as a specific inclusion or exclusion criterion (Barrington 2003; Cooper 1997; Ghazizdeh 2014; Shaw 2007; Soysal 2002). All studies excluded women with abnormal endometrial pathology or other uterine abnormalities such as polyps or fibroids. Other inclusion and exclusion criteria are detailed in the Included studies table.
Interventions
Comparisons in the included studies were as follows:
Endometrial resection versus oral medication (Cooper 1997).
Hysterectomy versus oral medication (Kupperman 2004).
Endometrial resection versus LNG‐IUS (Crosignani 1997; Ergun 2012; Ghazizdeh 2014; Istre 1998; Malak 2006).
Thermal balloon ablation versus LNG‐IUS (Barrington 2003; de Souza 2010; Shaw 2007; Soysal 2002; Talis 2006; Tam 2006).
Ablation using bipolar electrocauterisation (NovaSure) versus LNG‐IUS (Ghazizdeh 2014).
Hysterectomy versus LNG‐IUS (Hurskainen 2001; Sesti 2012).
In six studies, women in the surgical arm received transcervical endometrial resection with a loop or rollerball (where stated), a 'first‐generation' technique (Cooper 1997; Crosignani 1997; Ergun 2012; Ghazizdeh 2014; Istre 1998; Malak 2006); while in seven studies they received a 'second‐generation' technique, either thermal balloon ablation (Barrington 2003; de Souza 2010; Shaw 2007; Soysal 2002; Talis 2006; Tam 2006), or bipolar electrocauterisation (Ghazizdeh 2014). In two studies women in the surgical group underwent hysterectomy (Hurskainen 2001: Kupperman 2004).
Medical management in Cooper 1997 consisted of a minimum of three cycles of oral medication. This included progestogens, the combined oral contraceptive pill, tranexamic acid, danazol, hormone replacement therapy and non‐steroidal anti‐inflammatory drugs. In Kupperman 2004, all women had already tried cyclical medroxyprogesterone acetate and found it unsatisfactory. Ninety per cent of them were prescribed various forms of hormonal therapy, generally combined with a non‐steroidal anti‐inflammatory drug (in most cases naproxen sodium). Women dissatisfied with one medical therapy were encouraged to try other medical options. Medical management in the other nine studies consisted of LNG‐IUS, an intrauterine device releasing 20 μg per day of levonorgestrel (progesterone).
Outcomes
Primary review outcomes
Menstrual bleeding
Eleven studies reported menstrual blood loss. One measured blood loss objectively using the alkaline haematin method (Hurskainen 2001), and 10 used self rated bleeding scores, either the Pictorial Blood Loss Assessment Chart (PBAC) (Barrington 2003; Crosignani 1997; Ergun 2012; Istre 1998; Malak 2006; Sesti 2012; Shaw 2007; Soysal 2002; Talis 2006), or a zero to five scale (Cooper 1997).
Satisfaction rates
Satisfaction rates were the primary outcome in Cooper 1997 and were also reported by other studies (Crosignani 1997; Ghazizdeh 2014; Shaw 2007; Soysal 2002; Talis 2006).
Adverse events
Most studies reported adverse effects, including operative complications, complications with insertion of the LNG‐IUS (where applicable) and longer‐term adverse effects (for example, pelvic or abdominal pain, irregular bleeding, vaginal discharge, breast tenderness). Kupperman 2004 only reported events requiring hospitalisation. Two studies reported postoperative complications and adverse effects associated with 'treatment failure' (that is removal of the LNG‐IUS) (Shaw 2007; Talis 2006); one focused on adverse events in the LNG‐IUS group only (Tam 2006). Sesti 2012 reported only short‐term adverse events (occurring for up to 30 days postoperatively). Two studies failed to report clear data on adverse events (Ergun 2012; Ghazizdeh 2014), and a third did not report this outcome at all (de Souza 2010).
Secondary review outcomes
Nine studies reported detailed quality of life data (Cooper 1997; Crosignani 1997; de Souza 2010; Hurskainen 2001; Kupperman 2004; Malak 2006; Soysal 2002; Talis 2006; Tam 2006). For two studies this was the primary outcome (Hurskainen 2001; Kupperman 2004). Tools used to measure quality of life are listed in the table Characteristics of included studies.
All studies reported on requirement for additional surgery or medical treatment, and three analysed cost‐effectiveness in detail (Hurskainen 2001; Kupperman 2004; Talis 2006).
Excluded studies
We excluded 11 studies from the review. Four were non‐randomised (Barrington 1997; Ghazizadeh 2011; Romer 2000; Soysal 2005), one was partially randomised but separate data for the randomised group were not obtainable (Assaf 2000), four did not report the comparison of interest (Ergun 2012a; Reid 2005; Shabaan 2011; Shokeir 2013), one did not report the outcomes of interest (Lahteenmaki 1998), and one was discontinued due to poor enrolment (SMART 2000) (see Excluded studies).
Risk of bias in included studies
See Figure 2 for a summary of the risk of bias in individual studies and Figure 3 for a summary of each risk of bias item across all included studies.
2.
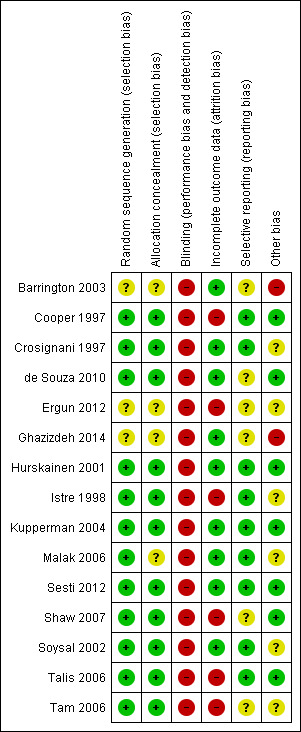
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
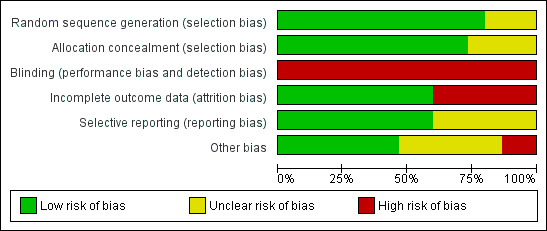
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
Twelve studies described satisfactory methods of sequence generation and we rated them as at low risk of bias in this domain. Three did not clearly describe the method used and we rated them as at unclear risk of bias (Barrington 2003; Ergun 2012; Ghazizdeh 2014)
Allocation concealment
Eleven studies described satisfactory methods of allocation concealment and we rated them as at low risk of bias in this domain. Four did not clearly describe the method used and we rated them as at unclear risk of bias (Barrington 2003; Ergun 2012; Ghazizdeh 2014; Malak 2006).
Blinding
No studies reported use of blinding, which would clearly be very difficult for most of these comparisons. In view of the subjective nature of our primary outcome measures, we rated all studies as at high risk of bias in this domain.
Incomplete outcome data
Nine studies analysed a high proportion of the women randomised (90% to 100%) and provided valid reasons for all or nearly all missing data. No study attempted to impute missing data. We rated six studies as at high risk of attrition bias: in Cooper 1997 18% of the women were missing from the analysis at five years without details of the reasons; in three studies over 25% of women in one or both arms were not included in the analysis (Ergun 2012; Shaw 2007; Tam 2006), and in Talis 2006 only 70% of the women randomised were analysed for PBAC scores at two years.
Selective reporting
We rated nine studies as at low risk of this bias as they reported on all expected outcomes, including those that were prespecified, and reported them systematically for both comparison groups based on prospectively collected data. We rated four studies as at unclear risk of this bias because they did not clearly report adverse events across both intervention groups (Barrington 2003; Ergun 2012; Ghazizdeh 2014; Shaw 2007). Two studies did not clearly specify a primary outcome or clearly report adverse events across both intervention groups and we rated them as at high risk of selective reporting (de Souza 2010; Tam 2006).
Other potential sources of bias
We rated five studies as at unclear risk of other bias (Crosignani 1997; Istre 1998; Malak 2006; Soysal 2002; Tam 2006); see Characteristics of included studies for details. We rated two as at high risk of bias due to differences between the groups at baseline (Barrington 2003; Ghazizdeh 2014), and we rated the remainder of the studies as at low risk of other potential bias.
Source of funding
Six studies were funded by government or tertiary institutional research grants or received no external funding (Cooper 1997; Crosignani 1997; Hurskainen 2001; Kupperman 2004; Shaw 2007; Tam 2006), though medicated intrauterine devices (where used) or surgical equipment were often supplied by the manufacturer. Two studies were commercially funded (de Souza 2010; Istre 1998). It was not stated how any of the other studies were funded.
Contact with study authors
We sought additional information from most of the principal investigators regarding the study design and results. We received replies from the principal investigators of eight studies (de Souza 2010; Ghazizdeh 2014; Hurskainen 2001; Istre 1998; Kupperman 2004; Shaw 2007; Talis 2006; Tam 2006). See Characteristics of included studies for more details on the methodology of all the included studies.
Effects of interventions
1. Surgery versus oral medication
Two studies made this comparison. They compared endometrial ablation (Cooper 1997) and hysterectomy (Kupperman 2004) versus oral medication.
PRIMARY OUTCOMES
1.1 Menstrual bleeding
1.1a Objective measures
Neither of the studies reported objective assessment of bleeding (such as use of the modified alkaline haematin method).
1.1b Subjective measures
One study of 187 women compared transcervical endometrial resection with oral medication and assessed menstrual bleeding at four months, two years and five years (Cooper 1997).
At four months, control of bleeding (cure or improvement to an acceptable level) was reported by more women in the surgical group than in the medical group (risk ratio (RR) 2.66, 95% confidence interval (CI) 1.94 to 3.64, 186 women). Mean self rated bleeding scores were reduced from baseline in both groups but scores were lower in the surgical group (mean difference (MD) ‐12.70, 95% CI ‐10.36 to ‐15.04, 186 women) (Figure 4; Analysis 1.1; Analysis 1.2).
4.
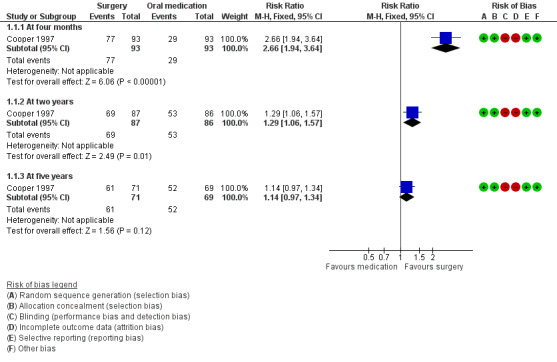
Forest plot of comparison: 1 Surgery versus oral medication, outcome: 1.1 Control of bleeding (cure or improvement to acceptable level).
1.1. Analysis.
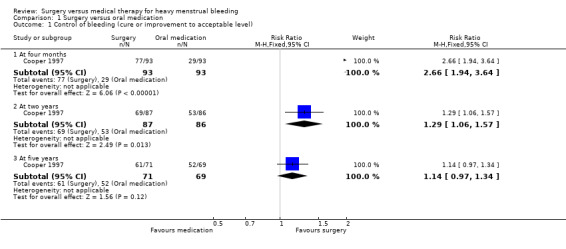
Comparison 1 Surgery versus oral medication, Outcome 1 Control of bleeding (cure or improvement to acceptable level).
1.2. Analysis.
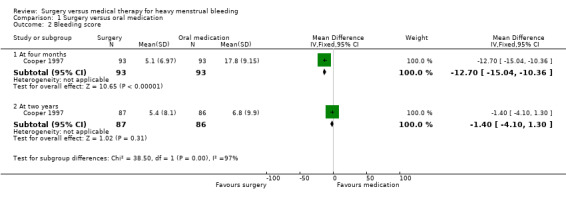
Comparison 1 Surgery versus oral medication, Outcome 2 Bleeding score.
At two years surgery was still rated significantly more effective than oral medication in control of bleeding (RR 1.29, 95% CI 1.06 to 1.57, 173 women), but there was no longer evidence of a difference between the groups in bleeding scores (MD ‐1.40, 95% CI ‐4.10 to 1.30, 173 women) (Figure 4; Analysis 1.1; Analysis 1.2).
At five years there was no longer evidence of a difference between the groups in control of bleeding (RR 1.14, 95% CI 0.97 to 1.34, 140 women) (Analysis 1.1).
In interpreting the two‐year and five‐year results for this study, it is relevant to bear in mind that analysis was by intention‐to‐treat and that by two years of follow‐up the majority of women in the medical group had received surgery (see below: 'Requirement for further surgery').
1.2 Satisfaction rate
The study comparing endometrial ablation with oral medication, Cooper 1997, reported on satisfaction levels at four months, two years and five years. Women in the surgical group reported a higher satisfaction rate at four months than women in the medical group (RR 2.80, 95% CI 1.96 to 3.99, 186 women) (Analysis 1.3). At six‐month follow‐up, in Kupperman 2004 women in the hysterectomy group reported higher levels of satisfaction with symptom resolution (P value ≤ 0.001) and overall health (P value = 0.006) than women in the medical group.
1.3. Analysis.
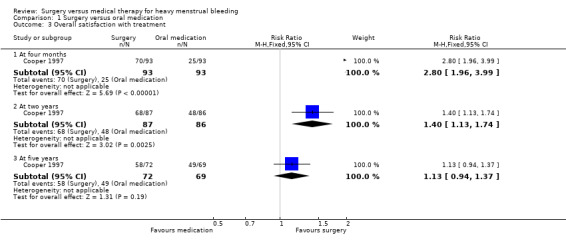
Comparison 1 Surgery versus oral medication, Outcome 3 Overall satisfaction with treatment.
In Cooper 1997, at two years there were still more women in the surgical arm than in the medical arm who were satisfied with their treatment (Analysis 1.3), but in Kupperman 2004 by this stage there was no evidence of a difference in satisfaction levels in the two groups (P value = 0.68). By five years there was no evidence of a difference between the groups in Cooper 1997 either (Analysis 1.3).
As noted above, in interpreting the two‐year and five‐year results for these studies, it is relevant to bear in mind that analysis was by intention‐to‐treat and that by two years of follow‐up the majority of women in the medical groups had received surgery.
1.3 Adverse effects
Both studies described adverse effects, though the level of detail varied.
Perioperative/short‐term adverse events
In Cooper 1997, six women undergoing endometrial resection (6%) had persistent uterine bleeding during surgery that required the temporary insertion of a uterine Foley catheter (Table 3).
1. Operative and postoperative complications in the surgical arm.
| Study ID | Operation | No. of operations | Operative problems | Postoperative/late problems |
| Cooper 1997 | Transcervical endometrial resection | 93 | Persistent uterine bleeding necessitating uterine catheterisation for 6 hours (6) | Nil |
| Crosignani 1997 | Transcervical endometrial resection | 35 | Nil | Nil |
| de Souza 2010 | Thermal balloon ablation | 28 | Nil reported | Nil reported |
| Ergun 2012 | Rollerball ablation | 31 | Nil reported | Nil reported |
| Ghazizdeh 2014 | Endometrial resection or ablation | 62 | Nil reported | Nil reported |
| Hurskainen 2001 | Hysterectomy: vaginal (30), abdominal (22), laparoscopic (57) | 109 | Bladder perforation (3), bowel perforation (1) | Occurred in a total of 33 women: wound infection (12), wound rupture (2), infected pelvic haematoma (6), postoperative fever (2), peritonitis (1), ileus (2), urinary retention (4), severe abdominal pain (3), vesicovaginal fistula (1), postoperative bleeding (2), ureter lesion (1) |
| Istre 1998 | Transcervical endometrial resection | 29 | Nil | None stated |
| Kupperman 2004 | Hysterectomy | 28 | 1 woman had superficial thermal injury to small bowel during lysis of adhesions | Occurred in a total of 4 women: 1 had postoperative fever 1 had hypovolaemia 6 days postoperatively, requiring salpo‐oophorectomy plus readmission 21 days postoperatively for haematemesis and oesophagitis 1 had a new seizure disorder 9 days postoperatively 1 had trachelectomy 15 months after supracervical hysterectomy, for persistent cyclic bleeding |
| Malak 2006 | Endometrial resection | 30 | All surgery uneventful | 1 woman had repeat surgery due to haematometra |
| Sesti 2012 | Laparoscopic supracervical hysterectomy | 36 | None reported | None reported |
| Shaw 2007 | Thermal balloon ablation | 33 | 1 technical failure with equipment ‐ surgery did not proceed | 15 women stayed overnight due to cramping lower abdominal pain. Use of a paracervical block was introduced following which only 1 woman needed an overnight stay. |
| Soysal 2002 | Thermal balloon ablation | 36 | 2 required cervical dilatation | Nil |
| Talis 2006 | Thermal balloon ablation | 39 | 1 required general anaesthetic | 5 women received post‐operative antibiotics for possible endometritis |
| Tam 2006 | Thermal balloon ablation | 15 | 3 women required additional surgery for endometrial polyps (2) or submucosal fibroid (1) | — |
In the hysterectomy group of Kupperman 2004, two women suffered perioperative complications (a superficial injury to the small bowel and a fever) and three had late complications necessitating readmission: one was hypovolaemic and required a laparotomy and salpingo‐oophorectomy (removal of the ovaries), one developed a new seizure disorder seven days postoperatively and a third required removal of the cervix due to persistent bleeding (Table 3).
Adverse events over follow‐up
Cooper 1997 reported fewer adverse effects in the surgical group than in the medical group at four months (RR 0.26, 95% CI 0.15 to 0.46, 186 women, Analysis 1.4). Symptoms included nausea, headaches and weight gain in the medical group and new pain in both groups. One woman in the medical arm who had been prescribed danazol suffered a cerebrovascular accident and one who had been prescribed the combined oral contraceptive pill developed hypertension.
1.4. Analysis.
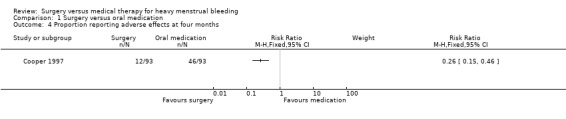
Comparison 1 Surgery versus oral medication, Outcome 4 Proportion reporting adverse effects at four months.
Kupperman 2004 reported that two women in the medical group required hospital admission for bleeding problems. One required dilatation and curettage for abnormal uterine bleeding at seven months post‐randomisation and the other required a blood transfusion and injection of gonadotrophin‐releasing hormone for haemorrhagic vaginal bleeding.
SECONDARY OUTCOMES
1.4 Quality of life and symptom control
Both studies comparing surgery with oral medication reported SF‐36 scores (Cooper 1997; Kupperman 2004). The SF‐36 change scores in both these studies had high standard deviations, indicating skewed distribution. Results are therefore recorded in additional tables rather than forest plots (see Table 4).
2. SF‐36: Surgery versus oral medication.
| Study | SF‐36 category | Time |
Surgical mean change |
Medical mean change |
Change difference | P value change difference |
| Cooper 1997 | See below P values calculated by Mann‐Whitney U test for difference in change between groups | |||||
| Physical function | 4 months | + 10.16 (SD 16.51) | + 4.84 (SD 16.72) | — | P value < 0.05 | |
| 2 years | + 5.00 (SD 18.97) | + 3.73 (SD 17.19) | — | P value = 0.65 | ||
| 5 years | + 7.75 (SD 16.39) | + 1.06 (SD 23.81) | — | P value = 0.10 | ||
| Social function | 4 months | + 17.44 (SD 16.51) | + 7.57 (SD 26.26) | — | P value < 0.05 | |
| 2 years | + 10.59 (SD 26.52) | + 3.94 (SD 25.26) | — | P value = 0.10 | ||
| 5 years | + 10.24 (SD 24.49) | + 2.96 (SD 27.22) | — | P value = 0.10 | ||
| Physical role | 4 months | + 32.26 (SD 38.23) | + 15.32 (SD 46.78) | — | P value < 0.01 | |
| 2 years | + 18.60 (SD 45.73) | + 12.95 (SD 44.58) | — | P value = 0.42 | ||
| 5 years | + 31.62 (SD 33.15) | + 15.14 (SD 39.77) | — | P value = 0.06 | ||
| Emotional role | 4 months | + 31.54 (SD 45.94) | + 8.96 (SD 49.93) | — | P value < 0.01 | |
| 2 years | + 22.48 (SD 50.47) | + 11.25 (SD 45.17) | — | P value = 0.13 | ||
| 5 years | + 33.81 (SD 34.11) | + 14.35 (SD 40.61) | — | P value = 0.02 | ||
| Mental health | 4 months | + 15.01 (SD 19.00) | + 4.78 (SD 16.69) | — | P value < 0.01 | |
| 2 years | + 9.98 (SD 19.14) | + 7.17 (SD 19.20) | — | P value = 0.35 | ||
| 5 years | + 13.26 (SD 16.94) | + 3.62 (SD 18.21) | — | P value = 0.01 | ||
| Energy/fatigue | 4 months | + 20.53 (SD 20.76) | + 7.07 (SD 20.23) | — | P value < 0.01 | |
| 2 years | + 14.58 (SD 21.96) | + 10.06 (SD 19.57) | — | P value = 0.17 | ||
| 5 years | + 17.31 (22.35) | + 10.62 (SD 18.79) | — | P value = 0.07 | ||
| Pain | 4 months | + 21.62 (SD 31.33) | + 8.84 (SD 26.39) | — | P value < 0.01 | |
| 2 years | + 12.34 (SD 27.20) | + 11.38 (SD 28.51) | — | P value = 0.82 | ||
| 5 years | + 14.81 (SD 25.35) | + 11.98 (SD 23.66) | — | P value = 0.6 | ||
| General health | 4 months | + 10.49 (SD 20.85) | ‐‐ 0.25 (SD 15.99) | — | P value = <0.01 | |
| 2 years | + 1.69 (SD 18.83) | ‐ 0.67 (SD 13.90) | — | P value = 0.36 | ||
| 5 years | + 6.97 (SD 23.10) | ‐3.88 (SD 20.13) | — | P value = 0.01 | ||
| Kupperman 2004 | Mental component summary | 6 months | + 8 | + 2 | 6 (95% CI 0.4 to 12) | P value = 0.04 |
| 2 years | +7 | +4 | 3 (95% CI ‐2 to 7) | P value = 0.25 | ||
| Physical component summary | 6 months | +6 | +3 | 3 (95% CI ‐2 to 8) | P value = 0.21 | |
| 2 years | +7 | +9 | ‐2 (95% CI ‐5 to 1) | P value = 0.19 |
CI: confidence interval SD: standard deviation
In Cooper 1997, at four months SF‐36 scores were improved in all or most categories for women in both groups, but all improvements were greater for women in the surgical arm (Table 4).
At two years SF‐36 scores continued to improve from baseline for women in the medical group but generally fell for women in the surgical group, with the result that there was no evidence of a difference between the groups in the change in scores from baseline. However, by this time 59% of women in the medical group had received surgery. At five years the surgical group had greater improvement from baseline levels in three out of eight SF‐36 categories, namely emotional role, mental health and general health (Table 4).
Similarly, women in the hysterectomy group in Kupperman 2004 reported greater improvements at six months in SF‐36 mental health scores (P value ≤ 0.05) than the medical group. At two years these improvements were maintained in the hysterectomy group but the scores for the medical group had also improved for most categories so that there were few differences between the groups. By then 53% of women in the medical group had had a hysterectomy.
1.5 Requirement for additional treatment for heavy menstrual bleeding
Requirement for additional surgical treatment
Fewer women in the surgical arms than in the medical arms of these studies required additional surgery (endometrial ablation or hysterectomy or both) within two years of their initial treatment (14% versus 58%): RR 0.25, 95% CI 0.15 to 0.39, two studies, 236 women, I2 = 39% (Analysis 1.5). In the study with five‐year follow‐up these figures were 26% and 77% respectively (RR 0.35, 95% CI 0.25 to 0.50, 187 women) with about 18% of women in each arm having had a hysterectomy (Cooper 1997) (Analysis 1.5). Most of these operative procedures were performed within the first 18 months (Kupperman 2004) or two years (Cooper 1997).
1.5. Analysis.
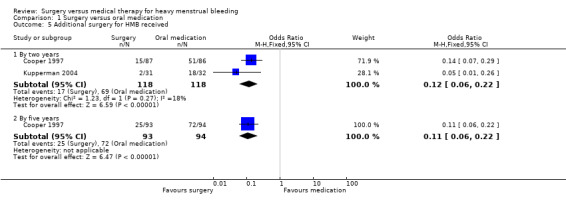
Comparison 1 Surgery versus oral medication, Outcome 5 Additional surgery for HMB received.
Requirement for additional medical treatment
The study of endometrial ablation versus oral medication reported this outcome: 7/87 (8%) of women in the surgical group were using medical therapy at two years (Cooper 1997).
1.6 Cost
Kupperman 2004 compared the cost of hysterectomy and oral medication by measuring relative resource use over a 24‐month follow‐up with assessments conducted three‐monthly. In the first 12 months of follow‐up, using intention‐to‐treat analysis, women in the hysterectomy group used significantly more resources (USD 6777 versus USD 4479, P value = 0.03) attributable mainly to use of inpatient care. During the second year of follow‐up resource use was roughly equivalent in the surgical and medical groups (USD 1360 versus USD 1338 respectively). Within two years there was a 53% rate of cross‐over to hysterectomy in the medical group, which accounted for the similarity in resource use. In 'as‐treated' analyses, mean total resource use for women in the medical group who did not subsequently receive hysterectomy was USD 2595 compared to USD 7024 for women in the hysterectomy group.
2. Surgery versus levonorgestrel‐intrauterine device (LNG‐IUS)
PRIMARY OUTCOMES
Thirteen studies compared surgery with LNG‐IUS. In two studies women in the surgical arm underwent hysterectomy (Hurskainen 2001; Sesti 2012). In the other 11 studies women in the surgical arm underwent conservative surgery: transcervical endometrial resection (Crosignani 1997; Ergun 2012; Ghazizdeh 2014; Istre 1998; Malak 2006), or ablation with either thermal balloon (Barrington 2003; de Souza 2010; Shaw 2007; Soysal 2002; Talis 2006; Tam 2006), or electrocautery (Ghazizdeh 2014). One study included two types of ablation (Ghazizdeh 2014).
Since hysterectomy is expected to have a 100% success rate in stopping menstrual bleeding and since adverse effects are commonly related to bleeding patterns, for these outcomes we considered it inappropriate to pool Hurskainen 2001 or Sesti 2012 with studies that used conservative surgery.
2.1 Menstrual bleeding
2.1a Objective measures
Hysterectomy versus LNG‐IUS
Hurskainen 2001 reported objective assessment of bleeding at one year, using the modified alkaline haematin method. When analysed by intention‐to‐treat, including in the LNG‐IUS group the 20% of women randomised to the LNG‐IUS group who subsequently had a hysterectomy, bleeding was controlled in all women in the hysterectomy group (107/107) and 90% (104/116) in the LNG‐IUS group (RR 1.11, 95% CI 1.05 to 1.19, one RCT, 223 women) (Analysis 2.1). When outcomes were analysed at one year by treatment received, no women in the hysterectomy group had any further menstrual bleeding while in the LNG‐IUS group 69% of women (80/116) had control of bleeding.
2.1. Analysis.
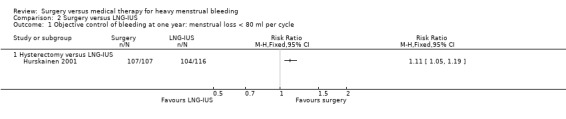
Comparison 2 Surgery versus LNG‐IUS, Outcome 1 Objective control of bleeding at one year: menstrual loss < 80 ml per cycle.
At one year, blood loss was measured objectively in all women in the LNG‐IUS group of this study who still had a LNG‐IUS in situ, apart from those whose bleeding was nil or negligible. This amounted to 25 women, only one of whom had a loss in excess of 80 ml. The mean loss for the 25 women was 13 ml (range 1 ml to 92 ml).
Conservative surgery versus LNG‐IUS
No studies of conservative surgery versus LNG‐IUS reported objective measures of blood loss.
2.1b Subjective measures
Hysterectomy versus LNG‐IUS
One study of hysterectomy versus LNG‐IUS reported this outcome, measured by pictorial blood loss assessment chart (PBAC) scores at one year (Sesti 2012). There was no evidence of a difference between the groups (MD 0.20, 95% CI ‐5.12 to 5.52, one study, 72 women) (Analysis 2.3). At two years, PBAC scores were lower in the surgical group (MD ‐52.70, 95% CI ‐76.50 to ‐28.90, one RCT, 72 women) (Analysis 2.4).
2.3. Analysis.
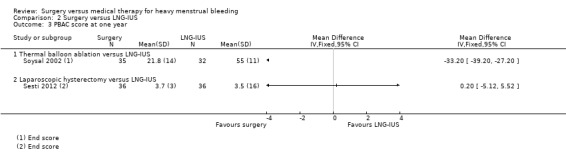
Comparison 2 Surgery versus LNG‐IUS, Outcome 3 PBAC score at one year.
2.4. Analysis.
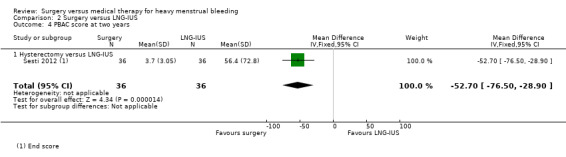
Comparison 2 Surgery versus LNG‐IUS, Outcome 4 PBAC score at two years.
Conservative surgery versus LNG‐IUS
Ten studies of conservative surgery versus LNG‐IUS reported this outcome. Measures used were self report of "well controlled bleeding" and PBAC scores. Duration of follow‐up ranged from six months to five years. Eight studies reported PBAC data that were unsuitable for meta‐analysis (see Table 5).
3. Mean or median PBAC scores over six months to two years follow‐up.
| Trial | Comparison | Baseline | At 3 months | At 6 months | At 1 year | At 2 years | Change from baseline |
| Barrington 2003 | Surgical: balloon ablation Medical: LNG‐IUS | Surgical: preoperative mean 122 (range 63 to 424) Medical: preoperative mean 107 (range 27 to 408) Difference between groups P value = 0.025 |
— | Surgical: mean 61 (range 0 to 424) Medical: mean 31 (range 0 to 100) Difference between groups P value = 0.690 | — | — | — |
| de Souza 2010 | Surgical: balloon ablation Medical: LNG‐IUS | Surgical: preoperative mean 419.7 +/‐ 72.1 Medical: preoperative mean 541.9 +/‐ 97.8 Difference between groups P value = 0.579 |
— | — | Statistically significant decrease from baseline in both groups (P value < 0.001); no significant difference between the groups | — | — |
| Crosignani 1997 | Surgical: endometrial resection Medical: LNG‐IUS |
Surgical: preoperative mean 203.2 +/‐ 77.4 Medical: preoperative mean 184.8 +/‐ 62.2 |
— | — | Decrease in PBAC score: Surgical: 38.8 +/‐ 37.1 Medical: 23.5 +/‐ 32.6 (P value = 0.15) |
— | Blood loss fell 89% from baseline in the surgical group and 79% from baseline in the LNG‐IUS group |
| Ergun 2012 | Surgical: roller ball endometrial ablation Medical: LNG‐IUS |
Surgical mean score 440 Medical mean score 480 (SDs not reported) |
— | — | Surgical mean score: 55 Medical mean score: 70 (SDs not reported) (P value > 0.05) |
— | — |
| Istre 1998 | Surgical: endometrial resection; Medical: LNG‐IUS | — | — | — | Surgical: median 8.5 (range 0 to 55) Medical: median 12 (range 0 to 97) No significant difference between groups | Surgical: Median 10 (range 0 to 175) Medical: Median 8.5 (range 0 to 128) No significant difference between groups | Decrease in both groups from baseline: P value < 0.0001 (Friedman's 2‐way ANOVA) Difference between groups: N/S (Wilcoxon rank sum test) |
| Malak 2006 | Surgical: endometrial resection; Medical: LNG‐IUS | Surgical: mean 346.8 (SD 143.6) Medical: mean 316.8 (SD 152.0) |
— | — | Surgical: Mean 42.2 (SD 30.4), median 45 (range 0 to 100) Medical: Mean 40.6 (SD 28.5), median 42 (range 0 to 95) No significant difference between groups |
— | — |
| Shaw 2007 | Surgical: balloon ablation Medical: LNG‐IUS |
Median (range): Surgical: 432 (126 to 1650) Medical: 450 (146 to 1200) |
Median score (range): Surgical: 184 (5 to 610) Medical: 172 (0 to 729) |
Median score (range): Surgical: 81 (0 to 440) Medical: 124 (0 to 610) |
Median score (range): Surgical: 62 (0 to 142) Medical: 26 (0 to 68) (P value < 0.001) |
— | — |
| Talis 2006 | Surgical: balloon ablation Medical: LNG‐IUS | — | Surgical: median 75.0 Medical: median 52.0 (P value = 0.452) | Median score: Surgical: 52.5 Medical: 32.0 (P value = 0.002) |
Surgical: 60.0 Medical: median 11.5 (P value = 0.002) | Surgical: Median 56.5 Medical: Median 12.0 (P value = 0.002) | — |
LNG‐IUS: levonorgestrel‐releasing intrauterine device N/S: non‐significant PBAC: pictorial blood loss assessment chart SD: standard deviation
Bleeding at up to one year (10 studies)
Five studies of conservative surgery versus LNG‐IUS measured the proportion of women who reported that their bleeding was well controlled by their primary treatment at one year (PBAC score < 75 per cycle or bleeding 'normal' or lighter) (Crosignani 1997; Istre 1998; Malak 2006; Soysal 2002; Tam 2006). Pooled findings favoured the surgical group (RR 1.19, 95% CI 1.07 to 1.32, five studies, 281 women, I2 = 15%) (Figure 5; Analysis 2.2).
5.
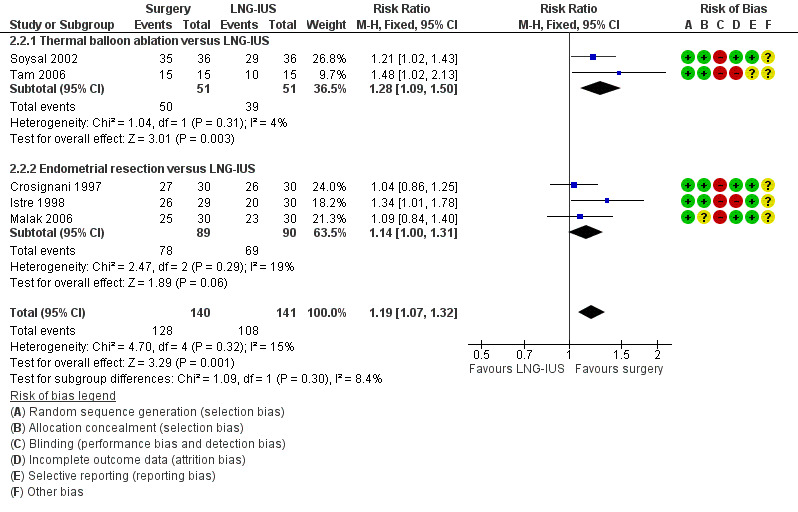
Forest plot of comparison: 2 Surgery versus LNG‐IUS, outcome: 2.2 Subjective control of bleeding at up to one year: PBAC =/< 75 per cycle with primary treatment.
2.2. Analysis.
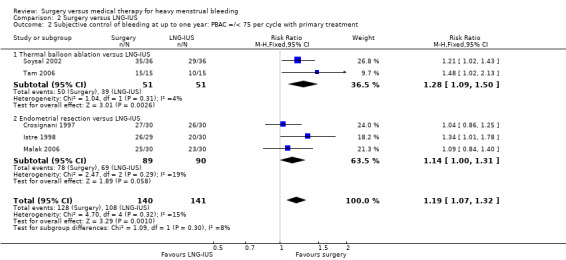
Comparison 2 Surgery versus LNG‐IUS, Outcome 2 Subjective control of bleeding at up to one year: PBAC =/< 75 per cycle with primary treatment.
Nine studies of conservative surgery versus LNG‐IUS reported PBAC scores at six months (Barrington 2003), or one year (Crosignani 1997; de Souza 2010; Ergun 2012; Istre 1998; Malak 2006; Shaw 2007; Soysal 2002; Talis 2006). All studies reported that both groups had significant reductions from baseline in PBAC scores. In the only one of these studies that reported data suitable for analysis, Soysal 2002, the mean PBAC score at one year was lower in the surgical group (MD ‐33.20, 95% CI ‐39.20 to ‐27.20, one RCT, 67 women) (Analysis 2.3). The other eight studies reported median values or skewed data and their data are reported in an additional table (Table 5). Their findings were inconsistent: five of the eight found no evidence of a difference between the groups in PBAC scores (Barrington 2003; de Souza 2010; Ergun 2012; Istre 1998; Malak 2006), one reported lower PBAC scores in the surgical group (Crosignani 1997), and two reported lower scores in the LNG‐IUS group (Shaw 2007; Talis 2006).
Bleeding at two years (one study)
One study compared conservative surgery versus LNG‐IUS and reported median PBAC scores at two years (Istre 1998). PBAC scores decreased in both groups, with no evidence of a difference between the groups (Table 5).
2.2 Satisfaction
Five studies reported satisfaction rates, all of which compared conservative surgery versus LNG‐IUS (Crosignani 1997; Ergun 2012; Ghazizdeh 2014; Shaw 2007; Soysal 2002; Talis 2006). Satisfaction rates were higher in the surgical group at one year (RR 1.16, 95% CI 1.04, to 1.28, six RCTs, 442 women, I2 = 27%) (Analysis 2.5), but there was no evidence of a difference between the groups at two years (RR 0.93, 95% CI 0.81 to 1.08, two RCTs, 117 women, I2 = 1%) (Analysis 2.6). Findings at one year were sensitive to the choice of statistical model and use of a random‐effects model showed no conclusive evidence of a difference between the groups (RR 1.13, 95% CI 1.00 to 1.27, six RCTs, 442 women, I2 = 4%).
2.5. Analysis.
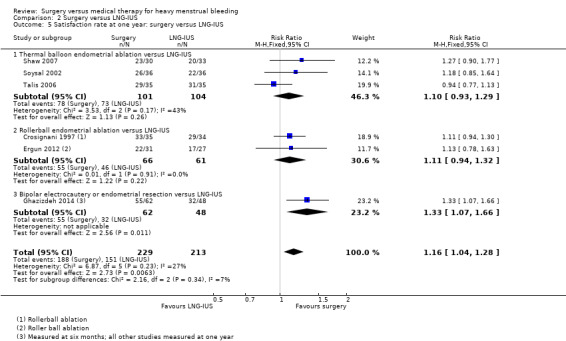
Comparison 2 Surgery versus LNG‐IUS, Outcome 5 Satisfaction rate at one year: surgery versus LNG‐IUS.
2.6. Analysis.
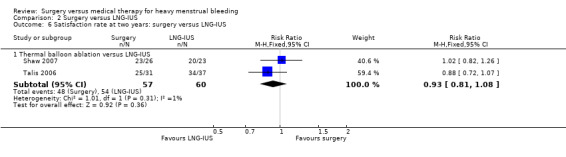
Comparison 2 Surgery versus LNG‐IUS, Outcome 6 Satisfaction rate at two years: surgery versus LNG‐IUS.
2.3 Adverse effects
All of the studies, apart from Barrington 2003 and de Souza 2010, described adverse effects, though the level of detail varied.
Hysterectomy versus LNG‐IUS
Perioperative/short‐term adverse effects
(For details see Table 3).
Hurskainen 2001 detailed 35 operative and postoperative complications that occurred among the 107 women in the surgical group who had a hysterectomy. These included perforations of the bladder and bowel, vesicovaginal fistula (creation of an abnormal passage between the vagina and the bladder), urinary retention, intestinal obstruction, postoperative bleeding, severe postoperative pain, peritonitis, fever, wound infection, wound rupture and infected pelvic haematoma (collection of blood) (Table 3). In the LNG‐IUS group, insertion of the device was easy for 73% of women but required local anaesthetic for 10% and was impossible for two women (1.7%). One woman had the device removed two hours after insertion due to severe pain.
Sesti 2012 reported that there were no postoperative complications in either group that necessitated readmission, blood transfusion or repeat surgery.
Adverse effects over follow‐up
(For details see Table 6).
4. Adverse effects (other than operative): surgery versus LNG‐IUS.
| Study | Surgical arm | Surgical adverse effects | IUS arm | IUS adverse effects |
| Crosignani 1997 | n = 35 | Weight gain (3), headache (3), decreased libido (2), pelvic pain (1), anxiety/depression (1) | n = 34 | Occasional heavy bleeding (3), irregular spotting (12), bloating (10), weight gain (8), breast pain (6), headache (4), pelvic pain (2), decreased libido (2), hair loss (2), acne (2), anxiety/depression (2), hypertension (1), leg pain (1) |
| de Souza 2010 | n = 28 | No adverse effects reported | n = 30 | No adverse effects reported |
| Ergun 2012 | n = 31 | 1 woman had endometrial collection due to synechia, which required drainage | n = 27 | 2 women requested removal of LNG‐IUS at 3 months due to bleeding; most common side effect was spotting, especially in the first 3 months |
| Ghazizdeh 2014 | n = 62 | In hysteroscopic resection group (n = 32): post‐treatment pain (1), spotting (1) No adverse effects in the ablation group |
n = 48 | No adverse effects reported |
| Hurskainen 2001 | n = 117 | No adverse effects reported during follow‐up (see Table 8 for operative and postoperative adverse effects) | n = 119 | Symptoms requiring discontinuation of treatment: intermenstrual bleeding (42), hormonal symptoms (18), lower abdominal pain (6 ‐ 2 of whom were diagnosed with diverticulitis), depression (2), recurrent thromboembolic disease (1), benign ovarian cyst (1) |
| Istre 1998 | n = 29 | New symptoms within first year: pelvic pain/inflammation (4), bleeding (3), vaginitis (1), genital ulceration (1), abdominal pain (1) Significant adverse events within 3 years (not necessarily treatment related): endometriosis (1), significant menstrual bleeding and pain (1), stroke 1.5 months post‐surgery (in hypertensive participant), pelvic inflammatory disease (3), adenomyosis (1), myometritis (1), abnormal Pap test (3) | n = 30 | New symptoms within first year: bleeding disorders (6), abdominal pain (4), breast tenderness (3), headache (2), acne (2), mood changes (1), pelvic pain or vaginal discharge (7) Significant adverse events within 3 years (not necessarily treatment‐related): severe oedema (1), uterine inflammation (3), pelvic inflammatory disease (2) partial expulsion (1) |
| Malak 2006 | n = 30 | 7 women reported 1 or more local adverse events: irregular bleeding/spotting (4), pelvic pain (3), vaginal discharge (4); 10 reported generalised symptoms: abdominal pain (5), breast tenderness (3), headache (2), acne (2), mood changes (1) | n = 30 | 9 women reported 1 or more local adverse events: pelvic pain and local tenderness (4), bleeding (5), vaginitis (2), genital ulceration (1). 2 reported generalised events: abdominal pain (1), haematometra requiring repeat ER (1) |
| Sesti 2012 | n = 36 | None reported | n = 36 | At 2 years bleeding problems were worse than at 3‐month follow‐up: 1 woman still had menorrhagia, while 5 reported ongoing intermenstrual spotting |
| Shaw 2007 | n = 33 | Adverse events requiring discontinuation of treatment (n = 7): dysmenorrhoea and bleeding (2), continuing menorrhagia (5) | n = 33 | Adverse events requiring discontinuation of treatment (n = 13): coil expulsion (2), prolonged bleeding/spotting (6), continuing menorrhagia (5) |
| Soysal 2002 | n = 36 | Mastalgia (1), weight gain (4), mood swings (1), bloating (2), dysmenorrhoea (2), lower abdominal pain ‐ haematometra (1) | n = 36 | Spotting (6), mastalgia (5), weight gain (10), mood swings (2), bloating (8), acne/greasy skin (7), nausea (4), headache (1), leg pain (1), spontaneous expulsion of LNG‐IUS (1) |
| Talis 2006 | n = 39 | Suspected endometritis requiring antibiotics (5), dysmenorrhoea (1) | n = 40 | Spontaneous expulsion (4), pain requiring removal of device (2), "unscheduled bleeding" (2), actinomycoses (1) |
| Tam 2006 | n = 15 | Adverse effects not mentioned in this group | n = 18 | Irregular spotting and/or persistent menorrhagia (5); no reports of breast discomfort or bloating |
ER: emergency room IUS: intrauterine device LNG‐IUS: levonorgestrel‐releasing intrauterine device
Sesti 2012 reported that 22% of women in the LNG‐IUS group had spotting every month over the first six months. Bleeding patterns improved over the first year but then deteriorated, and at two years bleeding problems were worse than at three‐month follow‐up.
Hurskainen 2001 reported that within five years, 60 women in the LNG‐IUS group (50%) had had the device removed for intermenstrual bleeding, heavy bleeding and/or hormonal symptoms. Fifty of these women subsequently underwent hysterectomy, of whom 15 (30%) experienced surgical complications (most commonly infection and severe abdominal pain). Measures of bone mineral density in this study suggested that hysterectomy may accelerate age‐related loss since women in the hysterectomy group, but not in the LNG‐IUS group, had a significant decrease in lumbar spine bone mineral density at five‐year follow‐up (Analysis 2.8).
2.8. Analysis.
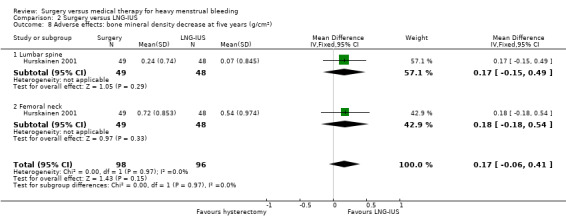
Comparison 2 Surgery versus LNG‐IUS, Outcome 8 Adverse effects: bone mineral density decrease at five years (g/cm2).
Hurskainen 2001 followed up 221 women (94% of randomised women) at 10 years. By this stage, 46% (55/119) of the women in the LNG‐IUS group had had a hysterectomy. The effects of the interventions were reported by treatment received. Women treated by hysterectomy were more likely to reports feelings of incomplete emptying than LNG‐IUS users, and stress urinary incontinence and urinary tract infections were also more common in the hysterectomy group. The hysterectomy group were also more likely to use medication for urinary incontinence. This study also investigated the effect of the interventions on markers of cardiovascular risk, and reported that women who had hysterectomy had higher levels of serum inflammatory markers and increased use of diabetes medication, compared to LNG‐IUS users.
Conservative surgery versus LNG‐IUS
Perioperative/short‐term adverse events
(For details see Table 3).
Four studies comparing conservative surgery with LNG‐IUS reported on adverse effects associated with the initial procedure (Crosignani 1997; Ghazizdeh 2014; Istre 1998; Soysal 2002). All surgical operations were straightforward and no complications occurred during insertion of the LNG‐IUS, though cervical dilatation was required by 4% of women to facilitate insertion of the intrauterine device and by 5% of women during balloon ablation.
Adverse effects over follow‐up
(For details see Table 6).
At one year, adverse effects were less common in the surgical group (RR 0.51, 95% CI 0.36 to 0.74, three studies, 201 women, I2 = 28%) (Figure 6; Analysis 2.7). The most commonly reported adverse effect was irregular bleeding and spotting. Adverse effects necessitated removal of the LNG‐IUS in two women (7%) in Ergun 2012, four women (13%) in Malak 2006 and five women (23%) in Tam 2006 in the LNG‐IUS group during the first year of follow‐up. At one year, spontaneous complete or partial expulsion of the LNG‐IUS had occurred in two women in Crosignani 1997, two in Tam 2006 and one in Istre 1998.
6.
Forest plot of comparison: 2 Surgery versus LNG‐IUS, outcome: 2.7 Proportion of women with adverse events at one year.
2.7. Analysis.
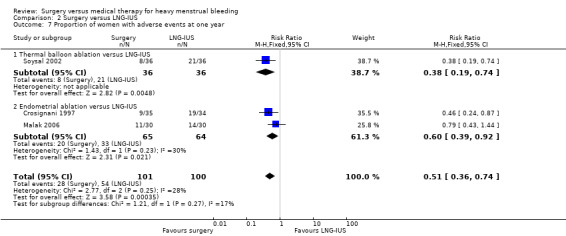
Comparison 2 Surgery versus LNG‐IUS, Outcome 7 Proportion of women with adverse events at one year.
By two years, Talis 2006 reported that five women in the surgical arm (13%) had experienced possible endometritis requiring antibiotics, five women in the LNG‐IUS group (13%) had required removal of the device due to pain, unscheduled bleeding or infection and four devices (10%) had been spontaneously expelled.
SECONDARY OUTCOMES
2.4 Quality of life and symptom control
Quality of life
Seven studies reported quality of life. All compared hysterectomy or conservative surgery versus LNG‐IUS. They used a variety of measures, including the EuroQol visual analogue scale (Hurskainen 2001), the Psychological General Wellbeing Index (de Souza 2010), and the SF‐36 (Crosignani 1997; Hurskainen 2001; Sesti 2012; Soysal 2002; Talis 2006; Tam 2006). They reported findings at up to one year (Crosignani 1997; de Souza 2010; Hurskainen 2001; Sesti 2012; Soysal 2002; Talis 2006; Tam 2006), two years (Talis 2006), five years (de Souza 2010; Hurskainen 2001), and 10 years (Hurskainen 2001).
We did not pool data on quality of life due to clinical and statistical heterogeneity and (in some cases) evidence of probable skew. Where the ratio of the mean to the standard deviation was less than two in any domain, we assumed skew and reported the study results in an additional table.
Hysterectomy versus LNG‐IUS
At one year of follow‐up, the smaller study of hysterectomy versus LNG‐IUS reported mixed findings, with better quality of life scores in the LNG‐IUS group for five of the eight SF‐36 domains (mental health, vitality, physical role, emotional role and social function), better quality of life in the surgical group for one domain (pain), and no evidence of a difference between the groups in other domains (Sesti 2012) (Analysis 2.11).
2.11. Analysis.
Comparison 2 Surgery versus LNG‐IUS, Outcome 11 SF36 score at one year: surgery versus LNG‐IUS.
In the larger study of hysterectomy versus LNG‐IUS (Hurskainen 2001), there was an improvement in quality of life in nearly all SF‐36 domains in both groups. At one year, women in the hysterectomy group had less pain than women in the LNG‐IUS group. Apart from this, there was no evidence of a difference between women in the two groups at one year, five years or 10 years in any of the SF‐36 domains (Table 7). Hurskainen 2001 also measured change in quality of life using the four‐item EuroQol visual analogue scale. There was an improvement in scores in both groups, with no evidence of a difference between them at one year (MD 0.00, 95% CI ‐0.05 to 0.05, 228 women), five years (MD 0.02, 95% CI ‐0.05 to 0.09, 232 women) or 10 years (MD ‐0.01, 95% CI ‐0.06 to 0.06, 221 women) (Analysis 2.9). Overall, quality of life scores improved during the first five years in both groups but between five and 10 years they diminished, and the scores returned close to the baseline level.
5. Quality of life: Hysterectomy versus LNG‐IUS.
| Study | Measure: change from baseline | Time |
Hysterectomy (n = 109) mean change (SD) |
LNG‐IUS (n = 116) mean change (SD) |
| Hurskainen 2001 | SF‐36 General health | 1 year | 6.2 (17.27) | 5.5 (16.48) |
| 5 years | 4.40 (18.60) | 3.60 (19.32) | ||
| 10 years | ‐4.5 (20.16) | ‐2.3 (18.73) | ||
| SFG‐36 Physical function | 1 year | 7.1 (17.27) | 4.8 (16.48) | |
| 5 years | ‐2.00 (19.66) | ‐1.40 (20.15) | ||
| 10 years | ‐3.8 (22.58) | ‐3.4 (22.31) | ||
| SF‐36 Emotional wellbeing | 1 year | 8.4 (18.35) | 8.1 (19.78) | |
| 5 years | 8.10 (17.78) | 8.40 (20.69) | ||
| 10 years | 3.2 (20.7) | 5.7 (23.54) | ||
| SF‐36 Vitality | 1 year | 12.5 (21.5) | 10.2 (23) | |
| 5 years | 10.00 (22.70) | 9.40 (22.90) | ||
| 10 years | 5.3 (25.26) | 6 (23) | ||
| SF‐36 Physical role limitation | 1 year | 18.6 (44.8) | 18.1 (40.1) | |
| 5 years | 10.80 (45.95) | 8.90 (41.67) | ||
| 10 years | 3.2 (48.11) | 8.2 (46.63) | ||
| SF‐36 Emotional role limitation | 1 year | 20 (41.5) | 15.8 (42.3) | |
| 5 years | 12.90 (17.78) | 16.20 (43.06) | ||
| 10 years | 4.9 (51.6) | 9.1 (56.19) | ||
| SF‐36 Social function | 1 year | 12.4 (22.6) | 11.8 (23) | |
| 5 years | 9.00 (24.89) | 8.70 (25.38) | ||
| 10 years | 1.8 (27.68) | 7.9 (29.7) | ||
| SF‐36 Bodily pain | 1 year | 21.1 (27.8) | 11.8 (27.2) | |
| 5 years | 13.40 (31.18) | 12.80 (27.04) | ||
| 10 years | 4 (48.11) | 8.2 (46.63) | ||
| General Health VAS | 5 years | 12.9 (190) | 16.2 (44.15) | |
| Anxiety (Spielberger state‐trait scale) | 5 years | ‐1.9 (24.66) | ‐2.4 (23.80) | |
| Depression (Beck scale) | 5 years | ‐1.4 (15.01) | ‐1.2 (18.39) | |
| Sexual satisfaction (higher score = more) | 5 years | ‐0.2 (6.29) | ‐0.7 (5.79) | |
| Sexual problems (higher score = more) | 5 years | ‐0.04 (3.01) | ‐0.02 (3.04) | |
| Sexual satisfaction with partner (higher score = more) | 5 years | ‐0.4 (3.55) | ‐0.7 (3.86) |
LNG‐IUS: levonorgestrel‐releasing intrauterine device SD: standard deviation VAS: visual analogue scale
2.9. Analysis.
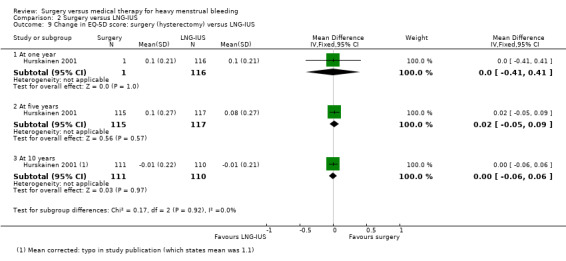
Comparison 2 Surgery versus LNG‐IUS, Outcome 9 Change in EQ‐5D score: surgery (hysterectomy) versus LNG‐IUS.
Conservative surgery versus LNG‐IUS
Four studies of conservative surgery versus LNG‐IUS reported SF‐36 scores at one year (Crosignani 1997; Soysal 2002; Talis 2006; Tam 2006). In most respects they found no evidence of a difference between the groups. Crosignani 1997 found no evidence of a difference between the groups in any of the eight SF‐36 domains (Table 8). Soysal 2002 reported higher scores in the surgical group for one SF‐36 domain (physical role limitation), but otherwise found no difference between the groups. Tam 2006 reported higher quality of life scores in the surgical group for three domains (general health, mental health, emotional role), but otherwise found no difference between the groups, and Talis 2006 assessed the SF‐36 "General health" domain and found no difference between the groups (Analysis 2.11).
6. SF‐36: Endometrial ablation versus LNG‐IUS.
| SF‐36 category | Time |
Surgical group Median score (IQR) |
Medical group Median score (IQR) |
Study |
| Physical function | 1 year | 75 (42.5 to ?) | 72.5 (53.7 to 91.2) | Soysal 2002 (n = 33 surgical, 32 medical) |
| 90 (71.9 to 94.7) | 85 (62.8 to 95) | Crosignani 1997 (n = 31 surgical, 31 medical) | ||
| Social function | 1 year | 50 (12.5 to 87.5) | 50 (3.7 to 96.8) | Soysal 2002 (n = 33 surgical, 32 medical) |
| 75 (56.2 to 87.5) | 75 (50 to 87.5) | Crosignani 1997 (n = 31 surgical, 31 medical) | ||
| Physical role | 1 year | 50 (‐25 to 125) | 25 (‐25 to 75) | Soysal 2002 (n = 33 surgical, 32 medical) |
| 100 (50 to 100) | 100 (50 to 100) | Crosignani 1997 (n = 31 surgical, 31 medical) | ||
| Emotional role | 1 year | 33.3 (‐33.3 to 99.9) | 33.3 (‐58.3 to 124.9) | Soysal 2002 (n = 33 surgical, 32 medical) |
| 100 (66.7 to 100) | 66.7 (33.3 to 100) | Crosignani 1997 (n = 31 surgical, 31 medical) | ||
| Mental health | 1 year | 52 (22 to 82) | 52 (25 to 79) | Soysal 2002 (n = 33 surgical, 32 medical) |
| 64 (46.7 to 68) | 60 (46 to 68) | Crosignani 1997 (n = 31 surgical, 31 medical) | ||
| Energy/fatigue | 1 year | 45 (10 to 80) | 45 (26.2 to 63.7) | Soysal 2002 (n = 33 surgical, 32 medical) |
| 55 (40 to 70) | 55 (47.5 to 65) | Crosignani 1997 (n = 31 surgical, 31 medical) | ||
| Pain | 1 year | 51 (20 to 82) | 51 (30 to 72) | Soysal 2002 (n = 33 surgical, 32 medical) |
| 72 (55 to 92) | 41 (41 to 84) | Crosignani 1997 (n = 31 surgical, 31 medical) | ||
| General health | 1 year | 47 (19.5 to 74.5) | 52 (25.5 to 78.5) | Soysal 2002 (n = 33 surgical, 32 medical) |
| 72.5 (64.5 to 77) | 65 (51 to 79.5) | Crosignani 1997 (n = 31 surgical, 31 medical) |
IQR: interquartile range LNG‐IUS: levonorgestrel‐releasing intrauterine device
At two years Talis 2006 again found no difference between the groups (Analysis 2.12).
2.12. Analysis.
Comparison 2 Surgery versus LNG‐IUS, Outcome 12 SF 36 score at 2 years: surgery versus LNG‐IUS.
Over five‐year follow‐up de Souza 2010 found no evidence of a change from baseline in either group nor any evidence of a difference between the groups in quality of life, measured with the Psychological General Wellbeing Index (Analysis 2.10).
2.10. Analysis.
Comparison 2 Surgery versus LNG‐IUS, Outcome 10 Final PGWBI score: thermal balloon ablation versus LNG‐IUS.
2.5 Requirement for additional surgical or medical treatment for heavy menstrual bleeding
Requirement for additional surgical treatment
At one year women having any type of surgical treatment were less likely than women who had a LNG‐IUS to require surgery for heavy menstrual bleeding in addition to their allocated treatment (RR 0.23, 95% CI 0.12 to 0.44, six studies, 540 women, I2 = 52%) (Analysis 2.13). None of the hysterectomy group required additional surgery while about 2% of women who had balloon ablation and 4% of women who had endometrial resection required extra surgery during the first year. Of women who had a LNG‐IUS, about 12% required surgery during the first year.
2.13. Analysis.
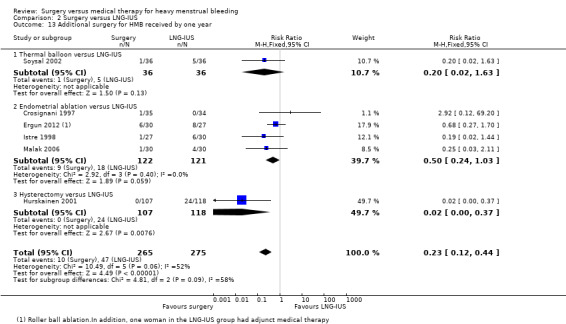
Comparison 2 Surgery versus LNG‐IUS, Outcome 13 Additional surgery for HMB received by one year.
At two years, data were available from two studies comparing conservative surgery versus LNG‐IUS. There was no evidence of a difference between the groups, though numbers were small (Shaw 2007; Talis 2006) (Analysis 2.14).
2.14. Analysis.
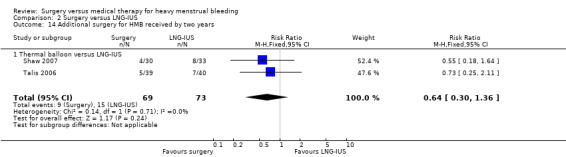
Comparison 2 Surgery versus LNG‐IUS, Outcome 14 Additional surgery for HMB received by two years.
At five years, Hurskainen 2001 reported that 50 of the 60 women who no longer had a LNG‐IUS in situ had had a hysterectomy (representing 42% of those randomised to LNG‐IUS) and one had had thermo‐ablation. By 10 years, 55 women in the LNG‐IUS group (46% of those randomised to LNG‐IUS) had had a hysterectomy.
Ghazizdeh 2014 noted that three women in the hysteroscopic endometrial resection group eventually underwent hysterectomy for heavy bleeding. There was apparently no requirement for additional surgery in the other two groups in this study.
Requirement for additional medical treatment
Very few women were reported as undergoing additional medical treatment. At one year in one study one woman in the surgical group (< 3%) who had persistent menorrhagia was receiving medical treatment (Crosignani 1997). At two years five women in the surgical group in Talis 2006 had had a LNG‐IUS inserted.
Two studies mentioned re‐insertion of the LNG‐IUS; this was required by five women in Hurskainen 2001 within the first year and one woman in Istre 1998 within three years. Of 57 women in Hurskainen 2001 who had the LNG‐IUS in situ at five years, eight had had a replacement.
2.6 Cost and resource use
Hurskainen 2001 compared the cost of hysterectomy and LNG‐IUS. Although 42% of the LNG‐IUS group subsequently underwent hysterectomy, the overall direct and indirect costs after five years were about 40% lower in the LNG‐IUS group. Sensitivity analyses using different cost estimates had little impact on these findings. At 10‐year follow‐up, the overall costs were lower in the LNG‐IUS group (USD 3423 per participant versus USD 4937), even though 46% of the LNG‐IUS group subsequently had surgery.
Talis 2006 reported the cost‐effectiveness of thermal balloon ablation versus LNG‐IUS. The expected direct and indirect costs of treatment were USD 1693 for ablation and USD 869 for the LNG‐IUS. The findings were robust to changes in key drivers of costs. Since the treatments appeared to result in similar quality of life outcomes (measured by the SF‐36) the authors concluded that the LNG‐IUS was more cost‐effective than thermal balloon ablation.
Heterogeneity
Heterogeneity was low or absent for most analyses, though there was moderate heterogeneity for comparisons between LNG‐IUS and conservative surgery for some SF‐36 measures and for two‐year satisfaction rates.
Sensitivity analyses and assessment of publication bias
Use of an alternative statistical model negated the statistical significance of one analysis (Analysis 2.5). Otherwise, sensitivity analyses for the primary outcomes did not substantially influence any of our findings. We could not construct a funnel plot to test for publication bias because there were insufficient studies for any one comparison.
Discussion
Summary of main results
A comparison of surgical versus medical treatments is complicated by the variety of treatments available within each modality, particularly with respect to medical approaches. The medical treatment in two of the studies comprised a wide range of oral hormonal and non‐hormonal medications. The medical treatment in the other studies was very different, consisting of an intrauterine device. As a result, the focus of this systematic review effectively split into two main comparisons: surgery versus oral medication and surgery versus levonorgestrel‐intrauterine device (LNG‐IUS).
Women in the surgical arms of these studies underwent a variety of operations that included the relatively simple procedure of endometrial ablation, the more specialised technique of endometrial resection and the radical option of hysterectomy. Despite this, the studies comparing different types of surgery versus LNG‐IUS reported fairly consistent findings, save for the inevitable disparity in outcomes measuring blood loss when hysterectomy comprised one of the comparisons.
Menstrual bleeding
Neither medical treatment nor conservative surgery can rival hysterectomy in achieving 100% cessation of bleeding. No other intervention considered in this review was effective for all women, but some were more effective than others. Endometrial resection was much more successful than oral medication in controlling bleeding at four months' follow‐up in Cooper 1997. As most of the women in this study (78%) had tried medical therapy unsuccessfully in the past, it is questionable whether the poor results achieved with oral medication are generalisable to women with heavy menstrual bleeding who newly present for treatment. Conservative surgery was more effective than LNG‐IUS in controlling bleeding at one year, but both approaches had high rates of success in all the relevant studies.
Satisfaction
Definition of treatment success is necessarily subjective and perhaps best based on a woman's satisfaction level and willingness to continue a particular therapy. Women who had transcervical endometrial resection or hysterectomy rated their satisfaction with treatment and their overall quality of life higher than women who had oral medication. However, when conservative surgery (endometrial ablation or resection) was compared with LNG‐IUS, rates of satisfaction were high in both groups and did not differ substantially. Similarly, studies that used multidimensional scales to measure quality of life found few differences between surgery and LNG‐IUS.
A very high proportion of women randomised to receive medical treatment subsequently required surgery. In Kupperman 2004, 53% of women in the medical group had had a hysterectomy by two years and in Cooper 1997 77% of women initially assigned oral medication had had surgery within five years. Seventeen per cent (47/275) of women in the LNG‐IUS arms of the six relevant studies had had surgery by one year.
Adverse effects
Adverse effects such as nausea, headaches and weight gain were reported by about half of the women who took oral medication and "bad side effects" were cited by 45% of the 60 women who found the treatment unacceptable at four months (Cooper 1997). Women with a LNG‐IUS also commonly reported adverse effects, often hormonal symptoms such as breast tenderness, bloating and intermenstrual bleeding. The LNG‐IUS group was also more likely to develop transient asymptomatic ovarian cysts.
Women who had endometrial resection or balloon ablation were overall less likely to experience adverse effects than women having medical treatment.
The adverse effects of hysterectomy were potentially life‐threatening. They included bladder and bowel perforations, infected pelvic haematomas and peritonitis. By treatment received, there were 39 complications among 131 women who had a hysterectomy. A substudy of Hurskainen 2001 evaluated the effect of hysterectomy or LNG‐IUS on ovarian function and concluded that hysterectomy but not LNG‐IUS appeared to alter intra‐ovarian blood flow and possibly impair ovarian function (Halmesmaki 2007). Moreover at 10‐year follow‐up, in an "as treated" analysis, stress urinary incontinence and urinary tract infections were more common in women who received a hysterectomy than in ongoing LNG‐IUS users and women who had hysterectomy had higher levels of markers for cardiovascular risk. The finding with respect to increased risk of urinary tract infections in the hysterectomy group persisted when the analysis was controlled for the occurrence of lower urinary tract symptoms at baseline (Heliovaara‐Peippo 2010).
Treatment choice
All the interventions considered in this review significantly reduced menstrual bleeding and improved health‐related quality of life from baseline and could be considered for treating menorrhagia. Oral medication may be effective and acceptable for some women, and worth trying before resorting to more invasive procedures, but probably will not suit the majority of women for long‐term use. The LNG‐IUS presents a suitable alternative to surgery for many women and although it reduces bleeding significantly less than surgical methods during the first year of use it has the advantage of being easily reversible. Conservative surgery and the LNG‐IUS both provide satisfactory treatment for the majority of women who perceive their menstrual blood loss as heavy, and neither precludes further intervention if needed. Our findings suggest that an individual woman's preferences and circumstances are the most important factor in treatment choice. Hysterectomy is the only treatment guaranteed to stop all menstrual bleeding but in view of its potential complications most women may be well advised to try a less radical treatment as first‐line therapy.
Hurskainen 2001 explored their data using a multiple regression model in order to identify predictors of outcome at one year. Women without objective menorrhagia (that is at least 80 ml blood loss per menstrual period) had a significantly better outcome with the LNG‐IUS than with hysterectomy in scores for health‐related quality of life and in partner sexual satisfaction. Neither age nor the presence of fibroids were predictive of outcome. This suggests that pretreatment assessment of menstrual blood loss may be useful for determining which women will require hysterectomy. This approach is complicated by the difficulty in achieving an objective assessment of menstrual blood loss.
Overall completeness and applicability of evidence
In the included studies the diagnosis of heavy menstrual bleeding was based on self report, with or without a bleeding score chart, and it is possible that many of the participants did not have objective menorrhagia (blood loss > 80 ml/cycle). There was notable disparity in the criteria used for menorrhagia even between studies that used the same measure; the three studies using pictorial blood loss assessment chart (PBAC) scores to determine eligibility for inclusion had thresholds ranging from 75 ml to 150 ml.
The benefits of oral medication as a first‐line treatment were not accurately reflected in these studies as the vast majority of women had already tried at least one oral medication without success.
More evidence comparing second‐generation surgical methods with intrauterine devices is required, and a multicentre randomised controlled trial (RCT) is currently underway in the Netherlands, comparing the cost‐effectiveness of endometrial ablation versus LNG‐IUS in women with menorrhagia (Herman 2013).
Quality of the evidence
None of the studies were blinded, and blinding would scarcely be feasible for these interventions. We judged that this created a high risk of bias for subjective review outcomes. Another common limitation in the studies was high risk of attrition bias, as six of the studies were missing data for 18% to 30% of randomised participants at follow‐up points ranging from one to five years.
The overall quality of the evidence for different comparisons ranged from very low to moderate. The main limitations were lack of blinding, attrition and imprecision. Moreover it was difficult to interpret study findings over follow‐up due to confounding associated with women who had been randomised to medical treatment subsequently undergoing surgery.
Potential biases in the review process
We were unaware of any potential biases in the review process.
Agreements and disagreements with other studies or reviews
Our findings were largely consistent with a health technology assessment of hysterectomy, endometrial ablation and LNG‐IUS for heavy menstrual bleeding (Bhattacharya 2011), which included comparisons of surgery versus LNG‐IUS, using individual participant data for a total of 730 women. They concluded that LNG‐IUS is potentially cheaper and more effective than first‐generation endometrial ablation techniques and that LNG‐IUS is associated with similar satisfaction rates to second‐generation endometrial ablation. They suggested that there is weak evidence to suggest that hysterectomy is preferable to LNG‐IUS in terms of satisfaction rates, and that hysterectomy is considered the most cost‐effective strategy. They concluded that owing to its invasive nature and risk of complications hysterectomy is considered a final option by gynaecological experts and consumers.
NICE guidelines review medical and surgical options for treatment of heavy menstrual bleeding and are broadly in agreement with our findings (NICE 2007). The guidelines support the use of LNG‐IUS as first‐line medical treatment, provided long‐term (at least 12 months) use is anticipated and that hormonal treatment is acceptable. With regard to surgery, they support the use of endometrial ablation as first or second‐line therapy where bleeding is having a severe impact on a woman's quality of life, and she does not want to conceive in the future. They recommend consideration the following second‐generation techniques: impedance‐controlled bipolar radiofrequency ablation (described in this review as bipolar electrocauterisation), fluid‐filled thermal balloon endometrial ablation, microwave endometrial ablation and free fluid thermal endometrial ablation. They suggest that first‐generation ablation techniques are appropriate provided hysteroscopic myomectomy is to be included in the procedure. NICE recommend against using hysterectomy as a routine first‐line treatment solely for heavy menstrual bleeding.
Authors' conclusions
Implications for practice.
Surgery, especially hysterectomy, reduces menstrual bleeding more than medical treatment at one year. There is no conclusive evidence of a difference in satisfaction rates between surgery and a levonorgestrel‐intrauterine device (LNG‐IUS), though adverse effects such as bleeding and spotting are more likely to occur with LNG‐IUS. Oral medication suits a minority of women in the long term, and a LNG‐IUS device provides a better alternative to surgery in most cases. A high proportion of women initially treated with oral medication, conservative surgery or LNG‐IUS require further intervention. Although hysterectomy is a definitive treatment for heavy menstrual bleeding, it can cause serious complications and most women may be well advised to try a less radical treatment as first‐line therapy. Both LNG‐IUS and conservative surgery appear to be safe, acceptable and effective.
Implications for research.
It is claimed that second‐generation techniques for endometrial ablation are safer and easier to perform than hysteroscopic methods (Lethaby 2013a), and further evidence comparing second‐generation surgical methods with intrauterine devices is required. A multicentre randomised controlled trial (RCT) is currently underway in the Netherlands, comparing the cost‐effectiveness of endometrial ablation versus LNG‐IUS in women with menorrhagia. We await the results with interest.
What's new
| Date | Event | Description |
|---|---|---|
| 28 January 2016 | New search has been performed | Updated with three new RCTs (Ergun 2012; Ghazizdeh 2014; Sesti 2012). One ongoing RCT identified (Herman 2013). 'Summary of findings' table added. Number of outcomes reduced. |
| 28 January 2016 | New citation required but conclusions have not changed | The conclusions of this review have not changed with the addition of 3 new studies. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 25 April 2010 | New search has been performed | Added four new RCTs (de Souza 2010; Malak 2006; Shaw 2007; Tam 2006); added new data for other RCTs (Hurskainen 2001; Talis 2006); changed from ORs to RRs to make results easier to interpret; added NNTs; updated background and discussion sections. |
| 6 November 2008 | Amended | Converted to new review format. |
| 9 December 2005 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
Thanks are expressed to the study investigators who kindly responded to our requests for additional information; also to members of the Editorial Office of the Cochrane Gynaecology and Fertility Group who assisted at all stages of the review.
Appendices
Appendix 1. CGF Specialised Register search strategy
14 January 2016
Keywords CONTAINS "menorrhagia" or "Menorrhagia‐Symptoms" or "heavy bleeding" or "heavy menstrual bleeding" or "heavy menstrual loss" or "dysfunctional uterine bleeding" or "dysfunctional uterine bleeding" or "dysfunctional uterine bleeding" or "excessive menstrual bleeding" or "excessive menstrual loss" or "iron deficiency anemia" or "abnormal bleeding" or "abnormal uterine bleeding" or "abnormal vaginal bleeding" AND Keywords CONTAINS "surgery‐gynaecological" or "Hysterectomy" or "Hysterectomy,abdominal" or "hysterectomy ‐laparoscopic" or "hysterectomy, laparoscopically assisted vaginal" or "Hysterectomy rate" or "Hysterectomy, subtotal" or "hysterectomy techniques" or "Hysterectomy, Vaginal" or "total abdominal hysterectomy" or "total abdominal hysterectomy" or "LAVH" or "TAH" or "transcervical endometrial resection" or "transcervical hysteroscopic endometrial coagulation" or "transcervical hysteroresection" or "transcervical resection" or "Laser Ablation" or "hysteroscopic endometrial resection" or "hysteroscopy" or "hysteroscopic " or "*Electrosurgery‐Methods" or "electrosurgery" or "rollerball" or "rollerball electroablation" or "thermal balloon" or "photodynamic therapy" or "microwave endometrial ablation" or "endometrial ablation" or "endometrial cryoblation" or "endometrial resection" or "endometrial resection, transcervical" or "NovaSure" AND Keywords CONTAINS "medical management" or "medical therapy" or "Prostaglandins" or "mefenamic acid" or "NSAID" or "NSAIDs" or "non steroidal" or "Flurbiprofen" or "Meclofenamic Acid" or "Ibuprofen" or "naproxen" or "Naproxen Sodium" or "diclofenac" or "GnRH a" or "GnRH analogue" or "GnRH analogue" or "GnRH analogues" or "GnRHa" or "Gonadorelin" or "gonadotrophin" or "Gonadotrophin releasing hormones" or "tranexamic acid" or "progestin" or "progestogen" or "progestogens" or "progestins" or "Norethisterone" or "Medroxyprogesterone Acetate" or "oral contraceptive" or "danazol" or "Levonorgestrel" or "LNG‐IUS" or "Mirena" or "antifibrinolytics"
Appendix 2. CENTRAL search strategy
5 March 2015. Updated 14 January 2016 with retrieval of no additional records.
1 exp Menorrhagia/ (244) 2 menorrhagia.tw. (328) 3 hypermenorrhoea.tw. (1) 4 excessive menstrua$.tw. (17) 5 dysfunctional uterine bleeding.tw. (113) 6 heavy menstrua$.tw. (93) 7 abnormal uterine bleeding.tw. (96) 8 abnormal menstrua$ bleeding.tw. (1) 9 iron deficient anaemia.tw. (2) 10 or/1‐9 (635) 11 exp endometrial ablation techniques/ or exp hysterectomy/ or exp hysterectomy, vaginal/ or exp hysteroscopy/ (1722) 12 hysterectom$.tw. (2552) 13 endometrial ablation.tw. (177) 14 hysteroscop$.tw. (545) 15 (TAH or LAVH or TCRE).tw. (136) 16 transcervical resection$.tw. (40) 17 laser ablation.tw. (257) 18 exp Electrosurgery/ (178) 19 electrosurgery.tw. (67) 20 (rollerball or thermal balloon).tw. (73) 21 (hyperthermia or thermotherap$).tw. (710) 22 photodynamic therap$.tw. (747) 23 exp Cryosurgery/ (247) 24 (cryoablation or microwave or laser$ or Cryosurger$).tw. (8732) 25 surgical treatment$.tw. (2854) 26 endometrial resection.tw. (72) 27 Balloon.tw. (2795) 28 (catheter ablation or radiofrequency or saline irrigation).tw. (1469) 29 exp Catheter Ablation/ (847) 30 ablation.tw. (2506) 31 or/11‐30 (21035) 32 (medical therap$ or medical treatment$).tw. (3808) 33 prostaglandin synthetase inhibitor$.mp. or PGSI$.tw. [mp=title, original title, abstract, MeSH headings, heading words, keyword] (75) 34 exp Mefenamic Acid/ (109) 35 Mefenamic Acid.tw. (192) 36 exp Anti‐Inflammatory Agents, Non‐Steroidal/ (13420) 37 Nonsteroidal anti inflammator$.tw. (1408) 38 NSAID$.tw. (2372) 39 Non steroidal anti inflammator$.tw. (1332) 40 flurbiprofen.tw. (555) 41 FLURBIPROFEN/ (337) 42 meclofenamic acid.tw. (9) 43 Meclofenamic Acid/ (47) 44 ibuprofen.tw. or IBUPROFEN/ (2128) 45 naproxen.tw. or NAPROXEN/ (1402) 46 diclofenac.tw. or DICLOFENAC/ (2725) 47 gonadotrophin‐releasing hormone analogue$.tw. (53) 48 tranexamic acid.tw. or Tranexamic Acid/ (723) 49 progestogen.tw. or Progestins/ (786) 50 norethisterone.tw. or Norethindrone/ (879) 51 medroxyprogesterone acetate.tw. or Medroxyprogesterone 17‐Acetate/ (1361) 52 Contraceptives, Oral, Combined/ (594) 53 danazol.tw. or DANAZOL/ (308) 54 Levonorgestrel.tw. or Levonorgestrel/ (906) 55 LNG‐IUS.tw. (86) 56 mirena.tw. (42) 57 antifibrinolytic.tw. or Antifibrinolytic Agents/ (549) 58 Combined oral contraceptive$.tw. (337) 59 or/32‐58 (26350) 60 59 and 10 and 31 (111)
Appendix 3. MEDLINE search strategy
5 March 2015, updated 14 January 2016 with retrieval of 8 more records.
1 exp Menorrhagia/ (3581) 2 menorrhagia.tw. (2686) 3 hypermenorrhoea.tw. (31) 4 excessive menstrua$.tw. (163) 5 dysfunctional uterine bleeding.tw. (779) 6 heavy menstrua$.tw. (512) 7 abnormal uterine bleeding.tw. (1382) 8 abnormal menstrua$ bleeding.tw. (36) 9 iron deficient anaemia.tw. (42) 10 or/1‐9 (6776) 11 exp endometrial ablation techniques/ or exp hysterectomy/ or exp hysterectomy, vaginal/ or exp hysteroscopy/ (28277) 12 hysterectom$.tw. (27351) 13 endometrial ablation.tw. (976) 14 hysteroscop$.tw. (4939) 15 (TAH or LAVH or TCRE).tw. (1213) 16 transcervical resection$.tw. (196) 17 laser ablation.tw. (4581) 18 exp Electrosurgery/ (3806) 19 electrosurgery.tw. (1177) 20 (rollerball or thermal balloon).tw. (305) 21 (hyperthermia or thermotherap$).tw. (22084) 22 photodynamic therap$.tw. (12018) 23 exp Cryosurgery/ (11040) 24 (cryoablation or microwave or laser$ or Cryosurger$).tw. (215571) 25 surgical treatment$.tw. (118658) 26 endometrial resection.tw. (270) 27 Balloon.tw. (48789) 28 (catheter ablation or radiofrequency or saline irrigation).tw. (26425) 29 exp Catheter Ablation/ (22294) 30 ablation.tw. (59556) 31 or/11‐30 (515895) 32 (medical therap$ or medical treatment$).tw. (57246) 33 prostaglandin synthetase inhibitor$.mp. or PGSI$.tw. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] (667) 34 exp Mefenamic Acid/ (942) 35 Mefenamic Acid.tw. (1055) 36 exp Anti‐Inflammatory Agents, Non‐Steroidal/ (159226) 37 Nonsteroidal anti inflammator$.tw. (12422) 38 NSAID$.tw. (18605) 39 Non steroidal anti inflammator$.tw. (11856) 40 flurbiprofen.tw. (1982) 41 FLURBIPROFEN/ (1640) 42 meclofenamic acid.tw. (284) 43 Meclofenamic Acid/ (934) 44 ibuprofen.tw. or IBUPROFEN/ (10885) 45 naproxen.tw. or NAPROXEN/ (5489) 46 diclofenac.tw. or DICLOFENAC/ (9539) 47 gonadotrophin‐releasing hormone analogue$.tw. (239) 48 tranexamic acid.tw. or Tranexamic Acid/ (2823) 49 progestogen.tw. or Progestins/ (11300) 50 norethisterone.tw. or Norethindrone/ (4586) 51 medroxyprogesterone acetate.tw. or Medroxyprogesterone 17‐Acetate/ (6294) 52 Contraceptives, Oral, Combined/ (4230) 53 danazol.tw. or DANAZOL/ (2820) 54 Levonorgestrel.tw. or Levonorgestrel/ (4851) 55 LNG‐IUS.tw. (471) 56 mirena.tw. (229) 57 antifibrinolytic.tw. or Antifibrinolytic Agents/ (5211) 58 Combined oral contraceptive$.tw. (1951) 59 or/32‐58 (266223) 60 59 and 10 and 31 (534) 61 randomized controlled trial.pt. (385614) 62 controlled clinical trial.pt. (88649) 63 randomized.ab. (310509) 64 placebo.tw. (162856) 65 clinical trials as topic.sh. (170973) 66 randomly.ab. (224859) 67 trial.ti. (133531) 68 (crossover or cross‐over or cross over).tw. (62761) 69 or/61‐68 (959243) 70 (animals not (humans and animals)).sh. (3896812) 71 69 not 70 (883409) 72 60 and 71 (113)
Appendix 4. EMBASE search strategy
5 March 2015, updated 14 January 2016 with retrieval of 19 more records.
1 exp Menorrhagia/ (7133) 2 menorrhagia.tw. (3897) 3 hypermenorrhoea.tw. (46) 4 excessive menstrua$.tw. (184) 5 dysfunctional uterine bleeding.tw. (993) 6 heavy menstrua$.tw. (804) 7 abnormal uterine bleeding.tw. (1994) 8 abnormal menstrual bleeding.tw. (47) 9 iron deficient anaemia.tw. (63) 10 or/1‐9 (10609) 11 exp hysterectomy/ or exp abdominal hysterectomy/ or exp hysterotomy/ or exp vaginal hysterectomy/ (49293) 12 exp endometrial ablation/ (1951) 13 exp hysteroscopy/ (8105) 14 hysterectom$.tw. (37166) 15 endometrial ablation.tw. (1521) 16 hysteroscop$.tw. (7921) 17 (TAH or LAVH or TCRE).tw. (1951) 18 transcervical resection$.tw. (287) 19 laser ablation.tw. (5009) 20 exp Electrosurgery/ (14369) 21 electrosurgery.tw. (1354) 22 (rollerball or thermal balloon).tw. (409) 23 (hyperthermia or thermotherap$).tw. (24955) 24 photodynamic therap$.tw. (14618) 25 exp Cryosurgery/ (8891) 26 (cryoablation or microwave or laser$ or Cryosurger$).tw. (217556) 27 surgical treatment$.tw. (137177) 28 endometrial resection.tw. (386) 29 Balloon.tw. (67239) 30 (catheter ablation or radiofrequency or saline irrigation).tw. (38251) 31 exp Catheter Ablation/ (22696) 32 ablation.tw. (81982) 33 or/11‐32 (605620) 34 (medical therap$ or medical treatment$).tw. (77649) 35 prostaglandin synthetase inhibitor$.mp. or PGSI$.tw. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (654) 36 exp Mefenamic Acid/ (4526) 37 Mefenamic Acid.tw. (1228) 38 exp nonsteroid antiinflammatory agent/ (436223) 39 Nonsteroidal anti inflammator$.tw. (14339) 40 NSAID$.tw. (29279) 41 Non steroidal anti inflammator$.tw. (15438) 42 flurbiprofen.tw. (2382) 43 FLURBIPROFEN/ (6485) 44 meclofenamic acid.tw. (295) 45 Meclofenamic Acid/ (2306) 46 ibuprofen.tw. or IBUPROFEN/ (38384) 47 naproxen.tw. or NAPROXEN/ (21900) 48 diclofenac.tw. or DICLOFENAC/ (31294) 49 gonadotrophin‐releasing hormone analogue$.tw. (273) 50 tranexamic acid.tw. or Tranexamic Acid/ (7462) 51 exp gestagen/ or progestogen.tw. (138408) 52 norethisterone.tw. or Norethindrone/ (7321) 53 medroxyprogesterone acetate.tw. or Medroxyprogesterone 17‐Acetate/ (15087) 54 exp oral contraceptive agent/ (52608) 55 danazol.tw. or DANAZOL/ (7558) 56 Levonorgestrel.tw. or Levonorgestrel/ (10234) 57 LNG‐IUS.tw. (708) 58 mirena.tw. (1202) 59 exp antifibrinolytic agent/ or antifibrinolytic.tw. (23726) 60 Combined oral contraceptive$.tw. (2110) 61 or/34‐60 (694326) 62 Clinical Trial/ (840570) 63 Randomized Controlled Trial/ (361087) 64 exp randomization/ (65027) 65 Single Blind Procedure/ (19568) 66 Double Blind Procedure/ (118089) 67 Crossover Procedure/ (41657) 68 Placebo/ (252907) 69 Randomi?ed controlled trial$.tw. (110098) 70 Rct.tw. (16005) 71 random allocation.tw. (1370) 72 randomly allocated.tw. (21607) 73 allocated randomly.tw. (1990) 74 (allocated adj2 random).tw. (720) 75 Single blind$.tw. (15294) 76 Double blind$.tw. (147582) 77 ((treble or triple) adj blind$).tw. (424) 78 placebo$.tw. (209034) 79 prospective study/ (277002) 80 or/62‐79 (1426279) 81 case study/ (30453) 82 case report.tw. (274917) 83 abstract report/ or letter/ (915864) 84 or/81‐83 (1215116) 85 80 not 84 (1387551) 86 10 and 33 and 61 and 85 (376)
Appendix 5. PsycINFO search strategy
5 March 2015 Updated 14 January 2016 with retrieval of no additional records. 1 exp menstrual disorders/ (1056) 2 menorrhagia.tw. (71) 3 hypermenorrhoea.tw. (1) 4 excessive menstrua$.tw. (5) 5 dysfunctional uterine bleeding.tw. (19) 6 heavy menstrua$.tw. (15) 7 abnormal uterine bleeding.tw. (11) 8 abnormal menstrual bleeding.tw. (1) 9 iron deficient anaemia.tw. (1) 10 or/1‐9 (1126) 11 exp hysterectomy/ or exp surgery/ (43862) 12 hysterectom$.tw. (697) 13 endometrial ablation.tw. (4) 14 hysteroscop$.tw. (12) 15 (TAH or LAVH or TCRE).tw. (18) 16 transcervical resection$.tw. (0) 17 laser ablation.tw. (50) 18 Electrosurg$.tw. (9) 19 (hyperthermia or thermotherap$).tw. (1130) 20 photodynamic therap$.tw. (17) 21 (cryoablation or microwave or laser$ or Cryosurg$).tw. (2580) 22 surgical treatment$.tw. (1626) 23 endometrial resection.tw. (4) 24 Balloon.tw. (644) 25 (catheter ablation or radiofrequency or saline irrigation).tw. (344) 26 ablation.tw. (3273) 27 or/11‐26 (52331) 28 exp "Medical Treatment (General)"/ (6661) 29 (medical therap$ or medical treatment$).tw. (6913) 30 (prostaglandin synthetase inhibitor$ or PGSI$).tw. (55) 31 Mefenamic Acid.tw. (13) 32 exp Anti Inflammatory Drugs/ (4482) 33 Nonsteroidal anti inflammator$.tw. (538) 34 NSAID$.tw. (680) 35 Non steroidal anti inflammator$.tw. (396) 36 flurbiprofen.tw. (42) 37 meclofenamic acid.tw. (9) 38 ibuprofen.tw. (347) 39 naproxen.tw. (148) 40 diclofenac.tw. (178) 41 gonadotrophin‐releasing hormone analogue$.tw. (4) 42 tranexamic acid.tw. (7) 43 progestogen.tw. (100) 44 (Progestin$ or progestagen$).tw. (564) 45 (norethisterone or Norethindrone).tw. (38) 46 (medroxyprogesterone acetate or Medroxyprogesterone 17‐Acetate).tw. (219) 47 exp Oral Contraceptives/ (782) 48 danazol.tw. (14) 49 Levonorgestrel.tw. (64) 50 LNG‐IUS.tw. (15) 51 mirena.tw. (9) 52 antifibrinolytic.tw. (4) 53 Combined oral contraceptive$.tw. (87) 54 or/28‐53 (19667) 55 10 and 27 and 54 (9)
Appendix 6. CINAHL
| # | Query (5 March 2015) (updated 14 January 2016 with retrieval of 11 more records) | Results |
| S47 | S34 AND S46 | 154 |
| S46 | S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 | 943,091 |
| S45 | TX allocat* random* | 4,203 |
| S44 | (MH "Quantitative Studies") | 13,053 |
| S43 | (MH "Placebos") | 9,088 |
| S42 | TX placebo* | 33,261 |
| S41 | TX random* allocat* | 4,203 |
| S40 | (MH "Random Assignment") | 38,671 |
| S39 | TX randomi* control* trial* | 83,653 |
| S38 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 754,896 |
| S37 | TX clinic* n1 trial* | 169,561 |
| S36 | PT Clinical trial | 77,361 |
| S35 | (MH "Clinical Trials+") | 183,965 |
| S34 | S9 AND S33 | 540 |
| S33 | S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 | 59,166 |
| S32 | TX Balloon | 7,257 |
| S31 | (MM "Catheter Ablation") | 7,213 |
| S30 | TX(catheter ablation or radiofrequency or saline irrigation) | 11,435 |
| S29 | TX endometrial resection | 59 |
| S28 | TX surgical therap* | 3,940 |
| S27 | TX surgical treatment* | 11,174 |
| S26 | (MM "Surgery, Gynecologic+") | 5,314 |
| S25 | TX (cryoablation or microwave or laser) | 15,411 |
| S24 | TX Cryosurgery | 1,142 |
| S23 | TX photodynamic therap* | 1,252 |
| S22 | TX(hyperthermia or thermotherap*) | 2,130 |
| S21 | TX (rollerball or thermal balloon) | 63 |
| S20 | TX Electrosurg* | 911 |
| S19 | (MM "Electrosurgery") | 490 |
| S18 | TX laser ablation | 479 |
| S17 | TX transcervical resection | 36 |
| S16 | TX (TAH or LAVH or TCRE) | 105 |
| S15 | TX hysteroscop* | 863 |
| S14 | TX hysterectom* | 5,243 |
| S13 | (MM "Hysteroscopy") | 428 |
| S12 | (MM "Hysterectomy") OR (MM "Hysterectomy, Vaginal") | 2,361 |
| S11 | TX endometri* ablation | 322 |
| S10 | (MM "Endometrial Ablation Techniques") | 94 |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 | 1,243 |
| S8 | TX abnormal menstrua* bleeding | 16 |
| S7 | TX abnormal uterine bleeding | 235 |
| S6 | TX hypermenorrhea | 10 |
| S5 | TX hypermenorrhoea | 1 |
| S4 | TX dysfunctional uterine bleeding | 106 |
| S3 | TX heavy menstrua* | 200 |
| S2 | TX Menorrhagia | 915 |
| S1 | (MM "Menorrhagia") | 515 |
Data and analyses
Comparison 1. Surgery versus oral medication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Control of bleeding (cure or improvement to acceptable level) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At four months | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.94, 3.64] |
| 1.2 At two years | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.06, 1.57] |
| 1.3 At five years | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.34] |
| 2 Bleeding score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At four months | 1 | 186 | Mean Difference (IV, Fixed, 95% CI) | ‐12.70 [‐15.04, ‐10.36] |
| 2.2 At two years | 1 | 173 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐4.10, 1.30] |
| 3 Overall satisfaction with treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At four months | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.8 [1.96, 3.99] |
| 3.2 At two years | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.13, 1.74] |
| 3.3 At five years | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.94, 1.37] |
| 4 Proportion reporting adverse effects at four months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 Additional surgery for HMB received | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 By two years | 2 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.06, 0.22] |
| 5.2 By five years | 1 | 187 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.06, 0.22] |
Comparison 2. Surgery versus LNG‐IUS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Objective control of bleeding at one year: menstrual loss < 80 ml per cycle | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Hysterectomy versus LNG‐IUS | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Subjective control of bleeding at up to one year: PBAC =/< 75 per cycle with primary treatment | 5 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.07, 1.32] |
| 2.1 Thermal balloon ablation versus LNG‐IUS | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.09, 1.50] |
| 2.2 Endometrial resection versus LNG‐IUS | 3 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.00, 1.31] |
| 3 PBAC score at one year | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Thermal balloon ablation versus LNG‐IUS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Laparoscopic hysterectomy versus LNG‐IUS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PBAC score at two years | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐52.70 [‐76.50, ‐28.90] |
| 4.1 Hysterectomy versus LNG‐IUS | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐52.70 [‐76.50, ‐28.90] |
| 5 Satisfaction rate at one year: surgery versus LNG‐IUS | 6 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.04, 1.28] |
| 5.1 Thermal balloon endometrial ablation versus LNG‐IUS | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.93, 1.29] |
| 5.2 Rollerball endometrial ablation versus LNG‐IUS | 2 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.94, 1.32] |
| 5.3 Bipolar electrocautery or endometrial resection versus LNG‐IUS | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.07, 1.66] |
| 6 Satisfaction rate at two years: surgery versus LNG‐IUS | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Thermal balloon ablation versus LNG‐IUS | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.08] |
| 7 Proportion of women with adverse events at one year | 3 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.36, 0.74] |
| 7.1 Thermal balloon ablation versus LNG‐IUS | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.19, 0.74] |
| 7.2 Endometrial ablation versus LNG‐IUS | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.92] |
| 8 Adverse effects: bone mineral density decrease at five years (g/cm2) | 1 | 194 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.06, 0.41] |
| 8.1 Lumbar spine | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.15, 0.49] |
| 8.2 Femoral neck | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.18, 0.54] |
| 9 Change in EQ‐5D score: surgery (hysterectomy) versus LNG‐IUS | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 At one year | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.41, 0.41] |
| 9.2 At five years | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.09] |
| 9.3 At 10 years | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.06, 0.06] |
| 10 Final PGWBI score: thermal balloon ablation versus LNG‐IUS | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐10.30 [‐26.54, 5.94] |
| 10.1 At five years | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐10.30 [‐26.54, 5.94] |
| 11 SF36 score at one year: surgery versus LNG‐IUS | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 General health | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Physical function | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Mental health | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Vitality | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Physical role limitation | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 Emotional role limitation | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.7 Social function | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.8 Bodily pain | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 SF 36 score at 2 years: surgery versus LNG‐IUS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 General health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Additional surgery for HMB received by one year | 6 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.12, 0.44] |
| 13.1 Thermal balloon versus LNG‐IUS | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.63] |
| 13.2 Endometrial ablation versus LNG‐IUS | 4 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.24, 1.03] |
| 13.3 Hysterectomy versus LNG‐IUS | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.37] |
| 14 Additional surgery for HMB received by two years | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.30, 1.36] |
| 14.1 Thermal balloon versus LNG‐IUS | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.30, 1.36] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrington 2003.
| Methods | Number randomised: 50 Losses to follow‐up: 4 (2 in each group) No power calculation described Source of funding: not reported Years: not reported | |
| Participants | Inclusion criteria: women with menorrhagia refractory to medical therapy Exclusion criteria: uterine cavity over 12 cm long, malignant or premalignant pathology (pre‐treatment endometrial biopsy taken) |
|
| Interventions | Surgery: thermal balloon ablation Medical treatment: LNG‐IUS |
|
| Outcomes | PBAC score Amenorrhoea | |
| Notes | No measures of variance or tests of significance were reported for the change from baseline in each group nor for the difference in change between the groups: we have made attempts to contact the author for more data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | All women received their allocated treatment. 44/50 analysed for primary outcome at 6 months. Reasons for withdrawal/dropout given. |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects/tolerability not reported. Follow‐up only 6 months. |
| Other bias | High risk | PBAC scores significantly higher in the surgical group at baseline |
Cooper 1997.
| Methods | Randomised controlled trial 86 eligible women (of 273) refused randomisation, in most cases due to preference for a particular treatment. They were followed up separately. Number randomised: 187 Number analysed: 186 at 4 months, 173 at 2 years, 143 at 5 years Failures to receive allocated treatment: 10 women in the medical group stopped treatment before 4‐month follow‐up Losses to follow‐up: 1 woman from the medical group was lost to follow‐up by 4 months; 14 were lost to follow‐up at 2 years (7 from each group); 43 were lost to follow‐up at 5 years (20 from the surgical group, 23 from the medical group). At 5 years, operative details were available for 176/187 women randomised. Analysis by intention‐to‐treat for women who completed follow‐up at each stage, with secondary analysis according to the number of medical treatments used prior to gynaecological referral. Power calculation: sample of 180 gives 80% power to detect a 20% difference in satisfaction rate at a 5% level of significance. This was achieved at 4 months but not at 2 or 5 years. After 4‐month follow‐up all recruits could request further and/or different treatment Source of funding: Scottish Office Department of Health Years: 1994‐95 | |
| Participants | Included: 187 women referred to gynaecologists at Aberdeen Royal Infirmary, Scotland for treatment of clinically diagnosed dysfunctional uterine bleeding (i.e. uterus < 10 weeks' pregnancy size and normal endometrial pathology) Mean age 42 years Family complete Excluded: women referred specifically for surgery | |
| Interventions | Surgical arm: injection of gonadotrophin‐releasing hormone analogue followed 5 weeks later by transcervical resection of endometrium using rollerball coagulation to fundus and cornua plus loop resection of cavity walls. Medical arm: 3 cycles of medical treatment not previously used by patient, as selected by senior gynaecologist Actual treatment received: 33% (31 women) received progestogens (prescribed only to women with heavy and irregular periods; days 12 to 25, or 5 to 25 if there was also dysmenorrhoea) 26% (24 women) received combined pill (second‐generation with 30 μg of estradiol) 23% (22 women) received tranexamic acid (1 g 4 times daily for first 5 days of period in women with regular periods, plus mefenamic acid 500 mg 3 times a day if there was associated dysmenorrhoea) 16% (15 women) received danazol (200 mg daily for 90 days) 2% (2 women) received hormone replacement therapy with a non‐steroidal anti‐inflammatory drug All women could request further and/or different treatment at 4‐month follow‐up | |
| Outcomes | Primary outcome: treatment satisfaction (direct question) Other outcomes: subjective relief of menstrual symptoms, bleeding score (1 to 5), pain score (1 to 5), anxiety and depression score (HADS) Health‐related quality of life: SF‐36, premenstrual symptoms Treatment acceptability (direct question and semantic differential technique) Preferred subsequent treatment Additional treatment received Haemoglobin level | |
| Notes | 14 did not complete 2‐year questionnaire but data on their surgical status obtained from hospital database 43 did not complete 5‐year questionnaire, but data on the surgical status of 32 of these obtained from hospital database |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation by serially numbered, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | High risk | 143/187 analysed at 5 years. Reasons for withdrawal/dropout given in 11 cases. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
Crosignani 1997.
| Methods | Randomised controlled trial 27 (of 97) women refused randomisation: 14 requested endometrial ablation, 9 requested hysterectomy, 4 lost to further contact. Number randomised: 70 Failures to receive allocated treatment: nil Losses to follow‐up: 1 woman in the medical group was lost to follow‐up after 6/12 check and excluded from further analysis Analysis by intention‐to‐treat for women who completed follow‐up Power calculation assumed that at 1 year there would be a 30 ml difference in mean blood loss between the 2 arms: this did not occur so power calculation invalidated Source of funding: Italian National Research Council; LNG‐IUSs supplied by Leiras Oy Pharmaceuticals | |
| Participants | 70 premenopausal women referred to a gynaecology clinic in Milan for hysterectomy for menorrhagia (diagnosed by history, haemoglobin and iron levels and PBAC score ≥ 100 month for 2 months as per menstrual diary) Mean age: 44 Normal uterus on hysteroscopy Normal endometrial pathology on biopsy Uterus =/< volume of 8‐week pregnancy, calculated by ultrasound Family complete, not breastfeeding Excluded: women < 38 years Use of oestrogens or progestogens in previous 3/12 Use of GnRH antagonists in previous 6/12 Recent use of other hormonal agents or drugs that could affect menstrual blood loss Serious concomitant illness Myoma > 3 cm diameter | |
| Interventions | Surgical arm: endometrial resection during early proliferative phase of cycle, using rollerball electrode for cornua and fundus, and loop for rest of cavity. All surgery performed by the same surgeon, who specialised in operative hysteroscopy. Medical arm: intrauterine device releasing 20 μg of levonorgestrel daily inserted within 7 days of start of menstruation. | |
| Outcomes | Primary outcome: reduction in menstrual bleeding at 1 year, measured by PBAC subjective bleeding score Other outcomes: amenorrhoea/oligomenorrhoea rates Health‐related quality of life: SF‐36 Treatment satisfaction Additional treatment received Adverse effects Haemoglobin level | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation by sequentially numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | 69/70 analysed. Reason for withdrawal/dropout given. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | SF‐36 not completed at baseline ‐ unclear whether groups were equivalent |
de Souza 2010.
| Methods | Randomised controlled trial
Single centre 84 women screened: 24 excluded, 2 withdrew consent Number randomised: 58 Number analysed: 58 at 1 month, 55 for most outcomes at 6 and 12 months Failures to receive allocated treatment: nil Losses to follow‐up: 3 by 12 months Analysis by intention‐to‐treat for all women who completed follow‐up Power calculation: 58 patients would detect a difference of over 40% between proportions and a difference of more than 1 standard deviation between the means of the 2 groups, with 90% power and 95% CI Trial conducted: 2005‐2007 Source of funding: Bayer Schering Pharma Laboratory |
|
| Participants | Women aged at least 35 years referred to a university clinic for treatment of menorrhagia refractory to medication, 3‐month washout period, regular menstrual cycles, menstrual blood loss over 80 ml according to PBAC, negative pregnancy test, uterine volume under 200 ml (length × width × height × 0.45), completed family Exclusion criteria: intracavitary abnormalities, pelvic inflammatory disease, suspected endometrial pathology, abnormal endometrial histology, abnormal cervical cytology, previous endometrial resection and ablation, any other pathology for which hysterectomy would be more appropriate |
|
| Interventions | Surgical arm: thermal balloon ablation under general anaesthetic in operating room, during days 1 to 15 of menstrual cycle Medical arm: levonorgestrel‐releasing intrauterine device, inserted during days 1 to 15 of menstrual cycle | |
| Outcomes | Menstrual blood loss measured by PBAC and Hb Bleeding pattern (amenorrhoea, intermenstrual bleeding) Quality of life measured by Psychological General Wellbeing Index (PGWBI) Outcomes measured at 1, 6 and 12 months Treatment failure (increase in blood loss, no increase in Hb) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered, opaque, sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | 55/58 women included in 6‐month and 12‐month analyses; exclusions explained |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome not clearly defined, adverse effects not reported |
| Other bias | Low risk | No other potential bias identified |
Ergun 2012.
| Methods | Randomised controlled trial Number randomised: 58 Failures to receive allocated treatment Losses to follow‐up: 16 Analysis by ITT: no Power calculation: not reported Source of funding: not stated |
|
| Participants | Included: women with abnormal uterine bleeding not responding to medical treatment (progesterone, oral contraceptive, NSAIDs etc.) aged over 35, regular menstrual cycle, score of 100 on PBAC Mean age: Not reported Excluded: pregnancy, pelvic infection, uterine abnormality, suspicious endometrial pathology on TVUS |
|
| Interventions | Surgical arm: rollerball endometrial ablation (n = 31) Medical arm: LNG‐IUS (n = 27) Actual treatment received: all |
|
| Outcomes | Blood loss: PBAC (end scores), satisfaction on a 5‐point scale, need for additional treatment | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised ..." |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | High risk | 58 patients randomised (31 in rollerball group and 27 in LNG‐IUS group). 7/31 (22.6%) withdrew from the rollerball group and 9/27 (33%) withdrew from the LNG‐IUS group, so only 72% (42/58) were included in the analysis. These latter numbers were used in the analysis with no method described for analysing missing data. |
| Selective reporting (reporting bias) | Unclear risk | The authors noted that they investigated adverse effects but these were not adequately reported |
| Other bias | Unclear risk | Not possible to determine how similar the groups were at baseline; no measure of statistical variation (e.g. SD) reported |
Ghazizdeh 2014.
| Methods | Randomised controlled trial Number randomised: 110 patients Failures to receive allocated treatment unclear – numbers who were analysed were not reported Losses to follow‐up unclear (for satisfaction, it looks as though 3 were lost to follow‐up in the HER group) Analysis by ITT: no Power calculation: not reported Source of funding: not reported |
|
| Participants | Included: consecutive women with menorrhagia. Patients were candidates for hysterectomy. They had all been treated with hormonal therapy for at least 6 months and had shown no response to this therapy. Mean age: 40 (35 to 45) Excluded: Patients who were pregnant or who were null‐gravid or primiparous, and those who had had an abnormal Pap smear, genital infection, hormonal disorder, hormonal treatment, anomalous uterus, any intra‐cavity disorder, coagulative disorder, or an abnormal endometrial biopsy were excluded. With regard to myomas, they only excluded those submucosal myomas that were > 2 cm and intramural myomas that moved the endometrial layer. A uterine cavity > 11 cm was also classified as an exclusion criterion. |
|
| Interventions | Surgical arm 1: hysteroscopic endometrial resection. Endometrial resection was done by monopolar loop resection with a depth of 3 mm to 5 mm, and rollerball resection with superficial cauterisation was applied to the cornual region (n = 32) Surgical arm 2: bipolar electrocauterisation (NovaSure) endometrial ablation (n = 30) Medical arm: Mirena (n = 48) Actual treatment received: appears to be as above |
|
| Outcomes | Treatment success (according to decreased blood loss and less interaction between bleeding and normal activity) ‐ measure unclear, data not used in analysis Complications (data not used as totals unclear) Resurgery Satisfaction |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "divided randomly into 3 groups ..." Big difference in group sizes. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | Satisfaction rates reported for 107/110 women (97%) |
| Selective reporting (reporting bias) | Unclear risk | The authors noted that they investigated adverse effects but these were not adequately reported |
| Other bias | High risk | Study reports contradictory statements about menorrhagia: 1. "The rate of menorrhagia was very high in 89.6% of patients in the Mirena group, 53.3% in the NovaSure group and 67.7% in the HER group (p = 0.005)." [This appears to refer to baseline]. 2. "Before treatment, the rate of menorrhagia was high in 100% of patients in the Mirena group, 96.7% in the NovaSure group, and 93.5% in the hysteroscopic endometrial resection group (p = 0.225)." Also there are very large discrepancies between the groups for other baseline characteristics |
Hurskainen 2001.
| Methods | Randomised controlled trial 5 centres 598 women screened, 362 not randomised (184 not eligible, 178 not willing) Number randomised: 236 Number analysed: 232 Failures to receive allocated treatment: 12 women: 2 in the LNG‐IUS group (IUS could not be inserted); 8 in the hysterectomy group (including 2 who had LNG‐IUS and 5 who cancelled surgery due to reduced blood loss or due to job or family situation). In 2 cases, women had hysterectomy 12 months after randomisation. Losses to follow‐up: 4 at 5 years (2 in the LNG‐IUS group, 2 in the hysterectomy group (1 died, 1 withdrew)) Analysis by intention‐to‐treat for all women who completed follow‐up Power calculation: 80% power to detect a 7.5% difference between groups at a 5% level of significance Trial conducted 1994‐2002 Source of funding: Academy of Finland, National Research and Development Center for Welfare and Health (STAKES), University research grants. LNG‐IUSs provided by Leiras Oy Pharmaceuticals | |
| Participants | Women referred to 1 of 5 university hospitals in Finland for treatment of menorrhagia
Age 35 to 49, menstruating, premenopausal, family complete, suitable for either treatment Exclusion criteria: Submucous fibroids, endometrial polyps, ovarian tumours or cysts > 5 cm diameter, cervical disease, urinary and bowel symptoms or pain caused by large fibroids, lack of indication for hysterectomy, metrorrhagia bleeding between periods) as main complaint Previous unsuccessful treatment with LNG‐IUS History of cancer Severe depression Acne (Assessed by history and by cervical smear, endometrial biopsy, transvaginal ultrasound and hysteroscopy if necessary to exclude any of the above) Mean age 43 49% had fibroids 59% had ≥ 80 ml blood loss |
|
| Interventions | Surgical arm: hysterectomy: abdominal (20%), vaginal (28%) or laparoscopic (52%), done (or supervised) by an experienced gynaecologist, with a mean waiting time of 6.7 months (range 12 days to 21 months) Medical arm: levonorgestrel‐releasing intrauterine device inserted at randomisation | |
| Outcomes | Primary outcome:
Health‐related quality of life by EuroQol EQ‐5D questionnaire Other outcomes: Quality of life by RAND 36‐item health survey Objective bleeding (alkaline haematin method), amenorrhoea/oligomenorrhoea rates Health‐related quality of life (RAND 36‐item health survey and EuroQol EQ‐5D questionnaire) Menopausal symptoms (Kupperman test of menopausal distress) General health on VAS (0 to 100) Anxiety (Finnish version of Spielberger State‐Trait Anxiety Inventory) Sexual functioning (McCoy Sex Scale) Adverse effects Cost‐effectiveness Haemoglobin |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done separately for each centre (drawn from a hat), using randomly varying clusters |
| Allocation concealment (selection bias) | Low risk | Allocated by numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | 232/236 analysed at 5 years (98%). Reasons for withdrawal/dropout given in 2 cases. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
Istre 1998.
| Methods | Randomised controlled trial Number randomised: 60 Number analysed: 52 at 1 year, 44 at 2 years, 40 at 3 years Failures to receive allocated treatment: 1 in surgical arm (refused treatment due to new relationship) Deviations from study protocol: 2 women in the LNG‐IUS group (1 had a PBAC score of 60 at baseline, 1 became postmenopausal between 12 and 36 months); 2 in the surgical group (2 became menopausal at 12 months, 1 of whom requested to withdraw from study) Losses to follow‐up: At 1 year: 9 women lost to follow‐up or excluded from all or some of the analyses: 3 in the surgical arm (1 who refused treatment, as above; 2 excluded ? due to ineligibility); 6 patients in the LNG‐IUS arm who discontinued treatment (3 for persistent irregular bleeding, 2 for pain, 1 for acne) At 2 years: 16 women lost to follow‐up At 3 years: 20 (?19 ‐ see notes) women lost to follow‐up or excluded from all or some analyses: 9 (?8 ‐ see notes) in the surgical arm (3 as above plus 6 who required further surgery), 11 from the LNG‐IUS arm (9 who discontinued due to side effects, 2 who withdrew from study) Analysis by intention‐to‐treat for primary outcome (i.e. whether primary treatment controlled bleeding). For other outcomes (e.g. bleeding rates) results were only reported for women who continued original allocated treatment and had no further intervention Power analysis: 15 participants per group calculated to give 80% power to detect a 15 ml difference between treatments at 5% significance Source of funding: Schering, Berlin. 2 trial investigators were employees of Schering. Years: enrolment 1993‐1995 | |
| Participants | 60 premenopausal women aged 30 to 49 years who had sought medical attention for heavy menstrual bleeding, referred by general practitioner for surgery to gynaecological outpatient clinic in Oslo specialising in operative hysteroscopy. Required to have a PBAC score > 75 for 2 months before randomisation.
Family complete
Regular uterine cavity ≤ 10 cm in length Exclusions: Breast feeding Current pregnancy Sub serous myoma > 40 mm diameter Use of hormonal medication within past 3 months History of thrombo‐embolic disease or liver disease Any abnormal intrauterine pathology Pelvic inflammatory disease within past 6 months or current infection Participants were initially prepared to undergo hysterectomy. 40% had unsuccessfully tried medical therapy. The rest had either refused conservative surgery or had had no previous treatment. Median age: 41 |
|
| Interventions | Surgical arm: endometrial resection with diathermy loop (regardless of day of menstrual cycle) under spinal block or general anaesthesia Medical arm: levonorgestrel‐releasing intrauterine device inserted within 7 days of start of menstruation | |
| Outcomes | Primary outcome: treatment success (defined as a PBAC subjective bleeding score ≤ 75 at 12 months, no re‐surgery in TCRE group, no removal of device in LNG‐IUS group)
amenorrhoea/oligomenorrhoea rates (bleeding diary)
Genital health: defined by the trialist as an "overall feeling of lower abdominal health") Quality of life on a VAS: hot flushes, sweating, sleeping problems, dyspareunia (pain on intercourse), vaginal dryness, urinary frequency, nervousness, depression, oedema, libido Additional treatment received Adverse effects |
|
| Notes | There are minor discrepancies in data concerning the number of losses to follow‐up at each time point Large number of withdrawals: 33% of participants did not complete 3‐year follow‐up NB LNG‐IUS not assessed: at the onset of the study, it was not commonly used in the USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed, opaque, sequentially numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | High risk | Large number of withdrawals in the LNG‐IUS group: 33% of participants did not complete 3‐year follow‐up, therefore not included in the analysis for the primary outcome (PBAC score) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | There are minor discrepancies in the data concerning the number of losses to follow‐up at each time point |
Kupperman 2004.
| Methods | Randomised controlled trial Multicentre Number of women screened for inclusion: 1557 (of whom 544 excluded as they did not meet the inclusion criteria) Number of women with abnormal uterine bleeding: 1013 Number of women eligible for randomisation: 184 (of whom 92 were in the original study group and had unsuccessfully been treated with cyclic MPA for 3 to 5 months and 92 became eligible in the second year of the trial, having been unsuccessfully treated with cyclic MPA for at least 3 months outside of the trial ‐ see notes) Number of women randomised: 63 (38 of 92 women in the original study group, plus 25* of 92 women who became eligible in the second year) Losses to follow‐up: 4 (2 in each group) for efficacy outcomes Number analysed: 59 for efficacy outcomes; 58 for resource use Failures to receive allocated treatment: 3/31 in the hysterectomy group did not undergo surgery. In the medical group all received medical treatment; 17 also received hysterectomy Analysis by intention‐to‐treat and 'as treated' Power calculation: 60 participants give 90% power to detect a 6.8% change in the SF‐36 mental health score, P value = 0.05 (the authors state that a 3 to 5‐point difference shows meaningful improvement). The original power calculation was scaled down to allow for a smaller sample size, due to recruitment difficulties Trial conducted August 1997 to December 2000 Source of funding: grant from the Agency for Health Care Research and Quality Years: August 1997 to December 2000 | |
| Participants | Inclusion criteria: Premenopausal women aged 31 to 49 with abnormal uterine bleeding (> 7 days of flow each month or heavy flow with haematocrit < 32%), recruited in clinical centres at Alabama or Tennessee Universities, USA, who were dissatisfied with medical treatment including a course of cyclic MPA for at least 3 months Exclusion criteria: Other causes of anaemia, FSH > 30, pregnancy, desire to maintain fertility, endocrinopathy, coagulation problems, treatment for abnormal bleeding with depo‐MPA or GnRH antagonist within the past 6 months, oral contraceptive or intrauterine device use within the past 3 months, contraindications to study medications, potential problems with subject compliance, participation in another trial, evidence of pelvic pathology for which hysterectomy or other specific directed therapy was indicated (e.g. neoplasia, cancer, hyperplasia, intrauterine polyps, submucous myomas) Recruitment strategy: mass mailing, medical records review, advertisements in local mass media, physician referrals Mean age: 41 Participant characteristics: 45% African American, 45% obese (BMI > 30) | |
| Interventions | Surgery:
Abdominal or vaginal hysterectomy as decided by gynaecologist. Prophylactic oophorectomy discouraged.
Medical treatment:
As decided by participating gynaecologist, who was told that "preferred" treatment was a combination of low‐dose oral contraceptives with 21 active days and 7 placebo days monthly and/or a cyclic prostaglandin synthetase inhibitor (PSI) for 5 days over the menstrual period. If therapy unsuccessful, another could be substituted. Women dissatisfied with one medical therapy were encouraged to try other medical options. Actual treatment received: Hysterectomy group: 10/28 abdominal hysterectomy, 18/28 vaginal hysterectomy Medical group: 29/32 hormonal treatment ± PSI (5/32 cyclic progestogen, 2/32 continuous progestogen, 3/32 prostaglandin 8/32 combined oestrogen and progestogen, 2/32 oestrogen only) inhibitor only. 17/32 women received PSI, most commonly naproxen (15/17) |
|
| Outcomes | Health‐related quality of life, measured by a range of instruments, the primary one being the mental component summary of SF‐36 but also including (among others) 12 items from the MOS mental health inventory, 2 from a health distress scale and complete sleep problems, 4‐item body attitudes questionnaire, 5 sexual functioning scales
SF‐36 physical component summary
Overall health, measured by EuroQol VAS and single‐item global health question
Single‐item ratings of symptom resolution and symptom satisfaction
Symptom resolution
Satisfaction Resource use over 2‐year follow‐up (inpatient and outpatient services, including all diagnostic and therapeutic procedures), using Diagnosis‐Related Groups, relative value units associated with Current Procedural Terminology codes: these assign relative weights and values to services, based on estimated average resource use |
|
| Notes | *25 women (of 92 screened) who had been treated unsuccessfully with MPA outside of the trial joined the trial in the second year because of difficulty recruiting participants from the original MPA study group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using randomly permuted blocks of sizes 4, 6 and 8 |
| Allocation concealment (selection bias) | Low risk | Allocated by sequenced randomisation envelopes opened in front of a witness |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | 59/63 analysed. Reasons for withdrawal/dropout given. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
Malak 2006.
| Methods | Randomised controlled trial Number randomised: 60 (30 in each group) Losses to follow‐up: nil for treatment success Number analysed: 60/60 for treatment success All women received their allocated treatment Analysis by ITT Power calculation reported Source of funding: not reported Years: not reported | |
| Participants | Inclusion criteria:
Premenopausal women aged 40 to 50 years with regular uterine cavity, no wish for pregnancy, spontaneous cycles, scheduled for hysterectomy for excessive uterine bleeding (PBAC score > 100 monthly) with or without dysmenorrhoea Exclusion criteria: Fibroid > 3 cm diameter, > 3 fibroids on ultrasound, possible malignancy or active liver disease, adnexal tumours/cysts, pelvic inflammatory disease with past 12 months. Participant characteristics: 80% had previous failed attempt at conservative medical treatment Mean age: 46 to 47 years Setting: Kasr El‐Aini Hospital, Cairo University |
|
| Interventions | LNG‐IUS, with only barrier contraceptive methods Endometrial resection |
|
| Outcomes | Efficacy: treatment choice at 6 months or at discontinuation Treatment success with primary intervention (PBAC score < 75 at 12 months), no removal of LNG‐IUS or repeat surgery Quality of life (by EuroQol ‐ visual analogue scale: EQ‐VAS) Adverse events |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a randomisation table" |
| Allocation concealment (selection bias) | Unclear risk | States sealed envelopes. Unclear whether sequentially numbered and opaque. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | No losses to follow‐up for primary outcome |
| Selective reporting (reporting bias) | Low risk | Reports all expected outcomes |
| Other bias | Unclear risk | Text inconsistent re nature of primary outcome ‐ states "the primary measure of efficacy was the woman's decision at six months or at discontinuation...": treatment decision is an outcome that applies to only one group. However, text goes on to propose a more detailed definition that applies to both groups. |
Sesti 2012.
| Methods | Randomised controlled trial Number randomised: 72 Failures to receive allocated treatment: nil Losses to follow‐up: nil Analysis by ITT: yes Power calculation: yes Source of funding: not reported |
|
| Participants | Included: women with HMB unresponsive to medical treatment; age 35 to 50 years, completed family; failed appropriate first‐line oral medical therapy; normal PAP smear; no pelvic pathology on US; normal endometrial biopsy; PBAC > or = 100 (average of 2 cycles) Mean age: 47.1 and 47.5 years Excluded: previous endometrial resection/ablation; previous insertion of LNG‐IUS; any uterine pathology on scan or hysteroscopy; any pathology where hysterectomy indicated; not fully investigated abnormal uterine bleeding; postmenopausal bleeding |
|
| Interventions | Surgical arm: hysterectomy (laparoscopic supracervical) Medical arm: LNG‐IUS Actual treatment received: as randomised |
|
| Outcomes | Primary outcome; effects on PBAC at 12 months (end scores) Other outcomes: changes in haemoglobin levels, presence of anaemia; bleeding frequency/length; quality of life; intensity of postoperative pain; early postoperative complications |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated list using serially numbered opaque sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "computer generated list using serially numbered opaque sealed envelopes" – "blindly allocated" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | 72 randomised and 72 included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other potential bias identified |
Shaw 2007.
| Methods | Randomised controlled trial Number randomised: 66 (33 in each group) Number analysed: 46/66 for PBAC at 12 months All women in LNG‐IUS group and 30/33 in surgical group received their allocated treatment Power calculation reported Source of funding: no specific external funding; 30 balloon treatment systems donated and research nurse part‐funded by educational grant (advised by email from author) Years: 2001‐2003 | |
| Participants | Inclusion criteria:
Women with idiopathic menorrhagia aged 25 to 49 years in whom prior appropriate oral medical treatment had failed. Family complete, normal histology, normal ultrasound (fibroids up to 2.5 cm OK), normal cervical smear, PBAC score > 120 (mean over 2 cycles) Exclusion criteria: Previous LNG‐IUS or endometrial resection/ablation, abnormal uterine bleeding, other pathology, submucous fibroid, uterine cavity < 7 cm or over 11 cm Mean age: 43 |
|
| Interventions | LNG‐IUS Thermal balloon ablation |
|
| Outcomes | Change in PBAC score at 12 months (median and range) Changes in Hb and ferritin at 6 months Patient satisfaction Hysterectomy rate at 2 years Treatment discontinuation (e.g. for adverse events, menorrhagia, LNG‐IUS expulsion) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated" |
| Allocation concealment (selection bias) | Low risk | "Sequentially sealed opaque envelope ...numbered 1 to 100" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | High risk | 46/66 analysed for PBAC score at 12 months; withdrawals/dropouts described |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects not systematically reported |
| Other bias | Low risk | No other potential bias identified |
Soysal 2002.
| Methods | Randomised controlled trial Number randomised: 72 Number analysed: 72 Failures to receive allocated treatment: none Losses to follow‐up: none Analysis by intention‐to‐treat for some outcomes only ‐ 6 women who had hysterectomy due to treatment failure (1 in the surgical group, 5 in the LNG‐IUS group) were not included in outcome measures for bleeding, satisfaction or haemoglobin Power calculation: 60 women required in each group to give 80% power to detect a 15 ml difference in blood loss at a 5% level of significance. (Objective loss to be estimated, based on PBAC score). Source of funding: not stated Years: 1999‐2001 | |
| Participants | Women aged over 40 complaining of menorrhagia who refused or did not respond to medical treatment, seen at university medical centre in Turkey Documented PBAC scores of > 150 for 2 consecutive months before randomisation Median age: 44 years Family complete Normal blood tests, transvaginal ultrasonography, hysteroscopy, endometrial suction biopsy or cervical smear examination No discrete intramural or subserous myomas > 2 cm diameter Regular uterine cavity ≤ 8‐week pregnancy and < 190 ml on ultrasonography Exclusions: Any medical disorder other than iron deficiency anaemia Abnormal intrauterine pathology | |
| Interventions | Surgical arm: thermal balloon ablation under local anaesthetic. Endometrium thinned preoperatively by monthly injections of GnRH analogue for 2 months before surgery Medical arm: levonorgestrel‐releasing intrauterine device inserted within 7 days of start of menstruation | |
| Outcomes | Primary outcome: menstrual blood flow reduction, measured by PBAC subjective bleeding score and haemoglobin Other outcomes: health‐related quality of life: SF‐36, HAD depression scale Treatment satisfaction Additional treatment received Adverse effects | |
| Notes | Standard deviations reported for PBAC scores are very low ‐ could these be standard errors? Unable to contact author for clarification. No repeat treatments given ‐ women for whom initial treatment failed (persistent or new symptoms, loss of intrauterine device, treatment unacceptable) had hysterectomy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation by serially numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | Low risk | All randomised women analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | Standard deviations reported for PBAC scores are very low ‐ could these be standard errors? Unable to contact author for clarification. |
Talis 2006.
| Methods | Randomised controlled trial Number screened: 177 of whom 71 were ineligible; 23/106 eligible women declined randomisation Number randomised: 83, of whom 4 were excluded as submucous fibroids were found at hysteroscopy Losses to follow‐up: 11 by 2 years (3 in the LNG‐IUS group, 8 in the ablation group) Number analysed: 79 for treatment success, 54 for PBAC at 2 years (excluded 11 failures and 2 exclusions from the LNG‐IUS group; 10 failures and 2 exclusions from the surgical group) Failures to receive allocated treatment: 4 excluded as above. All others received allocated treatment. Analysis was not by ITT Power calculation: for 80% power to detect a 50‐point difference in PBAC score, 30 women were needed for each arm Source of funding: Academic research grant Years: 1999‐2001 | |
| Participants | Included: women with self described HMB who had completed their family, aged 25 to 50 years, and had a regular cycle with discrete episodes of menstruation occurring every 3 to 6 weeks Excluded: women with ultrasound abnormalities (submucosal fibroids, intramural fibroids > 3 cm diameter, large subserosal fibroids, endometrial polyps); laboratory abnormalities (follicle‐stimulating hormone level (FSH) > 30 IU/l, adverse endometrial histology); or hysteroscopic abnormalities (submucosal fibroids, endometrial polyps), incidental adnexal abnormality on ultrasound, severe inter‐menstrual bleeding, severe dysmenorrhoea, severe pre‐menstrual pain, chronic pelvic pain, medical contraindications to either study treatment, previous endometrial ablation or resection, uninvestigated post‐coital bleeding or untreated abnormal cervical cytology | |
| Interventions | Diagnostic hysteroscopy followed by:
Surgical arm: balloon ablation (Thermachoice)
or
Medical arm: LNG‐IUS (Mirena) Both interventions under local anaesthetic if tolerated, or else under general anaesthetic |
|
| Outcomes | Primary: menstrual loss, measured by PBAC Satisfaction, quality of life and menstrual symptoms, measured by questionnaire Secondary: haemoglobin level Treatment side effects Treatment failure (for the LNG‐IUS this was confirmed expulsion, completed removal or the initiation of alternative therapy. For thermal balloon ablation this was the initiation of medication or the completion of alternative surgery, such as hysterectomy). Cost‐effectiveness: including direct and indirect costs of treatment, subsequent medical treatment, lost income and medical treatment for failed procedures: see publication for details of economic modelling | |
| Notes | Talis 2006 excluded women with treatment failure from the analysis: only 75% of women randomised were included in the analysis at 1 year, while only 58% were included at 2 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation in blocks of 20 |
| Allocation concealment (selection bias) | Low risk | Allocation by serially numbered, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | High risk | 58/83 (70%) analysed for PBAC at 2 years: treatment failures excluded |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
Tam 2006.
| Methods | Randomised controlled trial Number randomised: 44 (22 in each group) Losses to follow‐up: 1 Number analysed: 33/44 for PBAC at 12 months 18/22 women in the LNG‐IUS group and 15/22 in the surgical group received their allocated treatment Power calculation not reported Source of funding: no external funding (advised by email from author) Years: not stated | |
| Participants | Inclusion criteria:
Women aged over 40 years with a documented history of excessive menstrual bleeding for at least 3 months, family complete, prior oral medical treatment unsuccessful, not on any hormonal treatment Exclusion criteria: Uterus > 10 weeks' gravid size, submucosal fibroids, endometrial polyps, contraindications to interventions, possible malignancy Participant characteristics: Mean age: 44 years Recruited via outpatient gynaecology clinic Setting: university‐affiliated tertiary referral centre, Hong Kong |
|
| Interventions | LNG‐IUS Thermal balloon endometrial ablation (with endometrial priming) |
|
| Outcomes | Menstrual pattern at 1 year (self reported) Adverse effects Haemoglobin and iron status Health status: SF‐36 (using norms for Hong Kong Chinese) |
|
| Notes | Study authors report a statistically significant difference between the intervention groups in haemoglobin level at 1 year ‐ MDs not significantly different in RevMan analysis in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random number series" |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes (personal correspondence with author) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. Our primary review outcomes are subjective and therefore susceptible to bias related to lack of blinding. |
| Incomplete outcome data (attrition bias) Primary outcomes | High risk | 33/44 analysed at 1 year (reasons for withdrawals and dropouts given) |
| Selective reporting (reporting bias) | Unclear risk | Does not clearly specify a primary outcome. Adverse events not reported clearly across both groups. |
| Other bias | Unclear risk | Low rate of acceptance among eligible participants (44/73) |
BMI: body mass index FSH: follicle‐stimulating hormone GnRH: gonadotrophin‐releasing hormone HER: hysteroscopic endometrial resection HMB: heavy menstrual bleeding ITT: intention‐to‐treat LNG‐IUS: levonorgestrel‐releasing intrauterine device MD: mean difference MOS: Medical Outcomes Study MPA: medroxyprogesterone acetate NSAID: non‐steroidal anti‐inflammatory drug PBAC: pictorial blood loss assessment chart PSI: prostaglandin synthetase inhibitor SD: standard deviation TVUS: transvaginal ultrasound US: ultrasound VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Assaf 2000 | Only partially randomised; unable to obtain separate data for randomised group |
| Barrington 1997 | A prospective study of levonorgestrel intrauterine device only ‐ no control group |
| Ergun 2012a | Assesses tranexamic acid as an adjunct to surgery |
| Ghazizadeh 2011 | Described as randomised but states that women could choose their treatment |
| Lahteenmaki 1998 | Compares levonorgestrel intrauterine device to medical treatment with respect to proportion of women on each treatment cancelling their decision to have hysterectomy |
| Reid 2005 | Compares 2 medical treatments (LNG‐IUS and mefenamic acid) |
| Romer 2000 | Not randomised |
| Shabaan 2011 | Compares 2 medical interventions |
| Shokeir 2013 | Assesses gestagens as an adjunct to surgery |
| SMART 2000 | Closed due to poor enrolment |
| Soysal 2005 | Compares levonorgestrel intrauterine device with thermal balloon ablation in women with myoma‐related menorrhagia. Not randomised ‐ uses historical controls. |
LNG‐IUS: levonorgestrel‐ releasing intrauterine device
Characteristics of ongoing studies [ordered by study ID]
Herman 2013.
| Trial name or title | Herman 2013 |
| Methods | RCT |
| Participants | Women ≥ 34 years with heavy menstrual bleeding, PBAC score > 150 points. Planned sample size = 266 |
| Interventions | A strategy starting with a levonorgestrel‐releasing intrauterine device or a strategy starting with endometrial ablation |
| Outcomes | PBAC score at 24 months, patient satisfaction, complications, number of re‐interventions, menstrual bleeding pattern, quality of life, sexual function, sick leave and costs |
| Starting date | — |
| Contact information | m.herman@mmc.nl |
| Notes | — |
PBAC: pictorial blood loss assessment chart RCT: randomised controlled trial
TCRE: transcervical resection of the endometrium
Differences between protocol and review
In early versions of the review we reported menstrual loss as "change in menstrual loss". From 2016 onwards we combined change and end scores where studies reported continuous outcomes on the same scale (as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)), and we renamed the outcomes as simply "menstrual loss".
In the 2016 update, we endeavoured to reduce the number of primary outcomes and the number of outcomes overall, in line with current Cochrane policy that outcomes should be as few as possible and that primary outcomes should normally reflect at least one potential benefit and at least one potential area of harm. We achieved this by the following changes:
In earlier versions of the review quality of life and need for additional surgery were primary outcomes. From 2016 onwards these became secondary outcomes.
For the outcome "quality of life", we limited the measure to "self‐reported change in quality of life, recorded in a reproducible and validated format". Previously this outcome also included the measure "self reported change in premenstrual symptoms and dysmenorrhoea (painful periods) recorded in a reproducible and validated format".
We deleted the outcome "haemoglobin level" in line with the Cochrane policy of giving preference to patient‐reported outcomes rather than biochemical measures.
We reduced the number of time points for follow‐up to one year (or less), two years, five years and 10 years.
In 2016 we decided not to report numbers needed to treat to benefit (NNTBs) with our effect estimates, as these are misleading given the very high cross‐over rates between treatment arms.
Contributions of authors
Jane Marjoribanks: developed the background section, edited the objectives and selection criteria, searched for and selected the studies, extracted the data and wrote the text and update. Anne Lethaby: contributed to the background section, drafted the objectives and selection criteria, formulated the methods, searched for and selected the studies, extracted the data, checked study quality, provided statistical advice and commented on the drafts on several occasions. Cindy Farquhar: initiated and conceptualised the protocol and commented on the drafts.
Sources of support
Internal sources
University of Auckland, New Zealand.
External sources
No sources of support supplied
Declarations of interest
None known.
Cindy Farquhar and Anne Lethaby are authors of one of the included trials (Talis 2006). However they have no present or past affiliations or other involvement in any organization or entity with an interest in the review’s findings that might lead to a real or perceived conflict of interest.
Jane Marjoribanks has no present or past affiliations or other involvement in any organization or entity with an interest in the review’s findings that might lead to a real or perceived conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barrington 2003 {published data only}
- Barrington JW, Arunkalaivanan AS, Abdel‐Fattah M. Comparison between the levonorgestrel intrauterine system (LNG‐IUS) and thermal balloon ablation in the treatment of menorrhagia. European Journal of Obstetrics and Gynaecology and Reproductive Biology 2003;108:72‐4. [DOI] [PubMed] [Google Scholar]
Cooper 1997 {published data only}
- Cooper KG, Jack SA, Parkin DE, Grant AM. Five‐year follow up of women randomised to medical management or transcervical resection of the endometrium for heavy menstrual loss: clinical and quality of life outcomes. British Journal of Obstetrics and Gynaecology 2001;108:1222‐8. [DOI] [PubMed] [Google Scholar]
- Cooper KG, Parkin DE, Garratt AM, Grant AM. A randomised comparison of medical and hysteroscopic management in women consulting a gynaecologist for treatment of heavy menstrual loss. British Journal of Obstetrics and Gynaecology 1997;104:1360‐6. [DOI] [PubMed] [Google Scholar]
- Cooper KG, Parkin DE, Garratt AM, Grant AM. Two‐year follow up of women randomised to medical management or transcervical resection of the endometrium for heavy menstrual loss: clinical and quality of life outcomes. British Journal of Obstetrics and Gynaecology 1999;106:258‐65. [DOI] [PubMed] [Google Scholar]
Crosignani 1997 {published data only}
- Crosignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, Giorgi O. Levonorgestrel‐releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstetrics and Gynecology 1997;90:257‐63. [DOI] [PubMed] [Google Scholar]
de Souza 2010 {published data only}
- Silva‐Filho AL, Pereira Fde A, Souza SS, Loures LF, Rocha AP, Valadares CN, et al. Five‐year follow‐up of levonorgestrel‐releasing intrauterine system versus thermal balloon ablation for the treatment of heavy menstrual bleeding: a randomized controlled trial. Contraception 2013;87(4):409‐15. [DOI] [PubMed] [Google Scholar]
- Souza SS, Camargos AF, Rezende CP, Pereira FA, Araujo CA, Silva Filho AL. A randomized prospective trial comparing the levonorgestrel‐releasing intrauterine system with thermal balloon ablation for the treatment of heavy menstrual bleeding [Contraception]. 2010 81;3:226‐31. [DOI] [PubMed] [Google Scholar]
Ergun 2012 {published data only}
- Ergun B, Bastu E, Kuru O, Sen S, Kilic Y, Bastu E, et al. Comparison of rollerball endometrial ablation and levonorgestrel releasing intrauterine system in the management of abnormal uterine bleeding. International Journal of Gynecology and Obstetrics. Conference: 20th FIGO World Congress of Gynecology and Obstetrics Rome Italy: Conference publication. 2012.
- Ergun B, Kuru O, Sen S, Kilic Y. Comparison between roller‐ball endometrial ablation and levonorgestrel intrauterine system (LNG‐IUS) in the treatment of abnormal uterine bleeding. Turk Jinekoloji ve Obstetrik Dernegi Dergisi 2011;4:259‐63. [Google Scholar]
- Ergun B, Kuru O, Sen S, Kilic Y, Bastu E. Roller‐ball endometrial ablation versus levonorgestrel releasing intrauterine system in the management of abnormal uterine bleeding. Gineco.eu 2012;8(4):199‐201. [Google Scholar]
Ghazizdeh 2014 {published data only}
- Ghazizadeh S, Panahi Z, Ghanbari Z, Menshadi AT, Farahmandian T, Javadian P. Comparative efficacy of NovaSure, the levonorgestrel‐releasing intrauterine system, and hysteroscopic endometrial resection in the treatment of menorrhagia: a randomized clinical trial. Journal of Gynecologic Surgery 2014;30(4):215‐8. [Google Scholar]
Hurskainen 2001 {published data only}
- Elovainio M, Teperi J, Aalto A‐M, Grenman S, Kivel A, Kujansuu E, et al. Depressive symptoms as predictors of discontinuation of treatment of menorrhagia by levonorgestrel‐releasing intrauterine system. International Journal of Behavioral Medicine 2007;14(2):70–5. [DOI] [PubMed] [Google Scholar]
- Halmesmaki K, Hurskainen R, Teperi J, Grenman S, Kivela A, Kujansuu E, et al. The effect of hysterectomy or levonorgestrel‐releasing intrauterine system on sexual functioning among women with menorrhagia: a 5‐year randomised controlled trial. BJOG: an International Journal of Obstetrics and Gynaecology 2007;114:563‐8. [DOI] [PubMed] [Google Scholar]
- Halmesmaki K, Hurskainen R, Tiitinen A, Terperi J, Grenman S, Kivela A, et al. A randomized controlled trial of hysterectomy or levonorgestrel‐releasing intrauterine system in the treatment of menorrhagia ‐ effect on FSH levels and menopausal symptoms. Human Reproduction 2004;19(2):378‐82. [DOI] [PubMed] [Google Scholar]
- Halmesmaki KH, Hurskainen RA, Cacciatore B, Tiitinen A, Paavonen JA. Effect of hysterectomy or LNG‐IUS on serum inhibin B levels and ovarian blood flow. Maturitas 2007;57:279‐85. [DOI] [PubMed] [Google Scholar]
- Halmesmäki K, Paavonen J, Tuppurainen M, Hurskainen R. Randomized controlled trial of the effect of hysterectomy or LNG‐IUS use on bone mineral density: a five‐year follow‐up. Therapy 2006;3:509‐15. [Google Scholar]
- Heliovaara‐Peippo S, Halmesmaki K, Hurskainen R, Teperi J, Grenman S, Kivela A, et al. The effect of hysterectomy or levonorgestrel‐releasing intrauterine system on lower abdominal pain and back pain among women treated for menorrhagia: a five‐year randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2009;88(12):1389‐96. [DOI] [PubMed] [Google Scholar]
- Heliovaara‐Peippo S, Halmesmaki K, Hurskainen R, Teperi J, Grenman S, Kivela A, et al. The effect of hysterectomy or levonorgestrel‐releasing intrauterine system on lower urinary tract symptoms: a 10‐year follow‐up study of a randomised trial. BJOG: an International Journal of Obstetrics and Gynaecology 2010;117(5):602‐9. [DOI] [PubMed] [Google Scholar]
- Heliovaara‐Peippo S, Hurskainen R, Teperi J, Aalto AM, Grenman S, Halmesmaki K, et al. Quality of life and costs of levonorgestrel‐releasing intrauterine system or hysterectomy in the treatment of menorrhagia: a 10‐year randomized controlled trial. American Journal of Obstetrics and Gynecology 2013;209(6):535.e1‐14. [DOI] [PubMed] [Google Scholar]
- Hurskainen R, Teperi J, Rissanen P, Aalto A‐M, Grenman S, Kivela A, et al. Quality of life and cost‐effectiveness of levonorgestrel‐releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet 2001;357:273‐7. [DOI] [PubMed] [Google Scholar]
- Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivela A, et al. Clinical outcomes and costs with the levonorgestrel‐releasing intrauterine system or hysterectomy for the treatment of menorrhagia. JAMA 2004;291(12):1456‐63. [DOI] [PubMed] [Google Scholar]
- Inki P, Hurskainen R, Palo P, Ekholm E, Grenham S, Kivela A, et al. Comparison of ovarian cyst formation in women using the levonorgestrel‐releasing intrauterine system vs hysterectomy. Ultrasound in Obstetrics and Gynaecology 2002;20(4):381‐5. [DOI] [PubMed] [Google Scholar]
- Leminen H, Helio‐Vaara‐Peippo S, Halmesmaki K, Teperi J, Grenman S, Kivela A, et al. The effect of hysterectomy or levonorgestrel‐releasing intrauterine system on premenstrual symptoms in women treated for menorrhagia: secondary analysis of a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2012;91(3):318‐25. [DOI] [PubMed] [Google Scholar]
Istre 1998 {published and unpublished data}
- Istre O. Will the progesterone IUD replace the need for endometrial ablation?. www.oistre.com (accessed 15 January 2003).
- Istre O, Trolle B. Treatment of menorrhagia with the levonorgestrel intrauterine system versus endometrial resection. Fertility and Sterility 2001;76(2):304‐9. [DOI] [PubMed] [Google Scholar]
- Kittelson N, Istre O. A randomized study comparing levonorgestrel intrauterine system (LNG IUS) and transcervical resection of the endometrium (TCRE) in the treatment of menorrhagia: preliminary results. Gynaecological Endoscopy 1998;7:61‐5. [Google Scholar]
- Rauramo I, Elo I, Istre O. Long term treatment of idiopathic menorrhagia with the levonorgestrel intrauterine system versus endometrial resection. Personal communication 21 January 2003.
- Rauramo I, Elo I, Istre O. Long‐term treatment of menorrhagia with levonorgestrel intrauterine system versus endometrial resection. Obstetrics and Gynecology 2004;104(6):1314‐21. [DOI] [PubMed] [Google Scholar]
Kupperman 2004 {published data only}
- Kupperman M, Varner RE, Summitt RL, Learman LA, Ireland C, Vittinghoff E, et al. Effect of hysterectomy vs medical treatment on health‐related quality of life and sexual functioning. JAMA 2004;291(12):1447‐55. [DOI] [PubMed] [Google Scholar]
- Learman LA, Summitt RL, Varner E, Richter HE, Lin F, Ireland CC, et al for the Medicine and Surgery Research Group. Hysterectomy versus expanded medical treatment for abnormal uterine bleeding: clinical outcomes in the Medicine or Surgery Trial. Obstetrics and Gynecology 2004;103(5 Part 1):824‐33. [DOI] [PubMed] [Google Scholar]
- Showstack J, Lin F, Learman LA, Vittinghoff E, Kuppermann M, Varner RE, et al for the Ms Research Group. Randomized trial of medical treatment versus hysterectomy for abnormal uterine bleeding: resource use in the Medicine or Surgery (Ms) trial. American Journal of Obstetrics and Gynecology 2006;194:332‐8. [DOI] [PubMed] [Google Scholar]
- Varner RE, Ireland CE, Summitt RL, Richter HE, Learman LA, Vittinghoff E, et al. Medicine or surgery (Ms): a randomized clinical trial comparing hysterectomy and medical treatment in premenopausal women with abnormal uterine bleeding. Controlled Clinical Trials 2004;25:104‐18. [DOI] [PubMed] [Google Scholar]
Malak 2006 {published data only}
- Malak KA, Shawki O. Management of menorrhagia with the levonorgestrel intrauterine system versus endometrial resection. Gynecological Surgery 2006;3:275‐80. [Google Scholar]
Sesti 2012 {published data only}
- Sesti F, Piancatelli R, Pietropolli A, Ruggeri V, Piccione E. Levonorgestrel‐releasing intrauterine system versus laparoscopic supracervical hysterectomy for the treatment of heavy menstrual bleeding: a randomized study. Journal of Women's Health 2012;21(8):851‐7. [DOI] [PubMed] [Google Scholar]
Shaw 2007 {published data only}
- Shaw R, Symonds IM, Tamizian O, Chaplain J, Mukhopadhyay S. Randomised comparison of thermal balloon ablation and levonorgestrel intrauterine system in patients with idiopathic menorrhagia. Australian and New Zealand Journal of Obstetric and Gynaecology 2007;47:335‐40. [DOI] [PubMed] [Google Scholar]
Soysal 2002 {published data only}
- Soysal M, Soysal S, Ozer S. A randomized controlled trial of levonorgestrel releasing IUD and thermal balloon ablation in the treatment of menorrhagia. Zentralblatt fur Gynakologie 2002;124(4):213‐9. [DOI] [PubMed] [Google Scholar]
Talis 2006 {published data only (unpublished sought but not used)}
- Brown PM, Farquhar CM, Lethaby A, Sadler LC, Johnson NP. Cost‐effectiveness analysis of levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG: an International Journal of Obstetrics and Gynaecology 2006;113:797‐803. [DOI] [PubMed] [Google Scholar]
- Busfield RA, Farquhar CM, Sowter MC, Lethaby A, Sprecher M, Yu Y, et al. A randomised trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG: an International Journal of Obstetrics and Gynaecology 2006;113:257‐63. [DOI] [PubMed] [Google Scholar]
Tam 2006 {published data only}
- Tam WH, Yuen PM, Ng DPS, Lung PL, Lok IH, Rogers MS. Health status function after treatment with thermal balloon endometrial ablation and levonorgestrel intrauterine system for idiopathic menorrhagia: a randomised study. Gynecologic and Obstetric Investigation 2006;62:84‐8. [DOI] [PubMed] [Google Scholar]
- Wing HT, Pong MY, Ng DPS, Pui LL, Lok IH, Rogers MS. Health status function after treatment with thermal balloon endometrial ablation and levonorgestrel intrauterine system for idiopathic menorrhagia: a randomized study. Gynecologic and Obstetric Investigation 2006;62(2):84‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Assaf 2000 {published data only}
- Assaf A. Endometrial resection versus progesterone containing IUD for perimenopausal bleeding. 9th Annual Congress of International Society for Gynecologic Endoscopy/10th Annual Meeting of Australian Gynaecological Endoscopy Society, Queensland April 2000.
Barrington 1997 {published data only}
- Barrington JW, Bowen‐Simpkins P. The levonorgestrel intrauterine system in the management of menorrhagia. British Journal of Obstetrics and Gynaecology 1997;104(5):614‐6. [DOI] [PubMed] [Google Scholar]
Ergun 2012a {published data only}
- Ergun B, Bastu E, Ozsurmeli M, Celik C. Tranexamic acid: a potential adjunct to resectoscopic endometrial ablation. International Surgery 2012;97(4):310‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghazizadeh 2011 {published data only}
- Ghazizadeh S, Bakhtiari F, Rahmanpour H, Davari‐Tanha F, Ramezanzadeh F. A randomized clinical trial to compare levonorgestrel‐releasing intrauterine system (Mirena) vs trans‐cervical endometrial resection for treatment of menorrhagia. International Journal of Women's Health 2011;3:207‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lahteenmaki 1998 {published data only}
- Lahteenmaki P, Haukkamaa M, Puolakka J, Riikonen U, Sainio S, Suvisaari J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ 1998;316:1122‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2005 {published data only}
- Reid PC, Virtanen‐Kari S. Randomised comparative trial of the levonorgestrel intrauterine system and mefenamic acid for the treatment of idiopathic menorrhagia: a multiple analysis using total menstrual fluid loss, menstrual blood loss and pictorial blood loss assessment charts. BJOG: an International Journal of Obstetrics and Gynaecology 2005;112:1‐5. [DOI] [PubMed] [Google Scholar]
Romer 2000 {published data only}
- Romer T. Prospective comparison study of levonorgestrel IUD versus roller‐ball endometrial ablation in the management of refractory recurrent hypermenorrhea. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2000;90:27‐9. [DOI] [PubMed] [Google Scholar]
Shabaan 2011 {published data only}
- Shaaban MM, Zakherah MS, El‐Nashar SA, Sayed GH. Levonorgestrel‐releasing intrauterine system compared to low dose combined oral contraceptive pills for idiopathic menorrhagia: a randomized clinical trial. Contraception 2011;83(1):48‐54. [DOI] [PubMed] [Google Scholar]
Shokeir 2013 {published data only}
- Shokeir T, Eid M, Abdel‐Hady el‐S. Does adjuvant long‐acting gestagen therapy improve the outcome of hysteroscopic endometrial resection in women of low‐resource settings with heavy menstrual bleeding?. Journal of Minimally Invasive Gynecology 2013;20(2):222‐6. [DOI] [PubMed] [Google Scholar]
SMART 2000 {published data only}
- Rogerson L, Duffy S. Management of menorrhagia ‐ SMART study (Satisfaction with Mirena and Ablation: a Randomised Trial). BJOG: an International Journal of Obstetrics and Gynaecology 2000;107:1323‐6. [DOI] [PubMed] [Google Scholar]
Soysal 2005 {published data only}
- Soysal S, Soysal ME. The efficacy of levonorgestrel‐releasing intrauterine device in selected cases of myoma‐related menorrhagia: a prospective controlled trial. Gynecologic and Obstetric Investigation 2005;59(1):29‐35. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Herman 2013 {published data only}
- Herman MC, Brink MJ, Geomini PM, Meurs HS, Huirne JA, Eising HP, et al. Levonorgestrel releasing intrauterine system (Mirena) versus endometrial ablation (Novasure) in women with heavy menstrual bleeding: a multicentre randomised controlled trial. BMC Womens Health 2013;13(32):1472‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aberdeen 1999
- Aberdeen Endometrial Ablation Trials Group. A randomised trial of endometrial ablation versus hysterectomy for the treatment of dysfunctional uterine bleeding: outcome at four years. Aberdeen Endometrial Ablation Trials Group. British Journal of Obstetrics and Gynaecology 1999;106(4):360‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Beaumont 2007
- Beaumont HH, Augood C, Duckitt K, Lethaby A. Danazol for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001017.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharya 2011
- Bhattacharya S, Middleton LJ, Tsourapas A, Lee AJ, Champaneria R, Daniels JP, et al. International Heavy Menstrual Bleeding Individual Patient Data Meta‐analysis Collaborative Group. Hysterectomy, endometrial ablation and Mirena(R) for heavy menstrual bleeding: a systematic review of clinical effectiveness and cost‐effectiveness analysis. Health Technology Assessment 2011; Vol. 15, issue 19. [DOI] [PMC free article] [PubMed]
Brache 2002
- Brach V, Faundes A, Alvarez F, Cochon L. Nonmenstrual adverse events during use of implantable contraceptives for women: data from clinical trials. Contraception 2002;65:63‐74. [DOI] [PubMed] [Google Scholar]
Chimbira 1979
- Chimbira TH, Cope E, Anderson ABM, Bolton G. The effect of danazol on menorrhagia, coagulation mechanisms, haematological indices and body weight. British Journal of Obstetrics and Gynaecology 1979;86:46‐50. [DOI] [PubMed] [Google Scholar]
Chimbira 1980
- Chimbira TH, Anderson AB, Turnbull A. Relation between measured blood loss and patient's subjective assessment of loss, duration of bleeding, number of sanitary towels used, uterine weight and endometrial surface area. British Journal of Obstetrics and Gynaecology 1980;87(7):603‐9. [DOI] [PubMed] [Google Scholar]
Dragoman 2014
- Dragoman MV. The combined oral contraceptive pill ‐ recent developments, risks and benefits. Best Practice and Research Obstetrics and Gynaecology 2014;28:825‐34. [DOI] [PubMed] [Google Scholar]
Dunn 1999
- Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs 1999;57(6):1005‐32. [DOI] [PubMed] [Google Scholar]
Farquhar 2002
- Farquhar C, Steiner C. Hysterectomy rates in the United States 1990‐1997. Obstetrics and Gynecology 2002;99:229‐34. [DOI] [PubMed] [Google Scholar]
Farquhar 2005
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG: an International Journal of Obstetrics and Gynaecology 2005;112(7):956‐62. [DOI] [PubMed] [Google Scholar]
Farquhar 2009
- Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000154.pub2] [DOI] [PubMed] [Google Scholar]
Fergusson 2013
- Fergusson RJ, Lethaby A, Shepperd S, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD000329.pub2] [DOI] [PubMed] [Google Scholar]
Fraser 1991
- Fraser IS, McCarron G. Randomized trial of 2 hormonal and 2 prostaglandin‐inhibiting agents in women with a complaint of menorrhagia. Australia and New Zealand Journal of Obstetrics and Gynaecology 1991;31(1):66‐70. [DOI] [PubMed] [Google Scholar]
Frick 2009
- Frick KD, Clark MA, Steinwachs DM, Langenberg P, Stovall D, Munro MG, et al. STOP‐DUB Research Group. Financial and quality‐of‐life burden of dysfunctional uterine bleeding among women agreeing to obtain surgical treatment. Women's Health Issues: official publication of the Jacobs Institute of Women's Health 2009;19(1):70‐8. [DOI] [PubMed] [Google Scholar]
Hallberg 1964
- Hallberg L, Nilsson L. Determination of menstrual blood loss. Scandinavian Journal of Clinical Laboratory Investigations 1964;16:244‐8. [PubMed] [Google Scholar]
Hallberg 1966
- Hallberg L, Hogdahl A, Nilsson L, Rybo G. Menstrual blood loss ‐ a population study: variation at different ages and attempts to define normality. Acta Obstetricia et Gynecologica Scandinavica 1966;45:320‐51. [DOI] [PubMed] [Google Scholar]
Halmesmaki 2007
- Halmesmaki K, Hurskainen R, Teperi J, Grenman S, Kivela A, Kujansuu E, et al. The effect of hysterectomy or levonorgestrel‐releasing intrauterine system on sexual functioning among women with menorrhagia: a 5‐year randomised controlled trial. BJOG: an International Journal of Obstetrics and Gynaecology 2007;114:563–8. [DOI] [PubMed] [Google Scholar]
Heliovaara‐Peippo 2010
- Heliovaara‐Peippo S, Halmesmaki K, Hurskainen R, Teperi J, Grenman S, Kivela A, et al. The effect of hysterectomy or levonorgestrel‐releasing intrauterine system on lower urinary tract symptoms: a 10‐year follow‐up study of a randomised trial. BJOG: an International Journal of Obstetrics and Gynaecology 2010;117(5):602‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Higham 1990
- Higham JM, O'Brien PMS, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. British Journal of Obstetrics and Gynaecology 1990;97(8):734‐9. [DOI] [PubMed] [Google Scholar]
Higham 1999
- Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1999;82:73‐6. [DOI] [PubMed] [Google Scholar]
Irvine 1998
- Irvine GA, Campbell‐Brown MB, Lumsden MA, Heikkila A, Walker JJ, Cameron IT. Randomised comparative study of the levonorgestrel intrauterine device and norethisterone for the treatment of idiopathic menorrhagia. British Journal of Obstetrics and Gynaecology 1998;105(6):592‐8. [DOI] [PubMed] [Google Scholar]
Jack 2005
- Jack SA, Cooper KG, Seymour J, Graham W, Fitzmaurice A, Perez J. A randomised controlled trial of microwave endometrial ablation without endometrial preparation in the outpatient setting:patient acceptability, treatment outcome and costs. BJOG: an International Journal of Obstetrics and Gynaecology 2005;112:1109‐16. [DOI] [PubMed] [Google Scholar]
Lethaby 2008
- Lethaby A, Irvine GA, Cameron IT. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD001016.pub2] [DOI] [PubMed] [Google Scholar]
Lethaby 2009b
- Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD000249] [DOI] [PubMed] [Google Scholar]
Lethaby 2013
- Lethaby A, Duckitt K, Farquhar C. Non‐steroidal anti‐inflammatory drugs for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD000400.pub3] [DOI] [PubMed] [Google Scholar]
Lethaby 2013a
- Lethaby A, Penninx J, Hickey M, Garry R, Marjoribanks J. Endometrial resection and ablation techniques for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD001501.pub4] [DOI] [PubMed] [Google Scholar]
Lethaby 2015
- Lethaby A, Hussain M, Rishworth JR, Rees MC. Progesterone or progestogen‐releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD002126.pub3] [DOI] [PubMed] [Google Scholar]
Liu 2007
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health‐related quality of life, work impairment, and health‐care costs and utilization in abnormal uterine bleeding. Value in Health 2007;10(8):183‐94. [DOI] [PubMed] [Google Scholar]
Luukkainen 2001
- Luukkainen T, Pakarinen P, Toivonen J. Progestin‐releasing intrauterine systems. Seminars in Reproductive Medicine 2001;19(4):355‐63. [DOI] [PubMed] [Google Scholar]
New Ethicals 2000
- Adis International. New Ethicals Compendium. 7th Edition. Auckland: Adis International, 2000. [Google Scholar]
NICE 2005
- National Institute for Health and Care Excellence. Long‐acting reversible contraception. https://www.nice.org.uk/guidance/cg30/chapter/1‐recommendations 2005; Vol. NICE guidelines: CG30. [PubMed]
NICE 2007
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. http://bit.ly/1SQ8SSf 2007; Vol. NICE guidelines: CG44. [PubMed]
Nieboer 2009
- Nieboer TE, Johnson N, Lethaby A, Tavender E, Curr E, Garry R, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD003677.pub4] [DOI] [PubMed] [Google Scholar]
NZ Guidelines 1998
- National Health Committee Working Party. Guidelines for the Management of Heavy Menstrual Bleeding (http://www.nzgg.org.nz/library/gl_complete/gynae_hmb). Wellington, NZ: National Health Committee, 1998. [Google Scholar]
Opperman 1998
- Opperman J, Browning D, Child A, Laverty C, Fraser IS. Pregnancy following rollerball endometrial ablation. Gynaecological Endoscopy 1998;7(1):3‐7. [Google Scholar]
Overton 1997
- Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the MISTLETOE study. British Journal of Obstetrics and Gynaecology 1997;104:1351‐9. [DOI] [PubMed] [Google Scholar]
Preston 1995
- Preston JT, Cameron IT, Adams EJ, Smith SK. Comparative study of tranexamic acid and norethisterone in the treatment of ovulatory menorrhagia. British Journal of Obstetrics and Gynaecology 1995;102:401‐6. [DOI] [PubMed] [Google Scholar]
Reid 2000
- Reid PC, Coker A, Coltart R. Assessment of menstrual blood loss using a pictorial chart: a validation study. British Journal of Obstetrics and Gynaecology 2000;107(3):320‐2. [DOI] [PubMed] [Google Scholar]
RNZCGP 2002
- Hall J. Royal New Zealand College of General Practitioners Research Unit Database 1995‐6. Personal communication 19 July 2002.
Shapley 2004
- Shapley M, Jordan K, Peter PR. An epidemiological survey of symptoms of menstrual loss in the community. British Journal of General Practice 2004;54:359‐63. [PMC free article] [PubMed] [Google Scholar]
Tan 2013
- Tan YH, Lethaby A. Pre‐operative endometrial thinning agents before endometrial destruction for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010241.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Marjoribanks 2002
- Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD003855] [DOI] [Google Scholar]
Marjoribanks 2003
- Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003855] [DOI] [PubMed] [Google Scholar]
Marjoribanks 2006
- Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding.. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003855.pub2] [DOI] [PubMed] [Google Scholar]


