Abstract
Background
Although endometrial adenocarcinoma is a common gynaecological cancer, a comparatively small proportion of patients present with, or develop, recurrent or advanced disease. However, for those women whose disease does progress or recur the prognosis is poor and the best treatment is yet to be identified. Co‐morbidity, including obesity and cardiac disease, and concerns over toxicity have prevented more extensive studies of cytotoxic chemotherapy, although there are a number of active agents.
Objectives
To assess any benefits or adverse effects of cytotoxic chemotherapy in women with advanced, recurrent or metastatic endometrial adenocarcinoma.
Search methods
Systematic searches of MEDLINE, EMBASE, CENTRAL and the Cochrane Gynaecological Cancer specialist trials register were conducted to identify all eligible randomised controlled trials (RCTs).Databases were searched from 1966 to January 2012. Literature searches were supplemented with searches of relevant trials registers and conference proceedings.
Selection criteria
RCTs comparing chemotherapy versus another intervention (including different chemotherapy) in advanced disease were considered. Trials of adjuvant treatment or for sarcomatous tumours were excluded.
Data collection and analysis
Data were extracted from the papers by review authors and authors of included studies contacted for further information.
Main results
Fourteen eligible trials, which recruited patients between 1974 and 2005, were identified, eight of which compared 'more' with 'less' chemotherapy. Results from these eight trials, including 1519 patients, showed that treatment consisting of 'more' chemotherapy was associated with longer overall survival (OS) (hazard ratio (HR) 0.86; 95% confidence intervals (CI) 0.77 to 0.96; P = 0.005) and with longer progression‐free survival (PFS) (n = 1526; HR 0.82; 95% CI 0.74 to 0.90; P < 0.0001). However, serious acute toxicities were more common in women randomised to the more‐intense chemotherapy regimens.
There was no evidence to suggest that any particular doublet chemotherapy was better (or worse) than any other, or that any single‐agent chemotherapy was better (or worse) than another; however, data for these two comparisons were limited. There were no comparative trials of chemotherapy with endocrine therapy or best supportive care alone.
Authors' conclusions
This review suggests that more‐intense chemotherapy regimens may improve both OS and PFS for women with advanced or recurrent endometrial cancer. However, owing to inconsistencies between cytotoxic drug combinations that have been assessed in randomised trials to date, the optimum regimen has still to be defined. Future trials should aim to include measures of quality of life (QoL) and symptom control in addition to survival and progression outcomes.
Keywords: Female; Humans; Antineoplastic Agents; Antineoplastic Agents/adverse effects; Antineoplastic Agents/therapeutic use; Antineoplastic Combined Chemotherapy Protocols; Antineoplastic Combined Chemotherapy Protocols/therapeutic use; Endometrial Neoplasms; Endometrial Neoplasms/drug therapy; Endometrial Neoplasms/pathology; Neoplasm Recurrence, Local; Neoplasm Recurrence, Local/drug therapy; Neoplasm Recurrence, Local/pathology; Randomized Controlled Trials as Topic
Plain language summary
More chemotherapy helps women with advanced endometrial cancer; however, the best combination of chemotherapy drugs is still not clear
Using more chemotherapy drugs in combination seems to help women with advanced or recurrent endometrial cancer to live for longer and to delay the cancer from spreading or getting worse. However, giving these extra drugs may cause more serious short‐term side effects. We do not know what effect using more drugs has on long‐term side effects, control of symptoms or quality of life because they were poorly studied in the individual trials included in this review.
Background
Description of the condition
There were around 280,000 cases of endometrial adenocarcinoma worldwide in 2008 (Jemal 2011); however, there is wide geographical variation. Incidence tends to be highest in more developed areas (12.9 per 100,000 per year) where there were more than 33,000 deaths from the disease in 2008 (Jemal 2011).
Risk factors include chronic exposure to oestrogens such as exogenous unopposed oestrogen used as hormone replacement therapy (HRT), having few children, early onset of menstruation and late menopause. Obesity is a risk factor owing to conversion of precursor hormones to oestrogen in peripheral fat cells and rising rates of obesity in affluent countries may result in an increasing incidence of endometrial cancer. Diabetes seems to be a separate risk factor. Trials of adjuvant tamoxifen given to women being treated for breast cancer have shown that there is an increased risk of endometrial cancer from using tamoxifen (it is a weak oestrogen); an approximate doubling of the risk was seen with one to two years of therapy and a quadrupling of the risk was seen with five years of therapy (EBCTCG 1998).
Ninety per cent of uterine cancers are adenocarcinoma and arise in the endometrium (lining of the uterus). Histological grading is split into three categories; grade I being well differentiated and grade III poorly differentiated (differentiation refers to how abnormal the cells and tissue patterns are). The degree of spread of the tumour is classified according to the Federation of International Gynaecologists and Obstetricians (FIGO) staging system (FIGO 2009). Eighty per cent of women who present with endometrial cancer that has not spread from the uterus have good overall survival (OS). Despite this, certain features identify women with higher risk of recurrence: certain histological subtypes (e.g. papillary serous carcinoma, endometrioid carcinoma and clear cell carcinoma); high grade, deep invasion in the muscle of the uterus; tumour extending to the cervix; spread to lymphatic or blood vessels and extra‐uterine disease (Bremond 2001).
Description of the intervention
Optimum treatment for endometrial cancer depends on stage and grade. Almost 90% of women with endometrial cancer are treated by primary surgery with five‐year survival rates of over 70%. Adjuvant radiotherapy following surgery may be offered if the risk of local recurrence is high (grade III or stage Ib and above) and adjuvant treatments, such as endocrine therapy or chemotherapy, have been investigated in women at high risk of recurrence. Treatment failure, defined as recurrence or spread to other parts of the body (metastases), is common particularly in women with advanced‐stage disease at diagnosis or those with high‐risk features in the primary tumour. It is important to distinguish between isolated pelvic relapse and relapse where the cancer has become widespread. The former can be treated by radiotherapy or exenteration surgery, which is an extensive surgical procedure (Morris 1996). The latter requires palliative treatment. About one third of recurrences are localised to the pelvis and two‐thirds have spread outside the pelvis (Burke 1990).
Women with local recurrences not amenable to surgery or radical irradiation and those with more extensive spread are considered for systemic therapy, which may be endocrine therapy or cytotoxic chemotherapy, depending on grade and the women's general level of fitness (performance status). These women are not considered curable. The aim of systemic therapy should be palliation (relief) of symptoms, improvement in quality of life (QoL), delaying progression of the disease and extending OS. A Cochrane review of hormone therapy in advanced or recurrent disease (Kokka 2010) found no good evidence to suggest that hormone treatments improve survival for these women. A separate Cochrane review of adjuvant chemotherapy (after hysterectomy) for women presenting with earlier stages of endometrial cancer amenable to surgery showed that chemotherapy may improve survival and prolong disease‐free survival for these women (Johnson 2011).
How the intervention might work
Cytotoxic chemotherapy has been used for advanced disease. A number of drugs have shown anti‐tumour activity in Phase II clinical trials, including doxorubicin, epirubicin, cisplatin, carboplatin and paclitaxel. However, the optimum combination of drugs is still not known and the full effects of cytotoxic chemotherapy on survival, progression‐free survival (PFS), control of symptoms and QoL are not well established (Humber 2007).
Why it is important to do this review
The purpose of this review is to assess the potential role of cytotoxic chemotherapy where the cancer has spread to other parts of the body or where local cancer is no longer treatable with surgery or radiotherapy. It updates the original review carried out by Humber and colleagues (Humber 2007). More recently, interest in giving adjuvant chemotherapy to women with endometrial cancer at high risk of developing metastases has grown (Faust 2010, Johnson 2011). This review may therefore also provide useful insights as to which drugs or combinations of drugs may be appropriate for use in this setting.
Objectives
To assess:
the effectiveness of cytotoxic chemotherapy in women with advanced/recurrent/metastatic endometrial adenocarcinoma;
whether there is a most effective drug and/or combination;
the adverse effects of treatment;
the effect of treatment on QoL.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that met the eligibility criteria were considered for inclusion in this review.
Types of participants
We included trials that had randomised women with advanced/recurrent/metastatic endometrial adenocarcinoma (not amenable to potentially curative surgery or radical radiotherapy) who were suitable for cytotoxic chemotherapy.
We excluded trials accruing women who:
had chemotherapy in an adjuvant setting following potentially curative surgery;
had uterine carcinosarcoma or sarcoma (these types of cancer behave differently from the common endometrial cancer);
had earlier stages of endometrial cancer.
Types of interventions
Any cytotoxic chemotherapy versus:
placebo;
best supportive care;
alternative chemotherapy.
For the purposes of this review, cytotoxic chemotherapy is defined as a drug given with the intent of producing tumour regression as defined by World Health Organization (WHO) criteria for assessing response (Miller 1981).
Types of outcome measures
In the original review carried out by Humber and colleagues (Humber 2007), outcomes considered were OS, PFS, QoL, symptom control, acute toxicity and late toxicity. However, since there were insufficient data in that review to assess the effect of treatments on QoL, symptom control or late toxicity, this update also necessarily concentrated on:
OS;
PFS;
'serious' acute toxicity (grade 3 or greater).
Search methods for identification of studies
Electronic searches
The following electronic databases were searched:
the Cochrane Gynaecological Cancer Collaborative Review Group's Trial Register
the Cochrane Central Register of Controlled Trials (CENTRAL; Appendix 1)
MEDLINE: we developed a search strategy consisting of the highly sensitive search strategy (HSSS) for RCTs as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and terms related to the review topic (Appendix 2).
EMBASE (Appendix 3)
Databases were searched from 1966 to January 2012.
Searching other resources
For this update, systematic searches of electronic resources were supplemented by handsearching the reference lists of all published eligible trials; abstract searches from relevant conference proceedings, including annual meetings of the American Society of Clinical Oncology (2006 to 2011), and the biennial meetings of the International Gynecologic Cancer Society (2006 to 2010), the European Society for Medical Oncology (2006 to 2010) and the European Society of Gynecological Oncology (2007 to 2011).
Moreover, we searched trials registries including Clinicaltrials.gov, National Cancer Institute's database Physicians Data Query (PDQ at cancer.gov) and the World Health Organization International Clinical Trials Registry for ongoing and closed trials (www.who.int/ictrp/) (no time‐limits were placed on searches).
For the original review (2005) authors of relevant trials had been contacted to ask if they knew of further data that may or may not have been published. Papers in all languages were sought and translations carried out if necessary.
Data collection and analysis
Selection of studies
Trials identified by the searches were assessed by two review authors independently for inclusion.
Data extraction and management
Data was also extracted independently from the resulting trials by two review authors. Where there were disagreements between the two review authors these were resolved by discussion or if necessary, through consultation with a third review author. No attempt was made to blind review authors to article authors or journals. Data extracted and documented included:
method of randomisation and allocation concealment;
numbers of participants randomised to each arm of the trial;
number of participants excluded from the analysis in each arm of the trial;
whether there was an intention‐to‐treat (ITT) analysis;
length of follow‐up;
participant characteristics (age, histology, grade, extent of disease, previous therapies, performance status, whether disease lies in an area previously treated by radiotherapy and whether any other first‐line treatment had been received);
type of intervention (e.g. drug, dosage and administration regimen/frequency, planned number of cycles);
proportions of participants who received all, part or none of the planned treatment, or who experienced delays during treatment;
hazard ratio (HR) and its variance for survival and PFS or, if these were not reported, other relevant summary statistics or data from Kaplan‐Meier curves for their estimation (Parmar 1998; Tierney 2007);
response rates;
grades and type of acute toxicity.
Where insufficient data were available in the trial report, study authors were contacted and asked whether they could supply summary data from their trial for inclusion in this review.
Assessment of risk of bias in included studies
Each trial was assessed for risk of bias based on the primary outcome of OS using information from the published trial report supplemented with information supplied by study authors where available.
Measures of treatment effect
For meta‐analyses of the time‐to‐event outcomes of OS and PFS, the most appropriate statistic is the HR. If provided in a trial report or by the investigators, the HR and associated statistics were used directly in the meta‐analysis. Alternatively, they were estimated indirectly from other summary statistics or from data extracted from published Kaplan‐Meier curves using the methods described in Parmar 1998 and Tierney 2007.
Where sufficient data were reported, odds ratios (ORs) for each acute toxicity category were calculated from the total number of grade 3 and 4 events in each treatment arm. Where more than one type of toxicity was reported in a given category (e.g. nausea reported separately from vomiting), the most frequent was used.
Data synthesis
For the outcomes of OS and PFS, the estimated log HR and variance for individual trials were combined across all trials using a fixed‐effect model to give a pooled HR (Yusuf 1985). This represents the overall risk of an event on the research treatment versus control. Absolute differences in median survival and PFS were estimated using the HR and the average control group median survival or PFS ((median/HR) ‐ median).
For categories of acute toxicity, ORs were calculated for individual studies and were combined across all trials, using a fixed‐effect model and indicate the overall odds of a severe toxic event for each type of toxicity in the treatment arm versus the control arm.
Subgroup analysis and investigation of heterogeneity
Eligible trials were grouped within three main categories to facilitate interpretation of results, using the same main categories as in the original review (Humber 2005).
1. More‐ versus less‐intense chemotherapy
Single chemotherapy agent versus the same agent in combination with one or more additional chemotherapy agents.
Two chemotherapy agents versus the same two agents in combination with one or more additional agents.
Combination of any two‐agent chemotherapy agents with a combination of more than two chemotherapy agents.
2. Doublet chemotherapy versus alternative doublet chemotherapy
Two‐agents versus two agents (substitution of one of the agents for another, or in one trial, the same two‐agents on each arm but administered according to different timing regimens).
3. Single‐agent chemotherapy versus alternative single‐agent chemotherapy
A single‐agent versus a different single‐agent chemotherapy.
Results
Description of studies
Results of the search
A total of 3293 references were retrieved via the electronic searches, of which 14 were found to be eligible RCTs. No trials were excluded (Figure 1). Of the 14 eligible trial reports, three gave limited data on the primary end points of S and PFS (Horton 1978; Long 2006; Pawinski 1999). Further information was requested from all authors resulting in additional or updated data being obtained for five trials (Edmonson 1987; Horton 1982;Long 2006; Nomura 2010a; Pawinski 1999).
1.
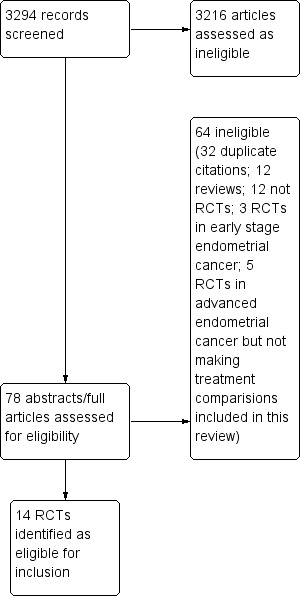
Study flow diagram.
Two further studies (NCT00052312; NCT00063999) were identified via the searches of trial registers; however, as both of these studies are listed as "Active, not recruiting" and no data are yet available, they have not been evaluated as part of this review.
Included studies
Comparison 1: Trials of more versus less chemotherapy
Eight of the eligible trials made a comparison of more‐intense versus less‐intense chemotherapy. These eight trials were grouped according to the treatment comparisons made as follows:
Cisplatin combined with other drugs compared with cisplatin as a single agent
Edmonson 1987: one small trial randomised women to receive cisplatin alone or cisplatin in combination with doxorubicin and cyclophosphamide. Thirty women with histologically confirmed, evaluable, progestin‐refractory, metastatic endometrial cancer were recruited into this study between May 1980 and September 1983. All patients randomised were treated and analysed for efficacy. Dose reductions were made for heavily pre‐irradiated patients. Patients progressing on cisplatin alone were allowed to cross‐over into the combination arm. Additional information on survival, PFS and toxicity from an updated analysis (April 2004) were supplied by the investigators for inclusion in this review.
Doxorubicin combined with other drugs compared with doxorubicin as a single agent
Thigpen 1994: 387 women with histologically documented, advanced or recurrent endometrial carcinoma, not amenable to surgery or radiotherapy were entered into this study between 1979 and 1985. They were randomised to receive treatment with doxorubicin alone or in combination with cyclophosphamide. All patients had been treated with progestins in the past, but none had received prior cytotoxic chemotherapy. A request for further information on the main outcomes was declined by the investigators (2004).
Aapro 2003: 177 patients with advanced, inoperable or recurrent endometrial cancer were recruited into this study between September 1988 and June 1994. They were randomly assigned to receive doxorubicin alone or in combination with cisplatin. One of the patients in the doxorubicin arm had received prior chemotherapy.
Thigpen 2004: 299 women with advanced or recurrent endometrial carcinoma were recruited into this study between December 1988 and December 1992. They were randomised to treatment with doxorubicin alone or in combination with cisplatin. None of the patients had been previously treated with chemotherapy.
Doxorubicin and cisplatin combined with other drugs compared with doxorubicin and cisplatin
Long 2006: 28 women with advanced or recurrent endometrial carcinoma were entered into this trial between March 1992 and December 1993. They were randomised to receive doxorubicin and cisplatin or doxorubicin, cisplatin, methotrexate and vinblastine. None of the patients had received prior chemotherapy.
Fleming 2004a: 273 women with either stage III to IV or recurrent endometrial cancer were registered for this study between December 1998 and August 2000. Women were randomised to receive treatment with doxorubicin and cisplatin or doxorubicin, cisplatin and paclitaxel (plus granulocyte colony‐stimulating factor, G‐CSF) support. None of the patients had received prior chemotherapy.
Three‐drug combination chemotherapy (plus hormones) compared with two‐drug combination chemotherapy (plus hormones)
Cohen 1984: 295 women with advanced or recurrent endometrial adenocarcinoma were recruited into this study between May 1977 and October 1979. Patients were required to have primary stage III, stage IV, recurrent or residual endometrial adenocarcinoma considered incurable by radiation or surgery alone or in combination. Patients were excluded if they had received prior cytotoxic chemotherapy. Patients were randomised to melphalan, 5‐fluorouracil (5‐FU) and megestrol or doxorubicin, cyclophosphamide, 5‐FU and megestrol.
Horton 1982: 131 women with either histologically confirmed stage III to IV, recurrent or metastatic adenocarcinoma of the uterine corpus, who were not candidates for surgery or radiotherapy, were recruited between January 1977 and June 1979. Patients were randomised to receive either cyclophosphamide, doxorubicin and megestrol or cyclophosphamide, doxorubicin, 5‐FU and megestrol. Additional data, from updated ITT analyses of acute toxicity, OS and PFS were supplied by the investigators for inclusion in this review (February 2011). One patient (in the control arm) had received chemotherapy previously.
Comparison 2: Trials comparing different chemotherapy doublets
Four of the 14 eligible trials compared different two‐drug regimens (or doublets) or different treatment scheduling of a common doublet (Gallion 2003). One three‐arm trial, comparing three separate chemotherapy doublets, was split into two separate comparisons (Nomura 2010a; Nomura 2010b) both including a common 'control' arm for the purposes of the review.
Trials comparing different two‐drug combinations
Fleming 2004b: 328 patients with stage III to IV or recurrent endometrial carcinoma were enrolled between August 1996 and November 1998. Prior treatment with hormones, but not chemotherapy, was permitted. Women were randomised to receive doxorubicin and cisplatin or doxorubicin, paclitaxel and G‐CSF support.
Nomura 2010a/Nomura 2010b: 90 eligible patients with primary stage III to IV or recurrent endometrial carcinoma were enrolled between February 2003 and May 2005. Women were randomised to receive one of three treatment regimens: docetaxel and cisplatin, docetaxel and carboplatin or paclitaxel and carboplatin. After randomisation two of the 90 patients were excluded (prior to treatment) as they did not have measurable lesions. Twenty‐nine patients had received prior chemotherapy treatment. Additional data on OS and PFS were provided by the investigators for inclusion in this update (January 2011). For the purposes of the review, we have made two separate comparisons of docetaxel and cisplatin versus docetaxel and carboplatin (Nomura 2010a) and of paclitaxel and carboplatin versus docetaxel and carboplatin (Nomura 2010b).
Weber 2003: 70 patients with advanced or recurrent endometrial carcinoma were enrolled between 1997 and 2002. Women were randomised to receive doxorubicin and cisplatin or carboplatin and paclitaxel. Additional data regarding OS and PFS were requested (January 2011); however, it was declined by the investigators.
Trials comparing different scheduling of the same two‐drug combination
Gallion 2003: 352 women with primary stage III to IV or recurrent endometrial carcinoma were entered between March 1993 and August 1996. Those who had received prior cytotoxic chemotherapy, more than one prior biological therapy or prior radiotherapy in the previous three months were not eligible. Women were randomised to receive doxorubicin and cisplatin, either as a standard‐timed or circadian‐timed schedule.
Comparison 3: Trials comparing different single agents
Two remaining eligible trials compared different single‐agent chemotherapies:
Horton 1978: 47 women with histologically confirmed endometrial cancer with measurable local extension or metastases not amenable to cure by surgery or radiotherapy were recruited into this study. The trial opened in 1974, but the closing date is unknown. Women were randomised to receive doxorubicin or cyclophosphamide. None of the patients had previously been treated with chemotherapy. Neither OS or PFS were reported and the investigators have confirmed that no further data are available (January 2011).
Pawinski 1999: 74 women with histologically confirmed adenocarcinoma of the endometrium and evidence of recurrent or metastatic disease were randomised to receive cyclophosphamide or ifosfamide between June 1987 and May 1994. Approximately half the patients had received prior chemotherapy. Additional ITT analyses of OS, PFS and acute toxicity were supplied by the investigators for inclusion in this review (February 2011).
Full details of the chemotherapy regimens used in the trials may be found in the Characteristics of included studies table.
Excluded studies
No eligible trials were excluded from this review. However, two trials were reported with insufficient outcome data to allow them to be included in the analyses (Horton 1978; Weber 2003).
Risk of bias in included studies
Allocation
Ten trials gave details of the method of randomisation; seven trials described the sequence generation (Aapro 2003; Fleming 2004b;Gallion 2003; Horton 1982;Long 2006;Nomura 2010a; Thigpen 2004). Three trials described the stratification factors used (Cohen 1984; Edmonson 1987; Horton 1978), giving little indication of biased allocation; however, details of allocation concealment were only described adequately in four reports (Fleming 2004a; Fleming 2004b;Gallion 2003;Thigpen 2004). This may reflect a potential bias or simply be because of inadequate reporting.
Blinding
Owing to the nature of the treatments being used, it was not possible for the participants and personnel in these trials to be blinded to the treatment allocations. The outcome assessment was also not blinded; however, for the primary outcome of OS, this is not likely to introduce bias.
Incomplete outcome data
Four studies did not exclude any randomised patients from the analyses (Horton 1982; Long 2006; Pawinski 1999; Weber 2003) and a further eight studies excluded only a small percentage of patients from the analyses (Aapro 2003; Edmonson 1987; Fleming 2004a; Fleming 2004b; Gallion 2003; Nomura 2010a; Nomura 2010b; Thigpen 1994; Thigpen 2004). Two studies (Cohen 1984; Horton 1978) excluded 13% and 15% of the total randomised patients from the reported analyses; however, in both cases, the exclusions were reported to be balanced by arm and therefore may be less likely to introduce bias in the results. Full details of these exclusions are given in the Characteristics of included studies table.
Selective reporting
None of the protocols for included studies were available to check the planned outcomes of the studies. However, all but two studies (Horton 1978; Weber 2003) reported the outcomes of interest for this review in sufficient detail to be included in the analyses.
Effects of interventions
The complete, partial and overall response rates; median PFS/interval and median survival for all RCTs are summarised in Table 1, Table 2 and Table 3.
1. Comparison 1: More versus less chemotherapy: response rates, progression‐free survival and overall survival.
| Study | Patients randomised (patients analysed) | Median age (years) (range) | Type of patients | ORR (%) | Median PFS (months) | Median OS (months) |
| Horton 1982 | ||||||
| Megestrol, cyclophosphamide, doxorubicin | 64 (64) | 63 (42‐85) | Stage III‐IV, recurrent or metastatic | 23% | NR | NR |
| Megestrol, cyclophosphamide, doxorubicin, 5‐FU | 67 (67) | 65 (37‐81) | 13% | NR | NR | |
| Aapro 2003 | ||||||
| Doxorubicin | 87 (87) | 63 (41‐76) | Advanced or recurrent | 17% | †7 | 7 |
| Doxorubicin, cisplatin | 90 (90) | 63 (40‐76) | 43% | †8 | 9 | |
| Cohen 1984 | ||||||
| Melphalan, 5‐FU, megestrol acetate | 146 (126) | 63 (33‐82) | Stage III‐IV or recurrent | 38%* | †6 | 11 |
| Doxorubicin, cyclophosphamide, 5‐FU, megestrol acetate | 149 (131) | 36%** | †5 | 10 | ||
| Edmonson 1987 | ||||||
| Cisplatin | 14 (14) | 64 (41‐78) | Progestin‐refractory, Stage III‐IV metastatic | 21% | 1.8 | 7.4 |
| Cisplatin, doxorubicin, cyclophosphamide | 16 (16) | 66 (52‐79) | 31% | 2.9 | 6.6 | |
| Fleming 2004a | ||||||
| Doxorubicin, cisplatin | 136 (129) | NR (≤ 50 to 81) | Stage III‐IV or recurrent | 34% | 5.3 | 12.3 |
| Doxorubicin, cisplatin, paclitaxel | 137 (134) | 57% | 8.3 | 15.3 | ||
| Long 2006 | ||||||
| Doxorubicin, cyclophosphamide | 15 (15) | 65 (49‐77) | Advanced, recurrent or metastatic | 26% | 6.2 | 15 |
| Doxorubicin, cyclophosphamide, methotrexate, vinblastine | 13 (13) | 67 (37‐79) | 69% | 6.9 | 15 | |
| Thigpen 1994 | ||||||
| Doxorubicin | 171 (132) | 65.1 (36‐90) | Advanced or recurrent | 22% | †3.2 | 6.9 |
| Doxorubicin, cyclophosphamide | 185 (144) | 65.0 (43‐83) | 30% | †3.9 | 7.3 | |
| Thigpen 2004 | ||||||
| Doxorubicin | 150 (150) | 66.9 (NS) | Stage III‐IV or recurrent | 25% | 5.7 | 9.0 |
| Doxorubicin, cisplatin | 131 (131) | 64.4 (NS) | 42% | 3.8 | 9.2 | |
5‐FU: 5‐fluorouracil; ORR: objective response rate; PFS: progression‐free survival, OS: overall survival; NR: not reported
* response based on 77 patients; ** response based on 78 patients
† Based on time to progression not PFS
2. Comparison 2: Doublet versus doublet chemotherapy: response rates, progression‐free survival and overall survival.
| Study | Patients randomised (patients analysed) |
Median age (years) (range) |
Type of patients | ORR (%) | Median PFS (months) | Median OS (months) |
| Fleming 2004b | ||||||
| Doxorubicin, cisplatin | 160 (157) | NR (≤ 60 ‐ ≥71)** | Stage III‐IV or recurrent | 40% | 7.2 | 12.6 |
| Doxorubicin, paclitaxel, filgrastim | 168 (160) | 43% | 6.0 | 13.6 | ||
| Gallion 2003 | ||||||
| Standard timed doxorubicin + cisplatin | 175 (169) | 65 (≤ 50 to 81) | Stage III‐IV, persistent or recurrent | 46% | 6.5 | 11.2 |
| Circadian timed doxorubicin + cisplatin | 177 (173) | 49% | 5.9 | 13.2 | ||
| Weber 2003 | ||||||
| Doxorubicin, cisplatin | 34 (29) | 64 (44‐75) | Advanced or recurrent | 27.6% | *6.7 | NR |
| Carboplatin, paclitaxel | 36 (34) | 35.3% | *7.7 | NR | ||
| Nomura 2010a | ||||||
| Docetaxel, carboplatin | 30 (29) | 66 (54‐73) | Advanced, recurrent or metastatic |
51.7% | 7.6 | 20.7 |
| Docetaxel, cisplatin | 30 (29) | 64 (39‐74) | 48.3% | 7.8 | 24 | |
| Nomura 2010b | ||||||
| Docetaxel, carboplatin | 30 (29) | 66 (54‐73) | Advanced, recurrent or metastatic |
51.7% | 7.6 | 20.7 |
| Paclitaxel, carboplatin | 30 (30) | 61 (49‐74) | 60.0% | 9.5 | 28.1 | |
ORR: objective response rate; PFS: progression‐free survival; OS overall survival; NR: not stated reported
* Time to progression (not PFS)
** Report states that age was not well balanced by treatment arm
3. Comparison 3: Single‐agent versus single‐agent chemotherapy: response rates, progression‐free survival and overall survival times.
| Study | Patients randomised (patients analysed) | Median age (years) (range) | Type of patients | ORR (%) | Median PFS (months) | Median OS (months) |
| Horton 1978 | ||||||
| Doxorubicin | 21 (21) | NR | Local extension or metastases | 19% | NR | NR |
| Cyclophosphamide | 19 (19) | 0% | NR | NR | ||
| Pawinski 1999 | ||||||
| Cyclophosphamide | 36 | 62.5 (45‐73) | Recurrent or metastatic |
7% | 1.6 | NR |
| Ifosfamide | 38 | 61.0 (40‐70) | 12% | 1.8 | NR | |
ORR: objective response rate; PFS: progression‐free survival; OS overall survival; NR: not stated reported
Comparison 1: trials of more versus less chemotherapy
Overall, the trials of more versus less chemotherapy tended to be well balanced with respect to well‐known prognostic factors such as age, stage and performance status with median age across all studies ranging from 63 to 67 years (Table 1). However, in one study (Edmonson 1987) a slightly higher proportion of women had received prior radiation in the treatment arm. In two studies, there was a higher proportion of poorly differentiated, grade III tumours in the control arm (Edmonson 1987; Thigpen 1994).
Although not pre‐specified as an outcome, the objective response rate tended to be improved in the 'more' chemotherapy arms, compared with less chemotherapy in all except two of the trials (Cohen 1984; Horton 1982; Table 1). However, these differences were only statistically significant for two of the trials (Fleming 2004a; Thigpen 2004).
Overall survival
For the main outcomes of OS, data were available for all eight trials, including 1519 patients. Across the trials there was a significant 14% reduction the risk of death with more chemotherapy compared with less chemotherapy (HR 0.86; 95% CI 0.77 to 0.96; P = 0.005; Analysis 1.1), with little evidence of heterogeneity (P = 0.90; I2 = 0%). This represents a potential absolute improvement in absolute survival of about 1.5 months (from 9 to 10.5 months).
1.1. Analysis.
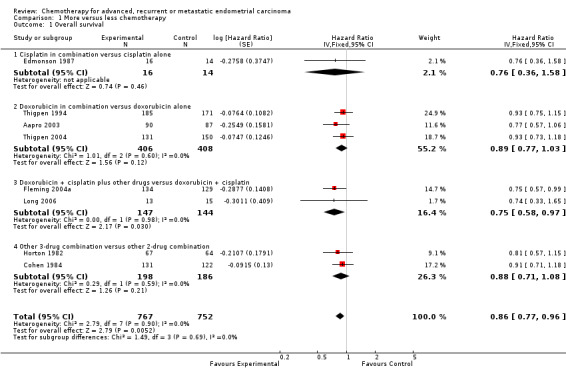
Comparison 1 More versus less chemotherapy, Outcome 1 Overall survival.
Despite differences in the drugs and combinations included in these trials, there was no suggestion that the results varied between subgroups (test for interaction P = 0.69). While the results for each subgroup tended towards favouring more treatment, only one of the subgroups had a significant treatment effect (doxorubicin and cisplatin in combination with other drugs compared with doxorubicin and cisplatin alone). However, as this result was based on only two trials, including 291 patients, it should be treated with caution.
Progression‐free survival
Progression data were available for all eight trials, including 1526 patients; however, three studies (Aapro 2003; Cohen 1984; Thigpen 1994) reported only time to progression and not PFS. While the results from these trials were combined with the PFS results of the remaining trials, they may give a somewhat different estimate of the effect of treatment, since deaths were censored rather than included as events. Overall the HR of 0.82 (95% CI 0.74 to 0.90) across trials shows a highly significant 18% relative improvement in the time to progression/PFS with the more‐intense regimens compared to the less‐intense regimens (P < 0.0001), which suggests an absolute improvement of approximately one month (from six to seven months).
The results across all trials tended to favour more chemotherapy and there was no strong evidence that the results varied either between individual trials (heterogeneity P = 0.23; I2 = 25%) or between trial subgroups (test for interaction P = 0.19). However, the trial subgroup that compared doxorubicin and cisplatin plus other drugs versus doxorubicin and cisplatin alone and that comparing doxorubicin in combination with other drugs versus doxorubicin alone had results that were significantly in favour of the more chemotherapy arm (Analysis 1.2).
1.2. Analysis.
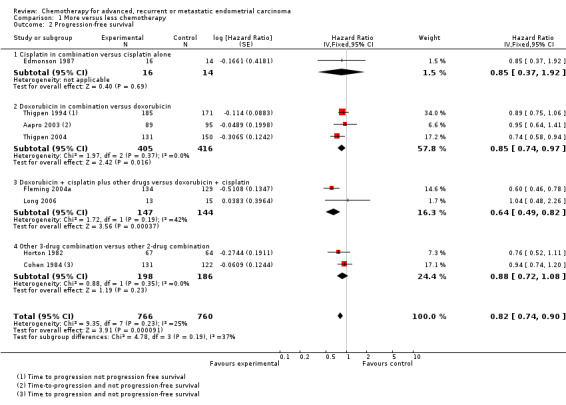
Comparison 1 More versus less chemotherapy, Outcome 2 Progression‐free survival.
Acute toxicity
To assess toxicity, three trials used the National Cancer Institute Common Toxicity Criteria (NCI CTC), either version 1 (Edmonson 1987; Long 2006), or version 2 (Fleming 2004a). Four trials used either the WHO/Eastern Cooperative Oncology Group (ECOG) (Aapro 2003; Thigpen 1994) or the Gynecologic Oncology Group (GOG) criteria (Cohen 1984; Thigpen 2004). One further trial did not state which scale was used (Horton 1982). However, as all trials used a 5‐point system where 0 was no toxicity and 5 was toxicity‐related death, it was appropriate to combine data across all trials.
Data on serious (i.e. grade 3 or 4) nausea and vomiting, diarrhoea/other GI toxicity, white blood cell toxicity, thrombocytopenia, anaemia and alopecia were available in sufficient detail for formal analyses. Grade 3‐4 cardiotoxicity, fever/infection, neurological toxicity, stomatatis/mucositis and renal/genitourinary toxicity were reported less frequently or for fewer trials, possibly as their occurrence depends on the individual drugs being given. Thus formal analyses of these types of toxicity would be unreliable and were not completed.
Across all trials, serious nausea and vomiting more than doubled with the more‐intense regimens compared with less‐intense chemotherapies (OR 2.64; 95% CI 1.71 to 4.09; P < 0.00001; Analysis 1.3). While the results appeared fairly consistent across all trials (heterogeneity P = 0.21; I2 = 32%), they did vary between the trial subgroups. For diarrhoea/other GI toxicities, although there were few grade 3 or 4 events overall (n = 31), there seemed to be an excess with more chemotherapy compared to less chemotherapy (OR 2.25; 95% CI 1.09 to 4.63; Analysis 1.4).
1.3. Analysis.
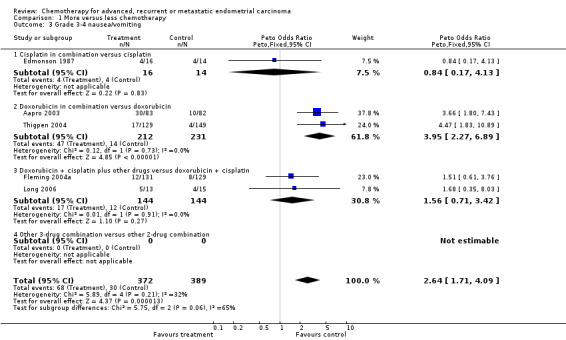
Comparison 1 More versus less chemotherapy, Outcome 3 Grade 3‐4 nausea/vomiting.
1.4. Analysis.
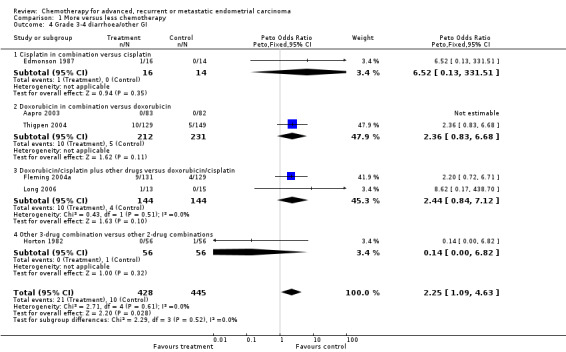
Comparison 1 More versus less chemotherapy, Outcome 4 Grade 3‐4 diarrhoea/other GI.
Results for serious white blood cell toxicities (including leukopenia, neutropenia and granulocytopenia) were much more variable from trial to trial (heterogeneity P < 0.0001; I2 = 89%), and by trial subgroup (test for interaction P < 0.00001) such that it did not seem reasonable to pool these results using either a fixed‐ or a random‐effects model (Analysis 1.5). The effects of more chemotherapy on serious thrombocytopenia were similarly varied between trials (heterogeneity P < 0.00001; I2 = 91%) and subgroups (test for interaction P < 0.0001; Analysis 1.6) and so results across all trials were not pooled.
1.5. Analysis.
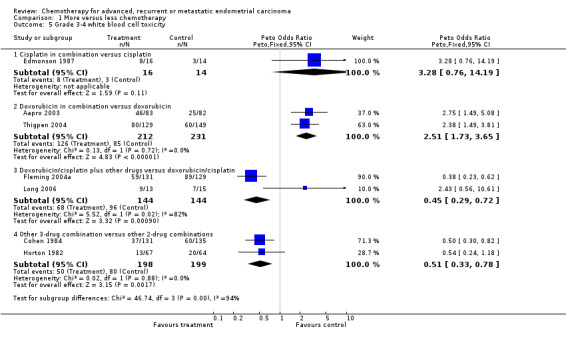
Comparison 1 More versus less chemotherapy, Outcome 5 Grade 3‐4 white blood cell toxicity.
1.6. Analysis.
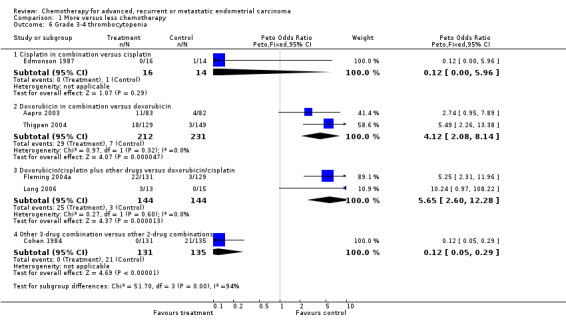
Comparison 1 More versus less chemotherapy, Outcome 6 Grade 3‐4 thrombocytopenia.
Although based on data from only one trial (Thigpen 2004; total number of events 35), serious anaemia was increased (OR 5.32; 95% CI 2.62 to 10.81; P < 0.0001) with more chemotherapy (Analysis 1.7). Serious alopecia was also increased with more chemotherapy (OR 1.86; 95% CI 1.01 to 3.42; P = 0.05; Analysis 1.8); however, this is based on data from only two trials and the events were dominated by one of those trials (Aapro 2003) that compared doxorubicin alone and in combination.
1.7. Analysis.
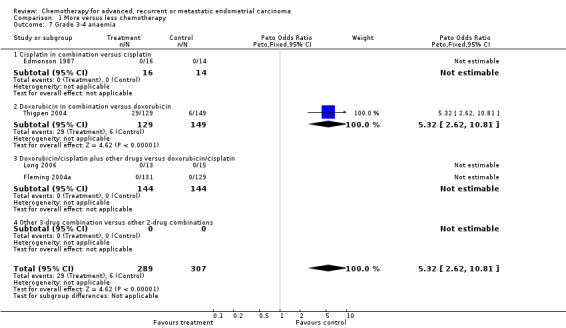
Comparison 1 More versus less chemotherapy, Outcome 7 Grade 3‐4 anaemia.
1.8. Analysis.
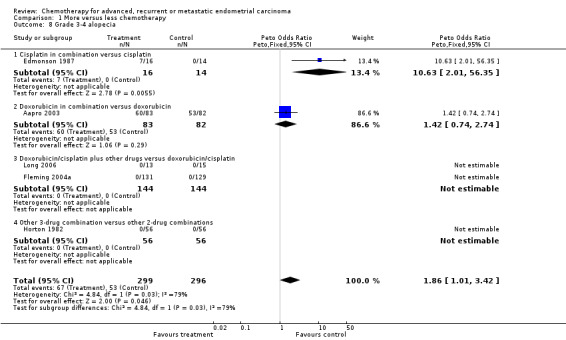
Comparison 1 More versus less chemotherapy, Outcome 8 Grade 3‐4 alopecia.
Across all trials, 19 patients were reported to have died owing to treatment‐related toxicity; however, not all trials reports whether these deaths were in the more or less chemotherapy arms. In addition, a number of trials reported that toxicity was a factor that led to protocol violations or discontinuation of treatment. Cohen 1984 stated that 35/107 patients with serious haematological toxicity had protocol violations; Aapro 2003 reported that 10% of patients receiving more chemotherapy and 2% of those receiving less chemotherapy stopped treatment due to "extensive toxicity" and Fleming 2004a reported that 32/134 patients (24%) receiving more chemotherapy and 12/129 patients (9%) receiving less chemotherapy discontinued treatment due to toxicity.
Comparison 2: trials comparing different chemotherapy doublets
Overall, the trials included in this comparison tended to be well balanced with respect to well‐known prognostic factors such as age, stage and performance status with median age across all studies ranging from 61 to 66 years (Table 2). However, one study (Fleming 2004b) reported that patients were not well balanced by age. There were no significant differences reported between the treatment arms for any of the trials in terms of objective response rates and both the median OS and PFS times were also similar between the treatment arms for all of the trials (Table 2). For one trial (Weber 2003) insufficient outcome data were available for formal analyses of any of the outcomes.
For the three remaining trials in this comparison, there was no evidence that any of the treatment comparisons made differed in terms of either OS (Analysis 2.1) or PFS (Analysis 2.2). Because the treatment comparisons being made between the studies varied widely in terms of the chemotherapy doublets being investigated, no overall effect was calculated.
2.1. Analysis.
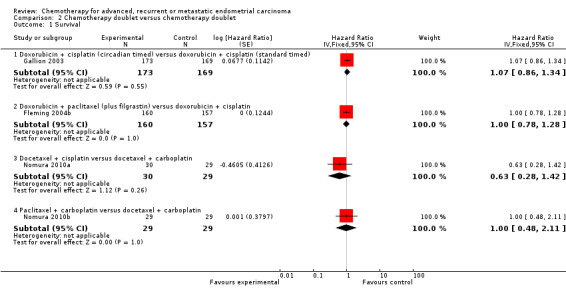
Comparison 2 Chemotherapy doublet versus chemotherapy doublet, Outcome 1 Survival.
2.2. Analysis.
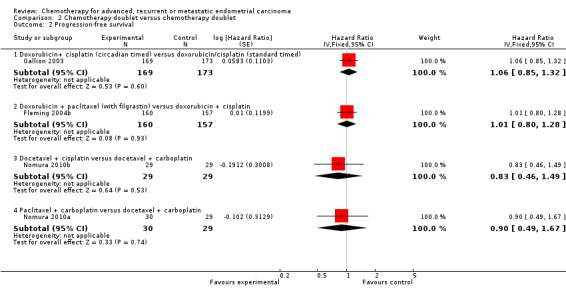
Comparison 2 Chemotherapy doublet versus chemotherapy doublet, Outcome 2 Progression‐free survival.
Acute toxicity was assessed using GOG criteria for two trials (Fleming 2004b; Gallion 2003) and using NCU CTC version 2 for one further trial (Nomura 2010a; Nomura 2010b). All three trials reported that the major toxicities in both arms were haematological (leukopenia or granulocytopenia). In two trials (Fleming 2004b; Nomura 2010a; Nomura 2010b), while the rates of grade 3 to 4 haematological toxicity were in excess of 70% overall, there was no significant difference between the treatment arms. In the third trial (Gallion 2003), rates were similarly high (> 70% overall); however, there was a significant difference in the rates of both leukopenia (P = 0.01) and granulocytopenia (P = 0.04) that favoured the circadian treatment schedule.
The most common serious non‐haematological toxicity for all three trials was gastrointestinal toxicity. Gallion 2003 and Nomura 2010a; Nomura 2010b reported levels of nausea and vomiting of 14% and 9% overall (respectively) and Fleming 2004b reported rates for all grade 3 to 4 gastrointestinal symptoms of 14%. No significant differences were reported between the treatment arms for any of the studies.
Across all four trials in this comparison, 17 patients were reported to have died due to treatment‐related toxicity, most commonly due to renal failure (four deaths) and sepsis (seven deaths). In addition, Fleming 2004b reported that 48 patients (30% of the total randomised) stopped treatment due to toxicity although the numbers stopping treatment were very similar on the two treatment arms.
Comparison 3: trials comparing different single agents
This comparison includes two trials (Horton 1978; Pawinski 1999) that compared different single‐agent chemotherapy regimens (Table 3). However, data for inclusion in this meta‐analysis were only available for one trial (Pawinski 1999) that randomised only 74 patients. There was no evidence of a difference in terms of OS (Analysis 3.1) or PFS (Analysis 3.2) between the treatment arms. The most commonly reported serious toxicities for this trial were nausea and vomiting, and alopecia. Grade 3 nausea and vomiting was reported for 20 patients (27%) overall, with no difference between the treatment arms. Grade 3 alopecia was reported for 12 patients (16% of the total randomised) receiving ifosfamide and six patients (8% of the total randomised) receiving cyclophosphamide.
3.1. Analysis.
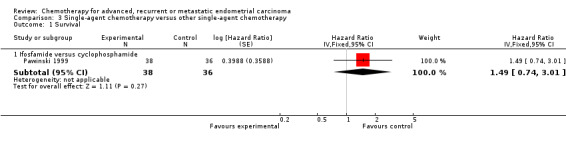
Comparison 3 Single‐agent chemotherapy versus other single‐agent chemotherapy, Outcome 1 Survival.
3.2. Analysis.
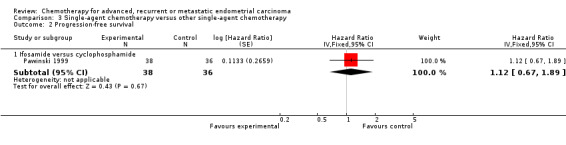
Comparison 3 Single‐agent chemotherapy versus other single‐agent chemotherapy, Outcome 2 Progression‐free survival.
No deaths were reported as being due to treatment‐related toxicity for either trial; however, toxicity was cited as the reason that three patients discontinued treatment in one trial (Pawinski 1999).
Discussion
Summary of main results
Our searches identified 14 RCTs, randomising 2525 women, of cytotoxic chemotherapy in advanced or recurrent endometrial adenocarcinoma. The apparent quality of these trials based largely on the trial reports was variable. For example, it is not known how randomisation occurred in a number of the studies and the method of concealment of allocation generally was not reported. Three trials randomised fewer than 50 patients (Edmonson 1987; Horton 1978; Long 2006) and so would only be sufficiently powered to detect a very large difference in the effectiveness between the two arms. Two studies were stopped prematurely (Aapro 2003; Long 2006). Aapro 2003 cited a dramatic fall in recruitment after the publication results of Thigpen 2004 in abstract form as the reason for early termination. Long 1995 stopped early after only accruing 28 patients, at a slow rate. Furthermore, some trials excluded relatively large numbers of patients after randomisation such that analyses were not always on an "intention‐to‐treat" basis. Therefore, the possibility of some bias in the estimates of treatment effect cannot be ruled out. Trials were grouped into three main comparisons according to the broad comparisons made. The majority (8 trials, 1571 women) compared more versus less chemotherapy and this review largely focused on the results of this comparison.
Across the eight trials comparing more‐ versus less‐intense chemotherapy, the results for both OS and PFS were consistent across the trial groups and in favour of the more‐intensive chemotherapy regimens. As the control arms for some of these trials are the comparators for others, it is not possible from these results to recommend a particular regimen. However, in terms of survival and PFS, three or more drug combinations including doxorubicin and cisplatin seemed better than the two‐drug combination of doxorubicin and cisplatin, which in turn appeared to improve upon single‐agent doxorubicin chemotherapy. However, more chemotherapy was generally associated with increased rates of serious acute toxicities, most commonly haematological and gastrointestinal. Survival benefits should therefore be considered alongside these increases in toxicity, in particular as advanced endometrial cancer tends to affect more elderly women often with other co‐morbidities. While two trials reported increases in alopecia, as may be expected with doxorubicin‐based regimens, there was a lack of alopecia data reported in trials using paclitaxel‐containing regimens, where alopecia may also be expected to be common.
The data does not allow any conclusions to be made about which patients may potentially gain the greatest benefits from chemotherapy. The randomised trials enrolled a heterogeneous group of patients in terms of prior therapies, which included hormonal agents, radiotherapy and cytotoxic agents, may have impacted on the response to chemotherapy in this advanced setting. The trials also included patients with both advanced and recurrent disease and there may be differences between these patient groups in terms of how well they respond to treatments. These and other factors including performance status and other co‐morbidities may impact on how much benefit a patient obtains from treatment as well as how they tolerate more‐intensive chemotherapy. There are no data regarding correlation between sites of metastatic disease and benefit (e.g. any metastases versus visceral metastases). There is therefore little evidence base from randomised studies to help guide initial choice of therapy, be it endocrine, chemotherapy or best supportive care.
There was no good evidence from the RCTs comparing other chemotherapy regimens or scheduling, that any were superior in terms of improved survival and PFS and/or reduced toxicity; however, insufficient data and inconsistent 'control' arm treatments between the trials comparing different two‐drug combinations makes any conclusion difficult. Moreover, these modestly sized trials are unlikely to be adequately powered to discriminate between the treatment arms. Only two trials compared single‐agent chemotherapies and these are possibly now outdated. While traditionally, doxorubicin has been the most common drug used in the treatment of endometrial cancer, more recently, paclitaxel and carboplatin have become more widely used, possibly as a result of their routine use in the treatment of ovarian and, increasingly, cervical cancer. Results of one of the currently active trials (NCT00063999) may help to determine whether this two‐drug combination improves on doxorubicin‐based treatment for women with advanced endometrial cancer.
As no QoL data were collected or reported in any of the trials, we cannot draw any conclusions on the potential of chemotherapy to palliate symptoms and improve QoL, although these are clearly important priorities for both patients and clinicians. Given the modest impact of chemotherapy on PFS and OS, collection of such patient‐reported data must be a priority in future research. Reduction in tumour volume may lead to symptomatic benefit, but because such gains may be offset by treatment‐related toxicity from treatment, it is sensible to try to assess effects on QoL in a systematic way in trials of palliative therapy. Reproducible scoring systems are available for assessing both symptoms and global QoL. Trials in other tumour types such as non‐small cell lung cancer have used such scoring systems and demonstrated significant improvements in QoL, even where survival benefits were small (Cullen 1999).
Authors' conclusions
Implications for practice.
More‐intensive cytotoxic chemotherapy regimens, with drugs including cisplatin, carboplatin, doxorubicin and taxanes may improve OS and PFS in women with advanced, metastatic or recurrent endometrial adenocarcinoma. However, the optimal treatment regimen is still unclear and benefits are at the expense of increased acute toxicity. These issues should be discussed with patients before they consent to chemotherapy and where possible, patients should be offered entry into well‐designed RCTs of treatment.
No single‐agent or combination chemotherapy stands out. However, our results suggest the combination of up to three drugs, including doxorubicin, may be a reasonable choice. Two active studies identified in our searches (NCT00052312; NCT00063999) are evaluating the use of the three‐drug combination of doxorubicin, cisplatin and paclitaxel, albeit that the comparators differ between the two studies, with one using doxorubicin and cisplatin (NCT00052312) and the other using paclitaxel and carboplatin (NCT00063999). Results of these two studies, once available, may help to define an optimal treatment regimen for women with advanced disease.
Implications for research.
Despite the involvement of over 2500 women in randomised trials, basic questions have not been answered. While the results of this review demonstrate improvements in survival and PFS with some of the more‐intensive chemotherapy regimens in women with advanced, recurrent or metastatic endometrial adenocarcinoma, there may remain a need to randomise women with advanced, recurrent and metastatic disease to receive chemotherapy, hormone therapy or best supportive care and to assessment properly the impact of treatment within subgroups of patients defined by the stage or extent of disease, prior treatments and baseline characteristics that may influence the response to treatment. In this way, patients most likely to benefit may be defined thus reducing unnecessary harms and side effects for those women less likely to benefit. Furthermore, QoL and symptom scores should supplement outcomes such as response, progression and OS.
What's new
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 27 March 2014 | Amended | Contact details updated. |
| 11 July 2012 | Amended | Author details amended. |
| 4 July 2012 | New citation required but conclusions have not changed | Literature searches updated up to January 2012. Four new studies added but conclusions not changed. |
| 10 May 2012 | New search has been performed | Text of the review revised. |
| 14 June 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank Jane Hayes, Information Manager, Cochrane Gynaecological Cancer Review Group for help conducting the searches. They would also like to thank Dr Aapro, Dr Long, Dr Coens (Pawinski), Dr Aoki (Nomura) and Dr Manola (Horton) for providing further information either for this update or for the original review.
The authors are also grateful to the authors of the original version of this review:
Caroline Humber who prepared the protocol, assisted with the searches, extracted data, sought and obtained unpublished data from investigators, wrote the text of the review and prepared the tables.
Mandy Collingwood, who assisted with editing the protocol, helped to prepare the search strategy and obtained relevant papers
John Green, who prepared the abstract and was involved in the development of the protocol and the subsequent review.
John Kirwan, who was involved in the development of the protocol and the subsequent review.
Chris Williams, who was involved in the development of the protocol and the subsequent review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Endometrial Neoplasms explode all trees #2 endometr* near/5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinom* or adenocarcinom*) #3 uter* and lining and (cancer* or tumor* or neoplas* or malignan* or carcinom* or adenocarcinom*) #4 (#1 OR #2 OR #3) #5 advanced or metasta* or recurren* #6 (#4 AND #5) #7 Any MeSH descriptor with qualifier: DH #8 chemotherap* #9 MeSH descriptor Antineoplastic Agents explode all trees #10 MeSH descriptor Antineoplastic Combined Chemotherapy Protocols explode all trees #11 cytotoxic* #12 doxorubicin* #13 adriamycin* #14 doxil* #15 rubex* #16 epirubicin* #17 pharm*rubicin* #18 ellence* #19 hydroxydoxorubicin* #20 hydroxydaunorubicin* #21 cyclophosphamide* #22 cytoxan* #23 neosar* #24 ctx* #25 cisplatin* #26 platinol* #27 cddp* #28 carboplatin* #29 paraplatin* #30 cbdca* #31 ifosfamide* #32 ifex* #33 isophosphamide* #34 paclitaxel* #35 taxol* #36 taxane* #37 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36) #38 (#6 AND #37)
Appendix 2. MEDLINE search strategy
Medline Ovid
1 exp Endometrial Neoplasms/ 2 (endometr* adj5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinom* or adenocarcinom*)).mp. 3 (uter* and lining and (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinom* or adenocarcinom*)).mp. 4 1 or 2 or 3 5 (advanced or metasta* or recurren*).mp. 6 4 and 5 7 drug therapy.fs. 8 chemotherap*.mp. 9 exp Antineoplastic Agents/ 10 exp Antineoplastic Combined Chemotherapy Protocols/ 11 cytotoxic*.mp. 12 doxorubicin*.mp. 13 adriamycin*.mp. 14 doxil*.mp. 15 rubex*.mp. 16 epirubicin*.mp. 17 pharm?rubicin*.mp. 18 ellence*.mp. 19 hydroxydoxorubicin*.mp. 20 hydroxydaunorubicin*.mp. 21 cyclophosphamide*.mp. 22 cytoxan*.mp. 23 neosar*.mp. 24 ctx*.mp. 25 cisplatin*.mp. 26 platinol*.mp. 27 cddp*.mp. 28 carboplatin*.mp. 29 paraplatin*.mp. 30 cbdca*.mp. 31 ifosfamide*.mp. 32 ifex*.mp.] 33 isophosphamide*.mp. 34 paclitaxel*.mp. 35 taxol*.mp. 36 taxane*.mp. 37 or/7‐36 38 6 and 37 39 randomized controlled trial.pt. 40 controlled clinical trial.pt. 41 randomized.ab. 42 placebo.ab. 43 drug therapy.fs. 44 randomly.ab. 45 trial.ab. 46 groups.ab. 47 or/39‐46 48 38 and 47 49 (animals not (humans and animals)).sh. 50 48 not 49
key: mp = title, original title, abstract, name of substance word, subject heading word fs = floating subheading ab = abstract pt = publication type sh = subject heading
Appendix 3. EMBASE search strategy
Embase Ovid
1 exp endometrium tumor/ 2 (endometr* adj5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinom* or adenocarcinom*)).mp. 3 (uter* and lining and (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinom* or adenocarcinom*)).mp. 4 1 or 2 or 3 5 (advanced or metasta* or recurren*).mp. 6 4 and 5 7 dt.fs. 8 chemotherap*.mp. 9 exp antineoplastic agent/ 10 cytotoxic*.mp. 11 doxorubicin*.mp. 12 adriamycin*.mp. 13 doxil*.mp. 14 rubex*.mp. 15 epirubicin*.mp. 16 pharm?rubicin*.mp. 17 ellence*.mp. 18 hydroxydoxorubicin*.mp. 19 hydroxydaunorubicin*.mp. 20 cyclophosphamide*.mp. 21 cytoxan*.mp. 22 neosar*.mp. 23 ctx*.mp. 24 cisplatin*.mp. 25 platinol*.mp. 26 cddp*.mp. 27 carboplatin*.mp. 28 paraplatin*.mp. 29 cbdca*.mp. 30 ifosfamide*.mp. 31 ifex*.mp. 32 isophosphamide*.mp. 33 paclitaxel*.mp. 34 taxol*.mp. 35 taxane*.mp. 36 or/7‐35 37 6 and 36 38 exp controlled clinical trial/ 39 randomized.ab. 40 placebo.ab. 41 dt.fs. 42 randomly.ab. 43 trial.ab. 44 groups.ab.# 45 or/38‐44 46 37 and 45
key: mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name fs = floating subheading ab = abstract
Data and analyses
Comparison 1. More versus less chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 8 | 1519 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.77, 0.96] |
| 1.1 Cisplatin in combination versus cisplatin alone | 1 | 30 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.36, 1.58] |
| 1.2 Doxorubicin in combination versus doxorubicin alone | 3 | 814 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.77, 1.03] |
| 1.3 Doxorubicin + cisplatin plus other drugs versus doxorubicin + cisplatin | 2 | 291 | Hazard Ratio (Fixed, 95% CI) | 0.75 [0.58, 0.97] |
| 1.4 Other 3‐drug combination versus other 2‐drug combination | 2 | 384 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.71, 1.08] |
| 2 Progression‐free survival | 8 | 1526 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 2.1 Cisplatin in combination versus cisplatin alone | 1 | 30 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.37, 1.92] |
| 2.2 Doxorubicin in combination versus doxorubicin | 3 | 821 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.74, 0.97] |
| 2.3 Doxorubicin + cisplatin plus other drugs versus doxorubicin + cisplatin | 2 | 291 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.49, 0.82] |
| 2.4 Other 3‐drug combination versus other 2‐drug combination | 2 | 384 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 3 Grade 3‐4 nausea/vomiting | 5 | 761 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.64 [1.71, 4.09] |
| 3.1 Cisplatin in combination versus cisplatin | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.17, 4.13] |
| 3.2 Doxorubicin in combination versus doxorubicin | 2 | 443 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.95 [2.27, 6.89] |
| 3.3 Doxorubicin + cisplatin plus other drugs versus doxorubicin + cisplatin | 2 | 288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.56 [0.71, 3.42] |
| 3.4 Other 3‐drug combination versus other 2‐drug combination | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Grade 3‐4 diarrhoea/other GI | 6 | 873 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.25 [1.09, 4.63] |
| 4.1 Cisplatin in combination versus cisplatin | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.52 [0.13, 331.51] |
| 4.2 Doxorubicin in combination versus doxorubicin | 2 | 443 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.36 [0.83, 6.68] |
| 4.3 Doxorubicin/cisplatin plus other drugs versus doxorubicin/cisplatin | 2 | 288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.44 [0.84, 7.12] |
| 4.4 Other 3‐drug combination versus other 2‐drug combinations | 1 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 5 Grade 3‐4 white blood cell toxicity | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Cisplatin in combination versus cisplatin | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.28 [0.76, 14.19] |
| 5.2 Doxorubicin in combination versus doxorubicin | 2 | 443 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.51 [1.73, 3.65] |
| 5.3 Doxorubicin/cisplatin plus other drugs versus doxorubicin/cisplatin | 2 | 288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.29, 0.72] |
| 5.4 Other 3‐drug combination versus other 2‐drug combinations | 2 | 397 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.33, 0.78] |
| 6 Grade 3‐4 thrombocytopenia | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cisplatin in combination versus cisplatin | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.00, 5.96] |
| 6.2 Doxorubicin in combination versus doxorubicin | 2 | 443 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.12 [2.08, 8.14] |
| 6.3 Doxorubicin/cisplatin plus other drugs versus doxorubicin/cisplatin | 2 | 288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.65 [2.60, 12.28] |
| 6.4 Other 3‐drug combination versus other 2‐drug combinations | 1 | 266 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.05, 0.29] |
| 7 Grade 3‐4 anaemia | 4 | 596 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.32 [2.62, 10.81] |
| 7.1 Cisplatin in combination versus cisplatin | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Doxorubicin in combination versus doxorubicin | 1 | 278 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.32 [2.62, 10.81] |
| 7.3 Doxorubicin/cisplatin plus other drugs versus doxorubicin/cisplatin | 2 | 288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Other 3‐drug combination versus other 2‐drug combinations | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Grade 3‐4 alopecia | 5 | 595 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.86 [1.01, 3.42] |
| 8.1 Cisplatin in combination versus cisplatin | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.63 [2.01, 56.35] |
| 8.2 Doxorubicin in combination versus doxorubicin | 1 | 165 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.74, 2.74] |
| 8.3 Doxorubicin/cisplatin plus other drugs versus doxorubicin/cisplatin | 2 | 288 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Other 3‐drug combination versus other 2‐drug combinations | 1 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Chemotherapy doublet versus chemotherapy doublet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival | 4 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Doxorubicin + cisplatin (circadian timed) versus doxorubicin + cisplatin (standard timed) | 1 | 342 | Hazard Ratio (Fixed, 95% CI) | 1.07 [0.86, 1.34] |
| 1.2 Doxorubicin + paclitaxel (plus filgrastin) versus doxorubicin + cisplatin | 1 | 317 | Hazard Ratio (Fixed, 95% CI) | 1.0 [0.78, 1.28] |
| 1.3 Docetaxel + cisplatin versus docetaxel + carboplatin | 1 | 59 | Hazard Ratio (Fixed, 95% CI) | 0.63 [0.28, 1.42] |
| 1.4 Paclitaxel + carboplatin versus docetaxel + carboplatin | 1 | 58 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.48, 2.11] |
| 2 Progression‐free survival | 4 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Doxorubicin+ cisplatin (circadian timed) versus doxorubicin/cisplatin (standard timed) | 1 | 342 | Hazard Ratio (Fixed, 95% CI) | 1.06 [0.85, 1.32] |
| 2.2 Doxorubicin + paclitaxel (with filgrastin) versus doxorubicin + cisplatin | 1 | 317 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.80, 1.28] |
| 2.3 Docetaxel + cisplatin versus docetaxel + carboplatin | 1 | 58 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.46, 1.49] |
| 2.4 Paclitaxel + carboplatin versus docetaxel + carboplatin | 1 | 59 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.49, 1.67] |
Comparison 3. Single‐agent chemotherapy versus other single‐agent chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Ifosfamide versus cyclophosphamide | 1 | 74 | Hazard Ratio (Fixed, 95% CI) | 1.49 [0.74, 3.01] |
| 2 Progression‐free survival | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Ifosamide versus cyclophosphamide | 1 | 74 | Hazard Ratio (Fixed, 95% CI) | 1.12 [0.67, 1.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aapro 2003.
| Methods | Randomised Phase II/III trial 1988 to 1994 | |
| Participants | 177 women with histologically confirmed advanced or recurrent adenocarcinoma of the endometrium not thought to be suitable for radiotherapy or endocrine therapy. No prior chemotherapy, radiotherapy or hormone therapy in previous 4 weeks. WHO performance status ≤2 | |
| Interventions | Arm 1: doxorubicin 60 mg/m2 IV every 28 days Arm 2: doxorubicin 60 mg/m2 IV, cisplatin 50 mg/m2 IV every 28 days | |
| Outcomes | OS Progression‐free interval Response Acute toxicity (WHO) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "During randomisation, patients were stratified according to institute, differentiation, type of disease and performance status using the minimisation technique" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 ineligible patients (7% of total randomised). Balanced by treatment arm. However, all patients were included in the analyses of efficacy |
| Selective reporting (reporting bias) | Low risk | No protocol available, but all outcomes of interest to review reported. Note: trial reports that PFS will be analysed; however, progression‐free interval is reported |
Cohen 1984.
| Methods | Prospective randomised trial 1977 to 1979 | |
| Participants | 295 women with primary stage III, stage IV or recurrent or residual endometrial adenocarcinoma incurable by radiotherapy or surgery. No prior chemotherapy | |
| Interventions | Arm 1: melphalan (7 mg/m2/day PO) plus 5‐FU (525 mg/m2/day IV) for 4 days, every 28 days, plus megace (180 mg/day PO), daily for 8 weeks Arm 2: doxorubicin (40 mg/m2 IV bolus), cyclophosphamide (400 mg/m2 IV bolus), 5‐FU (400 mg/m2 IV bolus) every 21 days, plus megace (180 mg/day orally) daily for 8 weeks | |
| Outcomes | OS Progression‐free interval Response Acute toxicity (GOG) |
|
| Notes | 63 patients assigned non‐randomly to arm 1 and analysed separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised... stratified on the basis of performance status, previous progestational therapy, measurable disease and stage" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 38 patients excluded (13% of total randomised) from the analysis. Exclusions balanced by treatment arm. Main reason in both arms was errors either in pathology or administration |
| Selective reporting (reporting bias) | Low risk | No protocol available; however, trial reports all outcomes of interest to the review (note: progression‐free interval not PFS) |
Edmonson 1987.
| Methods | Randomised Phase II study 1980 to 1983 | |
| Participants | 30 women with histologically confirmed, objectively evaluable endometrial carcinoma, refractory to progestin treatment. No prior chemotherapy. ECOG performance status ≤ 3 | |
| Interventions | Arm 1: cisplatin (60 mg/m2) IV every 21 days until progression Arm 2: cisplatin (40 mg/m2) IV, cyclophosphamide (400 mg/m2) IV, doxorubicin (40 mg/m2) IV every 28 days until progression | |
| Outcomes | OS PFS Response Acute toxicity (NCI CTC v1) |
|
| Notes | No excluded patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "assigned at random" and "stratified by PS [performance status], histology and stage" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in analyses supplied by investigator for use in this review |
| Selective reporting (reporting bias) | Low risk | Insufficient detail in trial report, but all outcomes of interest were supplied by the investigator |
Fleming 2004a.
| Methods | Randomised Phase III trial 1998 to 2000 | |
| Participants | 273 women with advance or recurrent endometrial cancer. No prior chemotherapy | |
| Interventions | Arm 1: doxorubicin (60 mg/m2) IV, cisplatin (50 mg/m2) IV every 21 days for 7 cycles Arm 2: doxorubicin (45 mg/m2) IV, cisplatin (50 mg/m2) IV, paclitaxel (160 mg/m2) IV every 21 days for 7 cycles plus G‐CSF support | |
| Outcomes | OS PFS Response Acute toxicity (NCI‐CTC v2) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned with equal probability of assignment to each treatment arm" |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation ‐ sequence concealed from institutes and patients |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 ineligible patients (4% or total randomised) excluded from the analyses, slightly imbalanced by arm but low numbers overall (7 vs 3) |
| Selective reporting (reporting bias) | Low risk | No protocol available but trial reports all outcomes of interest to the review (response, PFS and OS) |
Fleming 2004b.
| Methods | Randomised Phase III trial 1996 to 1998 | |
| Participants | 328 patients with primary stage III, stage IV or recurrent endometrial carcinoma. Prior hormone therapy permitted, but no prior chemotherapy. GOG performance status 0 to 2 | |
| Interventions | Arm 1: doxorubicin (60 mg/m2) IV, cisplatin (50 mg/m2) IV every 21 days for 7 cycles (max) or until progression or unacceptable toxicity Arm 2: doxorubicin (50 mg/m2) IV, paclitaxel (150 mg/m2) IV, filigastrim (5 μg/kg) day 3 to day 12 every 21 days for 7 cycles (max) or until progression or unacceptable toxicity | |
| Outcomes | OS PFS Response Acute toxicity (GOG) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation with equal probabilities balancing the sequence of assigned regimens within institutes and strata" |
| Allocation concealment (selection bias) | Low risk | Central allocation, with concealment of the treatment sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 ineligible patients (3% of total randomised) were excluded from the analyses. Slightly imbalance by arm (3 vs 8) but low numbers overall |
| Selective reporting (reporting bias) | Low risk | No protocol available but trial reports all outcomes of interest to the review (response, PFS and OS) |
Gallion 2003.
| Methods | Randomised Phase III trial 1993 to 1996 | |
| Participants | 352 women with histologically documented; primary stage II, IV or recurrent endometrial carcinoma. Prior hormonal therapy or biological response modifiers permitted, but had not received prior cytotoxic chemotherapy, more than 1 biological therapy or prior radiotherapy in previous 3 months | |
| Interventions | Arm 1: (standard time) doxorubicin (60 mg/m2) IV, cisplatin (60 mg/m2) IV every 21 days for 8 cycles Arm 2: (circadian time) doxorubicin (60 mg/m2) IV at 6 a.m., cisplatin (60 mg/m2) IV at 6 p.m. every 21 days for 8 cycles | |
| Outcomes | OS PFS Response Acute toxicity (GOG) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block design: computerised random number generator with a block length of 4 assignments |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation. Sequence of treatment assignments was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 ineligible patients (3% of the total randomised) excluded from the analyses, balanced by arm 1 |
| Selective reporting (reporting bias) | Low risk | No protocol available but trial reports all outcomes of interest to the review (response, PFS and OS) |
Horton 1978.
| Methods | Randomised Phase III trial 1974 to ? | |
| Participants | 47 women with histologically confirmed endometrial carcinoma with local extension or metastasis not amenable to cure and failed prior progestational therapy. No prior chemotherapy | |
| Interventions | Arm 1: cyclophosphamide (666 mg/m2) IV bolus every 21 days until progression Arm 2: doxorubicin (50 mg/m2) IV bolus every 21 days until max dose of 550 mg/m2 or progression |
|
| Outcomes | Survival Response Acute toxicity |
|
| Notes | Insufficient outcome data available to include this trial in the analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Central office |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7 patients (15%) were randomised but were excluded from the analysis for reasons of ineligibility (n = 3) or because no data were available (n = 4). It is unclear whether they received treatment |
| Selective reporting (reporting bias) | High risk | No protocol available. Trial reports median survival according to performance status only. OS and PFS not reported. Toxicity and response are reported. No data available from the trial investigators |
Horton 1982.
| Methods | Randomised trial 1977 to 1979 |
|
| Participants | 131 women with histologically confirmed stage III or IV recurrent or metastatic adenocarcinoma, not amenable to standard surgery or RT. Prior progestational treatment allowed | |
| Interventions | Arm 1: megestrol (3 x 80 mg/day PO); cyclophosphamide (400 mg/m2) and doxorubicin (40 mg/m2) IV bolus every 28 days Arm 2: megestrol (3 x 80 mg/day PO), cyclophosphamide (250 mg/m2) and doxorubicin (30 mg/m2) once, and 5‐FU 300 mg/m2 daily for 3 days (IV bolus) every 28 days |
|
| Outcomes | OS PFS Response Acute toxicity (ECOG scale) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly permuted blocks, within strata, balanced by institution" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients excluded from the analyses supplied for use in this review |
| Selective reporting (reporting bias) | Low risk | Paper reports detail insufficient for inclusion in analyses; however, investigator supplied all outcomes on request |
Long 2006.
| Methods | Randomised prospective trial 1992 to 1993 |
|
| Participants | 28 women with advanced, recurrent or metastatic endometrial carcinoma | |
| Interventions | Arm 1: doxorubicin (30 mg/m2), cisplatin (70 mg/m2) IV every 28 days for max 4 cycles Arm 2: methotrexate (30 mg/m2) IV, vinblastine (3 mg/m2) IV days 1, 15 and 22, plus doxorubicin (30 mg/m2) and cisplatin (70 mg/m2) day 2, every 28 days for max 4 cycles |
|
| Outcomes | Survival Progression‐free interval Response Toxicity (NCI‐CTC, v1) |
|
| Notes | "Due to slow accrual and termination of the Gynecological Program in the NCCTG, this trial was closed prior to completion of its accrual objectives." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dynamic balancing |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients excluded from analyses supplied by investigator for use in this review |
| Selective reporting (reporting bias) | Low risk | Trial reports response, PFS, OS and toxicity. Full details for all outcomes supplied by the investigator for use in this review |
Nomura 2010a.
| Methods | Randomised Phase II trial 2003 to 2005 |
|
| Participants | 90 women* with advanced, metastatic or recurrent endometrial carcinoma | |
| Interventions | Arm 1: docetaxel (70 mg/m2) IV and carboplatin (AUC = 6) every 21 days Arm 2: docetaxel (70 mg/m2) IV and cisplatin (60 mg/m2) IV every 21 days All regimen to be given until progression or adverse advents prohibit further treatment |
|
| Outcomes | OS PFS Response Acute toxicity (NCI‐CTC, v2) |
|
| Notes | 2 arms of a 3‐arm study with Nomura 2010b. *90 women randomised across all 3 arms, 30 per arm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation, stratified by prior taxane therapy and measurable lesions |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients (3% of total randomised) were excluded after randomisation and were not included in the analyses |
| Selective reporting (reporting bias) | Low risk | Trial reports response, adverse events, treatment completion rate and PFS only. OS and PFS supplied by authors upon request to enable analysis as two separate treatment comparisons within this review |
Nomura 2010b.
| Methods | Randomised Phase II trial 2003 to 2005 |
|
| Participants | 90 women* with advanced, metastatic or recurrent endometrial carcinoma | |
| Interventions | Arm 2: docetaxel (70 mg/m2) IV and cisplatin (60 mg/m2) IV every 21 days Arm 3: paclitaxel 180 mg/m2 and carboplatin (AUC = 6) IV on day 1, every 21 days All regimen to be given until progression or adverse advents prohibit further treatment |
|
| Outcomes | OS PFS Response Acute toxicity (NCI‐CTC, v2) |
|
| Notes | 2 arms of a 3‐arm study with Nomura 2010a. *90 women randomised across all 3 arms, 30 per arm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation, stratified by prior taxane therapy and measurable lesions |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient (2% of total randomised) was excluded after randomisation and were not included in the analyses |
| Selective reporting (reporting bias) | Low risk | Trial reports response, adverse events, treatment completion rate and PFS only. OS and PFS supplied by authors upon request to enable analysis as two separate treatment comparisons within this review |
Pawinski 1999.
| Methods | Randomised Phase II study 1987 to 1994 | |
| Participants | 74 women with recurrent or metastatic histologically confirmed endometrial carcinoma. Prior chemotherapy permitted, but not the study drugs | |
| Interventions | Arm 1: ifosfamide (5 g/m2) IV every 21 days until progression Arm 2: cyclophosphamide (1200 mg/m2) IV every 21 days until progression |
|
| Outcomes | OS Progression‐free interval Response rate Acute toxicity (GOG) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly given" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 patients (18% of the total randomised) were excluded from the analysis for reasons of ineligibility. It is unclear whether they received treatment; however, full ITT analyses were provided by investigators for this review |
| Selective reporting (reporting bias) | Low risk | Trial reports response rates and toxicity. Deaths and progressions were collected but not reported; however, survival and PFSl results were provided by the investigators upon request for use in this review |
Thigpen 1994.
| Methods | Randomised Phase III trial 1979 to 1985 | |
| Participants | 387 women with histologically documented advanced or recurrent endometrial adenocarcinoma, adenoacanthoma or adenosquamous carcinoma no longer amenable to control with surgery or radiotherapy; no prior chemotherapy | |
| Interventions | Arm 1: doxorubicin (60 mg/m2) IV every 21 days for 8 cycles Arm 2: doxorubicin (60 mg/m2) IV, cyclophosphamide (500 mg/m2) IV every 21 days for 8 cycles | |
| Outcomes | OS Progression‐free interval Response Acute toxicity (GOG) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31 patients (8% of the total randomised) were excluded from the analysis for reasons of ineligibility. 15 were excluded prior to treatment (8 from arm 1 and 15 from arm 2); 16 were treated and subsequently excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | No protocol available but trial reports all outcomes of interest to the review (note: progression‐free interval reported and not PFS) |
Thigpen 2004.
| Methods | Randomised Phase III trial 1988 to 1992 | |
| Participants | 299 women with advanced or recurrent endometrial carcinoma, no prior chemotherapy | |
| Interventions | Arm 1: doxorubicin (60 mg/m2) IV every 21 days for 8 cycles Arm 2: doxorubicin (60 mg/m2) IV, cisplatin (50 mg/m2) IV every 21 days for 8 cycles | |
| Outcomes | Survival PFS Response Acute toxicity (GOG) |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequentially drawn from pre‐allocated lists of treatments, randomly permuted and balanced within blocks" |
| Allocation concealment (selection bias) | Low risk | "All patients were registered centrally. The randomised treatment was not revealed until registration was complete" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 ineligible patients were randomised but excluded from the analyses (6% of the total randomised). Also, 3 not receiving the study agent excluded from the toxicity analysis |
| Selective reporting (reporting bias) | Low risk | Protocol not available but trial reports all outcomes of interest to the review |
Weber 2003.
| Methods | Randomised Phase II trial 1997 to 2002 |
|
| Participants | 70 women with recurrent or advanced endometrial carcinoma | |
| Interventions | Arm 1: doxorubicin (60 mg/m2) and cisplatin (50 mg/m2) every 21 days for 6 cycles Arm 2: carboplatin (AUC = 5) and paclitaxel (175 mg/m2) every 21 days for 6 cycles |
|
| Outcomes | OS PFS Response Acute Toxicity |
|
| Notes | Insufficient outcome data available to include this trial in the analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment not blinded, but for primary outcome of OS, unlikely to introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients excluded from analysis |
| Selective reporting (reporting bias) | High risk | No protocol available. Reported as an abstract only with insufficient detail for inclusion in review |
5‐FU: 5‐fluorouracil; AUC: area under curve; ECOG: Eastern Cooperative Oncology Group; G‐CSF: granulocyte colony‐stimulating factor; GOG: Gynecologic Oncology Group; ITT: intention to treat; IV: intravenous; max: maximum; NCCTG: North Central Cancer Treatment Group; NCI CTC: National Cancer Institute Common Toxicity Criteria; OS: overall survival; PFS: progression‐free survival; PO, orally; WHO: World Health Organization.
Characteristics of ongoing studies [ordered by study ID]
NCT00052312.
| Trial name or title | Doxorubicin and Cisplatin With or Without Paclitaxel in Treating Patients With Locally Advanced, Metastatic, and/or Relapsed Endometrial Cancer |
| Methods | Randomised Phase II trial |
| Participants | Patients With Locally Advanced, Metastatic, and/or Relapsed Endometrial Cancer |
| Interventions | Doxorubicin, paclitaxel and cisplatin versus doxorubicin and cisplatin |
| Outcomes | Progression free survival; overall survival and toxicity |
| Starting date | September 2002 |
| Contact information | Nicholas S. Reed, MD University of Glasgow |
| Notes |
NCT00063999.
| Trial name or title | Combination Chemotherapy in Treating Patients With Stage III, Stage IV, or Recurrent Endometrial Cancer |
| Methods | Randomized Phase III Trial |
| Participants | Patients with stage III, stage IV, or recurrent endometrial cancer. |
| Interventions | Doxorubicin, cisplatin, paclitaxel and filgrastim (G‐CSF) versus paclitaxel and carboplatin |
| Outcomes | Overall survival, Progression free survival |
| Starting date | August 2003 |
| Contact information | Philip J. DiSaia, Gynecologic Oncology Group |
| Notes |
Contributions of authors
CV appraised the updated search results for relevant eligible trials, extracted data from published trials, completed the final analyses of all outcomes and updated the text of the review for this update.
JT prepared the data extraction forms, extracted data and obtained unpublished data from investigators for the original review that was also used in this update. She also advised on the conduct of the analyses for this update, helped with interpretation of the results and preparation of the final manuscript.
SB appraised the updated search results for relevant eligible trials, assisted with data extraction, sought additional unpublished summary data from investigators and conducted preliminary survival and PFS analyses for this updated review.
PS helped with the interpretation of the results and with the preparation of the final manuscript.
Sources of support
Internal sources
Medical Research Council, UK.
NHS R&D, UK.
Macmillan Cancer Relief, UK.
University of Liverpool, UK.
University of Leicester, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Aapro 2003 {published data only}
- Aapro M, Bolis G, Chevallier B, Burg MEL, Poveda A, Oliveira C, et al. An EORTC‐GCCG randomized phase II trial of doxorubicin versus dox‐cisplatin (cDDP) in endometrial carcinoma. Proceedings of the Annual Meeting of the American Society of Clinical Oncology. 1994; Vol. 13, issue Abstract 885:275.
- Aapro MS, Wijk FH, Bolis G, Chevallier B, Burg MEL, Poveda A, et al on behalf of the EORTC‐GCCG. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC‐GCCG. Annals of Oncology 2003;14:441‐8. [DOI] [PubMed] [Google Scholar]
Cohen 1984 {published and unpublished data}
- Cohen CJ, Bruckner HW, Deppe G, Blessing JA, Homesley H, Lee JH, et al. Multidrug treatment of advanced and recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Obstetrics and Gynecology 1984;63(5):719‐26. [PubMed] [Google Scholar]
Edmonson 1987 {published and unpublished data}
- Edmonson JH, Krook JE, Hilton JF, Malkasian GD, Everson LK, Jefferies JA, et al. Randomized phase II studies of cisplatin and a combination of cyclophosphamide‐doxorubicin‐cisplatin (CAP) in patients with progestin‐refractory advanced endometrial carcinoma. Gynecologic Oncology 1987;28(1):20‐4. [DOI] [PubMed] [Google Scholar]
Fleming 2004a {published data only}
- Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2004;22(11):2159‐66. [DOI] [PubMed] [Google Scholar]
- Fleming GF, Brunetto VL, Mundt AJ, Burks RT, Look KY, Reid G. Randomised trial of doxorubicin (DOX) plus cisplatin (CIS) versus DOX plus CIS plus paclitaxel (TAX) in patients with advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group (GOG) study. Proceedings of American Society of Clinical Oncology. 2002; Vol. 21:202a Abstract 807.
Fleming 2004b {published data only}
- Fleming GF, Filiaci VL, Bentley RC, Herzog T, Vaccarello L, Gallion H. Phase III randomised trial of doxorubicin + cisplatin versus doxorubicin + 24‐h paclitaxel + filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Annals of Oncology 2004;15:1173‐8. [DOI] [PubMed] [Google Scholar]
Gallion 2003 {published data only}
- Gallion HH, Brunetto VL, Cibull M, Lentz SS, Reid G, Soper JT, et al. Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrence endometrial carcinoma: a Gynecologic Oncology Group study. Journal of Clinical Oncology 2003;21(20):3808‐13. [DOI] [PubMed] [Google Scholar]
Horton 1978 {published data only}
- Horton J, Begg CB, Arseneault J, Bruckner H, Creech R, Hahn RG. Comparison of adriamycin with cyclophosphamide in patients with advanced endometrial cancer. Cancer Treatment Reports 1978;62(1):159‐61. [PubMed] [Google Scholar]
Horton 1982 {published and unpublished data}
- Horton J, Elson P, Gordon P, Hahn R, Creech R. Combination chemotherapy for advanced endometrial cancer. An evaluation of three regimens. Cancer 1982;49:2241‐5. [DOI] [PubMed] [Google Scholar]
Long 2006 {published data only}
- Long HJ, Nelimark RA, Cha SS. Comparison of methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) vs doxorubicin and cisplatin (AC) in advanced endometrial carcinoma. Proceedings of the Annual Meeting of the American Society of Clinical Oncology. 1995; Vol. 14:282 Abstract 797.
- Long III H, Nelimark R, Podratz K, Suman V, Keeney G, Nikcevich D, et al. Phase III comparison of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) vs. doxorubicin and cisplatin (AC) in women with advanced primary or recurrent metastatic carcinoma of the uterine endometrium. Gynecologic Oncology 2006;100:501‐5. [DOI] [PubMed] [Google Scholar]
Nomura 2010a {published and unpublished data}
- Nomura H, Aoki D, Takahashi F, Katsumata N, Watanabe Y, Konishi I, et al. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: Japanese Gynecologic Oncology Group trial (JGOG2041). Journal of Clinical Oncology (ASCO Meeting Abstracts). 2008; Vol. 26:16526. [DOI] [PubMed]
- Nomura H, Aoki D, Takahashi F, Katsumata N, Watanabe Y, Konishi I, et al. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: a Japanese Gynecologic Oncology Group study (JGOG2041). Annals of Oncology 2011;22(3):636‐42. [DOI] [PubMed] [Google Scholar]
Nomura 2010b {published and unpublished data}
- Nomura H, Aoki D, Takahashi F, Katsumata N, Watanabe Y, Konishi I, et al. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: Japanese Gynecologic Oncology Group trial (JGOG2041). Journal of Clinical Oncology (ASCO Meeting Abstracts). 2008; Vol. 26:16526. [DOI] [PubMed]
- Nomura H, Aoki D, Takahashi F, Katsumata N, Watanabe Y, Konishi I, et al. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: a Japanese Gynecologic Oncology Group study (JGOG2041). Annals of Oncology 2011;22(3):636‐42. [DOI] [PubMed] [Google Scholar]
Pawinski 1999 {published and unpublished data}
- Pawinski A, Tumolo S, Hoesel G, Cervantes A, Oosterom AT, Hoctin Boes G, et al. Cyclophosphamide or ifosfamide in patients with advanced and/or recurrent endometrial carcinoma: a randomised phase II study of the EORTC Gynecological Cancer Cooperative Group. European Journal of Obstetrics and Gynecology and Reproductive Biology 1999;86:179‐83. [DOI] [PubMed] [Google Scholar]
Thigpen 1994 {published data only}
- Thigpen J, Blessing P, DiSaia P, Ehrlich C for the Gynecologic Oncology Group. A randomized comparison of adriamycin with or without cyclophosphamide in the treatment of advanced or recurrent endometrial carcinoma. Proceedings of the American Society of Clinical Oncology. 1985:115 Abstract C‐448.
- Thigpen JT, Blessing JA, DiSaia PJ, Yordan E, Carson LF, Evers C. A randomized comparison of doxorubicin alone versus doxorubicin plus cyclophosphamide in the management of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Journal of Clinical Oncology 1994;12(7):1408‐14. [DOI] [PubMed] [Google Scholar]
Thigpen 2004 {published data only}
- Thigpen JT, Brady MF, Homesley, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a Gynecologic Oncology Group Study (GOG). Journal of Clinical Oncology 2004;22(19):3902‐8. [DOI] [PubMed] [Google Scholar]
- Thigpen T, Blessing J, Homesley H, Malfetano J, DiSaia P, Yordan E. Phase III trial of doxorubicin ± cisplatin in advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group (GOG) study. Proceedings of the American Society of Clinical Oncology. 1993; Vol. 12:261 Abstract 830.
Weber 2003 {published data only}
- Weber B, Mayer F, Bougnoux P, Lesimple T, Joly F, Fabbro M, et al. What is the best chemotherapy regimen in recurrent or advanced endometrial carcinoma? Preliminary results. Proceedings of the American Society of Clinical Oncology. 2003; Vol. 22:Abstract 1819.
References to ongoing studies
NCT00052312 {unpublished data only}
- Reed NS (Investigator). Doxorubicin and cisplatin with or without paclitaxel in treating patients with locally advanced, metastatic and/or relapsed endometrial cancer. http://clinicaltrials.gov/ct2/show/record/NCT00052312.
NCT00063999 {unpublished data only}
- Miller D (Protocol chair). Randomized phase III trial of doxorubicin/cisplatin/paclitaxel and G‐CSF versus carboplatin/paclitaxel in patients with stage III and IV or recurrent endometrial cancer. http://clinicaltrials.gov/ct/show/NCT0006399.
Additional references
Bremond 2001
- Bremond A, Bataillard A, Thomas L, Achard JL, Fervers B, Fondrinier E, et al. Cancer of the endometrium. British Journal of Cancer 2001;84(supple 2):31‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burke 1990
- Burke TW, Heller PB, Woodward J, Davidson SA, Hoskins WJ, Park RC, et al. Treatment failure in endometrial carcinoma. Obstetrics and Gynaecology 1990;75:96‐101. [PubMed] [Google Scholar]
Cullen 1999
- Cullen MH, Woodroffe CM, Billingham LJ, Chetiyarwardana AD, Joshi R, Ferry D, et al. Mitomycin, ifosfamide and cisplatin (MIC) in unresectable non‐small cell lung cancer: effects on survival and quality of life. Journal of Clinical Oncology 1999;17(10):3188‐94. [DOI] [PubMed] [Google Scholar]
EBCTCG 1998
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. The Lancet 1998;351:1451‐67. [PubMed] [Google Scholar]
Faust 2010
- Faust G, Davies Q, Symonds P. Changes in the treatment of endometrial cancer. British Journal of Obstetrics and Gynaecology 2010;117:1043‐6. [DOI] [PubMed] [Google Scholar]
FIGO 2009
- Creasman W. Revised FIGO staging for carcinoma of the endometrium. International Journal of Gynaecology and Obstetrics 2009;105(2):109. [DOI] [PubMed] [Google Scholar]
Jemal 2011
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA: Cancer Journal for Clinicians 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
Johnson 2011
- Johnson N, Bryant A, Miles T, Hogberg T, Cornes P. Adjuvant chemotherapy for endometrial cancer after hysterectomy. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD003175.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kokka 2010
- Kokka F, Brockbank E, Oram D, Gallagher C, Bryant A. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD007926.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1981
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207‐14. [DOI] [PubMed] [Google Scholar]
Morris 1996
- Morris M, Alvarez RD, Kinney WK, Wilson TO. Treatment of recurrent adenocarcinoma of the endometrium with pelvic exenteration. Gynecologic Oncology 1996;60:288. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Progress in Cardiovascular Diseases 1985;27(5):335‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Humber 2005
- Humber CE, Tierney J, Symonds PR, Collingwood M, Kirwan JJ, Williams C, et al. Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003175.pub2] [DOI] [PubMed] [Google Scholar]
Humber 2007
- Humber CE, Tierney JF, Symonds RP, Collingwood M, Kirwan J, Williams C, et al. Chemotherapy for advanced, recurrent or metastatic endometrial cancer: a systematic review of Cochrane collaboration. Annals of Oncology 2007;18(3):409‐20. [DOI] [PubMed] [Google Scholar]


